Applications of Probiotic Constituents in Cosmetics
Abstract
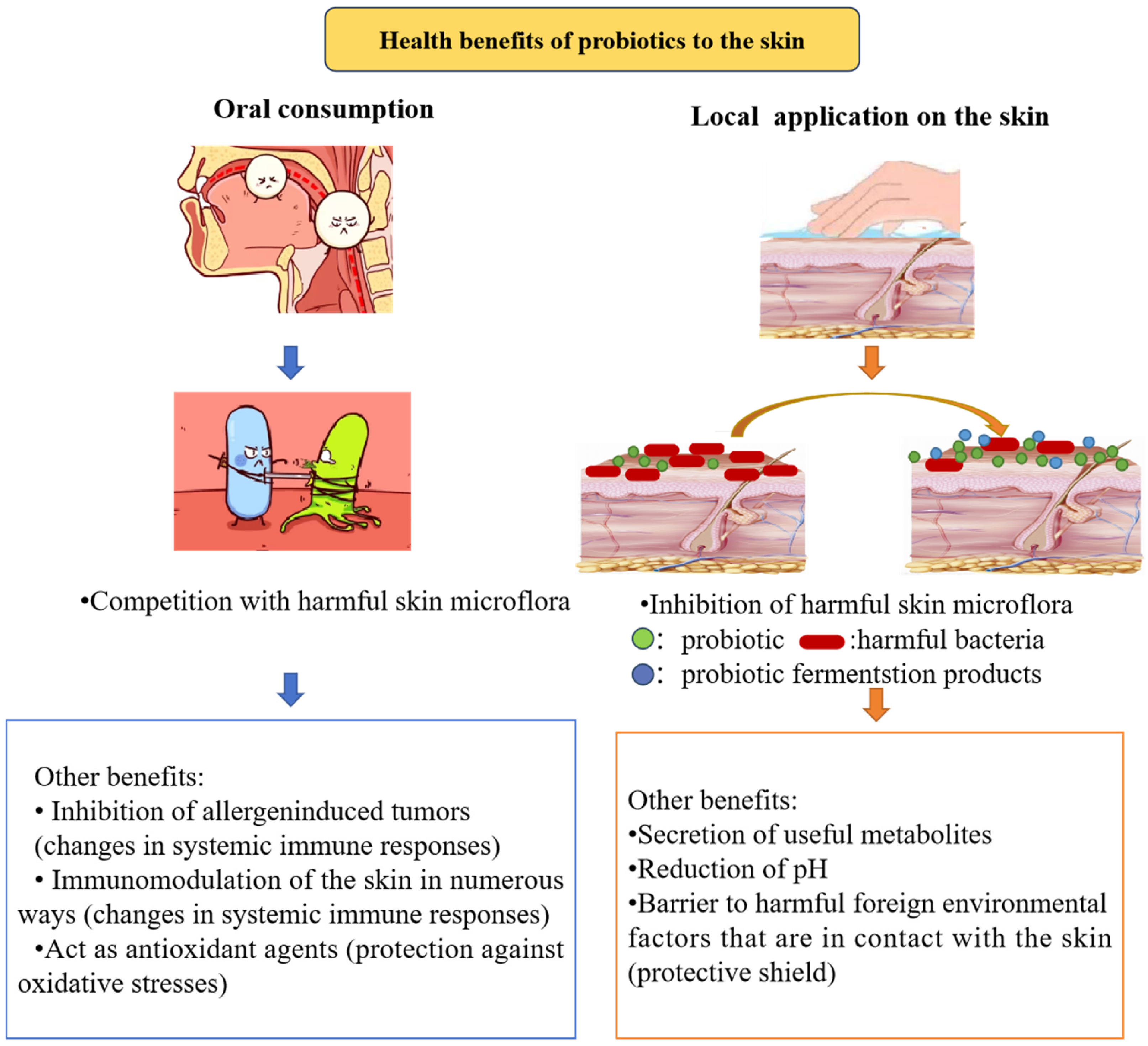
:1. Introduction
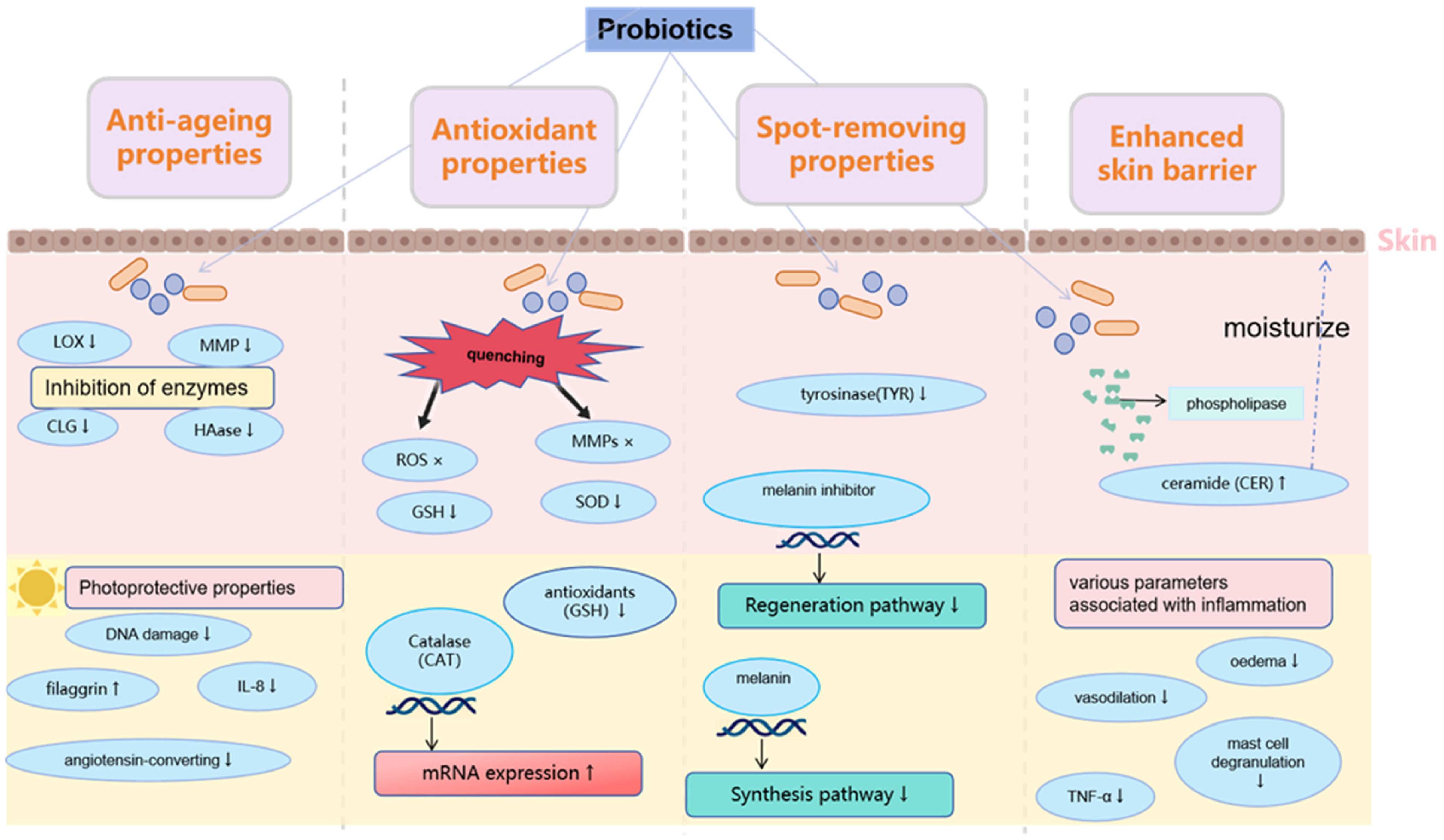
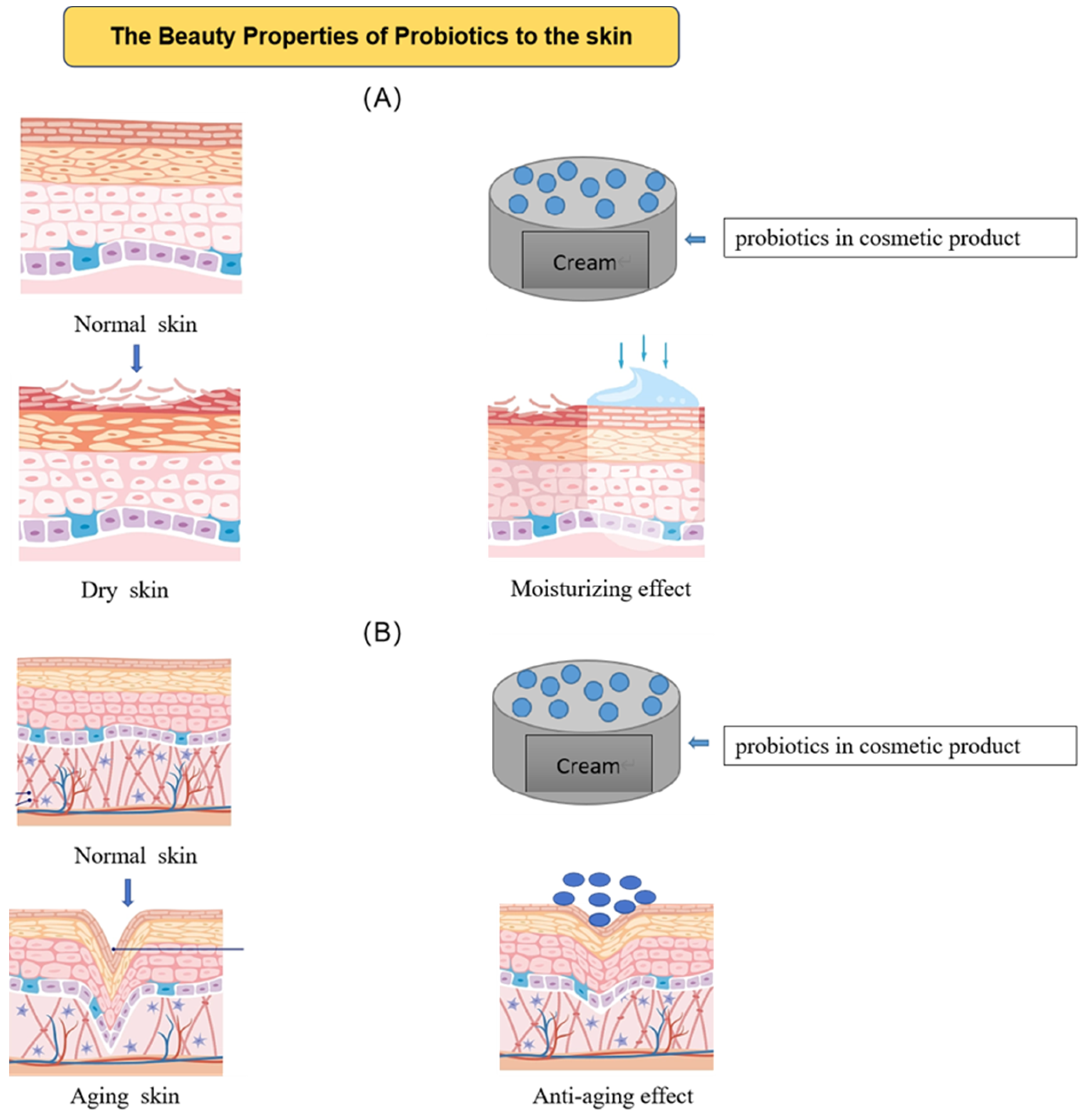
2. Application of Probiotics in Anti-Aging Cosmetics
2.1. Hydrate and Restore the Skin’s Barrier
2.2. Antioxidation
2.3. Other Anti-Aging Effects
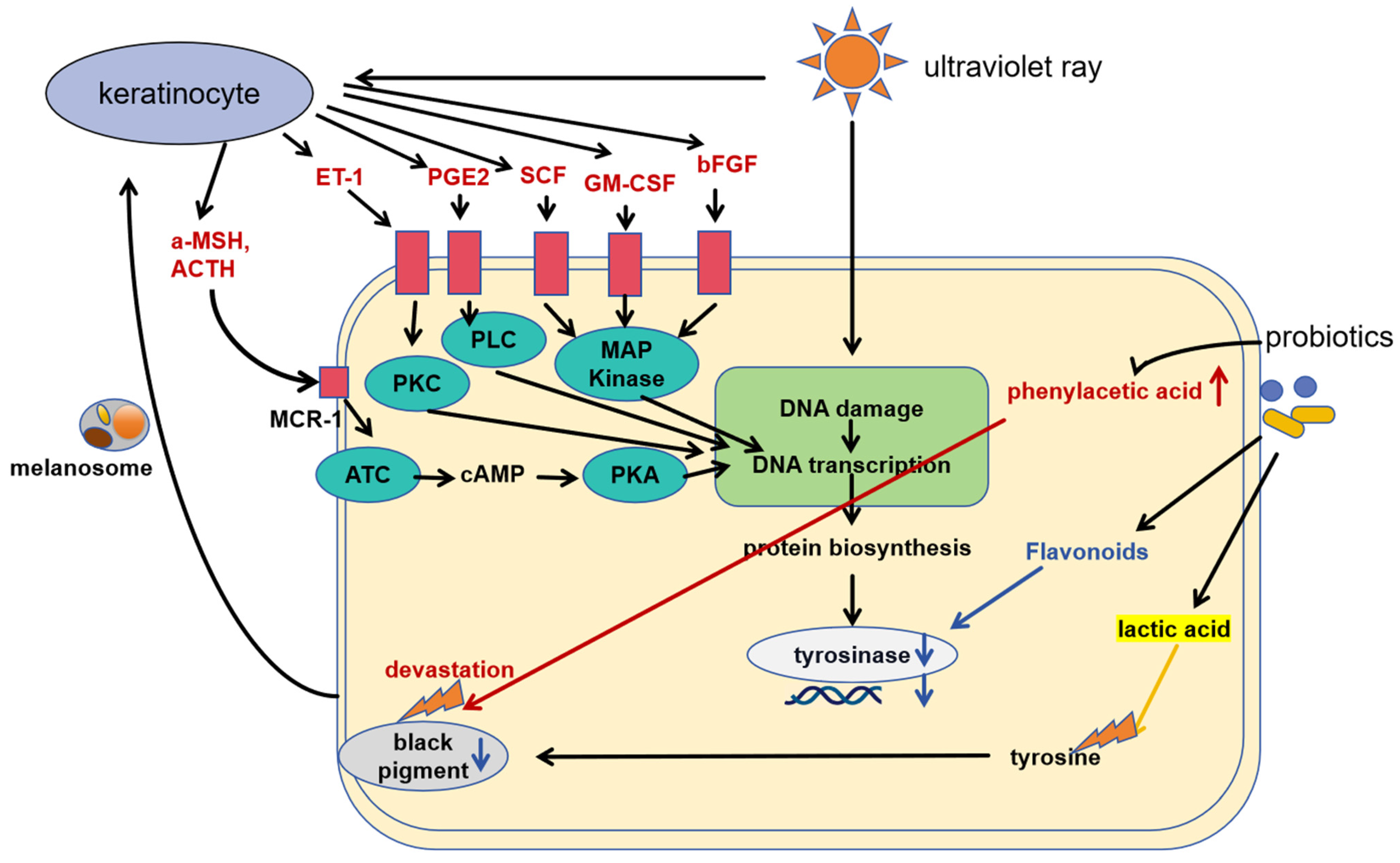
3. Using Probiotics to Whiten Skin and Get Rid of Freckles
3.1. Tyrosinase Activity Inhibition
3.2. The Role of Lipoteichoic Acid
3.3. Other Whitening Effects
4. Probiotics Used in Anti-Inflammatory Cosmetics
4.1. Cutaneous Inflammation
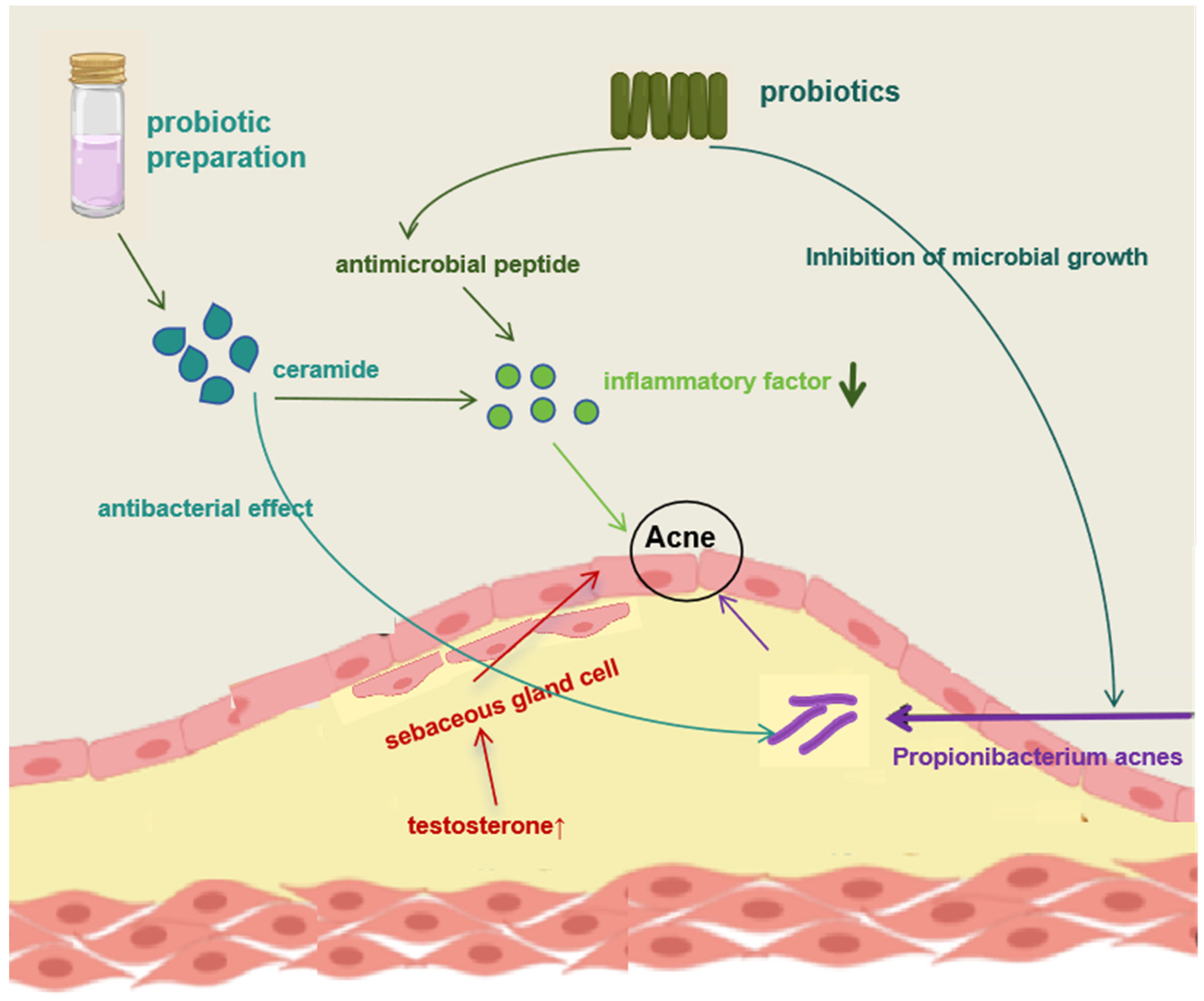
4.2. Acne
5. Application of Probiotic Fermentation
6. Conclusions and Prospects
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| FDA | Food and Drug Administration |
| ICCR | International Cosmetic Regulatory |
| CFUs/g | Colony-Forming Units/gram |
| WHO | World Health Organization |
| FDA | Food and Drug Administration |
| EFSA | European Food Safety Authority |
| S. thermophilus | Streptococcus thermophilus |
| L. plantarum | Lactobacillus plantarum |
| HK L-137 | Heat-inactivated Lactobacillus plantarum L-137 |
| HA | Hyaluronic acid |
| ROS | Reactive oxygen species |
| SOD | Superoxide dismutase |
| GSH | Glutathione |
| VE | Vitamin E |
| Q10 | Coenzyme Q10 |
| MMPs | Matrix metalloproteinases |
| AL | Lactobacillus acidophilus KCCM12625P |
| HDF | Human dermal fibroblasts |
| RL | Heat-killed Lactobacillus rhamnosus |
| NMN | β-Nicotinamide Mononucleotide |
| LAB | Lactic acid bacteria |
| CAT | Catalase |
| EPS | Exopolysaccharides |
| PL | Heat-killed Lacticaseibacillus paracasei |
| EVs | Extracellular vesicles |
| IL-10 | Interleukin 10 |
| IL-8 | Interleukin 8 |
| ACE | Angiotensin-converting enzyme |
| BL | Bifidobacterium longum |
| AD | Atopic dermatitis |
| HT La1 | Heat-treated cosmetic lotions containing Lactobacillus johnsonii NCC 533 |
| AE | Acanthopanax Korean root |
References
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Bermudez-Brito, M.; Plaza-Díaz, J.; Muñoz-Quezada, S.; Gómez-Llorente, C.; Gil, A. Probiotic mechanisms of action. Ann. Nutr. Metab. 2012, 61, 160–174. [Google Scholar] [CrossRef] [PubMed]
- Telesetsky, A. UN Food and Agriculture Organization: Exercising Legal Personality to Implement the UN Convention on the Law of the Sea. In Global Challenges and the Law of the Sea; Springer International Publishing: Cham, Switzerland, 2020. [Google Scholar]
- Puebla-Barragan, S.; Reid, G. Probiotics in Cosmetic and Personal Care Products: Trends and Challenges. Molecules 2021, 26, 1249. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef] [PubMed]
- Kober, M.M.; Bowe, W.P. The effect of probiotics on immune regulation, acne, and photoaging. Int. J. Womens Dermatol. 2015, 1, 85–89. [Google Scholar] [CrossRef]
- Krutmann, J. Pre-and probiotics for human skin. J. Dermatol. Sci. 2009, 54, 1–5. [Google Scholar] [CrossRef]
- Yu, J.; Ma, X.; Wang, X.; Cui, X.; Ding, K.; Wang, S.; Han, C. Application and mechanism of probiotics in skin care: A review. J. Cosmet. Dermatol. 2022, 21, 886–894. [Google Scholar] [CrossRef]
- Gao, T.; Wang, X.; Li, Y.; Ren, F. The Role of Probiotics in Skin Health and Related Gut-Skin Axis: A Review. Nutrients 2023, 15, 3123. [Google Scholar] [CrossRef]
- Duarte, M.; Oliveira, A.L.; Oliveira, C.; Pintado, M.; Amaro, A.; Madureira, A.R. Current postbiotics in the cosmetic market-an update and development opportunities. Appl. Microbiol. Biotechnol. 2022, 106, 5879–5891. [Google Scholar] [CrossRef]
- Karnwal, A.; Shrivastava, S.; Al-Tawaha, A.; Kumar, G.; Singh, R.; Kumar, A.; Mohan, A.; Yogita; Malik, T. Microbial Biosurfactant as an Alternate to Chemical Surfactants for Application in Cosmetics Industries in Personal and Skin Care Products: A Critical Review. Biomed. Res. Int. 2023, 2023, 2375223. [Google Scholar] [CrossRef]
- Gilchrest, B.A. Skin aging and photoaging: An overview. J. Am. Acad. Dermatol. 1989, 21, 610–613. [Google Scholar] [CrossRef]
- Teng, Y.; Huang, Y.; Danfeng, X.; Tao, X.; Fan, Y. The Role of Probiotics in Skin Photoaging and Related Mechanisms: A Review. Clin. Cosmet. Investig. Dermatol. 2022, 15, 2455–2464. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zhang, X.; Song, Y.; Zheng, B.; Wen, Z.; Gong, M.; Meng, L. Heat-Killed Lacticaseibacillus paracasei Ameliorated UVB-Induced Oxidative Damage and Photoaging and Its Underlying Mechanisms. Antioxidants 2022, 11, 1875. [Google Scholar] [CrossRef] [PubMed]
- Vinderola, G.; Sanders, M.E.; Salminen, S. The Concept of Postbiotics. Foods 2022, 11, 1077. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, J.; Ma, M.; Zhao, Y.; Song, Y.; Zheng, B.; Wen, Z.; Gong, M.; Meng, L. Heat-Killed Lactobacillus rhamnosus ATCC 7469 Improved UVB-Induced Photoaging via Antiwrinkle and Antimelanogenesis Impacts. Photochem. Photobiol. 2023. ahead-of-print. [Google Scholar] [CrossRef]
- Im, A.R.; Lee, B.; Kang, D.J.; Chae, S. Protective effects of tyndallized Lactobacillus acidophilus IDCC 3302 against UVB-induced photodamage to epidermal keratinocytes cells. Int. J. Mol. Med. 2019, 43, 2499–2506. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, E.F. Reactive oxygen species activate the human elastin promoter in a transgenic model of cutaneous photoaging. Dermatol. Surg. 2002, 28, 132–135. [Google Scholar] [CrossRef]
- Blanchet-Réthoré, S.; Bourdès, V.; Mercenier, A.; Haddar, C.H.; Verhoeven, P.O.; Andres, P. Effect of a lotion containing the heat-treated probiotic strain Lactobacillus johnsonii NCC 533 on Staphylococcus aureus colonization in atopic dermatitis. Clin. Cosmet. Investig. Dermatol. 2017, 10, 249–257. [Google Scholar] [CrossRef]
- Elmahdy, A.; Maibach, H.I. Textbook of Aging Skin; Springer International Publishing: Cham, Switzerland, 2017. [Google Scholar]
- Ra, J.; Lee, D.E.; Kim, S.H.; Jeong, J.W.; Ku, H.K.; Kim, T.Y.; Choi, I.D.; Jeung, W.; Sim, J.H.; Ahn, Y.T. Effect of oral administration of Lactobacillus plantarum HY7714 on epidermal hydration in ultraviolet B-irradiated hairless mice. J. Microbiol. Biotechnol. 2014, 24, 1736–1743. [Google Scholar] [CrossRef]
- Ishii, Y.; Sugimoto, S.; Izawa, N.; Sone, T.; Chiba, K.; Miyazaki, K. Oral administration of Bifidobacterium breve attenuates UV-induced barrier perturbation and oxidative stress in hairless mice skin. Arch. Dermatol. Res. 2014, 306, 467–473. [Google Scholar] [CrossRef]
- Imokawa, G.; Abe, A.; Jin, K.; Higaki, Y.; Kawashima, M.; Hidano, A. Decreased level of ceramides in stratum corneum of atopic dermatitis: An etiologic factor in atopic dry skin? J. Investig. Dermatol. 1991, 96, 523–526. [Google Scholar] [CrossRef]
- Kim, H.; Kim, H.R.; Jeong, B.J.; Lee, S.S.; Kim, T.R.; Jeong, J.H.; Lee, M.; Lee, S.; Lee, J.S.; Chung, D.K. Effects of oral intake of kimchi-derived Lactobacillus plantarum K8 lysates on skin moisturizing. J. Microbiol. Biotechnol. 2015, 25, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Kimoto-Nira, H.; Aoki, R.; Sasaki, K.; Suzuki, C.; Mizumachi, K. Oral intake of heat-killed cells of Lactococcus lactis strain H61 promotes skin health in women. J. Nutr. Sci. 2012, 1, e18. [Google Scholar] [CrossRef] [PubMed]
- Tulkens, J.; Vergauwen, G.; Van Deun, J.; Geeurickx, E.; Dhondt, B.; Lippens, L.; De Scheerder, M.A.; Miinalainen, I.; Rappu, P.; De Geest, B.G.; et al. Increased levels of systemic LPS-positive bacterial extracellular vesicles in patients with intestinal barrier dysfunction. Gut 2020, 69, 191–193. [Google Scholar] [CrossRef] [PubMed]
- Nakai, H.; Hirose, Y.; Murosaki, S.; Yoshikai, Y. Lactobacillus plantarum L-137 upregulates hyaluronic acid production in epidermal cells and fibroblasts in mice. Microbiol. Immunol. 2019, 63, 367–378. [Google Scholar] [CrossRef] [PubMed]
- McGrath, J.A.; Uitto, J. The filaggrin story: Novel insights into skin-barrier function and disease. Trends Mol. Med. 2008, 14, 20–27. [Google Scholar] [CrossRef]
- Shin, M.; Truong, V.L.; Lee, M.; Kim, D.; Kim, M.S.; Cho, H.; Jung, Y.H.; Yang, J.; Jeong, W.S.; Kim, Y. Investigation of phenyllactic acid as a potent tyrosinase inhibitor produced by probiotics. Curr. Res. Food Sci. 2023, 6, 100413. [Google Scholar] [CrossRef]
- Kim, S.; Seo, H.; Mahmud, H.A.; Islam, M.I.; Sultana, O.F.; Lee, Y.; Kim, M.; Song, H.Y. Melanin Bleaching and Melanogenesis Inhibition Effects of Pediococcus acidilactici PMC48 Isolated from Korean Perilla Leaf Kimchi. J. Microbiol. Biotechnol. 2020, 30, 1051–1059. [Google Scholar] [CrossRef]
- Kim, H.R.; Kim, H.; Jung, B.J.; You, G.E.; Jang, S.; Chung, D.K. Lipoteichoic acid isolated from Lactobacillus plantarum inhibits melanogenesis in B16F10 mouse melanoma cells. Mol. Cells 2015, 38, 163–170. [Google Scholar] [CrossRef]
- Tsai, W.H.; Chou, C.H.; Chiang, Y.J.; Lin, C.G.; Lee, C.H. Regulatory effects of Lactobacillus plantarum-GMNL6 on human skin health by improving skin microbiome. Int. J. Med. Sci. 2021, 18, 1114–1120. [Google Scholar] [CrossRef]
- Liu, W.S.; Kuan, Y.D.; Chiu, K.H.; Wang, W.K.; Chang, F.H.; Liu, C.H.; Lee, C.H. The extract of Rhodobacter sphaeroides inhibits melanogenesis through the MEK/ERK signaling pathway. Mar. Drugs 2013, 11, 1899–1908. [Google Scholar] [CrossRef] [PubMed]
- Khmaladze, I.; Butler, É.; Fabre, S.; Gillbro, J.M. Lactobacillus reuteri DSM 17938-A comparative study on the effect of probiotics and lysates on human skin. Exp. Dermatol. 2019, 28, 822–828. [Google Scholar] [CrossRef]
- Cogen, A.L.; Nizet, V.; Gallo, R.L. Skin microbiota: A source of disease or defence? Br. J. Dermatol. 2008, 158, 442–455. [Google Scholar] [CrossRef]
- Kimoto-Nira, H.; Sekiyama, Y.; Moriya, N. Towards application of water extract from heat-killed Lactococcus lactis H61 as a cosmetic ingredient. Lett. Appl. Microbiol. 2019, 68, 530–536. [Google Scholar] [CrossRef] [PubMed]
- Guéniche, A.; Bastien, P.; Ovigne, J.M.; Kermici, M.; Courchay, G.; Chevalier, V.; Breton, L.; Castiel-Higounenc, I. Bifidobacterium longum lysate, a new ingredient for reactive skin. Exp. Dermatol. 2010, 19, e1–e8. [Google Scholar] [CrossRef] [PubMed]
- Fujiki, T.; Hirose, Y.; Yamamoto, Y.; Murosaki, S. Enhanced immunomodulatory activity and stability in simulated digestive juices of Lactobacillus plantarum L-137 by heat treatment. Biosci. Biotechnol. Biochem. 2012, 76, 918–922. [Google Scholar] [CrossRef] [PubMed]
- Di Marzio, L.; Cinque, B.; De Simone, C.; Cifone, M.G. Effect of the lactic acid bacterium Streptococcus thermophilus on ceramide levels in human keratinocytes in vitro and stratum corneum in vivo. J. Investig. Dermatol. 1999, 113, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.Y.; Jeong, D.; Park, S.H.; Shin, K.K.; Hong, Y.H.; Kim, E.; Yu, Y.G.; Kim, T.R.; Kim, H.; Lee, J.; et al. Antiwrinkle and Antimelanogenesis Effects of Tyndallized Lactobacillus acidophilus KCCM12625P. Int. J. Mol. Sci. 2020, 21, 1620. [Google Scholar] [CrossRef]
- Im, A.R.; Lee, B.; Kang, D.J.; Chae, S. Skin Moisturizing and Antiphotodamage Effects of Tyndallized Lactobacillus acidophilus IDCC 3302. J. Med. Food 2018, 21, 1016–1023. [Google Scholar] [CrossRef]
- Zhou, X.; Du, H.H.; Ni, L.; Ran, J.; Hu, J.; Yu, J.; Zhao, X. Nicotinamide Mononucleotide Combined with Lactobacillus fermentum TKSN041 Reduces the Photoaging Damage in Murine Skin by Activating AMPK Signaling Pathway. Front. Pharmacol. 2021, 12, 643089. [Google Scholar] [CrossRef]
- Hong, Y.F.; Lee, H.; Jung, B.J.; Jang, S.; Chung, D.K.; Kim, H. Lipoteichoic acid isolated from Lactobacillus plantarum down-regulates UV-induced MMP-1 expression and up-regulates type I procollagen through the inhibition of reactive oxygen species generation. Mol. Immunol. 2015, 67, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Matsuguchi, T.; Takagi, A.; Matsuzaki, T.; Nagaoka, M.; Ishikawa, K.; Yokokura, T.; Yoshikai, Y. Lipoteichoic acids from Lactobacillus strains elicit strong tumor necrosis factor alpha-inducing activities in macrophages through Toll-like receptor 2. Clin. Vaccine Immunol. 2003, 10, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Jo, C.S.; Myung, C.H.; Yoon, Y.C.; Ahn, B.H.; Min, J.W.; Seo, W.S.; Lee, D.H.; Kang, H.C.; Heo, Y.H.; Choi, H.; et al. The Effect of Lactobacillus plantarum Extracellular Vesicles from Korean Women in Their 20s on Skin Aging. Curr. Issues Mol. Biol. 2022, 44, 526–540. [Google Scholar] [CrossRef]
- Kim, S.H.; Yoem, S.H.; Kim, J.H.; Hong, J.W.; Oh, Y.S.; Kim, J.W. Enhancement of TRP Gene Expression and UV Absorption by Bioconverted Chestnut Inner Shell Extracts Using Lactiplantibacillus plantarum. Molecules 2022, 27, 4940. [Google Scholar] [CrossRef] [PubMed]
- Boo, Y.C. Arbutin as a Skin Depigmenting Agent with Antimelanogenic and Antioxidant Properties. Antioxidants 2021, 10, 1129. [Google Scholar] [CrossRef] [PubMed]
- Saeedi, M.; Eslamifar, M.; Khezri, K. Kojic acid applications in cosmetic and pharmaceutical preparations. Biomed. Pharmacother. 2019, 110, 582–593. [Google Scholar] [CrossRef]
- Wohlrab, J.; Kreft, D. Niacinamide—Mechanisms of action and its topical use in dermatology. Skin Pharmacol. Physiol. 2014, 27, 311–315. [Google Scholar] [CrossRef] [PubMed]
- El-Nashar, H.A.S.; El-Din, M.I.G.; Hritcu, L.; Eldahshan, O.A. Insights on the Inhibitory Power of Flavonoids on Tyrosinase Activity: A Survey from 2016 to 2021. Molecules 2021, 26, 7546. [Google Scholar] [CrossRef]
- Liu, W.S.; Chen, M.C.; Chiu, K.H.; Wen, Z.H.; Lee, C.H. Amelioration of dextran sodium sulfate-induced colitis in mice by Rhodobacter sphaeroides extract. Molecules 2012, 17, 13622–13630. [Google Scholar] [CrossRef]
- Romagnani, S. Coming back to a missing immune deviation as the main explanatory mechanism for the hygiene hypothesis. J. Allergy Clin. Immunol. 2007, 119, 1511–1513. [Google Scholar] [CrossRef]
- Lopes, E.G.; Moreira, D.A.; Gullón, P.; Gullón, B.; Cardelle-Cobas, A.; Tavaria, F.K. Topical application of probiotics in skin: Adhesion, antimicrobial and antibiofilm in vitro assays. J. Appl. Microbiol. 2017, 122, 450–461. [Google Scholar] [CrossRef] [PubMed]
- Matsuura-Hachiya, Y.; Arai, K.Y.; Ozeki, R.; Kikuta, A.; Nishiyama, T. Angiotensin-converting enzyme inhibitor (enalapril maleate) accelerates recovery of mouse skin from UVB-induced wrinkles. Biochem. Biophys. Res. Commun. 2013, 442, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Wüthrich, B. Clinical aspects, epidemiology, and prognosis of atopic dermatitis. Ann. Allergy Asthma Immunol. 1999, 83, 464–470. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.H.; Choi, S.J.; Choi, H.I.; Choi, J.P.; Park, H.K.; Kim, E.K.; Kim, M.J.; Moon, B.S.; Min, T.K.; Rho, M.; et al. Lactobacillus plantarum-derived Extracellular Vesicles Protect Atopic Dermatitis Induced by Staphylococcus aureus-derived Extracellular Vesicles. Allergy Asthma Immunol. Res. 2018, 10, 516–532. [Google Scholar] [CrossRef] [PubMed]
- Di Marzio, L.; Centi, C.; Cinque, B.; Masci, S.; Giuliani, M.; Arcieri, A.; Zicari, L.; De Simone, C.; Cifone, M.G. Effect of the lactic acid bacterium Streptococcus thermophilus on stratum corneum ceramide levels and signs and symptoms of atopic dermatitis patients. Exp. Dermatol. 2003, 12, 615–620. [Google Scholar] [CrossRef] [PubMed]
- Muizzuddin, N.; Maher, W.; Sullivan, M.; Schnittger, S.; Mammone, T. Physiological effect of a probiotic on skin. J. Cosmet. Sci. 2012, 63, 385–395. [Google Scholar] [PubMed]
- Bowe, W.P.; Logan, A.C. Acne vulgaris, probiotics and the gut-brain-skin axis—Back to the future? Gut Pathog. 2011, 3, 1. [Google Scholar] [CrossRef]
- Fortuna, M.C.; Garelli, V.; Pranteda, G.; Romaniello, F.; Cardone, M.; Carlesimo, M.; Rossi, A. A case of Scalp Rosacea treated with low dose doxycycline and probiotic therapy and literature review on therapeutic options. Dermatol. Ther. 2016, 29, 249–251. [Google Scholar] [CrossRef]
- Kang, B.S.; Seo, J.G.; Lee, G.S.; Kim, J.H.; Kim, S.Y.; Han, Y.W.; Kang, H.; Kim, H.O.; Rhee, J.H.; Chung, M.J.; et al. Antimicrobial activity of enterocins from Enterococcus faecalis SL-5 against Propionibacterium acnes, the causative agent in acne vulgaris, and its therapeutic effect. J. Microbiol. 2009, 47, 101–109. [Google Scholar] [CrossRef]
- Park, M.J.; Bae, Y.S. Fermented Acanthopanax koreanum Root Extract Reduces UVB- and H2O2-Induced Senescence in Human Skin Fibroblast Cells. J. Microbiol. Biotechnol. 2016, 26, 1224–1233. [Google Scholar] [CrossRef]
- Shin, D.; Lee, Y.; Huang, Y.H.; Lim, H.W.; Jang, K.; Kim, D.D.; Lim, C.J. Probiotic fermentation augments the skin anti-photoaging properties of Agastache rugosa through up-regulating antioxidant components in UV-B-irradiated HaCaT keratinocytes. BMC Complement. Altern. Med. 2018, 18, 196. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.C.; Ng, S.C.; Chuang, H.L.; Wen, S.Y.; Kuo, C.H.; Mahalakshmi, B.; Huang, C.Y.; Kuo, W.W. Extracts of Jasminum sambac flowers fermented by Lactobacillus rhamnosus inhibit H2O2—And UVB-induced aging in human dermal fibroblasts. Environ. Toxicol. 2021, 36, 607–619. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.R.; Jeong, D.H.; Kim, S.; Lee, S.W.; Sin, H.S.; Yu, K.Y.; Jeong, S.I.; Kim, S.Y. Fermentation of Blackberry with L. plantarum JBMI F5 Enhance the Protection Effect on UVB-Mediated Photoaging in Human Foreskin Fibroblast and Hairless Mice through Regulation of MAPK/NF-κB Signaling. Nutrients 2019, 11, 2429. [Google Scholar] [CrossRef]
- Ha, J.H.; Kim, A.R.; Lee, K.S.; Xuan, S.H.; Kang, H.C.; Lee, D.H.; Cha, M.Y.; Kim, H.J.; An, M.; Park, S.N. Anti-Aging Activity of Lavandula angustifolia Extract Fermented with Pediococcus pentosaceus DK1 Isolated from Diospyros kaki Fruit in UVB-Irradiated Human Skin Fibroblasts and Analysis of Principal Components. J. Microbiol. Biotechnol. 2019, 29, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Y.; Hu, C.Y.; Chen, Y.H.; Li, Y.T.; Chung, Y.C. Submerged fermentation with Lactobacillus brevis significantly improved the physiological activities of Citrus aurantium flower extract. Heliyon 2022, 8, e10498. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.M.; Xu, P.F.; Cheng, M.Y.; Lei, S.N.; Liu, Q.L.; Wang, W. Optimization of Fermentation Process of Pomegranate Peel and Schisandra Chinensis and the Biological Activities of Fermentation Broth: Antioxidant Activity and Protective Effect against H2O2-induced Oxidative Damage in HaCaT Cells. Molecules 2021, 26, 3432. [Google Scholar] [CrossRef]
- Ikarashi, N.; Fukuda, N.; Ochiai, M.; Sasaki, M.; Kon, R.; Sakai, H.; Hatanaka, M.; Kamei, J. Lactobacillus helveticus-Fermented Milk Whey Suppresses Melanin Production by Inhibiting Tyrosinase through Decreasing MITF Expression. Nutrients 2020, 12, 2082. [Google Scholar] [CrossRef]
- Fu, H.; Zhang, Y.; An, Q.; Wang, D.; You, S.; Zhao, D.; Zhang, J.; Wang, C.; Li, M. Anti-Photoaging Effect of Rhodiola rosea Fermented by Lactobacillus plantarum on UVA-Damaged Fibroblasts. Nutrients 2022, 14, 2324. [Google Scholar] [CrossRef]

| Product ID | Cosmetic Effects (Manufacturer′s Declaration) | Type of Product | Cosmetic Ingredient List |
|---|---|---|---|
| 1 | Skin feels soft, moisturized, and revitalized. Restore skin barrier function and seal in moisture on the skin’s surface. Skin relief and a reduction in potential irritation. | Lotion | Lactobacillus ferment |
| 2 | Enhance wrinkles, unwind, and keep skin looking young. Enhance wrinkles, unwind, and keep skin looking young. | Lotion | Lactococcus ferment extract Yeast cytolytic extract |
| 3 | To stop dry air from the outside, stop water loss and create a water-locking barrier on the skin’s surface. | Lotion | Lactobacillus ferment hyaluronic acid |
| 4 | Maintain the balance between the skin’s water and oil content. Improved skin stability. Revitalize and repair skin. | Toner | Bifida ferment lysate * |
| 5 | Multidimensional repair of skin fragility and relieving skin discomfort. | Cream | Alteromonas Baumann ferment extract |
| 6 | Adjust skin and facial flora, inhibit acne inflammation root, improve redness and sensitivity, and enhance skin defense. | Cream | Lactobacillus extract |
| 7 | Balance skin micro-ecological environment and repair the micro-ecological barrier. Intensive moisture lock water and reduce skin moisture loss. | Cream | Leuconostoc ferment filtrate Prebiotics: pentavitin |
| 8 | Balance skin flora and improve skin health. | Cream | Lactobacillus/soymilk ferment filtrate |
| 9 | Balance skin flora and improve skin health. | Cleanser | Lactobacillus/soymilk ferment filtrate Candida bobicola/glucose/methyl Rapeseedate ferment |
| 10 | Improve skin antioxidant capacity. | Cream | Lactococcus ferment Bacillus ferment |
| 11 | Produce acne suppressor, help regulate the skin surface flora, and reduce the risk of acne. | Cream | Bifidobacterium longum, lysate |
| 12 | Repair skin barrier, and relieve redness and discomfort. | Mask | Vitreoscilla ferment |
| 13 | Promotes collagen regeneration and smoothes wrinkles. | Lotion | Bifidobacterium longum, lysate Yeast extract Lactococcus ferment |
| 14 | Reduce skin redness and sensitivity. Inhibiting skin oxidation factor. | Serum | PITERATM |
| 15 | Regulate skin flora, strengthen the micro-ecological barrier, and improve skin condition. | Lotion | Lactobacillus/soymilk ferment filtrate Bifidobacterium longum, lysate |
| 16 | Strengthen the skin barrier and improve the skin quality. Strengthen the muscle base, firm the skin, and delicate skin. Promote collagen regeneration. | Serum | Bifidobacterium longum, lysate Yeast extract Vitreoscilla ferment |
| 17 | Repair the skin barrier. | Serum | Bifidobacterium longum, lysate Yeast extract Lactobacillus |
| 18 | Maintain skin micro-ecological balance and adjust skin flora. | Serum | Lactobacillus/soymilk ferment filtrate |
| 19 | Prevent the imbalance of micro-ecological barriers. Balance bacteria symbiosis, improve skin redness. | Serum | Lactobacillus ferment lysate |
| 20 | Enhance the outer strength of the skin. | Mask | Lactobacillus |
| 21 | After fermentation, lactic acid bacteria present a protective film to prevent water evaporation, water tender, and shining through the skin, and strengthen the skin cuticle. | Cream | Lactococcus ferment lysate Bifida ferment extract |
| 22 | Activate skin micro-ecological activity and inhibit harmful bacteria reproduction, and the skin becomes soft and delicate. | Lotion | Lactobacillus |
| 23 | Balance the micro-ecological barrier to help reduce repeated breakouts. Balance water and oil, stabilize skin, and maintain skin health. | Gel Cream Toner | Lactobacillus Lactobacillus ferment Lactococcus ferment lysate Prebiotics: chicory root extract |
| 24 | Make the skin moist and tender. Maintain skin elasticity and permeability. | Cream Cleansing | Original nourishing ingredient: S.E. essence Bifidobacterium/soybean ferment Lactococcus/milk ferment |
| 25 | Balance skin micro-ecology and make skin healthy and delicate. | Serum Cream | NATURAL RETM: Lactobacillus complex extract |
| 26 | Stong microbial barrier. Maintain the balance of bacteria and stabilize the healthy state of the skin. | Cleansing Toner | Lactobacillus Lactococcus ferment lysate Lactobacillus/soymilk ferment filtrate Saccharomyces/rice ferment filtrate |
| 27 | Prebiotics: maintain skin micro-ecological health. Biostime: provides an environment for probiotics to grow. | Serum Cream | Inulin Alpha-glucan oligosaccharide Yeast ferment extract filtrate Lactobacillus/soymilk ferment lysate Lactobacillus/soybean extract ferment filtrate. Lactobacillus/punica granatum feuit ferment extract |
| 28 | Extracted from the fermentation of lactic acid bacteria, it helps to reduce the effects of toxins and maintain the balance and integrity of the skin microbiome. | Serum | Lactococcus ferment extract |
| 29 | Firming skin and energizing bacteria. | Serum | Plant-derived probiotics: chicory root |
| 30 | Reduce skin redness, acne, and sunburn. Restore skin elasticity. | Serum | Lactococcus lactis fermentation lysate |
| 31 | Maintain and counterbalance facial symbiotic bacteria. Help restore the dynamic balance of bacteria on the skin surface and create a stable skin microenvironment. | Cleaning Serum Cream | Lactobacillus/soymilk ferment filtrate Lactobacillus |
| 32 | Balance skin flora and prevent aging. | Serum | Bifida ferment lysate |
| 33 | Regulate skin pH. | Cleaning | Bifidobacterium longum, lysate Lactobacillus ferment Lactobacillus ferment lysate Streptococcus thermophilus ferment Lactobacillus/soybean extract ferment |
| 34 | Maintain the balance of bacteria and stabilize the healthy state of the skin. | Serum | Bifida ferment lysate |
| 35 | Accelerate collagen regeneration and effectively improve skin fullness. | Cream | Bifidobacterium longum, lysate |
| Activity | Experimental Model | Type of Probiotics /Active Constituent | Mechanism of Action/Effect | References | |
|---|---|---|---|---|---|
| In Vitro | In Vivo/Ex Vivo | ||||
| Antioxidant | Human dermal fibroblast (HDF) | Lipoteichoic acid isolated from Lactobacillus plantarum(LTA)
|
| [14] | |
| Normal human dermal fibroblast (NHDF) cells B16F10 murine melanoma cells | Heat-killed Lacticaseibacillus paracasei (PL) |
| [15] | ||
| Murine | Nicotinamide mononucleotide (NMN) combined with Lactobacillus fermentum TKSN041 |
| [16] | ||
| Mouse skin fibroblast (MSF) cells Human epidermal melanocytes (HEM) | Heat-killed L. rhamnosus ATCC 7469 (RL) |
| [17] | ||
| Human keratinocytes Human dermal fibroblasts B16F10 murine melanoma cells | Tyndallized Lactobacillus acidophilus KCCM12625P (AL) |
| [18] | ||
| Anti-aging | HS68 cells dermal fibroblast cells | Extracts of Jasminum sambac flowers fermented by Lactobacillus rhamnosus |
| [19] | |
| Streptococcus salivarium spp. Streptococcus thermophilus S244 | Significant increase in skin moisture (immediate and long-term). | [20] | |||
| Hs68 cells Human dermal fibroblasts | Hairless mice | Administered vehicle or L. plantarum HY7714
|
| [21,22] | |
| Human foreskin fibroblast (Hs68) | SKH-1 hairless mice | Fermented blackberry (FBB) by L. plantarum JBMI F5 -FBB pretreatment -FBB administration |
| [23] | |
| HaCaT cells | SKH-1 hairless mice Volunteers | Kimchi-derived L. plantarum K8 lysates
|
| [24,25] | |
| Double-blind, placebo-controlled trial Japanese women volunteers(aged 31–62 years) 8-week treatment | Heat-killed cells of Lactococcus lactis strain H61
|
| [24,25] | ||
| UVB-irradiated normal human epidermal keratinocytes (NHEKs) | Cosmetic preparation that contained Water extract from heat-killed L.lactis H61 |
| [26] | ||
| Primary epidermal cells | Hairless mice | Heat-killed L. plantarum L-137 |
| [27] | |
| Clinical trials | Extracellular vesicles (EVs) that were secreted from L. plantarum of women in their 20s (LpEVs) |
| [28] | ||
| Spot removing and whitening | Cultures of Bifidobacterium bifidum IDCC4201 and Lactiplantibacillus plantarum IDCC 3501
|
| [29] | ||
| Kimchi-derived Pediococcus acidilactici PMC48 |
| [30] | |||
| B16F10 mouse melanoma cells | Lipoteichoic acid (LTA) isolated from Lactobacillus plantarum (pLTA) |
| [31] | ||
| Clinical observation | L. plantarum-GMNL6
|
| [32] | ||
| Anti-melanogenic signaling pathway in α-melanocyte stimulating hormone (α-MSH)-treated B16F10 melanoma cells and zebrafish | Extracts of Rhodobacter sphaeroides (Lycogen™) |
| [33] | ||
| Anti- inflammatory | Twenty-seven AD patients and six healthy control subjects Staphylococcus aureus-induced mouse AD models | Lactobacillus plantarum-derived extracellular vesicles
|
| [34] | |
| Ex vivo skin models | Live and the lysate products of probiotic strain Lactobacillus reuteri DSM 17938 |
in a healthy (unstimulated) state of RHE. | [35] | ||
| Human keratinocytes | In the forearm skin of 11 atopic dermatitis (AD) patients stratum corneum 20 healthy elderly women. | An experimental cream containing sonicated Streptococcus thermophilus
|
| [36] | |
| Nerve cell cultures in vitro | Ex vivo human skin explant model Sixty-six female volunteers | Bifidobacterium longum sp. extract (BL)
|
| [37] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dou, J.; Feng, N.; Guo, F.; Chen, Z.; Liang, J.; Wang, T.; Guo, X.; Xu, Z. Applications of Probiotic Constituents in Cosmetics. Molecules 2023, 28, 6765. https://doi.org/10.3390/molecules28196765
Dou J, Feng N, Guo F, Chen Z, Liang J, Wang T, Guo X, Xu Z. Applications of Probiotic Constituents in Cosmetics. Molecules. 2023; 28(19):6765. https://doi.org/10.3390/molecules28196765
Chicago/Turabian StyleDou, Jiaxin, Ning Feng, Fangyu Guo, Zouquan Chen, Jie Liang, Ting Wang, Xueping Guo, and Zhenshang Xu. 2023. "Applications of Probiotic Constituents in Cosmetics" Molecules 28, no. 19: 6765. https://doi.org/10.3390/molecules28196765
APA StyleDou, J., Feng, N., Guo, F., Chen, Z., Liang, J., Wang, T., Guo, X., & Xu, Z. (2023). Applications of Probiotic Constituents in Cosmetics. Molecules, 28(19), 6765. https://doi.org/10.3390/molecules28196765






