Sustainable Applications of Nanopropolis to Combat Foodborne Illnesses
Abstract
:1. Introduction
2. Foodborne Illness as a Public Health Challenge
3. Propolis: Chemical Composition, Antioxidant, and Antimicrobial Properties
4. Main Chemical Agents Involved in the Bioactivity of Propolis
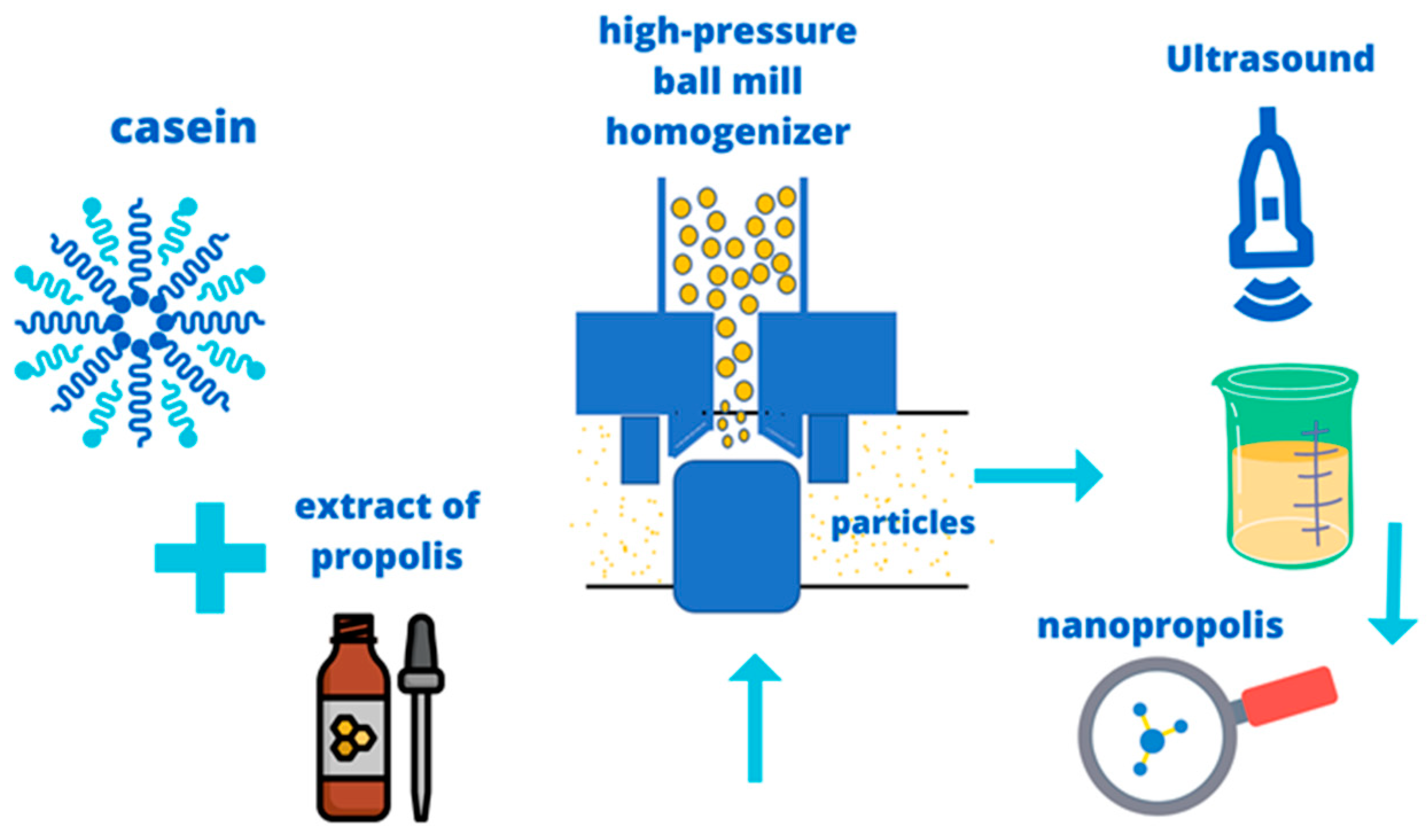
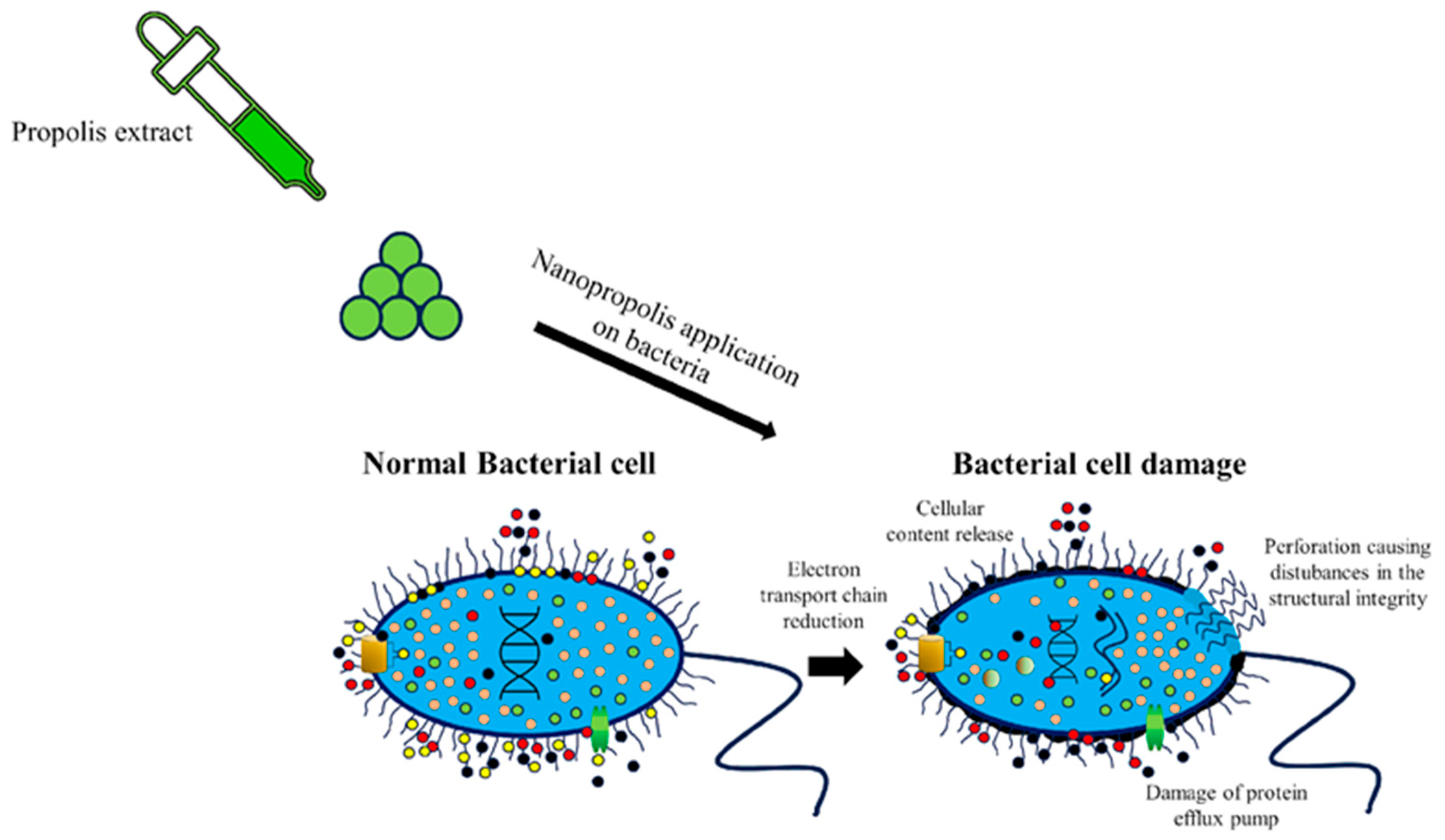
5. Methods of Synthesis of Nanopropolis and Its Antimicrobial Effects
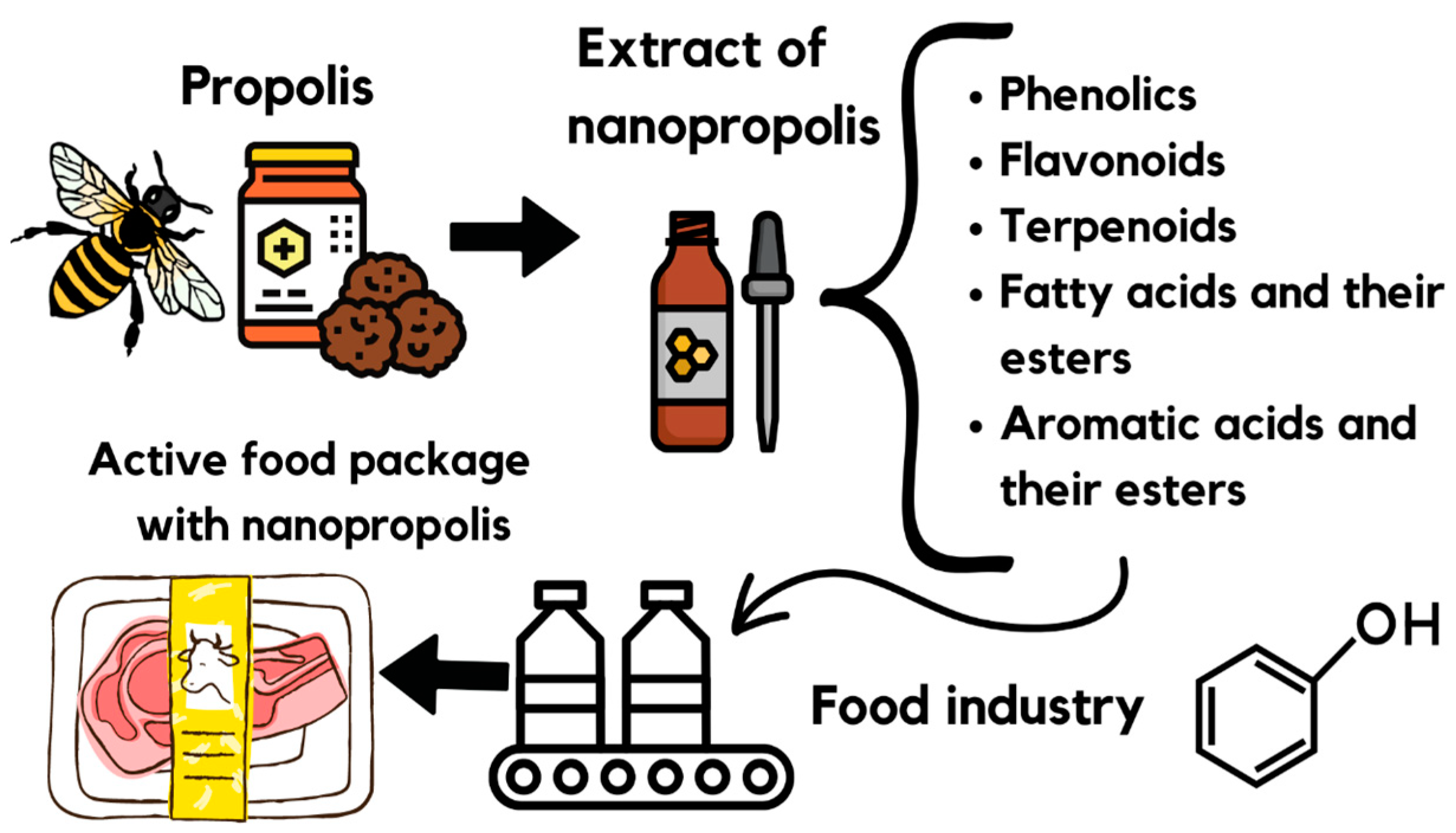
6. Advantages and Disadvantages of Using Nanopropolis in the Food Industry
7. Nano-Delivery Systems (NDSs) in Food
8. Safety in the Use of Nanopropolis in Food
9. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abid, H.; Mohd, J.; Ravi, P.S.; Shanay, R.; Rajiv, S. Applications of nanotechnology in medical field: A brief review. Glob. Health J. 2023, 7, 70–77. [Google Scholar] [CrossRef]
- Kamarudin, S.H.; Rayung, M.; Abu, F.; Ahmad, S.B.; Fadil, F.; Karim, A.A.; Norizan, M.N.; Sarifuddin, N.; Mat Desa, M.S.Z.; Mohd Basri, M.S.; et al. A review on antimicrobial packaging from biodegradable polymer composites. Polymers 2022, 14, 174. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Liao, W.; Xia, W. Recent advances in chitosan-based bioactive materials for food preservation. Food Hydrocoll. 2023, 140, 108612. [Google Scholar] [CrossRef]
- Mei, L.; Ji, Q.; Jin, Z.; Guo, T.; Yu, K.; Ding, W.; Liu, C.; Wu, Y.; Zhang, N. Nano-microencapsulation of tea seed oil via modified complex coacervation with propolis and phosphatidylcholine for improving antioxidant activity. LWT 2022, 163, 113550. [Google Scholar] [CrossRef]
- Sulaeman, A.; Mulyati, A.H. The bee propolis for preventing and healing non-communicable diseases. In Functional Foods and Nutraceuticals in Metabolic and Non-Communicable Diseases; Academic Press: Cambridge, MA, USA, 2022; pp. 465–479. [Google Scholar]
- Tavares, L.; Smaoui, S.; Lima, P.S.; de Oliveira, M.M.; Santos, L. Propolis: Encapsulation and application in the food and pharmaceutical industries. Trends Food Sci. Technol. 2022, 127, 169–180. [Google Scholar] [CrossRef]
- Tatli Seven, P.; Seven, I.; Gul Baykalir, B.; Iflazoglu Mutlu, S.; Salem, A.Z. Nanotechnology and nano-propolis in animal production and health: An overview. Ital. J. Anim. Sci. 2018, 17, 921–930. [Google Scholar] [CrossRef]
- da Silva Martins, L.H.; da Silva, S.B.; Bichara, C.M.G.; de Oliveira, J.R.A.; Santos Filho, A.F.; Alves, R.C.B.; Komesu, A.; Rai, M. Brazilian Medicinal Plant Extracts with Antimicrobial Action Against Microorganisms that Cause Foodborne Diseases. In Eco-Friendly Biobased Products Used in Microbial Diseases; CRC Press: Boca Raton, FL, USA, 2022; pp. 121–137. [Google Scholar]
- USDA. Food Safety and Inspection Service-U.S Departament of Agricultura. 2020. Available online: https://www.fsis.usda.gov/food-safety/foodborne-illness-and-disease#:~:text=Foodborne%20illness%20is%20a%20preventable,year%20in%20the%20United%20States (accessed on 28 April 2023).
- Zulqarnain, M.; Haitao, H.; Wen, L.; Cui, G.; Xia, W. Undercooked Leghorn Chicken is A Source of Campylobacter jejuni, An Infectious Agent for Causing Prosthetic Joint Infection and Diarrhea. J. Infect. Dis. Case Rep. 2022, 164, 2–4. [Google Scholar] [CrossRef]
- Chaidoutis, E.; Keramydas, D.; Papalexis, P.; Migdanis, A.; Migdanis, I.; Lazaris, A.C.; Kavantzas, N. Foodborne botulism: A brief review of cases transmitted by cheese products. Biomed. Rep. 2022, 16, 41. [Google Scholar] [CrossRef]
- Dolan, G.P.; Foster, K.; Lawler, J.; Amar, C.; Swift, C.; Aird, H.; Gorton, R. An epidemiological review of gastrointestinal outbreaks associated with Clostridium perfringens, North East of England, 2012–2014. Epidemiol. Infect. 2016, 144, 1386–1393. [Google Scholar] [CrossRef]
- Costa, D.; Razakandrainibe, R.; Basmaciyan, L.; Raibaut, J.; Delaunay, P.; Morio, F.; Gargala, G.; Villier, V.; Mouhajir, A.; Levy, B.; et al. A summary of cryptosporidiosis outbreaks reported in France and overseas departments, 2017–2020. Food Waterborne Parasitol. 2022, 27, e00160. [Google Scholar] [CrossRef]
- Cowley, L.A.; Dallman, T.J.; Fitzgerald, S.; Irvine, N.; Rooney, P.J.; McAteer, S.P.; Day, M.; Perry, N.T.; Bono, J.L.; Jenkins, C.; et al. Short-term evolution of Shiga toxin-producing Escherichia coli O157:H7 between two food-borne outbreaks. Microb. Genom. 2016, 2, e000084. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, S.; Chen, X.; Qu, C. Review controlling Listeria monocytogenes in ready-to-eat meat and poultry products: An overview of outbreaks, current legislations, challenges, and future prospects. Trends Food Sci. Technol. 2021, 116, 24–35. [Google Scholar] [CrossRef]
- Le, H.H.T.; Dalsgaard, A.; Andersen, P.S.; Nguyen, H.M.; Ta, Y.T.; Nguyen, T.T. Large-scale Staphylococcus aureus foodborne disease poisoning outbreak among primary school children. Microbiol. Res. 2021, 12, 43–52. [Google Scholar] [CrossRef]
- Brumfield, K.D.; Chen, A.J.; Gangwar, M.; Usmani, M.; Hasan, N.A.; Jutla, A.S.; Huq, A.; Colwell, R.R. Environmental Factors Influencing Occurrence of Vibrio parahaemolyticus and Vibrio vulnificus. Appl. Environ. Microbiol. 2023, 89, e00307-23. [Google Scholar] [CrossRef] [PubMed]
- Reddy, M.P.; Sulaiman, Z.I.; Askar, G. Vertebral Discitis Caused by Salmonella enterica serovar Montevideo Infection. IDCases 2023, 13, e01882. [Google Scholar] [CrossRef]
- Malek, M.; Barzilay, E.; Kramer, A.; Camp, B.; Jaykus, L.A.; Escudero-Abarca, B.; Derrick, G.; White, P.; Gerba, C.; Higgins, C.; et al. Outbreak of Norovirus infection among river rafters associated with packaged delicatessen meat, Grand Canyon, 2005. Clin. Infect. Dis. 2009, 48, 31–37. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Active Investigations of Multistate Foodborne Outbreaks | CDC. Available online: https://www.cdc.gov/foodsafety/outbreaks/lists/active-investigations.html (accessed on 28 August 2023).
- Bouaroura, A.; Segueni, N.; Diaz, J.G.; Bensouici, C.; Akkal, S.; Rhouati, S. Preliminary analysis of the chemical composition, antioxidant and anticholinesterase activities of Algerian propolis. Nat. Prod. Res. 2020, 34, 3257–3261. [Google Scholar] [CrossRef]
- Selvaraju, G.D.; Umapathy, V.R.; SumathiJones, C.; Cheema, M.S.; Jayamani, D.R.; Dharani, R.; Sneha, S.; Yamuna, M.; Gayathiri, E.; Yadav, S. Fabrication and characterization of surgical sutures with propolis silver nanoparticles and analysis of its antimicrobial properties. J. King Saud Univ. -Sci. 2022, 34, 102082. [Google Scholar] [CrossRef]
- Irigoiti, Y.; Navarro, A.; Yamul, D.; Libonatti, C.; Tabera, A.; Basualdo, M. The use of propolis as a functional food ingredient: A review. Trends Food Sci. Technol. 2021, 115, 297–306. [Google Scholar] [CrossRef]
- Pobiega, K.; Kraśniewska, K.; Gniewosz, M. Application of propolis in antimicrobial and antioxidative protection of food quality—A review. Trends Food Sci. Technol. 2019, 83, 53–62. [Google Scholar] [CrossRef]
- Almuhayawi, M.S. Propolis as a novel antibacterial agent. Saudi J. Biol. Sci. 2020, 27, 3079–3086. [Google Scholar] [CrossRef] [PubMed]
- Sahu, A.; Nayak, G.; Bhuyan, S.K.; Bhuyan, R.; Kar, D.; Kuanar, A. A comparative study on antioxidant activity of propolis ethanolic extract and oil from different agroclimatic regions of Eastern India. Biocatal. Agric. Biotechnol. 2023, 50, 102685. [Google Scholar]
- Fitria, A.; Hanifah, S.; Chabib, L.; Muhammad Uno, A.; Munawwarah, H.; Atsil, N.; Aditya Pohara, H.; Weuanggi, D.A.; Syukri, Y. Design and characterization of propolis extract loaded self-nano emulsifying drug delivery system as immunostimulant. Saudi Pharm. J. 2021, 29, 625–634. [Google Scholar] [CrossRef] [PubMed]
- Morissette, M.; Litim, N.; Di Paolo, T. Natural phytoestrogens: A class of promising neuroprotective agents for Parkinson disease. In Discovery and Development of Neuroprotective Agents from Natural Products; Elsevier: Amsterdam, The Netherlands, 2018; pp. 9–61. [Google Scholar]
- Chen, X.; Wan, W.; Ran, Q.; Ye, T.; Sun, Y.; Liu, Z.; Liu, X.; Shi, S.; Qu, C.; Zhang, C.; et al. Pinocembrin mediates antiarrhythmic effects in rats with isoproterenol-induced cardiac remodeling. Eur. J. Pharmacol. 2022, 920, 174799. [Google Scholar] [CrossRef]
- Mani, R.; Natesan, V. Chrysin: Sources, beneficial pharmacological activities, and molecular mechanism of action. Phytochemistry 2018, 145, 187–196. [Google Scholar] [CrossRef]
- Salehi, B.; Venditti, A.; Sharifi-Rad, M.; Kręgiel, D.; Sharifi-Rad, J.; Durazzo, A.; Lucarini, M.; Santini, A.; Souto, E.B.; Novellino, E.; et al. The therapeutic potential of apigenin. Int. J. Mol. Sci. 2019, 20, 1305. [Google Scholar] [CrossRef]
- Pagnan, A.L.; Pessoa, A.S.; Tokuhara, C.K.; Fakhoury, V.S.; Oliveira, G.S.N.; Sanches, M.L.R.; Inacio, K.K.; Ximenes, V.F.; Oliveira, R.C. Anti-tumour potential and selectivity of caffeic acid phenethyl ester in osteosarcoma cells. Tissue Cell 2022, 74, 101705. [Google Scholar] [CrossRef]
- Iannuzzi, C.; Liccardo, M.; Sirangelo, I. Overview of the Role of Vanillin in Neurodegenerative Diseases and Neuropathophysiological Conditions. Int. J. Mol. Sci. 2023, 24, 1817. [Google Scholar] [CrossRef]
- Ogut, E.; Armagan, K.; Gül, Z. The role of syringic acid as a neuroprotective agent for neurodegenerative disorders and future expectations. Metab. Brain Dis. 2022, 37, 859–880. [Google Scholar] [CrossRef]
- Widelski, J.; Okińczyc, P.; Suśniak, K.; Malm, A.; Bozhadze, A.; Jokhadze, M.; Korona-Głowniak, I. Correlation between chemical profile of Georgian propolis extracts and their activity against Helicobacter pylori. Molecules 2023, 28, 1374. [Google Scholar] [CrossRef]
- Feng, L.S.; Cheng, J.B.; Su, W.Q.; Li, H.Z.; Xiao, T.; Chen, D.A.; Zhang, Z.L. Cinnamic acid hybrids as anticancer agents: A mini-review. Arch. Der Pharm. 2022, 355, 2200052. [Google Scholar] [CrossRef] [PubMed]
- Muvhulawa, N.; Dludla, P.V.; Ziqubu, K.; Mthembu, S.X.; Mthiyane, F.; Nkambule, B.B.; Mazibuko-Mbeje, S.E. Rutin ameliorates inflammation and improves metabolic function: A comprehensive analysis of scientific literature. Pharmacol. Res. 2022, 178, 106163. [Google Scholar] [CrossRef] [PubMed]
- Shahinozzaman, M.; Basak, B.; Emran, R.; Rozario, P.; Obanda, D.N. Artepillin C: A comprehensive review of its chemistry, bioavailability, and pharmacological properties. Fitoterapia 2020, 147, 104775. [Google Scholar] [CrossRef] [PubMed]
- Kakkar, S.; Bais, S. A review on protocatechuic acid and its pharmacological potential. Int. Sch. Res. Not. 2014, 2014, 952943. [Google Scholar] [CrossRef]
- Naveed, M.; Hejazi, V.; Abbas, M.; Kamboh, A.A.; Khan, G.J.; Shumzaid, M.; Ahmad, F.; Babazadeh, D.; Xia, F.; Modarresi-Ghazani, F.; et al. Chlorogenic acid (CGA): A pharmacological review and call for further research. Biomed. Pharmacother. 2018, 97, 67–74. [Google Scholar] [CrossRef]
- Gong, G.; Guan, Y.Y.; Zhang, Z.L.; Rahman, K.; Wang, S.J.; Zhou, S.; Luan, X.; Zhang, H. Isorhamnetin: A review of pharmacological effects. Biomed. Pharmacother. 2020, 128, 110301. [Google Scholar] [CrossRef]
- Ramalingam, M.; Kim, H.; Lee, Y.; Lee, Y.I. Phytochemical and pharmacological role of liquiritigenin and isoliquiritigenin from radix glycyrrhizae in human health and disease models. Front. Aging Neurosci. 2018, 10, 348. [Google Scholar] [CrossRef]
- Tay, K.C.; Tan, L.T.H.; Chan, C.K.; Hong, S.L.; Chan, K.G.; Yap, W.H.; Pusparajah, P.; Lee, L.H.; Goh, B.H. Formononetin: A review of its anticancer potentials and mechanisms. Front. Pharmacol. 2019, 10, 820. [Google Scholar] [CrossRef] [PubMed]
- Eddouks, M.; Bidi, A.; Bouhali, B.E.; Zeggwagh, N.A. Insulin resistance as a target of some plant-derived phytocompounds. Stud. Nat. Prod. Chem. 2014, 43, 351–373. [Google Scholar]
- Barsola, B.; Kumari, P. Green synthesis of nano-propolis and nanoparticles (Se and Ag) from ethanolic extract of propolis, their biochemical characterization: A review. Green Process. Synth. 2022, 11, 659–673. [Google Scholar] [CrossRef]
- Pinheiro Machado, G.T.; Veleirinho, M.B.; Mazzarino, L.; Machado Filho, L.C.P.; Maraschin, M.; Cerri, R.L.A.; Kuhnen, S. Development of propolis nanoparticles for the treatment of bovine mastitis: In vitro studies on antimicrobial and cytotoxic activities. Can. J. Anim. Sci. 2019, 99, 713–723. [Google Scholar] [CrossRef]
- Zaleh, A.A.; Salehi-Vaziri, A.; Pourhajibagher, M.; Bahador, A. The synergistic effect of nano-propolis and curcumin-based photodynamic therapy on remineralization of white spot lesions: An ex vivo study. Photodiagnosis Photodyn. Ther. 2022, 38, 102789. [Google Scholar] [CrossRef] [PubMed]
- Afrouzan, H.; Amirinia, C.; Mirhadi, S.A.; Ebadollahi, A.; Vaseji, N.; Tahmasbi, G. Evaluation of antimicrobial activity of propolis and nanopropolis against Staphylococcus aureus and Candida albicans. Afr. J. Microbiol. Res. 2012, 6, 421–425. [Google Scholar]
- Hamdi, D.; Wijanarko, A.; Hermansyah, H.; Asih, S.C.; Sahlan, M. Production of nanopropolis using high pressure ball mill homogenizer. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2019; Volume 217, p. 012014. [Google Scholar]
- Prasetyo, R. Potensi nanopropolis lebah madu trigona spp asal pandeglang sebagai antibakteri. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2019. [Google Scholar]
- Seibert, J.B.; Bautista-Silva, J.P.; Amparo, T.R.; Petit, A.; Pervier, P.; dos Santos Almeida, J.C.; Azevedo, M.C.; Silveira, B.M.; Brandão, G.C.; de Souza, G.H.B.; et al. Development of propolis nanoemulsion with antioxidant and antimicrobial activity for use as a potential natural preservative. Food Chem. 2019, 287, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Hasan, A.E.Z.; Ambarsari, L.; Widjaja, W.K.; Prasetyo, R. Potency of nanopropolis stingless bee Trigona spp Indonesia as antibacterial agent. IOSR J. Pharm. 2014, 4, 01–09. [Google Scholar]
- Sabir, A. The inflammatory response on rat dental pulp following ethanolic extract of propolis (EEP) application. Maj Ked Gigi (Dent J) 2005, 38, 77–83. [Google Scholar] [CrossRef]
- Júnior, L.M.; Jamróz, E.; de Ávila Gonçalves, S.; da Silva, R.G.; Alves, R.M.V.; Vieira, R.P. Preparation and characterization of sodium alginate films with propolis extract and nano-SiO2. Food Hydrocoll. Health 2022, 2, 100094. [Google Scholar] [CrossRef]
- Shahabi, N.; Soleimani, S.; Ghorbani, M. Investigating functional properties of halloysite nanotubes and propolis used in reinforced composite film based on soy protein/basil seed gum for food packaging application. Int. J. Biol. Macromol. 2023, 231, 123350. [Google Scholar] [CrossRef]
- Soleimanifard, M.; Feizy, J.; Maestrelli, F. Nanoencapsulation of propolis extract by sodium caseinate-maltodextrin complexes. Food Bioprod. Process. 2021, 128, 177–185. [Google Scholar] [CrossRef]
- Madani, Z.; Sales, M.; Moghadamnia, A.A.; Kazemi, S.; Asgharpour, F. Propolis nanoparticle enhances antimicrobial efficacy against Enterococcus faecalis biofilms. S. Afr. J. Bot. 2022, 150, 1220–1226. [Google Scholar] [CrossRef]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; Rodriguez-Torres, M.D.P.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef] [PubMed]
- Luykx, D.M.; Peters, R.J.; van Ruth, S.M.; Bouwmeester, H. A review of analytical methods for the identification and characterization of nano delivery systems in food. J. Agric. Food Chem. 2008, 56, 8231–8247. [Google Scholar] [CrossRef] [PubMed]
- Augustin, M.A. The role of microencapsulation in the development of functional dairy foods. Aust. J. Dairy Technol. 2003, 58, 156. [Google Scholar]
- Chayed, S.; Winnik, F.M. In vitro evaluation of the mucoadhesive properties of polysaccharide-based nanoparticulate oral drug delivery systems. Eur. J. Pharm. Biopharm. 2007, 65, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Taylor, T.M.; Weiss, J.; Davidson, P.M.; Bruce, B.D. Liposomal nanocapsules in food science and agriculture. Crit. Rev. Food Sci. Nutr. 2005, 45, 587–605. [Google Scholar] [CrossRef]
- Onyeaka, H.; Passaretti, P.; Miri, T.; Al-Sharify, Z.T. The safety of nanomaterials in food production and packaging. Curr. Res. Food Sci. 2022, 5, 763–774. [Google Scholar] [CrossRef]
- Burdock, G.A. Review of the biological properties and toxicity of bee propolis (propolis). Food Chem. Toxicol. 1998, 36, 347–363. [Google Scholar] [CrossRef]
| Type of Microorganism | Type of Food | Symptoms | Current Outbreaks | Reference |
|---|---|---|---|---|
| Campylobacter jejuni | Shellfish, milk, chicken, and meat | Gastrointestinal disorders, diarrheal illness, and prosthetic joint infection (rare) | China | Zulqarnain et al. 2022 [10] |
| Clostridium botulinum | Cheese products, canned vegetables, canned meats | Neurotoxins, disease (neurological symptoms) | Chaidoutis et al. 2022 [11] | |
| Clostridium perfringens | Animals’ and humans’ intestinal tract, food such as raw meats, dehydrated soups, and sauces, raw vegetables, and spices | Diarrheal illness, necrotizing enteritis | England | Dolan et al. 2016 [12] |
| Cryptosporidium | Water, fruit, vegetable, and shellfish contamination, calves and cheese curds | Acute diarrhea, vomiting, nausea, and abdominal pain | France | Costa et al. 2022 [13] |
| Escherichia coli O157:H7 | Cattle and other ruminants and contaminated food or water | Severe bloody diarrhea and hemolytic uremic syndrome | Middle East/North Africa | Cowley et al. 2016 [14] |
| Listeria monocytogenes | Ready-to-eat meat and poultry products | Mild gastroenteritis and invasive listeriosis | USA, European Union, Australia, New Zealand | Zhang et al. 2021 [15] |
| Staphylococcus aureus | Dairy products (milk, cheese, and cream), meat, and fish | Sudden vomiting, diarrhea, nausea, malaise, abdominal cramps, pain, and prostration | Vietnam | Le et al. 2021 [16] |
| Vibrio spp. Vibrio parahaemolyticus and Vibrio vulnificus | Water and filter-fed shellfish, especially oysters | Cholera, severe extraintestinal infections, including necrotizing fasciitis and septicemia, and deaths | Japan and USA | Brumfield et al. 2023 [17] |
| Salmonella enterica serovars | Meat, poultry, fruits, and vegetables | Gastroenteritis, bacteremia, extraintestinal infection, infectious osteomyelitis, and discitis | USA | Reddy, Sulaiman and Askar 2023 [18] |
| Norovirus | Packaged delicatessen meat | Gastroenteritis, diarrhea or vomiting | USA | Malek et al. 2009 [19] |
| Phenolic Compound | Type | Chemical Structure | Biological Properties | Reference |
|---|---|---|---|---|
| Pinocembrin or 5,7-dihydroxyflavanone | Flavonoid/flavanone | 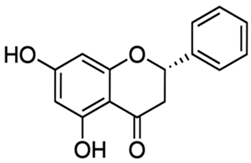 | Antioxidant, anti- inflammatory, and antibacterial activities | Chen et al. 2022 [29] |
| Chrysin or 5,7-dihydroxyflavone | Flavonoid/flavone | 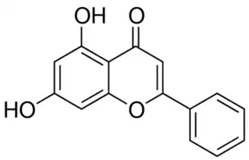 | Antioxidant, anti-inflammatory, anticancer, and antiviral activities | Mani and Natesan 2018 [30] |
| Apigenin or 4′,5,7-trihydroxyflavone | Flavonoid/flavone | 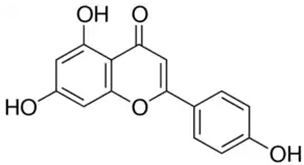 | Anti-inflammatory and antioxidant functions | Salehi et al. 2019 [31] |
| Caffeic acid phenethyl ester in (CA) | Phenolic acid/hydroxycinnamic acid | 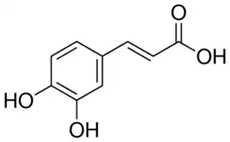 | Antioxidant and anticancer activities | Pagnan et al. 2022 [32] |
| Vanillin or 4-hydroxy-3-methoxybenzaldehyde or vanillic aldehyde | Phenolic acid/phenolic aldehyde | 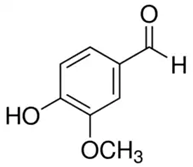 | Neuroprotective, antioxidant, and anti-inflammatory activities | Iannuzzi et al. 2023 [33] |
| Syringic acid (SA) or 3,5-Dimethoxy-4-hydroxybenzoic acid | Phenolic acid/benzoic acid derivative phenolic | 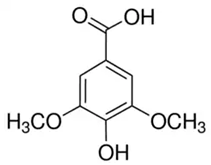 | Antioxidant, anti-inflammatory, and neuroprotective activities | Ogut et al. 2022 [34] |
| Pinobanksin or (2R,3R)-3,5,7-Trihydroxy-2-phenyl-2,3-dihydro-4H-chromen-4-one | Flavonoid/flavanone | 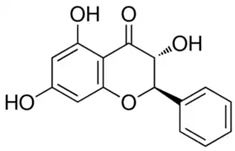 | Antimicrobial, antioxidant, and anti-inflammatory activities | Widelski et al. 2023 [35] |
| Cinnamic acid or (Z)-cinnamate or 3-phenyl-acrylate | Phenolic acid hydroxycinnamic acid | 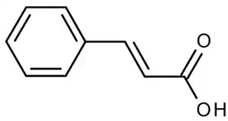 | Anticancer, antioxidant, and anti-inflammatory activities | Feng et al. 2022 [36] |
| Rutin or 3,3′,4′,5,7-Pentahydroxyflavone 3-rutinoside | Flavonoid/flavonol glycoside | 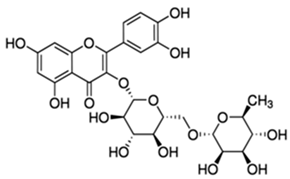 | Antioxidant and anti-inflammatory activities | Muvhulawa et al. 2022 [37] |
| Artepillin C (ARC) or (2E)-3-[4-Hydroxy-3,5-bis(3-methyl-2-buten-1-yl)phenyl]-2-propenoic acid | Phenolic acid/prenylated derivative of p-coumaric acid | 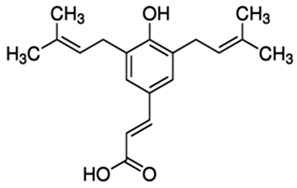 | Antioxidant, antimicrobial, anti-inflammatory, antidiabetic, neuroprotective, and gastroprotective activities | Shahinozzam et al. 2020 [38] |
| Protocatechuic acid or 3,4-Dihydroxybenzoic acid or protocatechuic acid | Phenolic acid/hydroxybenzoic acid | 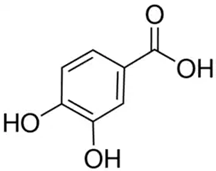 | Antioxidant activity, antibacterial activity, anticancer activity, antiulcer activity, antidiabetic activity, antiaging activity, antifibrotic activity, antiviral activity, anti-inflammatory activity, analgesic activity, antiatherosclerotic activity, cardiac activity, and hepatoprotective activity | Kakkar and Bais 2014 [39] |
| Chlorogenic acid (CGA) or 1,4,5-Trihydroxycyclohexanecarboxylic acid 3-(3,4-dihydroxycinnamate), 3-(3,4-Dihydroxycinnamoyl) quinic acid | Phenolic acid/hydroxycinnamic acid |  | Antioxidant, antibacterial, hepatoprotective, cardioprotective, anti-inflammatory, antipyretic, neuroprotective, antiobesity, antiviral, antimicrobial, and antihypertension activities; free radical scavenger; and a central nervous system stimulator | Naveed et al. 2018 [40] |
| Isorhamnetin or 3′-Methoxy-3,4′,5,7-tetrahydroxyflavone | Flavonoid/flavanone | 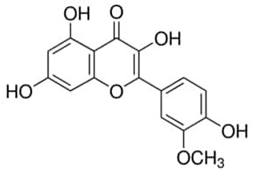 | Cardiovascular and cerebrovascular protection; antitumor, anti-inflammatory, and antioxidation activities; organ protection; and prevention of obesity | Gong et al. 2020 [41] |
| Liquiritigenin or 7,4′-Dihydroxyflavanone | Flavonoid/flavanone | 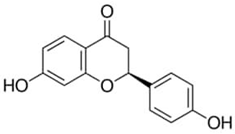 | Antidiabetic, anticancer, hepatoprotective, antibacterial, and anti-inflammatory activities; radical scavenging; neuroprotection against stroke; and memory-enhancing activities | Ramalingam et al. 2018 [42] |
| Formononetin or 7-Hydroxy-3-(4-methoxyphenyl)-4H-1-benzopyran-4-one | Flavonoid/O-methylated isoflavone | 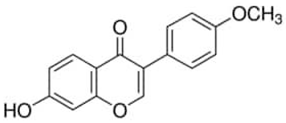 | Anticancer, antibacterial, anti-inflammatory, and antioxidant activities | Tay et al. 2019 [43] |
| Biochanin A or 4′-Methylgenistein, 5,7-Dihydroxy-4′-methoxyisoflavone | Flavonoid/O-methylated isoflavone |  | Anticancer, antibacterial, anti-inflammatory, antioxidant, and neuroprotective activities | Morissette et al. 2018 [28] |
| Myricetin or 3,3′,4′,5,5′,7-Hexahydroxyflavone | Flavonoid/flavonol | 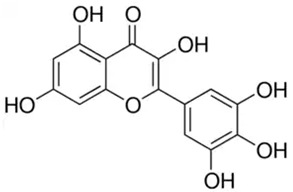 | Antidiabetic, anticancer, hepatoprotective, antibacterial, and anti-inflammatory activities | Eddouks et al. 2014 [44] |
| Obtaining the Extract | Method for Obtaining Nanopropolis | Nanoencapsulation Agent | Antimicrobial Action | Reference |
|---|---|---|---|---|
| Propolis with ethanol extract | Using the high-speed and high-pressure homogenization method, after homogenization, this material was evaporated to remove solvents, thus obtaining the propolis or nanopropolis nanoparticles. | - | not determined in this research | Tatli et al. 2018 [7] |
| - | A high-pressure ball mill homogenizer was used to produce nanosized particles, which were then subjected to sonication and separated using an ultrafiltration system, synthesizing the nanoparticles. | Casein micelles | not determined in this research | Barsola and Kumari 2022 [45] |
| Propolis with ethanol extract | Agitation via magnetic stirring and heating at 60 °C until complete solubilization were carried out to create nanopropolis suspensions; this study aimed at creating nanoparticles while altering the quantities of propolis extract to optimize the preparation conditions. | Soy lecithin | not determined in this research | Pinheiro Machado et al. 2019 [46] |
| Propolis with ethanol extract | The extracting solution was subjected to magnetic stirring for 7 days at room temperature; then, it was filtered with Wattman 4 paper to separate the impurities. For, to this solution, water in a 1:10 ratio was added for the purification of propolis. The suspension was submitted to an ultrasonic bath for 30 min until obtaining colloidal nanoprolis, with a pH adjusted to neutral. In the final step, the authors converted the colloidal nanopropolis into powder through the lyophilization process, thus obtaining nanopropolis particles. | Oil and surfactants | Not determined in this research | Zaleh et al. 2022 [47] |
| Propolis with ethanol extract | Obtained via the milling media method | Not mentioned | Staphylococcus aureus and Candida albicans | Afrouzan et al. 2012 [48] |
| Propolis with ethanol extract | High-pressure ball mill homogenizer | Casein micelle | Escherichia coli, Bacillus subtilis, and Staphylococcus aureus | Hamdi et al. 2019 [49] |
| Propolis with ethanol extract | High-speed homogenization technique and solvent evaporation | Casein micelle | B. subtilis, for S. aureus and E. coli | Prasetyo 2019 [50] |
| Material | Functionality | Advantages | Reference |
|---|---|---|---|
| Protein-Based Systems | Food proteins are frequently employed in manufactured foods since they are GRAS and have a high nutritional value. The most practical and often-utilized matrix in food applications is protein hydrogel. It is possible to create novel protein vehicles with increased delivery capabilities by shrinking the matrix size from micrometers to nanometers. | Protein-based NDSs can be made in two ways that are both relatively simple to prepare: the “top-down” approach, where structures are created by disassembling bulk materials, and the “bottom up” approach, where structures are created from molecules that are capable of self-assembly. Because they may form complexes with polysaccharides, lipids, or other biopolymers and a range of nutrients can be added, protein-based NDSs are particularly intriguing. | Augustin 2003 [60] |
| Polysaccharide- and Poly(lactic) Acid-Based Systems | Glycosidic linkages hold the monosaccharides (carbohydrates) in polymers called polysaccharides. These naturally occurring polymers, which can be found in pectin, guar gum, and insulin from plants, chitosan and chondroitin sulfate from animals, alginates from algae, and dextran from microbes, are often quite big and frequently branched. | To provide controlled release carriers for medications and proteins, homo- and copolymers made of poly (lactic acid) and poly (glycolic acid) are widely used because of their biodegradability and biocompatibility. These aliphatic polyester polymers are broken down by bulk ester bond hydrolysis. | Chayed and Winnik 2007 [61] |
| Lipid-Based Systems | These systems provide a few benefits over conventional encapsulation techniques, including the potential for industrial-scale production using natural components, targetability, and the capacity to entrap substances with various solubilities. | The structure of liposomes can include additional molecules like proteins or polymers in addition to one or more lipid and/or phospholipid bilayers. The ability of nanoliposomes to concurrently absorb and release two compounds with different solubilities is a key benefit. This indicates that these systems can tolerate both lipid-soluble and water-soluble molecules. | Taylor et al. 2005 [62] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bezerra, F.W.F.; Silva, J.d.M.e.; Fontanari, G.G.; Oliveira, J.A.R.d.; Rai, M.; Chisté, R.C.; Martins, L.H.d.S. Sustainable Applications of Nanopropolis to Combat Foodborne Illnesses. Molecules 2023, 28, 6785. https://doi.org/10.3390/molecules28196785
Bezerra FWF, Silva JdMe, Fontanari GG, Oliveira JARd, Rai M, Chisté RC, Martins LHdS. Sustainable Applications of Nanopropolis to Combat Foodborne Illnesses. Molecules. 2023; 28(19):6785. https://doi.org/10.3390/molecules28196785
Chicago/Turabian StyleBezerra, Fernanda Wariss Figueiredo, Jonilson de Melo e Silva, Gustavo Guadagnucci Fontanari, Johnatt Allan Rocha de Oliveira, Mahendra Rai, Renan Campos Chisté, and Luiza Helena da Silva Martins. 2023. "Sustainable Applications of Nanopropolis to Combat Foodborne Illnesses" Molecules 28, no. 19: 6785. https://doi.org/10.3390/molecules28196785
APA StyleBezerra, F. W. F., Silva, J. d. M. e., Fontanari, G. G., Oliveira, J. A. R. d., Rai, M., Chisté, R. C., & Martins, L. H. d. S. (2023). Sustainable Applications of Nanopropolis to Combat Foodborne Illnesses. Molecules, 28(19), 6785. https://doi.org/10.3390/molecules28196785








