Antibacterial Activity and the Mechanism of the Z-Scheme Bi2MoO6/Bi5O7I Heterojunction under Visible Light
Abstract
:1. Introduction
2. Results and Discussion
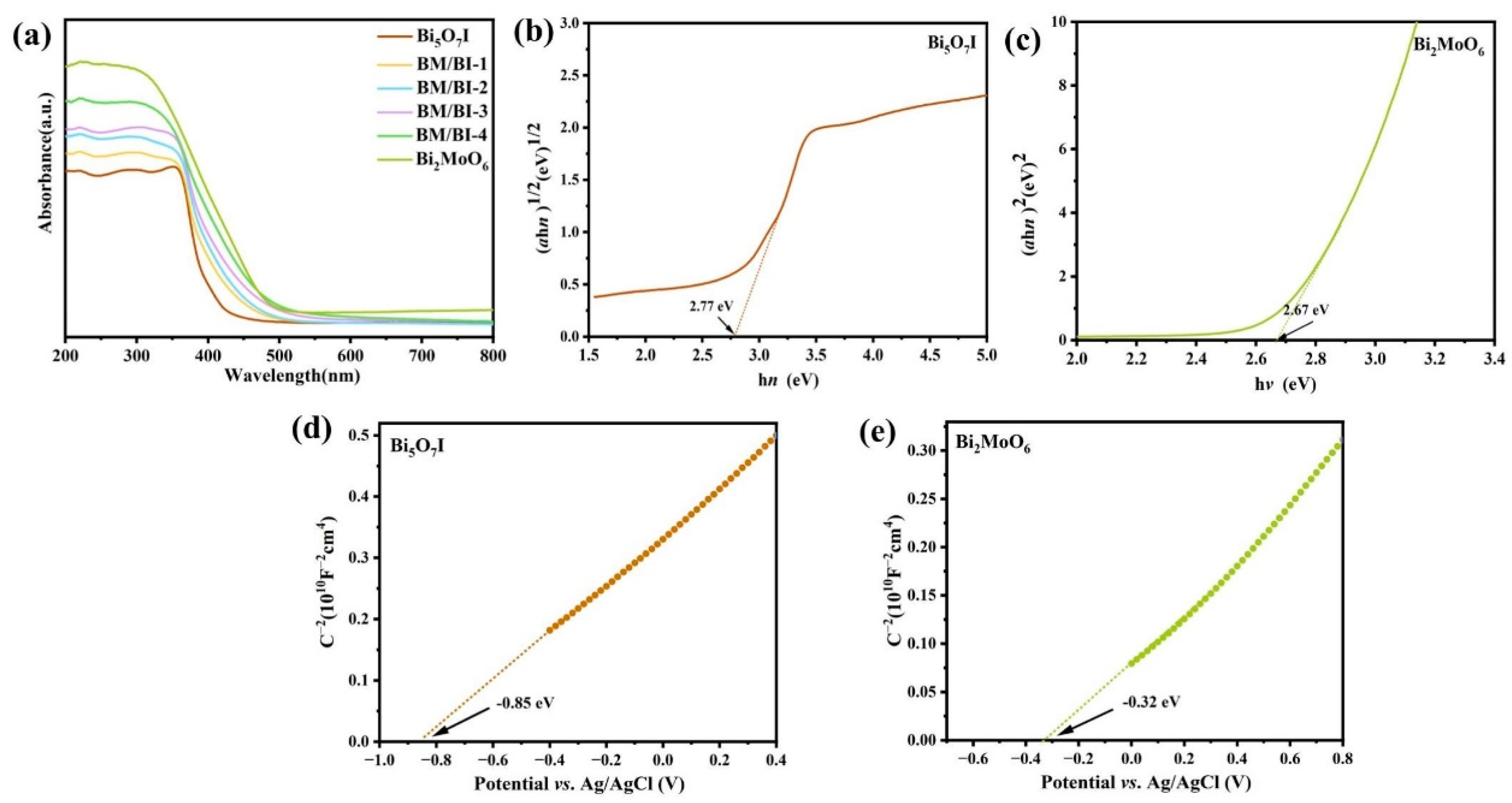
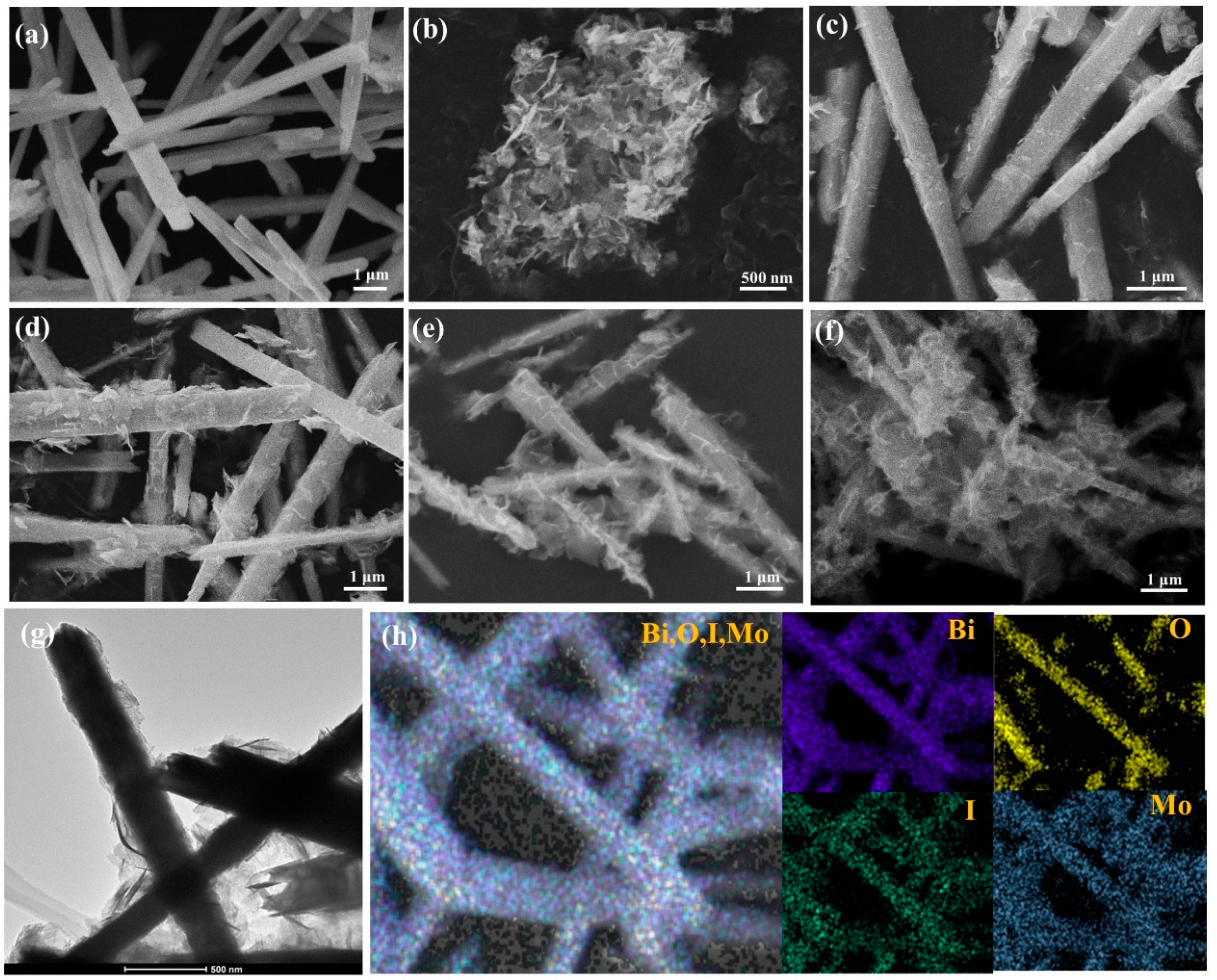
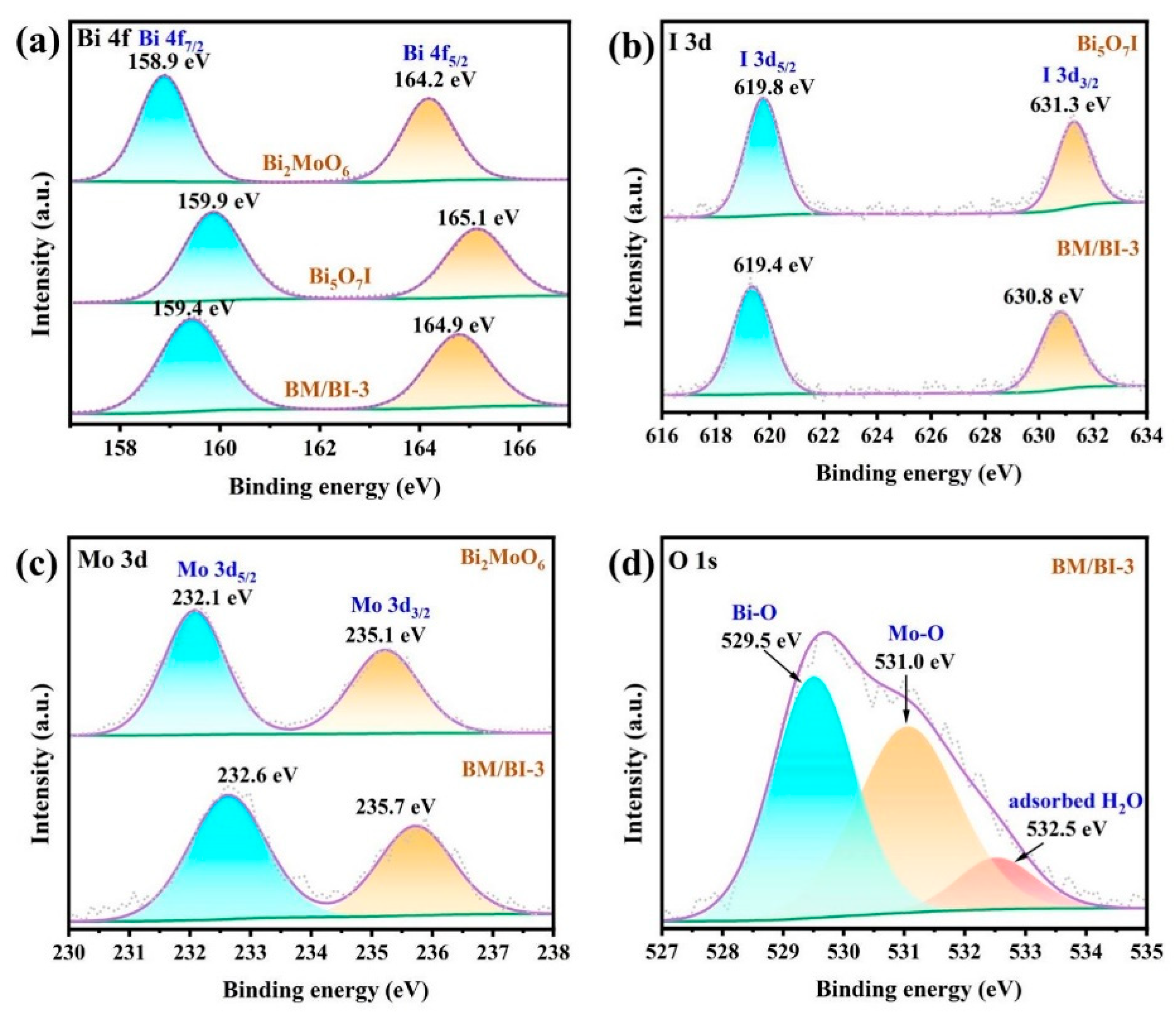
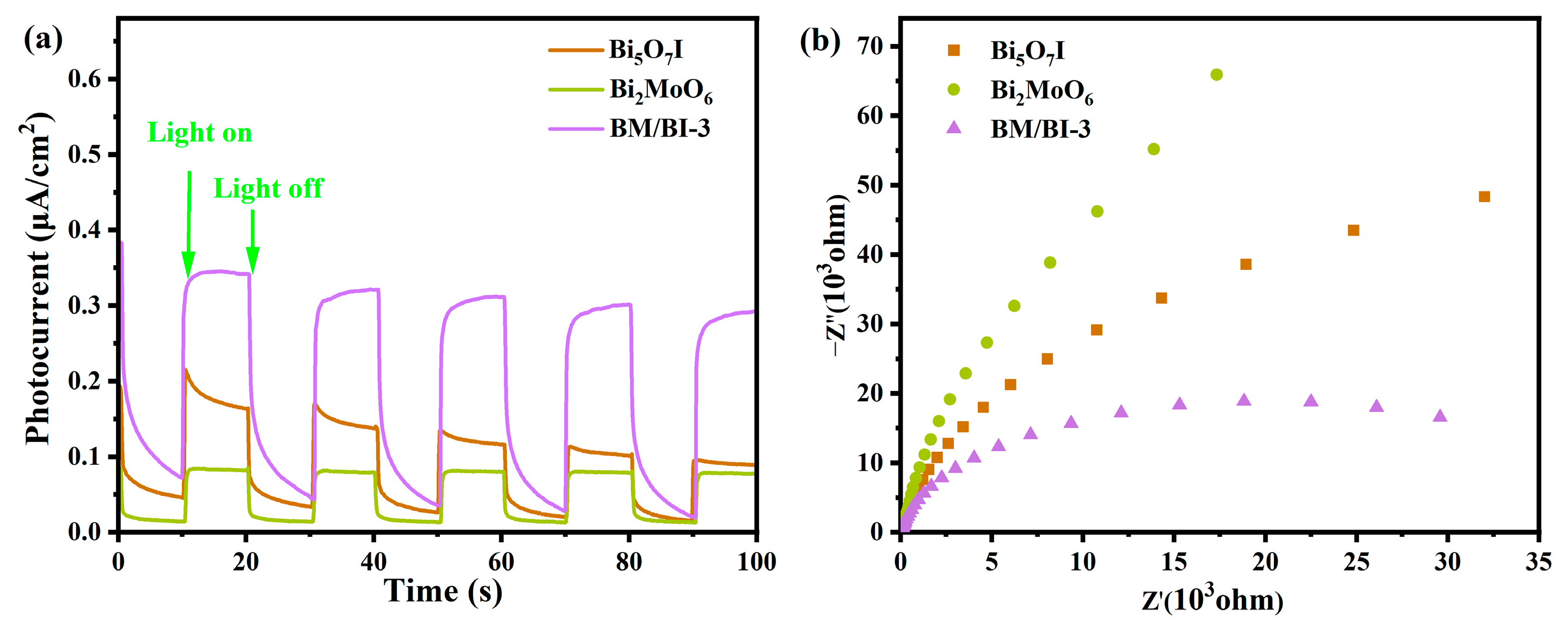
2.1. Material Characterization
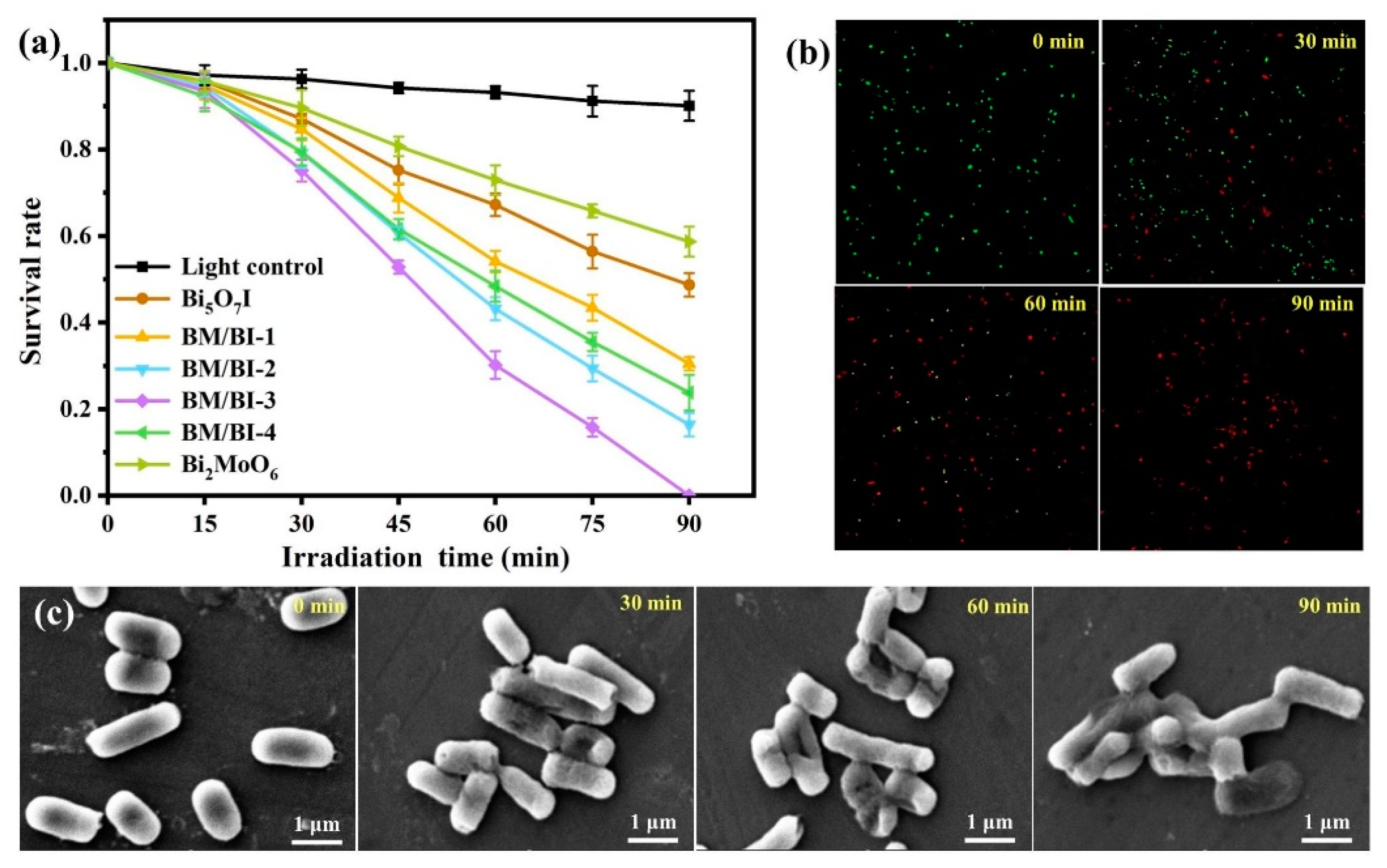
2.2. Photocatalytic Antibacterial Activity
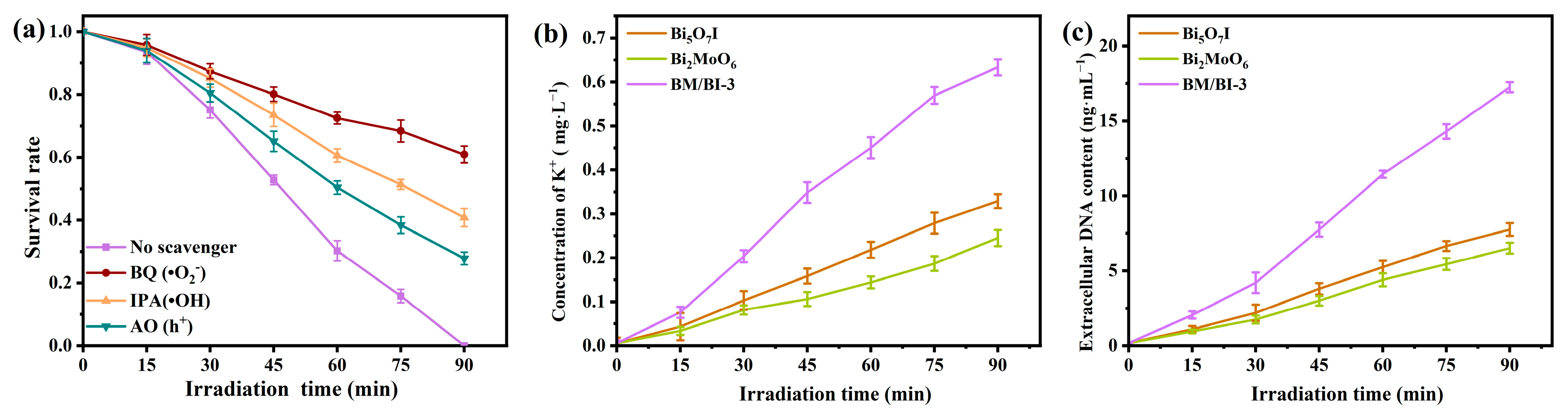
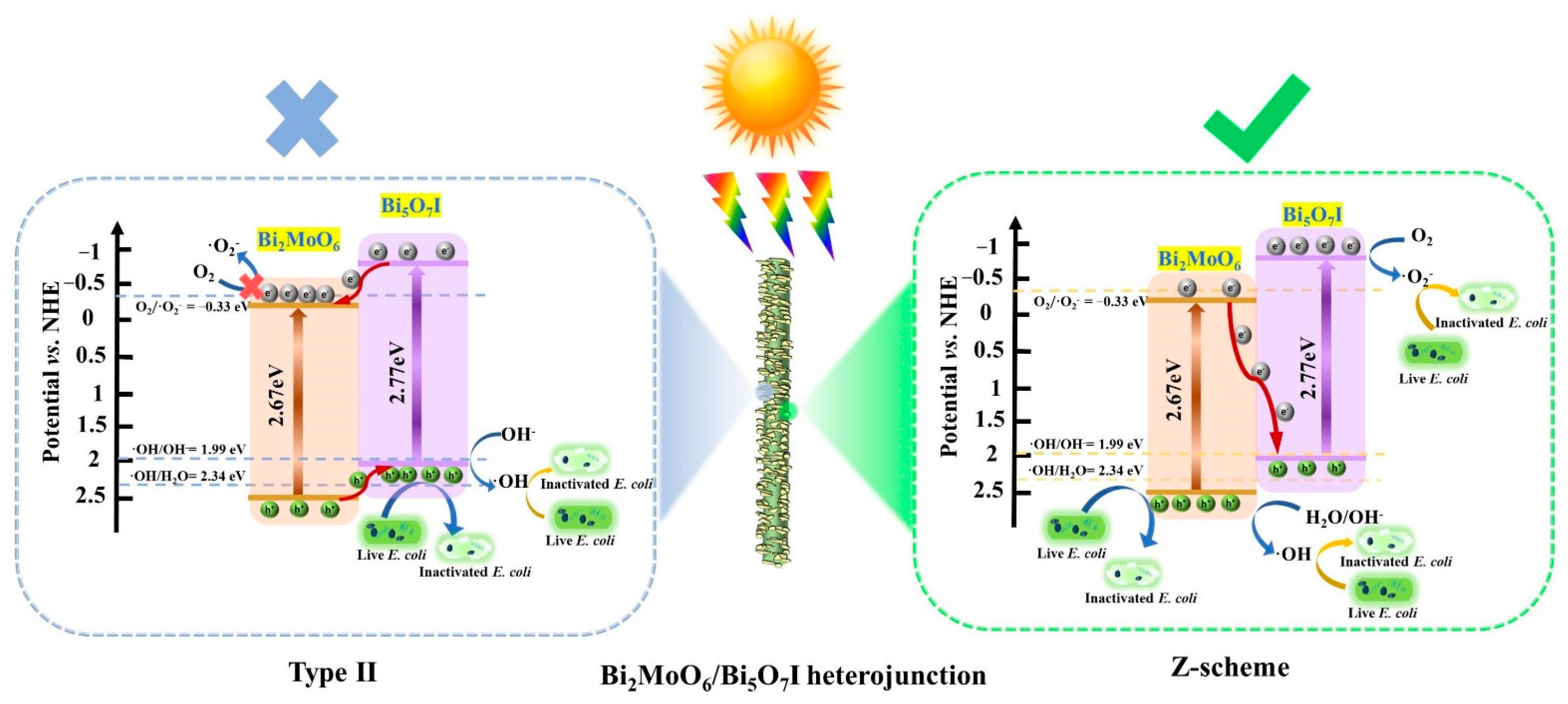
2.3. Mechanism of Improved Photocatalytic Antibacterial Activity for Bi2MoO6/Bi5O7I Heterojunction
3. Experiment Section
3.1. Synthesis of Materials
3.2. Characterization and Photoelectrochemical Measurement
3.3. Photocatalytic Inactivation of E. coli
3.4. Fluorescence Microscopy Assays and Microstructure of E. coli
3.5. Measurement of Intracellular Components Leakage
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Wang, Z.; Du, C.; Ding, D.; Chen, R.; Yang, S.; Cai, T. Recent advances in metal-free catalysts for the remediation of antibiotics, antibiotic resistant bacteria (ARB), and antibiotic resistant genes (ARGs). J. Mater. Chem. A 2022, 10, 15235–15266. [Google Scholar] [CrossRef]
- Wang, J.; Xu, S.; Zhao, K.; Song, G.; Zhao, S.; Liu, R. Risk control of antibiotics, antibiotic resistance genes (ARGs) and antibiotic resistant bacteria (ARB) during sewage sludge treatment and disposal: A review. Sci. Total Environ. 2023, 877, 162772. [Google Scholar] [CrossRef]
- Li, N.; Du, H.; Tan, M.; Yang, L.; Xue, B.; Zheng, S.; Wang, Q. Construction of Z-scheme CuBi2O4/MIL-88A(Fe) heterojunctions with enhanced LED light driven photocatalytic Cr(VI) reduction and antibacterial performance. Appl. Surf. Sci. 2023, 614, 156249. [Google Scholar] [CrossRef]
- Zhao, H.; Guan, X.; Zhang, F.; Huang, Y.; Xia, D.; Hu, L.; Ji, X.; Yin, R.; He, C. Rational design of a bismuth oxyiodide (Bi/BiO1-xI) catalyst for synergistic photothermal and photocatalytic inactivation of pathogenic bacteria in water. J. Mater. Sci. Technol. 2022, 100, 110–119. [Google Scholar] [CrossRef]
- Liu, H.; Ma, S.; Shao, L.; Liu, H.; Gao, Q.; Li, B.; Fu, H.; Fu, S.; Ye, H.; Zhao, F.; et al. Defective engineering in graphitic carbon nitride nanosheet for efficient photocatalytic pathogenic bacteria disinfection. Appl. Catal. B Environ. 2020, 261, 118201. [Google Scholar] [CrossRef]
- Sharma, K.; Dutta, V.; Sharma, S.; Raizada, P.; Hosseini-Bandegharaei, A.; Thakur, P.; Singh, P. Recent advances in enhanced photocatalytic activity of bismuth oxyhalides for efficient photocatalysis of organic pollutants in water: A review. J. Ind. Eng. Chem. 2019, 78, 1–20. [Google Scholar] [CrossRef]
- Alapi, T.; Veres, B.; Náfrádi, M.; Farkas, L.; Pap, Z.; Covic, A. Application of BiOX Photocatalyst to Activate Peroxydisulfate Ion-Investigation of a Combined Process for the Removal of Organic Pollutants from Water. Catalysts 2023, 13, 513. [Google Scholar] [CrossRef]
- Mera, A.C.; Martínez-de la Cruz, A.; Pérez-Tijerina, E.; Meléndrez, M.F.; Valdés, H. Nanostructured BiOI for air pollution control: Microwave-assisted synthesis, characterization and photocatalytic activity toward NO transformation under visible light irradiation. Mat. Sci. Semicon Proc. 2018, 88, 20–27. [Google Scholar] [CrossRef]
- Li, X.; Li, K.; Ding, D.; Yan, J.; Wang, C.; Carabineiro, S.A.C.; Liu, Y.; Lv, K. Effect of oxygen vacancies on the photocatalytic activity of flower-like BiOBr microspheres towards NO oxidation and CO2 reduction. Sep. Purif. Technol. 2023, 309, 123054. [Google Scholar] [CrossRef]
- Zhang, M.; Xie, F.; Zhang, L.; Jian, X.; Liu, J.; Zhang, X.; Wang, Y.; Li, R.; Fan, C. Efficient oxygen evolution photocatalyst of BiOBr: In situ light-induced formation of surface oxygen vacancies and application in water splitting. Mater. Lett. 2022, 321, 132416. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, W.; Sun, S.; Jiang, D.; Gao, E. Selective transport of electron and hole among {001} and {110} facets of BiOCl for pure water splitting. Appl. Catal. B Environ. 2015, 162, 470–474. [Google Scholar] [CrossRef]
- Ren, X.; Gao, M.; Zhang, Y.; Zhang, Z.; Cao, X.; Wang, B.; Wang, X. Photocatalytic reduction of CO2 on BiOX: Effect of halogen element type and surface oxygen vacancy mediated mechanism. Appl. Catal. B Environ. 2020, 274, 119063. [Google Scholar] [CrossRef]
- Gong, S.; Zhu, G.; Wang, R.; Rao, F.; Shi, X.; Gao, J.; Huang, Y.; He, C.; Hojamberdiev, M. Synergistically boosting highly selective CO2–to–CO photoreduction over BiOCl nanosheets via in-situ formation of surface defects and non-precious metal nanoparticles. Appl. Catal. B Environ. 2021, 297, 120413. [Google Scholar] [CrossRef]
- Zhang, N.; Li, L.; Shao, Q.; Zhu, T.; Huang, X.; Xiao, X. Fe-Doped BiOCl Nanosheets with Light-Switchable Oxygen Vacancies for Photocatalytic Nitrogen Fixation. ACS Appl. Energy Mater. 2019, 2, 8394–8398. [Google Scholar] [CrossRef]
- Li, H.; Shang, J.; Ai, Z.; Zhang, L. Efficient Visible Light Nitrogen Fixation with BiOBr Nanosheets of Oxygen Vacancies on the Exposed {001} Facets. J. Am. Chem. Soc. 2015, 137, 6393–6399. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, T.; Tao, X.; Qiu, G.; Li, C.; Li, B. Interfacial synergy of Pd sites and defective BiOBr for promoting the solar-driven selective oxidation of toluene. J. Mater. Chem. A 2020, 8, 17657–17669. [Google Scholar] [CrossRef]
- Contreras, D.; Melin, V.; Márquez, K.; Pérez-González, G.; Mansilla, H.D.; Pecchi, G.; Henríquez, A. Selective oxidation of cyclohexane to cyclohexanol by BiOI under visible light: Role of the ratio (1 1 0)/(0 0 1) facet. Appl. Catal. B Environ. 2019, 251, 17–24. [Google Scholar] [CrossRef]
- Cao, B.; He, D.; Rao, F.; Hassan, Q.U.; Cai, X.; Liu, L.; Xue, F.; Zhu, G. Constructing BiOI/CeO2 p-n heterojunction with enhanced NOx photo-oxidative removal under visible light irradiation. J. Alloys Compd. 2023, 934, 167962. [Google Scholar] [CrossRef]
- Wu, Z.; Jing, J.; Zhang, K.; Li, W.; Yang, J.; Shen, J.; Zhang, S.; Xu, K.; Zhang, S.; Zhu, Y. Epitaxial BiP5O14 layer on BiOI nanosheets enhancing the photocatalytic degradation of phenol via interfacial internal-electric-field. Appl. Catal. B Environ. 2022, 307, 121153. [Google Scholar] [CrossRef]
- Wang, J.; Li, S.; Yang, K.; Zhang, T.; Jiang, S.; Li, X.; Li, B. Bacitracin-Controlled BiOI/Bi5O7I Nanosheet Assembly and S-Scheme Heterojunction Formation for Enhanced Photocatalytic Performances. ACS Appl. Nano Mater. 2022, 5, 6736–6749. [Google Scholar] [CrossRef]
- Xiao, Y.; Wu, J.; Jia, T.; Li, T.; Wang, Z.; Qi, Y.; Liu, Q.; Qi, X.; He, P. Fabrication of BiOI nanosheets with exposed (001) and (110) facets with different methods for photocatalytic oxidation elemental mercury. Colloid Interface Sci. Commun. 2021, 40, 100357. [Google Scholar] [CrossRef]
- Ye, L.; Jin, X.; Ji, X.; Liu, C.; Su, Y.; Xie, H.; Liu, C. Facet-dependent photocatalytic reduction of CO2 on BiOI nanosheets. Chem. Eng. J. 2016, 291, 39–46. [Google Scholar] [CrossRef]
- Xiong, J.; Zeng, H.-Y.; Xu, S.; Peng, J.-F.; Liu, F.-Y.; Wang, L.-H. Enhancing the intrinsic properties of flower-like BiOI by S-doping toward excellent photocatalytic performances. J. Mater. Sci. Technol. 2022, 118, 181–189. [Google Scholar] [CrossRef]
- Xu, Y.; Yan, A.; Zhang, X.; Huang, F.; Li, D.; Zhao, X.; Weng, H.; Zhang, Z. Nb/Se Co-doped BiOI nanomaterials with exposed (110) facets for enhanced visible-light-driven photocatalytic activity. Chem. Commun. 2021, 57, 5774–5777. [Google Scholar] [CrossRef]
- Yang, J.; Su, H.; Wu, Y.; Li, D.; Zhang, D.; Sun, H.; Yin, S. Facile synthesis of kermesinus BiOI with oxygen vacancy for efficient hydrogen generation. Chem. Eng. J. 2021, 420, 127607. [Google Scholar] [CrossRef]
- Huang, J.; Zheng, X.; Liu, Y.; Wang, F.; Li, D.; Liu, H.; Li, R.; Chen, T.; Lv, W.; Liu, G. Integration of oxygen vacancies into BiOI via a facile alkaline earth ion-doping strategy for the enhanced photocatalytic performance toward indometacin remediation. J. Hazard. Mater. 2021, 412, 125147. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Li, Y.; Cheng, Z.; Peng, Y.; Shen, M.; Yang, S.; Zhang, Y. Crystal face-selective Bi4Ti3O12/BiOI heterojunction constructed for enhanced visible ligtht-driven photocatalytic activity. Appl. Surf. Sci. 2021, 552, 149507. [Google Scholar] [CrossRef]
- Huang, L.; Liu, J.; Li, Y.; Yang, L.; Wang, C.; Liu, J.; Li, H.; Huang, L. Enhancement of photocatalytic activity of Z-scheme BiO2-x/BiOI heterojunction through vacancy engineering. Appl. Surf. Sci. 2021, 555, 149665. [Google Scholar] [CrossRef]
- Yuan, Y.; Guo, R.T.; Hong, L.F.; Lin, Z.D.; Ji, X.Y.; Pan, W.G. Fabrication of a dual S-scheme Bi7O9I3/g-C3N4/Bi3O4Cl heterojunction with enhanced visible-light-driven performance for phenol degradation. Chemosphere 2022, 287, 132241. [Google Scholar] [CrossRef] [PubMed]
- Zuarez-Chamba, M.; Tuba-Guamán, D.; Quishpe, M.; Pazmiño, K.; Vizuete, K.; Debut, A.; Cisneros–Pérez, P.A.; Reinoso, C.; Santacruz, C.; Salgado, A. Synthesis of Bi4O5I2 microbars for pollutant degradation through a photocatalytic process. Mater. Lett. 2023, 336, 133888. [Google Scholar] [CrossRef]
- Xie, J.; Yin, S.; Lu, Z.; Hu, J.; Hao, A.; Cao, Y. BixOyIz with oxygen vacancies for boosting the photocatalytic oxidation of bisphenol A and tetracycline. New J. Chem. 2023, 47, 9271–9278. [Google Scholar] [CrossRef]
- Low, J.; Yu, J.; Jaroniec, M.; Wageh, S.; Al-Ghamdi, A.A. Heterojunction Photocatalysts. Adv. Mater. 2017, 29, 1601694. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, M.; Cheng, B.; Shao, Y. Recent advances in g-C3N4-based heterojunction photocatalysts. J. Mater. Sci. Technol. 2020, 56, 1–17. [Google Scholar] [CrossRef]
- Ju, P.; Hao, L.; Zhang, Y.; Sun, J.; Dou, K.; Lu, Z.; Liao, D.; Zhai, X.; Sun, C. In-situ topotactic construction of novel rod-like Bi2S3/Bi5O7I p-n heterojunctions with highly enhanced photocatalytic activities. J. Mater. Sci. Technol. 2023, 135, 126–141. [Google Scholar] [CrossRef]
- Shi, H.; Fan, J.; Zhao, Y.; Hu, X.; Zhang, X.; Tang, Z. Visible light driven CuBi2O4/Bi2MoO6 p-n heterojunction with enhanced photocatalytic inactivation of E. coli and mechanism insight. J. Hazard. Mater. 2020, 381, 121006. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Xu, J.; Chen, M. Synthesis of La2Ti2O7/Bi5O7I photocatalysts with improved photocatalytic activity for degradation of CIP under visible light. Sep. Purif. Technol. 2022, 282, 120004. [Google Scholar] [CrossRef]
- Li, S.; Wang, C.; Cai, M.; Yang, F.; Liu, Y.; Chen, J.; Zhang, P.; Li, X.; Chen, X. Facile fabrication of TaON/Bi2MoO6 core–shell S-scheme heterojunction nanofibers for boosting visible-light catalytic levofloxacin degradation and Cr(VI) reduction. Chem. Eng. J. 2022, 428, 131158. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, H.; Peng, J.; Guo, J.; Wang, B.; Ding, L.; Cao, X.; Chang, Y.; Liu, G. Synthesis of 2D/2D direct Z-scheme WO3/Bi5O7I heterojunctions for decomposition and detoxification of organic compounds under simulated sunlight. Appl. Surf. Sci. 2023, 614, 156050. [Google Scholar] [CrossRef]
- Liu, K.; Wang, L.; Fu, T.; Zhang, H.; Lu, C.; Tong, Z.; Yang, Y.; Peng, Y. Oxygen-functionalized Ti3C2 MXene/exfoliated montmorillonite supported S-scheme BiOBr/Bi2MoO6 heterostructures for efficient photocatalytic quinolone antibiotics degradation. Chem. Eng. J. 2023, 457, 141271. [Google Scholar] [CrossRef]
- Zhao, J.; Guan, X.; Zhang, C.; Xue, J.; Li, R.; Liu, J.; Zhang, X. Citric acid-assisted in-situ synthesis of a distinctive 3D flower-like spherical Bi2MoO6 nano-film and its enhanced CO2 photoreduction activity. Nano-Struct. Nano-Objects 2023, 33, 100939. [Google Scholar] [CrossRef]
- Li, J.; Yin, Y.; Liu, E.; Ma, Y.; Wan, J.; Fan, J.; Hu, X. In situ growing Bi2MoO6 on g-C3N4 nanosheets with enhanced photocatalytic hydrogen evolution and disinfection of bacteria under visible light irradiation. J. Hazard. Mater. 2017, 321, 183–192. [Google Scholar] [CrossRef]
- Sun, P.; Li, K.; Liu, X.; Wang, J.; Qiu, X.; Wei, W.; Zhao, J. Peptide-mediated Aqueous Synthesis of NIR-II Emitting Ag2S Quantum Dots for Rapid Photocatalytic Bacteria Disinfection. Angew. Chem. Int. Ed. Engl. 2023, 62, e202300085. [Google Scholar] [CrossRef]
- Lim, Q.F.; Yap, R.C.C.; Teng, C.P.; Yeo, J.C.C.; Tan, M.Y.; Toh, J.P.W.; Zhu, Q.; Thitsartarn, W.; He, C.; Liu, S.; et al. Electrospray-on-Electrospun Breathable, Biodegradable, and Robust Nanofibrous Membranes with Photocatalytic Bactericidal Activity. ACS Appl. Nano Mater. 2023, 6, 1828–1838. [Google Scholar] [CrossRef]
- Fu, Y.; Tan, M.; Guo, Z.; Hao, D.; Xu, Y.; Du, H.; Zhang, C.; Guo, J.; Li, Q.; Wang, Q. Fabrication of wide-spectra-responsive NA/NH2-MIL-125(Ti) with boosted activity for Cr(VI) reduction and antibacterial effects. Chem. Eng. J. 2023, 452, 139417. [Google Scholar] [CrossRef]
- Shi, H.; Wan, J.; Dong, X.; Xi, J.; Zhang, L.; Wang, W.; Zhang, X.; Shi, Y.; Tang, Z. Ag bridged step-scheme MoS2/Bi4O5Br2 heterojunction for enhanced visible light driven photocatalytic disinfection activity. Appl. Surf. Sci. 2023, 607, 155056. [Google Scholar] [CrossRef]
- Xiao, K.; Wang, T.; Sun, M.; Hanif, A.; Gu, Q.; Tian, B.; Jiang, Z.; Wang, B.; Sun, H.; Shang, J.; et al. Photocatalytic Bacterial Inactivation by a Rape Pollen-MoS2 Biohybrid Catalyst: Synergetic Effects and Inactivation Mechanisms. Environ. Sci. Technol. 2020, 54, 537–549. [Google Scholar] [CrossRef]
- Arasavilli, S.; Taksal, P.A.; Das, B.K.; Chowdhury, S.; Bhattacharya, J. Photocatalysis aided antibacterial properties of a green graphitic carbon-Cu2O-Ag2O composite: Towards an active non-persistent disinfectant. J. Water Process Eng. 2023, 55, 104231. [Google Scholar] [CrossRef]
- He, N.; Cao, S.; Gu, J.; Uddin, A.; Zhang, C.; Yu, Y.; Chen, H.; Jiang, F. Well-designed oxidized Sb/g-C3N4 2D/2D nanosheets heterojunction with enhanced visible-light photocatalytic disinfection activity. J. Colloid Interface Sci. 2022, 606, 1284–1298. [Google Scholar] [CrossRef] [PubMed]
- Zhan, H.; Zhou, Q.; Li, M.; Zhou, R.; Mao, Y.; Wang, P. Photocatalytic O2 activation and reactive oxygen species evolution by surface BN bond for organic pollutants degradation. Appl. Catal. B Environ. 2022, 310, 121329. [Google Scholar] [CrossRef]
- Lin, Z.; Ye, S.; Xu, Y.; Lin, X.; Qin, Z.; Bao, J.; Peng, H. Construction of a novel efficient Z-scheme BiVO4/EAQ heterojunction for the photocatalytic inactivation of antibiotic-resistant pathogens: Performance and mechanism. Chem. Eng. J. 2023, 453, 139747. [Google Scholar] [CrossRef]
- Zhang, Y.; Ma, F.; Ling, M.; Zheng, H.; Wu, Y.; Li, L. In-situ constructed indirect Z-type heterojunction by plasma Bi and BiO2-X–Bi2O2CO3 co-modified with BiOCl@Bi–MOF for enhanced photocatalytic efficiency toward antibiotics. Chem. Eng. J. 2023, 464, 142762. [Google Scholar] [CrossRef]
- Yang, H.; He, D.; Liu, C.; Zhang, T.; Qu, J.; Jin, D.; Zhang, K.; Lv, Y.; Zhang, Z.; Zhang, Y.N. Visible-light-driven photocatalytic disinfection by S-scheme alpha-Fe2O3/g-C3N4 heterojunction: Bactericidal performance and mechanism insight. Chemosphere 2022, 287, 132072. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, Z.; Li, J.; Wang, N.; Guo, W.; Zhang, K. Antibacterial Activity and the Mechanism of the Z-Scheme Bi2MoO6/Bi5O7I Heterojunction under Visible Light. Molecules 2023, 28, 6786. https://doi.org/10.3390/molecules28196786
Ma Z, Li J, Wang N, Guo W, Zhang K. Antibacterial Activity and the Mechanism of the Z-Scheme Bi2MoO6/Bi5O7I Heterojunction under Visible Light. Molecules. 2023; 28(19):6786. https://doi.org/10.3390/molecules28196786
Chicago/Turabian StyleMa, Zhanqiang, Juan Li, Nan Wang, Wei Guo, and Kaiyue Zhang. 2023. "Antibacterial Activity and the Mechanism of the Z-Scheme Bi2MoO6/Bi5O7I Heterojunction under Visible Light" Molecules 28, no. 19: 6786. https://doi.org/10.3390/molecules28196786
APA StyleMa, Z., Li, J., Wang, N., Guo, W., & Zhang, K. (2023). Antibacterial Activity and the Mechanism of the Z-Scheme Bi2MoO6/Bi5O7I Heterojunction under Visible Light. Molecules, 28(19), 6786. https://doi.org/10.3390/molecules28196786







