A Comprehensive Mini-Review on Lignin-Based Nanomaterials for Food Applications: Systemic Advancement and Future Trends
Abstract
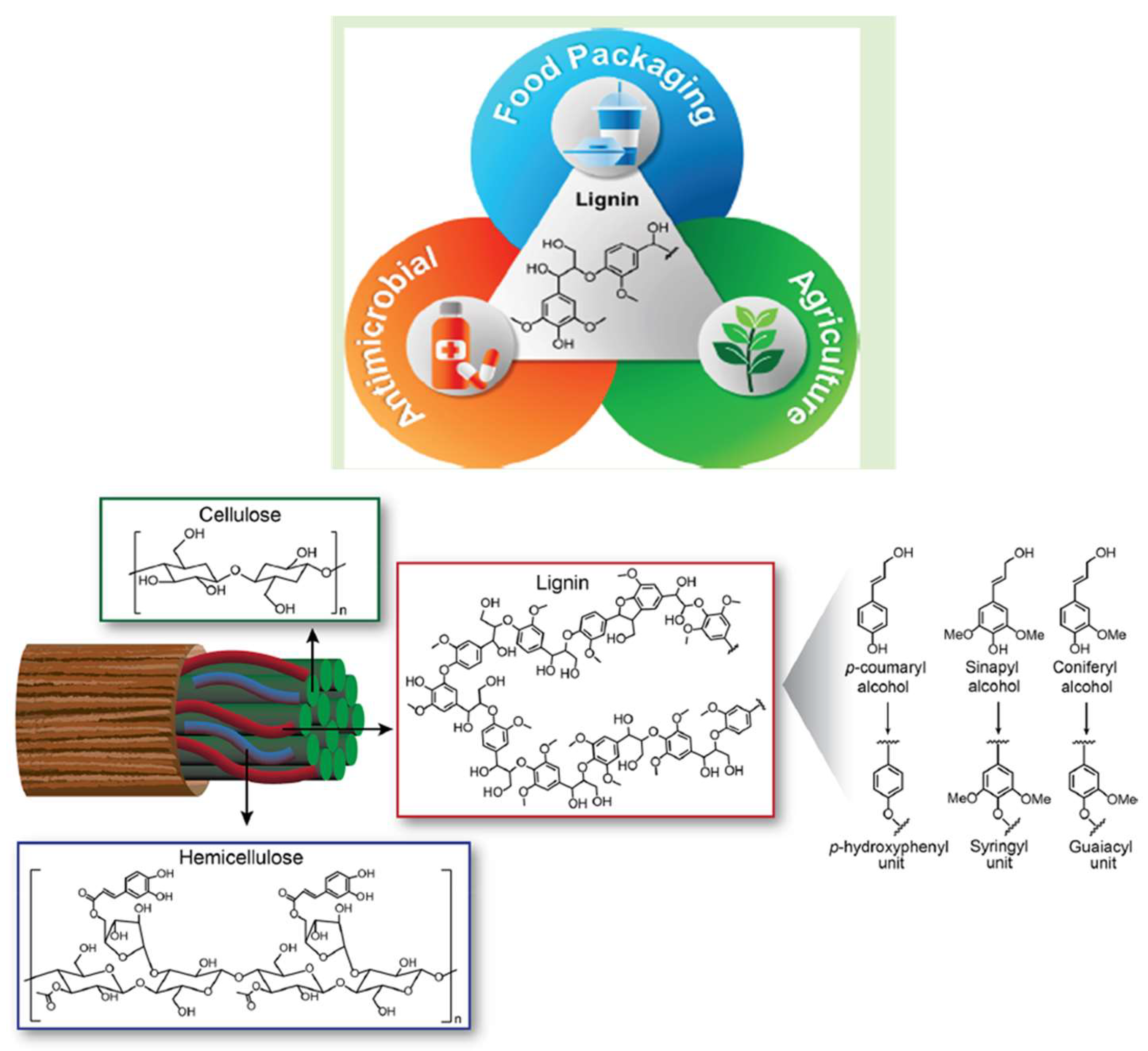
:1. Introduction
2. Biosynthesis of Mono- and Oligo-Lignans and Their Function
3. The Biosynthesis of Lignin Formation
4. Potential Monolignols
5. Opportunities and Obstacles for the Lignin-Valorization Industry in the Food Sector
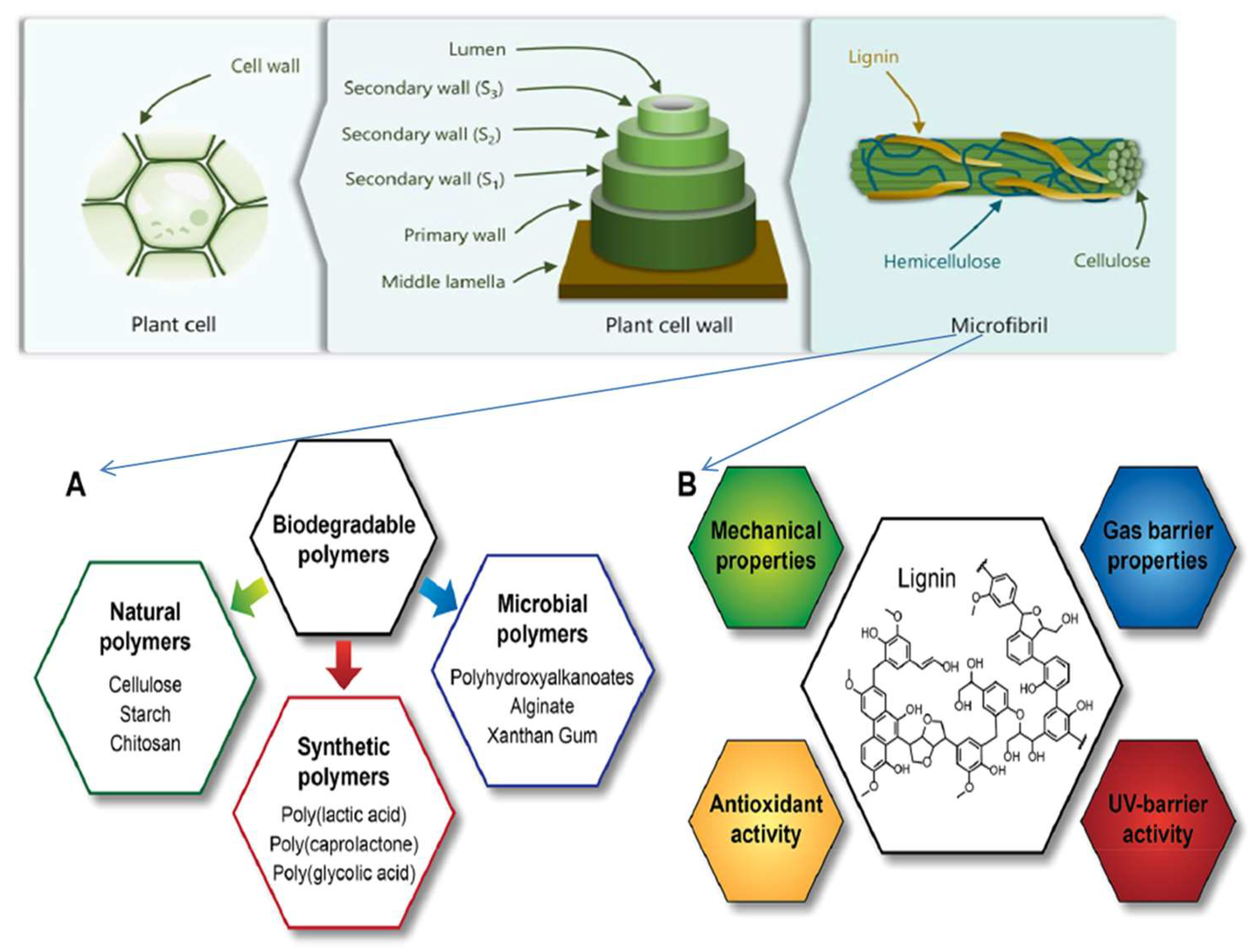
6. Lignin-Based Food Packaging
7. Food Packaging Barrier Concepts
8. Evaluation of Lignin-Based Biopolymer Recyclability
9. The Role of Lignin as an Antimicrobial Agent in Food Preservation
| Lignin Type | Concentration (mg/mL) | Inactivated Pathogen | Reference |
|---|---|---|---|
| DMSO Medium | |||
| Kraft lignin | 15 | Escherichia coli, Staphylococcus aureus, Pseudomonas aeruginosa, Salmonella enteritidis, Bacillus cereus | [60] |
| Pyrolytic lignin | 5 | Staphylococcus aureus, Escherichia coli | [61] |
| Organosolv/Kraft lignin | 1–20 | Aspergillus niger | [62] |
| Organosolv lignin | 0.48–0.025 | Candida parapsilosis, Candida krusei, Candida guilliermondii, Candida albicans, | [63] |
| 0.5, 5, 10 | Aspergillus niger, Saccharomyces cerevisiae | [64] | |
| Cell Culture Medium | |||
| Lignosulfonate | 70 nM–236.6 μM | HIV | [65] |
| 0–0.2 | HIV | [66] | |
| 0–0.5 | HIV, HSV | [67] | |
| Others | |||
| Kraft lignin | 100 | Listeria monocytogenes, Staphylococcus aureus | [68] |
| Lignin- | 0.05 | Encephalomyocarditis virus (EMV) | [69] |
| carbohydrate | 0.5 | Herpes simplex virus (HSV) | [70] |
| complex | 0.1, 2 | EMV, HSV | [71] |
| Ligno-sulfonate | 10 | Human immunodeficiency virus (HIV), HSV | [72] |
10. Challenges and Future Perspectives for the Application of Lignin as Antimicrobial Agent
| Type of Lignin | NP Preparation Method | Loading Strategy | Particle Size (nm) | Antimicrobial Activity | References |
|---|---|---|---|---|---|
| Spherical | |||||
| Kraft, acetylated | Solvent displacement | Entrapment | 160–1348 | S. aureus, S. epidermidis, and E. faecalis | [75,76] |
| Alkali | Solvent displacement | Emulsion | ~200 | Penicillium italicum | [77] |
| Lignosulfonate, PNMA-modified | Self-assembling, chemical reduction | Adsorption | 11 | S. aureus and E. coli | [78] |
| Kraft | Solvent displacement | Ion exchange | 60–200 | S. aureus, E. coli, and P. aeruginosa | [79] |
| Alkali | Emulsion evaporation | Entrapment | 117 | E. coli | [80] |
| Quasi-spherical | |||||
| Lignosulfonate, modified with an azo dye | Chemical reduction to form ZnO | Coating | 21–32 | S. haemolyticus, C. diphtheriae, B. cereus, R. ornithinolytica, S. typhimurium, S. paratyphi, A. fumigatus, A. penicilloides, C. albicans, C. coronatus, and M. cookei | [81] |
| Organosolv | Chemical reduction to form AgNPs | Adsorption | 28–54 | E. coli | [82] |
| Irregular | |||||
| Kraft | Coating of lignin on silica followed by chemical reduction to form AgNP | Adsorption | 30–36 | B. subtilis, S. aureus, P. aeruginosa, E. coli and K. pneumoniae | [83] |
| Kraft | Acid precipitation | Infusion | 40–70 | S. aureus, S. epidermidis, E. coli, and P. aeruginosa | [84] |
11. Conclusions and Prospects
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Singh, S. Energy Crisis and Climate Change: Global Concerns and Their Solutions. In Energy: Crises, Challenges and Solutions; Wiley: Hoboken, NJ, USA, 2021; pp. 1–17. [Google Scholar]
- ArrArrow, K.J. Global Climate Change: A Challenge to Policy. Econ. Voice 2007, 4, 1270. [Google Scholar] [CrossRef]
- Sheldon, R.A.; Brady, D. Green Chemistry, Biocatalysis, and the Chemical Industry of the Future. ChemSusChem 2022, 15, e202102628. [Google Scholar] [CrossRef] [PubMed]
- Pramanik, A.; Chaudhary, A.A.; Sinha, A.; Chaubey, K.K.; Ashraf, M.S.; Basher, N.S.; Rudayni, H.A.; Dayal, D.; Kumar, S. Nanocatalyst-Based Biofuel Generation: An Update, Challenges and Future Possibilities. Sustainability 2023, 15, 6180. [Google Scholar] [CrossRef]
- Baranwal, J.; Barse, B.; Fais, A.; Delogu, G.L.; Kumar, A. Biopolymer: A Sustainable Material for Food and Medical Applications. Polymers 2022, 14, 983. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Wang, C.-G.; Chee, P.L.; Qu, C.; Fok, A.Z.; Yong, F.H.; Ong, Z.L.; Kai, D. Strategies for lignin depolymerization and reconstruction towards functional polymers. Sustain. Energy Fuels 2023, 7, 2953–2973. [Google Scholar] [CrossRef]
- Malode, S.J.; Prabhu, K.K.; Mascarenhas, R.J.; Shetti, N.P.; Aminabhavi, T.M. Recent advances and viability in biofuel production. Energy Convers. Manag. X 2020, 10, 100070. [Google Scholar] [CrossRef]
- Lu, X.; Gu, X.; Shi, Y. A review on lignin antioxidants: Their sources, isolations, antioxidant activities and various applications. Int. J. Biol. Macromol. 2022, 210, 716–741. [Google Scholar] [CrossRef]
- Armah, E.K.; Chetty, M.; Rathilal, S.; Asante-Sackey, D.; Tetteh, E.K. Lignin: Value addition is key to profitable biomass biorefinery. In Handbook of Biofuels; Academic Press: Cambridge, MA, USA, 2022; pp. 233–247. [Google Scholar] [CrossRef]
- Liu, J.; Li, X.; Li, M.; Zheng, Y. Lignin biorefinery: Lignin source, isolation, characterization, and bioconversion. Adv. Bioenergy 2022, 7, 211–270. [Google Scholar] [CrossRef]
- Wei, S.; Li, Z.; Sun, Y.; Zhang, J.; Ge, Y.; Li, Z. A comprehensive review on biomass humification: Recent advances in pathways, challenges, new applications, and perspectives. Renew. Sustain. Energy Rev. 2022, 170, 112984. [Google Scholar] [CrossRef]
- Shelar, A.; Nile, S.H.; Singh, A.V.; Rothenstein, D.; Bill, J.; Xiao, J.; Chaskar, M.; Kai, G.; Patil, R. Recent Advances in Nano-Enabled Seed Treatment Strategies for Sustainable Agriculture: Challenges, Risk Assessment, and Future Perspectives. Nano-Micro Lett. 2023, 15, 1–37. [Google Scholar] [CrossRef]
- Boarino, A.; Klok, H.-A. Opportunities and Challenges for Lignin Valorization in Food Packaging, Antimicrobial, and Agricultural Applications. Biomacromolecules 2023, 24, 1065–1077. [Google Scholar] [CrossRef]
- Dixon, R.A.; Barros, J. Lignin biosynthesis: Old roads revisited and new roads explored. Open Biol. 2019, 9, 190215. [Google Scholar] [CrossRef]
- Zhang, L.; Larsson, A.; Moldin, A.; Edlund, U. Comparison of lignin distribution, structure, and morphology in wheat straw and wood. Ind. Crops Prod. 2022, 187, 115432. [Google Scholar] [CrossRef]
- Sun, R. Lignin Source and Structural Characterization. ChemSusChem 2020, 13, 4385–4393. [Google Scholar] [CrossRef] [PubMed]
- Salminen, J.P.; Wahala, K.; de Freitas, V.; Quideau, S. (Eds.) Recent Advances in Polyphenol Research; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2023; Volume 8. [Google Scholar]
- Holmgren, A.; Brunow, G.; Henriksson, G.; Zhang, L.; Ralph, J. Non-enzymatic reduction of quinone methides during oxidative coupling of monolignols: Implications for the origin of benzyl structures in lignins. Org. Biomol. Chem. 2006, 4, 3456–3461. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K. Lignin characterization: Size, and stability structure. In Natural Polyphenols from Wood: Tannin and Lignin—An Industrial Perspective; Elsevier: Amsterdam, The Netherlands, 2021; p. 147. [Google Scholar]
- Agustiany, E.A.; Rasyidur Ridho, M.; Rahmi, D.N.M.; Madyaratri, E.W.; Falah, F.; Lubis, M.A.R.; Fudholi, A.; Syamani, F.A.; Karungamye, P.; Sohail, A.; et al. Recent developments in lignin modification and its application in lignin-based green composites: A review. Polym. Compos. 2022, 43, 4848–4865. [Google Scholar] [CrossRef]
- Ruwoldt, J. A Critical Review of the Physicochemical Properties of Lignosulfonates: Chemical Structure and Behavior in Aqueous Solution, at Surfaces and Interfaces. Surfaces 2020, 3, 622–648. [Google Scholar] [CrossRef]
- Arvanitoyannis, I.S.; Bosnea, L.A. Recycling of polymeric materials used for food packaging: Current status and perspectives. Food Rev. Int. 2001, 17, 291–346. [Google Scholar] [CrossRef]
- Matthews, C.; Moran, F.; Jaiswal, A.K. A review on European Union’s strategy for plastics in a circular economy and its impact on food safety. J. Clean. Prod. 2020, 283, 125263. [Google Scholar] [CrossRef]
- Vostrejs, P.; Adamcová, D.; Vaverková, M.D.; Enev, V.; Kalina, M.; Machovsky, M.; Sourkova, M.; Marova, I.; Kovalcik, A. Active biodegradable packaging films modified with grape seeds lignin. RSC Adv. 2020, 10, 29202–29213. [Google Scholar] [CrossRef]
- Aadil, K.R.; Prajapati, D.; Jha, H. Improvement of physcio-chemical and functional properties of alginate film by Acacia lignin. Food Packag. Shelf Life 2016, 10, 25–33. [Google Scholar] [CrossRef]
- Ji, M.; Li, J.; Li, F.; Wang, X.; Man, J.; Li, J.; Zhang, C.; Peng, S. A biodegradable chitosan-based composite film reinforced by ramie fibre and lignin for food packaging. Carbohydr. Polym. 2022, 281, 119078. [Google Scholar] [CrossRef] [PubMed]
- Domenek, S.; Louaifi, A.; Guinault, A.; Baumberger, S. Potential of lignins as antioxidant additive in active biodegradable packaging materials. J. Polym. Environ. 2013, 21, 692–701. [Google Scholar] [CrossRef]
- Bhat, R.; Abdullah, N.; Din, R.H.; Tay, G.-S. Producing novel sago starch based food packaging films by incorporating lignin isolated from oil palm black liquor waste. J. Food Eng. 2013, 119, 707–713. [Google Scholar] [CrossRef]
- Shankar, S.; Reddy, J.P.; Rhim, J.-W. Effect of lignin on water vapor barrier, mechanical, and structural properties of agar/lignin composite films. Int. J. Biol. Macromol. 2015, 81, 267–273. [Google Scholar] [CrossRef]
- Guo, Y.; Tian, D.; Shen, F.; Yang, G.; Long, L.; He, J.; Song, C.; Zhang, J.; Zhu, Y.; Huang, C.; et al. Transparent Cellulose/Technical Lignin Composite Films for Advanced Packaging. Polymers 2019, 11, 1455. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.-Q.; Ye, D.-Z.; Tang, J.-B.; Zhang, L.-J.; Zhang, X. From waste to functional additives: Thermal stabilization and toughening of PVA with lignin. RSC Adv. 2016, 6, 13797–13802. [Google Scholar] [CrossRef]
- Silva, R.R.A.; Marques, C.S.; Arruda, T.R.; Teixeira, S.C.; de Oliveira, T.V. Biodegradation of Polymers: Stages, Measurement, Standards and Prospects. Macromol 2023, 3, 371–399. [Google Scholar] [CrossRef]
- Kai, D.; Tan, M.J.; Chee, P.L.; Chua, Y.K.; Yap, Y.L.; Loh, X.J. Towards lignin-based functional materials in a sustainable world. Green Chem. 2016, 18, 1175–1200. [Google Scholar] [CrossRef]
- Nagarajan, K.; Balaji, A.; Rajan, S.T.K.; Ramanujam, N. Preparation of bio-eco based cellulose nanomaterials from used disposal paper cups through citric acid hydrolysis. Carbohydr. Polym. 2020, 235, 115997. [Google Scholar] [CrossRef]
- Yang, W.; Weng, Y.; Puglia, D.; Qi, G.; Dong, W.; Kenny, J.M.; Ma, P. Poly (lactic acid)/lignin films with enhanced toughness and anti-oxidation performance for active food packaging. Int. J. Biol. Macromol. 2020, 144, 102–110. [Google Scholar] [CrossRef]
- Parvathy, G.; Sethulekshmi, A.S.; Jayan, J.S.; Raman, A.; Saritha, A. Lignin based nano-composites: Synthesis and applications. Process Saf. Environ. Prot. 2021, 145, 395–410. [Google Scholar]
- Chuensangjun, C.; Kanomata, K.; Kitaoka, T.; Chisti, Y.; Sirisansaneeyakul, S. Surface-modified cellulose nanofibers-graft-poly (lactic acid) s made by ring-opening polymerization of l-lactide. J. Polym. Environ. 2019, 27, 847–861. [Google Scholar] [CrossRef]
- Vahabi, H.; Brosse, N.; Abd Latif, N.; Fatriasari, W.; Solihat, N.; Hashim, R.; Saeb, M. Nanolignin in materials science and technology—Does flame retardancy matter? In Biopolymeric Nanomaterials; Elsevier: Amsterdam, The Netherlands, 2021; pp. 515–559. [Google Scholar]
- Kirschweng, B.; Tátraaljai, D.; Földes, E.; Pukánszky, B. Natural antioxidants as stabilizers for polymers. Polym. Degrad. Stab. 2017, 145, 25–40. [Google Scholar] [CrossRef]
- Anushikha; Gaikwad, K.K. Lignin as a UV blocking, antioxidant, and antimicrobial agent for food packaging applications. Biomass Convers. Biorefinery 2023, 1–13. [Google Scholar] [CrossRef]
- Cavallo, E.; He, X.; Luzi, F.; Dominici, F.; Cerrutti, P.; Bernal, C.; Foresti, M.L.; Torre, L.; Puglia, D. UV Protective, Antioxidant, Antibacterial and Compostable Polylactic Acid Composites Containing Pristine and Chemically Modified Lignin Nanoparticles. Molecules 2021, 26, 126. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Li, Q.; Wu, H.; Zhou, Z.; Feng, S.; Deng, P.; Zou, H.; Tian, D.; Lu, C. Sustainable Starch/Lignin Nanoparticle Composites Biofilms for Food Packaging Applications. Polymers 2023, 15, 1959. [Google Scholar] [CrossRef]
- Coles, R.; McDowell, D.; Kirwan, M.J. Food Packaging Technology; CRC Press: Boca Raton, FL, USA, 2003; Volume 5. [Google Scholar]
- Lambert, S.; Wagner, M. Environmental performance of bio-based and biodegradable plastics: The road ahead. Chem. Soc. Rev. 2017, 46, 6855–6871. [Google Scholar] [CrossRef]
- Versino, F.; Ortega, F.; Monroy, Y.; Rivero, S.; López, O.V.; García, M.A. Sustainable and bio-based food packaging: A review on past and current design innovations. Foods 2023, 12, 1057. [Google Scholar] [CrossRef]
- Polman, E.M.; Gruter, G.J.M.; Parsons, J.R.; Tietema, A. Comparison of the aerobic biodegradation of biopolymers and the corresponding bioplastics: A review. Sci. Total Environ. 2021, 753, 141953. [Google Scholar] [CrossRef]
- Sethupathy, S.; Morales, G.M.; Gao, L.; Wang, H.; Yang, B.; Jiang, J.; Sun, J.; Zhu, D. Lignin valorization: Status, challenges and opportunities. Bioresour. Technol. 2022, 347, 126696. [Google Scholar] [CrossRef]
- Mujtaba, M.; Lipponen, J.; Ojanen, M.; Puttonen, S.; Vaittinen, H. Trends and challenges in the development of bio-based barrier coating materials for paper/cardboard food packaging; a review. Sci. Total Environ. 2022, 851, 158328. [Google Scholar] [CrossRef]
- Löckner, C. Sustainable Food Packaging in Austria—A Multi-Criteria Mapping of Potential Implementation Options. Master’s Thesis, University of Graz, Graz, Austria, 2022. [Google Scholar]
- Goswami, T.K.; Mangaraj, S. Advances in polymeric materials for modified atmosphere packaging (MAP). In Multifunctional and Nanoreinforced Polymers for Food Packaging; Woodhead Publishing: Cambridge, UK, 2011; pp. 163–242. [Google Scholar]
- Asgher, M.; Qamar, S.A.; Bilal, M.; Iqbal, H.M. Bio-based active food packaging materials: Sustainable alternative to conventional petrochemical-based packaging materials. Food Res. Int. 2020, 137, 109625. [Google Scholar] [CrossRef] [PubMed]
- Priyadarshi, R.; Roy, S.; Ghosh, T.; Biswas, D.; Rhim, J.W. Antimicrobial nanofillers reinforced biopolymer composite films for active food packaging applications—A review. Sustain. Mater. Technol. 2022, 32, e00353. [Google Scholar] [CrossRef]
- Tiwari, P.; Bajpai, M.; Sharma, A. Antimicrobials from Medicinal Plants: Key Examples, Success Stories and Prospects in Tackling Antibiotic Resistance. Lett. Drug Des. Discov. 2023, 20, 420–438. [Google Scholar] [CrossRef]
- Cheynier, V.; Comte, G.; Davies, K.M.; Lattanzio, V.; Martens, S. Plant phenolics: Recent advances on their biosynthesis, genetics, and ecophysiology. Plant Physiol. Biochem. 2013, 72, 1–20. [Google Scholar] [CrossRef]
- Barapatre, A.; Aadil, K.R.; Jha, H. Synergistic antibacterial and antibiofilm activity of silver nanoparticles biosynthesized by lignin-degrading fungus. Bioresour. Bioprocess. 2016, 3, 8. [Google Scholar] [CrossRef]
- Cowan, M.M. Plant products as antimicrobial agents. Clin. Microbiol. Rev. 1999, 12, 564–582. [Google Scholar] [CrossRef] [PubMed]
- Morales, A.; Labidi, J.; Gullón, P.; Astray, G. Synthesis of advanced biobased green materials from renewable biopolymers. Curr. Opin. Green Sustain. Chem. 2021, 29, 100436. [Google Scholar] [CrossRef]
- Martínez, V.; Mitjans, M.; Pilar Vinardell, M. Pharmacological applications of lignins and lignins related compounds: An overview. Curr. Org. Chem. 2012, 16, 1863–1870. [Google Scholar] [CrossRef]
- Vinardell, M.P.; Mitjans, M. Lignins and their derivatives with beneficial effects on human health. Int. J. Mol. Sci. 2017, 18, 1219. [Google Scholar] [CrossRef]
- Lourençon, T.V.; de Lima, G.G.; Ribeiro, C.S.; Hansel, F.A.; Maciel, G.M.; da Silva, K.; Winnischofer, S.M.B.; de Muniz, G.I.B.; Magalhães, W.L. Antioxidant, antibacterial and antitumoural activities of kraft lignin from hardwood fractionated by acid precipitation. Int. J. Biol. Macromol. 2021, 166, 1535–1542. [Google Scholar] [CrossRef]
- Matos, M.; Claro, F.C.; Lima, T.A.; Avelino, F.; Hansel, F.A.; Maciel, G.M.; Lomonaco, D.; Magalhães, W.L. Acetone: Water fractionation of pyrolytic lignin improves its antioxidant and antibacterial activity. J. Anal. Appl. Pyrolysis 2021, 156, 105175. [Google Scholar] [CrossRef]
- Gordobil, O.; Herrera, R.; Yahyaoui, M.; İlk, S.; Kaya, M.; Labidi, J. Potential use of kraft and organosolv lignins as a natural additive for healthcare products. RSC Adv. 2018, 8, 24525–24533. [Google Scholar] [CrossRef]
- de Melo, C.M.L.; da Cruz Filho, I.J.; de Sousa, G.F.; de Souza Silva, G.A.; do Nascimento Santos, D.K.D.; da Silva, R.S.; de Sousa, B.R.; de Lima Neto, R.G.; do Carmo Alves de Lima, M.; de Moraes Rocha, G.J. Lignin isolated from Caesalpinia pulcherrima leaves has antioxidant, antifungal and immunostimulatory activities. Int. J. Biol. Macromol. 2020, 162, 1725–1733. [Google Scholar] [CrossRef] [PubMed]
- García, A.; Spigno, G.; Labidi, J. Antioxidant and biocide behaviour of lignin fractions from apple tree pruning residues. Ind. Crops Prod. 2017, 104, 242–252. [Google Scholar] [CrossRef]
- Oeyen, M.; Noppen, S.; Vanhulle, E.; Claes, S.; Myrvold, B.O.; Vermeire, K.; Schols, D. A unique class of lignin derivatives displays broad anti-HIV activity by interacting with the viral envelope. Virus Res. 2019, 274, 197760. [Google Scholar] [CrossRef]
- Qiu, M.; Wang, Q.; Chu, Y.; Yuan, Z.; Song, H.; Chen, Z.; Wu, Z. Lignosulfonic acid exhibits broadly anti-HIV-1 activity–potential as a microbicide candidate for the prevention of HIV-1 sexual transmission. PLoS ONE 2012, 7, e35906. [Google Scholar] [CrossRef]
- Gordts, S.C.; Ferir, G.; D’huys, T.; Petrova, M.I.; Lebeer, S.; Snoeck, R.; Andrei, G.; Schols, D. The low-cost compound lignosulfonic acid (LA) exhibits broad-spectrum anti-HIV and anti-HSV activity and has potential for microbicidal applications. PLoS ONE 2015, 10, e0131219. [Google Scholar] [CrossRef]
- Dong, X.; Dong, M.; Lu, Y.; Turley, A.; Jin, T.; Wu, C. Antimicrobial and antioxidant activities of lignin from residue of corn stover to ethanol production. Ind. Crops Prod. 2011, 34, 1629–1634. [Google Scholar] [CrossRef]
- Li, R.; Ouda, R.; Kimura, C.; Narita, R.; Nishimura, H.; Fujita, T.; Watanabe, T. Conversion of beech wood into antiviral lignin–carbohydrate complexes by microwave acidolysis. ACS Sustain. Chem. Eng. 2021, 9, 9248–9256. [Google Scholar] [CrossRef]
- Zhang, Y.; But, P.P.-H.; Ooi, V.E.-C.; Xu, H.-X.; Delaney, G.D.; Lee, S.H.; Lee, S.F. Chemical properties, mode of action, and in vivo anti-herpes activities of a lignin–carbohydrate complex from Prunella vulgaris. Antivir. Res. 2007, 75, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-B.; Yamagishi, C.; Hayashi, K.; Hayashi, T. Antiviral and immunostimulating effects of lignin-carbohydrate-protein complexes from Pimpinella anisum. Biosci. Biotechnol. Biochem. 2011, 75, 459–465. [Google Scholar] [CrossRef]
- Fukuchi, K.; Koshikawa, T.; Asai, D.; Inomata, M.; Sakagami, H.; Takemura, H.; Kanamoto, T.; Aimi, H.; Kikkawa, Y. Lignosulfonate Rapidly Inactivates Human Immunodeficiency and Herpes Simplex Viruses. Medicines 2021, 8, 56. [Google Scholar] [CrossRef] [PubMed]
- Cassoni, A.C.; Costa, P.; Vasconcelos, M.W.; Pintado, M. Systematic review on lignin valorization in the agro-food system: From sources to applications. J. Environ. Manag. 2022, 317, 115258. [Google Scholar] [CrossRef] [PubMed]
- Chaisuwan, W.; Phimolsiripol, Y.; Chaiyaso, T.; Techapun, C.; Leksawasdi, N.; Jantanasakulwong, K.; Rachtanapun, P.; Wangtueai, S.; Sommano, S.R.; You, S.; et al. The Antiviral Activity of Bacterial, Fungal, and Algal Polysaccharides as Bioactive Ingredients: Potential Uses for Enhancing Immune Systems and Preventing Viruses. Front. Nutr. 2021, 8, 772033. [Google Scholar] [CrossRef]
- Maldonado-Carmona, N.; Marchand, G.; Villandier, N.; Ouk, T.-S.; Pereira, M.M.; Calvete, M.J.F.; Calliste, C.A.; Żak, A.; Piksa, M.; Pawlik, K.J.; et al. Porphyrin-Loaded Lignin Nanoparticles Against Bacteria: A Photodynamic Antimicrobial Chemotherapy Application. Front. Microbiol. 2020, 11, 606185. [Google Scholar] [CrossRef]
- Maldonado-Carmona, N.; Ouk, T.-S.; Villandier, N.; Calliste, C.A.; Calvete, M.J.F.; Pereira, M.M.; Leroy-Lhez, S. Photophysical and Antibacterial Properties of Porphyrins Encapsulated inside Acetylated Lignin Nanoparticles. Antibiotics 2021, 10, 513. [Google Scholar] [CrossRef]
- Chen, L.; Shi, Y.; Gao, B.; Zhao, Y.; Jiang, Y.; Zha, Z.; Xue, W.; Gong, L. Lignin Nanoparticles: Green Synthesis in a γ-Valerolactone/Water Binary Solvent and Application to Enhance Antimicrobial Activity of Essential Oils. ACS Sustain. Chem. Eng. 2019, 8, 714–722. [Google Scholar] [CrossRef]
- Lü, Q.-F.; Zhang, J.-Y.; Yang, J.; He, Z.-W.; Fang, C.-Q.; Lin, Q. Self-Assembled Poly(N-methylaniline)-Lignosulfonate Spheres: From Silver-Ion Adsorbent to Antimicrobial Material. Chem. Eur. J. 2013, 19, 10935–10944. [Google Scholar] [CrossRef] [PubMed]
- Lintinen, K.; Luiro, S.; Figueiredo, P.; Sakarinen, E.; Mousavi, Z.; Seitsonen, J.; Rivière, G.N.S.; Mattinen, U.; Niemelä, M.; Tammela, P.; et al. Antimicrobial Colloidal Silver–Lignin Particles via Ion and Solvent Exchange. ACS Sustain. Chem. Eng. 2019, 7, 15297–15303. [Google Scholar] [CrossRef]
- Paudel, S.; Peña-Bahamonde, J.; Shakiba, S.; Astete, C.E.; Louie, S.M.; Sabliov, C.M.; Rodrigues, D.F. Prevention of infection caused by enteropathogenic E. coli O157:H7 in intestinal cells using enrofloxacin entrapped in polymer based nanocarriers. J. Hazard. Mater. 2021, 414, 125454. [Google Scholar] [CrossRef]
- Jose, L.M.; Kuriakose, S.; Mathew, T. Development of photoresponsive zinc oxide nanoparticle—Encapsulated lignin functionalized with 2-[(E)-(2-hydroxy naphthalen-1-yl) diazenyl] benzoic acid: A promising photoactive agent for antimicrobial photodynamic therapy. Photodiagn. Photodyn. Ther. 2021, 36, 102479. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.-F.; Xu, L.; Qin, X.-L. Efficient antibacterial silver nanoparticles composite using lignin as a template. J. Compos. Mater. 2014, 49, 2329–2335. [Google Scholar] [CrossRef]
- Klapiszewski, Ł.; Rzemieniecki, T.; Krawczyk, M.; Malina, D.; Norman, M.; Zdarta, J.; Majchrzak, I.; Dobrowolska, A.; Czaczyk, K.; Jesionowski, T. Kraft lignin/silica–AgNPs as a functional material with antibacterial activity. Colloids Surf. B Biointerfaces 2015, 134, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Richter, A.P.; Brown, J.S.; Bharti, B.; Wang, A.; Gangwal, S.; Houck, K.; Hubal, E.A.C.; Paunov, V.N.; Stoyanov, S.D.; Velev, O.D. An environmentally benign antimicrobial nanoparticle based on a silver-infused lignin core. Nat. Nanotechnol. 2015, 10, 817–823. [Google Scholar] [CrossRef]
| Process Type | Extraction Process Conditions | Solubility | Molecular Weight (kDa) | Poly-Dispersity | Impurities | Reference | |
|---|---|---|---|---|---|---|---|
| pH | Temperature | ||||||
| Sulfur process (Sulfur lignin) | |||||||
| Kraft lignin | NaOH, Na2S | 170 °C | Alkali | 0.1–3.0 | 2.5–3.5 | Sulfur (1–3%) and ash (1–2%) | [20] |
| Lignosulfonates | SO2, Na+/Ca+/Mg+/NH4+ | 140 °C | Water | 20–50 | 4.2–8.0 | Sulfur (3.5–8%) and ash (4–8%) | [20] |
| Sulfur-free process (Non-Sulfur lignin) | |||||||
| Organosolv lignin | acetic acid/formic acid/organic solvents | 150–200 °C | Wide range of Organic solvents | 0.5–4.0 | 1.3–4.0 | Carbohydrates (1–3%) and ash (1.7%) | [21] |
| Soda lignin | NaOH | 150–170 °C | Alkaline media pH > 10 | 0.8–3.0 | 2.5–3.5 | Carbohydrates (1.5–3%) and ash (0.7–2.3%) | [21] |
| Lignin Type | Polymer Matrix | Tensile Strength (%) | Elongation at Break (%) | Gas Barrier Properties | Antioxidant Activity | UV-Barrier Activity | References |
|---|---|---|---|---|---|---|---|
| Soda lignin | PHB/PHA | 85 ↑ | 77 ↓ | Yes/Present | Yes/Present | Yes/Present | [24] |
| Alginate | 74 ↓ | - | Yes/Present | Yes/Present | Yes/Present | [25] | |
| Chitosan | 66 ↑ | 26 ↓ | - | Yes/Present | - | [26] | |
| Poly(lactide) | 27 ↓ | 43 ↓ | - | Yes/Present | - | [27] | |
| Kraft lignin | Starch | 33 ↑ | 30 ↓ | Yes/Present | - | - | [28] |
| Agar | 15 ↑ | 18 ↓ | Yes/Present | - | Yes/Present | [29] | |
| Organosolv lignin | Cellulose | - | - | - | Yes/Present | Yes/Present | [30] |
| Lignosulfonate | Poly(vinyl alcohol) | 40 ↑ | 40 ↓ | - | - | - | [31] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chelliah, R.; Wei, S.; Vijayalakshmi, S.; Barathikannan, K.; Sultan, G.; Liu, S.; Oh, D.-H. A Comprehensive Mini-Review on Lignin-Based Nanomaterials for Food Applications: Systemic Advancement and Future Trends. Molecules 2023, 28, 6470. https://doi.org/10.3390/molecules28186470
Chelliah R, Wei S, Vijayalakshmi S, Barathikannan K, Sultan G, Liu S, Oh D-H. A Comprehensive Mini-Review on Lignin-Based Nanomaterials for Food Applications: Systemic Advancement and Future Trends. Molecules. 2023; 28(18):6470. https://doi.org/10.3390/molecules28186470
Chicago/Turabian StyleChelliah, Ramachandran, Shuai Wei, Selvakumar Vijayalakshmi, Kaliyan Barathikannan, Ghazala Sultan, Shucheng Liu, and Deog-Hwan Oh. 2023. "A Comprehensive Mini-Review on Lignin-Based Nanomaterials for Food Applications: Systemic Advancement and Future Trends" Molecules 28, no. 18: 6470. https://doi.org/10.3390/molecules28186470
APA StyleChelliah, R., Wei, S., Vijayalakshmi, S., Barathikannan, K., Sultan, G., Liu, S., & Oh, D.-H. (2023). A Comprehensive Mini-Review on Lignin-Based Nanomaterials for Food Applications: Systemic Advancement and Future Trends. Molecules, 28(18), 6470. https://doi.org/10.3390/molecules28186470








