Chemical Composition of Litsea pungens Essential Oil and Its Potential Antioxidant and Antimicrobial Activities
Abstract
:1. Introduction
2. Results and Discussions
2.1. The Composition of Essential Oils of L. pungens
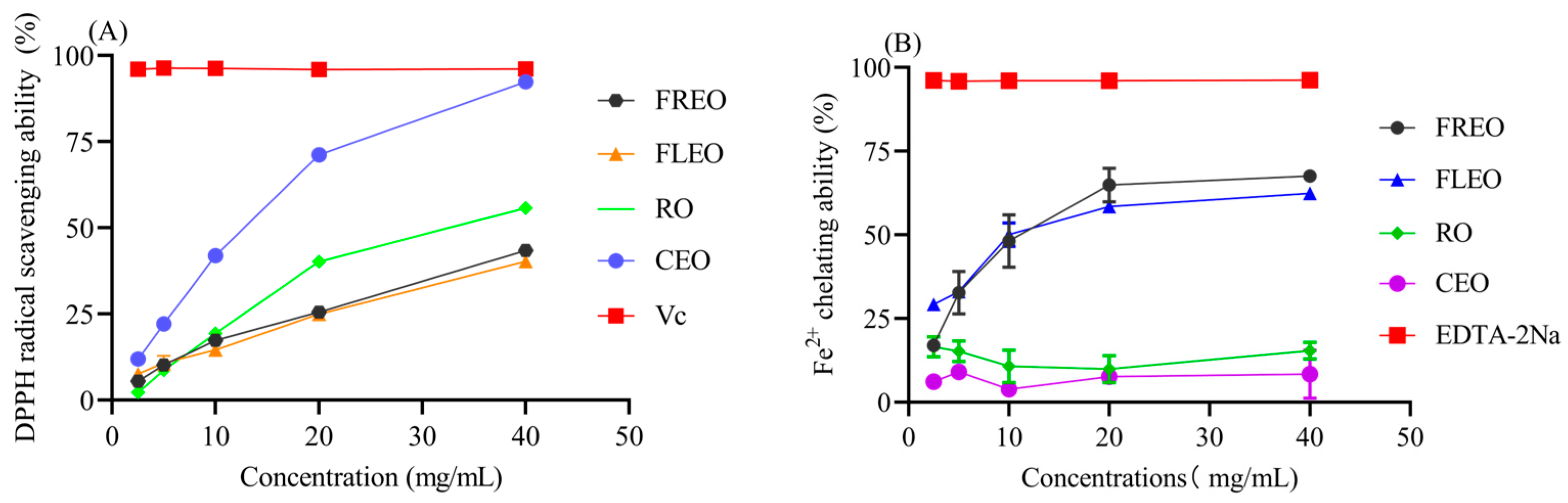
2.2. Antioxidant Ability
2.3. The CEO Protected CHO Cells against H2O2
2.4. Antimicrobial Activity
3. Materials and Methods
3.1. Plant Materials and Reagents
3.2. Extraction of L. pungens Essential Oil
3.3. Gas Chromatograph/Mass Spectrum (GC–MS) Analysis
3.4. Identification of Essential Oil Chemical Conponents
3.5. Antioxidant Assays
3.5.1. DPPH Clearance Ability
3.5.2. Fe2+ Chelating Ability
3.5.3. Cell Culture
3.5.4. Establishment of H2O2-Induced Model
3.5.5. Toxicity of Essential Oil on CHO Cells
3.5.6. Protection of Essential Oil on CHO Cells under H2O2 Stress
3.6. Antifungal Assay
3.7. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Che, C.T.; Zhang, H.J. Plant Natural Products for Human Health. Int. J. Mol. Sci. 2019, 20, 830. [Google Scholar] [CrossRef] [PubMed]
- Tuttolomondo, T.; La, B.S.; Licata, M.; Virga, G.; Leto, C.; Saija, A.; Trombetta, D.; Tomaino, A.; Speciale, A.; Napoli, E.M.; et al. Biomolecular Characterization of Wild Sicilian Oregano: Phytochemical Screening of Essential Oils and Extracts, and Evaluation of Their Antioxidant Activities. Chem. Biodivers. 2013, 10, 411–433. [Google Scholar] [CrossRef]
- Kaur, K.; Kaushal, S. Phytochemistry and Pharmacological Aspects of Syzygium aromaticum: A Review. J. Pharmacogn. Phytochem. 2019, 8, 398–406. [Google Scholar]
- Stringaro, A.; Colone, M.; Angiolella, L. Antioxidant, Antifungal, Antibiofifilm, and Cytotoxic Activities of Mentha spp. Essential Oils. Medicines 2018, 5, 112. [Google Scholar] [CrossRef] [PubMed]
- El Kharraf, S.; El-Guendouz, S.; Farah, A.; Bennani, B.C.; Mateus, M.C.; Miguel, M.G. Hydrodistillation and Simultaneous Hydrodistillation-steam Distillation of Rosmarinus Officinalis and Origanum compactum: Antioxidant, Anti-inflammatory, and Antibacterial Effect of the Essential Oils. Ind. Crops Prod. 2021, 168, 113591. [Google Scholar] [CrossRef]
- Wani, A.R.; Yadav, K.; Khursheed, A.; Rather, M. An Updated and Comprehensive Review of the Antiviral Potential of Essential Oils and Their Chemical Constituents with Special Focus on Their Mechanism of Action Against Various Influenza and Coronaviruses. Microb. Pathog. 2021, 152, 104620. [Google Scholar] [CrossRef]
- Zuo, X.; Gu, Y.U.; Wang, C.; Zhang, J.R.; Zhang, J.; Wang, G.Q.; Wang, F. A Systematic Review of the Anti-inflammatory and Immunomodulatory Properties of 16 Essential Oils of Herbs. Evid. Based Complement. Altern. Med. 2020, 26, 3793. [Google Scholar] [CrossRef]
- Wang, Y.T.; Zhu, L.; Zeng, D.; Long, W.; Zhu, S.M. Chemical Composition and Anti-inflammatory Activities of Essential Oil from Trachydium roylei. J. Food Drug Anal. 2016, 24, 602–609. [Google Scholar] [CrossRef]
- Zhang, Y.D.; Xin, C.; Cheng, C.L.; Wang, Z.Y. Antitumor Activity of Nanoemulsion Based on Essential Oil of Pinus koraiensis pinecones in MGC-803 Tumor-bearing Nude Mice. Arab. J. Chem. 2020, 13, 8226–8238. [Google Scholar] [CrossRef]
- El Ayeb-Zakhama, A.; Sakka-Rouis, L.; Bergaoui, A.; Flamini, G.; Ben, J.H.; Harzallah-Skhiri, F. Chemical Composition and Allelopathic Potential of Essential Oils from Tipuana tipu(Benth.)Kuntze Cultivated in Tunisia. Chem. Biodivers. 2016, 13, 309–318. [Google Scholar] [CrossRef]
- Kong, Q.B.; Zhou, L.J.; Wang, X.J.; Luo, S.Y.; Li, J.J.; Xiao, H.Y.; Ding, C.B. Chemical Composition and Allelopathic Effect of Essential Oil of Litsea pungens. Agronomy 2021, 11, 1115. [Google Scholar] [CrossRef]
- Chaieb, K.; Zmantar, T.; Ksouri, R.; Hajlaoui, H.; Mahdouani, K.; Abdelly, C.; Bakhrouf, A. Antioxidant Properties of the Essential Oil of Eugenia caryophyllata and Its Antifungal Activity against a Large Number of Clinical Candida Species. Mycoses 2007, 50, 403–406. [Google Scholar] [CrossRef] [PubMed]
- Visioli, F.; Poli, A.; Gall, C. Antioxidant and Other Biological Activities of Phenols from Olives and Olive Oil. Med. Res. Rev. 2002, 22, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Pu, D.D.; Shan, Y.M.; Duan, W.; Huang, Y.; Liang, L.; Yan, Y.; Hu, G. Characterization of the Key Aroma Compounds in the Fruit of Litsea pungens Hemsl. (LPH) by GC-MS/O, OAV, and Sensory Techniques. J. Food Qual. 2021, 2021, 6668606. [Google Scholar] [CrossRef]
- Kon, K.; Rai, M. Antibacterial Activity of Thymus vulgaris Essential Oil Alone and in Combination with Other Essential Oils. Nusant Biosci. 2012, 4, 2. [Google Scholar] [CrossRef]
- Zheng, K.W.; Li, W.; Fu, B.Q.; Fu, M.F.; Ren, Q.L.; Yang, F.; Qin, C. Physical, Antibacterial and Antioxidant Properties of Chitosan Films Containing Hardleaf Oatchestnut Starch and Litsea cubeba Oil. Int. J. Biol. Macromol. 2018, 118, 707–715. [Google Scholar] [CrossRef]
- Saikiaa, A.K.; Chetiaa, D.; D’Arrigob, M.; Smerigliob, A.; Stranoc, T.; Ruberto, G. Screening of Fruit and Leaf Essential Oils of Litsea cubeba Pers. from North-east India–chemical Composition and Antimicrobial Activity. J. Essent. Oil Res. 2013, 25, 330–338. [Google Scholar] [CrossRef]
- Vieira, A.J.; Beserra, F.P.; Souza, M.C.; Totti, B.M.; Rozza, A.L. Limonene: Aroma of Innovation in Health and Disease. Chem. Biol. Interact. 2018, 283, 97–106. [Google Scholar] [CrossRef]
- Thielman, J.; Muranyi, P. Review on the Chemical Composition of Litsea cubeba Essential Oils and the Bioactivity of Its Major Constituents Citral and Limonene. J. Essent. Oil Res. 2019, 31, 361–378. [Google Scholar] [CrossRef]
- Si, L.; Chen, Y.; Han, X.; Zhan, Z.; Tian, S.; Cui, Q.; Wang, Y. Chemical Composition of Essential Oils of Litsea cubeba Harvested from Its Distribution Areas in China. Molecules 2012, 17, 7057–7066. [Google Scholar] [CrossRef]
- Yang, Y.; Jiang, J.Z.; Luobu, Q.M.; Yan, X.J.; Zhao, J.X.; Yuan, H.Z.; Qin, Z.; Wang, M. The Fungicidal Terpenoids and Essential Oil from Litsea cubeba in Tibet. Molecules 2010, 15, 7075–7082. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.W.; Liu, Y.Q. Chemical Composition and Antibacterial Activity of Essential Oils from Different Parts of Litsea cubeba. Chem. Biodivers. 2010, 7, 229–235. [Google Scholar] [CrossRef] [PubMed]
- She, Q.H.; Li, W.S.; Jiang, Y.Y.; Wu, Y.C.; Zhou, Y.H.; Zhang, L. Chemical Composition, Antimicrobial Activity and Antioxidant Activity of Litsea cubeba Essential Oils in Different Months. Nat. Prod. Res. 2020, 34, 3285–3288. [Google Scholar] [CrossRef]
- Amiri, H. Volatile Constituents and Antioxidant Activity of Flowers, Stems and Leaves of Nasturtium officinale R. Br. Nat. Prod. Res. 2012, 26, 109–115. [Google Scholar] [CrossRef]
- Singh, H.P.; Kaur, S.; Negi, K.; Kumari, S.; Saini, V.; Batish, D.R.; Kohli, R.K. Assessment of In Vitro Antioxidant Activity of Essential Oil of Eucalyptus citriodora (lemon-scented Eucalyptus; Myrtaceae) and Its Major Constituents. LWT Food Sci. Technol. 2012, 48, 237–241. [Google Scholar] [CrossRef]
- Zeng, P.; Li, J.; Chen, Y.; Zhang, L. The Structures and Biological Functions of Polysaccharides from Traditional Chinese Herbs. Prog. Mol. Biol. Transl. Sci. 2019, 163, 423–444. [Google Scholar] [CrossRef]
- Faleiro, D.; Immich, S.M.; Majolo, F.; Mayer, L.; Ethur, E.M.; Goettert, M.I. GC/MS Analysis and Potential Cytotoxic Activity of Calyptranthes grandifolia (O. Berg), Calyptranthes tricona (D. Legrand) and Myrciaria plinioides (D. Legrand) Essential Oil in RAW264.7 and CHO-K1 cells. Biomed. Pharmacother. 2017, 89, 1431–1441. [Google Scholar] [CrossRef]
- Oboh, G.; Ademosun, A.O.; Odubanjo, O.V.; Akinbola, I.A. Antioxidative Properties and Inhibition of Key Enzymes Relevant to Type-2 Diabetes and Hypertension by Essential Oils from Black Pepper. Adv. Pharmacol. Sci. 2013, 2013, 926047. [Google Scholar] [CrossRef]
- Sies, H. Hydrogen Peroxide as a Central Redox Signaling Molecule in Physiological Oxidative Stress: Oxidative Eustress. Redox. Biol. 2017, 11, 613–619. [Google Scholar] [CrossRef]
- Majeed, M.; Hussain, A.I.; Chatha, S.A.S.; Khosa, M.K.K.; Kamal, G.M.; Kamal, M.A.; Zhang, X.; Liu, M.O. Ptimization Protocol for the Extraction of Antioxidant Components from Origanum vulgare Leaves Using Response Surface Methodology. Saudi J. Biol. Sci. 2016, 23, 389–396. [Google Scholar] [CrossRef]
- Knežević-Vukčević, J. Modulation of Genotoxicity and DNA Repair by Plant Monoterpenes Camphor, Eucalyptol and Thujone in Escherichia Coli and Mammalian Cells. Food Chem. Toxicol. 2011, 49, 2035–2045. [Google Scholar] [CrossRef]
- Nikolić, B.; Mitić-Ćulafić, D.; Vuković-Gačić, B.; Knezevic-Vukcevic, J. The Antimutagenic Effect of Monoterpenes Against UV-irradiation-, 4NQO-and t-BOOH-induced Mutagenesis in Coli. Arch. Biol. Sci. 2011, 63, 117–128. [Google Scholar] [CrossRef]
- Xanthis, V.; Fitsiou, E.; Voulgaridou, G.P.; Bogadakis, A.; Chlichlia, K.; Galanis, A.; Pappa, A. Antioxidant and Cytoprotective Potential of the Essential Oil Pistacia lentiscus var. chia and Its Major Components Myrcene and α-Pinene. Antioxidants 2021, 10, 127. [Google Scholar] [CrossRef] [PubMed]
- Katooli, N.; Maghsodlo, R.; Razavi, S.E. Evaluation of Eucalyptus Essential Oil Against Some Plant Pathogenic Fungi. J. Plant Breed. Crop Sci. 2011, 3, 41–43. Available online: http://www.academicjournals.org/jpbcs (accessed on 1 July 2023).
- Zhou, L.J.; Li, J.J.; Kong, Q.B.; Luo, S.Y.; Wang, J.; Feng, S.L.; Yuan, M.; Chen, T.; Yuan, S.; Ding, C. Chemical Composition, Antioxidant, Antimicrobial, and Phytotoxic Potential of Eucalyptus grandis × E. urophylla Leaves Essential Oils. Molecules 2021, 26, 1450. [Google Scholar] [CrossRef]
- Liu, X.X.; Chen, Q.B.; Wang, Z.H.; Xie, L.; Zhi, X. Phytotoxic Effects of Essential Oil from Eucalyptus grandis × E. urophylla on pathogenic fungi and pest insects. Front. For. China. 2008, 3, 232–236. [Google Scholar] [CrossRef]
- Chang, H.T.; Cheng, Y.H.; Wu, C.L.; Chang, S.T.; Chang, T.T.; Su, Y.C. Antifungal Activity of Essential Oil and Its Constituents from Calocedrus macrolepis var. formosana Florin Leaf Against Plant Pathogenic Fungi. Bioresour. Technol. 2008, 99, 6266–6270. [Google Scholar] [CrossRef]
- An, P.P.; Yang, X.B.; Yu, J.; Qi, J.; Ren, X.; Kong, Q. α-terpineol and Terpene-4-ol, the Critical Components of Tea Tree Oil, Exert Antifungal Activities in Vitro and in Vivo Against Aspergillus Niger in Grapes by Inducing Morphous Damage and Metabolic Changes of Fungus. Food Control 2019, 98, 42–53. [Google Scholar] [CrossRef]
- Sparkman, O.D. Identification of Essential Oil Components by Gas Chromatography/quadrupole mass Spectroscopy Robert P. Adams. J. Am. Soc. Mass. Spectrom. 2005, 16, 1902–1903. [Google Scholar] [CrossRef]
- Lucia, A.; Gonzalez, A.P.; Seccacini, E.; Licastro, S.; Zerba, E.; Masuh, H. Larvicidal Effect of Eucalyptus grandis Essential Oil and Turpentine and Their Major Components on Aedes Aegypti Larvae. J. Am. Mosq. Control Assoc. 2007, 23, 299–303. [Google Scholar] [CrossRef]
- Boulanouar, B.; Abdelaziz, G.; Aazza, S.; Gago, C.; Miguel, M.G. Antioxidant Activities of Eight Algerian Plant Extracts and Two Essential Oils. Ind. Crops Prod. 2013, 46, 85–96. [Google Scholar] [CrossRef]
- Karamać, M.; Gai, F.; Longato, E.; Meineri, G.; Janiak, M.A.; Amarowicz, R.; Peiretti, P.G. Antioxidant Activity and Phenolic Composition of Amaranth (Amaranthus caudatus) during Plant Growth. Antioxidants 2019, 8, 173. [Google Scholar] [CrossRef] [PubMed]
- Shen, P.; Yue, Y.; Zheng, J.; Park, Y. Caenorhabditis Elegans: A Convenient In Vivo Model for Assessing the Impact of Food Bioactive Compounds on Obesity, Aging, and Alzheimer’s Disease. Annu. Rev. Food Sci. Technol. 2018, 9, 1–22. [Google Scholar] [CrossRef] [PubMed]

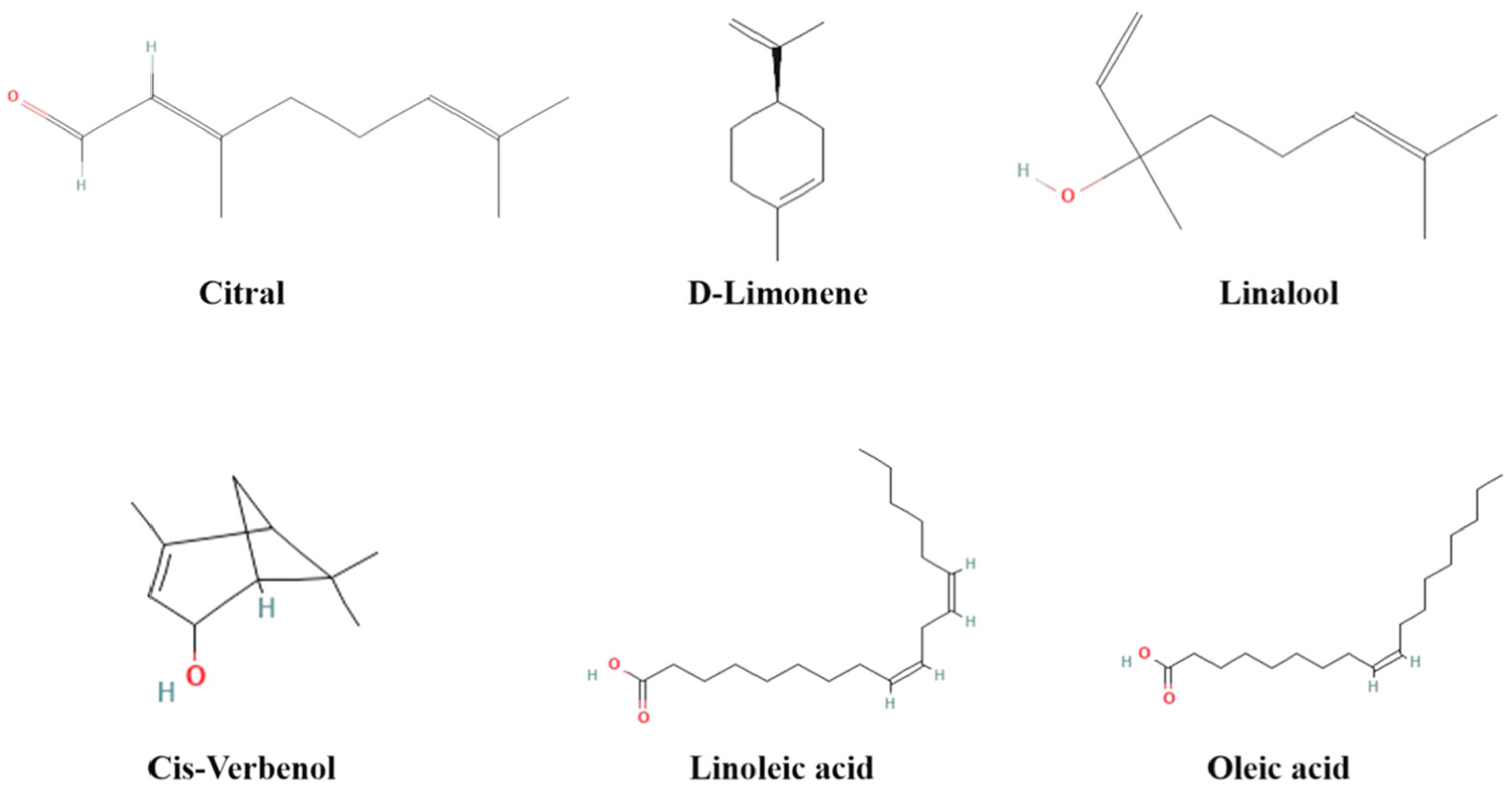
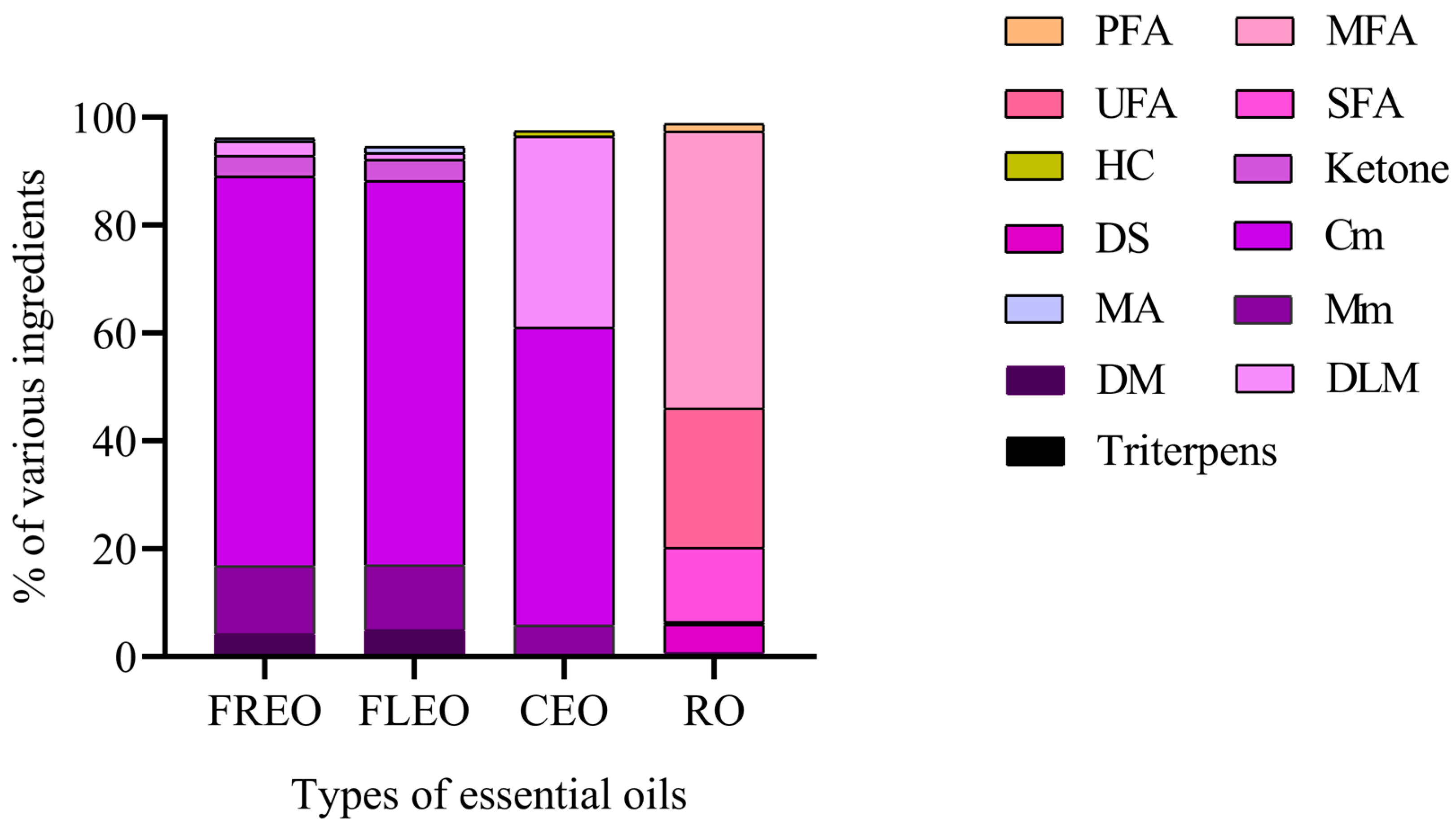

| Type of Essential Oil | No. | RT | RI | Relative Contents (%) | Identified Compounds | Type of Compounds |
|---|---|---|---|---|---|---|
| FREO | 1 | 6.399 | 922 | 2.00 | α-Pinene | DM |
| 2 | 6.749 | 943 | 0.90 | Camphene | DM | |
| 3 | 7.432 | 979 | 1.31 | β-pinene | DM | |
| 4 | 7.681 | 986 | 3.91 | 6-Methyl-5-hepten-2-one | Ketone | |
| 5 | 8.732 | 1019 | 12.54 | D-Limonene | Mm | |
| 6 | 8.791 | 1032 | 0.50 | Cineole | DLM | |
| 7 | 9.218 | 1037 | 0.90 | Ocimene | Cm | |
| 8 | 10.506 | 1099 | 2.30 | Linalool | Cm | |
| 9 | 11.824 | 1152 | 0.82 | Citronellal | Cm | |
| 10 | 12.542 | 1185 | 2.16 | Cis-Verbenone | DLM | |
| 11 | 12.756 | 1190 | 0.52 | α-Terpineol | MA | |
| 12 | 13.984 | 1210 | 68.23 | Citral | Cm | |
| Total contents percentage of identified compounds: 96.09% | ||||||
| Unidentified compounds: 3.91% | ||||||
| FLEO | 1 | 6.405 | 929 | 1.95 | α-Pinene | DM |
| 2 | 6.755 | 952 | 0.92 | Camphene | DM | |
| 3 | 7.444 | 979 | 2.11 | β-pinene | DM | |
| 4 | 7.693 | 986 | 3.87 | 6-Methyl-5-hepten-2-one | Ketone | |
| 5 | 8.744 | 1031 | 11.96 | D-Limonene | Mm | |
| 6 | 8.803 | 1032 | 0.70 | Cineole | DLM | |
| 7 | 10.518 | 1099 | 3.87 | Linalool | Cm | |
| 8 | 11.830 | 1153 | 0.73 | Citronellal | Cm | |
| 9 | 12.115 | 1168 | 0.61 | Cis-Verbennol | DLM | |
| 10 | 12.441 | 1177 | 0.43 | Terpinen-4-ol | MA | |
| 11 | 12.548 | 1190 | 0.68 | α-Terpineol | MA | |
| 12 | 14.774 | 1270 | 66.69 | Citral | Cm | |
| Total contents percentage of identified compounds: 94.52% | ||||||
| Unidentified compounds: 5.48% | ||||||
| CEO | 1 | 7.800 | 1013 | 1.05 | Hydrazinecarboxamide | HC |
| 2 | 8.738 | 1075 | 5.79 | D-Limonene | Mm | |
| 3 | 13.949 | 1142 | 35.36 | Cis-Verbenol | DLM | |
| 4 | 14.714 | 1276 | 55.29 | Citral | Cm | |
| Total contents percentage of identified compounds: 97.49% | ||||||
| Unidentified compounds: 2.51% | ||||||
| RO | 1 | 7.683 | 988 | 0.71 | α-Pinene | DM |
| 2 | 8.088 | 991 | 0.68 | Decanoic acid | SFA | |
| 3 | 9.086 | 1010 | 5.27 | β-Caryophyllene | DS | |
| 4 | 12.478 | 1189 | 0.51 | Epoxycaryophyllene | Triterpenes | |
| 5 | 16.688 | 1191 | 7.60 | Palmitic acid | SFA | |
| 6 | 18.355 | 1249 | 21.52 | Linoleic acid | UFA | |
| 7 | 18.432 | 1265 | 45.30 | Oleic acid | MFA | |
| 8 | 18.561 | 1279 | 1.39 | Linolenic acid | PFA | |
| 9 | 18.638 | 1286 | 3.80 | Stearic acid | SFA | |
| 10 | 20.846 | 1398 | 4.25 | Cis-11-Eicosenoic acid | UFA | |
| 11 | 21.219 | 1424 | 1.15 | Arachidic acid | SFA | |
| 12 | 24.862 | 1530 | 6.06 | Erucic acid | MFA | |
| 13 | 25.506 | 1597 | 0.51 | Docosanoic acid | SFA | |
| Total contents percentage of identified compounds: 98.75% | ||||||
| Unidentified compounds: 1.25% | ||||||
| Essential Oil Type | IR (%) | The Concentration of Essential Oil (mg/mL) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Strains | CK | CK1 | CK2 | 2.5 | 5 | 10 | 20 | ||
| FREO | Bacillus anthracis | 0 ± 0.00 f | 0 ± 0.00 f | 85.77 ± 2.85 b | 12.17 ± 1.95 e | 23.10 ± 2.42 d | 38.38 ± 2.00 c | 100 ± 0.00 a | |
| Fusarium oxysporium | 0 ± 0.00 f | 0 ± 0.00 f | 90.12 ± 2.45 b | 5.40 ± 1.72 e | 26.23 ± 2.97 d | 74.74 ± 1.25 c | 100 ± 0.00 a | ||
| FLEO | Bacillus anthracis | 0 ± 0.00 e | 0 ± 0.00 e | 85.65 ± 4.72 a | 5.62 ± 1.49 d | 7.41 ± 1.18 c | 13.03 ± 0.80 b | 86.05 ± 2.29 a | |
| Fusarium oxysporium | 0 ± 0.00 f | 0 ± 0.00 f | 90.12 ± 3.45 b | 6.63 ± 2.01 e | 19.96 ± 3.26 d | 42.90 ± 1.34 c | 92.45 ± 3.69 a | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, T.; Kong, Q.; Kuang, X.; Zhou, J.; Wang, H.; Zhou, L.; Yang, H.; Feng, S.; Ding, C. Chemical Composition of Litsea pungens Essential Oil and Its Potential Antioxidant and Antimicrobial Activities. Molecules 2023, 28, 6835. https://doi.org/10.3390/molecules28196835
Chen T, Kong Q, Kuang X, Zhou J, Wang H, Zhou L, Yang H, Feng S, Ding C. Chemical Composition of Litsea pungens Essential Oil and Its Potential Antioxidant and Antimicrobial Activities. Molecules. 2023; 28(19):6835. https://doi.org/10.3390/molecules28196835
Chicago/Turabian StyleChen, Tao, Qingbo Kong, Xuekun Kuang, Jiasi Zhou, Haizhou Wang, Lijun Zhou, Hongyu Yang, Shiling Feng, and Chunbang Ding. 2023. "Chemical Composition of Litsea pungens Essential Oil and Its Potential Antioxidant and Antimicrobial Activities" Molecules 28, no. 19: 6835. https://doi.org/10.3390/molecules28196835
APA StyleChen, T., Kong, Q., Kuang, X., Zhou, J., Wang, H., Zhou, L., Yang, H., Feng, S., & Ding, C. (2023). Chemical Composition of Litsea pungens Essential Oil and Its Potential Antioxidant and Antimicrobial Activities. Molecules, 28(19), 6835. https://doi.org/10.3390/molecules28196835








