Abstract
The chemical nature of intracellular labile iron pools (LIPs) is described. By virtue of the kinetic lability of these pools, it is suggested that the isolation of such species by chromatography methods will not be possible, but rather mass spectrometric techniques should be adopted. Iron-sensitive fluorescent probes, which have been developed for the detection and quantification of LIP, are described, including those specifically designed to monitor cytosolic, mitochondrial, and lysosomal LIPs. The potential of near-infrared (NIR) probes for in vivo monitoring of LIP is discussed.
1. Introduction
Iron-dependent proteins play a central role in oxygen transport, electron transport, redox reactions, and nucleotide synthesis. Iron is typically incorporated into proteins as heme, iron sulfur clusters, or directly into tertiary structure cavities [1]. As there is a heavy demand for iron in order to maintain normal metabolic processes, iron is efficiently conserved in the mammalian body. Indeed, mammalian iron excretion is highly controlled, and the iron content of sweat and urine is low. It has been estimated that once absorbed, the average iron atom spends approximately 10 years inside a human before being excreted. Thus, iron is located in a range of intracellular pools, some relatively kinetically inert such as ferritin and others, such as the “labile iron pool” (LIP), which is labile, thereby facilitating the transmembrane movement of iron and iron donation to apoenzymes. Bioavailable, cytosolic iron exists in the iron(II) (ferrous) state and loosely interacts with a range of counter ions [2]. Williams argued that the electrode potential of the cytosol favours iron(II) over iron(III), and because the iron(II)-binding constants for many cytosolic enzymes fall in the range 10−8–10−7 M [3], it requires a similar standing concentration of iron(II) in order to prevent dissociation from the enzyme. Fluorescent probe studies, using calcein [4] and Phen Green SK [5], have demonstrated that the majority of the cytosolic LIP is iron(II), which, in view of the 100-fold higher kinetic lability of iron(II) when compared with iron(III) [6], is a logical strategy. Iron(II) is generally the form involved in intracellular translocation, for instance, incorporation into iron-requiring enzymes, incorporation into ferritin, and transport across membranes by the divalent metal transporter 1 (DMT1) and ferroportin. This kinetically labile group of iron species, LIP, is estimated to exist in concentrations of the order of 1 µM [2].
Iron(II) is capable of forming a range of oxygen radicals under aerobic conditions, via Fenton chemistry [7,8] and so the level of LIP needs to be carefully controlled, thereby limiting the potential toxicity of this critically important iron pool. In mammals, cellular LIP homeostasis is achieved by two “Iron Regulatory Proteins” (IRP 1 and IRP 2), which control the iron uptake into the cell via transferrin receptor 1, as well as iron export via ferroportin and iron storage in ferritin. This network of feedback mechanisms is presented schematically in Figure 1. Both IRP 1 and IRP 2 are RNA-binding proteins, capable of binding to ‘iron response elements’ (IREs) on mRNA [9]. By binding to IREs they inhibit the translation or degradation of mRNAs, which encode proteins involved in iron storage and iron import, thereby influencing LIP levels [10]. With IRP 1, IREs compete for binding to the protein with the coenzyme 4Fe-4S cluster of aconitase (ACO 1). With IRP 2, the insertion of a di-iron centre into the F-box and leucine-rich repeat protein 5 (FBXL5) permits the formation of a ubiquitin ligase complex, which subsequently binds to IRP 2, inducing its breakdown by the proteasomal pathway. Thus, the assembly of these two iron centres, namely 4Fe-4S and Fe-O-Fe, creates an integrated iron sensing mechanism for LIP, which in turn is linked to many feedback loops.
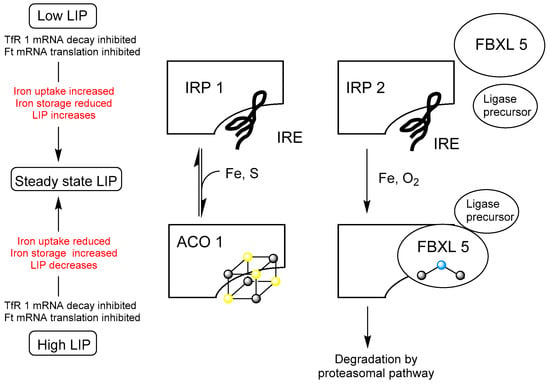
Figure 1.
Feedback mechanisms that control cellular iron homeostasis. IRP 1 and IRP 2 are RNA-binding proteins at low LIP levels, which are capable of binding IRE. IRE binding leads to an increase in LIP. At a certain LIP level, LIP contributes to the assembly of the [4Fe-4S] cluster, which binds to IRP 1 converting it to aconitase (ACO 1). Simultaneously, insertion of a diiron centre into a hem-erythrin-like domain of FBXL 5 stabilises the protein enabling it to form a complex ligase precursor, which induces degradation by the proteasomal pathway. ( , sulfur;
, sulfur;  , iron;
, iron;  , oxygen). This figure is adapted from Figure 2 in Kühn [9].
, oxygen). This figure is adapted from Figure 2 in Kühn [9].
 , sulfur;
, sulfur;  , iron;
, iron;  , oxygen). This figure is adapted from Figure 2 in Kühn [9].
, oxygen). This figure is adapted from Figure 2 in Kühn [9].
There are additional iron-sensing networks that are adopted in a wide range of organisms; another well-characterized example is based on iron- and oxygen-dependent prolyl hydroxylases [11]. The addition of a hydroxyl group to specific prolines within the Hypoxia-Inducible Factor (HIF), a transcription factor involved in hypoxia and iron metabolism, leads to their ubiquitination and subsequent breakdown [11]. The activity of prolyl hydroxylases is iron-dependent and so LIP levels influence the cytosolic levels of HIF.
2. Chemical Components of LIP
Many ligands previously associated with the cytosolic LIP chelate iron(III) but not iron(II) at pH 7.0; namely, amino acids [12], ATP/AMP [13], and inositol phosphates [14]. In contrast, citrate does bind iron(II) under cytoplasmic conditions and has been suggested to be the major component of LIP [15]. However, Fe2+·citrate is susceptible towards autoxidation at pH 7.0 [16], which renders citrate an unlikely iron(II) buffer. Based on the relatively strong interaction between iron(II), thiol-containing compounds, cysteine, and glutathione (GSH) have also been considered as possible cytosolic ligands for iron(II) [17]. Speciation plots based on the typical cytosolic levels of cysteine indicate that this ligand does not bind iron(II) sufficiently tightly to make a significant contribution to the LIP [17]. In contrast, GSH is present in the cytoplasm at a much higher concentration than that of cysteine, with the cytosolic level of GSH in human erythrocytes being 2.5 mM [18] and in rat liver being 8 mM [19]. Using the logK1 value for iron(II) of 5.12 [17], GSH is predicted to bind iron(II) to a considerable extent at pH 7.0 (Figure 2). GSH also rapidly reacts with hexaaquo·iron(III) converting it to hexaaquo·iron(II) (1) (Scheme 1) [20].
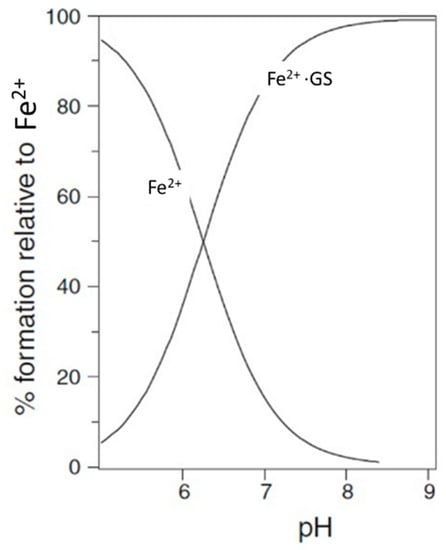
Figure 2.
Speciation plot of iron(II) (1 µM) with [GSH] = 2 mM.
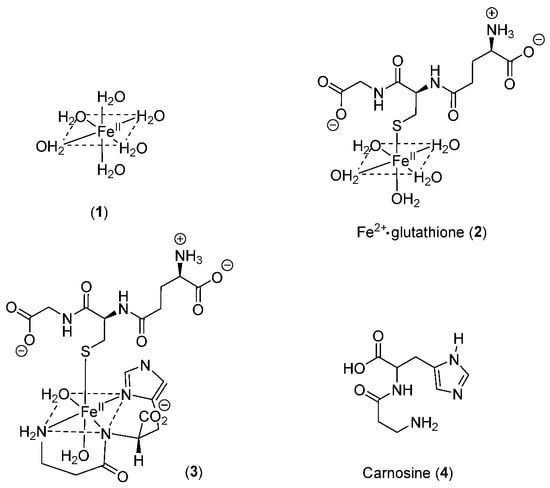
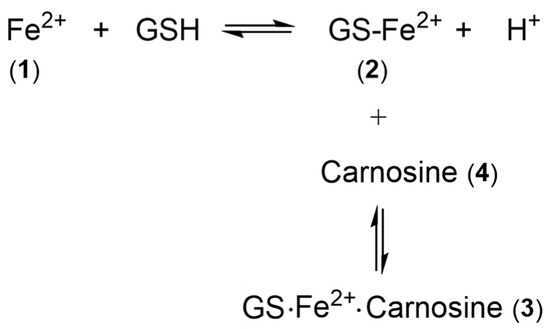
Scheme 1.
Structures of the aqueous, glutathione, and glutathione/carnosine complexes with iron(II). (1) hexaaquo·iron(II); (2) iron(II)glutathione; (3) iron(II)glutathione·carnosine; (4) carnosine.
GSH does not chelate iron(II) as, unlike cysteine, the bidentate coordination would require the formation of chelate rings containing either 9, 10, or 11 atoms. Such ring sizes are associated with unfavourable entropy differences. The non-chelating structure (2) (Scheme 1) agrees with that previously proposed for glutathione coordination of metal ions [21].
Over the concentration ranges of GSH (2–10 mM) and iron(II) (0.5–5 µM), the two major cytosolic iron(II) species are reliably predicted to be Fe2+·GS (2) and hexaaquo·Fe2+ (1), with the glutathione complex dominating. The concentration of [Fe(H2O)6]2+ (1) is linearly related to the total concentration of cytosolic iron(II) and is predicted to vary between 10−8 M and 5 × 10−7 M for a range of total iron(II) concentration between 1 µM and 10 µM. Clearly, the large excess of glutathione does not prevent [Fe2+]cytosol from acting as a potential signal for iron(II) sensors, such as the IRP 1/aconitase- and IRP 2/FBXL5-linked systems. Because of the high kinetic lability of iron(II), this system will be close to equilibrium and therefore at pH 7 the speciation plot depicted in Figure 2 will accurately reflect the cytosolic concentrations. Although autoxidation of cytosolic Fe2+·GS will occur at a slow rate, the resulting iron(III) will be rapidly reduced back to iron(II) (Figure 3), in a fashion similar to that of the reduction in methemoglobin.
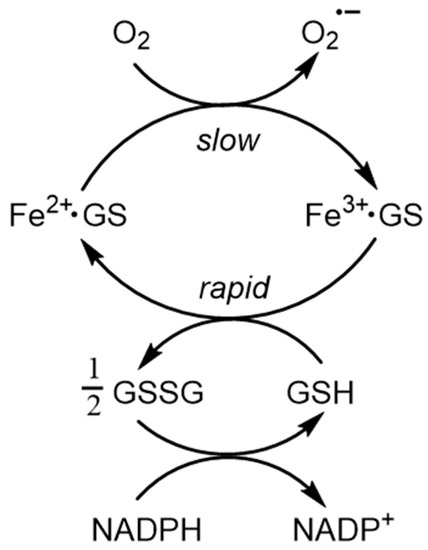
Figure 3.
The buffering of cytoplasmic iron(II) by GSH. Although there is predicted to be a slow rate of autoxidation of Fe2+·GS, the resulting Fe3+·GS will be rapidly reduced back to Fe2+·GS by the presence of the large excess of GSH.
Iron(II) binding by GSH offers a means by which the cytosol can distinguish iron(II) and manganese(II), both of which are present at similar levels [22]. The affinity of manganese(II) for GSH is markedly lower than that of iron(II), with the logK1 values being 2.7 [23] and 5.1 [17], respectively. This difference has a major effect on Mn(II) speciation, where citrate is found to be the dominant ligand, with GSH failing to bind even a trace of Mn(II) under cytoplasmic conditions [17].
As iron(II)glutathione, under physiological conditions, exists as a hydrated iron(II) cation with a single coordination site being occupied by monodentate glutathione (2), other low-molecular-weight ligands present in the cytosol can simultaneously coordinate to this ion, forming a ternary complex. The additional bidentate or tridentate ligand would need to possess a similar affinity for iron(II) as that of GSH in order to form an appreciable concentration of the ternary complex; the ligand would also need to be present at a relatively high concentration (1–10 mM). These requirements severely limit the number of such possibilities. Significantly, a group of widely distributed histidine-containing dipeptides, including carnosine (4) (Scheme 1), are capable of binding transition metals [24]. Indeed, it has been suggested that carnosine can compete for iron(II) with hypoxia-inducible factor 1 alpha (H1F-1α), an iron-dependent proline hydroxylase [25]. Furthermore, carnosine has previously been demonstrated to form mixed transition metal complexes with glutathione [26]. A likely structure for such a ternary complex of carnosine, glutathione, and iron(II) is indicated by 3 (Scheme 1).
Thus, LIP is likely to consist of several iron(II) complexes in equilibrium with each other (Scheme 2), with glutathione acting as a redox buffer locking iron into the iron(II) state. The concentrations of iron(II), glutathione, and carnosine will influence the relative concentrations of the LIP components (1), (2), and (3). In such a situation, it is important to establish which form of LIP generates an intracellular signal. Is this task limited to hexaaquo·iron(II) (1) or could GS·Fe2+ (2) and GS·Fe2+·carnosine (3) trigger some more specific intracellular events?
Scheme 2.
Equilibria between iron(II) (1), glutathione (GSH), and carnosine (4).
In all probability, it is only hexaaquo·Fe2+ (1) that provides the critical intracellular signal.
3. The Nature of the Labile Iron Pool in Different Intracellular Organelles
Mitochondrial Labile Iron Pool. Mitochondria are major sites for heme and iron-sulfur cluster synthesis in both plants and animals and, consequently, there is a constant iron influx into mitochondria as they export both heme and iron-sulfur clusters for use elsewhere in the cell. There are almost certainly multiple mechanisms for mitochondrial iron uptake. Mitoferrins 1 and 2 have, for instance, been linked to the delivery of iron to mitochondria in a range of organisms [27,28], with the accumulation being dependent on the mitochondrial membrane potential [29]. Significantly there is also a considerable movement of glutathione across mitochondrial membranes, facilitated by the dianionic exchangers; malate2−/HPO42− and 2-oxoglutarate2−/malate2− [30,31]. An interesting possible variant of such an exchange is for Fe2+·GS (3) to act as a substrate for this family of transporters. Indeed it has been suggested that iron(II) is transported into the mitochondrion as a complex rather than as inorganic iron [29].
The concentrations of citrate and glutathione are higher in the mitochondrial matrix than in the cytosol; namely [citrate] = 1.1–1.25 mM [32,33] and [GSH] = 11 mM [19]. Speciation analysis confirms that under these conditions, Fe2+·GS is the dominant form of iron(II) at pH 8.0 [2]. Indeed, a pH of 8.0 further favours the formation of Fe2+·GS (2) when compared to the cytosol. Furthermore, the mitochondrial ratio of [GSH]/[GSSG] is higher than that found in the cytosol [34].
In addition to an iron(II) buffering role, Fe2+·GS binds to glutaredoxins, proteins that are required for iron cluster assembly and heme biosynthesis [35]. Glutaredoxins are small proteins that possess a glutathione-binding site and a redox-active thiol function, both of which are located on the surface of the protein. These thiol functions typically catalyse thiol-disulphide exchange reactions. Some glutaredoxins are also capable of simultaneously binding iron and glutathione, forming [2Fe-2S] clusters, which are shared between two subunits of a homodimer [36,37]. These [2Fe-2S] clusters are transferred to acceptor proteins and are also involved in iron-regulatory protein (IRP) regulation [38]. The mitochondrion is the dominant location for iron-sulfur cluster synthesis [39], but the precise steps in [4Fe-4S] cluster biosynthesis are not completely defined [40], although glutathione has been demonstrated to possess a central role in the process [41,42]. The overall biogenesis occurs in two parts: The de novo assembly of a Fe-S cluster on a scaffold protein, such as the Iron-Sulfur Cluster Assembly Enzyme (ISCU), and then the subsequent transfer to target apoproteins. Glutaredoxins and glutathione are directly involved in these processes [36,43,44]. In the presence of glutathione, [2Fe-2S] clusters undergo a reversible exchange between apo-ISCU and glutathione, forming a complex [45]. Reductive coupling of two [2Fe-2S] clusters to form a single [4Fe-4S] cluster takes place on homodimeric cluster scaffold proteins [44] and the resulting cluster can, in turn, be transferred to apoproteins. Thus, it is conceivable that glutathione acts not only as a ligand for mononuclear iron(II) but also for [2Fe-2S] clusters.
Lysosomal Labile Iron Pool. The lysosome is involved in the breakdown of many iron-containing proteins, in particular ferritin. The digestion of ferritin generates a pool of ferric iron, which, when exposed to the acid pH range of most lysosomes (pH 4.5–5.5), will be complexed by citrate. Citrate levels in the lysosome typically fall in the range of 10–20 mM. Glutathione has a relatively low affinity for iron at pH 5.0 (Figure 2) and so the concentration of iron(II)glutathione is predicted to be much lower than that found in the cytosol. Thus, the predominant iron species in the lysosome is likely iron(III) coordinated with citrate.
Iron(III) citrate and other related complexes are capable of redox cycling in the lysosome and this can be reduced by intraliposomal iron chelation [46]. Iron(III) citrate is also a substrate for CytB reductase, reducing iron(III) to iron(II), thereby generating a substrate for DMT1. Thus, lysosomal LIP is recycled into the cytosol LIP in a controlled manner.
Nuclear Labile Iron Pool. The sequestering of genetic material within the nucleus of the eukaryotic cell provides the nucleus with the ability to regulate gene expression. Iron is an essential component for the expression of many genes [47,48] and of multiple enzymes involved in DNA metabolism [49], including both DNA synthesis and repair. Iron-dependent enzymes include DNA polymerase and primase, DNA-helicases, nucleases, glycosylases, and demethylases together with ribonucleotide reductases, many of which contain iron sulfur clusters [49].
The boundary between the nucleus and cytoplasm is a double membrane termed the nuclear envelope. This envelope is perforated with nuclear pore complex structures through which nucleocytoplasmic transfer occurs [50,51]. All passive transport across the nuclear envelope is through the nuclear pore complexes, which possess a central aqueous channel 9 nm in diameter. This channel permits the passive movement of molecules measuring less than 50 KDa [50]. Macromolecules larger than 50 KDa require combined assistance from nuclear localization signals and the nuclear pore complex. Thus, unless there are some extremely efficient transmembrane ion and solute pumps present in the nuclear envelope, the cytosolic and nucleus solute concentrations will be similar. Thus, hexaaquo·iron(II) and iron(II)glutathione are likely to be the major labile species in the nuclear pool.
4. Isolation and Characterization of Labile Iron Species
It should be clear from the previous discussion in this overview that in all probability, the LIP consists of a number of components and that these may differ between intracellular compartments by virtue of different pH values and concentrations of various iron-binding compounds in these various compartments. In the majority of compartments, namely cytosol, the mitochondrion, and the nucleus, the dominant redox state is iron(II), which is kinetically labile. This property renders it extremely difficult to isolate and purify such species from any biological matrix. Chromatography will not be possible due to the lability of iron(II)glutathione; iron dissociates from the complex and binds to other competing ligands including the stationary phase of the column. Mass spectrometry would appear to be the most promising method for the identification of labile iron species in biological matrices [52]. We are currently investigating such possibilities. At the present time, the most successful procedures for the quantification of LIP are fluorescent-based assays.
5. Iron-Sensitive Fluorescent Probes
The design of iron probes is typically based on bifunctional structures containing both a chelating agent and a fluorophore. A third function can be incorporated into the probe in order to influence subcellular localization [53,54]. For iron, the chelating agent can be selective for either iron(II) or iron(III). Probes to detect iron may contain either “turn-on” fluorophores, in which the binding of iron increases fluorescence, or “turn-off” fluorophores, in which the binding of iron quenches the fluorescence [55]. Although “turn-on” probes are preferred because they lead to more accurate quantification of iron, they have proven to be difficult to design and, at present, most currently available fluorescent iron probes are of the “turn-off” type. The chelation of iron by a probe leads to dynamic fluorescence quenching as both the quantum yield and the lifetime of the fluorescence decrease [56].
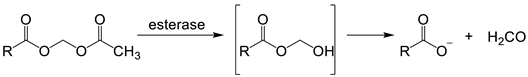
With ‘turn off’ probes, the concentration of LIP is determined by the decrease in fluorescence after correcting for photobleaching and leakage of the probe using either an in situ or an ex-situ calibration method [5,57]. Based on Equation (1), the equilibrium constant (Kprobe) can be used to quantify LIP, where LIP = [Fe2+] + [Fe2+·probe]. In principle, Kprobe, [Fe2+·probe], and [probe] can be determined. In order to minimise the efflux from the cell, the acetoxy methoxy group (AM) has been successfully introduced. AM enhances the membrane permeability of the probe, but cytosolic esterases rapidly cleave the AM esters to the more hydrophilic carboxylate function (Equation (2)). As a result, the now hydrophilic probe remains trapped within the cytosol [58].

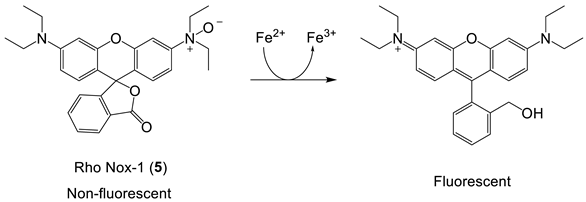



Rho Nox-1, an interesting iron(II) probe, has been investigated for the fluorescent-based detection of LIP [59,60]. The Fe2+-mediated deoxygenation of the N-oxide group on the fluorophore is reported to lead to an enhanced fluorescence, and as such, it is a “turn-on” probe, Equation (3). The mechanism of this deoxygenation reaction is not clear at the present time, and the reduction in the N-oxide may also be facilitated by thiols, including glutathione, which is present in the cytosol at high concentrations. More control studies are required before this class of probes can be reliably used to report LIP concentrations.
Cytosolic Probes. There are many fluorescent probes, selective for either iron(II) or iron(III), that have been previously described [61,62]. However, the properties of many of these probes have not been carefully investigated in cell culture systems. We discuss probes that have been investigated in some detail, namely calcein-AM (6), Phengreen SK diacetate (7), Fura-2AM (8), Rho Nox-1 (5), and Cytosense LITM.
Calcein-AM (6) is currently the most widely adopted fluorescent iron probe, having been studied in a wide range of cells [63,64]. Typical behaviour is presented in Figure 4 where a single HepG2 cell is monitored [65]. As iron(II), presented as iron(II) ammonium sulfate, enters the cytosol it quenches the calcein fluorescence. Upon the addition of Salicylaldehyde isonicotinoyl hydrazone (SIH, 9) (40–100 µM), the chelator gains rapid access to the cytosol where it out-competes calcein for iron(II) and consequently the fluorescence rapidly increases. When the behaviour of SIH (9) and deferoxamine (DFO) are compared in the presence of another cell line, it is clear that DFO fails to gain entry to the cytosol during the time scale of the study Figure 5 [63].
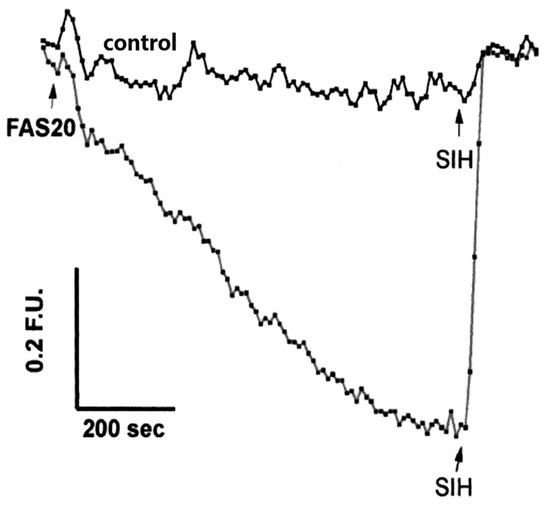
Figure 4.
LIP levels in a single human hepatoma HepG2 cell. Iron(II) ammonium sulfate (FAS, 20 µM) was added to half of the cells. SIH (100 µM) was added after the indicated time interval [65].
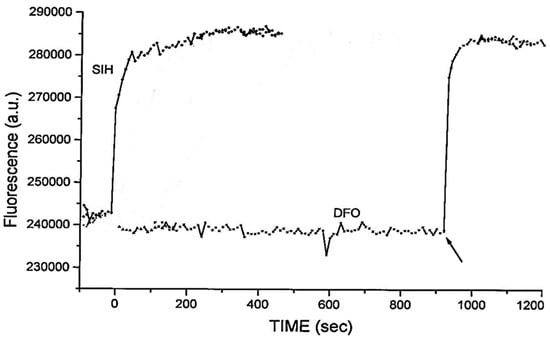
Figure 5.
Dequenching of calcein–iron complexes in K562 cells by extracellularly added chelators (100 μM) at zero time. Arrow indicates the addition of SIH (100 μM) [63].
As indicated by the discussion centred on Equation (1) using such probes, it is, in principle, possible to determine the concentration of LIP. The concentration of 6 can be determined from the fluorescence of a single cell or a suspension of cells. [Fe2+·6] is measured from the change in fluorescence upon the addition of SIH and the binding constant of iron(II) to the probe. However, the relevant binding constant for calcein is not certain; iron(II) certainly binds to the carboxylate functions of calcein, but physical measurements have demonstrated that iron also binds to phenolic groups on calcein, which can favour autoxidation of iron(II) to iron(III) [66]. Thus there is uncertainty regarding the chemical structure of the physiologically relevant iron complex that acts as a sink; indeed, a mixture of complexes likely form and thus uncertainty exists as to what iron(II)-binding constant to use. An additional limitation of calcein is that it is a substrate for a xenobiotic exporter protein, which facilitates leakage from cells.
Despite this limitation, calcein has been utilized extremely effectively to provide information on changes in LIP levels in a range of cell types. Thus, the rate of influx of iron chelators into cells can be directly compared [67] (Figure 6), the UVA-induced production of LIP can be monitored [68] (Figure 7), and the influence of LIP on the erythrocyte stage of Plasmodium falciparum can be monitored during cell culture (Figure 8) [69].
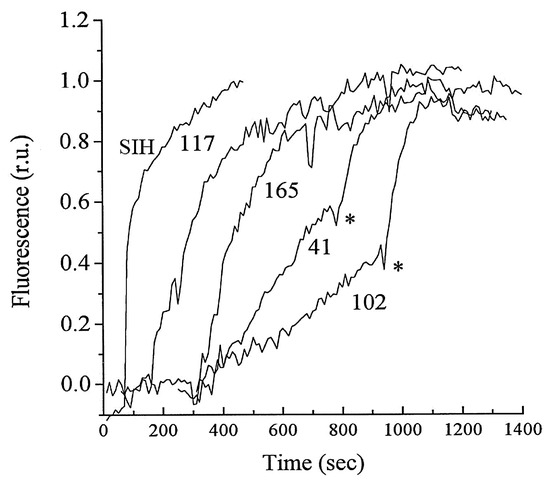
Figure 6.
Dequenching of calcein–iron complexes in K562 cells. Various chelators (100 μM) added at different time points, SIH (100 μM) marked by *. The chelators 102, 41, 165, and 117 are analogues of Deferiprone (DFP) [67].
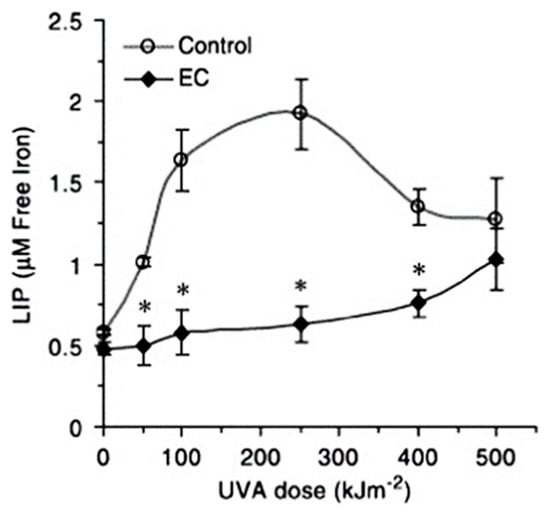
Figure 7.
UVA-induced production of LIP in skin fibroblasts. EC—epicatechin (30 μM) [68]. * = significant difference.
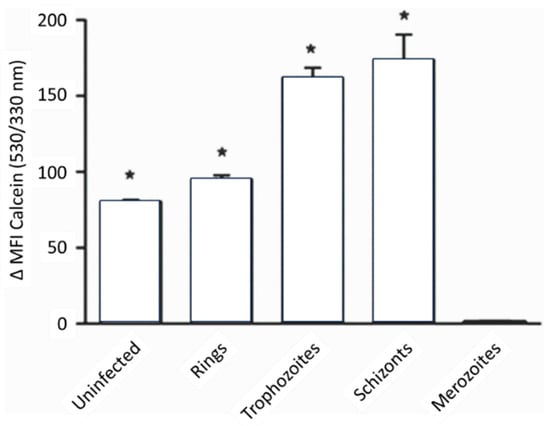
Figure 8.
LIP levels in various malaria parasite stages. The LIP increases as the parasite matures within the host red blood cell. The LIP of uninfected and different-stage parasitized RBCs was determined by assessing the fluorescence of calcein-loaded cells with the addition of the iron chelator, deferiprone (DFP) [69]. * = significant difference.
Phen green SK diacetate (7) is metabolized by cytosol esterases to Phen green SK (10), as shown in Equation (4), and hence is largely trapped in the cytosol. However, a small proportion of 10 gains access to the mitochondria and lysosomes [70]. Phen green SK diacetate (7) has been utilized to measure cytosolic LIP by using an ex-situ calibration method [70,71]. When this method was applied to isolated rat hepatocytes, a range of LIP values was obtained (Figure 9), with a large proportion falling in the range of 0.8–2.0 µM [71].
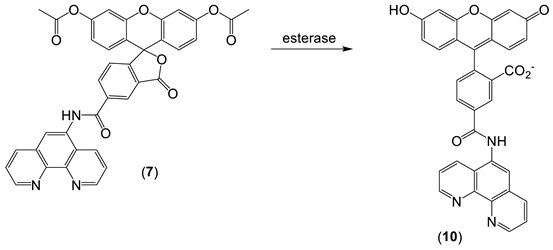

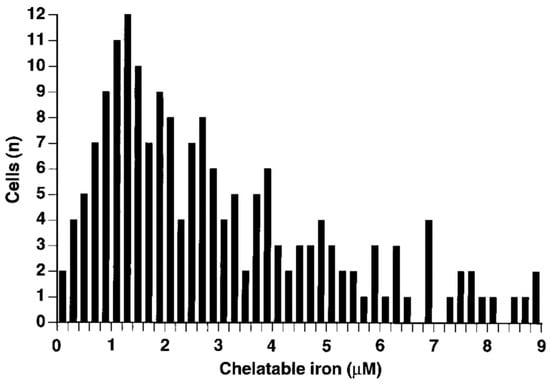
Figure 9.
Frequency distribution of the hepatocellular concentration of LIP. Cells were incubated with PG SK diacetate (20 μM) for 10 min. Bipyridyl (5 mM) was added and the resulting fluorescence was noted [71].
Rho Nox-1 (5) is reported to be selectively sensitive to the presence of iron(II) [59], and confocal fluorescence microscopy images of HepG2 cells incubated with (NH4)2Fe(SO4)2 register an increase in fluorescence intensity (Figure 10). As indicated in the earlier section on “Iron-sensitive fluorescent probes”, more control studies on this group of probes are urgently required. Stability studies are reported for coincubation with GSH, but only at 1 mM, although cytosolic GSH is at least 8 mM. It has not been established whether Rho Nox-1 (5) can detect iron(II) in the presence of physiological levels of GSH.
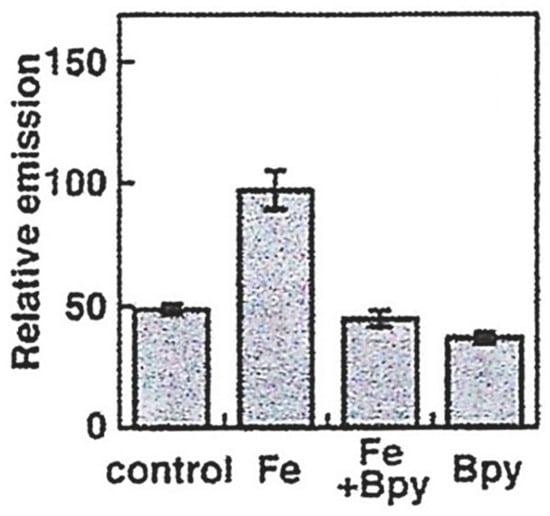
Figure 10.
Quantification of LIP in HepG2 cells supplemented with (NH4)2Fe(SO4)2 (100 µM) for 30 min at 37 °C. 2,2′ Bipyridyl (Bpy, 1 mM) was used to reduce the availability of iron(II) [59].
Hydroxypyridin-4-one (HOPO)-containing probes have been investigated for the quantification of LIP [54]. Under biological conditions, they are selective for iron, binding iron(II), and rapidly autoxidizing the metal to iron(III) (Equation (5)) in a similar fashion to that of DFO. CP655 (11) achieves rapid entry into cells without the necessity of using AM or acetyl esters. However, this ability to penetrate plasma membranes provides CP655 access to both lysosomes and mitochondria, thus providing a measure of cellular LIP as opposed to cytosolic LIP. A further limitation of this class of fluorescent probes is that they are not trapped intracellularly and will efflux once the incubation media is changed [72]. However, changes in LIP levels induced by the addition of iron(III) citrate and a competing chelator CP94, in a similar fashion to those reported for calcein and Phen green SK, can be monitored by changes in fluorescence (Figure 11).
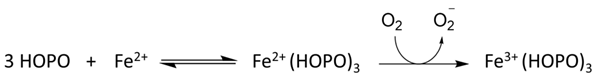
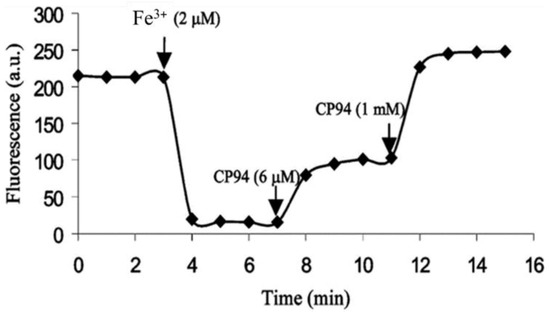
Figure 11.
Fluorescence of the hydroxypyridinone CP655 (6 μM) in primary hepatocytes, quenched by iron(III) (2 μM) and recovered in the presence of CP94 [72].
In principle, HOPO probes, which are linked to ester moieties, could be targeted to the cytosol in a similar manner to that of calcein AM (6). We have synthesized a range of such molecules and, as a result, identified Cytosense LI as being suitable for the quantification of cytosolic LIP. Time-lapse fluorescent microscopy demonstrates that Cytosense LI enters cells rapidly and responds to the addition of iron presented as a complex with 8-hydroxyquinoline (Figure 12). The determination of the concentration of LIP in primary skin fibroblast FEK4 cells was achieved by measuring the fluorescence difference of pairs of samples, namely Cytosense LI and DFO and conversion to [LIP] using an ex situ calibration curve (Figure 13). A value of 0.29 ± 0.02 µM was obtained for a mean of four determinations [73]. More control studies are required for Cytosense LI, but it appears to be a promising probe for cytosolic LIP measurement.
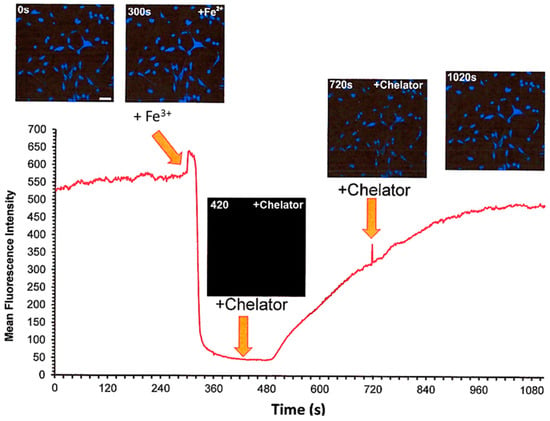
Figure 12.
Live cell imaging of the Cytosense LI response to the modulation of intracellular levels of labile iron. FEK4 cells were treated sequentially with 3 µM Fe3+ and 700 µM DFP. Fluorescence changes were monitored quantitatively using time-lapse microscopy. The trace represents the MEAN ± SD fluorescence from a field of view containing Ca 70 cells.
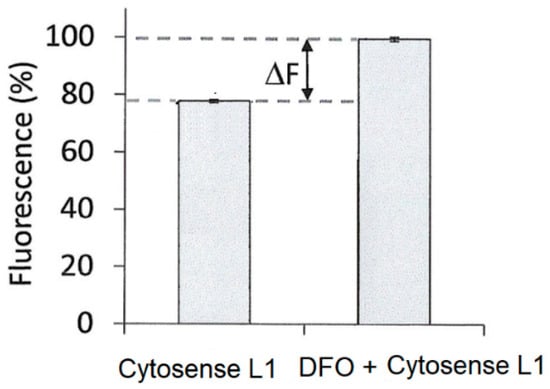
Figure 13.
Determination of LIP in FEK4 cells using Cytosense LI. The fluorescence of Cytosense LI ± DFO from three independent experiments is shown as a bar chart [73].
Endoperoxide reactivity-based probes have also been utilized to provide an estimate of cytosolic iron(II) levels [74,75]. They are reported to be selective for iron(II) via the Fenton reaction. However, to date, there have been no further reports on the application of this class of probes for monitoring iron(II) levels.
Mitochondrial Probes. As mitochondria are the main utilizers of iron in the cell, as well as being a major source of the superoxide anion, O2−•, iron homeostasis is critically important. An increase in the mitochondrial iron burden is likely to be linked to associated pathology, for instance with Friedreich’s ataxia where there is inefficient iron sulfur cluster synthesis [76]. The mitochondrial LIP is buffered to some extent by mitochondrial ferritin, but not in all tissues, for instance, liver mitochondria lack mitochondrial ferritin.
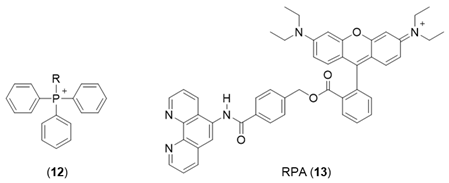
One widely adopted method of targeting molecules to the mitochondrial matrix is by attachment to delocalized lipophilic cations. By taking advantage of the substantial negative electrochemical potential maintained across the inner mitochondrion membrane (typically −200 mV), delocalized cations are able to cross the membrane and hence be accumulated with a distribution driven by the Nernst potential, typically in excess of 1000-fold. Triphenyl phosphonium salts (12) are such examples where the positive charge formally associated with the central phosphorus atom is delocalized over the three phenyl rings, resulting in a low electron density. This, together with the overall lipophilic character of the molecule, enables rapid penetration of membranes. This useful property of lipophilic cations has been utilized to target fluorescent iron sensors in the mitochondrion. Both Petrat’s [77] and Cabantchik’s groups [78,79] have utilized a derivative of rhodamine (RPA, 13) to monitor mitochondrial iron levels. Rhodamine is a positively charged fluorescent molecule where the single positive charge is delocalized on both nitrogen atoms and over the plane of the tricyclic structure. By adopting methods developed for the cytosol, estimates of the mitochondrial LIP were made. The values for mitochondria of different cells were found to be distributed over a wide concentration range, centred around 12 µM [77]. A limitation of this probe is the difficulty in reversing the quenched signal under physiological conditions [79] and thus the associated difficulty of unequivocally assigning the quenched signal to labile iron.
Largely as a result of the above limitation, a different approach has been introduced for monitoring the mitochondrial matrix LIP. A series of peptides have been prepared, members of which are capable of being targeted to and accumulated in mitochondria. These peptides contain alternate hydrophobic and basic amino acids and typically contain between 4 to 6 residues. Using this general design, a series of small fluorescent peptides have been synthesized that are selectively accumulated by mitochondria [80]. By incorporating an iron-chelating moiety into the molecule (14), it becomes possible to monitor the mitochondrial LIP. BP19 (14) has been utilized to monitor the mitochondrial LIP of cultured fibroblasts obtained from Friedreich’s ataxia patients [81]. The mean levels of mitochondrial LIP in Friedreich’s ataxia cells were found to be 1.11 ± 0.37 µM, whereas those from healthy donors were 0.17 ± 0.12 µM. This finding supports the observation of Rouault et al. [82] that there is an accumulation of redox-active labile iron in the mitochondria of Friedreich’s ataxia patients.
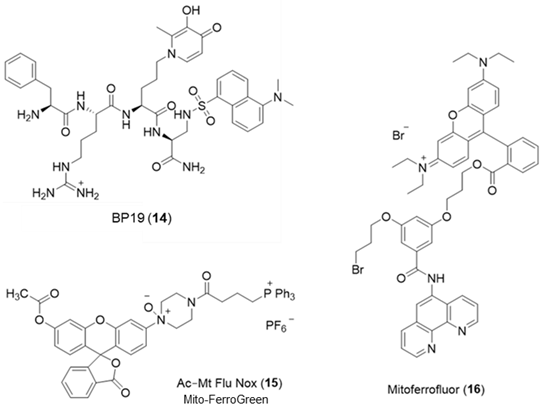
A mitochondria-targeted, NO-containing fluorescent probe Mito-FerroGreen (15) has been introduced for the measurement of mitochondrial LIP [83]. It contains the triphenyl phosphonium entity to facilitate mitochondrial targeting and an NO function, which renders the fluorescence of the probe iron(II) sensitive. The same limitations exist with this molecule as with Rho Nox-1 (5), namely more control studies are required to establish iron(II) selectivity. Another phenanthroline-based mitochondrial probe Mitoferrofluor (16) has been investigated for the detection of iron(II) [84]. This probe is also quenched by copper (II), but not at normal physiological copper levels. This probe can detect mitochondrial chelatable Fe2+ in both polarized and depolarized mitochondria.
Lysosomal targeting Probes. The endosomal/lysosomal compartment of cells contains a relatively high level of labile iron, largely resulting from the breakdown products of both mitochondria [85] and ferritin [86]. Chelation of this iron pool protects against oxidative stress-induced cellular damage [46,87]. The measurement of labile iron levels in the endosomal/lysosomal compartment of hepatocytes is difficult because of its small size, approximately 1% of the cell volume [88]. However, the larger lysosomes present in liver endothelial cells are amenable to measurement. Using Phen Green SK in combination with 1,10-phenanthroline, Petrat and coworkers determined a labile iron level for this intracellular compartment of 15.8 ± 4.1 µM [88].
The hydroxypyridinone SF34 (17) has been demonstrated to only be located in endosomal/lysosomal compartments in macrophages [89]. SF34 does not permeate membranes rapidly and so can be trapped within the endosomal/lysosomal compartment, which it enters by endocytosis. This probe has been utilized to compare the ability of different chelators to mobilize lysosomal LIP using single-cell flow cytometry [90].
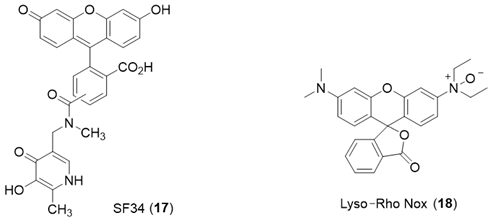
The Rho Nox-1 analogue, Lyso-Rho Nox (18), has been reported to be localized in the lysosomal compartment of human adenocarcinoma (MCF-7) cells, and this property has been utilized to report differences in lysosomal LIP levels [91].
6. NIR Probes
The development and application of near-infrared (NIR) technologies is also under investigation, in order to facilitate our understanding of the nature of cytosolic iron pools in vivo. This is primarily due to the unique properties of NIR light and its interaction with biological tissues. Namely, by exploiting a region of the electromagnetic spectrum, which is less absorbed and scattered (i.e., above 650 nm), NIR light is known to offer several advantages, such as increased tissue penetration, limited autofluorescence by endogenous molecules, and, thus, minimized background interference, along with reduced photobleaching of the fluorophores and photodamage to the tissues. All this leads to an enhanced signal-to-noise ratio by NIR agents and, thus, clearer and more reliable imaging results for in vivo imaging. In this context, NIR-based agents represent promising tools for the study of intracellular iron levels in organs and tissues, which would be challenging or impossible to access with shorter-wavelength probes (e.g., emitting in the ultraviolet-visible region). Iron-targeted NIR probes have been developed with this purpose in mind [92,93,94] to facilitate in vivo investigations of iron-related disorders, including neurodegeneration and other syndromes by the imbalance of iron homeostasis. Typically, these probes are designed in such a way that ensures high specificity for iron, exhibiting a change in their fluorescence properties upon binding to the metal, thereby enabling the monitoring of its dynamics in the cytosol with enhanced sensitivity and spatial resolution. Noteworthily, the ability to selectively detect iron(II) and/or iron(III) species is of great importance to the understanding of the roles of this metal in biological processes. This has the potential to facilitate the identification of new and tailored therapeutic interventions for diseases characterized by iron-overload or, in general, altered physiological levels of iron. To date, a limited number of NIR probes for iron(III) have been developed, and there is only a single report on the application of iron(II)-sensitive NIR probes for in vivo imaging [94,95,96].
We present below a selection of Fe-targeted NIR probes that would hold promise for further development in the field and possible clinical translation, such as DCI-Fe(II) (19), CAM (20), LS1 (21), and DFOm-NPs (22) (Figure 14). However, other agents with iron sensing mechanisms not fully defined or difficult to be adopted in in vivo settings have also been reported [97,98,99,100,101], along with bioluminescent and supramolecular systems [102,103], as well as technologies based on 2D materials, quantum dots, and metal-organic frameworks [104,105,106].
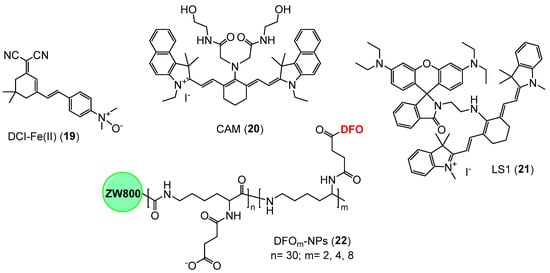
Figure 14.
Selected examples of NIR probes for the detection of iron(II) (i.e., 19) and iron(III) (i.e., 20–22) in in vivo systems.
In the context of iron(II)-targeted imaging, Zheng and collaborators developed the fluorescent probe DCI-Fe(II) (19) (Figure 14), which can sense the metal on the basis of N-oxide chemistry [107], being reported to be highly effective in the detection and imaging of iron(II) ions in real-time, both in cells and in vivo. In particular, this agent demonstrated a metal concentration-dependent NIR signal turn-on response (at 700 nm) coupled to a large Stokes shift (195 nm), along with a detection limit of 51 nM for iron(II). However, the selectivity of this probe for iron(II) remains to be established as indicated earlier in this review. Additionally, a far-red probe (i.e., 620 nm emission) was reported as an effective imaging probe for the detection of Fe(II) levels in mouse models of inflammation [101]. In a similar fashion, a caged D-aminoluciferin construct was recently developed as a bioluminescent reporter for visualizing iron pools in luciferase-expressing cells and mouse models, working through an iron-dependent uncaging mechanism, which is reported to enable sensitive and selective imaging of ferrous over ferric species [96,102].
With regard to iron(III) probes for in vivo imaging, Zhu et al. reported the synthesis of a cyanine-based NIR agent (20, CAM) (Figure 14) equipped with two N-(2-hydroxyethyl)acetamide groups to specifically chelate iron [108]. This agent functions as a “turn-off” probe, providing a decrease in fluorescence emission at 804 nm upon binding to iron(III) ions. Moreover, the probe demonstrated very high selectivity for iron(III) ions over a range of other metal ions, with a detection limit of approximately 8 μM, although its suitability for in vivo imaging would require further assessment in animal models. Li et al. introduced the iron-targeted NIR probe LS1 (21) (Figure 14), which utilises a cyanine-based fluorophore attached to the rhodamine B dye [109]. This probe is reported to produce a ratiometric response in detecting iron(III) ions, although it can also sense Cu(II) levels. Upon the addition of iron(III), the fluorescence intensity of 21 (Figure 14) at 777 nm decreases, while there is an increase in the intensity at 577 nm, which is due to the iron(III)-induced spirocyclic ring opening followed by the activation of a cyanine-mediated photoinduced electron transfer process. As with the NO-based probes, selectivity for iron needs to be carefully established before this agent (21) can be extensively used for in vivo imaging. In 2019, Kang and colleagues reported NIR probes coupled with deferoxamine (DFO) [110]. The design of these agents focuses on engineered bio-conjugation strategies to incorporate DFO into a backbone of ε-poly-L-lysine residues (DFOm-NPs, 22) (Figure 14), which in turn is attached to the zwitterionic NIR fluorophore ZW800-1C, in order to enable effective in vivo tracking. Analogues of such probes could have the potential for the detection and quantification of LIP in vivo.
These examples demonstrate the diversity and potential of NIR probes for the measurements of iron levels in vivo. The non-invasive nature and high sensitivity of these probes may offer valuable insights into the complexities of iron homeostasis and metabolism, as well as its implications in health and disease. As the field of bioimaging continues to advance, more elaborate and/or effective probes are likely to be developed.
7. Conclusions
This review provides information on the chemical nature of LIP and outlines the function of this important metabolic pool. The possible variation of LIP chemistry in the cytosol, mitochondria, lysosomes, and nucleus is discussed. The precise chemical structures of the major components need to be identified in biological matrices and this is likely to be achieved by ICP-MS techniques.
The potential of using iron-sensitive probes for the quantification of LIP is considered, and a range of applications of such probes is described. The quantification of LIP by the use of iron-specific fluorescent probes is currently under development, but it is now possible to monitor LIP levels in cytosolic, mitochondrial, and lysosomal pools of cells in suspension. Such measurements made under in vivo conditions will be more difficult, but NIR probes are under development for this purpose. Undoubtedly, the ability to visualize and quantify iron dynamics in living organisms is expected to have a significant impact on the development of targeted therapeutic interventions for iron-related pathological conditions.
Author Contributions
Formal analysis, R.C.H. and A.C.; investigation, R.C.H., C.P. and A.C.; writing—original draft preparation, R.C.H. and A.C.; writing—review and editing, R.C.H., C.P., Y.M. and A.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
R.C.H. and C.P. are both members of the Board of Directors of MINT Therapeutics Ltd. The views expressed in this article are the personal views of the authors and may not be understood or quoted as being made on behalf of or reflecting the position of the company or organisation with which the authors are affiliated. MINT Therapeutics Ltd. had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Maret, W.; Wedd, A. Binding, Transport and Storage of Metal Ions in Biological Cells; Royal Society of Chemistry: Cambridge, UK, 2014. [Google Scholar]
- Hider, R.C.; Kong, X. Iron speciation in the cytosol: An overview. Dalton Trans. 2013, 42, 3220–3229. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.J.P. Free manganese(II) and iron(II) cations can act as intracellular cell controls. FEBS Lett. 1982, 140, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Breuer, W.; Epsztejn, S.; Cabantchik, Z.I. Iron acquired from transferrin by K562 cells is delivered into a cytoplasmic pool of chelatable iron(II). J. Biol. Chem. 1995, 270, 24209–24215. [Google Scholar] [CrossRef]
- Petrat, F.; de Groot, H.; Sustmann, R.; Rauen, U. The chelatable iron pool in living cells: A methodically defined quantity. Biol. Chem. 2002, 383, 489–502. [Google Scholar] [CrossRef]
- Helm, L.; Merbach, A.E. Inorganic and bioinorganic solvent exchange mechanisms. Chem. Rev. 2005, 105, 1922–1959. [Google Scholar] [CrossRef]
- Koppenol, W.H. The centennial of the Fenton reaction. Free Rad. Biol. Med. 1993, 15, 645–651. [Google Scholar] [CrossRef] [PubMed]
- Ran, Y.; Moursy, M.; Hider, R.C.; Cilibrizzi, A. The colorimetric detection of the hydroxyl radical. Int. J. Mol. Sci. 2023, 24, 4162. [Google Scholar] [CrossRef]
- Kühn, L. Iron regulatory proteins and their role in controlling iron metabolism. Metallomics 2015, 7, 232–243. [Google Scholar] [CrossRef]
- Hentze, M.W.; Kühn, L.C. Molecular control of vertebrate iron metabolism: In RNA based circuits operated by iron, nitric oxide and oxidative stress. Proc. Natl. Acad. Sci. USA 1996, 93, 8175–8182. [Google Scholar] [CrossRef]
- Simpson, R.J.; Mckie, A.T. Iron and oxygen sensing: A tale of 2 interacting elements? Metallomics 2015, 7, 223–231. [Google Scholar] [CrossRef]
- Miller, J.P.G.; Perkins, D.J. Model experiments for the study of iron transfer from transferrin to ferritin. Eur. J. Biochem. 1969, 10, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Weaver, J.; Pollack, S. Low-Mr iron isolated from guinea pig reticulocytes as AMP-Fe and ATP-Fe complexes. Biochem. J. 1989, 261, 787–792. [Google Scholar] [CrossRef] [PubMed]
- Veiga, N.; Torres, J.; Mansell, D.; Freeman, S.; Dominguez, S.; Barker, C.J.; Diaz, A.; Kremer, C. “Chelatable iron pool”: Inositol 1,2,3-trisphosphate fulfils the conditions required to be a safe cellular iron ligand. J. Biol. Inorg. Chem. 2009, 14, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Morley, C.G.D.; Bezkorovainy, A. Identification of the iron chelate in hepatocyte cytosol. IRCS Med. Sci. 1983, 11, 1106–1107. [Google Scholar]
- Harris, D.C.; Aisen, P. Facilitation of Fe(II) autoxidation by Fe(III) complexing agents. Biochim. Biophys. Acta 1973, 329, 156–158. [Google Scholar] [CrossRef] [PubMed]
- Hider, R.C.; Kong, X.L. Glutathione: A key component of the cytoplasmic labile iron pool. Biometals 2011, 24, 1179–1187. [Google Scholar] [CrossRef]
- Kondo, T.; Dale, G.L.; Beutler, E. Thiol transport from human red blood cells. Methods Enzymol. 1995, 252, 72–82. [Google Scholar]
- Soboll, S.; Gründel, S.; Harris, J.; Kolb-Bachofen, V.; Ketterer, B.; Sies, H. The content of glutathione and glutathione S-transferases and the glutathione peroxidase activity in rat liver nuclei determined by a non-aqueous technique of cell fractionation. Biochem. J. 1995, 311, 889–894. [Google Scholar] [CrossRef]
- Hamed, M.Y.; Silver, J.; Wilson, M.T. Studies of the reactions of ferric iron with glutathione and some related thiols. Inorg. Chim. Acta 1983, 78, 1–11. [Google Scholar] [CrossRef]
- Fuhr, J.; Rabenstein, D. Nuclear magnetic resonance studies of the solution chemistry of metal complexes. IX binding of cadmium, zinc, lead, and mercury by glutathione. J. Am. Chem. Soc. 1973, 95, 6944–6948. [Google Scholar] [CrossRef]
- Ba, L.A.; Doering, M.; Burkholz, T.; Jacob, C. Metal trafficking: From maintaining the metal homeostasis to future drug design. Metallomics 2009, 1, 292–311. [Google Scholar] [CrossRef] [PubMed]
- Martin, H.B.; Edsall, J.T. The association of divalent cations with glutathione. J. Am. Chem. Soc. 1959, 81, 4044–4047. [Google Scholar] [CrossRef]
- Baran, E.J. Metal complexes of carnosine. Biochemistry 2000, 65, 789–797. [Google Scholar] [PubMed]
- Boakye, A.A.; Zhang, D.; Guo, L.; Zheng, Y.; Hoetker, D.; Zhao, J.; Posa, D.K.; Ng, C.K.; Zheng, H.; Kumar, A.; et al. Carnosine supplementation enhances post ischemic hind limb revascularization. Front. Physiol. 2019, 10, 751. [Google Scholar] [CrossRef]
- Brown, C.E.; Antholine, W.E. Chelation chemistry of carnosine. Evidence that mixed complexes may occur in vivo. J. Chem. Phys. 1979, 83, 3314–3319. [Google Scholar] [CrossRef]
- Shaw, G.C.; Cope, J.J.; Li, L.; Corson, K.; Hersey, C.; Ackermann, G.E.; Gwynn, B.; Lambert, A.J.; Wingert, R.A.; Traver, D.; et al. Mitoferrin is essential for erythroid iron assimilation. Nature 2006, 440, 96–100. [Google Scholar] [CrossRef]
- Paradkar, P.N.; Zumbrennen, K.B.; Paw, B.H.; Ward, D.M.; Kaplan, J. Regulation of mitochondrial iron import through differential turnover of mitoferrin 1 and mitoferrin 2. Mol. Cell Biol. 2009, 29, 1007–1016. [Google Scholar] [CrossRef]
- Atkinson, A.; Winge, D.R. Metal acquisition of availability in the mitochondria. Chem. Rev. 2009, 109, 4708–4721. [Google Scholar] [CrossRef]
- Chen, Z.; Lash, L.H. Evidence for mitochondrial uptake of glutathione by dicarboxylate and 2-oxoglutarate carriers. J. Pharmacol. Exp. Ther. 1998, 285, 608–618. [Google Scholar]
- Lash, L.H. Mitochondrial glutathione transport: Physiological, pathological and toxicological implications. Chem. Biol. Interact. 2006, 163, 54–67. [Google Scholar] [CrossRef]
- Bowman, E.J.; Ikuma, H.; Stein, H.J. Citric acid cycle activity in mitochondria isolated from mung bean hypocotyls. Plant Physiol. 1976, 58, 426–432. [Google Scholar] [CrossRef] [PubMed]
- Kauppinen, R.A.; Hiltunen, J.K.; Hassinen, I.E. Mitochondrial membrane potential, transmembrane difference in the NAD+ redox potential and the equilibrium of the glutamate-aspartate translocase in the isolated perfused rat heart. Biochim. Biophys. Acta 1982, 681, 286–291. [Google Scholar] [CrossRef] [PubMed]
- Go, Y.M.; Jones, D.P. Redox compartmentalization in eukaryotic cells. Biochim. Biophys. Acta 2008, 1780, 1273–1290. [Google Scholar] [CrossRef] [PubMed]
- Lillig, C.H.; Berndt, C.; Holmgren, A. Glutaredoxin systems. Biochim. Biophys. Acta 2008, 1780, 1304–1317. [Google Scholar] [CrossRef]
- Wells, W.W.; Yang, Y.; Deits, T.L.; Gran, Z.R. Thioltransferases. Adv. Enzymol. Relat. Areas Mol. Biol. 1993, 66, 149–201. [Google Scholar]
- Mesecke, N.; Mittler, S.; Eckers, E.; Herrmann, J.M.; Depante, M. Two novel monothiol glutaredoxins from Saccharomyces cerevisiae provide further insight into iron-sulfur cluster binding, oligomerization, and enzymatic activity of glutaredoxins. Biochemistry 2008, 47, 1452–1463. [Google Scholar] [CrossRef]
- Ehrensberger, K.M.; Bird, A.J. Hammering out details: Regulating metal levels in eukaryotes. Trends Biochem. Sci. 2011, 36, 524–531. [Google Scholar] [CrossRef]
- Lill, R.; Hoffmann, B.; Molik, S.; Pierik, A.J.; Rielzschel, N.; Stchling, O.; Uzarska, M.A.; Webert, H.; Wilbrecht, C.; Muhlenhoff, U. The role of mitochondria in cellular iron-sulfur protein biogenesis and iron metabolism. Biochim. Biophys. Acta 2012, 1823, 1491–1508. [Google Scholar] [CrossRef]
- Lill, R. Function and biogenesis of iron-sulphur proteins. Nature 2009, 460, 831–838. [Google Scholar] [CrossRef]
- Sipos, K.; Lange, H.; Fekete, Z.; Uilmann, P.; Lill, R.; Kispal, G. Maturation of cytosolic iron-sulfur proteins requires glutathione. J. Biol. Chem. 2002, 277, 26944–26949. [Google Scholar] [CrossRef]
- Sharma, A.K.; Pallesen, L.J.; Spang, R.J.; Walden, W.E. Cytosolic iron-sulfur cluster assembly (CIA) system: Factors, Mechanism, and relevance to cellular iorn regulation. J. Biol. Chem. 2010, 285, 26745–26751. [Google Scholar] [CrossRef] [PubMed]
- Mühlenhoff, U.; Molik, S.; Godoy, J.R.; Uzarska, M.A.; Richter, N.; Seubert, A.; Zhang, Y.; Stubbe, J.; Pierrel, F.; Herrers, E.; et al. Cytosolic monothiol glutaredoxins function in intracellular iron sensing and trafficking via their bound iron-sulfur cluster. Cell Metab. 2010, 12, 373–385. [Google Scholar] [CrossRef] [PubMed]
- Ye, H.; Rouault, T.A. Human iron-sulfur cluster assembly, cellular iron homeostasis, and disease. Biochemistry 2010, 49, 4945–4956. [Google Scholar] [CrossRef] [PubMed]
- Qi, W.; Li, J.; Chain, C.Y.; Pasquevich, G.A.; Pasquevich, A.F.; Cowan, J.A. Glutathione-complexes iron-sulfur clusters. Reaction intermediates and evidence for a template effect promoting assembly and stability. Chem. Commum. 2013, 49, 6313–6315. [Google Scholar] [CrossRef][Green Version]
- Kürz, T.; Gustafsson, B.; Brunk, U.T. Intralysosomal iron chelation protects against oxidative stress-induced cellular damage. FEBS J. 2006, 273, 3106–3117. [Google Scholar] [CrossRef]
- Babitt, J.L.; Huang, F.W.; Xia, Y.; Sidis, Y.; Andrews, N.C.; Lin, H.Y. Modulation of bone morphogenic protein signaling in vivo regulates systemic iron balance. J. Clin. Investig. 2007, 117, 1933–1939. [Google Scholar] [CrossRef]
- Yu, P.B.; Hong, C.C.; Sachidanandan, C.; Babitt, J.L.; Deng, D.Y.; Hoyng, S.A.; Lin, H.Y.; Bloch, K.D.; Peterson, R.T. Dorsomorphin inhibits BMP signals required for embryogenesis and iron metabolism. Nat. Chem. Biol. 2008, 4, 33–41. [Google Scholar] [CrossRef]
- Puig, S.; Ramos-Alonso, L.; Romero, A.M.; Martinez-Pastor, M.T. The elemental role of iron in DNA synthesis and repair. Metallomics 2017, 9, 1483–1500. [Google Scholar] [CrossRef]
- Macara, I.G. Transport into and out of the nucleus. Microbiol. Mol. Biol. Rev. 2001, 65, 570–594. [Google Scholar] [CrossRef]
- Yasuhura, N.; Takeda, E.; Inoue, H.; Kotera, I.; Yoneda, Y. Importin alpha/beta-mediated nuclear protein import is regulated in a cell cycle-dependent manner. Exp. Cell Res. 2004, 297, 285–293. [Google Scholar] [CrossRef]
- O’Keeffe, R.; Latunde-Dada, G.O.; Chen, Y.-L.; Kong, X.L.; Cilibrizzi, A.; Hider, R.C. Glutathione and the intracellular labile heme pool. Biometals 2021, 34, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Abbate, V.; Hider, R.C. Iron-sensitive fluorescent probes: Monitoring intracellular iron pools. Metallomics 2015, 7, 212–222. [Google Scholar] [CrossRef] [PubMed]
- Cilibrizzi, A.; Abbate, V.; Chen, Y.-L.; Ma, Y.; Zhou, T.; Hider, R.C. Hydroxypyridinone journey into metal chelation. Chem. Rev. 2018, 118, 7657–7701. [Google Scholar] [CrossRef] [PubMed]
- Maret, W. Analysing free zinc(II) ion concentrations in cell biology with fluorescent chelating molecules. Metallomics 2015, 7, 202–211. [Google Scholar] [CrossRef]
- Zander, M. Fluorimetrie; Springer: Berlin/Heidelberg, Germany, 1981. [Google Scholar]
- Alhawsah, B.; Yan, B.; Aydin, Z.; Niu, X.; Guo, M. Highly selective fluorescent probe with an ideal pH profile for the rapid and unambiguous determination of subcellular labile iron (III) pools in human cells. Anal. Lett. 2022, 55, 1954–1970. [Google Scholar] [CrossRef]
- Tenopoulou, M.; Kurz, T.; Doulias, P.T.; Galaris, D.; Brunk, U.T. Does the calcein-AM method assay the total cellular ‘labile iron pool’ or only a fraction of it? Biochem. J. 2007, 403, 261–266. [Google Scholar] [CrossRef]
- Hirayama, T.; Okuda, K.; Nagasawa, H. A highly selective turn-on fluorescent probe for iron(II) to visualize labile iron in living cells. Chem. Sci. 2013, 4, 1250–1256. [Google Scholar] [CrossRef]
- Hirayama, T.; Tsuboi, H.; Niwa, M.; Miki, A.; Kadota, S.; Ikeshita, Y.; Okua, K.; Nagasawa, H. A universal fluorogenic switch for Fe(II) ion based on N-oxide chemistry permits the visualization of intracellular redox equilibrium shift towards labile iron in hypoxic tumor cells. Chem. Sci. 2017, 8, 4858–4866. [Google Scholar] [CrossRef]
- Carter, K.P.; Young, A.M.; Palmer, A.E. Fluorescent sensors for measuring metal ions in living systems. Chem. Rev. 2014, 114, 4564–4601. [Google Scholar] [CrossRef]
- Hirayama, T.; Nagasawa, H. Chemical tools for detecting Fe ions. J. Clin. Biochem. Nutr. 2017, 60, 39–48. [Google Scholar] [CrossRef]
- Cabantchik, I.; Glickstein, H.; Milgram, P.; Breuer, W. A fluorescence assay for assessing chelation of intracellular iron in a membrane model system and in mammalian cells. Anal. Biochem. 1996, 233, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Glickstein, H.; El, R.B.; Shvartsman, M.; Cabantchik, I. Intracellular labile iron pools as direct targets of iron chelators: A fluorescence study of chelator action in living cells. Blood 2005, 106, 3242–3250. [Google Scholar] [CrossRef] [PubMed]
- Epsztejn, S.; Kakhlon, O.; Glickstein, H.; Breuer, W.; Cabantchik, Z.I. Fluorescence analysis of the labile iron pool of mammalian cells. Anal. Biochem. 1997, 248, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Thomas, F.; Serratrice, G.; Béguin, C.; Aman, E.S.; Pierre, J.L.; Fontecave, M.; Laulhère, J.P. Calcein as a fluorescent probe for ferric iron. Application to iron nutrition in plant cells. J. Biol. Chem. 1999, 274, 13375–13383. [Google Scholar] [CrossRef] [PubMed]
- Zanninelli, G.; Glickstein, H.; Breuer, W.; Milgram, P.; Brissot, P.; Hider, R.C.; Konijn, A.M.; Lipman, J.; Shanzer, A.; Cabantchik, Z.I. Chelation and Mobilization of Cellular Iron by Different Classes of Chelators. Mol. Pharmacol. 1997, 51, 842–852. [Google Scholar] [CrossRef]
- Basu-Modak, S.; Ali, D.; Gordon, M.; Polte, T.; Yiakouvaki, A.; Pourzand, C.; Rice-Evans, C.; Tyrrell, R.M. Suppression of UVA-mediated release of labile iron by epicatechin—A link to lysosomal protection. Free Radical Biol. Med. 2006, 41, 1197–1204. [Google Scholar] [CrossRef] [PubMed]
- Clark, M.; Fisher, N.C.; Kasthuri, R.; Hand, C.C. Parasite maturation and host serum iron influence the labile iron pool of erythrocyte stage Plasmodium falciparum. Br. J. Haematol. 2013, 161, 262–269. [Google Scholar] [CrossRef]
- Petrat, F.; Rauen, U.; de Groot, H. Determination of the chelatable iron pool of isolated rat hepatocytes by digital fluorescence microscopy using the fluorescent probe, phen green SK. Hepatology 1999, 29, 1171–1179. [Google Scholar] [CrossRef]
- Petrat, F.; de Groot, H.; Rauen, U. Determination of the chelatable iron pool of single intact cells by laser scanning microscopy. Arch. Biochem. Biophys. 2000, 376, 74–81. [Google Scholar] [CrossRef]
- Ma, Y.M.; de Groot, H.; Liu, Z.; Hider, R.C.; Petrat, F. Chelation and determination of labile iron in primary hepatocytes by pyridinone fluorescent probes. Biochem. J. 2006, 395, 49–55. [Google Scholar] [CrossRef]
- Hider, R.C.; Ma, Y.; Reelfs, O.; Cilibrizzi, A.; Pourzand, C. A Novel Fluorescent Probe with High Selectivity and Sensitivity for Quantitation and Imaging of Cytosolic Labile Iron Pool. Abstract in European Iron Club Conference. 2022, p. 0101. Available online: https://researchportal.bath.ac.uk/en/publications/a-novel-fluorescent-probe-with-high-selectivity-and-sensitivity-f (accessed on 16 July 2022).
- Aron, A.T.; Loehr, M.O.; Bogena, J.; Chang, C.J. An endoperoxide reactivity-based FRET probe for ratiometric fluorescence imaging of labile iron pools in living cells. J. Am. Chem. Soc. 2016, 138, 14338–14346. [Google Scholar] [CrossRef]
- Spangler, B.; Morgan, C.W.; Fontaine, S.D.; Vander Wal, M.N.; Chang, C.J.; Wells, J.A.; Renslo, A.R. A reactivity-based probe of the intracellular labile ferrous iron pool. Nat. Chem. Biol. 2016, 12, 680–685. [Google Scholar] [CrossRef]
- Monfort, B.; Want, K.; Gervason, S.; D’Autréaux, B. Recent advances in the elucidation of Frataxin biochemical function open novel perspectives for the Treatment of Friedreich’s Ataxia. Front. Neurosci. 2022, 16, 838335. [Google Scholar] [CrossRef]
- Petrat, F.; Weisheit, D.; Lensen, M.; de Groot, H.; Sustmann, R.; Rauen, U. Selective determination of mitochondrial chelatable iron in viable cells with a new fluorescent sensor. Biochem. J. 2002, 362, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Shvartsman, M.; Fibach, E.; Cabantchik, Z.I. Transferrin-iron routing to the cytosol and mitochondria as studied by live and real-time fluorescence. Biochem. J. 2010, 429, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Cabantchik, Z.I. Labile iron in cells and body fluids: Physiology, pathology, and pharmacology. Front. Pharmacol. 2014, 5, 45. [Google Scholar] [CrossRef] [PubMed]
- Cilibrizzi, A.; Pourzand, C.; Abbate, V.; Reelfs, O.; Versari, L.; Floresta, G.; Hider, R. The synthesis and properties of mitochondrial targeted iron chelators. Biometals 2023, 36, 321–337. [Google Scholar] [CrossRef] [PubMed]
- Reelfs, O.; Abbate, V.; Cilibrizzi, A.; Pook, M.A.; Hider, R.C.; Pourzand, C. The role of mitochondrial labile iron in Friedreich’s ataxia skin fibroblasts sensitivity to ultraviolet A. Metallomics 2019, 11, 656–665. [Google Scholar] [CrossRef]
- Rouault, T.A. Mitochondrial iron overload: Causes and consequences. Curr. Opin. Genet. Dev. 2016, 38, 31–37. [Google Scholar] [CrossRef]
- Hirayama, T.; Kadota, S.; Niwa, M.; Nagasawa, H. A mitochondria-targeted fluorescent probe for selective detection of mitochondrial labile Fe(II). Metallomics 2018, 10, 794–801. [Google Scholar] [CrossRef]
- Kholmukhamedov, A.; Li, L.; Lindsey, C.C.; Hu, J.; Nieminen, A.-L.; Takemoto, K.; Beeson, G.C.; Beneker, C.M.; McInnes, C.; Beeson, C.C.; et al. A new fluorescent sensor mitoferrofluor indicates the presence of chelatable iron in polarized and depolarized mitochondria. J. Biol. Chem. 2022, 298, 102336. [Google Scholar] [CrossRef] [PubMed]
- Kurz, T.; Terman, A.; Gustafsson, B.; Brunk, U.T. Lysosomes and oxidative stress in aging and apoptosis. Biochim. Biophys. Acta 2008, 1780, 1291–1303. [Google Scholar] [CrossRef] [PubMed]
- Kidane, T.Z.; Sauble, E.; Linder, M.C. Release of iron from ferritin requires lysosomal activity. Am. J. Physiol. Cell Physiol. 2006, 291, C445–C455. [Google Scholar] [CrossRef] [PubMed]
- .Kurz, T.; Terman, A.; Gustafsson, B.; Brunk, U.T. Lysosomes in iron metabolism, ageing and apoptosis. Histochem. Cell. Biol. 2008, 129, 389–406. [Google Scholar] [CrossRef] [PubMed]
- Petrat, F.; de Groot, H.; Rauen, U. Subcellular distribution of chelatable iron: A laser scanning microscopic study in isolated hepatocytes and liver endothelial cells. Biochem. J. 2001, 356, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Fakih, S.; Podinovskaia, M.; Kong, X.; Collins, H.L.; Schaible, U.E.; Hider, R.C. Targeting the Lysosome: Fluorescent Iron(III) Chelators To Selectively Monitor Endosomal/Lysosomal Labile Iron Pools. J. Med. Chem. 2008, 51, 4539–4552. [Google Scholar] [CrossRef]
- Fakih, S.; Podinovskaia, M.; Kong, X.; Schaible, U.E.; Collins, H.L.; Hider, R.C. Monitoring intracellular labile iron pools: A novel fluorescent iron(III) sensor as a potential non-invasive diagnosis tool. J. Pharm. Sci. 2009, 98, 2212–2226. [Google Scholar] [CrossRef]
- Hirayama, T.; Miki, A.; Nagasawa, H. Organelle-specific analysis of labile Fe(II) during ferroptosis by using a cocktail of various colour organelle-targeted fluorescent probes. Metallomics 2019, 11, 111–117. [Google Scholar] [CrossRef]
- Sun, Y.; Sun, P.; Guo, W. Fluorescent probes for iron, heme, and related enzymes. Coord. Chem. Rev. 2021, 429, 213645. [Google Scholar] [CrossRef]
- Lu, M.; Wang, Y.; Li, Y.; Li, Z.; Xu, S.; Yao, C. A colorimetric and fluorescent chemosensor of Fe3+ based on an asymmetrical squarylium dye. J. Appl. Spectrosc. 2018, 85, 341–348. [Google Scholar] [CrossRef]
- Wu, D.; Chen, L.; Lee, W.; Ko, G.; Yin, J.; Yoon, J. Recent progress in the development of organic dye based near-infrared fluorescence probes for metal ions. Coord. Chem. Rev. 2018, 354, 74–97. [Google Scholar] [CrossRef]
- Zheng, X.; Cheng, W.; Ji, C.; Zhang, J.; Yin, M. Detection of metal ions in biological systems: A review. Rev. Anal. Chem. 2020, 39, 231–246. [Google Scholar] [CrossRef]
- Hirayama, T. Fluorescent probes for the detection of catalytic Fe(II) ion. Free Rad. Biol. Med. 2019, 133, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Cong, T.; Liang, Q.; Li, Z.; Xu, S. Dual colorimetric and fluorescent chemosensor of Fe3+ and Cu2+ based on 2,5-bis[(4-carboxylic-piperidylamino)thiophenyl] -croconine. Tetrahedron 2015, 71, 5478–5483. [Google Scholar] [CrossRef]
- Qu, X.; Liu, Q.; Ji, X.; Chen, H.; Zhou, Z.; Shen, Z. Enhancing the Stokes’ shift of BODIPY dyes via through-bond energy transfer and its application for Fe3+-detection in live cell imaging. Chem. Commun. 2012, 48, 4600–4602. [Google Scholar] [CrossRef]
- Shen, B.-X.; Qian, Y. A novel triphenylamine-BODIPY dendron: Click synthesis, near-infrared emission and a multi-channel chemodosimeter for Hg2+ and Fe3+. J. Mater. Chem. B 2016, 4, 7549–7559. [Google Scholar] [CrossRef] [PubMed]
- Vijay, N.; Wu, S.P.; Velmathi, S. Turn on fluorescent chemosensor containing rhodamine B fluorophore for selective sensing and in vivo fluorescent imaging of Fe3+ ions in HeLa cell line and zebrafish. J. Photochem. Photobiol. A Chem. 2019, 384, 112060. [Google Scholar] [CrossRef]
- Dong, B.; Song, W.; Lu, Y.; Tian, M.; Kong, X.; Mehmood, A.H.; Lin, W. Live cell-specific fluorescent probe for the detection of labile Fe(II) and the evaluation of esterase activity in live animals. Sens. Actuators B Chem. 2020, 305, 127470. [Google Scholar] [CrossRef]
- Aron, A.T.; Heffern, M.C.; Lonergan, Z.R.; Vander Wal, M.N.; Blank, B.R.; Spangler, B.; Zhangd, Y.; Park, H.M.; Stahl, A.; Renslo, A.R.; et al. In vivo bioluminescence imaging of labile iron accumulation in a murine model of Acinetobacter baumannii infection. Proc. Natl. Acad. Sci. USA 2017, 114, 12669–12674. [Google Scholar] [CrossRef]
- Yao, Q.F.; Lü, B.Z.; Ji, C.D.; Cai, Y.; Yin, M.Z. Supramolecular host-guest system as ratiometric Fe3+ ion sensor based on water-soluble pillar[5]arene. ACS Appl. Mater. Interfaces 2017, 9, 36320–36326. [Google Scholar] [CrossRef]
- Wang, M.; Guo, L.; Cao, D. Porous organic polymer nanotubes as luminescent probe for highly selective and sensitive detection of Fe3+. Sci China Chem. 2017, 60, 1090–1097. [Google Scholar] [CrossRef]
- Zhang, Q.; Sun, Y.; Liu, M.L.; Liu, Y. Selective detection of Fe3+ ions based on fluorescence MXene quantum dots via a mechanism integrating electron transfer and inner filter effect. Nanoscale 2020, 12, 1826–1832. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Yue, D.; Huang, Y.; Zhang, J.; Wang, Z.; Yang, D.; Qian, G. Photo-induced electron transfer in a metal–organic framework: A new approach towards a highly sensitive luminescent probe for Fe3+. Chem. Commun. 2019, 55, 11231. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.R.; Feng, S.M.; Gong, S.Y.; Xia, Q.F.; Feng, G.Q. In vivo imaging of Fe2+ using an easily obtained probe with a large stokes shift and bright strong lipid droplet-targetable near-infrared fluorescence. Sens. Actuators B Chem. 2020, 309, 127796. [Google Scholar] [CrossRef]
- Zhu, M.; Shi, C.; Xu, X.; Guo, Z.; Zhu, W. Near-infrared cyanine-based sensor for Fe3+ with high sensitivity: Its intracellular imaging application in colorectal cancer cells. RSC Adv. 2016, 6, 100759–100764. [Google Scholar] [CrossRef]
- Li, S.; Zhang, D.; Xie, X.; Ma, S.; Liu, Y.; Xu, Z.; Gao, Y.; Ye, Y. A novel solvent-dependently bifunctional NIR absorptive and fluorescent ratiometric probe for detecting Fe3+/Cu2+ and its application in bioimaging. Sens. Actuators B Chem. 2016, 224, 661–667. [Google Scholar] [CrossRef]
- Kang, H.; Han, M.; Xue, J.; Baek, Y.; Chang, J.O.; Hu, S.; Nam, H.Y.; Jo, M.J.; El Fakhri, G.; Hutchens, M.P.; et al. Renal clearable nanochelators for iron overload therapy. Nat. Commun. 2019, 10, 5134. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).