Dissolution Behavior of Fluoroalkylated Diazonaphthoquinone and Its Blends with Fluorinated Copolymers under UV Irradiation
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis of Materials
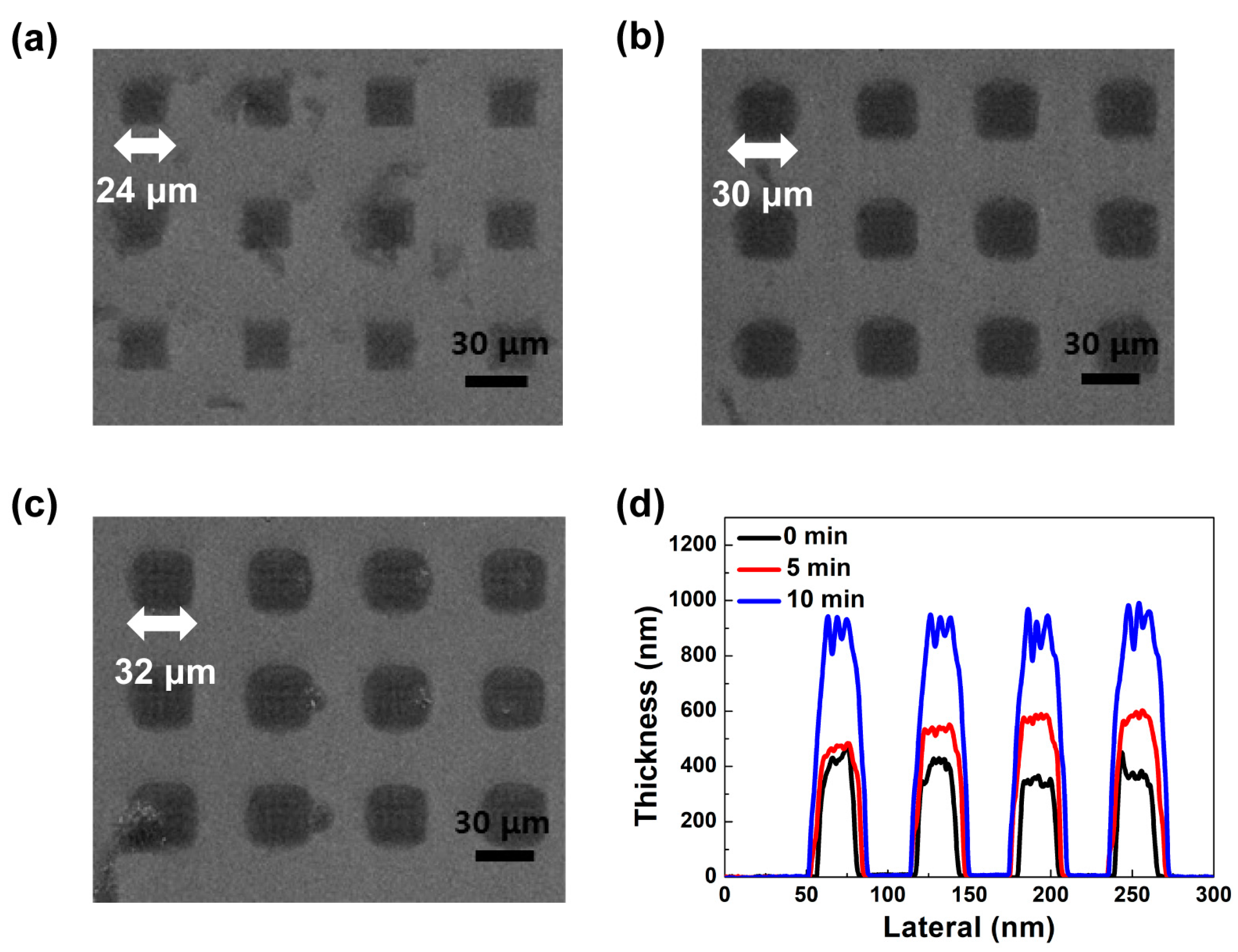
2.2. Photolithographic Evaluation
2.3. Mechanism Study
2.3.1. FT-IR Spectra
2.3.2. Influence of Moisture in Films
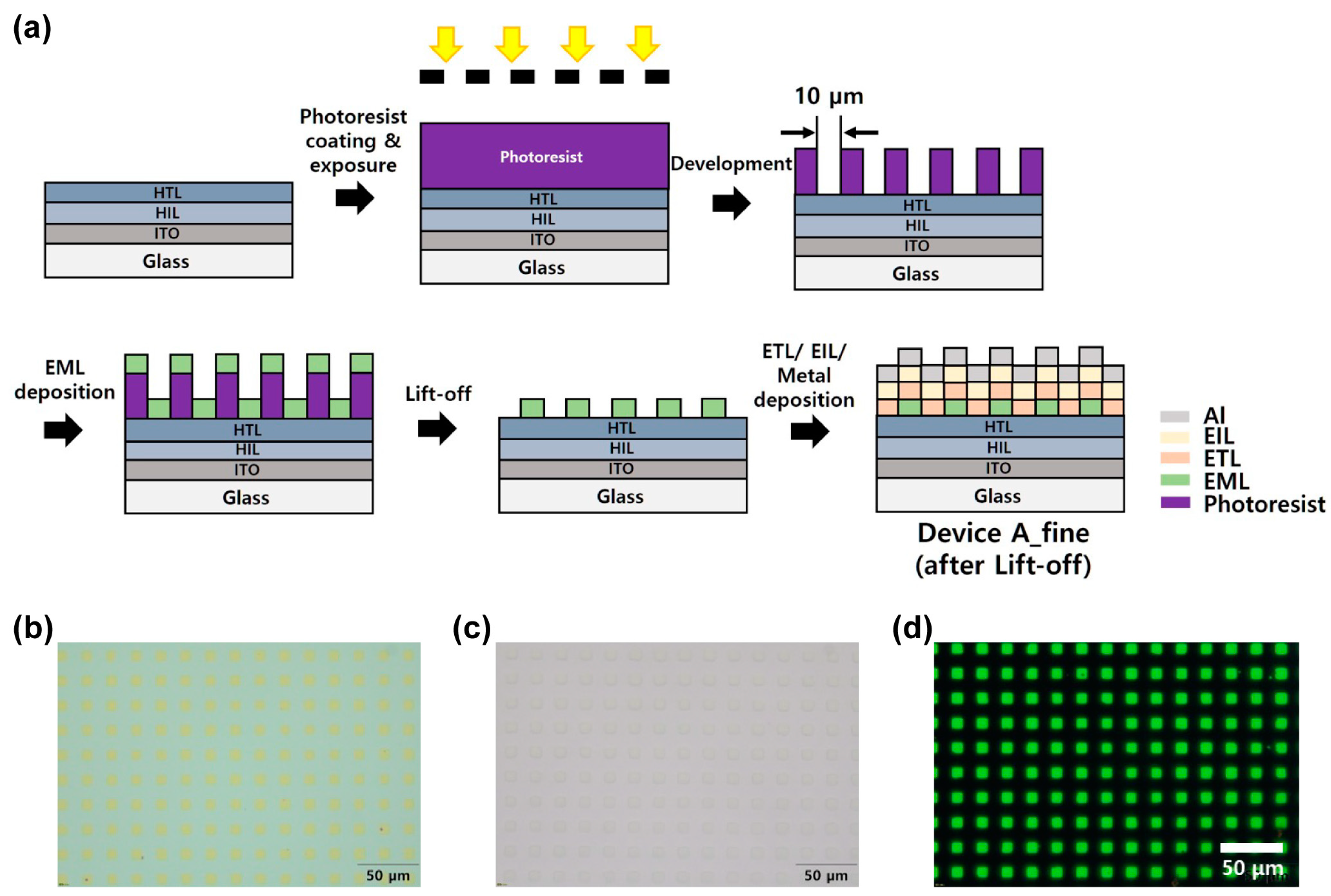
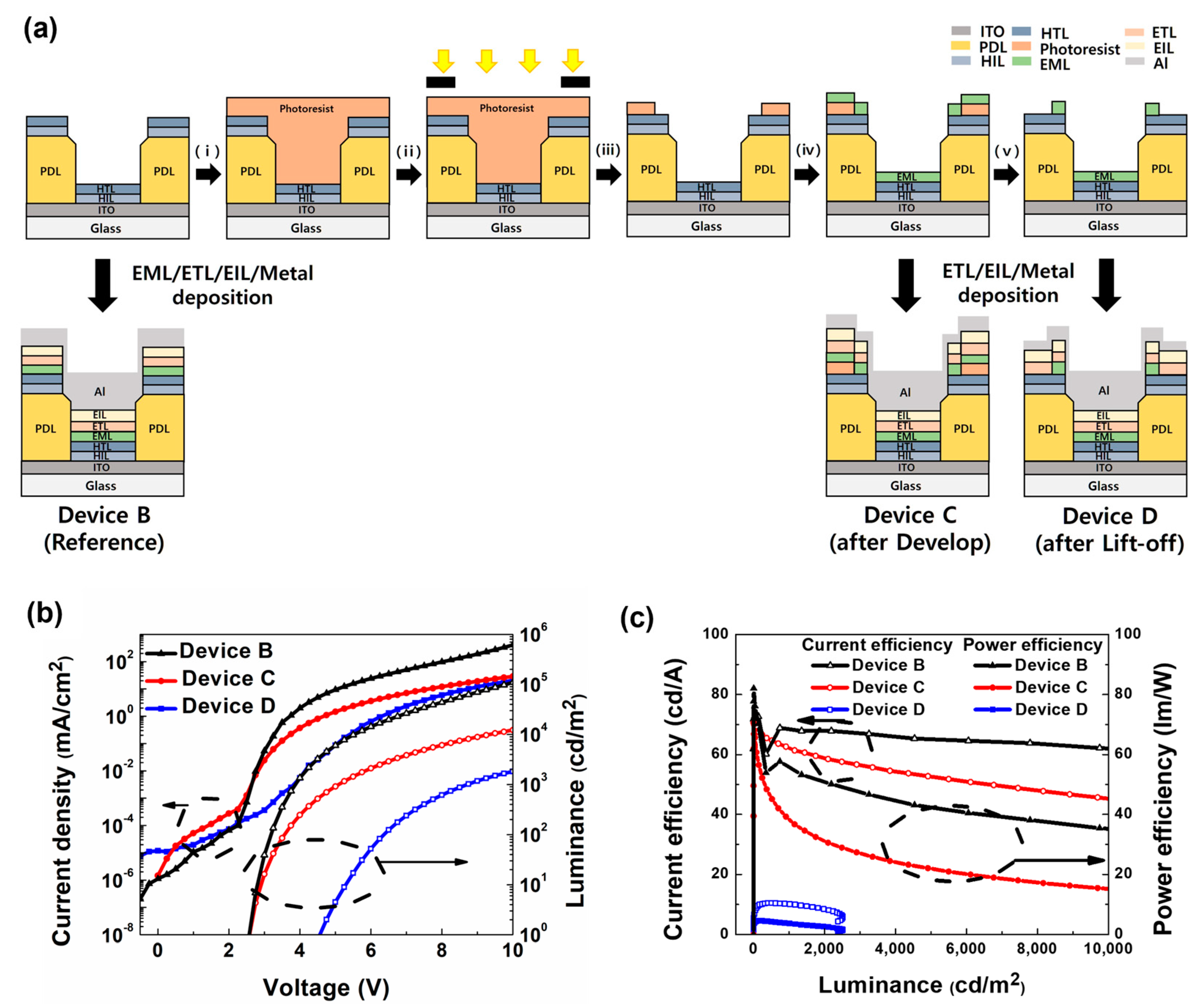
2.4. Fabrication and Performance Evaluation of OLED Pixels
3. Experimental Section
3.1. Materials
3.2. Characterization
3.3. Synthesis of Materials
3.3.1. Synthesis of Fluorinated DNQ Derivative (RF2D1)
Monoacetylphloroglucinol (1)
Difluoroalkylated Phenol (2)
Difluoroalkylated Diazonaphthoquinone (RF2D1, 3)
3.3.2. Synthesis of Copolymer
Raft Copolymer of FOMA and IBMA (PFI-X)
3.4. Lithographic Evaluation
3.4.1. RF2D1 Film
3.4.2. Mixture Films of RF2D1 + PFI–X (X = 0.25, 0.5, 0.75)
3.5. Fabrication of Micropatterned OLED Pixels
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Boher, P.; Leroux, T.; Collomb-Patton, V.; Bignon, T. Optical characterization of OLED displays. J. Soc. Inf. Disp. 2015, 23, 429–437. [Google Scholar] [CrossRef]
- Geffroy, B.; Le Roy, P.; Prat, C. Organic light-emitting diode (OLED) technology: Materials, devices and display technologies. Polym. Int. 2006, 55, 572–582. [Google Scholar] [CrossRef]
- Yang, H.I.; Naveen, K.R.; Cho, S.M.; Kim, J.Y.; Jung, Y.H.; Kwon, J.H. Systematic investigation on polymer layer selection for flexible thin film encapsulation. Org. Electron. 2023, 115, 106761. [Google Scholar] [CrossRef]
- Lee, H.S.; Jang, S.; Noh, J.; Jeon, H.; Choi, B.-S.; Jeon, Y.M.; Song, K.; Song, J.; Chu, H.Y.; Kim, S. In 28-4L: Late-News Paper: An Ultra High Density 1.96” UHD 2250ppi Display. SID Symp. Dig. Tech. Pap. 2017, 48, 403–405. [Google Scholar] [CrossRef]
- Ghosh, A.; Donoghue, E.P.; Khayrullin, I.; Ali, T.; Wacyk, L.; Tice, K.; Vazan, F.; Prache, O.; Wang, Q.; Sziklas, L. 18-1: Invited Paper: Ultra-High-Brightness 2K x 2K Full-Color OLED Microdisplay Using Direct Patterning of OLED Emitters. SID Symp. Dig. Tech. Pap. 2017, 48, 226–229. [Google Scholar] [CrossRef]
- Lih, J.J.; Chao, C.I.; Lee, C.C. Novel pixel design for high-resolution AMOLED displays with a shadow mask. J. Soc. Inf. Disp. 2007, 15, 3–7. [Google Scholar] [CrossRef]
- Kwon, J.H. RGB color patterning for AMOLED TVs. Inf. Disp. 2013, 29, 12–15. [Google Scholar] [CrossRef]
- Shin, H.Y.; Suh, M.C. A study on full color organic light emitting diodes with blue common layer under the patterned emission layer. Org. Electron. 2014, 15, 2932–2941. [Google Scholar] [CrossRef]
- Haskal, E.; Buechel, M.; Dijksman, J.; Duineveld, P.; Meulenkamp, E.; Mutsaers, C.; Sempel, A.; Snijder, P.; Vulto, S.; Van de Weijer, P. 21.1: Ink Jet Printing of Passive-Matrix Polymer Light Emitting Displays. SID Symp. Dig. Tech. Pap. 2002, 33, 776–779. [Google Scholar] [CrossRef]
- Hu, Z.; Yin, Y.; Ali, M.U.; Peng, W.; Zhang, S.; Li, D.; Zou, T.; Li, Y.; Jiao, S.; Chen, S.-J. Inkjet printed uniform quantum dots as color conversion layers for full-color OLED displays. Nanoscale 2020, 12, 2103–2110. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.-g.; Koo, Y.; Ha, J.; Lee, C. 41-2: Invited Paper: Recent Developments in Inkjet-printed OLEDs for High Resolution, Large Area Applications. SID Symp. Dig. Tech. Pap. 2020, 51, 591–594. [Google Scholar] [CrossRef]
- Lee, S.T.; Chin, B.D.; Kim, M.H.; Kang, T.M.; Song, M.W.; Lee, J.H.; Kim, H.D.; Chung, H.K.; Wolk, M.B.; Bellmann, E. 29.3: A Novel Patterning Method for Full-Color Organic Light-Emitting Devices: Laser Induced Thermal Imaging (LITI). SID Symp. Dig. Tech. Pap. 2004, 35, 1008–1011. [Google Scholar] [CrossRef]
- Suh, M.C.; Chin, B.D.; Kim, M.H.; Kang, T.M.; Lee, S.T. Enhanced luminance of blue light-emitting polymers by blending with hole-transporting materials. Adv. Mater. 2003, 15, 1254–1258. [Google Scholar] [CrossRef]
- Takakuwa, A.; Misaki, M.; Yoshida, Y.; Yase, K. Micropatterning of emitting layers by microcontact printing and application to organic light-emitting diodes. Thin Solid. Film. 2009, 518, 555–558. [Google Scholar] [CrossRef]
- Li, J.; Xu, L.; Tang, C.W.; Shestopalov, A.A. High-resolution organic light-emitting diodes patterned via contact printing. ACS Appl. Mater. Interfaces 2016, 8, 16809–16815. [Google Scholar] [CrossRef]
- Gather, M.C.; Koehnen, A.; Falcou, A.; Becker, H.; Meerholz, K. Solution-processed full-color polymer organic light-emitting diode displays fabricated by direct photolithography. Adv. Funct. Mater. 2007, 17, 191–200. [Google Scholar] [CrossRef]
- Balocco, C.; Majewski, L.; Song, A. Non-destructive patterning of conducting-polymer devices using subtractive photolithography. Org. Electron. 2006, 7, 500–507. [Google Scholar] [CrossRef]
- Huang, J.; Xia, R.; Kim, Y.; Wang, X.; Dane, J.; Hofmann, O.; Mosley, A.; De Mello, A.; De Mello, J.; Bradley, D. Patterning of organic devices by interlayer lithography. J. Mater. Chem. 2007, 17, 1043–1049. [Google Scholar] [CrossRef]
- Jang, W.; Lee, M.; Kweon, H.; Park, H.W.; Yang, J.; Kim, S.; Jo, H.; Lee, C.; Cho, J.H.; Kwak, K. Tetrabranched photo-crosslinker enables micrometer-scale patterning of light-emitting super yellow for high-resolution OLEDs. ACS Photonics 2021, 8, 2519–2528. [Google Scholar] [CrossRef]
- Yamamoto, H.; Kozawa, T.; Tagawa, S. Study on dissolution behavior of polymer-bound and polymer-blended photo acid generator (PAG) resists by using quartz crystal microbalance (QCM) method. Microelectron. Eng. 2014, 129, 65–69. [Google Scholar] [CrossRef]
- Zakhidov, A.A.; Lee, J.K.; Fong, H.H.; DeFranco, J.A.; Chatzichristidi, M.; Taylor, P.G.; Ober, C.K.; Malliaras, G.G. Hydrofluoroethers as orthogonal solvents for the chemical processing of organic electronic materials. Adv. Mater. 2008, 20, 3481–3484. [Google Scholar] [CrossRef]
- Choi, Y.M.; Shin, H.Y.; Son, J.; Park, C.; Park, K.-W.; Lee, J.-K.; Jung, B.J. Two-color pixel patterning for high-resolution organic light-emitting displays using photolithography. Micromachines 2020, 11, 650. [Google Scholar] [CrossRef] [PubMed]
- Krotkus, S.; Ventsch, F.; Kasemann, D.; Zakhidov, A.A.; Hofmann, S.; Leo, K.; Gather, M.C. Photo-patterning of highly efficient state-of-the-art phosphorescent OLEDs using orthogonal hydrofluoroethers. Adv. Opt. Mater. 2014, 2, 1043–1048. [Google Scholar] [CrossRef]
- Son, J.; Oh, H.-T.; Kwon, O.J.; Lim, J.-M.; Jung, H.; Jung, B.J.; Hwang, D.-H.; Lee, C.; Lee, J.-K.; Yoon, J.G. Highly soluble fluorous alkyl ether-tagged imaging materials for the photo-patterning of organic light-emitting devices. J. Mater. Chem. C 2017, 5, 926–930. [Google Scholar] [CrossRef]
- Son, J.; Shin, H.Y.; Choi, Y.M.; Chae, S.G.; Park, C.; Jung, B.J.; Lee, J.-K. Descumming fluorous solution for photolithographic patterning of organic light-emitting diodes. Microelectron. Eng. 2020, 227, 111324. [Google Scholar] [CrossRef]
- Son, J.; Roh, H.; Shin, H.Y.; Park, K.-W.; Park, C.; Park, H.; Lee, C.; Kwak, J.; Jung, B.J.; Lee, J.-K. Photo-cleavable perfluoroalkylated copolymers for tailoring quantum dot thin films. Polym. Chem. 2020, 11, 6624–6631. [Google Scholar] [CrossRef]
- Park, K.-W.; Choi, S.; Choi, Y.M.; Kim, G.; Ku, Y.; Son, J.; Lee, J.-K.; Jung, B.J.; Kim, M. Photoinduced Solubility Modulation in the Copolymers of Fluoroalkyl, Spiropyranyl, and Isobornyl Methacrylates. ACS Appl. Polym. Mater. 2023, 5, 3447–3456. [Google Scholar] [CrossRef]
- Reiser, A.; Huang, J.; He, X.; Yeh, T.; Jha, S.; Shih, H.; Kim, M.; Han, Y.; Yan, K. The molecular mechanism of novolak–diazonaphthoquinone resists. Eur. Polym. J. 2002, 38, 619–629. [Google Scholar] [CrossRef]
- Nakayama, T.; Ueda, M. A new positive-type photoresist based on mono-substituted hydroquinone calix [8] arene and diazonaphthoquinone. J. Mater. Chem. 1999, 9, 697–702. [Google Scholar] [CrossRef]
- Andraos, J.; Chiang, Y.; Huang, C.; Kresge, A.; Scaiano, J. Flash photolytic generation and study of ketene and carboxylic acid enol intermediates formed by the photolysis of diazonaphthoquinones in aqueous solution. J. Am. Chem. Soc. 1993, 115, 10605–10610. [Google Scholar] [CrossRef]
- Vleggaar, J.; Huizer, A.; Kraakman, P.; Nijssen, W.; Visser, R.; Varma, C. Photoinduced Wolff-rearrangement of 2-diazo-1-naphthoquinones: Evidence for the participation of a carbene intermediate. J. Am. Chem. Soc. 1994, 116, 11754–11763. [Google Scholar] [CrossRef]
- Suwa, M.; Kamogawa, M.; Fujii, M.; Nishiyama, T.; Kozuka, H. Intermolecular interactions between sol–gel-derived random-structure oligomeric silsesquioxanes and a diazonaphthoquinone derivative. Jpn. J. Appl. Phys. 2021, 60, 051002. [Google Scholar] [CrossRef]
- Wallraff, G.; Hinsberg, W.D. Lithographic imaging techniques for the formation of nanoscopic features. Chem. Rev. 1999, 99, 1801–1822. [Google Scholar] [CrossRef] [PubMed]
- Furuta, A.; Hanabata, M. Novolak structures suitable for high resolution positive photoresists and application. J. Photopolym. Sci. Technol. 1989, 2, 383–390. [Google Scholar] [CrossRef]
- Li, Q.; Cao, Z.; Wang, G. Diazonaphthoquinone-based amphiphilic polymer assemblies for NIR/UV light-and pH-responsive controlled release. Polym. Chem. 2018, 9, 463–471. [Google Scholar] [CrossRef]
- Zacharovas, S.J.; Bakanas, R.; Adlienė, D.; Šeperys, R.; Narmontas, P.; Jankauskaitė, V. Holograms recording with pulsed laser on diazonaphthoquinone-novolac-based photoresists and their nanocomposites. Opt. Eng. 2014, 53, 097101. [Google Scholar] [CrossRef]
- Bratton, D.; Ayothi, R.; Deng, H.; Cao, H.B.; Ober, C.K. Diazonaphthoquinone molecular glass photoresists: Patterning without chemical amplification. Chem. Mater. 2007, 19, 3780–3786. [Google Scholar] [CrossRef]
- Wolpert, D.; Schade, M.; Brixner, T. Femtosecond midinfrared study of the photoinduced Wolff rearrangement of diazonaphthoquinone. J. Chem. Phys. 2008, 129, 094504. [Google Scholar] [CrossRef]
- Omote, T.; Mochizuki, H.; Koseki, K.I.; Yamaoka, T. Fluorine-containing photoreactive polyimide. 7. Photochemical reaction of pendant 1, 2-naphthoquinone diazide moieties in novel photoreactive polyimides. Macromolecules 1990, 23, 4796–4802. [Google Scholar] [CrossRef]
- Alvey, L.J.; Meier, R.; Soós, T.; Bernatis, P.; Gladysz, J.A. Syntheses and carbonyliridium complexes of unsymmetrically substituted fluorous trialkylphosphanes: Precision tuning of electronic properties, including insulation of the perfluoroalkyl groups. Eur. J. Inorg. Chem. 2000, 2000, 1975–1983. [Google Scholar] [CrossRef]
- Moad, G.; Chong, Y.; Postma, A.; Rizzardo, E.; Thang, S.H. Advances in RAFT polymerization: The synthesis of polymers with defined end-groups. Polymer 2005, 46, 8458–8468. [Google Scholar] [CrossRef]
- Forrest, S.R.; Bradley, D.D.; Thompson, M.E. Measuring the efficiency of organic light-emitting devices. Adv. Mater. 2003, 15, 1043–1048. [Google Scholar] [CrossRef]
- Baker, M.B.; Ferreira, R.B.; Tasseroul, J.; Lampkins, A.J.; Al Abbas, A.; Abboud, K.A.; Castellano, R.K. Selective and sequential aminolysis of benzotrifuranone: Synergism of electronic effects and ring strain gradient. J. Org. Chem. 2016, 81, 9279–9288. [Google Scholar] [CrossRef] [PubMed]




| PFI-X | FOMA:IBMA | Mnb (g/mol) | PDI b | Tgc (°C) | |
|---|---|---|---|---|---|
| Feed Ratio | Composition a | ||||
| PFI-0.25 | 1:0.25 | 1:0.15 | 8200 | 1.16 | 27 |
| PFI-0.5 | 1:0.5 | 1:0.38 | 8100 | 1.26 | 33 |
| PFI-0.75 | 1:0.75 | 1:0.67 | 8100 | 1.29 | 42 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, G.; Kang, S.-E.; Kim, D.H.; Won, J.-I.; Ku, Y.; Son, J.; Lee, J.-K.; Jung, B.J. Dissolution Behavior of Fluoroalkylated Diazonaphthoquinone and Its Blends with Fluorinated Copolymers under UV Irradiation. Molecules 2023, 28, 6784. https://doi.org/10.3390/molecules28196784
Kim G, Kang S-E, Kim DH, Won J-I, Ku Y, Son J, Lee J-K, Jung BJ. Dissolution Behavior of Fluoroalkylated Diazonaphthoquinone and Its Blends with Fluorinated Copolymers under UV Irradiation. Molecules. 2023; 28(19):6784. https://doi.org/10.3390/molecules28196784
Chicago/Turabian StyleKim, Gayoung, Sae-Eun Kang, Doo Hong Kim, Jong-In Won, Yejin Ku, Jongchan Son, Jin-Kyun Lee, and Byung Jun Jung. 2023. "Dissolution Behavior of Fluoroalkylated Diazonaphthoquinone and Its Blends with Fluorinated Copolymers under UV Irradiation" Molecules 28, no. 19: 6784. https://doi.org/10.3390/molecules28196784
APA StyleKim, G., Kang, S.-E., Kim, D. H., Won, J.-I., Ku, Y., Son, J., Lee, J.-K., & Jung, B. J. (2023). Dissolution Behavior of Fluoroalkylated Diazonaphthoquinone and Its Blends with Fluorinated Copolymers under UV Irradiation. Molecules, 28(19), 6784. https://doi.org/10.3390/molecules28196784







