Simultaneous Determination of 54 Pesticides in Proso Millet Using QuEChERS with Liquid Chromatography-Tandem Mass Spectrometry (LC–MS/MS)
Abstract
1. Introduction
2. Results and Discussion
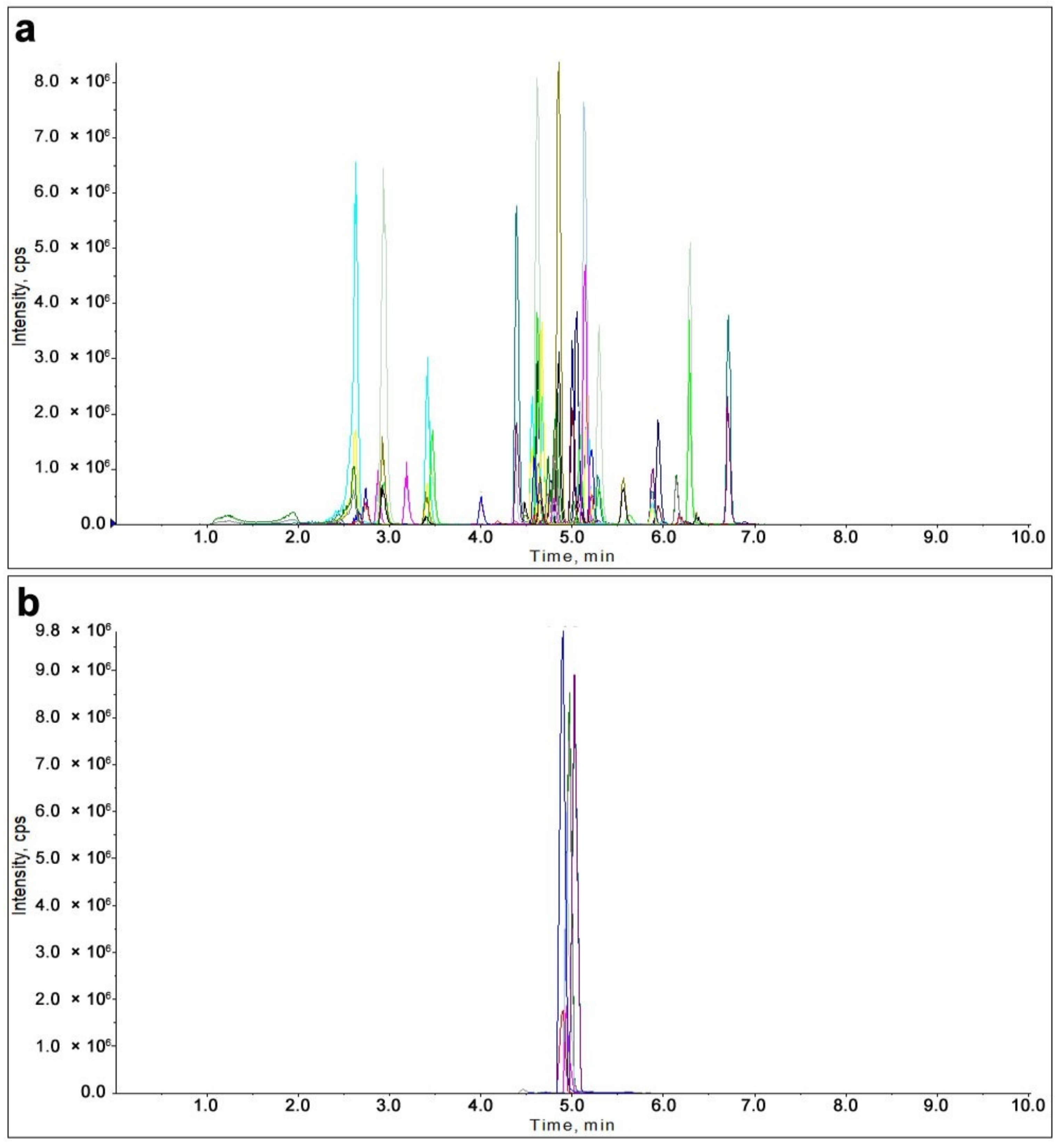
2.1. Optimization of Instrumental Conditions
2.2. Optimization of QuEChERS Method
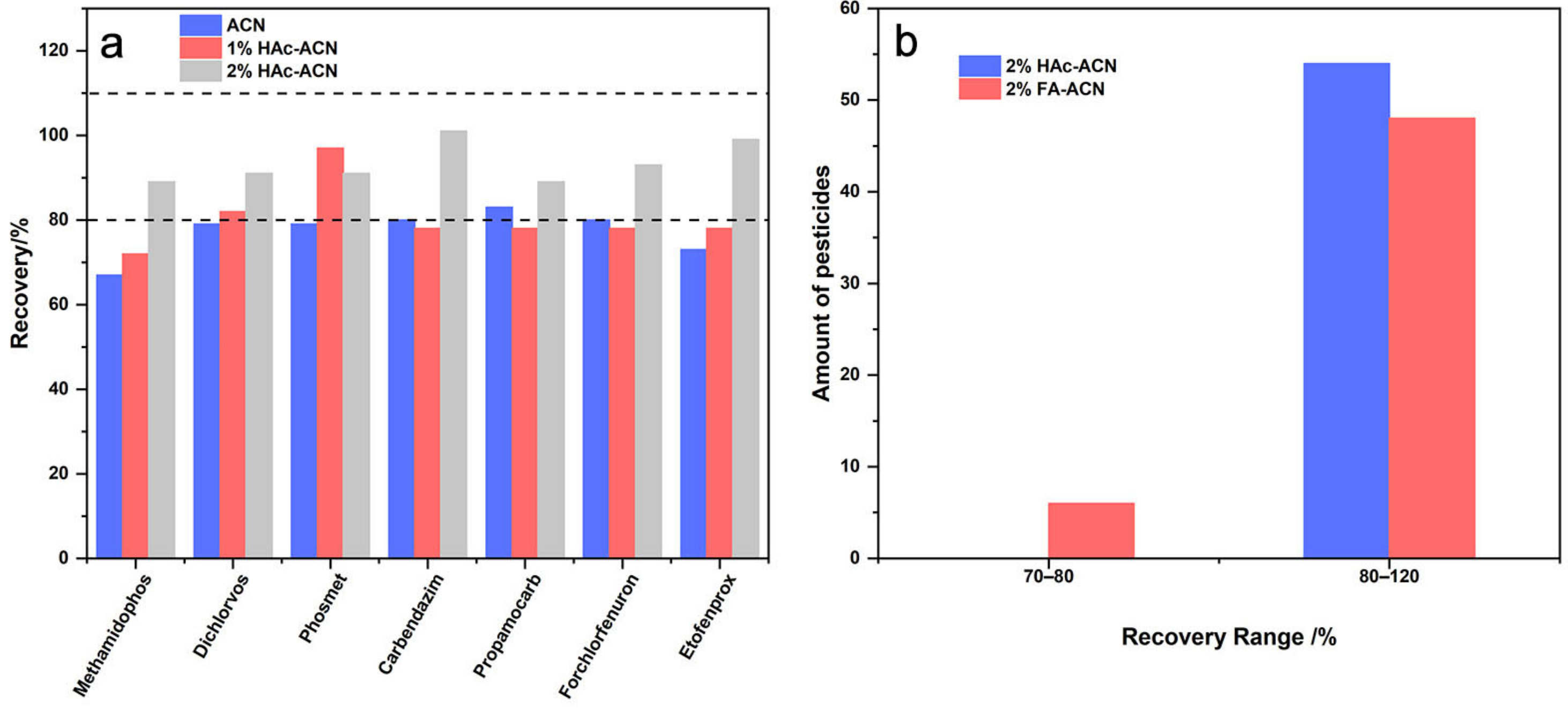
2.2.1. Extraction
2.2.2. Clean-Up
2.3. Method Validation
2.4. Real Sample Analysis
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Sample Pretreatment
3.3. LC–MS/MS Analysis
3.4. Method Validation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Ren, J.; Ma, C.; Li, M.; Dang, Y.; Yu, X.; Du, S. Physicochemical, structural and functional properties of non-waxy and waxy proso millet protein. Foods 2023, 12, 1116. [Google Scholar] [CrossRef] [PubMed]
- Zheng, N.-N.; Yun, T.-T.; Yao, Y.; Ren, G.-X.; Wang, C.-L.; Qi, W.-T. Comparative analysis of nutritional and functional components in different breeds of proso millet. Sci. Technol. Food Ind. 2017, 1, 348–359. [Google Scholar]
- Lu, R.; Tian, Q.; Sun, N.; Qiao, Z.-J.; Zhang, L.-Z.; Niu, W.; Shan, L.; Ji, P.-S. Determination of phenolic compounds in proso millet by high performance liquid chromatography. Food Sci. 2014, 35, 152–155. [Google Scholar]
- Nishizawa, N.; Fudamoto, Y. The elevation of plasma concentration of high-density lipoprotein chloesterol in mice fed with protein from proso millet. Biosci. Biotechnol. Biochem. 1995, 59, 333–335. [Google Scholar] [CrossRef]
- Kumari, K.S.; Thayumanavan, B. Characterization of starches of proso, foxtail, barnyard, kodo and little millets. Plant Foods Hum. Nutr. 1998, 53, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Denery-Papini, S.; Nicolas, Y.; Popineau, Y. Efficiency and limitation of immunochemical assays for the testing of gluten-free foods. J. Cereal Sci. 1999, 30, 121–131. [Google Scholar] [CrossRef]
- Nishizawa, N.; Sato, D.; Ito, Y.; Nagasawa, T.; Hatakeyama, Y.; Choi, M.-R.; Choi, Y.-Y.; Wei, Y.M. Effects of dietary protein of proso millet on liver injury induced by D-galactosamine in rats. Biosci. Biotechnol. Biochem. 2002, 66, 92–96. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Jung, M.W.; Lee, J.; Lee, J.; Shin, Y.; Kim, J.H. Dissipation of the insecticide cyantraniliprole and its metabolite IN-J9Z38 in proso millet during cultivation. Sci. Rep. 2019, 9, 11648. [Google Scholar] [CrossRef] [PubMed]
- Song, M.-H.; Yu, J.-W.; Keum, Y.-S.; Lee, J.-H. Dynamic modeling of pesticide residue in proso millet under multiple application situations. Environ. Pollut. 2023, 334, 121993. [Google Scholar] [CrossRef]
- Wang, F.Y.; Li, X.; Yu, S.M.; He, S.H.; Cao, D.T.; Yao, S.J.; Fang, H.; Yu, Y.L. Chemical factors affecting uptake and translocation of six pesticides in soil by maize (Zea mays L.). J. Hazard. Mater. 2021, 405, 124269. [Google Scholar] [CrossRef]
- Ju, C.; Dong, S.; Zhang, H.; Yao, S.; Wang, F.; Cao, D.; Xu, S.; Fang, H.; Yu, Y. Subcellular distribution governing accumulation and translocation of pesticides in wheat (Triticum aestivum L.). Chemosphere 2020, 248, 126024. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Shen, J.-S.; Bai, H.-H.; Guo, L.; Tang, J.-J.; Jiang, Y.-B.; Xie, J.-W. A photoluminescent nanocrystal-based signaling protocol highly sensitive to nerve agents and highly toxic organophosphate pesticides. Analyst 2009, 134, 2153–2157. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Anastassiades, M.; Lehotay, S.J.; Stajnbaher, D.; Schenck, F. Fast and easy multiresidue method employing acetonitrile extraction/partitioning and “dispersive solid-phase extraction” for the determination of pesticide residues in produce. J. AOAC Int. 2003, 86, 412–431. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Han, M.; Dai, X.H.; Yang, X.F.; Yi, S. A multi-residue method for the determination of 124 pesticides in rice by modified QuEChERS extraction and gas chromatography–tandem mass spectrometry. Food Chem. 2013, 138, 1198–1205. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Wang, L.; Peng, Y.; Luo, M.; Wang, W.; Liu, X. Multiresidue analysis of over 200 pesticides in cereals using a QuEChERS and gas chromatography–tandem mass spectrometry-based method. Food Chem. 2015, 169, 372–380. [Google Scholar] [CrossRef] [PubMed]
- Kolberg, D.I.; Prestes, O.D.; Adaime, M.B.; Zanella, R. Development of a fast multiresidue method for the determination of pesticides in dry samples (wheat grains, flour and bran) using QuEChERS based method and GC-MS. Food Chem. 2011, 165, 1436–1442. [Google Scholar] [CrossRef]
- Ruan, H.; Rong, W.; Ma, Y.; Ji, W.; Liu, H.; Song, N. Determination of 34 pesticides residues in rice, proso millet and wheat with QuEChERS-online gel permeation chromatography-gas chromatography-mass spectrometry. Chin. J. Chromatogr. 2013, 31, 1211–1217. [Google Scholar] [CrossRef] [PubMed]
- Demir, E.; Göktug, Ö.; Inam, R.; Doyduk, D. Development and characterization of iron (III) phthalocyanine modified carbon nanotube paste electrodes and application for determination of fluometuron herbicides as an electrochemical sensor. J. Electroanal. Chem. 2021, 895, 115389. [Google Scholar] [CrossRef]
- Krone, N.; Hughes, B.A.; Lavery, G.G.; Stewart, P.M.; Arlt, W.; Shackleton, C.H. Gas chromatography/mass spectrometry (GC/MS) remains a pre-eminent discovery tool in clinical steroid investigations even in the era of fast liquid chromatography tandem mass spectrometry (LC/MS/MS). J. Steroid Biochem. Mol. Biol. 2010, 121, 496–504. [Google Scholar] [CrossRef]
- Jiao, W.; Xiao, Y.; Qian, X.; Tong, M.; Hu, Y.; Hou, R.; Hua, R. Optimized combination of dilution and refined QuEChERS to overcome matrix effects of six types of tea for determination eight neonicotinoid insecticides by ultra performance liquid chromatography–electrospray tandem mass spectrometry. Food Chem. 2016, 210, 26–34. [Google Scholar] [CrossRef]
- Field, J.K.; Euerby, M.R.; Haselmann, K.F.; Petersson, P. Investigation into reversed-phase chromatography peptide separation systems Part IV: Characterisation of mobile phase selectivity differences. J. Chromatogr. A 2021, 1641, 461986. [Google Scholar] [CrossRef] [PubMed]
- McCalley, D.V. Effect of buffer on peak shape of peptides in reversed-phase high performance liquid chromatography. J. Chromatogr. A 2004, 1038, 77–84. [Google Scholar] [CrossRef] [PubMed]
- EN15662-2018; Foods of Plant Origin—Determination of Pesticide Residues Using GC-MS and/or LC-MS/MS Following Acetonitrile Extration/Partitioning and Clean-Up by Dispersive SPE—QuEChERS-Method. Polski Komitet Normalizacyjny: Warsaw, Poland, 2008.
- Chiesa, L.M.; Labella, G.F.; Panseri, S.; Britti, D.; Galbiati, F.; Villa, R.; Arioli, F. Accelerated solvent extraction by using an ‘in-line’ clean-up approach for multiresidue analysis of pesticides in organic honey. Food Addit. Contam. Part A Chem. Anal. Control. Expo. Risk Assess. 2017, 34, 809–818. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Sapozhnikova, Y.; Lehotay, S.J. Method validation for 243 pesticides and environmental contaminants in meats and poultry by tandem mass spectrometry coupled to low-pressure gas chromatography and ultrahigh-performance liquid chromatography. Food Control 2016, 66, 270–282. [Google Scholar] [CrossRef]
- Li, P.P.; Cheng, J.; Le, Y. Determination of 46 pesticide residues in sulfur-containing vegetables using solid-phase extraction and gas chromatography-triple quadrupole mass spectrometry. J. Instrum. Anal. 2015, 34, 421–427. [Google Scholar]
- Wang, S.W.; Wang, X.N.; He, Q.; Lin, H.D.; Chang, H.; Sun, H.B.; Liu, Y.P. Simultaneous Determination of Seven Pesticides and Metabolite Residues in Litchi and Longan through High-Performance Liquid Chromatography-Tandem Mass Spectrometry with Modified QuEChERS. Molecules 2022, 27, 5737. [Google Scholar] [CrossRef] [PubMed]
- Ajibola, A.S.; Tislera, S.; Zwiener, C. Simultaneous determination of multiclass antibiotics in sewage sludge based on QuEChERS extraction and liquid chromatography-tandem mass spectrometry. Anal. Methods 2020, 12, 576–586. [Google Scholar] [CrossRef]
- Hou, X.; Qiao, T.; Zhao, Y.; Liu, D. Dissipation and safety evaluation of afidopyropen and its metabolite residues in supervised cotton field. Ecotox. Environ. Safe 2019, 180, 227–233. [Google Scholar] [CrossRef] [PubMed]
- NY/T 788-2018; Guideline for the Testing of Pesticide Residues in Crops. Ministry of Agriculture and Rural Affairs of the People’s Republic of China: Beijing, China, 2018. Available online: https://www.sdtdata.com/fx/fmoa/tsLibCard/169332.html (accessed on 20 July 2023).
- SANTE/11312/2021; Guidance Document on Analytical Quality Control and Method Validation Procedures for Pesticide Residues and Analysis in Food and Feed. European Commission: Brussels, Belgium, 2017. Available online: https://www.accredia.it/en/documento/guidance-sante-11312-2021-analytical-quality-control-and-method-validation-procedures-for-pesticide-residues-analysis-in-food-and-feed/ (accessed on 29 August 2023).
- Chen, X.X.; He, S.; Gao, Y.M.; Ma, Y.C.; Hu, J.Y.; Liu, X.L. Dissipation behavior, residue distribution and dietary risk assessment of field-incurred boscalid and pyraclostrobin in grape and grape field soil via MWCNTs-based QuEChERS using an RRLC-QqQ-MS/MS technique. Food Chem. 2019, 274, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Morais, E.H.d.C.; Collins, C.H.; Jardim, I.C.S.F. Pesticide determination in sweet peppers using QuEChERS and LC–MS/MS. Food Chem. 2018, 249, 77–83. [Google Scholar] [CrossRef]
- Pareja, L.; Cesio, V.; Heinzen, H.; Fernández-Alba, A.R. Evaluation of various QuEChERS based methods for the analysis of herbicides and other commonly used pesticides in polished rice by LC–MS/MS. Talanta 2011, 83, 1613–1622. [Google Scholar] [CrossRef]
- GB 2763-2021; National Food Safety Standard-Maximum Residue Limits for Pesticides in Food. Ministry of Agriculture and Rural Affairs of the People’s Republic of China: Beijing, China, 2021. Available online: https://www.sdtdata.com/fx/fmoa/tsLibCard/183688.html (accessed on 28 May 2023).

| No. | Pesticide | Category 1 | RT | Parent Ion | Product Ion 2 | DP | CE |
|---|---|---|---|---|---|---|---|
| Positive Mode | |||||||
| 1 | Methamidophos | I | 2.34 | 142.0 | 94.0 *; 125.0 | 57 | 19; 18 |
| 2 | Phorate | I | 5.54 | 261.0 | 75.0 *; 47.0 | 51 | 21; 53 |
| 3 | Omethoate | I | 2.76 | 214.0 | 183.0 *; 109.0 | 60 | 16; 36 |
| 4 | Dichlorvos | I | 4.62 | 221.0 | 109.0 *; 127.0 | 70 | 23; 27 |
| 5 | Triazophos | I | 5.07 | 314.1 | 162.1 *; 119.1 | 80 | 24; 50 |
| 6 | Dimethoate | I | 3.71 | 230.0 | 199.0 *; 125.0 | 56 | 13; 29 |
| 7 | Chlorpyrifos | I | 6.11 | 349.9 | 197.9 *; 97.0 | 75 | 28; 45 |
| 8 | Acephate | I | 2.68 | 184.0 | 143.0 *; 125.0 | 50 | 12; 25 |
| 9 | Malathion | I | 5.03 | 331.0 | 127.0 *; 99.0 | 70 | 16; 32 |
| 10 | Phosalone | I | 5.48 | 368.0 | 182.0 *; 322.0 | 76 | 20; 13 |
| 11 | Phosmet | I | 4.90 | 318.0 | 160.0 *; 133.0 | 61 | 17; 49 |
| 12 | Isocarbophos | I | 5.20 | 231.0 | 121.0 *; 109.0 | 100 | 26; 38 |
| 13 | Diazinon | I | 5.42 | 305.1 | 169.0 *; 153.1 | 90 | 28; 28 |
| 14 | Profenofos | I | 5.81 | 372.9 | 302.9 *; 344.9 | 80 | 25; 18 |
| 15 | Phorate Sulfone | I | 4.78 | 293.0 | 97.0 *; 115.0 | 65 | 50; 35 |
| 16 | Phorate Sulfoxide | I | 4.74 | 277.0 | 199.0 *; 153.0 | 25 | 13; 19 |
| 17 | Tau-Fluvalinate | I | 6.53 | 503.1 | 208.1 *; 181.1 | 61 | 16; 38 |
| 18 | Iprodione | F | 5.26 | 330.0 | 245.0 *; 288.0 | 30 | 20; 18 |
| 19 | Deltamethrin | I | 6.37 | 523.0 | 280.9 *; 506.0 | 55 | 23; 16 |
| 20 | Fenpropathrin | I | 6.11 | 350.2 | 125.1 *; 97.1 | 85 | 23; 46 |
| 21 | Triadimefon | F | 5.07 | 294.1 | 197.1 *; 225.1 | 70 | 21; 17 |
| 22 | Aldicarb | I | 4.30 | 116.1 | 89.0 *; 70.0 | 25 | 14; 14 |
| 23 | Aldicarb Sulfone | I | 2.90 | 240.1 | 148.0 *; 166.1 | 30 | 17; 16 |
| 24 | Aldicarb Sulfoxide | I | 2.82 | 207.1 | 132.0 *; 89.0 | 55 | 9; 20 |
| 25 | Carbofuran | I | 4.63 | 222.1 | 165.1 *; 123.0 | 70 | 16; 29 |
| 26 | 3-Hydroxy Carbofuran | I | 3.63 | 238.1 | 181.1 *; 163.1 | 70 | 16; 18 |
| 27 | Methomyl | I | 3.05 | 163.1 | 88.0 *; 106.0 | 38 | 12; 14 |
| 28 | Carbaryl | I | 4.69 | 202.1 | 145.1 *; 127.1 | 56 | 15; 40 |
| 29 | Carbendazim | F | 3.06 | 192.1 | 160.1 *; 132.1 | 80 | 25; 41 |
| 30 | Phoxim | I | 5.41 | 299.1 | 129.0 *; 153.0 | 55 | 18; 10 |
| 31 | Pyridaben | A | 6.47 | 365.1 | 309.1 *; 147.1 | 77 | 17; 34 |
| 32 | Pyrimethanil | F | 4.97 | 200.1 | 183.1 *; 168.1 | 30 | 33; 40 |
| 33 | Difenoconazole | F | 5.55 | 406.1 | 251.0 *; 337.0 | 105 | 35; 24 |
| 34 | Acetamiprid | I | 3.64 | 223.1 | 126.0 *; 99.0 | 65 | 28; 60 |
| 35 | Imidacloprid | I | 3.38 | 256.1 | 175.1 *; 209.1 | 45 | 27; 22 |
| 36 | Dimethomorph | F | 5.01 | 388.1 | 301.1 *; 165.1 | 105 | 29; 43 |
| 37 | Pendimethalin | H | 6.16 | 282.1 | 212.1 *; 194.1 | 40 | 15; 28 |
| 38 | Azoxystrobin | F | 4.86 | 404.1 | 372.1 *; 344.1 | 80 | 20; 34 |
| 39 | Thiamethoxam | I | 3.10 | 292.0 | 211.1 *; 181.1 | 30 | 16; 30 |
| 40 | Chlorfluazuron | I | 6.32 | 540.0 | 382.9 *; 384.9 | 70 | 30; 30 |
| 41 | Prochloraz | F | 5.45 | 376.2 | 308.0 *; 266.0 | 20 | 15; 22 |
| 42 | Chlorbenzuron | I | 5.33 | 309.0 | 156.0 *; 139.0 | 50 | 18; 40 |
| 43 | Diflubenzuron | I | 5.24 | 311.0 | 158.0 *; 141.0 | 45 | 20; 49 |
| 44 | Propamocarb | F | 2.77 | 189.2 | 102.1 *; 74.0 | 70 | 24; 34 |
| 45 | Forchlorfenuron | R | 4.83 | 248.1 | 129.0 *; 93.0 | 50 | 23; 47 |
| 46 | Etofenprox | I | 6.90 | 394.2 | 177.1 *; 107.0 | 30 | 19; 59 |
| 47 | Chlorantraniliprole | I | 4.84 | 484.0 | 285.9 *; 452.9 | 45 | 19; 25 |
| 48 | Pyraclostrobin | F | 5.38 | 388.1 | 194.1 *; 163.1 | 50 | 18; 36 |
| 49 | Metalaxyl | F | 4.82 | 280.2 | 220.1 *; 192.1 | 75 | 18; 24 |
| 50 | Paclobutrazol | R | 5.02 | 294.1 | 70.0 *; 125.0 | 90 | 40; 45 |
| Negative Mode | |||||||
| 51 | Fipronil | I | 5.17 | 434.9 | 330.0 *; 250.0 | −25 | −24; −38 |
| 52 | Fipronil Desulfinyl | I | 5.12 | 387.0 | 351.0 *; 282.0 | −30 | −19; −47 |
| 53 | Fipronil Sulfone | I | 5.26 | 450.9 | 414.9 *; 282.0 | −28 | −26; −38 |
| 54 | Fipronil Sulfide | I | 5.20 | 418.9 | 262.0 *; 383.0 | −20 | −35; −22 |
| Pesticide | R2 | LOQ μg/kg | ME (%) | Recovery, % (RSD, %) | ||
|---|---|---|---|---|---|---|
| 0.01 mg/kg | 0.1 mg/kg | 0.2 mg/kg | ||||
| Methamidophos | 0.9996 | 3.3 | −5.7 | 86 (2) | 89 (2) | 89 (2) |
| Phorate | 0.9900 | 2.0 | 6.8 | 95 (17) | 85 (19) | 105 (5) |
| Omethoate | 0.9987 | 0.50 | −18.0 | 98 (3) | 100 (2) | 94 (6) |
| Dichlorvos | 0.9932 | 3.3 | −18.5 | 93 (3) | 91 (6) | 95 (4) |
| Triazophos | 0.9900 | 2.0 | −36.4 | 103 (3) | 102 (6) | 107 (3) |
| Dimethoate | 0.9975 | 1.7 | −9.8 | 101 (3) | 101 (2) | 98 (2) |
| Chlorpyrifos | 0.9970 | 4.0 | −29.0 | 94 (5) | 98 (5) | 100 (5) |
| Acephate | 0.9986 | 6.7 | −8.0 | 99 (5) | 92 (7) | 98 (3) |
| Malathion | 0.9905 | 1.0 | −45.7 | 99 (7) | 98 (17) | 100 (5) |
| Phosalone | 0.9977 | 2.5 | −38.4 | 99 (11) | 94 (10) | 104 (6) |
| Phosmet | 0.9939 | 10.0 | −43.4 | 92 (5) | 91 (11) | 96 (6) |
| Isocarbophos | 0.9943 | 5.0 | −48.0 | 103 (4) | 101 (3) | 101 (1) |
| Diazinon | 0.9976 | 3.3 | −17.0 | 101 (4) | 106 (6) | 100 (2) |
| Profenofos | 0.9994 | 3.3 | −32.0 | 103 (8) | 100 (7) | 101 (7) |
| Phorate Sulfone | 0.9904 | 0.67 | −32.3 | 105 (3) | 100 (6) | 101 (7) |
| Phorate Sulfoxide | 0.9929 | 0.50 | −21.7 | 106 (8) | 102 (5) | 108 (1) |
| Tau-Fluvalinate | 0.9909 | 0.33 | −32.1 | 100 (11) | 93 (9) | 99 (11) |
| Iprodione | 0.9989 | 2.0 | −41.5 | 102 (8) | 106 (2) | 97 (2) |
| Deltamethrin | 0.9960 | 8.3 | −28.6 | 104 (8) | 104 (5) | 92 (14) |
| Fenpropathrin | 0.9956 | 2.0 | −29.7 | 97 (12) | 104 (6) | 104 (4) |
| Triadimefon | 0.9994 | 5.0 | −10.7 | 107 (4) | 96 (4) | 103 (2) |
| Aldicarb | 0.9981 | 0.50 | 1.7 | 103 (11) | 100 (4) | 105 (2) |
| Aldicarb Sulfone | 0.9994 | 3.0 | −10.2 | 107 (7) | 102 (4) | 100 (6) |
| Aldicarb Sulfoxide | 0.9972 | 0.033 | −11.9 | 104 (10) | 100 (9) | 97 (8) |
| Carbofuran | 0.9968 | 1.0 | −32.4 | 105 (8) | 109 (2) | 104 (3) |
| 3-HydroxyCarbofuran | 0.9991 | 2.0 | −26.1 | 103 (3) | 104 (3) | 100 (5) |
| Methomyl | 0.9999 | 0.67 | 3.3 | 98 (5) | 105 (3) | 105 (1) |
| Carbaryl | 0.9946 | 5.0 | −30.2 | 95 (11) | 96 (13) | 105 (4) |
| Carbendazim | 0.9995 | 0.67 | −26.5 | 99 (2) | 101 (2) | 96 (2) |
| Phoxim | 0.9982 | 10.0 | −24.3 | 99 (3) | 93 (1) | 102 (3) |
| Pyridaben | 0.9981 | 1.0 | −43.3 | 102 (8) | 114 (4) | 107 (1) |
| Pyrimethanil | 0.9986 | 2.5 | −34.9 | 104 (8) | 94 (5) | 100 (4) |
| Difenoconazole | 0.9970 | 2.0 | −31.7 | 106 (5) | 109 (4) | 107 (3) |
| Acetamiprid | 0.9987 | 0.50 | −23.4 | 100 (2) | 101 (2) | 107 (3) |
| Imidacloprid | 0.9978 | 2.0 | −44.3 | 104 (3) | 101 (3) | 96 (3) |
| Dimethomorph | 0.9970 | 3.3 | −4.4 | 100 (6) | 96 (5) | 96 (4) |
| Pendimethalin | 0.9955 | 10.0 | 30.6 | 95 (6) | 100 (6) | 94 (11) |
| Azoxystrobin | 0.9983 | 2.5 | −0.8 | 93 (10) | 99 (7) | 104 (4) |
| Thiamethoxam | 0.9998 | 1.0 | −14.1 | 100 (5) | 105 (5) | 100 (5) |
| Chlorfluazuron | 0.9998 | 0.25 | −39.4 | 106 (5) | 99 (5) | 95 (7) |
| Prochloraz | 0.9976 | 0.50 | 21.0 | 90 (20) | 99 (4) | 96 (9) |
| Chlorbenzuron | 0.9972 | 1.0 | −40.9 | 102 (5) | 103 (3) | 103 (5) |
| Diflubenzuron | 0.9993 | 2.0 | −39.6 | 102 (7) | 101 (3) | 106 (2) |
| Propamocarb | 0.9974 | 0.40 | 10.1 | 83 (2) | 89 (3) | 90 (5) |
| Forchlorfenuron | 0.9982 | 2.0 | −67.3 | 98 (3) | 93 (4) | 96 (2) |
| Etofenprox | 0.9947 | 1.0 | 6.9 | 102 (7) | 99 (5) | 100 (5) |
| Chlorantraniliprole | 0.9980 | 2.0 | −57.2 | 98 (8) | 104 (5) | 85 (4) |
| Pyraclostrobin | 0.9924 | 1.0 | −29.0 | 103 (8) | 98 (5) | 101 (3) |
| Metalaxyl | 0.9964 | 1.0 | −40.0 | 105 (4) | 102 (4) | 100 (4) |
| Paclobutrazol | 0.9963 | 3.3 | −54.0 | 104 (7) | 101 (4) | 102 (3) |
| Fipronil | 0.9946 | 1.0 | 58.6 | 99 (1) | 103 (4) | 103 (1) |
| Fipronil Desulfinyl | 0.9935 | 2.0 | −2.7 | 105 (1) | 102 (2) | 106 (1) |
| Fipronil Sulfone | 0.9958 | 2.0 | −4.9 | 102 (1) | 105 (2) | 105 (1) |
| Fipronil Sulfide | 0.9936 | 1.0 | 23.0 | 106 (1) | 103 (1) | 106 (1) |
| Sample No. | Pesticide | Concentration (mg/kg) | MRL (mg/kg) |
|---|---|---|---|
| 7 | Aldicarb sulfone | 0.022 | NA 1 |
| 25 | Imidacloprid | 0.011 | NA 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, C.; Ren, P.; Qi, Y.; Yang, Y.; Qin, S. Simultaneous Determination of 54 Pesticides in Proso Millet Using QuEChERS with Liquid Chromatography-Tandem Mass Spectrometry (LC–MS/MS). Molecules 2023, 28, 6575. https://doi.org/10.3390/molecules28186575
Ding C, Ren P, Qi Y, Yang Y, Qin S. Simultaneous Determination of 54 Pesticides in Proso Millet Using QuEChERS with Liquid Chromatography-Tandem Mass Spectrometry (LC–MS/MS). Molecules. 2023; 28(18):6575. https://doi.org/10.3390/molecules28186575
Chicago/Turabian StyleDing, Chao, Pengcheng Ren, Yanli Qi, Yanmei Yang, and Shu Qin. 2023. "Simultaneous Determination of 54 Pesticides in Proso Millet Using QuEChERS with Liquid Chromatography-Tandem Mass Spectrometry (LC–MS/MS)" Molecules 28, no. 18: 6575. https://doi.org/10.3390/molecules28186575
APA StyleDing, C., Ren, P., Qi, Y., Yang, Y., & Qin, S. (2023). Simultaneous Determination of 54 Pesticides in Proso Millet Using QuEChERS with Liquid Chromatography-Tandem Mass Spectrometry (LC–MS/MS). Molecules, 28(18), 6575. https://doi.org/10.3390/molecules28186575





