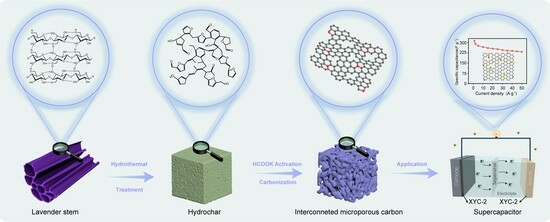
Constructing Interconnected Microporous Structures in Carbon by Homogeneous Activation as a Sustainable Electrode Material for High-Performance Supercapacitors
Abstract
:1. Introduction
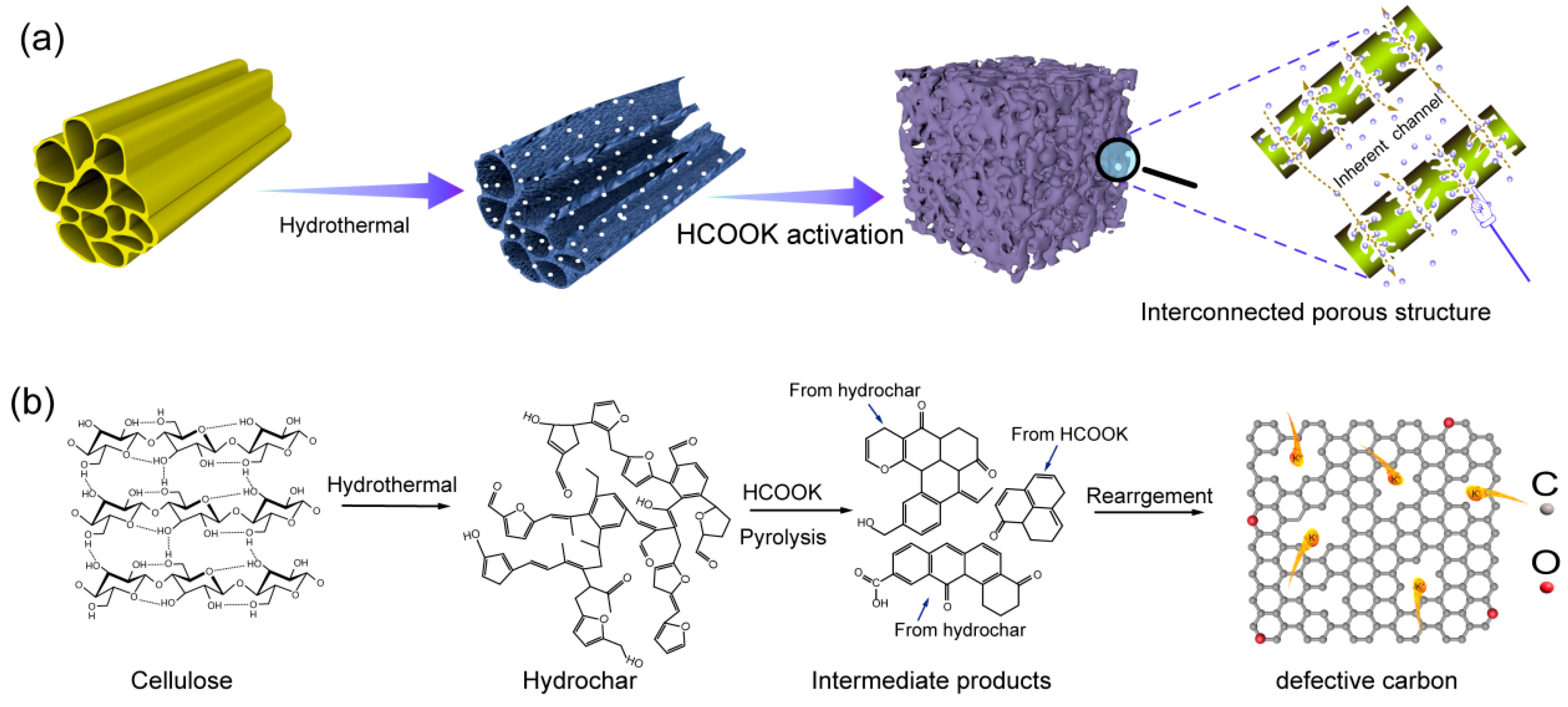
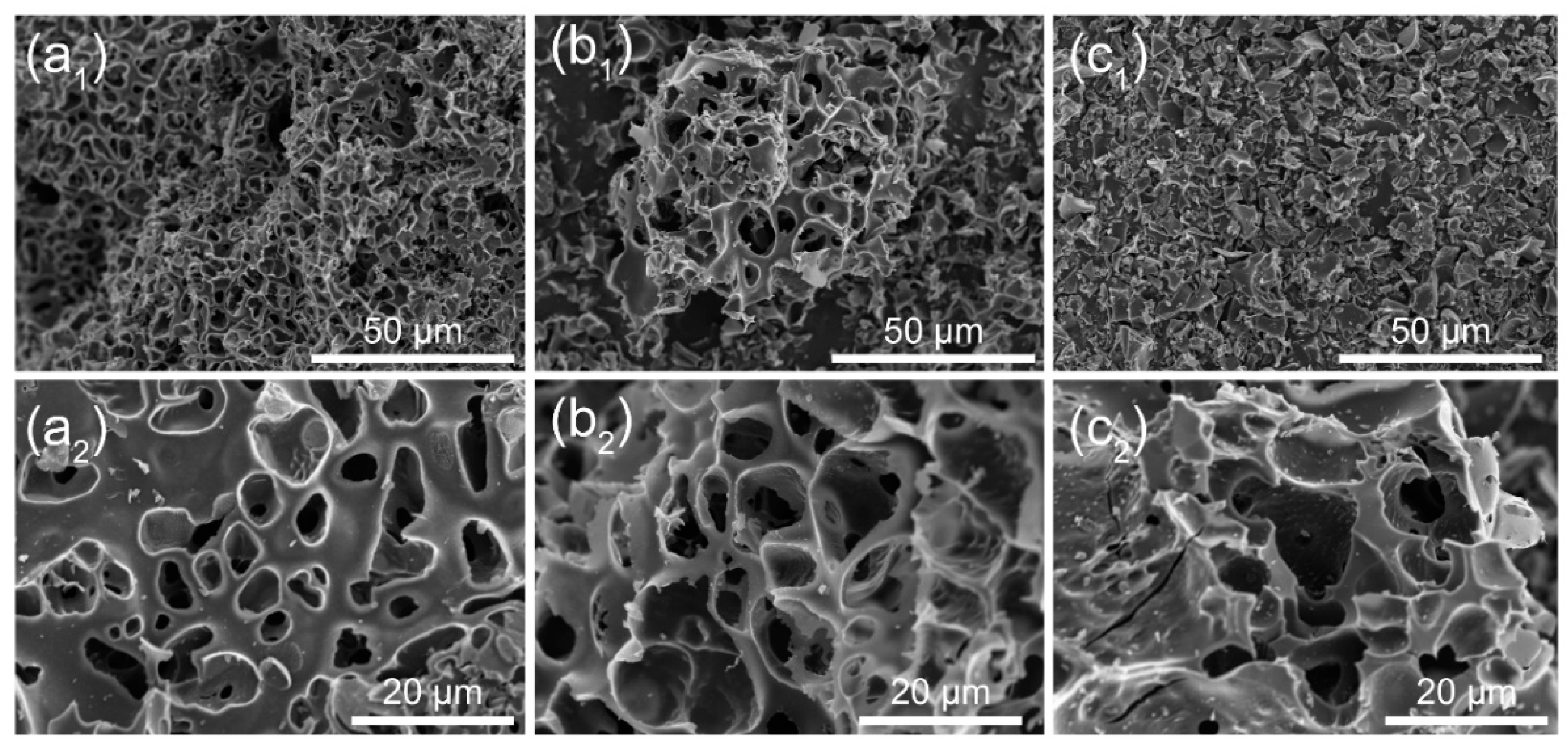
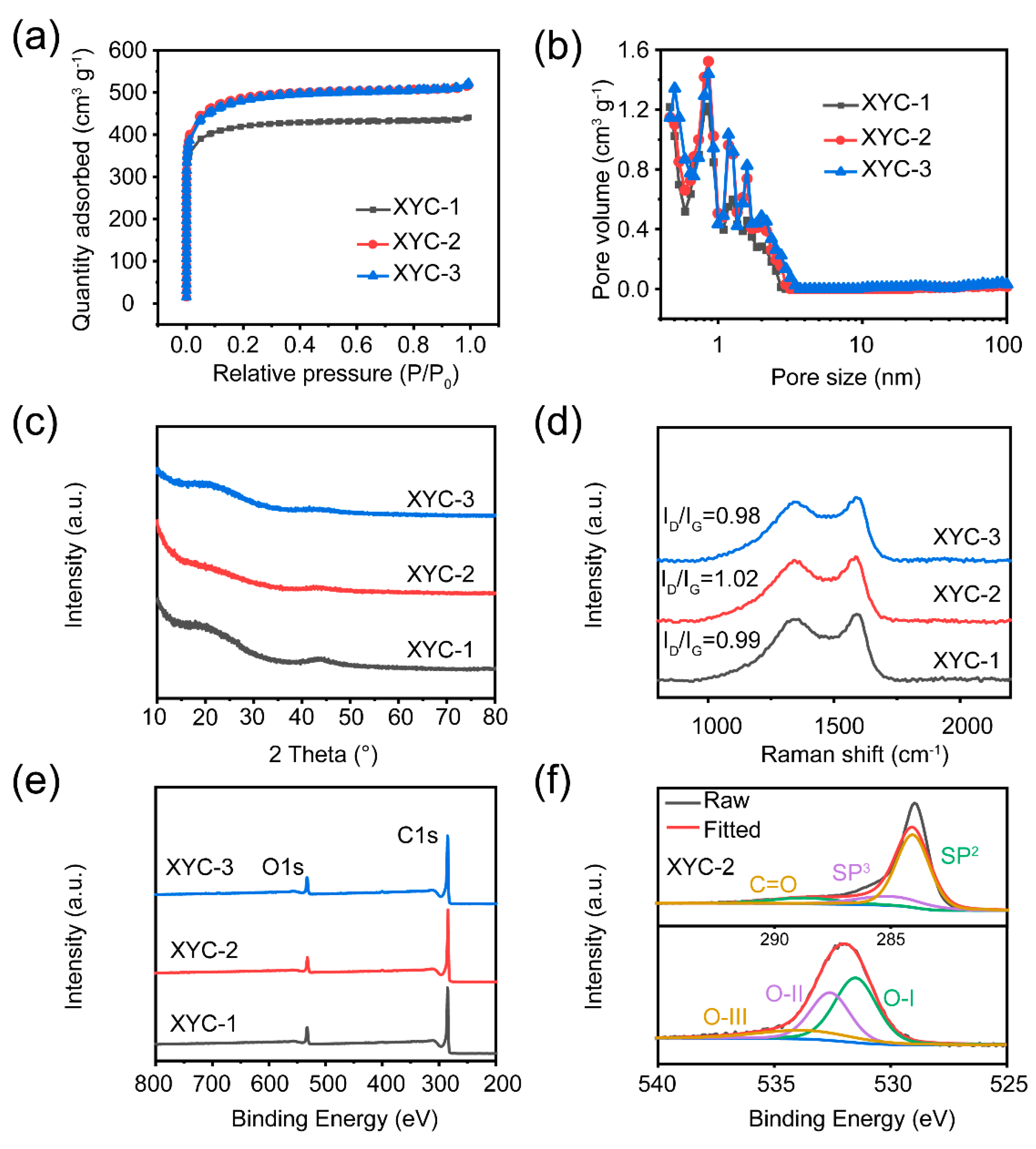
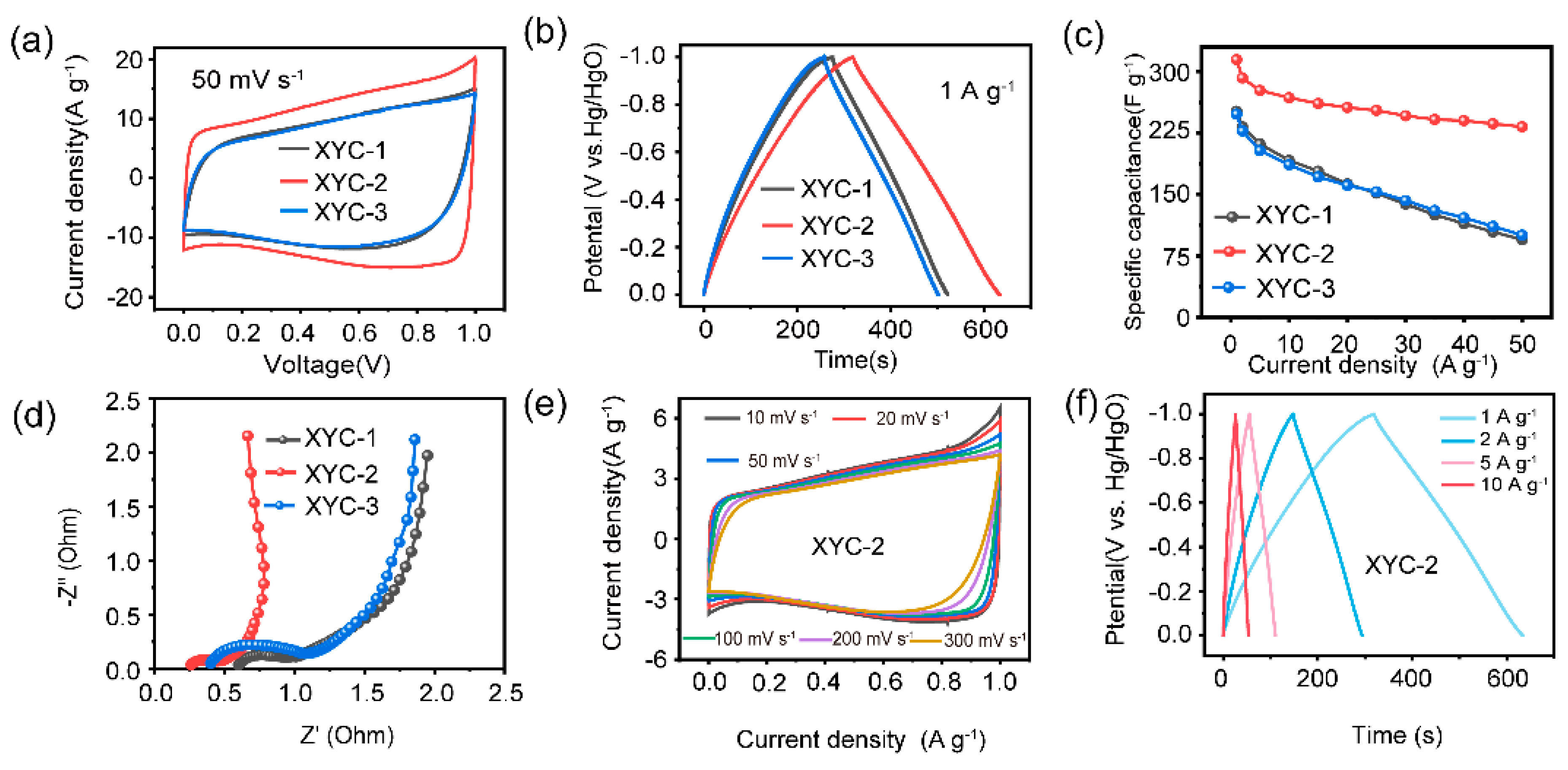
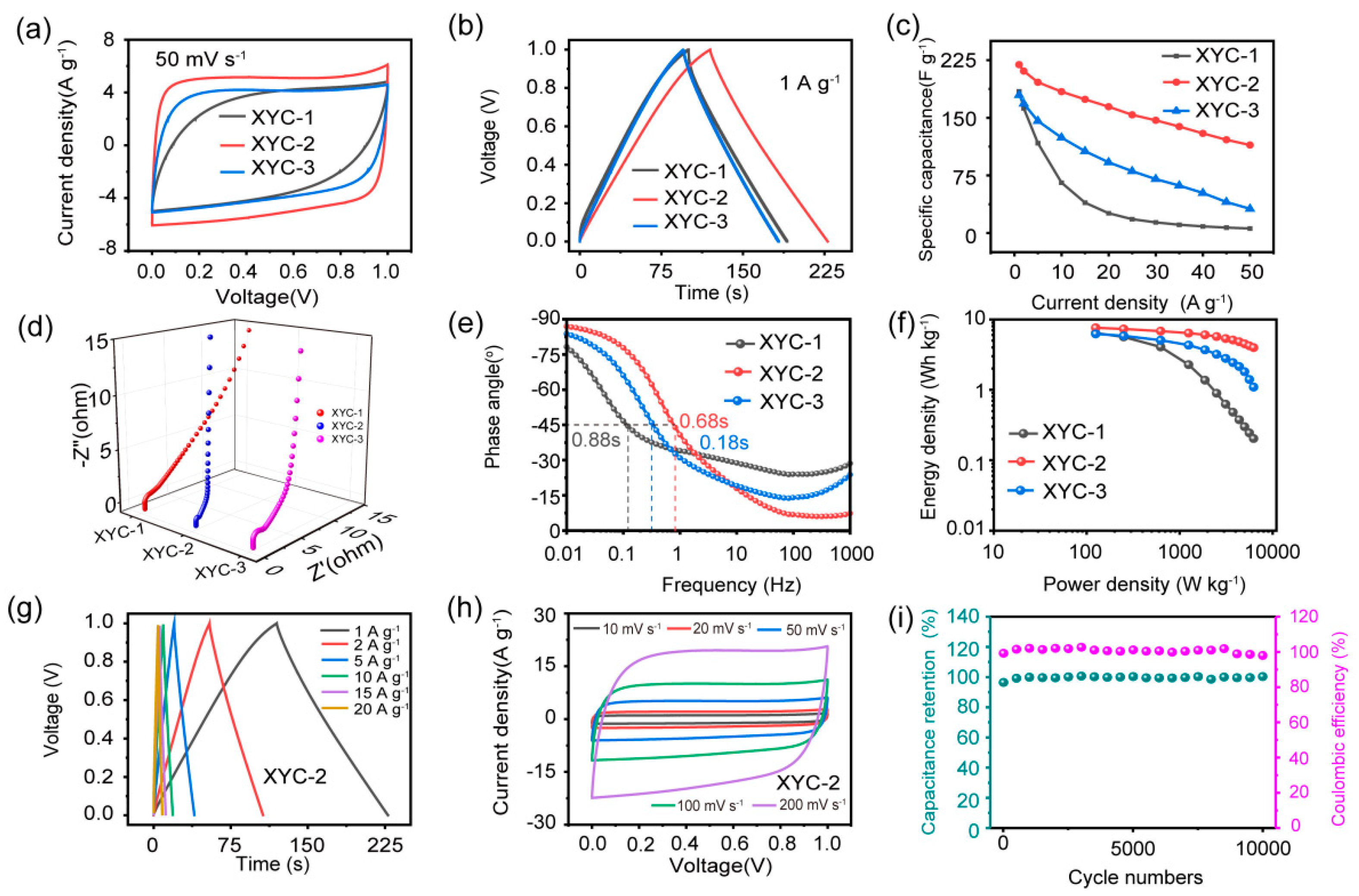
2. Results and Discussion
3. Materials and Methods
3.1. Electrochemical Measurements
3.2. Structural Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Zhao, Z.; Xia, K.; Hou, Y.; Zhang, Q.; Ye, Z.; Lu, J. Designing flexible, smart and self-sustainable supercapacitors for portable/wearable electronics: From conductive polymers. Chem. Soc. Rev. 2021, 50, 12702–12743. [Google Scholar] [CrossRef] [PubMed]
- Guo, N.; Liu, A.; Luo, W.; Ma, R.; Yan, L.; Ai, L.; Xu, M.; Wang, L.; Jia, D. Hybrid nanoarchitectonics of coal-derived carbon with oxidation-induced morphology-selectivity for high-performance supercapacitor. J. Colloid Interface Sci. 2023, 639, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Dar, M.A.; Dinagaran, S.; Govindarajan, D.; Ahamed, S.R.; Habib, F.; Siva, C.; Moholkar, A.V.; Ahmad, Z.; Yatoo, M.A. Snx-0MnxS nanomaterial based electrodes for future-generation supercapacitor and data storage devices. J. Alloys Compd. 2023, 958, 170523. [Google Scholar] [CrossRef]
- Yan, J.; Li, S.; Lan, B.; Wu, Y.; Lee, P.S. Rational Design of Nanostructured Electrode Materials toward Multifunctional Supercapacitors. Adv. Funct. Mater. 2020, 30, 1902564. [Google Scholar] [CrossRef]
- Kumar, R.; Joanni, E.; Sahoo, S.; Shim, J.J.; Matsuda, A.; Tan, W.K.; Singh, R.K. An overview of recent progress in nanostructured carbon-based supercapacitor electrodes: From zero to bi-dimensional materials. Carbon 2022, 193, 298–338. [Google Scholar] [CrossRef]
- Wang, F.; Wu, X.; Yuan, X.; Liu, Z.; Zhang, Y.; Fu, L.; Zhu, Y.; Zhou, Q.; Wu, Y.; Huang, W. Latest advances in supercapacitors: From new electrode materials to novel device designs. Chem. Soc. Rev. 2017, 46, 6816–6854. [Google Scholar] [CrossRef]
- Sahoo, S.; Kumar, R.; Joanni, E.; Singh, R.K.; Shim, J.-J. Advances in pseudocapacitive and battery-like electrode materials for high performance supercapacitors. J. Mater. Chem. A 2022, 10, 13190–13240. [Google Scholar]
- Ma, R.; Luo, W.; Yan, L.; Guo, C.; Ding, X.; Gong, X.; Jia, D.; Xu, M.; Ai, L.; Guo, N.; et al. Constructing the quinonyl groups and structural defects in carbon for supercapacitor and capacitive deionization applications. J. Colloid Interface Sci. 2023, 645, 685–693. [Google Scholar] [CrossRef]
- Yan, L.; Liu, A.; Ma, R.; Guo, C.; Ding, X.; Feng, P.; Jia, D.; Xu, M.; Ai, L.; Guo, N.; et al. Regulating the specific surface area and porous structure of carbon for high performance supercapacitors. Appl. Surf. Sci. 2023, 615, 156267. [Google Scholar] [CrossRef]
- Yin, B.; Zhang, J.; Su, Y.; Huan, Y.; Hao, L.; Wang, C.; Hu, X.; Wei, T. A universal charge-compensating strategy for high-energy-density pseudocapacitors. J. Energy Chem. 2023, 78, 333–339. [Google Scholar] [CrossRef]
- Yang, M.; Song, W.J. One-pot two-step synthesis of micro- and mesoporous organic fibrils for efficient pseudocapacitors. J. Mater. Chem. A 2022, 10, 17511–17519. [Google Scholar] [CrossRef]
- Yang, N.; Yu, S.; Zhang, W.; Cheng, H.M.; Simon, P.; Jiang, X. Electrochemical Capacitors with Confined Redox Electrolytes and Porous Electrodes. Adv. Mater. 2022, 34, e2202380. [Google Scholar] [CrossRef]
- Shang, Z.; An, X.; Zhang, H.; Shen, M.; Baker, F.; Liu, Y.; Liu, L.; Yang, J.; Cao, H.; Xu, Q.; et al. Houttuynia-derived nitrogen-doped hierarchically porous carbon for high-performance supercapacitor. Carbon 2020, 161, 62–70. [Google Scholar] [CrossRef]
- Ouyang, T.; Zhang, T.; Wang, H.; Yang, F.; Yan, J.; Zhu, K.; Ye, K.; Wang, G.; Zhou, L.; Cheng, K.; et al. High-throughput fabrication of porous carbon by chemical foaming strategy for high performance supercapacitor. Chem. Eng. J. 2018, 352, 459–468. [Google Scholar] [CrossRef]
- Chen, B.; Wu, D.; Wang, T.; Yuan, F.; Jia, D. Rapid preparation of porous carbon by flame burning carbonization method for supercapacitor. Chem. Eng. J. 2023, 462, 142163. [Google Scholar] [CrossRef]
- Xu, T.; Wang, Y.; Liu, K.; Zhao, Q.; Liang, Q.; Zhang, M.; Si, C. Ultralight MXene/carbon nanotube composite aerogel for high-performance flexible supercapacitor. Adv. Compos. Hybrid Mater. 2023, 6, 108. [Google Scholar] [CrossRef]
- Zheng, Y.; Tian, Y.; Sarwar, S.; Luo, J.; Zhang, X. Carbon nanotubes decorated NiSe2 nanosheets for high-performance supercapacitors. J. Power Sources 2020, 452, 227793. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, D.; Lau, A.; Ma, T.; Lin, H.; Jia, B. Hybridized Graphene for Supercapacitors: Beyond the Limitation of Pure Graphene. Small 2021, 17, 2007311. [Google Scholar] [CrossRef]
- Nithya, V.D. A review on holey graphene electrode for supercapacitor. J. Energy Storage 2021, 44, 103380. [Google Scholar] [CrossRef]
- Zhai, Z.; Ren, B.; Xu, Y.; Wang, S.; Zhang, L.; Liu, Z. Nitrogen self-doped carbon aerogels from chitin for supercapacitors. J. Power Sources 2021, 481, 228976. [Google Scholar] [CrossRef]
- Zhuo, H.; Hu, Y.; Chen, Z.; Zhong, L. Cellulose carbon aerogel/PPy composites for high-performance supercapacitor. Carbohydr. Polym. 2019, 215, 322–329. [Google Scholar] [CrossRef]
- Yang, Z.; Jia, Y.; Niu, Y.; Zhang, Y.; Zhang, C.; Li, P.; Zhu, M.; Li, Q. One-step wet-spinning assembly of twisting-structured graphene/carbon nanotube fiber supercapacitor. J. Energy Chem. 2020, 51, 434–441. [Google Scholar] [CrossRef]
- Shi, L.; Ye, J.; Lu, H.; Wang, G.; Lv, J.; Ning, G. Flexible all-solid-state supercapacitors based on boron and nitrogen-doped carbon network anchored on carbon fiber cloth. Chem. Eng. J. 2021, 410, 128365. [Google Scholar] [CrossRef]
- Zhu, J.; Wang, L.; Gan, X.; Tang, T.; Qin, F.; Luo, W.; Li, Q.; Guo, N.; Zhang, S.; Jia, D.; et al. Graphene quantum dot inlaid carbon nanofibers: Revealing the edge activity for ultrahigh rate pseudocapacitive energy storage. Energy Stor. Mater. 2022, 47, 158–166. [Google Scholar] [CrossRef]
- Vijayakumar, M.; Bharathi Sankar, A.; Sri Rohita, D.; Rao, T.N.; Karthik, M. Conversion of Biomass Waste into High Performance Supercapacitor Electrodes for Real-Time Supercapacitor Applications. ACS Sustain. Chem. Eng. 2019, 7, 17175–17185. [Google Scholar] [CrossRef]
- Rawat, S.; Mishra, R.K.; Bhaskar, T. Biomass derived functional carbon materials for supercapacitor applications. Chemosphere 2022, 286, 131961. [Google Scholar] [CrossRef]
- Yang, X.; Sun, G.; Wang, F.; Li, X.; Zhang, Z.; Zhen, Y.; Wang, D.; Gao, X.; Fu, F.; Chi, R. Rational design of dense microporous carbon derived from coal tar pitch towards high mass loading supercapacitors. J. Colloid Interface Sci. 2023, 646, 228–237. [Google Scholar] [CrossRef]
- Mishra, D.; Zhou, R.; Hassan, M.M.; Hu, J.; Gates, I.; Mahinpey, N.; Lu, Q. Bitumen and asphaltene derived nanoporous carbon and nickel oxide/carbon composites for supercapacitor electrodes. Sci. Rep. 2022, 12, 4095. [Google Scholar] [CrossRef]
- Liu, X.; Liu, C.-F.; Xu, S.; Cheng, T.; Wang, S.; Lai, W.-Y.; Huang, W. Porous organic polymers for high-performance supercapacitors. Chem. Soc. Rev. 2022, 51, 3181–3225. [Google Scholar] [CrossRef]
- Liu, W.; Ulaganathan, M.; Abdelwahab, I.; Luo, X.; Chen, Z.; Tan, S.J.R.; Wang, X.; Liu, Y.; Geng, D.; Bao, Y.; et al. Two-Dimensional Polymer Synthesized via Solid-State Polymerization for High-Performance Supercapacitors. ACS Nano 2018, 12, 852–860. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, L.; Zhang, J. A review of electrode materials for electrochemical supercapacitors. Chem. Soc. Rev. 2012, 41, 797–828. [Google Scholar] [CrossRef] [PubMed]
- Arico, A.S.; Bruce, P.; Scrosati, B.; Tarascon, J.M.; Van, S.W. Nanostructured materials for advanced energy conversion and storage devices. Nat. Mater. 2005, 4, 366–377. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Li, J.; Jiang, Z.; Shi, M.; Sheng, R.; Liu, Z.; Zhang, S.; Cao, Y.; Wei, T.; Fan, Z. Large-surface-area activated carbon with high density by electrostatic densification for supercapacitor electrodes. Carbon 2021, 175, 281–288. [Google Scholar] [CrossRef]
- Dong, X.L.; Li, W.C.; Jiang, B.; Zhou, Y.-Q.; Lu, A.-H. Using inorganic dynamic porogens for preparing high-surface-area capacitive carbons with tailored micropores. J. Mater. Chem. A 2019, 7, 687–692. [Google Scholar] [CrossRef]
- Zhang, J.; Tang, T.; Gan, X.; Yuan, R.; Li, Q.; Zhu, L.; Guo, N.; Zhu, J.; Li, Y.; Zhang, S.; et al. Schiff base reaction induced densification of chitosan-derived microporous carbon for compact capacitive energy storage. Chem. Eng. J. 2023, 470, 144257. [Google Scholar] [CrossRef]
- Luo, W.; Guo, N.; Wang, L.; Jia, D.; Xu, M.; Zhang, S.; Ai, L.; Sheng, R.; Feng, S.; Gong, X.; et al. Homogeneous activation induced by bacterial cellulose nanofibers to construct interconnected microporous carbons for enhanced capacitive storage. J. Colloid Interface Sci. 2023, 636, 33–41. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, T.; Chen, X.; Wu, D. Agar-based porous electrode and electrolyte for flexible symmetric supercapacitors with ultrahigh energy density. J. Power Sources 2021, 507, 230252. [Google Scholar] [CrossRef]
- Guo, N.; Luo, W.; Zhou, D.; Xu, M.; Qiu, D.; Gao, A.; Ma, R.; Sheng, R.; Jia, D.; Wang, L. In-situ expansion strategy towards hierarchical mesoporous carbon: Formation mechanism and application in supercapacitors. Int. J. Energy Res. 2022, 46, 7249–7260. [Google Scholar] [CrossRef]
- Fu, F.; Yang, D.; Zhang, W.; Wang, H.; Qiu, X. Green self-assembly synthesis of porous lignin-derived carbon quasi-nanosheets for high-performance supercapacitors. Chem. Eng. J. 2020, 392, 123721. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, S.; Li, X.; Li, L.; Bao, L.; Zhang, N.; Peng, J.; Li, X. Synergistically optimizing electronic structure and reducing ions transport resistance by oxygen functional groups and defects in carbon for superior sodium capture and potassium storage capability. Carbon 2021, 181, 323–334. [Google Scholar] [CrossRef]
- Huo, S.; Song, X.; Zhao, Y.; Ni, W.; Wang, H.; Li, K. Insight into the significant contribution of intrinsic carbon defects for the high-performance capacitive desalination of brackish water. J. Mater. Chem. A 2020, 8, 19927–19937. [Google Scholar] [CrossRef]
- Niu, J.; Shao, R.; Liang, J.; Dou, M.; Li, Z.; Huang, Y.; Wang, F. Biomass-derived mesopore-dominant porous carbons with large specific surface area and high defect density as high performance electrode materials for Li-ion batteries and supercapacitors. Nano Energy 2017, 36, 322–330. [Google Scholar] [CrossRef]
- Ma, X.; Wu, Q.; Wang, W.; Lu, S.; Xiang, Y.; Aurbach, D. Mass-producible polyhedral macrotube carbon arrays with multi-hole cross-section profiles: Superb 3D tertiary porous electrode materials for supercapacitors and capacitive deionization cells. J. Mater. Chem. A 2020, 8, 16312–16322. [Google Scholar] [CrossRef]
- Gong, X.; Zhang, S.; Luo, W.; Guo, N.; Wang, L.; Jia, D.; Zhao, Z.; Feng, S.; Jia, L. Enabling a Large Accessible Surface Area of a Pore-Designed Hydrophilic Carbon Nanofiber Fabric for Ultrahigh Capacitive Deionization. ACS Appl. Mater. Interfaces 2020, 12, 49586–49595. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Wang, J.; Guo, Z.; Xie, F.; Liu, H.; Yadegari, H.; Tebyetekerwa, M.; Ryan, M.P.; Hu, Y.S.; Titirici, M.M. The Role of Hydrothermal Carbonization in Sustainable Sodium-Ion Battery Anodes. Adv. Energy Mater. 2022, 12, 2200208. [Google Scholar] [CrossRef]
- Kim, D.; Jin, X.; Cho, Y.; Lim, J.; Yan, B.; Ko, D.; Kim, D.K.; Piao, Y. Facile preparation of N-doped porous carbon nanosheets derived from potassium citrate/melamine for high-performance supercapacitors. J. Electroanal. Chem. 2021, 892, 115302. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Ma, R.; Chen, F.; Wang, D.; Zhang, H.; Lu, C. Constructing Interconnected Microporous Structures in Carbon by Homogeneous Activation as a Sustainable Electrode Material for High-Performance Supercapacitors. Molecules 2023, 28, 6851. https://doi.org/10.3390/molecules28196851
Li H, Ma R, Chen F, Wang D, Zhang H, Lu C. Constructing Interconnected Microporous Structures in Carbon by Homogeneous Activation as a Sustainable Electrode Material for High-Performance Supercapacitors. Molecules. 2023; 28(19):6851. https://doi.org/10.3390/molecules28196851
Chicago/Turabian StyleLi, Huijie, Rui Ma, Feifei Chen, Danting Wang, Hongmin Zhang, and Chunyang Lu. 2023. "Constructing Interconnected Microporous Structures in Carbon by Homogeneous Activation as a Sustainable Electrode Material for High-Performance Supercapacitors" Molecules 28, no. 19: 6851. https://doi.org/10.3390/molecules28196851
APA StyleLi, H., Ma, R., Chen, F., Wang, D., Zhang, H., & Lu, C. (2023). Constructing Interconnected Microporous Structures in Carbon by Homogeneous Activation as a Sustainable Electrode Material for High-Performance Supercapacitors. Molecules, 28(19), 6851. https://doi.org/10.3390/molecules28196851