Study on the Thermal Stabilizing Process of Layered Double Hydroxides in PVC Resin
Abstract
1. Introduction
2. Results and Discussion
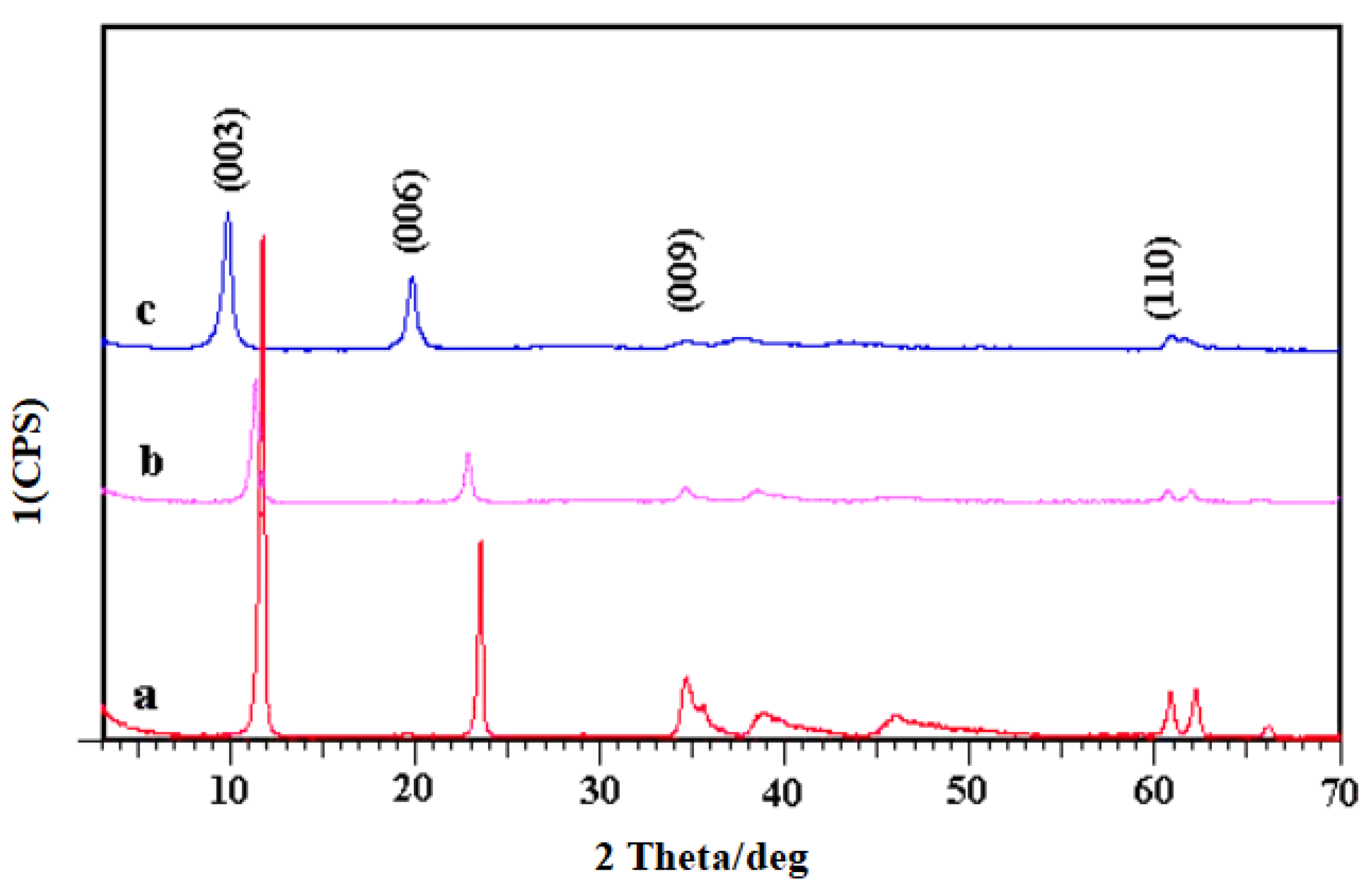
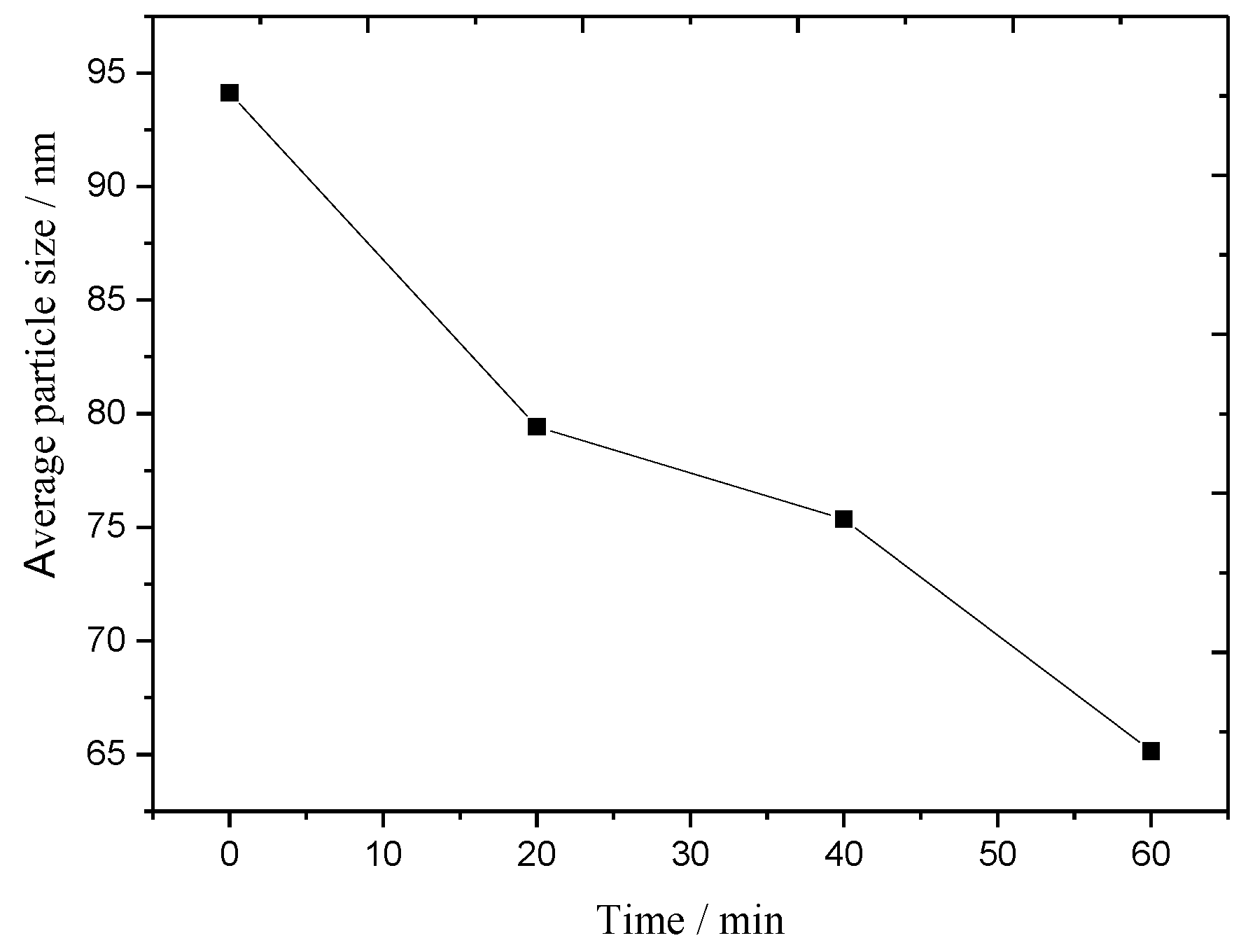
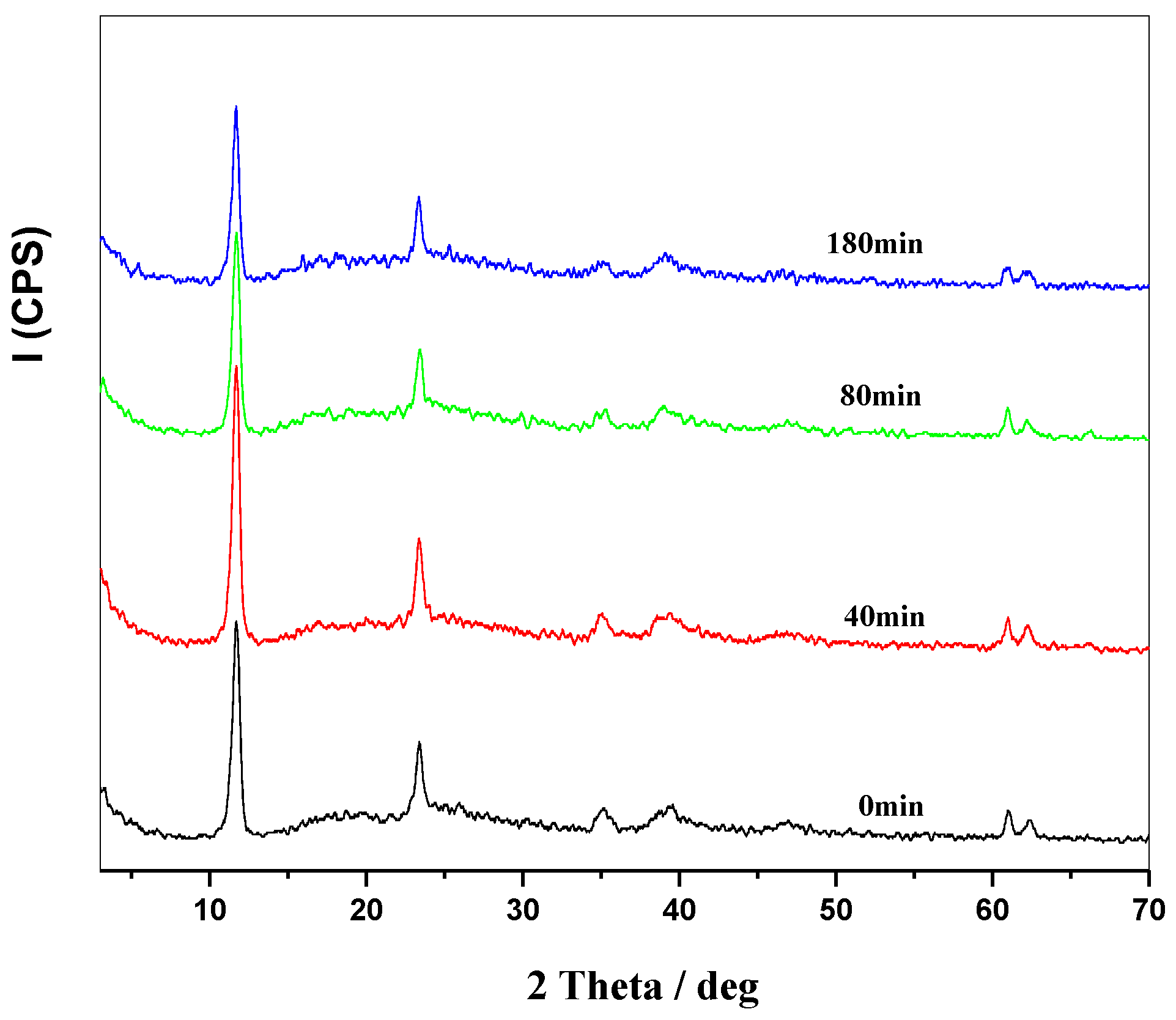
2.1. Structure of MgAl-LDHs
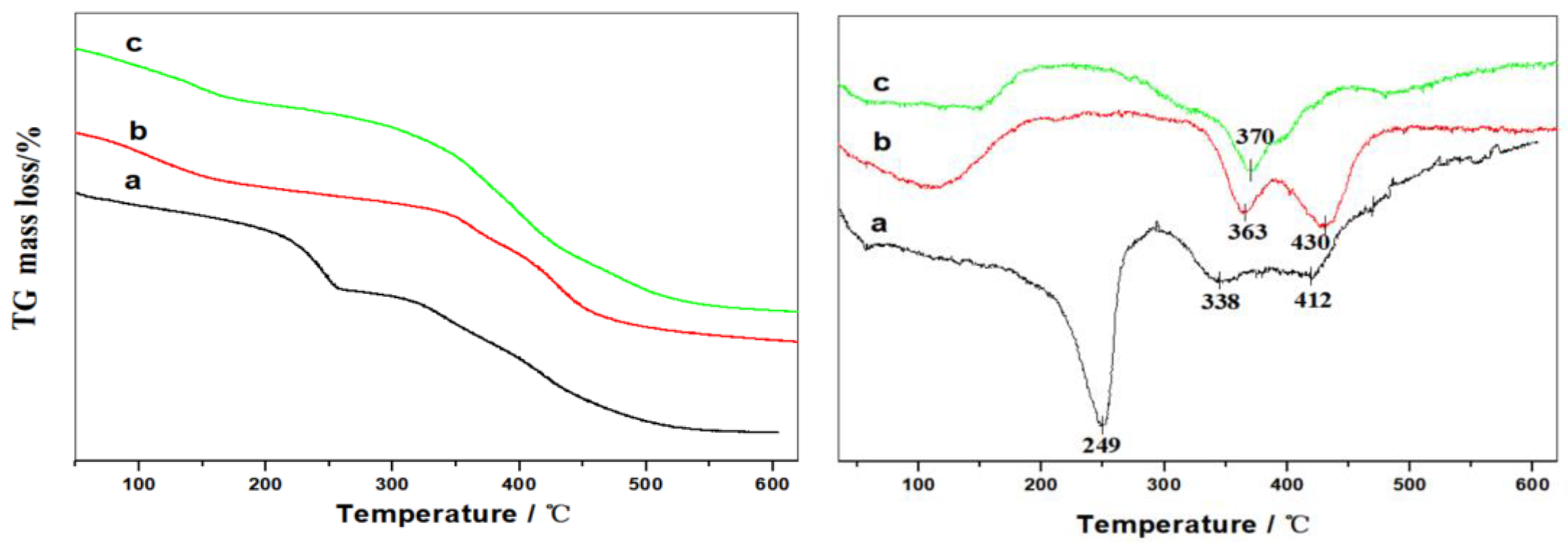
2.2. Thermal Analysis of MgAl-LDHs
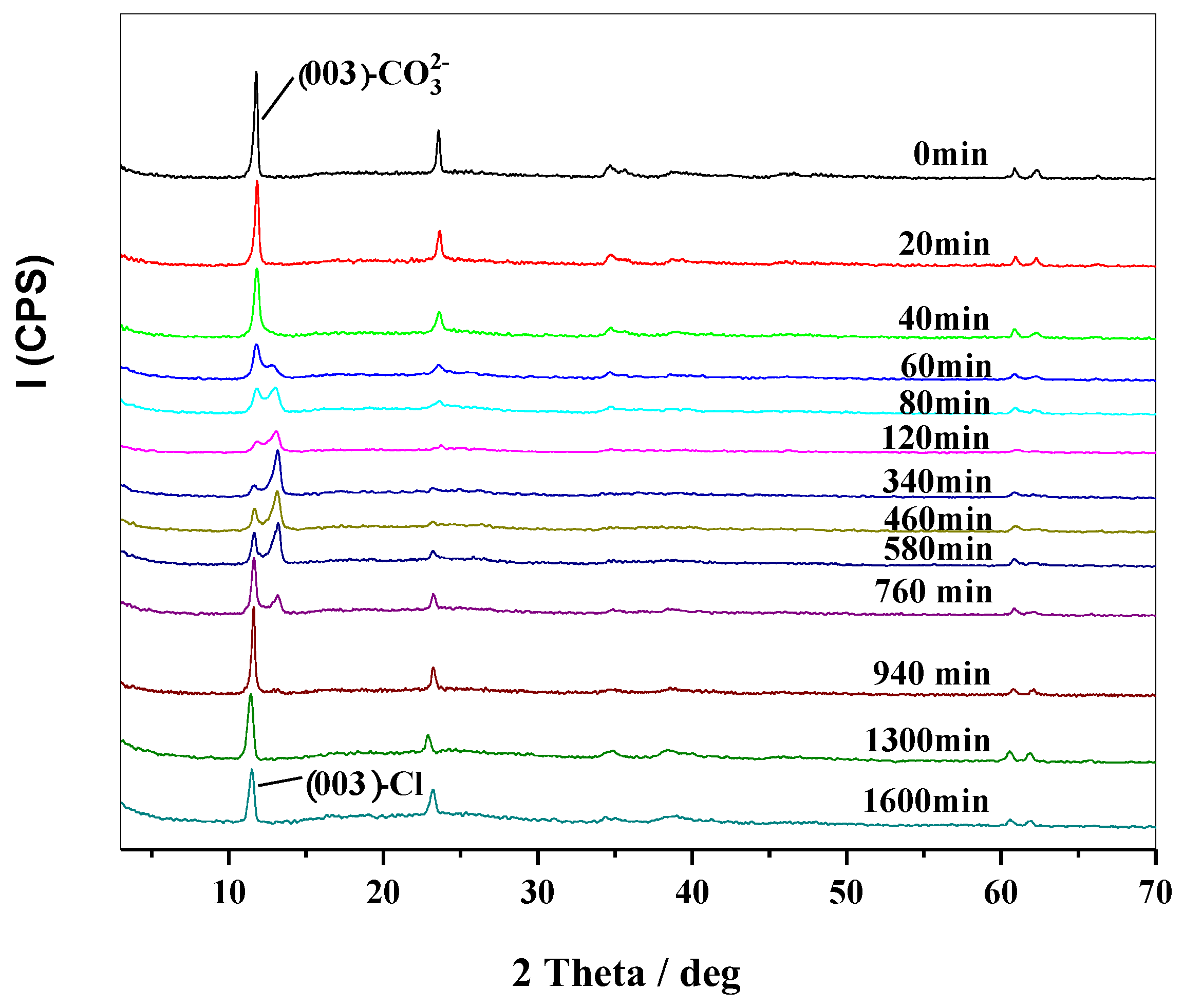
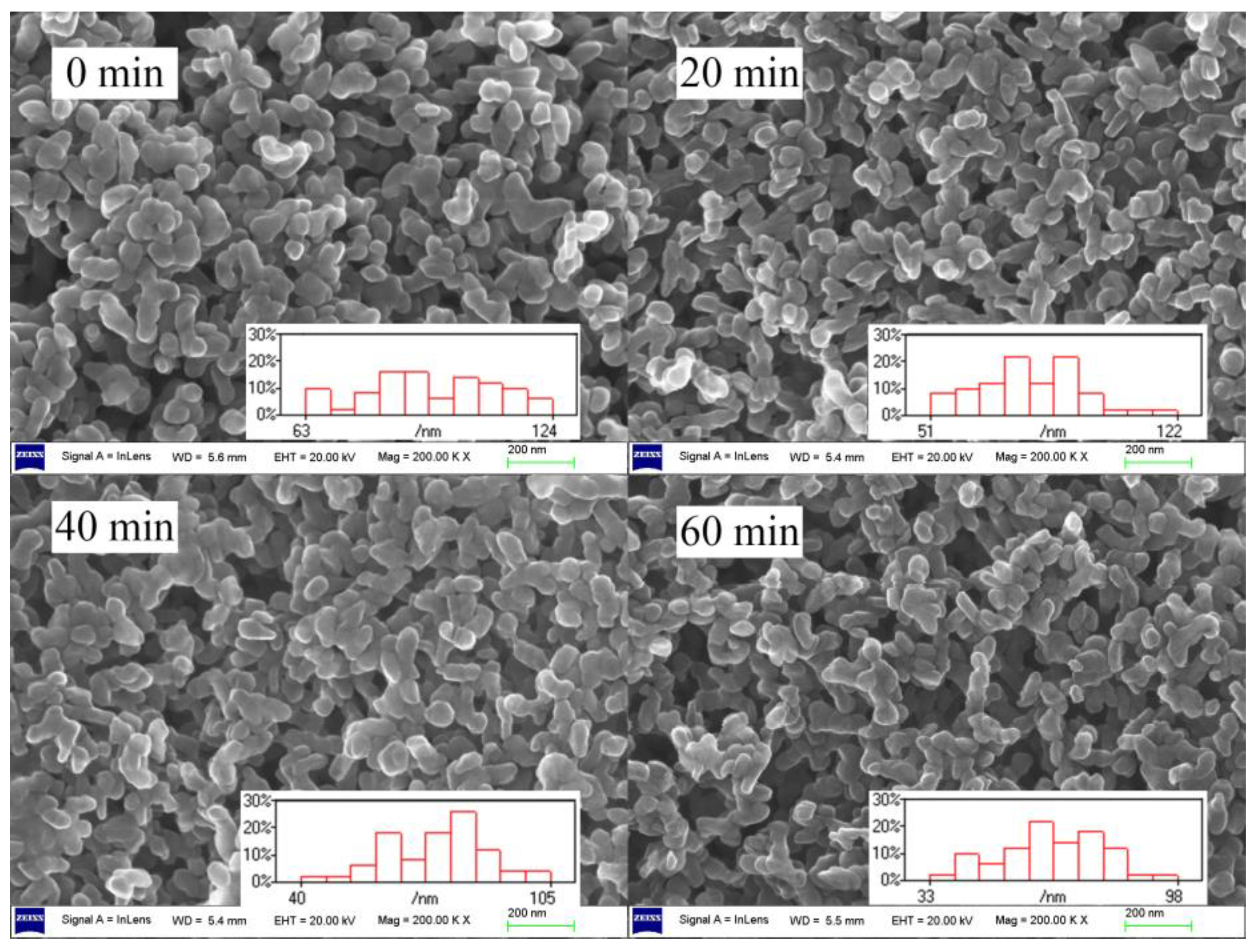
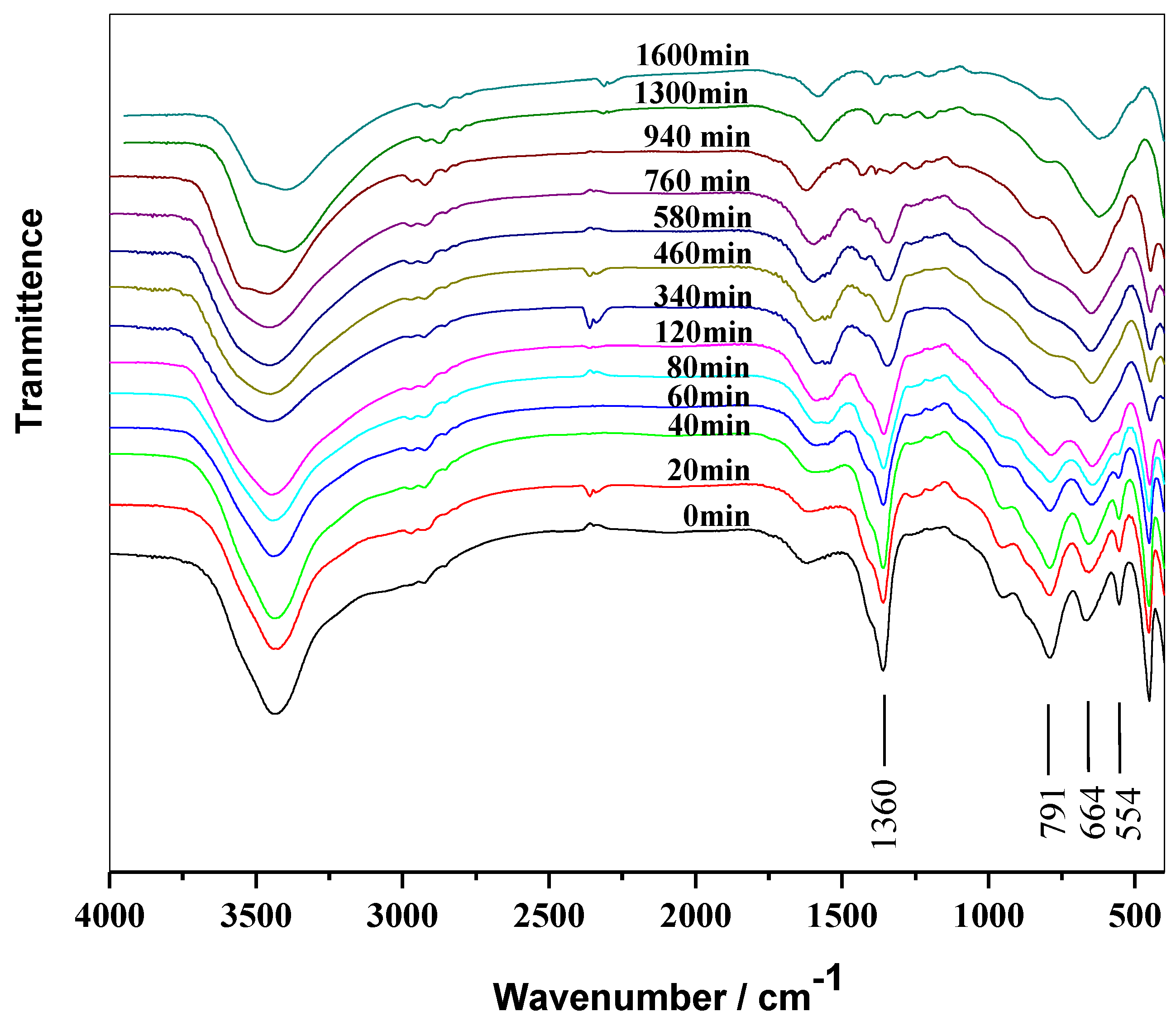
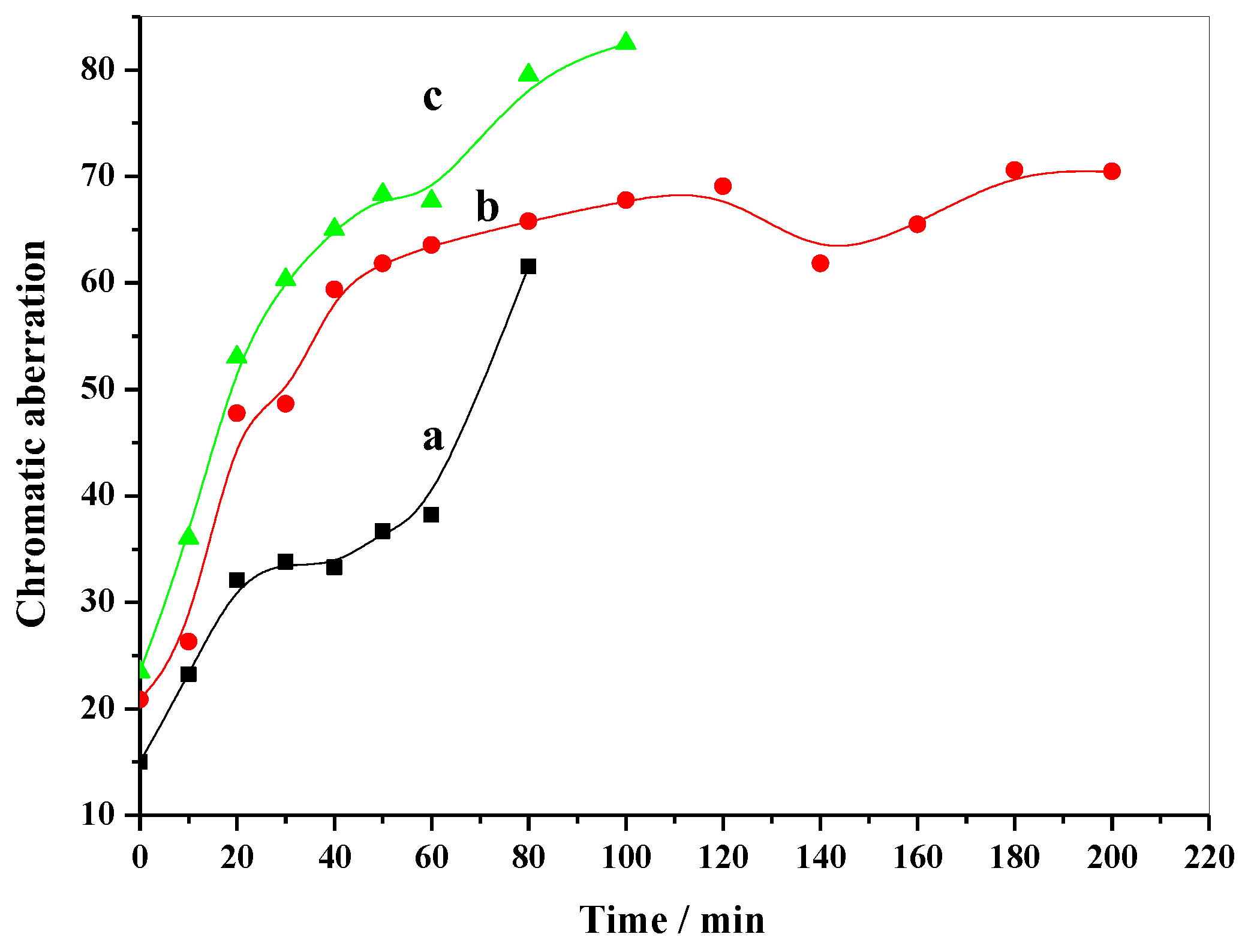
2.3. Thermal Stabilizing Process of MgAl-CO3-LDHs in PVC
2.4. Thermal Stabilizing Process of MgAl-NO3-LDHs in PVC
2.5. Thermal Stabilizing Process of MgAl-Cl-LDHs in PVC
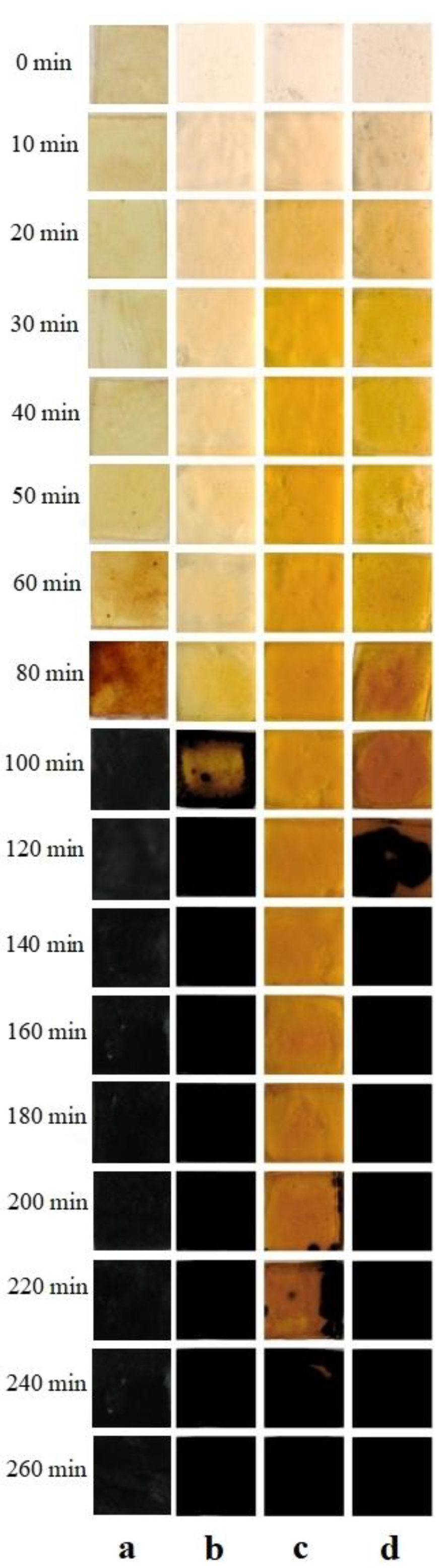
2.6. Thermal Stabilizing Effect of Different MgAl-LDHs in PVC
3. Materials and Methods
3.1. Materials
3.2. Preparation of LDHs
3.3. Characterization of Samples
3.4. Thermal Stability Testing of PVC Strips
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wilczewski, S.; Skórczewska, K.; Tomaszewska, J.; Osial, M.; Dąbrowska, A.; Nikiforow, K.; Jenczyk, P.; Grzywacz, H. Graphene Modification by Curcuminoids as an Effective Method to Improve the Dispersion and Stability of PVC/Graphene Nanocomposites. Molecules 2023, 28, 3383. [Google Scholar] [CrossRef] [PubMed]
- Klempova, S.; Oravec, M.; Vizarova, K. Analysis of thermally and UV–Vis aged plasticized PVC using UV–Vis, ATR-FTIR and Raman spectroscopy. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2023, 294, 122541. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Han, W.; Hu, X.; Li, D.; Ma, X.; Liu, H.; Liu, L.; Lu, W.; Liu, S. Pentaerythritol p-hydroxybenzoate ester-based zinc metal alkoxides as multifunctional antimicrobial thermal stabilizer for PVC. Polym. Degrad. Stabil. 2020, 181, 109340. [Google Scholar] [CrossRef]
- Zhang, D.; Yao, S.; Li, S.; Wang, J.; Yao, Y. A novel La-containing additive for the long-term thermal stabilization of poly(vinyl chloride). Polym. Degrad. Stabil. 2017, 144, 187–194. [Google Scholar] [CrossRef]
- Dong, T.; Li, D.; Li, Y.; Han, W.; Zhang, L.; Xie, G.; Sunarso, J.; Liu, S. Design and synthesis of polyol ester-based zinc metal alkoxides as a bi-functional thermal stabilizer for poly(vinyl chloride). Polym. Degrad. Stabil. 2019, 159, 125–132. [Google Scholar] [CrossRef]
- Meng, W.; Dong, Y.; Li, J.; Cheng, L.; Zhang, H.; Wang, C.; Jiao, Y.; Xu, J.; Hao, J.; Qu, H. Bio-based phytic acid and tannic acid chelate-mediated interfacial assembly of Mg(OH)2 for simultaneously improved flame retardancy, smoke suppression and mechanical properties of PVC. Composites Part. B Engineering 2020, 188, 107854. [Google Scholar] [CrossRef]
- Wang, M.; Wu, T.; Bu, Q.; Song, X.; Li, M.; Yuan, S. Rare earth Ce based metal organic framework as efficient synergistic thermal stabilizer for PVC: Preparation and thermal stabilization behavior. Thermochim. Acta 2022, 718, 179365. [Google Scholar] [CrossRef]
- Miyata, S.; Kuroda, M. Method for Inhibiting the Thermal or Ultraviolet Degradation of Thermoplastic Resin and Thermoplastic Resin Composition Having Stability to Thermal or Ultraviolet Degradation. U.S. Patent US4299759, 10 November 1981. [Google Scholar]
- Yan, J.; Yang, Z. Intercalated hydrotalcite-like materials and their application as thermal stabilizers in poly (vinyl chloride). J. Appl. Polym. Sci. 2017. [Google Scholar] [CrossRef]
- Fan, L.; Yang, L.; Lin, Y.; Fan, G.; Li, F. Enhanced thermal stabilization effect of hybrid nanocomposite of Ni–Al layered double hydroxide/carbon nanotubes on polyvinyl chloride resin—ScienceDirect. Polym. Degrad. Stabil. 2020, 176, 109153. [Google Scholar] [CrossRef]
- Dinari, M.; Roghani, N. Effect of triazine based silane coupling agent modified LDH on the thermal and mechanical properties of PVC based nanocomposites. J. Polym. Res. 2021, 28, 320. [Google Scholar] [CrossRef]
- Aisawa, S.; Nakada, C.; Hirahara, H.; Takahashi, N.; Narita, E. Preparation of dipentaerythritol-combined layered double hydroxide particle and its thermostabilizing effect for polyvinyl chloride. Appl. Clay Sci. 2019, 180, 105205. [Google Scholar] [CrossRef]
- Melánová, K.; Kopecká, K.; Beneš, L.; Kutálek, P.; Knotek, P.; Zmrhalová, Z.; Svoboda, J. Functionalization of ZnAl-Layered Double Hydroxide with Ensulizole and Its Application as a UV-Protective Agent in a Transparent Polymer Coating. Molecules 2023, 28, 6262. [Google Scholar] [CrossRef] [PubMed]
- Koba-Ucun, O.; Ölmez Hanci, T.; Arslan-Alaton, I.; Arefi-Oskoui, S.; Khataee, A.; Kobya, M.; Orooji, Y. Toxicity of Zn-Fe Layered Double Hydroxide to Different Organisms in the Aquatic Environment. Molecules 2021, 26, 395. [Google Scholar] [CrossRef]
- Yi, S.; Yang, Z.H.; Wang, S.W.; Liu, D.R.; Wang, S.Q.; Liu, Q.Y.; Chi, W.W. Effects of MgAlCe-CO3 layered double hydroxides on the thermal stability of PVC resin. J. Appl. Polym. Sci. 2010, 119, 2620–2626. [Google Scholar] [CrossRef]
- Gao, Z.; Lu, L.; Shi, C.; Qian, X. The effect of OCoAl-LDH and OCoFe-LDH on the combustion behaviors of polyvinyl chloride. Polym. Advan Technol. 2020, 31, 675–685. [Google Scholar] [CrossRef]
- Nia, S.B.; Pooresmaeil, M.; Namazi, H. Carboxymethylcellulose/layered double hydroxides bio-nanocomposite hydrogel: A controlled amoxicillin nanocarrier for colonic bacterial infections treatment. Int. J. Biol. Macromol. 2020, 155, 1401–1409. [Google Scholar]
- Cao, T.; Xu, K.; Chen, G.; Guo, C.Y. Poly(ethylene terephthalate) nanocomposites with a strong UV-shielding function using UV-absorber intercalated layered double hydroxides. Rsc Adv. 2013, 3, 6282–6285. [Google Scholar] [CrossRef]
- Izbudak, B.; Cecen, B.; Anaya, I.; Miri, A.K.; Bal-Ozturk, A.; Karaoz, E. Layered double hydroxide-based nanocomposite scaffolds in tissue engineering applications. Rsc Adv. 2021, 11, 30237–30252. [Google Scholar] [CrossRef]
- Ali, J.; Li, D.; Shahzad, A.; Wajid Ullah, M.; Ifthikar, J.; Asif, M.; Yanan, C.; Lei, X.; Chen, Z.; Wang, S. MoS4-LDH: A dual centre Fe-based layered double hydroxide catalyst for efficient atrazine removal and peroxymonsulfate activation. Chem. Eng. J. 2023, 456, 141161. [Google Scholar] [CrossRef]
- Ali, S.; Marwat, M.A.; Khan, M.F.; Adam, K.M.; Din, Z.U.; Karim, M.R.A.; Khan, S. Ti3SiC2-coupled NiCoMn LDH nanocomposites as positive electrode for high performance supercapacitors. J. Alloy Compd. 2023, 956, 170229. [Google Scholar] [CrossRef]
- Xie, Y.; Gu, Y.; Meng, J.; Yan, X.; Chen, Y.; Guo, X.; Lang, W. Ultrafast separation of oil/water mixtures with layered double hydroxide coated stainless steel meshes (LDH-SSMs). J. Hazard. Mater. 2020, 398, 122862. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Lu, Z.; Shao, M.; Yan, D.; Wei, M.; Evans, D.G.; Duan, X. Flexible hierarchical nanocomposites based on MnO2 nanowires/CoAl hydrotalcite/carbon fibers for high-performance supercapacitors. Rsc Adv. 2013, 3, 1045–1049. [Google Scholar] [CrossRef]
- Cong, X.; Shang, Y.; Zhao, L.; Jiang, H.; Tian, W.; Wu, J.; Zhang, S.; Tian, D.; Lu, S.; Tan, Y. Binary heterojunctions of Mg/Al-LDH and carbon-based matrixes derived from self-assembly synthesis for inhibition of pyrene photopolymerizing: Elucidation of LDHs protecting bitumen against UV aging. Colloids Surf. A Physicochem. Eng. Asp. 2023, 677, 132313. [Google Scholar] [CrossRef]
- Labuschagne, F.J.; Molefe, D.M.; Focke, W.W.; Van der Westhuizen, I.; Wright, H.C.; Royeppen, M.D. Heat stabilising flexible PVC with layered double hydroxide derivatives. Polym. Degrad. Stabil. 2015, 113, 46–54. [Google Scholar] [CrossRef]
- Wen, X.; Yang, Z.; Yan, J.; Xie, X. Green preparation and characterization of a novel heat stabilizer for poly (vinyl chloride)–hydrocalumites. Rsc Adv. 2015, 5, 32020–32026. [Google Scholar] [CrossRef]
- Runjuan, W.; Zhanhong, Y.; Hongyan, C.; Youwang, H.U.; Ji’An, D. Zn-Al-La hydrotalcite-like compounds as heating stabilizer in PVC resin. J. Rare Earth 2012, 30, 895–902. [Google Scholar]
- Guo, Y.; Zhang, Q.; Hu, Q.; Tian, W.; Leroux, F.; Tang, P.; Li, D.; Feng, Y. Size-dependent effect of MgAl-layered double hydroxides derived from Mg(OH)2 on thermal stability of poly (vinyl chloride). Mater. Today Commun. 2021, 29, 102851. [Google Scholar] [CrossRef]
- Van der Ven, L.; Van Gemert, M.; Batenburg, L.F.; Keern, J.J.; Gielgens, L.H.; Koster, T.; Fischer, H.R. On the action of hydrotalcite-like clay materials as stabilizers in polyvinylchloride. Appl. Clay Sci. 2000, 17, 25–34. [Google Scholar] [CrossRef]
- Lin, Y.; Li, D.; Evans, D.; Duan, X. Modulating effect of Mg-Al-CO3 layered double hydroxides on the thermal stability of PVC resin. Polym. Degrad. Stabil. 2005, 88, 286–293. [Google Scholar] [CrossRef]
- Wang, M.; Bao, W.; Wang, J.; Wang, K.; Xu, J.; Chen, H.; Xia, X. A green approach to the synthesis of novel “Desert rose stone”-like nanobiocatalytic system with excellent enzyme activity and stability. Sci. Rep. 2014, 4, 6606. [Google Scholar] [CrossRef]
- Busetto, C.; Del Piero, G.; Manara, G.; Trifiro, F.; Vaccari, A. Catalysts for low-temperature methanol synthesis. Preparation of Cu-Zn-Al mixed oxides via hydrotalcite-like precursors. J. Catal. 1984, 85, 260–266. [Google Scholar] [CrossRef]
- Vulic, T.; Reitzmann, A.; Ranogajec, J.; Marinkovic-Neducin, R. The influence of synthesis method and Mg-Al-Fe content on the thermal stability of layered double hydroxides. J. Therm. Anal. Calorim. 2012, 110, 227–233. [Google Scholar] [CrossRef]
- Gu, Z.; Thomas, A.C.; Xu, Z.P.; Campbell, J.H.; Lu, G.Q.M. In Vitro Sustained Release of LMWH from MgAl-layered Double Hydroxide Nanohybrids. Chem. Mater. 2008, 20, 3715–3722. [Google Scholar] [CrossRef]
- Palmer, S.J.; Grand, L.M.; Frost, R.L. The synthesis and spectroscopic characterisation of hydrotalcite formed from aluminate solutions. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2011, 79, 156–160. [Google Scholar] [CrossRef][Green Version]
- Zhao, Y.; Li, F.; Zhang, R.; Evans, D.G.; Duan, X. Preparation of layered double-hydroxide nanomaterials with a uniform crystallite size using a new method involving separate nucleation and aging steps. Chem. Mater. 2002, 14, 4286–4291. [Google Scholar] [CrossRef]
- Feng, Y.; Li, D.; Li, C.; Wang, Z.; Evans, D.G.; Duan, X. Synthesis of Cu-containing layered double hydroxides with a narrow crystallite-size distribution. Clay Clay Miner. 2003, 51, 566–569. [Google Scholar] [CrossRef]
- ISO/DIS 305; Plastics—Determination of Thermal Stability of Poly(vinyl chloride), Related Chlorine-Containing Homopolymers and Copolymers and Their Compounds—Discoloration Method. ISO: Geneva, Switzerland, 2019.

| Theoretical HCl Absorption Capacities (mmol HCl/g LDH) | NO3− | CO32− | Cl− |
|---|---|---|---|
| Layers | 21.77 | 24.63 | 24.09 |
| Interlayer | / | 4.10 | / |
| Total | 21.77 | 28.7 | 24.09 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rao, Z.; Li, K.; Liu, P.; Lin, Y.; Lyu, X. Study on the Thermal Stabilizing Process of Layered Double Hydroxides in PVC Resin. Molecules 2023, 28, 7792. https://doi.org/10.3390/molecules28237792
Rao Z, Li K, Liu P, Lin Y, Lyu X. Study on the Thermal Stabilizing Process of Layered Double Hydroxides in PVC Resin. Molecules. 2023; 28(23):7792. https://doi.org/10.3390/molecules28237792
Chicago/Turabian StyleRao, Zhi, Kaitao Li, Pingli Liu, Yanjun Lin, and Xiang Lyu. 2023. "Study on the Thermal Stabilizing Process of Layered Double Hydroxides in PVC Resin" Molecules 28, no. 23: 7792. https://doi.org/10.3390/molecules28237792
APA StyleRao, Z., Li, K., Liu, P., Lin, Y., & Lyu, X. (2023). Study on the Thermal Stabilizing Process of Layered Double Hydroxides in PVC Resin. Molecules, 28(23), 7792. https://doi.org/10.3390/molecules28237792






