Cadmium Toxicity and Health Effects—A Brief Summary
Abstract
:1. Introduction
2. Sources of Cadmium
3. Exposure and Accumulation of Cadmium in the Human Body—Pathological Effects
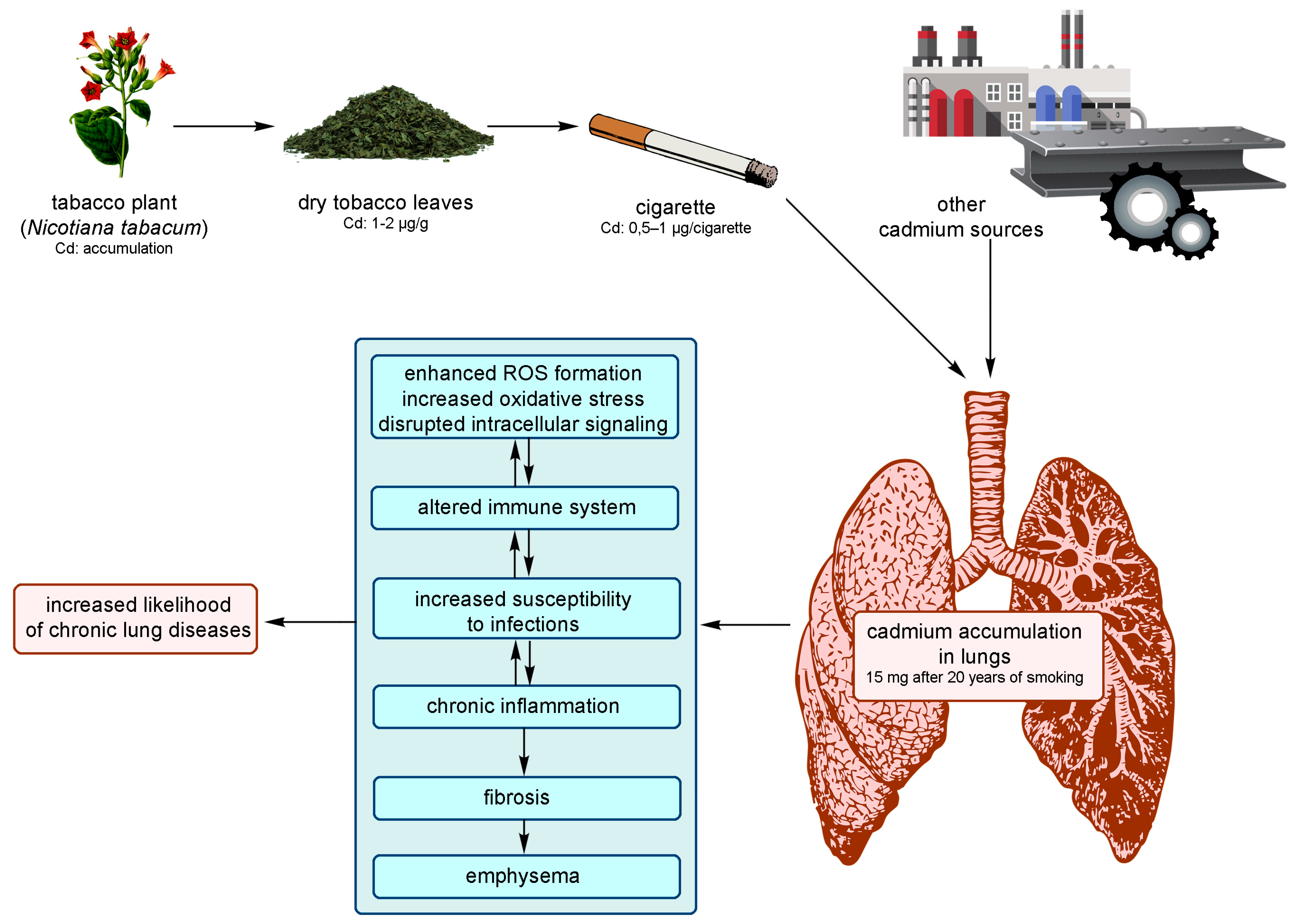
3.1. Pathological Effects in the Respiratory System
3.2. Pathological Effects in the Nephrological System
- –
- Stage 1: Cadmium accumulates in the renal cortex by binding to MT. The concentration of Cd excreted in the urine is proportional to the content of this element in the kidneys; its concentration in the urine reflects past exposure.
- –
- Stage 2: With high exposure and depletion of MT stores in the kidneys, the urinary cadmium concentration reflects both current and past exposure.
- –
- Stage 3: When the renal tubules are damaged, the kidneys lose the ability to reabsorb cadmium, which results in significant urinary excretion of this element; this reflects ongoing exposure and its elimination from the kidneys.
3.3. Pathological Effects in the Circulatory System
3.4. Pathological Effects in the Skeletal System
3.5. Pathological Effects in the Reproductive System
3.6. Pathological Effects in the Nervous System
3.7. Cell Cycle
3.8. Carcinogenic Effect of Cadmium
4. Environmentally Degrading Effects of Cadmium
- –
- transformation of heavy metals into forms inaccessible to plants (soil liming; increasing the amount of organic matter; vaping with a combination of organic matter);
- –
- partial removal of heavy metals from the soil (phytoremediation; introduction of natural and synthetic adsorbents into the soil; cultivation of industrial crops; extraction and flushing of the soil; removal of contaminated soil);
- –

5. Accumulation of Cadmium in Tobacco Smokers
6. Accumulation of Cadmium in Food
7. Prevention and Monitoring of Cadmium Poisoning
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- John, E. The Elements, 2nd ed.; Clarendon Press: Oxford, UK, 1991. [Google Scholar]
- Qing, Y.; Yang, J.; Zhu, Y.; Li, Y.; Zheng, W.; Wu, M.; He, G. Dose-response evaluation of urinary cadmium and kidney injury biomarkers in Chinese residents and dietary limit standards. Environ. Health 2021, 20, 75. [Google Scholar] [CrossRef] [PubMed]
- Zając-Nędza, M.; Langauer-Lewowicka, H. Kadm. Mark Kazimierz (Pod Red.): Choroby Zawodowe; Wydawnictwo Lekarskie PZWL: Warszawa, Poland, 2001; pp. 195–199. [Google Scholar]
- Balali-Mood, M.; Naseri, K.; Tahergorabi, Z.; Khazdair, M.R.; Sadeghi, M. Toxic Mechanisms of Five Heavy Metals: Mercury, Lead, Chromium, Cadmium, and Arsenic. Front. Pharmacol. 2021, 12, 643972. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, H.R.; Dennis, S.; Fitzpatrick, S. Cadmium: Mitigation strategies to reduce dietary exposure. J. Food Sci. 2020, 85, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Ruczaj, A.; Brzóska, M.M. Environmental exposure of the general population to cadmium as a risk factor of the damage to the nervous system: A critical review of current data. J. Appl. Toxicol. 2023, 43, 66–88. [Google Scholar] [CrossRef] [PubMed]
- Järup, L. Hazards of heavy metal contamination. Br. Med. Bull. 2003, 68, 167–182. [Google Scholar] [CrossRef]
- Järup, L.; Akesson, A. Current status of cadmium as an environmental health problem. Toxicol. Appl. Pharmacol. 2009, 238, 201–208. [Google Scholar] [CrossRef]
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Heavy metal toxicity and the environment. Mol. Clin. Environ. Toxicol. 2012, 101, 133–164. [Google Scholar]
- Sienko Michell, J.; Plane Robert, A. Chemistry Principles and Applications, 3rd ed.; McGraw-Hill: New York, NY, USA, 1980. [Google Scholar]
- Charkiewicz, A.E.; Omeljaniuk, W.J.; Orywal, K.; Czygier, M.; Szmitkowski, M.; Mroczko, B.; Maslach, D.; Szpak, A. Concentration of selected elements and antioxidative potential in a group of males working in the metal industry. Am. J. Men. Health 2019, 13, 1557988319851954. [Google Scholar] [CrossRef]
- Ganguly, K.; Levänen, B.; Palmberg, L.; Åkesson, A.; Lindén, A. Cadmium in tobacco smokers: A neglected link to lung disease? Eur. Respir. Rev. 2018, 27, 170122. [Google Scholar] [CrossRef]
- World Health Organization. WHO Estimates of the Global Burden of Foodborne Diseases: Foodborne Disease Burden Epidemiology Reference Group 2007–2015; World Health Organization: Geneva, Switzerland, 2015. [Google Scholar]
- Kayiranga, A.; Li, Z.; Isabwe, A.; Ke, X.; Simbi, C.H.; Ifon, B.E.; Yao, H.; Wang, B.; Sun, X. The Effects of Heavy Metal Pollution on Collembola in Urban Soils and Associated Recovery Using Biochar Remediation: A Review. Int. J. Environ. Res. Public Health 2023, 20, 3077. [Google Scholar] [CrossRef]
- Mannino, D.M.; Holguin, F.; Greves, H.M.; Savage-Brown, A.; Stock, A.L.; Jones, R.L. Urinary cadmium levels predict lower lung function in current and former smokers: Data from the Third National Health and Nutrition Examination Survey. Thorax 2004, 59, 194–198. [Google Scholar] [CrossRef] [PubMed]
- Handal, A.J.; Harlow, S.D. Employment in the Ecuadorian cut-flower industry and the risk of spontaneous abortion. BMC Int. Health Hum. Rights 2009, 9, 25. [Google Scholar] [CrossRef] [PubMed]
- Genchi, G.; Sinicropi, M.S.; Lauria, G.; Carocci, A.; Catalano, A. The Effects of Cadmium Toxicity. Int. J. Environ. Res. Public Health 2020, 17, 3782. [Google Scholar] [CrossRef] [PubMed]
- Omeljaniuk, W.; Socha, K.; Soroczynska, J.; Charkiewicz, A.E.; Laudanski, T.; Kulikowski, M.; Kobylec, E.; Szpak, A.; Borawska, M.H. Cadmium and lead in women who miscarried. Clin. Lab. 2018, 64, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Charkiewicz, A.E.; Jamiołkowski, J.; Pędzinski, B.; Krzyzak, M.; Maslach, D.; Szpak, A.; Omeljaniuk, W.J. Changes in dietary patterns and the nutritional status in men in the metallurgical industry in Poland over a 21-year period. Ann. Nutr. Metab. 2018, 72, 161–171. [Google Scholar] [CrossRef]
- Wuana, R.A.; Okieimen, F.E. Heavy Metals in Contaminated Soils: A Review of Sources, Chemistry, Risks and Best Available Strategies for Remediation. ISRN Ecol. 2011, 2011, 1–20. [Google Scholar] [CrossRef]
- Nejabat, M.; Kahe, H.; Shirani, K.; Ghorbannejad, P.; Hadizadeh, F.; Karimi, G. Health risk assessment of heavy metals via dietary intake of wheat in Golestan Province, Iran. Hum. Ecol. Risk Assess. 2017, 23, 1193–1201. [Google Scholar] [CrossRef]
- Lech, T.; Sadlik, J.K. Cadmium concentration in human autopsy tissues. Biol. Trace Elem. Res. 2017, 179, 172–177. [Google Scholar] [CrossRef]
- Nawrot, T.S.; Martens, D.S.; Hara, A.; Plusquin, M.; Vangronsveld, J.; Roels, H.A.; Staessen, J.A. Association of total cancer and lung cancer with environmental exposure to cadmium: The meta-analytical evidence. Cancer Causes Control 2015, 26, 1281–1288. [Google Scholar] [CrossRef]
- Julin, B.; Wolk, A.; Johansson, E.; Andersson, S.O.; Andrén, O.; Akesson, A. Dietary cadmium exposure and prostate cancer incidence: A population-based prospective cohort study. Br. J. Cancer 2012, 107, 895–900. [Google Scholar] [CrossRef]
- Wang, B.; Du, Y. Cadmium and its neurotoxic effects. Oxid. Med. Cell. Longev. 2013, 2013, 898034. [Google Scholar] [CrossRef] [PubMed]
- Syeda, T.; Cannon, J.R. Environmental exposures and the etiopathogenesis of Alzheimer’s disease: The potential role of BACE1 as a critical neurotoxic target. J. Biochem. Mol. Toxicol. 2021, 35, e22694. [Google Scholar] [CrossRef] [PubMed]
- Scherer, G.; Barkemeyer, H. Cadmium concentrations in tobacco and tobacco smoke. Ecotoxicol. Environ. Saf. 1983, 7, 71–78. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer (IARC) Monographs—Cadmium; IARC: Lyon, France, 1993.
- Paschal, D.C.; Burt, V.; Caudill, S.P.; Gunter, E.W.; Pirkle, J.L.; Sampson, E.J.; Miller, D.T.; Jackson, R.J. Exposure of the U.S. population aged 6 years and older to cadmium: 1988–1994. Arch. Environ. Contam. Toxicol. 2000, 38, 377–383. [Google Scholar] [CrossRef]
- Nordberg, M.; Nordberg, G.F. Metallothionein and Cadmium Toxicology—Historical Review and Commentary. Biomolecules 2022, 12, 360. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Fagerberg, B.; Sallsten, G.; Borné, Y.; Hedblad, B.; Engström, G.; Barregard, L.; Andersson, E.M. Smoking-induced risk of future cardiovascular disease is partly mediated by cadmium in tobacco: Malmö Diet and Cancer Cohort Study. Environ. Health 2019, 18, 56. [Google Scholar] [CrossRef] [PubMed]
- Fagerberg, B.; Barregard, L.; Sallsten, G.; Forsgard, N.; Ostling, G.; Persson, M.; Borné, Y.; Engström, G.; Hedblad, B. Cadmium exposureand atherosclerotic carotid plaques-results from the Malmodiet and Cancer study. Environ. Res. 2015, 136, 67–74. [Google Scholar] [CrossRef]
- Czeczot, H.; Majewska, M. Cadmium–exposure and its effects on health. Toksykologia 2010, 66, 243–250. [Google Scholar]
- Kasperczyk, A.; Ostałowska, A.; Grucka-Mamczar, E.; Birkner, E. A comparison of cadmium, zinc and selenium concentrations in human blood and seminal plasma. Bromat. Chem. Toksykol. XLI 2008, 1, 81–87. [Google Scholar]
- Marlowe, M.; Cossairt, A.; Moon, C. Main and interaction effects of metallic toxins on classroom behavior. J. Abnorm. Child Psychol. 1985, 13, 185–198. [Google Scholar] [CrossRef]
- Hart, R.P.; Rose, C.S.; Hamer, R.M. Neuropsychological effects of occupational exposure to cadmium. J. Clin. Exp. Neuropsychol. 1989, 11, 933–943. [Google Scholar] [CrossRef] [PubMed]
- Rani, A.; Kumar, A.; Lal, A.; Pant, M. Cellular mechanisms of cadmium-induced toxicity: A review. Int. Environ. Health Res. 2014, 24, 378–399. [Google Scholar] [CrossRef] [PubMed]
- Bertin, G.; Averbeck, D. Cadmium: Cellular effects, modifications of biomolecules, modulation of DNA repair and genotoxic consequences (a review). Biochimie 2006, 88, 1549–1559. [Google Scholar] [CrossRef]
- Peng, L.; Huang, Y.; Zhang, J.; Peng, Y.; Lin, X.; Wu, K.; Huo, X. Cadmium exposure and the risk of Brest cancer in Chaoshan population of southeast China. Environ. Sci. Pollut. Res. Int. 2015, 22, 19870–19878. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Wang, X.; Huo, X.; Xu, X.; Lin, K.; Zhang, J.; Huang, Y.; Wu, K. Blood cadmium burden and the risk of nasopharyngeal carcinoma: A case-control study in Chinese Chaoshan population. Environ. Sci. Pollut. Res. Int. 2015, 22, 12323–12331. [Google Scholar] [CrossRef]
- Feki-Tounsi, M.; Hamza-Chaffai, A. Cadmium as a possible cause of bladder cancer: A review of accumulated evidence. Environ. Sci. Pollut. Res. 2014, 18, 10561–10573. [Google Scholar] [CrossRef]
- Chen, C.; Xun, P.; Nishio, M.; Sekikawa, A.; He, K. Cadmium exposure and risk of pancreatic cancer: A meta-analysis of prospective cohort studies and case-control studies among individuals without occupational exposure history. Environ. Sci. Pollut. Res. 2015, 22, 17465–17474. [Google Scholar] [CrossRef]
- Song, J.K.; Luo, H.; Yin, X.H.; Huang, G.L.; Luo, S.Y.; Lin, D.R.; Yuan, D.B.; Zhang, W.; Zhu, J.G. Association between cadmium exposure and renal cancer risk: A meta-analysis of observational studies. Sci. Rep. 2015, 5, 17976. [Google Scholar] [CrossRef]
- Tarhonska, K.; Lesicka, M.; Janasik, B.; Roszak, J.; Reszka, E.; Braun, M.; Kołacińska-Wow, A.; Jabłońska, E. Cadmium and breast cancer-Current state and research gaps in the underlying mechanisms. Toxicol. Lett. 2022, 361, 29–42. [Google Scholar] [CrossRef]
- Lin, H.C.; Hao, W.M.; Chu, P.H. Cadmium and cardiovascular disease: An overview of pathophysiology, epidemiology, therapy, and predictive value. Rev. Port. Cardiol. 2021, 40, 611–617. [Google Scholar] [CrossRef] [PubMed]
- Bernhard, D.; Rossmann, A.; Henderson, B.; Kind, M.; Seubert, A.; Wick, G. Increased serumcadmium and strontium levels in young smokers: Effects onarterial endothelial cell gene transcription. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 833–838. [Google Scholar] [CrossRef] [PubMed]
- Neumeister, B.; Böhm, B.O. Klinikleitfaden Labordiagnostik; Urban & Fischer Verlag/Elsevier GmbH: Munich, Germany, 2015. [Google Scholar]
- Borné, Y.; Fagerberg, B.; Persson, M.; Östling, G.; Söderholm, M.; Hedblad, B.; Sallsten, G.; Barregard, L.; Engström, G. Cadmiumcarotid atherosclerosis, and incidence of ischemicstroke. J. Am. Heart Assoc. 2017, 6, e006415. [Google Scholar] [CrossRef] [PubMed]
- Charkiewicz, A.E.; Garley, M.; Ratajczak-Wrona, W.; Nowak, K.; Jabłońska, E.; Maślach, D.; Omeljaniuk, W.J. Profile of new vascular damage biomarkers in middle-aged men with arterial hypertension. Adv. Med. Sci. 2021, 66, 185–191. [Google Scholar] [CrossRef]
- Charkiewicz, A.E.; Garley, M.; Ratajczak-Wrona, W.; Jabłońska, E.; Miltyk, W.; Motyka, J.; Omeljaniuk, W.J. The diagnostic potential of novel biomarkers of hypertension in men. Arch. Med. Sci. 2022, 18, 1666–1671. [Google Scholar] [CrossRef] [PubMed]
- Akesson, A.; Bjellerup, P.; Lundh, T.; Lidfeldt, J.; Nerbrand, C.; Samsioe, G.; Skerfving, S.; Vahter, M. Cadmium-induced effects on bone in a population-based study of women. Environ Health Perspect. 2006, 114, 830–834. [Google Scholar] [CrossRef] [PubMed]
- Staessen, J.A.; Roels, H.A.; Emelianov, D.; Kuznetsova, T.; Thijs, L.; Vangronsveld, J.; Fagard, R. Environmental exposure to cadmium, forearm bone density, and risk of fractures: Prospective population study. Public Health and Environmental Exposure to Cadmium (PheeCad) Study Group. Lancet 1999, 353, 1140–1144. [Google Scholar] [CrossRef]
- Kjellström, T. Itai-itai disease. In Cadmium and Health: A Toxicological and Epidemiological Appraisal Vol II. Effects and Response; Friberg, L., Elinder, C.G., Kjellstrom, T., Nordberg, G., Eds.; CRC Press: Boca Raton, FL, USA, 1986; pp. 257–290. [Google Scholar]
- Kippler, M.; Ekström, E.C.; Lönnerdal, B.; Goessler, W.; Akesson, A.; El Arifeen, S.; Persson, L.A.; Vahter, M. Influence of iron and zinc status on cadmium accumulation in Bangladeshi women. Toxicol. Appl. Pharmacol. 2007, 222, 221–226. [Google Scholar] [CrossRef]
- Bah, H.A.F.; Martinez, V.O.; dos Santos, N.R.; Gomes Junior, E.A.; Costa, D.O.; Pires, E.M.; Santana, J.V.A.; Cerqueira, F.d.S.; Menezes-Filho, J.A. Determinants of Exposure to Potentially Toxic Metals in Pregnant Women of the DSAN-12M Cohort in the Recôncavo Baiano, Brazil. Int. J. Environ. Res. Public Health 2023, 20, 2949. [Google Scholar] [CrossRef]
- Tinkov, A.A.; Filippini, T.; Ajsuvakovae, O.P.; Skalnaya, M.G.; Aasethf, J.; Bjørklundh, G.; Gatiatulinai, E.R.; Popova, E.V.; Nemereshinai, O.N.; Huangk, P.T.; et al. Cadmium and atherosclerosis: Areview of toxicological mechanisms and a meta-analysis of epidemiologic studies. Environ. Res. 2018, 162, 240–260. [Google Scholar] [CrossRef]
- IARC. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans-Beryllium, Cadmium, Mercury, and Exposures in the Glass Manufacturing Industry. In Summary of Data Reported and Evaluation; International Agency for Cancer Research-World Health Organization: Geneva, Switzerland, 1997; Volume 58. [Google Scholar]
- Mezynska, M.; Brzóska, M.M. Environmental exposure to cadmium-a risk for health of the general population in industrialized countries and preventive strategies. Environ. Sci. Pollut. Res. Int. 2018, 25, 3211–3232. [Google Scholar] [CrossRef]
- Giaginis, C.; Gatzidou, E.; Theocharis, S. DNA repair systems as targets of cadmium toxicity. Toxicol. Appl. Pharmacol. 2006, 213, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Xu, X.; Wang, L.; Han, J.; Katuwal, H.B.; Jiao, S.; Qiu, G. Human Dietary Exposure to Heavy Metals via Rice in Nepal. Int. J. Environ. Res. Public Health 2023, 20, 4134. [Google Scholar] [CrossRef] [PubMed]
- Zakaria, Z.; Zulkafflee, N.S.; Mohd Redzuan, N.A.; Selamat, J.; Ismail, M.R.; Praveena, S.M.; Tóth, G.; Abdull Razis, A.F. Understanding Potential Heavy Metal Contamination, Absorption, Translocation and Accumulation in Rice and Human Health Risks. Plants 2021, 10, 1070. [Google Scholar] [CrossRef] [PubMed]
- Wieczorek, J.; Baran, A.; Urbański, K.; Mazurek, R.; Klimowicz-Pawlas, A. Assessment of the pollution and ecological risk of lead and cadmium in soils. Environ. Geochem. Health 2018, 40, 2325–2342. [Google Scholar] [CrossRef] [PubMed]
- Charkiewicz, A.E.; Backstrand, J.R. Lead Toxicity and Pollution in Poland. Int. J. Environ. Res. Public Health 2020, 17, 4385. [Google Scholar] [CrossRef]
- EEA Report. 2018. Available online: https://www.eea.europa.eu/data-and-maps/figures/annual-mean-cadmium-concentrations-4 (accessed on 1 December 2020).
- Clemens, S.; Aarts, M.; Thomine, S.; Verbruggen, M. Plant science: The key to preventing slow cadmium poisoning. Trends Plant Sci. 2013, 18, 92–99. [Google Scholar] [CrossRef]
- Smith, D.B.; Cannon, W.F.; Woodruff, L.G.; Solano Federico Kilburn, J.E.; Fey, D.L. Geochemical and Mineralogical Data for Soils of the Conterminous United States; U.S. Geological Survey Data Series 801. 2013; 19p. Available online: https://pubs.usgs.gov/ds/801/ (accessed on 7 April 2022).
- Rosada, J.; Przewocka, M. Remediation and reclamation of agricultural lands covered by emissions of metallurgy industry. Zeszyty Naukowe; Inżynieria Środowiska/Uniwersytet Zielonogórski: Zielona Góra, Poland, 2017; Volume 168, pp. 69–82. [Google Scholar]
- Garbisu, C.; Alkorta, I. Basic Concepts on Heavy Metal Soil Bioremediation. Eur. J. Miner. Process. Environ. Prot. 2003, 3, 58–66. [Google Scholar]
- Available online: https://echa.europa.eu/pl/registration-dossier/-/registered-dossier/15342/7/1 (accessed on 28 August 2022).
- Satarug, S.; Đorđević, A.B.; Yimthiang, S.; Vesey, D.A.; Gobe, G.C. The NOAEL Equivalent of Environmental Cadmium Exposure Associated with GFR Reduction and Chronic Kidney Disease. Toxics 2022, 10, 614. [Google Scholar] [CrossRef]
- Joint FAO/WHO Expert Committee on Food Additives. Safety Evaluation of Certain Food Additives and Contaminants: Prepared by the Seventy-Third Meeting of the Joint FAO/WHO Expert Committee on Food Additives (JECFA). In Proceedings of the Meeting, 73th & World Health Organization, Geneva, Switzerland, 9–14 November 2020; World Health Organization: Geneva, Switzerland, 2011; pp. 305–374. Available online: https://apps.who.int/iris/handle/10665/44521 (accessed on 1 September 2022).
- Available online: https://blogs.edf.org/health/2020/04/07/best-practices-for-reducing-cadmium-in-food-new-review-from-fda-scientists/ (accessed on 7 April 2020).
- Agency for Toxic Substances & Disease Registry (ATSDR). Toxicological Profile for Cadmium; U.S. Department of Health and Human Services, Public Health Service: Atlanta, GA, USA, 2012.
- Krzywy, I.; Krzywy, E.; Peregud-Pogorzelski, J.; Łuksza, K.; Brodkiewicz, A. Cadmium-is there something to fear? Ann. Acad. Med. Stetin. 2011, 57, 49–63. [Google Scholar]
- Jakubowski, M. Cadmium and its inorganic compounds-calculated as Cd. Documentation of Occupational Exposure Limits. Podstawy Metod. Oceny Sr. Pr. 2012, 2, 111–146. [Google Scholar]
- Ghoochani, M.; Rastkari, N.; Yunesian, M.; Nodehi, R.N.; Mesdaghinia, A.; Houshiarra, A.; Shamsipour, M.; Dehghani, M.H. What do we know about exposure of Iranians to cadmium? Findings from a systematic review. Environ. Sci. Pollut. Res. 2018, 25, 1033–1043. [Google Scholar] [CrossRef] [PubMed]
- Gratão, P.L.; Polle, A.; Lea, P.; Azevedo, R.A. Making the life of heavy metal-stressed plants a little easier. Funct. Plant Biol. 2005, 32, 481–494. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Lei, Q.; Cheng, Y.; Xie, Y.; Qian, H.; Guo, Y.; Chen, Y.; Yao, W. Study on the removal of cadmium in rice using microbial fermentation method. J. Food Sci. 2017, 82, 1467–1474. [Google Scholar] [CrossRef] [PubMed]
- Panel, E.C. Scientific opinion of the panel on contaminants in the food chain on a request from the European Commission on cadmium in food. EFSA J. 2009, 980, 1–139. [Google Scholar]
- Satarug, S.; Garrett, S.H.; Sens, M.A.; Sens, D.A. Cadmium, environmental exposure, and health outcomes. Environ. Health Perspect. 2010, 118, 182–190. [Google Scholar] [CrossRef]
- Spungen, J.H. Children’s exposures to lead and cadmium: FDA total diet study 2014–16. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess 2019, 36, 893–903. [Google Scholar] [CrossRef]
- Praveena, S.M.; Omar, N.A. Heavy metal exposure from cooked rice grain ingestion and its potential health risks to humans from total and bioavailable forms analysis. Food Chem. 2017, 235, 203–211. [Google Scholar] [CrossRef]
- Liu, L.; Han, J.; Xu, X.; Xu, Z.; Abeysinghe, K.S.; Atapattu, A.J.; De Silva, P.; Lu, Q.; Qiu, G. Dietary exposure assessment of cadmium, arsenic, and lead in market rice from Sri Lanka. Environ. Sci. Pollut. Res. 2020, 27, 42704–42712. [Google Scholar] [CrossRef]
- Meharg, A.A.; Norton, G.; Deacon, C.; Williams, P.; Adomako, E.E.; Price, A.; Zhu, Y.; Li, G.; Zhao, F.J.; McGrath, S.; et al. Variation in rice cadmium related to human exposure. Environ. Sci. Technol. 2013, 47, 5613–5618. [Google Scholar] [CrossRef]
- Rahman, M.A.; Rahman, M.M.; Reichman, S.M.; Lim, R.P.; Naidu, R. Heavy metals in Australian grown and imported rice and vegetables on sale in Australia: Health hazard. Ecotoxicol. Environ. Saf. 2014, 100, 53–60. [Google Scholar] [CrossRef]
- Elinder, C.G.; Järup, L. Cadmium exposure and health risks: Recent findings. Ambio 1996, 25, 370. [Google Scholar]
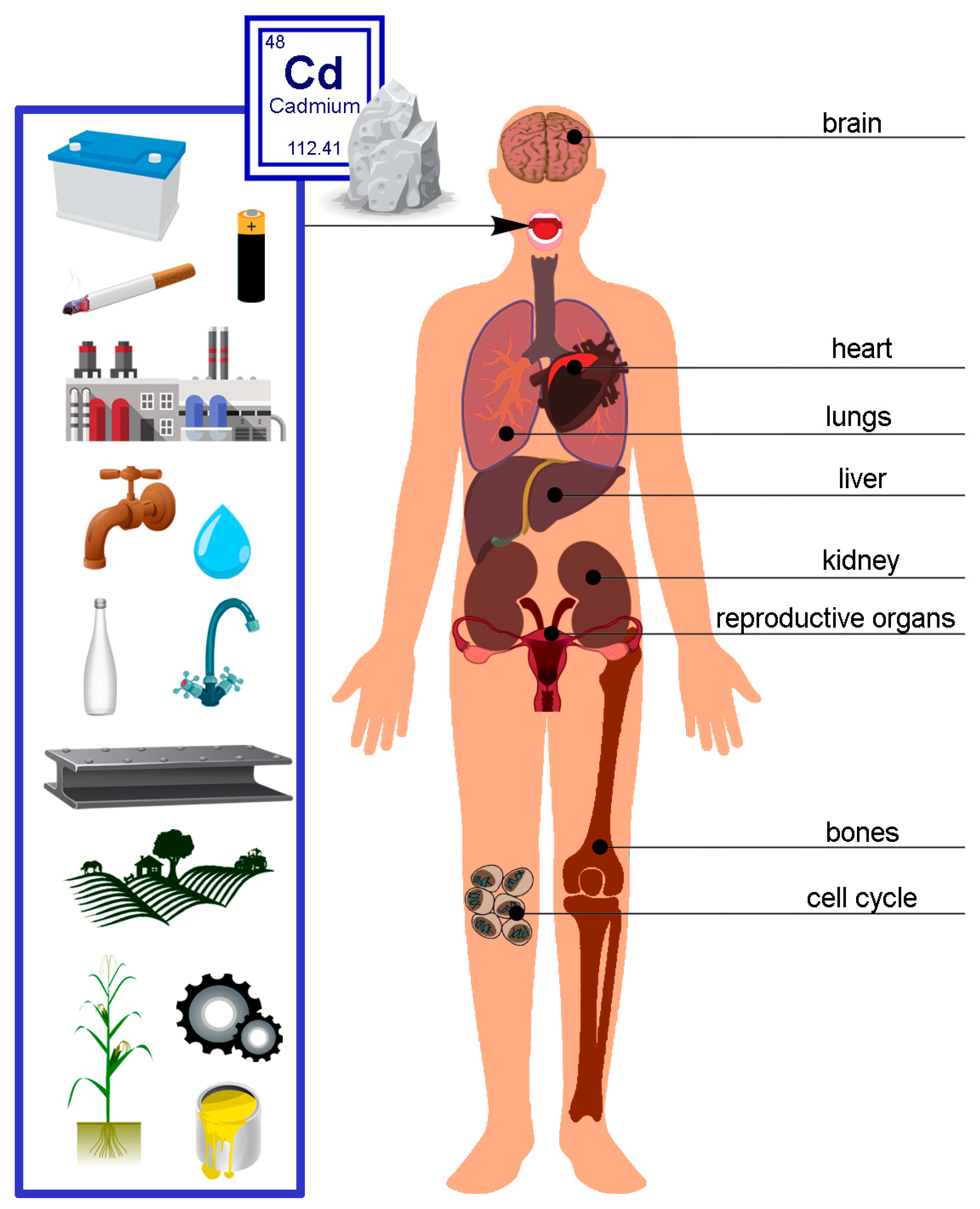
| Human Body | Effects of Exposure to Cadmium Poisoning |
|---|---|
| Respiratory system |
|
| Nephrological system |
|
| Circulatory system |
|
| Skeletal system |
|
| Reproductive system |
|
| Nervous system |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Charkiewicz, A.E.; Omeljaniuk, W.J.; Nowak, K.; Garley, M.; Nikliński, J. Cadmium Toxicity and Health Effects—A Brief Summary. Molecules 2023, 28, 6620. https://doi.org/10.3390/molecules28186620
Charkiewicz AE, Omeljaniuk WJ, Nowak K, Garley M, Nikliński J. Cadmium Toxicity and Health Effects—A Brief Summary. Molecules. 2023; 28(18):6620. https://doi.org/10.3390/molecules28186620
Chicago/Turabian StyleCharkiewicz, Angelika Edyta, Wioleta Justyna Omeljaniuk, Karolina Nowak, Marzena Garley, and Jacek Nikliński. 2023. "Cadmium Toxicity and Health Effects—A Brief Summary" Molecules 28, no. 18: 6620. https://doi.org/10.3390/molecules28186620
APA StyleCharkiewicz, A. E., Omeljaniuk, W. J., Nowak, K., Garley, M., & Nikliński, J. (2023). Cadmium Toxicity and Health Effects—A Brief Summary. Molecules, 28(18), 6620. https://doi.org/10.3390/molecules28186620