Abstract
To investigate the volatile components of Schisandra chinensis (Turcz.) Bail (commonly known as northern Schisandra) of different colors and to explore their similarities and differences, to identify the main flavor substances in the volatile components of the branch exudates of northern schisandra, and finally to establish a fingerprint map of the volatile components of the dried fruits and branch exudates of northern Schisandra of different colors, we used GC-IMS technology to analyze the volatile components of the dried fruits and branch exudates of three different colors of northern Schisandra and established a fingerprint spectra. The results showed that a total of 60 different volatile chemical components were identified in the branch exudates and dried fruits of Schisandra. The components of germplasm resources with different fruit colors were significantly different. The ion mobility spectrum and OPLS-DA results showed that white and yellow fruits were more similar compared to red fruits. The volatile components in dried fruits were significantly higher than those in branch exudates. After VIP (variable importance in projection) screening, 41 key volatile substances in dried fruits and 30 key volatile substances in branch exudates were obtained. After screening by odor activity value (OAV), there were 24 volatile components greater than 1 in both dried fruits and branch exudates. The most important contributing volatile substance was 3-methyl-butanal, and the most important contributing volatile substance in white fruit was (E)-2-hexenal.
1. Introduction
Schisandra chinensis (Tuncz.) Baill. is a perennial woody vine of the Schisandra family mainly produced in eastern Russia, northeastern China, Korea, and other places. Its fruit is a traditional Chinese medicinal material, commonly known as “Northern Schisandra”. Schisandra contains a variety of medicinal active ingredients, mainly including lignans, polysaccharides, volatile oils, triterpenes, organic acids, etc. [1]. The 2020 edition of the Chinese Pharmacopoeia records 100 prescriptions related to Schisandra, which are usually used to treat various diseases, such as asthma and cardiovascular diseases [2,3]. The volatile oil of Schisandra can also improve cognitive dysfunction in mice [4]. The volatile chemical constituents of Schisandra, a significant part of which is Schisandra essential oil, have been extensively studied for their pharmacological activity. Experimental validation has shown that Schisandra essential oil has anti-inflammatory and neuroprotective effects [5]. The volatile components of Schisandra can significantly alleviate neuronal damage and apoptosis, protecting the hippocampal and cortical structures [6]. These volatile components also alleviate skeletal muscle cell damage in mice [7] and have shown potential resistance to atherosclerosis [8]. Schisandra not only has a pleasant aroma but also has high potential pharmacological activity, making it of great research value in both culinary and medicinal applications. The dried fruits of Schisandra chinensis contain about 20% volatile oil, which mainly consists of terpene compounds [9].
Gas chromatography–ion mobility spectrometry (GC-IMS) is a sensitive and rapid gas phase separation detection technology that has emerged in recent years. It combines ion mobility spectrometry with gas chromatography to generate a two-dimensional spectrum of volatile compounds. This is based on the retention characteristics of the gas chromatography column and the ion mobility rate of the ion mobility spectrometry detector, providing a more convenient, faster, and accurate method of analysis [10]. Furthermore, the sample does not require complex concentration and enrichment, which helps maintain the stability of flavor substances. As such, GC-IMS can be widely used for distinguishing volatile components and isomers, analyzing trace components, and facilitating rapid on-site detection [11]. It is commonly used for odor detection in various fields, such as food and environmental pollution, and is very suitable for the rapid detection of volatile components [12]. GC-IMS technology can be applied to the identification of volatile organic compounds in traditional Chinese medicine materials, and the measurement results can be used as a reference for quality evaluation and variety selection. The University of Chinese Academy of Sciences applied GC-IMS technology to measure the effect of different drying methods on the volatile oil components of wolfberry [13]; Tianjin University of Traditional Chinese Medicine used GC-IMS technology to study the fingerprint of Asarum volatile oil and its chemical pattern recognition [14]; Zhejiang Institute of Food and Drug Inspection established a fingerprint of volatile substances in Aurantium using GC-IMS technology and established a new method for identifying Aurantium [15]. Heilongjiang University of Traditional Chinese Medicine applied GC-IMS technology to measure the volatile oil components of Schisandra, and the results showed that the composition of Schisandra volatile components was complex, including terpenes, aromatics, aliphatics, and other compounds [6].
According to the “Flora of China”, Schisandra is a small red berry that is nearly spherical or obovate in shape, with a diameter of 6–8 mm. Most Schisandra berries are red or dark red, and people often use the depth of the red color as a sensory indicator to evaluate the quality of Schisandra. As a result, the color of Schisandra berries has been subjected to directional selection pressure during natural evolution. White and yellow germplasms, as freely segregating offspring, are very precious germplasm resources. The Schisandra National Forest Germplasm Bank of the Institute of Special Products of the Chinese Academy of Agricultural Sciences has collected white and yellow Schisandra germplasm resources and used SRAP technology to analyze the genetic rules of fruit color. The results show that the genotype of red Schisandra is dominant homozygous, white is recessive homozygous, and yellow fruit is heterozygous [16]. However, there have been no reports on the differences in volatile components between different fruit colors. In this study, we measured the volatile components of dried fruits and branch exudates of three different fruit colors of Schisandra and established a fingerprint map of volatile components of different fruit colors of Schisandra. Using principal component analysis, cluster analysis, OAV value analysis, OPLS-DA analysis and other methods, we screened the key aroma components of Schisandra and qualitatively analyzed the key volatile components of different fruit colors of Schisandra fruits. This lays a foundation for germplasm identification, quality evaluation, variety selection, and development of volatile compounds in dried fruits and branch exudates.
2. Results and Discussion
2.1. Analysis of Ion Migration Spectra of Schisandra chinensis Branch Sap and Dried Fruits of Different Colors
Using GC-IMS technology to analyze the volatile components of branch sap and dried fruits of three different colors, the two-dimensional ion migration spectra of volatile organic compounds obtained are shown in Figure 1 and Figure 2. The horizontal axis represents the ion migration time, and the vertical axis represents the gas chromatographic retention time. The red vertical line at 1.0 on the horizontal axis is the RIP (reaction ion peak), and the dots shown in the picture are the volatile organic compounds detected in the sample. From white to red, the deeper the color, the higher the relative concentration of volatile organic compounds.
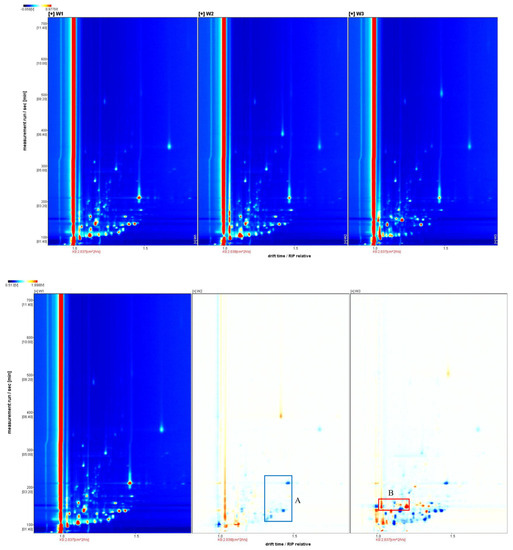
Figure 1.
Two-dimensional ion migration spectra (top) and differential spectra (bottom) of white, yellow, and red Schisandra chinensis branch sap. A is the substance that is less in the W2 sample compared to the other samples, B is the substance that is increased in the W3 sample compared to the other samples.
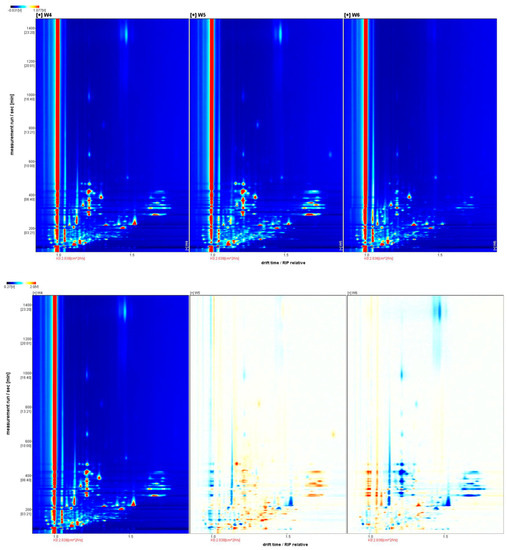
Figure 2.
Twodimensional ion migration spectra (top) and differential spectra (bottom) of white, yellow, and red Schisandra chinensis dried fruits.
2.1.1. Ion Migration Spectra Analysis of White, Yellow, and Red Fruit Branch Sap
Figure 1 shows that the peak positions of W1 and W2 in the three ion migration spectra are close, and the composition of W3 is significantly different from that of W1 and W2. Taking W1 as a reference and subtracting the signal peaks of W1 in the remaining spectra, a differential spectrum can be obtained. In the differential spectrum, blue dots indicate that the corresponding volatile organic compounds in this sample are reduced compared to those at the same position in W1. Red dots indicate that this substance contains more than W1, and the deeper the color, the greater the difference. In the differential spectrum, it can be found that compared with W1, except for some positions where the content of W2 is reduced, there are fewer volatile organic compound difference points. The content difference of volatile organic compounds in W3 is more significant, and there are more difference substances compared with W1 and W2.
2.1.2. Ion Migration Spectra Analysis of White, Yellow, and Red Schisandra chinensis Dried Fruits
The ion migration spectra of dried fruits of different colors are shown in Figure 2. It can be seen that there are more points separated in the dried fruit spectra than in the branch sap spectra. By judging the peak positions, it can be preliminarily seen that the components of W4 and W5 are more similar and significantly different from those of W6. The differential spectrum shows that there are more blue dots in W6, indicating that the relative content of some volatile chemical substances in W4 and W5 is higher than that in W6.
2.2. Qualitative Analysis of Volatile Chemical Substances in White, Yellow, and Red Fruit Branch Sap and Dried Fruits
The VoCal software 0.4.03built into the flavor analyzer is used to analyze the ion migration spectra. The NIST and IMS databases built into the software are used to organize and compare the original data in the GC-IMS spectra to obtain the fingerprint spectra of the sample’s volatile chemical substances. The experiment uses 4-methyl-2-pentanol as an internal standard. By comparing the actual retention index and ion drift time of different substances with the database, it is possible to compare volatile chemical substances. The peak volume of volatile chemical substances is calculated, and the concentration of chemical substances can be obtained by calculating using the internal standard method, achieving quantification of chemical substances in different samples. Volatile compounds that are not identified are represented by numerical codes.
As shown in Figure 3 and Figure 4, the difference in the content of each substance can be intuitively seen from the depth of the colors on the fingerprint spectra. The establishment of fingerprint spectra visualizes the spectral data and directly helps in the directional selection of germplasm resources rich in special chemical substances (odor thresholds: compilations of odor threshold values in air, water, and other media (edition 2011)). Fingerprint spectra can also be used as a reference for the evaluation and identification of Schisandra chinensis germplasm resources in the future. Consistent with the results of the migration nursery, the number and types of volatile substances in Schisandra chinensis dried fruits are significantly higher than those in Schisandra chinensis branch sap, indicating that the aroma of Schisandra chinensis dried fruits is richer than that of branch sap. W1 and W2 have high similarity, W4 and W5 have high similarity, and the fingerprint spectra of white and yellow fruits are more similar.
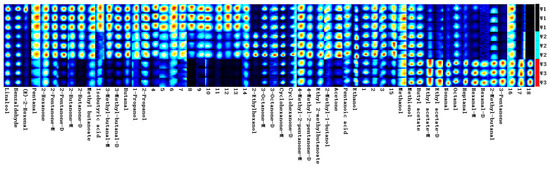
Figure 3.
Fingerprint spectra of white, yellow, and red Schisandra chinensis branch sap.
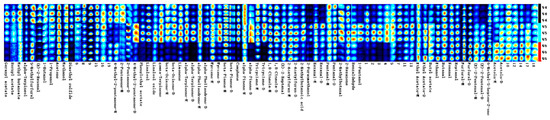
Figure 4.
Fingerprint spectra of white, yellow, and red Schisandra chinensis dried fruits.
2.3. Classification of Volatile Chemical Substances in Branch Sap and Dried Fruits
The volatile compounds in dried fruits and branch exudates can be classified into alkenes, ketones, aldehydes, alcohols, esters, and others (as shown in Figure 5). Overall, the amount of volatile substances detected in dried fruits is higher than that in branch exudates. Among them, alkenes, alcohols, ketones, and aldehydes are present in high amounts in dried fruits. The volatile components in branch exudates are mainly alcohols, ketones, and aldehydes. Alkenes are the high-content volatile substances in dried fruits. The total amount of volatile substances in white and yellow fruits is relatively high, both in dried fruits and branch exudates. The content of alkenes is the highest in W4 and W5. In the corresponding W1 and W2, the content of ketones is the highest, followed by alcohols. The content of alcohols is relatively high in W3. The qualitative analysis results are shown in Table 1. A total of 83 volatile compounds were identified.
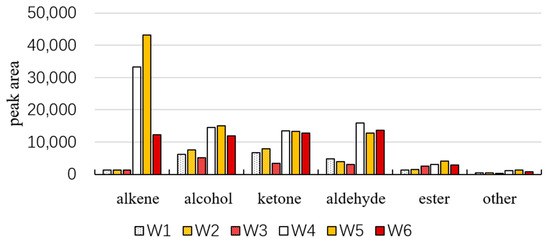
Figure 5.
Peak area of different types of compounds in Schisandra.

Table 1.
Volatile chemical substances detected in samples.
2.4. Principal Component Analysis and OPLS-DA Analysis of Samples
The peak volume data of monomers and dimers of the same chemical substance detected were merged and the experimental data obtained from six injections were analyzed by principal component analysis (PCA-X), orthogonal partial least squares discriminant analysis (OPLS-DA) and 200 times permutation test using SIMCA 14.0 software. The branch exudates and Schisandra dried fruits were divided into two groups, and the VIP values of each component were calculated. SPSS 27 software was used to perform one-way ANOVA analysis and homogeneity of variance test on the chemical components of branch exudates and Schisandra dried fruits, respectively, to obtain p values. Volatile components with special contributions were screened using p value < 0.05 and VIP value > 1 as the criteria for further data analysis.
A total of 60 different volatile chemical components were identified in Schisandra branch exudates and dried fruits using GC-IMS. With 60 common components as dependent variables and dried fruits, branch exudates and different fruit colors as independent variables divided into six groups, PCA-X and OPLS-DA analysis of samples of Schisandra dried fruits and branch exudates of different colors can achieve effective differentiation.
The volatile chemical components obtained were processed using PCA, resulting in the data shown in Figure 6. The R2X (1) = 0.648 and the R2X (2) = 0.13, indicating that the model samples have good reliability. It can be observed that there is a good distinction between the dried fruits and branch exudates, and the differences between different groups are also noticeable. The data obtained from PCA was further processed and analyzed. In the OPLS-DA analysis (Figure 7 and Figure 8), the independent variable fitting index R2X = 0.972, the dependent variable fitting index R2Y = 0.992, and the model prediction index Q2 = 0.965 are all greater than 0.5, and the fitting result is acceptable [17]. Then, 200 permutation analyses were performed on all experimental data to verify whether the model is effective (Figure 9). As shown in the figure above, the intersection of the Q2 regression line with the vertical axis is less than 0, indicating that the model is effective, that there is no overfitting, and that the analysis is effective.
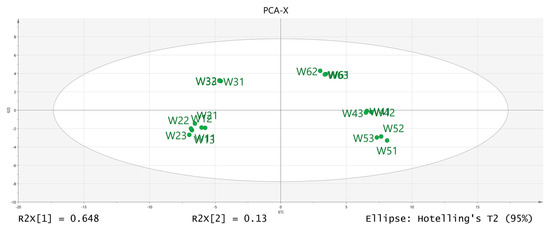
Figure 6.
Results of PCA-X (principal component analysis) analysis.
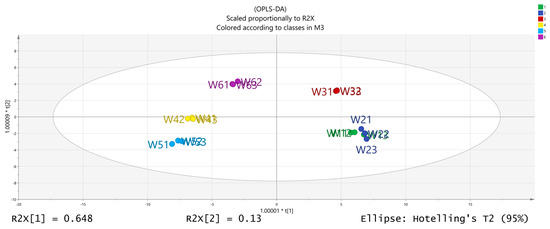
Figure 7.
Results of OPLS-DA (orthogonal partial least squares discriminant analysis) analysis.
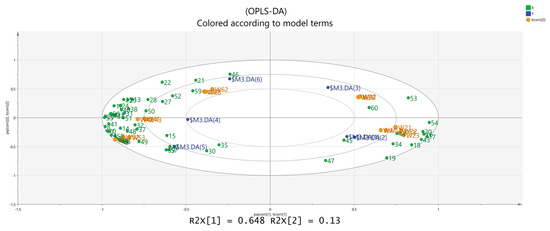
Figure 8.
Biplot of OPLS-DA analysis.
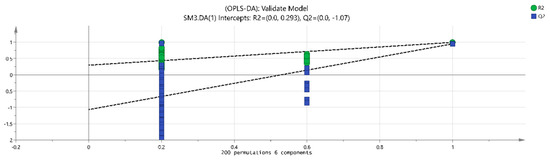
Figure 9.
Permutation retention.
The results show that there are significant differences in the main component composition of Schisandra of different colors. The biplot shows that the dried fruit samples are more closely related to more volatile components, indicating that the flavor of Schisandra dried fruits is richer and the main flavor components of branch exudates are significantly different from those of dried fruits.
Schisandra branch exudates and dried fruits were divided into two groups and OPLS-DA analysis was performed separately. The VIP values of the odor components of Schisandra branch exudates and dried fruits were calculated separately to screen key volatile components. At the same time, SPSS software was used to perform one-way ANOVA analysis to calculate the significance of single components. Lists with p < 0.05 and VIP > 1 were screened separately for further discussion(Table 2).

Table 2.
Different volatile components of Schisandra branch exudates and dried fruits of different colors.
The screening results show that there are 30 key volatile substances in branch exudates and 41 key volatile substances in dried fruits in terms of content. The VIP values show that the chemical substances with the greatest contribution in the branch exudate group are: 2-ethylhexanol (1.234), ethanol (1.219), acetone (1.193), 2-hexanone (1.189), and 1-propanol (1.186); in the dried fruit group, the key substances with the greatest contribution are: (E)-2-heptenal (1.159), 5-methylfurfural (1.158), cyclohexanone (1.154), (E)-2-hexenal (1.153), and 2-acetylfuran (1.153). The results indicate that the aroma composition of Schisandra dried fruits and branch exudates is complex, with many chemical substances contributing significantly, and there is a significant difference between the volatile substances of branch exudates and dried fruits.
In order to further visually display the contribution of different aroma component contents to different samples, ORIGIN was used to perform differential analysis on the aroma of Schisandra branch exudates and dried fruits. According to the criteria of p < 0.05 and VIP > 1, flavor substances were screened and classified [18]. The peak area was normalized, and cluster heat maps were made separately. The red part in the figure can directly show the classification according to the different component contents and can also see the highest and most prominent volatile substances (Figure 10 and Figure 11).
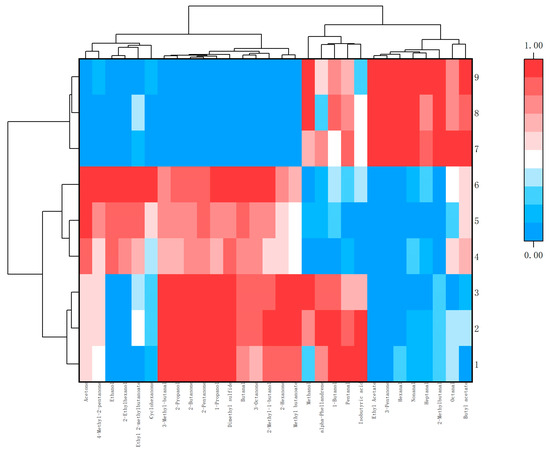
Figure 10.
Cluster heat map of differential aroma components in branch exudates.
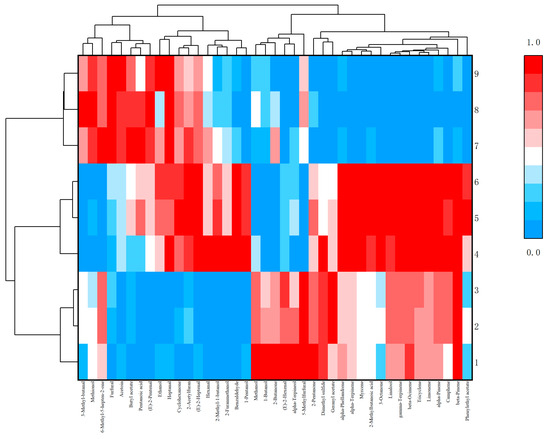
Figure 11.
Cluster heat map of differential aroma components in dried fruits.
2.5. Analysis of OAV Values of Schisandra chinensis Branch Exudates and Dried Fruit and Screening of Key Flavor Substances
VIP values can only indicate the contribution of volatile components, and do not fully represent the composition of odors. The true olfactory composition of Schisandra chinensis branch exudates and dried fruit cannot be determined solely by VIP values. The introduction of odor activity values (OAV) is necessary to determine the aroma characteristics of Schisandra chinensis branch exudates and dried fruit. Calculating OAV values can more intuitively show the contribution of a single component to the overall aroma. It is generally believed that: OAV > 1 can be considered to have a certain impact on the aroma of the sample, and OAV > 10 proves that the aroma component has a great impact on the aroma of the sample. Calculate the OAV values and standard deviations of each aroma component in the branch exudates and Schisandra chinensis dried fruit, and screen according to the standard of OAV > 1, and then perform correlation analysis.
The internal standard method is used to quantify the chemical substances detected in six groups of samples. The internal standard is 4-methyl-2-pentanol with a concentration of 198 ppb and a signal peak volume of 504.95. Therefore, the intensity of each signal peak is about 0.392 ppb. The detected chemical substances are analyzed and calculated to finally obtain the concentration and standard deviation of three repeated injections of each chemical substance. Schisandra chinensis dried fruit selects the air threshold, and Schisandra chinensis branch exudates selects the water threshold. A total of 57 components can query the threshold value and calculate according to the method described above.
After screening, W1 has 15 aroma components with OAV values greater than 1 and 8 aroma components greater than 10; W2 has 15 aroma components with OAV values greater than 1 and 8 aroma components greater than 10; W3 has 14 aroma components with OAV values greater than 1 and 8 aroma components greater than 10; W4 has 24 aroma components with OAV values greater than 1 and 12 aroma components greater than 10; W5 has a total of 22 aroma components with OAV values greater than 1 and 14 aroma components greater than 10; W6 has a total of 22 aroma components with OAV values greater than 1 and 10 aroma components greater than 10. Except for the white dried fruit, the main contributing aroma is (E)-2-hexenal, and the main contributing aroma of the remaining samples is all 3-methyl-butanal (Table 3).

Table 3.
Odor activity values (OAV) of Schisandra chinensis branch exudates and dried fruit of different fruit colors.
2.6. Discussion
Through the screening of OAV values, the key flavor substance in white fruit dried fruit is (E)-2-hexenal. This substance is also the main aroma-contributing substance of Chinese jujubes, with a strong plant aroma (fresh green, multi-leaf, rich fruit flavor) flavor characteristics [4] (http://www.thegoodscentscompany.com/search2.html, accessed on 28 March 2023). Both Schisandra chinensis dried fruit and branch exudates show that 3-Methyl-butanal is the key flavor substance of Schisandra chinensis. This substance is the main aroma component of green tea that has been killed and has elegant peach, chocolate, and fat aromas [19]. It can be used as a characteristic volatile label substance for Schisandra chinensis identification. 1,8-cineole in dried fruit is also a major contributing aroma substance with a cool mint and camphor smell. It is the main aroma component of Australian grape wine and is also used as a food additive and spice [20]. At the same time, chemical substances such as β-ocimene and myrcene with high OAV values have also been found in traditional Chinese medicines such as qianhu and have obvious aromatic odors (Surendran et al. 2021; Jovanović et al. 2015 [21]). There are a total of 24 volatile chemical substances with OAV > 1 in Schisandra chinensis dried fruit and branch exudates. The aroma substances are rich and have high potential for tea, seasoning, and spice development.
3. Materials and Methods
3.1. Experimental Materials
Six-year-old Schisandra trees were used as materials. White fruit germplasm (3N2S2), yellow fruit germplasm (variety “Jinwuwei No. 1”) and red fruit germplasm (variety “Yanzhihong”) were all collected from the Schisandra National Forest Germplasm Resource Bank of the Institute of Special Products of the Chinese Academy of Agricultural Sciences (Figure 12). Three clonal plants were selected for each resource. The three different-colored Schisandra fruits were harvested when they were fully ripe in September 2019. After removing the stems and branches in the laboratory, they were dried in the shade at 22 °C. They were completely dried after one month and then vacuum-sealed and stored in a refrigerator at 4 °C, respectively. In March of the following spring, branch exudates from the above germplasms were collected, placed in 50 mL centrifuge tubes, and labeled as white juice, yellow juice, and red juice. Three parallel samples were set up for each sample. W1, W2, and W3 are branch exudates of white-fruit, yellow-fruit, and red-fruit Schisandra, respectively. W4, W5, and W6 are dried fruits of white-fruit, yellow-fruit, and red-fruit Schisandra, respectively.

Figure 12.
White, yellow, and red Schisandra fruits.
3.2. Experimental Methods
3.2.1. Sample Pre-Treatment
Take 1 mL of fresh branch exudate and place it in a 20 mL headspace vial. Incubate at 50 °C for 15 min before sampling. Take 1 g of the sample and crush it, then place it in a 20 mL headspace vial. Incubate at 50 °C for 15 min before sampling.
3.2.2. GC-IMS Conditions
The FlavourSpec® flavor analyzer (GAS Company, Jinan, China) was used in the experiment. The operating conditions of the detector were as follows: input voltage: 220 V, frequency 50 Hz, ambient temperature 22 °C, humidity 42%, and the gas used was high-purity nitrogen (99.999%). The detector’s ionization source was a tritium source (H3), with the type of radiation being beta radiation. The radiation energy of the ionization source was 6.5 KeV. The length of the drift tube was 98 mm, and the voltage of the drift tube was 5000 V.
Gas phase–ion mobility spectrometry unit: analysis time 25 min; column type: MXT-5, length 15 m, inner diameter 0.53 mm, film thickness 1 μm; column temperature 60 °C; carrier gas/drift gas: N2; IMS temperature: 45 °C.
Automatic headspace sampler unit: sample volume 400 μL; incubation time: 15 min; incubation temperature: 50 °C; sample needle temperature: 55 °C; incubation speed: 500 rpm.
The initial carrier gas flow rate is set to 2 mL/min for 0–2 min, and the carrier gas flow rate is increased to 100 mL/min for 2–20 min. The carrier gas flow rate is maintained at 100 mL/min for 20–25 min until the end.
3.2.3. Data Processing Conditions
Preliminary analysis: Use the VOCal analysis software that comes with the FlavourSpec® flavor analyzer. The VOCal software can be used to analyze the spectra output from the instrument and perform qualitative and quantitative data analysis. The built-in IMS and NIST databases can be used to compare and qualitatively identify volatile substances. After establishing a standard curve for the identified substances, quantitative analysis can be performed. The database is equipped with three plug-ins: Reporter, Gallery Plot, and Dynamic PCA. The Reporter plug-in can directly compare spectral differences in two-dimensional, three-dimensional and other aspects; the Gallery Plot plug-in can compare the output fingerprint spectra and quantitatively and intuitively compare the differences in volatile substances between different samples; the Dynamic PCA plug-in can perform dynamic principal component analysis and similarity analysis to quickly identify unknown samples [22].
Quantitative calculation of chemical components:
Ci is the calculated mass concentration of the chemical component, in μg/L; Cis is the mass concentration of the internal standard substance. The internal standard substance used in the experiment is 4-methyl-2-pentanol, with a concentration of 198 μg/L; Ai is the signal peak volume of the chemical component; Ais is the signal peak volume of the internal standard substance.
3.2.4. OAV Value Analysis
The OAV analysis method will be used to analyze and screen the main aroma components. The calculation of the OAV value is related to the threshold value of the main aroma component itself. The selection of the threshold value mainly refers to the book “ODOUR THRESHOLDS”. Due to different years and different statistical methods, there may be differences in the same substance. The principle for selecting the threshold value in this experiment is: the air threshold value is selected for Schisandra dried fruit, and the water threshold value is selected for Schisandra branch exudate. All threshold values are selected from the latest data (odor thresholds: compilations of odor threshold values in air, water and other media).
Calculation of OAV value:
C—the amount of substance, in units of mg/m3 (in air), mg/kg (in water); OT—the threshold value of the substance itself.
3.2.5. OPLS-DA Analysis
OPLS-DA analysis, also known as orthogonal partial least squares discriminant analysis, is used to observe the clustering of samples based on principal component analysis (PCA) data analysis. The OPLS-DA analysis model is then used to screen VIP values, identify differential variables, and locate key differential volatile substances. This part was used by SIMCA and SPSS.
4. Conclusions
This study applied GC-IMS technology for the first time to analyze volatile chemical substances in Schisandra chinensis branch exudates and dried fruit of different colors. The volatile substance fingerprint of white-, yellow-, and red-fruit Schisandra chinensis branch exudates and dried fruit was established. This fingerprint can effectively distinguish Schisandra chinensis dried fruit and branch exudates of different colors and can be directly used for quality characteristic evaluation and germplasm identification.
Different algorithms, such as PCA-X, one-way ANOVA, OPLS-DA, and OAV were used to compare and analyze the volatile components of Schisandra chinensis. The results showed significant differences in content among the aroma components of Schisandra chinensis branch exudates and dried fruit. The content and number of high-content types of aroma components in Schisandra chinensis dried fruit are higher than those in branch exudate samples. Comparing between different fruit colors, it was found that the aroma composition of white fruit and yellow fruit Schisandra chinensis is closer, with red fruit Schisandra chinensis significantly different from yellow fruit and white fruit.
The experiment screened out key volatile chemical substances of dried fruits and branch exudates through VIP value and p value joint screening. The key volatile substances of overripe fruits are more abundant than those of branch exudates, with complex composition and significant differences. These findings highlight the importance of considering both plant part and maturity stage in analyzing volatile components, providing valuable insights for future research in plant chemistry.
Author Contributions
S.F., Y.Y. (Yiming Yang) and X.H. cultivated and provided the germplasm resources; T.T., N.S. and Y.G. were responsible for the data collection and processing of the paper; Y.Y. (Yiping Yan) and P.X. were responsible for data analysis and the writing of the entire paper; special thanks to researcher W.L., who was responsible for the revision of the paper and team organization and project support. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by Central Public-Interest Scientific Institution Basal Research Fund (No. 1610342023012) and Science and Technology Development Plan Project of Jilin Province, China (20200404011YY).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All related data and methods are presented in this paper. Additional inquiries should be addressed to the corresponding author.
Acknowledgments
Thanks to the Fruit Research Team of the Special Research Institute of the Chinese Academy of Agricultural Sciences for providing germplasm resources cultivation and experimental equipment; thanks to Xu for all the help in writing the paper; thanks to Xulin Zhang, Bowei Sun, and Hang Xu of the institute for their suggestions and help in revision; thanks to Jinli Wen, Yanli He, and Weiyu Cao for their technical support.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Not applicable.
References
- Liu, H.; Guo, J.; Wang, Z.; Chen, Y.; Wu, G.; Yang, B.; Kuang, H. Analysis of Terpenes, Aromatic and Aliphatic Compounds in Schisandra chinensis Essential Oil. Chem. Eng. 2016, 30, 27–29+32. [Google Scholar] [CrossRef]
- Olas, B. Cardioprotective Potential of Berries of Schisandra chinensis Turcz. (Baill.), Their Components and Food Products. Nutrients 2023, 15, 592. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Xiao, Q.; Liu, J.; Mu, X.; Zhang, J.; Qi, Y.; Zhang, B.; Xiao, P.; Liu, H. Chemical Characterization and Potential Mechanism of the Anti-Asthmatic Activity of a Subfraction from Schisandra chinensis Fruit Extract. J. Agric. Food Chem. 2022, 70, 5015–5025. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Song, J.; Bi, J.; Meng, X.; Wu, X. Characterization of Volatile Profile from Ten Different Varieties of Chinese Jujubes by HS-SPME/GC–MS Coupled with E-Nose. Food Res. Int. 2018, 105, 605–615. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Zhang, X.; Ren, F.; Yan, T.; Wu, B.; Bi, K.; Bi, W.; Jia, Y. Essential Oil of Schisandra chinensis Ameliorates Cognitive Decline in Mice by Alleviating Inflammation. Food Funct. 2019, 10, 5827–5842. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.; Yang, Y.; Peng, J.; Zhang, Y.; Wu, B.; He, B.; Jia, Y.; Yan, T. Schisandra chinensis (Turcz.) Baill. Essential Oil Exhibits Antidepressant-like Effects and against Brain Oxidative Stress through Nrf2/HO-1 Pathway Activation. Metab. Brain Dis. 2022, 37, 2261–2275. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.S.; Han, M.H.; Kim, G.-Y.; Kim, C.M.; Chung, H.Y.; Hwang, H.J.; Kim, B.W.; Choi, Y.H. Schisandrae Semen Essential Oil Attenuates Oxidative Stress-Induced Cell Damage in C2C12 Murine Skeletal Muscle Cells through Nrf2-Mediated Upregulation of HO-1. Int. J. Mol. Med. 2015, 35, 453–459. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.-W.; Kim, J.W.; Ku, S.K.; Kim, S.G.; Kim, K.Y.; Kim, G.-Y.; Hwang, H.J.; Kim, B.W.; Chung, H.Y.; Kim, C.M.; et al. Essential Oils Purified from Schisandrae Semen Inhibits Tumor Necrosis Factor-α-Induced Matrix Metalloproteinase-9 Activation and Migration of Human Aortic Smooth Muscle Cells. BMC Complement. Altern. Med. 2015, 15, 7. [Google Scholar] [CrossRef]
- Teng, H.; Lee, W.Y. Antibacterial and Antioxidant Activities and Chemical Compositions of Volatile Oils Extracted from Schisandra chinensis Baill. Seeds Using Simultaneous Distillation Extraction Method, and Comparison with Soxhlet and Microwave-Assisted Extraction. Biosci. Biotechnol. Biochem. 2014, 78, 79–85. [Google Scholar] [CrossRef]
- Xie, H.; Meng, L.; Guo, Y.; Xiao, H.; Jiang, L.; Zhang, Z.; Song, H.; Shi, X. Effects of Volatile Flavour Compound Variations on the Varying Aroma of Mangoes “Tainong” and “Hongyu” during Storage. Molecules 2023, 28, 3693. [Google Scholar] [CrossRef]
- Wen, J.; Wang, Y.; Cao, W.; He, Y.; Sun, Y.; Yuan, P.; Sun, B.; Yan, Y.; Qin, H.; Fan, S.; et al. Comprehensive Evaluation of Ten Actinidia Arguta Wines Based on Color, Organic Acids, Volatile Compounds, and Quantitative Descriptive Analysis. Foods 2023, 12, 3345. [Google Scholar] [CrossRef] [PubMed]
- Vautz, W.; Franzke, J.; Zampolli, S.; Elmi, I.; Liedtke, S. On the Potential of Ion Mobility Spectrometry Coupled to GC Pre-Separation—A Tutorial. Anal. Chim. Acta 2018, 1024, 52–64. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Hu, N.; Wang, H. Comparison of the Effects of Different Drying Methods on Volatile Substances in Goji Berries from Qaidam Based on GC-IMS. Res. Dev. Nat. Prod. 2022, 34, 1989–1998+2033. [Google Scholar] [CrossRef]
- Chai, S.; Miao, X.; Yao, G.; He, J. Establishment of GC-MS Fingerprint of Volatile Oil in Asarum from Different Origins and Its Chemical Pattern Recognition Study. Mod. Drugs Clin. 2023, 38, 71–76. [Google Scholar]
- Fang, C.; He, J.; Xiao, Q.; Chen, B.; Zhang, W. Development of the Volatile Fingerprint of Qu Aurantii Fructus by HS-GC-IMS. Molecules 2022, 27, 4537. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Li, C.; Xu, P.; Li, X.; Wang, Z.; Zhao, Y.; Ai, J. Identification of SRAP Markers Related to Fruit Color in Schisandra chinensis. Biotechnology 2015, 25, 147–150. [Google Scholar] [CrossRef]
- Shao, S.; Xu, M.; Lin, Y.; Chen, X.; Fang, D.; Cai, J.; Wang, J.; Jin, S.; Ye, N. Analysis of Aroma Differences in Tieguanyin Oolong Tea from Different Origins Based on Electronic Nose and HS-SPME-GC-MS Technology. Food Sci. 2023, 44, 232–239. [Google Scholar]
- Yun, J.; Cui, C.; Zhang, S.; Zhu, J.; Peng, C.; Cai, H.; Yang, X.; Hou, R. Use of Headspace GC/MS Combined with Chemometric Analysis to Identify the Geographic Origins of Black Tea. Food Chem. 2021, 360, 130033. [Google Scholar] [CrossRef]
- Wang, H.; Hua, J.; Jiang, Y.; Yang, Y.; Wang, J.; Yuan, H. Influence of Fixation Methods on the Chestnut-like Aroma of Green Tea and Dynamics of Key Aroma Substances. Food Res. Int. 2020, 136, 109479. [Google Scholar] [CrossRef]
- Capone, D.L.; Van Leeuwen, K.; Taylor, D.K.; Jeffery, D.W.; Pardon, K.H.; Elsey, G.M.; Sefton, M.A. Evolution and Occurrence of 1,8-Cineole (Eucalyptol) in Australian Wine. J. Agric. Food Chem. 2011, 59, 953–959. [Google Scholar] [CrossRef]
- Jovanović, O.P.; Zlatković, B.K.; Jovanović, S.Č.; Petrović, G.; Stojanović, G.S. Composition of Peucedanum Longifolium Waldst. & Kit. Essential Oil and Volatiles Obtained by Headspace. J. Essent. Oil Res. 2015, 27, 182–185. [Google Scholar] [CrossRef]
- Cao, W.; Shu, N.; Wen, J.; Yang, Y.; Jin, Y.; Lu, W. Characterization of the Key Aroma Volatile Compounds in Nine Different Grape Varieties Wine by Headspace Gas Chromatography–Ion Mobility Spectrometry (HS-GC-IMS), Odor Activity Values (OAV) and Sensory Analysis. Foods 2022, 11, 2767. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).