Abstract
Despite the various biological activities exhibited by water chestnut (the fruit of the Trapa genus), the phenolic compounds present in its extract require comprehensive characterization. Accordingly, we analyzed a 80% methanol extract of commercially available water chestnut and identified a new hydrolyzable tannin dimer termed trapadin A. Additionally, 22 known compounds, including 10 hydrolyzable tannin monomers and 2 dimers, were also detected in the extract. Spectroscopic and chemical methods were used to elucidate the structure of trapadin A, revealing it to be a hydrolyzable tannin dimer formed from units of tellimagrandin II and 1,2,3,6-tetra-O-galloyl-β-d-glucose. Moreover, the 1,1-diphenyl-2-picrylhydrazyl radical scavenging activity assay used to determine the half-maximal effective concentration values for the 23 compounds isolated from water chestnut indicated significant radical scavenging activity associated with hydrolyzable tannins. Notably, trapadin A, the new hydrolyzable tannin dimer, exhibited the highest activity value among the tested compounds.
1. Introduction
The water chestnut is the fruit of the aquatic plant Trapa genus, which includes several species such as T. japonica, T. incisa, and T. bispinosa. It thrives in swamps and lakes across Asia and features a hard walnut-like shell. In Japan, particularly in the Kyushu region, the edible starchy portion of the water chestnut is boiled and consumed along with the husks, yielding a tea that is considered to have stomachic and nourishing effects [1]. Notably, water chestnut primarily consists of carbohydrates, with lipids, proteins, and carbohydrates constituting 0.5, 5.8, and 40.6 g per 100 g, respectively. Additionally, this fruit is rich in vitamin B1 and folic acid [2]. Water chestnuts from T. japonica and T. bispinosa also reportedly contain hydrolyzable tannins [1,3,4,5]. Biological studies have indicated diverse health benefits associated with water chestnut, including antioxidant [1,6], antidiabetic [5,7,8], anti-obesity [9,10], anti-glycation [5], anti-inflammatory [11,12], antiadipogenic [6], and hepatic protective effects [13]. Furthermore, it has demonstrated inhibitory activity against α-amylase [14] and α-glucosidase [3,5,14]. Recently, water chestnut fruit extract has garnered attention for its potential in skin improvement [15] and as a treatment for alopecia [16], positioning it as a promising cosmeceutical and beauty food.
Considering the presence of diverse polyphenolic compounds in water chestnuts that exert various biological activities, a comprehensive investigation to identify its characteristic phenolic compounds is warranted. Moreover, antioxidant function is a typical biological activity of polyphenols [17]. Antioxidants in foods contribute to the maintenance of health and reduce the risk of various age-related diseases [18]. The contribution of their activity has been attributed to their chemical structure [17]. Therefore, detailed structural elucidation of the polyphenol activity in foods is important.
Accordingly, we isolated, identified, and characterized phenolic compounds from commercially available water chestnuts. Additionally, 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical scavenging activity assays were performed for the obtained compounds.
2. Results and Discussion
2.1. Isolation and Characterization of Phenolic Constituents
An 80% aqueous methanol (MeOH) extract of whole water chestnut was concentrated to obtain a water (H2O) extract. The H2O extracts were separately chromatographed using Diaion HP-20, YMC GEL ODS-AQ, MCI-gel CHP-20P, and Sephadex LH-20 with MeOH–H2O or ethanol (EtOH)–MeOH in a stepwise gradient mode. The fractions with similar high-performance liquid chromatography (HPLC) chromatograms were merged and further purified using column chromatography to obtain trapadin A (1), gallic acid (2) [19], methyl gallate (3) [19], vanillic acid (4) [19], brevifolincarboxylic acid (5) [20], ellagic acid (6) [21], urolithin A (7) [22], isourolithin A (8) [13], urolithin B (9) [11], urolithin M6 (10) [22], 1,2-di-O-galloyl-β-d-glucose (11) [23,24], 1,6-di-O-galloyl-β-d-glucose (12) [25], 1,2,3-tri-O-galloyl-β-d-glucose (13) [5,26], 1,2,6-tri-O-galloyl-β-d-glucose (14) [5,26], 1,2,3,6-tetra-O-galloyl-β-d-glucose (15) [5,26], 1,2,3,4,6-penta-O-galloyl-β-d-glucose (16) [24,27], 1,6-di-O-galloyl-2-O-p-coumaroyl-β-d-glucose (17) [24], 1,6-di-O-galloyl-2-O-caffeoyl-β-d-glucose (18) [28], tellimagrandin I (19) [26,29], tellimagrandin II (20) [5,26], cornusiin A (21) [30], rugosin D (22) [31], and (7′S,8′R)-dihydrodehydrodiconiferyl alcohol-9′-O-β-d-glucose (23) [5] (Figure 1). The known compounds 2–23 were identified by direct HPLC comparison with authentic standards and/or by comparing their spectral data with those reported in the literature. To the best of our knowledge, vanillic acid (4), brevifolincarboxylic acid (5), urolithin A (7), urolithin M6 (10), and 1,6-di-O-galloyl-2-O-p-coumaroyl-β-d-glucose (17) were isolated for the first time from water chestnut in this study.
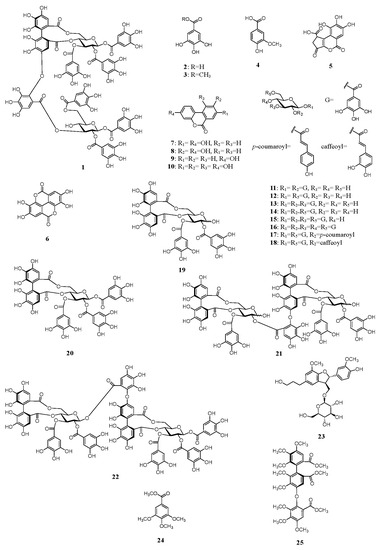
Figure 1.
Structures of compounds 1–25.
Trapadin A (1) was isolated as a light-brown amorphous powder. Its molecular formula was assigned as C75H56O48 based on high-resolution electrospray ionization mass spectrometry (HR-ESI-MS) results (m/z 1747.1838 [M + Na]+ calculated for C75H56O48 + Na: 1747.1833). The ultra-violet (UV) spectrum showed absorption maxima at 217 and 279 nm. Its 1H nuclear magnetic resonance (NMR) spectrum (500 MHz, in MeOH-d4) showed that it comprised six galloyl (Gal) groups (δ 7.10, 7.03, 7.01, 6.99, 6.91, 6.84 (each 2H, s)), a valoneoyl (Val) group (δ 6.95 (Val H-6″), 6.60 (Val H-3), 6.21 (Val H-3′)), and two sets of sugar proton signals (Table 1). The presence of two β-glucose units with the 4C1 conformation in 1 was indicated by two anomeric proton signals at δ 6.03 (d, J = 8.0 Hz) and δ 6.04 (d, J = 8.0 Hz) and the coupling pattern assigned using 1H–1H correlation spectroscopy (COSY). The presence of a free hydroxyl group at the C-4 of glucose II was also based on the appearance of H-1–H-3 and H-6 signals in a lower field (δ 6.04–4.37) than the H-4 signal (δ 3.65), as shown in Table 1. The 13C NMR spectrum (126 MHz, in MeOH-d4), exhibiting 12 carbon signals (δ 94.2, 93.8, 72.4, 72.7, 73.9, 76.7, 71.7, 69.7, 73.5, 76.9, 63.9, and 65.0) in the aliphatic region, assignable to two glucose units, also supported their existence (Table 1). The NMR data were similar to those of woodfordin A [32] and cornusiin G [33], which are dimeric tannins formed from units of tellimagrandin II and 1,2,3,6-tetra-O-galloyl-β-d-glucose. The linking position for each unit was determined using key heteronuclear multiple-bond connectivity (HMBC) correlations from glucose I H-4 (δ 5.09) and Val H-3′ (δ 6.21) to Val C-7′ (δ 169.0), from Val H-3′ (δ 6.21) to Val C-4′ (δ 147.9), from glucose I H-6 (δ 3.87, 5.38) and Val H-3 (δ 6.60) to Val C-7 (δ 169.4), from glucose II H-3 (δ 5.51) and Val H-6″ (δ 6.95) to Val C-7″ (δ 166.8), and from Val H-6″ (δ 6.95) to Val C-2″ (δ 137.1). The proton signals of the galloyl groups were correlated through each ester carbonyl carbon with the proton signals of each glucose, as shown in Figure 2; therefore, the galloyl groups were assigned to be at O-1, -2, and -3 on glucose I and at O-1, -2, and -6 on glucose II. The absolute configuration of the valoneoyl group in 1 was determined; a positive Cotton effect ([θ]224 +1.8 × 105) in the short wavelength of the circular dichroism (CD) spectrum of 1 (in MeOH) indicated the (S)-configuration of the Val group [30,34]. Methylation of 1 followed by methanolysis yielded a methyl tri-O-methylgallate (24) and a trimethyl octa-O-methylvaloneate (25) (Figure 1). Furthermore, the (S)-configuration of the Val group of 1 was supported by a positive Cotton effect ([θ]219 + 0.5 × 105) in the CD spectrum of 25, similarly to that observed in 1 [35].

Table 1.
1H and 13C NMR data for glucose residues of compound 1 measured in MeOH-d4.
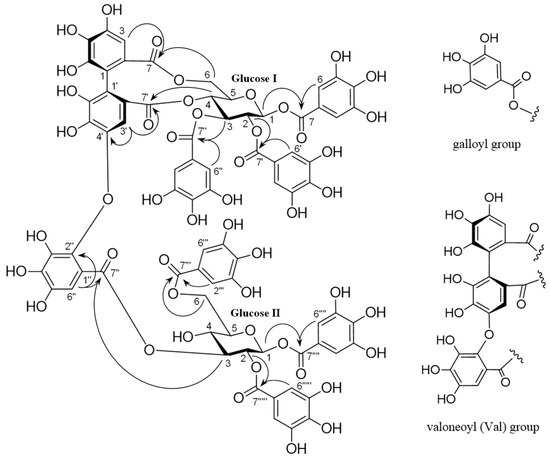
Figure 2.
Key heteronuclear multiple-bond connectivity (HMBC) correlations (H→C) of 1.
Two carbonyls of the hexahydroxydiphenoyl (HHDP) part of the Val group were located at O-4 and O-6 of the glucose I core in 1, which indicated the existence of two regioisomers. Orientation of the Val group in 1 was found to be of the isorugosin type, similar to that in cornusiin G, based on a comparison of the 1H NMR signal (δ 6.60) for Val H-3 and the 13C NMR signal (δ 147.9) for Val C-4″ of 1 with those of the rugosin and isorugosin types [31,33,36,37]. The sugar unit obtained following acid hydrolysis of 1 was identified as D-glucose based on HPLC analysis of the derivatives prepared by the reaction with L-cysteine methyl ester and o-tolyl isothiocyanate according to a previously reported method [35]. Based on these data, the structure of trapadin A was established as 1.
2.2. DPPH Radical Scavenging Activity
Several methods for evaluating antioxidant activity based on their principles and simplicity have been reported. Among these, the DPPH assay showed relatively high measurement accuracy for single compounds [38]. The DPPH method has extremely high reproducibility and is effective as a standard test method [39]. Therefore, in this study, the antioxidant activity was evaluated using the DPPH method.
DPPH radical scavenging activity assays were performed for compounds 1–23 (Table 2). The degree of activity was indicated by the half-maximal effective concentration (EC50) values. Compounds 4 and 7–9 were inactive (EC50: >100 μM), as evidenced by their antioxidant activity, and none of them had adjacent phenolic hydroxyl groups in its molecules. All the other compounds had adjacent phenolic hydroxyl groups in their molecules, and the greater the number, the stronger the activity. The EC50 values showed notable activity for hydrolyzable tannins. The EC50 values of hydrolyzable tannin monomers and dimers were between 5.06 and 12.5 µM and 3.48 and 3.91 µM, respectively, and the dimer with a higher number of phenolic hydroxyl groups in the molecule exhibited enhanced activity. Among these values, the EC50 value of trapadin A (1), the new compound, was 3.14 µM, demonstrating the highest activity. Therefore, we concluded that hydrolyzable tannins, especially dimers, contribute enormously to the antioxidant activity of water chestnuts. Therefore, further investigation of hydrolyzable tannin oligomers in water chestnuts is necessary.

Table 2.
DPPH radical scavenging activities of compounds 1–23.
3. Experimental Section
3.1. General
Optical rotations were measured using a JASCO-P-1020 digital polarimeter (JASCO Corporation, Tokyo, Japan). The UV spectra were recorded using a Shimadzu UVmini-1240 (Shimadzu Corporation, Kyoto, Japan). The HR-ESI-MS spectra were obtained using a micrOTOF-Q mass spectrometer (Bruker Daltonics, Billerica, MA, USA) with MeOH as the solvent. The NMR spectra were recorded using a Bruker AVANCE500 instrument (Bruker Biospin, Billerica, MA, USA; 500 and 126 MHz for 1H and 13C, respectively), and chemical shifts were expressed as parts per million (ppm) relative to those of the solvents (MeOH-d4 (δH 3.30; δC 49.0) and acetone-d6 (δH 2.04; δC 29.8)) on a tetramethylsilane scale. The standard pulse sequences programmed for the instrument (AVANCE500) were used for each 2D measurement (COSY, HSQC, and HMBC). The JCH was set at 10 Hz for HMBC analysis. Column chromatography was performed using Diaion HP-20, MCI-gel CHP-20P (Mitsubishi Chemical Co., Tokyo, Japan), Chromatorex ODS (Fuji Silysia Chemical Ltd., Aichi, Japan), Sephadex LH-20 (Cytiva, Tokyo, Japan), and YMC GEL ODS (YMC Co. Ltd., Kyoto, Japan) columns. The reversed-phase (RP) HPLC conditions were as follows: Condition 1—column, YMC-pack ODS AQ-3C2 (5 µm, 150 × 2.0 mm i.d., YMC Co., Ltd., Kyoto, Japan); mobile phase, 10 mmol/L phosphate buffer–acetonitrile (9:1); column temperature, 40 °C; flow rate, 0.2 mL/min; detection wavelength, 280 nm. Condition 2—column, L-column ODS (5 µm, 150 × 2.1 mm i.d., Chemicals Evaluation and Research Institute, Tokyo, Japan); mobile phase, solvent A was 0.1% formic acid in water, and solvent B was 0.1% formic acid in acetonitrile (0–30 min, 0–50% B in A; 30–35 min, 50–85% B in A; 35–40 min, 85% B in A; 40–50 min, 85–100% B in A); injection volume, 3 µL; column temperature, 40 °C; flow rate, 0.3 mL/min; and detection wavelength, 200–400 nm. Condition 3—column, L-column ODS (5 µm, 150 × 2.1 mm i.d.); mobile phase, 0.1% formic acid in water–0.1% formic acid in acetonitrile (75:25); column temperature, 35 °C; flow rate, 0.3 mL/min; detection wavelength, 250 nm. The normal-phase (NP) HPLC condition was as follows: Condition 4—column, SILICA SG80 (5 µm, 150 × 2.0 mm i.d., Shiseido Co., Ltd., Tokyo, Japan); mobile phase, n-hexane–MeOH–tetrahydrofran–formic acid (55:33:11:1) containing oxalic acid (450 mg/L); column temperature, room temperature; flow rate, 0.3 mL/min; detection wavelength, 280 nm. Analytical and preparative thin-layer chromatography (TLC) was performed on TLC Silica gel 60F254 plates (Merck, Darmstadt, Germany).
3.2. Materials
The water chestnuts (lot. nos. 06047F209 and 06047D280, the fruit of Trapa bispinosa) used for the phytochemical investigation were purchased from Nakajima Shoyaku Ltd. (Kyoto, Japan). All other reagents used were of special or analytical grade.
3.3. Extraction and Isolation
Water chestnuts (625 g) were homogenized in 80% MeOH (MeOH–H2O (8:2), 6.4 L); the homogenate was filtered and concentrated to yield 20 g of residue. A portion (17 g) of the residue suspended in H2O was separated using column chromatography on a Diaion HP-20 column with an aqueous MeOH in a stepwise gradient mode as follows: 0:100→10:90→20:80→30:70→50:50→100:0.
A 10% MeOH eluate (700 mg) was separated using column chromatography over MCI-gel CHP-20P with aqueous MeOH to obtain brevifolincarboxylic acid (5, 4.7 mg), 1,2-di-O-galloyl-β-d-glucose (11, 5.3 mg), and 1,6-di-O-galloyl-β-d-glucose (12, 2.1 mg). A 30% MeOH eluate (393.7 mg) was separated using column chromatography over Sephadex LH-20 with EtOH and/or YMC GEL ODS with aqueous MeOH to obtain methyl gallate (3, 8.1 mg), 1,2,3-tri-O-galloyl-β-d-glucose (13, 10.5 mg), tellimagrandin I (19, 15.0 mg), and cornusiin A (21, 7.4 mg). A 50% MeOH eluate (700 mg) was separated using column chromatography over Sephadex LH-20 with EtOH and/or YMC GEL ODS and/or MCI-gel CHP-20P with aqueous MeOH to obtain gallic acid (2, 4.1 mg), vanillic acid (4, 3.2 mg), ellagic acid (6, 3.9 mg), 1,2,6-tri-O-galloyl-β-d-glucose (14, 41.0 mg), 1,2,3,6-tetra-O-galloyl-β-d-glucose (15, 146.2 mg), 1,2,3,4,6-penta-O-galloyl-β-d-glucose (16, 9.4 mg), tellimagrandin II (20, 104.6 mg), 1,6-di-O-galloyl-2-O-p-coumaroyl-β-d-glucose (17, 11.3 mg), 1,6-di-O-galloyl-2-O-caffeoyl-β-d-glucose (18, 1.8 mg), (7′S,8′R)-dihydrodehydrodiconiferyl alcohol-9′-O-β-d-glucose (23, 4.6 mg), rugosin D (22, 19.0 mg), and trapadin A (1, 26.5 mg). A MeOH eluate (800 mg) was separated using column chromatography over Sephadex LH-20 and/or MCI-gel CHP-20P with aqueous MeOH to obtain urolithin A (7, 3.8 mg), isourolithin A (8, 0.8 mg), urolithin B (9, 5.4 mg), and urolithin M6 (10, 1.1 mg). These compounds were identified following direct comparison with authentic standards or by comparing their spectral data with those reported in the literature. The physical and spectral data of the new compound 1 are as follows.
Trapadin A (1): A light-brown amorphous powder. HR-ESI-MS m/z: 1747.1838 ([M + Na]+, calcd. for C75H56O48 + Na: 1747.1833). UV λmax (MeOH) nm (log ε): 217 (5.22), 279 (4.88). [α]D22 +14° (c = 0.2, MeOH). CD (MeOH) [α] (nm) +1.8 × 105 (224), +0.4 × 105 (238), −0.5 × 105 (258), +0.6 × 105 (280),1H-NMR (500 MHz, MeOH-d4) δ: 7.10, 7.03, 7.01, 6.99, 6.91, 6.84 (each 2H, s, galloyl-H), 6.95 (1H, s, Val H-6″), 6.60 (1H, s, Val H-3), 6.21 (1H, s, Val H-3′), glucose protons data are provided in Table 1. 13C-NMR (126 MHz, MeOH-d4) δ: 169.4 (Val C-7), 169.0 (Val C-7′), 168.4, 167.3, 167.2, 166.9, 166.4, 166.3 (galloyl C-7), 166.8 (Val C-7″), 147.9 (Val C-4′), 146.54, 146.48, 146.44, 146.37, 146.34, 146.02 (galloyl C-3 and C-5), 145.98 (Val C-4), 145.4, 145.1 (Val C-6, C-6′), 143.8 (Val C-5″), 141.2 (Val C-4″), 140.78 (2C), 140.66, 140.26, 140.23, 139.96, 139.92 (galloyl C-4, Val C-3″), 137.8 (Val C-5′), 137.4 (Val C-5), 137.1 (Val C-2″), 126.03, 126.01 (Val C-2, C-2′), 121.3, 120.6, 120.4, 120.3, 119.9, 119.7 (galloyl C-1), 117.9 (Val C-1′), 116.2 (Val C-1), 114.9 (Val C-1″), 110.69, 110.68, 110.62, 110.5 (3C), 110.4, 110.3 (galloyl C-2 and C-6, Val C-6″), 108.4 (Val C-3), 105.9 (Val C-3′), glucose carbon data are provided in Table 1.
3.4. Methylation of Compound 1 Followed by Methanolysis
A solution of compound 1 (4 mg) in EtOH (2 mL) was treated with (trimethylsilyl)diazomethane in hexane solution (2 mL) at room temperature overnight. After solvent removal, the residue was directly methanolyzed without further purification using 0.2% sodium methoxide in MeOH (2 mL) at room temperature for 12 h. After acidification with a few drops of 10% hydrochloric acid, the reaction mixture was evaporated, and the residue was purified using preparative TLC (n-hexane–acetone (1:1)) to yield methyl tri-O-methylgallate (24, 1.7 mg, HR-ESI-MS m/z: 249.0752 (M + Na)+, calcd. for C11H14O5 + Na: 249.0733) and trimethyl octa-O-methylvaloneate (25; 1.0 mg, HR-ESI-MS m/z: 683.1958 (M + Na)+, calcd. for C32H36O15 + Na: 638.1946, CD (MeOH) [θ] (nm): +0.5 × 105 (219), −0.3 × 105 (249). 1H-NMR (in acetone-d6) δ: 7.38, 7.31, 6.90 (each 1H, s), 4.02, 3.95, 3.94, 3.93, 3.86, 3.76, 3.75, 3.62, 3.55, 3.54, 3.41 (each 3H, s)), which were identified by comparison with an authentic sample.
3.5. Determination of the Sugar Configuration of Compound 1
The sugar configuration was determined using a previously described method [35]. Compound 1 (1.0 mg) was hydrolyzed by heating in 1 mol/L hydrochloric acid (0.2 mL) and neutralized using Amberlite IRA400 (Organo Corporation, Tokyo, Japan). After evaporation, the residue was dissolved in pyridine (0.2 mL) containing L-cysteine methyl ester hydrochloride (1.0 mg) and heated at 60 °C for 1 h. o-Tolyl isothiocyanate (1.0 mg) in pyridine (0.2 mL) was then added to each mixture, followed by direct analysis using RP-HPLC (Condition 3). The peak from compound 1 coincided with that of the derivative similarly prepared from an authentic D-glucose sample.
3.6. DPPH Radical Scavenging Activities of Compounds 1–23
The DPPH radical scavenging activity of each compound was determined using the DPPH Antioxidant Assay Kit (Dojin Laboratories, Kumamoto, Japan) following the manufacturer’s instructions [39,40]. The sample solution, assay buffer, and DPPH working solution were mixed in 96-well plates and incubated in the dark at 25 °C for 30 min. The absorbance was measured at 517 nm using an Infinite F200 microplate reader (Tecan Group Ltd., Mannedorf, Switzerland). The EC50 was determined via regression line analysis, and Trolox was used as the positive control. All experiments were performed in triplicate.
4. Conclusions
In conclusion, we successfully isolated a new hydrolyzable tannin dimer, trapadin A (1), from water chestnut, along with 22 known compounds. Through the application of spectroscopic and chemical methods, the structure of trapadin A (1) was elucidated, revealing a hydrolyzable tannin dimer formed from units of tellimagrandin II and 1,2,3,6-tetra-O-galloyl-β-d-glucose. Furthermore, the determined EC50 values in the DPPH radical scavenging assay revealed substantial activity for hydrolyzable tannins, with trapadin A (1), the new hydrolyzable tannin dimer, exhibiting the highest activity value. The findings of this study suggest that the hydrolyzable tannin dimers are responsible for the antioxidant activity of the water chestnut.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules28186563/s1, Figure S1: 1H-NMR spectrum of compound 1; Figure S2: 1H-1H COSY spectrum of compound 1; Figure S3: 13C-NMR spectrum of compound 1; Figure S4: HSQC spectrum of compound 1; Figure S5: HMBC spectrum of compound 1; Figure S6: Selected HMBC correlations of compound 1; Table S1: NMR data of woodfordin A and cornusiin G, which are dimeric hydrolyzable tannins formed from units of tellimagrandin II and 1,2,3,6-tetra-O-galloyl-β-d-glucose; spectral data of compounds 2–23.
Author Contributions
Conceptualization, Y.A.; Formal analysis, T.U. and Y.A.; Funding acquisition, Y.A.; Investigation, T.U., Y.M. and Y.A.; Methodology, T.U., M.Y. and Y.A.; Project administration, Y.A.; Resources, H.I.; Validation, T.U. and Y.A.; Visualization, T.U. and Y.A.; Writing—original draft preparation, T.U. and Y.A.; Writing—review and editing, M.Y., H.I. and Y.A. All authors have read and agreed to the published version of the manuscript.
Funding
A portion of this work was supported by JSPS KAKENHI grant number JP20K07120.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article and Supplementary Materials.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Not available.
References
- Shindo, K.; Kuroki, E.; Toyoda, M. Antioxidative compounds contained in the seed with hard shell of Trapa japonica flerov. and its herbal tea. Nippon Kasei Gakkaishi 2013, 64, 353–359. [Google Scholar]
- Ministry of Education, Culture, Sports, Science and Technology. Standard Tables of Composition in Japan 2015, 7th Revised Version; Ministry of Education, Culture, Sports, Science and Technology: Tokyo, Japan, 2015. [Google Scholar]
- Kawabe, S.; Ganeko, N.; Ito, H. Ellagitannin dimers from the pericarps of Trapa japonica. Shouyakugaku Zasshi 2017, 71, 53–54. [Google Scholar]
- Nonaka, G.; Matsumoto, Y.; Nishioka, I. Trapain, a new hydrolyzable tannin from Trapa japonica Flerov. Chem. Pharm. Bull. 1981, 29, 1184–1187. [Google Scholar] [CrossRef]
- Iwaoka, Y.; Suzuki, S.; Kato, N.; Hayakawa, C.; Kawabe, S.; Ganeko, N.; Uemura, T.; Ito, H. Characterization and identification of bioactive polyphenols in the Trapa bispinosa Roxb. pericarp extract. Molecules 2021, 26, 5802. [Google Scholar] [CrossRef]
- Lee, D.; Lee, O.-H.; Choi, G.; Kim, J.D. Antioxidant and anti-adipogenic activities of Trapa japonica shell extract cultivated in Korea. Prev. Nutr. Food Sci. 2017, 22, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.-J.; Lee, S.-K.; Song, J.-H.; Kim, M.-E.; Kim, M.-J.; Jang, J.-S.; Lee, J.-H.; Kim, J.-I. Water chestnut (Trapa japonica Flerov) exerts inhibitory effect on postprandial glycemic response in rats and free radical scavenging activity in vitro. Food Sci. Biotechnol. 2009, 18, 808–812. [Google Scholar]
- Kang, H.-G.; Bashir, K.M.I.; Kim, K.-Y.; Shin, S.; Choi, M.-W.; Hong, E.-J.; Choi, S.-H.; Kim, J.-W.; Choi, J.-S.; Ku, S.-K. Evaluation of dose-dependent obesity and diabetes-related complications of water chestnut (fruit of Trapa japonica) extracts in type II obese diabetic mice induced by 45% kcal high-fat diet. Medicina 2022, 58, 189. [Google Scholar] [CrossRef]
- Park, S.-J.; Lee, M.; Kim, K.-Y.; Shin, S.; Choi, M.-W.; Hong, E.-J.; Lee, J. Trapa japonica Flerov extract prevents obesity by regulating adipogenesis and lipolysis in differentiated 3T3-L1 cells. Appl. Sci. 2022, 12, 290. [Google Scholar] [CrossRef]
- Kim, M.J.; Im, K.R.; Yoon, K.-S. Trapa japonica Flerov extract attenuates lipid accumulation through downregulation of adipogenic transcription factors in 3T3-L1 cells. Am. J. Mol. Biol. 2015, 5, 32–41. [Google Scholar] [CrossRef]
- Kim, Y.-S.; Hwang, J.-W.; Jang, J.-H.; Son, S.; Seo, I.-B.; Jeong, J.-H.; Kim, E.-H.; Moon, S.H.; Jeon, B.-T.; Park, P.-J. Trapa japonica pericarp extract reduces LPS-induced inflammation in macrophages and acute lung injury in mice. Molecules 2016, 21, 392. [Google Scholar] [CrossRef]
- Kim, B.; Kim, J.E.; Choi, B.-K.; Kim, H.-S. Anti-inflammatory effects of water chestnut extract on cytokine responses via nuclear factor-κB-signaling pathway. Biomol. Ther. 2015, 23, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-S.; Kim, E.-K.; Hwang, J.-W.; Seo, I.-B.; Jang, J.-H.; Son, S.; Jeong, J.-H.; Moon, S.H.; Jeon, B.-T.; Park, P.-J. Characterization of the antioxidant fraction of Trapa japonica pericarp and its hepatic protective effects in vitro and in vivo. Food Funct. 2016, 7, 1689–1699. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, M.; Yasutake, K.; Hino, M.; Ohwatari, H.; Ohmagari, N.; Takedomi, K.; Tanaka, T.; Nonaka, G. Inhibitory effects of polyphenols from water chestnut (Trapa japonica) husk on glycolytic enzymes and postprandial blood glucose elevation in mice. Food Chem. 2014, 165, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Nam, G.-H.; Kawk, H.-W.; Kim, S.-Y.; Kim, Y.-M. Solvent fractions of fermented Trapa japonica fruit extract stimulate collagen synthesis through TGF-β1/GSK-3β/β-catenin pathway in human dermal fibroblasts. J. Cosmet. Dermatol. 2020, 19, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Nam, G.-H.; Jo, K.-J.; Park, Y.-S.; Kawk, H.W.; Yoo, J.-G.; Jang, J.D.; Kang, S.M.; Kim, S.-Y.; Kim, Y.-M. Bacillus/Trapa japonica fruit extract ferment filtrate enhances human hair follicle dermal papilla cell proliferation via the Akt/ERK/GSK-3β signaling pathway. BMC Complement. Altern. Med. 2019, 19, 104. [Google Scholar] [CrossRef]
- Bešlo, R.; Golubić, N.; Rastija, V.; Agić, D.; Karnaš, M.; Šubarić, D.; Lučić, B. Antioxidant activity, metabolism, and bioavailability of polyphenols in the diet of animals. Antioxidants 2023, 12, 1141. [Google Scholar] [CrossRef]
- Puruteanu, L.L.; Bailey, D.S.; Grádinaru, A.C.; Jäntschi, L. The biochemistry and effectiveness of antioxidants in food, fruits, and marine algae. Antioxidants 2023, 12, 860. [Google Scholar] [CrossRef]
- SDBSWeb. National Institute of Advanced Industrial Science and Technology. Available online: https://sdbs.db.aist.go.jp (accessed on 10 February 2023).
- Saijyo, R.; Nonaka, G.; Nishioka, I. Tannins and related compounds. LΧΧΧVII. Isolation and characterization of four new hydrolyzable tannins from the leaves of Mallotus repandus. Chem. Pharm. Bull. 1989, 37, 2624–2630. [Google Scholar] [CrossRef]
- Bai, N.; He, K.; Roller, M.; Zheng, B.; Chen, X.; Shao, Z.; Peng, T.; Zheng, Q. Active compounds from Lagerstroemia speciosa, insulin-like glucose uptake-stimulatory/inhibitory and adipocyte differentiation-inhibitory activities in 3T3-L1 cells. J. Agric. Food Chem. 2008, 56, 11668–11674. [Google Scholar] [CrossRef]
- Ito, H.; Iguchi, A.; Hatano, T. Identification of urinary and intestinal bacterial metabolites of ellagitannin geraniin in rats. J. Agric. Food Chem. 2008, 56, 393–400. [Google Scholar] [CrossRef]
- Ishimatsu, M.; Tanaka, T.; Nonaka, G.; Nishioka, I.; Nishizawa, M.; Yamagishi, T. Tannins and related compounds. LXXV. Isolation and characterization of novel diastereoisomeric ellagitannins, nupharins A and B, and their homologues from Nuphar japonicum DC. Chem. Pharm. Bull. 1989, 37, 129–134. [Google Scholar] [CrossRef]
- Kashiwada, Y.; Nonaka, G.; Nishioka, I.; Yamagishi, T. Galloyl and hydroxycinnamoyl glucoses from rhubarb. Phytochemistry 1988, 27, 1473–1477. [Google Scholar] [CrossRef]
- Nonaka, G.; Nishioka, I. Tannins and related compounds. X. Rhubarb (2): Isolation and structures of a glycerol gallate, gallic acid glucoside gallates, galloylglucoses and isolindleyin. Chem. Pharm. Bull. 1983, 31, 1652–1658. [Google Scholar] [CrossRef]
- Yoshida, T.; Hatano, T.; Okuda, T.; Memon, M.U.; Shingu, T.; Inoue, K. Spectral and chromatographic analyses of tannins. I. 13C Nuclear magnetic resonance spectra of hydrolysable tannins. Chem. Pharm. Bull. 1984, 32, 1790–1799. [Google Scholar] [CrossRef]
- Kiss, A.K.; Derwińska, M.; Dawidowska, A.; Naruszewicz, M. Novel biological properties of Oenothera paradoxa defatted seed extracts: Effects on metallopeptidase activity. J. Agric. Food Chem. 2008, 56, 7845–7852. [Google Scholar] [CrossRef]
- Huang, H.-C.; Chao, C.-L.; Liaw, C.-C.; Hwang, S.-Y.; Kuo, Y.-H.; Chang, T.-C.; Chao, C.H.; Chen, C.-J.; Kuo, Y.-H. Hypoglycemic constituents isolated from Trapa natans L. pericarps. J. Agric. Food Chem. 2016, 64, 3794–3803. [Google Scholar] [CrossRef]
- Hatano, T.; Yoshida, T.; Shingu, T.; Okuda, T. 13C Nuclear magnetic resonance spectra of hydrolyzable tannins. II. Tannins forming anomer mixtures. Chem. Pharm. Bull. 1988, 36, 2925–2933. [Google Scholar] [CrossRef]
- Hatano, T.; Ogawa, N.; Kira, R.; Yasuhara, T.; Okuda, T. Tannins of Cornaceous plants. I. Cornusiins A, B and C, dimeric monomeric and trimeric hydrolyzable tannins from Cornus officinalis, and orientation of valoneoyl group in related tannins. Chem. Pharm. Bull. 1989, 37, 2083–2090. [Google Scholar] [CrossRef]
- Hatano, T.; Ogawa, N.; Shingu, T.; Okuda, T. Tannins of Rosaceous plants. IX. Rugosins D, E, F and G, dimeric and trimeric hydrolyzable tannins with valoneoyl group(s), from flower petals of Rosa rugosa THUNB. Chem. Pharm. Bull. 1990, 38, 3341–3346. [Google Scholar] [CrossRef]
- Yoshida, T.; Chou, T.; Nitta, A.; Okuda, T. Woodfordins A, B and C, dimeric hydrolyzable tannins from Woodfordia fruticose flowers. Hetelocycles 1989, 29, 2267–2271. [Google Scholar]
- Hatano, T.; Yasuhara, T.; Abe, R.; Okuda, T. A galloylated monoterpene glucoside and dimeric hydrolysable tannin from Cornus officinalis. Phytochemistry 1990, 29, 2975–2978. [Google Scholar] [CrossRef]
- Okuda, T.; Yoshida, T.; Hatano, T.; Koga, T.; Toh, N.; Kuriyama, K. Circular dichroism of hydrolysable tannins-I ellagitannins and gallotannins. Tetrahedron Lett. 1982, 23, 3937–3940. [Google Scholar] [CrossRef]
- Tanaka, T.; Nakashima, T.; Ueda, T.; Tomii, K.; Kouno, I. Facile discrimination of aldose enantiomers by reversed-phase HPLC. Chem. Pharm. Bull. 2007, 55, 899–901. [Google Scholar] [CrossRef] [PubMed]
- Hatano, T.; Ogawa, N.; Yasuhara, T.; Okuda, T. Tannins of Rosaceous plants. VIII. Hydrolyzable tannins monomers having a valoneoyl group from flower petals of Rosa rugosa Thunb. Chem. Pharm. Bull. 1990, 38, 3308–3313. [Google Scholar] [CrossRef]
- Ito, H.; Yamaguchi, K.; Kim, T.-H.; Khennouf, S.; Gharzouli, K.; Yoshida, T. Dimeric and trimeric hydrolyzable tannins from Quercus coccifera and Quercus suber. J. Nat. Prod. 2002, 65, 339–345. [Google Scholar] [CrossRef]
- Shimamura, T.; Matsuura, R.; Tokuda, T.; Sugumoto, N.; Yamazaki, T.; Matsufuji, H.; Matsui, T.; Matsumoto, K.; Ukeda, H. Comparison of conventional antioxidants assays for evaluating potencies of natural antioxidants as food additives by collaborative srudy. Nippon Shokuhin Kagaku Kogaku Kaishi 2007, 54, 482–487. [Google Scholar] [CrossRef]
- Shimamura, T.; Sumikura, Y.; Yamazaki, T.; Tada, A.; Kashiwagi, T.; Ishikawa, H.; Matsui, T.; Sugimoto, N.; Akiyama, H.; Ukeda, H. Applicability of the DPPH assay for evaluating the antioxidant capacity of food additives-Inter-laboratory evaluation study. Anal. Sci. 2014, 30, 717–721. [Google Scholar] [CrossRef]
- Uchikura, T.; Kitano, T.; Yoshimura, M.; Amakura, Y. Characterization of phenolic constituents in hazelnut kernels. Biosci. Biotechnol. Biochem. 2023, 87, 688–695. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).