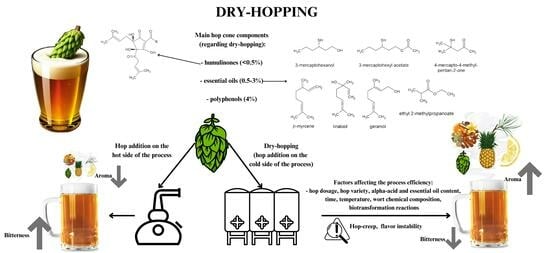
Effects of Dry-Hopping on Beer Chemistry and Sensory Properties—A Review
Abstract
:1. Introduction
2. Chemical Composition of Hops
| Compound Group | Typical Content (% (w/w)) |
|---|---|
| Resins | 15–30 |
| Essential oils | 0.5–3 |
| Proteins | 15 |
| Monosaccharides | 2 |
| Polyphenols | 4.3–14 |
| Pectins | 2 |
| Amino acids | 0.1 |
| Waxes and steroids | trace-25 |
| Ash | 8 |
| Moisture | 10 |
| Cellulose and others | 40–43 |
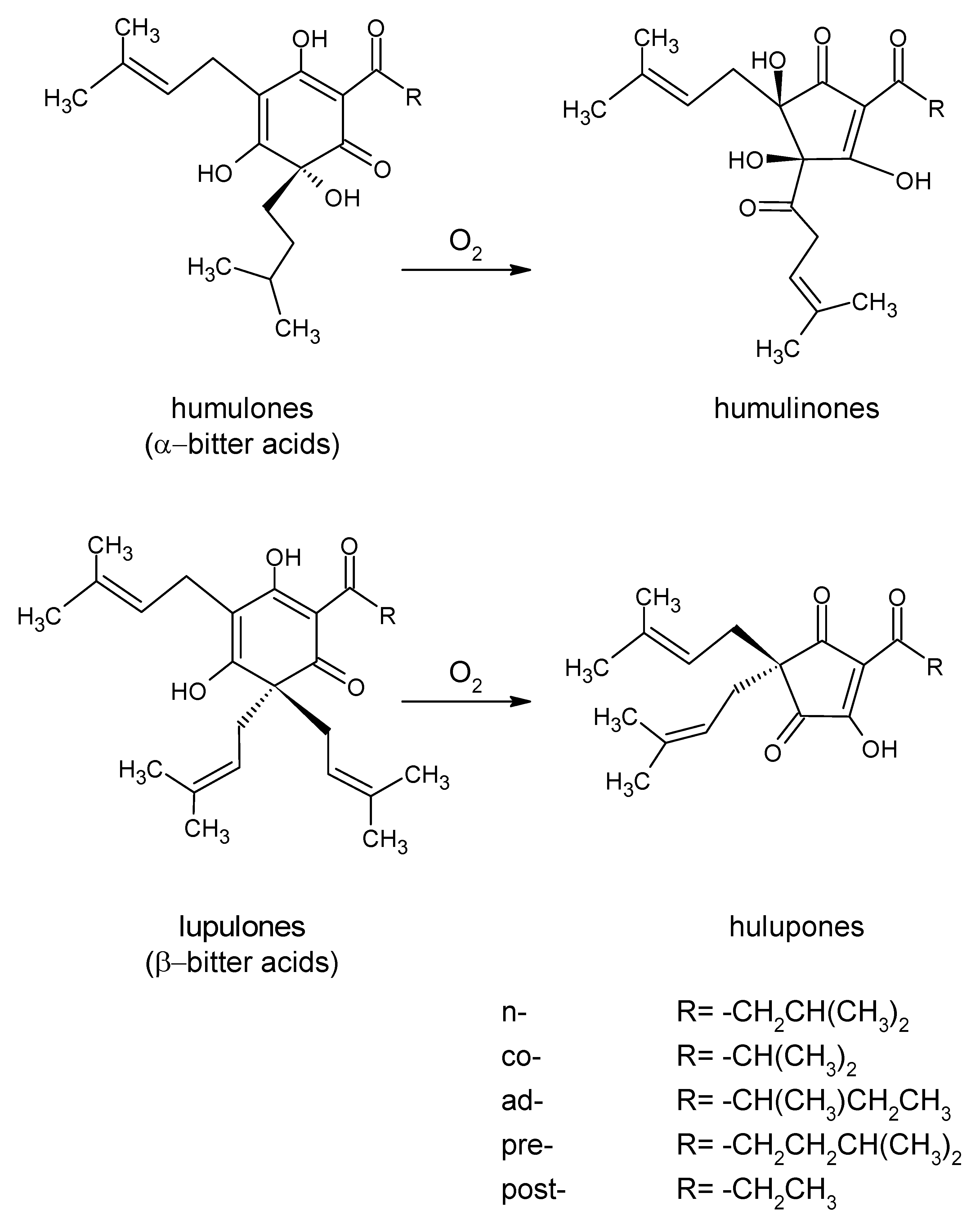
2.1. Hop Resins
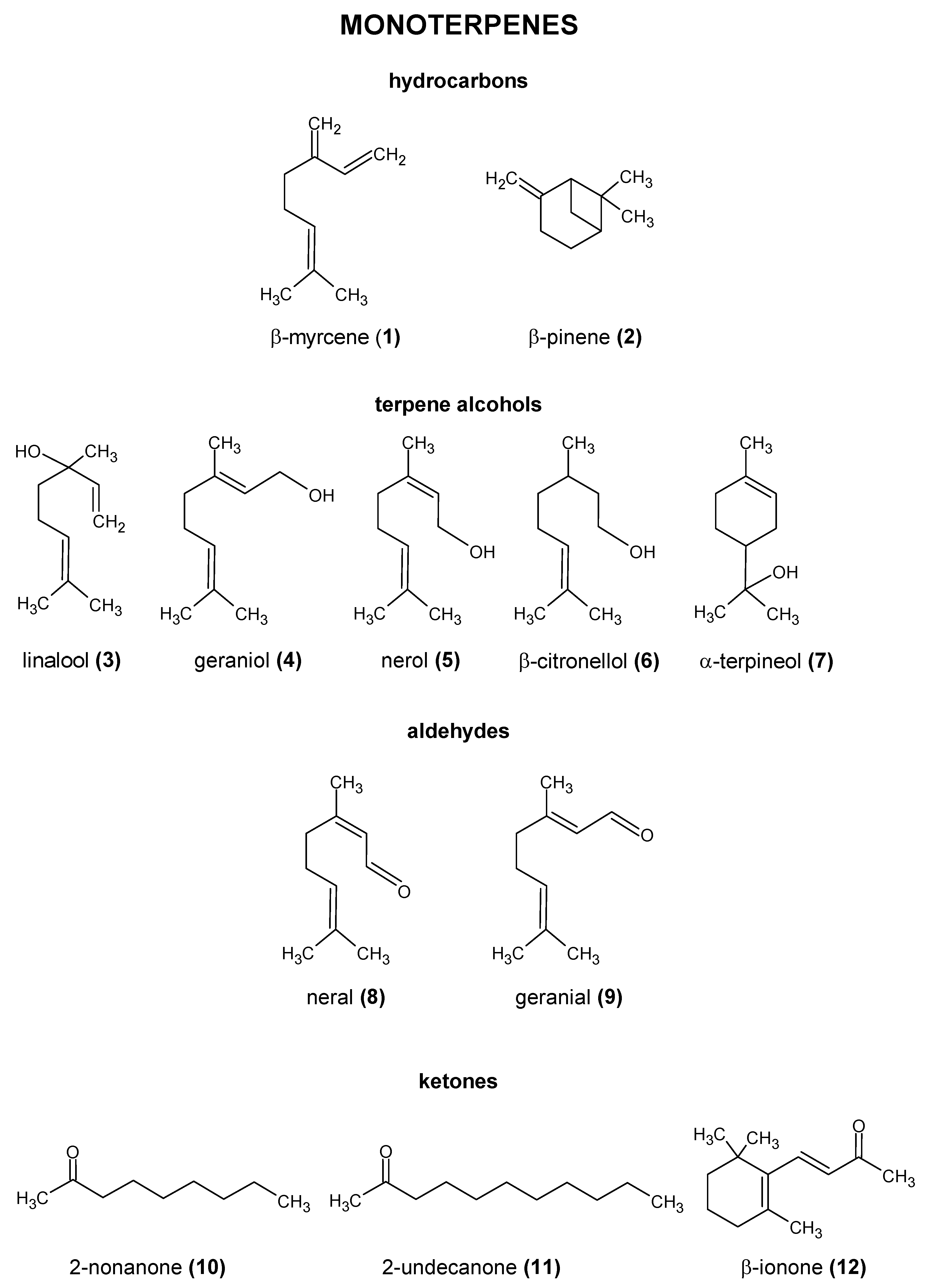
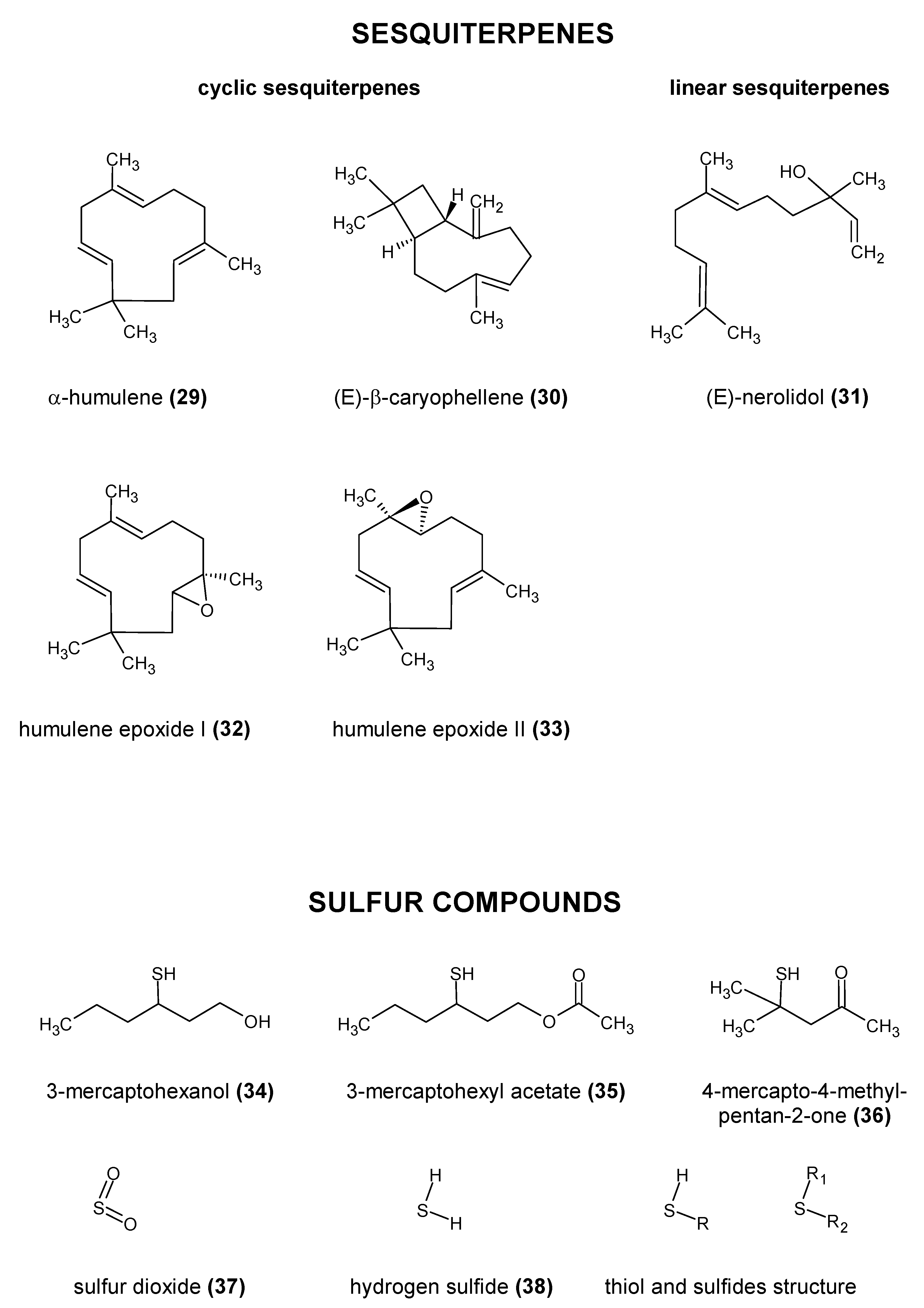
2.2. Essential Oils

| Compound | Aroma | Aroma Threshold | Example of Concentrations (±SD *) in Dry-Hopped Beer (the Methods Used for Analysis) | Reference |
| Hydrocarbons | ||||
| α-humulene (29) | spicy and woody | 450 µg/L, 120 µg/L | 5.2 (±0.5) µg/L (Trap GC-MS) 0.4 (±0.04)–1.2 (±0.41) μg/L (HS-GC-MS) | [51,52,53,54] |
| β-myrcene (1) | herbaceous, resinous, green, balsamic, fresh hops | 350 µg/L, 30–200 µg/L, 9.5 µg/L | 79.7 (±2.8) μg/L (Trap GC-MS) 0.3 (±0.12)–15.9 (±2.58) μg/L (HS-GC-MS) 117.4–863.6 μg/L (HS-SPME-GC-MS) | [51,52,53,54,55,56] |
| β-pinene (2) | turpentine odor with a dry, woody, or resinous aroma | 140 µg/L | 15.4 (±3.3)–89.4 (±19.7) μg/L (HS-GC-SPME) | [50,57,58] |
| (E)-caryophyllene (30) | spicy and woody | 230 µg/L | 2.3 (±0.2) μg/L (Trap GC-MS) 0.2 (±0.06)–0.3 (±0.12) μg/L (SPE-GC-MS) | [51,52,53,54] |
| Terpene Alcohols | ||||
| α-terpineol (7) | lilac odor with a sweet taste reminiscent of peach on dilution | 330 μg/L, 450 μg/L | 25.4 (±6.4) μg/L (Trap GC-MS) 0.2 (±0.07)–2.8 (±0.15) μg/L (HS-GC-MS) | [36,54,55,57,59] |
| β-citronellol (6) | lemon/lime-like | 8 μg/L, 40 μg/L | 26.3 (±3.5) μg/L (Trap GC-MS) 1.2 (±0.05)–1.8 (±0.29) μg/L | [36,42,54,55] |
| geraniol (4) | floral/rose-like | 4–5 μg/L, 40 μg/L | 265 (±45.8) μg/L (Trap GC-MS) 30.9 (±0.92)–72.8 (±0.17) μg/L (HS-GC-MS) | [36,41,54,55] |
| linalool (3) | floral/lavender/coriander/citrus-like flavor | 8 μg/L | 155 (±8.0) μg/L (Trap GC-MS) 33.4 (±0.80)–36.4 (±0.87) μg/L (HS-GC-MS) | [42,54,55] |
| nerol (5) | fresh, sweet, rose-like | 300 μg/L, 80 μg/L | 5.2 (±0.10)–7.7 (±0.25) μg/L (SPME-GC-MS) | [36,57,59] |
| Sulfur Compounds | ||||
| 3-mercaptohexanol (3MH) (34) | rhubarb and grapefruit | 55 ng/L | 29–475 ng/L (LC-MS/MS) | [55,60] |
| 3-mercaptohexyl acetate (3MHA) (35) | passion fruit and grapefruit | 4 ng/L | 4–10 ng/L (LC-MS/MS) | [55,60] |
| 4-mercapto-4-methylpentan-2-one (4MMP) (36) | blackcurrant and passion-fruit-like | 1.5 ng/L | 31–40 ng/L (LC-MS/MS) | [55,60] |
| Esters | ||||
| ethyl 2-methyl- butanoate (16) | green-fruity, apple-like | 1.1 μg/L | 1.1–1.9 μg/L (SPME-GC-MS) | [56,57] |
| ethyl 3-methyl- butanoate (17) | fruity, vinous, apple-like | 2 μg/L | 1.5–1.8 μg/L (SPME-GC-MS) | [56,57] |
| ethyl 2-methyl- propanoate (18) | fruity | 6.3 μg/L | 11.8–18.7 μg/L (SPME-GC-MS) | [56,57] |
| ethyl 4-methyl- pentanoate (19) | fruity | 1.0 μg/L | 0.6–0.9 μg/L (SPME-GC-MS) | [56,57] |
| ethyl heptanoate (20) | fruity, reminiscent of cognac, wine/brandy | 400 μg/L | 1.6–8.8 μg/L (SPME-GC-MS) | [57,61,62] |
| isoamyl acetate (21) | fruity, banana, sweet, fragrant | 1200 μg/L | 275 (±54.6)–(330 (±11.6) μg/L (Trap GC-MS) | [54,55,57,63] |
| isoamyl isobutyrate (22) | fruity, apricot, pineapple | N/A ** | 0.6–16.5 μg/L (SPME-GC-MS) | [57,62] |
| isobutyl isobutyrate (23) | pineapple | N/A | 0.4–54 μg/L (SPME-GC-MS) | [57,61] |
| 2-methylbutyl isobutyrate (2-methylbutyl 2-methylpropanoate) (24) | fruity | 78 μg/L | 1.6–103.6 μg/L (SPME-GC-MS) 41–198 μg/L (GC-FID) 24–87 µg/L (HS-SPME-GC-MS) | [57,61,63,64,65] |
| 2-methylbutyl 2-methylbutyrate (25) | fruity, floral, banana and pineapple | N/A | 0.13–0.3 μg/L (SPME-GC-MS) | [66] |
| methyl geranate (26) | fruity, floral, waxy, herbal, citrus/sweet, candy | N/A | 1.3–7.6 µg/L (SPME-GC-MS) 8–192 µg/L (HS-SPME-GC-MS) | [1,65,66] |
| 2-methylbutylpropanoate (27) | sweet, fruity, rum-like | N/A | 41–198 µg/L (SPME-GC-MS) | [66] |
| isoamyl butyrate (28) | fruity, green apple-like, and apricot | N/A | N/A | [66] |
| Ketones | ||||
| 2-nonanone (10) | rue, rose and tea-like | 5–200 μg/L | 6.9 (±0.30) μg/L (Trap GC-MS) 0.1–2.7 µg/L (SPME-GC-MS) | [54,56,57] |
| 2-undecanone (11) | rue odor with a sweet flavor reminiscent of peach | 7 μg/L, 400 μg/L | 4.4 (±0.30) μg/L (Trap GC-MS) 0.1–6.0 µg/L (SPME-GC-MS) | [54,56,57] |
| β-ionone (12) | violet-like, fruity, woody | N/A | ≤0.1 μg/L (SPME-GC-MS) | [57,61] |
2.3. Polyphenols
3. Extraction of Compounds during the Process
3.1. Volatile Compounds
3.2. Bitter Compounds
3.3. Other Compounds
4. Parameters Affecting the Extraction Process
4.1. Hop Dose and Alpha Content
4.2. Time and Temperature
4.3. Early vs. Late Dry-Hopping
4.4. Chemical Composition of Wort
4.5. Other Factors
5. Chemical Changes during the Process
6. Problems
6.1. Hop Creep
6.2. Haze Formation
6.3. Other Problems
6.3.1. Grassy Flavor
6.3.2. Potential of Infection
6.3.3. Flavor Stability
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Lafontaine, S.R.; Shellhammer, T.H. Impact of static dry-hopping rate on the sensory and analytical profiles of beer. J. Inst. Brew. 2018, 124, 434–442. [Google Scholar] [CrossRef]
- Praet, T.; van Opstaele, F.; Baert, J.; Aerts, G.; de Cooman, L. Comprehensive characterisation of the hop-derived sesquiterpenoid fingerprint of American kettle hopped lager beers. Brew. Sci. 2014, 67, 187–194. [Google Scholar]
- Schönberger, C.; Kostelecky, T. 125th Anniversary review: The role of hops in brewing. J. Inst. Brew. 2011, 117, 259–267. [Google Scholar] [CrossRef]
- Steele, M. IPA: Brewing Techniques, Recipes, and the Evolution of India Pale Ale; Brewers Publications: Boulder, CO, USA, 2012. [Google Scholar]
- Sterowski, R. Did Hodgson Invent Dry Hopping? 2010. Available online: https://refreshingbeer.blogspot.com/2010/01/did-hodgson-invent-dry-hopping.html (accessed on 14 August 2023).
- Healey, J. The Hops List: 265 Beer Hop Varietes from around the World; Blurb. Reischling Press, Inc.: Tukwila, DC, USA, 2016. [Google Scholar]
- Hanke, S.; Herrmann, M.; Rückerl, J.; Schönberger, C.; Back, W. Hop Volatile Compounds (Part II): Transfer rates of hop compounds from hop pellets to wort and beer. Brew. Sci. 2008, 61, 140–147. [Google Scholar]
- Almaguer, C.; Schönberger, C.; Gastl, M.; Arendt, E.K.; Becker, T. Humulus lupulus—A story that begs to be told. A review. J. Inst. Brew. 2014, 120, 289–314. [Google Scholar] [CrossRef]
- Lafontaine, S.; Varnum, S.; Roland, A.; Delpech, S.; Dagan, L.; Vollmer, D.; Kishimoto, T.; Shellhammer, T. Impact of harvest maturity on the aroma characteristics and chemistry of Cascade hops used for dry-hopping. Food Chem. 2019, 278, 228–239. [Google Scholar] [CrossRef]
- Nickerson, B.G.; Williams, A.P.; Haunold, A. Varietal differences in the proportions of cohumulone, adhumulone, and humulone in hops. J. Am. Soc. Brew. Chem. 1986, 44, 91–94. [Google Scholar] [CrossRef]
- Oladokun, O.; James, S.; Cowley, T.; Smart, K.; Hort, J.; Cook, D. Dry-hopping: The effects of temperature and hop variety on the bittering profiles and properties of resultant beers. Brew. Sci. 2017, 70, 187–196. [Google Scholar] [CrossRef]
- Carbone, K.; Gervasi, F. An Updated Review of the Genus Humulus: A Valuable Source of Bioactive Compounds for Health and Disease Prevention. Plants 2022, 11, 3434. [Google Scholar] [CrossRef]
- Baker, G.A.; Danenhower, T.M.; Force, L.J.; Petersen, K.J.; Betts, T.A. HPLC Analysis of α- and β-Acids in Hops. J. Chem. Educ. 2008, 85, 954. [Google Scholar] [CrossRef]
- Maye, J.P.; Smith, R.; Steiner, S.S. A natural foam enhancer from hops. In Proceedings of the Poster World Brewing Congress; Portland, OR, USA, 28 July–1 August 2012. [Google Scholar]
- Fritsch, A.; Shellhammer, T.H. Alpha-acids do not contribute bitterness to lager beer. J. Am. Soc. Brew. Chem. 2007, 65, 26–28. [Google Scholar] [CrossRef]
- Maye, J.P.; Smith, R. Hidden Secrets of the New England IPA. MBBA Tech. Q. 2018, 55, 88–92. [Google Scholar] [CrossRef]
- Hauser, D.G.; Lafontaine, S.R.; Shellhammer, T.H. Extraction efficiency of dry-hopping. J. Am. Soc. Brew. Chem. 2019, 77, 188–198. [Google Scholar] [CrossRef]
- Liu, Y.; Lu, N.; Jiang, T. Synthesis, characterization, crystal structure, and antioxidant activity of hexahydro-β-acids. J. Mol. Struct. 2019, 1175, 721–727. [Google Scholar] [CrossRef]
- Taniguchi, Y.; Matsukura, Y.; Ozaki, H.; Nishimura, K.; Shindo, K. Identification and quantification of the oxidation products derived from α-acids and β-acids during storage of hops (Humulus lupulus L.). J. Agric. Food Chem. 2013, 61, 3121–3130. [Google Scholar] [CrossRef] [PubMed]
- Maye, J.P.; Leker, J.; Smith, R. Preparation of dicyclohexylamine humulinones and dicyclohexylamine hulupones. J. Am. Soc. Brew. Chem. 2016, 74, 57–60. [Google Scholar] [CrossRef]
- Algazzali, V.; Shellhammer, T. Bitterness intensity of oxidized hop acids: Humulinones and hulupones. J. Am. Soc. Brew. Chem. 2016, 74, 36–43. [Google Scholar] [CrossRef]
- Hahn, C.D.; Lafontaine, S.R.; Pereira, C.B.; Shellhammer, T.H. Evaluation of nonvolatile chemistry affecting sensory bitterness intensity of highly hopped beers. J. Agric. Food Chem. 2018, 66, 3505–3513. [Google Scholar] [CrossRef]
- Almaguer, C.; Gastl, M.; Arendt, E.K.; Becker, T. Comparative study of the contribution of hop (Humulus lupulus L.) hard resins extracted from different hop varieties to beer quality parameters. J. Am. Soc. Brew. Chem. 2015, 73, 115–123. [Google Scholar] [CrossRef]
- Cocuzza, S.; Gmeinwieser, S.; Helmschrott, K.; Peifer, F.; Zarnkov, M. How alcohol content in dry-hopped beer affects final beer composition-a model study. Brew. Sci. 2022, 75, 44–53. [Google Scholar] [CrossRef]
- Haseleu, G.; Intelmann, D.; Hofmann, T. Structure determination and sensory evaluation of novel bitter compounds formed from β-acids of hop (Humulus lupulus L.) upon wort boiling. Food Chem. 2009, 116, 71–81. [Google Scholar] [CrossRef]
- Dostálek, P.; Karabín, M.; Jelínek, L. Hop phytochemicals and their potential role in metabolic syndrome prevention and therapy. Molecules 2017, 22, 1761. [Google Scholar] [CrossRef]
- Krofta, K.; Hervert, J.; Mikyška, A.; Dušek, M. Hop beta acids—From cones to beer. Acta Hortic. 2019, 1236, 15–22. [Google Scholar] [CrossRef]
- Aberl, A.; Coelhan, M. Determination of volatile compounds in different hop varieties by headspace-trap GC/MS—In comparison with conventional hop essential oil analysis. J. Agric. Food Chem. 2012, 60, 2785–2792. [Google Scholar] [CrossRef] [PubMed]
- Preedy, V.R. (Ed.) Beer in Health and Disease Prevention; Academic Press: Burlington, MA, USA, 2009. [Google Scholar] [CrossRef]
- Rutnik, K.; Knez Hrnčič, M.; Jože Košir, I. Hop essential oil: Chemical composition, extraction, analysis, and applications. Food Rev. Int. 2022, 38, 529–551. [Google Scholar] [CrossRef]
- Nickerson, B.G.; Van Engel, L.E. Hop aroma component profile and the aroma unit. J. Am. Soc. Brew. Chem. 1992, 50, 77–81. [Google Scholar] [CrossRef]
- Sharpe, F.R.; Laws, D.R.J. The essential oil of hops a review. J. Inst. Brew. 1981, 87, 96–107. [Google Scholar] [CrossRef]
- Nance, M.R.; Setzer, N.W. Volatile components of aroma hops (Humulus lupulus L.) commonly used in beer brewing. J. Brew. Distill. 2011, 2, 16–22. [Google Scholar]
- Holt, S.; Miks, M.H.; de Carvalho, B.T.; Foulquie-Moreno, M.R.; Thevelein, J.M. The molecular biology of fruity and floral aromas in beer and other alcoholic beverages. FEMS Microbiol. Rev. 2019, 43, 193–222. [Google Scholar] [CrossRef]
- Janish, S. Dry hop best practices: Using science as a guide for process and recipe development. MBAA Tech. Q. 2021, 58, 59–65. [Google Scholar] [CrossRef]
- Takoi, K.; Koie, K.; Itoga, Y.; Katayama, Y.; Shimase, M.; Nakayama, Y.; Watari, J. Biotransformation of hop-derived monoterpene alcohols by lager yeast and their contribution to the flavor of hopped beer. J. Agric. Food Chem. 2010, 58, 5050–5058. [Google Scholar] [CrossRef] [PubMed]
- De Almeida, N.E.C.; de Aguiar, I.; Cardoso, D.R. Mechanism of hop-derived terpenes oxidation in beer. J. Braz. Chem. Soc. 2015, 26, 2362–2368. [Google Scholar] [CrossRef]
- Karabín, M.; Hudcová, T.; Jelínek, L.; Dostálek, P. Biologically active compounds from hops and prospects for their use. Compr. Rev. Food Sci. Food Saf. 2016, 15, 542–567. [Google Scholar] [CrossRef] [PubMed]
- Knez Hrnčič, M.; Španinger, E.; Košir, I.J.; Knez, Ž.; Bren, U. Hop compounds: Extraction techniques, chemical analyses, antioxidative, antimicrobial, and anticarcinogenic effects. Nutrients 2019, 11, 257. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Wang, X.; Yuan, A.; Liu, J.; Li, Z.; Xie, D.; Zhang, H.; Luo, W.; Xu, H.; Liu, J.; et al. Chemical constituents and bioactivities of hops (Humulus lupulus L.) and their effects on beer- related microorganisms. Food Energy Secur. 2022, 11, e367. [Google Scholar] [CrossRef]
- Lafontaine, S.; Caffrey, A.; Dailey, J.; Varnum, S.; Hale, A.; Eichler, B.; Dennenlöhr, J.; Schubert, C.; Knoke, L.; Lerno, L.; et al. Evaluation of variety, maturity, and farm on the concentrations of monoterpene diglycosides and hop volatile/nonvolatile composition in five Humulus lupulus cultivars. J. Agric. Food Chem. 2021, 69, 4356–4370. [Google Scholar] [CrossRef]
- Cibaka Kankolongo, M.-L.; Ferreira, C.S.; Decourrière, L.; Lorenzo-Alonso, C.-J.; Bodart, E.; Collin, S. Dry hopping with the dual-purpose varieties Amarillo, Citra, Hallertau Blanc, Mosaic, and Sorachi Ace: Minor contribution of hop terpenol glucosides to beer flavors. J. Am. Soc. Brew. Chem. 2017, 75, 122–129. [Google Scholar] [CrossRef]
- Brendel, S.; Hofmann, T.; Granvogl, M. Dry-hopping to modify the aroma of alcohol-free beer on a molecular level—Loss and transfer of odor-active compounds. J. Agric. Food Chem. 2020, 68, 8602–8612. [Google Scholar] [CrossRef]
- Cibaka, M.-L.K.; Decourrière, L.; Lorenzo-Alonso, C.-J.; Bodart, E.; Robiette, R.; Collin, S. 3-Sulfanyl-4-methylpentan-1-ol in dry-hopped beers: First evidence of glutathione S-conjugates in hop (Humulus lupulus L.). J. Agric. Food Chem. 2016, 64, 8572–8582. [Google Scholar] [CrossRef]
- Sharp, D.C.; Steensels, J.; Shellhammer, T.H. The effect of hopping regime, cultivar and β-glucosidase activity on monoterpene alcohol concentrations in wort and beer. J. Inst. Brew. 2017, 123, 185–191. [Google Scholar] [CrossRef]
- Takoi, K. Flavor compounds contributing to the characteristic flavor of new aroma hop cultivars. BRAUWELT Int. 2013, 31, 86–91. [Google Scholar]
- Olšovská, J.; Vrzal, T.; Štěrba, K.; Slabý, M.; Kubizniaková, P.; Čejka, P. The chemical profiling of fatty acids during the brewing process. J. Sci. Food Agric. 2019, 99, 1772–1779. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, C.S.; Collin, S. Fate of hop and fermentation odorants in commercial Belgian dry-hopped beers over 2 years of bottle storage: Key-role of oxidation and hop esterases. J. Am. Soc. Brew. Chem. 2021, 79, 259–271. [Google Scholar] [CrossRef]
- Forster, A.; Gahr, A. On the fate of certain hop substances during dry hopping. Brew. Sci. 2013, 66, 94–103. [Google Scholar]
- Haslbeck, K.; Minkenberg, D.; Coelhan, M. Investigations into the transfer rate of volatile compounds in dry hopping using an octanol-water partition coefficient model. J. Am. Soc. Brew. Chem. 2018, 76, 169–177. [Google Scholar] [CrossRef]
- Bordiga, M.; Nollet, L.M.L. Food Aroma Evolution during Food Processing, Cooking, and Aging; CRC Press: Boca Raton, FL, USA, 2020; ISBN 978-1-138-33824-1. [Google Scholar]
- Rutnik, K.; Ocvirk, M.; Košir, I.J. Impact of hop freshness on dry hopped beer quality. Foods 2022, 11, 1310. [Google Scholar] [CrossRef]
- Salamon, V.R.; Dabija, A.; Ferencz, Á.; Tankó, G.; Ciocan, E.M.; Codină, G.G. The effect of dry hopping efficiency on β-myrcene dissolution into beer. Plants 2022, 11, 1043. [Google Scholar] [CrossRef]
- Schmidt, C.; Biendl, M. Hop aroma characterization of dry hopped beers using headspace-trap GC-MS. Acta Hortic. 2019, 1236, 7–13. [Google Scholar] [CrossRef]
- Haslbeck, K.; Bub, S.; Schönberger, C.; Zarnkow, M.; Jacob, F.; Coelhan, M. On the fate of β-myrcene during fermentation-the role of stripping and uptake of hop oil components by brewer’s yeast in dry-hopped wort and beer. Brew. Sci. 2017, 70, 159–169. [Google Scholar] [CrossRef]
- Schnaitter, M.; Kell, A.; Kollmannsberger, H.; Schüll, F.; Gastl, M.; Becker, T. Scale-up of dry hopping trials: Importance of scale for aroma and taste perceptions. Chem. Ing. Tech. 2016, 88, 1955–1965. [Google Scholar] [CrossRef]
- Burdock, A.G. Fenaroli’s Handbook of Flavor Ingredients, 6th ed.; CRC Press: Boca Raton, FL, USA, 2009; ISBN 9781420090772. [Google Scholar]
- Feng, S. Aroma-Active Compounds in “Centennial”, “Citra” and “Nelson Sauvin” Hop Varieties and Their Aroma Contribution to Dry-Hopped Beer. Master’s Thesis, Oregon State University, Corvallis, OR, USA, 4 June 2014. [Google Scholar]
- Takoi, K.; Itoga, Y.; Koie, K.; Takayanagi, J.; Kaneko, T.; Watanabe, T.; Matsumoto, I.; Nomura, M. Systematic analysis of behaviour of hop-derived monoterpene alcohols during fermentation and new classification of geraniol-rich flavour hops. Brew. Sci. 2017, 70, 177–186. [Google Scholar]
- Michel, M.; Haslbeck, K.; Ampenberger, F.; Meier-Dörnberg, T.; Stretz, D.; Hutzler, M.; Coelhan, M.; Jacob, F.; Liu, Y. Screening of brewing yeast β-lyase activity and release of hop volatile thiols from precursors during fermentation. Brew. Sci. 2019, 72, 179–186. [Google Scholar] [CrossRef]
- Takoi, K.; Tokita, K.; Sanekata, A.; Usami, Y.; Itoga, Y.; Koie, K.; Matsumoto, I.; Nakayama, Y. Varietal Difference of Hop-Derived Flavour Compounds in Late-Hopped/Dry-Hopped Beers. Brew. Sci. 2016, 69, 1–7. [Google Scholar]
- Tan, Y.; Siebert, K.J. Quantitative structure-activity relationship modeling of alcohol, ester, aldehyde, and ketone flavor thresholds in beer from molecular features. J. Agric. Food Chem. 2004, 52, 3057–3064. [Google Scholar] [CrossRef]
- Rettberg, N.; Schubert, C.; Dennenlöhr, J.; Thörner, S.; Knoke, L.; Maxminer, J. Instability of hop-derived 2-methylbutyl isobutyrate during aging of commercial pasteurized and unpasteurized ales. J. Am. Soc. Brew. Chem. 2020, 78, 175–184. [Google Scholar] [CrossRef]
- Forster, A.; Gahr, A. A Comparison of the Analytical and Brewing Characteristics of Cascade and Comet Hop Varieties as Grown in Yakima (USA) and Hallertau (Germany). Brew. Sci. 2014, 67, 137–148. [Google Scholar] [CrossRef]
- McCabe, A.K.; Keyes, J.K.; Hemetsberger, H.; Kurr, C.V.; Albright, B.; Ward, M.G.; McKinley, M.L.; Breezley, S.J.; Cole, C.A. Aroma Profile Development in Beer Fermented with Azacca, Idaho-7, and Sultana Hops. Molecules 2023, 28, 5802. [Google Scholar] [CrossRef]
- Kemp, O.; Hofmann, S.; Braumann, I.; Jensen, S.; Fenton, A.; Oladokun, O. Changes in key hop-derived compounds and their impact on perceived dry-hop flavour in beers after storage at cold and ambient temperature. J. Inst. Brew. 2021, 127, 367–384. [Google Scholar] [CrossRef]
- Cibaka, M.-L.K.; Gros, J.; Nizet, S.; Collin, S. Quantitation of selected terpenoids and mercaptans in the dual-purpose hop varieties Amarillo, Citra, Hallertau Blanc, Mosaic, and Sorachi Ace. J. Agric. Food Chem. 2015, 63, 3022–3030. [Google Scholar] [CrossRef]
- Cordente, A.G.; Capone, L.D.; Curtin, D.C. Unravelling glutathione conjugate catabolism in Saccharomyces cerevisiae: The role of glutathione/dipeptide transporters and vacuolar function in the release of volatile sulfur compounds 3-mercaptohexan-1-ol and 4-mercapto-4-methylpentan-2-one. Appl. Microbiol. Biotechnol. 2015, 99, 9709–9722. [Google Scholar] [CrossRef]
- Chenot, C.; Collin, S.; Suc, L.; Roland, A. Unusual profile of thiol precursors in special alts: First evidence of chemical glutathione-/γGluCys- and CysGly-/Cys- conversions. J. Am. Soc. Brew. Chem. 2023; in press. Published online 7 April 2023. [Google Scholar] [CrossRef]
- Bonnaffoux, H.; Roland, A.; Schneider, R.; Cavelier, F. Spotlight on release mechanisms of volatile thiols in beverages. Food Chem. 2021, 339, 127628. [Google Scholar] [CrossRef] [PubMed]
- Chenot, C.; Collin, S. Ability of the Mandarina Bavaria hop variety to release free odorant polyfunctional thiols in late-hopped beers. J. Inst. Brew. 2021, 127, 140–148. [Google Scholar] [CrossRef]
- Molitor, W.R.; Roop, I.J.; Denby, M.C.; Depew, J.C.; Liu, S.D.; Stadulis, E.S.; Shellhammer, H.T. The sensorial and chemical changes in beer brewed with yeast genetically modified to release polyfunctional thiols from malt and hops. Fermentation 2022, 8, 370. [Google Scholar] [CrossRef]
- Zhao, H.; Li, H.; Sun, G.; Yang, B.; Zhao, M. Assessment of endogenous antioxidative compounds and antioxidant activities of lager beers. J. Sci. Food Agric. 2013, 93, 910–917. [Google Scholar] [CrossRef]
- McLaughlin, I.R.; Lederer, C.; Shellhammer, T.H. Bitterness-modifying properties of hop polyphenols extracted from spent hop material. J. Am. Soc. Brew. Chem. 2008, 66, 174–183. [Google Scholar] [CrossRef]
- Goiris, K.; Jaskula-Goiris, B.; Syryn, E.; Van Opstaele, F.; De Rouck, G.; Aerts, G.; De Cooman, L. The flavoring potential of hop polyphenols in beer. J. Am. Soc. Brew. Chem. 2014, 72, 135–142. [Google Scholar] [CrossRef]
- Gribkova, I.N.; Kharlamova, L.N.; Lazareva, I.V.; Zakharov, M.A.; Zakharova, V.A.; Kozlov, V.I. The influence of hop phenolic compounds on dry hopping beer quality. Molecules 2022, 27, 740. [Google Scholar] [CrossRef]
- Jaskula-Goiris, B.; Goiris, K.; Syryn, E.; Van Opstaele, F.; De Rouck, G.; Aerts, G.; De Cooman, L. The use of hop polyphenols during brewing to improve flavor quality and stability of Pilsner beer. J. Am. Soc. Brew. Chem. 2014, 72, 175–183. [Google Scholar] [CrossRef]
- Gasiński, A.; Kawa-Rygielska, J.; Paszkot, J.; Pietrzak, W.; Śniegowska, J.; Szumny, A. Second life of hops: Analysis of beer hopped with hop pellets previously used to dry-hop a beer. LWT 2022, 159, 113186. [Google Scholar] [CrossRef]
- Haslbeck, K.; Bub, S.; von Kamp, K.; Michel, M.; Zarnkow, M.; Hutzler, M.; Coelhan, M. The influence of brewing yeast strains on monoterpene alcohols and esters contributing to the citrus flavour of beer. J. Inst. Brew. 2018, 124, 403–415. [Google Scholar] [CrossRef]
- Takoi, K.; Itoga, Y.; Takayanagi, J.; Matsumoto, I.; Nakayama, Y. Control of hop aroma impression of beer with blend-hopping using geraniol-rich hop and new hypothesis of synergy among hop-derived flavour compounds. Brew. Sci. 2016, 69, 85–93. [Google Scholar]
- Wéber, N. Optimization of Super Dry Hopping for Medium and Large-Scale Applications. Master’s Thesis, Université Catholique de Louvain, Ottignies-Louvain-la-Neuve, Belgium, 2019. [Google Scholar]
- Kohles, M.; Gutsch, F.; Zarnkow, M.; Jacob, F.; Novy, R.; Schönberger, C. An approach to develop an external dry hopping method by restoring the aroma transfer through dilution. Brew. Sci. 2021, 74, 151–159. [Google Scholar] [CrossRef]
- Tusha, K.; Nešpor, J.; Jelínek, L.; Vodičková, H.; Kinčl, T.; Dostálek, P. Effect of Czech hop varieties on aroma of dry-hopped lager beer. Foods 2022, 11, 2520. [Google Scholar] [CrossRef] [PubMed]
- Habschied, K.; Košir, I.J.; Krstanović, V.; Kumrić, G.; Mastanjević, K. Beer Polyphenols—Bitterness, Astringency, and Off-Flavors. Beverages 2021, 7, 38. [Google Scholar] [CrossRef]
- Oladokun, O.; James, S.; Cowley, T.; Dehrmann, F.; Smart, K.; Hort, J.; Cook, D. Perceived bitterness character of beer in relation to hop variety and the impact of hop aroma. Food Chem. 2017, 230, 215–224. [Google Scholar] [CrossRef]
- Maye, J.P.; Smith, R. Dry hopping and its effects on the International Bitterness Unit test and beer bitterness. MBAA Tech. Q. 2016, 53, 134–136. [Google Scholar] [CrossRef]
- Smith, R.J.; Maye, J.P.; Wilson Hopsteiner, R.J.H.; Yakima, W.A. Formation of humulinones in hops and hop pellets and its implication for dry-hopped beers. In Proceedings of the ASBC Conference, La Quina, CA, USA, 14–17 June 2015. [Google Scholar]
- Ferreira, C.S.; Thibault de Chanvalon, E.; Bodart, E.; Collin, S. Why humulinones are key bitter constituents only after dry hopping: Comparison with other Belgian styles. J. Am. Soc. Brew. Chem. 2018, 76, 236–246. [Google Scholar] [CrossRef]
- Parkin, E.; Shellhammer, T. Toward understanding the bitterness of dry-hopped beer. J. Am. Soc. Brew. Chem. 2017, 75, 363–368. [Google Scholar] [CrossRef]
- Marques, L.; Espinosa, M.H.; Andrews, W.; Foster, R.T. Advancing flavor stability improvements in different beer types using novel electron paramagnetic resonance area and forced beer aging methods. J. Am. Soc. Brew. Chem. 2017, 75, 35–40. [Google Scholar] [CrossRef]
- Von Terzi, K.G.B. Influences on the Concentration of Hop Flavor Components during Dry Hopping. Ph.D. Thesis, TUM School of Life Sciences, Technischen Universität München, Munich, Germany, 30 December 2021. [Google Scholar]
- Maye, J.P.; Smith, R.; Leker, J. Dry hopping and its effect on beer bitterness, the IBU test, and pH. Brauwelt Int. 2018, 2018, 25–29. [Google Scholar]
- Mitter, W.; Cocuzza, S. Dry hopping—A study of various parameters. Consequences of the Applied Dosing Method. Supplement to the article “Revival of a Process (Dry Hopping—Basics and Techniques)”. Brew. Beverage Ind. Int. 2013, 4, 70–74. [Google Scholar]
- Titus, B.M.; Lerno, L.A.; Beaver, J.W.; Byrnes, N.K.; Heymann, H.; Oberholster, A. Impact of dry hopping on beer flavor stability. Foods 2021, 10, 1264. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, H.P. A study of Factors Affecting the Extraction of Flavor when Dry Hopping Beer. Master’s Thesis, Oregon State University, Corvallis, OR, USA, 7 August 2012. Available online: https://ir.library.oregonstate.edu/concern/graduate_thesis_or_dissertations/rx913t14h (accessed on 19 August 2023).
- Reglitz, K.; Lemke, N.; Steinhaus, M.; Hanke, S. On the behavior of the important hop odorant 4-mercapto-4-methylpentan-2-one (4MMP) during dry hopping and during storage of dry hopped beer. Brew. Sci. 2018, 71, 96–99. [Google Scholar] [CrossRef]
- Ferreira, C.S.; Simon, M.; Collin, S. Why catechin and epicatechin from early hopping impact the color of aged dry-hopped beers while flavan-3-ol oligomers from late and dry hopping increase colloidal instability. J. Am. Soc. Brew. Chem. 2023, 81, 255–264. [Google Scholar] [CrossRef]
- Vollmer, M.D.; Algazzali, V.; Shellhammer, H.T. Aroma properties of lager beer dry-hopped with oxidized hops. J. Am. Soc. Brew. Chem. 2017, 75, 22–26. [Google Scholar] [CrossRef]
- Takoi, K. Behaviour of hop-derived branched-chain fatty acids during fermentation and their sensory effect on hopped beer flavours. Brew. Sci. 2019, 72, 196–206. [Google Scholar] [CrossRef]
- Takoi, K. Do branched chain fatty acids invariably produce off-flavours? BRAUWELT Int. 2021, 39, 74–77. [Google Scholar]
- Dietz, C.; Cook, D.; Wilson, C.; Oliveira, P.; Ford, R. Exploring the multisensory perception of terpene alcohol and sesquiterpene rich hop extracts in lager style beer. Food Res. Int. 2021, 148, 110598. [Google Scholar] [CrossRef]
- Takoi, K.; Itoga, Y.; Koie, K.; Kosugi, T.; Shimase, M.; Katayama, Y.; Nakayama, Y.; Watari, J. The contribution of geraniol metabolism to the citrus flavour of beer: Synergy of geraniol and β-citronellol under coexistence with excess linalool. J. Inst. Brew. 2010, 116, 251–260. [Google Scholar] [CrossRef]
- Bruner, J.; Marcus, A.; Fox, G. Dry-hop creep potential of various Saccharomyces yeast species and strains. Fermentation 2021, 7, 66. [Google Scholar] [CrossRef]
- King, A.J.; Dickinson, J.R. Biotransformation of hop aroma terpenoids by ale and lager yeasts. FEMS Yeast Res. 2003, 3, 53–62. [Google Scholar] [CrossRef]
- Carrau, M.F.; Medina, K.; Boido, E.; Farina, L.; Gaggero, C.; Dellacassa, E.; Versini, G.; Henschke, A.P. De novo synthesis of monoterpenes by Saccharomyces cerevisiae wine yeasts. FEMS Microbiol. Lett. 2005, 243, 107–115. [Google Scholar] [CrossRef]
- Brendel, S.; Hofmann, T.; Granvogl, M. Hop-induced formation of ethyl esters in dry-hopped beer. Food Prod. Process. Nutr. 2020, 2, 18. [Google Scholar] [CrossRef]
- Forster, A.; Gahr, A.; Opstaele, F. On the transfer rate of geraniol with dry hopping. Brew. Sci. 2014, 67, 60. [Google Scholar]
- Chenot, C.; Thibault de Chanvalon, E.; Janssens, P.; Collin, S. Modulation of the sulfanylalkyl acetate/alcohol ratio and free thiol release from cysteinylated and/or glutathionylated sulfanylalkyl alcohols in beer under different fermentation conditions. J. Agric. Food Chem. 2021, 69, 6005–6012. [Google Scholar] [CrossRef] [PubMed]
- Nizet, S.; Peeters, F.; Gros, J.; Collin, S. Chapter 43—Odorant Polyfunctional Thiols Issued from Bottle Beer Refermentation. In Flavour Science; Ferreira, V., Lopez, R., Eds.; Academic Press: San Diego, CA, USA, 2014; pp. 227–230. [Google Scholar]
- Chenot, C.; Donck, W.; Janssens, P.; Collin, S. Malt and hop as Sources of thiol S-conjugates: Thiol-releasing property of lager yeast during fermentation. J. Agric. Food Chem. 2022, 70, 3272–3279. [Google Scholar] [CrossRef] [PubMed]
- Werrie, P.-Y.; Deckers, S.; Fauconnier, M.-L. Brief insight into the underestimated role of hop amylases on beer aroma profiles. J. Am. Soc. Brew. Chem. 2022, 80, 66–74. [Google Scholar] [CrossRef]
- Kirkpatrick, R.K.; Shellhammer, H.T. Evidence of dextrin hydrolyzing enzymes in Cascade hops (Humulus lupulus). J. Agric. Food Chem. 2018, 66, 9121–9126. [Google Scholar] [CrossRef]
- Bruner, J.; Williams, J.; Fox, G. Further exploration of hop creep variability with Humulus lupulus cultivars and proposed method for determination of secondary fermentation. MBAA Tech. Q. 2020, 57, 169–176. [Google Scholar] [CrossRef]
- Kirkendall, A.J.; Mitchell, A.C.; Chadwick, R.L. The freshening power of Centennial hops. J. Am. Soc. Brew. Chem. 2018, 76, 178–184. [Google Scholar] [CrossRef]
- Cottrell, T.M. A search for diastatic enzymes endogenous to Humulus lupulus and produced by microbes associated with pellet hops driving “Hop Creep” of dry hopped beer. J. Am. Soc. Brew. Chem. 2022, 81, 435–447. [Google Scholar] [CrossRef]
- Rubottom, N.L.; Lafontaine, R.S.; Hauser, G.D.; Pereira, C.; Shellhammer, H.T. Hop kilning temperature sensitivity of dextrin-reducing enzymes in hops. J. Am. Soc. Brew. Chem. 2022, 80, 75–83. [Google Scholar] [CrossRef]
- Rubottom, L.N.; Shellhammer, T.H. Evaluating the impact of high and low kilning temperatures on popular American aroma hops. J. Am. Soc. Brew. Chem. 2023, in press. [Google Scholar] [CrossRef]
- Kirkpatrick, R.K.; Shellhammer, H.T. A Cultivar-based screening of hops for dextrin degrading enzymatic potential. J. Am. Soc. Brew. Chem. 2018, 76, 247–256. [Google Scholar] [CrossRef]
- Stokholm, A. Dry-Hop Induced Refermentation: An Overview and an Investigation of Agronomic Influences on Hop Diastatic Potential. Master’s Thesis, Oregon State University, Corvallis, OR, USA, 16 December 2020. [Google Scholar]
- Wang, Y.; Ye, L. Haze in beer: Its formation and alleviating strategies, from a protein-polyphenol complex angle. Foods 2021, 10, 3114. [Google Scholar] [CrossRef]
- Steiner, E.; Becker, T.; Gastrl, M. Turbidity and haze formation in beer—Insights and overview. J. Inst. Brew. 2010, 116, 360–368. [Google Scholar] [CrossRef]
- Sadosky, P.; Schwarz, P.B.; Horsley, R.D. Effect of arabinoxylans, β-glucans, and dextrins on the Viscosity and membrane filterability of a beer model solution. J. Am. Soc. Brew. Chem. 2002, 60, 153–162. [Google Scholar] [CrossRef]
- Speers, R.A.; Jin, Y.-L.; Paulson, A.T.; Stewart, R.J. Effects of β-glucan, shearing and environmental factors on the turbidity of wort and beer. J. Inst. Brew. 2003, 109, 236–244. [Google Scholar] [CrossRef]
- Gramshaw, J.W. Phenolic constituents of beer and brewing materials. II. The role of polyphenols in the formation of non-biological haze. J. Inst. Brew. 1967, 73, 455–472. [Google Scholar] [CrossRef]
- Mikyška, A.; Hrabák, M.; Hašková, D.; Šrogl, J. The role of malt and hop polyphenols in beer quality, flavour and haze stability. J. Inst. Brew. 2002, 108, 78–85. [Google Scholar] [CrossRef]
- Ye, L.; Huang, Y.; Li, M.; Li, C.; Zhang, G. The chemical components in malt associated with haze formation in beer. J. Inst. Brew. 2016, 122, 524–529. [Google Scholar] [CrossRef]
- Mastanjević, K.; Krstanović, V.; Lukinac, J.; Jukić, M.; Vulin, Z.; Mastanjević, K. Beer—The importance of colloidal stability (non-biological haze). Fermentation 2018, 4, 91. [Google Scholar] [CrossRef]
- Leiper, K.A.; Stewart, G.G.; McKeown, J.P. Beer polypeptides and silica gel. Part, I. Polypeptides involved in haze formation. J. Inst. Brew. 2003, 109, 57–72. [Google Scholar] [CrossRef]
- Li, H.J.; Deinzer, M.L. Structural identification and distribution of proanthocyanidins in 13 different hops. J. Agric. Food Chem. 2006, 54, 4048–4056. [Google Scholar] [CrossRef]
- Dvořáková, M.; Douanier, M.; Jurková, M.; Kellner, V.; Dostálek, P. Comparison of antioxidant activity of barley (Hordeum vulgare L.) and malt extracts with the content of free phenolic compounds measured by high performance liquid chromatography coupled with CoulArray detector. J. Inst. Brew. 2008, 114, 150–159. [Google Scholar] [CrossRef]
- Fărcaş, A.; Tofană, M.; Socaci, S.A.; Bor, A.M. Preliminary study on antioxidant activity and polyphenols content in discharged waste from beer production. J. Agroaliment. Proc. Technol. 2013, 19, 319–324. [Google Scholar]
- Li, H.-J.; Deinzer, M.L. 32—Proanthocyanidins in Hops. In Beer in Health and Disease Prevention; Preedy, V.R., Ed.; Academic Press: Burlington, NJ, USA, 2009; pp. 333–348. [Google Scholar] [CrossRef]
- Gąsior, J.; Kawa-Rygielska, J.; Kucharska, A.Z. Carbohydrates profile, polyphenols content and antioxidative properties of beer worts produced with different dark malts varieties or roasted barley grains. Molecules 2020, 25, 3882. [Google Scholar] [CrossRef]
- Olšovská, J.; Dušek, M.; Zušťáková, V.; Mikyška, A. The proanthocyanidin profile in beer and its raw materials. Kvas. Prum. 2015, 61, 296–304. [Google Scholar] [CrossRef]
- Steiner, E.; Gastl, M.; Becker, T. Protein changes during malting and brewing with focus on haze and foam formation: A review. Eur. Food Res. Technol. 2011, 232, 191–204. [Google Scholar] [CrossRef]
- Benucci, I.; Mazzocchi, C.; Lombardelli, C.; Esti, M. Phenolic-degrading enzymes: Effect on haze active phenols and chill haze in India Pale Ale beer. Foods 2022, 12, 77. [Google Scholar] [CrossRef] [PubMed]
- Di Domenico, M.; Feola, A.; Ambrosio, P.; Pinto, F.; Galasso, G.; Zarrelli, A.; Di Fabio, G.; Porcelli, M.; Scacco, S.; Inchingolo, F.; et al. Antioxidant effect of beer polyphenols and their bioavailability in dental-derived stem cells (D-dSCs) and human intestinal epithelial lines (Caco-2) cells. Stem Cells Int. 2020, 2020, 8835813. [Google Scholar] [CrossRef] [PubMed]
- Kallmeyer, M. The Role of Polyphenols in Beer Haze Formation. Available online: https://draymans.com/the-role-of-polyphenols-in-beer-haze-formation/ (accessed on 18 August 2023).
- Huismann, M.; Gormley, F.; Dzait, D.; Willoughby, N.; Stewart, K.; Speers, A.R.; Maskell, L.D. Unfilterable beer haze. Part II: Identifying suspect cell wall proteins. J. Am. Soc. Brew. Chem. 2022, 80, 26–34. [Google Scholar] [CrossRef]
- Huismann, M.; Gormley, F.; Dzait, D.; Speers, A.R.; Maskell, L.D. Unfilterable beer haze. Part I: The investigation of an India Pale Ale haze. J. Am. Soc. Brew. Chem. 2022, 80, 17–25. [Google Scholar] [CrossRef]
- Bolcato, C.; Tague, E.; Hakimi, A.; Horn, D.; Lopez-Ferrer, D.; Burns, L.; Shaner, L. A Journey into the Hazy Beer Proteome: How Does Dry Hopping Alter the Proteomic Landscape of Beer? ASMS. 2021. Available online: https://lcms.labrulez.com/labrulez-bucket-strapi-h3hsga3/sub227_PO_66121_ASMS_2021_Poster_Bolcato_D_Fbranf_cbk_FINAL_7d2a284bdd/sub227-PO66121-ASMS2021-Poster-Bolcato-DFbranf-cbk-FINAL.pdf (accessed on 18 August 2023).
- Siebert, K.J. Haze in Beverages. Adv. Food Nutr. Res. 2009, 57, 53–86. [Google Scholar] [CrossRef]
- Oliver, G.; Colicchio, T. The Oxford Companion to Beer, 1st ed.; Oxford University Press Inc.: Oxford, UK, 2012. [Google Scholar]
- Palmer, J.J. How to Brew: Everything You Need to Know to Brew Great Beer Every Time, 4th ed.; Brewers Publication: Boulder, CO, USA, 2017. [Google Scholar]
- Demireva, Z.N. Studies on the fatty acids composition of hop extract. J. Inst. Brew. 1995, 101, 437–438. [Google Scholar] [CrossRef]
- Cao, J.; Deng, L.; Zhu, X.M.; Fan, Y.; Hu, J.N.; Li, J.; Deng, Z.Y. Novel approach to evaluate the oxidation state of vegetable oils using characteristic oxidation indicators. J. Agric. Food Chem. 2014, 62, 12545–12552. [Google Scholar] [CrossRef]
- Fauconnier, M.L.; Mpambara, A.; Delcarte, J.; Jacques, P.; Thonart, P.; Marlier, M. Conversion of green note aldehydes into alcohols by yeast alcohol dehydrogenase. Biotechnol. Lett. 1999, 21, 629–633. [Google Scholar] [CrossRef]
- Vincenti, S.; Mariani, M.; Alberti, J.-C.; Jacopini, S.; Brunini-Bronzini de Caraffa, V.; Berti, L.; Maury, J. Biocatalytic synthesis of natural green leaf volatiles using the lipoxygenase metabolic pathway. Catalysts 2019, 9, 873. [Google Scholar] [CrossRef]
- Hongsoongnern, P.; Chambers, E., IV. A lexicon for green odor or flavor and characteristics of chemicals associated with green. J. Sens. Stud. 2008, 23, 205–221. [Google Scholar] [CrossRef]
- Saint-Eve, A.; Déléris, I.; Aubin, E.; Semon, E.; Feron, G.; Rabillier, J.-M.; Ibarra, D.; Guichard, E.; Souchon, I. Influence of composition (CO2 and sugar) on aroma release and perception of mint-flavored carbonated beverages. J. Agric. Food Chem. 2009, 57, 5891–5898. [Google Scholar] [CrossRef] [PubMed]
- Steinhaus, M.; Schieberle, P. Comparison of the most odor-active compounds in fresh and dried hop cones (Humulus lupulus L. variety spalter select) based on GC-olfactometry and odor dilution techniques. J. Agric. Food Chem. 2000, 48, 1776–1783. [Google Scholar] [CrossRef] [PubMed]
- Wietstock, C.P.; Methner, J.F. Formation of aldehydes by direct oxidative degradation of amino acids via hydroxyl and ethoxy radical attack in buffered model solutions. Brew. Sci. 2013, 66, 104–113. [Google Scholar]
- Kolek, J.; Patakova, P.; Junkova, P.; Krofta, K.; Hynek, R.; Dostalek, P. Isolation and identification of Pantoea agglomerans from the inflated bag with dried hop pellets stored under a modified atmosphere. J. Appl. Microbiol. 2021, 131, 281–287. [Google Scholar] [CrossRef]
- Van Vuuren, H.J.J.; Cosser, K.; Prior, B.A. The influence of Enterobacter agglomerans on beer flavour. J. Inst. Brew. 1980, 86, 31–33. [Google Scholar] [CrossRef]
- Ferreira, C.S.; Collin, S. Fate of bitter compounds through dry-hopped beer aging. Why cis-humulinones should be as feared as trans-isohumulones? J. Am. Soc. Brew. Chem. 2020, 78, 103–113. [Google Scholar] [CrossRef]
- Mikyška, A.; Dušek, M.; Slabý, M. How does fermentation, filtration and stabilization of beer affect polyphenols with health benefits. Kvas. Prum. 2019, 65, 120–126. [Google Scholar] [CrossRef]
- Guan, X.; Nie, C.; Guo, Y.; Zhang, J. Changes of hop-derived aroma compounds in India Pale Ale during brewing and storage. Int. J. Food Eng. 2019, 5, 50–57. [Google Scholar] [CrossRef]
- Drexler, G.; Bailey, B.; Schönberger, C.; Gahr, A.; Newman, R.; Pöschl, M.; Geiger, E. The influence of hop harvest date on flavor stability in dry-hopped beers. MBAA Tech. Q. 2010, 47, 1–7. [Google Scholar] [CrossRef]
- Borremans, Y.; Van Opstaele, F.; Van Holle, A.; Van Nieuwenhove, J.; Jaskula-Goiris, B.; De Clippeleer, J.; Naudts, D.; De Keukeleire, D.; De Cooman, L.; Aerts, G. Analytical and sensory assessment of the flavour stability impact of dry-hopping in single-hop beers. In Proceedings of the 10th Trends in Brewing, Ghent, Belgium, 1–4 April 2012. [Google Scholar]
- Barnette, M.B.; Shellhammer, H.T. Evaluating the impact of dissolved oxygen and aging on dry-hopped aroma stability in beer. J. Am. Soc. Brew. Chem. 2019, 77, 179–187. [Google Scholar] [CrossRef]
- Mikyška, A.; Krofta, K. Assessment of changes in hop resins and polyphenols during long-term storage. J. Inst. Brew. 2012, 118, 269–279. [Google Scholar] [CrossRef]
- Nizet, S.; Gros, J.; Peeters, F.; Chaumont, S.; Robiette, R.; Collin, S. First evidence of the production of odorant polyfunctional thiols by bottle refermentation. J. Am. Soc. Brew. Chem. 2013, 71, 15–22. [Google Scholar] [CrossRef]
- Tran, T.T.H.; Cibaka, M.-L.K.; Collin, S. Polyfunctional thiols in fresh and aged Belgian special beers: Fate of hop S-Cysteine conjugates. J. Am. Soc. Brew. Chem. 2015, 73, 61–70. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klimczak, K.; Cioch-Skoneczny, M.; Duda-Chodak, A. Effects of Dry-Hopping on Beer Chemistry and Sensory Properties—A Review. Molecules 2023, 28, 6648. https://doi.org/10.3390/molecules28186648
Klimczak K, Cioch-Skoneczny M, Duda-Chodak A. Effects of Dry-Hopping on Beer Chemistry and Sensory Properties—A Review. Molecules. 2023; 28(18):6648. https://doi.org/10.3390/molecules28186648
Chicago/Turabian StyleKlimczak, Krystian, Monika Cioch-Skoneczny, and Aleksandra Duda-Chodak. 2023. "Effects of Dry-Hopping on Beer Chemistry and Sensory Properties—A Review" Molecules 28, no. 18: 6648. https://doi.org/10.3390/molecules28186648
APA StyleKlimczak, K., Cioch-Skoneczny, M., & Duda-Chodak, A. (2023). Effects of Dry-Hopping on Beer Chemistry and Sensory Properties—A Review. Molecules, 28(18), 6648. https://doi.org/10.3390/molecules28186648