Thermal-Induced Performance Decay of the State-of-the-Art Polymer: Non-Fullerene Solar Cells and the Method of Suppression
Abstract
:1. Introduction
2. Results and Discussion
2.1. Performance and Thermal Stability of the PM6:Y6 Cells
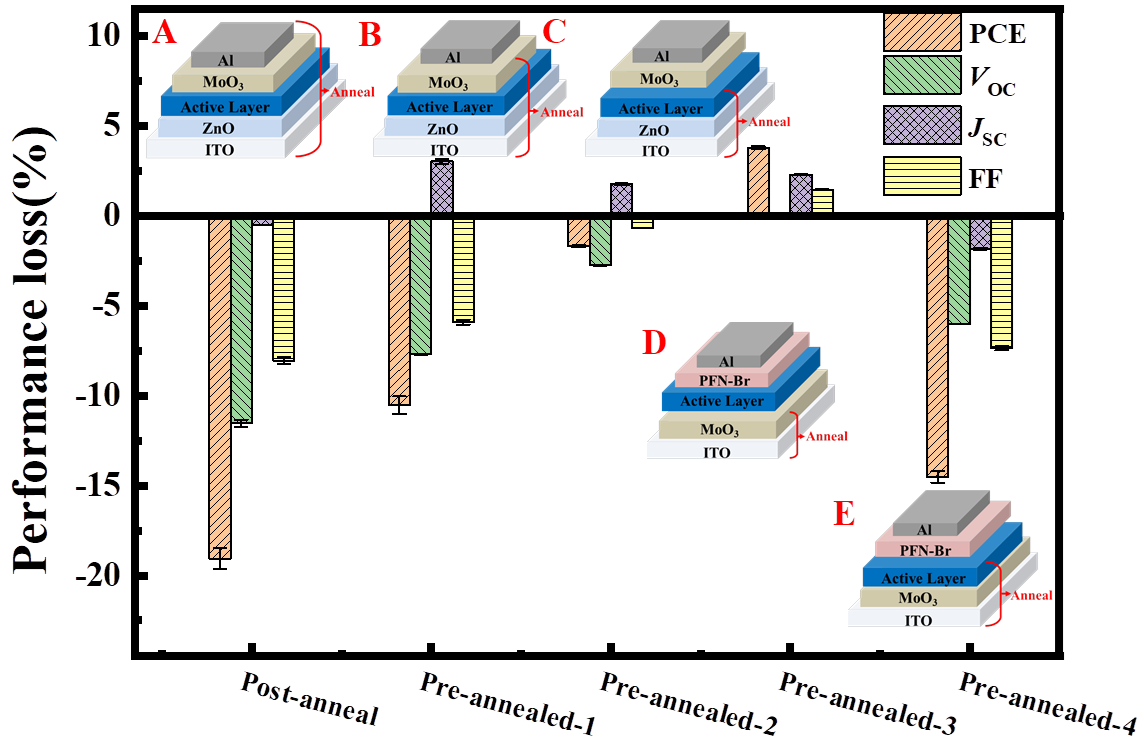
2.2. Identifying the Interface Degradation of the Cells
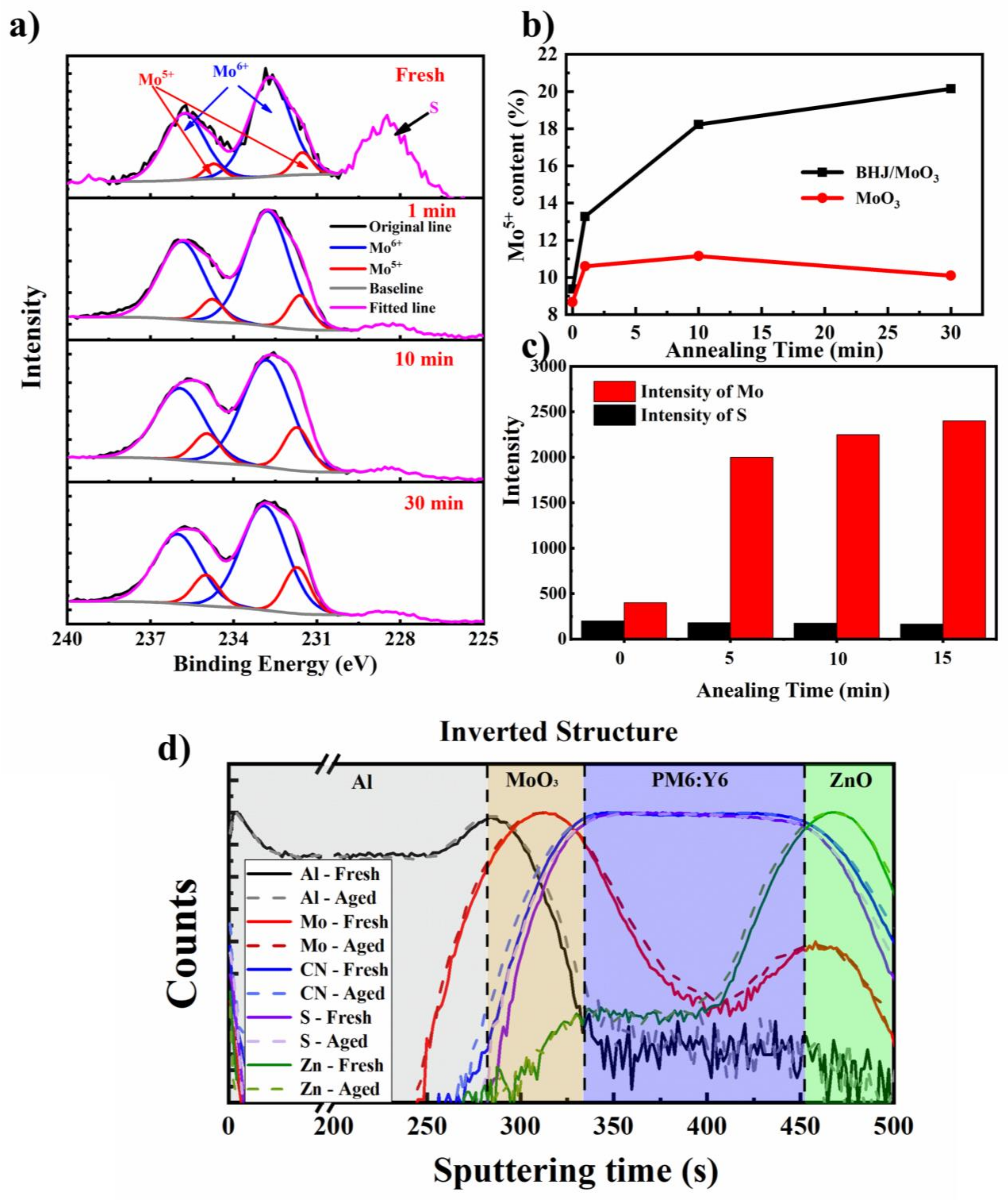
2.3. Interactions at the Polymer/MoO3 Interface upon Thermal Annealing
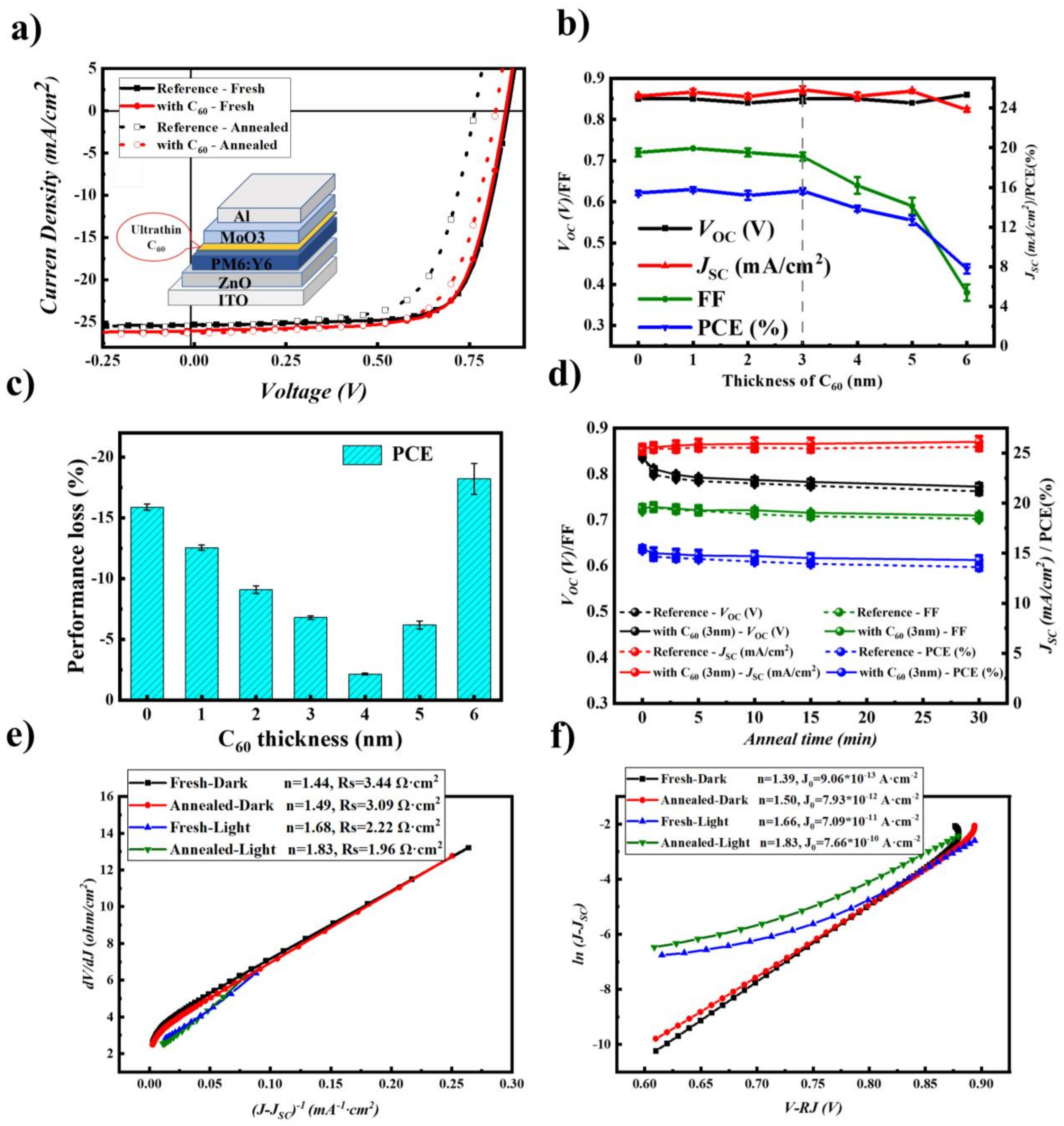
2.4. Stability Improvement by Inserting a C60 Layer
3. Materials and Methods
3.1. Materials
3.2. Organic Solar Cells Device Fabrication Methods
3.3. Organic Solar Cells Performance Characterizations
3.4. Instruments and Measurement
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Wang, Y.W.; Lee, J.H.; Hou, X.Y.; Labanti, C.; Yan, J.; Mazzolini, E.; Parhar, A.; Nelson, J.; Kim, J.S.; Li, Z. Recent Progress and Challenges toward Highly Stable Nonfullerene Acceptor-Based Organic Solar Cells. Adv. Energy Mater. 2021, 11, 2003002. [Google Scholar] [CrossRef]
- Zhao, F.W.; Zhang, H.T.; Zhang, R.; Yuan, J.; He, D.; Zou, Y.P.; Gao, F. Emerging Approaches in Enhancing the Efficiency and Stability in Non-Fullerene Organic Solar Cells. Adv. Energy Mater. 2020, 10, 2002746. [Google Scholar] [CrossRef]
- Wadsworth, A.; Moser, M.; Marks, A.; Little, M.S.; Gasparini, N.; Brabec, C.J.; Baran, D.; McCulloch, I. Critical Review of the Molecular Design Progress in Non-fullerene Electron Acceptors Towards Commercially Viable Organic Solar Cells. Chem. Soc. Rev. 2019, 48, 1596–1625. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Zheng, M.; Xu, J.Q.; Li, C.; Yan, J.; Zhou, G.Q.; Zhong, W.K.; Hao, T.Y.; Song, J.L.; Xue, X.N.; et al. Single-junction Organic Solar Cells with Over 19% Efficiency Enabled by a Refined Double-fibril Network Morphology. Nat. Mater. 2022, 21, 656–663. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Son, H.J. Intrinsic Photo-Degradation and Mechanism of Polymer Solar Cells: The Crucial Role of Non-fullerene Acceptors. J. Mater. Chem. A 2019, 7, 25830–25837. [Google Scholar] [CrossRef]
- Jiang, Y.Y.; Sun, L.L.; Jiang, F.Y.; Xie, C.; Hu, L.; Dong, X.Y.; Qin, F.; Liu, T.F.; Hu, L.; Jiang, X.S.; et al. Photocatalytic Effect of ZnO on the Stability of Nonfullerene Acceptors and its Mitigation by SnO2 for Nonfullerene Organic Solar Cells. Mater. Horiz. 2019, 6, 1438–1443. [Google Scholar] [CrossRef]
- Ryu, S.Y.; Ha, N.Y.; Ahn, Y.H.; Park, J.Y.; Lee, S. Effects of Oxygen Vacancies in a Zinc Oxide Electron Transport Layer on Long-Term Degradation and Short-Term Photo-Induced Changes in the Operation Characteristics of Organic Solar Cells. ACS Appl. Energy Mater. 2022, 5, 9668–9675. [Google Scholar] [CrossRef]
- Liu, B.W.; Han, Y.F.; Li, Z.R.; Gu, H.M.; Yan, L.P.; Lin, Y.; Luo, Q.; Yang, S.F.; Ma, C.-Q. Visible Light-Induced Degradation of Inverted Polymer:Nonfullerene Acceptor Solar Cells: Initiated by the Light Absorption of ZnO Layer. Sol. RRL 2021, 5, 2000638. [Google Scholar] [CrossRef]
- Han, Y.F.; Dong, H.L.; Pan, W.; Liu, B.W.; Chen, X.Z.; Huang, R.; Li, Z.Y.; Li, F.S.; Luo, Q.; Zhang, J.Q.; et al. An Efficiency of 16.46% and a T80 Lifetime of Over 4000 h for the PM6:Y6 Inverted Organic Solar Cells Enabled by Surface Acid Treatment of the Zinc Oxide Electron Transporting Layer. ACS Appl. Mater. Interfaces 2021, 13, 17869–17881. [Google Scholar] [CrossRef]
- Liu, B.W.; Su, X.; Lin, Y.; Li, Z.R.; Yan, L.P.; Han, Y.F.; Luo, Q.; Fang, J.; Yang, S.F.; Tan, H.W.; et al. Simultaneously Achieving Highly Efficient and Stable Polymer:Non-Fullerene Solar Cells Enabled By Molecular Structure Optimization and Surface Passivation. Adv. Sci. 2022, 9, 2104588. [Google Scholar] [CrossRef]
- Hu, L.; Jiang, Y.Y.; Sun, L.L.; Xie, C.; Qin, F.; Wang, W.; Zhou, Y.H. Significant Enhancement of Illumination Stability of Nonfullerene Organic Solar Cells via an Aqueous Polyethylenimine Modification. J. Phys. Chem. Lett. 2021, 12, 2607–2614. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Xiao, J.Y.; Zhang, G.C.; Wei, L.; Jiao, X.C.; Yip, H.L.; Cao, Y. Interface-enhanced Organic Solar Cells with Extrapolated T-80 Lifetimes of over 20 Years. Sci. Bull. 2020, 65, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Hong, L.; Yao, H.F.; Cui, Y.; Yu, R.N.; Lin, Y.W.; Chen, T.W.; Xu, Y.; Qin, J.Z.; Hsu, C.S.; Ge, Z.Y.; et al. Simultaneous Improvement of Efficiency and Stability of Organic Photovoltaic Cells by using a Cross-Linkable Fullerene Derivative. Small 2021, 17, 2101133. [Google Scholar] [CrossRef] [PubMed]
- Duan, L.P.; Uddin, A. Progress in Stability of Organic Solar Cells. Adv. Sci. 2020, 7, 1903259. [Google Scholar] [CrossRef]
- Aydin, E.; Allen, T.G.; De Bastiani, M.; Xu, L.J.; Avila, J.; Salvador, M.; Van Kerschaver, E.; De Wolf, S. Interplay Between Temperature and Bandgap Energies on the Outdoor Performance of Perovskite/silicon Tandem Solar Cells. Nat. Energy 2020, 5, 851–859. [Google Scholar] [CrossRef]
- Lee, D.; Kim, J.; Park, G.; Bae, H.W.; An, M.; Kim, J.Y. Enhanced Operating Temperature Stability of Organic Solar Cells with Metal Oxide Hole Extraction Layer. Polymers 2020, 12, 992. [Google Scholar] [CrossRef]
- Fu, Z.Y.; Xu, M.; Sheng, Y.S.; Yan, Z.B.; Meng, J.; Tong, C.H.; Li, D.; Wan, Z.N.; Ming, Y.; Mei, A.Y.; et al. Encapsulation of Printable Mesoscopic Perovskite Solar Cells Enables High Temperature and Long-Term Outdoor Stability. Adv. Funct. Mater. 2019, 29, 1809129. [Google Scholar] [CrossRef]
- Luo, C.Y.; Zuo, J.D. Property of POE, EVA and EPDM Encapsulating Paraffin as Form Stable Phase Change Materials. Mater. Res. Innov. 2013, 17, S58–S61. [Google Scholar] [CrossRef]
- Cho, H.W.; An, N.G.; Park, S.Y.; Shin, Y.S.; Lee, W.; Kim, J.Y.; Song, S. Thermally Durable Nonfullerene Acceptor with Nonplanar Conjugated Backbone for High-Performance Organic Solar Cells. Adv. Energy Mater. 2020, 10, 1903585. [Google Scholar] [CrossRef]
- Yu, L.Y.; Qian, D.P.; Marina, S.; Nugroho, F.A.A.; Sharma, A.; Hultmark, S.; Hofmann, A.I.; Kroon, R.; Benduhn, J.; Smilgies, D.M.; et al. Diffusion-Limited Crystallization: A Rationale for the Thermal Stability of Non-Fullerene Solar Cells. ACS Appl. Mater. Interfaces 2019, 11, 21766–21774. [Google Scholar] [CrossRef]
- Yang, W.; Luo, Z.; Sun, R.; Guo, J.; Wang, T.; Wu, Y.; Wang, W.; Guo, J.; Wu, Q.; Shi, M.; et al. Simultaneous Enhanced Efficiency and Thermal Stability in Organic Solar Cells from a Polymer Acceptor Additive. Nat. Commun. 2020, 11, 1218. [Google Scholar] [CrossRef] [PubMed]
- Chang, B.; Cheng, H.W.; Lin, Y.C.; Wang, H.C.; Chen, C.H.; Nguyen, V.T.; Yang, Y.; Wei, K.H. Incorporating Indium Selenide Nanosheets into a Polymer/Small Molecule Binary Blend Active Layer Enhances the Long-Term Stability and Performance of Its Organic Photovoltaics. ACS Appl. Mater. Interfaces 2020, 12, 55023–55032. [Google Scholar] [CrossRef] [PubMed]
- Han, J.H.; Bao, F.; Huang, D.; Wang, X.C.; Yang, C.M.; Yang, R.Q.; Jian, X.G.; Wang, J.Y.; Bao, X.C.; Chu, J.H. A Universal Method to Enhance Flexibility and Stability of Organic Solar Cells by Constructing Insulating Matrices in Active Layers. Adv. Funct. Mater. 2020, 30, 2003654. [Google Scholar] [CrossRef]
- Liang, S.Y.; Li, S.Y.; Zhang, Y.N.; Li, T.; Zhou, H.X.; Jin, F.; Sheng, C.A.X.; Ni, G.; Yuan, J.Y.; Ma, W.L.; et al. Efficient Hole Transfer via Delocalized Excited State in Small Molecular Acceptor: A Comparative Study on Photodynamics of PM6:Y6 and PM6:ITIC Organic Photovoltaic Blends. Adv. Funct. Mater. 2021, 31, 2102764. [Google Scholar] [CrossRef]
- Kim, T.; Younts, R.; Lee, W.; Lee, S.; Gundogdu, K.; Kim, B.J. Impact of the Photo-induced Degradation of Electron Acceptors on the Photophysics, Charge Transport and Device Performance of All-polymer and Fullerene-polymer Solar Cells. J. Mater. Chem. A 2017, 5, 22170–22179. [Google Scholar] [CrossRef]
- Yin, Y.T.; Pan, X.; Andersson, M.R.; Lewis, D.A.; Andersson, G.G. Mechanism of Organic Solar Cell Performance Degradation upon Thermal Annealing of MoOx. ACS Appl. Energy Mater. 2020, 3, 366–376. [Google Scholar] [CrossRef]
- Sachs-Quintana, I.T.; Heumuller, T.; Mateker, W.R.; Orozco, D.E.; Cheacharoen, R.; Sweetnam, S.; Brabec, C.J.; McGehee, M.D. Electron Barrier Formation at the Organic-Back Contact Interface is the First Step in Thermal Degradation of Polymer Solar Cells. Adv. Funct. Mater. 2014, 24, 3978–3985. [Google Scholar] [CrossRef]
- Lv, J.; Tang, H.; Huang, J.M.; Yan, C.Q.; Liu, K.; Yang, Q.G.; Hu, D.Q.; Singh, R.; Lee, J.; Lu, S.R.; et al. Additive-induced Miscibility Regulation and Hierarchical Morphology Enable 17.5% Binary Organic Solar Cells. Energy Environ. Sci. 2021, 14, 3044–3052. [Google Scholar] [CrossRef]
- Guo, Q.; Guo, Q.; Geng, Y.F.; Tang, A.L.; Zhang, M.J.; Du, M.Z.; Sun, X.N.; Zhou, E.J. Recent Advances in PM6:Y6-based Organic Solar Cells. Mater. Chem. Front. 2021, 5, 3257–3280. [Google Scholar] [CrossRef]
- Zeiske, S.; Sandberg, O.J.; Zarrabi, N.; Li, W.; Meredith, P.; Armin, A. Direct observation of trap-assisted recombination in organic photovoltaic devices. Nat. Commun. 2021, 12, 3603. [Google Scholar] [CrossRef]
- Peike, C.; Hädrich, I.; Weiß, K.-A.; Dürr, I.; Ise, F. Overview of PV Module Encapsulation Materials. Photovolt. Int. 2013, 19, 85–92. [Google Scholar]
- Shi, J.J.; Dong, J.; Lv, S.T.; Xu, Y.Z.; Zhu, L.F.; Xiao, J.Y.; Xu, X.; Wu, H.J.; Li, D.M.; Luo, Y.H.; et al. Hole-conductor-free Perovskite Organic Lead Iodide Heterojunction Thin-film Solar Cells: High Efficiency and Junction Property. Appl. Phys. Lett. 2014, 104, 063901. [Google Scholar] [CrossRef]
- Photodetectors and Solar Cells; Physics of Semiconductor Devices; John Wiley & Sons: Hoboken, NJ, USA, 2006; pp. 663–742.
- Hegedus, S.S.; Shafarman, W.N. Thin-film Solar Cells: Device Measurements and Analysis. Prog. Photovolt. 2004, 12, 155–176. [Google Scholar] [CrossRef]
- Hermerschmidt, F.; Savva, A.; Georgiou, E.; Tuladhar, S.M.; Durrant, J.R.; McCulloch, I.; Bradley, D.D.; Brabec, C.J.; Nelson, J.; Choulis, S.A. Influence of the hole transporting layer on the thermal stability of inverted organic photovoltaics using accelerated-heat lifetime protocols. ACS Appl. Mater. Interfaces 2017, 9, 14136–14144. [Google Scholar] [CrossRef]
- Gu, H.M.; Yan, L.P.; Saxena, S.; Shi, X.L.; Zhang, X.N.; Li, Z.R.; Luo, Q.; Zhou, H.Q.; Yang, Y.Z.; Liu, X.G.; et al. Revealing the Interfacial Photoreduction of MoO3 with P3HT from the Molecular Weight-Dependent “Burn-In” Degradation of P3HT:PC61BM Solar Cells. ACS Appl. Energy Mater. 2020, 3, 9714–9723. [Google Scholar] [CrossRef]
- Greenbank, W.; Hirsch, L.; Wantz, G.; Chambon, S. Interfacial Thermal Degradation in Inverted Organic Solar Cells. Appl. Phys. Lett. 2015, 107, 263301. [Google Scholar] [CrossRef]
- Greenbank, W.; Rolston, N.; Destouesse, E.; Wantz, G.; Hirsch, L.; Dauskardt, R.; Chambon, S. Improved Mechanical Adhesion and Electronic Stability of Organic Solar Cells with Thermal Ageing: The Role of Diffusion at the Hole Extraction Interface. J. Mater. Chem. A 2017, 5, 2911–2919. [Google Scholar] [CrossRef]
- Fleisch, T.H.; Mains, G.J. An XPS Study of the UV Reduction and Photochromism of MoO3 and WO3. J. Chem. Phys. 1982, 76, 780–786. [Google Scholar] [CrossRef]
- Yin, Y.T.; Sibley, A.; Quinton, J.S.; Lewis, D.A.; Andersson, G.G. Dipole Formation at the MoO3/Conjugated Polymer Interface. Adv. Funct. Mater. 2018, 28, 1802825. [Google Scholar] [CrossRef]
- Pranav, M.; Benduhn, J.; Nyman, M.; Hosseini, S.M.; Kublitski, J.; Shoaee, S.; Neher, D.; Leo, K.; Spoltore, D. Enhanced Charge Selectivity via Anodic-C60 Layer Reduces Nonradiative Losses in Organic Solar Cells. ACS Appl. Mater. Interfaces 2021, 13, 12603–12609. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qin, X.; Yu, X.; Li, Z.; Fang, J.; Yan, L.; Wu, N.; Nyman, M.; Österbacka, R.; Huang, R.; Li, Z.; et al. Thermal-Induced Performance Decay of the State-of-the-Art Polymer: Non-Fullerene Solar Cells and the Method of Suppression. Molecules 2023, 28, 6856. https://doi.org/10.3390/molecules28196856
Qin X, Yu X, Li Z, Fang J, Yan L, Wu N, Nyman M, Österbacka R, Huang R, Li Z, et al. Thermal-Induced Performance Decay of the State-of-the-Art Polymer: Non-Fullerene Solar Cells and the Method of Suppression. Molecules. 2023; 28(19):6856. https://doi.org/10.3390/molecules28196856
Chicago/Turabian StyleQin, Xingxing, Xuelai Yu, Zerui Li, Jin Fang, Lingpeng Yan, Na Wu, Mathias Nyman, Ronald Österbacka, Rong Huang, Zhiyun Li, and et al. 2023. "Thermal-Induced Performance Decay of the State-of-the-Art Polymer: Non-Fullerene Solar Cells and the Method of Suppression" Molecules 28, no. 19: 6856. https://doi.org/10.3390/molecules28196856
APA StyleQin, X., Yu, X., Li, Z., Fang, J., Yan, L., Wu, N., Nyman, M., Österbacka, R., Huang, R., Li, Z., & Ma, C.-Q. (2023). Thermal-Induced Performance Decay of the State-of-the-Art Polymer: Non-Fullerene Solar Cells and the Method of Suppression. Molecules, 28(19), 6856. https://doi.org/10.3390/molecules28196856







