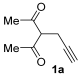
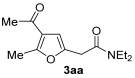
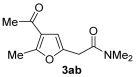
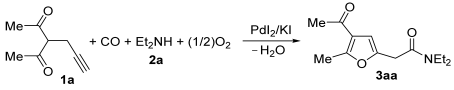Abstract
2-Propargyl-1,3-dicarbonyl compounds have been carbonylated under oxidative conditions and with the catalysis of the PdI2/KI catalytic system to selectively afford previously unreported 2-(4-acylfuran-2-yl)acetamides in fair to good yields (54–81%) over 19 examples. The process takes place under relatively mild conditions and occurs via a mechanistic pathway involving Csp-H activation by oxidative monoamincarbonylation of the terminal triple bond of the substrates with formation of 2-ynamide intermediates, followed by 5-exo-dig O-cyclization (via intramolecular conjugate addition of the in situ formed enolate to the 2-ynamide moiety) and aromative isomerization.
1. Introduction
Functionalized furans are a very important class of heterocyclic derivatives [1], known to possess important biological activities (see, for example, references [2,3]) and being useful precursors for further transformations (for a review, see reference [4]).
Among the synthetic methods available to prepare multisubstituted furans (for recent reviews, see references [5,6,7]), transition metal-catalyzed cyclization (TMCC) of suitable acyclic precursors is particularly attractive (for recent reviews, see references [8,9]; for a book, see reference [10]; for a recent example, see reference [11]). By this approach, the final compound with the desired substitution pattern can be obtained in one synthetic step starting from readily available substrates.
On the other hand, carbon monoxide is a very important C-1 building block in organic synthesis (for a recent book on carbonylation chemistry in organic synthesis, see reference [12]). In fact, CO can be installed in a large variety of organic substrates under different conditions, including transition-metal catalysis, to give high value–added carbonyl compounds (carbonylation reactions), including carbonylated heterocycles (for selected book chapters and reviews on metal-catalyzed carbonylation reactions, also leading to carbonylated heterocycles, see references [12,13,14,15,16,17,18,19,20,21,22,23]). Accordingly, the combination between TMCC and catalytic carbonylation with the appropriate acyclic precursor may represent an excellent entry to the direct synthesis of carbonyl-functionalized furan derivatives [12].
In particular, the catalytic system based on PdI2 in conjunction with an excess of KI, developed by our research group [24,25] (for a recent review, see reference [26], has proved very valuable for the realization of several important carbonylative cyclization processes, particularly under oxidative conditions, with the one-step formation of carbonylated heterocyclic derivatives. Among the different routes that the PdI2/KI catalytic system is able to promote, the Csp-H activation by oxidative monoalkoxycarbonylation of the terminal triple bond, disclosed by our group in 2001 for the catalytic synthesis of 2-ynamides from simple alkyl- or arylacetylenes (Scheme 1a) [27,28], is particularly significant. In fact, this reactivity has been successfully employed for the synthesis of a plethora of heterocyclic derivatives, when applied to terminal alkyne substrates bearing a nucleophilic group in suitable position for the occurrence of an intramolecular conjugate addition to the initially formed 2-ynamide intermediate (Scheme 1b) (for a review, see reference [26]; for a very recent example, see reference [29]).
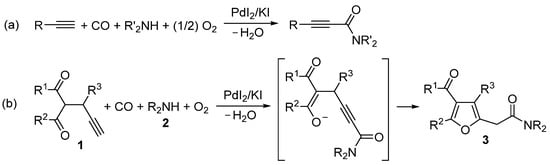
Scheme 1.
PdI2/KI-catalyzed oxidative monoaminocarbonylation of: (a) Simple alkyl- and arylacetylenes to give 2-ynamides [27,28] and (b) This work: 2-propargyl-1,3-dicarbonyl compounds 1 with amines 2 to 2-(4-acylfuran-2-yl)acetamides 3.
In this work, we report a new application of this concept, which allows the direct, multicomponent synthesis of previously unreported 2-(4-acylfuran-2-yl)acetamides 3 starting from readily available 2-propargyl-1,3-dicarbonyl compounds 1, carbon monoxide, and a secondary amine 2. In this case, the initially formed 2-ynamide intermediate I (formed by 2-promoted Csp-H palladation of 1 followed by CO insertion and nucleophilic displacement by 2) undergoes 5-exo-dig intramolecular conjugate addition from the oxygen of enolate moiety resulting from C-2 deprotonation by the basic amine, to give intermediate II, whose aromative isomerization eventually leads to 2-(4-acylfuran-2-yl)acetamides 3 (Scheme 2). It is worth noting that, as seen in the reaction of simple alkyl- and arylacetylenes (Scheme 1a) [27,28], the method is not applicable to the use of primary amines, as these compounds preferentially undergo oxidative carbonylation to ureas under our reaction conditions [30,31]. Moreover, as already seen for simple 1-alkynes [27,28], secondary amines of relatively low nucleophilicity (such as alkylarylamines of diarylamines) are not sufficiently reactive to give the reaction.
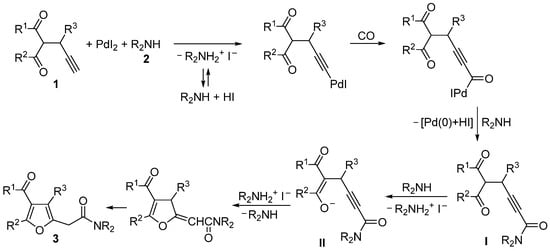
Scheme 2.
Mechanistic route leading to 2-(4-acylfuran-2-yl)acetamides 3 by PdI2/KI-catalyzed oxidative aminocarbonylation of 2-propargyl-1,3-dicarbonyl compounds 1 with secondary amines 2 through the formation of 2-ynamide intermediates I and II.
This approach, therefore, allows the direct synthesis of a new subclass of furan derivatives (2-(4-acylfuran-2-yl)acetamides 3) by the catalytic assembly of very simple building blocks (2-propargyl-1,3-dicarbonyl compounds 1, amines 2, CO, and O2), with formation of water as benign coproduct.
2. Results and Discussion
We started our investigation using 3-(prop-2-yn-1-yl)pentane-2,4-dione 1a as model substrate. The initial reaction was carried out in MeCN (0.10 mmol of 1a per mL of MeCN) at 100 °C in the presence of PdI2 (1 mol%), KI (KI:PdI2 molar ratio = 100) and Et2NH 2a (3 equiv) under 20 atm (at 25 °C) of a 4:1 mixture CO-air. After 15 h, 1a conversion was complete, and, after column chromatography purification, 2-(4-acetyl-5-methylfuran-2-yl)-N,N-diethylacetamide 3aa was recovered in 61% yield (based on starting 1a), in perfect agreement with our work hypothesis (Table 1, entry 1).

Table 1.
PdI2-catalyzed oxidative aminocarbonylation of 3-(prop-2-yn-1-yl)pentane-2,4-dione 1a under different conditions a.
After a brief optimization study, in which we varied some reaction parameters (such as solvent, amount of KI and amine, substrate concentration, and total pressure; Table 1), a 72% isolated yield of 3aa was achieved under conditions similar to those of the first experiment, but with a higher substrate concentration (0.22 mmol of 1a per mL of MeCN) and using 4 equiv of Et2NH 2a (Table 2, entry 1). The reaction could also be performed with a lower catalyst loading (0.33 mol% PdI2, maintaining the KI:PdI2 molar ratio = 100), with an acceptable 55% isolated yield of 3aa (Table 2, entry 2).

Table 2.
Synthesis of 2-(4-acylfuran-2-yl)acetamides 3 by PdI2/KI-catalyzed oxidative aminocarbonylation of 2-propargyl-1,3-dicarbonyl compounds 1 with secondary amines 2 a.
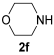
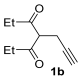
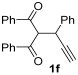
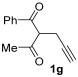
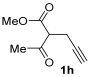
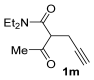
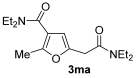
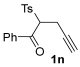
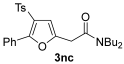
The reaction was then extended to other secondary amines 2b–f (Table 2, entries 3–11) and different 2-propynyl-1,3-diketones 1b–g (Table 2, entries 12–18). Similar results with respect to the parent reactions (Table 2, entries 1 and 2) were observed with other simple dialkylamines, such as Me2NH 2b (Table 2, entries 3 and 4), Bu2NH 2c (Table 2, entries 5 and 6), and even with sterically hindered N-ethylcyclohexylamine 2d (Table 2, entries 7 and 8). Interestingly, more sterically demanding diisopropylamine 2e was also reactive, and led to the formation of the corresponding furanacetamide 3ae in 54% yield with 1 mol% catalyst (Table 2, entry 9). A cyclic amine like morpholine 2f reacted well and delivered the corresponding product 3af in either 74% yield (with 1 mol% PdI2; Table 2, entry 10) or 61% yield (with 0.33 mol% catalyst; Table 2, entry 11). With Et2NH 2a or morpholine 2f as the amine, extension of the protocol to other symmetrical 2-propargyl-1,3-diketones 1b–e also led to good results, the corresponding furans being obtained in 66–81% yields with 1 mol% catalyst (Table 2, entries 12, 14, 16, 17) and 58–68% yields with 0.33 mol% catalyst (Table 2, entries 13, 15, 18). Only in the presence of a phenyl group α to the triple bond, as in 1,3-diphenyl-2-(1-phenylprop-2-yn-1-yl)propane-1,3-dione 1f, a lower product yield was observed, probably for steric reasons (54%, Table 2, entry 19). Clearly, the use of an unsymmetrical diketonic substrate, such as 1-phenyl-2-(prop-2-yn-1-yl)butane-1,3-dione 1g, afforded a mixture of isomeric products 3gf and 3gf′, which could be separated in 48% and 25% isolated yields, respectively (Table 2, entry 20). On the other hand, a selective process was observed with β-keto esters 1h–l, owing to the higher nucleophilicity of the ketonic oxygen with respect to the estereal one [32], with selective formation of alkyl 5-(2-(dialkylamino)-2-oxoethyl)-2-alkylfuran-3-carboxylates (Table 2, entries 21–30). The method also worked nicely with 2-acetyl-N,N-diethylpent-4-ynamide 1m and 1-phenyl-2-tosylpent-4-yn-1-one 1n, with formation of 5-(2-(diethylamino)-2-oxoethyl)-N,N-diethyl-2-methylfuran-3-carboxamide 3ma and N,N-dibutyl-2-(5-phenyl-4-tosylfuran-2-yl)acetamide 3nc, respectively, in 57–72% yields (Table 2, entries 31–33).
3. Materials and Methods
3.1. General Experimental Methods
Melting points were measured with a Leitz Laborlux 12 POL polarizing optical microscope (Leitz Italia GmbH/Srl, Lana, BZ, Italy) and are uncorrected. 1H NMR and 13C NMR spectra were recorded at 25 °C in CDCl3 at 500 MHz and 125 MHz, respectively, with Me4Si as internal standard using Bruker DPX Avance 300 and Bruker DPX Avance 500 NMR Spectrometers (Bruker Italia s.r.l., Milano, Italy); chemical shifts (δ) and coupling constants (J) are given in ppm and in Hz, respectively. IR spectra were taken with a JASCO FT-IR 4200 spectrometer (Jasco Europe s.r.l., Cremella, Lecco, Italy). All reactions were analyzed by TLC on silica gel 60 F254 and by GC-MS analysis using a Shimadzu QP-2010 GC–MS apparatus (Shimadzu Italia s.r.l., Milano, Italy) at 70 eV ionization voltage equipped with a 95% methyl polysiloxane-5% phenyl polysiloxane capillary column (30 m × 0.25 mm, 0.25 μm). Column chromatography was performed on silica gel 60 (Merck, 70–230 mesh; Merck Life Science s.r.l., Milano, Italy). Evaporation refers to the removal of solvent under reduced pressure. The HRMS spectra were taken on an Agilent 1260 Infinity UHD accurate-mass Q-TOF-MS mass spectrometer (Agilent Technologies, Santa Clara, CA, USA), equipped with an electrospray ion source (ESI) operated in dual ion mode. A total of 10 μL of the sample solutions (CH3OH) were introduced by continuous infusion at a flow rate of 200 L min−1 with the aid of a syringe pump. Experimental conditions were performed as follows: capillary voltage, 4000 V; nebulizer pressure, 20 psi; flow rate of drying gas, 10 L/min; temperature of sheath gas, 325 °C; flow rate of sheath gas, 10 L/min; skimmer voltage, 60 V; OCT1 RF Vpp, 750 V; fragmentor voltage, 170 V. The spectra data were recorded in the m/z range of 100–1000 Da in a centroid pattern of full-scan MS analysis mode. The MS/MS data of the selected compounds were obtained by regulating diverse collision energy (18–45 eV).
3.2. Preparation of Substrates
Substrates 1 were prepared and characterized as described in Supplementary Materials. All other materials were commercially available and were used without further purification.
3.3. General Procedure for the Synthesis of 2-(4-Acylfuran-2-yl)acetamides 3
A 35 mL stainless steel autoclave was charged in the presence of air with PdI2 (1.2 mg, 3.3 × 10−3 mmol, or 3.6 mg, 1.0 × 10−2 mmol; see Table 1), KI (55 mg, 0.33 mmol or 166 mg, 1.0 mmol), a solution of 1 [1.0 mmol; 1a, 138 mg; 1b, 166 mg; 1c, 150 mg; 1d, 152 mg; 1e, 262 mg; 1f, 338 mg; 1g, 200 mg; 1h, 154 mg; 1i, 168 mg; 1j, 196 mg; 1k, 196 mg; 1l, 230 mg; 1m, 195 mg; 1n, 312 mg] in anhydrous CH3CN (5.0 mL), and the amine 2 [4.0 mmol; 2a, 292 mg; 2b, 180 mg (2 mL of a 2 M solution in THF); 2c, 516 mg; 2d, 508 mg; 2e, 404 mg; 2f, 348 mg]. The autoclave was sealed and, while the mixture was stirred, the autoclave was pressurized with CO (16 atm) and air (up to 20 atm). After being stirred at 100 °C for 15 h, the autoclave was cooled, degassed, and opened. After evaporation of the solvent, products 3 were purified by column chromatography on silica gel using as eluent hexane-AcOEt from 8:2 to 6:4.
3.3.1. 2-(4-Acetyl-5-methylfuran-2-yl)-N,N-diethylacetamide (3aa)
Yield: 170 mg, starting from 138 mg of 1a (72%) (Table 1, entry 1). Yellow oil. IR (film): ν = 1667 (s), 1651 (s), 1566 (m), 1435 (m), 1404 (w), 1234 (m), 1134 (w), 949 (m), 795 (w) cm−1; 1H NMR (500 MHz, CDCl3): δ = 6.45 (s, 1 H), 3.67 (s, 2 H), 3.44–3.35 (m, 4 H), 2.55 (s, 3 H), 2.37 (s, 3 H), 1.20 (t, J = 7.1, 3 H), 1.15 (t, J = 7.1, 3 H); 13C NMR (125 MHz, CDCl3): δ = 194.2, 167.3, 157.7, 147.2, 122.2, 108.3, 42.5, 40.5, 33.2, 29.1, 14.34, 14.31, 12.9; GC-MS (EI): m/z = 237 (M+, 10), 137 (9), 100 (100), 72 (71); HRMS (ESI—TOF) m/z: [M + Na]+ Calcd. for C13H19NNaO3+ 260.1257; Found 260.1256.
3.3.2. 2-(4-Acetyl-5-methylfuran-2-yl)-N,N-dimethylacetamide (3ab)
Yield: 140 mg, starting from 138 mg of 1a (67%) (Table 1, entry 3). Yellow oil, IR (film): ν = 1686 (s), 1651 (s), 1557 (m), 1508 (w), 1396 (m), 1234 (m), 1138 (m), 1061 (w), 945 (m) cm−1; 1H NMR (500 MHz, CDCl3): δ = 6.44 (s, 1 H), 3.69 (s, 2 H), 3.10 (s, 3 H), 2.99 (m, 3 H), 2.55 (s, 3 H), 2.37 (s, 3 H); 13C NMR (125 MHz, CDCl3): δ = 194.2, 168.3, 157.8, 146.9, 122.3, 108.4, 37.7, 35.7, 33.4, 29.1, 14.3; GC-MS (EI): m/z = 209 (M+, 17), 137 (22), 72 (100); HRMS (ESI-TOF) m/z: [M + Na]+ Calcd. for C11H15NNaO3+ 232.0944; Found 232.0948.
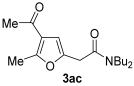
3.3.3. 2-(4-Acetyl-5-methylfuran-2-yl)-N,N-dibutylacetamide (3ac)
Yield: 219 mg, starting from 138 mg of 1a (75%) (Table 1, entry 5). Yellow oil. IR (film): ν = 1678 (s), 1643 (s), 1570 (m), 1454 (m), 1431 (m), 1373 (w), 1223 (m), 1138 (w), 1114 (w), 945 (m) cm−1; 1H NMR (500 MHz, CDCl3): δ = 6.44 (s, 1 H), 3.67 (s, 2 H), 3.37–3.32 (m, 2 H), 3.31–3.26 (m, 2 H), 2.55 (s, 3 H), 2.37 (s, 3 H), 1.61–1.49 (m, 4 H), 1.40–1.27 (m, 4 H), 0.96 (t, J = 7.3, 3 H), 0.92 (t, J = 7.3, 3 H); 13C NMR (125 MHz, CDCl3): δ = 194.2, 167.7, 157.7, 147.3, 122.3, 108.3, 48.3, 46.1, 33.3, 33.2, 31.3, 29.8, 29.1, 20.3, 20.1, 14.4, 13.9; GC-MS (EI): m/z = 293 (M+, 4), 156 (38), 137 (11), 100 (27), 57 (100); HRMS (ESI-TOF) m/z: [M + Na]+ Calcd. for C17H27NNaO3+ 316.1883; Found 316.1898.
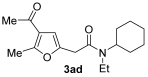
3.3.4. 2-(4-Acetyl-5-methylfuran-2-yl)-N-cyclohexyl-N-ethylacetamide (Mixture of Rotamers A + B, Deriving from Hindered Rotation around the Amide Bond: A/B ca 1.2 by 1H NMR) (3ad)
Yield: 215 mg, starting from 138 mg of 1a (74%) (Table 1, entry 7). Yellow oil. IR (film): ν = 1674 (m), 1643 (s), 1570 (w), 1427 (m), 1369 (w), 1234 (m), 1103 (w), 895 (w) cm−1; 1H NMR (500 MHz, CDCl3): δ = 6.45 [s, 1 H (A or B)], 6.44 [s, 1 H (B or A)], 4.31 [tt, J = 11.7, 3.5, 1 H (B)], 3.71 [s, 2 H (A)], 3.66 [s, 2 H (B)], 3.62 [tt, J = 11.7, 3.5, 1 H (A)], 3.37–3.27 [m, 2 H (A) + 2 H (B)], 2.56 [s, 3 H (A)], 2.55 [s, 3 H (B)], 2.37 [s, 3 H (A) + 3 H (B)], 1.90–1.60 [m, 4 H (A) + 4 H (B)], 1.56–1.22 [m, 6 H (A) + 6 H (B)], 1.23 [t, J = 7.2, 3 H (B)], 1.15 [t, J = 7.0, 3 H (A)]; 13C NMR (125 MHz, CDCl3): δ = 194.1 (A + B), 167.8 (B), 167.2 (A), 157.6 (A + B), 147.5 (A + B), 122.2 (A + B), 108.2 (A + B), 58.0 (A), 57.9 (B), 54.4 (A), 54.3 (B), 38.4 (B), 36.9 (A), 34.1 (A), 33.5 (B), 31.8 (A), 30.9 (B), 29.1 (A + B), 25.9 (A + B), 25.6 (B), 25.2 (A), 16.8 (A + B), 14.8 (A), 14.3 (B); GC-MS (EI): m/z = 291 (M+, 3), 154 (49), 137 (14), 123 (4), 95 (8), 83 (100); HRMS (ESI-TOF) m/z: [M + Na]+ Calcd. for C17H25NNaO3+ 314.1727; Found 314.1712.
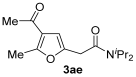
3.3.5. 2-(4-Acetyl-5-methylfuran-2-yl)-N,N-diisopropylacetamide (3ae)
Yield: 143 mg, starting from 138 mg of 1a (54%) (Table 1, entry 9). Yellow oil. IR (film): ν = 1651 (s), 1643 (s), 1566 (m), 1454 (m), 1369 (m), 1339 (w), 1231 (w), 1211 (m), 1041 (w), 945 (w) cm−1; 1H NMR (500 MHz, CDCl3): δ = 6.43 (s, 1 H), 4.03 (heptuplet, J = 6.6, 1 H), 3.66 (s, 2 H), 3.57–3.44 (m, 1 H), 2.57 (s, 3 H), 2.37 (s, 3 H), 1.40 (d, J = 6.7), 1.21 (d, J = 6.6); 13C NMR (125 MHz, CDCl3): δ = 194.2, 166.9, 152.6, 147.6, 122.3, 108.2, 49.3, 46.1, 35.5, 29.1, 20.9, 20.6, 20.5, 14.4, 14.3; GC-MS (EI): m/z = 265 (M+, 2), 137 (19), 128 (53), 96 (7), 86 (94), 43 (100); HRMS (ESI-TOF) m/z: [M + Na]+ Calcd. for C15H23NNaO3+ 288.1570; Found 288.1561.
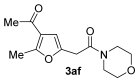
3.3.6. 2-(4-Acetyl-5-methylfuran-2-yl)-1-morpholinoethan-1-one (3af)
Yield: 186 mg, starting from 138 mg of 1a (74%) (Table 1, entry 10). Yellow oil. IR (film): ν = 1667 (s), 1651 (s), 1566 (m), 1435 (m), 1366 (w), 1273 (w), 1227 (m), 1111 (m), 1042 (w), 964 (w), 772 (m) cm−1; 1H NMR (500 MHz, CDCl3): δ = 6.45 (s, 1 H), 3.74–3.62 (m, 6 H), 3.70 (s, 2 H), 3.57–3.53 (m, 2 H), 2.56 (s, 3 H), 2.38 (s, 3 H); 13C NMR (125 MHz, CDCl3): δ = 194.0, 166.8, 157.8, 146.5, 122.3, 108.5, 66.7, 66.5, 46.6, 42.3, 33.1, 29.1, 14.3; GC-MS (EI): m/z = 251 (M+, 20), 137 (33), 114 (100), 70 (76); HRMS (ESI-TOF) m/z: [M + Na]+ Calcd. for C13H17NNaO4+ 274.1050; Found 274.1050.
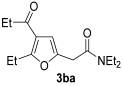
3.3.7. N,N-Diethyl-2-(5-ethyl-4-propionylfuran-2-yl)acetamide (3ba)
Yield: 214 mg, starting from 166 mg of 1b (81%) (Table 1, entry 12). Colorless oil. IR (film): ν = 1674 (s), 1647 (s), 1562 (m), 1458 (m), 1431 (w), 1254 (w), 1219 (m), 1134 (w), 1011 (w), 926 (m) cm−1; 1H NMR (500 MHz, CDCl3): δ = 6.45 (s, br, 1 H), 3.68 (d, J = 0.7, 2 H), 3.42 (q, J = 7.1, 2 H), 3.39 (q, J = 7.1, 2 H), 2.99 (q, J = 7.5, 2 H), 2.72 (q, J = 7.3, 2 H), 1.22 (t, J = 7.5, 3 H), 1.19 (t, J = 7.1, 3 H), 1.15 (t, J = 7.1, 3 H), 1.13 (t, J = 7.3, 3 H); 13C NMR (125 MHz, CDCl3): δ = 197.2, 167.4, 162.5, 147.1, 120.8, 107.8, 42.5, 40.5, 34.4, 33.4, 21.6, 14.3, 12.9, 12.1, 7.9; GC-MS (EI): m/z = 265 (M+, 16), 165 (5), 100 (100), 72 (47); HRMS (ESI-TOF) m/z: [M + Na]+ Calcd. for C15H23NNaO3+ 288.1570; Found 288.1576.
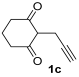
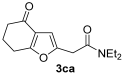
3.3.8. N,N-Diethyl-2-(4-oxo-4,5,6,7-tetrahydrobenzofuran-2-yl)acetamide (3ca)
Yield: 167 mg, starting from 150 mg of 1c (67%) (Table 1, entry 14). Yellow oil. IR (film): ν = 1682 (s), 1667 (s), 1582 (w), 1442 (m), 1366 (w), 1219 (m), 1111 (m), 1003 (m), 772 (w) cm−1; 1H NMR (500 MHz, CDCl3): δ = 6.44 (s, 1 H), 3.70 (s, 2 H), 3.43–3.33 (m, 4 H), 2.86 (t, J = 6.3, 2 H), 2.47 (t, J = 6.3, 2 H), 2.16 (quintuplet, J = 6.3, 2 H), 1.20 (t, J = 7.1, 3 H), 1.14 (t, J = 7.1, 3 H); 13C NMR (125 MHz, CDCl3): δ = 194.4, 167.1, 166.8, 149.9, 122.1, 104.3, 42.5, 40.5, 37.6, 33.5, 23.4, 22.6, 14.3, 12.9; GC-MS (EI): m/z = 249 (M+, 10), 149 (6), 100 (100), 72 (50); HRMS (ESI-TOF) m/z: [M + Na]+ Calcd. for C14H19NNaO3+ 272.1257; Found 272.1261.
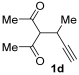
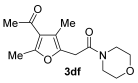
3.3.9. 2-(4-Acetyl-3,5-dimethylfuran-2-yl)-1-morpholinoethan-1-one (3df)
Yield: 175 mg, starting from 152 mg of 1d (66%) (Table 1, entry 16). Yellow solid, mp 90–91 °C. IR (film): ν = 1655 (s), 1558 (w), 1416 (m), 1354 (w), 1304 (w), 1231 (m), 1115 (m), 1072 (w), 1034 (w), 972 (m), 848 (m) cm−1; 1H NMR (500 MHz, CDCl3): δ = 3.69–3.62 (m, 6 H), 3.63 (s, 2 H), 3.57–3.53 (m, 2 H), 2.53 (s, 3 H), 2.42 (s, 3 H), 2.16 (s, 3 H); 13C NMR (125 MHz, CDCl3): δ = 194.8, 167.1, 157.3, 142.8, 123.3, 117.2, 66.8, 66.6, 46.5, 42.4, 31.3, 30.9, 15.3, 10.6; GC-MS (EI): m/z = 265 (M+, 26), 222 (3), 151 (100), 133 (6), 114 (40), 70 (30); HRMS (ESI-TOF) m/z: [M + Na]+ Calcd. for C14H19NNaO4+ 288.1206; Found 288.1216.
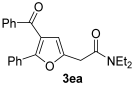
3.3.10. 2-(4-Benzoyl-5-phenylfuran-2-yl)-N,N-diethylacetamide (3ea)
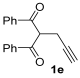
Yield: 245 mg, starting from 262 mg of 1e (68%) (Table 1, entry 17). Colorless oil. IR (film): ν = 1647 (s), 1551 (w), 1485 (m), 1447 (m), 1381 (w), 1261 (m), 1227 (m), 1134 (w), 1072 (w), 887 (m), 729 (m), 694 (m) cm−1; 1H NMR (500 MHz, CDCl3): δ = 7.86–7.80 (m, 2 H), 7.68–7.63 (m, 2 H), 7.51–7.45 (m, 1 H), 7.38–7.33 (m, 2 H), 7.30–7.23 (m, 3 H), 6.53 (t, br, 1 H), 3.81 (d, J = 0.7, 2 H), 3.46–3.38 (m, 4 H), 1.22 (t, J = 7.1. 3 H), 1.16 (t, J = 7.1, 3 H); 13C NMR (125 MHz, CDCl3): δ = 191.7, 167.1, 155.3, 148.5, 138.1, 132.8, 129.7, 128.9, 128.3, 127.7, 127.5, 121.9, 111.6, 42.6, 40.6, 33.6, 14.5, 13.0; GC-MS (EI): m/z = 361 (M+, 17), 261 (14), 105 (21), 100 (100), 77 (18); HRMS (ESI-TOF) m/z: [M + Na]+ Calcd. for C23H23NNaO3+ 384.1570; Found 384.1580.
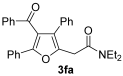
3.3.11. 2-(4-Benzoyl-3,5-diphenylfuran-2-yl)-N,N-diethylacetamide (3fa)
Yield: 235 mg, starting from 338 mg of 1f (54%) (Table 1, entry 19). Colorless oil. IR (film): ν = 1647 (s), 1597 (w), 1489 (w), 1447 (m), 1381 (w), 1335 (w), 1254 (m), 1126 (w), 1077 (w), 899 (m), 733 (m), 694 (m) cm−1; 1H NMR (500 MHz, CDCl3): δ = 7.85–7.77 (m, 2 H), 7.62–7.56 (m, 2 H), 7.41–7.10 (m, 11 H), 3.80 (s, 2 H), 3.46 (q, J = 7.1, 2 H), 3.35 (q, J = 7.1, 2 H), 1.18 (t, J = 7.1. 3 H), 1.15 (t, J = 7.1, 3 H); 13C NMR (125 MHz, CDCl3): δ = 193.6, 167.6, 151.9, 145.5, 137.3, 133.3, 131.6, 129.90, 129.71, 129.3, 128.43, 128.36, 128.25, 127.4, 126.5, 125.7, 121.8, 42.4, 40.7, 32.1, 14.3, 13.1; GC-MS (EI): m/z = 437 (M+, 40), 337 (67), 259 (2), 202 (3), 105 (100), 100 (77), 72 (31); HRMS (ESI-TOF) m/z: [M + Na]+ Calcd. for C29H27NNaO3+ 460.1883; Found 460.1901.
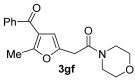
3.3.12. 2-(4-Benzoyl-5-methylfuran-2-yl)-1-morpholinoethan-1-one (3gf)
Yield: 150 mg, starting from 200 mg of 1g (48%) (Table 1, entry 20). Colorless oil. IR (film): ν = 1647 (s), 1566 (m), 1447 (m), 1273 (w), 1231 (m), 1115 (m), 1038 (w), 903 (m), 729 (m) cm−1; 1H NMR (500 MHz, CDCl3): δ = 7.81–7.75 (m, 2 H), 7.58–7.52 (m, 1 H), 7.49–7.43 (m, 2 H), 6.41 (s, 1 H), 3.72 (s, 2 H), 3.71–3.63 (m, 6 H), 3.57–3.53 (m, 2 H), 2.50 (s, 3 H); 13C NMR (125 MHz, CDCl3): δ = 191.1, 166.8, 159.0, 146.4, 139.1, 132.2, 128.9, 128.4, 121.4, 109.9, 66.8, 66.6, 46.7, 42.4, 33.3, 14.2; GC-MS (EI): m/z = 313 (M+, 35), 270 (2), 199 (42), 114 (100), 105 (11), 70 (55); HRMS (ESI-TOF) m/z: [M + Na]+ Calcd. for C18H19NNaO4+ 336.1206; Found 336.1209.
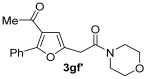
3.3.13. 2-(4-Acetyl-5-phenylfuran-2-yl)-1-morpholinoethan-1-one (3gf′)
Yield: 78 mg, starting from 200 mg of 1g (25%) (Table 1, entry 20). Colorless oil. IR (film): ν = 1651 (s), 1543 (w), 1447 (m), 1381 (w), 1273 (w), 1234 (m), 1115 (s) cm−1; 1H NMR (500 MHz, CDCl3): δ = 7.87–7.80 (m, 2 H), 7.48–7.40 (m, 3 H), 6.64 (t, br, J = 0.7, 1 H), 3.80 (d, J = 0.7, 2 H), 3.73–3.64 (m, 6 H), 3.60–3.56 (m, 2 H), 2.38 (s, 3 H); 13C NMR (125 MHz, CDCl3): δ = 193.8, 166.6, 156.1, 147.8, 129.8, 129.7, 128.5, 128.3, 123.2, 110.5, 66.8, 66.6, 46.7, 42.4, 33.3, 29.8; GC-MS (EI): m/z = 313 (M+, 45), 199 (100), 181 (6), 157 (5), 128 (14), 114 (98), 105 (13), 70 (61); HRMS (ESI-TOF) m/z: [M + Na]+ Calcd. for C18H19NNaO4+ 336.1206; Found 336.1217.
3.3.14. Methyl 5-(2-(Diethylamino)-2-oxoethyl)-2-methylfuran-3-carboxylate (3ha)
Yield: 177 mg, starting from 154 mg of 1h (70%) (Table 1, entry 21). Yellow oil. IR (film): ν = 1713 (s), 1651 (s), 1582 (w), 1451 (m), 1396 (w), 1219 (m), 1088 (m), 995 (w), 779 (m) cm−1; 1H NMR (500 MHz, CDCl3): δ = 6.43 (s, 1 H), 3.79 (s, 3 H), 3.65 (s, 2 H), 3.40 (q, J = 7.1, 2 H), 3.36 (q, J = 7.1, 2 H), 2.54 (s, 3 H), 1.18 (t, J = 7.1), 1.14 (t, J = 7.1, 3 H); 13C NMR (125 MHz, CDCl3): δ = 167.3, 164.5, 158.7, 147.3, 114.1, 108.4, 51.2, 42.5, 40.5, 33.5, 14.3, 13.7, 12.9; GC-MS (EI): m/z = 253 (M+, 9), 222 (5), 153 (4), 121 (12), 100 (100), 72 (55); HRMS (ESI-TOF) m/z: [M + Na]+ Calcd. for C13H19NNaO4+ 276.1206; Found 276.1218.
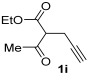
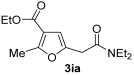
3.3.15. Ethyl 5-(2-(Diethylamino)-2-oxoethyl)-2-methylfuran-3-carboxylate (3ia)
Yield: 184 mg, starting from 168 mg of 1i (69%) (Table 1, entry 23). Colorless oil. IR (film): ν = 1713 (s), 1647 (s), 1578 (w), 1466 (m), 1381 (m), 1215 (s), 1099 (w), 1061 (m), 783 (m) cm−1; 1H NMR (500 MHz, CDCl3): δ = 6.43 (s, 1 H), 4.26 (q, J = 7.1), 3.64 (s, 2 H), 3.45–3.32 (m, 4 H), 2.53 (s, 3 H), 1.32 (t, J = 7.1. 3 H), 1.18 (t, J = 7.1, 3 H), 1.14 (t, J = 7.1, 3 H); 13C NMR (125 MHz, CDCl3): δ = 167.4, 164.1, 158.4, 147.3, 114.6, 108.5, 60.0, 42.5, 40.5, 33.5, 14.38, 14.36, 13.7, 12.9; GC-MS (EI): m/z = 267 (M+, 30), 222 (23), 167 (10), 139 (10), 121 (22), 100 (100), 72 (99); HRMS (ESI-TOF) m/z: [M + Na]+ Calcd. for C14H21NNaO4+ 290.1363; Found 290.1368.
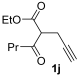
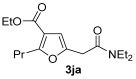
3.3.16. Ethyl 5-(2-(Diethylamino)-2-oxoethyl)-2-propylfuran-3-carboxylate (3ja)
Yield: 200 mg, starting from 196 mg of 1j (68%) (Table 1, entry 25). Colorless oil. IR (film): ν = 1713 (s), 1647 (s), 1578 (w), 1462 (m), 1431 (m), 1381 (w), 1215 (m), 1096 (m), 1050 (m), 779 (m) cm−1; 1H NMR (500 MHz, CDCl3): δ = 6.45 (s, br, 1 H), 4.26 (q, J = 7.1, 2 H), 3.66 (d, J = 0.6, 2 H), 3.42–3.34 (m, 4 H), 2.93 (t, J = 7.4, 2 H), 1.68 (sextuplet, J = 7.4, 2 H), 1.32 (t, J = 7.1, 3 H), 1.17 (t, J = 7.1, 3 H), 1.13 (t, J = 7.1, 3 H), 0.94 (t, J = 7.4, 3 H); 13C NMR (125 MHz, CDCl3): δ = 167.3, 164.0, 162.4, 147.2, 114.2, 108.3, 60.0, 42.5, 40.4, 33.6, 29.5, 21.6, 14.3, 13.8, 13.7, 12.9; GC-MS (EI): m/z = 295 (M+, 6), 250 (3), 195 (3), 100 (100), 72 (37); HRMS (ESI-TOF) m/z: [M + Na]+ Calcd. for C16H25NNaO4+ 318.1676; Found 318.1683.
3.3.17. Ethyl 5-(2-(Diethylamino)-2-oxoethyl)-2-isopropylfuran-3-carboxylate (3ka)
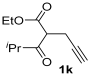
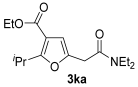
Yield: 209 mg, starting from 196 mg of 1k (71%) (Table 1, entry 27). Colorless oil. IR (film): ν = 1713 (s), 1647 (s), 1578 (w), 1381 (m), 1458 (m), 1365 (w), 1215 (m), 1130 (w), 1099 (w), 1061 (m), 783 (m) cm−1; 1H NMR (500 MHz, CDCl3): δ = 6.44 (s, 1 H), 4.26 (q, J = 7.1, 2 H), 3.73 (heptuplet, J = 7.0, 1 H), 3.67 (s, 2H), 3.45–3.35 (m, 4 H), 1.32 (t, J = 7.1. 3 H), 1.25 (d, J = 7.0, 6 H), 1.17 (t, J = 7.1, 3 H), 1.14 (t, J = 7.1, 3 H); 13C NMR (125 MHz, CDCl3): δ = 167.3, 166.4, 164.0, 146.9, 112.5, 108.2, 59.9, 42.5, 40.4, 33.7, 27.2, 20.78, 20.75, 14.3, 12.9; GC-MS (EI): m/z = 295 (M+, 7), 250 (4), 195 (3), 125 (2), 100 (100), 72 (38); HRMS (ESI-TOF) m/z: [M + Na]+ Calcd. for C16H25NNaO4+ 318.1676; Found 318.1675.
3.3.18. Benzyl 5-(2-(Diethylamino)-2-oxoethyl)-2-methylfuran-3-carboxylate (3la)
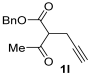
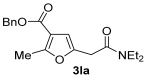
Yield: 220 mg, starting from 230 mg of 1l (67%) (Table 1, entry 29). Colorless oil. IR (film): ν = 1713 (s), 1647 (s), 1585 (w), 1454 (m), 1431 (m), 1381 (w), 1365 (w), 1219 (m), 1076 (m) cm−1; 1H NMR (500 MHz, CDCl3): δ = 7.41–7.29 (m, 5 H), 6.47 (s, 1 H), 5.25 (s, 2 H), 3.64 (s, 2 H), 3.39 (q, J = 7.1, 2 H), 3.35 (q, J = 7.1, 2 H), 2.55 (s, 3 H), 1.17 (t, J = 7.1, 3 H), 1.13 (t, J = 7.1, 3 H); 13C NMR (125 MHz, CDCl3): δ = 167.4, 163.9, 159.0, 147.4, 136.4, 128.6, 128.2, 128.1, 114.2, 108.6, 65.9, 42.5, 40.5, 33.5, 14.4, 14.0, 13.0; GC-MS (EI): m/z = 329 (M+, 5), 212 (6), 194 (2), 100 (100), 91 (18), 72 (35); HRMS (ESI-TOF) m/z: [M + Na]+ Calcd. for C19H23NNaO4+ 352.1519; Found 352.1543.
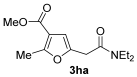
3.3.19. 5-(2-(Diethylamino)-2-oxoethyl)-N,N-diethyl-2-methylfuran-3-carboxamide (3ma)
Yield: 168 mg, starting from 195 mg of 1m (57%) (Table 1, entry 31). Colorless oil, IR (film): ν = 1632 (s), 1454 (m), 1381 (w), 1362 (w), 1269 (w), 1072 (w), cm−1; 1H NMR (300 MHz, CDCl3): δ = 6.13 (s, 1 H), 3.66 (s, 2 H), 3.48–3.32 (m, 8 H), 2.34 (s, 3 H), 1.23–1.10 (m, 12 H); 13C NMR (125 MHz, CDCl3): δ = 167.5, 165.8, 152.0, 146.9, 117.4, 107.4, 42.5, 40.3, 39.2, 33.7, 14.3, 12.9; GC-MS (EI): m/z = 294 (M+, 10), 222 (5), 194 (6), 152 (7), 123 (12), 100 (100), 72 (56); HRMS (ESI-TOF) m/z: [M + Na]+ Calcd. for C16H26N2NaO3+ 317.1836; Found 317.1837.
3.3.20. N,N-Dibutyl-2-(5-phenyl-4-tosylfuran-2-yl)acetamide (3nc)
Yield: 336 mg, starting from 312 mg of 1n (72%) (Table 1, entry 32). Yellow oil. IR (film): ν = 1643 (s), 1597 (w), 1551 (w), 1485 (m), 1454 (m), 1319 (s), 1254 (w), 1215 (w), 1153 (s), 1103 (m), 930 (w), 814 (w), 698 (m) cm−1; 1H NMR (500 MHz, CDCl3): δ = 7.85–7.81 (m, 2 H), 7.69–7.65 (m, 2 H), 7.42–7.38 (m, 3 H), 7.20–7.16 (m, 2 H), 6.64 (t, J = 0.8, 1 H), 3.73 (d, J = 0.8, 2 H), 3.36–3.31 (m, 2 H), 3.28–3.24 (m, 2 H), 2.35 (s, 3 H), 1.59–1.48 (m, 4 H), 1.36–1.22 (m, 4 H), 0.93 (t, J = 7.4, 3 H), 0.91 (t, J = 7.4, 3 H); 13C NMR (125 MHz, CDCl3): δ = 166.8, 154.1, 148.9, 144.1, 139.0, 129.8, 129.6, 128.7, 128.4, 128.2, 127.2, 110.3, 48.3, 46.1, 33.4, 31.3, 29.7, 21.5, 20.2, 20.1, 13.8; GC-MS (EI): m/z = 467 (M+, 10), 311 (9), 253 (2), 207 (8), 156 (100), 128 (11), 100 (39), 57 (68); HRMS (ESI-TOF) m/z: [M + Na]+ Calcd. for C27H33NNaO4S+ 490.2023; Found 490.2037.
3.4. Synthesis of 2-(4-Benzoyl-5-phenylfuran-2-yl)-N,N-diethylacetamide (3ea) in Larger Scale
A 250 mL stainless steel autoclave was charged in the presence of air with PdI2 (8.6 mg, 2.4 × 10−2 mmol), KI (398 mg, 2.4 mmol), a solution of 1,3-diphenyl-2-(prop-2-yn-1-yl)propane-1,3-dione 1e (627 mg, 2.4 mmol) in anhydrous CH3CN (12 mL), and diethylamine 2a (700 mg, 9.6 mmol). The autoclave was sealed and, while the mixture was stirred, the autoclave was pressurized with CO (16 atm) and air (up to 20 atm). After being stirred at 100 °C for 15 h, the autoclave was cooled, degassed, and opened. After evaporation of the solvent, product 3ea were purified by column chromatography on silica gel using as eluent hexane-AcOEt from 8:2 to 6:4 (yield: 586 mg, 68%).
4. Conclusions
In conclusion, we have reported the synthesis of a previously unreported subclass of furan derivatives (2-(4-acylfuran-2-yl)acetamides) by a direct catalytic carbonylative approach starting from readily available building blocks (2-propargyl-1,3-dicarbonyl compounds, secondary amines, and oxygen). The process is catalyzed by the simple PdI2/KI catalytic system and takes place through an ordered sequence of steps, involving: PdI2/KI-catalyzed oxidative monaminocarbonylation of the terminal triple bond of the substrate to give the corresponding 2-ynamide intermediate; base-induced enolization and 5-exo-dig O-cyclization via intramolecular conjugate addition to the 2-ynamide moiety; double bond shift with aromative isomerization.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules28196764/s1, Preparation and characterization of substrates, Copies of HRMS, 1H NMR, and 13C NMR spectra for substrates 1b, 1d, 1k, 1m and products 3aa, 3ab, 3ac, 3ad, 3ae, 3af, 3ba, 3ca, 3df, 3ea, 3fa, 3gf, 3gf′, 3ha, 3ia, 3ja, 3ka, 3la, 3ma, 3nc. References [33,34,35,36,37,38,39,40,41] are cited in Supplementary Materials.
Author Contributions
Conceptualization: B.G. and L.V.; methodology: I.Z., L.V., T.P., R.A., M.A.C., R.M. and B.G.; validation: I.Z., L.V., T.P., R.A., M.A.C. and R.M.; investigation: I.Z., L.V., T.P., R.A., M.A.C. and R.M.; writing—original draft preparation: B.G.; writing—review and editing: B.G.; supervision: B.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding authors.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds 3aa, 3ab, 3ac, 3ad, 3ae, 3af, 3ba, 3ca, 3df, 3ea, 3fa, 3gf, 3gf′, 3ha, 3ia, 3ja, 3ka, 3la, 3ma, and 3nc are available from the authors.
References
- Furan Derivatives: Recent Advances and Applications; Khan, A.; Rahman, M.M.; Ramesh, M.; Khan, S.; Asiri, A.M. (Eds.) IntechOpen: London, UK, 2022. [Google Scholar]
- Alizadeh, M.; Jalal, M.; Hamed, K.; Saber, A.; Kheirouri, S.; Tabrizi, F.P.F.; Kamari, N. Recent Updates on Anti-Inflammatory and Antimicrobial Effects of Furan Natural Derivatives. J. Inflamm. Res. 2020, 13, 451–463. [Google Scholar] [CrossRef]
- Sperry, J.B.; Wright, D.L. Furans, Thiophenes and Related Heterocycles in Drug Discovery. Curr. Opin. Drug Discov. Dev. 2005, 8, 723–740. [Google Scholar] [CrossRef]
- Zhang, B.; Huo, L.J. Recent Advances of Furan and Its Derivatives Based Semiconductor Materials for Organic Photovoltaics. Small Methods 2021, 5, 2100493. [Google Scholar] [CrossRef]
- Deepthi, A.; Babu, B.P.; Balachandran, A.L. Synthesis of Furans—Recent Advances. Org. Prep. Proced. Int. 2019, 51, 409–442. [Google Scholar] [CrossRef]
- Zhang, W.; Xu, W.; Zhang, F.; Li, Y. Recent Progress in Synthesis of Polysubstituted Furans. Chin. J. Org. Chem. 2019, 39, 1277–1283. [Google Scholar] [CrossRef]
- Duc, D.X. Recent Progress in the Synthesis of Furan. Mini-Rev. Org. Chem. 2019, 16, 422–452. [Google Scholar] [CrossRef]
- Nejrotti, S.; Prandi, C. Gold Catalysis and Furans: A Powerful Match for Synthetic Connections. Synthesis 2021, 53, 1046–1060. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, C.; Li, S. Progress in Cyclizations of 4-Acetylenic Ketones: Synthesis of Furans and Pyrroles. ChemistrySelect 2020, 5, 8656–8668. [Google Scholar] [CrossRef]
- Wu, X.-F. Transition Metal Catalyzed Furans Synthesis; Elsevier: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Pandey, S.; Shukla, R.K.; Volla, C.M.R. Access to Polysubstituted Furan Derivatives via Cascade Oxypalladation and Hydrocarbofunctionalization of Unactivated Alkenes. Org. Lett. 2023, 25, 4694–4699. [Google Scholar] [CrossRef]
- Carbon Monoxide in Organic Synthesis: Carbonylation Chemistry; Gabriele, B. (Ed.) Wiley-VCH: Weinheim, Germany, 2021; ISBN 978-3527347957. [Google Scholar]
- Gabriele, B. Chapter 3—Synthesis of Heterocycles by Palladium-Catalyzed Carbonylative Reactions. In Advances in Transition-Metal Mediated Heterocyclic Synthesis; Solé, D., Fernández, I., Eds.; Academic Press-Elsevier: London, UK, 2018; pp. 55–127. [Google Scholar]
- Feng, J.-B.; Wu, X.-F. Palladium-Catalyzed Synthesis of Heterocycles. In Advances in Heterocyclic Chemistry; Scriven, E.F.V., Ramsden, C.A., Eds.; Academic Press: Cambridge, MA, USA, 2017; Volume 121, pp. 207–246. [Google Scholar]
- Transition Metal Catalyzed Carbonylative Synthesis of Heterocycles; Wu, X.-F.; Beller, M. (Eds.) Topics in Heterocyclic Chemistry; Springer: Cham, Switzerland, 2016; Volume 42. [Google Scholar]
- Yin, Z.; Xu, J.; Wu, X.-F. No Making without Breaking: Nitrogen-Centered Carbonylation Reactions. ACS Catal. 2020, 10, 6510–6531. [Google Scholar] [CrossRef]
- Peng, J.-B.; Wu, F.-P.; Wu, X.-F. First-row Transition-Metal-Catalyzed Carbonylative Transformations of Carbon Electrophiles. Chem. Rev. 2019, 119, 2090–2127. [Google Scholar] [CrossRef]
- Ma, K.; Martin, B.S.; Yin, X.; Dai, M. Natural Product Syntheses via Carbonylative Cyclizations. Nat. Prod. Rep. 2019, 36, 174–219. [Google Scholar] [CrossRef]
- Perrone, S.; Troisi, L.; Salomone, A. Heterocycle Synthesis through Pd-Catalyzed Carbonylative Coupling. Eur. J. Org. Chem. 2019, 2019, 4626–4643. [Google Scholar] [CrossRef]
- Albano, G.; Aronica, L.A. Potentiality and Synthesis of O- and N-Heterocycles: Pd-Catalyzed Cyclocarbonylative Sonogashira Coupling as a Valuable Route to Phthalans, Isochromans, and Isoindolines. Eur. J. Org. Chem. 2017, 2017, 7204–7221. [Google Scholar] [CrossRef]
- Shen, C.R.; Wu, X.-F. Palladium-Catalyzed Carbonylative Multicomponent Reactions. Chem. Eur. J. 2017, 23, 2973–2987. [Google Scholar] [CrossRef]
- Wu, X.-F.; Neumann, H.; Beller, M. Synthesis of Heterocycles via Palladium-Catalyzed Carbonylations. Chem. Rev. 2013, 113, 1–35. [Google Scholar] [CrossRef]
- Gabriele, B.; Mancuso, R.; Salerno, G. Oxidative Carbonylation as a Powerful Tool for the Direct Synthesis of Carbonylated Heterocycles. Eur. J. Org. Chem. 2012, 2012, 6825–6839. [Google Scholar] [CrossRef]
- Gabriele, B.; Costa, M.; Salerno, G.; Chiusoli, G.P. A New Synthesis of Trimethyl Aconitate by Palladium-Catalysed Triple Carbonylation of Propynyl Alcohol. J. Chem. Soc. Chem. Commun. 1992, 1992, 1007–1008. [Google Scholar] [CrossRef]
- Gabriele, B.; Costa, M.; Salerno, G.; Chiusoli, G.P. An Efficient and Selective Palladium-Catalysed Oxidative Dicarbonylation of Alkynes to Alkyl- or Aryl-maleic Esters. J. Chem. Soc. Perkin Trans. 1994, 1, 83–87. [Google Scholar] [CrossRef]
- Mancuso, R.; Della Ca’, N.; Veltri, L.; Ziccarelli, I.; Gabriele, B. PdI2-Based Catalysis for Carbonylation Reactions: A Personal Account. Catalysts 2019, 9, 610. [Google Scholar] [CrossRef]
- Gabriele, B.; Salerno, G.; Veltri, L.; Costa, M. Synthesis of 2-Ynamides by Direct Palladium-Catalyzed Oxidative Aminocarbonylation of Alk-1-ynes. J. Organomet. Chem. 2001, 622, 84–88. [Google Scholar] [CrossRef]
- Ziccarelli, I.; Mancuso, R.; Giacalone, F.; Calabrese, C.; La Parola, V.; De Salvo, A.; Della Ca’, N.; Gruttadauria, M.; Gabriele, B. Heterogenizing Palladium Tetraiodide Catalyst for Carbonylation Reactions. J. Catal. 2022, 413, 1098–1110. [Google Scholar] [CrossRef]
- Veltri, L.; Amuso, R.; Prestia, T.; Vitale, P.; Gabriele, B. A Multicomponent Approach to Imidazo[2,1-b]thiazole Derivatives by Sequential PdI2/KI-Catalyzed Deprotective Oxidative Aminocarbonylation—Dearomative Cyclization—Aromatization. Eur. J. Org. Chem. 2022, 2022, e202200916. [Google Scholar] [CrossRef]
- Gabriele, B.; Salerno, G.; Mancuso, R.; Costa, M. Efficient Synthesis of Ureas by Direct Palladium-Catalyzed Oxidative Carbonylation of Amines. J. Org. Chem. 2004, 69, 4741–4750. [Google Scholar] [CrossRef]
- Mancuso, R.; Della Ca’, N.; Fini, F.; Carfagna, C.; Gabriele, B. Catalytic Oxidative Carbonylation of Amino Moieties to Ureas, Oxamides, 2-Oxazolidinones, and Benzoxazolones. ChemSusChem 2015, 8, 2204–2211. [Google Scholar] [CrossRef]
- Pickersgill, F.; Marchington, A.P.; Rayner, C.M. Selective Alkylation of β-Ketoester Enolates Using O-Methyl Aminosulfoxonium Salts; the First Example of C-alkylation Using Sulfoxonium Salt Electrophiles. J. Chem. Soc. Chem. Commun. 1994, 1994, 2597–2598. [Google Scholar] [CrossRef]
- Misztalewska, I.; Wilczewska, A.Z.; Wojtasik, O.K.; Markiewicz, H.; Kuchlewski, P.; Majcher, A.M. New Acetylacetone-Polymer Modified Nanoparticles as Magnetically Separable Complexing Agents. RSC Adv. 2015, 5, 100281–100289. [Google Scholar] [CrossRef]
- Chang, M.-Y.; Cheng, Y.-C.; Lu, W.-J. Bi(OTf)3-Mediated Cycloisomerization of γ-Alkynyl Arylketones: Application to the Synthesis of Substituted Furans. Org. Lett. 2015, 17, 1264–1267. [Google Scholar] [CrossRef]
- Schneider, L.M.; Schmiedel, V.M.; Pecchioli, T.; Lentz, D.; Merten, C.; Christmann, M. Asymmetric Synthesis of Carbocyclic Propellanes. Org. Lett. 2017, 19, 2310–2313. [Google Scholar] [CrossRef]
- Gree, R.; Park, H.; Paquette, L.A. Regio- and Stereoselective 1,2 Wagner-Meerwein Shifts during Trifluoroacetic acid Catalyzed Isomerization of Unsymmetrically Substituted Tricyclo[3.2.0.02,4]heptanes. J. Am. Chem. Soc. 1980, 102, 4397–4403. [Google Scholar] [CrossRef]
- Sanz, R.; Miguel, D.; Martínez, A.; Álvarez-Gutiérrez, J.M.; Rodriguez, F. Brønsted Acid Catalyzed Propargylation of 1,3-Dicarbonyl Derivatives. Synthesis of Tetrasubstituted Furans. Org. Lett. 2007, 9, 727–730. [Google Scholar] [CrossRef] [PubMed]
- Ruengsangtongkul, S.; Chaisan, N.; Thongsornkleeb, C.; Tummatorn, J.; Ruchirawat, S. Rate Enhancement in CAN-Promoted Pd(PPh3)2Cl2-Catalyzed Oxidative Cyclization: Synthesis of 2-Ketofuran-4-carboxylate Esters. Org. Lett. 2019, 21, 2514–2517. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wang, J.; Hu, X.; Shen, K.; Wang, W.; Chu, Y.; Lin, L.; Liu, X.; Feng, X. Catalytic Asymmetric Roskamp Reaction of α-Alkyl-α-diazoesters with Aromatic Aldehydes: Highly Enantioselective Synthesis of α-Alkyl-β-keto Esters. J. Am. Chem. Soc. 2010, 132, 8532–8533. [Google Scholar] [CrossRef]
- Barabe, F.; Levesque, P.; Korobkov, I.; Barriault, L. Synthesis of Fused Carbocycles via a Selective 6-Endo Dig Gold(I)-Catalyzed Carbocyclization. Org. Lett. 2011, 13, 5580–5583. [Google Scholar] [CrossRef]
- Katrun, P.; Songsichan, T.; Soorukram, D.; Pohmakotr, M.; Reutrakul, V.; Kuhakarn, C. o-Iodoxybenzoic Acid (IBX)–Iodine Mediated One-Pot Deacylative Sulfonylation of 1,3-Dicarbonyl Compounds: A Synthesis of β-Carbonyl Sulfones. Synthesis 2017, 49, 1109–1121. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).