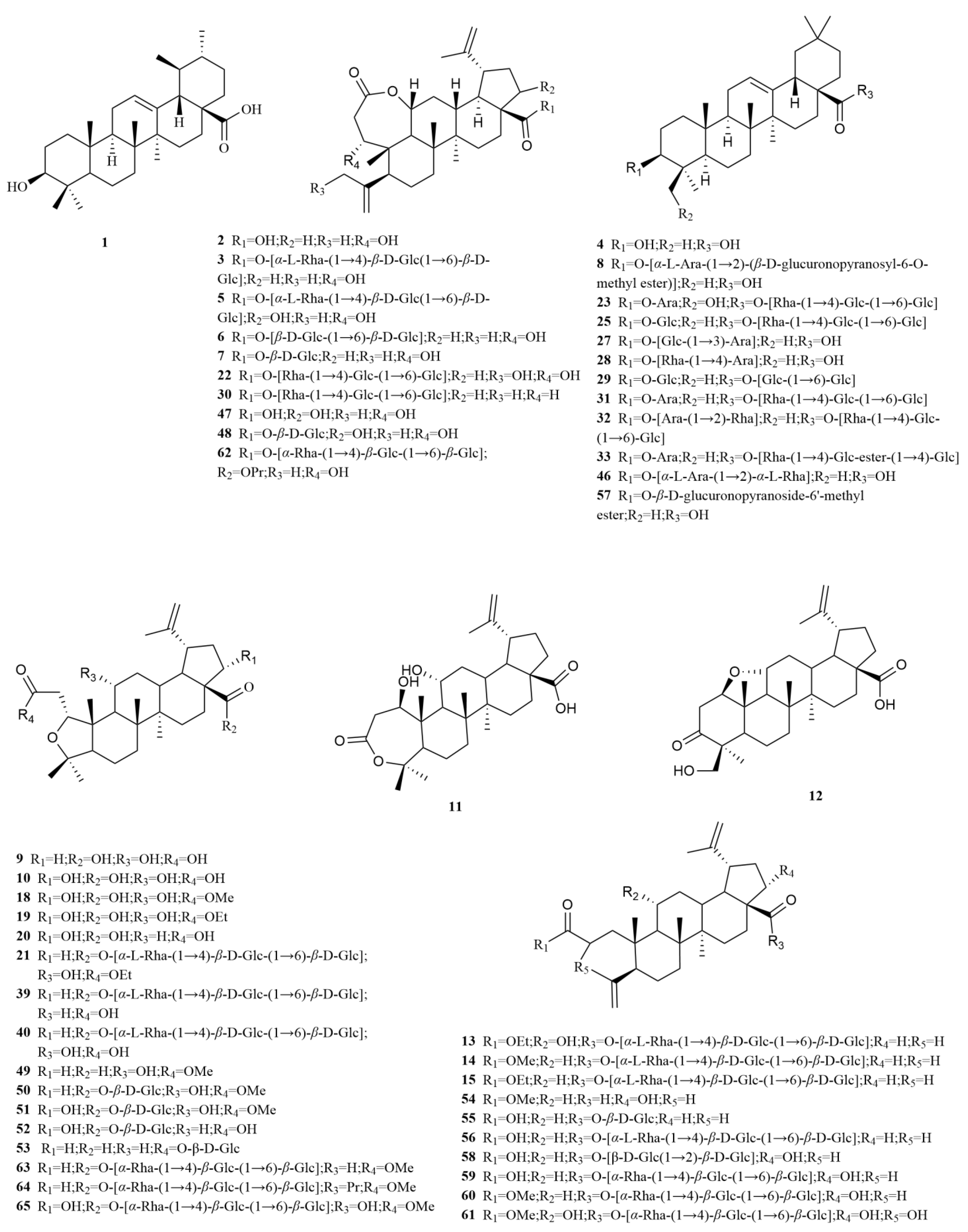
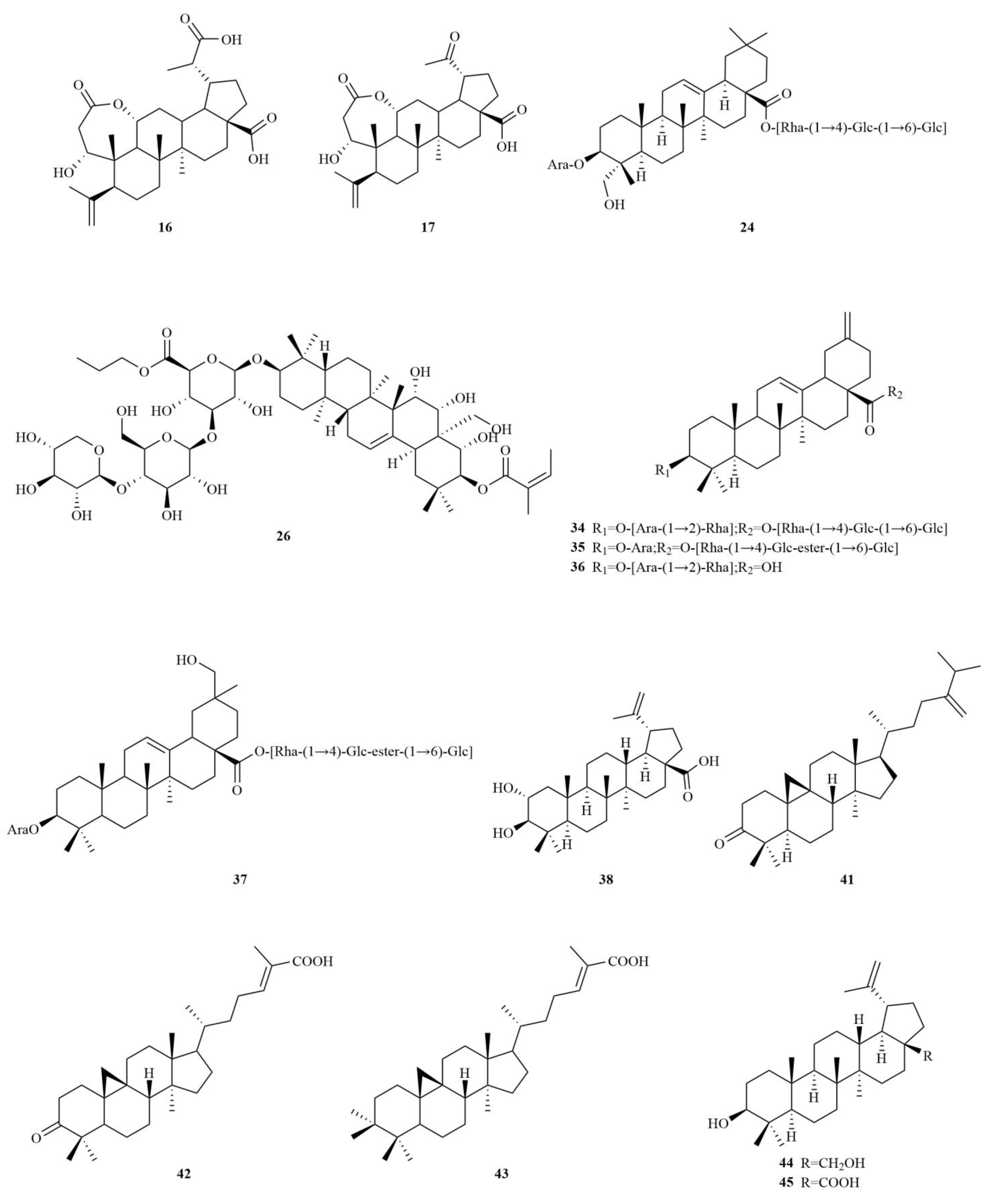
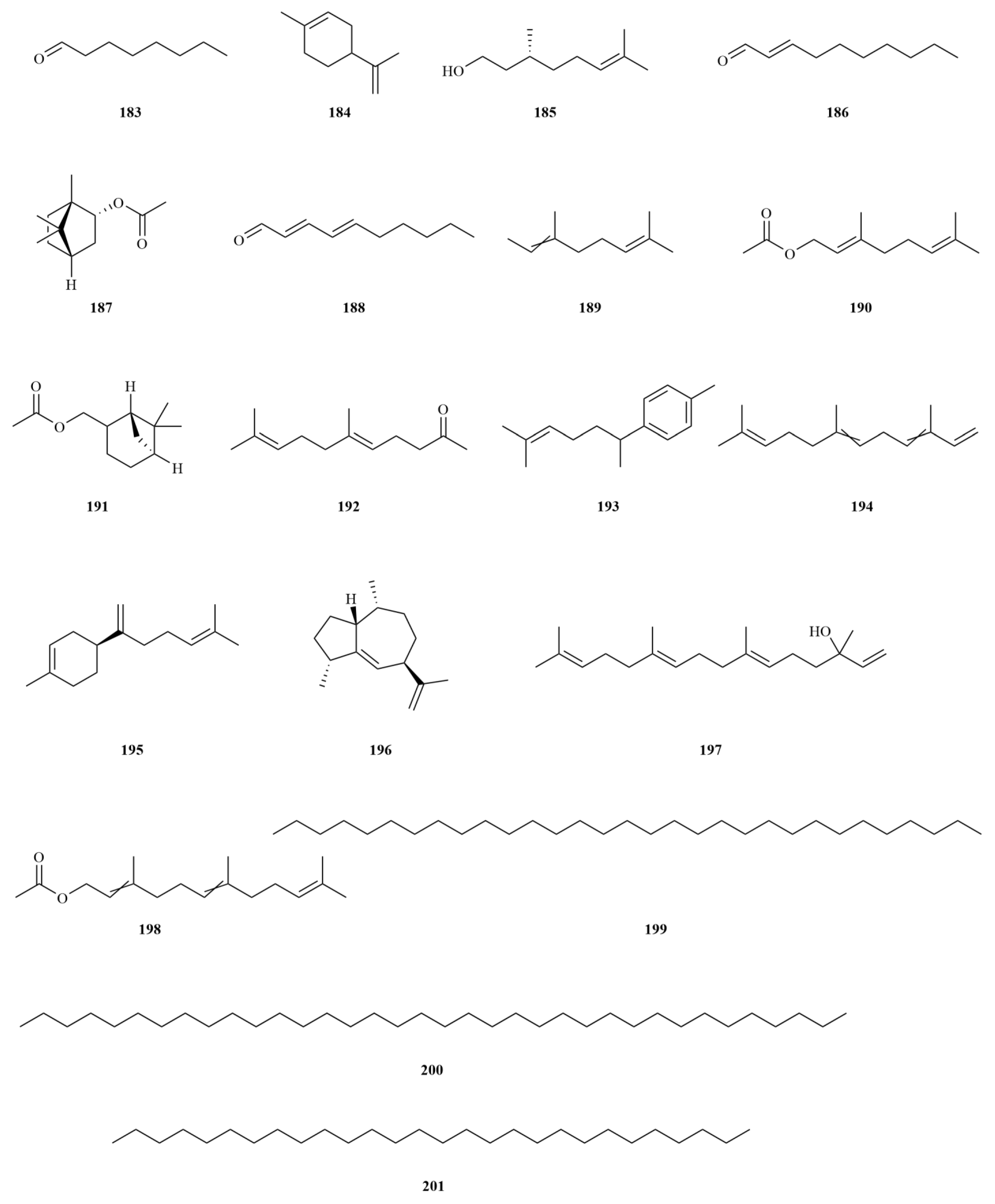
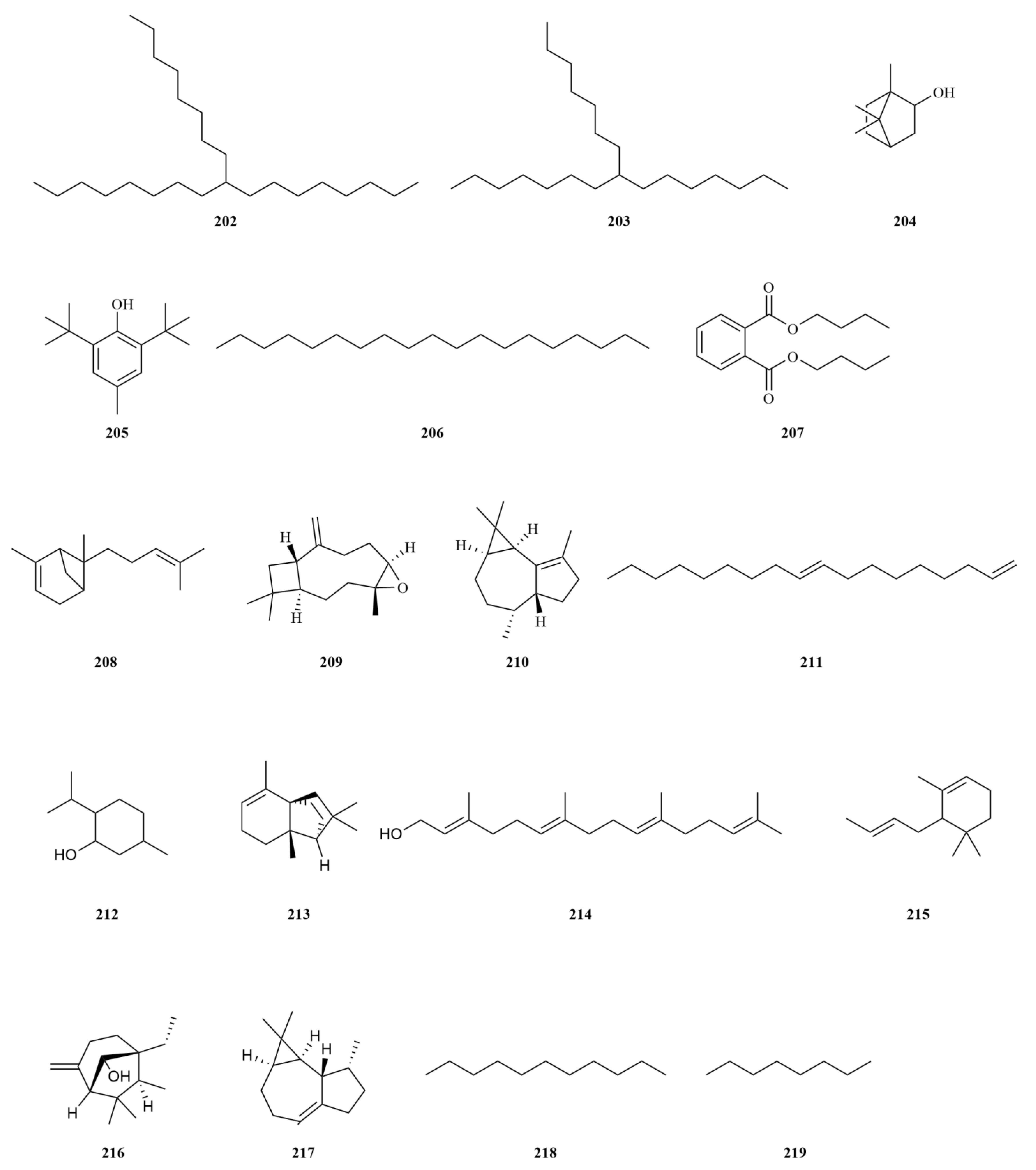
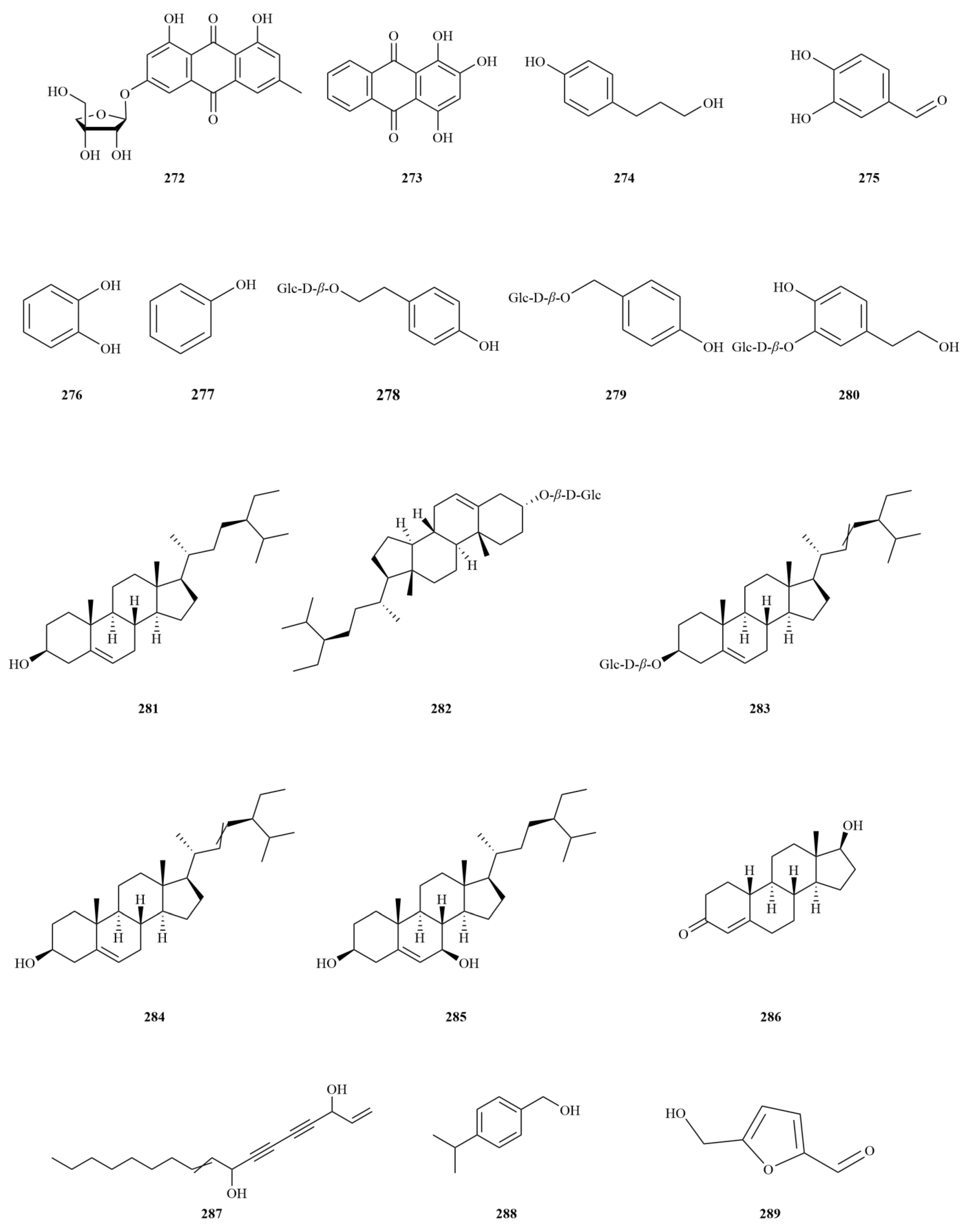
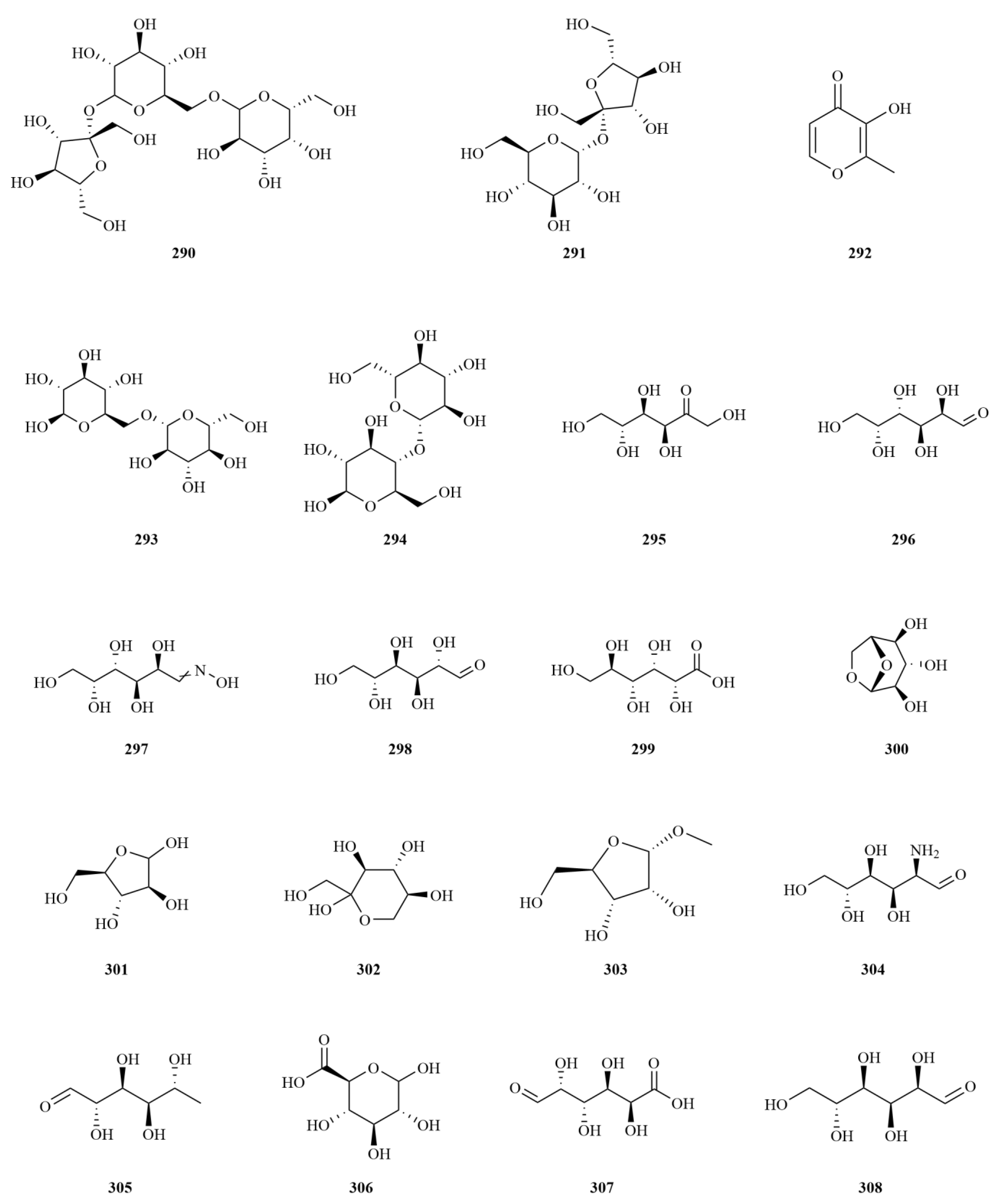
2.1. Terpenoids
E. sessiliflorus primarily consists of triterpenoids and their saponins, with smaller amounts of monoterpenoids and sesquiterpenoids. The triterpenoids are predominantly isolated from the fruits and leaves, leading to the identification of seventy-six triterpenoids and their saponins (
Table 1,
Figure 2). These compounds mainly belong to the oleanane-type, lupane-type, and 3,4-seco-lupane triterpenoid categories. Notably, the 3,4-seco-lupane triterpenoids are unique to the Eleutherococcus genus, with the first 3,4-seco-lupane triterpenoid saponin being isolated in 1985 [
14]. Later, this type of compound has been discovered in various Eleutherococcus species. Within
E. sessiliflorus, thirty-six 3,4-seco-lupane triterpenoids have been isolated and identified, including compounds like chiisanogenin (
2), chiisanoside (
3), elesesterpene C (
13), elesesterpene D (
14), elesesterpene H (
18), elesesterpene I (
19) [
15,
16]. Chiisanogenin and chiisanoside produced significant anti-inflammatory effects at doses of 10 and 30 mg/kg, with the effect of chiisanogenin being superior to chiisanoside [
17].
Among these triterpenoids, elesesterpenes A-K, isolated from the leaves, have garnered significant attention due to their remarkable anti-inflammatory activity. Moreover, these compounds exhibit significant anti-proliferative effects on various human cancer cell lines, including hepatocellular carcinomas (HepG2), lung adenocarcinoma (A549), and glioblastoma (LN229) [
16]. On the other hand, acanthosessiliosides A-O, isolated from the fruits, have demonstrated inhibitory effects on lipopolysaccharide-induced RAW264.7 cells. Additionally, some of these compounds have exhibited inhibitory activities against six different human cancer cell lines, including colon adenocarcinoma, breast adenocarcinoma, ovarian adenocarcinoma, cervix adenocarcinoma, hepatoma, and melanoma [
7,
9]. The lupane-type compounds in
E. sessiliflorus include betulin (
44) and betulinic acid (
45). Additionally, the oleanane-type compounds comprise oleanolic acid (
4), 3-O-[(α-L-arabinopyranosyl)-(1→2)]-[β-D-glucuronopyranosyl-6-O-methyl ester]-olean-12-ene-28-olic acid (
8), hederacoside D (
23) [
18,
19,
20] (
Table 1,
Figure 2), and numerous others. In cell-based studies, betulin, betulinic acid, and oleanolic acid potentiated
β-cell function and mass and enhanced hepatic insulin sensitivity to regulate blood sugar [
21].
Furthermore, the first identified ursane-type triterpenoid in
E. sessiliflorus is ursolic acid (
1), predominantly found in fruits [
22]. Ursolic acid (100 mg/kg) reduces myocardial damage by inhibiting oxidative stress [
23]. Triterpenoids have been extensively studied and have demonstrated remarkable pharmacological effects, including anti-inflammatory, antioxidant, anti-platelet aggregation, vasodilatory, and cardioprotective properties. Furthermore, pharmacokinetic studies have contributed to elucidating the mechanisms of action of
E. sessiliflorus and have guided its clinical applications [
24]. In comparison, research on monoterpenoids within
E. sessiliflorus is relatively limited. Six monoterpenoids have been isolated from the ethanol extract of fruits, comprising five acyclic monoterpenoids and one cyclic monoterpenoid [
25]. Additionally, two sesquiterpenoids, curcumenol (
72), and nootkatone (
73), have been identified from the leaves [
20]. Curcumenol (2.5–20 μM) possesses potential anti-inflammatory activities by diminishing pro-inflammatory mediators and cytokines and suppressing the expression of regulatory proteins [
26]. Nootkatone (10 mg/kg) could inhibit acute and chronic inflammatory responses in mice [
27].
Table 1.
Terpenoids isolated from Eleutherococcus sessiliflorus.
Table 1.
Terpenoids isolated from Eleutherococcus sessiliflorus.
| NO. | Name | Formula | The exact Theoretical Molecular Weight | Source | Characterization Method | Refs. |
|---|
| 1 | Ursolic acid | C30H48O3 | 456.3603 | fruits, leaves | IR, 13C-NMR, 1H-NMR | [22,28] |
| 2 | Chiisanogenin | C30H44O5 | 484.3189 | fruits, leaves | IR, 13C-NMR, 1H-NMR | [15] |
| 3 | Chiisanoside | C48H74O19 | 954.4824 | fruits, leaves | IR, 13C-NMR, 1H-NMR | [15] |
| 4 | Oleanolic acid | C30H48O3 | 456.3603 | fruits, leaves, roots | HPLC, HPLC-MS | [18,28,29] |
| 5 | 22α-Hydroxychiisanoside | C48H74O20 | 970.4773 | fruits | HPLC-MS, 13C-NMR, 1H-NMR | [30] |
| 6 | Divaroside | C43H68O15 | 824.4558 | leaves | HPLC | [10] |
| 7 | Sessiloside-A1 | C36H54O10 | 646.3717 | leaves | HPLC, UV, IR, HR-MS | [10,31] |
| 8 | 3-O-[(α-L-Arabinopyranosyl)-(1→2)]-[β-D-glucuronopyranosyl-6-O-methyl ester]-olean-12-ene-28-olic acid | C42H66O13 | 778.4503 | fruits | MS, 13C-NMR, 1H-NMR, DEPT, HMBC, HMQC, NOESY | [19] |
| 9 | (1R,11α)-1,4-Epoxy-11-hydroxy-3,4-secolupane-20(30)-ene-3,28-Dioic acid | C30H46O6 | 502.3294 | fruits | MS, 13C-NMR, 1H-NMR, DEPT, HMBC, HMQC, NOESY | [19] |
| 10 | (1R,11α,22α)-1,4-Epoxy-11,22-hydroxy-3,4-secolupane-20(30)-ene-3,28-dioic acid | C30H46O7 | 518.3244 | fruits | MS, 13C-NMR, 1H-NMR, DEPT, HMBC, HMQC, NOESY | [19] |
| 11 | Elesesterpene A | C30H46O6 | 502.3294 | leaves | X-ray, 13C-NMR, 1H-NMR | [16] |
| 12 | Elesesterpene B | C31H48O5 | 500.3502 | leaves | X-ray, 13C-NMR, 1H-NMR | [16] |
| 13 | Elesesterpene C | C50H80O19 | 984.5294 | leaves | X-ray, 13C-NMR, 1H-NMR | [16] |
| 14 | Elesesterpene D | C49H78O18 | 954.5188 | leaves, fruits | HR-MS, X-ray, 13C-NMR, 1H-NMR | [9,16] |
| 15 | Elesesterpene E | C50H80O18 | 968.5345 | leaves | X-ray, 13C-NMR, 1H-NMR | [16] |
| 16 | Elesesterpene F | C30H44O7 | 516.3087 | leaves | X-ray, 13C-NMR, 1H-NMR | [16] |
| 17 | Elesesterpene G | C29H42O6 | 486.2981 | leaves | X-ray, 13C-NMR, 1H-NMR | [16] |
| 18 | Elesesterpene H | C31H48O7 | 532.3400 | leaves | X-ray, 13C-NMR, 1H-NMR | [16] |
| 19 | Elesesterpene I | C32H50O7 | 546.3557 | leaves | X-ray, 13C-NMR, 1H-NMR | [16] |
| 20 | Elesesterpene J | C30H46O6 | 502.3294 | leaves | X-ray, 13C-NMR, 1H-NMR | [16] |
| 21 | Elesesterpene K | C50H80O20 | 1000.5243 | leaves | X-ray, 13C-NMR, 1H-NMR | [16] |
| 22 | 24-Hydroxychiisanoside | C48H74O20 | 970.4773 | leaves | UPLC-Q-TOF-MS | [20] |
| 23 | Hederacoside D | C53H86O22 | 1074.5611 | leaves | UPLC-Q-TOF-MS | [20] |
| 24 | Cauloside D | C53H86O22 | 1074.5611 | leaves | UPLC-Q-TOF-MS | [20] |
| 25 | Nipponoside B | C54H88O22 | 1088.5767 | leaves | UPLC-Q-TOF-MS | [20] |
| 26 | Saniculoside N | C55H88O22 | 1100.5767 | leaves | UPLC-Q-TOF-MS | [20] |
| 27 | Guaianin N | C41H66O12 | 750.4554 | leaves | UPLC-Q-TOF-MS | [20] |
| 28 | Eleutheroside I | C41H66O11 | 734.4605 | leaves | UPLC-Q-TOF-MS | [20] |
| 29 | Hemsgiganoside B | C48H76O19 | 956.4981 | leaves | UPLC-Q-TOF-MS | [20] |
| 30 | 1-Deoxyisochiisanoside | C48H76O19 | 956.4981 | leaves | UPLC-Q-TOF-MS | [20] |
| 31 | Ciwujianoside C3 | C53H86O21 | 1058.5662 | leaves | UPLC-Q-TOF-MS | [20] |
| 32 | Anhuienside C | C53H86O21 | 1058.5662 | leaves | UPLC-Q-TOF-MS | [20] |
| 33 | Ciwujianoside D1 | C55H88O22 | 1100.5767 | leaves | UPLC-Q-TOF-MS | [20] |
| 34 | Ciwujianoside B | C58H92O25 | 1188.5928 | leaves | UPLC-Q-TOF-MS | [20] |
| 35 | Ciwujianoside D2 | C54H84O22 | 1084.5454 | leaves | UPLC-Q-TOF-MS | [20] |
| 36 | Ciwujianoside E | C40H62O11 | 718.4292 | leaves | UPLC-Q-TOF-MS | [20] |
| 37 | Ciwujianoside D3 | C55H88O23 | 1116.5716 | leaves | UPLC-Q-TOF-MS | [20] |
| 38 | Alphitolic acid | C30H48O4 | 472.3553 | leaves | NMR, MS | [32] |
| 39 | 11-Deoxyisochiisanoside | C48H76O19 | 956.4981 | leaves | IR, 13C-NMR, 1H-NMR | [33] |
| 40 | Isochiisanoside | C48H76O19 | 956.4981 | leaves | IR, 13C-NMR, 1H-NMR | [33] |
| 41 | 3-Oxo-24-methylenecycloartan | C31H50O | 438.3862 | fruits | MS, 13C-NMR, 1H-NMR | [14] |
| 42 | Schizandronic acid | C30H46O3 | 454.3447 | fruits | MS, 13C-NMR, 1H-NMR | [14] |
| 43 | Isomangiferolic acid | C32H52O2 | 468.3967 | fruits | MS, 13C-NMR, 1H-NMR | [14] |
| 44 | Betulin | C30H50O2 | 442.3811 | fruits | MS, 13C-NMR, 1H-NMR | [14] |
| 45 | Betulinic acid | C30H48O3 | 456.3603 | fruits | MS, 13C-NMR, 1H-NMR | [14] |
| 46 | Eleutheroside K | C41H66O11 | 734.4605 | fruits | MS, 13C-NMR, 1H-NMR | [14] |
| 47 | 22-α-Hydroxychiisanogenin | C30H44O6 | 500.3138 | fruits | MS, 13C-NMR, 1H-NMR | [14] |
| 48 | Acanthosessilioside F | C36H54O11 | 662.3666 | fruits | MS, 13C-NMR, 1H-NMR, DEPT, COSY, NOESY, HSQC, HMBC | [7] |
| 49 | Acanthosessiligenin II | C31H48O6 | 516.3451 | fruits | MS, 13C-NMR, 1H-NMR, DEPT, COSY, NOESY, HSQC, HMBC | [7] |
| 50 | Acanthosessilioside B | C37H58O11 | 678.3979 | fruits | MS, 13C-NMR, 1H-NMR, DEPT, COSY, NOESY, HSQC, HMBC | [7] |
| 51 | Acanthosessilioside C | C37H58O12 | 694.3928 | fruits | MS, 13C-NMR, 1H-NMR, DEPT, COSY, NOESY, HSQC, HMBC | [7] |
| 52 | Acanthosessilioside E | C36H56O11 | 664.3823 | fruits | MS, 13C-NMR, 1H-NMR, DEPT, COSY, NOESY, HSQC, HMBC | [7] |
| 53 | Acanthosessilioside D | C36H56O10 | 648.3873 | fruits | MS, 13C-NMR, 1H-NMR, DEPT, COSY, NOESY, HSQC, HMBC | [7] |
| 54 | Acanthosessiligenin I | C31H48O5 | 500.3502 | fruits | MS, 13C-NMR, 1H-NMR, DEPT, COSY, NOESY, HSQC, HMBC | [7] |
| 55 | Acanthosessilioside A | C36H56O9 | 632.3924 | fruits | MS, 13C-NMR, 1H-NMR, DEPT, COSY, NOESY, HSQC, HMBC | [7] |
| 56 | Sessiloside | C48H76O20 | 972.4930 | leaves, fruits | UPLC-MS, IR, 13C-NMR, 1H-NMR | [9,33] |
| 57 | Calenduloside E 6′-methyl ester | C37H58O9 | 646.4081 | fruits | IR, 13C-NMR, 1H-NMR, COSY, HSQC, HMBC | [34] |
| 58 | Acanthosessilioside G | C42H66O15 | 810.4402 | fruits | UPLC-MS, 13C-NMR, 1H-NMR, IR | [9] |
| 59 | Acanthosessilioside H | C48H76O19 | 956.4981 | fruits | UPLC-MS, 13C-NMR, 1H-NMR, IR | [9] |
| 60 | Acanthosessilioside I | C49H78O19 | 970.5137 | fruits | UPLC-MS, 13C-NMR, 1H-NMR, IR | [9] |
| 61 | Acanthosessilioside O | C49H78O21 | 1002.5036 | fruits | UPLC-MS, 13C-NMR, 1H-NMR, IR | [9] |
| 62 | Acanthosessilioside K | C51H80O20 | 1012.5243 | fruits | UPLC-MS, 13C-NMR, 1H-NMR, IR | [9] |
| 63 | Acanthosessilioside L | C49H78O19 | 970.5137 | fruits | UPLC-MS, 13C-NMR, 1H-NMR, IR | [9] |
| 64 | Acanthosessilioside M | C52H84O20 | 1028.5556 | fruits | UPLC-MS, 13C-NMR, 1H-NMR, IR | [9] |
| 65 | Acanthosessilioside N | C49H78O21 | 1002.5036 | fruits | UPLC-MS, 13C-NMR, 1H-NMR, IR | [9] |
| 66 | (2E)-3,7-Dimethylocta-2,6-dienoate-6-O-α-L-arabinopyranosyl-(1→6)-β-D-glucopyranoside | C21H34O11 | 462.2101 | fruits | IR, HR-MS, 13C-NMR, 1H-NMR, DEPT, HMBC, HMQC, NOESY | [25] |
| 67 | (3Z,6E)-3,7-Dimethyl-3,6-octadiene-1,2,8-triol | C10H18O3 | 186.1256 | fruits | IR, HR-MS, 13C-NMR, 1H-NMR, DEPT, HMBC, HMQC, NOESY | [25] |
| 68 | (6E)-7-Methyl-3-methylene-6-octene-1,2,8-triol | C10H18O3 | 186.1256 | fruits | IR, HR-MS, 13C-NMR, 1H-NMR, DEPT, HMBC, [HMQC, NOESY | [25] |
| 69 | Kenposide A | C21H36O10 | 448.2308 | fruits | IR, HR-MS, 13C-NMR, 1H-NMR, DEPT, HMBC, HMQC, NOESY | [25] |
| 70 | Sacranoside B | C21H36O10 | 448.2308 | fruits | IR, HR-MS, 13C-NMR, 1H-NMR, DEPT, HMBC, HMQC, NOESY | [25] |
| 71 | 1-O-[(S)-Oleuropeyl]-β-D-glucopyranose | C16H26O8 | 346.1628 | fruits | IR, HR-MS, 13C-NMR, 1H-NMR, DEPT, HMBC, HMQC, NOESY | [25] |
| 72 | Curcumenol | C15H22O2 | 234.1620 | leaves | UPLC-MS | [20] |
| 73 | Nootkatone | C15H22O | 218.1671 | leaves | UPLC-MS | [20] |
| 74 | Icariside B1 | C19H30O8 | 386.1941 | fruits | MS, 13C-NMR, 1H-NMR | [14] |
| 75 | Icariside B2 | C19H30O8 | 386.1941 | fruits | MS, 13C-NMR, 1H-NMR | [14] |
| 76 | (6R,7E,9R)-9-Hydorxy-4,7-megastigmadien-3-one-9-O-β-D-apiofuranosyl-(1–6)-β-D-glucopyranoside | C24H38O11 | 502.2414 | fruits | MS, 13C-NMR, 1H-NMR | [14] |
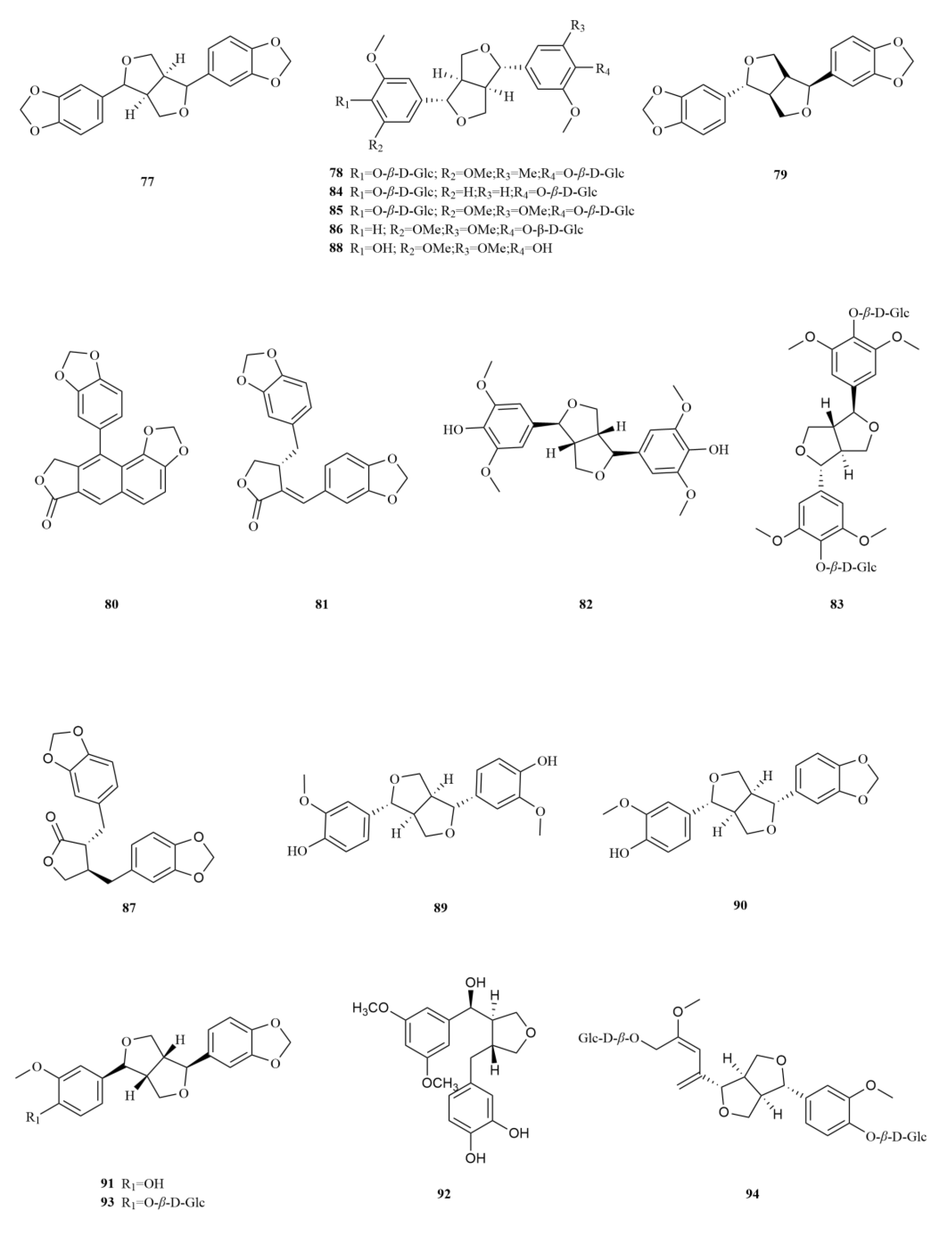
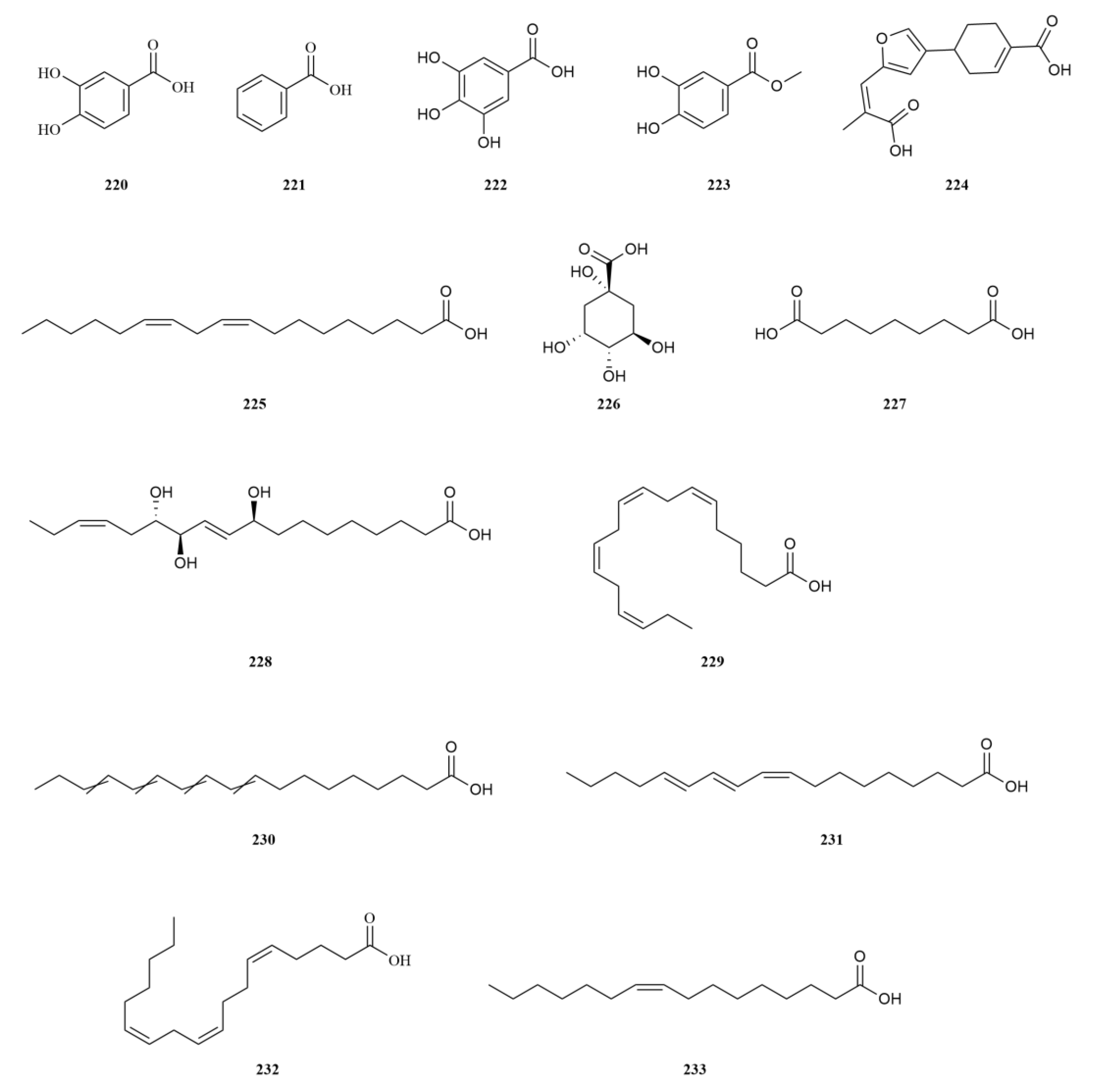
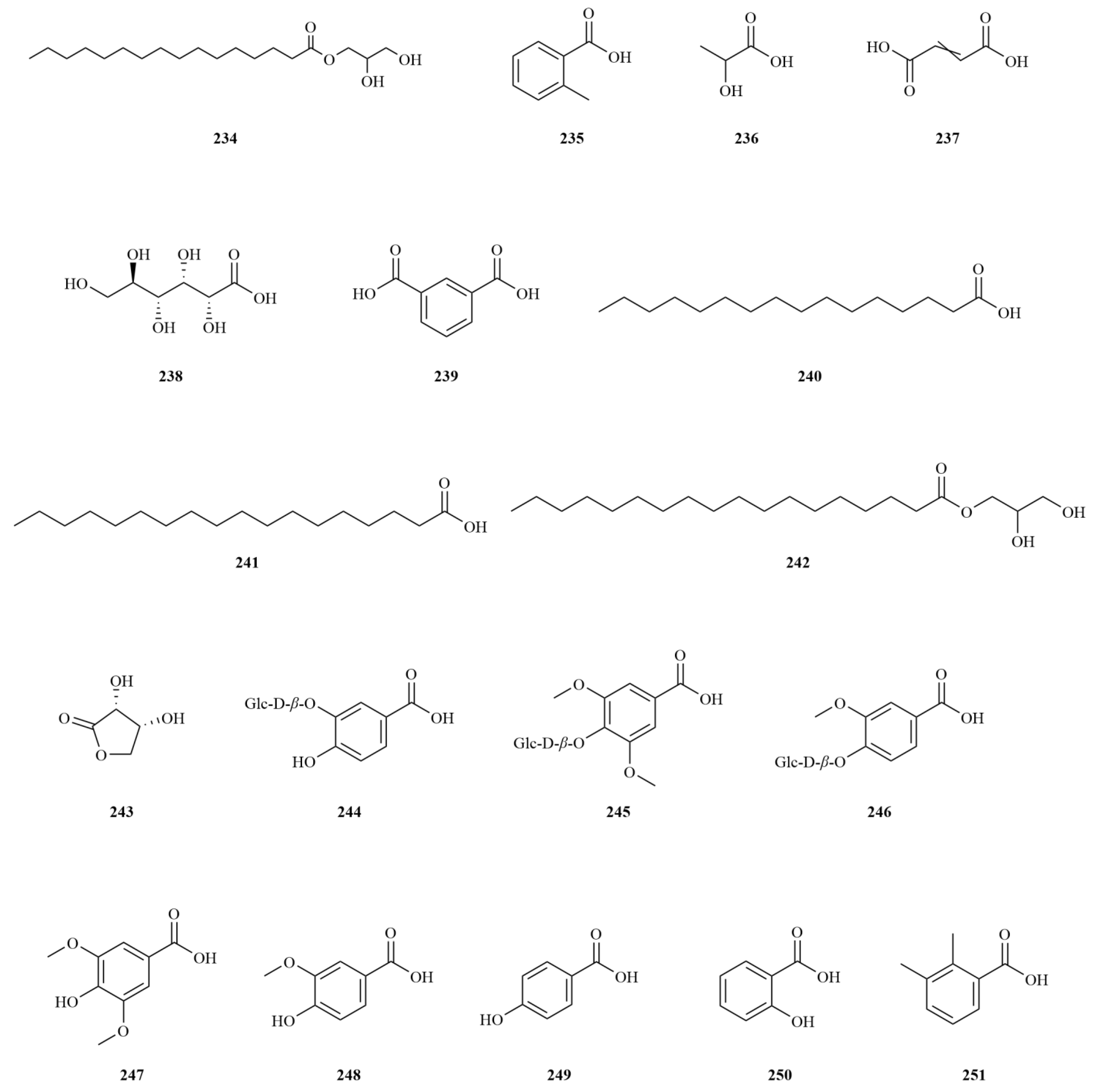
2.2. Phenylpropanoids
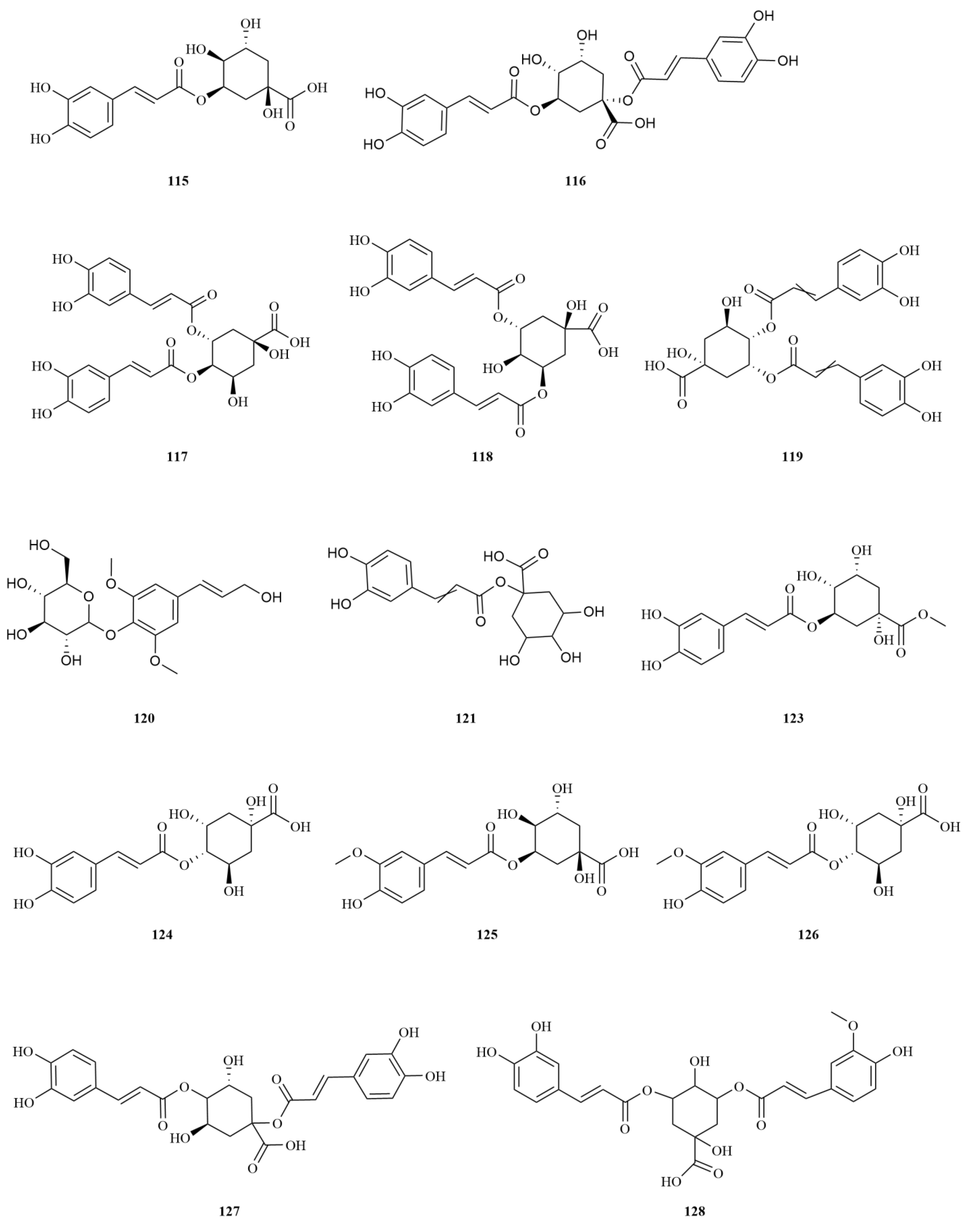
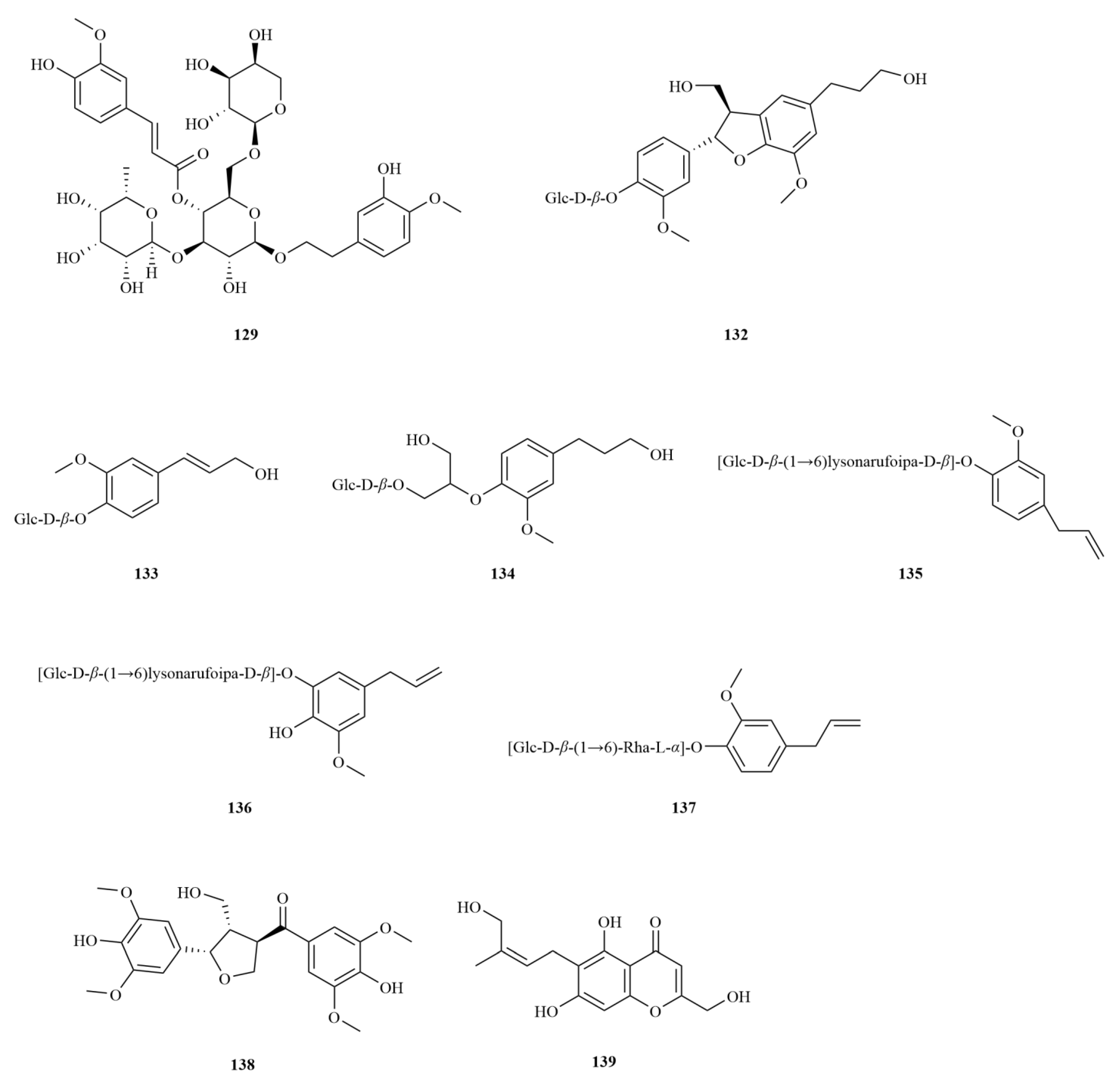
E. sessiliflorus is rich in phenylpropanoids, with a total of sixty-three compounds identified, including simple phenylpropanoids, lignans, and coumarins (
Table 2,
Figure 3). A notable discovery in
E. sessiliflorus was scoparone (lignans), which was isolated for the first time from its fruits, marking a novel finding within the
Eleutherococcus genus [
22]. Scoparone has anti-inflammatory, analgesic, and anti-coagulant effects. Recent research has found that it (500 μM) inhibits breast cancer cell viability through the NF-
κB signaling pathway [
35].
Starting with the roots of
E. sessiliflorus, eight phenylpropanoid compounds were successfully isolated from the ethyl acetate fraction of the 70% ethanol extract. Among these compounds, lignans like (+)-episesamin (
79), helioxanthin (
80), and (−)-syringaresinol (
82), alongside caffeic acid methyl ester (
111) and
p-hydrocoumaric acid (
112) were found [
36]. It is important to highlight that both (+)-episesamin and caffeic acid methyl ester represent newly discovered compounds within the
Eleutherococcus genus. Moving on to the fruits, a total of nine lignan compounds were isolated from the ethanol extract. Among them, acanthosessilin A (
92), a novel lignan, was identified for the first time in the
Eleutherococcus genus. Additionally, other compounds like (+)-piperitol (
90), (+)-xanthoxylol (
91), and simplexoside (
93) were observed, further enhancing our understanding of the repertoire of lignans in
E. sessiliflorus. Interestingly, hinokinin (
87) and (+)-pinoresinol (
89) (
Table 2,
Figure 3) were also first discovered within
E. sessiliflorus [
37]. Hinokinin (20 or 40 mg/kg) protects against high-fat diet/streptozotocin-induced cardiac injury in mice by alleviating oxidative stress, inflammation, and apoptosis [
38].
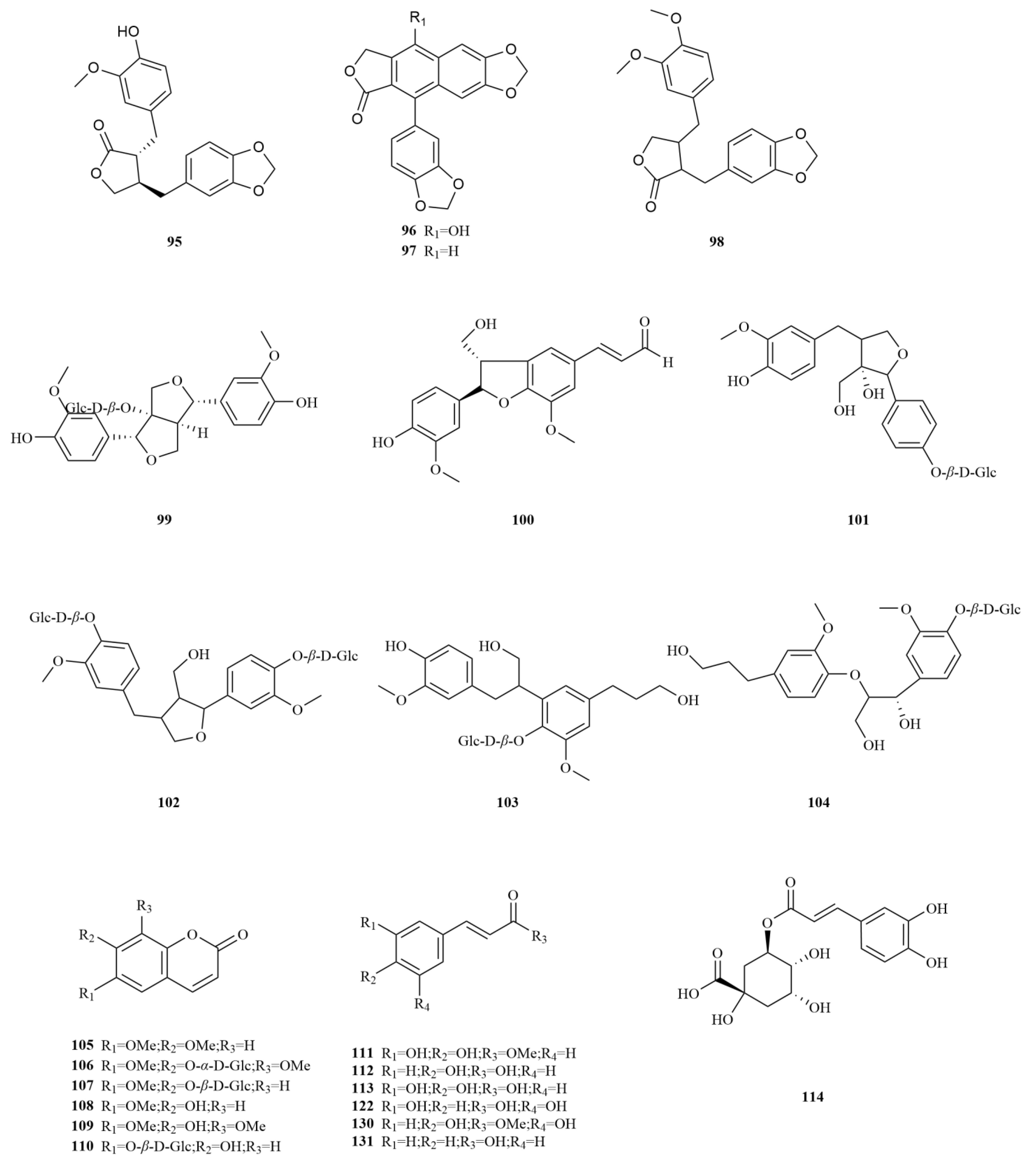
A comparative analysis was conducted to investigate the differences in the chemical composition among the roots of
E. sessiliflorus,
Eleutherococcus nodiflorus, and
E. senticosus. Notably, the compounds taiwanin C (
97) and taiwanin E (
96) (
Table 2,
Figure 3) were found to be unique to
E. sessiliflorus, highlighting its distinct chemical profile. Normal oral cells N28 and oral cancer cells T28 were treated with different concentrations of taiwanin C at 0, 1, 5, 10, 30, and 60 μM. Results showed the therapeutic potential of taiwanin C against arecoline-induced oral cancer and no significant cytotoxicity for normal oral cells [
39]. Taiwanin E (0.5, 1, 5, 10, and 20 μM) inhibits cell migration in human lovo colon cancer cells by suppressing MMP-2/9 expression through the p38 MAPK pathway [
40]. Moreover, a comparative analysis was carried out to examine the variation in the chemical composition among the roots of
E. sessiliflorus,
Eleutherococcus nodiflorus, and
E. senticosus. It was discovered that fourteen components were common to
E. senticosus and
E. sessiliflorus. A comparative analysis was conducted to explore the differences in chemical composition among the roots of
E. sessiliflorus,
Eleutherococcus nodiflorus, and
E. senticosus. Interestingly, it was observed that
E. senticosus contained various phenolic glycosides that were not detected in both
E. sessiliflorus and
Eleutherococcus nodiflorus, suggesting a higher similarity in chemical composition between
E. sessiliflorus and
Eleutherococcus nodiflorus [
6]. This finding supports considering
E. sessiliflorus as a potential substitute for
Eleutherococcus nodiflorus.To examine the changes in content, a study employed HPLC to simultaneously measure six components in the green and mature fruits of
E. sessiliflorus. The study observed that as the fruits of
E. sessiliflorus matured, there was an increasing trend in the content of (−)-pinoresinol-4,4′-di-O-
β-D-glucopyranoside (
84), acanthoside D (
85), acanthoside B (
86), and scopolin (
107) (
Table 2,
Figure 3). This suggests a gradual elevation in the concentration of active compounds during fruit growth, indicating the potential for higher medicinal properties [
41]. Another investigation focused on determining the content of eleutheroside E and eleutheroside B in different parts (roots, stems, leaves, and fruits) of
E. sessiliflorus through a 50% methanol extract. The results revealed that the fruits and stems had the highest contents of eleutheroside B and E. Importantly, no cytotoxic effects were observed on the normal cell line (DC2.4), and the roots extract exhibited a 23% inhibition rate on the stomach cancer cell line (SNU-719), highlighting its potential as a health food [
42]. Furthermore, since the initial discovery of scoparone (
105), a coumarin in
E. sessiliflorus fruits, subsequent research has identified six coumarins from various parts of the plant, including the roots, stems, fruits, and leaves. Among these, seopoletin (
108) and isofraxidin (
109) (
Table 2,
Figure 3) have been identified in
E. sessiliflorus, with contents of 30.5 μg/g and 7.90 μg/g, respectively [
43]. Isofraxidin (3 and 15 mg/kg) possesses significant analgesic and anti-inflammatory activities that may be mediated by regulating pro-inflammatory cytokines, TNF-
α and the phosphorylation of p38 and ERK1/2 [
44].
Coumarins exhibit a diverse range of pharmacological effects, including but not limited to anti-inflammatory, anti-coagulant, antimicrobial, anticancer, antihypertensive, antituberculous, anticonvulsant, and antihyperglycemic activities. Additionally, they possess antioxidant and neuroprotective properties [
45]. Notably, scoparone demonstrates anti-inflammatory, antioxidant, anti-apoptotic, anti-fibrotic, and lipid-lowering properties, making it a compound with multiple potential therapeutic benefits [
46]. On the other hand, esculin (
110), another coumarin found in
E. sessiliflorus, exhibits anti-diabetic effects, promoting improvements in pancreatic damage, enhanced insulin secretion, and glucose homeostasis. In addition, esculin, a coumarin compound found in
E. sessiliflorus, has several therapeutic properties, including anticancer, antibacterial, antiviral, neuroprotective, antithrombotic, and ophthalmic effects [
47] (
Table 2,
Figure 3).
Simple phenylpropanoids in
E. sessiliflorus have been extensively studied in fruits and roots (
Table 2,
Figure 3). Two specific phenylpropanoids,
p-hydroxycoumaric acid (
112) and caffeic acid (
113), were identified in the 70% ethanol extract of the fruits [
48]. Both
p-Hydroxycoumaric acid and caffeic acid showed good antioxidant capacities [
49]. Furthermore, 3,5-dihydroxycinnamic acid (
122) was also isolated from the same extract, marking its first-time identification in
E. sessiliflorus. This compound displayed potent ABTS and DPPH radical scavenging abilities, indicating its strong antioxidant properties [
50]. Additionally, HPLC analysis was conducted to determine the content of seven organic acids (caffeic acid, chlorogenic acid, neochlorogenic acid, 1,3-dicaffeoylquinic acid, isochlorogenic acid A-C (
113–
119) in the root at different growth stages ranging from four to eight years. The content of each organic acid increased with the age of the plant, with the 8th year showing the highest levels. Notably, chlorogenic acid was the most abundant [
51].
Table 2.
Phenylpropanoids isolated from Eleutherococcus sessiliflorus.
Table 2.
Phenylpropanoids isolated from Eleutherococcus sessiliflorus.
| NO. | Name | Formula | Exact Theoretical Molecular Weight | Source | Characterization method | Refs. |
|---|
| 77 | (−)-Sesamin | C20H18O6 | 354.1103 | fruits, roots | IR, 13C-NMR, 1H-NMR, MS | [22,36] |
| 78 | Liriodendrin | C34H46O18 | 742.2684 | stem barks | Unspecified | [52] |
| 79 | (+)-Episesamin | C20H18O6 | 354.1103 | roots | 13C-NMR, 1H-NMR, MS | [36] |
| 80 | Helioxanthin | C20H12O6 | 348.0634 | roots | 13C-NMR, 1H-NMR, MS | [36] |
| 81 | Savinin | C20H16O6 | 352.0947 | roots, leaves | UPLC-MS, 13C-NMR, 1H-NMR, MS | [20,36] |
| 82 | (−)-Syringaresinol | C22H26O8 | 418.1628 | roots | 13C-NMR, 1H-NMR, MS | [36] |
| 83 | Eleutheroside E | C34H46O18 | 742.2684 | roots, stems, fruits | HPLC | [42] |
| 84 | (−)-Pinoresinol-4,4′-di-O-β-D-glucopyranoside | C32H42O16 | 682.2473 | fruits | HPLC | [41] |
| 85 | Acanthoside D | C34H46O18 | 742.2684 | fruits, roots, root barks | UPLC- MS, HPLC, 13C-NMR, 1H-NMR | [6,41,53] |
| 86 | Acanthoside B | C28H36O13 | 580.2156 | fruits, roots, root barks | UPLC- MS, HPLC, 13C-NMR, 1H-NMR | [6,41,53] |
| 87 | Hinokinin | C20H18O6 | 354.1103 | fruits | HR-MS, 13C-NMR, 1H-NMR, DEPT, COSY, HSQC, HMBC, NOESY, IR | [37] |
| 88 | (+)-Syringaresinol | C22H26O8 | 418.1628 | fruits | HR-EI-MS, 13C-NMR, 1H-NMR, DEPT, COSY, HSQC, HMBC, NOESY, IR | [37] |
| 89 | (+)-Pinoresinol | C20H22O6 | 358.1416 | fruits, roots | 13C-NMR, 1H-NMR, MS, HR-MS, DEPT, COSY, HSQC, HMBC, NOESY, IR | [36,37] |
| 90 | (+)-Piperitol | C20H20O6 | 356.1260 | fruits | HR-MS, 13C-NMR, 1H-NMR, DEPT, COSY, HSQC, HMBC, NOESY, IR | [37] |
| 91 | (+)-Xanthoxylol | C20H20O6 | 356.1260 | fruits | HR-MS, 13C-NMR, 1H-NMR, DEPT, COSY, HSQC, HMBC, NOESY, IR | [37] |
| 92 | Acanthosessilin A | C20H24O6 | 360.1573 | fruits | HR-EI-MS, 13C-NMR, 1H-NMR, DEPT, COSY, HSQC, HMBC, NOESY, IR | [37] |
| 93 | Simplexoside | C26H30O11 | 518.1788 | fruits, roots | UPLC- MS, HR-MS, 13C-NMR, 1H-NMR, DEPT, COSY, HSQC, HMBC, NOESY, IR | [6,37] |
| 94 | (+)-Pinoresinol di-O-β-D-glucopyranoside | C32H42O16 | 682.2473 | roots | UPLC-MS | [6] |
| 95 | Pluviatolide | C20H20O6 | 356.1260 | roots | UPLC-MS | [6] |
| 96 | Taiwanin E | C20H12O7 | 364.0583 | roots | UPLC-MS | [6] |
| 97 | Taiwanin C | C20H12O6 | 348.0634 | roots | UPLC-MS | [6] |
| 98 | 3-(3,4-Dimethoxybenzyl)-2-(3,4-methylenedioxybenzyl) butyrolactone | C21H22O6 | 370.1416 | roots | UPLC-MS | [6] |
| 99 | (+)-l-Hydroxypinoresinol-l-O-β-D-glucoside | C26H32O12 | 536.1894 | fruits | MS, 13C-NMR, 1H-NMR | [14] |
| 100 | Balanophonin | C20H20O6 | 356.1260 | fruits | MS, 13C-NMR, 1H-NMR | [14] |
| 101 | Berchemol-4’-O-β-D-glucoside | C26H34O12 | 538.2050 | fruits | MS, 13C-NMR, 1H-NMR | [14] |
| 102 | Lariciresinol-4,4’-di-O-β-D-glucopyranoside | C32H34O11 | 594.2101 | fruits | MS, 13C-NMR, 1H-NMR | [14] |
| 103 | Icariside E3 | C26H36O11 | 524.2258 | fruits | MS, 13C-NMR, 1H-NMR | [14] |
| 104 | (7S,8R)-Erythro-7,9,9’-trihydroxy-3,3’-dimethoxy-8-O-4’-neolignan-4-O-β-D-glucopyranosideerythro | C26H36O12 | 540.2207 | fruits | MS, 13C-NMR, 1H-NMR | [14] |
| 105 | Scoparone | C11H10O4 | 206.0579 | fruits | IR, 13C-NMR, 1H-NMR | [22] |
| 106 | Isofraxidin-7-O-α-D-glucoside | C13H12O5 | 248.0685 | stems | IR, 13C-NMR, 1H-NMR | [54] |
| 107 | Scopolin | C16H18O9 | 354.0951 | fruits | HPLC | [41] |
| 108 | Scopoletin | C10H8O4 | 192.0423 | fruits | HPLC-MS, 13C-NMR, 1H-NMR | [30] |
| 109 | Isofraxidin | C11H10O5 | 222.0528 | roots, fruits, leaves | UPLC-MS, HPLC-MS, HPLC | [20,29,43] |
| 110 | Esculin | C15H16O9 | 340.0794 | leaves | UPLC-MS | [20] |
| 111 | Caffeic acid methyl ester | C10H10O4 | 194.0579 | roots | 13C-NMR, 1H-NMR, MS | [36] |
| 112 | p-Hydrocoumaric acid | C9H10O3 | 166.0630 | roots, fruits | 13C-NMR, 1H-NMR, MS | [36,48] |
| 113 | Caffeic acid | C9H8O4 | 180.0423 | roots, fruits | 13C-NMR, 1H-NMR, MS, HPLC | [48,51] |
| 114 | Chlorogenic acid | C16H18O9 | 354.0951 | roots | HPLC | [51] |
| 115 | Neochlorogenic acid | C16H18O9 | 354.0951 | roots | HPLC | [51] |
| 116 | 1,3-Dicaffeoylquinic acid | C25H24O12 | 516.1268 | roots | HPLC | [51] |
| 117 | Isochlorogenic acid B | C25H24O12 | 516.1268 | roots | HPLC | [51] |
| 118 | Isochlorogenic acid A | C25H24O12 | 516.1268 | roots | HPLC | [51] |
| 119 | Isochlorogenic acid C | C25H24O12 | 516.1268 | roots | HPLC | [51] |
| 120 | Syringin | C17H24O9 | 372.1420 | root barks, stems | HPLC | [42,55] |
| 121 | Caffeoylquinic acid | C16H18O9 | 354.0951 | roots, leaves | UPLC-MS, UPLC-MS | [6,20] |
| 122 | 3,5-Dihydroxycinnamic acid | C9H8O4 | 180.0423 | fruits | NMR, MS, IR | [50] |
| 123 | Chlorogenic acid methyl ester | C17H20O9 | 368.1107 | root barks | 13C-NMR, 1H-NMR | [53] |
| 124 | Cryptochlorogenic acid | C16H18O9 | 354.0951 | leaves | UPLC-MS | [20] |
| 125 | 3-Feruloylquinic acid | C17H20O9 | 368.1107 | leaves | UPLC-MS | [20] |
| 126 | 4-Feruloylquinic acid | C17H20O9 | 368.1107 | leaves | UPLC-MS | [20] |
| 127 | 1,4-Dicaffeoylquinic acid | C25H24O12 | 516.1268 | leaves | UPLC-MS | [20] |
| 128 | 3-Feruloyl-5-caffeoylquinic acid | C26H26O12 | 530.1424 | leaves | UPLC-MS | [20] |
| 129 | Angoroside C | C36H48O19 | 784.2790 | leaves | UPLC-MS | [20] |
| 130 | Ferulic acid | C10H10O4 | 194.0579 | leaves, roots | UPLC-MS, HPLC-MS | [20,29] |
| 131 | Cinnamic acid | C9H8O2 | 148.0524 | roots | HPLC-MS | [29] |
| 132 | (7S,8R) Dihydrodehydrodiconiferyl alcohol 4-O-β-D-glucopyranoside | C26H34O11 | 522.2101 | fruits | MS, 13C-NMR, 1H-NMR | [14] |
| 133 | Coniferin | C16H22O8 | 342.1315 | fruits | MS, 13C-NMR, 1H-NMR | [14] |
| 134 | 4-O-(2-O-β-D-Glucopyranosyl-1-hydroxymethylethly)-dihydroconiferyl alcohol | C19H30O10 | 418.1839 | fruits | MS, 13C-NMR, 1H-NMR | [14] |
| 135 | 1-Allyl-3-methoxyhenyl-6-O-β-D-apiofuranosyl-(1′′-6′)-β-D-glucopyranoside | C21H30O11 | 458.1788 | fruits | MS, 13C-NMR, 1H-NMR | [14] |
| 136 | Hovetrichoside G | C21H30O12 | 474.1737 | fruits | MS, 13C-NMR, 1H-NMR | [14] |
| 137 | Eugenyl β-rutinoside | C22H32O11 | 472.1945 | fruits | MS, 13C-NMR, 1H-NMR | [14] |
| 138 | Ciwujiatone | C22H26O9 | 434.1577 | root barks | 13C-NMR, 1H-NMR | [53] |
| 139 | Cnidimol D | C15H16O6 | 292.0947 | leaves | UPLC-MS | [20] |
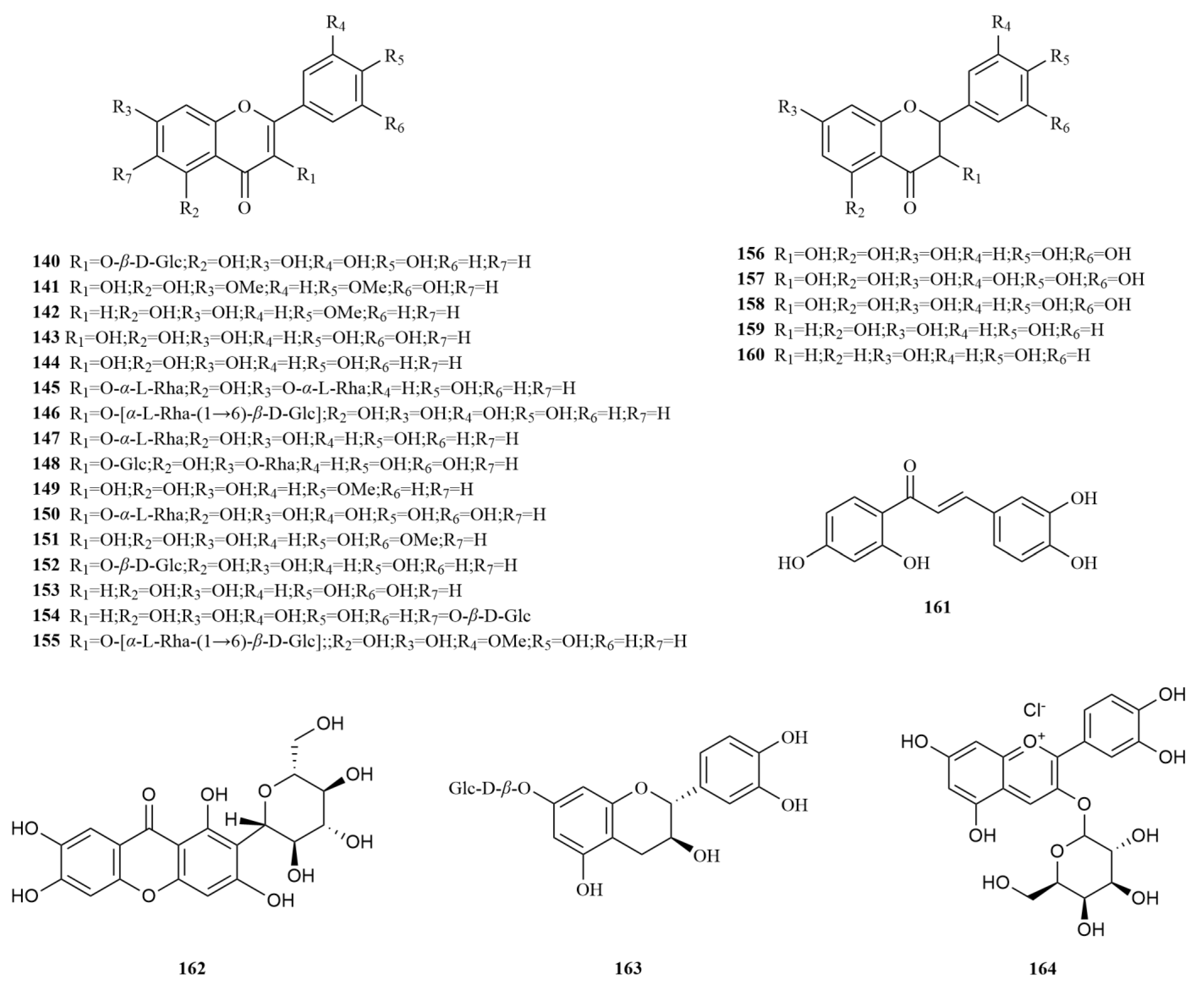
2.3. Flavonoids
To date, twenty-five flavonoids have been identified from
E. sessiliflorus (
Table 3,
Figure 4). These include three flavones, thirteen flavonols, two flavanones, three flavanonols, one chalcone (butein, (
162)), one biphenylketone (mangiferin, (
163)), one flavan-3-ol (catechin-7-O-
β-glucopyranoside, (
164)), and one anthocyanin (cyanidin 3-xylosyl-galactoside, (
165)) [
14,
20,
32,
56]. Butein is a promising anticancer molecule, and its major modes of action in different cancer cells are apoptosis and interference with cell cycle [
57]. Mangiferin (40, 80, and 120 mg/kg) decreased macrophage phagocytosis but increased NK cell activities in vivo. Meanwhile, it increased the survival rate of leukemia mice in vivo [
58]. Six flavonoids have been isolated and purified from the 70% extract of
E. sessiliflorus leaves. Among them, dihydromyricetin (
158), taxifolin (
159), and butein (
161) were identified for the first time within the
Eleutherococcus genus. Furthermore, researchers confirmed that these compounds exhibited relatively weak cytotoxic activity against the cell line A549 (IC
50 < 88.2 μM), suggesting their potential as advantageous candidates for anticancer drugs [
32].
E. sessiliflorus is recognized as a novel functional food with a rich content of protein, fiber, and minerals within its fruits. Presently,
E. sessiliflorus is being cultivated in Poland and has gained appreciation from vegetarian enthusiasts [
59]. Additionally, an analysis of flavonoid content was conducted across four
Eleutherococcus species, namely
Eleutherococcus henryi, Eleutherococcus koreanum, E. senticosus and
E. sessiliflorus. Notably, both the roots and stems and the fruits of
E. senticosus and
E. sessiliflorus exhibit elevated levels of total flavonoids. Moreover, hyperin is the most abundant flavonoid within
E. sessiliflorus [
60,
61]. Hyperin has proven effective in various domains, such as anti-inflammatory, antibacterial, antiviral, neuroprotective, antidepressant, and organ-protective properties. Its broad application in the field of anti-tumor treatments is particularly noteworthy, showing efficacy against lung cancer, cervical cancer, gastric cancer, colorectal cancer, pancreatic cancer, breast cancer, and ovarian cancer [
62]. Therefore, the flavonoid-rich
E. sessiliflorus presents a promising avenue in the domains of medicinal natural products, dietary supplements, and beverages, serving as a potential source of agricultural and industrial innovation.
Table 3.
Flavonoids isolated from Eleutherococcus sessiliflorus.
Table 3.
Flavonoids isolated from Eleutherococcus sessiliflorus.
| NO. | Name | Formula | Exact Theoretical Molecular Weight | Source | Characterization Method | Refs. |
|---|
| 140 | Hyperin | C21H20O12 | 464.0955 | fruits, roots | IR, 13C-NMR, 1H-NMR, HPLC-MS | [22,29] |
| 141 | Ombuin | C17H14O7 | 330.0740 | stems | IR, 13C-NMR, 1H-NMR | [54] |
| 142 | Acacetin | C16H12O5 | 284.0685 | stems | IR, 13C-NMR, 1H-NMR | [54] |
| 143 | Quercetin | C15H10O7 | 302.0427 | stems, fruits, roots, leaves | HPLC-MS, MS, IR, 13C-NMR, 1H-NMR, HPLC | [29,32,54,61] |
| 144 | Kaempferol | C15H10O6 | 286.0477 | stems, roots, | HPLC-MS, IR, 13C-NMR, 1H-NMR | [29,54] |
| 145 | Kaempferitrin | C27H30O14 | 578.1636 | stems | IR, 13C-NMR, 1H-NMR | [54] |
| 146 | Rutin | C27H30O16 | 610.1534 | stems, roots, fruits | HPLC | [60,61] |
| 147 | Afzelin | C21H20O10 | 432.1056 | stems, roots, fruits | HPLC | [60,61] |
| 148 | Antoside | C29H36O15 | 624.2054 | roots | UPLC-MS | [6] |
| 149 | Kaempferide | C16H12O6 | 300.0634 | fruits | HPLC | [63] |
| 150 | Myricitrin | C21H20O12 | 464.0955 | leaves | UPLC-MS | [20] |
| 151 | Isorhamnetin | C16H12O7 | 316.0583 | leaves | UPLC-MS | [20] |
| 152 | Astragalin | C21H20O11 | 448.1006 | leaves | UPLC-MS | [20] |
| 153 | Luteolin | C15H10O6 | 286.0477 | roots | HPLC-MS | [29] |
| 154 | Isooirenitn | C21H20O11 | 448.1006 | fruits | 13C-NMR, 1H-NMR | [14] |
| 155 | Isorhamnetin-3-O-rutinoside | C28H32O16 | 624.1690 | fruits | 13C-NMR, 1H-NMR | [14] |
| 156 | Catechin | C15H14O6 | 290.0790 | leaves, roots | UPLC-MS, HPLC-MS | [20,29] |
| 157 | Dihydromyricetin | C15H12O8 | 320.0532 | leaves | 13C-NMR, 1H-NMR, MS | [32] |
| 158 | Taxifolin | C15H12O7 | 304.0583 | leaves | 13C-NMR, 1H-NMR, MS | [32] |
| 159 | Naringenin | C15H12O5 | 272.0685 | leaves | 13C-NMR, 1H-NMR, MS | [32] |
| 160 | Liquiritigenin | C15H12O4 | 256.0736 | leaves | 13C-NMR, 1H-NMR, MS | [32] |
| 161 | Butein | C15H12O5 | 272.0685 | leaves | 13C-NMR, 1H-NMR, MS | [32] |
| 162 | Mangiferin | C19H18O11 | 422.0849 | leaves | UPLC-MS | [20] |
| 163 | Catechin-7-O-β-glucopyranoside | C21H24O11 | 452.1319 | fruits | 13C-NMR, 1H-NMR | [14] |
| 164 | Cyanidin-3-xylosyl-galactoside | C21H21ClO11 | 484.0772 | fruits | 13C-NMR, 1H-NMR | [56] |
2.6. Nitrogenous Compounds
Nitrogenous compounds are vital molecules widely distributed in nature and hold significant biological importance. Many organic compounds containing nitrogen exhibit notable biological activities, including alkaloids and amino acids. In the case of
E. sessiliflorus, twenty nitrogenous compounds have been identified, with the majority being found in the leaves (
Table 6,
Figure 7). The discovery of sessiline (
252) in the fruits has expanded our understanding of nitrogenous compounds in
E. sessiliflorus [
70]. Advanced techniques such as ultra-performance liquid chromatography-mass spectrometry have been employed to identify eight nitrogenous compounds [
20]. Additionally, four alkaloids have been identified, namely perlolyrine (
264), flazine (
265), 1-methyl-1,2,3,4-tetrahydro-
β-carboline-3-carboxylic acid (
266), and adenosine (
269) [
14]. Perlolyrine (20 mg/kg) has a strong anti-platelet effect and a certain degree of antithrombotic effect [
71]. Adenosine (10 mg/L in distilled water) induces the activation of AMPK in skeletal muscle and mitigates insulin resistance in mice with high-fat diet-induced diabetes [
72].
One noteworthy nitrogenous compound is palmitoylethanolamide (
253), an endogenous mediator. It has demonstrated favorable tolerability and lacks adverse effects on the body. Palmitoylethanolamide exhibits a broad spectrum of effects, including anti-inflammatory, analgesic, antibacterial, immunomodulatory, and neuroprotective properties [
73]. Another compound of interest is oleamide (
255), which has shown efficacy in sleep enhancement, temperature regulation, and analgesia [
74]. However, research on the amino acids present in
E. sessiliflorus has been limited. Only five amino acids have been identified in this plant, namely ethanolamine (
263), 3-hydroxy-L-proline (
267),
γ-aminobutyric acid (
268), phenylalanine (
270), and L-norvaline (
271) [
14,
20,
67,
75]. L-Norvaline (250 mg/L in animals’ water) reverses cognitive decline and synaptic loss in a murine model of Alzheimer’s disease [
76].
Table 6.
Nitrogenous compounds isolated from Eleutherococcus sessiliflorus.
Table 6.
Nitrogenous compounds isolated from Eleutherococcus sessiliflorus.
| NO. | Name | Formula | Exact Theoretical Molecular Weight | Source | Characterization Method | Refs. |
|---|
| 252 | Sessiline | C10H11NO4 | 209.0688 | fruits | IR, 13C-NMR, 1H-NMR, COSY | [70] |
| 253 | Palmitoylethanolamide | C18H37NO2 | 299.2824 | leaves | UPLC-MS | [20] |
| 254 | Hexadecanamide | C16H33NO | 255.2562 | leaves | UPLC-MS | [20] |
| 255 | Oleamide | C18H35NO | 281.2719 | leaves | UPLC-MS | [20] |
| 256 | Pheophorbide A | C35H36N4O5 | 592.2686 | leaves | UPLC-MS | [20] |
| 257 | Pyropheophorbide A | C33H34N4O3 | 534.2631 | leaves | UPLC-MS | [20] |
| 258 | Stearamide | C18H37NO | 283.2875 | leaves | UPLC-MS | [20] |
| 259 | L-2-Benzylaminooctanol | C15H25NO | 235.1936 | leaves | GC-MS | [67] |
| 260 | Isoquinolinium | C9H8N+ | 130.0651 | leaves | GC-MS | [67] |
| 261 | Cadaverine | C5H14N2 | 102.1157 | leaves | GC-MS | [67] |
| 262 | n-Butylamine | C4H11N | 73.0891 | leaves | GC-MS | [67] |
| 263 | Ethanolamine | C2H7NO | 61.0528 | leaves | GC-MS | [67] |
| 264 | Perlolyrine | C16H12N2O2 | 264.0899 | fruits | MS, 13C-NMR, 1H-NMR | [14] |
| 265 | Flazine | C17H12N2O4 | 308.0797 | fruits | MS, 13C-NMR, 1H-NMR | [14] |
| 266 | 1-Methyl-1,2,3,4-tetrahydro-β-carboline-3-carboxylic acid | C13H14N2O2 | 230.1055 | fruits | MS, 13C-NMR, 1H-NMR | [14] |
| 267 | 3-Hydroxy-L-proline | C5H9O3N | 131.0582 | fruits | MS, 13C-NMR, 1H-NMR | [14] |
| 268 | γ-Aminobutyric acid | C4H9NO2 | 103.0633 | aerial part | HPLC | [75] |
| 269 | Adenosine | C10H13N5O4 | 267.0968 | leaves, fruits | MS, 13C-NMR, 1H-NMR, UPLC-MS | [14,20] |
| 270 | Phenylalanine | C9H11NO2 | 165.0790 | leaves | UPLC-MS | [20] |
| 271 | L-Norvaline | C5H11NO2 | 117.0790 | leaves | GC-MS | [67] |