Selectivity of Sol-Gel and Hydrothermal TiO2 Nanoparticles towards Photocatalytic Degradation of Cationic and Anionic Dyes
Abstract
:1. Introduction
2. Results and Discussion
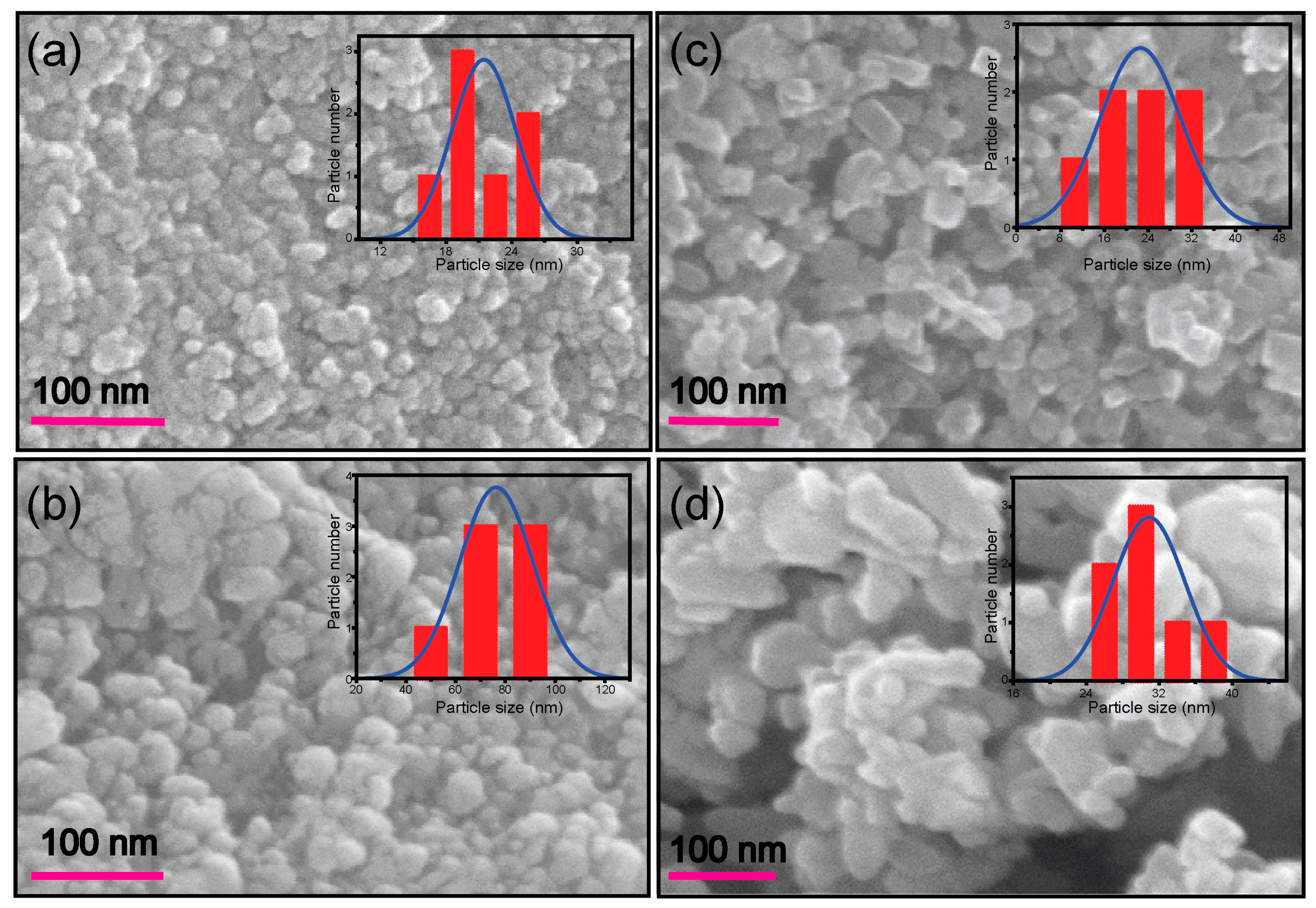
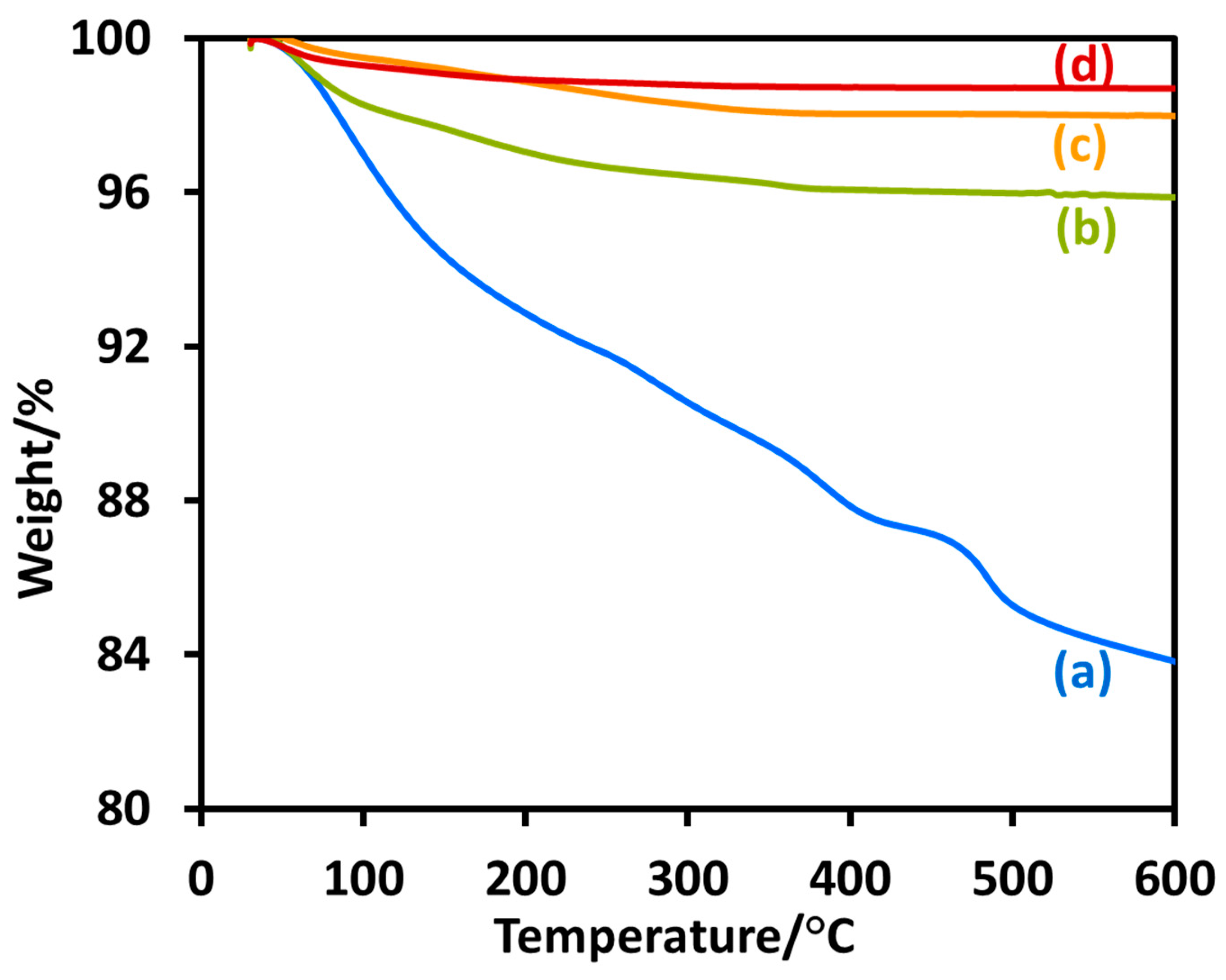
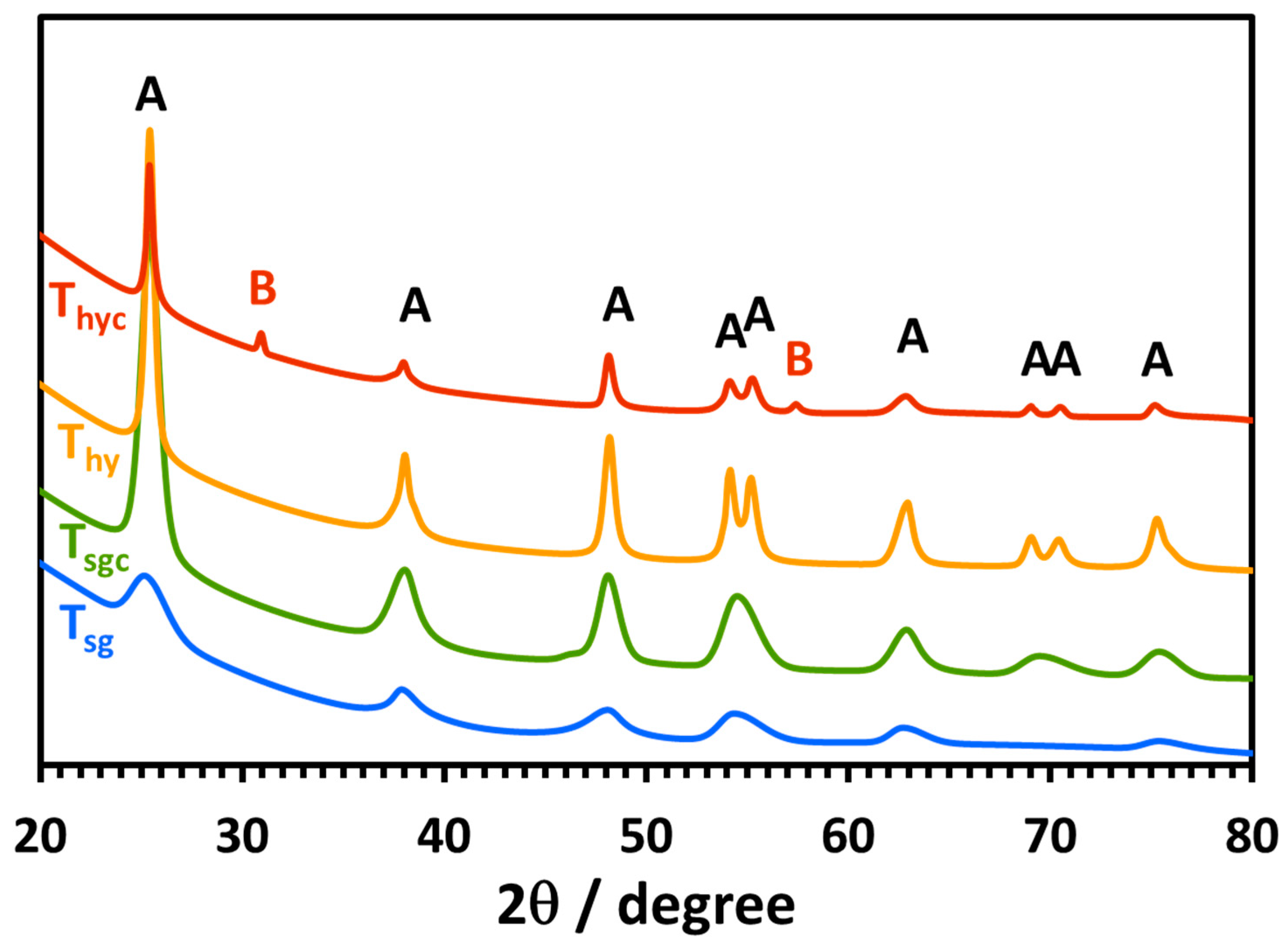
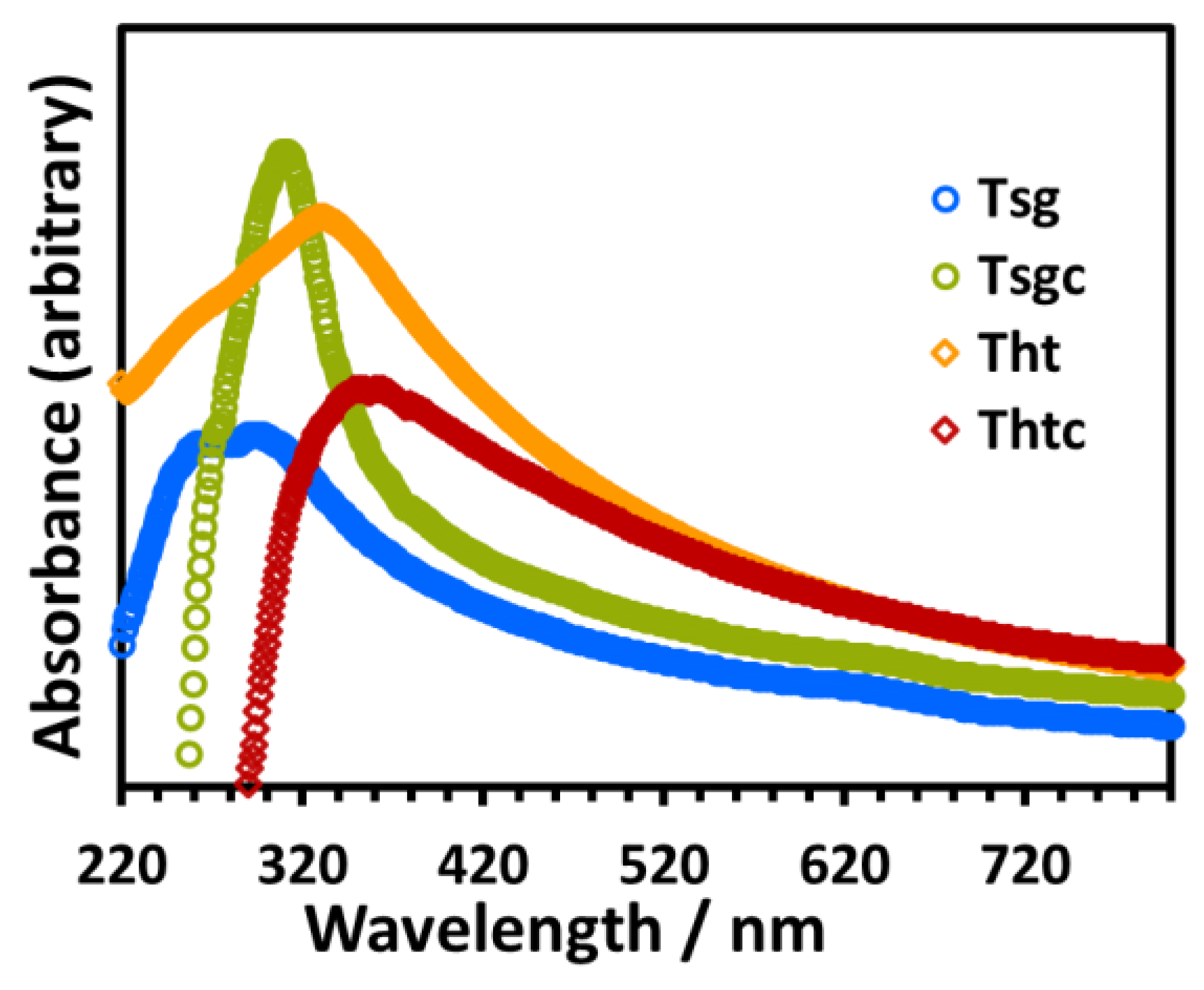
2.1. Morphological, Structural, Photophysical, and Thermal Analysis of TiO2 Nanoparticles
2.2. Photocatalytic Degradation Study
2.2.1. Control Experiment
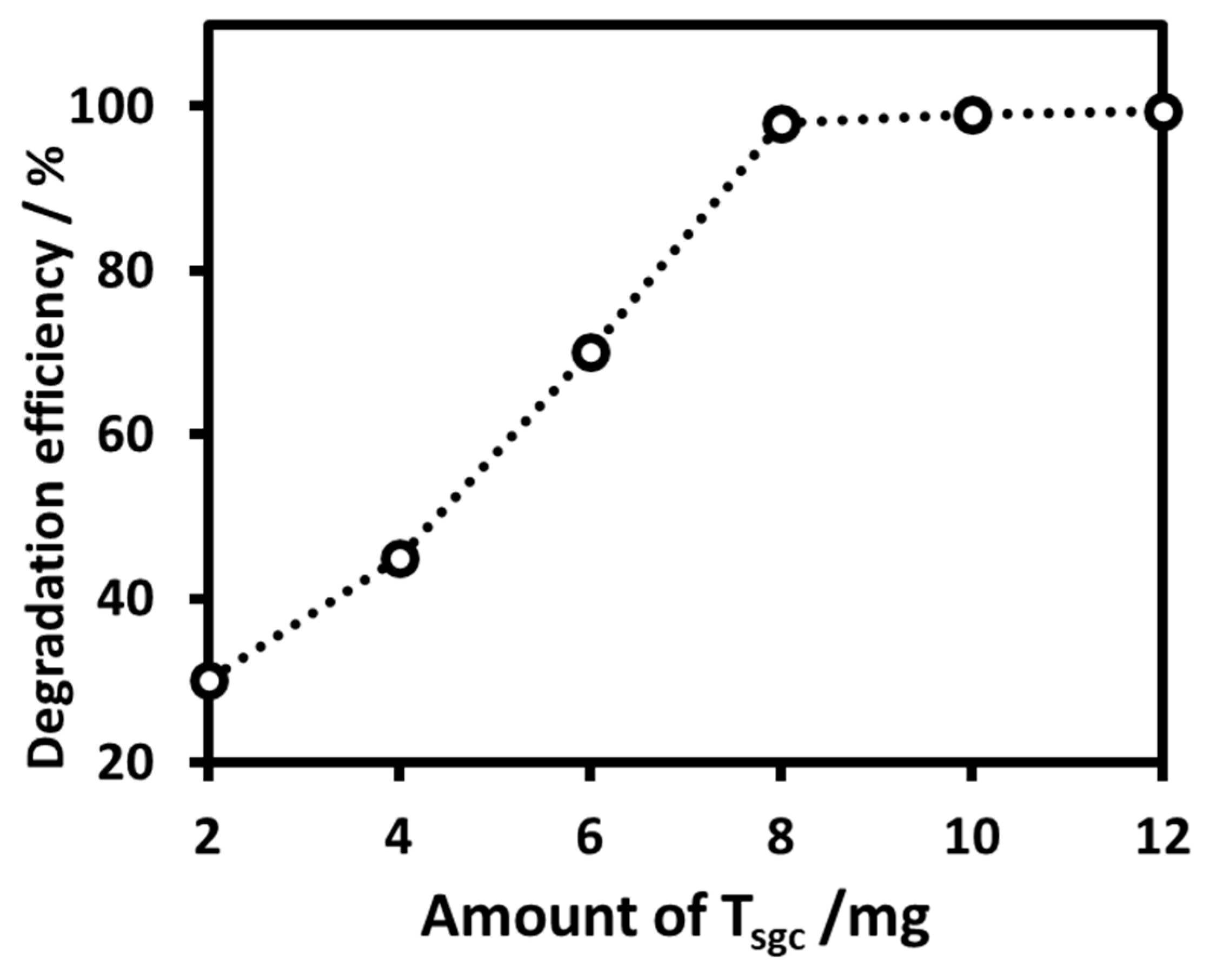
2.2.2. Determination of the Optimum Dose of the Nanoparticles
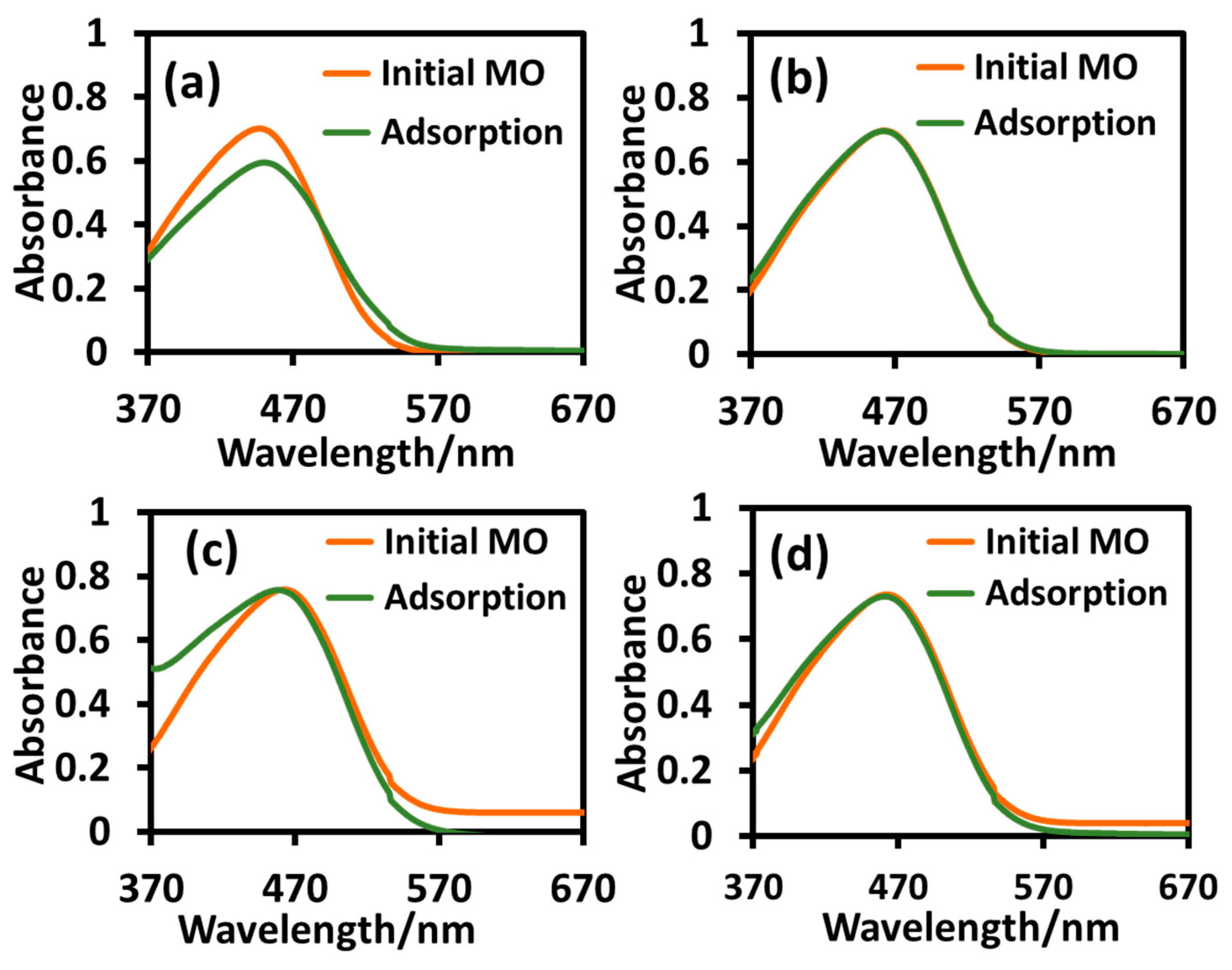
2.2.3. Adsorption Study
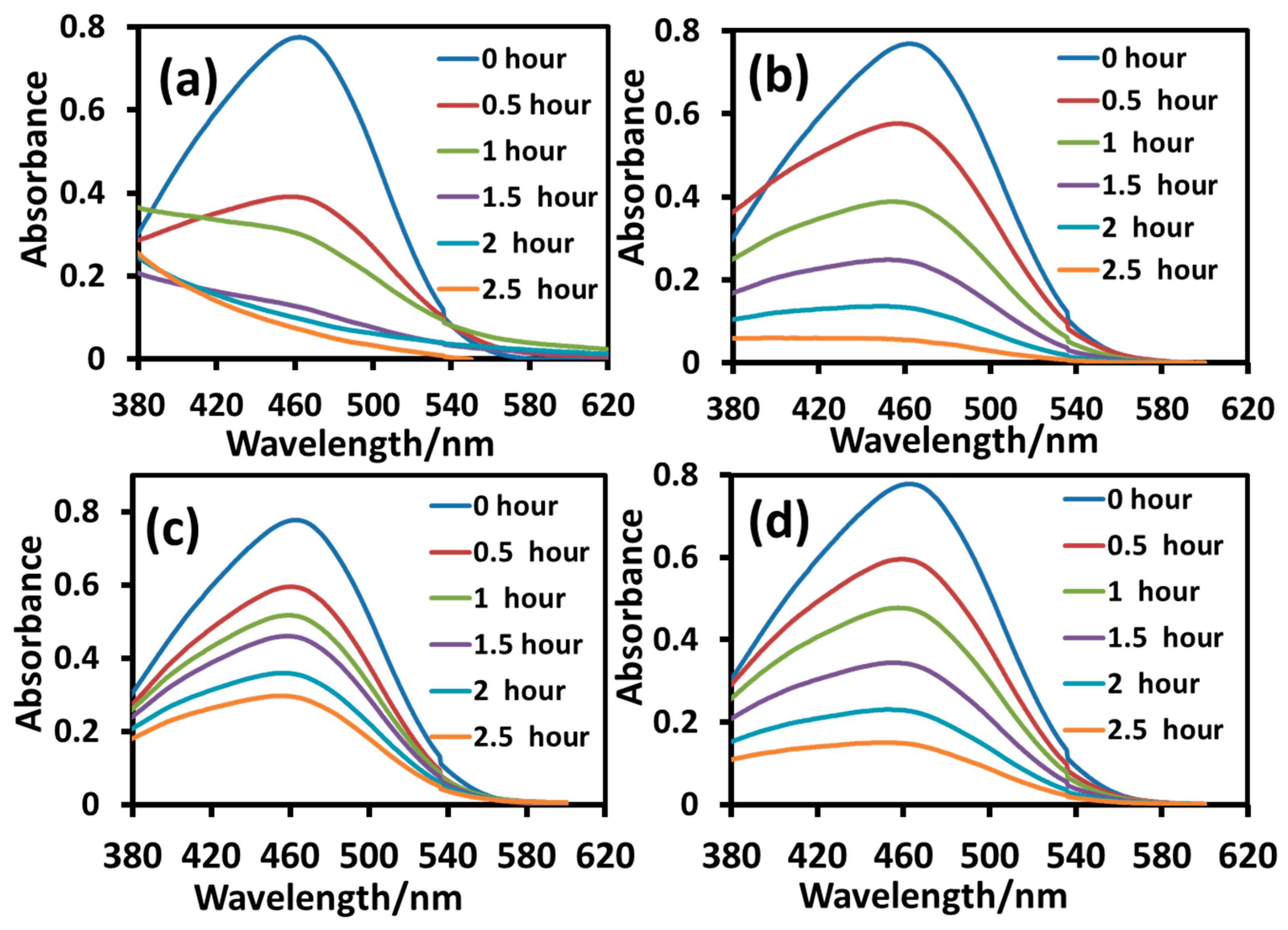
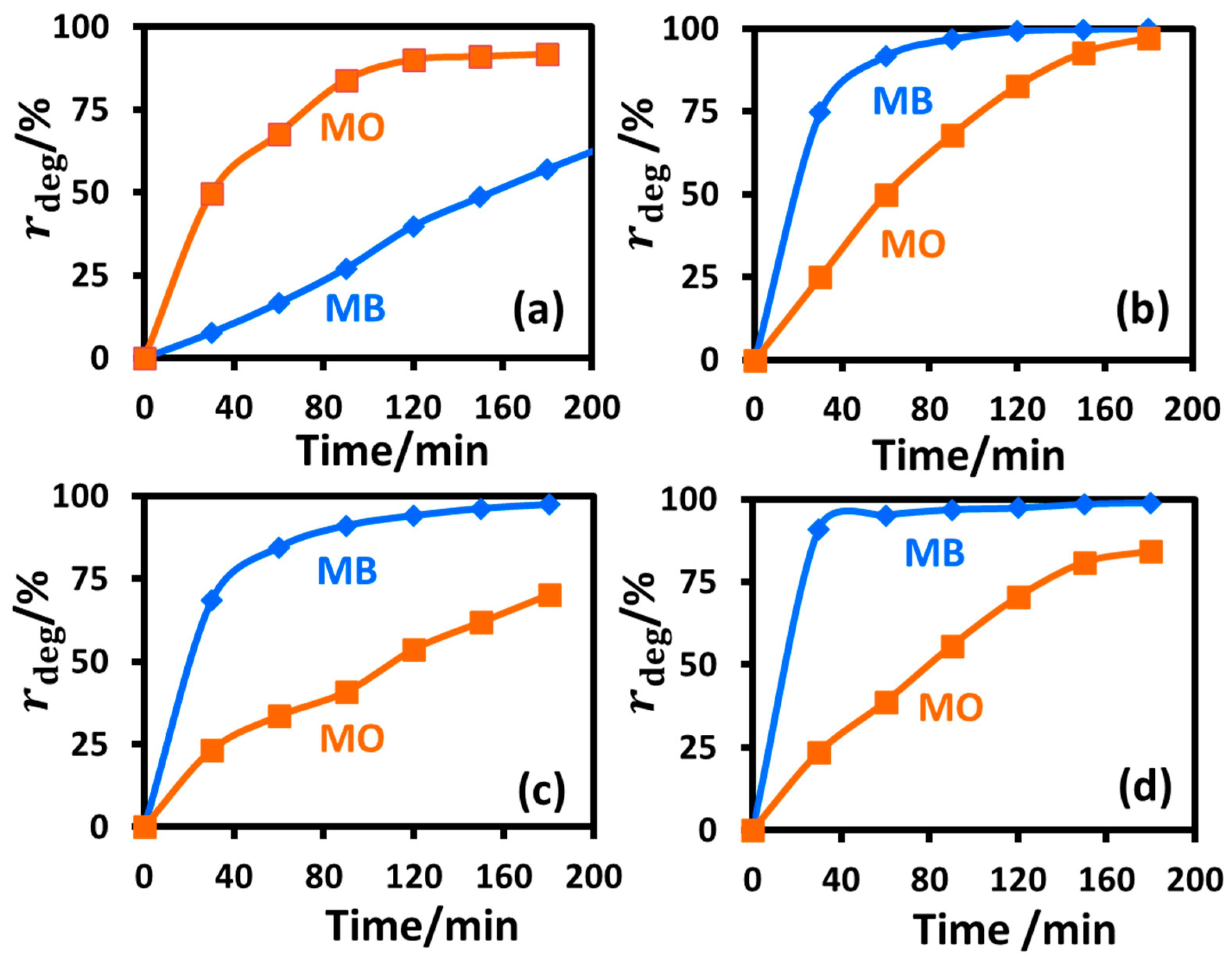
2.2.4. Photocatalytic Degradation of Cationic MB Dye under UV-B Irradiation
2.2.5. Photocatalytic Degradation of Anionic MO Dye under UV-B Irradiation
2.2.6. Degradation Study in the Absence of Light
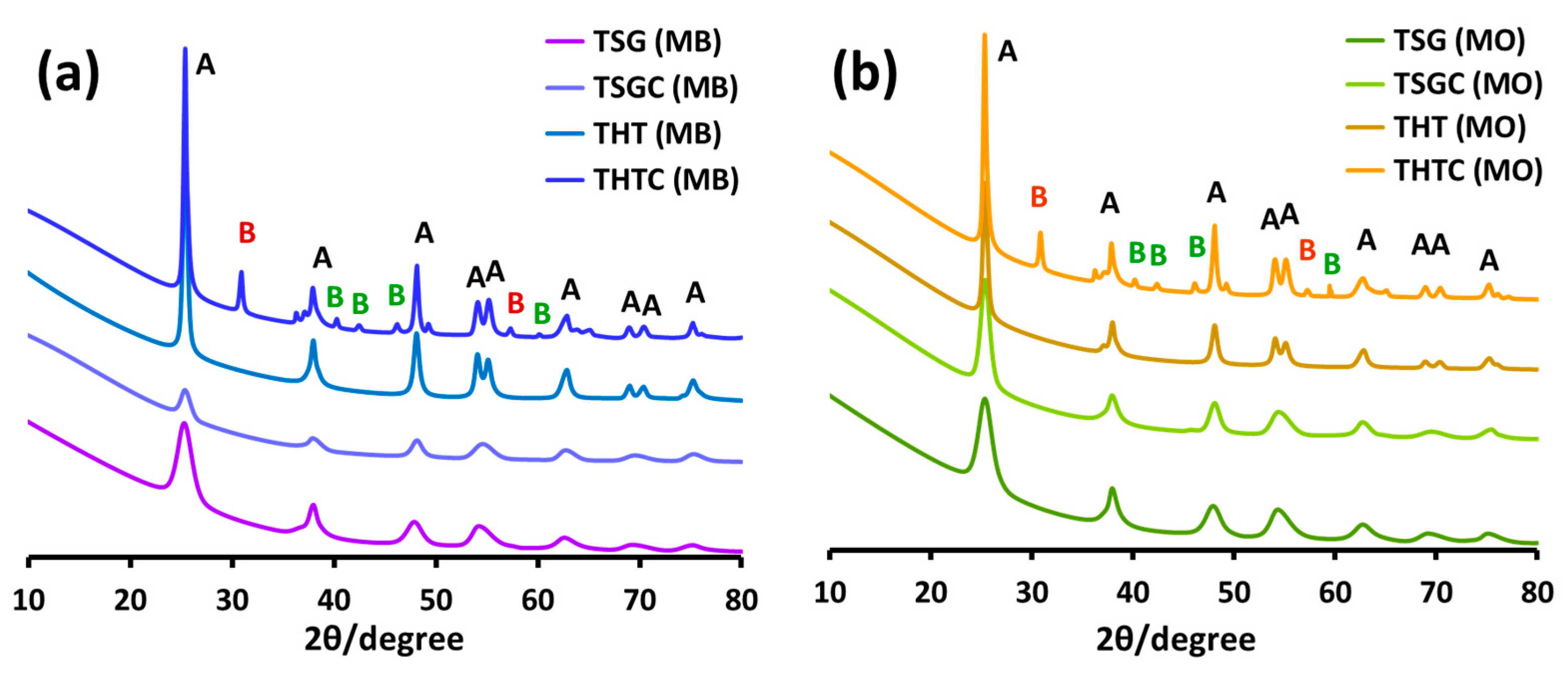
2.2.7. XRD Analysis of the Recovered Nanoparticles
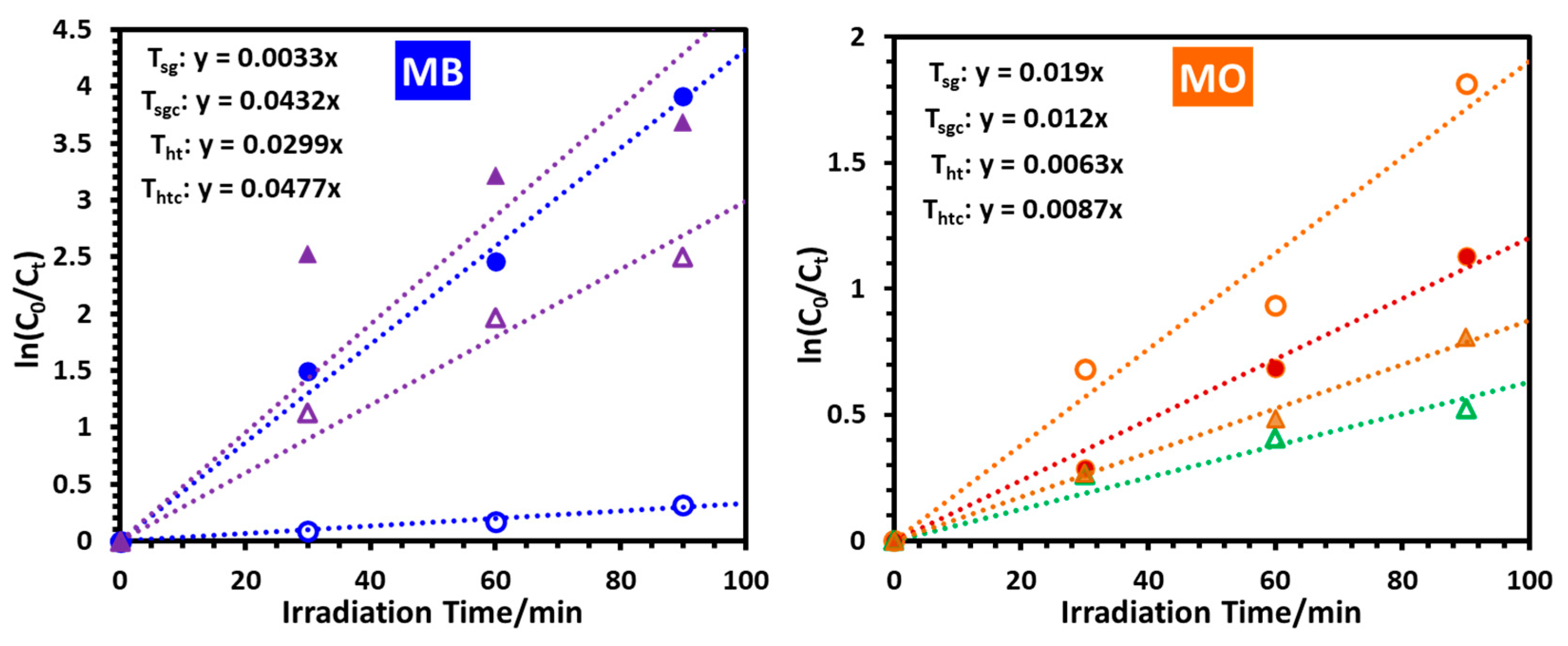
2.2.8. Selectivity of the Prepared Nanoparticles towards Catalyzing the Photodegradation of MB and MO under UV-B Irradiation
3. Materials and Methods
3.1. Materials
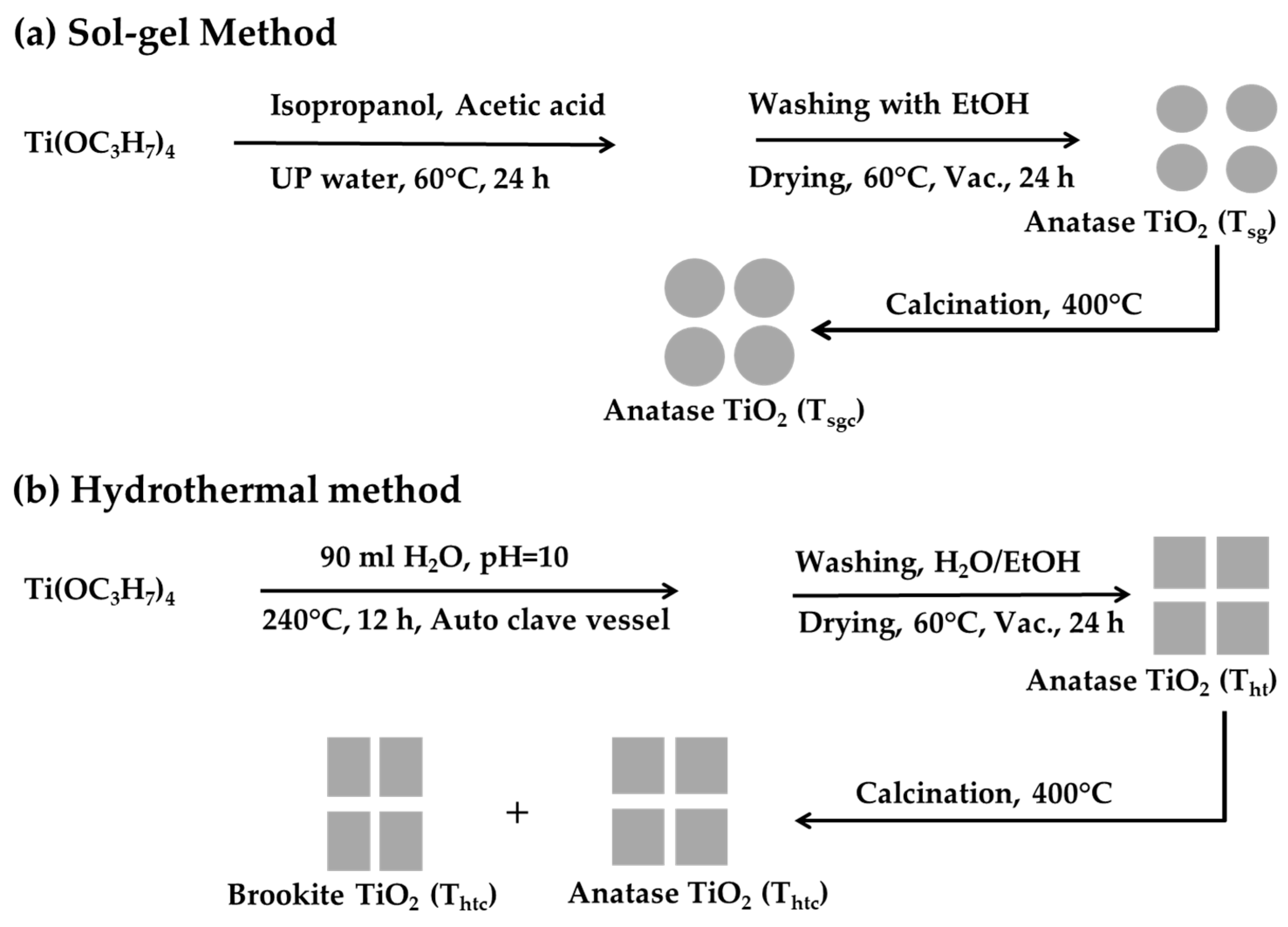
3.2. Preparation of the TiO2 Nanoparticles
3.2.1. Preparation of TiO2 Nanoparticles by Sol-Gel Technique
3.2.2. Preparation of TiO2 Nanoparticles by Hydrothermal Technique
3.3. Instruments
3.4. Adsorption and Degradation Experiments
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Zümriye, A. Application of biosorption for the removal of organic pollutants: A review. Process Biochem. 2005, 40, 997–1026. [Google Scholar]
- Tim, R.; Geoff, M.M.; Roger, M.; Poonam, N. Remediation of dyes in textile effluent: A critical review on current treatment technologies with a proposed alternative. Bioresour. Technol. 2001, 77, 247–255. [Google Scholar]
- Pearce, C.I.; Lioyd, J.R.; Guitrie, J.T. The removal of color from textile wastewater using whole bacterial cells: A review. Dyes Pigm. 2003, 58, 179. [Google Scholar] [CrossRef]
- Hossan, M.S.; Ochiai, B. Preparation of TiO2-Poly(3-Chloro-2-Hydroxypropyl Methacrylate) Nanocomposite for Selective Adsorption and Degradation of Dyes. Technologies 2018, 6, 92. [Google Scholar] [CrossRef]
- Hossan, M.S.; Ochiai, B. Detailed Study on Rapid Removal of Cationic Dyes Using TiO2-poly(3-chloro-2-hydroxypropyl methacrylate) Nanocomposite. J. Electrochem. Soc. 2019, 166, B3240–B3245. [Google Scholar] [CrossRef]
- Ali, I.; Suhali, M.; Alothman, Z.A.; Alwarthan, A. Recent advances in syntheses, properties and applications of TiO2 nanostructures. RSC Adv. 2018, 8, 30125–30147. [Google Scholar] [CrossRef]
- Noman, M.T.; Ashraf, M.A.; Ali, A. Synthesis and applications of nano-TiO2: A review. Environ. Sci. Pollut. Res. 2019, 26, 3262–3291. [Google Scholar] [CrossRef]
- O’Regan, B.; Grätzel, M. Low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films. Nature 1991, 353, 737–740. [Google Scholar] [CrossRef]
- Akter, J.; Hanif, M.A.; Islam, M.A.; Sapkota, K.P.; Hahn, J.R. Selective growth of Ti3+/TiO2/CNT and Ti3+/TiO2/C nanocomposite for enhanced visible-light utilization to degrade organic pollutants by lowering TiO2-bandgap. Sci. Rep. 2021, 11, 9490. [Google Scholar] [CrossRef]
- Kominami, H.; Murakami, S.; Kato, J.; Kera, Y.; Ohtani, B. Correlation Between Some Physical Properties of Titanium Dioxide Particles and Their Photocatalytic Activity for Some Probe Reactions in Aqueous Systems. J. Phys. Chem. B 2002, 106, 10501–10507. [Google Scholar] [CrossRef]
- Abazari, R.; Mahjoub, A.R.; Sanati, S. A facile and efficient preparation of anatase titania nanoparticles in micelle nanoreactors: Morphology, structure, and their high photocatalytic activity under UV light illumination. RSC Adv. 2014, 4, 56406–56414. [Google Scholar] [CrossRef]
- Wu, N.-L.; Wang, S.-Y.; Rusakova, I.A. Inhibition of Crystallite Growth in the Sol-Gel Synthesis of Nanocrystalline Metal Oxides. Science 1999, 285, 1375–1377. [Google Scholar] [CrossRef]
- Takasu, Y.; Mizutani, S.; Kumagai, M.; Sawaguchi, S.; Murakami, Y. Ti-V-W-O/Ti Oxide Electrodes as Candidates for Electrochemical Capacitors. Electrochem. Solid-State Lett. 1999, 2, 1–2. [Google Scholar] [CrossRef]
- Cheng, Y.; Zhang, M.; Yao, G.; Yang, L.; Tao, J.; Gong, Z.; He, G.; Sun, Z. Band gap manipulation of cerium doping TiO2 nanopowders by hydrothermal method. J. Alloys Compd. 2016, 662, 179–184. [Google Scholar] [CrossRef]
- Cheng, H.H.; Chen, S.S.; Yang, S.Y.; Liu, H.M.; Lin, K.S. Sol-Gel Hydrothermal Synthesis and Visible Light Photocatalytic Degradation Performance of Fe/N Codoped TiO2 Catalysts. Materials 2018, 11, 939–948. [Google Scholar] [CrossRef] [PubMed]
- Ángel-Sanchez, K.D.; Vázquez-Cuchillo, O.; Aguilar-Elguezabal, A.; Cruz-López, A.; Gómez, A.H. Photocatalytic degradation of 2,4-dichlorophenoxyacetic acid under visible light: Effect of synthesis route. Mater. Chem. Phys. 2013, 13, 423–430. [Google Scholar] [CrossRef]
- Albano, C.; Sarmiento, Y.; González, G. Synthesis and Characterization of Nanostructures: MWCNTf/TiO2 and MWCNTf/TiO2/Hap. Macromol. Symp. 2012, 321, 76–79. [Google Scholar] [CrossRef]
- Roman, A.G.; Zuñiga-Islas, C.; Quiroga-González, E. Immobilization of doped TiO2 nanostructures with Cu or In inside of macroporous silicon using the solvothermal method: Morphological, structural, optical and functional properties. Ceram. Int. 2020, 46, 1137–1147. [Google Scholar] [CrossRef]
- Nam, C.T.; Yang, W.-D.; Duc, L.M. Solvothermal Synthesis of TiO2 Photocatalysts in Ketone Solvents with Low Boiling Points. J. Nanomat. 2013, 2013, 627385. [Google Scholar] [CrossRef]
- Wu, J.-M. Low-temperature preparation of titania nanorods through direct oxidation of titanium with hydrogen peroxide. Cryst. Growth 2004, 269, 347–355. [Google Scholar] [CrossRef]
- Wu, J.-M.; Hayakawa, S.; Tsuru, K.; Osaka, A. Nanocrystalline Titania Made from Interactions of Ti with Hydrogen Peroxide Solutions Containing Tantalum Chloride. Cryst. Growth Des. 2002, 2, 147–149. [Google Scholar] [CrossRef]
- Djerdj, I.; Tonej, A.M.; Bijelic, M.; Vranesa, V.; Turkovic, A. Transmission electron microscopy studies of nanostructured TiO2 films on various substrates. Vacuum 2005, 80, 371–378. [Google Scholar] [CrossRef]
- Corradi, A.B.; Bondioli, F.; Focher, B. Conventional and Microwave-Hydrothermal Synthesis of TiO2 Nanopowders. J. Am. Ceram. Soc. 2005, 88, 2639–2641. [Google Scholar] [CrossRef]
- Russo, P.A.; Lima, S.; Rebuttini, V.; Pillinger, M.; Willinger, M.G.; Pinna, N.; Valente, A.A. Microwave-assisted coating of carbon nanostructures with titanium dioxide for the catalytic dehydration of D-xylose into furfural. RSC Adv. 2013, 3, 2595–2603. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, C.-C.; Zakaria, R.; Ying, J.Y. Role of Particle Size in Nanocrystalline TiO2–Based Photocatalysts. J. Phys. Chem. B 1998, 102, 10871–10878. [Google Scholar] [CrossRef]
- Zhang, H.; Finnegan, M.; Banfield, J.F. Preparing Single-Phase Nanocrystalline Anatase from Amorphous Titania with Particle Sizes Tailored by Temperature. Nano Lett. 2001, 1, 81–85. [Google Scholar] [CrossRef]
- Kavan, L.; Grätzel, M.; Gilbert, S.E.; Klemenz, C.; Scheel, H.J. Electrochemical and Photoelectrochemical Investigation of Single-Crystal Anatase. J. Am. Chem. Soc. 1996, 118, 6716–6723. [Google Scholar] [CrossRef]
- Moritz, T.; Reiss, J.; Diesner, K.; Su, D.; Chemseddine, A. Nanostructured Crystalline TiO2 through Growth Control and Stabilization of Intermediate Structural Building Units. J. Phys. Chem. B 1997, 101, 8052–8053. [Google Scholar] [CrossRef]
- Zhang, Z.H.; Zhong, X.H.; Liu, S.H. Aminolysis Route to Monodisperse Titania Nanorods with Tunable Aspect Ratio. Angew. Chem. Int. Ed. 2005, 44, 3466–3470. [Google Scholar] [CrossRef]
- Liu, S.; Wang, W.; Chen, J.; Li, J.G.; Li, X.; Sun, X.; Dong, Y. Foamed single-crystalline anatase nanocrystals exhibiting enhanced photocatalytic activity. J. Mater. Chem. A 2015, 3, 17837–17848. [Google Scholar] [CrossRef]
- Wu, J.-J.; Yu, C.-C. Aligned TiO2 Nanorods and Nanowalls. J. Phys. Chem. B 2004, 108, 3377–3379. [Google Scholar] [CrossRef]
- Zulmajdi, S.L.N.; Zamri, N.I.I.; Mahadi, A.H.; Rosli, M.Y.H.; Ja’afar, F.; Yasin, H.M.; Kusrini, E.; Hobley, J.; Usman, A. Sol-gel Preparation of Different Crystalline Phases of TiO2 Nanoparticles for Photocatalytic Degradation of Methylene Blue in Aqueous Solution. Am. J. Nanomater 2019, 7, 39–45. [Google Scholar] [CrossRef]
- Srinivasu, P.; Singh, S.P.; Islam, A.; Han, L. Novel Approach for the Synthesis of Nanocrystalline Anatase Titania and Their Photovoltaic Application. Adv. OptoElectron. 2011, 2011, 539382. [Google Scholar] [CrossRef]
- Scarpelli, F.; Mastropietro, T.F.; Poerio, T.; Godbert, N. Mesoporous TiO2 Thin Films: State of the Art. In Titanium Dioxide-Material for a Sustainable Environment; IntechOpen: London, UK, 2018; Chapter 3; pp. 57–80. [Google Scholar]
- Koelsch, M.; Cassaignon, S.; Guillemoles, J.F.; Jolivet, J.P. Comparison of optical and electrochemical properties of anatase and brookite TiO2 synthesized by the sol-gel method. Thin Solid Film. 2002, 403–404, 312–319. [Google Scholar] [CrossRef]
- Patterson, A.L. The Scherrer Formula for X-Ray Particle Size Determination. Phys. Rev. 1939, 56, 978–982. [Google Scholar] [CrossRef]
- Ohtani, B.; Prieto-Mahaney, O.O.; Li, D.; Abe, R. What is Degussa (Evonik) P25? Crystalline composition analysis, reconstruction from isolated pure particles and photocatalytic activity test. J. Photochem. Photobiol. A Chem. 2010, 216, 179–182. [Google Scholar] [CrossRef]
- Manzoli, M.; Freyria, F.; Blangetti, N.; Bonelli, B. Brookite, a sometimes under evaluated TiO2 polymorph. RSC Adv. 2022, 12, 3322–3334. [Google Scholar] [CrossRef]
- Hanif, M.A.; Lee, I.; Akter, J.; Islam, M.A.; Zahid, A.A.S.M.; Sapkota, K.P.; Hahn, J.R. Enhanced photocatalytic and antibacterial performance of ZnO nanoparticles prepared by an efficient thermolysis method. Catalysts 2019, 9, 608. [Google Scholar] [CrossRef]
- Kandiel, T.A.; Robben, L.; Alkaim, A.; Bahnemann, D. Brookite versus anatase TiO2 photocatalysts: Phase transformations and photocatalytic activities. Photochem. Photobiol. Sci. 2013, 12, 602–609. [Google Scholar] [CrossRef]
- Buckeridge, J.; Butler, K.; Catlow, C.; Logsdail, A.; Scanlon, D.; Shevlin, S.; Woodley, S.; Sokol, A.; Walsh, A. Polymorph Engineering of TiO2: Demonstrating How Absolute Reference Potentials are Determined by Local Coordination. Chem. Mater. 2015, 27, 3844–3851. [Google Scholar] [CrossRef]
- Jawale, N.S.; Arbuj, S.S.; Umarji, G.G.; Rane, S.B. Synthesis of Anatase/Brookite Mixed Phase TiO2 Nanostructures and its Photocatalytic Performance Study. ChemistrySelect 2021, 6, 8861–8867. [Google Scholar] [CrossRef]
- Hanif, M.A.; Akter, J.; Kim, Y.S.; Kim, H.G.; Hahn, J.R.; Kwac, L.K. Highly Efficient and Sustainable ZnO/CuO/g-C3N4 Photocatalyst for Wastewater Treatment under Visible Light through Heterojunction Development. Catalysts 2022, 12, 151. [Google Scholar] [CrossRef]
- Hanif, M.A.; Akter, J.; Lee, I.; Islam, M.A.; Sapkota, K.P.; Abbas, H.G.; Hahn, J.R. Formation of chemical heterojunctions between ZnO nanoparticles and single walled carbon nanotubes for synergistic enhancement of photocatalytic activity. J. Photochem. Photobiol. A Chem. 2021, 413, 113260. [Google Scholar] [CrossRef]






| Name of Samples | λ/% | l (± Error)/nm |
|---|---|---|
| Tsg | 17.8 | 4.39 ± 0.19 |
| Tsgc | 37.1 | 6.19 ± 0.56 |
| Tht | 31.2 | 15.99 ± 0.91 |
| Thtc | 17.0 | 19.88 ± 1.87 |
| Name of Sample | λ/% | % Change in λ | l (±Error)/nm |
|---|---|---|---|
| Tsg (MB) | 34.2 | +92 | 4.97 ± 0.65 |
| Tsgc (MB) | 22.1 | −40 | 5.98 ± 0.51 |
| Tht (MB) | 40.9 | +31 | 16.00 ± 0.91 |
| Thtc (MB) | 44.0 | +159 | 23.48 ± 1.64 |
| Tsg (MO) | 34.2 | +92 | 5.21 ± 0.56 |
| Tsgc (MO) | 31.5 | −15 | 6.77 ± 0.52 |
| Tht (MO) | 29.8 | −4.5 | 15.83 ± 0.86 |
| Thtc (MO) | 37.4 | +120 | 22.08 ± 1.56 |
| Sample Name | kMB × 103/min−1 | kMO × 103/min−1 | t1/2(MB)/min | t1/2(MO)/min |
|---|---|---|---|---|
| Tsg | 3.3 | 19.0 | 210 | 36.5 |
| Tsgc | 43.2 | 12.0 | 16.0 | 57.8 |
| Tht | 29.9 | 6.3 | 23.2 | 110 |
| Thtc | 47.7 | 8.7 | 14.5 | 79.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Islam, M.T.; Roni, M.N.P.; Ali, M.Y.; Islam, M.R.; Hossan, M.S.; Rahman, M.H.; Zahid, A.A.S.M.; Alam, M.N.E.; Hanif, M.A.; Akhtar, M.S. Selectivity of Sol-Gel and Hydrothermal TiO2 Nanoparticles towards Photocatalytic Degradation of Cationic and Anionic Dyes. Molecules 2023, 28, 6834. https://doi.org/10.3390/molecules28196834
Islam MT, Roni MNP, Ali MY, Islam MR, Hossan MS, Rahman MH, Zahid AASM, Alam MNE, Hanif MA, Akhtar MS. Selectivity of Sol-Gel and Hydrothermal TiO2 Nanoparticles towards Photocatalytic Degradation of Cationic and Anionic Dyes. Molecules. 2023; 28(19):6834. https://doi.org/10.3390/molecules28196834
Chicago/Turabian StyleIslam, Md. Torikul, Md. Nahid Parvez Roni, Md. Yunus Ali, Md. Robiul Islam, Md. Shamim Hossan, M. Habibur Rahman, A. A. S. Mostofa Zahid, Md. Nur E Alam, Md. Abu Hanif, and M. Shaheer Akhtar. 2023. "Selectivity of Sol-Gel and Hydrothermal TiO2 Nanoparticles towards Photocatalytic Degradation of Cationic and Anionic Dyes" Molecules 28, no. 19: 6834. https://doi.org/10.3390/molecules28196834
APA StyleIslam, M. T., Roni, M. N. P., Ali, M. Y., Islam, M. R., Hossan, M. S., Rahman, M. H., Zahid, A. A. S. M., Alam, M. N. E., Hanif, M. A., & Akhtar, M. S. (2023). Selectivity of Sol-Gel and Hydrothermal TiO2 Nanoparticles towards Photocatalytic Degradation of Cationic and Anionic Dyes. Molecules, 28(19), 6834. https://doi.org/10.3390/molecules28196834









