Compositing Benzothieno[3,2-b]Benzofuran Derivatives with Single-Walled Carbon Nanotubes for Enhanced Thermoelectric Performance
Abstract
:1. Introduction
2. Results and Discussion
2.1. Structural Analyses of BTBF, BTBF-Br, and BTBF-2Br
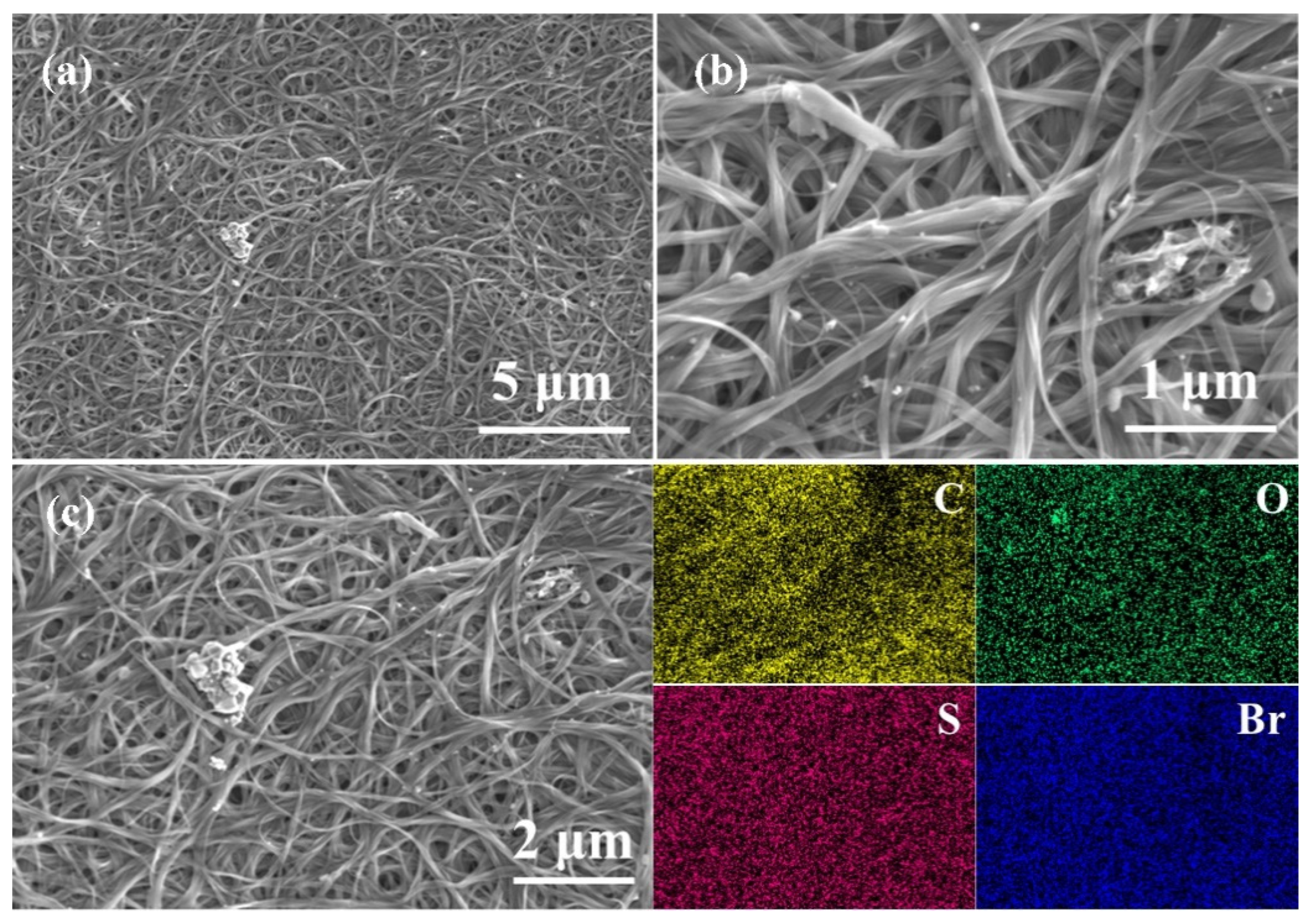
2.2. Morphological Observation and XPS Analyses of BTBF/SWCNT, BTBF-Br/SWCNT, and BTBF-2Br/SWCNT Composites
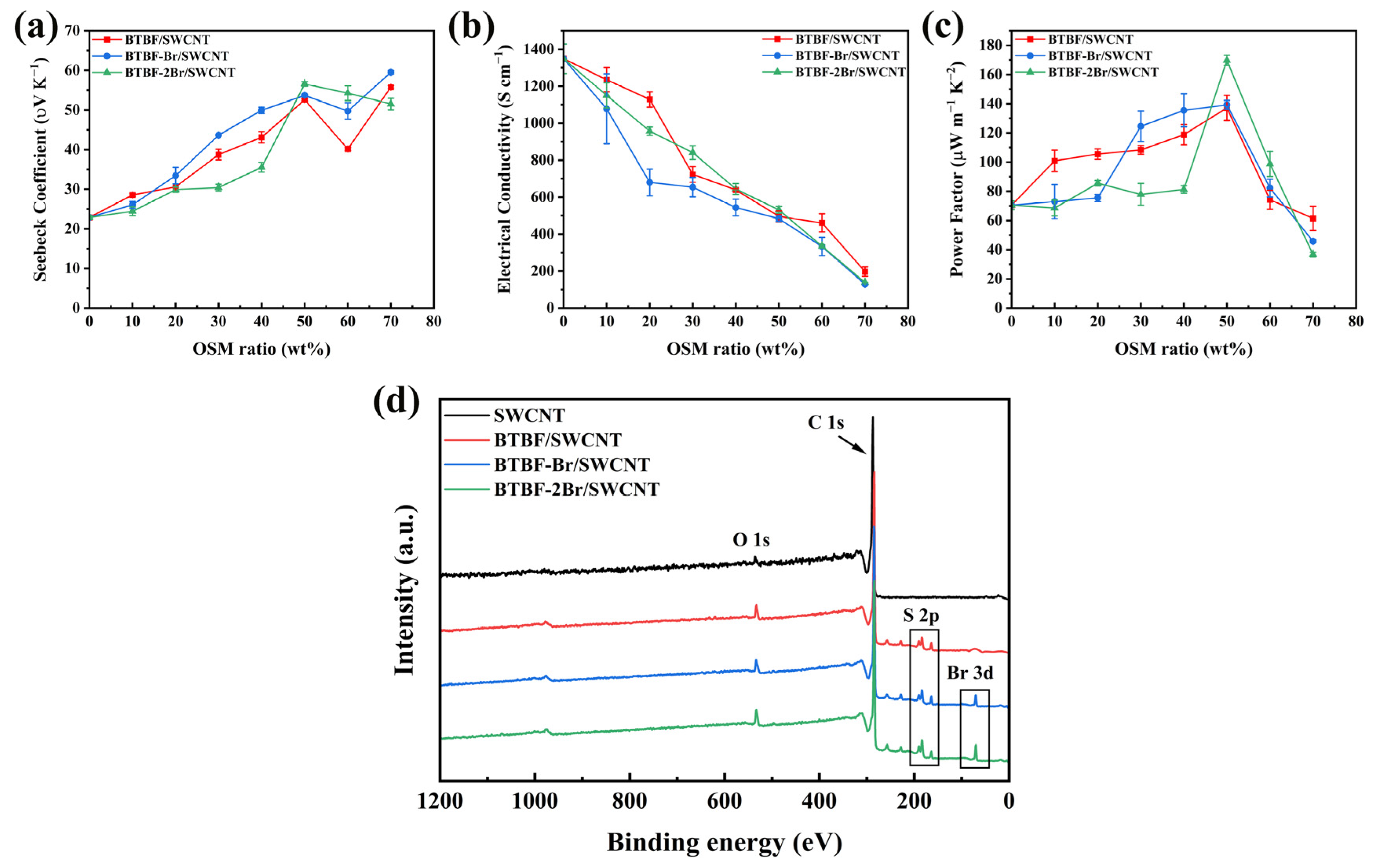
2.3. Thermoelectric Properties of BTBF/SWCNT, BTBF-Br/SWCNT, and BTBF-2Br/SWCNT Composites
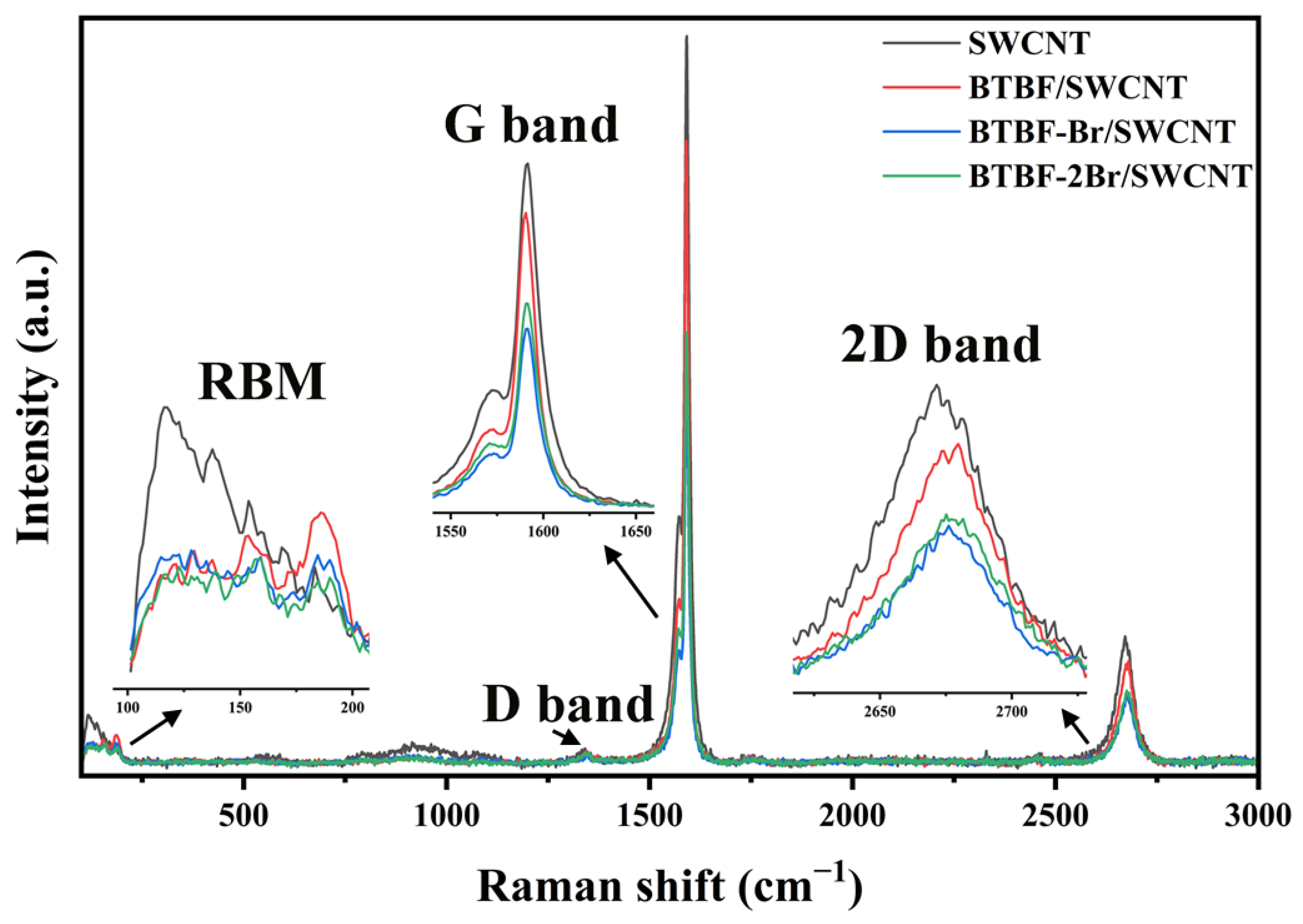
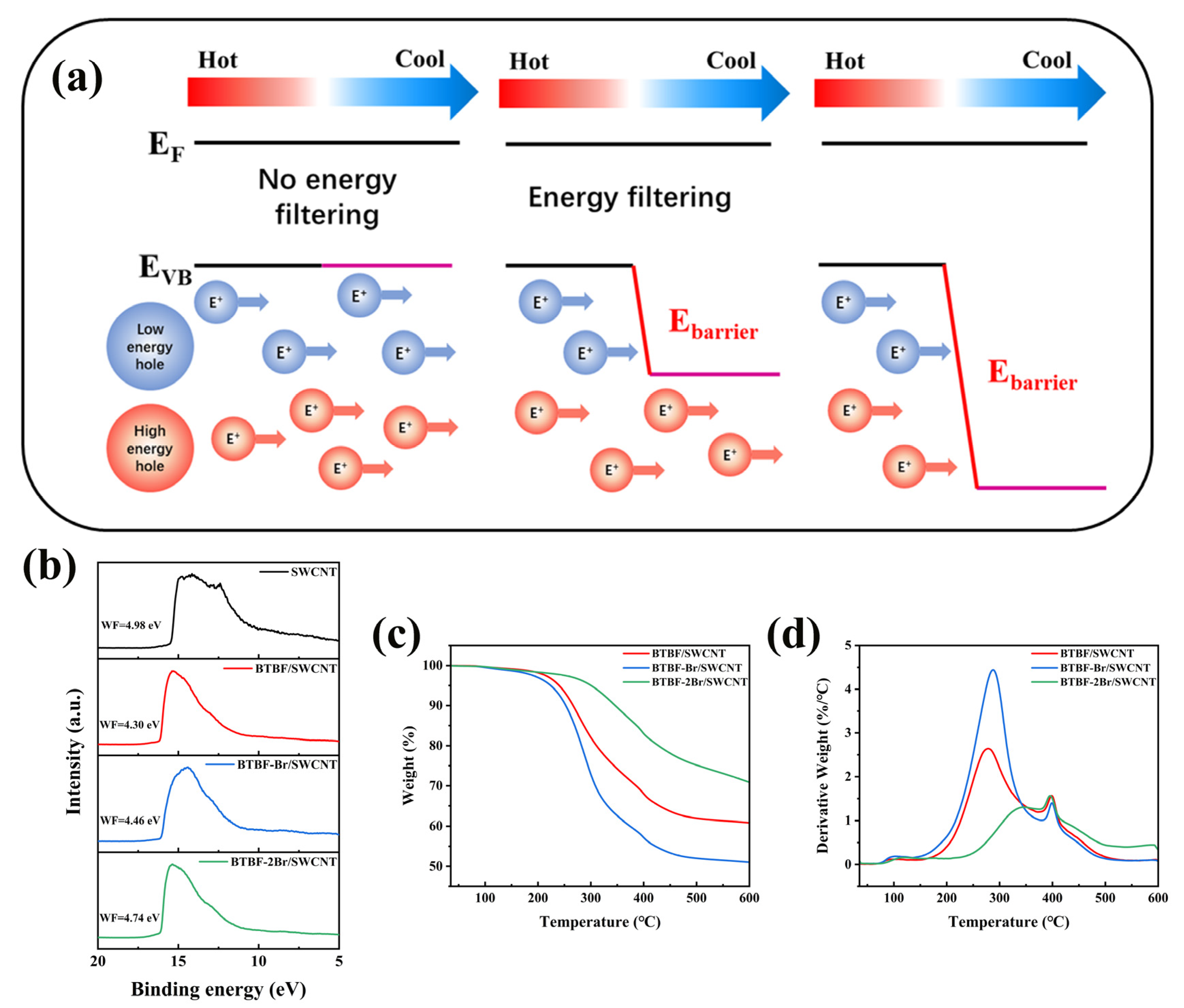
2.4. Raman and Ultraviolet Photoelectron Spectral Investigation of BTBF/SWCNT, BTBF-Br/SWCNT, and BTBF-2Br/SWCNT Composites
2.5. Thermal Stability and Flexibility of BTBF/SWCNT, BTBF-Br/SWCNT, and BTBF-2Br/SWCNT Composites
3. Experimental Section
3.1. Chemicals and Materials
3.2. Preparation of Composite Films
3.3. Analyses and Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Chow, J.; Kopp, R.J.; Portney, P.R. Energy resources and global development. Science 2003, 302, 1528–1531. [Google Scholar] [CrossRef] [PubMed]
- Brantut, J.P.; Grenier, C.; Meineke, J.; Stadler, D.; Krinner, S.; Kollath, C.; Esslinger, T.; Georges, A. A thermoelectric heat engine with ultracold atoms. Science 2013, 342, 713–715. [Google Scholar] [CrossRef] [PubMed]
- Russ, B.; Glaudell, A.; Urban, J.J.; Chabinyc, M.L.; Segalman, R.A. Organic thermoelectric materials for energy harvesting and temperature control. Nat. Rev. Mater. 2016, 1, 16050. [Google Scholar] [CrossRef]
- Du, Y.; Shen, S.Z.; Cai, K.; Casey, P.S. Research progress on polymer–inorganic thermoelectric nanocomposite materials. Prog. Polym. Sci. 2012, 37, 820–841. [Google Scholar] [CrossRef]
- Vining, C.B. An inconvenient truth about thermoelectrics. Nat. Mater. 2009, 8, 83–85. [Google Scholar] [CrossRef] [PubMed]
- Mo, J.-H.; Kim, J.-Y.; Kang, Y.H.; Cho, S.Y.; Jang, K.-S. Carbon Nanotube/Cellulose Acetate Thermoelectric Papers. ACS Sustain. Chem. Eng. 2018, 6, 15970–15975. [Google Scholar] [CrossRef]
- Liu, J.; Qiu, L.; Portale, G.; Koopmans, M.; Ten Brink, G.; Hummelen, J.C.; Koster, L.J.A. N-type organic thermoelectrics: Improved power factor by tailoring host-dopant miscibility. Adv. Mater. 2017, 29, 1701641. [Google Scholar] [CrossRef]
- Jiang, B.; Yu, Y.; Cui, J.; Liu, X.; Xie, L.; Liao, J.; Zhang, Q.; Huang, Y.; Ning, S.; Jia, B.; et al. High-entropy-stabilized chalcogenides with high thermoelectric performance. Science 2021, 371, 830–834. [Google Scholar] [CrossRef]
- Biswas, K.; He, J.; Zhang, Q.; Wang, G.; Uher, C.; Dravid, V.P.; Kanatzidis, M.G. Strained endotaxial nanostructures with high thermoelectric figure of merit. Nat. Chem. 2011, 3, 160–166. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Tritt, T.M. Advances in thermoelectric materials research: Looking back and moving forward. Science 2017, 357, eaak9997. [Google Scholar] [CrossRef]
- Gayner, C.; Kar, K.K. Recent advances in thermoelectric materials. Prog. Mater. Sci. 2016, 83, 330–382. [Google Scholar] [CrossRef]
- Venkatasubramanian, R.; Siivola, E.; Colpitts, T.; O’quinn, B. Thin-film thermoelectric devices with high room-temperature figures of merit. Nature 2001, 413, 597–602. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.; Wu, M.; He, D.; Pei, Y.; Wu, C.F.; Wu, X.; Yu, H.; Zhu, F.; Wang, K.; Chen, Y.; et al. 3D charge and 2D phonon transports leading to high out-of-plane ZT in n-type SnSe crystals. Science 2018, 360, 778–783. [Google Scholar] [CrossRef]
- Nandihalli, N. Imprints of interfaces in thermoelectric materials. Crit. Rev. Solid State 2023, 48, 361–410. [Google Scholar] [CrossRef]
- Nandihalli, N.; Liu, C.J.; Mori, T. Polymer based thermoelectric nanocomposite materials and devices: Fabrication and characteristics. Nano Energy 2020, 78, 105186. [Google Scholar] [CrossRef]
- Yin, S.; Lu, W.; Wu, X.; Luo, Q.; Wang, E.; Guo, C.Y. Enhancing thermoelectric performance of polyaniline/single-walled carbon nanotube composites via dimethyl sulfoxide-mediated electropolymerization. ACS Appl. Mater. Interfaces 2021, 13, 3930–3936. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Yin, S.; Guo, C.-Y. Self-healable and robust PE/PEDOT/SWCNT thermoelectric composites. ACS Appl. Mater. Interfaces 2022, 14, 32056–32065. [Google Scholar] [CrossRef]
- Yin, S.; Lu, W.; Wu, R.; Fan, W.; Guo, C.Y.; Chen, G. Poly(3,4-ethylenedioxythiophene)/Te/single-walled carbon nanotube composites with high thermoelectric performance promoted by electropolymerization. ACS Appl. Mater. Interfaces 2020, 12, 3547–3553. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Guo, C.-Y.; Chen, G. Flexible films of poly(3,4-ethylenedioxythiophene)/carbon nanotube thermoelectric composites prepared by dynamic 3-phase interfacial electropolymerization and subsequent physical mixing. J. Mater. Chem. A 2018, 6, 12275–12280. [Google Scholar] [CrossRef]
- Fan, W.; Liang, L.; Zhang, B.; Guo, C.-Y.; Chen, G. PEDOT thermoelectric composites with excellent power factors prepared by 3-phase interfacial electropolymerization and carbon nanotube chemical doping. J. Mater. Chem. A 2019, 7, 13687–13694. [Google Scholar] [CrossRef]
- Lu, W.; Yin, S.; Wu, X.; Luo, Q.; Wang, E.; Cui, L.; Guo, C.-Y. Aniline–pyrrole copolymers formed on single-walled carbon nanotubes with enhanced thermoelectric performance. J. Mater. Chem. C 2021, 9, 2898–2903. [Google Scholar] [CrossRef]
- Lee, W.; Hong, C.T.; Kwon, O.H.; Yoo, Y.; Kang, Y.H.; Lee, J.Y.; Cho, S.Y.; Jang, K.S. Enhanced thermoelectric performance of bar-coated SWCNT/P3HT thin films. ACS Appl. Mater. Interfaces 2015, 7, 6550–6556. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Sun, Y.; Xu, W.; Zhu, D. Organic thermoelectric materials: Emerging green energy materials converting heat to electricity directly and efficiently. Adv. Mater. 2014, 26, 6829–6851. [Google Scholar] [CrossRef]
- Bin, J.K.; Cho, N.S.; Hong, J.I. New host material for high-performance blue phosphorescent organic electroluminescent devices. Adv. Mater. 2012, 24, 2911–2915. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.; Lim, J.; Lee, J.-K.; Hong, J.-I. Oligothiophene-modified silver/silica core–shell nanoparticles for inhibiting open-circuit voltage drop and aggregation in polymer solar cells. J. Mater. Chem. A 2014, 2, 15357–15364. [Google Scholar] [CrossRef]
- Li, X.; Yu, Z.; Zhou, H.; Yang, F.; Zhong, F.; Mao, X.; Li, B.; Xin, H.; Gao, C.; Wang, L. Promoting the thermoelectric performance of single-walled carbon nanotubes by inserting discotic liquid-crystal molecules. ACS Sustain. Chem. Eng. 2021, 9, 1891–1898. [Google Scholar] [CrossRef]
- Jeon, Y.; Jang, J.G.; Kim, S.H.; Hong, J.-I. Twisted small organic molecules for high thermoelectric performance of single-walled carbon nanotubes/small organic molecule hybrids through mild charge transfer interactions. J. Mater. Chem. C 2021, 9, 8483–8488. [Google Scholar] [CrossRef]
- Wang, D.; Li, J.; Yang, K.; Wang, Y.; Jeong, S.Y.; Chen, Z.; Liao, Q.; Li, B.; Woo, H.Y.; Deng, X.; et al. Terminal cyano-functionalized fused bithiophene imide dimer-based n-type small molecular semiconductors: Synthesis, structure-property correlations, and thermoelectric performances. ACS Appl. Mater. Interfaces 2023, 15, 9714–9725. [Google Scholar] [CrossRef]
- Gao, C.; Chen, G. In situ oxidation synthesis of p-type composite with narrow-bandgap small organic molecule coating on single-walled carbon nanotube: Flexible film and thermoelectric performance. Small 2018, 14, e1703453. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, Y.; Zhou, X.; Gao, Y.; Gao, C.; Wang, L. High performance p-type organic thermoelectric materials based on metalloporphyrin/single-walled carbon nanotube composite films. J. Power Sources 2019, 423, 152–158. [Google Scholar] [CrossRef]
- Nie, X.; Li, X.; Huang, Y.; Wu, J.; Yang, F.; Zhong, F.; Hong, X.; Gao, C.; Wang, L. High performance of p-type and n-type thermoelectric materials based on liquid crystal mixture and single-walled carbon nanotube composites. Compos. Commun. 2021, 27, 100873. [Google Scholar] [CrossRef]
- Zhou, Y.; Yin, X.; Liu, Y.; Zhou, X.; Wan, T.; Wang, S.; Gao, C.; Wang, L. Significantly enhanced power factors of p-type carbon nanotube-based composite films by tailoring the peripheral substituents in porphyrin. ACS Sustain. Chem. Eng. 2019, 7, 11832–11840. [Google Scholar] [CrossRef]
- Kim, T.-H.; Jang, J.G.; Hong, J.-I. Enhanced thermoelectric performance of SWNT/organic small molecule (OSM) hybrid materials by tuning of the energy level of OSMs. J. Mater. Chem. C 2020, 8, 12795–12799. [Google Scholar] [CrossRef]
- Yin, X.; Peng, Y.; Luo, J.; Zhou, X.; Gao, C.; Wang, L.; Yang, C. Tailoring the framework of organic small molecule semiconductors towards high-performance thermoelectric composites via conglutinated carbon nanotube webs. J. Mater. Chem. A 2018, 6, 8323–8330. [Google Scholar] [CrossRef]
- Kumari, A.; Ghosh, A.; Mehta, B.R.; Sinha, A. Synergetic enhancement of Seebeck coefficients and electrical conductivity in flexible liquid crystal composites. ACS Sustain. Chem. Eng. 2023, 11, 4226–4236. [Google Scholar] [CrossRef]
- Luo, H.C.; Li, F.Y.; Zhang, Y.N.; Zhang, H.X.; Eglitis, R.I.; Jia, R. Theoretical study on (n,n)-nanotubes rolled-up from B/N substituted Me-graphene. Crystals 2023, 13, 829. [Google Scholar] [CrossRef]
- Kim, T.H.; Hong, J.I. Energy level modulation of small molecules enhances thermoelectric performances of carbon nanotube-based organic hybrid materials. ACS Appl. Mater. Interfaces 2022, 14, 55627–55635. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Huang, H.; Gao, C.; Liu, D.; Wang, L. Novel butterfly-shaped organic semiconductor and single-walled carbon nanotube composites for high performance thermoelectric generators. Mater. Horiz. 2021, 8, 1207–1215. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, Z.; Huang, H.; Wang, D.; Liu, D.; Wang, L. Organic radical compound and carbon nanotube composites with enhanced electrical conductivity towards high-performance p-type and n-type thermoelectric materials. J. Mater. Chem. A 2020, 8, 24675–24684. [Google Scholar] [CrossRef]
- Takimiya, K.; Osaka, I.; Mori, T.; Nakano, M. Organic semiconductors based on [1]benzothieno [3,2-b][1]benzothiophene substructure. Acc. Chem. Res. 2014, 47, 1493–1502. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, H.; Nakamura, E. Design and functions of semiconducting fused polycyclic furans for optoelectronic applications. Acc. Chem. Res. 2017, 50, 396–406. [Google Scholar] [CrossRef]
- Chen, D.; Yuan, D.; Zhang, C.; Wu, H.; Zhang, J.; Li, B.; Zhu, X. Ullmann-type intramolecular C–O reaction toward thieno[3,2-b]furan derivatives with up to six fused rings. J. Org. Chem. 2017, 82, 10920–10927. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Huang, J.; Li, C.; Jiang, Y.; Li, B.; Qi, T.; Zhu, X. One-pot synthesis and property study on thieno[3,2-b]furan compounds. RSC Adv. 2019, 9, 7123–7127. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, R.A.; Babu, S.A.; Nitha, P.R.; Krishnan, J.; John, J. Synthesis of benzothienobenzofurans via annulation of electrophilic benzothiophenes with phenols. Org. Lett. 2021, 23, 1814–1819. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; He, Y.; Guo, S.; Ali, M.U.; Zhao, C.; Zhu, Y.; Wang, T.; Wang, Y.; Miao, J.; Wei, G.; et al. Multifunctional benzo[4,5]thieno[3,2-b]benzofuran derivative with high mobility and luminescent properties. ACS Appl. Mater. Interfaces 2021, 13, 12250–12258. [Google Scholar] [CrossRef]
- Anthony, J.E.; Facchetti, A.; Heeney, M.; Marder, S.R.; Zhan, X. n-Type organic semiconductors in organic electronics. Adv. Mater. 2010, 22, 3876–3892. [Google Scholar] [CrossRef]
- Zhang, L.; Jin, J.; Huang, S.; Tan, B.; Luo, J.; Wang, D.; Liu, D.; Wang, L. Cross-conjugated spiro molecules and single-walled carbon nanotubes composite for high-performance organic thermoelectric materials and generators. Chem. Eng. J. 2021, 426, 131859. [Google Scholar] [CrossRef]
- Guan, X.; Ouyang, J. Enhancement of the Seebeck coefficient of organic thermoelectric materials via energy filtering of charge carriers. CCS Chem. 2021, 3, 2415–2427. [Google Scholar] [CrossRef]
- Lu, N.; Li, L.; Liu, M. A review of carrier thermoelectric-transport theory in organic semiconductors. Phys. Chem. Chem. Phys. 2016, 18, 19503–19525. [Google Scholar] [CrossRef]
- Ai, L.; Ajibola, I.Y.; Li, B. Copper-mediated construction of benzothieno[3,2-b]benzofurans by intramolecular dehydrogenative C-O coupling reaction. RSC Adv. 2021, 11, 36305–36309. [Google Scholar] [CrossRef] [PubMed]
- Ai, L.; Xie, X.; Li, B.; Wang, Y. Pure blue emitters based on benzo[4,5]thieno-S,S-dioxide-[3,2-b]benzofuran with high thermal stability. Dye. Pigment. 2023, 213, 111153. [Google Scholar] [CrossRef]

| Film Name | n (cm−3) | μ (cm2 V−1 s−1) | σ (S cm−1) |
|---|---|---|---|
| BTBF/SWCNT | 1.48 × 1020 | 15.8 | 496.05 |
| BTBF-Br/SWCNT | 9.43 × 1019 | 17.0 | 483.47 |
| BTBF-2Br/SWCNT | 2.94 × 1020 | 11.0 | 530.86 |
| Bending Cycle | S (μV K−1) | σ (S cm−1) | PF (μW m−1 K−2) |
|---|---|---|---|
| 0 | 59.51 | 530.86 | 169.70 |
| 25 | 56.68 | 495.50 | 159.18 |
| 50 | 55.13 | 468.45 | 142.37 |
| 100 | 54.07 | 399.01 | 116.65 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Ai, L.; Luo, Q.; Wu, X.; Li, B.; Guo, C.-Y. Compositing Benzothieno[3,2-b]Benzofuran Derivatives with Single-Walled Carbon Nanotubes for Enhanced Thermoelectric Performance. Molecules 2023, 28, 6519. https://doi.org/10.3390/molecules28186519
Li Y, Ai L, Luo Q, Wu X, Li B, Guo C-Y. Compositing Benzothieno[3,2-b]Benzofuran Derivatives with Single-Walled Carbon Nanotubes for Enhanced Thermoelectric Performance. Molecules. 2023; 28(18):6519. https://doi.org/10.3390/molecules28186519
Chicago/Turabian StyleLi, Yiyang, Liankun Ai, Qunyi Luo, Xin Wu, Baolin Li, and Cun-Yue Guo. 2023. "Compositing Benzothieno[3,2-b]Benzofuran Derivatives with Single-Walled Carbon Nanotubes for Enhanced Thermoelectric Performance" Molecules 28, no. 18: 6519. https://doi.org/10.3390/molecules28186519
APA StyleLi, Y., Ai, L., Luo, Q., Wu, X., Li, B., & Guo, C.-Y. (2023). Compositing Benzothieno[3,2-b]Benzofuran Derivatives with Single-Walled Carbon Nanotubes for Enhanced Thermoelectric Performance. Molecules, 28(18), 6519. https://doi.org/10.3390/molecules28186519







