China Medicinal Plants of the Ampelopsis grossedentata—A Review of Their Botanical Characteristics, Use, Phytochemistry, Active Pharmacological Components, and Toxicology
Abstract
:1. Botanical Description
2. Use
2.1. Traditional Uses
2.2. Modern Uses
3. Phytochemistry
3.1. Flavonoids
3.2. Phenols
3.3. Steroids and Terpenoids
3.4. Water-Soluble Polysaccharide
3.5. Volatile Components and Other Compounds
4. Pharmacological Properties
4.1. Anti-Inflammatory and Analgesia
4.2. Anti-Oxidation
4.3. Reduction of Blood Sugar, Blood Pressure, and Blood Lipid Levels
4.4. Liver and Kidney Protection
4.5. Tumor Suppression and Anti-Tumor Activity
4.6. Antibacterial
5. Toxicology
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zheng, X.J.; Zhang, W.T.; Xie, L.Y. Advances in the pharmacological effects of Ampelopsis. Fujian J. Med. 2013, 35, 152–154. [Google Scholar]
- Li, Y.J.; Zhong, Z.X. An introduction to the research and application of Ampelopsis grossedentata. Chin. Ethn. Folk. Med. 2013, 22, 26–27. [Google Scholar]
- Liu, J.X.; Zhou, T.D. Biopharmacological study of Ampelopsis grossedentata. Chin. Herb. Med. 1999, 6, 459–463. [Google Scholar]
- Chen, Z.Z. Wild natural tea-like plant substitute tea-wild Ampelopsis grossedentata. Guangxi Trop. Agric. 2003, 3, 27–28. [Google Scholar]
- Food and Drug Administration of Guangxi Zhuang Autonomous Region. Quality Standard of Zhuang Medicine in Guangxi Zhuang Autonomous Region; Guangxi Science and Technology Press: Nanning, China, 2008; Volume 1. [Google Scholar]
- Lai, F. The current situation of research and development of sweet tea resources in China and the direction of development in the new century. Tea Newsl. 2003, 3, 25–28. [Google Scholar]
- Yang, Z.G. Calling tea is not “tea” of Ampelopsis grossedentata. Life World 2019, 8, 74–83. [Google Scholar] [CrossRef]
- Hu, S.H. Ying Shan Zheng Yao; China Chinese Medicine Publishing House: Beijing, China, 2009. [Google Scholar]
- Liu, S.S. Cao Mu Bian Fang; Chongqing Publishing House: Chongqing, China, 2009. [Google Scholar]
- Xiong, L.Z.; Lin, L.P.; Xu, W.; Huang, Z.H. The literature examination and microscopic identification of Hakka Ampelopsis grossedentata. Fujian Tradit. Chin. Med. 2022, 53, 26–29. [Google Scholar]
- Song, W.W.; Dai, Q.L.; Huang, M.E.; Lei, F.X. A preliminary investigation of the folk herb “Ampelopsis grossedentata”. Hunan J. Tradit. Chin. Med. 1996, 5, 41. [Google Scholar]
- Wei, J.F.; Huang, X.C. Identification of the original plant of Guangxi sweet tea. Chin. Med. Mater. 1996, 1, 16–17. [Google Scholar]
- Song, B.Z.; Wan, D.R. Survey of common botanicals (Vitaceae) of the Tujia family in Hubei Province. Chin. J. Ethn. Folk. Med. 2003, 2, 117–118. [Google Scholar]
- Cao, T.R. Daily beverage plants of the Hmong compatriots. J. Plants 1994, 2, 12–13. [Google Scholar]
- Jiang, C.W. Research progress on the efficacy of Yao medicine Ampelopsis grossedentata. Zhuang Yao Med. Natly. Res. 2021, 1, 52–55+183. [Google Scholar]
- Yang, H. Research on the chemical composition and pharmacology of Yao nationality Ampelopsis grossedentata. Fujian Tea 2020, 42, 9–10. [Google Scholar]
- Yang, S.L.; Guo, S.R.; Zheng, P.C. JiNuo Nationality Medicine; Yunnan Science and Technology Press: Kunming, China, 2001; p. 300. [Google Scholar]
- Xu, L.J.; Ma, P.; Xiao, W.; Peng, Y.; He, C.N.; Xiao, P.G. Preliminary investigation on the ancient and modern application history of different tea-Ampelopsis grossedentata. China Mod. Tradit. Chin. Med. 2012, 14, 62–66. [Google Scholar]
- Chen, X.W.; Dong, B.P.; Chen, J.M.; Xiao, C.F.; Li, B.S.; Luo, D.S. Research on the medicinal value of Ampelopsis grossedentata for drinking. Hunan Agric. Sci. 2007, 6, 180–181. [Google Scholar]
- Li, J.C.; Li, S.Y.; Wang, Y.; Wang, W.N. Chemical composition, pharmacological effects and quality marker (Q-marker) prediction analysis of Ampelopsis grossedentata. J. Southwest Univ. Natl. (Nat. Sci. Ed.) 2021, 47, 254–266. [Google Scholar]
- Pan, L.H.; Luo, S.Z. Research on the application of Ampelopsis grossedentata in soft drinks. Beverag. Ind. 2005, 1, 37–38. [Google Scholar]
- Luo, A.L. Study on the Hepatoprotective Effect of a Compound Formula of Pueraria Lobata, Ampelopsis grossedentata and Corn Oligopeptide on Alcohol in Mice. Master’s Thesis, Shanghai Jiaotong University, Shanghai, China, 2019. [Google Scholar]
- Zhang, S.X.; Gao, M.; Liang, L.L.; Cheng, Y.; Tang, G.Y. Development of soy milk Ampelopsis grossedentata composite beverage. Food Res. Dev. 2016, 37, 78–80. [Google Scholar]
- Deng, F.L. Pharmacological Study of Ampelopsis grossedentata Ginseng Capsules. Master’s Thesis, Hubei College of Traditional Chinese Medicine, Wuhan, China, 2009. [Google Scholar]
- Li, D.M.; Li, C.L.; Wen, Y. Development of Ampelopsis grossedentata compound bagged tea and its in vitro hypoglycemic effect. Mod. Food Sci. Technol. 2022, 38, 228–236. [Google Scholar]
- Zheng, C.; Zeng, J.H.; Gu, C.Q.; Wei, X.C. Development of health Ampelopsis grossedentata beverage. J. Guangzhou Univ. (Nat. Sci. Ed.) 2008, 7, 34–39. [Google Scholar]
- Wang, D.Y.; Liu, J.M.; Zhang, J.T.; Zheng, S.J. Research on the chemical composition of the Ampelopsis grossedentata. Subtrop. Plant Lett. 1998, 2, 39–44. [Google Scholar]
- Wang, D.Y.; Xu, S.Y. Isolation and structure determination of grossedentatasin. J. Zhangzhou Norm. Coll. (Nat. Sci. Ed.) 1996, 2, 62–64+67. [Google Scholar]
- Wang, D.Y. Isolation and structure determination of grossedentataside. J. Zhangzhou Norm. Coll. (Nat. Sci. Ed.) 1999, 4, 42–45. [Google Scholar]
- Zhang, Y.S.; Yang, W.L.; Cui, C. Study on the chemical composition of Ampelopsis grossedentata. Chin. Herb. Med. 2003, 5, 21–22. [Google Scholar]
- He, G.X.; Pei, G.; Zhou, T.D.; Zhou, X.X. Determination of total flavonoids and dihydromyricetin in Ampelopsis grossedentata. Chin. J. Tradit. Chin. Med. 2000, 7, 39–41. [Google Scholar]
- Wang, J.; He, L.; Zheng, N. Dihydromyricetin in Ampelosis grossedentata collected from different habitats. Chin. Tradit. Patent. Med. 2014, 36, 145–147. [Google Scholar]
- Zhang, J.Y.; Chen, Y.; Luo, H.Q. Recent update on the pharmacological effects and mechanisms of dihydromyricetin. Front. Pharmacol. 2018, 9, 1204. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.J.; Liu, Y.; Liu, J.B.; Nong, G.; Liu, W. Research progress on chemical composition and pharmacological activity of Ampelopsis grossedentata. Chin. Pat. Med. 2022, 44, 2595–2601. [Google Scholar]
- Qin, J.P.; Xu, X.Q.; Li, J.J. Study on the chemical composition of Guangxi Yao Ampelosis grossedentata. Nat. Prod. Res. Dev. 1997, 4, 41–43. [Google Scholar]
- He, G.X.; Pei, G.; Du, F.L.; Ou, Y.W.; Li, B. Study on the chemical composition of Ampelosis grossedentata. China Mod. Tradit. Chin. Med. 2007, 12, 11–13. [Google Scholar]
- Zhang, Y.S. Isolation and Identification of Chemical Constituents of the Tea-Like Plant Ampelosis grossedentata and Study of Dihydromyricetin Extraction Technology. Master’s Thesis, Hunan Agricultural University, Changsha, China, 2001. [Google Scholar]
- Fu, M.; Li, X.Y.; Wang, D.Y.; Guo, M.Q. Study of flavonoids in the leaves of Ampelosis grossedentata. Chin. J. Pharm. Sci. 2015, 50, 574–578. [Google Scholar]
- Zhou, T.D.; Zhou, X.X. Isolation, structural identification and pharmacological activity of dihydroflavonol from Ampelosis grossedentata. Chin. J. Pharmacol. 1996, 8, 10–13. [Google Scholar]
- Bai, X.X.; Xia, G.P.; Zhao, N.X.; Dong, H.L.; Shao, Z.Y.; Han, Y.M. Phenolic chemical composition of Ampelosis grossedentata from Zhangjiajie. Chin. Med. Mater. 2013, 36, 65–67. [Google Scholar]
- Wang, D.Y.; Zheng, Z.Z.; Xu, S.Y.; Zheng, S.Z. Four new isoflavones from Ampelopsis grossedentata. J. Asian Nat. Prod. Res. 2002, 4, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.F.; Ying, L.; Sun, D.; Zhang, S.K.; Zhu, Y.J.; Xu, P. Supercritical carbon dioxide extraction of bioactive compounds from Ampelopsis grossedentata stems: Process optimization and antioxidant activity. Int. J. Mol. Sci. 2011, 12, 6856–6870. [Google Scholar] [CrossRef]
- Yuan, A.X.; Huang, X.M.; Chen, J. The chemical composition of explicit dental snake grapes. Chin. J. Tradit. Chin. Med. 1998, 6, 39–40+63. [Google Scholar]
- Du, Q.Z.; Chen, P.; Jerz, G.; Winterhalter, P. Preparative separation of flavonoid glycosides in leaves extract of Ampelopsis grossedentata using high-speed counter-current chromatography. J. Chromatogr. A 2004, 1040, 147–149. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, L.L.; Li, R.; Wang, Y. Study on the chemical composition of Ampelopsis grossedentata. Chin. Med. Mater. 2002, 4, 254–256. [Google Scholar]
- Zhang, Y.S.; Yang, W.L.; Xiong, H.P. Analysis of the chemical composition of volatile oil of Ampelopsis grossedentata. J. Hunan Agric. Univ. (Nat. Sci. Ed.) 2001, 2, 100–101. [Google Scholar]
- Zhang, S.X.; Ao, K.H.; Zeng, Q.H.; Liu, Y. Determination of chemical composition in volatile oil of Ampelopsis grossedentata by gas chromatography-mass spectrometry. China Brew. 2014, 33, 140–143. [Google Scholar]
- Lai, M.L.; Yu, J.P. Analysis of cyclic hydrocarbons in commercial Ampelopsis grossedentata by SPME-GC/MS. J. Mt. Agric. Biol. 2014, 33, 92–94. [Google Scholar]
- Wang, H.F.; You, X.Q. Gas chromatographic determination of aroma composition in Ampelopsis grossedentata. Nat. Prod. Res. Dev. 1996, 4, 47–50. [Google Scholar]
- Zhou, X.X.; Zhou, T.D.; Tan, C.S. The effect of dihydroyangenin bark on the contractile response of smooth muscle of rabbit thoracic aortic strips. Mod. Appl. Pharmacol. 1997, 2, 8–11+68. [Google Scholar]
- Liu, J.X.; Zhou, T.D. Experimental study on the effect of Yao Ampelopsis grossedentata on rabbit intestinal smooth muscle and detoxification. Chin. J. Ethn. Med. 1998, 2, 44–45. [Google Scholar]
- Qin, J.P.; Tan, J.N.; Ou, Y.; Lu, S.Y. Study on the method of determination of dihydroyangme barkin in Guangxi Yao Ampelopsis grossedentata. Chin. J. New Drugs 2004, 10, 915–917. [Google Scholar]
- Pan, X.Y. Isolation and Identification of Polysaccharide from Ampelopsis grossedentata and Its Tablet Preparation. Master’s Thesis, Dalian Ocean University, Dalian, China, 2019. [Google Scholar]
- Luo, Z.Y.; Cheng, C.; Li, W.; Wu, M.C. Isolation and purification of water-soluble polysaccharides from Ampelopsis grossedentata. Food Sci. 2007, 1, 151–154. [Google Scholar]
- Wang, Y.; Bian, X.; Park, J.; Ying, L.; Qian, L.; Xu, P. Physicochemical properties, in vitro antioxidant activities and inhibitory potential against α-glucosidase of polysaccharides from Ampelopsis grossedentata leaves and stems. Molecules 2011, 16, 7762–7772. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.M.; Fu, M. Extraction process and content study of polysaccharides from Ampelopsis grossedentata. J. Huaihua Coll. 2011, 30 (Suppl. S1), 39–42. [Google Scholar]
- Wu, J.; Zhao, F.T.; Fan, K.J.; Zhang, J.; Xu, B.X.; Wang, Q.S.; Tang, T.T.; Wang, T.Y. Dihydromyricetin Inhibits Inflammation of Fibroblast-Like Synoviocytes through Regulation of Nuclear Factor-κB Signaling in Rats with Collagen-Induced Arthritis. J. Pharmacol. Exp. Ther. 2019, 368, 218–228. [Google Scholar] [CrossRef]
- Wu, J.; Fan, K.J.; Wang, Q.S.; Xu, B.X.; Cai, Q.; Wang, T.Y. Dihydromyricetin protects the knee joints of rats with collagen-induced arthritis by inhibition of NF-κB signaling and osteoclastic bone resorption. Food Funct. 2020, 11, 6251–6264. [Google Scholar] [CrossRef]
- Jia, R.; Ma, J.; Meng, W.; Wang, N. Dihydromyricetin inhibits caerulin-induced TRAF3-p38 signaling activation and acute pancreatitis response. Biochem. Biophys. Res. Commun. 2018, 503, 1696–1702. [Google Scholar] [CrossRef]
- Wang, R.; Pi, J.; Su, X.; Liu, J.; Zeng, X.; Wong, I.; Huang, L.; Zhou, H.; Cai, J.; Li, T.; et al. Dihydromyricetin suppresses inflammatory responses in vitro and in vivo through inhibition of IKKβ activity in macrophages. Scanning 2016, 38, 901–912. [Google Scholar] [CrossRef]
- Chen, Y.L.; Zhang, Y.L.; Dai, Y.C.; Tang, Z.P. Systems pharmacology approach reveals the antiinflammatory effects of Ampelopsis grossedentata on dextran sodium sulfate-induced colitis. World J. Gastroenterol. 2018, 24, 1398–1409. [Google Scholar] [CrossRef]
- Gao, J.; Liu, B.; Ning, Z.; Zhao, R.; Zhang, A.; Wu, Q. Characterization and antioxidant activity of flavonoid-rich extracts from leaves of Ampelopsis grossedentata. J. Food Biochem. 2009, 33, 808–820. [Google Scholar] [CrossRef]
- Gao, Q.; Ma, R.; Chen, L.; Shi, S.; Cai, P.; Zhang, S.; Xiang, H. Antioxidant profiling of vine tea (Ampelopsis grossedentata): Off-line coupling heart-cutting HSCCC with HPLC–DAD–QTOF-MS/MS. Food Chem. 2017, 225, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Xie, K.; He, X.; Chen, K.; Chen, J.; Sakao, K.; Hou, D.X. Antioxidant Properties of a Traditional Vine Tea, Ampelopsis grossedentata. Antioxidants 2019, 8, 295. [Google Scholar] [CrossRef] [PubMed]
- Ou, X.H.; Ye, Y.; Huang, Q.J.; Liu, H.G.; Song, Y.F. Study on antioxidant activity of Ampelopsis grossedentata. Nat. Prod. Res. Dev. 2013, 25, 245–248. [Google Scholar]
- Ye, L.; Wang, H.; Duncan, S.E.; Eigel, W.N.; O’Keefe, S.F. Antioxidant activities of Vine Tea (Ampelopsis grossedentata) extract and its major component dihydromyricetin in soybean oil and cooked ground beef. Food Chem. 2015, 172, 416–422. [Google Scholar] [CrossRef]
- Wang, E.H.; Qin, Z.H.; Yang, L.S.; Lu, F.X.; Qin, T. Antioxidant activity evaluation of dihydromyricetin from Ampelopsis grossedentata on Guizhou traditional sausage. Food Sci. Technol. 2017, 42, 128–132. [Google Scholar]
- Xu, Y.; Wang, W.Z.; Zhou, Y.F. A study of Ampelopsis grossedentata flavonoids improving blood lipid levels in rats by influencing the AMPK signaling pathway. Chongqing Med. 2022, 51, 1626–1630+1637. [Google Scholar]
- Ran, L.; Wang, X.; Lang, H.; Xu, J.; Wang, J.; Liu, H.; Mi, M.; Qin, Y. Ampelopsis grossedentata supplementation effectively ameliorates the glycemic control in patients with type 2 diabetes mellitus. Eur. J. Clin. Nutr. 2019, 73, 776–782. [Google Scholar] [CrossRef] [PubMed]
- Xiang, J.; Lv, Q.; Yi, F.; Song, Y.; Le, L.; Jiang, B.; Xu, L.; Xiao, P. Dietary Supplementation of Vine Tea Ameliorates Glucose and Lipid Metabolic Disorder via Akt Signaling Pathway in Diabetic Rats. Molecules 2019, 24, 1866. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.P.; Wang, S.; An, F.X.; Ge, Z.W.; Lan, Y.; Zhang, Z. Study on the hypotensive effect of Ampelopsis grossedentata. China Mod. Drug Appl. 2013, 7, 229–230. [Google Scholar]
- Fan, L.; Qu, X.; Yi, T.; Peng, Y.; Jiang, M.; Miao, J.; Xiao, P. Metabolomics of the Protective Effect of Ampelopsis grossedentata and Its Major Active Compound Dihydromyricetin on the Liver of High-Fat Diet Hamster. Evid. Based Complement. Altern. Med. 2020, 2020, 3472578. [Google Scholar] [CrossRef]
- Ma, E.X.; Li, S.Y.; Cheng, X.Y.; Wang, W.N.; Wang, Y.; Wang, Y.; Li, J.C. Study on the anti-hyperuricemia and renal function protection effect of total flavonoids of Ampelopsis grossedentata. Chin. Pharmacol. Clin. 2021, 37, 80–85. [Google Scholar]
- Wu, S.H.; Yu, H.J.; Yu, T.; Zheng, Z.G.; Ke, D.; Zhao, L. Study on the hypo-ureic acid effect of Ampelopsis grossedentata extract. Food Ind. Sci. Technol. 2021, 42, 350–355. [Google Scholar]
- Shimizu, Y.; Sakurada, T.; Matsuoka, S.; Yui, K.; Hosoi, T. Vine Tea (Ampelopsis grossedentata) Extract Suppresses Postprandial Uric Acid Levels via Both Inhibition of Xanthine Oxidase and Facilitation of Uric Acid Excretion. Curr. Dev. Nutr. 2020, 4 (Suppl. S2), 474. [Google Scholar] [CrossRef]
- Tong, H.; Zhang, X.; Tan, L.; Jin, R.; Huang, S.; Li, X. Multitarget and promising role of dihydromyricetin in the treatment of metabolic diseases. Eur. J. Pharmacol. 2020, 870, 172888. [Google Scholar] [CrossRef]
- Chen, S.; Yu, J.P. Experimental study on the anti-inflammatory and antibacterial effects of total flavonoids of Ampelopsis grossedentata. J. Guiyang Coll. Tradit. Chin. Med. 2013, 35, 1–3. [Google Scholar]
- Zhang, Q.Y.; Li, R.; Zeng, G.F.; Liu, B.; Liu, J.; Shu, Y.; Liu, Z.K.; Qiu, Z.D.; Wang, D.J.; Miao, H.L.; et al. Dihydromyricetin inhibits migration and invasion of hepatoma cells through regulation of MMP-9 expression. World J. Gastroenterol. 2014, 20, 10082–10093. [Google Scholar] [CrossRef]
- Li, Y.; Kumar, P.S.; Tan, S.; Huang, C.; Xiang, Z.; Qiu, J.; Tan, X.; Luo, J.; He, M. Anticancer and antibacterial flavonoids from the callus of Ampelopsis grossedentata; a new weapon to mitigate the proliferation of cancer cells and bacteria. RSC Adv. 2022, 12, 24130–24138. [Google Scholar] [CrossRef]
- Li, X.; Yang, Z.S.; Cai, W.W.; Deng, Y.; Chen, L.; Tan, S.L. Dihydromyricetin Inhibits Tumor Growth and Epithelial-Mesenchymal Transition through regulating miR-455-3p in Cholangiocarcinoma. J. Cancer 2021, 12, 6058–6070. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Yin, G.; Jiang, M.; Tu, B.; Li, Z.; Wang, Y. Dihydromyricetin Exhibits Antitumor Activity in Nasopharyngeal Cancer Cell Through Antagonizing Wnt/β-catenin Signaling. Integr. Cancer Ther. 2021, 20, 1534735421991217. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Y.; Lu, Y.; Xu, Q.; Sun, D.; Liang, X.; Li, X.; Li, Y. Inhibitory effect of dihydromyricetin on the proliferation of JAR cells and its mechanism of action. Oncol. Lett. 2020, 20, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Chen, X.; Yuan, D.; Yi, Y.; Luo, Y. Golgi reassembly and stacking protein 65 downregulation is required for the anti-cancer effect of dihydromyricetin on human ovarian cancer cells. PLoS ONE 2019, 14, e0225450. [Google Scholar] [CrossRef]
- Wu, J.; Xiao, Z.; Li, H.; Zhu, N.; Gu, J.; Wang, W.; Liu, C.; Wang, W.; Qin, L. Present Status, Challenges, and Prospects of Dihydromyricetin in the Battle against Cancer. Cancers 2022, 14, 3487. [Google Scholar] [CrossRef]
- Liang, H.; He, K.; Li, T.; Cui, S.; Tang, M.; Kang, S.; Ma, W.; Song, L. Mechanism and antibacterial activity of vine tea extract and dihydromyricetin against Staphylococcus aureus. Sci. Rep. 2020, 10, 21416. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.N.; Wang, F.; Yuan, Y.T.; Liu, J.; Liu, Y.Z.; Yi, X. Antibacterial Activity and Mode of Action of Dihydromyricetin from Ampelopsis grossedentata Leaves against Food-Borne Bacteria. Molecules 2019, 24, 2831. [Google Scholar] [CrossRef]
- Zhan, X.S.; Liu, J.X.; Zhu, Z.; Tang, J.J.; Zhang, J.B.; Yin, S.H.; Zhang, M.Q. In vitro inhibition of two aquatic pathogenic bacteria by Ampelopsis grossedentata powder and dihydromyricetin. Fish. Res. 2022, 44, 162–168. [Google Scholar]
- Umair, M.; Sultana, T.; Zhu, X.Y.; Senan, A.M.; Saqib, J.; Labiba, K.; Muhammad, A.; Mian, A.M.; Dhama, K.; Al-Areqi, N.A.; et al. LC-ESI-QTOF/MS characterization of antimicrobial compounds with their action mode extracted from vine tea (Ampelopsis grossedentata) leaves. Food Sci. Nutr. 2022, 10, 422–435. [Google Scholar] [CrossRef]
- Zhong, Z.X.; Zhou, G.F.; Chen, X.F.; Qin, J.P. Long-term toxicity test of total flavonoids of Guangxi Ampelopsis grossedentata. Shizhen Natl. Med. 2003, 4, 193–195. [Google Scholar]
- Chen, Y.Q. Extraction and Isolation of Flavonoids and Dihydromyricetin from Garcinia Cambogia, Its Hypolipidemic Effect and Safety Evaluation of Ampelopsis grossedentata. Ph.D. Thesis, Huazhong Agricultural University, Wuhan, China, 2007. [Google Scholar]



| Nationality/Region | Nickname | Site of Use | Role of Tradition |
|---|---|---|---|
| Fujian Hakka | Ampelopsis grossedentata | Stem and leaf | Clearing heat and moisturizing the lung, anti-inflammation and detoxification, reducing blood pressure and fat, and eliminating fatigue [10]. |
| SHE-Minority in Sanming, Fujian Province | AG | Young stems and leaves | Heat stroke, mouth sores, aphonia, toothache, equine dental sores, and foot eczema [11]. |
| Guangxi (Zhuang nationality) | Sweet tea | Leaves and shoots | Good medicine for clearing heat and moisturizing the lung, eliminating phlegm and cough, and stopping bleeding and swelling [12]. |
| Hubei (Tujia Family) | Musty Tea | Leaves and shoots | Drinking tea can prevent and treat hypertension, and external application of fresh plants can treat Carbuncle swelling [13]. |
| Xiangxi (Hmong) | AG | Young leaf | Cool and quench thirst, as one of the teas for “oil tea” [14]. |
| Yao nationality | Tian Po tea, AG | The whole plant was used as medicine | Treatment of throat swelling and pain, cold and fever, icteric hepatitis, sore boils, anti-inflammatory, antibacterial, reducing three high, liver protection, liver protection, antioxidant, anti-tumor [15,16]. |
| Fanjing Mountain area, Guizhou province | AG, Sweet tea, White tea, Bang Bang tea | Tender stem and leaves | Prevention and treatment of hypertension, treatdamp-heat dysentery, pruritus of the skin, and ulcer or ulcer. It has the functions of nourishing the liver and kidney, moistening the lungs, relieving coughs, relieving drowsiness, and promoting sobriety [6]. |
| JiNuo nationality | AG | ★ | Chewing and swallowing to treat toothache [17]. |
| Dong nationality | AG | The whole plant was used as medicine | Beverage tea and treat skin and external diseases [18]. |
| LaHu nationality | AG | ★ | Daily consumption of tea [18]. |
| Hengdong County, Hunan Province | AG | ★ | People used to treat cuts, falls, swollen gums, oral ulcers, gastric ulcers, influenza, pneumonia, hypertension, diabetes, osteoporosis, hemorrhoids, constipation, anti-drinking poisoning, and cardiovascular diseases [19]. |
| Yingde city, Lianzhou city, Guangdong province | Wild AG, AG, White tea, Lai Li tea, Nectar tea | ★ | Treat colds and fevers, sore throats, icteric hepatitis, sore boils, hypertension, hyperlipidemia, etc. [20]. |
| Active Component | Molecular Formula | Distribution | References | |
|---|---|---|---|---|
| Flavonoids | ||||
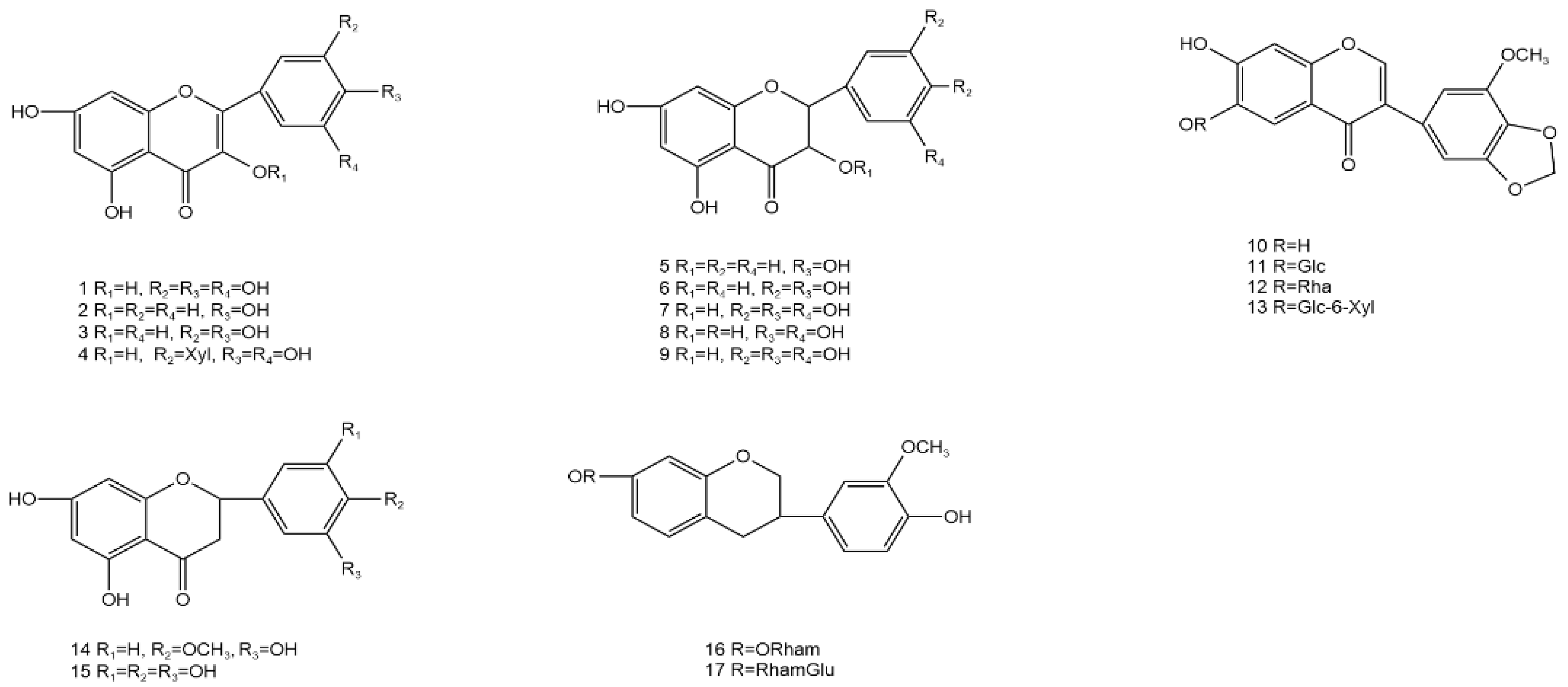
| 1 | Myricetin | C15H10O8 | tender stem and leaf | [35] |
| 2 | Kaempferol | C15H10O6 | stem and leaf | [36] |
| 3 | Quercetin | C15H10O7 | tender stem and leaf | [37] |
| 4 | Myricetin-3′-O-β-D-xylopyranoside | C15H9O7 | leaf | [38] |
| 5 | Dihydrokaempferol | C15H12O6 | leaf | [38] |
| 6 | Dihydroquercetin | C15H12O7 | stem and leaf | [36] |
| 7 | Dihydromyricetin | C15H12O8 | tender stem and leaf | [39] |
| 8 | Taxifolin | C15H12O7 | stem and leaf | [27] |
| 9 | (2R,3S)-5,7,3′,4′,5′-pentahydroxyflavanonol | C15H12O8 | stem and leaf | [40] |
| 10 | 6,7-dihydroxy-3′-methoxy-4′,5′-methylenedioxyisoflavone | C17H12O7 | stem and leaf | [41] |
| 11 | 6,7-dihydroxy-3′-methoxy-4, 5′-methylenedioxyisoflavone 6-O-β-D-glucopyranoside | C17H11O6 | stem and leaf | [41] |
| 12 | 6,7-dihydroxy-3′-methoxy-4′,5′-methylenedioxyisoflavone 6-O-α-L-rhamnopyranoside | C17H11O6 | stem and leaf | [41] |
| 13 | 6,7-dihydroxy-3′-methoxy-4′,5′-methylenedioxyisoflavone 6-O-β-D-xylopyranosyl-(1-6)-β-D-glucopyranoside | C17H11O6 | stem and leaf | [41] |
| 14 | Hesperetin | C15H13O6 | stem and leaf | [36] |
| 15 | 5,7,3′,4′,5′-pentahydroxyflavanone | C15H12O7 | stem and leaf | [40] |
| 16 | Grossedentatasin | C16H15O3 | stem and leaf | [28] |
| 17 | Grossedentataside | C16H15O3 | stem and leaf | [28] |
| 18 | Luteolin | C15H10O6 | stem | [42] |
| 19 | Vitexin | C15H9O5 | stem | [42] |
| 20 | Myricetrin | C15H9O7 | aerial part | [43] |
| 21 | Rutinum | C15H9O6 | tender stem and leaf | [37] |
| 22 | Myricetin-3-O-β-D-galactopyranoside | C15H9O7 | stem and leaf | [42] |
| 23 | Apigenin | C15H10O5 | stem and leaf | [36] |
| 24 | 5,7-dihydroxy-3′4′-dihydroxyflavone-3-O-6″-rhamnose | C15H9O6 | leaf | [44] |
| 25 | 5,7-dihydroxy-3′4′5′-trihydroxyflavone-3-O-6″-rhamnose | C15H9O7 | leaf | [44] |
| 26 | Afzelechin | C15H9O6 | stem and leaf | [27,38] |
| 27 | Astragalin | C15H9O5 | stem and leaf | [27,38] |
| 28 | Quercetin-3-O-α-L-rhamnopyranoside | C15H9O6 | stem and leaf | [27,38] |
| 29 | Quercetin-3-O-β-D-glucoside | C15H9O6 | stem and leaf | [27,38] |
| 30 | Myricetin-3-O-β-D- galactoside | C15H9O7 | stem and leaf | [27,38] |
| 31 | Bellidifolin | C14H10O6 | stem and leaf | [43] |
| Phenols | ||||
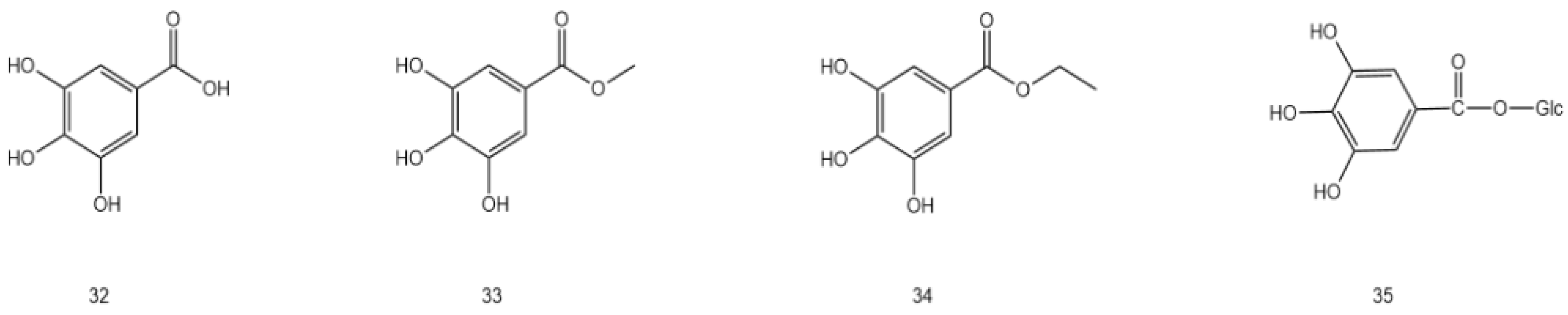
| 32 | Gallic acid | C7H6O5 | tender stem and leaf | [45] |
| 33 | Gallicin | C8H8O5 | stem and leaf | [27] |
| 34 | Ethyl gallate | C9H10O5 | tender stem and leaf | [30] |
| 35 | Gallic-β-D-glucose | C7H5O5 | tender stem and leaf | [30] |
| Steroids and terpenoids | ||||
| 36 | Stigmasterol | C29H48O | tender stem and leaf | [45] |
| 37 | β-sitosterol | C29H50O | tender stem and leaf | [45] |
| 38 | Oleanolic acid | C30H48O3 | stem and leaf | [36] |
| 39 | Ambrein | C30H52O | aerial part | [43] |
| Volatile components and other compounds | ||||
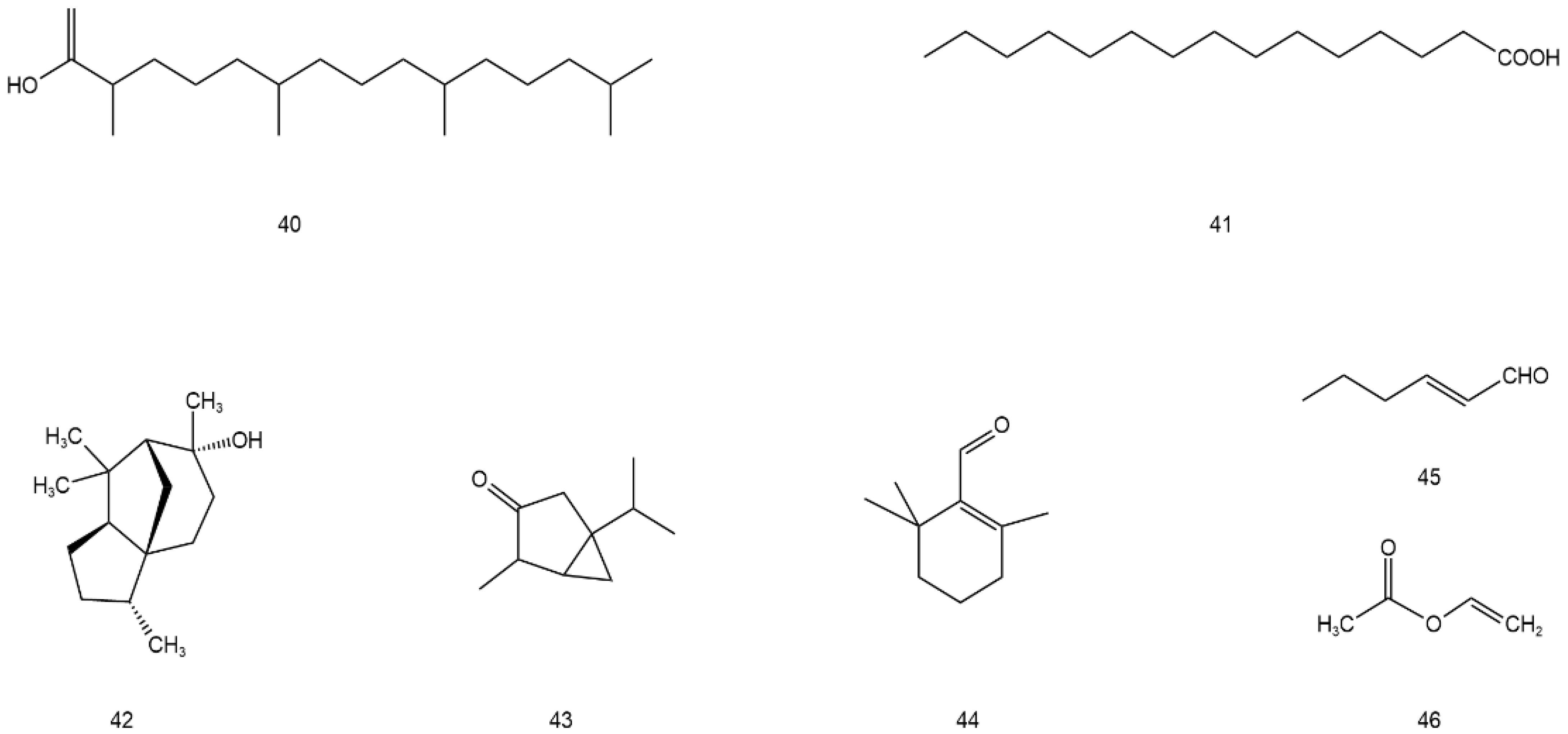
| 40 | Phytol | C20H40O | tender stem and leaf | [46] |
| 41 | n-Hexadecanoic acid | C15H30O2 | tender stem and leaf | [46] |
| 42 | Cedrol | C15H26O | tender stem and leaf | [46] |
| 43 | β-thujone | C10H16O | stem and leaf | [47] |
| 44 | β-cyclocitral | C10H16O | stem and leaf | [48] |
| 45 | (E)-2-hexenal | C6H10O | stem and leaf | [49] |
| 46 | (Z)-3-hexenyl hexanote | C4H6O2 | stem and leaf | [49] |
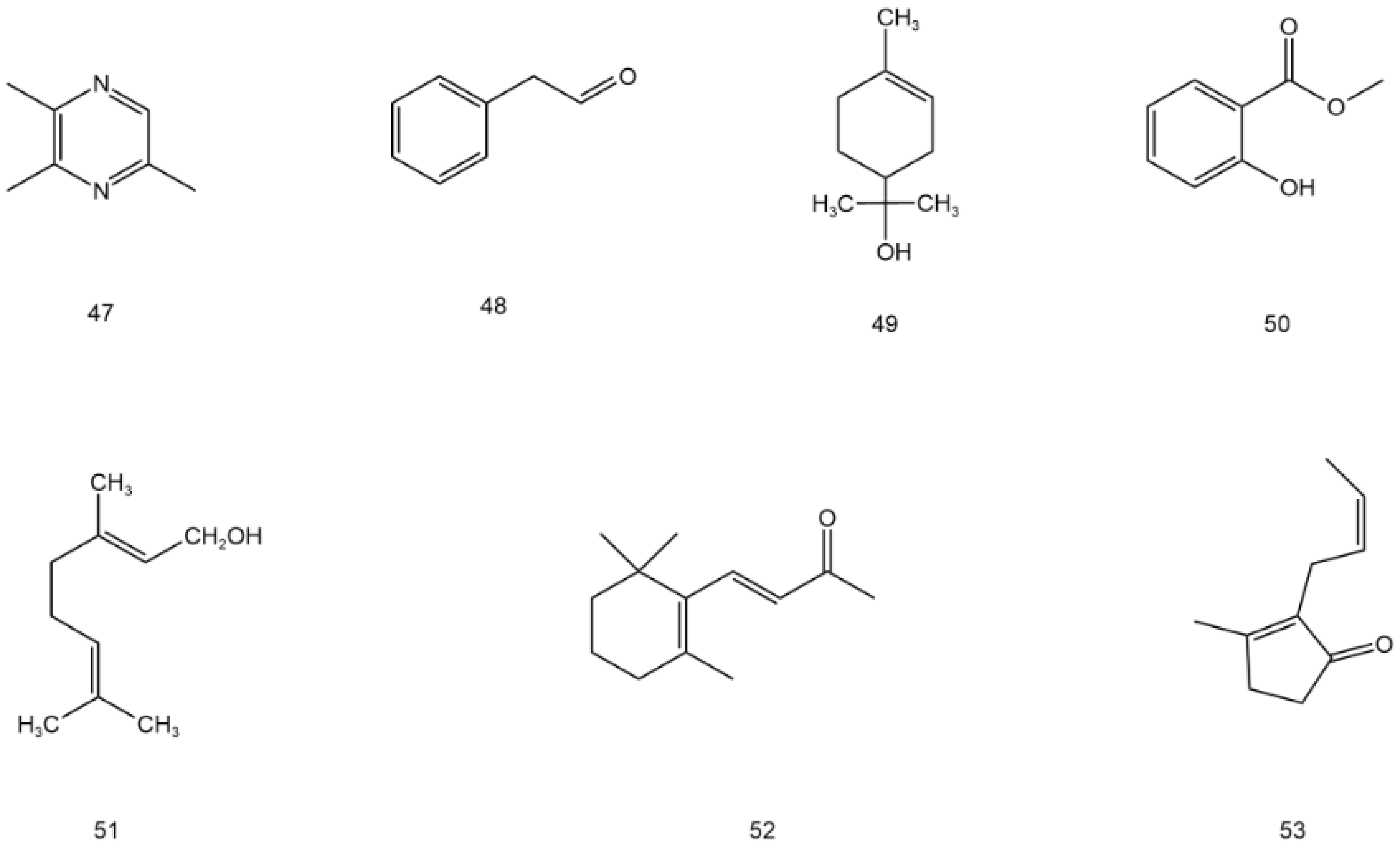
| 47 | Trimethyl pyrazine | C7H10N2 | stem and leaf | [49] |
| 48 | Phenylacetaldehyde | C8H8O | stem and leaf | [49] |
| 49 | α-terpinol | C10H20O | stem and leaf | [49] |
| 50 | Methyl salicylate | C8H8O3 | stem and leaf | [49] |
| 51 | Geraniol | C10H18O | stem and leaf | [49] |
| 52 | β-ionone | C13H20O | stem and leaf | [49] |
| 53 | (Z)-jasmone | C10H14O | stem and leaf | [49] |
| 54 | 6,10,14-trimethyl-2-pen-tadecanone | C18H36O | stem and leaf | [49] |
| 55 | Nerolidol | C15H26O | stem and leaf | [49] |
| 56 | Palmitic acid | C15H30O2 | stem and leaf | [49] |
| 57 | Emodin | C17H14O3 | stem and leaf | [49] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, R.-R.; Li, X.; Cao, Y.-H.; Peng, X.; Liu, G.-F.; Liu, Z.-K.; Yang, Z.; Liu, Z.-Y.; Wu, Y. China Medicinal Plants of the Ampelopsis grossedentata—A Review of Their Botanical Characteristics, Use, Phytochemistry, Active Pharmacological Components, and Toxicology. Molecules 2023, 28, 7145. https://doi.org/10.3390/molecules28207145
Wu R-R, Li X, Cao Y-H, Peng X, Liu G-F, Liu Z-K, Yang Z, Liu Z-Y, Wu Y. China Medicinal Plants of the Ampelopsis grossedentata—A Review of Their Botanical Characteristics, Use, Phytochemistry, Active Pharmacological Components, and Toxicology. Molecules. 2023; 28(20):7145. https://doi.org/10.3390/molecules28207145
Chicago/Turabian StyleWu, Rong-Rong, Xiang Li, Yu-Hang Cao, Xiong Peng, Gao-Feng Liu, Zi-Kui Liu, Zi Yang, Zhao-Ying Liu, and Yong Wu. 2023. "China Medicinal Plants of the Ampelopsis grossedentata—A Review of Their Botanical Characteristics, Use, Phytochemistry, Active Pharmacological Components, and Toxicology" Molecules 28, no. 20: 7145. https://doi.org/10.3390/molecules28207145
APA StyleWu, R.-R., Li, X., Cao, Y.-H., Peng, X., Liu, G.-F., Liu, Z.-K., Yang, Z., Liu, Z.-Y., & Wu, Y. (2023). China Medicinal Plants of the Ampelopsis grossedentata—A Review of Their Botanical Characteristics, Use, Phytochemistry, Active Pharmacological Components, and Toxicology. Molecules, 28(20), 7145. https://doi.org/10.3390/molecules28207145






