Abstract
Polyphenols are the largest group of phytochemicals with important biological properties. Their presence in conveniently available low-cost sources, such as agri-food by-products, has gained considerable attention in their recovery and further exploitation. Retrieving polyphenols in a green and sustainable way is crucial. Recently, deep eutectic solvents (DESs) have been identified as a safe and environmentally benign medium capable of extracting polyphenols efficiently. This review encompasses the current knowledge and applications of DESs and assisted technologies to extract polyphenols from agri-food by-products. Particular attention has been paid to fundamental mechanisms and potential applications in the food, cosmetic, and pharmaceutical industries. In this way, DESs and DESs-assisted with advanced techniques offer promising opportunities to recover polyphenols from agri-food by-products efficiently, contributing to a circular and sustainable economy.
1. Introduction
The agricultural and food industries generate large amounts of by-products throughout the food supply chain. The Food and Agricultural Organization (FAO) estimates that 1.3 billion tons of food are lost or wasted globally each year [1]. The disposal of these agri-food by-products costs food manufacturers a lot and has adverse impacts on the environment. On the other hand, these by-products are abundant, low-cost, and renewable sources of high-value-added compounds. Valorizing agri-food by-products into high-value-added compounds provides a unique roadmap for realizing the Sustainable Development Goals (SDGs) 12 (Responsible Consumption and Production) of the United Nations. Among the high-value-added compounds, polyphenols have attracted increasing attention due to their multiple health benefits.
Polyphenols are broadly present in the plant kingdom and play various important roles in growth and development processes. The protective role of polyphenols against radical oxygen and reactive nitrogen species, UV light, and plant pathogens results in a variety of beneficial bioactivities, including antioxidant, antimicrobial, anticarcinogenic, and antidiabetic activities [2]. Polyphenols have one or more aromatic rings carrying one or more hydroxyl groups. From the chemical structure standpoint, as shown in Figure 1, polyphenols are classified into two subgroups: flavonoids and nonflavonoids [3]. Flavonoids, including flavonols, flavanones, and condensed tannins, are built around a C6-C3-C6 carbon skeleton. Nonflavonoids include phenolic acids, stilbenes, gallotannins, ellagitannins, and lignins.
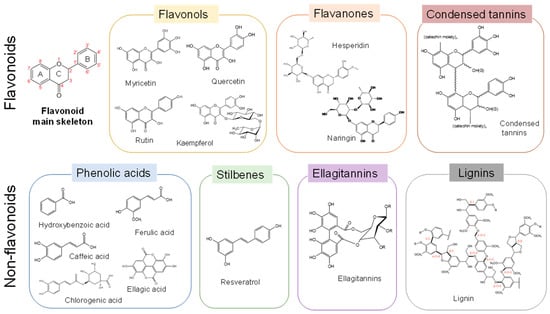
Figure 1.
Classification and structure of polyphenols.
The efficient extraction of polyphenols from various agri-food by-products is a preliminary and pivotal step. The most common strategies are solid–liquid extraction (SLE) and liquid−liquid extraction. The traditionally used solvents, such as acetone, methanol, ethanol, or their mixtures, are organic. Traditional solvents no longer meet green and sustainable development due to drawbacks such as high costs, difficult synthesis techniques, high toxicity, and poor recoverability [4]. With the rise of green and sustainable techniques, researchers are focusing their efforts on searching for new alternative solvents to replace traditional media. Deep eutectic solvents (DESs) have recently acquired popularity for extracting and valorizing polyphenols due to its superior ability to solubilize polyphenols [3,4,5]. DESs align with the 12 principles of green chemistry and has many remarkable advantages, such as low cost, ease of synthesis, tunable characteristics, good recyclability, and high biodegradability [6]. DESs are claimed to be green and safe, especially for natural DESs (NADESs) [4,7,8]. NADESs are composed of naturally occurring components and are generally recognized as safe (GRAS) [4]. The use of NADESs is allowed in food and pharmaceutical formulations [8].
Given the escalating demand for polyphenols for advanced industrial applications, this review provides a compilation of the newest information on the green extraction of polyphenols using DESs and advanced assisted technologies. It focuses on the recent advances in polyphenol-rich agri-food by-products, polyphenols extraction using DESs, improvement of extraction via assisted technologies, and applications in the food, cosmetic, and pharmaceutical industries. In addition, the future perspectives for addressing the challenges of polyphenol extraction using DESs are presented.
2. Polyphenol-Rich Agri-Food By-Products
A wide range of agri-food by-products are rich in polyphenols. Much research has been conducted to extract phenolic compounds from these agri-food by-products using DESs, including fruit by-products, vegetable by-products, tree nut by-products, cereal by-products, oil-bearing crop by-products, and other agri-food by-products.
2.1. Fruit By-Products
Fruits have a crucial role in our daily diet. Due to their perishable nature, fresh fruits are processed into various products. During the industrial process, tremendous amounts of by-products and waste are generated. These by-products are rich in different valuable compounds. Table 1 lists some recent applications of DESs and assisted techniques for extracting polyphenols from fruit by-products.

Table 1.
Some examples of recent applications of DESs and assisted techniques for extracting polyphenols from fruit by-products.
Apple is one of the largest fruit producers in the world. The major by-product generated during the apple juicing process is apple pomace. It is comprised of peels and flesh (90%), seeds (2~4%), and stems (1%), and these by-products are rich in polyphenols. It has been reported that DESs can efficiently extract quercetin glycosides, kaempferol, catechin, and procyanidins from apple pomace [9,10,53]. Extracts rich in these compounds exhibit cardioprotective, anticancer, and antimicrobial biological activities.
Citrus fruits, such as orange, mandarin, lemon, grapefruit, pomelo, lime, etc., are widely grown and consumed across the globe. Tremendous by-products are produced during the industrial production of juice, jellies, candied fruits, and jams. Various flavonoids (e.g., hesperidin and naringin) and phenolic acids have been recovered from citrus by-products using DESs [11,12,13,14,15,16]. For instance, naringin can be used as a food antioxidant or ingredient to treat obesity and diabetes. In addition, these compounds can be used as food additives to impart bitter taste [54].
Grape is one of the most widely cultivated fruits. According to the statistical data of FAO, the annual production of grapes is up to 75 million tons, and about 80% is used to manufacture wines and their derivatives. Consequently, a large quantity of grape by-products is globally produced each year. The principal by-product of the grape wine industry is grape pomace, consisting of residual pulp, skins, stems, and seeds. Grape pomace is a good source of polyphenols such as lignin, condensed tannins (proanthocyanidins), anthocyanins (e.g., malvidin-3-O-monoglucoside), phenolic acids, catechin and epicatechin derivatives, and hydroxytyrosol. These compounds possess various health benefits, including antimicrobial, anti-inflammatory, anticarcinogenic, cardioprotective, and neuroprotective activities [54].
Various berries, including blueberry, cranberry, raspberry, mulberry, sea buckthorn berry, and sour cherry, are used to manufacture juice, wine, and derivative products to extend shelf-life and economic value chain. Consequently, millions of tons of berry by-products are annually generated in the world. These berry by-products comprise leaves and pomace during harvest, juicing, and fermentation and are rich sources of phenolic compounds, especially anthocyanins. ChCl:malic acid efficiently extracted anthocyanins from Brazilian berries, and the anthocyanin-rich extracts showed promising antidiabetic and anti-obesity potential [26]. By using DESs, plenty of anthocyanins have been recovered from grape skin [17,18,19,20], grape pomace [21,22,23,24], red wine lees [25], jaboticaba pomace [27], blueberry pomace [26,28,29,30], blueberry wine residues [31], and other berries pomace [32,33,34,35,36].
Other fruits, such as pear, peach, pomegranate, mango, mangosteen, and date, are widely grown and much-consumed fruits. They can be consumed as fresh fruit or processed products. Industrial processing results in huge amounts of by-products; for example, the processing of pomegranate juice generates high quantities of peels and seeds. Various phenolic compounds, for example, ellagitannins, have been extracted using DESs from pomegranate peels, mesocarps, and seeds [37,38,39,40,41]. Mangiferin is the prominent phenolic compound in the DESs extract from mango peel, which showed excellent antioxidant capacity to protect sunflower oil from oxidation [42].
2.2. Vegetable By-Products
Onion, potato, and tomato are important vegetables that can be processed into various dehydrated, powdered, fried, or canned products. Consequently, huge amounts of by-products are produced during their industrial processing. These by-products are mainly comprised of peel, skin, and pomace. As shown in Table 2, they are excellent natural resources for many valuable polyphenols. Onion solid by-products contain high amounts of flavonoids such as quercetin, kaempferol, and myricetin [55,56,57,58,59,60]. Quercetin is a naturally occurring flavonoid that has cardioprotective, neuroprotective, anticoagulant, anti-inflammatory, anticarcinogenic, and antioxidant activities [60]. It has been reported that the NADESs extract from onion peel showed higher antioxidant capacity than standard ascorbic acid [57]. Tomato is rich in flavones like chlorogenic acid, rutin, and quercetin [44,61]. Numerous phenolic compounds have been extracted from onion and tomato by-products using DESs, such as quercetin [58,60], kaempferol glycosides [59], and anthocyanins [62]. Additionally, DESs show high extraction efficiency in retrieving phenolic compounds from other vegetable by-products, like kale waste [63], bitter melon leaves [64], pepper leaves [65], and lotus leaves [66].

Table 2.
Some examples of recent applications of DESs and assisted techniques for extracting polyphenols from vegetable by-products.
2.3. Tree Nut By-Products
In the last decades, the consumption of tree nuts (such as Carya cathayensis Sarg, chestnut, and hazelnut) has boosted sharply worldwide due to their nutritional worth and health benefits. Their industrial processing generates vast amounts of underexploited by-products enriched in high-valued phenolic compounds, like ellagic acid, gallic acid, catechin hydrate, procyanidin, and myricetrin. As listed in Table 3, ever-growing studies have successfully used DESs to extract various phenolic compounds from these tree nut by-products [67,68,69,70,71]. For example, ChCl:malic acid extracted 20 phytochemicals from Carya cathayensis Sarg. peels, majorly including catechin, procyanidin B1 and B3, 2,3-dihydroxybenzoic acid, pinocembrin, and myricetrin. Additionally, the NADESs extract possessed the maximum content of phenolic compounds and antioxidant activity, as well as α-glucosidase and α-amylase inhibitory effects [70]. A high content of ellagic acid (4.64 mg/g) with high purity (85.6%) can be extracted using ChCl:n-propanol from chestnut shells [68].

Table 3.
Some examples of recent applications of DESs and assisted techniques for extracting polyphenols from tree nut by-products.
2.4. Cereal By-Products
Wheat, rice, foxtail millet, and buckwheat are important and highly consumed cereals worldwide. They serve as very efficient and major sources of human energy and nutrition. The industrial processing (like milling and brewing) of cereals produces enormous amounts of bran, husk, hull, and spent grain. For example, about 35 million tons of wet brewer spent grain are produced annually during the worldwide manufacture of beer [72]. As shown in Table 4, these by-products are major sources of natural rutin, flavonoids, and anthocyanins. As a green and sustainable extraction medium, DESs have extracted polyphenols from wheat barn [73] and brewer spent grain [72], anthocyanin from black rice bran [74], and rutin from tartary buckwheat hull [75]. The polyphenol-rich NADESs extract from foxtail millet bran was mainly composed of coumaric acid, apigenin-C-dihexoside, and p-coumaroylspermidine, and showed high acetylcholinesterase inhibitory activity [76].

Table 4.
Some examples of recent applications of DESs and assisted techniques for extracting polyphenols from cereal by-products.
2.5. Oil-Bearing Crop By-Products
Olive, soybean, peanut, sunflower, and rapeseed are the primary oil-bearing crops in the world. Most of them are used to produce edible vegetable oils via physical pressing or solvent extraction. During these industrial processes, very large quantities of by-products are produced, including olive oil pomace, olive tree leaves, sunflower disks, and peanut hulls. As listed in Table 5, oleuropein, flavones (luteolin-7-glucoside and luteolin), isoflavone (daidzein, genistein, and puerarin), flavonols (rutin and quercetin), flavan-3-ols (catechin), phenolic acids (chlorogenic acid), and substituted phenols (hydroxytyrosol) are rich in these by-products [78,79,80,81,82,83,84,85,86]. One of the most bioactive molecules possessing anti-inflammatory and antiplatelet effects is hydroxytyrosol [80,87,88,89]. Another bioactive molecule with anti-hyperglycemia, cholesterol-lowering, anticarcinogenic, antidiabetic, and antiallergic activities is isoflavone. They have been efficiently extracted using DESs from soy molasses and kudzu roots [82].

Table 5.
Some examples of recent applications of DESs and assisted techniques for extracting polyphenols from oil-bearing crop by-products.
2.6. Other Agri-Food By-Products
Other agri-food by-products, such as cocoa and coffee, are also excellent resources for polyphenols. The coffee production chain generally consists of eight process units: planting, harvesting the cocoa beans, drying, milling, tasting, roasting, grinding, and brewing. Approximately 10.5 million tons of cocoa by-products were generated globally during the 2020/21 season [90]. As shown in Table 6, procyanidins and chlorogenic acids have been efficiently extracted from cocoa by-products [91,92] and spent coffee grounds [93,94] using various DESs as the extraction media. In addition, xanthohumol, which possesses a variety of bioactivities, has been sustainably and simply recovered from spent hops using ChCl-based DESs [95].

Table 6.
Some examples of recent applications of DESs and assisted techniques for extracting polyphenols from other agri-food by-products.
3. Use of DESs as Green Solvents in the Extraction of Polyphenols from Agri-Food By-Products
3.1. DESs and Its Mechanism of Polyphenol Extraction
DESs are liquid eutectic mixtures of hydrogen bond acceptors (HBAs) and hydrogen bond donors (HBDs) (Figure 2). The establishment of hydrogen bonds (H-bonds) results in the formation of a homogeneous eutectic mixture after a simple stir of these components under mild conditions. DESs are becoming increasingly important in the agri-food industry for producing clean-labeled products that consumers demand [7]. DESs can be prepared using a wide range of chemicals. Choline chloride (ChCl) usually functions as the HBA, while carbohydrates, alcohols, acids, amides, and phenolic compounds function as HBDs. As shown in Table 1, Table 2, Table 3, Table 4, Table 5 and Table 6, ChCl-based DESs are the optimum solvents for efficiently extracting polyphenols. In general, the polarity, diffusivity, viscosity, and conductivity are crucial physicochemical properties of DESs that influence the extraction yield of polyphenols. This review does not cover the physicochemical properties of DESs, as readers are advised to refer to the related content in our previous study [6].
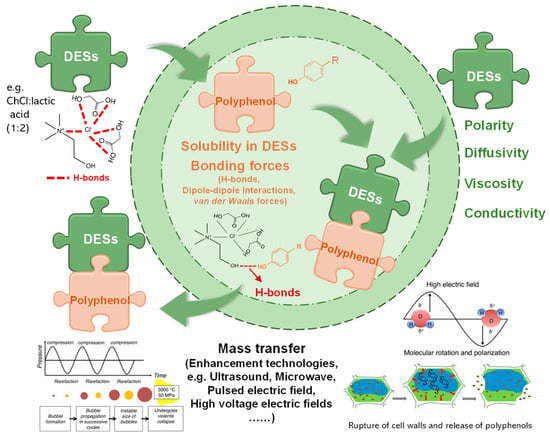
Figure 2.
Simplified mechanisms of polyphenol dissolution in DESs media.
The extraction of polyphenol molecules from agri-food by-products can be considered an SLE process [96]. According to the principle of SLE, the extraction process of polyphenols using DESs includes mass transfer from a solid to a liquid phase and polyphenol dissolution in DESs (as shown in Figure 2). DESs are good solvents with rare solvation properties [3] and can highly solvate polyphenol due to its similar polarities and chemical interactions, such as H-bonds, dipole–dipole interactions, and van der Waals forces. The H-bonds interactions between DESs and polyphenol are so strong (stronger than water solute) that they outweigh the other polyphenol−polyphenol electrostatic forces. The van der Waals interactions become weaker than the steric hindrance of the ether groups, resulting in aromatic ring dispersion in DESs. The high dissolution of polyphenols and diffusivity of DESs facilitate polyphenol molecules’ diffusion outside of plant cells. The affinity between polyphenols and DESs can be theoretically elucidated via a conductor-like screening model for real solvents (COSMO-RS) analysis [15,21,27,87].
The effect of DESs on the cell structure of agri-food by-products can be intuitively revealed via scanning electron microscopy (SEM) and field emission SEM (FE-SEM). SEM images of orange peel cell structures after ChCl-based DESs extraction showed a higher disintegration level than raw material, suggesting that ChCl-based DESs were efficient for the dissolution of cell wall structure [16]. Figure 3A,B show the microstructure of mulberry leaves before and after extraction with DESs using SEM [35]. As shown in Figure 3A, the cell wall structure showed integrity before extraction; however, when treated with ChCl:citric acid, the cell walls were entirely damaged (Figure 3B). A SEM image of ChCl:MA soaked Carya cathayensis Sarg. peels exhibited rough and rugae structures on its outer surface, which was ascribed to the partial erosion and penetration of DESs on the cell wall [69]. More pronounced damage was observed for olive leaves with DESs than ethanol [86]. The FE-SEM images of the pre- and post-extraction Pyrus ussuriensis leaves are shown in Figure 3C,D [45]. As can be observed in Figure 3C, there was no solvent penetration on the surface of the Pyrus ussuriensis leaves before extraction. However, sufficient solvent penetrations were observed in the FE-SEM images of Pyrus ussuriensis leaves after ChCl:glutaric acid extraction (Figure 3D). The high solubility of polyphenols in the DESs facilitated the penetration of the solvent, which led to structural changes in the overall leaf surface.
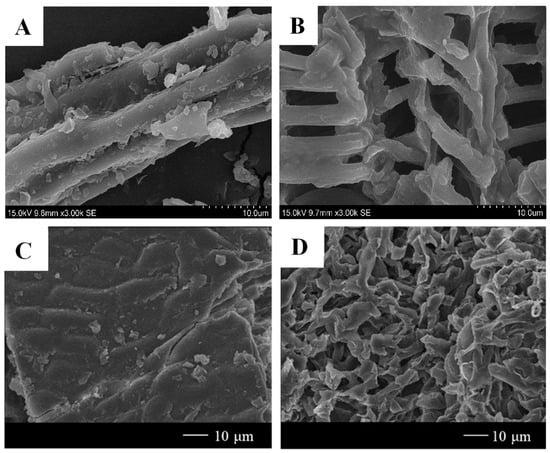
Figure 3.
SEM images of mulberry leaves before DESs extraction (A) and after DESs extraction (B) and FE-SEM images of Pyrus ussuriensis leaves surface before DESs extraction (C) and after DESs extraction (D) (reproduced from [35,45] with permissions from Elsevier license number 1400088-1).
Furthermore, mass transfer and kinetic studies corroboratively elucidate the extraction mechanism of polyphenols using DESs. A mass transfer result confirmed an efficient contact between polyphenols and DESs according to the high Biot number values [47]. Fick’s model successfully forecasted the extraction kinetics of tannic acid using an ultrasound-assisted DESs extraction method and revealed that diffusivity was the controlling factor [57]. Kinetic studies showed that the extraction diffusivity of anthocyanin in NADESs (1.063 × 10−12) was markedly higher than in water (0.835 × 10−12) [74]. Moreover, it has been reported that DESs extracted more polyphenols with higher molecular weights and more diverse phenolic compounds than methanol from mangosteen peel [43]. Quantum chemical calculation combined with molecular dynamic simulation revealed that the high extraction efficiency of ChCl:malic acid was due to the large solvent accessible surface area, long lifetime of H-bonds between ChCl:malic acid and extract, and low intermolecular interaction energy [70]. In summary, DESs can serve as an efficient solvent for the extraction of polyphenols.
3.2. Process for the Green Extraction of Polyphenols
In recent years, numerous research studies involved in extracting polyphenols using various DESs have emerged (Table 1, Table 2, Table 3, Table 4, Table 5 and Table 6). The extraction yield of polyphenols is usually determined by the total phenolic content (TPC), which is expressed as a mg gallic acid equivalent (GAE) per gram of dry weight. In addition, the extraction yield of flavonoid and anthocyanin is determined by total flavonoid content (TFC) and total anthocyanin content (TAC), respectively. The composition of phenolic compounds is commonly measured by HPLC-DAD, HPLC-PDA, or HPLC-MS. The higher polyphenol yield of DESs than conventional organic solvents suggests that DESs are efficient solvents for extracting polyphenols. Figure 4 shows the schematic process for the green extraction of polyphenols from agri-food by-products.
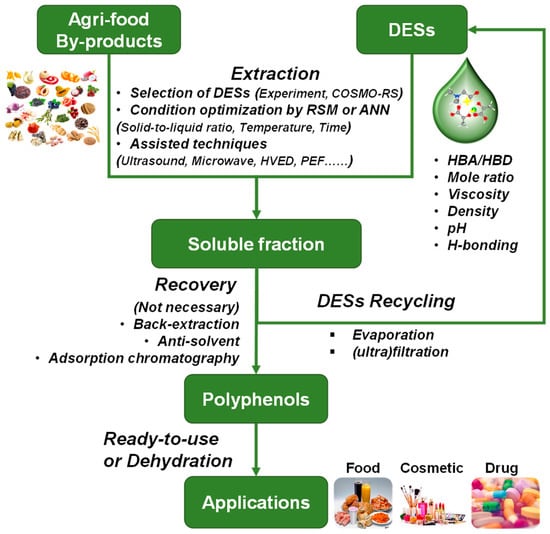
Figure 4.
Schematic process for the green and sustainable extraction of polyphenols from agri-food by-products.
The milled agri-food by-products and selected DESs are mixed at a certain solid-to-liquid ratio. Then, the mixture is heated to the preset temperature in a specific device and maintained for a certain time. The selection of the optimum solvent for phenolic compounds is a crucial step that can be achieved using experimental methods or theoretical simulations. COSMOtherm is an in silico approach based on COSMO-RS and can predict the solubility of the target compound in a wide range of solvents [15,21,27,87]. Compared to the experimental method, COSMOtherm is much more time- and labor-saving. In general, the chemical nature of HBAs and HBDs, their molar ratio, viscosity, density, pH, and H-bonding network in the DESs are crucial factors that influence the extraction yield of polyphenols. The extraction conditions (namely solid-to-liquid ratio, water content, temperature and time, and technical parameters of assisted technologies) are usually optimized to maximize the extraction yield of polyphenols. The optimization process can be experimentally conducted and optimized using the response surface method (RSM) and/or artificial neural network (ANN) methods [32,74].
After the extraction process, the resultant polyphenol-rich liquid is taken out to recover polyphenols using various strategies, including back-extraction [13], the addition of anti-solvent [75], and adsorption chromatography with microporous resins [49]. However, in some cases, such as when using NADESs as the extraction medium, the recovery process is not necessary. Furthermore, NADESs can be used as a storage system to stabilize polyphenols or as a formulation system to deliver polyphenols distribution in drug and cosmetic products [96]. At the same time, the DESs-rich supernatant is collected and recycled through anti-solvent evaporation or liquid phase (ultra)filtration. It has been reported that the recycled DESs still maintains an excellent performance in extracting polyphenols with acceptable efficiency [35]. Finally, the recovered polyphenol is ready to use [24,91] or dried into a final product. In summary, this scheme improves the extraction yield of polyphenols, reduces the generation of waste, minimizes the use of chemicals, and paves the way for a sustainable and circular bio-economy.
4. Assisted Technologies of Polyphenols Extraction with DESs
Apart from the proper selection of DESs, the extraction process can be improved using assisted technologies. As listed in Table 1, Table 2, Table 3, Table 4, Table 5 and Table 6, various techniques, including microwave, ultrasound, pulsed electric field (PEF), high-voltage electric discharge (HVED), and infrared, have already been combined with DESs to improve the extraction yield of polyphenols. Additionally, the joint use of these assisted techniques and DESs can shorten the extraction time, reduce solvent consumption, and curtail the operation cost.
4.1. Ultrasound
Ultrasound is the mostly-used and simplest assisted extraction technique due to the simple requirement of common equipment—ultrasonic bath. Ultrasound-assisted extraction (UAE) is based on the cavitation process generated by compression and rarefaction cycles related to the propagation of ultrasounds through the by-products [54]. As shown in Figure 2, micro-bubbles cavitation, microjets shooting on the surface, and severe agitation caused by mechano-acoustic effects during ultrasonication enhance the micro-pores for greater surface area contact with DESs [6]. As listed in Table 1, Table 2, Table 3, Table 4, Table 5 and Table 6, UAE has efficiently extracted various polyphenols from a wide range of agri-food by-products.
Ultrasound power [25,29,30,31,39,76], intensity [10], duty cycle [10,57], and amplitude [43,74] are critical parameters that influence the extraction efficiency. The ultrasonic power significantly affected the extraction yield of anthocyanins, whose content increased with increased ultrasonic power [25]. As shown in Figure 5A, the yield of anthocyanins using DESs was significantly enhanced with increasing ultrasound power and reached the maximum value at 300 W [31], whereas, as the ultrasound power increased above 300 W, anthocyanin yield decreased significantly. A similar trend was observed for the DESs-based UAE of anthocyanins from blueberry pomace [30]. Moreover, similar trends were also reported for the DESs-based UAE of polyphenols from foxtail millet bran [76], apple pomace [10], and pomegranate peel [39]. This was attributed to excessive heat generation of high ultrasound power, which led to the degradation of polyphenols. Rashid and co-workers studied the effect of acoustic intensity on the extraction of polyphenols, and the results showed that increasing the acoustic intensity from 20 to 83.1 W/cm2 increased the percentage increase in polyphenols extraction by 60~73.8% for the three tested DESs [10]. This was because of the amplification of ultrasonic waves, which resulted in the intensification of cavitation effect. During the travel of huge amplitude ultrasonic waves via DESs system, higher acoustic intensity led to energetic cavity collapse and shock waves formation. Finally, these actions resulted in interfacial turbulence, outer material disintegration, energy dissipation, and diffusion.
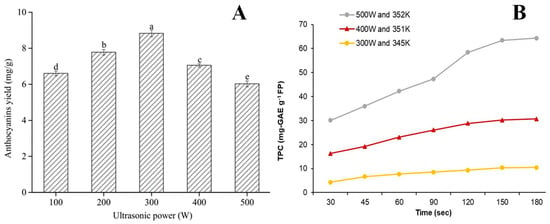
Figure 5.
Effects of ultrasonic power on anthocyanins yield (A) and microwave power TPC (B) (taken from Refs. [31,47] with permission of Wiley, permissions license ID number: 1400450-1). Note: Different lowercase letters indicate significant differences between groups, p < 0.05.
Pulse mode ultrasound is recommended to recover polyphenols as it avoids the cumulative thermal effect during the extraction process. Therefore, the duty cycle of pulse mode ultrasound should be carefully chosen. The extraction yield of tannic acid from onion peel significantly decreased with the increase in the duty cycle [57]. However, a different trend was reported for retrieving polyphenols from apple pomace [10]. The extraction yield of polyphenols increased with increasing duty cycle from 20% to 75%. Additionally, the yield of polyphenols is also affected by ultrasound amplitude. The extraction yield of proanthocyanidin from mangosteen peel was positively affected by ultrasound amplitude [43]. A significant increase in anthocyanin content was observed when raising the amplitude level to 21.25% [74]. However, higher amplitude levels caused a negative effect on anthocyanin content due to the chemical degradation of anthocyanins.
4.2. Microwave
The fundamental principle of microwave-assisted extraction (MAE) is dielectric heating [54]. A microwave is a propagating electromagnetic wave that interacts with polar molecules (e.g., DESs). The rotation and polarization of polar molecules in DESs promote the penetration of the DESs into biomass and provide energy to activate the bond-breaking required for the dissolution of polyphenols [6]. MAE intensifies the DESs extraction process as a result of heat and increased mass transfer [54]: (1) penetration of DESs into the biomass matrix; (2) solubilization and/or breakdown of the cellulose, hemicellulose, lignin, and polyphenols; (3) transport of the solubilized compounds from the insoluble biomass matrix to the bulk DESs phase; and (4) separation of the DESs liquid phase and residual solid phase.
As listed in Table 1, Table 2, Table 3, Table 4, Table 5 and Table 6, a high extraction yield of polyphenols has been achieved by synergistically using microwave and DESs from various agri-food by-products. It has been reported that MAE efficiently extracted polyphenols (especially anthocyanin) from sour cherry peels, requiring less than 5 min [48]. Generally, MAE extraction is time-saving and energy-saving. A thermodynamic study indicated that DESs-based MAE was an efficient, endothermic, and spontaneous system for extracting polyphenols from sour cherry pomace [47]. Microwave power is a critical parameter that influences the extraction efficiency of polyphenols. Panic and co-workers revealed that the extraction of TAC from grape pomace increased with increasing microwave power [23]. Similar trends were observed for the extraction of polyphenols from mango peel [42], sour cherry peels [47], and onion skin [56]. As shown in Figure 5B, increasing microwave power (300, 400, and 500 W) steadily increased the yields of polyphenols from sour cherry peels [47].
4.3. Other or Combined Technologies
Other assisted techniques, such as infrared, PEF, and HVED, have intensified the extraction process. Rajha and co-workers extracted polyphenols from pomegranate peels using three assisted methods, namely conventional solid–liquid, ultrasound, and infrared [38]. Results showed that infrared obtained the utmost concentration of polyphenols (152 mg/g). This was ascribed to the high absorption of infrared radiation wavelengths by DESs and polyphenols. In addition, during the infrared extraction process, polyphenols were excited in different ways, such as stretching, bending, and twisting, and consequently, the extraction was improved. In order to increase the extraction yields of polyphenols from grapefruit peels [11] and pomegranate seeds [37], HVED was used as the assisted technology. Results showed that HVED significantly enhanced the diffusivity of polyphenols compared to the control. HVED may cause electrohydraulic discharges, accompanied by multiple secondary phenomena, including high-amplitude pressure shock waves, strong liquid turbulence, bubbles cavitation, UV radiations, and free radicals. These actions result in the disruption of complex structures and an increase in mass transfer, hence promoting extraction efficiencies. By jointly using PEF and DESs, the extraction efficiencies of rutin and quercetin in noni pomace were significantly higher than those of conventional organic extraction [52]. During PEF treatment, the electroporation of cell walls occurs when cells are subjected to an applied voltage with an associated electric field greater than the critical transmembrane potential, increasing the release of polyphenols.
Moreover, a combined strategy using ultrasound and microwave with NADESs has been developed to extract anthocyanins from grape pomace [23] and polyphenols from olive and grape pomace [24]. The extraction processes were carried out under microwave power of 300 W coupling with ultrasound power of 50 W for 10 min. The adopted simultaneous ultrasound/microwave-assisted extraction (SUMAE) achieved the highest anthocyanin yields with reduced energy consumption. This was attributed to the fact that double irradiation of ultrasound and microwave has synergistic effects on the extraction process: ultrasound ruptures the cells, and microwave promotes the release of polyphenols into DESs.
4.4. Comparison of These Assisted Technologies
Many studies reported that MAE was more efficient than UAE when coupling with DESs to extract polyphenols from agri-food by-products. For example, the extraction kinetic models of polyphenols from mulberry leaves confirmed that MAE (600 W, 60 °C, 20 min) was more efficient than UAE (250 W, 66 °C, 35 min) [36], MAE (500 W, 15 min) yielded more anthocyanin than UAE (500 W, 30 min) from blueberry peel [28], and MAE (180 W within 30 s) was more efficient than UAE (30 W, 50 °C) in the extraction of polyphenols from sour cherry pomace [48]. This may be ascribed to the fact that the power of applied microwave irradiation was higher than that of ultrasound, and microwave could reduce DESs viscosity more than ultrasound. In addition, HVED showed better performance than ultrasound in extracting polyphenols [37]. It has been reported that higher Zpolyphenol values were obtained via HVED than via ultrasound. In the applied ultrasound energy input (400–3600 kJ/kg), the polyphenol normalized content (Zpolyphenols) increased with the energy input. In the case of HVED energy input, Zpolyphenols values significantly increased in the range of 27–267 kJ/kg. Beyond 267 kJ/kg, higher HVED energy inputs resulted in lower Zpolyphenols, probably owing to the degradation of polyphenols. One possible reason is that high-energy HVED generates radical species that oxidize the extracted polyphenols.
5. Applications of Polyphenol-Rich DESs Extract in the Food, Cosmetic, and Pharmaceutical Industry
DESs, particularly NADESs, are GRAS solvents and can be used in the food, cosmetic, and pharmaceutical industries [7,8]. NADESs are promising alternatives for producing biocompatible, ready-to-use extracts with specific biological activity without the need for extensive and costly downstream purification processes. The polyphenol-rich DESs extracts can be used as ready-to-use ingredients or additives for food products, cosmetic emulsions, and active packaging films/coatings (Figure 6).
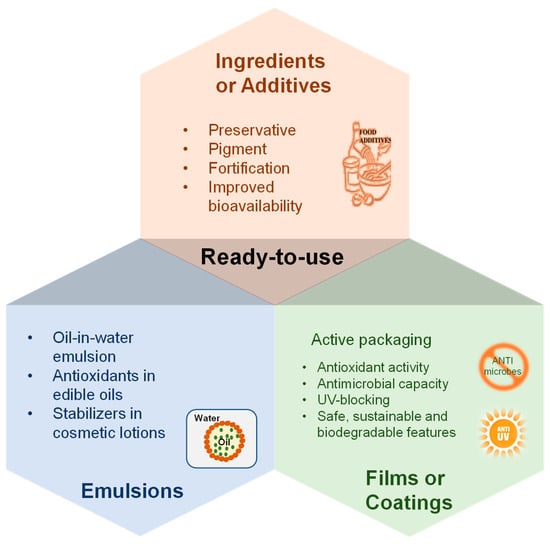
Figure 6.
Applications of polyphenol-rich DESs extract in the food, cosmetic, and pharmaceutical industry.
5.1. Ready-to-Use Ingredients or Additives for Products
Recent research on polyphenol-rich extracts using NADESs revealed excellent antioxidant and antiproliferative activities and low cytotoxicity [19,24,33,91,97]. They play the role of preservatives, pigments, and fortifications. For example, NADESs extract from mango peel has been found to retard the oxidation of sunflower oil [42] and soybean crude oil [50] and increase the induction time, indicating its potential as a natural preservative in edible oils. Chocolate milk was fortified by a polyphenol-rich NADESs extract from cocoa by-products [91], and the electronic tongue result showed that the fortified chocolate milk had sensory acceptability within 10% of NADESs extract. Moreover, NADESs improved the bioavailability of polyphenol [24], anthocyanin [19,97], isoflavone [82], and rutin [98] in cells or rats compared to their aqueous solutions. Consequently, the polyphenol-rich NADESs extracts could serve as a delivery agent or administration vehicle in the pharmaceutical industry. Non-compartmental pharmacokinetic results showed that NADESs enhanced the bioavailability of anthocyanins by 140% compared to methanol:water:formic acid solvent [97]. By delaying gastric chyme neutralization, NADESs improved the stability of phenolic compounds during in vitro digestion. These findings imply that in addition to being an environmentally benign solvent for polyphenols extraction, NADESs can be employed as a ready-to-use vehicle for improving oral absorption of anthocyanins. As a concluding remark, the polyphenol-rich NADESs extracts are readily to be used as ingredients or additives for various products.
5.2. Food and Cosmetic Emulsions
Apart from the production of nutraceuticals or functional foods and pharmaceutical formulations, polyphenol-rich NADESs extract can also be used in cosmetic products. Polyphenol-rich NADESs extract can prevent lipid oxidation in oil-in-water (O/W) emulsions [77,81]. The Rancimat test showed that the presence of NADESs extract in the O/W emulsion increased the induction time and antimicrobial effect by 10-fold compared to that prepared with water [81]. Increasing the lipophilization of polyphenols leads to a positive change in their capacity to stabilize lipids against oxidation in O/W emulsions [77]. The incorporation of NADESs extract in O/W emulsion resulted in lower peroxide value and 2-thiobarbituric acid reactive substances, indicating its antioxidant action on lipids. The obtained stable O/W emulsions with DESs extract suggest its feasibility in food formulations. Based on the beneficial impact on keratinocyte growth, it is highly suggested that the polyphenol-rich NADESs extract can be readily used in the cosmetic industry [21,22]. Its use in cosmetic formulations brings at least three benefits: (1) protecting skin cells against oxidative stress and inflammation, (2) stimulating cell growth and regeneration, and (3) stabilizing products for longer shelf-life. Cosmetic emulsion with the addition of DESs extracts from tomato pomace showed satisfactory physicochemical characteristics [61]. NADESs could serve as a solvent to obtain a phenolic-rich extract that could be readily applicable to cosmetic formulations.
5.3. Active Packaging Films or Coatings
Additionally, the polyphenol-rich NADESs extract can be used to fabricate active packaging films or coatings for food applications (Table 7). NADESs function as plasticizer agents, improving the flexibility of film or coating. At the same time, the phenolic compounds in NADESs extract, such as anthocyanins, render films/coatings with multiple functional properties, including antioxidant, antimicrobial, UV-blocking, and pH-sensitive properties. For example, the incorporation of anthocyanin-rich NADESs extract into polyvinyl alcohol-based film reduced its glass transition temperature, transparency, and Young’s modulus, whereas it increased its water vapor permeability, elasticity, and water solubility [99]. The incorporation of polyphenol-rich NADESs extract into coatings achieved an in vitro antimicrobial activity of 72% against Monilinia fructicola [100]. These films and/or coatings containing polyphenol-rich DESs extracts can be used as pH indicators for evaluating food quality during storage [99] and active packaging to extend shelf-life [101,102,103]. For example, the chitosan/zein films containing Rosa roxburghii Tratt leaves extract showed better antioxidant and antibacterial activities, effectively inhibited the growth of foodborne pathogens, and extended the shelf lives of blueberries and fresh-cut cherry tomatoes [102]. Overall, these findings suggest that polyphenol-rich NADESs extract could be incorporated into films or coatings for active packing in the food industry.

Table 7.
Films or coatings containing polyphenol-rich NADESs extracts for food packaging applications.
6. Concluding Remarks and Future Perspectives
Polyphenols, which are ubiquitous in a huge variety of agri-food by-products, can provide plentiful beneficial effects for human health. This review timely reports the ongoing progress on the green extraction of polyphenols using DESs and assisted technologies. Polyphenol-rich agri-food by-product resources, extraction mechanisms, assisted technologies, and applications were highlighted. Concerning further research on the application of polyphenol-rich DESs extracts in the food, cosmetic and pharmaceutical industry, the following challenges should be addressed: (1) although many researchers have evidenced that polyphenol-rich DESs extracts is benign for humans due to its natural presence in various foods and the non-cytotoxicity of DESs, an in-depth eco-toxicological and cyto-toxicological profile is required; (2) when using polyphenol-rich DESs extract as a food ingredient or additive, the recommended daily intake (RDI) and its effect on sensory acceptance must be taken in account; (3) more research is needed to understand the mechanism underlying the extraction and biological activity of polyphenol-rich DESs extracts, such as the extraction efficiency, specificity, kinetics, thermodynamics, and the relationship between structure and bioactivity; and (4) elimination of the drawbacks of DESs (high viscosity and low vapor pressure) via assisted technologies to realize industrial application. In the near future, it is highly expected that polyphenol-rich DESs extracts will expand rapidly in industrial applications.
Funding
This research was funded by National Natural Science Foundation of China (22308130) and the General Project of Natural Science Research in Colleges and Universities of Jiangsu (21KJB550010). And the APC was funded by HX20230309.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
ANN: artificial neural network, C3GE: cyanidin-3-O-glucoside equivalent, CAE: caffeic acid equivalent, ChCl: choline chloride, COSMO-RS: Conductor-like Screening Model for Real Solvents, DESs: deep eutectic solvents, ECE: epicatechin equivalent, FAE: ferulic acid equivalent, FAO: Food and Agricultural Organization, GAE: gallic acid equivalent, GRAS: generally recognized as safe, HBAs: hydrogen bond acceptors, HBDs: hydrogen bond donors, HVED: high-voltage electric discharge, HVEDAE: high voltage electric discharge assisted extraction, M3GE: malvidin-3-glucoside equivalent, MAE: microwave-assisted extraction, NADESs: natural deep eutectic solvents, O/W: oil-in-water, PEF: pulsed electric field, RSM: response surface methodology, RtE: rutin equivalent, SDGs: Sustainable Development Goals, SEM: scanning electron microscope, SLE: solid-liquid extraction, SUMAE: simultaneous ultrasound/microwave-assisted extraction, TAC: total anthocyanin content, TFC: total flavonoid content, TPC: total phenolic content, UAE: ultrasound-assisted extraction.
References
- FAO. The State of Food and Agriculture 2019 Moving Forward on Food Loss and Waste Reduction; United Nations: Rome, Italy, 2019; ISBN 9789210046268. [Google Scholar]
- Brglez Mojzer, E.; Knez Hrncic, M.; Skerget, M.; Knez, Z.; Bren, U. Polyphenols: Extraction Methods, Antioxidative Action, Bioavailability and Anticarcinogenic Effects. Molecules 2016, 21, 901. [Google Scholar] [CrossRef]
- Ruesgas-Ramon, M.; Figueroa-Espinoza, M.C.; Durand, E. Application of Deep Eutectic Solvents (DES) for Phenolic Compounds Extraction: Overview, Challenges, and Opportunities. J. Agric. Food Chem. 2017, 65, 3591–3601. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Fakayode, O.A.; Ren, M.; Li, H.; Liang, J.; Zhou, C. Green and sustainable extraction of lignin by deep eutectic solvent, its antioxidant activity, and applications in the food industry. Crit. Rev. Food Sci. Nutr. 2023. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Hu, T.; Gu, J. Edge-cloud cooperation driven self-adaptive exception control method for the smart factory. Adv. Eng. Inform. 2022, 51, 101493. [Google Scholar] [CrossRef]
- Zhou, M.; Fakayode, O.A.; Ahmed Yagoub, A.E.; Ji, Q.; Zhou, C. Lignin fractionation from lignocellulosic biomass using deep eutectic solvents and its valorization. Renew. Sust. Energ. Rev. 2022, 156, 111986. [Google Scholar] [CrossRef]
- Misan, A.; Nadpal, J.; Stupar, A.; Pojic, M.; Mandic, A.; Verpoorte, R.; Choi, Y.H. The perspectives of natural deep eutectic solvents in agri-food sector. Crit. Rev. Food Sci. Nutr. 2020, 60, 2564–2592. [Google Scholar] [CrossRef]
- Benvenutti, L.; Zielinski, A.A.F.; Ferreira, S.R.S. Which is the best food emerging solvent: IL, DES or NADES? Trends Food. Sci. Technol. 2019, 90, 133–146. [Google Scholar] [CrossRef]
- Moni Bottu, H.; Mero, A.; Husanu, E.; Tavernier, S.; Pomelli, C.S.; Dewaele, A.; Bernaert, N.; Guazzelli, L.; Brennan, L. The ability of deep eutectic solvent systems to extract bioactive compounds from apple pomace. Food Chem. 2022, 386, 132717. [Google Scholar] [CrossRef]
- Rashid, R.; Mohd Wani, S.; Manzoor, S.; Masoodi, F.A.; Masarat Dar, M. Green extraction of bioactive compounds from apple pomace by ultrasound assisted natural deep eutectic solvent extraction: Optimisation, comparison and bioactivity. Food Chem. 2023, 398, 133871. [Google Scholar] [CrossRef]
- El Kantar, S.; Rajha, H.N.; Boussetta, N.; Vorobiev, E.; Maroun, R.G.; Louka, N. Green extraction of polyphenols from grapefruit peels using high voltage electrical discharges, deep eutectic solvents and aqueous glycerol. Food Chem. 2019, 295, 165–171. [Google Scholar] [CrossRef]
- Jokić, S.; Šafranko, S.; Jakovljević, M.; Cikoš, A.-M.; Kajić, N.; Kolarević, F.; Babić, J.; Molnar, M. Sustainable Green Procedure for Extraction of Hesperidin from Selected Croatian Mandarin Peels. Processes 2019, 7, 469. [Google Scholar] [CrossRef]
- Xu, M.; Ran, L.; Chen, N.; Fan, X.; Ren, D.; Yi, L. Polarity-dependent extraction of flavonoids from citrus peel waste using a tailor-made deep eutectic solvent. Food Chem. 2019, 297, 124970. [Google Scholar] [CrossRef]
- Kalogiouri, N.P.; Palaiologou, E.; Papadakis, E.N.; Makris, D.P.; Biliaderis, C.G.; Mourtzinos, I. Insights on the impact of deep eutectic solvents on the composition of the extracts from lemon (Citrus limon L.) peels analyzed by a novel RP-LC–QTOF-MS/MS method. Eur. Food Res. Technol. 2022, 248, 2913–2927. [Google Scholar] [CrossRef]
- Gomez-Urios, C.; Vinas-Ospino, A.; Puchades-Colera, P.; Lopez-Malo, D.; Frigola, A.; Esteve, M.J.; Blesa, J. Sustainable Development and Storage Stability of Orange By-Products Extract Using Natural Deep Eutectic Solvents. Foods 2022, 11, 2457. [Google Scholar] [CrossRef] [PubMed]
- Ozturk, B.; Parkinson, C.; Gonzalez-Miquel, M. Extraction of polyphenolic antioxidants from orange peel waste using deep eutectic solvents. Sep. Purif. Technol. 2018, 206, 1–13. [Google Scholar] [CrossRef]
- Jeong, K.M.; Zhao, J.; Jin, Y.; Heo, S.R.; Han, S.Y.; da Yoo, E.; Lee, J. Highly efficient extraction of anthocyanins from grape skin using deep eutectic solvents as green and tunable media. Arch. Pharm. Res. 2015, 38, 2143–2152. [Google Scholar] [CrossRef] [PubMed]
- Cvjetko Bubalo, M.; Curko, N.; Tomasevic, M.; Kovacevic Ganic, K.; Radojcic Redovnikovic, I. Green extraction of grape skin phenolics by using deep eutectic solvents. Food Chem. 2016, 200, 159–166. [Google Scholar] [CrossRef]
- Radošević, K.; Ćurko, N.; Gaurina Srček, V.; Cvjetko Bubalo, M.; Tomašević, M.; Kovačević Ganić, K.; Radojčić Redovniković, I. Natural deep eutectic solvents as beneficial extractants for enhancement of plant extracts bioactivity. LWT-Food Sci. Technol. 2016, 73, 45–51. [Google Scholar] [CrossRef]
- Dabetić, N.; Todorović, V.; Panić, M.; Radojčić Redovniković, I.; Šobajić, S. Impact of Deep Eutectic Solvents on Extraction of Polyphenols from Grape Seeds and Skin. Appl. Sci. 2020, 10, 4830. [Google Scholar] [CrossRef]
- Panic, M.; Gunjevic, V.; Radosevic, K.; Cvjetko Bubalo, M.; Ganic, K.K.; Redovnikovic, I.R. COSMOtherm as an Effective Tool for Selection of Deep Eutectic Solvents Based Ready-To-Use Extracts from Grasevina Grape Pomace. Molecules 2021, 26, 4722. [Google Scholar] [CrossRef]
- Punzo, A.; Porru, E.; Silla, A.; Simoni, P.; Galletti, P.; Roda, A.; Tagliavini, E.; Samori, C.; Caliceti, C. Grape Pomace for Topical Application: Green NaDES Sustainable Extraction, Skin Permeation Studies, Antioxidant and Anti-Inflammatory Activities Characterization in 3D Human Keratinocytes. Biomolecules 2021, 11, 1181. [Google Scholar] [CrossRef] [PubMed]
- Panic, M.; Gunjevic, V.; Cravotto, G.; Radojcic Redovnikovic, I. Enabling technologies for the extraction of grape-pomace anthocyanins using natural deep eutectic solvents in up-to-half-litre batches extraction of grape-pomace anthocyanins using NADES. Food Chem. 2019, 300, 125185. [Google Scholar] [CrossRef] [PubMed]
- Panic, M.; Radic Stojkovic, M.; Kraljic, K.; Skevin, D.; Radojcic Redovnikovic, I.; Gaurina Srcek, V.; Radosevic, K. Ready-to-use green polyphenolic extracts from food by-products. Food Chem. 2019, 283, 628–636. [Google Scholar] [CrossRef] [PubMed]
- Bosiljkov, T.; Dujmić, F.; Cvjetko Bubalo, M.; Hribar, J.; Vidrih, R.; Brnčić, M.; Zlatic, E.; Radojčić Redovniković, I.; Jokić, S. Natural deep eutectic solvents and ultrasound-assisted extraction: Green approaches for extraction of wine lees anthocyanins. Food Bioprod. Proces. 2017, 102, 195–203. [Google Scholar] [CrossRef]
- Benvenutti, L.; Zielinski, A.A.F.; Ferreira, S.R.S. Pressurized aqueous solutions of deep eutectic solvent (DES): A green emergent extraction of anthocyanins from a Brazilian berry processing by-product. Food Chem. X 2022, 13, 100236. [Google Scholar] [CrossRef]
- Benvenutti, L.; Sanchez-Camargo, A.D.P.; Zielinski, A.A.F.; Ferreira, S.R.S. NADES as potential solvents for anthocyanin and pectin extraction from Myrciaria cauliflora fruit by-product: In silico and experimental approaches for solvent selection. J. Mol. Liq. 2020, 315, 113761. [Google Scholar] [CrossRef]
- Grillo, G.; Gunjevic, V.; Radosevic, K.; Redovnikovic, I.R.; Cravotto, G. Deep Eutectic Solvents and Nonconventional Technologies for Blueberry-Peel Extraction: Kinetics, Anthocyanin Stability, and Antiproliferative Activity. Antioxidants 2020, 9, 1069. [Google Scholar] [CrossRef]
- Zhang, X.J.; Liu, Z.T.; Chen, X.Q.; Zhang, T.T.; Zhang, Y. Deep eutectic solvent combined with ultrasound technology: A promising integrated extraction strategy for anthocyanins and polyphenols from blueberry pomace. Food Chem. 2023, 422, 136224. [Google Scholar] [CrossRef]
- Fu, X.; Wang, D.; Belwal, T.; Xie, J.; Xu, Y.; Li, L.; Zou, L.; Zhang, L.; Luo, Z. Natural deep eutectic solvent enhanced pulse-ultrasonication assisted extraction as a multi-stability protective and efficient green strategy to extract anthocyanin from blueberry pomace. LWT-Food Sci. Technol. 2021, 144, 111220. [Google Scholar] [CrossRef]
- Xue, H.; Tan, J.; Li, Q.; Tang, J.; Cai, X. Ultrasound-Assisted Deep Eutectic Solvent Extraction of Anthocyanins from Blueberry Wine Residues: Optimization, Identification, and HepG2 Antitumor Activity. Molecules 2020, 25, 5456. [Google Scholar] [CrossRef]
- Alrugaibah, M.; Yagiz, Y.; Gu, L. Use natural deep eutectic solvents as efficient green reagents to extract procyanidins and anthocyanins from cranberry pomace and predictive modeling by RSM and artificial neural networking. Sep. Purif. Technol. 2021, 255, 117720. [Google Scholar] [CrossRef]
- Teslic, N.; Santos, F.; Oliveira, F.; Stupar, A.; Pojic, M.; Mandic, A.; Pavlic, B.; Kljakic, A.C.; Duarte, A.R.C.; Paiva, A.; et al. Simultaneous Hydrolysis of Ellagitannins and Extraction of Ellagic Acid from Defatted Raspberry Seeds Using Natural Deep Eutectic Solvents (NADES). Antioxidants 2022, 11, 254. [Google Scholar] [CrossRef]
- Vázquez-González, M.; Fernández-Prior, Á.; Bermúdez Oria, A.; Rodríguez-Juan, E.M.; Pérez-Rubio, A.G.; Fernández-Bolaños, J.; Rodríguez-Gutiérrez, G. Utilization of strawberry and raspberry waste for the extraction of bioactive compounds by deep eutectic solvents. LWT-Food Sci. Technol. 2020, 130, 109645. [Google Scholar] [CrossRef]
- Zhou, P.; Wang, X.; Liu, P.; Huang, J.; Wang, C.; Pan, M.; Kuang, Z. Enhanced phenolic compounds extraction from Morus alba L. leaves by deep eutectic solvents combined with ultrasonic-assisted extraction. Ind. Crop. Prod. 2018, 120, 147–154. [Google Scholar] [CrossRef]
- Gao, M.-Z.; Cui, Q.; Wang, L.-T.; Meng, Y.; Yu, L.; Li, Y.-Y.; Fu, Y.-J. A green and integrated strategy for enhanced phenolic compounds extraction from mulberry (Morus alba L.) leaves by deep eutectic solvent. Microchem. J. 2020, 154, 104598. [Google Scholar] [CrossRef]
- Hernández-Corroto, E.; Boussetta, N.; Marina, M.L.; García, M.C.; Vorobiev, E. High voltage electrical discharges followed by deep eutectic solvents extraction for the valorization of pomegranate seeds (Punica granatum L.). Innov. Food Sci. Emerg. 2022, 79, 103055. [Google Scholar] [CrossRef]
- Rajha, H.N.; Mhanna, T.; El Kantar, S.; El Khoury, A.; Louka, N.; Maroun, R.G. Innovative process of polyphenol recovery from pomegranate peels by combining green deep eutectic solvents and a new infrared technology. LWT-Food Sci. Technol. 2019, 111, 138–146. [Google Scholar] [CrossRef]
- Kim, H.J.; Yoon, K.Y. Optimization of ultrasound-assisted deep eutectic solvent extraction of bioactive compounds from pomegranate peel using response surface methodology. Food Sci. Biotechnol. 2023. [Google Scholar] [CrossRef]
- Bertolo, M.R.V.; Martins, V.C.A.; Plepis, A.M.G.; Bogusz, S. Utilization of pomegranate peel waste: Natural deep eutectic solvents as a green strategy to recover valuable phenolic compounds. J. Clean. Prod. 2021, 327, 129471. [Google Scholar] [CrossRef]
- Kyriakidou, A.; Makris, D.P.; Lazaridou, A.; Biliaderis, C.G.; Mourtzinos, I. Physical Properties of Chitosan Films Containing Pomegranate Peel Extracts Obtained by Deep Eutectic Solvents. Foods 2021, 10, 1262. [Google Scholar] [CrossRef]
- Pal, C.B.T.; Jadeja, G.C. Microwave-assisted extraction for recovery of polyphenolic antioxidants from ripe mango (Mangifera indica L.) peel using lactic acid/sodium acetate deep eutectic mixtures. Food Sci. Technol. Int. 2020, 26, 78–92. [Google Scholar] [CrossRef] [PubMed]
- Plaza, M.; Domínguez-Rodríguez, G.; Sahelices, C.; Marina, M.L. A Sustainable Approach for Extracting Non-Extractable Phenolic Compounds from Mangosteen Peel Using Ultrasound-Assisted Extraction and Natural Deep Eutectic Solvents. Appl. Sci. 2021, 11, 5625. [Google Scholar] [CrossRef]
- Fernandez, M.L.A.; Espino, M.; Gomez, F.J.V.; Silva, M.F. Novel approaches mediated by tailor-made green solvents for the extraction of phenolic compounds from agro-food industrial by-products. Food Chem. 2018, 239, 671–678. [Google Scholar] [CrossRef]
- Lee, J.W.; Park, H.Y.; Park, J. Enhanced Extraction Efficiency of Flavonoids from Pyrus ussuriensis Leaves with Deep Eutectic Solvents. Molecules 2022, 27, 2798. [Google Scholar] [CrossRef] [PubMed]
- Şahin, S.; Bilgin, M. Valorization of peach (Prunus persica L.) waste into speciality products via green methods. Biomass Convers. Bior. 2021, 12, 123–132. [Google Scholar] [CrossRef]
- Kurtulbaş, E. Prediction of mass transfer and kinetic behavior during the extraction of high added-value products from sour cherry (Prunus cerasus L.) peels. J. Food Process. Pres. 2022, 46, e16401. [Google Scholar] [CrossRef]
- Popovic, B.M.; Micic, N.; Potkonjak, A.; Blagojevic, B.; Pavlovic, K.; Milanov, D.; Juric, T. Novel extraction of polyphenols from sour cherry pomace using natural deep eutectic solvents—Ultrafast microwave-assisted NADES preparation and extraction. Food Chem. 2022, 366, 130562. [Google Scholar] [CrossRef]
- Cui, Q.; Liu, J.-Z.; Wang, L.-T.; Kang, Y.-F.; Meng, Y.; Jiao, J.; Fu, Y.-J. Sustainable deep eutectic solvents preparation and their efficiency in extraction and enrichment of main bioactive flavonoids from sea buckthorn leaves. J. Clean. Prod. 2018, 184, 826–835. [Google Scholar] [CrossRef]
- Leal, F.C.; Farias, F.O.; do Amaral, W.; Toci, A.T.; Mafra, M.R.; Igarashi-Mafra, L. Green Solvents to Value Annona muricata L. Leaves as Antioxidants Source: Process Optimization and Potential as a Natural Food Additive. Waste Biomass Valori. 2021, 13, 1233–1241. [Google Scholar] [CrossRef]
- Kehili, M.; Isci, A.; Thieme, N.; Kaltschmitt, M.; Zetzl, C.; Smirnova, I. Microwave-assisted deep eutectic solvent extraction of phenolics from defatted date seeds and its effect on solubilization of carbohydrates. Biomass Convers. Bior. 2022. [Google Scholar] [CrossRef]
- Li, J.; Chen, W.; Niu, D.; Wang, R.; Xu, F.-Y.; Chen, B.-R.; Lin, J.-W.; Tang, Z.-S.; Zeng, X.-A. Efficient and green strategy based on pulsed electric field coupled with deep eutectic solvents for recovering flavonoids and preparing flavonoid aglycones from noni-processing wastes. J. Clean. Prod. 2022, 368, 133019. [Google Scholar] [CrossRef]
- Hu, T.; Wang, W.; Gu, J.; Xia, Z.; Zhang, J.; Wang, B. Research on Apple Object Detection and Localization Method Based on Improved YOLOX and RGB-D Images. Agronomy 2023, 13, 1816. [Google Scholar] [CrossRef]
- Panzella, L.; Moccia, F.; Nasti, R.; Marzorati, S.; Verotta, L.; Napolitano, A. Bioactive Phenolic Compounds from Agri-Food Wastes: An Update on Green and Sustainable Extraction Methodologies. Front. Nutr. 2020, 7, 60. [Google Scholar] [CrossRef]
- Pal, C.B.T.; Jadeja, G.C. Microwave-assisted deep eutectic solvent extraction of phenolic antioxidants from onion (Allium cepa L.) peel: A Box-Behnken design approach for optimization. J. Food Sci. Technol. 2019, 56, 4211–4223. [Google Scholar] [CrossRef]
- Shang, X.-c.; Zhang, Y.-q.; Zheng, Y.-f.; Li, Y. Temperature-responsive deep eutectic solvents as eco-friendly and recyclable media for microwave extraction of flavonoid compounds from waste onion (Allium cepa L.) skins. Biomass Convers. Bior. 2022. [Google Scholar] [CrossRef]
- Sukor, N.F.; Selvam, V.P.; Jusoh, R.; Kamarudin, N.S.; Rahim, S.A. Intensified DES mediated ultrasound extraction of tannic acid from onion peel. J. Food Eng. 2021, 296, 110437. [Google Scholar] [CrossRef]
- Stefou, I.; Grigorakis, S.; Loupassaki, S.; Makris, D.P. Development of sodium propionate-based deep eutectic solvents for polyphenol extraction from onion solid wastes. Clean Technol. Envir. 2019, 21, 1563–1574. [Google Scholar] [CrossRef]
- Pal, C.B.T.; Jadeja, G.C. Deep eutectic solvent-based extraction of polyphenolic antioxidants from onion (Allium cepa L.) peel. J. Sci. Food Agric. 2019, 99, 1969–1979. [Google Scholar] [CrossRef]
- Ciardi, M.; Ianni, F.; Sardella, R.; Di Bona, S.; Cossignani, L.; Germani, R.; Tiecco, M.; Clementi, C. Effective and Selective Extraction of Quercetin from Onion (Allium cepa L.) Skin Waste Using Water Dilutions of Acid-Based Deep Eutectic Solvents. Materials 2021, 14, 6465. [Google Scholar] [CrossRef]
- Vasyliev, G.; Lyudmyla, K.; Hladun, K.; Skiba, M.; Vorobyova, V. Valorization of tomato pomace: Extraction of value-added components by deep eutectic solvents and their application in the formulation of cosmetic emulsions. Biomass Convers. Bior. 2022, 12 (Suppl. S1), 95–111. [Google Scholar] [CrossRef]
- Grillo, G.; Tabasso, S.; Capaldi, G.; Radosevic, K.; Radojcic-Redovnikovic, I.; Gunjevic, V.; Calcio Gaudino, E.; Cravotto, G. Food-Waste Valorisation: Synergistic Effects of Enabling Technologies and Eutectic Solvents on the Recovery of Bioactives from Violet Potato Peels. Foods 2023, 12, 2214. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Liang, Y.N.; Stuckey, D.C.; Hu, X. Single-step extraction of bioactive compounds from cruciferous vegetable (kale) waste using natural deep eutectic solvents. Sep. Purif. Technol. 2023, 317, 123677. [Google Scholar] [CrossRef]
- Zannou, O.; Pashazadeh, H.; Ghellam, M.; Ali Redha, A.; Koca, I. Enhanced ultrasonically assisted extraction of bitter melon (Momordica charantia) leaf phenolic compounds using choline chloride-acetic acid–based natural deep eutectic solvent: An optimization approach and in vitro digestion. Biomass Convers. Bior. 2022. [Google Scholar] [CrossRef]
- Aviles-Betanzos, K.A.; Oney-Montalvo, J.E.; Cauich-Rodriguez, J.V.; Gonzalez-Avila, M.; Scampicchio, M.; Morozova, K.; Ramirez-Sucre, M.O.; Rodriguez-Buenfil, I.M. Antioxidant Capacity, Vitamin C and Polyphenol Profile Evaluation of a Capsicum chinense By-Product Extract Obtained by Ultrasound Using Eutectic Solvent. Plants 2022, 11, 2060. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Yan, Z.; Li, L.; Zhang, L.; Zhao, M.; Yi, H.; Wang, Z.; Li, G.; Wang, Z.; Li, M.; et al. Green Extraction of Phenolic Compounds from Lotus (Nelumbo nucifera Gaertn) Leaf Using Deep Eutectic Solvents: Process Optimization and Antioxidant Activity. Separations 2023, 10, 272. [Google Scholar] [CrossRef]
- González-Rivera, J.; Mero, A.; Husanu, E.; Mezzetta, A.; Ferrari, C.; D’Andrea, F.; Bramanti, E.; Pomelli, C.S.; Guazzelli, L. Combining acid-based deep eutectic solvents and microwave irradiation for improved chestnut shell waste valorization. Green Chem. 2021, 23, 10101–10115. [Google Scholar] [CrossRef]
- An, J.-Y.; Wang, L.-T.; Lv, M.-J.; Wang, J.-D.; Cai, Z.-H.; Wang, Y.-Q.; Zhang, S.; Yang, Q.; Fu, Y.-J. An efficiency strategy for extraction and recovery of ellagic acid from waste chestnut shell and its biological activity evaluation. Microchem. J. 2021, 160, 105616. [Google Scholar] [CrossRef]
- Fu, X.; Wang, D.; Belwal, T.; Xu, Y.; Li, L.; Luo, Z. Sonication-synergistic natural deep eutectic solvent as a green and efficient approach for extraction of phenolic compounds from peels of Carya cathayensis Sarg. Food Chem. 2021, 355, 129577. [Google Scholar] [CrossRef]
- Fu, X.; Belwal, T.; He, Y.; Xu, Y.; Li, L.; Luo, Z. UPLC-Triple-TOF/MS characterization of phenolic constituents and the influence of natural deep eutectic solvents on extraction of Carya cathayensis Sarg. peels: Composition, extraction mechanism and in vitro biological activities. Food Chem. 2022, 370, 131042. [Google Scholar] [CrossRef]
- Bener, M.; Sen, F.B.; Onem, A.N.; Bekdeser, B.; Celik, S.E.; Lalikoglu, M.; Asci, Y.S.; Capanoglu, E.; Apak, R. Microwave-assisted extraction of antioxidant compounds from by-products of Turkish hazelnut (Corylus avellana L.) using natural deep eutectic solvents: Modeling, optimization and phenolic characterization. Food Chem. 2022, 385, 132633. [Google Scholar] [CrossRef]
- Lopez-Linares, J.C.; Campillo, V.; Coca, M.; Lucas, S.; Garcia-Cubero, M.T. Microwave-assisted deep eutectic solvent extraction of phenolic compounds from brewer’s spent grain. J. Chem. Technol. Biot. 2021, 96, 481–490. [Google Scholar] [CrossRef]
- Mouratoglou, E.; Malliou, V.; Makris, D.P. Novel Glycerol-Based Natural Eutectic Mixtures and Their Efficiency in the Ultrasound-Assisted Extraction of Antioxidant Polyphenols from Agri-Food Waste Biomass. Waste Biomass Valori. 2016, 7, 1377–1387. [Google Scholar] [CrossRef]
- Thakur, R.; Gupta, V.; Dhar, P.; Deka, S.C.; Das, A.B. Ultrasound-assisted extraction of anthocyanin from black rice bran using natural deep eutectic solvents: Optimization, diffusivity, and stability. J. Food Process. Pres. 2022, 46, e16309. [Google Scholar] [CrossRef]
- Huang, Y.; Feng, F.; Jiang, J.; Qiao, Y.; Wu, T.; Voglmeir, J.; Chen, Z.G. Green and efficient extraction of rutin from tartary buckwheat hull by using natural deep eutectic solvents. Food Chem. 2017, 221, 1400–1405. [Google Scholar] [CrossRef]
- Zheng, B.; Yuan, Y.; Xiang, J.; Jin, W.; Johnson, J.B.; Li, Z.; Wang, C.; Luo, D. Green extraction of phenolic compounds from foxtail millet bran by ultrasonic-assisted deep eutectic solvent extraction: Optimization, comparison and bioactivities. LWT-Food Sci. Technol. 2022, 154, 112740. [Google Scholar] [CrossRef]
- Santos, M.C.B.; Barouh, N.; Baréa, B.; Villeneuve, P.; Bourlieu-Lacanal, C.; Ferreira, M.S.L.; Durand, E. Sequential one-pot NaDES assisted extraction and biotransformation of rice bran: A new strategy to boost antioxidant activity of natural extracts. Process Biochem. 2022, 117, 110–116. [Google Scholar] [CrossRef]
- Wu, J.; Su, M.; Hu, A.; Wang, H. Extraction and recovery of chlorogenic acid from sunflower disks using a high-efficiency system composed of deep eutectic solvents and macroporous resins. J. Food Process. Pres. 2022, 46, e16856. [Google Scholar] [CrossRef]
- Balaraman, H.B.; Sivasubramaniyam, A.; Rathnasamy, S.K. High selective purification of Quercetin from Peanut hull using protic deep eutectic mixture based liquid–liquid microextraction. Microchem. J. 2020, 152, 104444. [Google Scholar] [CrossRef]
- Chanioti, S.; Tzia, C. Extraction of phenolic compounds from olive pomace by using natural deep eutectic solvents and innovative extraction techniques. Innov. Food Sci. Emerg. 2018, 48, 228–239. [Google Scholar] [CrossRef]
- Pontes, P.V.d.A.; Czaikoski, A.; Almeida, N.A.; Fraga, S.; Rocha, L.d.O.; Cunha, R.L.; Maximo, G.J.; Batista, E.A.C. Extraction optimization, biological activities, and application in O/W emulsion of deep eutectic solvents-based phenolic extracts from olive pomace. Food Res. Int. 2022, 161, 111753. [Google Scholar] [CrossRef]
- Duru, K.C.; Slesarev, G.P.; Aboushanab, S.A.; Kovalev, I.S.; Zeidler, D.M.; Kovaleva, E.G.; Bhat, R. An eco-friendly approach to enhance the extraction and recovery efficiency of isoflavones from kudzu roots and soy molasses wastes using ultrasound-assisted extraction with natural deep eutectic solvents (NADES). Ind. Crop. Prod. 2022, 182, 114886. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, Y.; Gu, J.; Wang, J. A Proactive Manufacturing Resources Assignment Method Based on Production Performance Prediction for the Smart Factory. IEEE T. Ind. Inform. 2022, 18, 46–55. [Google Scholar] [CrossRef]
- Athanasiadis, V.; Grigorakis, S.; Lalas, S.; Makris, D.P. Highly Efficient Extraction of Antioxidant Polyphenols from Olea europaea Leaves Using an Eco-friendly Glycerol/Glycine Deep Eutectic Solvent. Waste Biomass Valori. 2018, 9, 1985–1992. [Google Scholar] [CrossRef]
- Unlu, A.E. Green and Non-conventional Extraction of Bioactive Compounds from Olive Leaves: Screening of Novel Natural Deep Eutectic Solvents and Investigation of Process Parameters. Waste Biomass Valori. 2021, 12, 5329–5346. [Google Scholar] [CrossRef] [PubMed]
- de Almeida Pontes, P.V.; Ayumi Shiwaku, I.; Maximo, G.J.; Caldas Batista, E.A. Choline chloride-based deep eutectic solvents as potential solvent for extraction of phenolic compounds from olive leaves: Extraction optimization and solvent characterization. Food Chem. 2021, 352, 129346. [Google Scholar] [CrossRef]
- Zurob, E.; Cabezas, R.; Villarroel, E.; Rosas, N.; Merlet, G.; Quijada-Maldonado, E.; Romero, J.; Plaza, A. Design of natural deep eutectic solvents for the ultrasound-assisted extraction of hydroxytyrosol from olive leaves supported by COSMO-RS. Sep. Purif. Technol. 2020, 248, 117054. [Google Scholar] [CrossRef]
- Mir-Cerda, A.; Granados, M.; Saurina, J.; Sentellas, S. Green Extraction of Antioxidant Compounds from Olive Tree Leaves Based on Natural Deep Eutectic Solvents. Antioxidants 2023, 12, 995. [Google Scholar] [CrossRef]
- Plaza, A.; Tapia, X.; Yañez, C.; Vilches, F.; Candia, O.; Cabezas, R.; Romero, J. Obtaining Hydroxytyrosol from Olive Mill Waste Using Deep Eutectic Solvents and Then Supercritical CO2. Waste Biomass Valori. 2020, 11, 6273–6284. [Google Scholar] [CrossRef]
- da Silva, M.R.; Jelley, R.E.; Carneiro, R.L.; Fedrizzi, B.; Weber, C.C.; Funari, C.S. Green solvents for the selective extraction of bioactive compounds from by-products of the coffee production chain. Innov. Food Sci. Emerg. 2023, 86, 103365. [Google Scholar] [CrossRef]
- Manuela, P.; Drakula, S.; Cravotto, G.; Verpoorte, R.; Hruškar, M.; Radojčić Redovniković, I.; Radošević, K. Biological activity and sensory evaluation of cocoa by-products NADES extracts used in food fortification. Innov. Food Sci. Emerg. 2020, 66, 102514. [Google Scholar] [CrossRef]
- Ruesgas-Ramon, M.; Suarez-Quiroz, M.L.; Gonzalez-Rios, O.; Barea, B.; Cazals, G.; Figueroa-Espinoza, M.C.; Durand, E. Biomolecules extraction from coffee and cocoa by- and co-products using deep eutectic solvents. J. Sci. Food Agric. 2020, 100, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Fanali, C.; Della Posta, S.; Dugo, L.; Gentili, A.; Mondello, L.; De Gara, L. Choline-chloride and betaine-based deep eutectic solvents for green extraction of nutraceutical compounds from spent coffee ground. J. Pharm. Biomed. Anal. 2020, 189, 113421. [Google Scholar] [CrossRef] [PubMed]
- García-Roldán, A.; Piriou, L.; Jauregi, P. Natural deep eutectic solvents as a green extraction of polyphenols from spent coffee ground with enhanced bioactivities. Front. Plant Sci. 2023, 13, 1072592. [Google Scholar] [CrossRef] [PubMed]
- Grudniewska, A.; Popłoński, J. Simple and green method for the extraction of xanthohumol from spent hops using deep eutectic solvents. Sep. Purif. Technol. 2020, 250, 117196. [Google Scholar] [CrossRef]
- Lu, W.; Liu, S. Choline chloride–based deep eutectic solvents (Ch-DESs) as promising green solvents for phenolic compounds extraction from bioresources: State-of-the-art, prospects, and challenges. Biomass Convers. Bior. 2020, 12, 2949–2962. [Google Scholar] [CrossRef]
- da Silva, D.T.; Smaniotto, F.A.; Costa, I.F.; Baranzelli, J.; Muller, A.; Somacal, S.; Monteiro, C.S.; Vizzotto, M.; Rodrigues, E.; Barcia, M.T.; et al. Natural deep eutectic solvent (NADES): A strategy to improve the bioavailability of blueberry phenolic compounds in a ready-to-use extract. Food Chem. 2021, 364, 130370. [Google Scholar] [CrossRef]
- Faggian, M.; Sut, S.; Perissutti, B.; Baldan, V.; Grabnar, I.; Dall’Acqua, S. Natural Deep Eutectic Solvents (NADES) as a Tool for Bioavailability Improvement: Pharmacokinetics of Rutin Dissolved in Proline/Glycine after Oral Administration in Rats: Possible Application in Nutraceuticals. Molecules 2016, 21, 1531. [Google Scholar] [CrossRef]
- Thakur, R.; Gupta, V.; Ghosh, T.; Das, A.B. Effect of anthocyanin-natural deep eutectic solvent (lactic acid/fructose) on mechanical, thermal, barrier, and pH-sensitive properties of polyvinyl alcohol based edible films. Food Packag. Shelf 2022, 33, 100914. [Google Scholar] [CrossRef]
- Boiteux, J.; Espino, M.; Azcarate, S.; Silva, M.F.; Gomez, F.J.V.; Pizzuolo, P.; Fernandez, M.L.A. NADES blend for bioactive coating design as a sustainable strategy for postharvest control. Food Chem. 2023, 406, 135054. [Google Scholar] [CrossRef]
- Braham, F.; Amaral, L.; Biernacki, K.; Carvalho, D.O.; Guido, L.F.; Magalhaes, J.; Zaidi, F.; Souza, H.K.S.; Goncalves, M.P. Phenolic Extraction of Moringa oleifera Leaves in DES: Characterization of the Extracts and Their Application in Methylcellulose Films for Food Packaging. Foods 2022, 11, 2641. [Google Scholar] [CrossRef]
- Zhao, P.; Wang, J.; Yan, X.; Cai, Z.; Fu, L.; Gu, Q.; Liu, L.; Jin, H.; Fu, Y. Functional chitosan/zein films with Rosa roxburghii Tratt leaves extracts prepared by natural deep eutectic solvents. Food Packag. Shelf 2022, 34, 101001. [Google Scholar] [CrossRef]
- Mostafa, H.; Airouyuwaa, J.O.; Hamed, F.; Wang, Y.; Maqsood, S. Structural, mechanical, antioxidant and antibacterial properties of soy protein isolate (SPI)-based edible food packaging films as influenced by nanocellulose (NC) and green extracted phenolic compounds from date palm leaves. Food Packag. Shelf 2023, 38, 101124. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).