Aroma Difference Analysis of Partridge Tea (Mallotus oblongifolius) with Different Drying Treatments Based on HS-SPME-GC-MS Technique
Abstract
:1. Introduction
2. Results and Discussion
2.1. Appearance of Partridge Tea under Different Drying Methods
| Parameters | OD | ID | HAD | LTD |
|---|---|---|---|---|
| L* | 38.95 ± 3.33 b | 40.56 ± 3.83 b | 47.28 ± 1.90 a | 48.14 ± 3.07 a |
| a* | 11.69 ± 2.09 b | 11.91 ± 1.44 b | 5.55 ± 2.25 c | 16.95 ± 2.00 a |
| b* | 5.72 ± 3.46 b | 5.52 ± 2.41 b | 13.86 ± 4.29 a | 1.11 ± 0.64 c |
2.2. Classification of Volatile Compounds in Partridge Tea
2.3. Identification of Volatile Compounds in Partridge Tea
2.4. Orthogonal Partial Least Squares Discriminant Analysis
3. Materials and Methods
3.1. Sample Preparation
3.2. Volatile Extraction via HS-SPME Procedure
3.3. Separation of Volatile Organic Compounds via GC-MS
3.4. Identification and Relative Quantification of Volatile Organic Compounds via GC-MS
3.5. Determination of Color Differences Using a Colorimeter
3.6. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Chen, H. Flora of Hainan (Volume II); Science Press: Beijing, China, 1956; Volume 156. [Google Scholar]
- Yan, W.; Li, J. Research Progress of Mallotus obongifolius in Hainan. Chin. Agric. Sci. Bull. 2016, 28, 200–204. [Google Scholar]
- Yan, W.; Li, J.; Zheng, D.; Friedman, C.; Wang, H. Analysis of genetic population structure and diversity in Mallotus oblongifolius using ISSR and SRAP markers. Peer J. 2019, 7, e7173. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhang, X.; Huo, D.; Cao, C.; Li, Y.; Liang, Y.; Li, B.; Li, L. Preliminary characterization, antioxidant and α-glucosidase inhibitory activities of polysaccharides from Mallotus furetianus. Carbohydr. Polym. 2019, 215, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, L.; Mariana, A.V.; Wu, Y.; Antônio Esmerino, L.; Azevedo, L.; Zhang, L.; Granato, D. Optimizing the extraction of bioactive compounds from pu-erh tea (Camellia sinensis var. assamica) and evaluation of antioxidant, cytotoxic, antimicrobial, antihemolytic, and inhibition of α-amylase and α-glucosidase activities. Food Res. Int. 2020, 137, 109430. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhang, W.; Wang, R.; Li, C.; Lin, X.; Wang, L. Screening and identification of natural α-glucosidase and α-amylase inhibitors from partridge tea (Mallotus furetianus Muell-Arg) and in silico analysis. Food Chem. 2022, 388, 133004. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, L.; Luo, Y. Composition Analysis by UPLC-PDA-ESI (-)-HRMS and Antioxidant Activity Using Saccharomyces cerevisiae Model of Herbal Teas and Green Teas from Hainan. Molecules 2018, 23, 2550. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Ding, Y.; Cheng, Y.; Chen, Y.; Tang, Y.; Wu, X.; Cheng, Y. Efficacy and Mechanism of Mallotus furetianus Müll. Arg. Extract on Nonalcoholic Fatty Liver Disease. Evid.-Based Complement. Altern. Med. Ecam 2022, 2022, 4897463. [Google Scholar] [CrossRef]
- Ng, Z.X.; Than, M.J.Y.; Yong, P.H. Peperomia pellucida (L.) Kunth herbal tea: Effect of fermentation and drying methods on the consumer acceptance, antioxidant and anti-inflammatory activities. Food Chem. 2021, 344, 128738. [Google Scholar] [CrossRef]
- Seremet Ceclu, L.; Botez, E.; Nistor, O.V.; Andronoiu, D.G.; Mocanu, G.D. Effect of different drying methods on moisture ratio and rehydration of pumpkin slices. Food Chem. 2016, 195, 104–109. [Google Scholar] [CrossRef]
- Yan, Z.; Zhou, Z.; Jiao, Y.; Huang, J.; Yu, Z.; Zhang, D.; Chen, Y.; Ni, D. Hot-Air Drying Significantly Improves the Quality and Functional Activity of Orange Black Tea Compared with Traditional Sunlight Drying. Foods 2023, 12, 1913. [Google Scholar] [CrossRef]
- Ciurzyńska, A.; Lenart, A.; Kawka, P. Influence of chemical composition and structure on sorption properties of freeze-dried pumpkin. Dry. Technol. 2013, 31, 655–665. [Google Scholar] [CrossRef]
- Imaniar, D.I.; Karyadi, J.N.; Marfu’ah, S.; Akbar, M.A. Physical quality change of rose tea during freeze drying. IOP Conf. Ser. Earth Environ. Sci. 2019, 365, 012035. [Google Scholar] [CrossRef]
- Wang, D.; Javed, H.U.; Shi, Y.; Naz, S.; Ali, S.; Duan, C.Q. Impact of Drying Method on the Evaluation of Fatty Acids and Their Derived Volatile Compounds in ‘Thompson Seedless’ Raisins. Molecules 2020, 25, 608. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Hao, Q.; Qiao, X.; Li, M.; Qiu, Z.; Zheng, Z.; Zhang, B. An evaluation of different pretreatment methods of hot-air drying of garlic:drying characteristics, energy consumption and quality properties. LWT 2023, 180, 114685. [Google Scholar] [CrossRef]
- Kittibunchakul, S.; Temviriyanukul, P.; Chaikham, P.; Kemsawasd, V. Effects of freeze drying and convective hot-air drying on predominant bioactive compounds, antioxidant potential and safe consumption of maoberry fruits. LWT 2023, 184, 114992. [Google Scholar] [CrossRef]
- Zhao, R.; Xiao, H.; Liu, C.; Wang, H.; Wu, Y.; Ben, A.; Wang, Y. Dynamic changes in volatile and non-volatile flavor compounds in lemon flavedo during freeze-drying and hot-air drying. LWT 2023, 175, 114510. [Google Scholar] [CrossRef]
- Fang, Q.; Luo, W.; Zheng, Y.; Ye, Y.; Hu, M.; Zheng, X.; Lu, J.; Liang, Y.; Ye, J. Identification of Key Aroma Compounds Responsible for the Floral Ascents of Green and Black Teas from Different Tea Cultivars. Molecules 2022, 27, 2809. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, Y.; Yan, F.; Tang, Y.; Yu, B.; Chen, B.; Lu, L.; Yuan, L.; Wu, Z.; Chen, H. Monitoring Changes in the Volatile Compounds of Tea Made from Summer Tea Leaves by GC-IMS and HS-SPME-GC-MS. Foods 2022, 12, 146. [Google Scholar] [CrossRef]
- Lin, J.; Zhang, P.; Pan, Z.; Xu, H.; Luo, Y.; Wang, X. Discrimination of oolong tea (Camellia sinensis) varieties based on feature extraction and selection from aromatic profiles analysed by HS-SPME/GC-MS. Food Chem. 2013, 141, 259–265. [Google Scholar] [CrossRef]
- Richter, T.M.; Eyres, G.T.; Silcock, P.; Bremer, P.J. Comparison of four extraction methods for analysis of volatile hop-derived aroma compounds in beer. J. Sep. Sci. 2017, 40, 4366–4376. [Google Scholar] [CrossRef]
- Schlumpberger, P.; Stübner, C.A.; Steinhaus, M. Development and evaluation of an automated solvent-assisted flavour evaporation (aSAFE). Eur. Food Res. Technol. 2022, 248, 2591–2602. [Google Scholar] [CrossRef]
- Barghamadi, A.; Mehrdad, M.; Sefidkon, F.; Yamini, Y.A.; Khajeh, M. Comparison of the Volatiles of Achillea millefolium L. Obtained by Supercritical Carbon Dioxide Extraction and Hydrodistillation Methods. J. Essent. Oil Res. 2009, 21, 259–263. [Google Scholar] [CrossRef]
- Carazzone, C.; Rodríguez, J.P.G.; Gonzalez, M.; López, G.-D. Volatilomics of Natural Products: Whispers from Nature. In Metabolomics-Methodology and Applications in Medical Sciences and Life Sciences; IntechOpen: London, UK, 2021; Volume 11, p. 13. ISBN 0000957720. [Google Scholar]
- Lytou, A.E.; Panagou, E.Z.; Nychas, G.E. Volatilomics for food quality and authentication. Curr. Opin. Food Sci. 2019, 28, 88–95. [Google Scholar] [CrossRef]
- Zheng, X.; Hu, T.; Xie, H.; Ou, X.; Huang, J.; Wang, C.; Liu, Z.; Li, Q. Characterization of the key odor-active compounds in different aroma types of Fu brick tea using HS-SPME/GC-MSO combined with sensory-directed flavor analysis. Food Chem. 2023, 426, 136527. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Liu, Z.; Chen, W.; Lan, X.; Zhan, S.; Sun, Y.; Su, W.; Lin, C.; Ni, L. Comparative study of the volatile fingerprints of roasted and unroasted oolong tea by sensory profiling and HS-SPME-GC-MS. Curr. Res. Food Sci. 2023, 6, 100442. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Huang, Y.; Chen, Y.; Zhu, M.; He, C.; Li, Z.; Wang, Y.; Liu, Z. Characteristic fingerprints and change of volatile organic compounds of dark teas during solid-state fermentation with Eurotium cristatum by using HS-GC-IMS, HS-SPME-GC-MS, E-nose and sensory evaluation. LWT 2022, 169, 113925. [Google Scholar] [CrossRef]
- Lv, S.; Wu, Y.; Zhou, J.; Lian, M.; Li, C.; Xu, Y.; Liu, S.; Wang, C.; Meng, Q. The study of fingerprint characteristics of Dayi Pu-Erh tea using a fully automatic HS-SPME/GC-MS and combined chemometrics method. PLoS ONE 2014, 9, e116428. [Google Scholar] [CrossRef]
- Cuevas, F.J.; Moreno-Rojas, J.M.; Ruiz-Moreno, M.J. Assessing a traceability technique in fresh oranges (Citrus sinensis L. Osbeck) with an HS-SPME-GC-MS method. Towards a volatile characterisation of organic oranges. Food Chem. 2017, 221, 1930–1938. [Google Scholar] [CrossRef]
- Worley, B.; Powers, R. PCA as a practical indicator of OPLS-DA model reliability. Curr. Metabolomics 2016, 4, 97–103. [Google Scholar] [CrossRef]
- Jeong, S.; Park, S.H.; Kim, C. Simulation of Morphology Changes in Drying Leaves. Comput. Graph. Forum 2013, 32, 204–215. [Google Scholar] [CrossRef]
- Bhatta, S.; Stevanovic Janezic, T.; Ratti, C. Freeze-Drying of Plant-Based Foods. Foods 2020, 9, 87. [Google Scholar] [CrossRef] [PubMed]
- Drynan, J.W.; Clifford, M.N.; Obuchowicz, J.; Kuhnert, N. The chemistry of low molecular weight black tea polyphenols. Nat. Prod. Rep. 2010, 27, 417–462. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Goodner, K.L.; Park, J.; Choi, J.; Talcott, S.T. Changes in antioxidant phytochemicals and volatile composition of Camellia sinensis by oxidation during tea fermentation. Food Chem. 2011, 129, 1331–1342. [Google Scholar] [CrossRef]
- Li, J.; Shao, Y.; Yao, Y.; Yu, Y.; Cao, G.; Zou, H.; Yan, Y. A novel quality evaluation method for magnolia bark using electronic nose and colorimeter data with multiple statistical algorithms. J. Tradit. Chin. Med. Sci. 2020, 7, 221–227. [Google Scholar] [CrossRef]
- Zong, X.; Sheng, X.; Li, L.; Zan, J.; Jiang, Y.; Zou, H.; Shen, S.; Yuan, H. Rapid Detection of Moisture Content in the Processing of Longjing Tea by Micro-Near-Infrared Spectroscopy and a Portable Colorimeter Based on a Data Fusion Strategy. Horticulturae 2022, 8, 1007. [Google Scholar] [CrossRef]
- Chen, Y.; Hu, B.; Xing, J.; Li, C. Endophytes: The novel sources for plant terpenoid biosynthesis. Appl. Microbiol. Biotechnol. 2021, 105, 4501–4513. [Google Scholar] [CrossRef] [PubMed]
- Amirzakariya, B.Z.; Shakeri, A. Bioactive terpenoids derived from plant endophytic fungi: An updated review (2011–2020). Phytochemistry 2022, 197, 113130. [Google Scholar] [CrossRef]
- Yang, W.; Chen, X.; Li, Y.; Guo, S.; Wang, Z.; Yu, X. Advances in Pharmacological Activities of Terpenoids. Nat. Prod. Commun. 2020, 15, 1934578X20903555. [Google Scholar] [CrossRef]
- Qin, S.; Li, J.; Kareem, A.; Wang, Y. GC-MS Analysis of Volatile Components in Partridge Tea (Mallotus obongifolius). HortScience Horts 2019, 54, 1288–1292. [Google Scholar] [CrossRef]
- Bosco, A.; Golsteyn, R.M. Emerging Anti-Mitotic Activities and Other Bioactivities of Sesquiterpene Compounds upon Human Cells. Molecules 2017, 22, 459. [Google Scholar] [CrossRef]
- Fidyt, K.; Fiedorowicz, A.; Strządała, L.; Szumny, A. β-caryophyllene and β-caryophyllene oxide-natural compounds of anticancer and analgesic properties. Cancer Med. 2016, 5, 3007–3017. [Google Scholar] [CrossRef]
- Dahham, S.S.; Tabana, Y.M.; Iqbal, M.A.; Ahamed, M.B.; Ezzat, M.O.; Majid, A.S.; Majid, A.M. The Anticancer, Antioxidant and Antimicrobial Properties of the Sesquiterpene β-Caryophyllene from the Essential Oil of Aquilaria crassna. Molecules 2015, 20, 11808–11829. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, F.; Yu, J.; Jin, J. β-Bourbonene attenuates proliferation and induces apoptosis of prostate cancer cells. Oncol. Lett. 2018, 16, 4519–4525. [Google Scholar] [CrossRef]

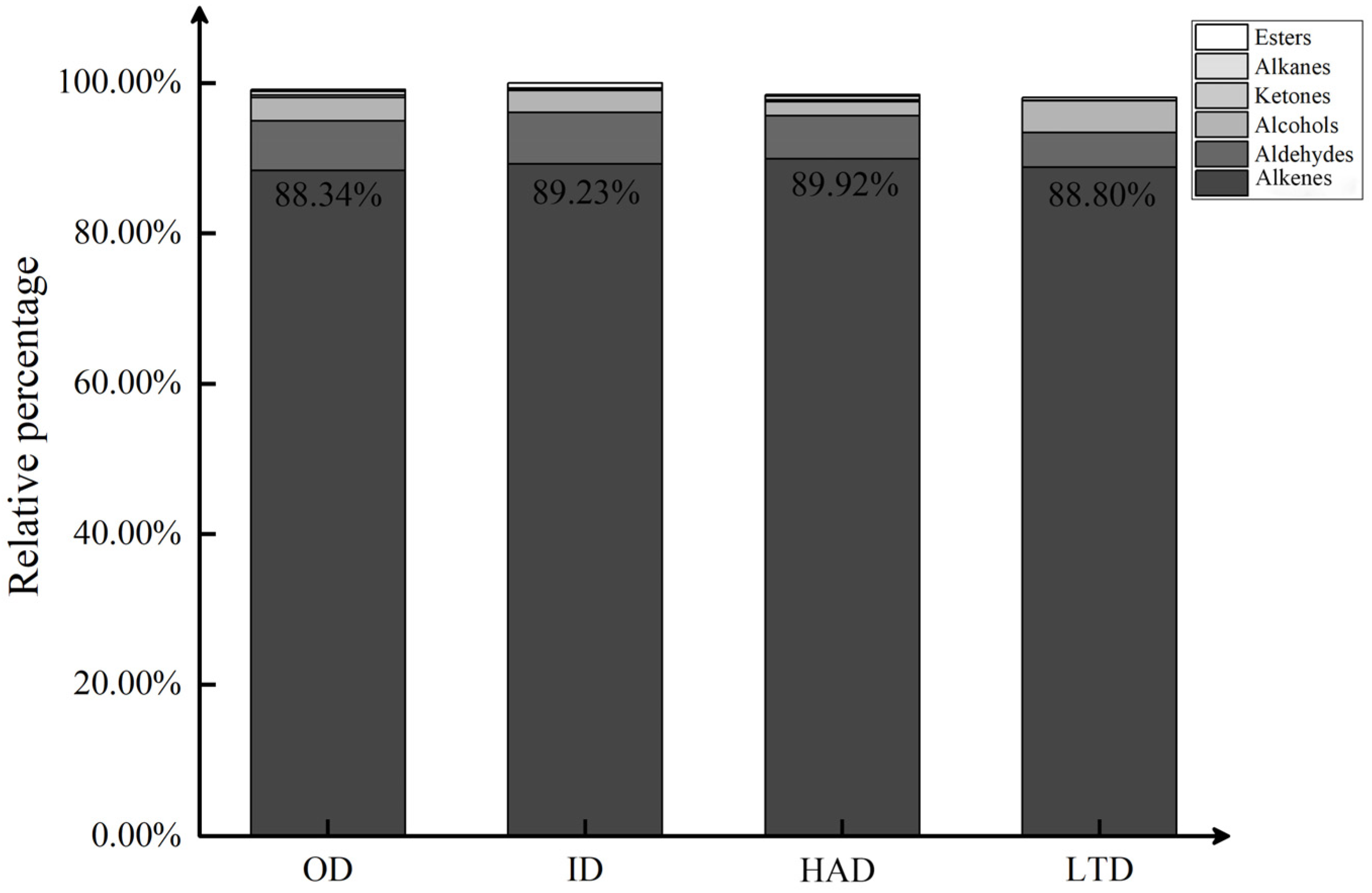
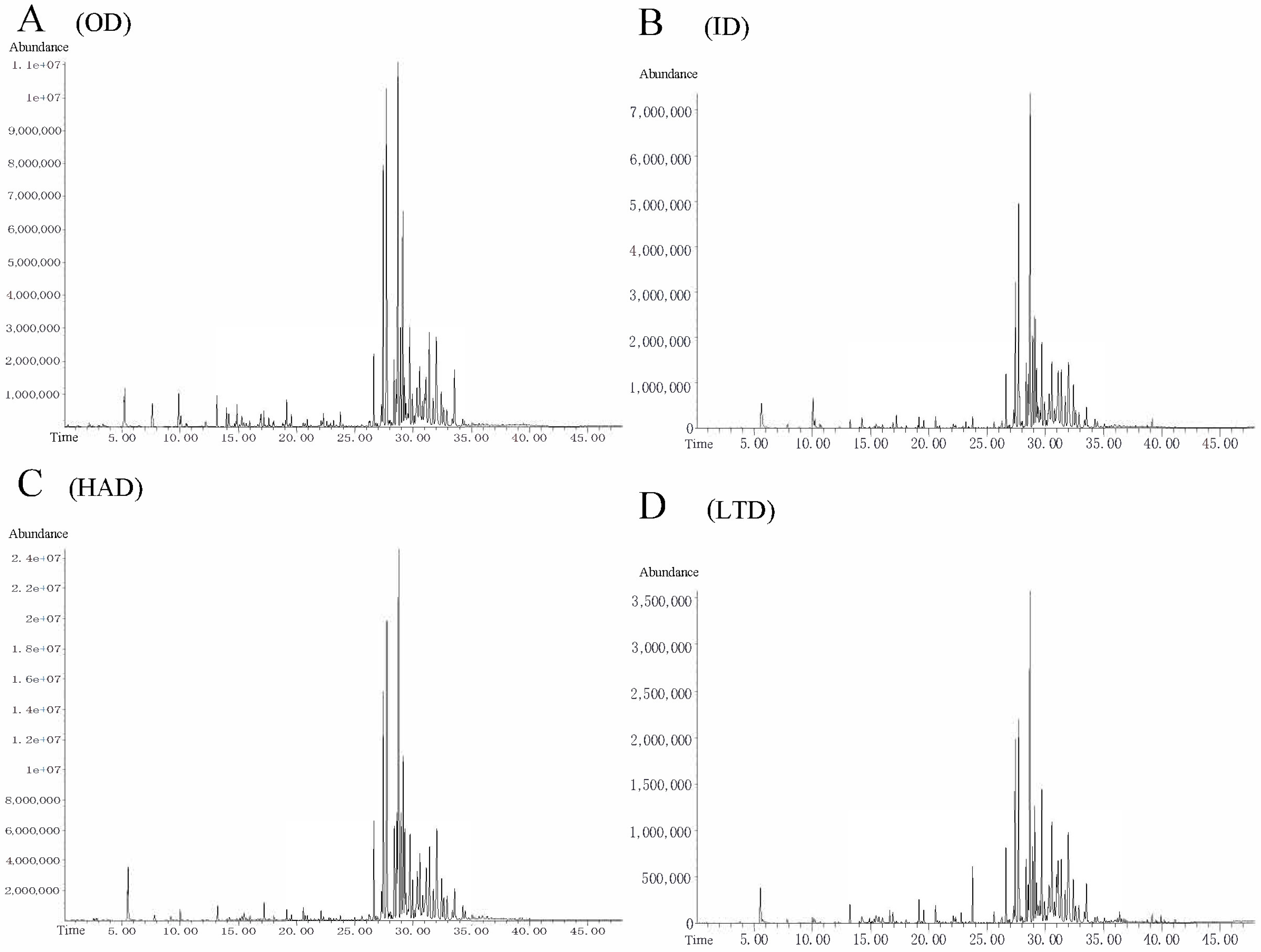
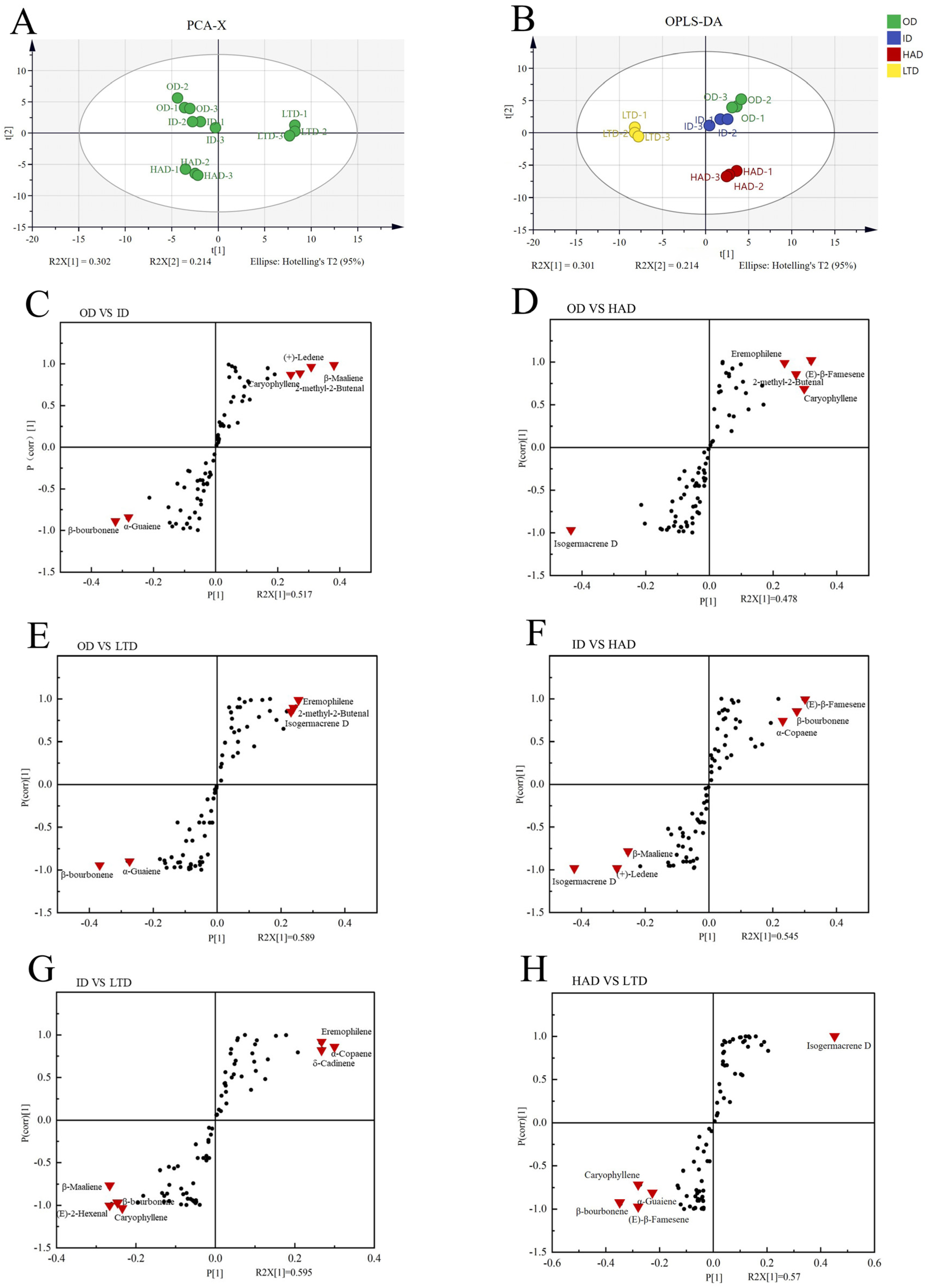
| No. | Compounds | Formula | RT | RI | OD (%) | ID (%) | HAD (%) | LTD (%) |
|---|---|---|---|---|---|---|---|---|
| 1 | 2-Methyl-2-butenal | C5H8O | 5.24 | 737 | 0.42 ± 0.73 b | 2.18 ± 0.21 a | 2.58 ± 0.96 a | 2.22 ± 0.33 a |
| 2 | cis-2-Pentenol | C5H10O | 6.75 | 769 | 0.02 ± 0.03 a | 0.03 ± 0.05 a | 0.05 ± 0.02 a | Nd a |
| 3 | Hexanal | C6H12O | 7.62 | 800 | 0.94 ± 0.14 a | 0.42 ± 0.15 b | 0.33 ± 0.09 bc | 0.19 ± 0.00 c |
| 4 | Furfural | C5H4O2 | 9.21 | 835 | Nd b | Nd b | 0.25 ± 0.05 a | Nd b |
| 5 | (E)-2-Hexenal | C6H10O | 9.88 | 850 | 1.06 ± 0.31 b | 1.94 ± 0.29 a | 0.58 ± 0.20 c | 0.21 ± 0.02 c |
| 6 | 3-Hexen-1-ol | C6H12O | 10.18 | 858 | Nd c | Nd c | 0.08 ± 0.04 b | 0.14 ± 0.00 a |
| 7 | (E)-2-Hexen-1-ol | C6H12O | 10.50 | 861 | 0.09 ± 0.03 b | 0.19 ± 0.03 a | 0.03 ± 0.06 bc | Nd c |
| 8 | Sorbaldehyde | C6H8O | 12.19 | 913 | 0.23 ± 0.04 a | 0.05 ± 0.09 b | Nd b | Nd b |
| 9 | α-Pinene | C10H16 | 13.16 | 939 | 0.63 ± 0.36 a | 0.79 ± 0.42 a | 0.46 ± 0.06 a | 0.88 ± 0.13 a |
| 10 | (Z)-2-Heptenal | C7H12O | 13.97 | 958 | 0.20 ± 0.35 a | 0.02 ± 0.04 a | 0.10 ± 0.04 a | Nd a |
| 11 | (E)-2-Heptenal | C7H12O | 13.98 | 962 | 0.49 ± 0.09 a | 0.05 ± 0.08 b | 0.03 ± 0.06 b | 0.09 ± 0.01 b |
| 12 | Benzaldehyde | C7H6O | 14.17 | 966 | 0.53 ± 0.21 a | 0.56 ± 0.10 a | 0.19 ± 0.04 b | 0.52 ± 0.02 a |
| 13 | β-Pinene | C10H16 | 14.84 | 974 | Nd b | 0.09 ± 0.03 a | 0.01 ± 0.02 b | Nd b |
| 14 | Matsutake alcohol | C8H16O | 14.88 | 976 | 0.54 ± 0.12 a | 0.16 ± 0.08 b | 0.11 ± 0.07 b | Nd b |
| 15 | Prenylacetone | C8H14O | 15.10 | 980 | 0.09 ± 0.08 a | 0.02 ± 0.03 a | 0.08 ± 0.02 a | 0.02 ± 0.03 a |
| 16 | (E,E)-2,4-Heptadienal | C7H10O | 15.97 | 1007 | 0.21 ± 0.08 a | 0.15 ± 0.01 a | 0.18 ± 0.03 a | 0.23 ± 0.02 a |
| 17 | 2-Ethylhexanol | C8H18O | 16.65 | 1015 | Nd b | Nd b | Nd b | 0.59 ± 0.09 a |
| 18 | Limonene | C10H16 | 16.69 | 1030 | 0.07 ± 0.03 a | 0.07 ± 0.02 a | 0.05 ± 0.01 a | Nd b |
| 19 | Benzenemethanol | C7H8O | 16.89 | 1033 | 0.25 ± 0.05 b | 0.28 ± 0.09 b | 0.11 ± 0.01 c | 0.58 ± 0.01 a |
| 20 | Benzeneacetaldehyde | C8H8O | 17.18 | 1042 | 0.51 ± 0.17 a | 0.44 ± 0.10 a | 0.58 ± 0.14 a | 0.08 ± 0.01 b |
| 21 | β-Ocimene | C10H16 | 17.32 | 1044 | Nd b | 0.04 ± 0.01 a | 0.04 ± 0.01 a | Nd b |
| 22 | (E)-2-Octenal | C8H14O | 17.61 | 1056 | 0.23 ± 0.03 a | 0.08 ± 0.03 b | 0.06 ± 0.01 b | Nd c |
| 23 | 3,5-Octadien-2-one | C8H12O | 18.04 | 1081 | 0.20 ± 0.04 a | 0.13 ± 0.01 b | 0.17 ± 0.02 ab | Nd c |
| 24 | Linalool | C10H18O | 19.05 | 1098 | 0.16 ± 0.03 ab | 0.20 ± 0.09 a | 0.08 ± 0.02 bc | 0.02 ± 0.03 c |
| 25 | Nonanal | C9H18O | 19.14 | 1102 | 0.74 ± 0.12 ab | 0.60 ± 0.17 bc | 0.41 ± 0.10 c | 0.87 ± 0.09 a |
| 26 | Benzeneethanol | C8H10O | 19.55 | 1114 | 0.36 ± 0.07 b | 0.23 ± 0.12 bc | 0.21 ± 0.04 c | 0.52 ± 0.03 a |
| 27 | Cucumber aldehyde | C9H14O | 20.74 | 1151 | 0.03 ± 0.06 b | Nd b | 0.15 ± 0.02 a | Nd b |
| 28 | (E)-2-Nonenal | C9H16O | 20.91 | 1161 | 0.23 ± 0.05 a | 0.03 ± 0.06 c | 0.15 ± 0.02 b | Nd c |
| 29 | 1-Dodecene | C12H24 | 21.85 | 1192 | 0.26 ± 0.00 a | 0.14 ± 0.09 a | 0.20 ± 0.10 a | 0.23 ± 0.02 a |
| 30 | Myrtenol | C10H16O | 22.22 | 1194 | 0.16 ± 0.04 a | 0.14 ± 0.04 a | 0.03 ± 0.00 b | 0.16 ± 0.04 a |
| 31 | Decanal | C10H20O | 22.31 | 1200 | 0.40 ± 0.04 a | 0.16 ± 0.04 b | 0.07 ± 0.12 b | 0.19 ± 0.02 b |
| 32 | (E,E)-2,4-Nonadienal | C9H14O | 22.60 | 1214 | 0.21 ± 0.08 a | 0.01 ± 0.02 b | Nd b | Nd b |
| 33 | β-Cyclocitral | C10H16O | 22.92 | 1218 | 0.11 ± 0.02 ab | 0.12 ± 0.03 a | 0.08 ± 0.01 b | 0.02 ± 0.03 c |
| 34 | cis-3-Hexenyl valerate | C11H20O2 | 23.18 | 1235 | 0.21 ± 0.06 b | 0.47 ± 0.20 a | 0.11 ± 0.06 b | Nd b |
| 35 | Butanoic acid, 3-methyl-, hexyl ester | C11H22O2 | 23.33 | 1244 | Nd b | 0.06 ± 0.02 a | Nd b | Nd b |
| 36 | Geraniol | C10H18O | 23.76 | 1250 | 0.37 ± 0.06 b | 0.67 ± 0.19 b | 0.17 ± 0.03 b | 1.30 ± 0.48 a |
| 37 | (E)-2-Decenal | C10H18O | 23.98 | 1260 | 0.07 ± 0.01 a | Nd b | Nd b | Nd b |
| 38 | α-Citral | C10H16O | 24.24 | 1269 | 0.01 ± 0.02 b | 0.07 ± 0.01 a | Nd b | Nd b |
| 39 | δ-Elemene | C15H24 | 26.29 | 1316 | 0.25 ± 0.04 b | 0.41 ± 0.08 a | 0.20 ± 0.01 b | 0.39 ± 0.04 a |
| 40 | α-Cubebene | C15H24 | 26.63 | 1348 | 2.33 ± 0.07 a | 2.34 ± 0.23 a | 2.37 ± 0.47 a | 2.55 ± 0.22 a |
| 41 | 3-Methyl-tridecane | C14H30 | 26.94 | 1371 | 0.02 ± 0.04 a | 0.03 ± 0.06 a | 0.13 ± 0.04 a | 0.04 ± 0.08 a |
| 42 | Ylangene | C15H24 | 27.29 | 1372 | 0.90 ± 0.05 b | 0.86 ± 0.05 b | 1.04 ± 0.02 a | 0.88 ± 0.11 b |
| 43 | α-Copaene | C15H24 | 27.44 | 1376 | 6.28 ± 1.61 a | 5.68 ± 0.73 a | 7.71 ± 1.41 a | 8.22 ± 1.14 a |
| 44 | β-Bourbonene | C15H24 | 27.72 | 1382 | 11.50 ± 1.11 a | 8.96 ± 0.14 b | 11.39 ± 1.23 a | 7.34 ± 0.36 b |
| 45 | Cyperene | C15H24 | 28.16 | 1390 | 0.11 ± 0.01 a | 0.13 ± 0.07 a | 0.15 ± 0.02 a | 0.03 ± 0.05 b |
| 46 | α-Gurjenene | C15H24 | 28.37 | 1401 | 0.77 ± 1.33 a | 0.02 ± 0.03 a | 1.39 ± 2.41 a | 0.87 ± 1.30 a |
| 47 | β-Maaliene | C15H24 | 28.38 | 1413 | Nd b | 3.14 ± 0.27 a | 0.84 ± 1.45 b | 0.89 ± 1.54 b |
| 48 | α-Santalene | C15H24 | 28.55 | 1420 | 1.73 ± 0.15 a | 2.38 ± 0.11 a | 2.67 ± 0.76 a | 1.95 ± 0.54 a |
| 49 | Caryophyllene | C15H24 | 28.71 | 1428 | 13.82 ± 0.69 b | 15.28 ± 0.07 ab | 16.98 ± 2.77 a | 13.66 ± 0.27 b |
| 50 | Calarene | C15H24 | 28.93 | 1434 | 4.09 ± 0.32 a | 4.38 ± 0.08 a | 3.49 ± 1.26 a | 3.39 ± 0.19 a |
| 51 | Isogermacrene D | C15H24 | 28.94 | 1439 | 4.79 ± 0.77 b | 5.17 ± 0.75 b | Nd c | 6.53 ± 0.52 a |
| 52 | α-Guaiene | C15H24 | 29.12 | 1440 | 7.06 ± 1.07 a | 5.03 ± 0.13 bc | 6.52 ± 1.24 ab | 4.49 ± 0.25 c |
| 53 | Aromandendrene | C15H24 | 29.26 | 1441 | 2.07 ± 0.31 b | 2.80 ± 0.31 a | 2.58 ± 0.44 ab | 1.87 ± 0.30 b |
| 54 | α-Caryophyllene | C15H24 | 29.72 | 1452 | 6.02 ± 1.55 a | 4.39 ± 1.00 a | 4.33 ± 0.39 a | 5.01 ± 0.81 a |
| 55 | Alloaromadendrene | C15H24 | 29.94 | 1454 | 1.64 ± 0.35 a | 1.67 ± 0.25 a | 1.31 ± 0.17 a | 1.22 ± 0.22 a |
| 56 | β-Copaene | C15H24 | 30.10 | 1459 | 0.98 ± 0.14 a | 0.89 ± 0.13 a | 0.41 ± 0.02 b | 0.97 ± 0.01 a |
| 57 | Germacrene d | C15H24 | 30.12 | 1461 | 0.36 ± 0.12 a | 0.29 ± 0.05 a | 0.29 ± 0.06 a | 0.35 ± 0.13 a |
| 58 | γ-Gurjunene | C15H24 | 30.26 | 1471 | 0.11 ± 0.20 b | 0.44 ± 0.12 a | Nd b | Nd b |
| 59 | γ-Muurolene | C15H24 | 30.34 | 1474 | 2.45 ± 0.48 a | 2.34 ± 0.13 a | 2.70 ± 0.26 a | 2.78 ± 0.54 a |
| 60 | (E)-β-Famesene | C15H24 | 30.58 | 1476 | Nd b | Nd b | 2.51 ± 0.21 a | Nd b |
| 61 | β-Selinene | C15H24 | 30.81 | 1479 | 1.35 ± 0.23 a | 1.38 ± 0.20 a | 1.07 ± 0.29 a | 0.94 ± 0.06 a |
| 62 | Eremophilene | C15H24 | 31.02 | 1486 | Nd b | Nd b | 1.35 ± 0.08 a | 1.95 ± 0.77 a |
| 63 | (+)-Ledene | C15H24 | 31.11 | 1489 | 2.87 ± 0.42 b | 5.05 ± 0.34 a | 2.59 ± 0.19 b | 4.20 ± 0.92 a |
| 64 | α-Bulnesene | C15H24 | 31.40 | 1503 | 4.03 ± 0.21 a | 3.34 ± 0.54 a | 3.50 ± 0.52 a | 3.14 ± 0.34 a |
| 65 | γ-Cadinene | C15H24 | 31.72 | 1507 | 1.21 ± 0.33 b | 1.50 ± 0.19 ab | 1.24 ± 0.09 b | 1.84 ± 0.27 a |
| 66 | δ-Cadinene | C15H24 | 32.00 | 1508 | 4.97 ± 0.82 b | 4.45 ± 1.25 b | 5.37 ± 0.32 ab | 6.61 ± 0.25 a |
| 67 | Cubenene | C15H24 | 32.43 | 1512 | 1.55 ± 0.05 a | 2.01 ± 0.57 a | 1.56 ± 0.30 a | 1.94 ± 0.36 a |
| 68 | Selina-4(15),7(11)-diene | C15H24 | 32.62 | 1544 | 0.85 ± 0.04 a | 0.95 ± 0.13 a | 1.11 ± 0.22 a | 0.94 ± 0.05 a |
| 69 | α-Calacorene | C15H20 | 32.88 | 1546 | Nd b | Nd b | Nd b | 0.80 ± 0.04 a |
| 70 | Selina-3,7(11)-diene | C15H24 | 32.90 | 1550 | 0.75 ± 0.08 ab | 0.81 ± 0.17 ab | 1.11 ± 0.25 a | 0.34 ± 0.58 b |
| 71 | E-Nerolidol | C15H26O | 33.37 | 1551 | 0.48 ± 0.29 a | 0.48 ± 0.06 a | 0.47 ± 0.07 a | 0.45 ± 0.10 a |
| 72 | Germacrene B | C15H24 | 33.57 | 1556 | 2.44 ± 0.22 a | 1.98 ± 0.68 ab | 1.30 ± 0.45 b | 1.36 ± 0.47 b |
| 73 | (+)-Spathulenol | C15H24O | 34.27 | 1557 | 0.51 ± 0.27 a | 0.38 ± 0.05 a | 0.41 ± 0.09 a | 0.25 ± 0.01 a |
| 74 | Caryophyllene oxide | C15H24O | 34.47 | 1561 | 0.48 ± 0.19 a | 0.11 ± 0.20 bc | 0.38 ± 0.01 ab | Nd c |
| 75 | Ledol | C15H26O | 35.09 | 1565 | 0.14 ± 0.04 a | 0.15 ± 0.04 a | 0.12 ± 0.03 a | 0.18 ± 0.04 a |
| 76 | α-Corocalene | C15H20 | 35.49 | 1629 | 0.02 ± 0.02 b | 0.02 ± 0.03 b | 0.01 ± 0.01 b | 0.07 ± 0.01 a |
| 77 | Cadalene | C15H18 | 36.80 | 1674 | 0.03 ± 0.03 b | 0.01 ± 0.02 b | 0.02 ± 0.02 b | 0.09 ± 0.01 a |
| 78 | Guaiazulene | C15H18 | 38.77 | 1772 | 0.05 ± 0.05 a | 0.03 ± 0.03 a | 0.03 ± 0.01 a | 0.07 ± 0.02 a |
| 79 | Isopropyl myristate | C17H34O2 | 39.18 | 1823 | 0.02 ± 0.04 b | 0.15 ± 0.13 ab | 0.07 ± 0.02 b | 0.25 ± 0.03 a |
| 80 | Neophytadiene | C20H38 | 39.41 | 1837 | Nd a | Nd a | 0.04 ± 0.00 a | Nd a |
| 81 | Hexahydrofarnesyl acetone | C18H36O | 39.51 | 1847 | 0.07 ± 0.06 a | Nd a | Nd a | 0.04 ± 0.06 a |
| 82 | Diisobutyl phthalate | C16H22O4 | 39.92 | 1868 | Nd a | Nd a | Nd a | 0.09 ± 0.09 a |
| Sample Name | Year | Drying Method | Temperature | Location |
|---|---|---|---|---|
| OD | 2023 | Outdoor sun drying | 30–35 °C | Hainan, China |
| ID | 2023 | Indoor shade drying | 25 ± 2 °C | |
| HAD | 2023 | Hot-air drying | 70 °C | |
| LTD | 2023 | Low-temperature drying | 1. Temperature down to −25 °C lyophilization for 4 h; 2. temperature down to −30 °C lyophilization for 10 h; 3. temperature up to −25 °C lyophilization for 2 h; 4. temperature up to −5 °C lyophilization for 5 h; 5. temperature up to 15 °C drying for 5 h; 6. temperature up to 30 °C drying for 6 h, drying completed. | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gui, X.; Feng, X.; Tang, M.; Li, J. Aroma Difference Analysis of Partridge Tea (Mallotus oblongifolius) with Different Drying Treatments Based on HS-SPME-GC-MS Technique. Molecules 2023, 28, 6836. https://doi.org/10.3390/molecules28196836
Gui X, Feng X, Tang M, Li J. Aroma Difference Analysis of Partridge Tea (Mallotus oblongifolius) with Different Drying Treatments Based on HS-SPME-GC-MS Technique. Molecules. 2023; 28(19):6836. https://doi.org/10.3390/molecules28196836
Chicago/Turabian StyleGui, Xinxin, Xueping Feng, Minqiang Tang, and Juanling Li. 2023. "Aroma Difference Analysis of Partridge Tea (Mallotus oblongifolius) with Different Drying Treatments Based on HS-SPME-GC-MS Technique" Molecules 28, no. 19: 6836. https://doi.org/10.3390/molecules28196836
APA StyleGui, X., Feng, X., Tang, M., & Li, J. (2023). Aroma Difference Analysis of Partridge Tea (Mallotus oblongifolius) with Different Drying Treatments Based on HS-SPME-GC-MS Technique. Molecules, 28(19), 6836. https://doi.org/10.3390/molecules28196836







