Comprehensive Analysis of the Chemical and Bioactivity Profiles of Endemic Crataegus turcicus Dönmez in Comparison with Other Crataegus Species
Abstract
1. Introduction
2. Results
2.1. Results of Total Phenolic (TPCs), Flavonoid (TFCs), Proanthocyanidin (TPACs), and Anthocyanin (TACs) Contents
2.2. HPTLC
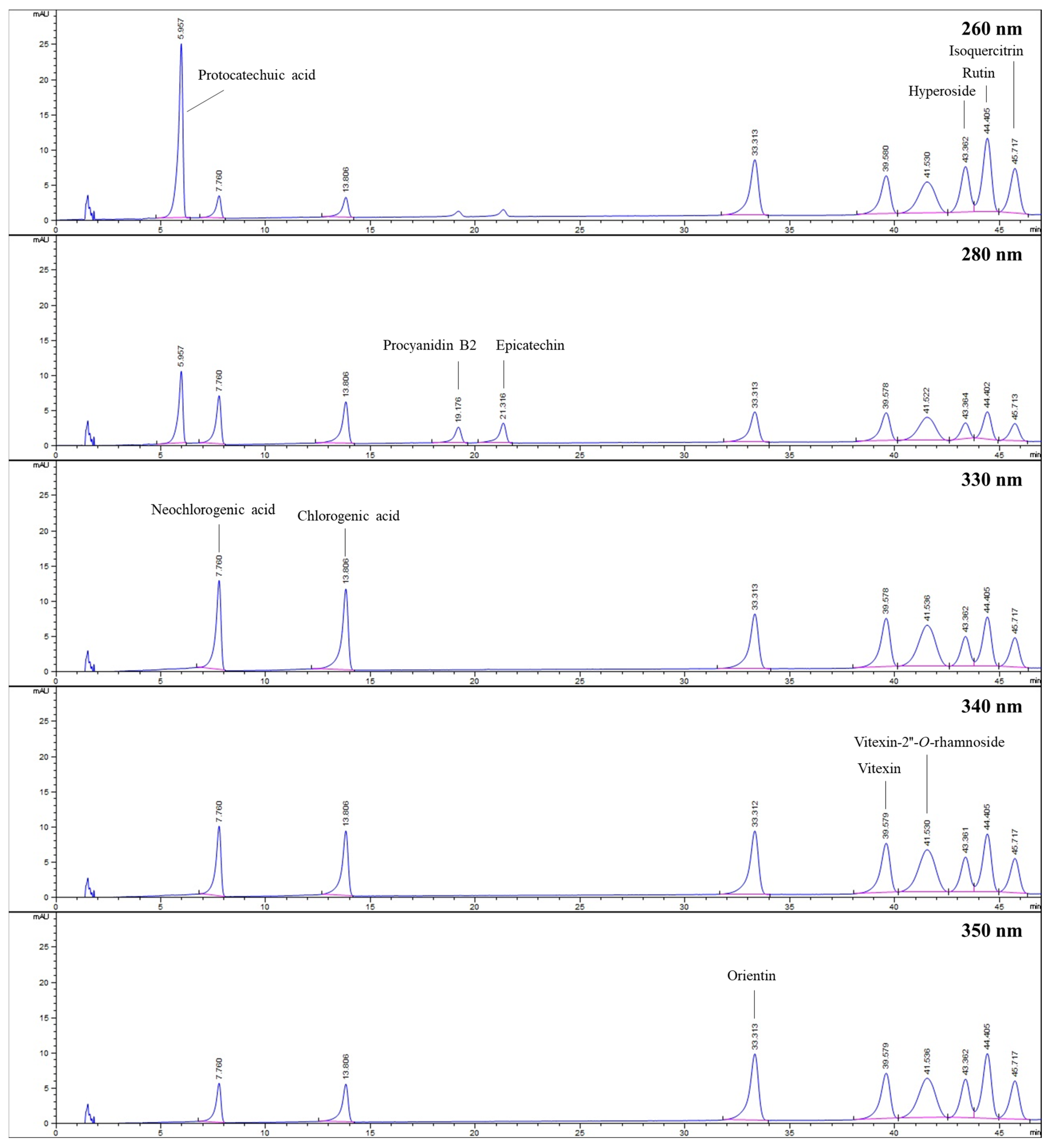
2.3. HPLC
2.4. Bioactivity Assays
2.4.1. Total Antioxidant Content
2.4.2. Anti-Inflammatory Activity
Effects of Crataegus Extracts on the Cell Viability of RAW 264.7 Macrophages
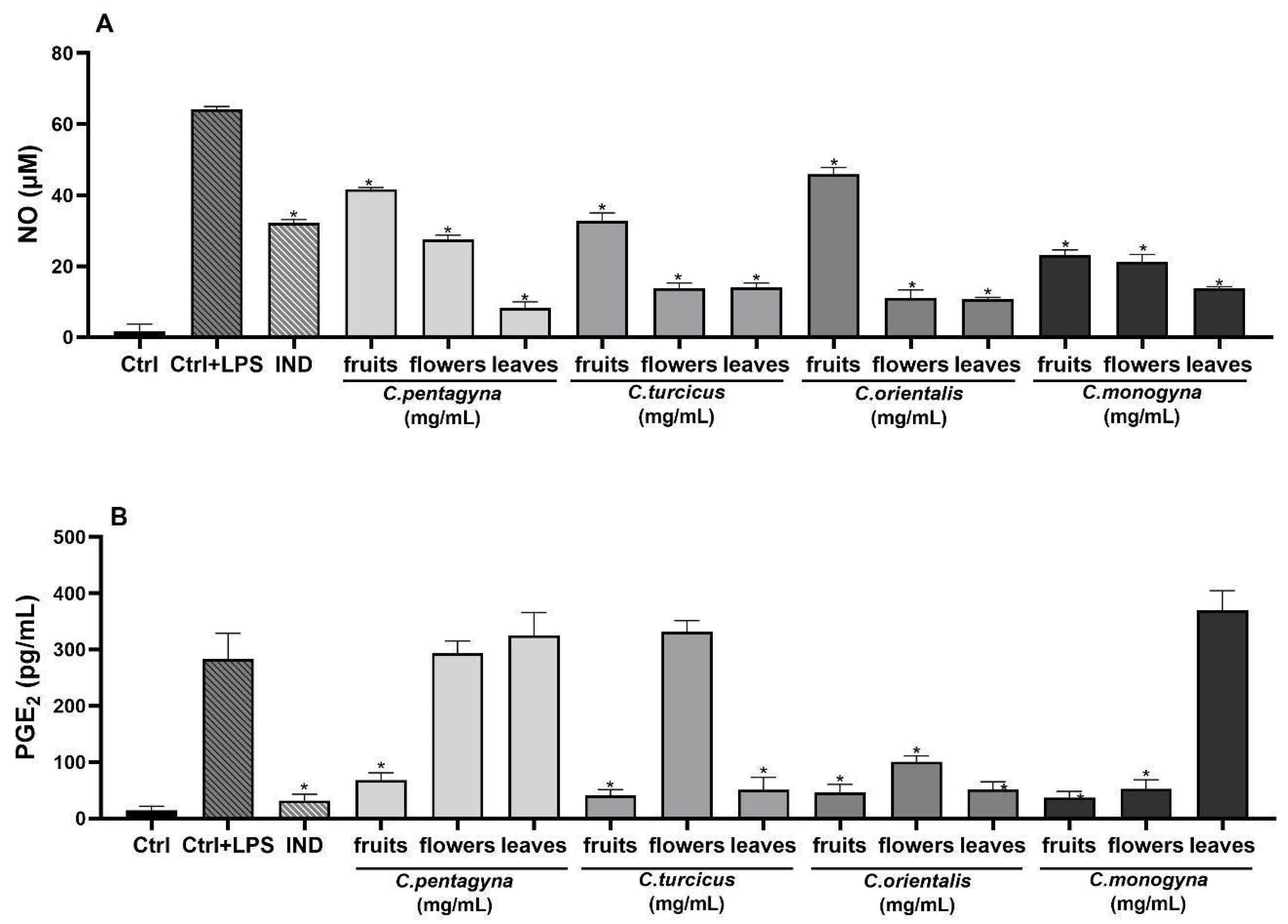
Nitric Oxide Production in LPS-Stimulated RAW 264.7 Cells
Evaluation of Analgesic Activity (PGE2 Assay)
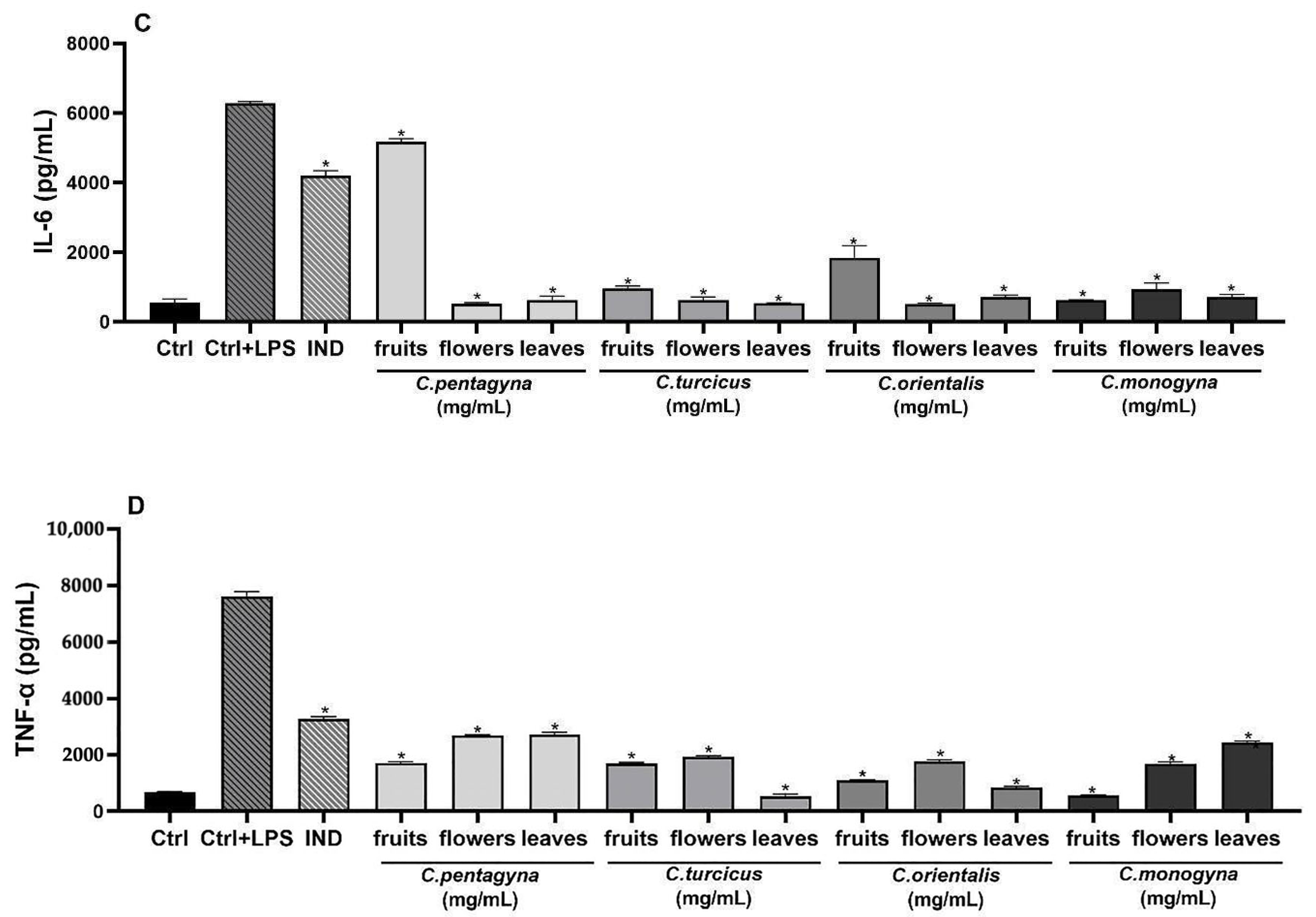
Interleukin (IL)-6-Releasing Inhibition Assay
Tumor Necrosis Factor alpha (TNF-α)
3. Discussion
4. Materials and Methods
4.1. Materials
4.1.1. Plant Material
4.1.2. Chemicals
4.2. Methods
4.2.1. Sample Extraction and Preparation of Sample Solution
4.2.2. Preparation of Standard Solutions
4.2.3. Total Phenolic Content (TPC) Assay
4.2.4. Total Flavonoid Content (TFC) Assay
4.2.5. Total Proanthocyanidin Content (TPAC) Assay
4.2.6. Total Anthocyanin Content (TAC) Assay
4.2.7. HPTLC Method
4.2.8. HPLC Method
4.3. Bioactivity Assays
4.3.1. Antioxidant Activity
DPPH Radical-Scavenging Activity Assay
ABTS Radical-Scavenging Activity Assay
CUPRAC Assay
FRAP Assay
4.3.2. Anti-Inflammatory Activity
Cell Viability Assay
Evaluation of Anti-Inflammatory Activity
Enzyme-Linked Immunosorbent Assay (ELISA)
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Zuo, L.; Prather, E.R.; Stetskiv, M.; Garrison, D.E.; Meade, J.R.; Peace, T.I.; Zhou, T. Inflammaging and oxidative stress in human diseases: From molecular mechanisms to novel treatments. Int. J. Mol. Sci. 2019, 20, 4472. [Google Scholar] [CrossRef] [PubMed]
- Ambriz-Pérez, D.L.; Leyva-López, N.; Gutierrez-Grijalva, E.P.; Heredia, J.B. Phenolic compounds: Natural alternative in inflammation treatment. A Review. Cogent Food Agric. 2016, 2, 1131412. [Google Scholar] [CrossRef]
- Rice-Evans, C.; Miller, N.; Paganga, G. Antioxidant properties of phenolic compounds. Trends Plant Sci. 1997, 2, 152–159. [Google Scholar] [CrossRef]
- Yang, B.; Liu, P. Composition and health effects of phenolic compounds in hawthorn (Crataegus spp.) of different origins. J. Sci. Food Agric. 2012, 92, 1578–1590. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Kallio, H.; Yang, B. Phenolic compounds in hawthorn (Crataegus grayana) fruits and leaves and changes during fruit ripening. J. Agric. Food Chem. 2011, 59, 11141–11149. [Google Scholar] [CrossRef]
- Alirezalu, A.; Salehi, P.; Ahmadi, N.; Sonboli, A.; Aceto, S.; Hatami Maleki, H.; Ayyari, M. Flavonoids profile and antioxidant activity in flowers and leaves of hawthorn species (Crataegus spp.) from different regions of Iran. Int. J. Food Prop. 2018, 21, 452–470. [Google Scholar] [CrossRef]
- Nazhand, A.; Lucarini, M.; Durazzo, A.; Zaccardelli, M.; Cristarella, S.; Souto, S.B.; Silva, A.M.; Severino, P.; Souto, E.B.; Santini, A. Hawthorn (Crataegus spp.): An updated overview on its beneficial properties. Forests 2020, 11, 564. [Google Scholar] [CrossRef]
- Edwards, J.E.; Brown, P.N.; Talent, N.; Dickinson, T.A.; Shipley, P.R. A review of the chemistry of the genus Crataegus. Phytochemistry 2012, 79, 5–26. [Google Scholar] [CrossRef]
- Ninfali, P.; Antonini, E.; Frati, A.; Scarpa, E.-S. C-Glycosyl flavonoids from Beta vulgaris cicla and betalains from Beta vulgaris rubra: Antioxidant, anticancer and antiinflammatory activities—A review. Phytother. Res. 2017, 31, 871–884. [Google Scholar] [CrossRef]
- Pirmoghani, A.; Salehi, I.; Moradkhani, S.; Karimi, S.A.; Salehi, S. Effect of Crataegus extract supplementation on diabetes induced memory deficits and serum biochemical parameters in male rats. IBRO Rep. 2019, 7, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, S.; Deepalakshmi, P.D.; Parasakthy, K.; Devaraj, H.; Devaraj, S.N. Effect of tincture of Crataegus on the LDL-receptor activity of hepatic plasma membrane of rats fed an atherogenic diet. Atherosclerosis 1996, 123, 235–241. [Google Scholar] [CrossRef]
- Lin, Y.; Vermeer, M.A.; Trautwein, E.A. Triterpenic acids present in hawthorn lower plasma cholesterol by inhibiting intestinal ACAT activity in hamsters. Evid. Based Compl. Alt. Med. 2011, 2011, 801272. [Google Scholar] [CrossRef]
- Jayalakshmi, R.; Thirupurasundari, C.J.; Devaraj, S.N. Pretreatment with alcoholic extract of shape Crataegus oxycantha (AEC) activates mitochondrial protection during isoproterenol—Induced myocardial infarction in rats. Mol. Cell. Biochem. 2006, 292, 59–67. [Google Scholar] [CrossRef]
- Hanus, M.; Lafon, J.; Mathieu, M. Double-blind, randomised, placebo-controlled study to evaluate the efficacy and safety of a fixed combination containing two plant extracts (Crataegus oxyacantha and Eschscholtzia californica) and magnesium in mild-to-moderate anxiety disorders. Curr. Med. Res. Opin. 2004, 20, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Zick, S.M.; Gillespie, B.; Aaronson, K.D. The effect of Crataegus oxycantha special extract WS 1442 on clinical progression in patients with mild to moderate symptoms of heart failure. Eur. J. Heart Fail. 2008, 10, 587–593. [Google Scholar] [CrossRef] [PubMed]
- Tadić, V.M.; Dobrić, S.; Marković, G.M.; Ðorđević, S.M.; Arsić, I.A.; Menković, N.R.; Stević, T. Anti-inflammatory, gastroprotective, free-radical-scavenging, and antimicrobial activities of hawthorn berries ethanol extract. J. Agric. Food Chem. 2008, 56, 7700–7709. [Google Scholar] [CrossRef]
- Benmalek, Y.; Yahia, O.A.; Belkebir, A.; Fardeau, M.-L. Anti-microbial and anti-oxidant activities of Illicium verum, Crataegus oxyacantha ssp monogyna and Allium cepa red and white varieties. Bioengineered 2013, 4, 244–248. [Google Scholar] [CrossRef]
- Sokół-Łętowska, A.; Oszmiański, J.; Wojdyło, A. Antioxidant activity of the phenolic compounds of hawthorn, pine and skullcap. Food Chem. 2007, 103, 853–859. [Google Scholar] [CrossRef]
- European Medicines Agency. Hawthorn Leaf and Flower, Crataegus spp., Folium cum Flore. Available online: https://www.ema.europa.eu/en/medicines/herbal/crataegi-folium-cum-flore (accessed on 17 June 2016).
- Council of Europe. Hawthorn Leaf and Flower. In European Pharmacopoeia; Council of Europe: Strasbourg, France, 2010; Volume 8, p. 1432. [Google Scholar]
- Dönmez, A.; Dönmez, E. Crataegus turcicus (Rosaceae), a new species from NE Turkey. Ann. Bot. Fenn. 2005, 42, 61–65. [Google Scholar]
- Idriss, H.T.; Naismith, J.H. TNF alpha and the TNF receptor superfamily: Structure-function relationship(s). Microsc. Res. Tech. 2000, 50, 184–195. [Google Scholar] [CrossRef] [PubMed]
- Bardakci, H.; Celep, E.; Gözet, T.; Kan, Y.; Kırmızıbekmez, H. Phytochemical characterization and antioxidant activities of the fruit extracts of several Crataegus taxa. S. Afr. J. Bot. 2019, 124, 5–13. [Google Scholar] [CrossRef]
- Catrinel, F.; Bedreag, G.; Trifan, A.; Bucur, L.; Arcus, M.; Tebrencu, C.; Miron, A.; Costache, I. Chemical and antioxidant studies on Crataegus pentagyna leaves and flowers. Rom. Biotechnol. Lett. 2014, 19, 9859–9867. [Google Scholar]
- Barros, L.; Carvalho, A.M.; Ferreira, I.C.F.R. Comparing the composition and bioactivity of Crataegus Monogyna flowers and fruits used in folk medicine. Phytochem. Anal. 2011, 22, 181–188. [Google Scholar] [CrossRef]
- Froehlicher, T.; Hennebelle, T.; Martin-Nizard, F.; Cleenewerck, P.; Hilbert, J.-L.; Trotin, F.; Grec, S. Phenolic profiles and antioxidative effects of hawthorn cell suspensions, fresh fruits, and medicinal dried parts. Food Chem. 2009, 115, 897–903. [Google Scholar] [CrossRef]
- Katanić Stanković, J.S.; Mićanović, N.; Grozdanić, N.; Kostić, A.Ž.; Gašić, U.; Stanojković, T.; Popović-Djordjević, J.B. Polyphenolic Profile, Antioxidant and Antidiabetic Potential of Medlar (Mespilus germanica L.), Blackthorn (Prunus spinosa L.) and Common Hawthorn (Crataegus monogyna Jacq.) Fruit Extracts from Serbia. Horticulturae 2022, 8, 1053. [Google Scholar] [CrossRef]
- Bubueanu, C.; Grigore, A.; Pirvu, L.C. HPTLC fingerprint use, an important step in plant-derived products quality control. Sci. Pap. Ser. B Hortic. 2015, 59, 437–442. [Google Scholar]
- Khokhlova, K.O.; Zdoryk, O.A.; Sydora, N.V.; Shatrovska, V.I. Chromatographic profiles analysis of fruits of Crataegus L. genus by high-performance thin-layer chromatography. Eur. Pharm. J. 2019, 66, 45–51. [Google Scholar] [CrossRef][Green Version]
- Alirezalu, A.; Ahmadi, N.; Salehi, P.; Sonboli, A.; Alirezalu, K.; Mousavi Khaneghah, A.; Barba, F.J.; Munekata, P.E.S.; Lorenzo, J.M. Physicochemical characterization, antioxidant activity, and phenolic compounds of hawthorn (Crataegus spp.) fruits species for potential use in food applications. Foods 2020, 9, 436. [Google Scholar] [CrossRef]
- Stoenescu, A.-M.; Trandafir, I.; Cosmulescu, S. Determination of phenolic compounds using HPLC-UV method in wild Fruit species. Horticulturae 2022, 8, 84. [Google Scholar] [CrossRef]
- Pavlovic, J.; Mitić, S.; Mitić, M.; Kocić, G.; Pavlović, A.; Tošić, S. Variation in the phenolic compounds profile and antioxidant activity in different parts of hawthorn (Crataegus pentagyna Willd.) during harvest periods. Pol. J. Food Nutr. Sci. 2019, 69, 367–378. [Google Scholar] [CrossRef]
- Luís, Â.; Domingues, F.; Duarte, A.P. Bioactive compounds, RP-HPLC analysis of phenolics, and antioxidant activity of some Portuguese shrub species extracts. Nat. Prod. Commun. 2011, 6, 1934578X1100601219. [Google Scholar] [CrossRef]
- Sagaradze, V.A.; Babaeva, E.Y.; Ufimov, R.A.; Trusov, N.A.; Kalenikova, E.I. Study of the variability of rutin, vitexin, hyperoside, quercetin in “Crataegi folium cum flore” of hawthorn (Crataegus L.) species from Russian flora. J. Appl. Res. Med. Aromat. Plants 2019, 15, 100217. [Google Scholar] [CrossRef]
- Rocchetti, G.; Senizza, B.; Zengin, G.; Mahomodally, M.F.; Senkardes, I.; Lobine, D.; Lucini, L. Untargeted metabolomic profiling of three Crataegus species (hawthorn) and their in vitro biological activities. J. Sci. Food Agric. 2020, 100, 1998–2006. [Google Scholar] [CrossRef] [PubMed]
- Martín-García, B.; Razola-Díaz, M.d.C.; Gómez-Caravaca, A.M.; Benítez, G.; Verardo, V. Setup of an ultrasonic-assisted extraction to obtain high phenolic recovery in Crataegus monogyna leaves. Molecules 2021, 26, 4536. [Google Scholar] [CrossRef]
- Özyürek, M.; Bener, M.; Güçlü, K.; Dönmez, A.; Süzgeç, S.; Pırıldar, S.; Mericli, A.; Apak, R. Evaluation of antioxidant activity of Crataegus species collected from different regions of Turkey. Rec. Nat. Prod. 2012, 6, 263–277. [Google Scholar]
- Bor, Z. Antinociceptive, Anti-Inflammatory, Antithrombotic and Antioxidant Effects of the Ethanol Extract of Crataegus orientalis. Master’s Thesis, Anadolu University, Eskişehir, Turkey, 2010. [Google Scholar]
- Šavikin, K.P.; Krstić-Miloševič, D.B.; Menkovič, N.R.; Beara, I.N.; Mrkonjić, Z.O.; Pijevijakušić, D.S. Crataegus orientalis leaves and berries: Phenolic profiles, antioxidant and anti-inflammatory activity. Nat. Prod. Commun. 2017, 12, 159–162. [Google Scholar] [CrossRef] [PubMed]
- Pelvan, E.; Serhatlı, M.; Karaoğlu, Ö.; Karadeniz, B.; Pembeci Kodolbaş, C.; Aslı Öncü, N.; Çakırca, G.; Damarlı, E.; Başdoğan, G.; Mergen Duymaz, G.; et al. Development of propolis and essential oils containing oral/throat spray formulation against SARS-CoV-2 infection. J. Func. Foods 2022, 97, 105225. [Google Scholar] [CrossRef]
- Guzelmeric, E.; Ugurlu, P.; Celik, C.; Sen, N.B.; Helvacıoglu, S.; Charehsaz, M.; Erdogan, M.; Ockun, M.A.; Kırmızıbekmez, H.; Aydın, A.; et al. Myrtus communis L. (Myrtle) plant parts: Comparative assessment of their chemical compositions, antioxidant, anticancer, and antimutagenic activities. S. Afr. J. Bot. 2022, 150, 711–720. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Viticult. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Degirmencioglu, H.; Güzelmeriç, E.; Yuksel, P.; Kırmızıbekmez, H.; Deniz, I.; Yesilada, E. A new type of anatolian propolis: Evaluation of its chemical composition, activity profile and botanical origin. Chem. Biodivers. 2019, 16, e1900492. [Google Scholar] [CrossRef]
- Sun, B.; Ricardo-da-Silva, J.M.; Spranger, I. Critical factors of vanillin assay for catechins and proanthocyanidins. J. Agric. Food Chem. 1998, 46, 4267–4274. [Google Scholar] [CrossRef]
- Lee, J.; Durst, R.W.; Wrolstad, R.E. Determination of total monomeric anthocyanin pigment content of fruit juices, beverages, natural colorants, and wines by the pH differential method: Collaborative study. J. AOAC Int. 2005, 88, 1269–1278. [Google Scholar] [CrossRef] [PubMed]
- Ying, X.; Wang, R.; Xu, J.; Zhang, W.; Li, H.; Zhang, C.; Li, F. HPLC determination of eight polyphenols in the leaves of Crataegus pinnatifida Bge. var. major. J. Chromatogr. Sci. 2009, 47, 201–205. [Google Scholar] [CrossRef] [PubMed]
- Blois, M.S. Antioxidant determinations by the use of a stable free radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Apak, R.; Güçlü, K.; Özyürek, M.; Karademir Çelik, S. Novel total antioxidant capacity index for dietary polyphenols and vitamins C and E, using their cupric ion reducing capability in the presence of neocuproine: CUPRAC method. J. Agric. Food Chem. 2004, 52, 7970–7981. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Karaoğlu, Ö.; Serhatlı, M.; Pelvan, E.; Karadeniz, B.; Demirtas, I.; Çakırca, G.; Sipahi, H.; Özhan, Y.; Karapınar, G.; Charehsaz, M.; et al. Chewable tablet with herbal extracts and propolis arrests Wuhan and Omicron variants of SARS-CoV-2 virus. J. Funct. Food 2023, 105, 105544. [Google Scholar] [CrossRef]
- Okur, M.E.; Karadağ, A.E.; Üstündağ Okur, N.; Özhan, Y.; Sipahi, H.; Ayla, Ş.; Daylan, B.; Demirci, B.; Demirci, F. In vivo wound healing and in vitro anti-inflammatory activity evaluation of Phlomis russeliana extract gel formulations. Molecules 2020, 25, 2695. [Google Scholar] [CrossRef] [PubMed]
- Okur, M.E.; Karadağ, A.E.; Özhan, Y.; Sipahi, H.; Ayla, Ş.; Daylan, B.; Kültür, Ş.; Demirci, B.; Demirci, F. Anti-inflammatory, analgesic and in vivo-in vitro wound healing potential of the Phlomis rigida Labill. extract. J. Ethnopharmacol. 2021, 266, 113408. [Google Scholar] [CrossRef] [PubMed]
- Erdoğan, M.; Konya, R.; Özhan, Y.; Sipahi, H.; Çinbilgel, İ.; Masullo, M.; Piacente, S.; Kırmızıbekmez, H. Secondary metabolites from Scutellaria brevibracteata subsp. subvelutina and their in vitro anti-inflammatory activities. S. Afr. J. Bot. 2021, 139, 12–18. [Google Scholar] [CrossRef]

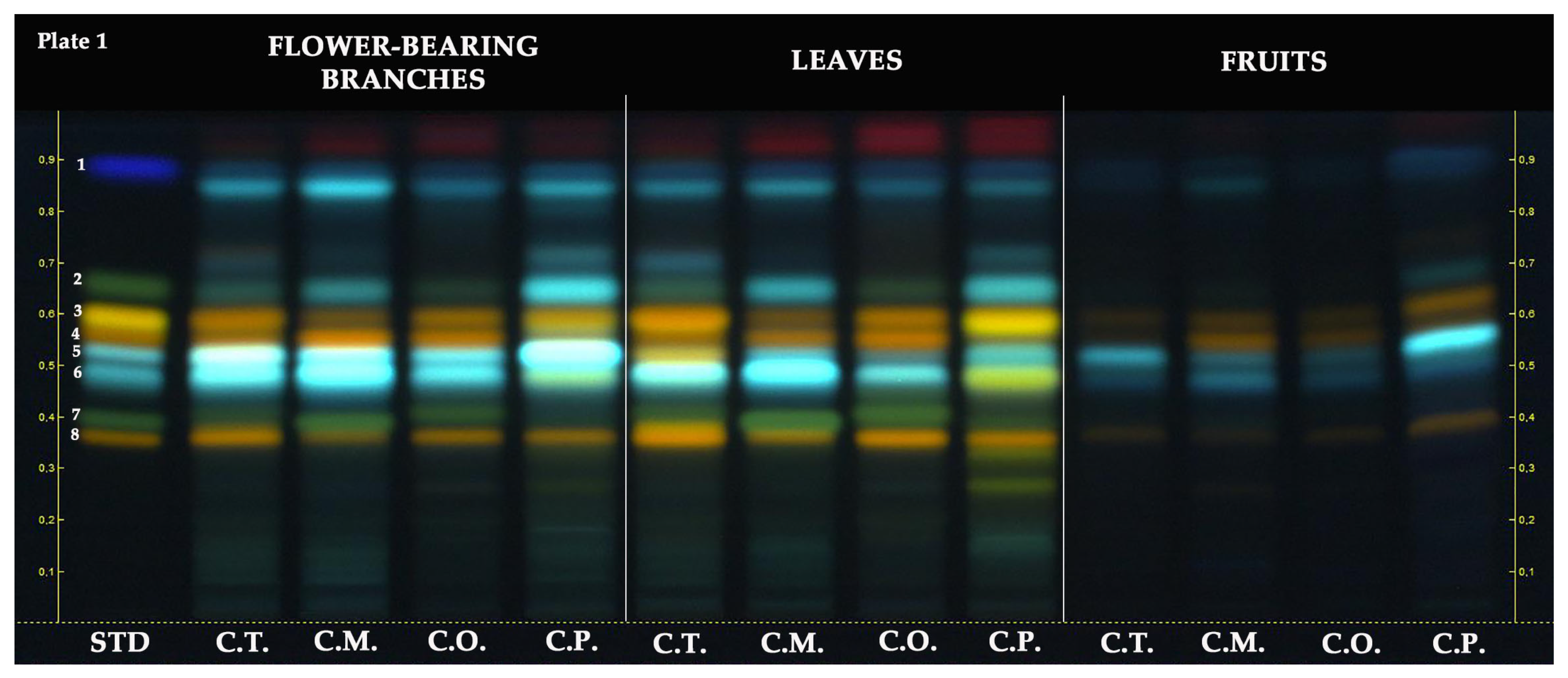
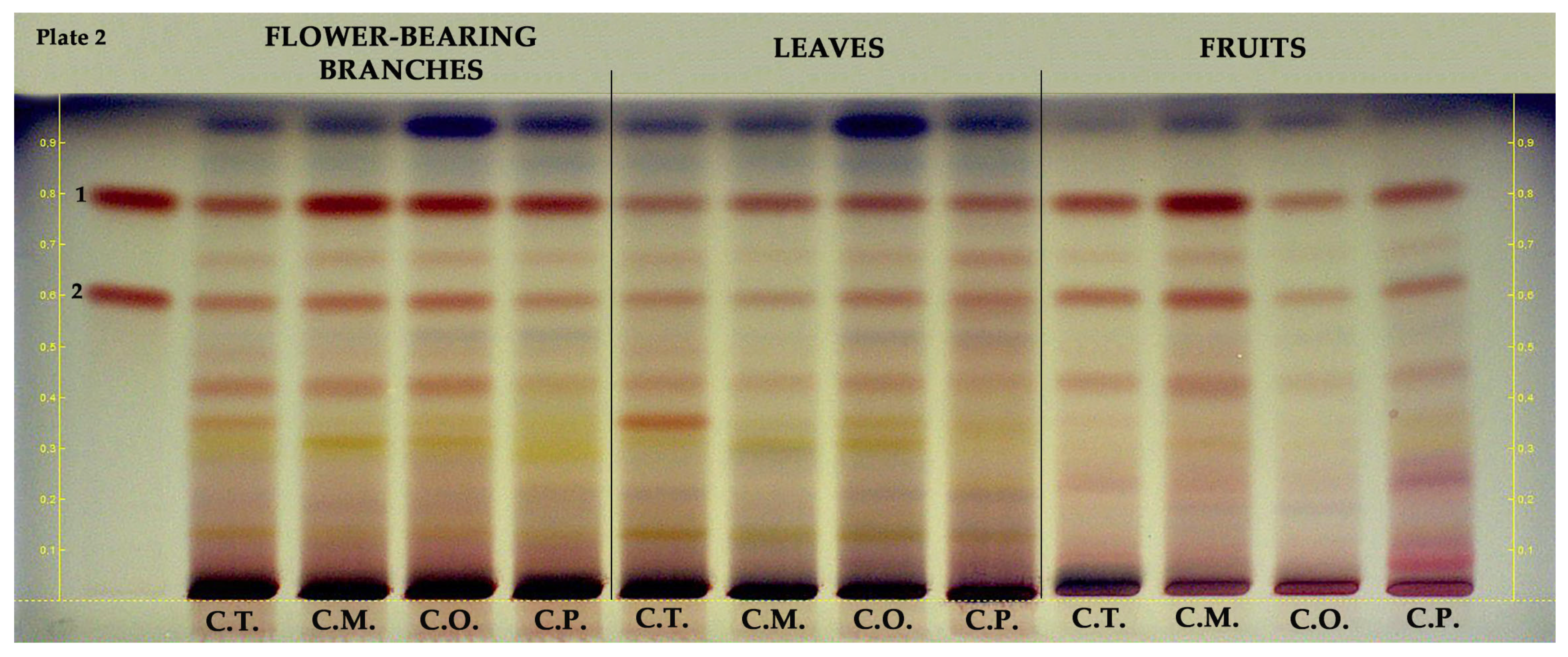
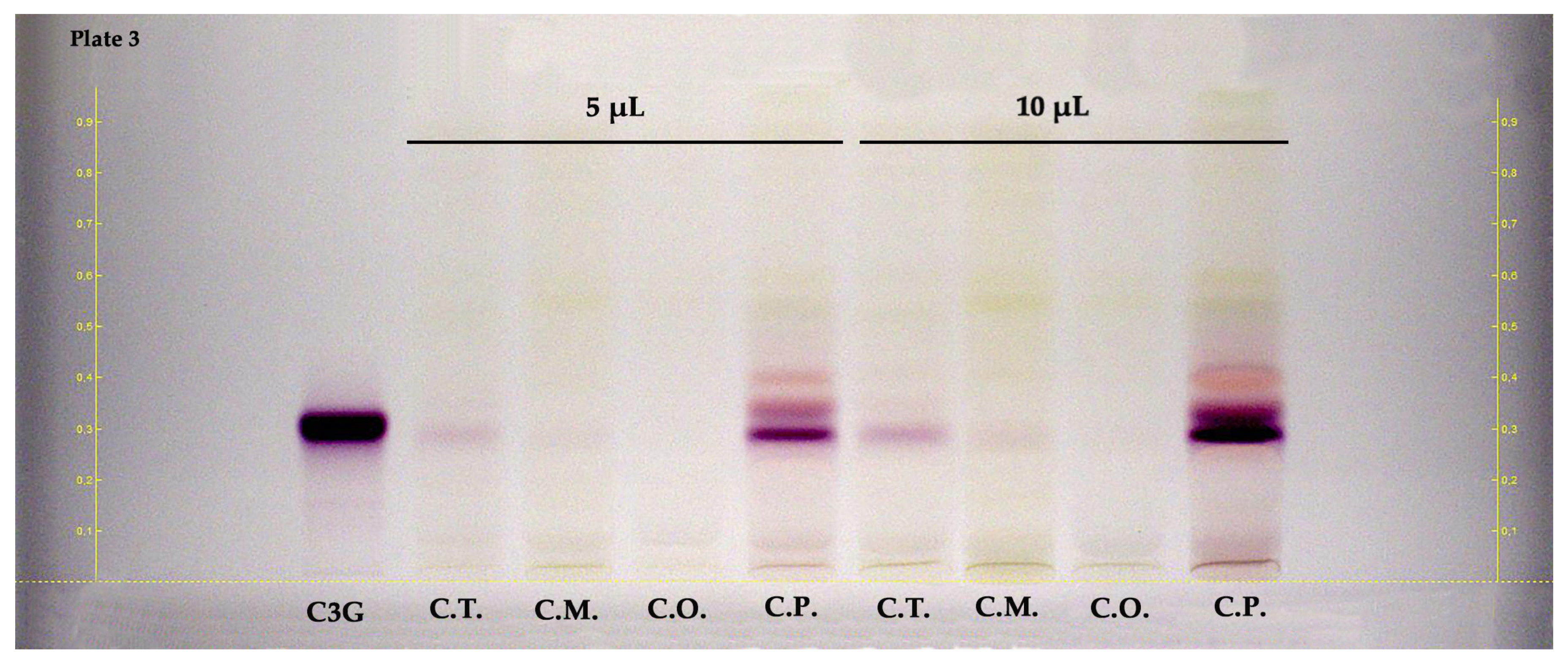
| Samples | Parts | TPC 1 | TFC 2 | TPAC 3 | TAC 4 |
|---|---|---|---|---|---|
| C. turcicus | flower-bearing branches | 239.09 ± 9.77 DE | 22.15 ± 1.37 CD | 30.73 ± 0.54 A | - |
| leaves | 227.57 ± 12.05 DE | 34.26 ± 2.77 B | 20.98 ± 0.05 C | - | |
| fruits | 69.43 ± 5.9 G | 1.54 ± 0.17 F | 12.96 ± 0.62 F | 6.47 ± 0.45 B | |
| C. monogyna | flower-bearing branches | 301.77 ± 21.8 CD | 20.49 ± 0.69 D | 30.11 ± 1.14 A | - |
| leaves | 169.66 ± 12.34 EF | 20.54 ± 1.33 D | 17.22 ± 1.31 DE | - | |
| fruits | 116.24 ± 10.83 FG | 6.08 ± 0.53 EF | 15.16 ± 0.43 EF | 2.02 ± 2.02 C | |
| C. orientalis | flower-bearing branches | 287.92 ± 37.02 CD | 20.72 ± 0.53 D | 28.69 ± 3.26 A | - |
| leaves | 874.06 ± 74.94 A | 37.47 ± 3.48 B | 20.01 ± 0.47 CD | - | |
| fruits | 42.12 ± 4.71 G | 8.37 ± 0.98 E | 7.46 ± 0.50 G | 0.24 ± 0.24 D | |
| C. pentagyna | flower-bearing branches | 368.94 ± 7.78 BC | 26.92 ± 1.18 C | 24.67 ± 1.07 B | - |
| leaves | 394.66 ± 35.05 B | 63.67 ± 4.71 A | 20.17 ± 1.20 CD | - | |
| fruits | 91.00 ± 2.77 FG | 9.87 ± 0.67 E | 18.76 ± 0.51 CD | 15.45 ± 15.45 A |
| Flower-Bearing Branches | Leaves | Fruits | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C.T. | C.M. | C.O. | C.P. | C.T. | C.M. | C.O. | C.P. | C.T. | C.M. | C.O. | C.P. | |
| Protocatechuic acid | 0.15 ± 0.01 H | 0.18 ± 0.01 H | 0.39 ± 0.01 F | 0.43 ± 0.03 E | 0.35 ± 0.01 G | 0.77 ± 0.00 B | 0.55 ± 0.01 C | 0.88 ± 0.00 A | 0.04 ± 0.01 I | n.d. | n.d. | 0.49 ± 0.01 D |
| Neochlorogenic acid | 18.18 ± 0.56 C | 21.90 ± 0.06 B | 7.82 ± 0.22 DE | 50.32 ± 4.02 A | n.d. | 4.64 ± 0.04 EF | 3.26 ± 0.07 FG | 4.64 ± 0.03 EF | 6.90 ± 0.02 DE | 1.26 ± 0.01 FG | 0.86 ± 0.04 G | 9.17 ± 0.16 D |
| Chlorogenic acid | 21.61 ± 0.61 E | 45.52 ± 0.50 A | 12.12 ± 0.13 G | 15.69 ± 0.75 F | 29.60 ± 0.04 D | 40.89 ± 0.44 B | 13.73 ± 0.39 G | 32.35 ± 0.02 C | 0.93 ± 0.01 H | 2.14 ± 0.07 H | 0.55 ± 0.00 H | 0.71 ± 0.01 H |
| Procyanidin B2 | 3.67 ± 0.07 F | 7.15 ± 0.05 B | 5.48 ± 0.19 D | 6.44 ± 0.04 C | 1.81 ± 0.09 I | 2.03 ± 0.09 HI | 2.92 ± 0.17 G | 1.97 ± 0.10 HI | 4.56 ± 0.05 E | 11.65 ± 0.17 A | 3.24 ± 0.14 G | 2.29 ± 0.16 H |
| Epicatechin | 3.59 ± 0.03 FG | 17.56 ± 0.27 A | 11.78 ± 0.75 C | 13.83 ± 0.11 B | 2.83 ± 0.10 G | 5.95 ± 0.11 E | 3.82 ± 0.51 F | 7.30 ± 0.02 D | 7.92 ± 0.06 D | 13.24 ± 0.18 B | 2.89 ± 0.12 G | 13.29 ± 0.21 B |
| Orientin | 0.08 ± 0.07 D | n.d. | n.d. | 1.54 ± 0.02 B | 0.47 ± 0.03 C | n.d. | n.d. | 13.49 ± 0.03 A | n.d. | n.d. | n.d. | n.d. |
| Vitexin | 0.90 ± 0.03 E | n.d. | 1.09 ± 0.08 D | 0.30 ± 0.05 F | 3.61 ± 0.14 B | n.d. | 1.77 ± 0.01 C | 4.98 ± 0.02 A | n.d. | n.d. | n.d. | n.d. |
| Vitexin-2″-O-rhamnoside | 4.92 ± 0.18 G | 26.50 ± 0.70 C | 13.93 ± 0.10 E | 1.70 ± 0.09 H | 12.83 ± 0.14 F | 76.03 ± 0.36 A | 49.41 ± 0.92 B | 19.63 ± 0.02 D | n.d. | n.d. | n.d. | n.d. |
| Hyperoside | 7.81 ± 0.22 E | 17.29 ± 0.01 A | 11.54 ± 0.31 C | 2.84 ± 0.03 H | 4.41 ± 0.22 G | 10.09 ± 0.55 D | 16.25 ± 0.29 B | n.d. | 0.45 ± 0.00 J | 5.61 ± 0.15 F | 2.23 ± 0.01 HI | 1.68 ± 0.02 I |
| Rutin | 1.83 ± 0.08 C | 0.34 ± 0.03 G | 1.33 ± 0.11 DE | 1.80 ± 0.01 CD | 5.27 ± 0.32 B | 1.67 ± 0.10 CD | 5.82 ± 0.25 A | 6.03 ± 0.37 A | 0.84 ± 0.00 F | n.d. | n.d. | 0.96 ± 0.02 EF |
| Isoquercitrin | n.d. | 3.07 ± 0.11 F | 6.30 ± 0.24 C | 5.12 ± 0.18 D | n.d. | 4.03 ± 0.02 E | 11.09 ± 0.23 B | 12.94 ± 0.06 A | n.d. | 2.18 ± 0.00 G | 1.28 ± 0.45 H | 2.02 ± 0.02 G |
| Samples | Parts | DPPH | ABTS | FRAP | CUPRAC |
|---|---|---|---|---|---|
| mg TE/g | |||||
| C. turcicus | flowering branches | 466.59 ± 8.97 A | 380.78 ± 20.11 BCD | 304.48 ± 6.89 B | 755.42 ± 16.76 CD |
| leaves | 346.65 ± 7.80 C | 365.47 ± 25.53 CD | 226.50 ± 8.66 D | 692.83 ± 39.22 D | |
| fruits | 129.93 ± 4.17 F | 130.78 ± 4.63 G | 105.51 ± 6.30 H | 277.03 ± 20.28 G | |
| C. monogyna | flowering branches | 319.29 ± 12.60 CD | 457.27 ± 38.03 A | 371.52 ± 9.04 A | 852.09 ± 21.16 A |
| leaves | 243.63 ± 8.18 E | 289.05 ± 10.26 E | 162.66 ± 3.33 F | 568.10 ± 10.41 E | |
| fruits | 132.89 ± 20.32 F | 215.97 ± 24.11 F | 128.81 ± 11.67 G | 359.33 ± 11.57 F | |
| C. orientalis | flowering branches | 383.98 ± 19.93 B | 400.34 ± 14.59 ABC | 271.72 ± 5.84 C | 779.56 ± 41.05 BC |
| leaves | 313.59 ± 13.11 CD | 334.43 ± 15.68 DE | 198.72 ± 7.68 E | 596.73 ± 32.51 E | |
| fruits | 70.04 ± 2.70 G | 119.45 ± 11.95 G | 66.52 ± 3.28 I | 222.13 ± 11.82 GH | |
| C. pentagyna | flowering branches | 411.07 ± 3.34 B | 442.72 ± 32.10 AB | 272.86 ± 9.44 C | 847.94 ± 30.56 AB |
| leaves | 296.23 ± 10.91 D | 397.07 ± 29.02 ABCD | 217.48 ± 11.91 DE | 553.50 ± 28.91 E | |
| fruits | 160.02 ± 14.22 F | 148.89 ± 5.34 G | 163.89 ± 2.24 F | 180.87 ± 0.61 H | |
| Samples | Groups | Concentration (mg/mL) | Cell Viability (%) | Nitrite Level (µM) | Nitrite Inhibition (%) |
|---|---|---|---|---|---|
| Ctrl | 117.13 ± 1.75 | 2.15 ± 2.27 | - | ||
| Ctrl + LPS | 101.12 ± 2.14 | 64.17 ± 0.84 | - | ||
| Indomethacin | 100 µM | 92.92 ± 1.47 | 32.20 ± 0.95 * | 49.83 ± 1.39 | |
| C. turcicus | flower-bearing branches | 0.125 | 115.61 ± 0.73 | 63.49 ± 1.18 | 1.06 ± 0.88 |
| 0.25 | 107.82 ± 1.93 | 61.33 ± 0.81 | 4.42 ± 0.58 | ||
| 0.5 | 104.06 ± 1.47 | 55.04 ± 1.03 | 14.22 ± 2.23 | ||
| 1 | 102.15 ± 1.47 | 13.86 ± 1.41 * | 78.39 ± 2.31 | ||
| leaves | 0.125 | 107.50 ± 0.71 | 61.15 ± 1.77 | 4.71 ± 2.90 | |
| 0.25 | 95.32 ± 2.83 | 54.11 ± 0.49 | 15.67 ± 1.18 | ||
| 0.5 | 83.70 ± 1.07 | 48.01 ± 1.15 * | 25.19 ± 1.62 | ||
| 1 | 79.97 ± 3.61 | 13.99 ± 1.30 * | 78.19 ± 2.20 | ||
| fruits | 0.125 | 117.56 ± 0.94 | 61.58 ± 1.98 | 4.05 ± 2.13 | |
| 0.25 | 110.97 ± 1.72 | 58.62 ± 2.53 | 8.65 ± 1.05 | ||
| 0.5 | 102.83 ± 1.64 | 43.06 ± 0.88 * | 32.90 ± 0.98 | ||
| 1 | 96.66 ± 4.04 | 32.75 ± 2.26 * | 48.94 ± 3.99 | ||
| C. monogyna | flower-bearing branches | 0.125 | 108.40 ± 1.18 | 58.99 ± 1.37 | 8.09 ± 1.12 |
| 0.25 | 99.89 ± 0.63 | 55.41 ± 1.70 | 13.66 ± 2.45 | ||
| 0.5 | 93.50 ± 1.21 | 50.22 ± 3.70 | 21.77 ± 1.93 | ||
| 1 | 86.20 ± 0.65 | 21.27 ± 2.08 * | 66.87 ± 2.13 | ||
| leaves | 0.125 | 106.40 ± 0.23 | 59.67 ± 0.98 | 7.02 ± 0.48 | |
| 0.25 | 96.79 ± 0.98 | 52.51 ± 1.57 | 18.16 ± 2.33 | ||
| 0.5 | 89.85 ± 1.44 | 43.00 ± 0.32 * | 32.98 ± 1.32 | ||
| 1 | 84.30 ± 2.10 | 13.80 ± 0.47 * | 78.49 ± 0.92 | ||
| fruits | 0.125 | 107.77 ± 1.53 | 57.69 ± 1.23 | 10.09 ± 2.50 | |
| 0.25 | 101.11 ± 0.84 | 47.63 ± 3.80 * | 25.75 ± 1.42 | ||
| 0.5 | 91.91 ± 1.48 | 37.51 ± 2.68 * | 41.52 ± 1.83 | ||
| 1 | 85.39 ± 2.06 | 23.12 ± 1.49 * | 63.98 ± 2.05 | ||
| C. orientalis | flower-bearing branches | 0.125 | 114.39 ± 1.55 | 56.64 ± 1.19 | 11.71 ± 2.95 |
| 0.25 | 110.37 ± 1.04 | 51.52 ± 0.67 | 19.71 ± 1.21 | ||
| 0.5 | 103.35 ± 2.97 | 44.54 ± 0.60 * | 30.58 ± 1.23 | ||
| 1 | 95.53 ± 0.95 | 11.09 ± 2.23 * | 82.71 ± 2.59 | ||
| leaves | 0.125 | 108.88 ± 0.69 | 63.12 ± 0.60 | 1.63 ± 1.42 | |
| 0.25 | 102.43 ± 1.15 | 58.06 ± 2.15 | 9.51 ± 2.13 | ||
| 0.5 | 98.61 ± 1.63 | 54.67 ± 1.03 | 14.81 ± 1.32 | ||
| 1 | 93.55 ± 1.12 | 10.65 ± 0.57 * | 83.40 ± 0.78 | ||
| fruits | 0.125 | 110.72 ± 0.89 | 60.72 ± 2.19 | 5.40 ± 2.55 | |
| 0.25 | 104.69 ± 1.64 | 52.57 ± 0.65 | 18.07 ± 1.86 | ||
| 0.5 | 100.34 ± 1.76 | 51.02 ± 0.47 | 20.48 ± 1.04 | ||
| 1 | 90.10 ± 0.26 | 45.96 ± 1.85 * | 28.38 ± 2.62 | ||
| C. pentagyna | flower-bearing branches | 0.125 | 112.26 ± 1.32 | 58.49 ± 0.75 | 8.84 ± 1.97 |
| 0.25 | 109.34 ± 2.51 | 53.06 ± 1.60 | 17.33 ± 1.47 | ||
| 0.5 | 99.33 ± 0.92 | 48.62 ± 2.51 * | 24.23 ± 2.06 | ||
| 1 | 83.23 ± 0.52 | 27.44 ± 1.28 * | 57.23 ± 2.05 | ||
| leaves | 0.125 | 115.10 ± 1.54 | 48.68 ± 3.06 | 24.18 ± 1.78 | |
| 0.25 | 108.45 ± 1.64 | 43.62 ± 1.19 | 32.02 ± 2.14 | ||
| 0.5 | 99.85 ± 1.98 | 30.22 ± 1.77 * | 52.88 ± 2.28 | ||
| 1 | 88.03 ± 1.29 | 8.19 ± 1.79 * | 87.24 ± 2.81 | ||
| fruits | 0.125 | 106.00 ± 0.84 | 63.19 ± 0.67 | 1.54 ± 0.42 | |
| 0.25 | 100.88 ± 1.77 | 60.41 ± 1.47 | 5.87 ± 1.67 | ||
| 0.5 | 96.09 ± 0.88 | 54.48 ± 1.34 | 15.10 ± 2.01 | ||
| 1 | 85.62 ± 1.37 | 41.70 ± 0.49 * | 35.01 ± 0.99 |
| Crataegus Species | Parts | Collection Month | Collection Place | Altitude | Herbarium Number |
|---|---|---|---|---|---|
| Crataegus turcicus Dönmez | Flower-bearing branches | June 2020 | Şavşat, Artvin | 1650 m | ARTH 13587 |
| Leaves | May 2020 | ||||
| Fruits | October 2020 | ||||
| Crataegus monogyna Jacq. | Flower-bearing branches | May 2020 | Ataşehir, Istanbul | 195 m | YEF 20048 |
| Leaves | May 2020 | ||||
| Fruits | September 2020 | ||||
| Crataegus orientalis Pall. ex M.Bieb. | Flower-bearing branches | May 2020 | Ardanuç, Artvin | 1650 m | ARTH 13585 |
| Leaves | May 2020 | ||||
| Fruits | October 2020 | ||||
| Crataegus pentagyna Waldst. & Kit. ex Willd. | Flower-bearing branches | May 2020 | Ardanuç, Artvin | 1035 m | KATO 19304 |
| Leaves | May 2020 | ||||
| Fruits | October 2020 |
| Plate No. | Investigated Compounds | Sub-Classes | Applied Concentration | Applied Volume | Mobile Phase | Derivatization Reagents | Visualization |
|---|---|---|---|---|---|---|---|
| 1 | Protocatechuic acid | Phenolic acid | 50 µg/mL | 10 µL | 5:3:1:1 (v/v) | NP/PEG | 366 nm |
| Vitexin | Flavone C-glycoside | ||||||
| Isoquercitrin | Flavonol glycoside | ||||||
| Orientin | Flavone C-glycoside | ||||||
| Hyperoside | Flavonol glycoside | ||||||
| Neochlorogenic acid | Phenolic acid | ||||||
| Chlorogenic acid | Phenolic acid | ||||||
| Vitexin-2″-O-rhamnoside | Flavone C-glycoside | ||||||
| Rutin | Flavonol glycoside | ||||||
| 2 | Epicatechin | Flavanol | 100 µg/mL | 5 µL | 10:1:4 (v/v) | vanillin- sulfuric acid | white light |
| Procyanidin B2 | |||||||
| 3 | Cyanidin 3-O-glucoside | Anthocyanin | 160 µg/mL | 2 µL | 5:3:1:1 (v/v) | – | white light |
| Standards | Linearity Range (µg/mL) | Calibration Equation A | r2 | LOQ (µg/mL) | LOD (µg/mL) |
|---|---|---|---|---|---|
| Protocatechuic acid | 5–50 | y = 34.362x + 20.86 | 0.9995 | 0.50 | 0.15 |
| Neochlorogenic acid | 5–50 | y = 21.462x − 2.5439 | 0.9992 | 0.09 | 0.03 |
| Chlorogenic acid | 5–50 | y = 24.027x − 3.9582 | 0.9999 | 1.45 | 0.43 |
| Procyanidin B2 | 5–50 | y = 4.5678x − 2.239 | 0.9977 | 1.19 | 0.36 |
| Epicatechin | 5–50 | y = 6.096x + 0.953 | 0.9997 | 1.28 | 0.38 |
| Orientin | 5–50 | y = 25.761x + 13.279 | 0.999 | 0.49 | 0.15 |
| Vitexin | 5–50 | y = 24.314x + 12.074 | 0.9973 | 0.64 | 0.19 |
| Vitexin-2″-O-rhamnoside | 10–100 | y = 16.009x + 8.6479 | 0.9995 | 2.51 | 0.75 |
| Hyperoside | 5–50 | y = 18.395x − 8.2302 | 0.9981 | 0.03 | 0.01 |
| Rutin | 10–100 | y = 13.511x − 3.8909 | 0.9995 | 1.08 | 0.32 |
| Isoquercitrin | 5–50 | y = 19.323x + 4.1729 | 0.9997 | 0.61 | 0.18 |
| Standards | Theoretical Value | Amount Found | % |
|---|---|---|---|
| Protocatechuic acid | 12.5 | 12.23 ± 0.15 | 97.85 |
| 6.25 | 6.02 ± 0.06 | 96.26 | |
| 3.125 | 2.78 ± 0.09 | 88.91 | |
| Neochlorogenic acid | 12.5 | 12.99 ± 0.46 | 103.93 |
| 6.25 | 7.00 ± 0.15 | 112.03 | |
| 3.125 | 3.36 ± 0.04 | 107.47 | |
| Chlorogenic acid | 12.5 | 12.38 ± 0.42 | 99.04 |
| 6.25 | 6.04 ± 0.13 | 96.64 | |
| 3.125 | 3.23 ± 0.09 | 103.38 | |
| Procyanidin B2 | 12.5 | 12.16 ± 0.37 | 97.27 |
| 6.25 | 6.74 ± 0.15 | 107.79 | |
| 3.125 | 3.23 ± 0.02 | 103.25 | |
| Epicatechin | 12.5 | 12.29 ± 0.28 | 98.28 |
| 6.25 | 5.88 ± 0.18 | 94.13 | |
| 3.125 | 2.87 ± 0.03 | 91.78 | |
| Orientin | 12.5 | 12.02 ± 0.24 | 96.18 |
| 6.25 | 5.76 ± 0.14 | 92.22 | |
| 3.125 | 3.11 ± 0.15 | 99.48 | |
| Vitexin | 12.5 | 12.23 ± 0.21 | 97.82 |
| 6.25 | 5.76 ± 0.08 | 92.21 | |
| 3.125 | 2.58 ± 0.03 | 81.06 | |
| Vitexin-2″-O-rhamnoside | 25 | 24.25 ± 0.89 | 97.02 |
| 12.5 | 12.01 ± 0.03 | 96.07 | |
| 6.25 | 6.36 ± 0.16 | 101.73 | |
| Hyperoside | 12.5 | 13.45 ± 0.23 | 107.56 |
| 6.25 | 6.08 ± 0.08 | 97.36 | |
| 3.125 | 3.56 ± 0.18 | 113.94 | |
| Rutin | 25 | 26.05 ± 0.77 | 104.19 |
| 12.5 | 12.17 ± 0.19 | 97.40 | |
| 6.25 | 6.26 ± 0.05 | 100.21 | |
| Isoquercitrin | 12.5 | 12.27 ± 0.34 | 98.17 |
| 6.25 | 6.76 ± 0.09 | 108.14 | |
| 3.125 | 2.82 ± 0.12 | 90.13 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Turnalar Ülger, T.; Oçkun, M.A.; Guzelmeric, E.; Sen, N.B.; Sipahi, H.; Özhan, Y.; Kan, Y.; Yesilada, E. Comprehensive Analysis of the Chemical and Bioactivity Profiles of Endemic Crataegus turcicus Dönmez in Comparison with Other Crataegus Species. Molecules 2023, 28, 6520. https://doi.org/10.3390/molecules28186520
Turnalar Ülger T, Oçkun MA, Guzelmeric E, Sen NB, Sipahi H, Özhan Y, Kan Y, Yesilada E. Comprehensive Analysis of the Chemical and Bioactivity Profiles of Endemic Crataegus turcicus Dönmez in Comparison with Other Crataegus Species. Molecules. 2023; 28(18):6520. https://doi.org/10.3390/molecules28186520
Chicago/Turabian StyleTurnalar Ülger, Tansu, Mehmet Ali Oçkun, Etil Guzelmeric, Nisa Beril Sen, Hande Sipahi, Yağmur Özhan, Yüksel Kan, and Erdem Yesilada. 2023. "Comprehensive Analysis of the Chemical and Bioactivity Profiles of Endemic Crataegus turcicus Dönmez in Comparison with Other Crataegus Species" Molecules 28, no. 18: 6520. https://doi.org/10.3390/molecules28186520
APA StyleTurnalar Ülger, T., Oçkun, M. A., Guzelmeric, E., Sen, N. B., Sipahi, H., Özhan, Y., Kan, Y., & Yesilada, E. (2023). Comprehensive Analysis of the Chemical and Bioactivity Profiles of Endemic Crataegus turcicus Dönmez in Comparison with Other Crataegus Species. Molecules, 28(18), 6520. https://doi.org/10.3390/molecules28186520






