In Situ Construction of Near-Infrared Response Hybrid Up-Conversion Photocatalyst for Degrading Organic Dyes and Antibiotics
Abstract
:1. Introduction
2. Result
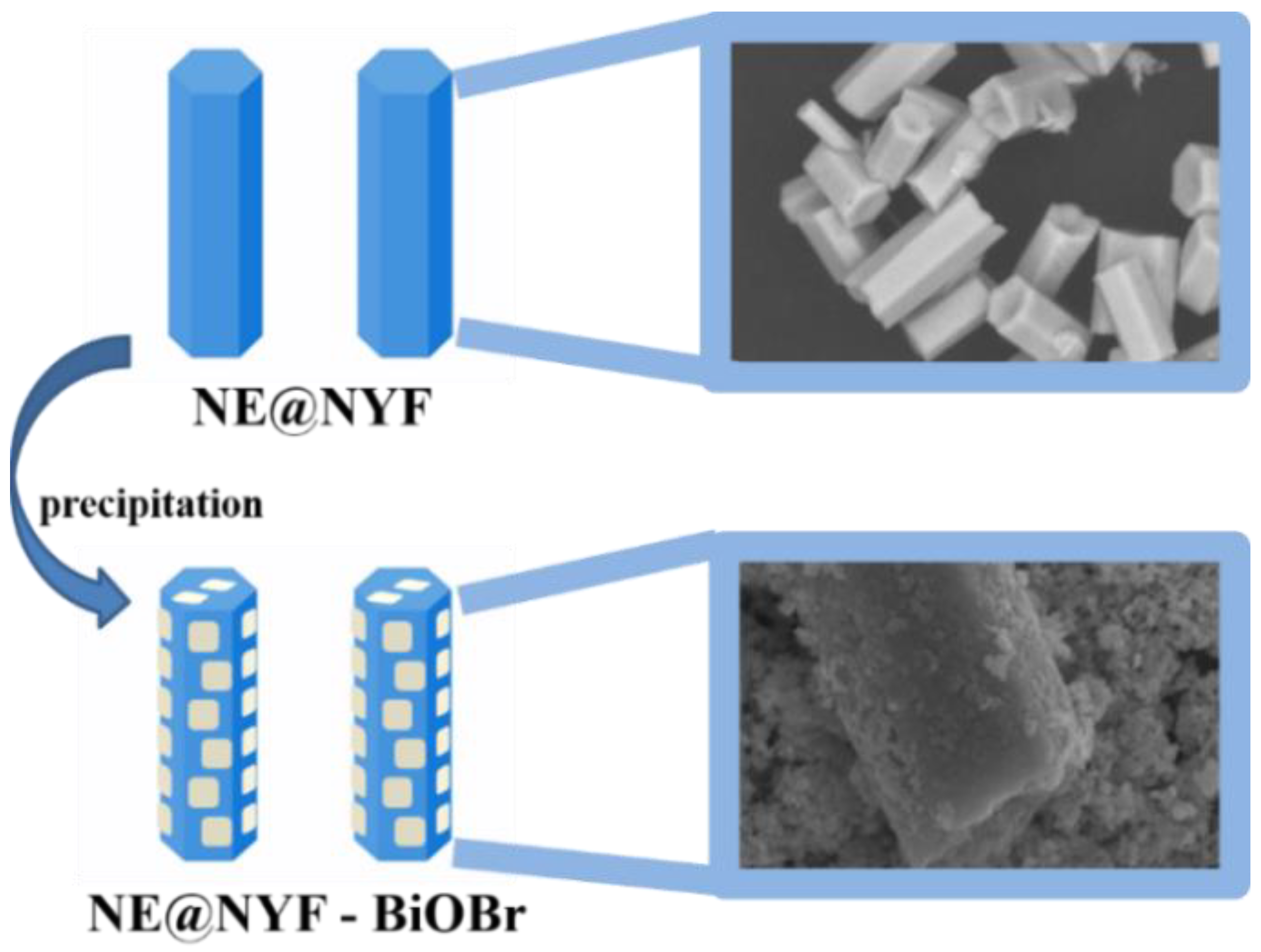
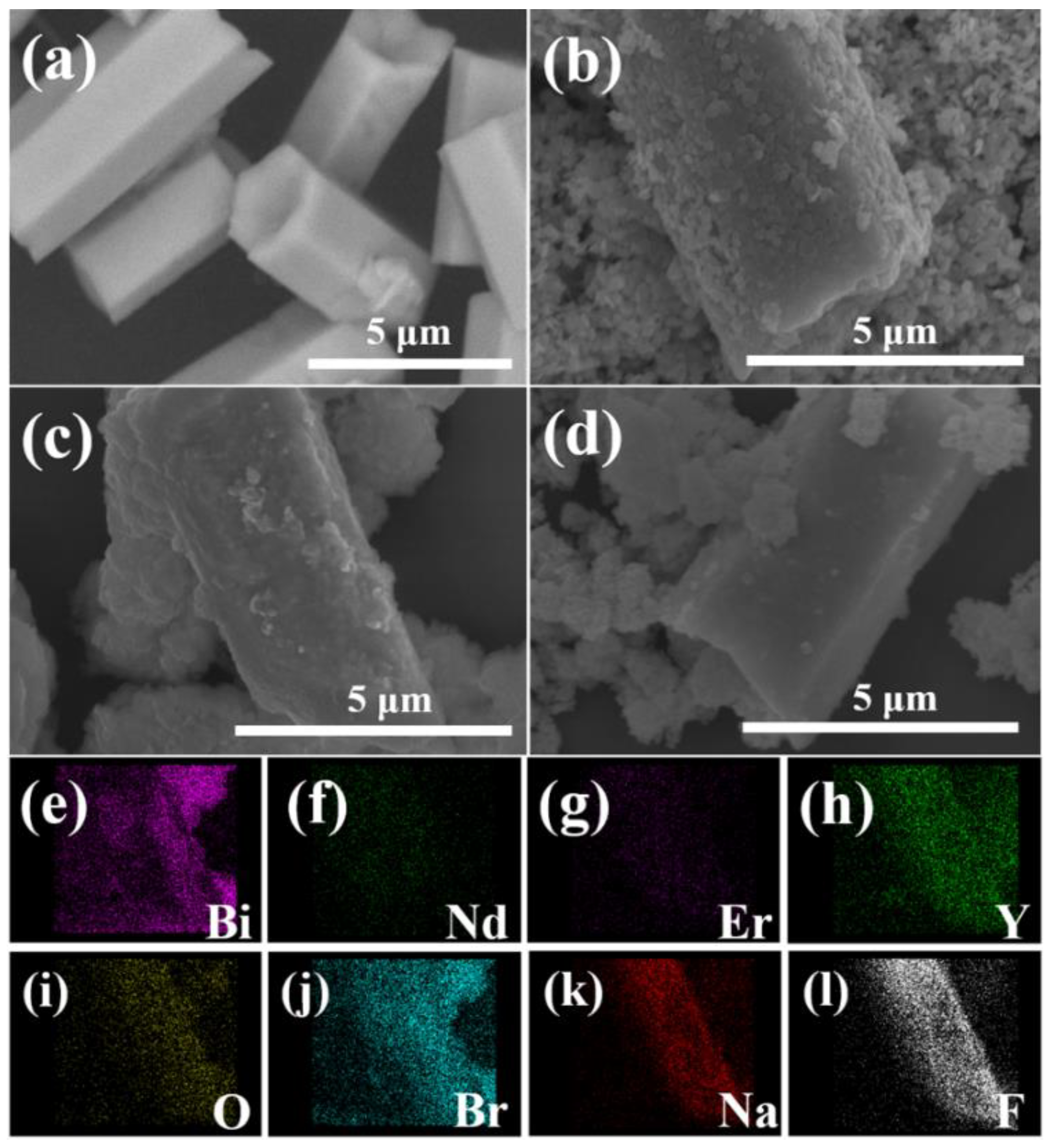
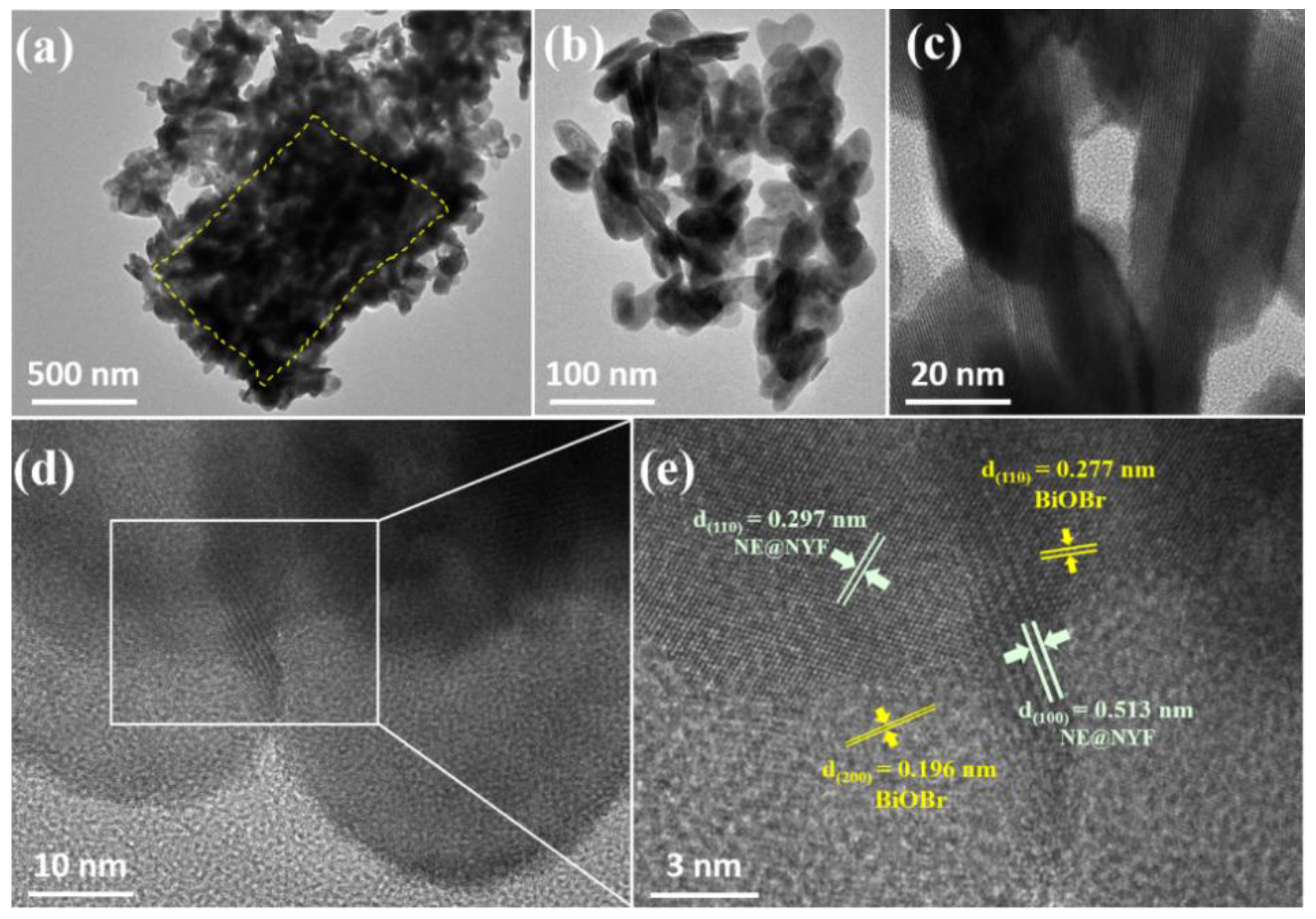
2.1. Structure and Morphology Characterizations
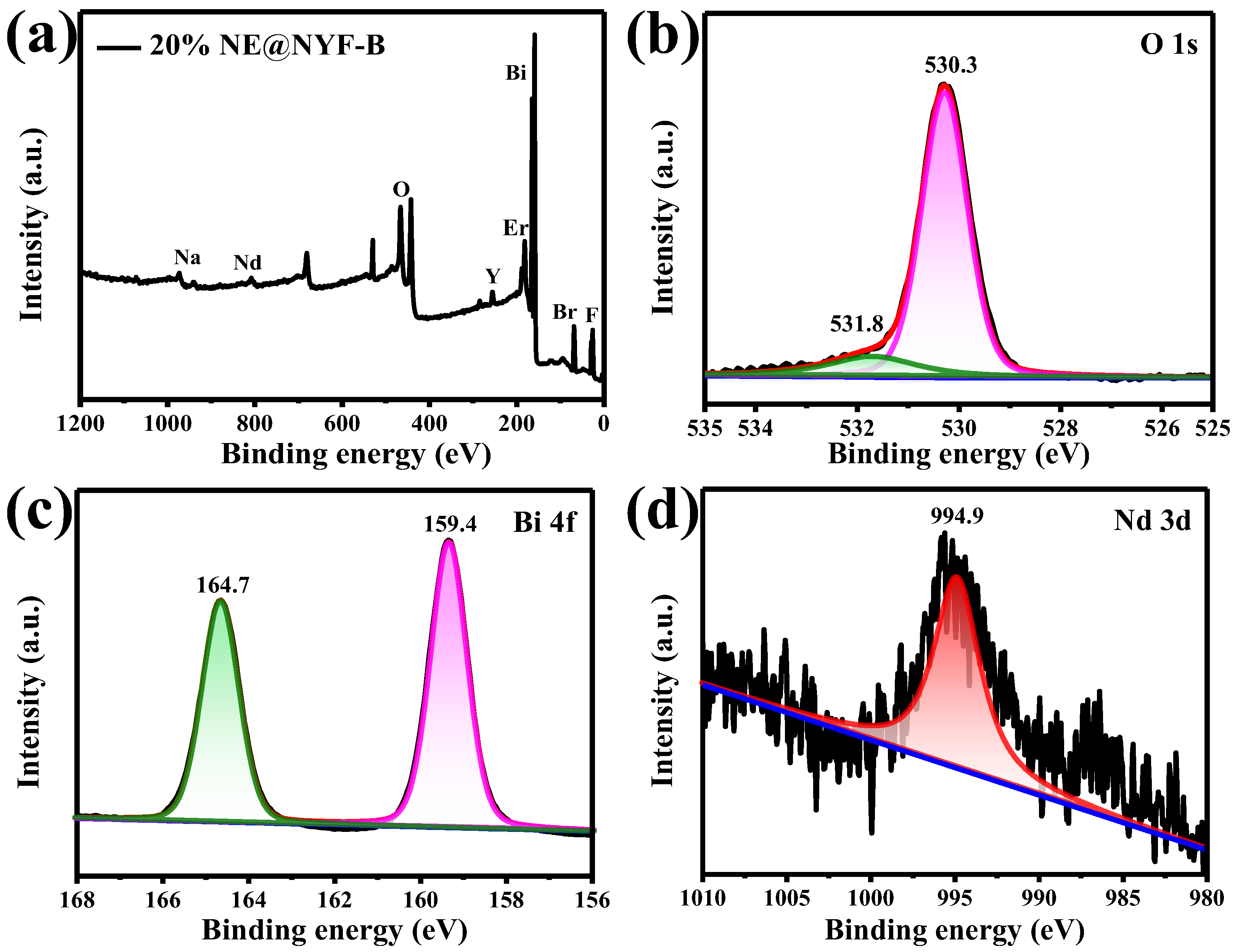
2.2. XPS Analysis
2.3. Optical Properties
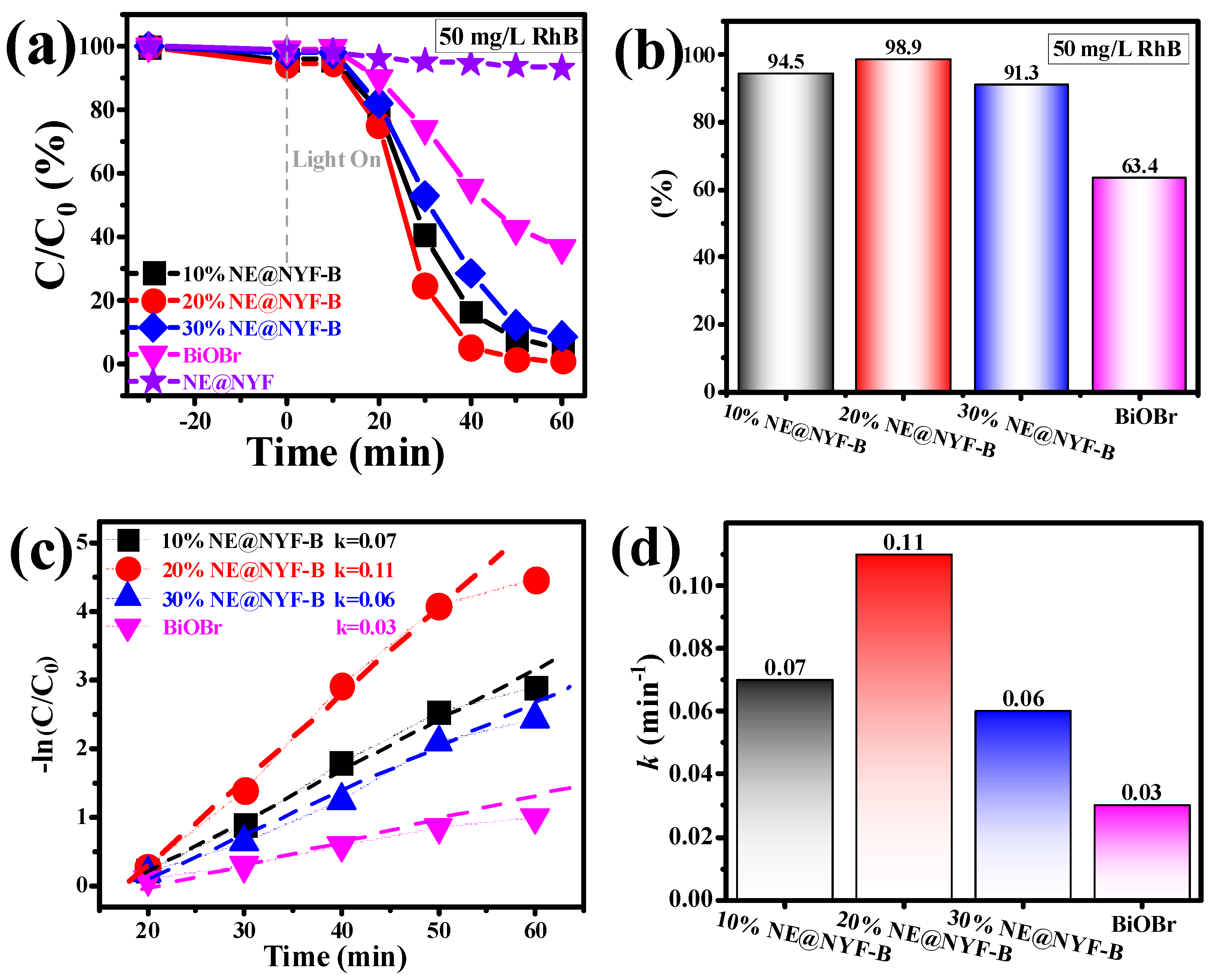
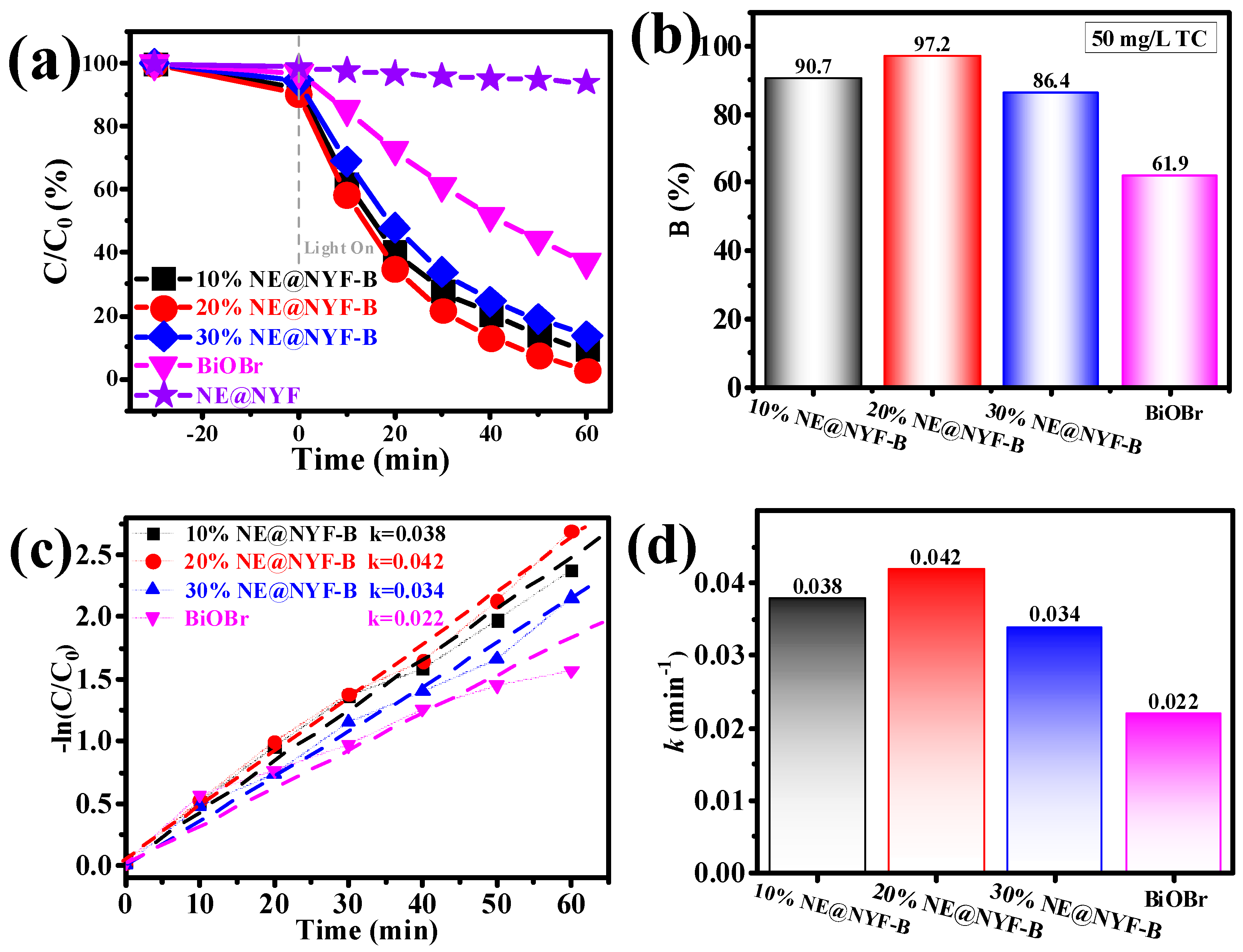
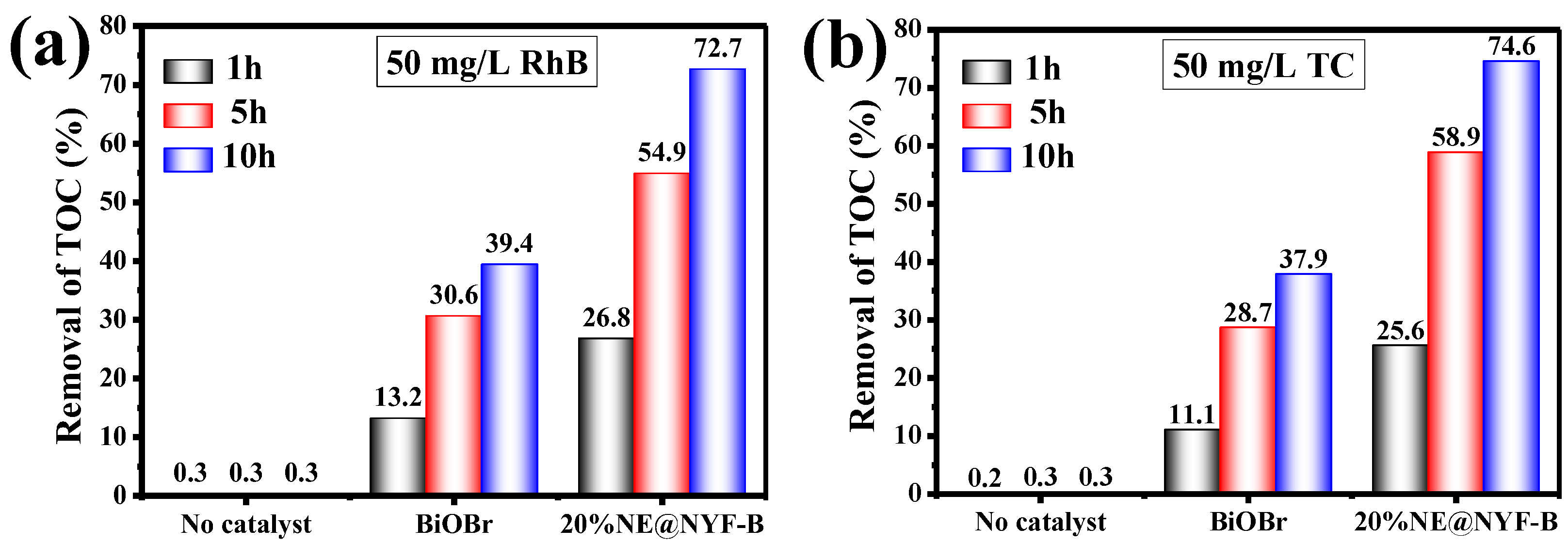
2.4. Photocatalytic Performance
2.5. Conductivity and Charge Transfer Efficiency
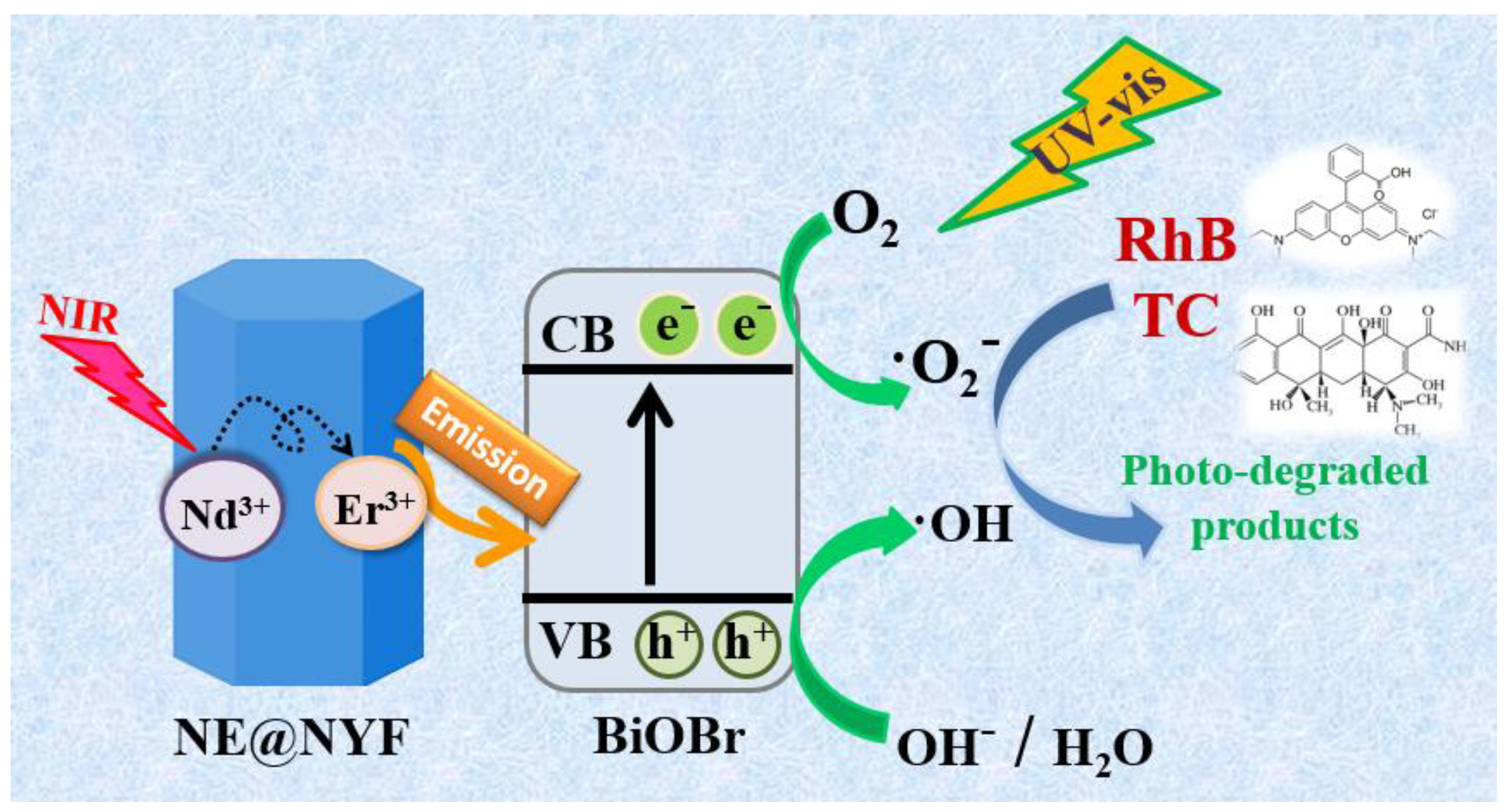
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Materials and Methods
4.2.1. Synthesis of Nd3+, Er3+@NaYF4
4.2.2. Synthesis of Nd3+, Er3+@NaYF4-BiOBr
4.3. Characterization
4.4. Photoelectrochemical Measurements
4.5. Photocatalytic Activity Test
4.6. Quenching Experiments
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Levinson, R.; Berdahl, P.; Akbari, H. Solar spectral optical properties of pigments—Part I: Model for deriving scattering and absorption coefficients from transmittance and reflectance measurements. Sol. Energy Mater. Sol. Cells 2005, 89, 319–349. [Google Scholar] [CrossRef]
- Tian, B.; Fernandez-Bravo, A.; Najafiaghdam, H.; Torquato, N.A.; Altoe, M.V.P.; Teitelboim, A.; Tajon, C.A.; Tian, Y.; Borys, N.J.; Barnard, E.S.; et al. Low irradiance multiphoton imaging with alloyed lanthanide nanocrystals. Nat. Commun. 2018, 9, 3082. [Google Scholar] [CrossRef] [PubMed]
- Askes, S.H.C.; Bonnet, S. Solving the oxygen sensitivity of sensitized photon upconversion in life science applications. Nat. Rev. Chem. 2018, 2, 437–452. [Google Scholar] [CrossRef]
- Richards, B.S.; Hudry, D.; Busko, D.; Turshatov, A.; Howard, I.A. Photon Upconversion for Photovoltaics and Photocatalysis: A Critical Review. Chem. Soc. Rev. 2021, 121, 9165–9195. [Google Scholar] [CrossRef] [PubMed]
- Fischer, S.; Ivaturi, A.; Jakob, P.; Krämer, K.W.; Martin-Rodriguez, R.; Meijerink, A.; Richards, B.; Goldschmidt, J.C. Upconversion solar cell measurements under real sunlight. Opt. Mater. 2018, 84, 389–395. [Google Scholar] [CrossRef]
- Huang, X.; Han, S.; Huang, W.; Liu, X. Enhancing solar cell efficiency: The search for luminescent materials as spectral converters. Chem. Soc. Rev. 2013, 42, 173–201. [Google Scholar] [CrossRef]
- Duan, L.; Zhu, H.; Li, M.; Zhao, X.; Wang, Y.; Zhang, Y.; Yu, L. Atom substitution method to construct full-solar-spectrum absorption MoSeS/TiO2 nanotube arrays for highly efficient hydrogen evolution. J. Alloys Compd. 2021, 889, 161694. [Google Scholar] [CrossRef]
- Ibrahim, A.A.; Salama, R.S.; El-Hakam, S.A.; Khder, A.S.; Ahmed, A.I. Synthesis of sulfated zirconium supported MCM-41 composite with high-rate adsorption of methylene blue and excellent heterogeneous catalyst. Colloids Surf. A Physicochem. Eng. Asp. 2021, 616, 126361. [Google Scholar] [CrossRef]
- Tian, Q.; Yao, W.; Wu, W.; Liu, J.; Wu, Z.; Liu, L.; Dai, Z.; Jiang, C. Efficient UV–Vis-NIR Responsive Upconversion and Plasmonic-Enhanced Photocatalyst Based on Lanthanide-Doped NaYF4/SnO2/Ag. ACS Sustain. Chem. Eng. 2017, 5, 10889–10899. [Google Scholar] [CrossRef]
- Shuai, Y.; Jun, S.; Linchun, C.; Dong, W.; Xiao, P.; Junle, Q. Research on Photoluminescence of Nd3+Doped NaYF4:Yb,Er/Tm Upconversion Nanoparticles. Acta Opt. Sin. 2015, 35, 0816005. [Google Scholar] [CrossRef]
- Zhang, Y.; Lan, X.; Park, S.H.; Wang, L.; Liu, D.; Shi, J. Rational design of efficient near-infrared photon conversion channel via dual-upconversion process for superior photocatalyst. Carbon 2020, 169, 111–117. [Google Scholar] [CrossRef]
- Zhou, L.; Chen, Z.; Dong, K.; Yin, M.; Ren, J.; Qu, X. DNA-mediated construction of hollow upconversion nanoparticles for protein harvesting and near-infrared light triggered release. Adv. Mater. 2014, 26, 2424–2430. [Google Scholar] [CrossRef]
- Nie, Z.; Ke, X.; Li, D.; Zhao, Y.; Zhu, L.; Qiao, R.; Zhang, X.L. NaYF4:Yb,Er,Nd@NaYF4:Nd Upconversion Nanocrystals Capped with Mn:TiO2 for 808 nm NIR-Triggered Photocatalytic Applications. J. Phys. Chem. C 2019, 123, 22959–22970. [Google Scholar] [CrossRef]
- Wu, Z.; Yuan, X.; Zeng, G.; Jiang, L.; Zhong, H.; Xie, Y.; Wang, H.; Chen, X.; Wang, H. Highly efficient photocatalytic activity and mechanism of Yb3+/Tm3+ codoped In2S3 from ultraviolet to near infrared light towards chromium (VI) reduction and rhodamine B oxydative degradation. Appl. Catal. B Environ. 2018, 225, 8–21. [Google Scholar] [CrossRef]
- Kumar, A.; Reddy, K.L.; Kumar, S.; Kumar, A.; Sharma, V.; Krishnan, V. Rational Design and Development of Lanthanide-Doped NaYF4@CdS-Au-RGO as Quaternary Plasmonic Photocatalysts for Harnessing Visible-Near-Infrared Broadband Spectrum. ACS Appl. Mater. Interfaces 2018, 10, 15565–15581. [Google Scholar] [CrossRef]
- Li, G.; Huang, S.; Zhu, N.; Yuan, H.; Ge, D. Near-infrared responsive upconversion glass-ceramic@BiOBr heterojunction for enhanced photodegradation performances of norfloxacin. J. Hazard. Mater. 2021, 403, 123981. [Google Scholar] [CrossRef]
- Reddy, K.L.; Kumar, S.; Kumar, A.; Krishnan, V. Wide spectrum photocatalytic activity in lanthanide-doped upconversion nanophosphors coated with porous TiO2 and Ag-Cu bimetallic nanoparticles. J. Hazard. Mater. 2019, 367, 694–705. [Google Scholar] [CrossRef]
- Huang, S.; Zhu, N.; Lou, Z.; Gu, L.; Miao, C.; Yuan, H.; Shan, A. Near-infrared photocatalysts of BiVO4/CaF2:Er3+, Tm3+, Yb3+ with enhanced upconversion properties. Nanoscale 2014, 6, 1362–1368. [Google Scholar] [CrossRef]
- Chen, Y.; Zhu, G.; Hojamberdiev, M.; Gao, J.; Zhu, R.; Wang, C.; Wei, X.; Liu, P. Three-dimensional Ag2O/Bi5O7I p-n heterojunction photocatalyst harnessing UV-vis-NIR broad spectrum for photodegradation of organic pollutants. J. Hazard. Mater. 2018, 344, 42–54. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Gu, L.; Miao, C.; Lou, Z.; Zhu, N.; Yuan, H.; Shan, A. Near-infrared photocatalyst of Er3+/Yb3+ codoped (CaF2@TiO2) nanoparticles with active-core/active-shell structure. J. Mater. Chem. A 2013, 1, 7874. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Y.; Wang, J.; Fan, Y.; Xiao, T.; Yin, Z.; Wang, T.; Qiu, J.; Song, Z. Enhancement of solar-driven photocatalytic activity of oxygen vacancy-rich Bi/BiOBr/Sr2LaF7:Yb3+,Er3+ composites through synergetic strategy of upconversion function and plasmonic effect. J. Environ. Sci. 2022, 115, 76–87. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Luo, Y.; Fu, S.; Lv, X.; He, Q.; Ji, F.; Xu, X. Visible-to-UVC driven upconversion photocatalyst sterilization efficiency and mechanisms of beta-NaYF4: Pr(3+), Li(+)@BiOCl with a core-shell structure. J. Environ. Manag. 2021, 288, 112394. [Google Scholar] [CrossRef]
- Shi, X.; Chen, X.; Chen, X.; Zhou, S.; Lou, S.; Wang, Y.; Yuan, L. PVP assisted hydrothermal synthesis of BiOBr hierarchical nanostructures and high photocatalytic capacity. Chem. Eng. J. 2013, 222, 120–127. [Google Scholar] [CrossRef]
- Yan, Y.; Yang, H.; Yi, Z.; Xian, T. NaBH4-Reduction Induced Evolution of Bi Nanoparticles from BiOCl Nanoplates and Construction of Promising Bi@BiOCl Hybrid Photocatalysts. Catalysts 2019, 9, 795. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Y.; Zhou, C.; Huo, D.; Zhang, R.; Wang, L. Hydroxyl-regulated BiOI nanosheets with a highly positive valence band maximum for improved visible-light photocatalytic performance. Appl. Catal. B Environ. 2020, 268, 118390. [Google Scholar] [CrossRef]
- Yan, Y.; Yang, H.; Yi, Z.; Wang, X.; Li, R.; Xian, T. Evolution of Bi Nanowires from BiOBr Nanoplates through a NaBH4 Reduction Method with Enhanced Photodegradation Performance. Environ. Eng. Sci. 2020, 37, 64–77. [Google Scholar] [CrossRef]
- Thomas, V.; Sofin, R.P.G.P.S.; Allen, M.; Thomas, H. Crystal Field Parameters and Optical Parameters of Nd3+ in Sodium Bismuth Silicate Glass. SQU J. Sci. 2015, 20, 70–76. [Google Scholar] [CrossRef]
- Xu, W.; Qi, H.; Zheng, L.; Zhang, Z.; Cao, W. Multifunctional nanoparticles based on the Nd3+/Yb3+ codoped NaYF4. Opt. Lett. 2015, 40, 5678–5681. [Google Scholar] [CrossRef]
- Singh, S.; Nannuri, S.H.; George, S.D.; Chakraborty, S.; Sharma, A.; Misra, S.K. Enhanced Visible/NIR driven catalytic activity in presence of neodymium (Nd3+), for Yb3+ and Tm3+ doped NaYF4 nanoparticles. J. Environ. Chem. Eng. 2021, 9, 105813. [Google Scholar] [CrossRef]
- Reszczyńska, J.; Grzyb, T.; Sobczak, J.W.; Lisowski, W.; Gazda, M.; Ohtani, B.; Zaleska, A. Lanthanide co-doped TiO2: The effect of metal type and amount on surface properties and photocatalytic activity. Appl. Surf. Sci. 2014, 307, 333–345. [Google Scholar] [CrossRef]
- Huang, H.; Li, H.; Wang, Z.; Wang, P.; Zheng, Z.; Liu, Y.; Dai, Y.; Li, Y.; Huang, B. Efficient near-infrared photocatalysts based on NaYF4:Yb3+,Tm3+@NaYF4:Yb3+,Nd3+@TiO2 core@shell nanoparticles. Chem. Eng. J. 2019, 361, 1089–1097. [Google Scholar] [CrossRef]
- Tian, Q.; Yao, W.; Wu, W.; Jiang, C. NIR light-activated upconversion semiconductor photocatalysts. Nanoscale Horiz. 2019, 4, 10–25. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.; Zhang, Y.; Ding, W.; Yu, L.; Zhou, X.; Lawrence Wu, C.-M. Preparation and photoelectrochemical properties of BiFeO3/BiOI composites. RSC Adv. 2020, 10, 26658–26663. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Choi, J.W.; Kim, W.; Lee, R.; Woo, H.C.; Shin, J.; Kim, H.; Son, Y.J.; Jo, J.Y.; Lee, H.; et al. Improvement of perovskite crystallinity by omnidirectional heat transfer via radiative thermal annealing. RSC Adv. 2019, 9, 14868–14875. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, L.; Park, S.H.; Kong, X.; Lan, X.; Song, Z.; Shi, J. Single near-infrared-laser driven Z-scheme photocatalytic H2 evolution on upconversion material@Ag3PO4@black phosphorus. Chem. Eng. J. 2019, 375, 121967. [Google Scholar] [CrossRef]
- Yin, D.; Zhao, F.; Zhang, L.; Zhang, X.; Liu, Y.; Zhang, T.; Wu, C.; Chen, D.; Chen, Z. Greatly enhanced photocatalytic activity of semiconductor CeO2 by integrating with upconversion nanocrystals and graphene. RSC Adv. 2016, 6, 103795–103802. [Google Scholar] [CrossRef]
- Liyanage, A.D.; Perera, S.D.; Tan, K.; Chabal, Y.; Balkus, K.J. Synthesis, Characterization, and Photocatalytic Activity of Y-Doped CeO2 Nanorods. ACS Catal. 2014, 4, 577–584. [Google Scholar] [CrossRef]
- Yassin, A.Y.; Abdelghany, A.M.; Salama, R.S.; Tarabiah, A.E. Structural, Optical and Antibacterial Activity Studies on CMC/PVA Blend Filled with Three Different Types of Green Synthesized ZnO Nanoparticles. J. Inorg. Organomet. Polym. Mater. 2023, 33, 1855–1867. [Google Scholar] [CrossRef]
- Lee, S.Y.; Lee, G.; Jun, Y.-S.; Park, Y.I. Visible/near-infrared driven highly efficient photocatalyst based on upconversion nanoparticles/g-C3N4 nanocomposite. Appl. Surf. Sci. 2020, 508, 144839. [Google Scholar] [CrossRef]
- Tan, L.; Li, D.; Zhang, L.; Xu, L.; Zhao, Y.; Zhu, L.; Qiao, R. Preparation of Multishell-Structured NaYF4:Yb,Tm,Nd@NaYF4:Yb,Nd@SiO2@ZnO Nanospheres with Effective NIR-Induced Photocatalytic Activity. J. Phys. Chem. C 2020, 124, 18081–18090. [Google Scholar] [CrossRef]
- Wang, J.; Wang, H.; Zuo, S.; Jin, X.; Zheng, B.; Deng, R.; Liu, W.; Wang, J. Synergistic effects of lanthanide surface adhesion and photon-upconversion for enhanced near-infrared responsive photodegradation of organic contaminants in wastewater. Environ. Sci. Nano 2020, 7, 3333–3342. [Google Scholar] [CrossRef]
- Yao, X.; Jiang, X.; Zhang, D.; Lu, S.; Wang, M.; Pan, S.; Pu, X.; Liu, J.; Cai, P. Achieving improved full-spectrum responsive 0D/3D CuWO4/BiOBr:Yb3+,Er3+ photocatalyst with synergetic effects of up-conversion, photothermal effect and direct Z-scheme heterojunction. J. Colloid Interface Sci. 2023, 644, 95–106. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Park, M.; Kim, H.Y.; Ding, B.; Park, S.-J. A facile ultrasonic-assisted fabrication of nitrogen-doped carbon dots/BiOBr up-conversion nanocomposites for visible light photocatalytic enhancements. Sci. Rep. 2017, 7, 45086. [Google Scholar] [CrossRef] [PubMed]
- Toby, B.H. EXPGUI, a graphical user interface for GSAS. J. Appl. Crystallogr. 2001, 34, 210–213. [Google Scholar] [CrossRef]




| Materials | Concentration Targets | Efficiency | Removal of TOC | Time | Ref. |
|---|---|---|---|---|---|
| NYF@C@Ag3PO4 | 10 mg/L CIP | 69.64% | / | 18 h | [11] |
| OVs-Bi/BiOBr/Sr2LaF7:Yb3+, Er3+ | 10 mg/L BPA | 81% | / | 180 min | [21] |
| UCNPs/g-C3N4 | 30 μM MO | ~70% | / | 60 min | [39] |
| UCN@SiO2@ZnO | 5 mg/L RhB | 61.2% | / | 250 min | [40] |
| UCNP@TiO2 | 20 μg/L RhB | ~98% | 66% | 300 min | [41] |
| CuWO4/BiOBr:Yb3+,Er3 | 20 mg/L TC | 93.9% | / | 60 min | [42] |
| N-CDs/BiOBr | 10 mg/L RhB | 89.3% | / | 50 min | [43] |
| Nd3+, Er3+@NYF-BiOBr | 50 mg/L RhB 50 mg/L TC | 98.9% 97.2% | 72.7% 74.6% | 60 min | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, L.; Wang, Y.; Su, X.; Liu, C.; Xue, K.; Luo, H.; Zhang, Y.; Zhu, H. In Situ Construction of Near-Infrared Response Hybrid Up-Conversion Photocatalyst for Degrading Organic Dyes and Antibiotics. Molecules 2023, 28, 6674. https://doi.org/10.3390/molecules28186674
Yu L, Wang Y, Su X, Liu C, Xue K, Luo H, Zhang Y, Zhu H. In Situ Construction of Near-Infrared Response Hybrid Up-Conversion Photocatalyst for Degrading Organic Dyes and Antibiotics. Molecules. 2023; 28(18):6674. https://doi.org/10.3390/molecules28186674
Chicago/Turabian StyleYu, Lianqing, Yankun Wang, Xinhai Su, Chong Liu, Kehui Xue, Huihua Luo, Yaping Zhang, and Haifeng Zhu. 2023. "In Situ Construction of Near-Infrared Response Hybrid Up-Conversion Photocatalyst for Degrading Organic Dyes and Antibiotics" Molecules 28, no. 18: 6674. https://doi.org/10.3390/molecules28186674
APA StyleYu, L., Wang, Y., Su, X., Liu, C., Xue, K., Luo, H., Zhang, Y., & Zhu, H. (2023). In Situ Construction of Near-Infrared Response Hybrid Up-Conversion Photocatalyst for Degrading Organic Dyes and Antibiotics. Molecules, 28(18), 6674. https://doi.org/10.3390/molecules28186674






