Heterointerface Engineered Core-Shell Fe2O3@TiO2 for High-Performance Lithium-Ion Storage
Abstract
:1. Introduction
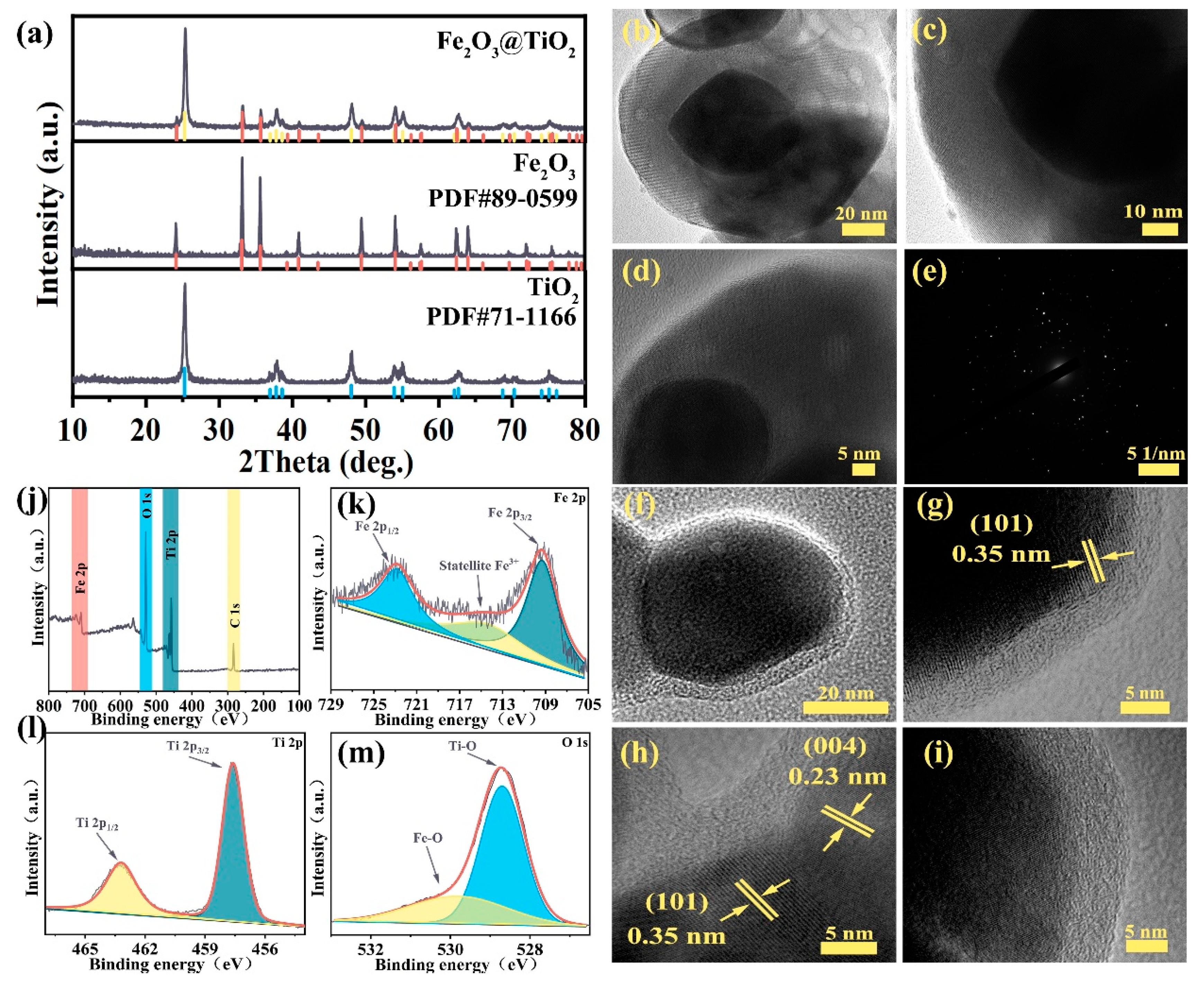
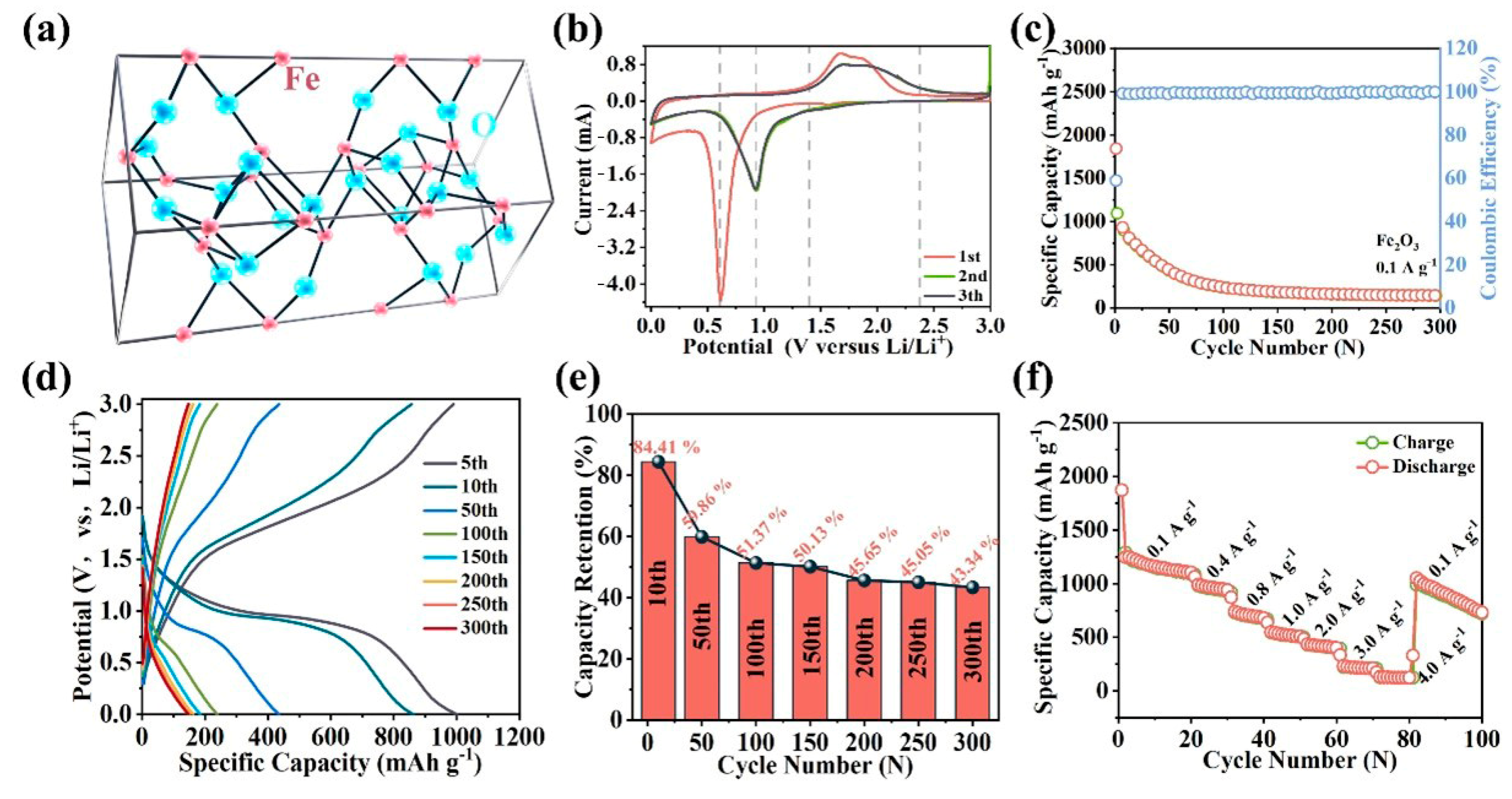
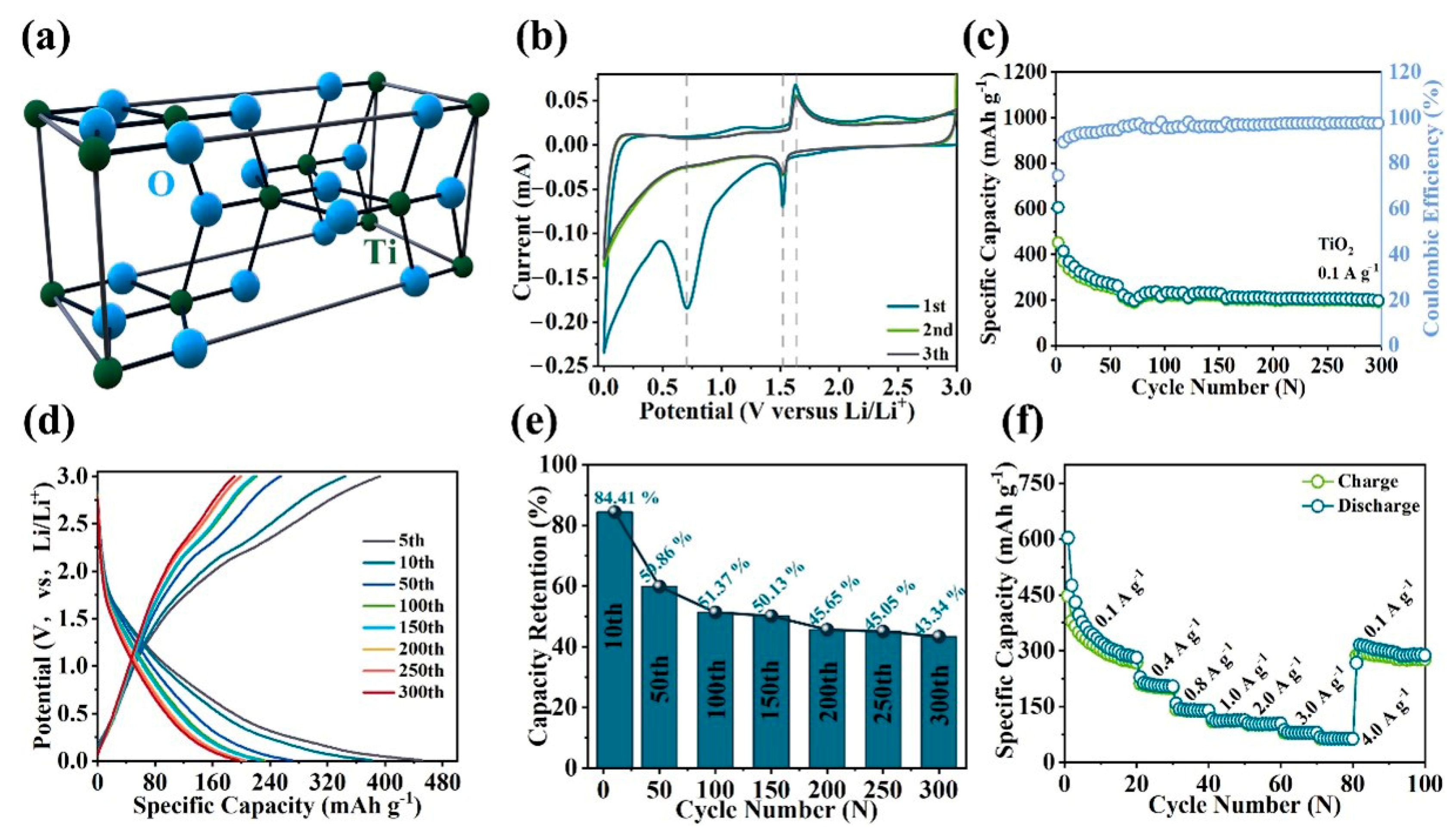
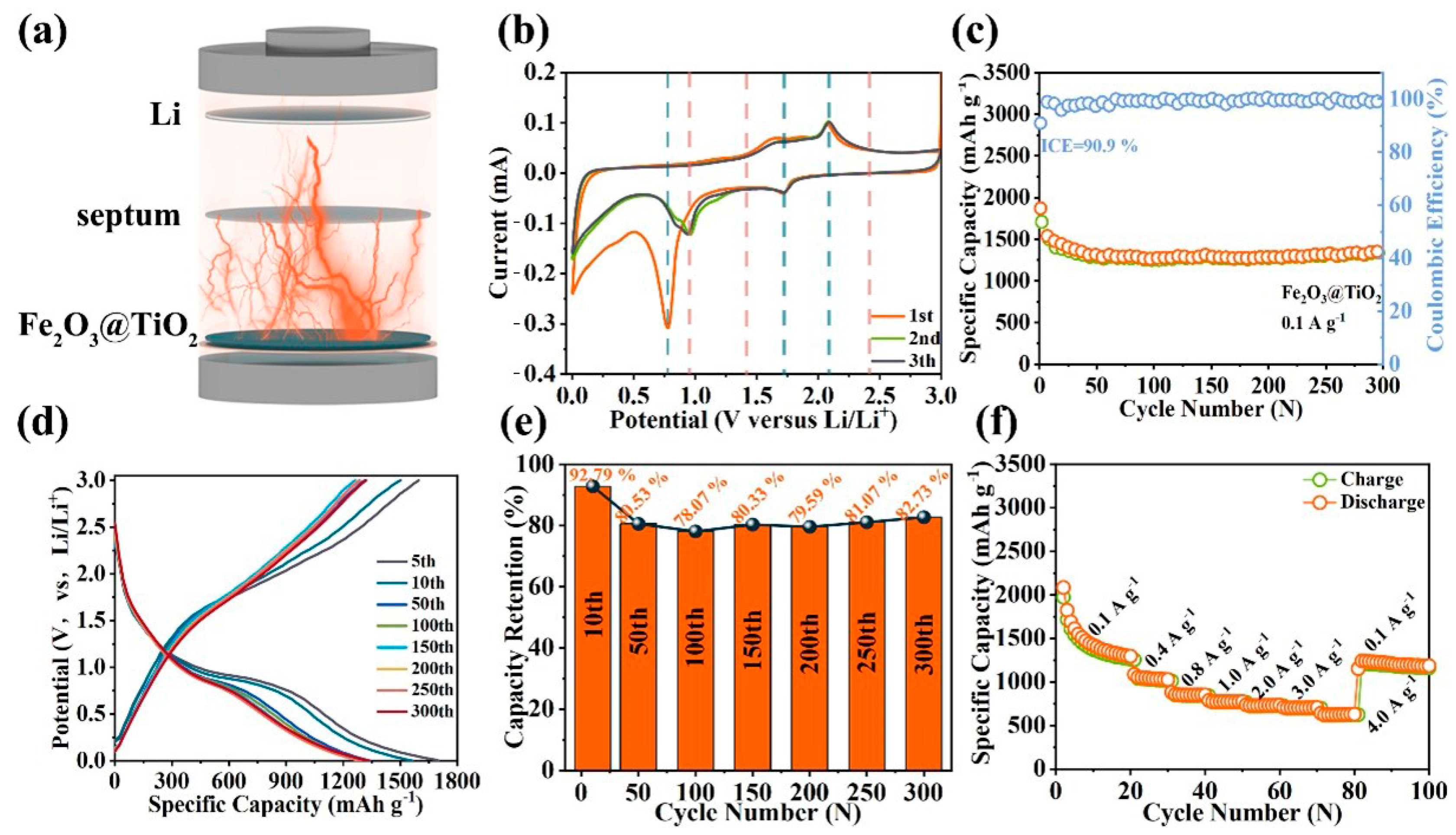
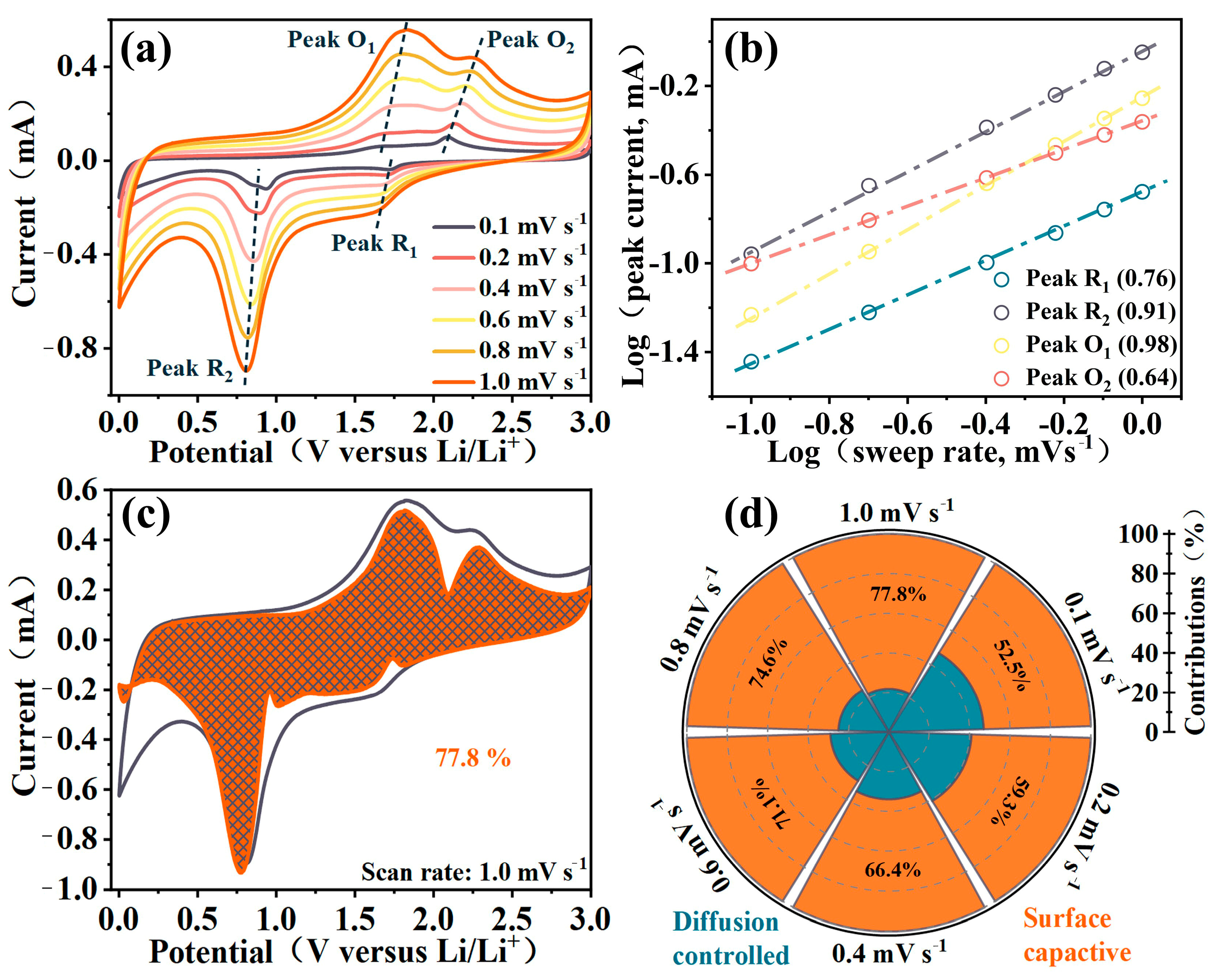
2. Results
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Sun, C.; Chen, F.; Tang, X.; Zhang, D.; Zheng, K.; Zhu, G.; Bin Shahid, U.; Liu, Z.; Shao, M.; Wang, J. Simultaneous interfacial interaction and built-in electric field regulation of GaZnON@NG for high-performance lithium-ion storage. Nano Energy 2022, 99, 107369. [Google Scholar] [CrossRef]
- Zhao, L.; Ning, Y.; Dong, Q.; Ullah, Z.; Zhu, P.; Zheng, S.; Xia, G.; Zhu, S.; Li, Q.; Liu, L. Longer cycle life and higher discharge voltage of a small molecular indanthrone resulting from the extended conjugated framework. J. Power Sources 2023, 556, 232518. [Google Scholar] [CrossRef]
- Mostafa, M.M.M.; Alshehri, A.A.; Salama, R.S. High performance of supercapacitor based on alumina nanoparticles derived from Coca-Cola cans. J. Energy Storage 2023, 64, 107168. [Google Scholar] [CrossRef]
- Yan, Z.; Li, J.; Chen, Q.; Chen, S.; Luo, L.; Chen, Y. Synthesis of CoSe2/Mxene Composites Using as High-performance Anode Materials for Lithium-ion Batteries. Adv. Compos. Hybrid Mater. 2022, 5, 2977–2987. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, Y.; Das, P.; Wu, Z.-S. Recent Advances in Interface Engineering and Architecture Design of Air-Stable and Water-Resistant Lithium Metal Anodes. Energy Fuels 2021, 35, 12902–12920. [Google Scholar] [CrossRef]
- Ma, J.; Zheng, S.; Zhou, F.; Zhu, Y.; Das, P.; Huang, R.; Zhang, L.; Wang, X.; Wang, H.; Cui, Y.; et al. All 3D printing lithium metal batteries with hierarchically and conductively porous skeleton for ultrahigh areal energy density. Energy Storage Mater. 2023, 54, 304–312. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhao, X.; Zhou, Y.; Liu, S.; Fang, G.; Liang, S. Towards establishing uniform metrics for evaluating the safety of lithium metal batteries. Adv. Powder Mater. 2023, 2, 100139. [Google Scholar] [CrossRef]
- Ji, X.; Zhang, Y.; Cao, M.; Gu, Q.; Wang, H.; Yu, J.; Guo, Z.-H.; Zhou, X. Advanced inorganic/polymer hybrid electrolytes for all-solid-state lithium batteries. J. Adv. Ceram. 2022, 11, 835–861. [Google Scholar] [CrossRef]
- Huang, J.; Dai, Q.; Cui, C.; Ren, H.; Lu, X.; Hong, Y.; Woo Joo, S. Cake-like porous Fe3O4@C nanocomposite as high-performance anode for Li-ion battery. J. Electroanal. Chem. 2022, 918, 116508. [Google Scholar] [CrossRef]
- Miao, Z.; Li, D.; Zhao, L.; Gao, K.; Sun, W.; Zhao, W.; Yuan, L.; Zhang, H.; Li, Z.; Wang, Y.-J.; et al. Domain-limited Growth Strategy to Construct Fe3C@C@CNTs Heterogeneous Interfaces for Multi-functional High-Performance Lithium-ion Storage and Microwave Absorption. J. Alloys Compd. 2023, 967, 171650. [Google Scholar] [CrossRef]
- Yang, Z.; Zhu, P.; Ullah, Z.; Zheng, S.; Yu, S.; Zhu, S.; Liu, L.; Li, Q. Synchronous Light Harvesting and Energy Storing Organic Cathode Material 1,4-Dihydroxyanthraquinone for Lithium-Ion Batteries. Chem. Eng. J. 2023, 468, 143787. [Google Scholar] [CrossRef]
- Li, Z.; Gao, K.; Han, Y.; Ding, S.; Cui, Y.; Hu, M.; Zhao, J.; Zhang, M.; Meng, A.; Yun, J.; et al. Atomic insights of electronic states engineering of GaN nanowires by Cu cation substitution for highly efficient lithium ion battery. J. Energy Chem. 2022, 67, 46. [Google Scholar] [CrossRef]
- Kiran, L.; Aydinol, M.K.; Ahmad, A.; Shah, S.S.; Bahtiyar, D.; Shahzad, M.I.; Eldin, S.M.; Bahajjaj, A.A.A. Flowers Like a-MoO3/CNTs/PANI Nanocomposites as Anode Materials for High-Performance Lithium Storage. Molecules 2023, 28, 3319. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.L.; Wang, L.; Sun, D.Y.; Meng, S.; Xu, D.D.; He, Z.X.; Dong, X.Y.; Li, Y.F.; Jin, Y.C. Aggregation-Morphology-Dependent Electrochemical Performance of Co3O4 Anode Materials for Lithium-Ion Batteries. Molecules 2019, 24, 3149. [Google Scholar] [CrossRef]
- Liu, Y.L.; Yang, R.F.; Li, X.X.; Yang, W.S.; Lin, Y.W.; Zhang, G.Q.; Wang, L.J. Nb2O5 Coating to Improve the Cyclic Stability and Voltage Decay of Li-Rich Cathode Material for Lithium-Ion Battery. Molecules 2023, 28, 3890. [Google Scholar] [CrossRef]
- Sun, C.; Xu, X.; Cui, C.; Chen, F.; Wang, Y.; Chen, S.; Shao, M.; Wang, J. High-Quality Epitaxial N Doped Graphene on SiC with Tunable Interfacial Interactions via Electron/Ion Bridges for Stable Lithium-Ion Storage. Nano Micro Lett. 2023, 15, 202. [Google Scholar] [CrossRef]
- Li, L.; Xie, F.; Wu, H.; Zhu, Y.; Zhang, P.; Li, Y.; Li, H.; Zhao, L.; Zhu, G. N-Doped Porous Carbon-Nanofiber-Supported Fe3C/Fe2O3 Nanoparticles as Anode for High-Performance Supercapacitors. Molecules 2023, 28, 5751. [Google Scholar] [CrossRef]
- Li, H.; Jiang, J.; Huang, J.; Wang, Y.; Peng, Y.; Zhang, Y.; Hwang, B.-J.; Zhao, J. Investigation of the Na Storage Property of One-Dimensional Cu2–xSe Nanorods. ACS Appl. Mater. Interfaces 2018, 10, 13491–13498. [Google Scholar] [CrossRef]
- Chen, J.S.; Zhu, T.; Yang, X.H.; Yang, H.G.; Lou, X.W. Top-Down Fabrication of α-Fe2O3 Single-Crystal Nanodiscs and Microparticles with Tunable Porosity for Largely Improved Lithium Storage Properties. J. Am. Chem. Soc. 2010, 132, 13162–13164. [Google Scholar] [CrossRef]
- Qi, X.; Zhang, H.-B.; Xu, J.; Wu, X.; Yang, D.; Qu, J.; Yu, Z.-Z. Highly Efficient High-Pressure Homogenization Approach for Scalable Production of High-Quality Graphene Sheets and Sandwich-Structured α-Fe2O3/Graphene Hybrids for High-Performance Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2017, 9, 11025–11034. [Google Scholar] [CrossRef]
- Wang, W.; Feng, Y.; Zhang, S.; Wang, M.; Song, W.; Yue, L.; Ge, M.; Mi, J. Facile Premixed Flame Synthesis C@Fe2O3/SWCNT as Superior Free-standing Anode for Lithium-ion Batteries. J. Alloys Compd. 2022, 905, 164247. [Google Scholar] [CrossRef]
- He, D.; Sun, M.; Cao, D.; Ding, Y.; Chen, H.; He, G. Flexible Free-Standing Fe2O3 Nanoparticle/Carbon Shells/Graphene Films for Advanced Lithium-Ion Batteries. ACS Appl. Nano Mater. 2022, 5, 5017–5024. [Google Scholar] [CrossRef]
- Xu, J.-S.; Zhu, Y.-J. Monodisperse Fe3O4 and γ-Fe2O3 Magnetic Mesoporous Microspheres as Anode Materials for Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2012, 4, 4752–4757. [Google Scholar] [CrossRef] [PubMed]
- Al-Thabaiti, S.A.; Mostafa, M.M.M.; Ahmed, A.I.; Salama, R.S. Synthesis of copper/chromium metal organic frameworks-Derivatives as an advanced electrode material for high-performance supercapacitors. Ceram. Int. 2023, 49, 5119–5129. [Google Scholar] [CrossRef]
- Zhang, Y.; Ding, Z.; Foster, C.W.; Banks, C.E.; Qiu, X.; Ji, X. Oxygen Vacancies Evoked Blue TiO2(B) Nanobelts with Efficiency Enhancement in Sodium Storage Behaviors. Adv. Funct. Mater. 2017, 27, 1700856. [Google Scholar] [CrossRef]
- Xia, T.; Zhang, W.; Li, W.J.; Oyler, N.A.; Liu, G.; Chen, X.B. Hydrogenated Surface Disorder Enhances Lithium ion Battery Performance. Nano Energy 2013, 2, 826–835. [Google Scholar] [CrossRef]
- Wang, L.; Yang, H.; Shu, T.; Xin, Y.; Chen, X.; Li, Y.; Li, H.; Hu, X. Nanoengineering S-Doped TiO2 Embedded Carbon Nanosheets for Pseudocapacitance-Enhanced Li-Ion Capacitors. ACS Appl. Energy Mater. 2018, 1, 1708–1715. [Google Scholar] [CrossRef]
- Hao, Z.; Chen, Q.; Dai, W.; Ren, Y.; Zhou, Y.; Yang, J.; Xie, S.; Shen, Y.; Wu, J.; Chen, W.; et al. Oxygen-Deficient Blue TiO2 for Ultrastable and Fast Lithium Storage. Adv. Energy Mater. 2020, 10, 1903107. [Google Scholar] [CrossRef]
- Li, J.; Wen, W.; Xu, G.; Zou, M.; Huang, Z.; Guan, L. Fe-added Fe3C carbon nanofibers as anode for Li ion batteries with excellent low-temperature performance. Electrochim. Acta 2015, 153, 300. [Google Scholar] [CrossRef]
- Cao, L.; Liang, X.; Ou, X.; Yang, X.; Li, Y.; Yang, C.; Lin, Z.; Liu, M. Heterointerface Engineering of Hierarchical Bi2S3/MoS2 with Self-Generated Rich Phase Boundaries for Superior Sodium Storage Performance. Adv. Funct. Mater. 2020, 30, 1910732. [Google Scholar] [CrossRef]
- Kresse, G.; Hafner, J. Ab Initio Molecular Dynamics for Open-shell Transition Metals. Phys. Rev. B 1993, 48, 13115–13118. [Google Scholar] [CrossRef]
- Kresse, G.; Hafner, J. Ab initio molecular dynamics for liquid metals. Phys. Rev. B 1993, 47, 558–561. [Google Scholar] [CrossRef] [PubMed]
- Pian, C.; Peng, W.; Ren, H.; Ma, C.; Su, Y.; Ti, R.; Chen, X.; Zhu, L.; Liu, J.; Sun, X.; et al. Robust α-Fe2O3@TiO2 Core–Shell Structures with Tunable Buffer Chambers for High-Performance Lithium Storage. Front. Chem. 2022, 10, 866369. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Li, Y.; Xie, H.; Zuo, Z.; Chu, P. High electrochemical performance Li-ion batteries based on the porous carbon@Fe2O3 composite anode. Ionics 2022, 28, 4943–4947. [Google Scholar] [CrossRef]
- Su, Q.; Xie, D.; Zhang, J.; Du, G.; Xu, B. In Situ Transmission Electron Microscopy Observation of the Conversion Mechanism of Fe2O3/Graphene Anode during Lithiation–Delithiation Processes. ACS Nano 2013, 7, 9115–9121. [Google Scholar] [CrossRef]
- Huang, Y.; Xu, Z.; Mai, J.; Lau, T.-K.; Lu, X.; Hsu, Y.-J.; Chen, Y.; Lee, A.C.; Hou, Y.; Meng, Y.S.; et al. Revisiting the Origin of Cycling Enhanced Capacity of Fe3O4 Based Nanostructured Electrode for Lithium ion Batteries. Nano Energy 2017, 41, 426–433. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Y.; Chen, Y.; Yousaf, M.; Wu, H.; Cao, A.; Han, R.P.S. Reticulate Dual-Nanowire Aerogel for Multifunctional Applications: A High-Performance Strain Sensor and a High Areal Capacity Rechargeable Anode. Adv. Funct. Mater. 2019, 29, 1807467. [Google Scholar] [CrossRef]
- Zheng, H.; Yang, Y.; Liu, X.; Guo, Z.; Feng, C. Controllable synthesis of FeVO4@TiO2 nanostructures as anode for lithium ion battery. J. Nanopart. Res. 2017, 19, 243. [Google Scholar] [CrossRef]
- Hayashi, A.; Hama, S.; Morimoto, H.; Tatsumisago, M.; Minami, T. Preparation of Li2S–P2S5 Amorphous Solid Electrolytes by Mechanical Milling. J. Am. Ceram. Soc. 2001, 84, 477–479. [Google Scholar] [CrossRef]
- Pu, X.; Zhao, D.; Fu, C.; Chen, Z.; Cao, S.; Wang, C.; Cao, Y. Understanding and Calibration of Charge Storage Mechanism in Cyclic Voltammetry Curves. Angew. Chem. Int. Ed. 2021, 60, 21310–21318. [Google Scholar] [CrossRef]
- Chen, M.; Chao, D.; Liu, J.; Yan, J.; Zhang, B.; Huang, Y.; Lin, J.; Shen, Z.X. Rapid Pseudocapacitive Sodium-Ion Response Induced by 2D Ultrathin Tin Monoxide Nanoarrays. Adv. Funct. Mater. 2017, 27, 1606232. [Google Scholar] [CrossRef]
- Chen, C.; Wen, Y.; Hu, X.; Ji, X.; Yan, M.; Mai, L.; Hu, P.; Shan, B.; Huang, Y. Na+ intercalation pseudocapacitance in graphene-coupled titanium oxide enabling ultra-fast sodium storage and long-term cycling. Nat. Commun. 2015, 6, 6929. [Google Scholar] [CrossRef]
- Xia, X.; Chao, D.; Zhang, Y.; Zhan, J.; Zhong, Y.; Wang, X.; Wang, Y.; Shen, Z.X.; Tu, J.; Fan, H.J. Generic Synthesis of Carbon Nanotube Branches on Metal Oxide Arrays Exhibiting Stable High-Rate and Long-Cycle Sodium-Ion Storage. Small 2016, 12, 3048–3058. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Yu, X.-Y.; Paik, U. Etching-in-a-Box: A Novel Strategy to Synthesize Unique Yolk-Shelled Fe3O4@Carbon with an Ultralong Cycling Life for Lithium Storage. Adv. Energy Mater. 2016, 6, 1502318. [Google Scholar] [CrossRef]
- Wang, L.; Chen, Q.; Zhu, Y.; Qian, Y. Graphene-wrapped Fe2O3 nanorings for Li ion battery anodes. Chin. Sci. Bull. 2014, 59, 4271–4273. [Google Scholar] [CrossRef]
- Lin, Y.; Sun, L.; Hu, J.; Tan, H.; Xie, F.; Qu, Y.; Wang, K.; Zhang, Y. Ti-doped Fe2O3/carbon cloth anode with oxygen vacancies and partial rGO encapsulation for flexible lithium ion batteries. J. Alloys Compd. 2022, 924, 166441. [Google Scholar] [CrossRef]
- Luo, Y.; Luo, J.; Jiang, J.; Zhou, W.; Yang, H.; Qi, X.; Zhang, H.; Fan, H.J.; Yu, D.Y.W.; Li, C.M.; et al. Seed-assisted synthesis of highly ordered TiO2@α-Fe2O3 core/shell arrays on carbon textiles for lithium-ion battery applications. Energy Environ. Sci. 2012, 5, 6559–6566. [Google Scholar] [CrossRef]
- Lv, X.; Deng, J.; Sun, X. Cumulative effect of Fe2O3 on TiO2 nanotubes via atomic layer deposition with enhanced lithium ion storage performance. Appl. Surf. Sci. 2016, 369, 314–319. [Google Scholar] [CrossRef]
- Chen, M.; Li, W.; Shen, X.; Diao, G. Fabrication of Core–Shell α-Fe2O3@ Li4Ti5O12 Composite and Its Application in the Lithium Ion Batteries. ACS Appl. Mater. Interfaces 2014, 6, 4514–4523. [Google Scholar] [CrossRef]
- Su, L.; Zhong, Y.; Zhou, Z. Role of Transition Metal Nanoparticles in the Extra Lithium Storage Capacity of Transition Metal Oxides: A Case Study of Hierarchical Core–shell Fe3O4@C and Fe@C Microspheres. J. Mater. Chem. A 2013, 1, 15158–15166. [Google Scholar] [CrossRef]
- Ding, R.; Zhang, J.; Zhang, J.; Li, Z.; Wang, C.; Chen, M. Core-shell Fe2N@amorphous carbon nanocomposite-filled 3D graphene framework: An additive-free anode material for lithium-ion batteries. Chem. Eng. J. 2019, 360, 1063–1070. [Google Scholar] [CrossRef]
- Li, Y.; Fu, Y.; Chen, S.; Huang, Z.; Wang, L.; Song, Y. Porous Fe2O3/Fe3O4@Carbon octahedron arrayed on three-dimensional graphene foam for lithium-ion battery. Compos. Part B 2019, 171, 130–137. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miao, Z.; Gao, K.; Li, D.; Gao, Z.; Zhao, W.; Li, Z.; Sun, W.; Wang, X.; Zhang, H.; Wang, X.; et al. Heterointerface Engineered Core-Shell Fe2O3@TiO2 for High-Performance Lithium-Ion Storage. Molecules 2023, 28, 6903. https://doi.org/10.3390/molecules28196903
Miao Z, Gao K, Li D, Gao Z, Zhao W, Li Z, Sun W, Wang X, Zhang H, Wang X, et al. Heterointerface Engineered Core-Shell Fe2O3@TiO2 for High-Performance Lithium-Ion Storage. Molecules. 2023; 28(19):6903. https://doi.org/10.3390/molecules28196903
Chicago/Turabian StyleMiao, Zeqing, Kesheng Gao, Dazhi Li, Ziwei Gao, Wenxin Zhao, Zeyang Li, Wei Sun, Xiaoguang Wang, Haihang Zhang, Xinyu Wang, and et al. 2023. "Heterointerface Engineered Core-Shell Fe2O3@TiO2 for High-Performance Lithium-Ion Storage" Molecules 28, no. 19: 6903. https://doi.org/10.3390/molecules28196903
APA StyleMiao, Z., Gao, K., Li, D., Gao, Z., Zhao, W., Li, Z., Sun, W., Wang, X., Zhang, H., Wang, X., Sun, C., Zhu, Y., & Li, Z. (2023). Heterointerface Engineered Core-Shell Fe2O3@TiO2 for High-Performance Lithium-Ion Storage. Molecules, 28(19), 6903. https://doi.org/10.3390/molecules28196903





