Comparison of the Chemical Composition and Antioxidant Properties of Propolis from Urban Apiaries
Abstract
:1. Introduction
2. Results
2.1. Chemical Composition of Urban Propolis Extracts
2.2. Antioxidant Activity of Urban Propolis Extracts
3. Discussion
4. Materials and Methods
4.1. Urban Propolis Sample
4.2. Preparation of Urban Propolis Extracts
4.3. Phenolic Compounds Determined by HPLC-DAD
4.3.1. Reagents and Standards
4.3.2. Chromatography Conditions
4.3.3. Preparation of Standard Solutions
4.4. Antioxidant Analysis of Urban Propolis Extract
4.4.1. Determination of the Antioxidant Capacity against DPPH and ABTS Radicals
4.4.2. Determination of the Antioxidant Capacity of FRAP Assay
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Wagh, V.D. Propolis: A Wonder Bees Product and Its Pharmacological Potentials. Adv. Pharmacol. Sci. 2013, 2013, 308249. [Google Scholar] [CrossRef]
- Ristivojević, P.; Trifković, J.; Andrić, F.; Milojković-Opsenica, D. Poplar-type propolis: Chemical composition, botanical origin and biological activity. Nat. Prod. Commun. 2015, 10, 1869–1876. [Google Scholar] [CrossRef]
- Machado, C.S.; Mokochinski, J.B.; de Lira, T.O.; Oliveira, F.d.C.E.d.; Cardoso, M.V.; Ferreira, R.G.; Sawaya, A.C.H.F.; Ferreira, A.G.; Pessoa, C.; Cuesta-Rubio, O.; et al. Comparative Study of Chemical Composition and Biological Activity of Yellow, Green, Brown, and Red Brazilian Propolis. Evid. Based Complement. Altern. Med. 2016, 2016, 6057650. [Google Scholar] [CrossRef]
- Sforcin, J.M.; Bankova, V. Propolis: Is there a potential for the development of new drugs? J. Ethnopharmacol. 2011, 133, 253–260. [Google Scholar] [CrossRef]
- Shahinozzaman, M.; Obanda, D.N.; Tawata, S. Chemical composition and pharmacological properties of Macaranga-type Pacific propolis: A review. Phytother. Res. 2021, 35, 207–222. [Google Scholar] [CrossRef]
- Moore, L.J.; Kosut, M. Buzz: Urban Beekeeping and the Power of the Bee; NYU Press: New York, NY, USA, 2013. [Google Scholar]
- Lorenz, S.; Stark, K. Saving the honeybees in Berlin? A case study of the urban beekeeping boom. Environ. Sociol. 2015, 1, 116–126. [Google Scholar] [CrossRef]
- Ropars, L.; Dajoz, I.; Fontaine, C.; Muratet, A.; Geslin, B. Wild pollinator activity negatively related to honey bee colony densities in urban context. PLoS ONE 2019, 14, e0222316. [Google Scholar] [CrossRef]
- Roest, P.B. Bees in the D: A Message of Conservation from an Urban Environment. Challenges 2019, 10, 19. [Google Scholar] [CrossRef]
- Stevenson, P.C.; Bidartondo, M.I.; Blackhall-Miles, R.; Cavagnaro, T.R.; Cooper, A.; Geslin, B.; Koch, H.; Lee, M.A.; Moat, J.; O’hanlon, R.; et al. The state of the world’s urban ecosystems: What can we learn from trees, fungi, and bees? Plants People Planet 2020, 2, 482–498. [Google Scholar] [CrossRef]
- Smith, T.J.; Saunders, M.E. Honey bees: The queens of mass media, despite minority rule among insect pollinators. Insect Conserv. Divers. 2016, 9, 384–390. [Google Scholar] [CrossRef]
- Alqarni, A.S.; Rushdi, A.I.; Owayss, A.A.; Raweh, H.S.; El-Mubarak, A.H.; Simoneit, B.R.T. Organic Tracers from Asphalt in Propolis Produced by Urban Honey Bees, Apis mellifera Linn. PLoS ONE 2015, 10, e0128311. [Google Scholar] [CrossRef] [PubMed]
- Cox-Foster, D.L.; Conlan, S.; Holmes, E.C.; Palacios, G.; Evans, J.D.; Moran, N.A.; Quan, P.-L.; Briese, T.; Hornig, M.; Geiser, D.M.; et al. A Metagenomic Survey of Microbes in Honey Bee Colony Collapse Disorder. Science 2007, 318, 283–287. [Google Scholar] [CrossRef] [PubMed]
- Nicewicz, Ł.; Nicewicz, A.W.; Kafel, A.; Nakonieczny, M. Set of stress biomarkers as a practical tool in the assessment of multistress effect using honeybees from urban and rural areas as a model organism: A pilot study. Environ. Sci. Pollut. Res. 2021, 28, 9084–9096. [Google Scholar] [CrossRef]
- Adaškevičiūtė, V.; Kaškonienė, V.; Kaškonas, P.; Barčauskaitė, K.; Maruška, A. Comparison of Physicochemical Properties of Bee Pollen with Other Bee Products. Biomolecules 2019, 9, 819. [Google Scholar] [CrossRef] [PubMed]
- Nicewicz, A.W.; Nicewicz, Ł.; Pawłowska, P. Antioxidant capacity of honey from the urban apiary: A comparison with honey from the rural apiary. Sci. Rep. 2021, 11, 9695. [Google Scholar] [CrossRef]
- Bankova, V. Chemical diversity of propolis and the problem of standardization. J. Ethnopharmacol. 2005, 100, 114–117. [Google Scholar] [CrossRef]
- Puspha, T.C.; Reddy, M.S. Pharmacological potentials of Propolis in urban landscapes. GSC Biol. Pharm. Sci. 2019, 08, 134–138. [Google Scholar] [CrossRef]
- Hodel, K.V.S.; Machado, B.A.S.; Santos, N.R.; Costa, R.G.; Menezes-Filho, J.A.; Umsza-Guez, M.A. Metal Content of Nutritional and Toxic Value in Different Types of Brazilian Propolis. Sci. World J. 2020, 2020, 4395496. [Google Scholar] [CrossRef]
- Yong, H.; Liu, J. Active packaging films and edible coatings based on polyphenol-rich propolis extract: A review. Compr. Rev. Food Sci. Food Saf. 2021, 20, 2106–2145. [Google Scholar] [CrossRef]
- Pobiega, K.; Kraśniewska, K.; Przybył, J.L.; Bączek, K.; Żubernik, J.; Witrowa-Rajchert, D.; Gniewosz, M. Growth Biocontrol of Foodborne Pathogens and Spoilage Microorganisms of Food by Polish Propolis Extracts. Molecules 2019, 24, 2965. [Google Scholar] [CrossRef]
- Pobiega, K.; Przybył, J.L.; Żubernik, J.; Gniewosz, M. Prolonging the Shelf Life of Cherry Tomatoes by Pullulan Coating with Ethanol Extract of Propolis During Refrigerated Storage. Food Bioprocess Technol. 2020, 13, 1447–1461. [Google Scholar] [CrossRef]
- Pobiega, K.; Igielska, M.; Włodarczyk, P.; Gniewosz, M. The use of pullulan coatings with propolis extract to extend the shelf life of blueberry (Vaccinium corymbosum) fruit. Int. J. Food Sci. Technol. 2021, 56, 1013–1020. [Google Scholar] [CrossRef]
- Gniewosz, M.; Pobiega, K.; Kraśniewska, K.; Synowiec, A.; Chaberek, M.; Galus, S. Characterization and Antifungal Activity of Pullulan Edible Films Enriched with Propolis Extract for Active Packaging. Foods 2022, 11, 2319. [Google Scholar] [CrossRef] [PubMed]
- Woźniak, M.; Sip, A.; Mrówczyńska, L.; Broniarczyk, J.; Waśkiewicz, A.; Ratajczak, I. Biological Activity and Chemical Composition of Propolis from Various Regions of Poland. Molecules 2023, 28, 141. [Google Scholar] [CrossRef]
- Miłek, M.; Ciszkowicz, E.; Tomczyk, M.; Sidor, E.; Zaguła, G.; Lecka-Szlachta, K.; Pasternakiewicz, A.; Dżugan, M. The Study of Chemical Profile and Antioxidant Properties of Poplar-Type Polish Propolis Considering Local Flora Diversity in Relation to Antibacterial and Anticancer Activities in Human Breast Cancer Cells. Molecules 2022, 27, 725. [Google Scholar] [CrossRef]
- Guzelmeric, E.; Sipahi, H.; Özhan, Y.; Hamitoğlu, M.; Helvacıoğlu, S.; Düz, G.; Akyıldız, I.E.; Yaman, B.K.; Hazar, M.; Dilsiz, S.A.; et al. Comprehensive estrogenic/anti-estrogenic, anticancer, mutagenic/anti-mutagenic, and genotoxic/anti-genotoxic activity studies on chemically characterized black poplar and Eurasian aspen propolis types. J. Pharm. Biomed. Anal. 2023, 226, 115241. [Google Scholar] [CrossRef] [PubMed]
- Wołosiak, R.; Drużyńska, B.; Derewiaka, D.; Piecyk, M.; Majewska, E.; Ciecierska, M.; Worobiej, E.; Pakosz, P. Verification of the Conditions for Determination of Antioxidant Activity by ABTS and DPPH Assays—A Practical Approach. Molecules 2022, 27, 50. [Google Scholar] [CrossRef] [PubMed]
- Siheri, W.; Alenezi, S.; Tusiimire, J.; Watson, D.G. Bee Products—Chemical and Biological Properties. In The Chemical and Biological Properties of Propolis; Alvarez-Suarez, J., Ed.; Springer: Cham, Switzerland, 2017; pp. 137–178. [Google Scholar] [CrossRef]
- Ebiloma, G.U.; Ichoron, N.; Siheri, W.; Watson, D.G.; Igoli, J.O.; De Koning, H.P. The Strong Anti-Kinetoplastid Properties of Bee Propolis: Composition and Identification of the Active Agents and Their Biochemical Targets. Molecules 2020, 25, 5155. [Google Scholar] [CrossRef]
- Cao, X.-P.; Chen, Y.-F.; Zhang, J.-L.; You, M.-M.; Wang, K.; Hu, F.-L. Mechanisms underlying the wound healing potential of propolis based on its in vitro antioxidant activity. Phytomedicine 2017, 34, 76–84. [Google Scholar] [CrossRef]
- Fathi Hafshejani, S.; Lotfi, S.; Rezvannejad, E.; Mortazavi, M.; Riahi-Madvar, A. Correlation between total phenolic and flavonoid contents and biological activities of 12 ethanolic extracts of Iranian propolis. Food Sci. Nutr. 2023, 11, 4308–4325. [Google Scholar] [CrossRef]
- Paula, V.B.; Estevinho, L.M.; Cardoso, S.M.; Dias, L.G. Comparative Methods to Evaluate the Antioxidant Capacity of Propolis: An Attempt to Explain the Differences. Molecules 2023, 28, 4847. [Google Scholar] [CrossRef] [PubMed]
- Tumbarski, Y.; Todorova, M.; Topuzova, M.; Gineva, G.; Yanakieva, V.; Ivanov, I.; Petkova, N. Comparative Study on Physicochemical, Antioxidant and Antimicrobial Properties of Propolis Collected from Different Regions of Bulgaria. J. Apic. Sci. 2023, 67, 37–56. [Google Scholar] [CrossRef]
- Wiktor, A.; Chadzynska, M.; Rybak, K.; Dadan, M.; Witrowa-Rajchert, D.; Nowacka, M. The Influence of Polyols on the Process Kinetics and Bioactive Substance Content in Osmotic Dehydrated Organic Strawberries. Molecules 2022, 27, 1376. [Google Scholar] [CrossRef] [PubMed]
- Xiao, F.; Xu, T.; Lu, B.; Liu, R. Guidelines for antioxidant assays for food components. Food Front. 2020, 1, 60–69. [Google Scholar] [CrossRef]

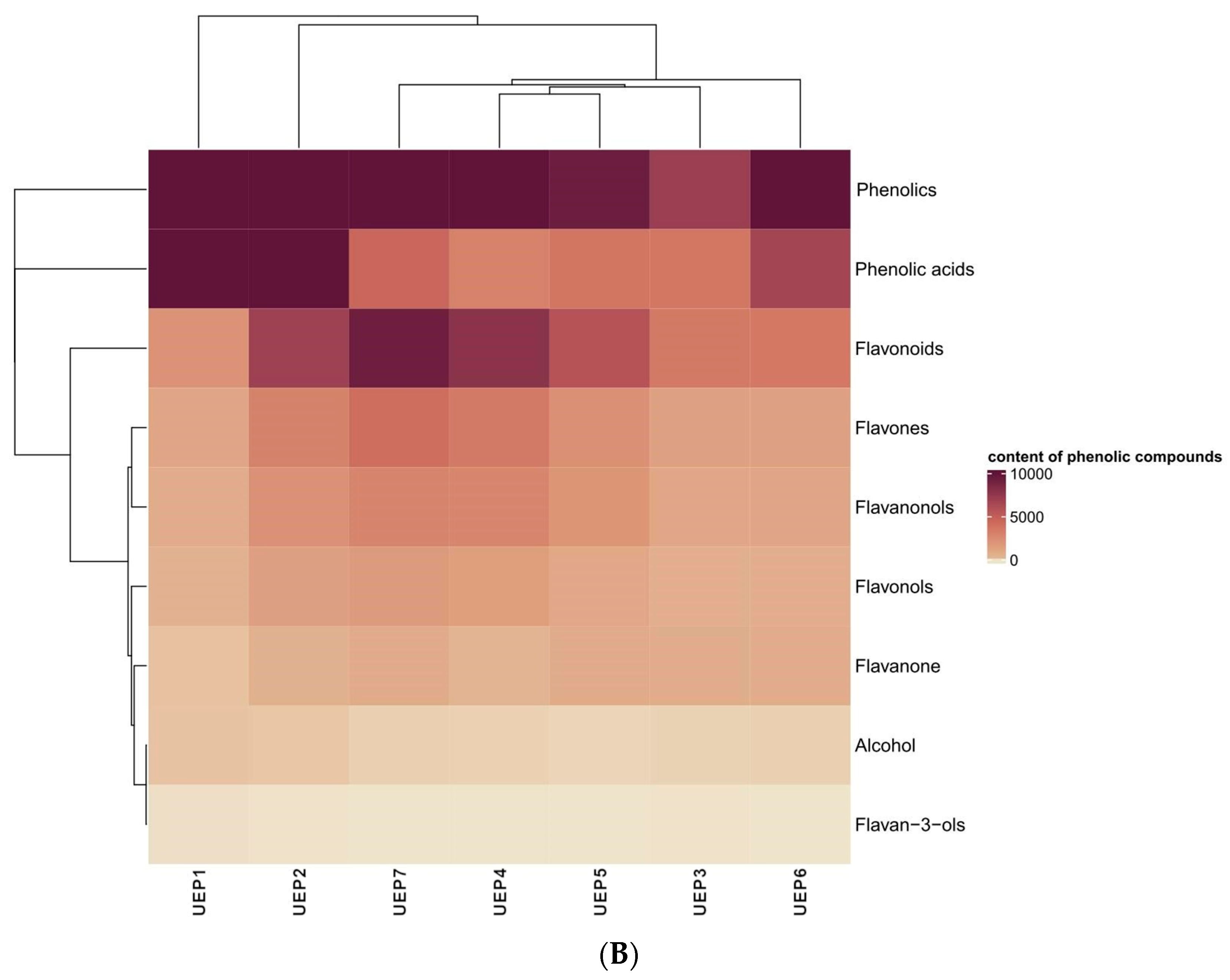


| Lp. | Compound | UEP 1 | UEP 2 | UEP 3 | UEP 4 | UEP 5 | UEP 6 | UEP 7 | |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 3,4-Dihydroxybenzoic acid | 3,4Ba | 18.82 ± 0.04 | 0.00 ± 0.00 | 4.11 ± 0.01 | 8.46 ± 0.00 | 5.13 ± 0.01 | 5.68 ± 0.01 | 7.61 ± 0.05 |
| 2 | (+)-Catechin | Cat | 0.54 ± 0.04 | 0.09 ± 0.01 | 0.19 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| 3 | 4-Hydroxybenzoic acid | 4hBa | 589.99 ± 12.31 | 202.33 ± 4.38 | 66.55 ± 0.83 | 100.83 ± 2.60 | 241.93 ± 2.02 | 166.47 ± 2.58 | 68.05 ± 2.17 |
| 4 | 3,4-Dihydroxycinnamic acid | 3,4Ca | 2039.50 ± 65.42 | 875.55 ± 4.54 | 474.50 ± 6.59 | 1035.53 ± 32.95 | 539.75 ± 23.29 | 441.47 ± 12.42 | 968.90 ± 38.20 |
| 5 | 4-Hydroxy-3-Methoxybenzoic acid | 4h3mBa | 99.89 ± 1.54 | 117.51 ± 0.39 | 53.98 ± 1.18 | 18.89 ± 0.22 | 52.88 ± 1.96 | 122.75 ± 2.92 | 19.45 ± 0.29 |
| 6 | 4-Hydroxy-3,5-Dimethoxybenzoic acid | 4h3,5mBa | 1007.43 ± 18.44 | 361.69 ± 7.37 | 177.18 ± 3.80 | 234.85 ± 5.02 | 181.48 ± 2.25 | 403.11 ± 11.04 | 213.59 ± 3.82 |
| 7 | 4-Hydroxycinnamic acid | 4hCa | 5643.57 ± 172.92 | 4218.83 ± 45.63 | 2494.79 ± 76.09 | 989.34 ± 24.55 | 2334.70 ± 35.99 | 3212.36 ± 93.14 | 1815.47 ± 43.87 |
| 8 | 4-Hydroxy-3-Methoxycinnamic acid | 4h3mCa | 5348.30 ± 145.74 | 4 152.01 ± 60.07 | 265.15 ± 8.67 | 142.55 ± 4.09 | 233.67 ± 1.00 | 2 071.96 ± 27.08 | 986.70 ± 40.01 |
| 9 | Ellagic acid dihydrate | Ea | 176.79 ± 2.13 | 12.81 ± 0.09 | 1.43 ± 0.00 | 1.42 ± 0.03 | 1.87 ± 0.02 | 9.04 ± 0.11 | 56.22 ± 1.71 |
| 10 | Cichoric acid | Cia | 84.29 ± 1.73 | 56.50 ± 0.62 | 17.74 ± 0.25 | 19.87 ± 0.32 | 37.56 ± 1.60 | 64.81 ± 2.33 | 21.68 ± 0.34 |
| 11 | 3,4-dimetoxycinnamic acid | 3,4mCa | 269.14 ± 7.30 | 160.58 ± 0.75 | 19.65 ± 0.12 | 255.73 ± 7.89 | 23.68 ± 0.17 | 54.76 ± 1.66 | 251.79 ± 5.23 |
| 12 | Cinnamyl alcohol | CiEtOH | 20.42 ± 0.51 | 11.79 ± 0.28 | 4.26 ± 0.06 | 4.49 ± 0.31 | 3.15 ± 0.08 | 5.94 ± 0.10 | 5.12 ± 0.21 |
| 13 | Cinnamic acid | Ca | 312.63 ± 7.40 | 96.98 ± 2.04 | 20.26 ± 0.23 | 110.39 ± 1.31 | 26.94 ± 0.52 | 105.93 ± 2.94 | 40.98 ± 1.34 |
| 14 | 4-Methoxycinnamic acid | 4mCa | 50.05 ± 1.47 | 25.77 ± 0.82 | 5.95 ± 0.11 | 79.10 ± 2.41 | 2.27 ± 0.10 | 7.45 ± 0.17 | 62.04 ± 2.68 |
| 15 | Quercetin | Qu | 77.15 ± 2.00 | 118.29 ± 3.74 | 47.82 ± 1.42 | 215.41 ± 6.95 | 58.18 ± 2.59 | 60.36 ± 2.41 | 104.25 ± 3.41 |
| 16 | Pinobanksin | PinB | 360.35 ± 11.88 | 581.94 ± 1.55 | 288.15 ± 4.03 | 687.32 ± 81.39 | 463.26 ± 21.47 | 268.38 ± 5.21 | 825.30 ± 40.81 |
| 17 | Apigenin | Api | 140.82 ± 4.22 | 413.96 ± 7.49 | 203.29 ± 3.80 | 302.48 ± 7.87 | 197.56 ± 1.41 | 175.60 ± 4.41 | 366.40 ± 5.79 |
| 18 | Kaempferol | Kam | 110.49 ± 6.89 | 284.08 ± 3.25 | 32.41 ± 1.48 | 241.40 ± 7.83 | 180.45 ± 1.33 | 151.64 ± 3.46 | 285.76 ± 3.91 |
| 19 | Isorhamnetin | isRh | 11.11 ± 0.23 | 22.28 ± 0.47 | 10.71 ± 0.10 | 41.73 ± 3.30 | 12.36 ± 0.18 | 11.48 ± 0.29 | 29.92 ± 1.52 |
| 20 | 5,7-Dihydroxyflavone | 5,7hFl | 430.33 ± 14.15 | 1857.32 ± 71.14 | 575.93 ± 12.23 | 1338.46 ± 75.94 | 703.37 ± 17.99 | 615.75 ± 20.03 | 2127.38 ± 63.68 |
| 21 | Pinocembrin | PinC | 286.15 ± 6.75 | 1524.08 ± 56.92 | 630.58 ± 21.43 | 2026.67 ± 52.88 | 1441.33 ± 36.19 | 746.47 ± 28.76 | 2022.86 ± 44.32 |
| 22 | Methyl-4’-Apigenin | 4mApi | 389.65 ± 20.13 | 409.34 ± 10.23 | 292.31 ± 10.07 | 1771.91 ± 46.71 | 1217.90 ± 15.34 | 349.04 ± 4.94 | 1325.88 ± 38.07 |
| 23 | Galangin | Gal | 160.83 ± 5.26 | 983.10 ± 43.44 | 475.33 ± 14.29 | 933.42 ± 68.34 | 637.95 ± 26.25 | 409.61 ± 13.80 | 1235.31 ± 37.98 |
| 24 | O-methylated flavone | OraA | 41.84 ± 1.07 | 281.04 ± 7.46 | 195.28 ± 1.50 | 46.93 ± 1.20 | 87.35 ± 2.07 | 131.82 ± 3.39 | 242.83 ± 4.44 |
| 25 | (+/−)-Pinostrobin | PinS | 24.45 ± 0.78 | 442.96 ± 5.31 | 690.19 ± 12.55 | 270.78 ± 9.41 | 727.94 ± 9.90 | 636.73 ± 28.27 | 746.98 ± 35.41 |
| Alcohol | 20.42 | 11.79 | 4.26 | 4.49 | 3.15 | 5.94 | 5.12 | ||
| Flavan-3-ols | 0.54 | 0.09 | 0.19 | 0.00 | 0.00 | 0.00 | 0.00 | ||
| Flavanone | 24.45 | 442.96 | 690.19 | 270.78 | 727.94 | 636.73 | 746.98 | ||
| Flavanonols | 646.50 | 2106.02 | 918.72 | 2713.99 | 1904.59 | 1014.84 | 2848.16 | ||
| Flavones | 1002.65 | 2961.65 | 1266.81 | 3459.78 | 2206.18 | 1272.20 | 4062.48 | ||
| Flavonols | 359.58 | 1407.75 | 566.26 | 1431.96 | 888.94 | 633.10 | 1655.24 | ||
| Total Phenolic acids | 15,640.41 | 10,280.55 | 3601.29 | 2996.95 | 3681.86 | 6665.79 | 4512.47 | ||
| Total Flavonoids | 2033.72 | 6918.47 | 3442.18 | 7876.50 | 5727.65 | 3556.87 | 9312.87 | ||
| Total Phenolics | 17,674.13 | 17,199.02 | 7043.47 | 10,873.45 | 9409.51 | 10,222.66 | 13,825.34 |
| UEP 1 * | UEP 2 | UEP 3 | UEP 4 | UEP 5 | UEP 6 | UEP 7 | |
|---|---|---|---|---|---|---|---|
| [mg Te/mL] | |||||||
| ABTS | 23.21 ± 1.30 b | 26.64 ± 0.58 bc | 47.91 ± 0.93 d | 33.47 ± 1.94 c | 16.80 ± 0.74 a | 44.23 ± 0.42 d | 51.53 ± 2.29 e |
| DPPH | 7.82 ± 0.45 a | 11.71 ± 0.49 b | 19.93 ± 0.49 d | 19.18 ± 0.45 d | 7.54 ± 0.44 a | 22.13 ± 0.45 e | 17.19 ± 1.23 c |
| FRAP | 13.30 ± 0.22 b | 15.97 ± 0.28 c | 28.82 ± 0.60 e | 23.49 ± 0.19 d | 10.93 ± 0.46 a | 29.55 ± 0.38 ef | 27.41 ± 0.86 de |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pobiega, K.; Kot, A.M.; Przybył, J.L.; Synowiec, A.; Gniewosz, M. Comparison of the Chemical Composition and Antioxidant Properties of Propolis from Urban Apiaries. Molecules 2023, 28, 6744. https://doi.org/10.3390/molecules28186744
Pobiega K, Kot AM, Przybył JL, Synowiec A, Gniewosz M. Comparison of the Chemical Composition and Antioxidant Properties of Propolis from Urban Apiaries. Molecules. 2023; 28(18):6744. https://doi.org/10.3390/molecules28186744
Chicago/Turabian StylePobiega, Katarzyna, Anna M. Kot, Jarosław L. Przybył, Alicja Synowiec, and Małgorzata Gniewosz. 2023. "Comparison of the Chemical Composition and Antioxidant Properties of Propolis from Urban Apiaries" Molecules 28, no. 18: 6744. https://doi.org/10.3390/molecules28186744
APA StylePobiega, K., Kot, A. M., Przybył, J. L., Synowiec, A., & Gniewosz, M. (2023). Comparison of the Chemical Composition and Antioxidant Properties of Propolis from Urban Apiaries. Molecules, 28(18), 6744. https://doi.org/10.3390/molecules28186744










