Plasma Polymerization of Precipitated Silica for Tire Application
Abstract
:1. Introduction
2. Results
2.1. Characterization of Plasma-Treated Silica
2.1.1. Thermo-Gravimetric Analysis (TGA) Results
2.1.2. Diffuse Reflectance Infrared Fourier Transform Spectroscopy (DRIFTs) Analysis
2.1.3. X-ray Photoelectron Spectroscopy (XPS) Analysis
2.1.4. Transmission Electron Microscopy (TEM) Analysis
2.2. Properties of Silica-Filled Rubber Compounds
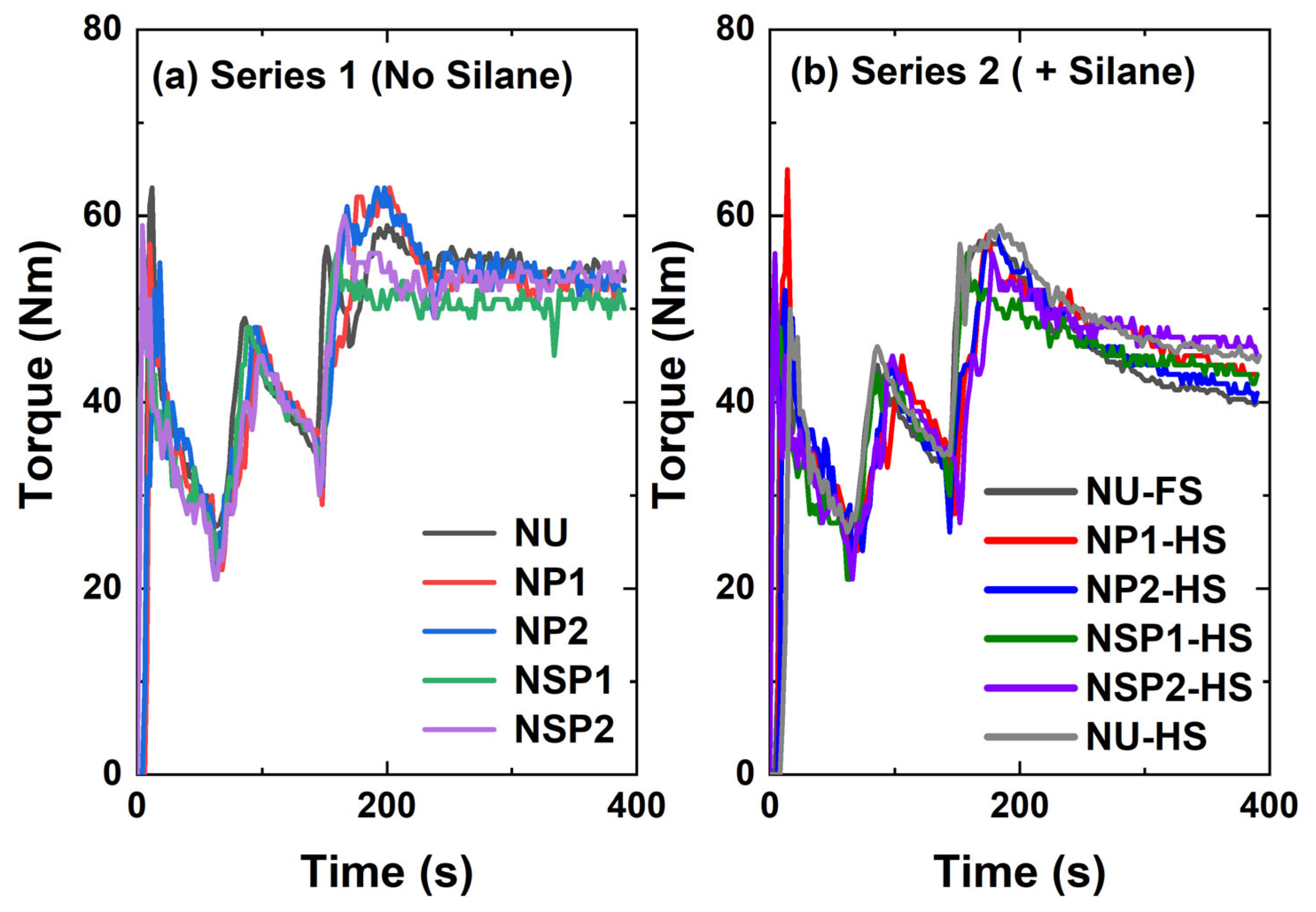
2.2.1. Mixing Behavior
2.2.2. Filler-Filler Interaction
2.2.3. Silica Filler Dispersion in NR Matrix
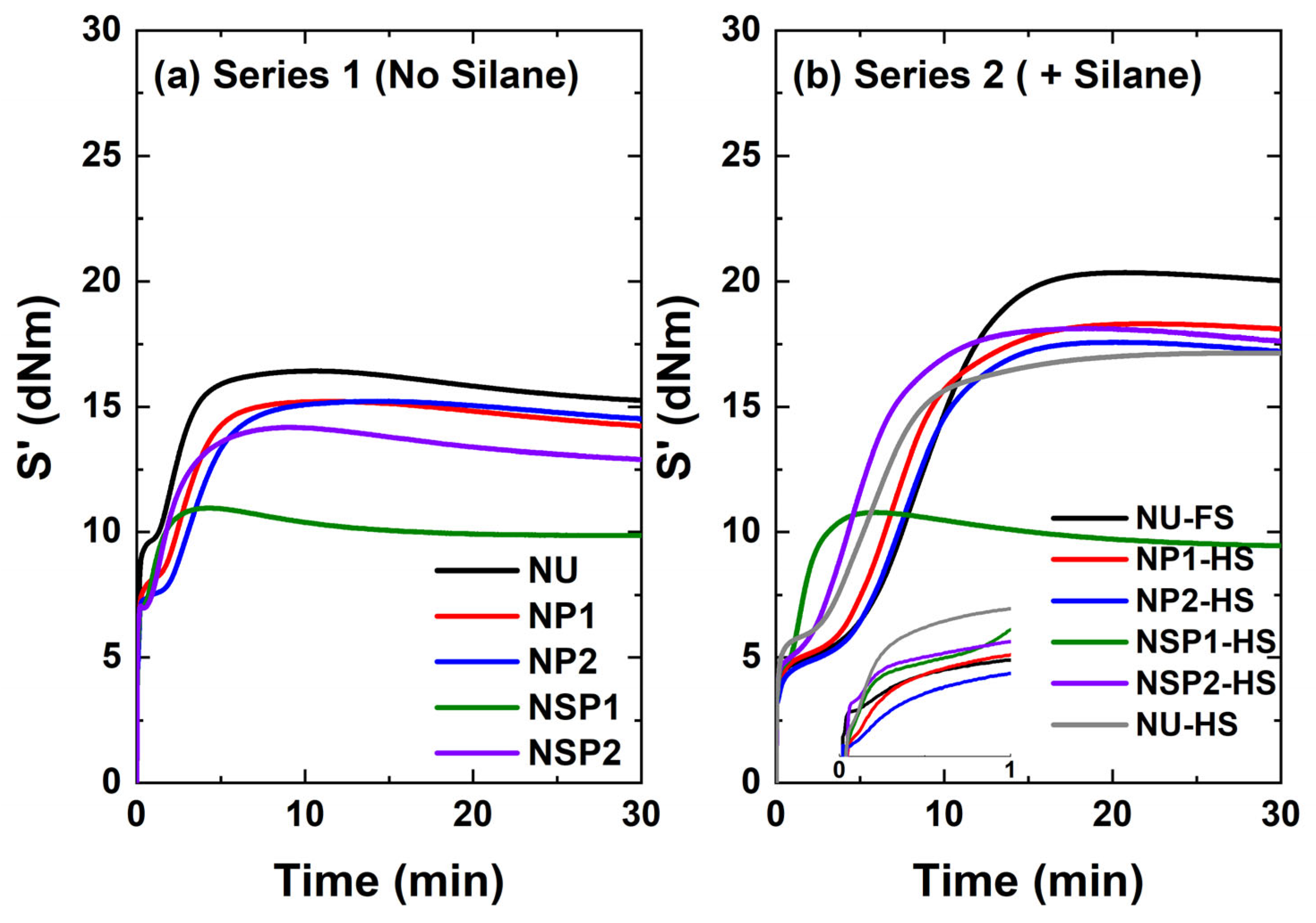
2.2.4. Cure Behavior of PD-Treated Silica-Filled Compounds
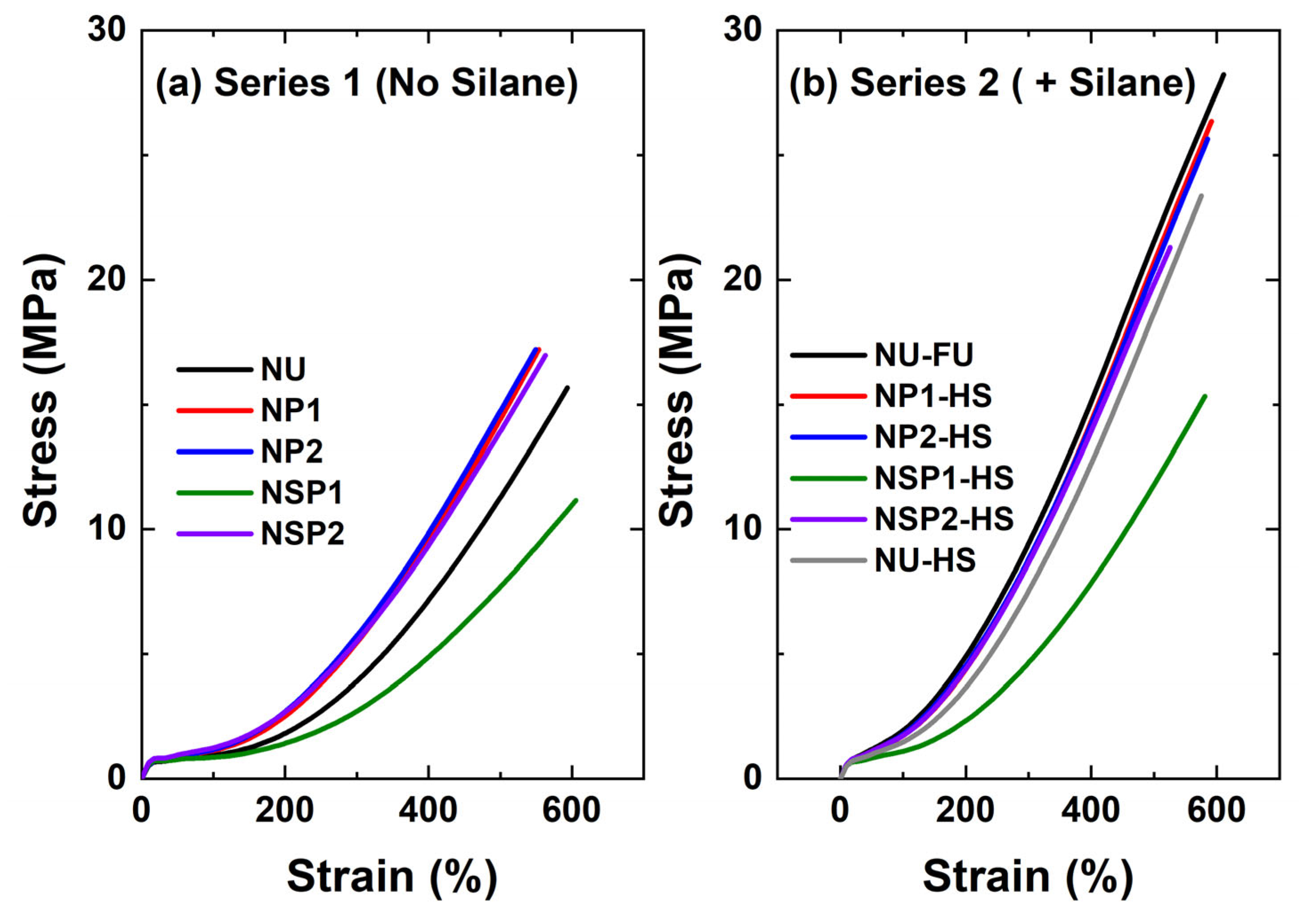
2.2.5. Mechanical Properties of PD-Treated Silica-Filled Compounds
2.2.6. Dynamic Mechanical Properties PD-Treated Silica-Filled Compounds
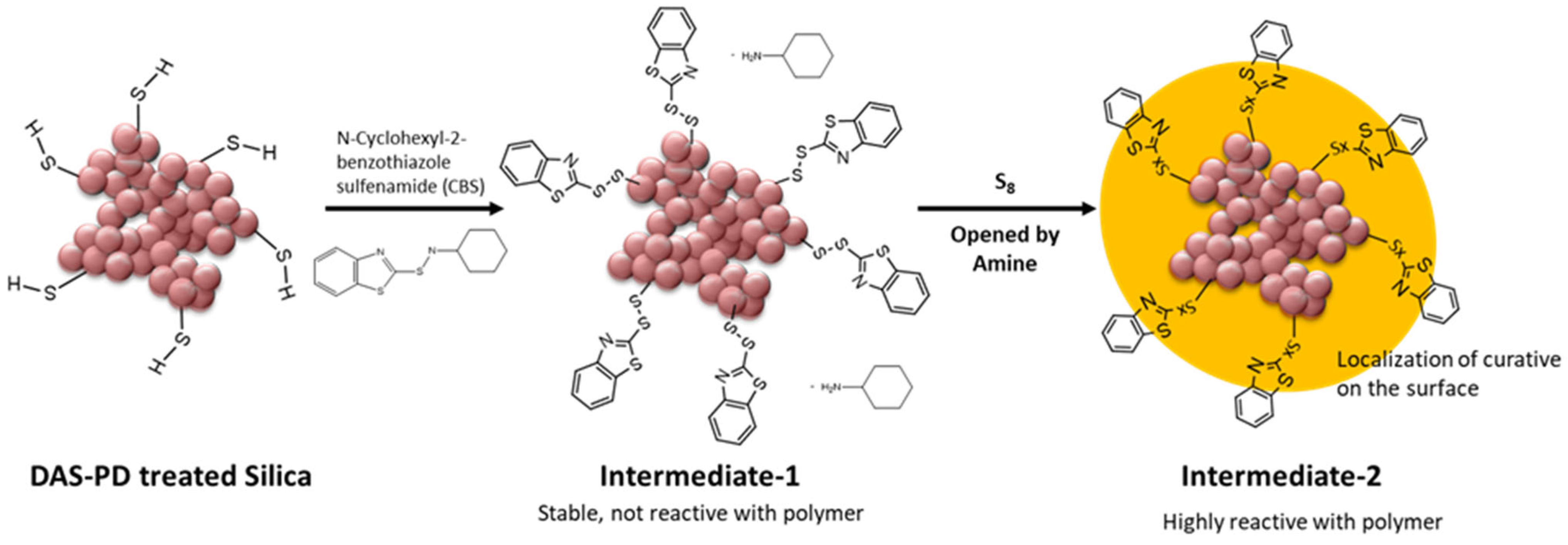
3. Discussion
4. Materials and Methods
4.1. Materials for PD
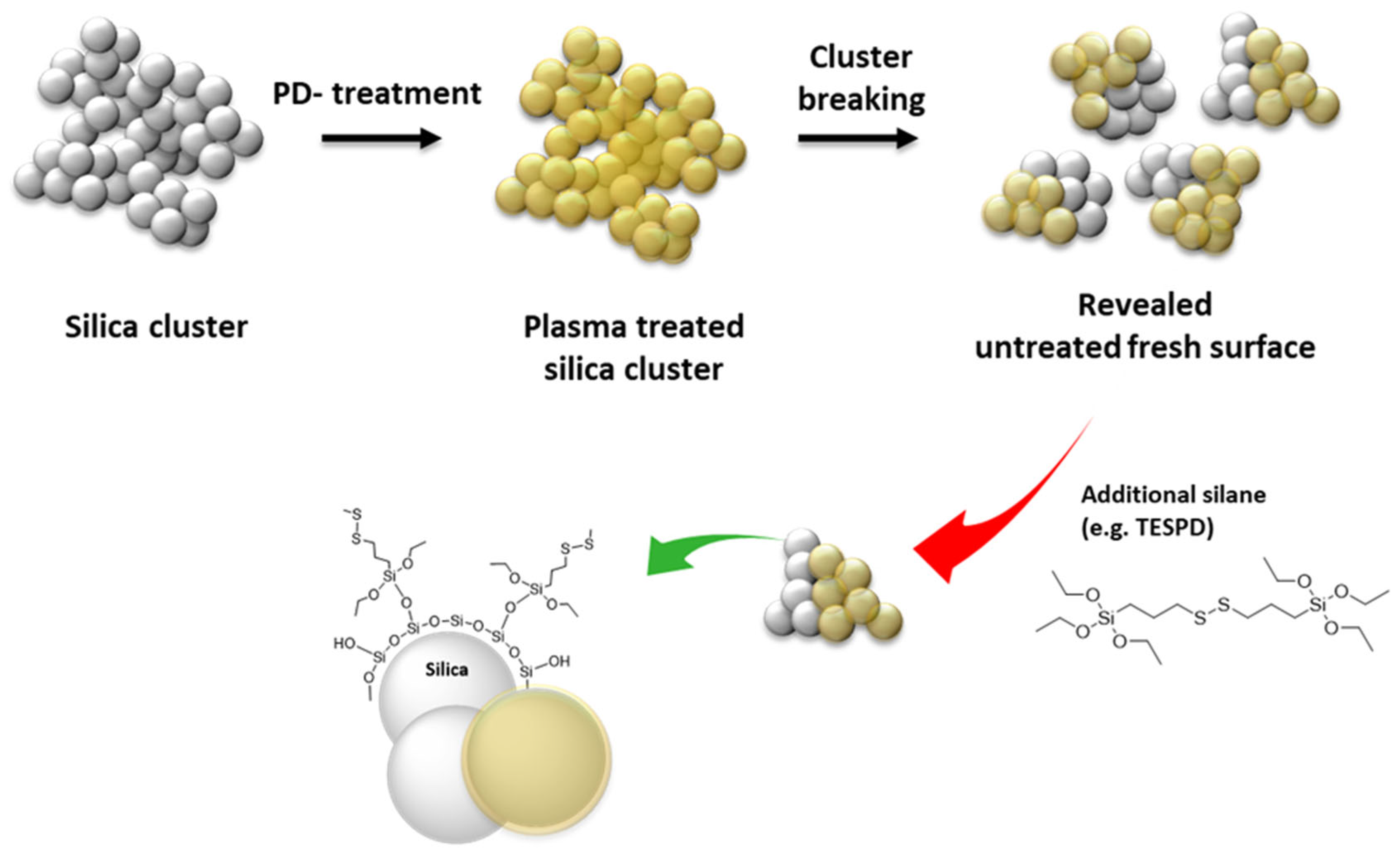
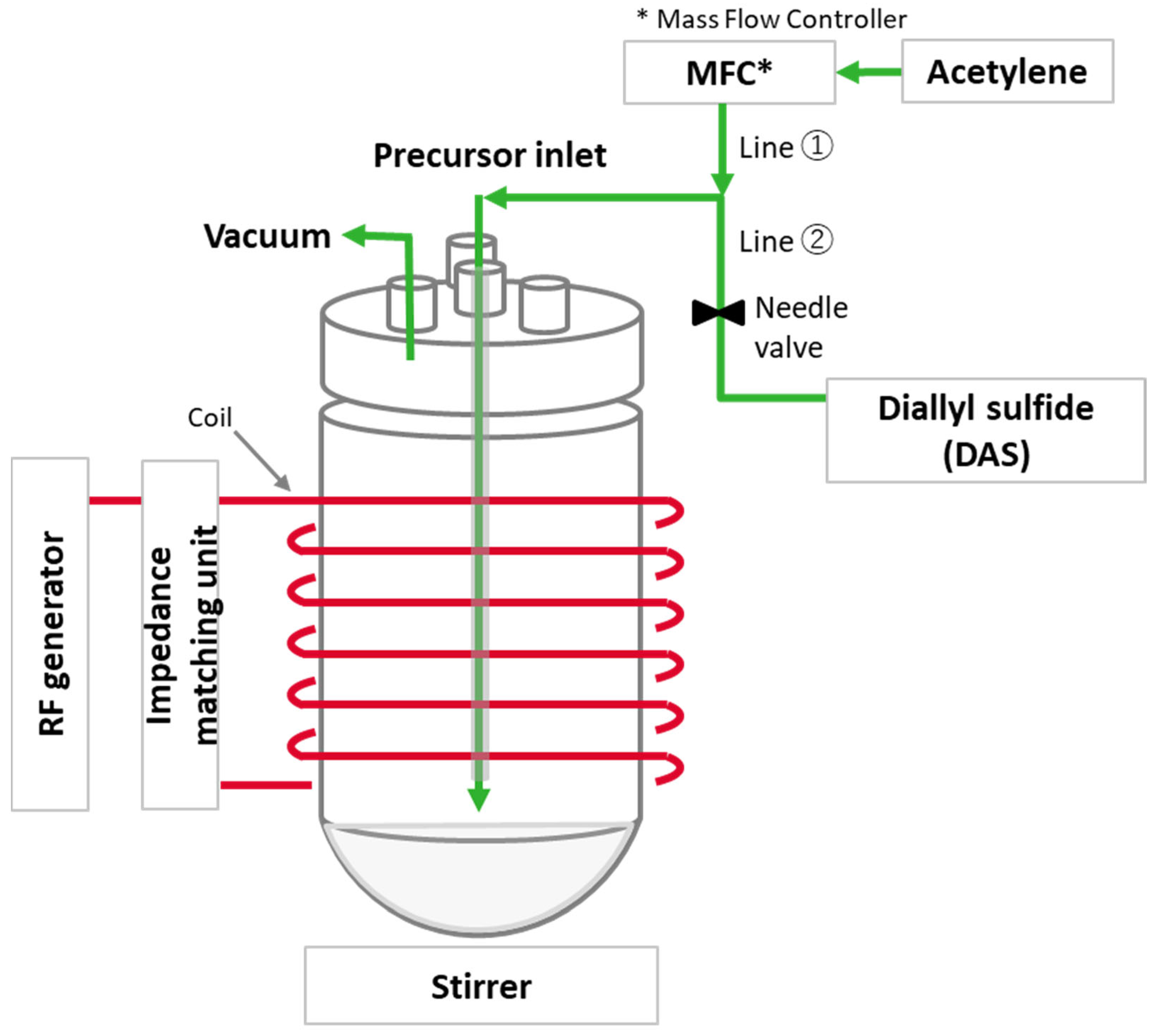
4.2. Preparation of PD-Treated Silica
4.3. Characterization of PD-Treated Silica
4.4. Preparation and Evaluation of PD-Treated Silica-Filled NR Compound
- (1)
- Series 1 (No Silane):
- (2)
- Series 2 (+Silane):
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
Abbreviation
| 6PPD | N-(1,3-dimethyl butyl)-N’-phenyl-p-phenylenediamine |
| ASTM | American Society for Testing and Materials Current |
| BET | Brunauer-Emmett-Teller surface area |
| CBS/CZ | N-Cyclohexyl-2-benzothiazole sulfenamide |
| CTAB | Cetyl trimethyl ammonium bromide |
| DAS | Diallyl Sulfide |
| DC | Direct current |
| DMA | Dynamic Mechanical Analyzer |
| DPG | 1,3-Diphenylguanidine |
| DRIFTs | Diffuse Reflectance Infrared Fourier Transform Spectroscopy |
| EF-TEM | Energy Filtered-Transmission Electron Microscopy |
| FT-IR | Fourier Transform Infrared spectroscopy |
| G′ | Shear storage modulus |
| G″ | Shear loss modulus |
| KBr | Potassium bromide |
| MFC | Mass Flow Controller |
| NR | Natural Rubber |
| PD | Plasma polymerization deposition |
| PHR | Parts per Hundred of Rubber |
| RF | Radio Frequency |
| RPA | Rubber Process Analyzer |
| RR | Rolling Resistance |
| S | Sulfur |
| SA | Stearic Acid |
| Tan δ | Loss modulus (G″)/Storage modulus (G′) |
| TDAE | Treated Distillate Aromatic Extracted Oil |
| TEM | Transmission Electron Microscopy |
| TESPD | bis-(3-triethoxysilyl-propyl)disulfide |
| TGA | ThermoGravimetric Analysis |
| VOCs | Volatile Organic Compounds |
| XPS | X-ray Photoelectron Spectroscopy |
| ZnO | Zinc oxide |
References
- Rauline, R. Copolymer Rubber Composition with Silica Filler, Tires Having a Base of Said Composition and Method of Preparing Same. U.S. Patent 5,227,425, 13 July 1993. [Google Scholar]
- Klockmann, O.; Hasse, A. A new rubber silane for future requirements: Lower rolling resistance; Lower VOCs. Kautsch. Gummi Kunstst. 2007, 60, 82–84. [Google Scholar]
- Hunsche, A.; Görl, U.; Koban, H.G.; Lehmann, T. Investigations on the reaction silica/organosilane and organosilane/polymer. Part 2: Kinetic aspects of the silica-organosilane reaction. Kautsch. Gummi Kunstst. 1998, 51, 525–533. [Google Scholar]
- Sarkawi, S.S. Nano-Reinforcement of Tire Rubbers: Silica-Technology for Natural Rubber: Exploring the Influence of Non-Rubber Constituents on the Natural Rubber-Silica System. Ph.D. Thesis, University of Twente, Enschede, The Netherlands, 2013. [Google Scholar]
- Zhang, C.; Tang, Z.; Guo, B.; Zhang, L. Significantly improved rubber-silica interface via subtly controlling surface chemistry of silica. Compos. Sci. Technol. 2018, 156, 70–77. [Google Scholar] [CrossRef]
- Zaborski, M.; Kosmalska, A.; Gulinski, J. Silica modified by use of organosilanes as a filler for carboxylated butadiene-acrylonitrile rubber. Kautsch. Gummi Kunstst. 2005, 58, 354–357. [Google Scholar]
- Ou, Y.-C.; Yu, Z.-Z.; Vidal, A.; Donnet, J.B. Effects of alkylation of silica filler on rubber reinforcement. Rubber Chem. Technol. 1994, 67, 834–844. [Google Scholar] [CrossRef]
- Ansarifar, A.; Wang, L.; Ellis, R.J.; Kirtley, S.P. The reinforcement and crosslinking of styrene butadiene rubber with silanized precipitated silica nanofiller. Rubber Chem. Technol. 2006, 79, 39–54. [Google Scholar] [CrossRef]
- Nah, C.; Huh, M.-Y.; Rhee, J.M.; Yoon, T.-H. Plasma surface modification of silica and its effect on properties of styrene-butadiene rubber compound. Polym. Int. 2002, 51, 510–518. [Google Scholar] [CrossRef]
- Tiwari, M.; Datta, R.N.; Talma, A.G.; Noordermeer, J.W.M.; Dierkes, W.K.; Van Ooij, W.J. Comparative study of plasma-thiophene and-acetylene coated silica in SBR and EPDM reinforcement. Rubber Chem. Technol. 2009, 82, 473–491. [Google Scholar] [CrossRef]
- Dierkes, W.; Tiwari, M.; Guo, R.; Datta, R.; Talma, A.; Noordermeer, J.W.M.; Van Ooij, W.J. Overcoming incompatibility problems in elastomer blends by tailored surface properties of rubber additives. Rubber Chem. Technol. 2013, 86, 1–27. [Google Scholar] [CrossRef]
- Mathew, T.; Datta, R.N.; Dierkes, W.K.; Noordermeer, J.W.M.; Van Ooij, W.J. A comparative investigation of surface modification of carbon black and silica by plasma polymerization. Rubber Chem. Technol. 2008, 81, 209–226. [Google Scholar] [CrossRef]
- Saramolee, P.; Trubmusik, S.; Sunthondecha, T.; Nisoa, M.; Johns, J. Effect of plasma-polymerised acetylene-coated silica on the compound properties of natural rubber composites. Heliyon 2021, 7, e08120. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Rytöluoto, I.; Anyszka, R.; Mahtabani, A.; Saarimäki, E.; Lahti, K.; Paajanen, M.; Dierkes, W.; Blume, A. Surface Modification of Fumed Silica by Plasma Polymerization of Acetylene for PP/POE Blends Dielectric Nanocomposites. Polymers 2019, 11, 1957. [Google Scholar] [CrossRef]
- Bernal-Ortega, P.; Anyszka, R.; Morishita, Y.; di Ronza, R.; Blume, A. Comparison between SBR Compounds Filled with In-Situ and Ex-Situ Silanized Silica. Polymers 2021, 13, 281. [Google Scholar] [CrossRef]
- Ngeow, Y.W.; Chapman, A.V.; Heng, J.Y.; Williams, D.R.; Mathys, S.; Hull, C.D. Characterization of silica modified with silanes by using thermogravimetric analysis combined with infrared detection. Rubber Chem. Technol. 2019, 92, 237–262. [Google Scholar] [CrossRef]
- Yasuda, H.; Lamaze, C.E. Polymerization of styrene in an electrodeless glow discharge. J. Appl. Polym. Sci. 1971, 15, 2277–2292. [Google Scholar] [CrossRef]
- Hoyos, B.E.A.; Romano, R.M. Experimental and theoretical conformational studies on diallyl sulfide. J. Mol. Struct. 2019, 1182, 54–62. [Google Scholar] [CrossRef]
- Yasuda, H.; Bumgarner, M.O.; Hillman, J.J. Polymerization of organic compounds in an electrodeless glow discharge. IV. Hydrocarbons in a closed system. J. Appl. Polym. Sci. 1975, 19, 531–543. [Google Scholar] [CrossRef]
- Blume, A.; El-Roz, M.; Thibault-Starzyk, F. Infrared study of the silica/silane reaction. Kautsch. Gummi Kunstst 2013, 10, 63–70. [Google Scholar]
- Friedrich, J. Plasma Polymerization Mechanism—Reviewed from a chemical point of view. Plasma Process. Polym. 2011, 8, 783–802. [Google Scholar] [CrossRef]
- Ben Haddada, M.; Blanchard, J.; Casale, S.; Krafft, J.-M.; Vallée, A.; Méthivier, C.; Boujday, S. Optimizing the immobilization of gold nanoparticles on functionalized silicon surfaces: Amine-vs thiol-terminated silane. Gold Bull. 2013, 46, 335–341. [Google Scholar] [CrossRef]
- Cano-Serrano, E.; Campos-Martin, J.M.; Fierro, J.L.G. Sulfonic acid-functionalized silica through quantitative oxidation of thiol groups. Chem. Commun. 2003, 2, 246–247. [Google Scholar] [CrossRef]
- Langlois, C.T.; Oikawa, T.; Bayle-Guillemaud, P.; Ricolleau, C. Energy-filtered electron microscopy for imaging core–shell nanostructures. J. Nanopart. Res. 2008, 10, 997–1007. [Google Scholar] [CrossRef]
- Sengloyluan, K.; Sahakaro, K.; Dierkes, W.K.; Noordermeer, J.W.M. Reinforcement efficiency of silica in dependence of different types of silane coupling agents in natural rubber-based tire compounds. Kautsch. Gummi Kunstst. 2016, 69, 44–53. [Google Scholar]
- Ten Brinke, J.W. Silica Reinforced Tyre Rubbers: Mechanistic Aspects of the Role of Coupling Agents. Ph.D. Thesis, University of Twente, Enschede, The Netherlands, 2002. [Google Scholar]
- Luginsland, H.-D.; Röben, C. The development of sulphur-functional silanes as coupling agents in silica-reinforced rubber compounds. Their historical development over several decades. Int. Polym. Sci. Technol. 2016, 43, 1–6. [Google Scholar] [CrossRef]
- Ten Brinke, J.W.; Debnath, S.C.; Reuvekamp, L.A.; Noordermeer, J.W. Mechanistic aspects of the role of coupling agents in silica–rubber composites. Compos. Sci. Technol. 2003, 63, 1165–1174. [Google Scholar] [CrossRef]
- Mihara, S.; Datta, R.N.; Noordermeer, J.W. Flocculation in silica reinforced rubber compounds. Rubber Chem. Technol. 2009, 82, 524–540. [Google Scholar] [CrossRef]
- York, W.M. In Improved Rolling Resistance with Equivalent Wet Traction through Competitive Inhibition; Efficiency Cure Accelerator/Mercapto-Type Silane Combination. In Proceedings of the Tire Technology Conference and Expo, Cologne, Germany, 10–12 February 2015. [Google Scholar]
- Klockmann, O.; Hahn, J.; Scheerer, H. New developments in the field of silicone rubber. In Proceedings of the International Rubber Conference 2009, Nuremberg, Germany, 29 June–2 July 2009. [Google Scholar]
- Ladouce-Stelandre, L.; Bomal, Y.; Flandin, L.; Labarre, D. Dynamic mechanical properties of precipitated silica filled rubber: Influence of morphology and coupling agent. Rubber Chem. Technol. 2003, 76, 145–159. [Google Scholar] [CrossRef]
- Luginsland, H.D. Reactivity of the sulfur chains of the tetrasulfane silane Si 69 and the disulfane silane TESPD. Kautsch. Gummi Kunststoffe 2000, 53, 10–19. [Google Scholar]
- Wang, M.-J. Effect of polymer-filler and filler-filler interactions on dynamic properties of filled vulcanizates. Rubber Chem. Technol. 1998, 71, 520–589. [Google Scholar] [CrossRef]
- Wang, M.-J. The role of filler networking in dynamic properties of filled rubber. Rubber Chem. Technol. 1999, 72, 430–448. [Google Scholar] [CrossRef]
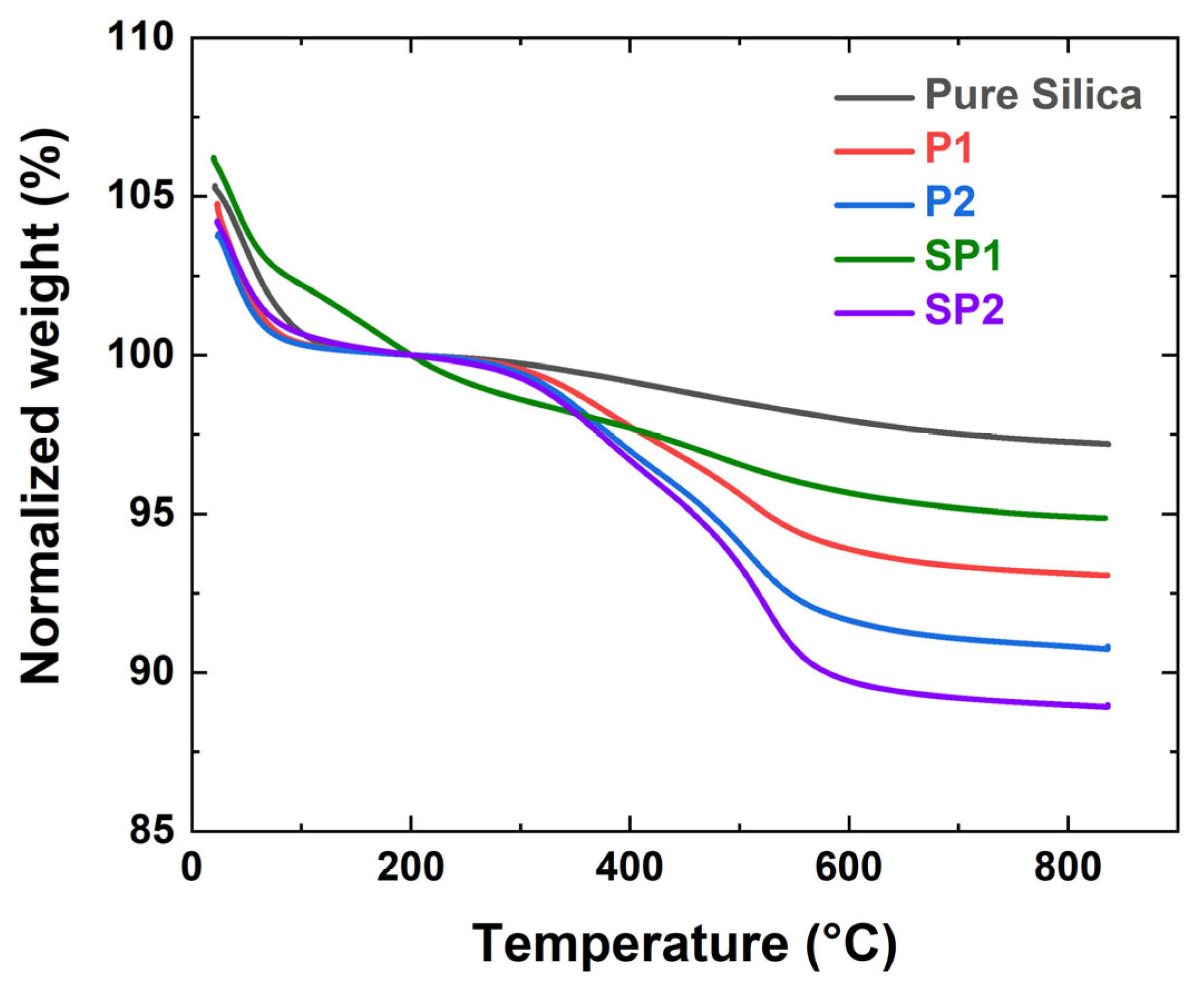
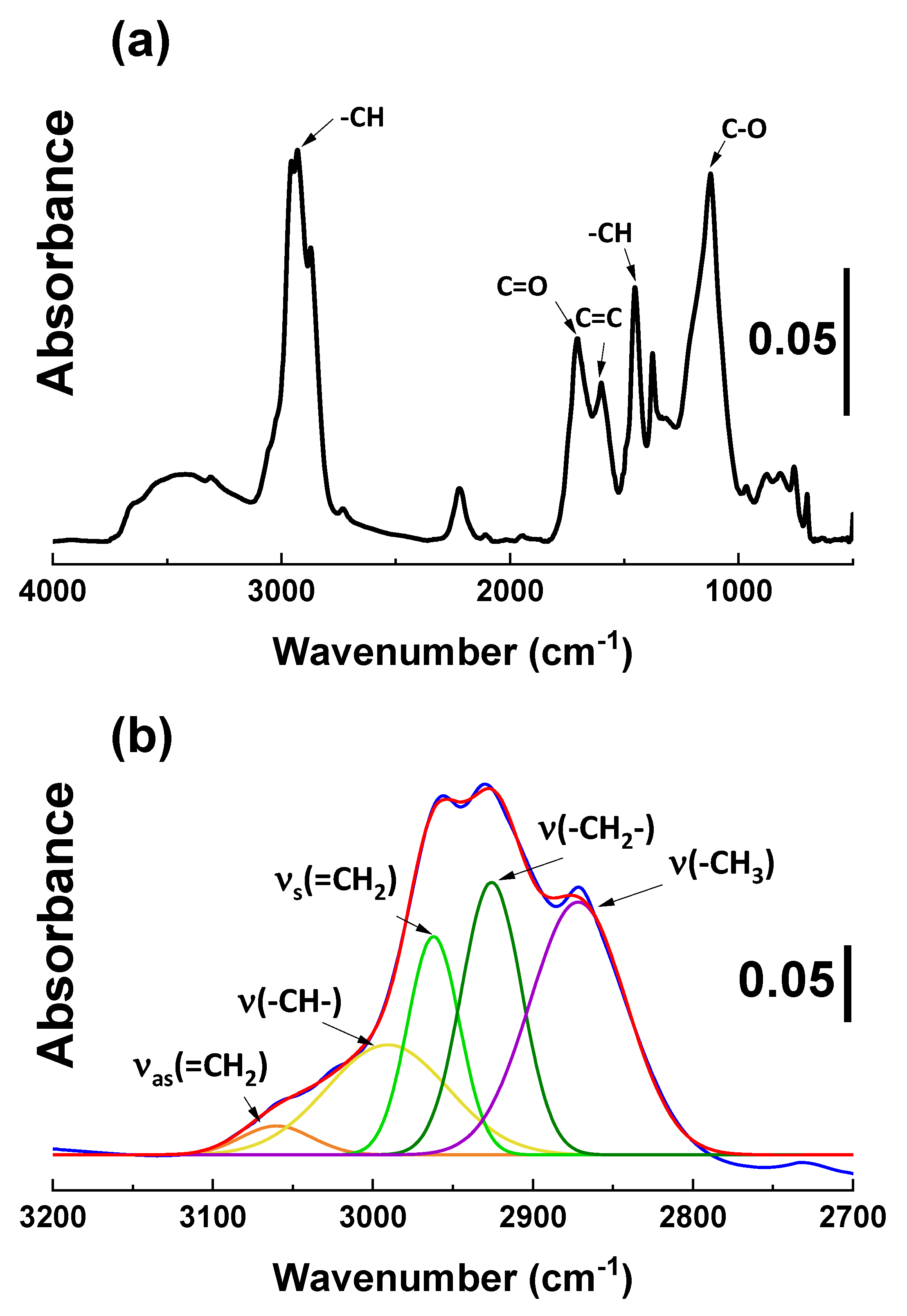
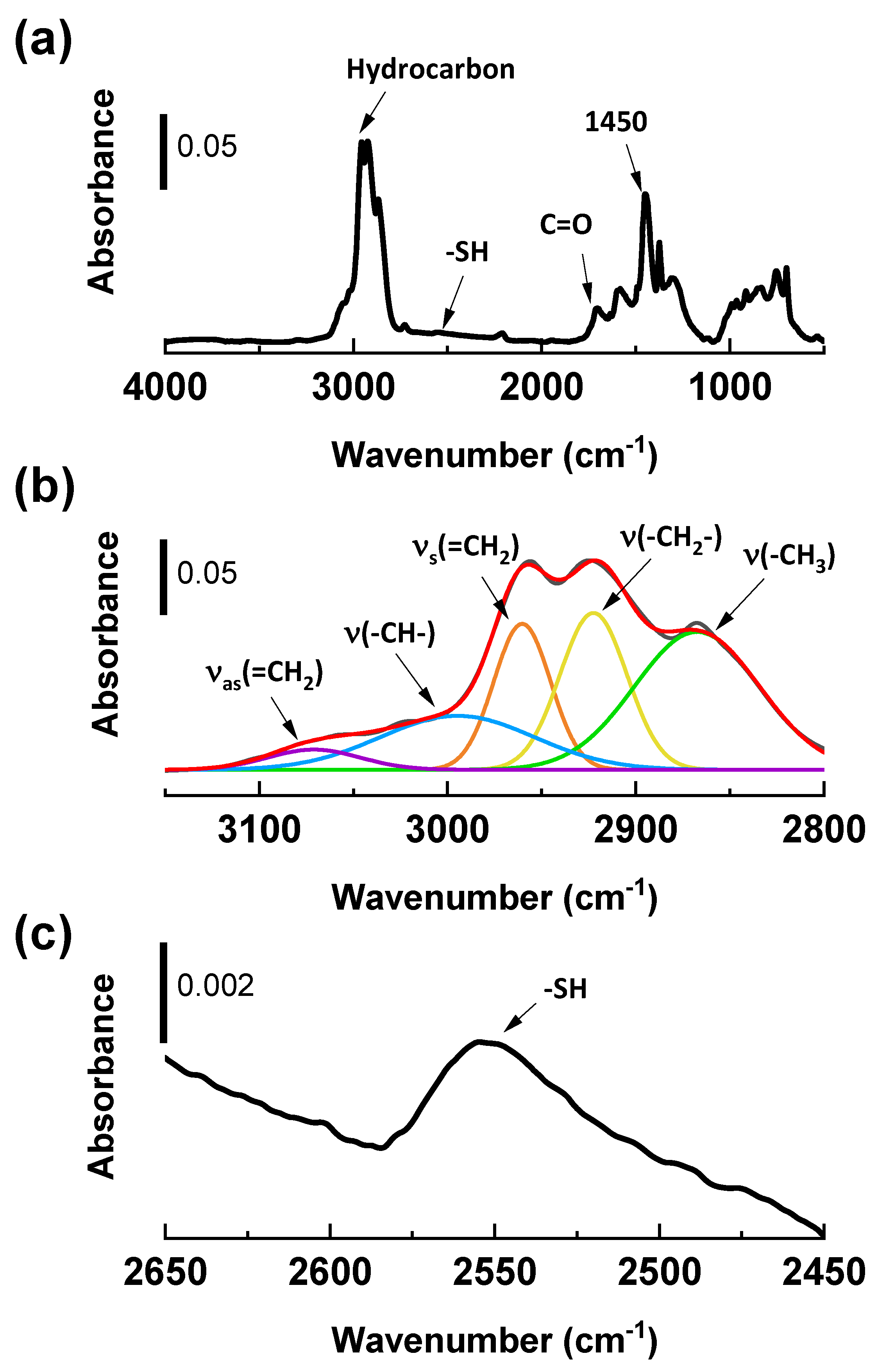
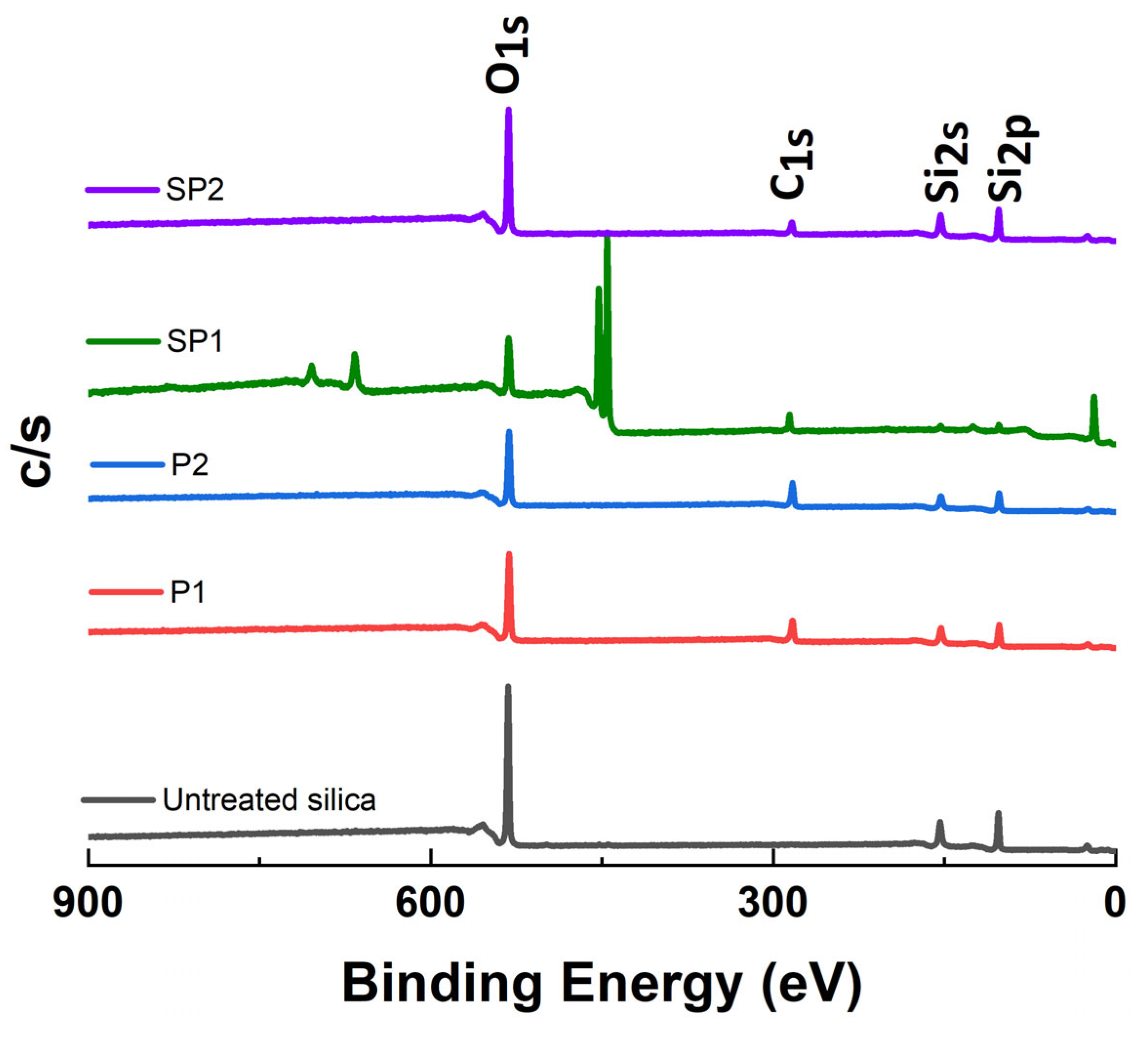

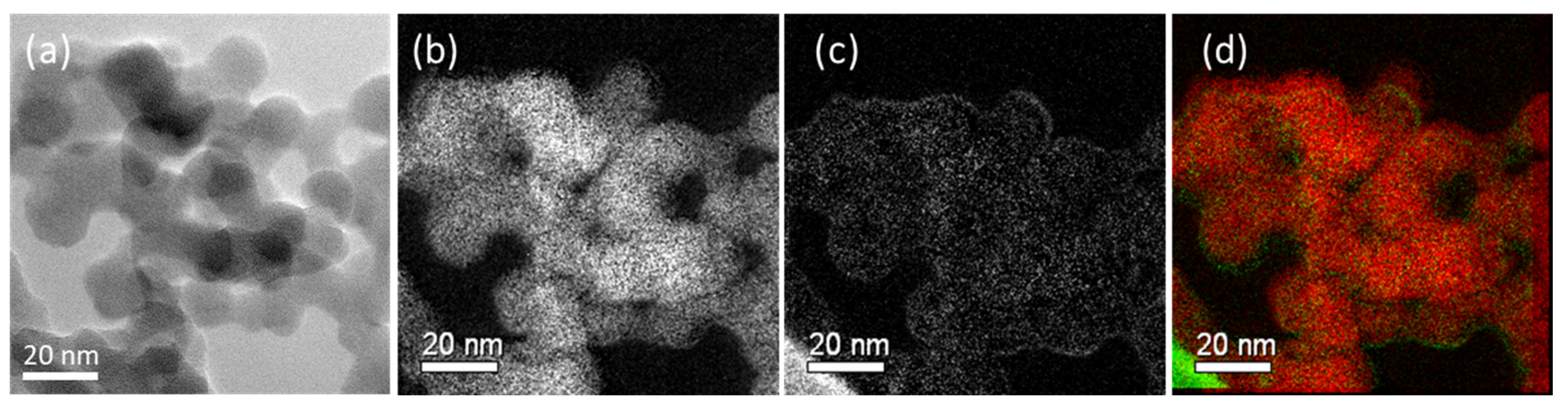

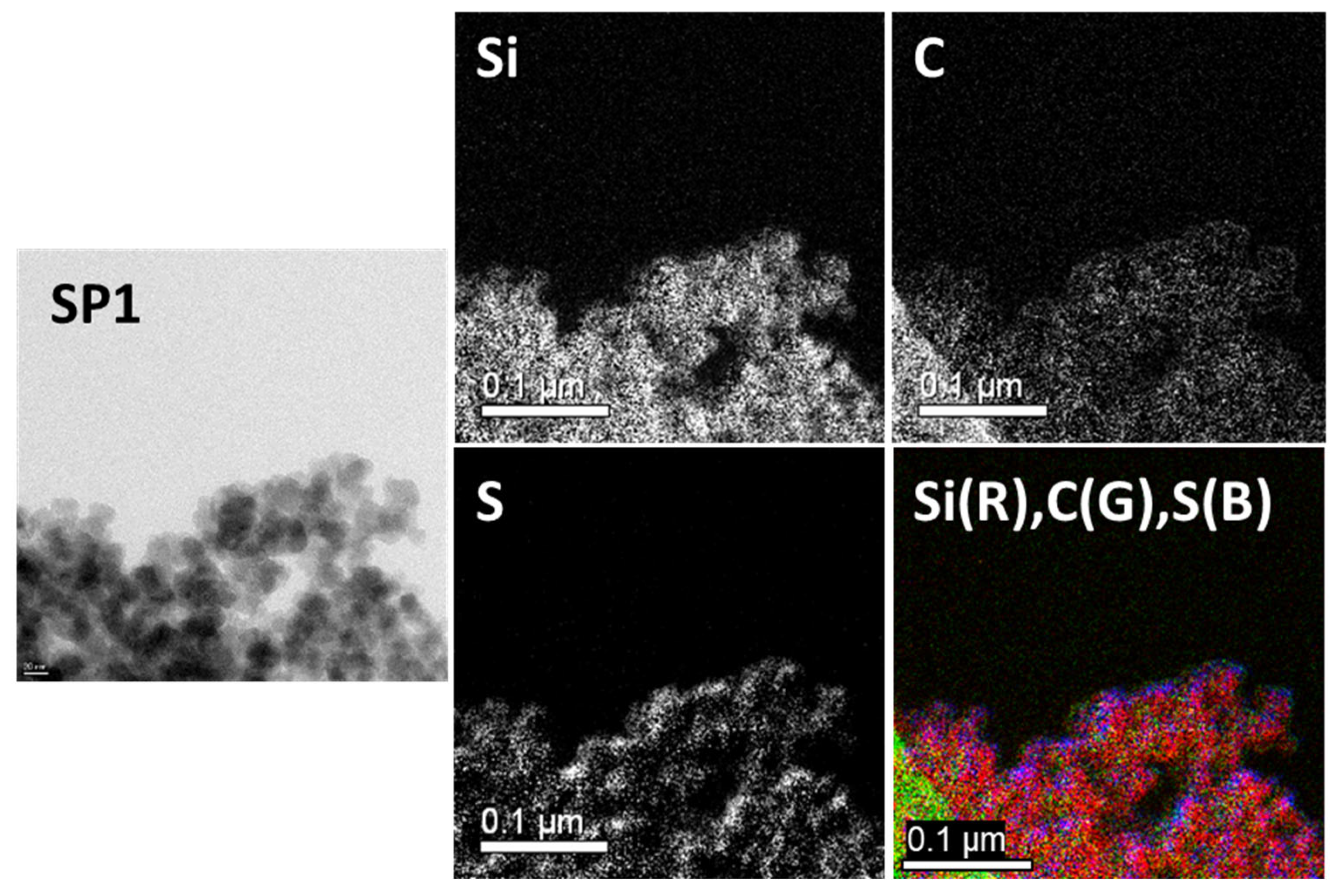
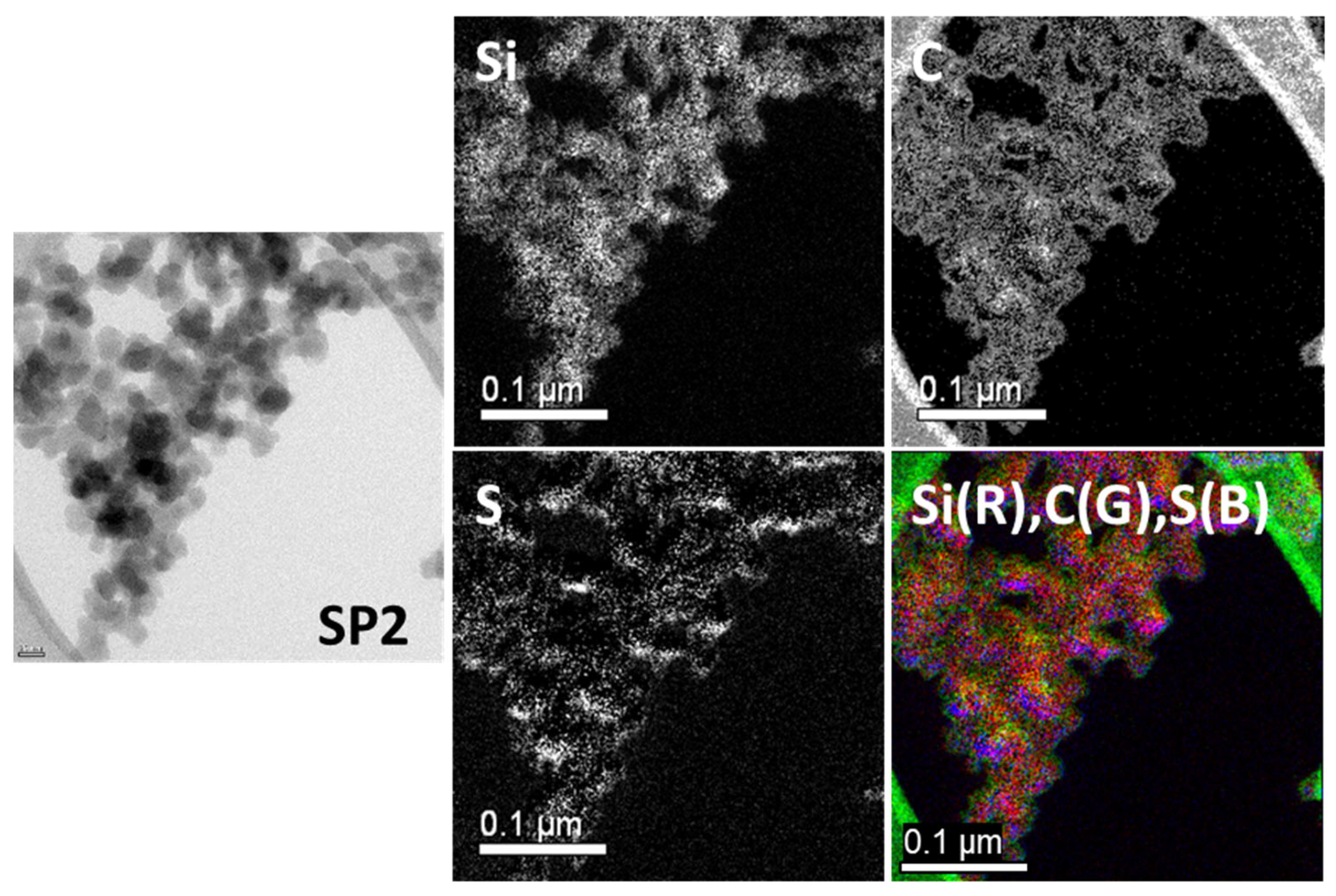

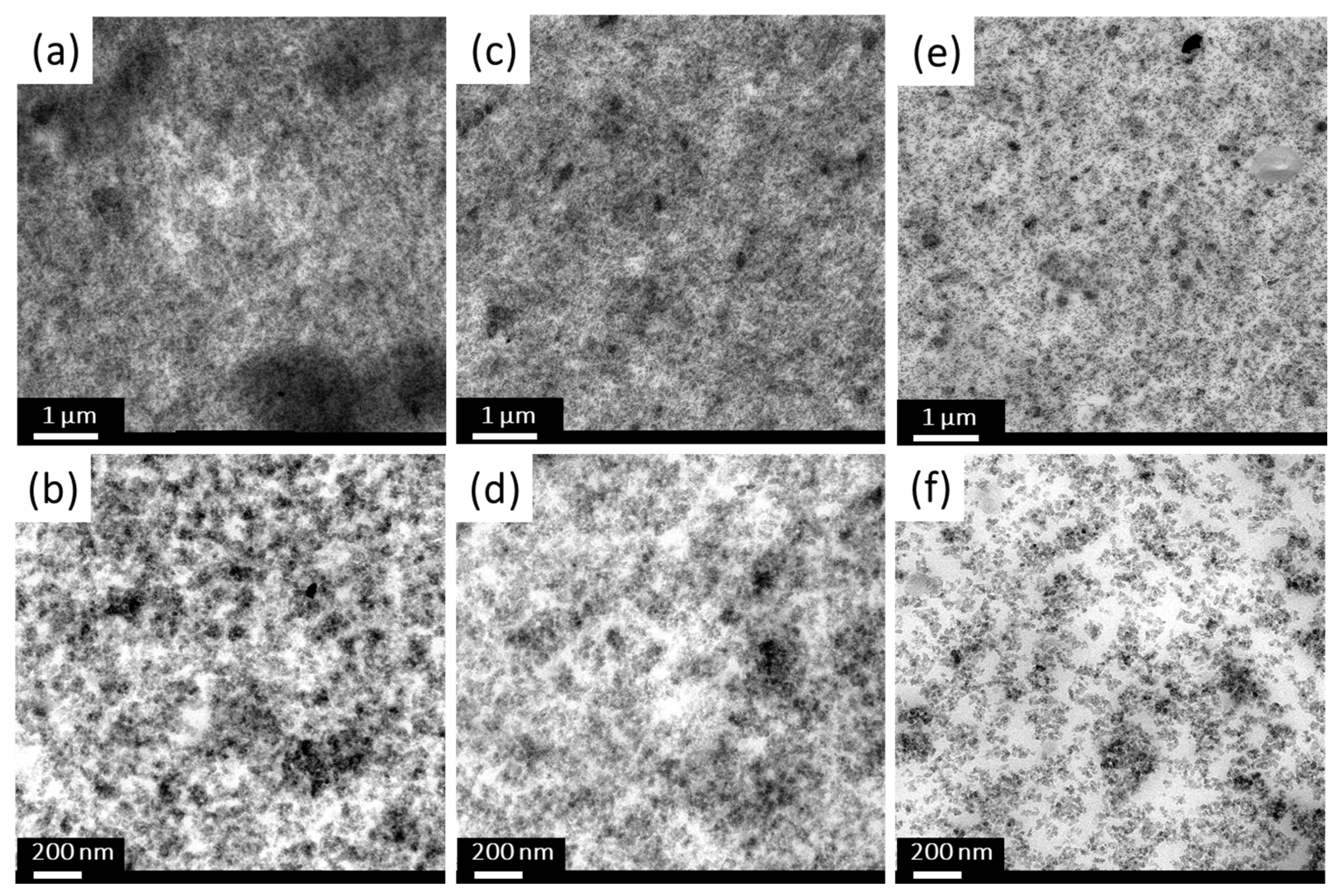
| Sample Code | Weight Change (200~850 °C) (%) | Degree of Deposition (%) | Estimated Number of Deposited Carbon Layers |
|---|---|---|---|
| Pure silica | 2.9 ± 0.1 | - | - |
| P1 | 7.0 ± 0.1 | 4.1 ± 0.1 | 1.1 |
| P2 | 9.3 ± 0.2 | 6.5 ± 0.2 | 1.5 |
| SP1 | 5.1 ± 0.1 | 2.2 ± 0.1 | 0.6 |
| SP2 | 11.1 ± 0.1 | 8.2 ± 0.1 | 1.9 |
| Sample | Series 1 (No Silane) | Series 2 (+Silane) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| NU | NP1 | NP2 | NSP1 | NSP2 | NUFS | NP1HS | NP2HS | NSP1HS | NSP2HS | NUHS | |
| ∆G′0.56–100% (MPa) | 3.1 | 2.9 | 2.4 | 2.8 | 3.0 | 1.3 | 1.7 | 1.3 | 1.8 | 1.9 | 1.8 |
| Sample | Elemental Composition | |||
|---|---|---|---|---|
| C | O | Si | S | |
| % | % | % | % | |
| Pure silica | 1.1 ± 0.5 | 69.8 ± 0.5 | 29.1 ± 0.9 | - |
| SP1 | 6.3 ± 0.7 | 65.4 ± 0.6 | 27.6 ± 0.3 | 0.7 ± 0.1 |
| SP2 | 15.8 ± 1.2 | 59.2 ± 0.6 | 24.8 ± 0.6 | 0.2 ± 0.1 |
| Compound Code | M100 | M300 | Tensile Strength | Elongation at Break | |
|---|---|---|---|---|---|
| MPa | MPa | MPa | % | ||
| Series 1 (No silane) | NU | 1.0 ± 0.1 | 3.9 ± 0.2 | 15.7 ± 1.0 | 593 ± 20 |
| NP1 | 1.1 ± 0.1 | 5.5 ± 0.2 | 17.2 ± 1.5 | 554 ± 21 | |
| NP2 | 1.2 ± 0.1 | 5.7 ± 0.1 | 17.2 ± 0.9 | 549 ± 20 | |
| NSP1 | 0.9 ± 0.1 | 2.7 ± 0.3 | 11.2 ± 0.7 | 605 ± 24 | |
| NSP2 | 1.2 ± 0.1 | 5.5 ± 0.2 | 17.0 ± 1.9 | 563 ± 33 | |
| Series 2 (+silane) | NU-FS | 1.9 ± 0.1 | 9.4 ± 0.4 | 28.2 ± 0.9 | 611 ± 24 |
| NP1-HS | 1.7 ± 0.1 | 8.7 ± 0.4 | 26.4 ± 0.6 | 592 ± 11 | |
| NP2-HS | 1.8 ± 0.1 | 8.8 ± 0.5 | 25.7 ± 1.5 | 586 ± 26 | |
| NSP1-HS | 1.1 ± 0.1 | 4.7 ± 0.2 | 15.3 ± 0.6 | 581 ± 19 | |
| NSP2-HS | 1.7 ± 0.1 | 8.7 ± 0.3 | 21.3 ± 0.7 | 526 ± 13 | |
| NU-HS | 1.5 ± 0.1 | 7.5 ± 0.6 | 23.4 ± 2.6 | 575 ± 32 | |
| Sample | Series 1 (No Silane) | Series 2 (+Silane) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| NU | NP1 | NP2 | NSP1 | NSP2 | NUFS | NP1HS | NP2HS | NSP1HS | NSP2HS | NUHS | |
| Tan δ @ 0 °C | 0.092 | 0.105 | 0.106 | 0.084 | 0.100 | 0.115 | 0.104 | 0.113 | 0.104 | 0.106 | 0.114 |
| Index | 100 | 114 | 115 | 92 | 109 | 125 | 113 | 123 | 113 | 116 | 124 |
| Sample | Series 1 (No Silane) | Series 2 (+Silane) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| NU | NP1 | NP2 | NSP1 | NSP2 | NUFS | NP1HS | NP2HS | NSP1HS | NSP2HS | NUHS | |
| Tan δ @ 60 °C | 0.169 | 0.193 | 0.188 | 0.219 | 0.194 | 0.142 | 0.161 | 0.153 | 0.220 | 0.184 | 0.150 |
| Index | 100 | 86 | 89 | 70 | 85 | 116 | 105 | 109 | 70 | 91 | 111 |
| Precursor | Molecular Formula | Molecular Weight (g/mol) | Boiling Point (°C) | Vapor Pressure @ 20 °C |
|---|---|---|---|---|
| Acetylene | C2H2 | 26 | −84 | - |
| Diallyl sulfide (DAS) | C6H10S | 114 | 138 | 9 mbar |
| Sample (1) | Silica (g) | RF Power (W) | Precursor | Treatment Time (h) | |
|---|---|---|---|---|---|
| Type | Monomer Pressure | ||||
| P 1 | 40 | 300 | Acetylene | 30 sccm (2) | 12 |
| P 2 | 40 | 450 | Acetylene | 50 sccm | 12 |
| SP1 | 40 | 450 | DAS | 0.6 mbar (3) | 12 |
| SP2 | 40 | 450 | 1st-Acetylene | 50 sccm | 10 |
| 2nd-DAS | 0.6 mbar | 2 | |||
| P 3 (4) | - | 450 | Acetylene | 50 sccm | 2 |
| SP3 (4) | - | 450 | DAS | 0.6 mbar | 2 |
| Mixing | Sample Code (1) | Series 1 (No Silane) | ||||
|---|---|---|---|---|---|---|
| NU | NP1 | NP2 | NSP1 | NSP2 | ||
| Step 1 | NR (SIR 20) | 100 | 100 | 100 | 100 | 100 |
| Silica | 55 | - | - | - | - | |
| P1 | - | 57.3 | - | - | - | |
| P2 | - | - | 58.5 | - | - | |
| SP1 | - | - | - | 56.2 | - | |
| SP2 | - | - | - | - | 59.5 | |
| ZnO/SA/6PPD /TDAE Oil | 2.5/1.0/2.0/8.0 | |||||
| Step 2 | Sulfur/CBS/DPG | 1.4/1.7/1.0 | ||||
| Mixing | Sample Code (1) | Series 2 (+Silane) | |||||
|---|---|---|---|---|---|---|---|
| NUFS | NP1HS | NP2HS | NSP1HS | NSP2HS | NUHS | ||
| Step 1 | NR (SIR 20) | 100 | 100 | 100 | 100 | 100 | 100 |
| Silica | 55 | - | - | - | - | 55 | |
| P1 | - | 57.3 | - | - | - | - | |
| P2 | - | - | 58.5 | - | - | - | |
| SP1 | - | - | - | 56.2 | - | - | |
| SP2 | - | - | - | - | 59.5 | - | |
| TESPD | 4.5 | 2.3 | 2.3 | 2.3 | 2.3 | 2.3 | |
| ZnO/SA/6PPD /TDAE Oil (2) | 2.5/1.0/2.0/8.0 | ||||||
| Step 2 | Sulfur/CBS/DPG | 1.4/1.7/1.0 | |||||
| Step 1, initial conditions: 80 °C and 70 rpm | |
| Time (mm: ss) | Action |
| 0:00 | The addition of rubber, mastication |
| 1:20 | Addition of 1/2 filler and silane (for Series 2) |
| 2:40 | Addition of 1/2 filler, TDAE Oil, and chemicals |
| 3:50 | Adjusted rotor revolution to keep the temperature at 150 °C |
| 6:30 | Discharge |
| Step 2, initial conditions: 60 °C and 60 rpm (24 h. after step 1) | |
| Time (mm: ss) | Action |
| 0:00 | Addition of masterbatch from Step 1, mastication |
| 1:00 | Addition of curatives (sulfur, CBS (1), and DPG (2)) |
| 2:40 | Discharge |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.; Dierkes, W.K.; Blume, A.; Talma, A.; Van Ommen, J.R.; Courtois, N.; Davin, J.; Recker, C.; Schoeffel, J. Plasma Polymerization of Precipitated Silica for Tire Application. Molecules 2023, 28, 6646. https://doi.org/10.3390/molecules28186646
Kim S, Dierkes WK, Blume A, Talma A, Van Ommen JR, Courtois N, Davin J, Recker C, Schoeffel J. Plasma Polymerization of Precipitated Silica for Tire Application. Molecules. 2023; 28(18):6646. https://doi.org/10.3390/molecules28186646
Chicago/Turabian StyleKim, Sunkeun, Wilma K. Dierkes, Anke Blume, Auke Talma, J. Ruud Van Ommen, Nicolas Courtois, Julian Davin, Carla Recker, and Julia Schoeffel. 2023. "Plasma Polymerization of Precipitated Silica for Tire Application" Molecules 28, no. 18: 6646. https://doi.org/10.3390/molecules28186646
APA StyleKim, S., Dierkes, W. K., Blume, A., Talma, A., Van Ommen, J. R., Courtois, N., Davin, J., Recker, C., & Schoeffel, J. (2023). Plasma Polymerization of Precipitated Silica for Tire Application. Molecules, 28(18), 6646. https://doi.org/10.3390/molecules28186646







