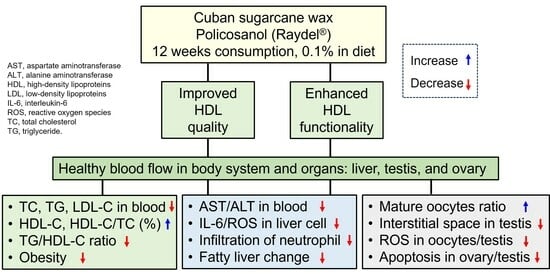
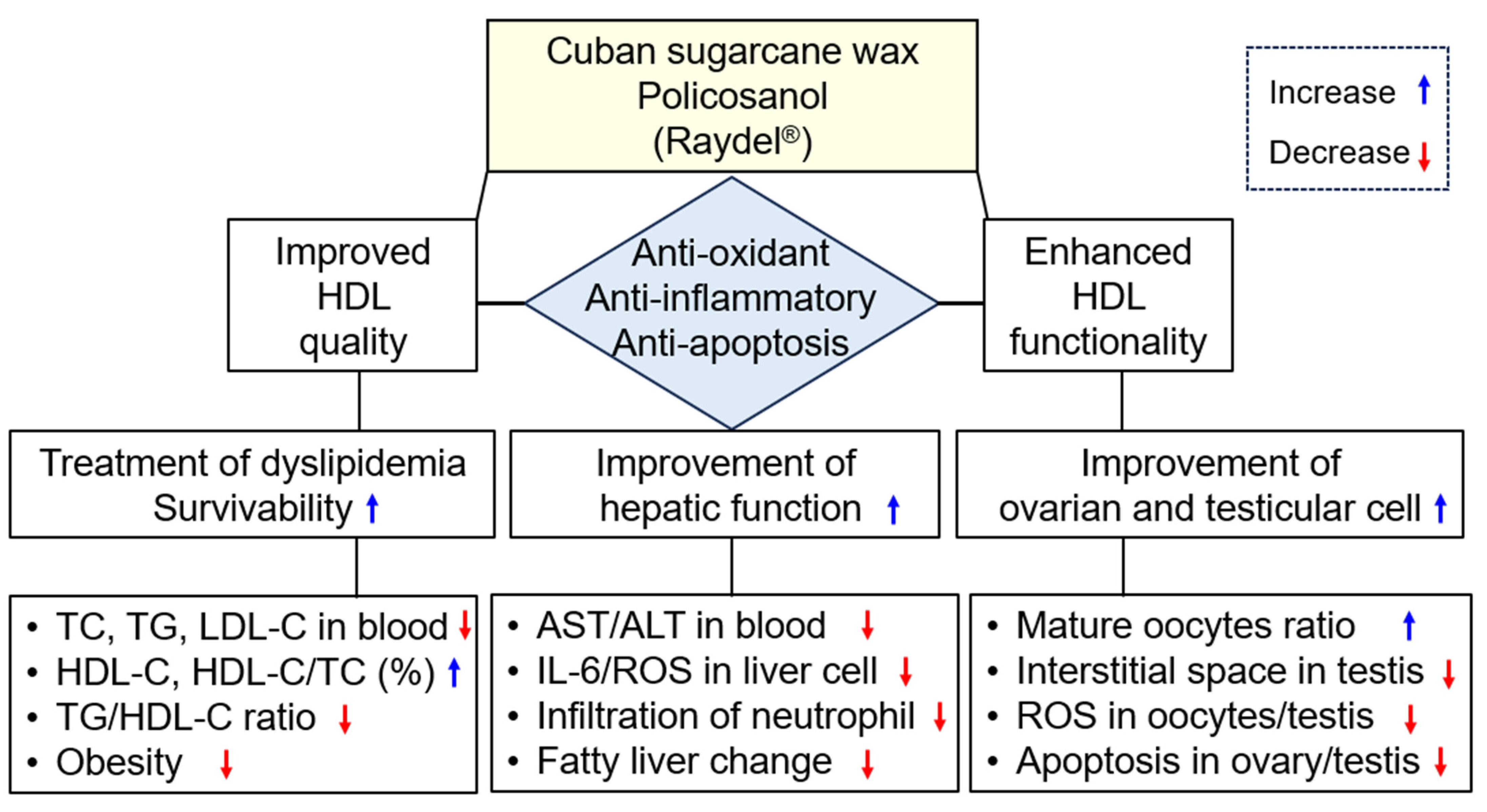
Cuban Policosanol (Raydel®) Potently Protects the Liver, Ovary, and Testis with an Improvement in Dyslipidemia in Hyperlipidemic Zebrafish: A Comparative Study with Three Chinese Policosanols
Abstract
1. Introduction
2. Results
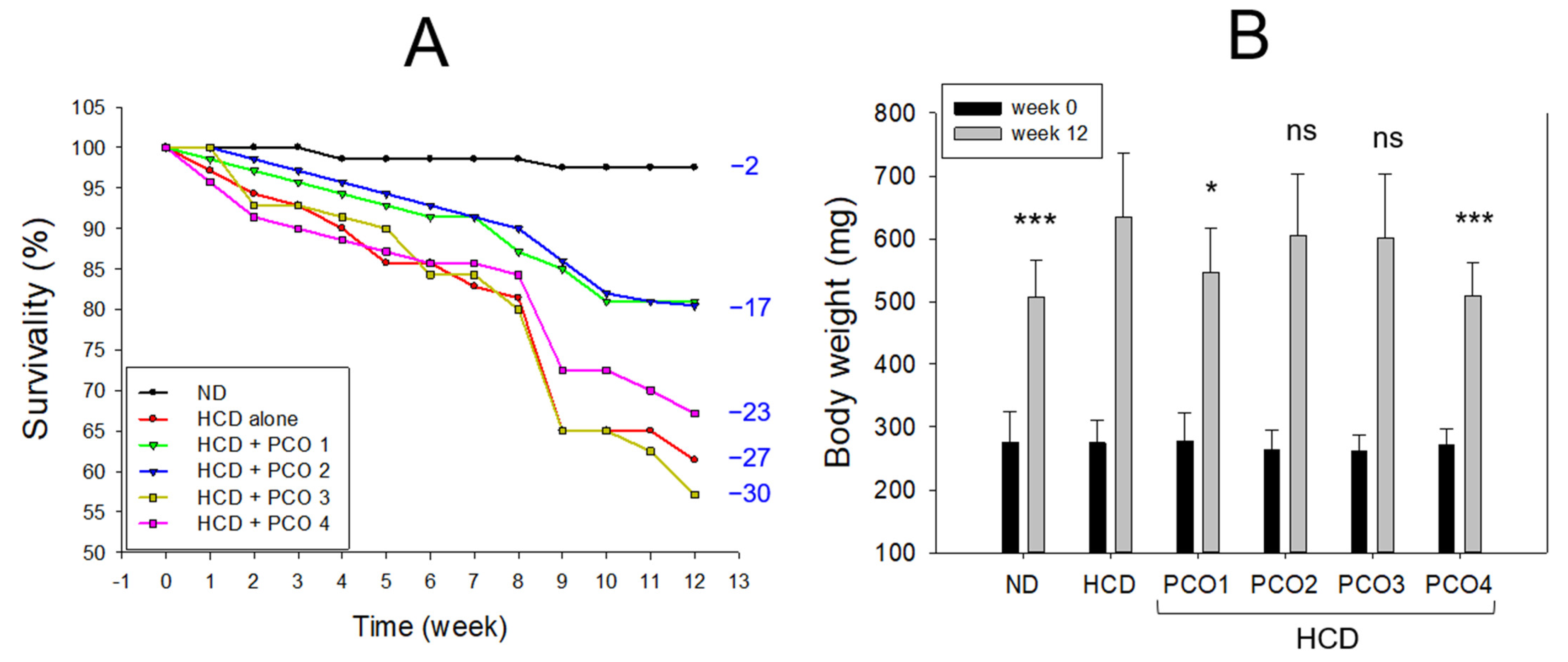
2.1. Change in Survivability and Body Weight
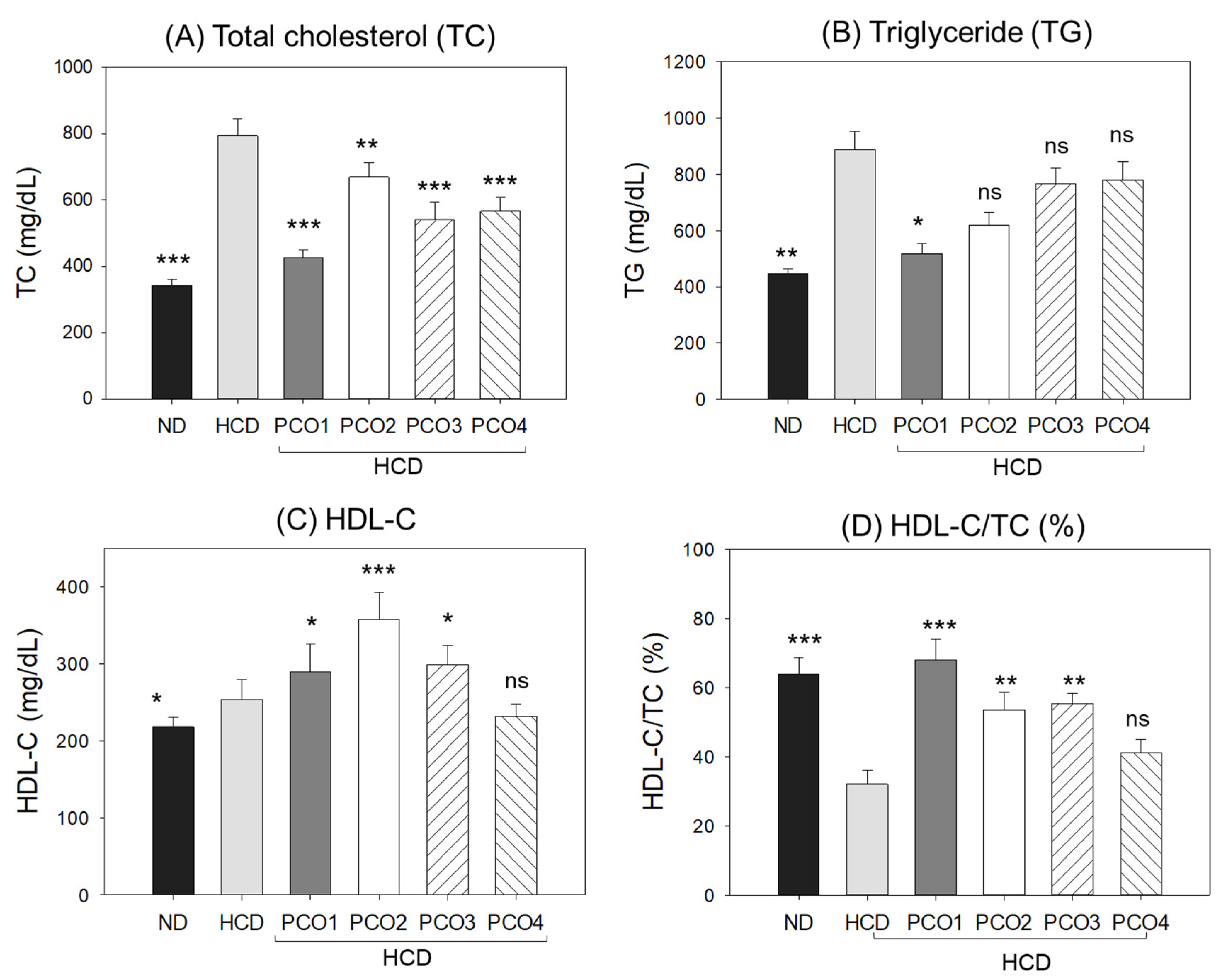
2.2. Change in Blood Lipid Levels
2.3. Change in Hepatic Function Parameters
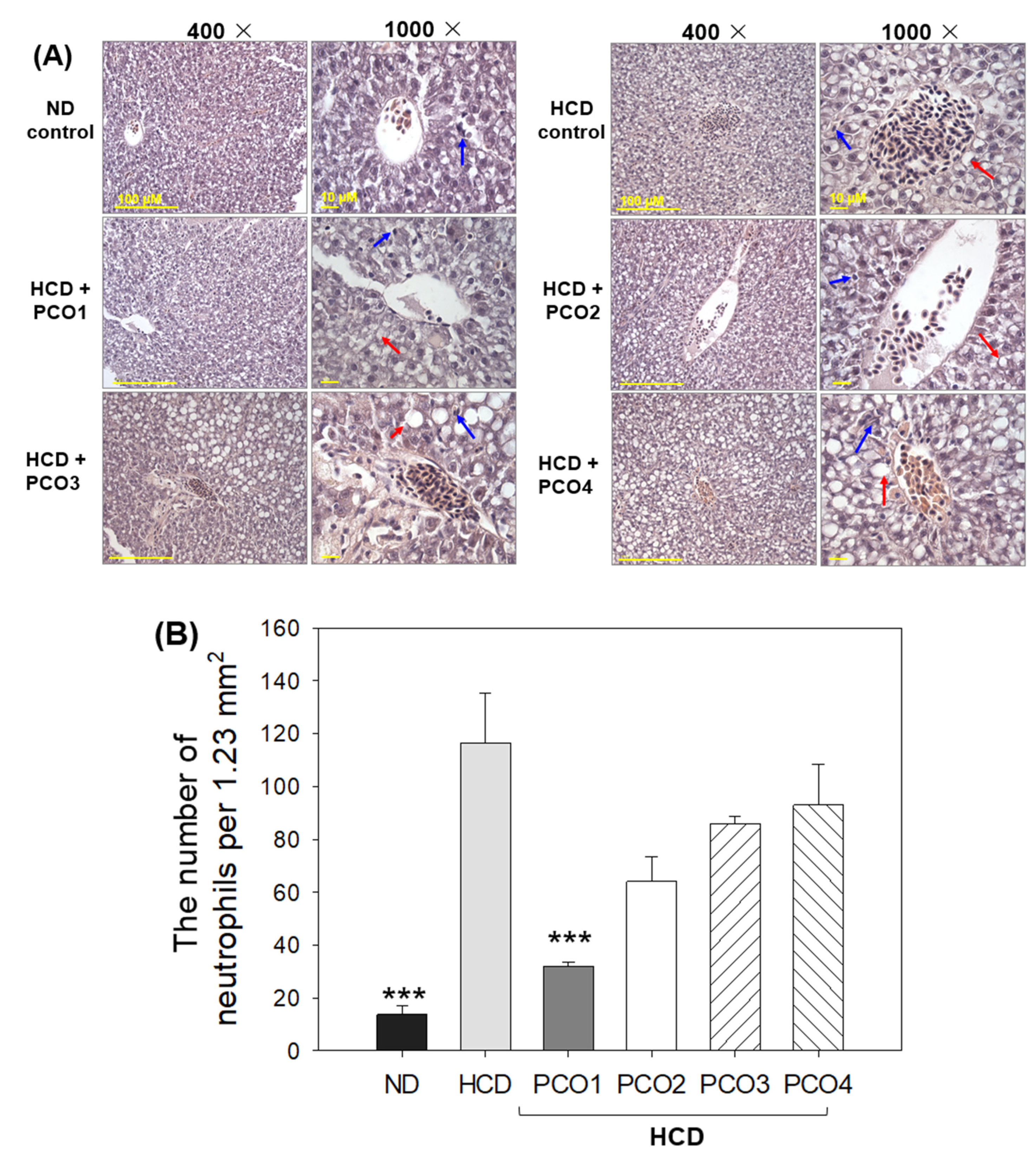
2.4. Analysis of the Liver Tissue (H&E Staining)
2.5. Fatty Liver Changes and ROS Production
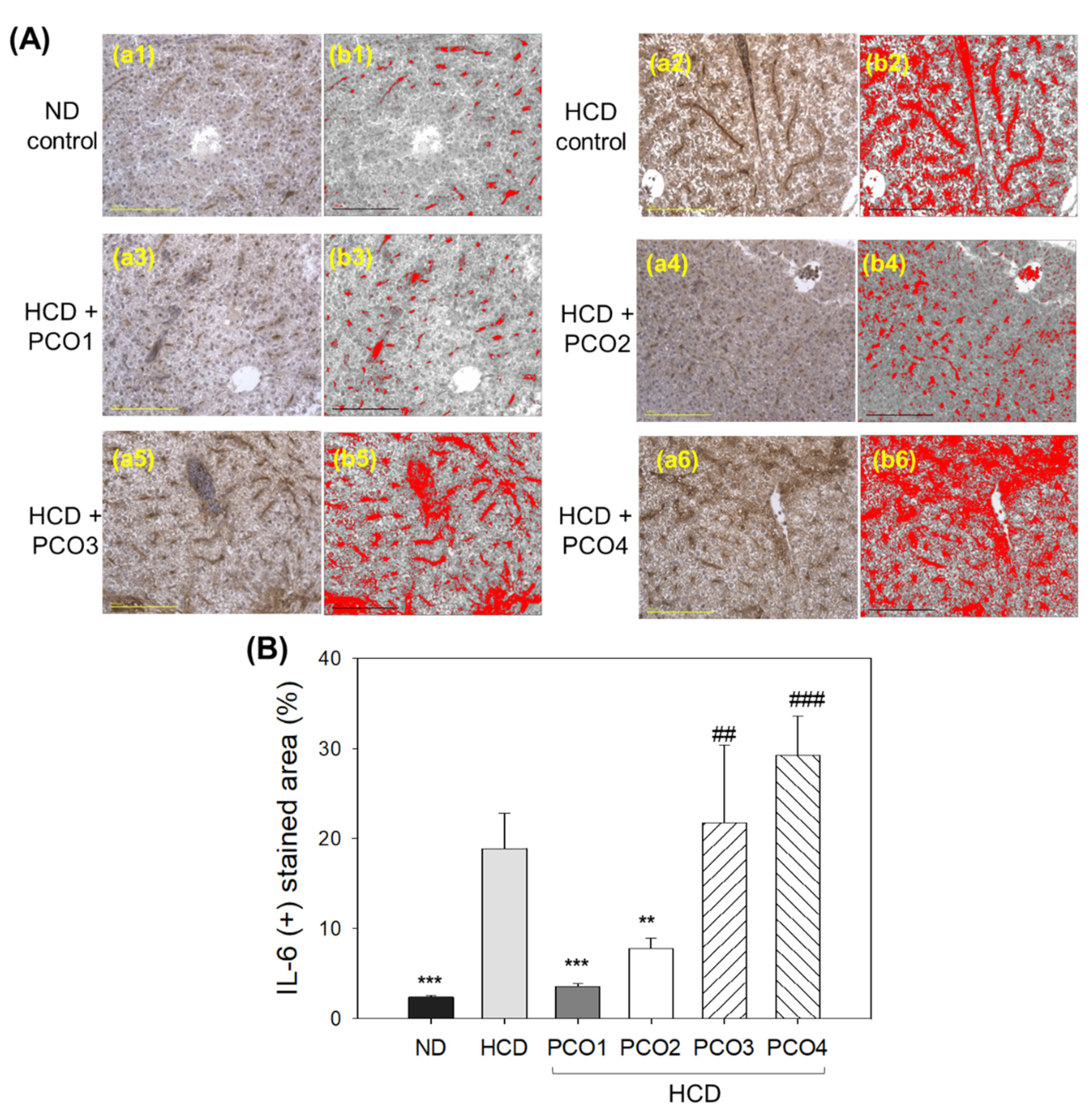
2.6. IL-6 Production in Liver Tissue (Immunohistochemistry)
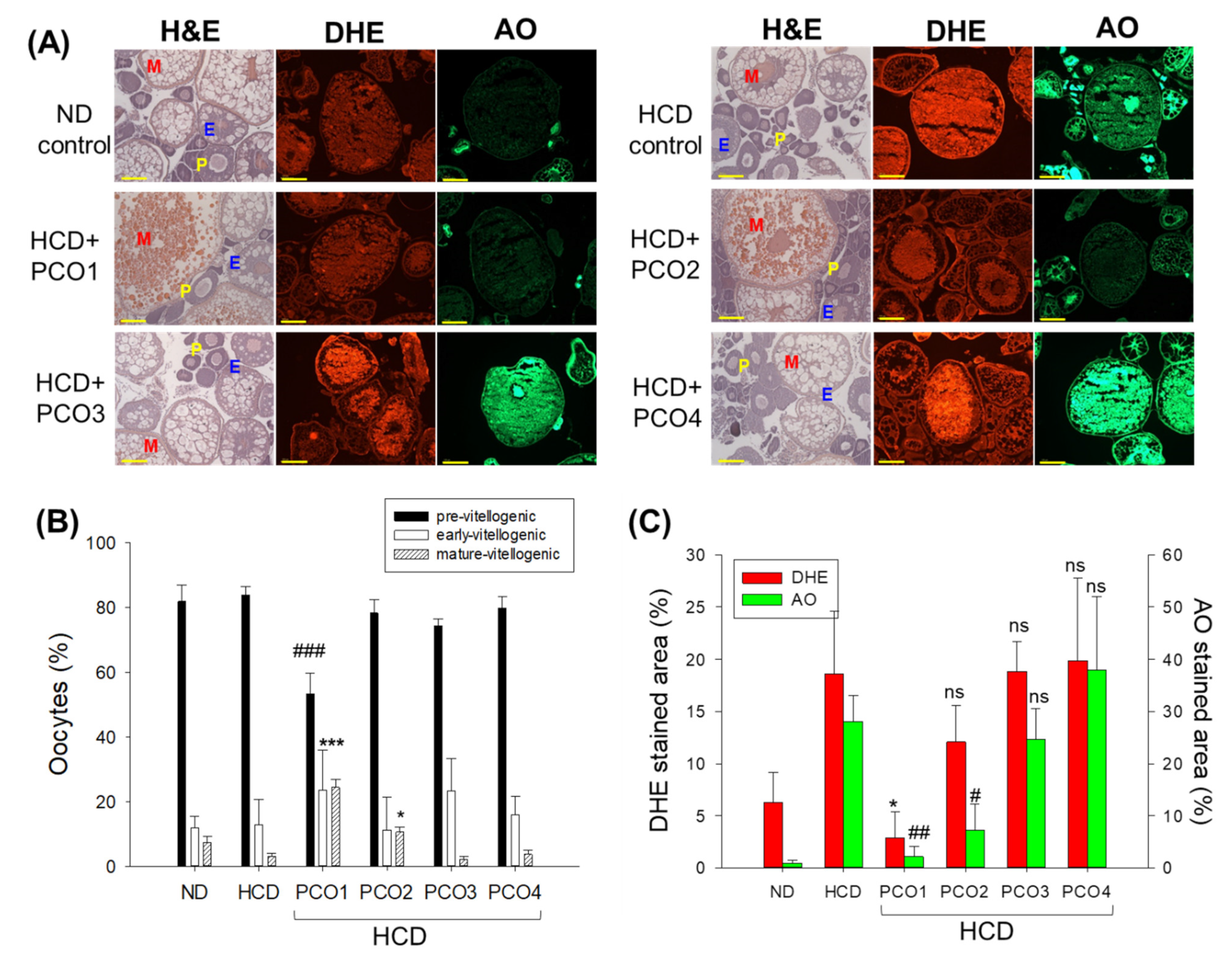
2.7. Examination of Ovarian Tissue Section
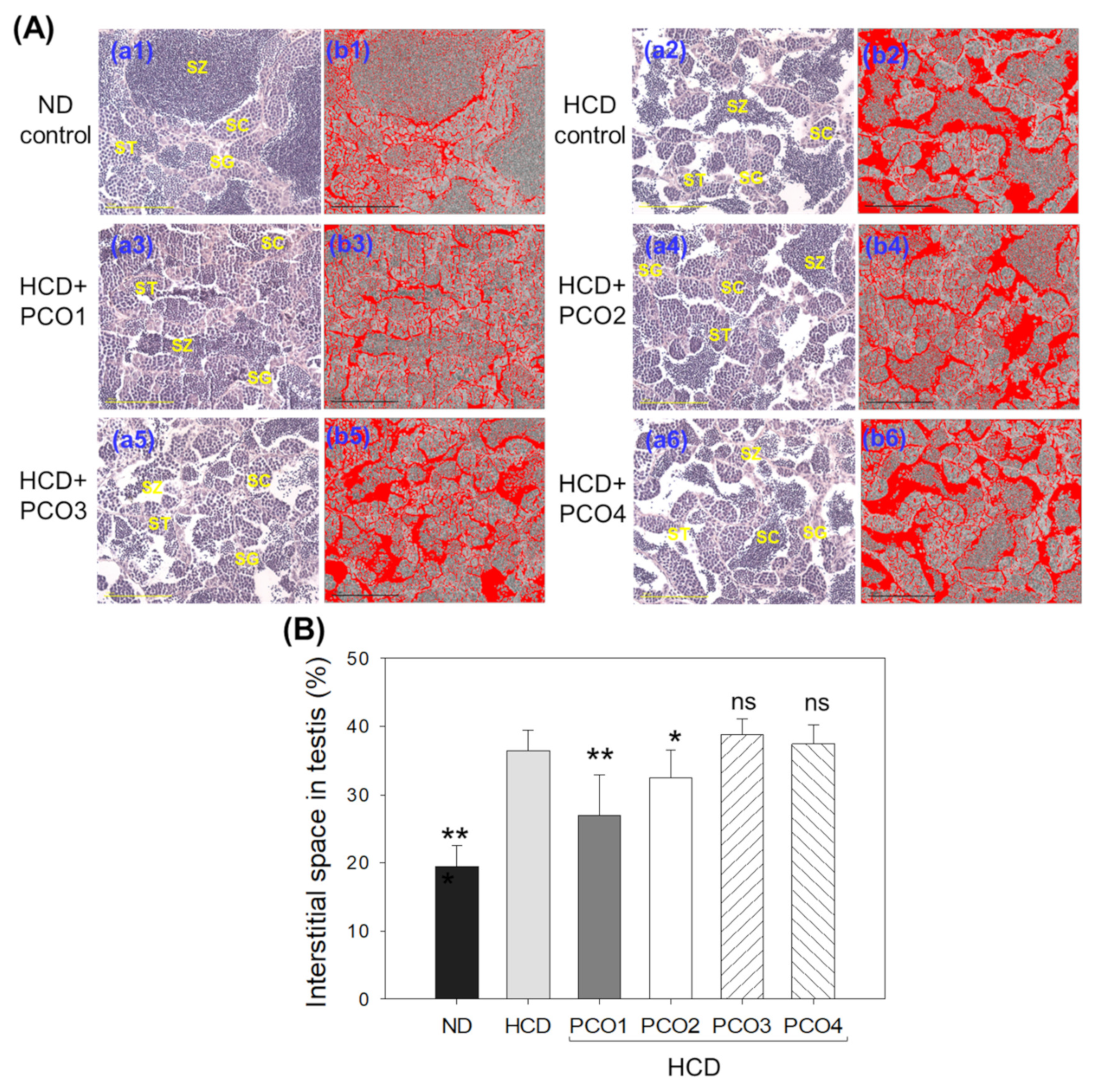
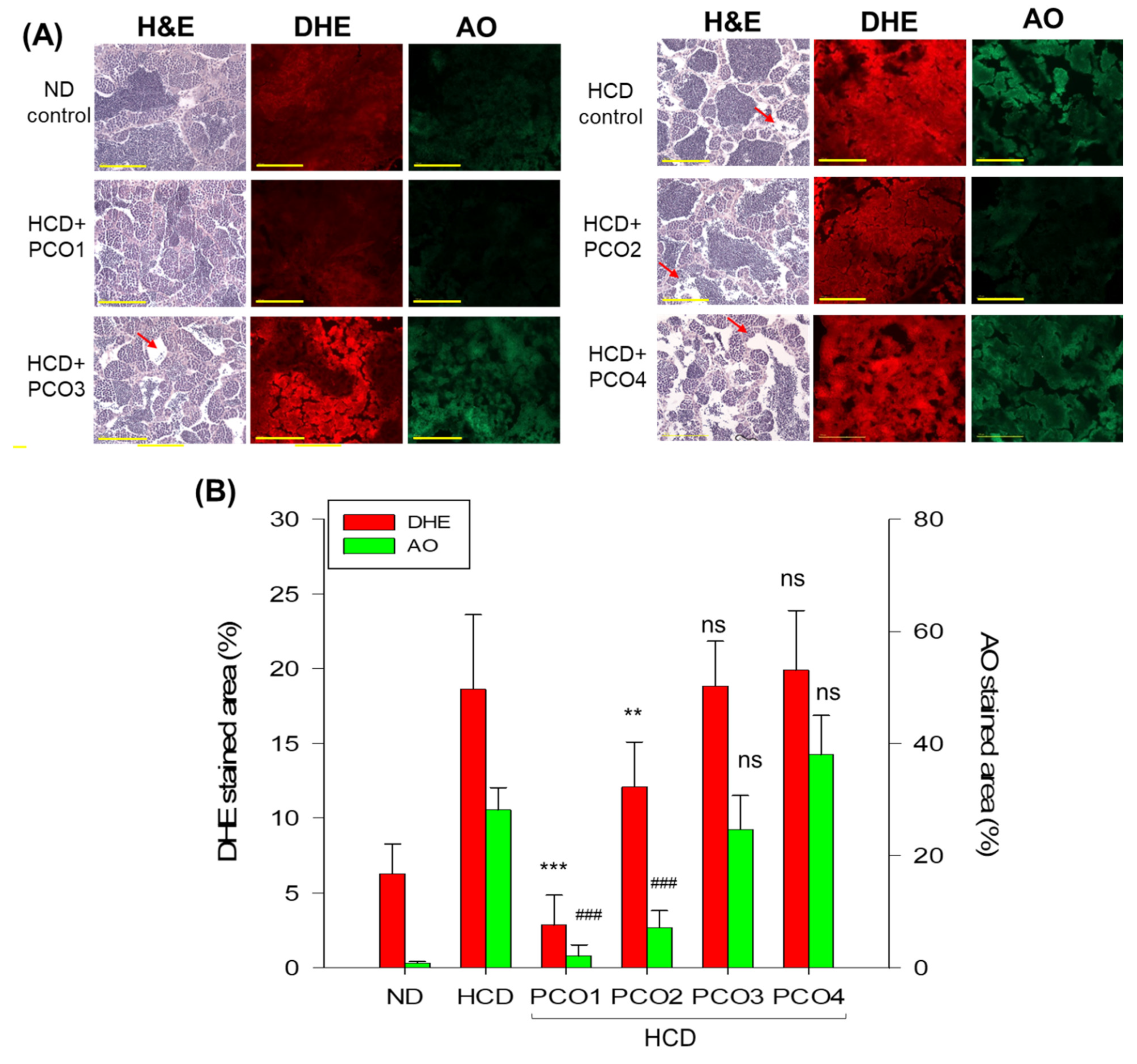
2.8. Analysis of Testis Tissue Section
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Zebrafish Maintenance and Policosanol Supplementation
4.3. Blood Collection and Analysis
4.4. Analysis of Hepatic Tissue
4.5. Analysis of Ovarian Tissue
4.6. Analysis of Testes Tissue
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Cho, K.-H. The Current Status of Research on High-Density Lipoproteins (HDL): A Paradigm Shift from HDL Quantity to HDL Quality and HDL Functionality. Int. J. Mol. Sci. 2022, 23, 3967. [Google Scholar] [CrossRef]
- Milman, S.; Atzmon, G.; Crandall, J.; Barzilai, N. Phenotypes and genotypes of high density lipoprotein cholesterol in exceptional longevity. Curr. Vasc. Pharmacol. 2014, 12, 690–697. [Google Scholar] [CrossRef]
- Gotto, A.M., Jr. Low high-density lipoprotein cholesterol as a risk factor in coronary heart disease: A working group report. Circulation 2001, 103, 2213–2218. [Google Scholar] [CrossRef]
- Franczyk, B.; Gluba-Brzózka, A.; Ciałkowska-Rysz, A.; Ławiński, J.; Rysz, J. The Impact of Aerobic Exercise on HDL Quantity and Quality: A Narrative Review. Int. J. Mol. Sci. 2023, 24, 4653. [Google Scholar] [CrossRef]
- Tangvarasittichai, S. Oxidative stress, insulin resistance, dyslipidemia and type 2 diabetes mellitus. World J. Diabetes 2015, 6, 456–480. [Google Scholar] [CrossRef]
- Cipriani, S.; Simon, J.A. Sexual Dysfunction as a Harbinger of Cardiovascular Disease in Postmenopausal Women: How Far Are We? J. Sex. Med. 2022, 19, 1321–1332. [Google Scholar] [CrossRef]
- Nikoobakht, M.; Pourkasmaee, M.; Nasseh, H. The relationship between lipid profile and erectile dysfunc-tion. Urol. J. 2005, 2, 40–44. [Google Scholar]
- Verit, F.F.; Zeyrek, F.Y.; Zebitay, A.G.; Akyol, H. Cardiovascular risk may be increased in women with unexplained infertility. Clin. Exp. Reprod. Med. 2017, 44, 28–32. [Google Scholar] [CrossRef]
- Luna-Castillo, K.P.; Lin, S.; Muñoz-Valle, J.F.; Vizmanos, B.; López-Quintero, A.; Márquez-Sandoval, F. Functional Food and Bioactive Compounds on the Modulation of the Functionality of HDL-C: A Narrative Review. Nutrients 2021, 13, 1165. [Google Scholar] [CrossRef]
- Askarpour, M.; Ghaedi, E.; Roshanravan, N.; Hadi, A.; Mohammadi, H.; Symonds, M.E.; Miraghajani, M. Policosanol supplementation significantly improves blood pressure among adults: A systematic review and meta-analysis of randomized controlled trials. Complement. Ther. Med. 2019, 45, 89–97. [Google Scholar] [CrossRef]
- Arruzazabala, M.; Carbajal, D.; Mas, R.; Garcia, M.; Fraga, V. Effects of Policosanol on platelet aggregation in rats. Thromb. Res. 1993, 69, 321–327. [Google Scholar] [CrossRef]
- Valdes, S.; Arruzazabala, M.L.; Fernandez, L.; Más, R.; Carbajal, D.; Aleman, C.; Molina, V. Effect of policosanol on platelet aggregation in healthy volunteers. Int. J. Clin. Pharmacol. Res. 1996, 16, 67–72. [Google Scholar]
- Venturelli, A.; Brighenti, V.; Mascolo, D.; Pellati, F. A new strategy based on microwave-assisted technology for the extraction and purification of beeswax policosanols for pharmaceutical purposes and beyond. J. Pharm. Biomed. Anal. 2019, 172, 200–205. [Google Scholar] [CrossRef]
- Wong, W.T.; Ismail, M.; Tohit, E.R.; Abdullah, R.; Zhang, Y.-D. Attenuation of Thrombosis by Crude Rice (Oryza sativa) Bran Policosanol Extract: Ex Vivo Platelet Aggregation and Serum Levels of Arachidonic Acid Metabolites. Evid. -Based Complement. Altern. Med. 2016, 2016, 7343942. [Google Scholar] [CrossRef]
- Ishaka, A.; Umar Imam, M.; Mahamud, R.; Zuki, A.B.; Maznah, I. Characterization of rice bran wax policosanol and its nanoemulsion formulation. Int. J. Nanomed. 2014, 9, 2261–2269. [Google Scholar] [CrossRef]
- Gong, J.; Qin, X.; Yuan, F.; Hu, M.; Chen, G.; Fang, K.; Wang, D.; Jiang, S.; Li, J.; Zhao, Y.; et al. Efficacy and safety of sugarcane policosanol on dyslipidemia: A meta-analysis of randomized controlled trials. Mol. Nutr. Food Res. 2018, 62, 1700280. [Google Scholar] [CrossRef]
- Cho, K.-H.; Kim, S.-J.; Yadav, D.; Kim, J.Y.; Kim, J.R. Consumption of Cuban Policosanol Improves Blood Pressure and Lipid Profile via Enhancement of HDL Functionality in Healthy Women Subjects: Randomized, Double-Blinded, and Placebo-Controlled Study. Oxidative Med. Cell. Longev. 2018, 2018, 4809525. [Google Scholar] [CrossRef]
- Lee, S.; Lee, G.S.; Moon, J.H.; Jung, J. Policosanol suppresses tumor progression in a gastric cancer xenograft model. Toxicol. Res. 2022, 38, 567–575. [Google Scholar] [CrossRef]
- Kim, J.-H.; Lim, D.-K.; Suh, Y.-H.; Chang, K.-A. Long-Term Treatment of Cuban Policosanol Attenuates Abnormal Oxidative Stress and Inflammatory Response via Amyloid Plaques Reduction in 5xFAD Mice. Antioxidants 2021, 10, 1321. [Google Scholar] [CrossRef]
- Cho, K.-H.; Baek, S.H.; Nam, H.-S.; Kim, J.-E.; Kang, D.-J.; Na, H.; Zee, S. Cuban Sugar Cane Wax Alcohol Exhibited Enhanced Antioxidant, Anti-Glycation and Anti-Inflammatory Activity in Reconstituted High-Density Lipoprotein (rHDL) with Improved Structural and Functional Correlations: Comparison of Various Policosanols. Int. J. Mol. Sci. 2023, 24, 3186. [Google Scholar] [CrossRef]
- Li, X.; Zhou, L.; Zheng, Y.; He, T.; Guo, H.; Li, J.; Zhang, J. Establishment of a non-alcoholic fatty liver disease model by high fat diet in adult zebrafish. Anim. Models Exp. Med. 2023. advance online publication. [Google Scholar] [CrossRef]
- Fang, L.; Liu, C.; Miller, Y.I. Zebrafish models of dyslipidemia: Relevance to atherosclerosis and angiogenesis. Transl. Res. J. Lab. Clin. Med. 2014, 163, 99–108. [Google Scholar] [CrossRef]
- Stoletov, K.; Fang, L.; Choi, S.-H.; Hartvigsen, K.; Hansen, L.F.; Hall, C.; Pattison, J.; Juliano, J.; Miller, E.R.; Almazan, F.; et al. Vascular lipid accumulation, lipoprotein oxidation, and macrophage lipid uptake in hypercholesterolemic zebrafish. Circ. Res. 2009, 104, 952–960. [Google Scholar] [CrossRef]
- Lee, E.-Y.; Yoo, J.-A.; Lim, S.-M.; Cho, K.-H. Anti-Aging and Tissue Regeneration Ability of Policosanol Along with Lipid-Lowering Effect in Hyperlipidemic Zebrafish via Enhancement of High-Density Lipoprotein Functionality. Rejuvenation Res. 2016, 19, 149–158. [Google Scholar] [CrossRef]
- Ayad, B.; Omolaoye, T.S.; Louw, N.; Ramsunder, Y.; Skosana, B.T.; Oyeipo, P.I.; Du Plessis, S.S. Oxidative Stress and Male Infertility: Evidence from a Research Perspective. Front. Reprod. Health 2022, 4, 822257. [Google Scholar] [CrossRef]
- Gao, Y.; Zou, Y.; Wu, G.; Zheng, L. Oxidative stress and mitochondrial dysfunction of granulosa cells in polycystic ovarian syndrome. Front. Med. 2023, 10, 1193749. [Google Scholar] [CrossRef]
- Su, Y.; He, L.; Hu, Z.; Li, Y.; Zhang, Y.; Fan, Z.; Zhao, K.; Zhang, H.; Liu, C. Obesity Causes Abrupt Changes in the Testicular Microbiota and Sperm Motility of Zebrafish. Front. Immunol. 2021, 12, 639239. [Google Scholar] [CrossRef]
- Knauff, E.A.; Westerveld, H.E.; Goverde, A.J.; Eijkemans, M.J.; Valkenburg, O.; van Santbrink, E.J.; Fauser, B.C.; van der Schouw, Y.T. Lipid profile of women with premature ovarian failure. Menopause 2008, 15, 919–923. [Google Scholar] [CrossRef]
- Whitfield, M.; Guiton, R.; Rispal, J.; Acar, N.; Kocer, A.; Drevet, J.R.; Saez, F. Dyslipidemia alters sperm maturation and capacitation in LXR-null mice. Reproduction 2017, 154, 827–842. [Google Scholar] [CrossRef]
- Cho, K.-H.; Kim, J.-E.; Komatsu, T.; Uehara, Y. Protection of Liver Functions and Improvement of Kidney Functions by Twelve Weeks Consumption of Cuban Policosanol (Raydel®) with a Decrease of Glycated Hemoglobin and Blood Pressure from a Randomized, Placebo-Controlled, and Double-Blinded Study with Healthy and Middle-Aged Japanese Participants. Life 2023, 13, 1319. [Google Scholar] [CrossRef]
- Cho, K.-H.; Nam, H.-S.; Baek, S.-H.; Kang, D.-J.; Na, H.; Komatsu, T.; Uehara, Y. Beneficial Effect of Cuban Policosanol on Blood Pressure and Serum Lipoproteins Accompanied with Lowered Glycated Hemoglobin and Enhanced High-Density Lipoprotein Functionalities in a Randomized, Placebo-Controlled, and Double-Blinded Trial with Healthy Japanese. Int. J. Mol. Sci. 2023, 24, 5185. [Google Scholar] [CrossRef] [PubMed]
- Zardoya, R.; Tula, L.; Castaño, G.; Más, R.; Illnait, J.; Fernández, J.C.; Díaz, E.; Fernández, L. Effects of policosanol on hypercholesterolemic patients with abnormal serum biochemical indicators of hepatic function. Curr. Ther. Res. 1996, 57, 568–577. [Google Scholar] [CrossRef]
- Yan, F.; Zhao, Q.; Li, Y.; Zheng, Z.; Kong, X.; Shu, C.; Liu, Y.; Shi, Y. The role of oxidative stress in ovarian aging: A review. J. Ovarian Res. 2022, 15, 100. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.-Y.; Cho, K.-H. High-dose consumption of NaCl resulted in severe degradation of lipoproteins associated with hyperlipidemia, hyperglycemia, and infertility via impairment of testicular spermatogenesis. Toxicol. Res. 2016, 5, 557–569. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cho, K.-H.; Yadav, D.; Kim, S.-J.; Kim, J.-R. Blood Pressure Lowering Effect of Cuban Policosanol is Accompanied by Improvement of Hepatic Inflammation, Lipoprotein Profile, and HDL Quality in Spontaneously Hypertensive Rats. Molecules 2018, 23, 1080. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, M.D.; Sánchez, M.; García, H. Multigeneration reproduction study of policosanol in rats. Toxicol. Lett. 1997, 90, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, M.D.; García, H. Teratogenic and reproductive studies of policosanol in the rat and rabbit. Teratog. Carcinog. Mutagen. 1994, 14, 107–113. [Google Scholar] [CrossRef]
- Long, L.; Wu, S.; Sun, J.; Wang, J.; Zhang, H.; Qi, G. Effects of octacosanol extracted from rice bran on blood hormone levels and gene expressions of glucose transporter protein-4 and adenosine monophosphate protein kinase in weaning piglets. Anim. Nutr. 2015, 1, 293–298. [Google Scholar] [CrossRef]
- Klisic, A.; Isakovic, A.; Kocic, G.; Kavaric, N.; Jovanovic, M.; Zvrko, E.; Skerovic, V.; Ninic, A. Relationship between Oxidative Stress, Inflammation and Dyslipidemia with Fatty Liver Index in Patients with Type 2 Diabetes Mellitus. Exp. Clin. Endocrinol. Diabetes Off. J. Ger. Soc. Endocrinol. Ger. Diabetes Assoc. 2018, 126, 371–378. [Google Scholar] [CrossRef]
- Noa, M.; Más, R.; Mendoza, S.; Gámez, R.; Mendoza, N.; González, J. Policosanol prevents bone loss in ovariectomized rats. Drugs Under Exp. Clin. Res. 2004, 30, 117–123. [Google Scholar]
- Kim, K.M.; Lim, Y.J.; Jang, W.G. Policosanol Stimulates Osteoblast Differentiation via Adenosine Monophosphate-Activated Protein Kinase-Mediated Expression of Insulin-Induced Genes 1 and 2. Cells 2023, 12, 1863. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Z.; Liu, J.; Niu, K.-M.; Lin, C.; Tu, Y.; Liu, Y.; Cai, L.; Liu, H.; Ouyang, K. Integrated Metagenomics and Metabolomics to Reveal the Effects of Policosanol on Modulating the Gut Microbiota and Lipid Metabolism in Hyperlipidemic C57BL/6 Mice. Front. Endocrinol. 2021, 12, 722055. [Google Scholar] [CrossRef] [PubMed]
- Gonnella, F.; Konstantinidou, F.; Di Berardino, C.; Capacchietti, G.; Peserico, A.; Russo, V.; Barboni, B.; Stuppia, L.; Gatta, V. A Systematic Review of the Effects of High-Fat Diet Exposure on Oocyte and Follicular Quality: A Molecular Point of View. Int. J. Mol. Sci. 2022, 23, 8890. [Google Scholar] [CrossRef] [PubMed]
- Ruebel, M.L.; Cotter, M.; Sims, C.R.; Moutos, D.M.; Badger, T.M.; Cleves, M.A.; Shankar, K.; Andres, A. Obesity Modulates Inflammation and Lipid Metabolism Oocyte Gene Expression: A Single-Cell Transcriptome Perspective. J. Clin. Endocrinol. Metab. 2017, 102, 2029–2038. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Luo, F.; Lin, Q. Policosanol: Extraction and biological functions. J. Funct. Foods 2019, 57, 351–360. [Google Scholar] [CrossRef]
- Harrabi, S.; Ferchichi, A.; Bacheli, A.; Fellah, H. Policosanol composition, antioxidant and anti-arthritic activities of milk thistle (Silybium marianum L.) oil at different seed maturity stages. Lipids Health Dis. 2018, 17, 82. [Google Scholar] [CrossRef] [PubMed]
- Nusslein-Volhard, C.; Dahm, R. Zebrafish: A Practical Approach, 1st ed.; Oxford University Press: Oxford, UK, 2002. [Google Scholar]
- National Research Council of the National Academy of Sciences. Guide for the Care and Use of Laboratory Animals; National Academy Press: Washington, DC, USA, 2010. [Google Scholar]
- Percie du Sert, N.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. PLoS Biol. 2020, 18, e3000410. [Google Scholar] [CrossRef]
- Owusu-Ansah, E.; Yavari, A.; Mandal, S.; Banerjee, U. Distinct mitochondrial retrograde signals control the G1-S cell cycle checkpoint. Nat. Genet. 2008, 40, 356–361. [Google Scholar] [CrossRef]
- Patel, U.N.; Patel, U.D.; Khadayata, A.V.; Vaja, R.K.; Modi, C.M.; Patel, H.B. Long-term exposure of the binary mixture of cadmium and mercury damages the developed ovary of adult zebrafish. Environ. Sci. Pollut. Res. Int. 2022, 29, 44928–44938. [Google Scholar] [CrossRef]
- Sutha, J.; Anila, P.A.; Gayathri, M.; Ramesh, M. Long term exposure to tris (2-chloroethyl) phosphate (TCEP) causes alterations in reproductive hormones, vitellogenin, antioxidant enzymes, and histology of gonads in zebrafish (Danio rerio): In vivo and computational analysis. Comp. Biochem. Physiol. Toxicol. Pharmacol. CBP 2022, 254, 109263. [Google Scholar] [CrossRef]
- Sabaliauskas, N.A.; Foutz, C.A.; Mest, J.R.; Budgeon, L.R.; Sidor, A.T.; Gershenson, J.A.; Joshi, S.B.; Cheng, K.C. High-throughput zebrafish histology. Methods 2006, 39, 246–254. [Google Scholar] [CrossRef]
- Koc, N.D.; Teksöz, N.; Ural, M.; Akbulut, C. Histological structure of zebrafish (Danio rerio, Ham-ilton, 1822) testicles. Elixir Aquacult. 2012, 46, 8117–8120. [Google Scholar]


| ND | HCD | HCD | HCD | HCD | HCD | ||
|---|---|---|---|---|---|---|---|
| Groups | Control (n = 70) | Control (n = 70) | PCO1 Raydel Sugarcane Wax Alcohol (n = 70) | PCO2 Xi’an Natural Sugarcane (n = 70) | PCO3 Xi’an Realin Sugarcane (n = 70) | PCO4 Shaanxi Rice Bran (n = 70) | |
| Diet composition (%) | Tetrabits 1 | 100 | 96 | 95.9 | 95.9 | 95.9 | 95.9 |
| Cholesterol (%, wt/wt) | - | 4 | 4 | 4 | 4 | 4 | |
| PCO (%, wt/wt) | - | - | 0.1 | 0.1 | 0.1 | 0.1 | |
| Octacosanol in PCO (mg/g) 2 | - | - | 692 | 356 | 69 | 492 | |
| Final total PCO amount (mg) 2 | 982 | 610 | 592 | 518 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, K.-H.; Kim, J.-E.; Baek, S.H. Cuban Policosanol (Raydel®) Potently Protects the Liver, Ovary, and Testis with an Improvement in Dyslipidemia in Hyperlipidemic Zebrafish: A Comparative Study with Three Chinese Policosanols. Molecules 2023, 28, 6609. https://doi.org/10.3390/molecules28186609
Cho K-H, Kim J-E, Baek SH. Cuban Policosanol (Raydel®) Potently Protects the Liver, Ovary, and Testis with an Improvement in Dyslipidemia in Hyperlipidemic Zebrafish: A Comparative Study with Three Chinese Policosanols. Molecules. 2023; 28(18):6609. https://doi.org/10.3390/molecules28186609
Chicago/Turabian StyleCho, Kyung-Hyun, Ji-Eun Kim, and Seung Hee Baek. 2023. "Cuban Policosanol (Raydel®) Potently Protects the Liver, Ovary, and Testis with an Improvement in Dyslipidemia in Hyperlipidemic Zebrafish: A Comparative Study with Three Chinese Policosanols" Molecules 28, no. 18: 6609. https://doi.org/10.3390/molecules28186609
APA StyleCho, K.-H., Kim, J.-E., & Baek, S. H. (2023). Cuban Policosanol (Raydel®) Potently Protects the Liver, Ovary, and Testis with an Improvement in Dyslipidemia in Hyperlipidemic Zebrafish: A Comparative Study with Three Chinese Policosanols. Molecules, 28(18), 6609. https://doi.org/10.3390/molecules28186609