Aquatic Peptide: The Potential Anti-Cancer and Anti-Microbial Activity of GE18 Derived from Pathogenic Fungus Aphanomyces invadans
Abstract
:1. Introduction
2. Results
2.1. In Silico Investigation of the Protein and GE18 Peptide
2.2. Peptide Toxicity Analysis
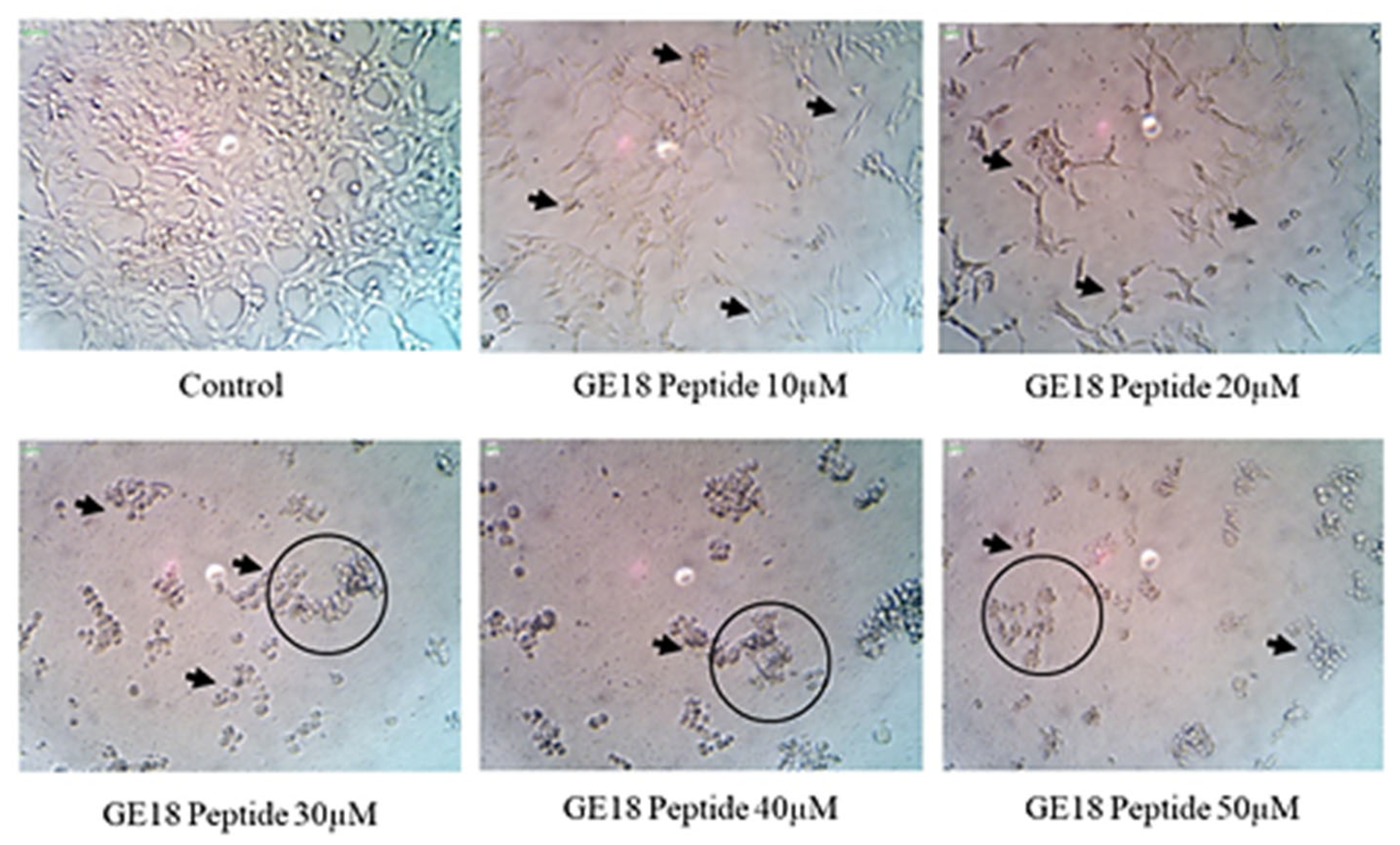
2.2.1. Effect of Peptide Toxicity on L6 Cells
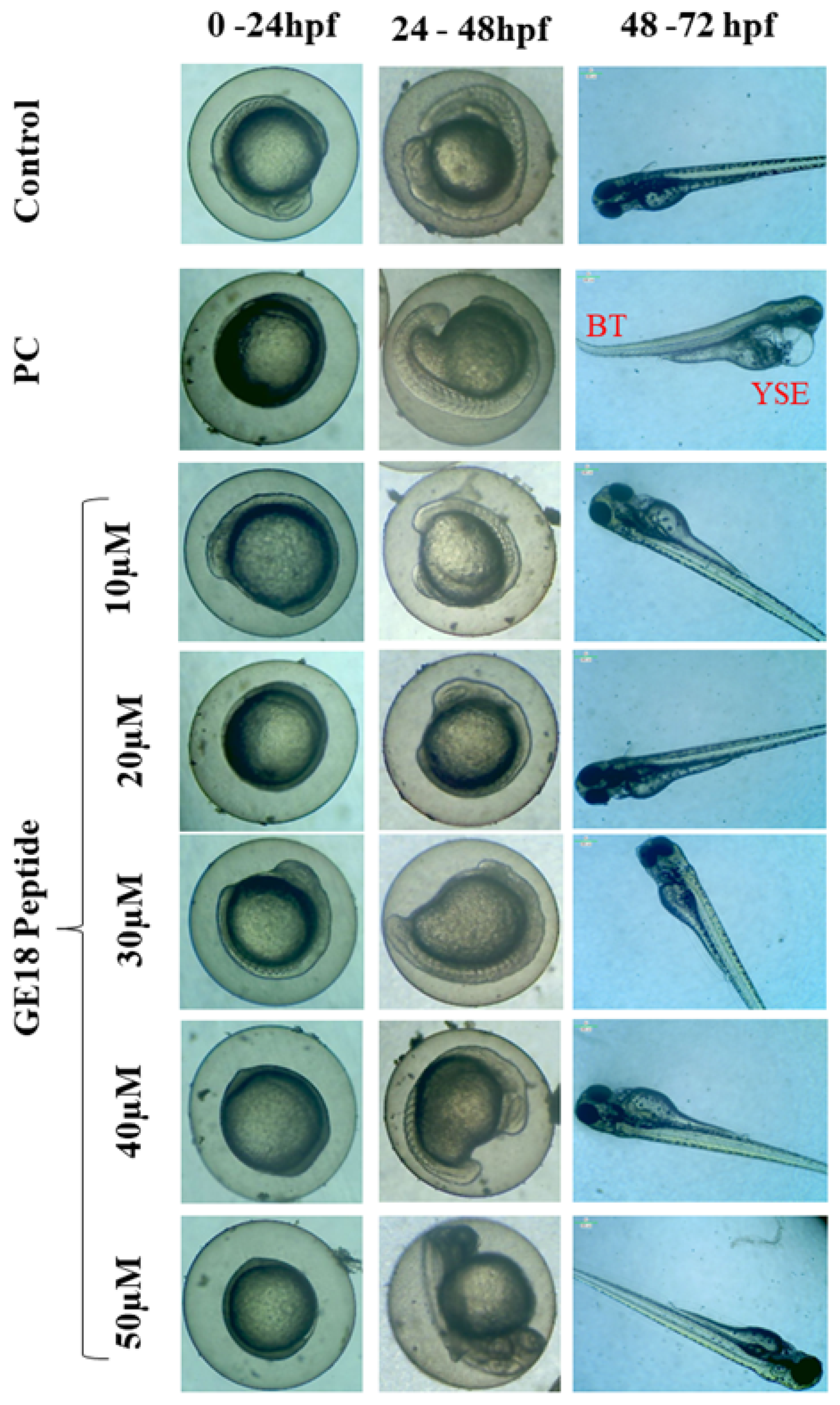
2.2.2. Effect of Peptide Toxicity on Zebrafish Larvae
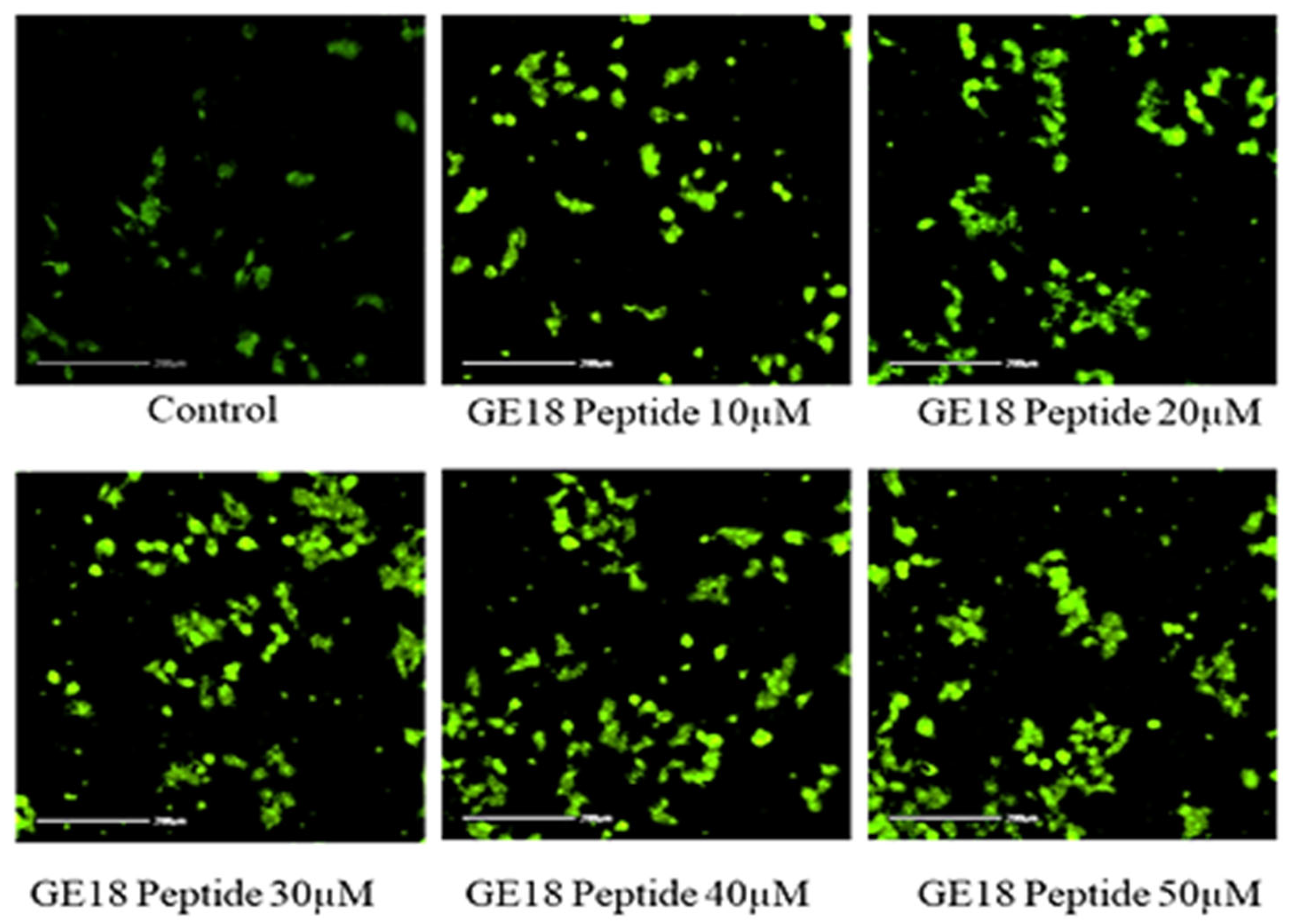
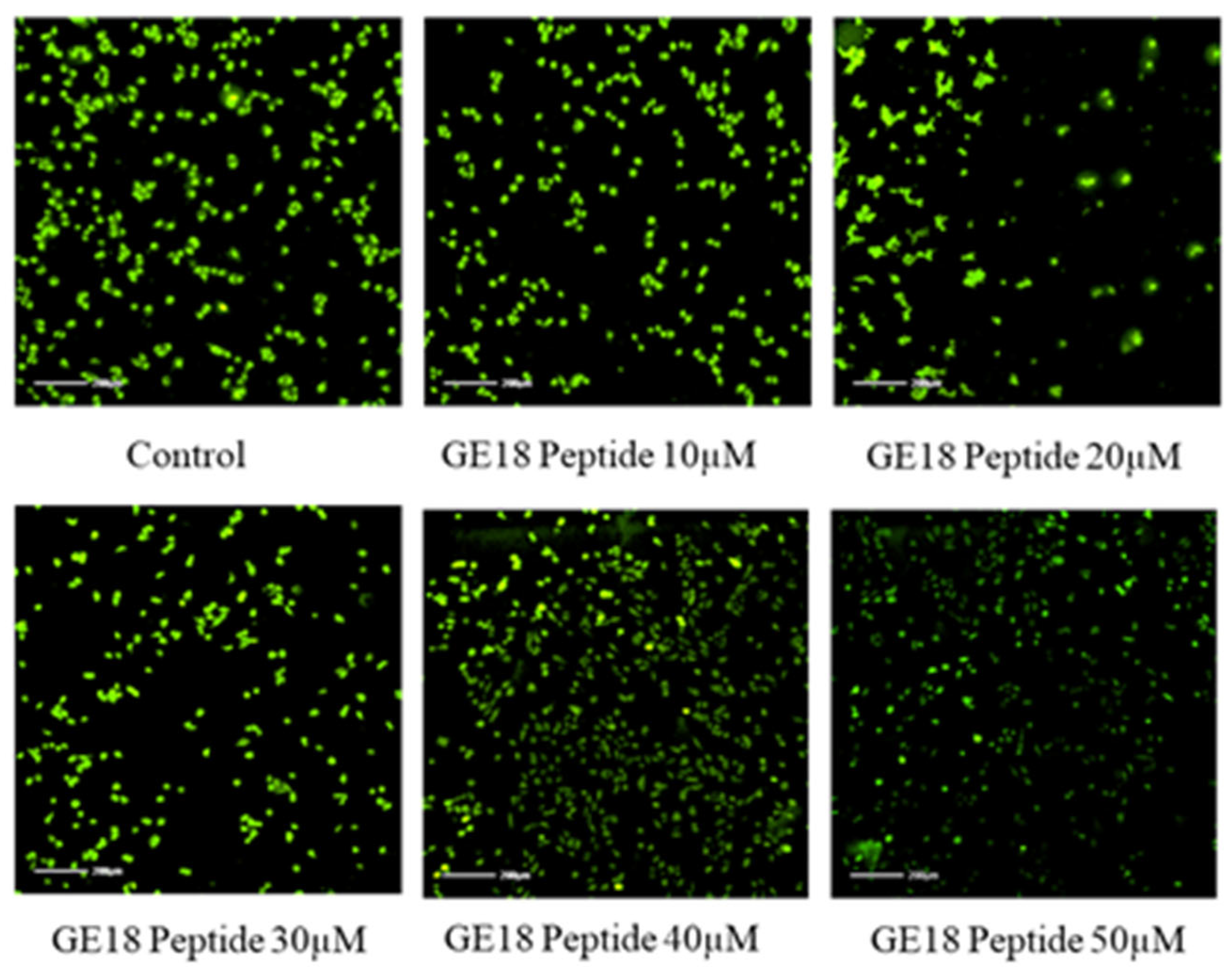
2.2.3. Effect of Peptide Toxicity on Zebrafish Larvae by Fluorescence Staining
2.3. In Vitro Anti-Cancer Activity of GE18
2.3.1. Anti-Proliferative Activity of GE18 on MCF-7 Cells
2.3.2. Morphology and Apoptosis
2.3.3. ROS Generation Potency
2.3.4. Mitochondrial Membrane Potential
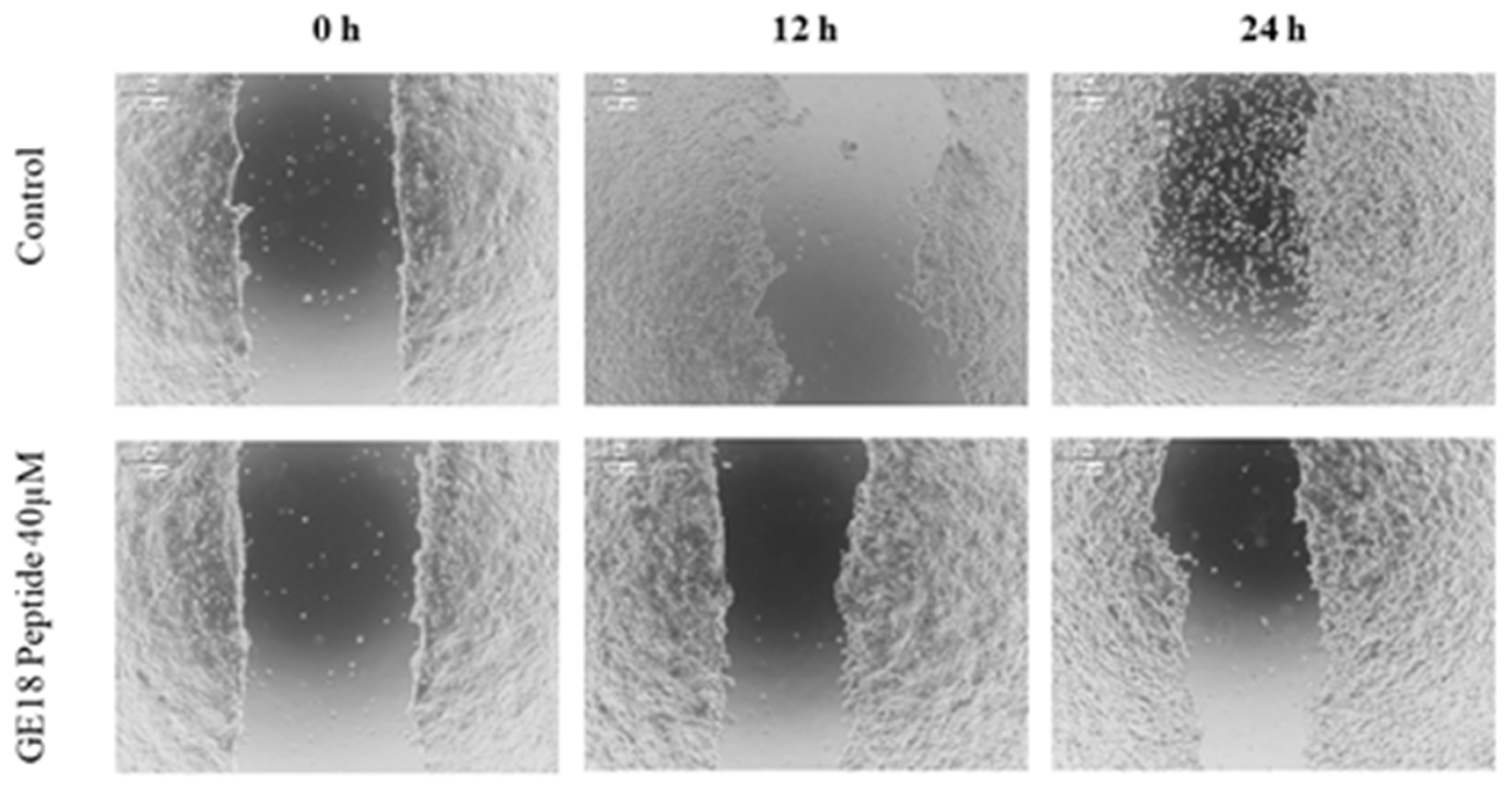
2.3.5. Scratch Assay
2.3.6. qPCR Analysis
2.4. Evaluation of Anti-Microbial Activity of GE18
2.4.1. MIC
2.4.2. Anti-Bacterial Activity
2.4.3. Inhibition of Biofilm Formation
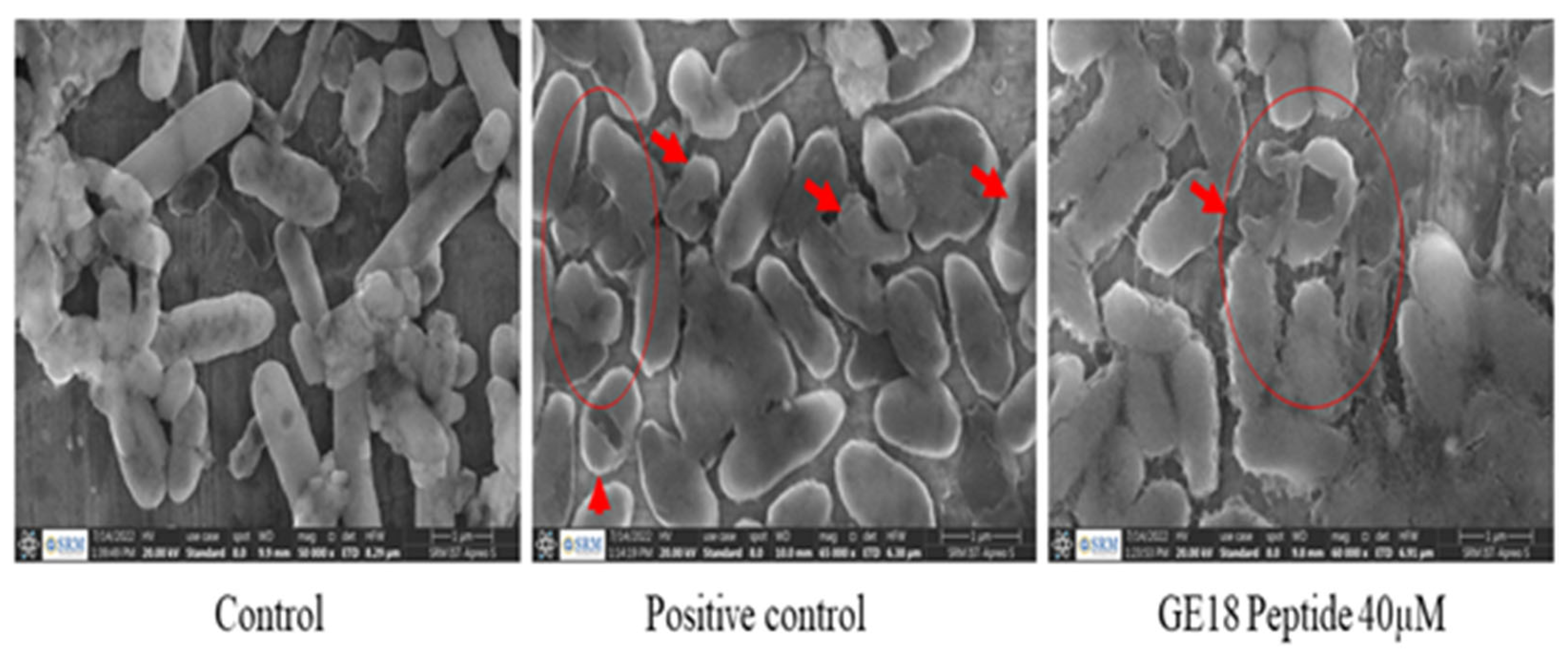
2.4.4. FE-SEM Analysis
3. Materials and Methods
3.1. In Silico Analysis of Properties of the Peptide
3.2. GE18 Peptide Toxicity Analysis
3.2.1. Maintenance of Cell Culture
3.2.2. In Vitro Toxicity Assessment in Myoblast Cells
3.2.3. In Vivo Toxicity Assessment in Zebrafish Embryos
3.3. Fluorescent Staining Assays
3.4. In Vitro Anti-Cancer Potency of GE18 Peptide
3.4.1. Assessment of Anti-Proliferative Activity
3.4.2. Cytotoxicity Assessment by LDH Leakage Assay
3.4.3. Morphological and Apoptosis Staining
3.4.4. ROS Generation
3.4.5. Rhodamine 123 Staining
3.4.6. Cell Migration Assay
3.4.7. In Vitro Anti-Cancer Gene Expression
3.5. Anti-Microbial Potential of GE18
3.5.1. MIC Radial Diffusion Assay
3.5.2. Time Kill Kinetics Assay
3.5.3. Protein Leakage Assay and Release of Intercellular Components
3.5.4. Biofilm Inhibition Assay
3.5.5. Morphology Analysis
3.5.6. Statistical Study
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Li, C.M.; Haratipour, P.; Lingeman, R.G.; Perry, J.J.P.; Gu, L.; Hickey, R.J.; Malkas, L.H. Novel Peptide Therapeutic Approaches for Cancer Treatment. Cells 2021, 10, 2908. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, H.; Ghosh, D.; Williams, R.O., 3rd. Just How Prevalent Are Peptide Therapeutic Products? A Critical Review. Int. J. Pharm. 2020, 587, 119491. [Google Scholar] [CrossRef] [PubMed]
- Bitler, B.G.; Schroeder, J.A. Anti-Cancer Therapies that Utilize Cell Penetrating Peptides. Recent Pat. Anti-Cancer Drug Discov. 2010, 5, 99–108. [Google Scholar] [CrossRef]
- Bray, F.; Laversanne, M.; Weiderpass, E.; Soerjomataram, I. The ever-increasing importance of cancer as a leading cause of premature death worldwide. Cancer 2021, 127, 3029–3030. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Mikaelian, A.G.; Traboulay, E.; Zhang, X.M.; Yeritsyan, E.; Pedersen, P.L.; Ko, Y.H.; Matalka, K.Z. Pleiotropic anticancer properties of scorpion venom peptides: Rhopalurus princeps venom as an anticancer agent. Drug Des. Dev. Ther. 2020, 14, 881–893. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Mant, C.T.; Farmer, S.W.; Hancock, R.E.; Vasil, M.L.; Hodges, R.S. Rational Design of α-Helical Anti-microbial Peptides with Enhanced Activities and Specificity/Therapeutic Index. J. Biol. Chem. 2005, 280, 12316–12329. [Google Scholar] [CrossRef]
- Hoskin, D.W.; Ramamoorthy, A. Studies on anticancer activities of antimicrobial peptides. Biochim. Biophys. Acta (BBA)-Biomembr. 2008, 1778, 357–375. [Google Scholar] [CrossRef]
- Mader, J.S.; Hoskin, D.W. Cationic antimicrobial peptides as novel cytotoxic agents for cancer treatment. Expert Opin. Investig. Drugs 2006, 15, 933–946. [Google Scholar] [CrossRef]
- Bevers, E.M.; Comfurius, P.; Zwaal, R.F.A. Regulatory Mechanisms in Maintenance and Modulation of Transmembrane Lipid Asymmetry: Pathophysiological Implications. Lupus 1996, 5, 480–487. [Google Scholar] [CrossRef]
- Yin, W.-B.; Baccile, J.A.; Bok, J.W.; Chen, Y.; Keller, N.P.; Schroeder, F.C. A Nonribosomal Peptide Synthetase-Derived Iron(III) Complex from the Pathogenic Fungus Aspergillus fumigatus. J. Am. Chem. Soc. 2013, 135, 2064–2067. [Google Scholar] [CrossRef] [PubMed]
- Kumaresan, V.; Pasupuleti, M.; Arasu, M.V.; Al-Dhabi, N.A.; Arshad, A.; Amin, S.M.N.; Yusoff, F.M.; Arockiaraj, J. A comparative transcriptome approach for identification of molecular changes in Aphanomyces invadans infected Channa striatus. Mol. Biol. Rep. 2018, 45, 2511–2523. [Google Scholar] [CrossRef]
- Velayutham, M.; Sarkar, P.; Rajakrishnan, R.; Kuppusamy, P.; Juliet, A.; Arockiaraj, J. Antiproliferation of MP12 derived from a fungus, Aphanomyces invadans virulence factor, cysteine-rich trypsin inhibitor on human laryngeal epithelial cells, and in vivo zebrafish embryo model. Toxicon 2022, 210, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Ravichandran, G.; Kumaresan, V.; Bhatt, P.; Arasu, M.V.; Al-Dhabi, N.A.; Arockiaraj, J. A Cumulative Strategy to Predict and Characterize Antimicrobial Peptides (AMPs) from Protein Database. Int. J. Pept. Res. Ther. 2017, 23, 281–290. [Google Scholar] [CrossRef]
- Roy, S.; Maheshwari, N.; Chauhan, R.; Sen, N.K.; Sharma, A. Structure prediction and functional characterization of secondary metabolite proteins of Ocimum. Bioinformation 2011, 6, 315–319. [Google Scholar] [CrossRef] [PubMed]
- Sannasimuthu, A.; Kumaresan, V.; Anilkumar, S.; Pasupuleti, M.; Ganesh, M.-R.; Mala, K.; Paray, B.A.; Al-Sadoon, M.K.; Albeshr, M.F.; Arockiaraj, J. Design and characterization of a novel Arthrospira platensis glutathione oxido-reductase-derived antioxidant peptide GM15 and its potent anti-cancer activity via caspase-9 mediated apoptosis in oral cancer cells. Free. Radic. Biol. Med. 2019, 135, 198–209. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Ye, X.; Sakurai, T.; Mu, Z.; Wei, L. ToxIBTL: Prediction of peptide toxicity based on information bottleneck and transfer learning. Bioinformatics 2022, 38, 1514–1524. [Google Scholar] [CrossRef]
- Velayutham, M.; Ojha, B.; Issac, P.K.; Lite, C.; Guru, A.; Pasupuleti, M.; Arasu, M.V.; Al-Dhabi, N.A.; Arockiaraj, J. NV14 from serine O-acetyltransferase of cyanobacteria influences the antioxidant enzymes in vitro cells, gene expression against H2O2 and other responses in vivo zebrafish larval model. Cell Biol. Int. 2021, 45, 2331–2346. [Google Scholar] [CrossRef]
- Murugan, R.; Rajesh, R.; Guru, A.; Haridevamuthu, B.; Almutairi, B.O.; Almutairi, M.H.; Juliet, A.; Renganayagi, S.; Gopinath, P.; Arockiaraj, J. Deacetylepoxyazadiradione Derived from Epoxyazadiradione of Neem (Azadirachta indica A. Juss) Fruits Mitigates LPS-Induced Oxidative Stress and Inflammation in Zebrafish Larvae. Chem. Biodivers. 2022, 19, e202200041. [Google Scholar] [CrossRef]
- Velayutham, M.; Sarkar, P.; Sudhakaran, G.; Al-Ghanim, K.A.; Maboob, S.; Juliet, A.; Guru, A.; Muthupandian, S.; Arockiaraj, J. Anti-Cancer and Anti-Inflammatory Activities of a Short Molecule, PS14 Derived from the Virulent Cellulose Binding Domain of Aphanomyces invadans, on Human Laryngeal Epithelial Cells and an In Vivo Zebrafish Embryo Model. Molecules 2022, 27, 7333. [Google Scholar] [CrossRef]
- Kathiravan, A.; Manjunathan, T.; Velusamy, M.; Guru, A.; Arockiaraj, J.; Jhonsi, M.A.; Gopinath, P. Nano-sized aggregation induced emissive probe for highly sensitive hypochlorous acid detection. Dye. Pigment. 2023, 210, 111016. [Google Scholar] [CrossRef]
- Sudhakaran, G.; Prathap, P.; Guru, A.; Rajesh, R.; Sathish, S.; Madhavan, T.; Arasu, M.V.; Al-Dhabi, N.A.; Choi, K.C.; Gopinath, P.; et al. Anti-inflammatory role demonstrated both in vitro and in vivo models using nonsteroidal tetranortriterpenoid, Nimbin (N1) and its analogs (N2 and N3) that alleviate the domestication of alternative medicine. Cell Biol. Int. 2022, 46, 771–791. [Google Scholar] [CrossRef]
- Haridevamuthu, B.; Guru, A.; Murugan, R.; Sudhakaran, G.; Pachaiappan, R.; Almutairi, M.H.; Almutairi, B.O.; Juliet, A.; Arockiaraj, J. Neuroprotective effect of Biochanin a against Bisphenol A-induced prenatal neurotoxicity in zebrafish by modulating oxidative stress and locomotory defects. Neurosci. Lett. 2022, 790, 136889. [Google Scholar] [CrossRef]
- Sudhakaran, G.; Rajesh, R.; Murugan, R.; Velayutham, M.; Guru, A.; Boopathi, S.; Muthupandian, S.; Gopinath, P.; Arockiaraj, J. Nimbin analog N2 alleviates high testosterone induced oxidative stress in CHO cells and alters the expression of Tox3 and Dennd1a signal transduction pathway involved in the PCOS zebrafish. Phytotherapy Res. 2022, 37, 1449–1461. [Google Scholar] [CrossRef]
- Guru, A.; Manjunathan, T.; Sudhakaran, G.; Juliet, A.; Gopinath, P.; Arockiaraj, J. 6-Gingerdione Reduces Apoptotic Conditions in HepG2 Cells and Inhibits Inflammatory Cytokine Gene Expression in Alcoholic Liver Injured Zebrafish Larvae. Chem. Biodivers. 2023, 20, e202200959. [Google Scholar] [CrossRef]
- Abdullah, A.-S.H.; Mohammed, A.S.; Abdullah, R.; Mirghani, M.E.S.; Al-Qubaisi, M. Cytotoxic effects of Mangifera indica L. kernel extract on human breast cancer (MCF-7 and MDA-MB-231 cell lines) and bioactive constituents in the crude extract. BMC Complement. Altern. Med. 2014, 14, 199. [Google Scholar] [CrossRef]
- Al-Qubaisi, M.; Rozita, R.; Yeap, S.-K.; Omar, A.-R.; Ali, A.-M.; Alitheen, N.B. Selective Cytotoxicity of goniothalamin against hepatoblastoma HepG2 cells. Molecules 2011, 16, 2944–2959. [Google Scholar] [CrossRef]
- Prabha, N.; Sannasimuthu, A.; Kumaresan, V.; Elumalai, P.; Arockiaraj, J. Intensifying the Anticancer Potential of Cationic Peptide Derived from Serine Threonine Protein Kinase of Teleost by Tagging with Oligo Tryptophan. Int. J. Pept. Res. Ther. 2019, 26, 75–83. [Google Scholar] [CrossRef]
- Umayaparvathi, S.; Meenakshi, S.; Vimalraj, V.; Arumugam, M.; Sivagami, G.; Balasubramanian, T. Antioxidant activity and anticancer effect of bioactive peptide from enzymatic hydrolysate of oyster (Saccostrea cucullata). Biomed. Prev. Nutr. 2014, 4, 343–353. [Google Scholar] [CrossRef]
- Sarkar, P.; Raju, S.V.; Velayutham, M.; Guru, A.; Pasupuleti, M.; Al Olayan, E.M.; Boushra, A.F.; Juliet, A.; Arockiaraj, J. A synthetic antioxidant molecule, GP13 derived from cysteine desulfurase of spirulina, Arthrospira platensis exhibited anti-diabetic activity on L6 rat skeletal muscle cells through GLUT-4 pathway. J. King Saud Univ. -Sci. 2023, 35, 102450. [Google Scholar] [CrossRef]
- Issac, P.K.; Karan, R.; Guru, A.; Pachaiappan, R.; Arasu, M.V.; Al-Dhabi, N.A.; Choi, K.C.; Harikrishnan, R.; Raj, J.A. Insulin signaling pathway assessment by enhancing antioxidant activity due to morin using in vitro rat skeletal muscle L6 myotubes cells. Mol. Biol. Rep. 2021, 48, 5857–5872. [Google Scholar] [CrossRef]
- Ahamed, M.; Alhadlaq, H. Nickel nanoparticle-induced dose-dependent cyto-genotoxicity in human breast carcinoma MCF-7 cells. OncoTargets Ther. 2014, 7, 269–280. [Google Scholar] [CrossRef] [PubMed]
- Al-Dabbagh, B.; Elhaty, I.A.; Al Hrout, A.; Al Sakkaf, R.; El-Awady, R.; Ashraf, S.S.; Amin, A. Antioxidant and anticancer activities of Trigonella foenum-graecum, Cassia acutifolia and Rhazya stricta. BMC Complement. Altern. Med. 2018, 18, 240. [Google Scholar] [CrossRef]
- Al-Harbi, L.N.; Subash-Babu, P.; Binobead, M.A.; Alhussain, M.H.; AlSedairy, S.A.; Aloud, A.A.; Alshatwi, A.A. Potential Metabolite Nymphayol Isolated from Water Lily (Nymphaea stellata) Flower Inhibits MCF-7 Human Breast Cancer Cell Growth via Upregulation of Cdkn2a, pRb2, p53 and Downregulation of PCNA mRNA Expressions. Metabolites 2020, 10, 280. [Google Scholar] [CrossRef]
- McLean, D.T.; Lundy, F.T.; Timson, D.J. IQ-motif peptides as novel anti-microbial agents. Biochimie 2013, 95, 875–880. [Google Scholar] [CrossRef] [PubMed]
- Raju, S.V.; Sarkar, P.; Pasupuleti, M.; Abbasi, A.M.; Al-Farraj, D.A.; Elshikh, M.S.; Elumalai, P.; Harikrishnan, R.; Rahman, M.A.; Arockiaraj, J. Antibacterial Activity of RM12, a Tachykinin Derivative, against Pseudomonas aeruginosa. Int. J. Pept. Res. Ther. 2021, 27, 2571–2581. [Google Scholar] [CrossRef]
- Raji, P.; Samrot, A.V.; Keerthana, D.; Karishma, S. Antibacterial Activity of Alkaloids, Flavonoids, Saponins and Tannins Mediated Green Synthesised Silver Nanoparticles Against Pseudomonas aeruginosa and Bacillus subtilis. J. Clust. Sci. 2019, 30, 881–895. [Google Scholar] [CrossRef]
- Chen, H.; Wang, B.; Gao, D.; Guan, M.; Zheng, L.; Ouyang, H.; Chai, Z.; Zhao, Y.; Feng, W. Broad-spectrum antibacterial activity of carbon nanotubes to human gut bacteria. Small 2013, 9, 2735–2746. [Google Scholar] [CrossRef] [PubMed]
- Soon, T.N.; Chia, A.Y.Y.; Yap, W.H.; Tang, Y.-Q. Anticancer Mechanisms of Bioactive Peptides. Protein Pept. Lett. 2020, 27, 823–830. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.; Liu, D.; Yang, Y. Anti-cancer peptides: Classification, mechanism of action, reconstruction and modification. Open Biol. 2020, 10, 200004. [Google Scholar] [CrossRef]
- Jenssen, H.; Hamill, P.; Hancock, R.E.W. Peptide Antimicrobial Agents. Clin. Microbiol. Rev. 2006, 19, 491–511. [Google Scholar] [CrossRef]
- Dennison, S.; Whittaker, M.; Harris, F.; Phoenix, D. Anticancer α-helical peptides and structure/function relationships underpinning their interactions with tumour cell membranes. Curr. Protein Pept. Sci. 2006, 7, 487–499. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Zhu, W.; Ding, L.; Ye, X.; Gao, H.; Tai, X.; Wu, Z.; Qian, Y.; Ruan, X.; Li, J.; et al. Bldesin, the first functionally characterized pathogenic fungus defensin with Kv1.3 channel and chymotrypsin inhibitory activities. J. Biochem. Mol. Toxicol. 2018, 33, e22244. [Google Scholar] [CrossRef]
- Bai, C.-Z.; Feng, M.-L.; Hao, X.-L.; Zhao, Z.-J.; Li, Y.-Y.; Wang, Z.-H. Anti-tumoral effects of a trypsin inhibitor derived from buckwheat in vitro and in vivo. Mol. Med. Rep. 2015, 12, 1777–1782. [Google Scholar] [CrossRef] [PubMed]
- Sarzaeem, A.; ZARE, M.A.; Moradhaseli, S.; Morovvati, H.; Lotfi, M. Cytotoxic effect of ICD-85 (venom-derived peptides) on HeLa cancer cell line and normal LK cells using MTT assay. Arch. Iran. Med. 2012, 15, 696–701. [Google Scholar]
- Pannkuk, E.L.; Risch, T.S.; Savary, B.J. Isolation and identification of an extracellular subtilisin-like serine protease secreted by the Bat Pathogen Pseudogymnoascus destructans. PLoS ONE 2015, 10, e0120508. [Google Scholar] [CrossRef] [PubMed]
- Felício, M.R.; Silva, O.N.; Gonçalves, S.; Santos, N.C.; Franco, O.L. Peptides with Dual Antimicrobial and Anticancer Activities. Front. Chem. 2017, 5, 5. [Google Scholar] [CrossRef] [PubMed]
- Chiangjong, W.; Chutipongtanate, S.; Hongeng, S. Anticancer peptide: Physicochemical property, functional aspect and trend in clinical application (Review). Int. J. Oncol. 2020, 57, 678–696. [Google Scholar] [CrossRef]
- Lite, C.; Guru, A.; Juliet, M.; Arockiaraj, J. Embryonic exposure to butylparaben and propylparaben induced developmental toxicity and triggered anxiety-like neurobehavioral response associated with oxidative stress and apoptosis in the head of zebrafish larvae. Environ. Toxicol. 2022, 37, 1988–2004. [Google Scholar] [CrossRef]
- Haridevamuthu, B.; Manjunathan, T.; Guru, A.; Kumar, R.S.; Rajagopal, R.; Kuppusamy, P.; Juliet, A.; Gopinath, P.; Arockiaraj, J. Hydroxyl containing benzo[b]thiophene analogs mitigates the acrylamide induced oxidative stress in the zebrafish larvae by stabilizing the glutathione redox cycle. Life Sci. 2022, 298, 120507. [Google Scholar] [CrossRef]
- Prabha, N.; Guru, A.; Harikrishnan, R.; Gatasheh, M.K.; Hatamleh, A.A.; Juliet, A.; Arockiaraj, J. Neuroprotective and antioxidant capability of RW20 peptide from histone acetyltransferases caused by oxidative stress-induced neurotoxicity in in vivo zebrafish larval model. J. King Saud Univ. -Sci. 2022, 34, 101861. [Google Scholar] [CrossRef]
- Kang, M.-C.; Cha, S.H.; Wijesinghe, W.; Kang, S.-M.; Lee, S.-H.; Kim, E.-A.; Song, C.B.; Jeon, Y.-J. Protective effect of marine algae phlorotannins against AAPH-induced oxidative stress in zebrafish embryo. Food Chem. 2013, 138, 950–955. [Google Scholar] [CrossRef]
- Guru, A.; Velayutham, M.; Arockiaraj, J. Lipid-Lowering and Antioxidant Activity of RF13 Peptide From Vacuolar Protein Sorting-Associated Protein 26B (VPS26B) by Modulating Lipid Metabolism and Oxidative Stress in HFD Induced Obesity in Zebrafish Larvae. Int. J. Pept. Res. Ther. 2022, 28, 74. [Google Scholar] [CrossRef]
- Sudhakaran, G.; Prathap, P.; Guru, A.; Haridevamuthu, B.; Murugan, R.; Almutairi, B.O.; Almutairi, M.H.; Juliet, A.; Gopinath, P.; Arockiaraj, J. Reverse pharmacology of Nimbin-N2 attenuates alcoholic liver injury and promotes the hepatoprotective dual role of improving lipid metabolism and downregulating the levels of inflammatory cytokines in zebrafish larval model. Mol. Cell. Biochem. 2022, 477, 2387–2401. [Google Scholar] [CrossRef]
- Zarandi, P.K.; Mirakabadi, A.Z.; Sotoodehnejadnematalahi, F. Cytotoxic and anti-cancer effects of ICD-85 (Venom derived peptides) in human breast adenocarcinoma and normal human dermal fibroblasts. Iran. J. Pharm. Res. 2019, 18, 232–240. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, L.; Liu, Y.; Han, P.; Hong, D.; Li, S.; Ma, A.; Jia, Y. Brevilaterin B from Brevibacillus laterosporus has selective antitumor activity and induces apoptosis in epidermal cancer. World J. Microbiol. Biotechnol. 2022, 38, 201. [Google Scholar] [CrossRef]
- Shaibani, M.E.; Heidari, B.; Khodabandeh, S.; Shahangian, S.S. Production and Fractionation of Rocky Shore Crab (Grapsus albacarinous) Protein Hydrolysate by Ultrafiltration Membrane: Assessment of Antioxidant and Cytotoxic Activities. J. Aquat. Food Prod. Technol. 2021, 30, 339–352. [Google Scholar] [CrossRef]
- Karanam, G.; Arumugam, M.K. Reactive oxygen species generation and mitochondrial dysfunction for the initiation of apoptotic cell death in human hepatocellular carcinoma HepG2 cells by a cyclic dipeptide Cyclo(-Pro-Tyr). Mol. Biol. Rep. 2020, 47, 3347–3359. [Google Scholar] [CrossRef]
- Siddiqui, M.A.; Alhadlaq, H.A.; Ahmad, J.; Al-Khedhairy, A.A.; Musarrat, J.; Ahamed, M. Copper Oxide Nanoparticles Induced Mitochondria Mediated Apoptosis in Human Hepatocarcinoma Cells. PLoS ONE 2013, 8, e69534. [Google Scholar] [CrossRef]
- Yi, C.; Li, G.; Wang, W.; Sun, Y.; Zhang, Y.; Zhong, C.; Stovall, D.B.; Li, D.; Shi, J.; Sui, G. Disruption of YY1-EZH2 interaction using synthetic peptides inhibits breast cancer development. Cancers 2021, 13, 2402. [Google Scholar] [CrossRef]
- Al-Khayal, K.; Alafeefy, A.; Vaali-Mohammed, M.-A.; Mahmood, A.; Zubaidi, A.; Al-Obeed, O.; Khan, Z.; Abdulla, M.; Ahmad, R. Novel derivative of aminobenzenesulfonamide (3c) induces apoptosis in colorectal cancer cells through ROS generation and inhibits cell migration. BMC Cancer 2017, 17, 4. [Google Scholar] [CrossRef]
- Rasaratnam, K.; Nantasenamat, C.; Phaonakrop, N.; Roytrakul, S.; Tanyong, D. A novel peptide isolated from garlic shows anticancer effect against leukemic cell lines via interaction with Bcl-2 family proteins. Chem. Biol. Drug Des. 2021, 97, 1017–1028. [Google Scholar] [CrossRef]
- Batoni, G.; Maisetta, G.; Brancatisano, F.L.; Esin, S.; Campa, M. Use of Antimicrobial Peptides Against Microbial Biofilms: Advantages and Limits. Curr. Med. Chem. 2011, 18, 256–279. [Google Scholar] [CrossRef]


| Gene | Primer Sequence | Reference |
|---|---|---|
| Bcl-2 | Forward: 5′-GTGGATGACTGAGTACCT-3′ Reverse: 5′-CCAGGAGAAATCAAACAGAG-3′ | [34] |
| BAX | Forward: 5′-TCAGGATGCGTCCACCAAGAAG-3′ Reverse: 5′-TGTGTCCACGGCGGCAATCATC-3′ | |
| p53 | Forward: 5′-CCTCAGCATCTTATCCGAGTGG-3′ Reverse: 5′-TGGATGGTGGTACAGTCAGAGC-3′ | |
| Caspase-3 | Forward: 5′-ACATGGAAGCGAATCAATGGACTC-3′ Reverse: 5′-AAGGACTCAAATTCTGTTGCCACC-3′ | |
| Caspase-9 | Forward: 5′-GCTCTTCCTTTGTTCATC- 3′ Reverse: 5′-CTCTTCCTCCACTGTTCA-3′ | |
| GAPDH (internal control) | Forward: 5′-GTCTCCTCTGACTTCAACAGCG-3′ Reverse: 5′-ACCACCCTGTTGCTGTAGCCAA-3′ |
| Microorganism | Activity |
|---|---|
| Aeromonas hydrophila MTCC 1739 | − |
| Bacillus cereus ATCC 2106 | + |
| B. subtilis ATCC 6051 | + |
| Escherichia coli ATCC 9637 | − |
| Klebsiella pneumoniae ATCC 27736 | − |
| Pseudomonas aeruginosa ATCC 25668 | ++++ |
| Staphylococcus aureus ATCC 33592 | ++ |
| Serratia marcescens MTCC 3124 | − |
| Salmonella enterica MTCC 1166 | − |
| Vibrio harvey MTCC 7954 | + |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Velayutham, M.; Priya, P.S.; Sarkar, P.; Murugan, R.; Almutairi, B.O.; Arokiyaraj, S.; Kari, Z.A.; Tellez-Isaias, G.; Guru, A.; Arockiaraj, J. Aquatic Peptide: The Potential Anti-Cancer and Anti-Microbial Activity of GE18 Derived from Pathogenic Fungus Aphanomyces invadans. Molecules 2023, 28, 6746. https://doi.org/10.3390/molecules28186746
Velayutham M, Priya PS, Sarkar P, Murugan R, Almutairi BO, Arokiyaraj S, Kari ZA, Tellez-Isaias G, Guru A, Arockiaraj J. Aquatic Peptide: The Potential Anti-Cancer and Anti-Microbial Activity of GE18 Derived from Pathogenic Fungus Aphanomyces invadans. Molecules. 2023; 28(18):6746. https://doi.org/10.3390/molecules28186746
Chicago/Turabian StyleVelayutham, Manikandan, P. Snega Priya, Purabi Sarkar, Raghul Murugan, Bader O. Almutairi, Selvaraj Arokiyaraj, Zulhisyam Abdul Kari, Guillermo Tellez-Isaias, Ajay Guru, and Jesu Arockiaraj. 2023. "Aquatic Peptide: The Potential Anti-Cancer and Anti-Microbial Activity of GE18 Derived from Pathogenic Fungus Aphanomyces invadans" Molecules 28, no. 18: 6746. https://doi.org/10.3390/molecules28186746
APA StyleVelayutham, M., Priya, P. S., Sarkar, P., Murugan, R., Almutairi, B. O., Arokiyaraj, S., Kari, Z. A., Tellez-Isaias, G., Guru, A., & Arockiaraj, J. (2023). Aquatic Peptide: The Potential Anti-Cancer and Anti-Microbial Activity of GE18 Derived from Pathogenic Fungus Aphanomyces invadans. Molecules, 28(18), 6746. https://doi.org/10.3390/molecules28186746











