Abstract
Antibody engineering has developed into a wide-reaching field, impacting a multitude of industries, most notably healthcare and diagnostics. The seminal work on developing the first monoclonal antibody four decades ago has witnessed exponential growth in the last 10–15 years, where regulators have approved monoclonal antibodies as therapeutics and for several diagnostic applications, including the remarkable attention it garnered during the pandemic. In recent years, antibodies have become the fastest-growing class of biological drugs approved for the treatment of a wide range of diseases, from cancer to autoimmune conditions. This review discusses the field of therapeutic antibodies as it stands today. It summarizes and outlines the clinical relevance and application of therapeutic antibodies in treating a landscape of diseases in different disciplines of medicine. It discusses the nomenclature, various approaches to antibody therapies, and the evolution of antibody therapeutics. It also discusses the risk profile and adverse immune reactions associated with the antibodies and sheds light on future applications and perspectives in antibody drug discovery.
1. Introduction
Antibodies, or immunoglobulins, are the effector molecules of the humoral immune response. They are antigen-neutralizing molecules, produced by plasma B-cells in our body, that protect us by destroying pathogens and preventing their propagation. The main functions performed by antibodies are neutralization, opsonization, activation of the complement pathway, and antibody-dependent cell-mediated cytotoxicity. With the advancement of contemporary molecular science, their natural potential has been harnessed to develop therapeutics to treat diseases ranging from cancer to autoimmune diseases.
Antibody engineering is regarded as a rich seam, and its scope is quite evident, as seen by the great variety of engineered antibodies on the market. As a means of natural defense, our immune systems produce antibodies to help us circumvent infections and diseases. Today, therapeutic antibodies are the most well-characterized and potent weapons used by physicians to fight many diseases. Protein engineering has been extensively applied to modify the properties and functions of antibodies [1]. The landmark work in developing the first monoclonal antibodies [2] and producing the first FDA-approved antibody, muromonab-CD3 (Orthoclone, OKT3, developed for acute transplant rejection) [3], paved the way for more than 100 therapeutic and diagnostic antibodies.
The ‘big 5’ that drive the therapeutic antibody industry are infliximab, adalimumab, trastuzumab, bevacizumab, and rituximab. Infliximab and adalimumab are specific for tumor necrosis factor (TNF) and are used for treating Crohn’s disease, rheumatoid arthritis, and plaque psoriasis. Trastuzumab and bevacizumab are specific to human epidermal growth factor receptor 2 (HER2) and vascular endothelial growth factor A (VEGFA), respectively, and are used for the treatment of different cancers. Lastly, rituximab is a CD20-specific antibody used for both rheumatoid arthritis and non-Hodgkin’s lymphoma. Antibodies generate revenue in the millions, accounting for the highest-earning category in biological drugs. The main indications for monoclonal antibodies are cancer and autoimmune diseases, collectively making up ~78% of the total antibody sales in the US [4].
1.1. Antibody Structure and Its Types
A fully assembled and functional antibody (with a molecular weight of ~150 kDa) consists of four polypeptide chains, i.e., two identical light and two identical heavy chains (Figure 1). The sizes of the light and heavy chains are 25 and 50 kDa, respectively. The two chains are joined to each other by covalent disulfide linkages, along with some non-covalent interactions like hydrophobic, ionic, salt bridging, and hydrogen bonding interactions that contribute to the assembly of the structure. The light and heavy chains further comprise two (a variable, VL and a constant, CL) and four immunoglobulin (a variable, VH and three constants, CH1, CH2, and CH3) domains, respectively. The variable domains comprise the amino terminals of both chains. The amino terminal varies and changes in specificity according to the antigen. These variable domains (VH and VL) assemble to form the antigen binding site. In the variable domain, the region that exhibits hypervariability is called the complementarity determining region (CDR), and the region in the variable domain that remains largely the same or unchanged is called the framework region (FW). There are three CDRs each in the heavy and light chains. They are present in connecting loops projecting out of the β strands, arranged in two sheets to form a β sandwich with Greek-key topology. The HCDR3 (heavy-chain complementarity determining region 3) is the most variable. The region beyond the variable domain is called the constant region, comprising the immunoglobulin domains (one, CL and three, CH1, CH2, and CH3). The antibody comprises six immunoglobulin domains (two in the light chain and four in the heavy chain), namely, variable light (VL), constant light (CL), variable heavy (VH), and constant heavy (CH1, CH2, and CH3). The general structure of this domain consists of a sandwich of two β sheets composed of antiparallel β strands connected to each other with a disulfide bond.
There are various versions of antibodies, ranging from completely unaltered antibodies either from mice (murine) or humans to altered versions like chimeric, humanized, and fragmented antibodies produced via protein engineering approaches (Figure 1). Murine monoclonal antibodies, derived from mice and rats of the Muridae family, are IgGs that kicked off the modern development of therapeutic antibodies. They have a similar structure to human antibodies, with two heavy chains and two light chains forming a Y-shaped molecule [5]. Despite similarities, there are differences in the sequence and glycosylation of the constant domains, which lead to an immunogenic response. The antibody also contains distinct CDR-H3 regions in the variable heavy domain, contributing to differences in amino acid composition and immune response [6]. Chimeric antibodies often contain human constant domains and murine variable domains [7]. Humanized antibodies are largely human antibodies with murine hypervariable regions that underwent targeted mutations grafted onto the variable domains [8,9].
Camelid antibodies, derived from camels, llamas, and alpacas, are notably composed of only a heavy-chain variable domain (VHH), lacking the CH1 chain found in human antibodies [10]. Owing to their small size, these are often termed nanobodies, and they are an example of the fragmented antibodies pursued by antibody designers [11]. Like the variable new antigen receptor (VNAR) [12] found in sharks, the single VHH domain was found to exhibit the same affinity as an entire human antibody [13]. The high affinity of VHH for a large repertoire of sequences may be due to a long CDR3 loop with hypervariable regions slightly different from those in mice and humans [14]. It has been thought that the paratopes of camelid antibodies can uniquely bind epitopes on concave surfaces, which is distinct from the binding of human antibodies [15]. This may be attributed to the presence of a non-canonical disulfide bond in addition to the conserved or canonical disulfide bond. The non-canonical disulfide bond between CDR1 and CDR3 is a distinguishing feature of VHHs and is hypothesized to reduce the entropy associated with the immobilization of the CDR3 loop upon antigen binding [16].
Examples of antibody fragments are the single-chain fragment variable (scFv) that comprises a variable heavy and variable light domain (VH + VL) connected by a linker; the fragment antibody (Fab), which has a variable part and a constant heavy domain 1 from both light and heavy chains (VH + CH1 and VL + CL); or the single domain variable heavy domain (VHH) from camelids. These antibodies can also be modified by linking them to radionuclides, enzymes, drugs, or toxins or by adding elements (bispecific antibodies or CAR-T) to them to help improve their effector responses. The next section provides more details about these modification methods.
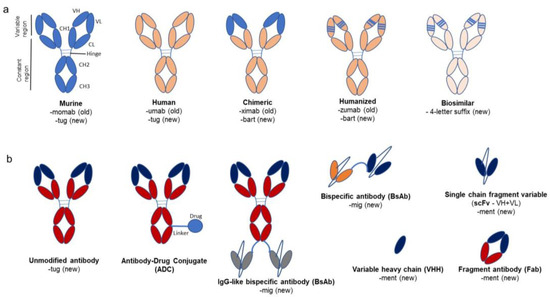
Figure 1.
Overview of monoclonal antibodies and their variants with old and new naming conventions. (a) shows murine, human, chimeric, and humanized antibodies. The chimeric antibody has a variable region of murine origin (light blue) and a constant region sourced from a human antibody (coral orange). The humanized antibody is mostly human except for the complementarity-determining regions (CDRs, light blue lines) or hypervariable regions, which are of murine origin. The old and new nomenclature for antibodies are mentioned beneath each picture. The murine and human antibodies are unaltered, so they contain the stem “tug” compared to the chimeric and humanized antibodies, which carry the stem “bart”. The biosimilars have a random 4-letter suffix. (b) shows the variants of antibodies available, viz. antibody-drug conjugate (ADC), bispecific antibodies, fragment antibodies (with variable (VL + VH) and constant domain 1 (CH1 and CL) from both heavy and light chains), and single-chain fragment antibodies like single-chain fragment variable (scFv − VH + VL) and variable heavy domain (VHH). This figure is based on a paper by Wang et al. (2022) [17].
1.2. Types of Therapy with Antibodies
Antibodies have been used in different ways to target the diseases listed in Figure 2 (adapted from Carter 2001 [18], and Lu et al. 2020 [19]). These fall into three main categories viz., naked antibody, immunoconjugate, and multistep targeting. Naked antibodies kill the target cell by antibody-dependent cell-mediated cytotoxicity (ADCC) or complement-dependent cytotoxicity (CDC). Immunoconjugates have antibodies tagged with a radionuclide, drug, toxin, cytokine, or enzyme. In radioimmunotherapy, radioactively labeled murine antibodies specific to cellular antigens are utilized to target lymphomas and radio-sensitive malignancies. Murine antibodies are chosen based on their high immunogenicity and rapid tumor clearance, thereby limiting radiation exposure. I-131-Tositumomab is one such example and has been used in the treatment of non-Hodgkin’s lymphoma [20]. Another immunoconjugate therapy is antibody-directed enzyme prodrug therapy (ADEPT), which utilizes cancer antibodies linked to drug-activating enzymes to target malignant cells. A nontoxic prodrug, a substrate for the enzyme, is administered and is cleaved by the enzyme to release an inactivating component from the prodrug, generating a potent cytotoxic agent or molecule that can kill cancer cells. There has been limited success in clinical trials of ADEPT [21]. Antibodies are directly linked to drugs to generate antibody-drug conjugates (ADCs), which have the potential to kill the target cells (e.g., cancerous cells), sparing normal cells. So far, twelve ADCs have received market approval (all for oncotherapies), and several investigational ADCs are currently in pre-clinical and clinical trials. Additionally, antibodies are conjugated to liposomes (forming immunoliposomes), which carry the drug or therapeutic nucleotides through the body. Immunoliposomes are effectively used in vivo to direct tumor-suppressing genes into tumors, utilizing an antibody fragment targeted to the human transferrin receptor. Gene delivery through immunoliposomes has been accomplished in brain and breast cancer tissue [22]. The third category, bispecific antibodies, consists of antibodies engineered to have specificity for two ligands or receptors, therefore enhancing their therapeutic effect. Bispecific antibodies help mediate antibody-engaged effector functions [23]. Finally, CAR-T involves the insertion of a gene for a chimeric T-cell receptor antibody that targets specific markers on T-cells in a way such that the engineered cells target and kill the cancer cell [24]. CAR-T antibodies have received a great deal of attention recently, owing to their clinical benefits and success with cancer patients [25,26].
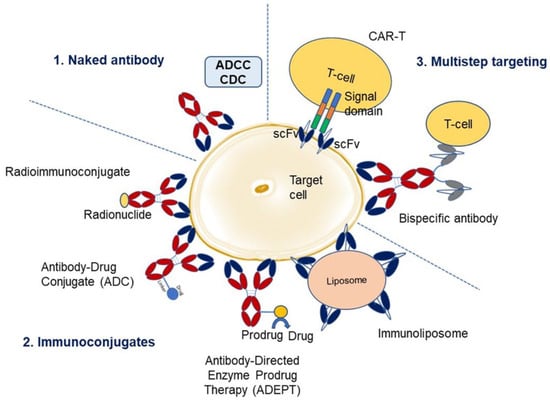
Figure 2.
Overview of monoclonal antibody therapies. There are three main categories. The first category utilizes naked antibody and kills the target cell by antibody-dependent cell-mediated cytotoxicity (ADCC) and complement-dependent cytotoxicity (CDC). The second category comprises immunoconjugates where an antibody is linked to a radionuclide (radioimmunoconjugate), drug (antibody-drug conjugate), enzyme (antibody-directed enzyme prodrug therapy, ADEPT), or liposome (immunoliposome). The third category utilizes a multistep targeting approach where the antibodies are engineered to be specific either to the chimeric T-cell signaling domain/receptor (CAR-T) or T-cell marker (bispecific antibodies), along with specificity towards target cell for enhanced effector function (adapted from Carter 2001 [18], and Lu et al. 2020 [19]).
1.3. Evolution of Antibody Therapeutics
Production of therapeutic antibody drugs has evolved from the use of murine antibodies, first created via hybridoma technology, to the latest humanized or human versions using transgenic human-mice platforms [17]. The first monoclonal antibodies developed in mouse models [2,3] were found to be immunogenic due to their foreign origin and produced a strong human anti-mouse antibody (HAMA) response in patients [27], which was observed as neurotoxicity (post-HAMA response) in patients who received muromonab-CD3 [28]. To circumvent these problems, antibodies were designed as chimeric variants, which were structures like human antibodies that also retained binding properties due to the use of murine variable regions. The first United States (US) Federal Drug Administration (FDA)-approved chimeric antibody, Abciximab (an anti-GPIIb/IIIa Fab, approved in 1994), was targeted towards platelet aggregation [29] and displayed a resolved HAMA response. However, it needed further optimization, as the variable region was of murine origin and could elicit immunogenicity. This was followed by efforts to produce a humanized antibody, where the complementary-determining regions (CDRs) were grafted onto the human antibody scaffold [30,31]. Daclizumab (an anti-IL2 receptor antibody for transplant rejection) and bevacizumab (an anti-vascular endothelial growth factor (VEGF) antibody for metastatic colorectal cancer) are the two known humanized antibodies approved by the FDA in 1997 and 2004, respectively [32]. In 1990, Sir Gregory P. Winter utilized phage display technology to produce fully human mAbs (suffix-umab) [33]. Adalimumab (Humira), an anti-tumor necrosis factor α (TNFα) antibody, is a completely human mAb approved by the FDA in 2002 for the treatment of cancer and autoimmunity [34,35,36,37,38]. Finally, efforts were directed toward producing fully human antibodies using humanized transgenic mice platforms [39]. Examples include panitumumab and nivolumab, which target the epidermal factor receptor (EGFR) antibody [40] and the programmed cell death protein-1 (PD1), respectively [41,42].
1.4. Nomenclature of Antibodies
The names of antibodies provide information about the intended target, source (host), modifications, and conjugation to other molecules. The WHO published guidelines from the International Nonproprietary Name (INN) in 2014, 2017, and 2021 describing the classification of naming mAbs [43,44,45]. The nomenclature consists of a prefix, a substem (initially proposed as two and later reduced to one), and a stem (suffix). The prefix is random, as it provides a unique identity to the antibody. The substem indicates the target (e.g., “ci” for cardiovascular, “ba” for bacterial, “os” for bone, “ta” for tumor, etc.) and host (e.g., “o” for murine, “xi” for chimeric, “zu” for humanized, and “nu” for fully human). The second part of the substem, which revealed information about the host, has been eliminated after 2017, applicable only to the antibodies produced after 2017. Until 2021, the stem “mab” was used for all the antibodies and their fragments. However, after the revisions in 2021, the stem was divided into four groups. Group 1 uses the stem “tug” for full-length unmodified immunoglobulins, like IgG, IgA, IgM, IgE, etc., that might occur normally in the immune system. Group 2 uses “bart” for full-length antibodies artificial, which contain engineered constant domains with point mutations for any reason like glycation or altered complement binding. Group 3 uses “mig” for multi-immunoglobulins (including bi- and multi-specific monoclonal antibodies), regardless of the format type (full length or fragments). Finally, group 4 uses the stem “ment” for monospecific antibody fragments that lack an Fc region. Biosimilar mAbs have a core name with the reference drug followed by a meaningless and unique four-letter suffix separated by a hyphen. For example, adalimumab biosimilars have distinguishing suffixes like -atto, -adbm, -adaz, -bwwd, and -afzb [46].
1.5. Databases for Therapeutic Antibodies
There are various databases of therapeutic antibodies that are either approved or under regulatory review. The antibody society can be accessed to receive a comprehensive review of therapeutic antibodies in the European Union (EU) and the United States (US) [47]. Figure 3 (adapted from the antibody society website, www.antibodysociety.org/resources/approved-antibodies, accessed on 30 June 2023) shows the number of antibody therapeutics in cancer and non-cancer areas that have been granted first approval either in the US or the EU. The Oxford Protein Informatics Group has helpful resources for people studying therapeutic antibodies [48]. The Therapeutic Structural Antibody database (Thera-SAbDab) is a database from OPIG [49] that provides information on immunotherapeutic variable domain sequences and their corresponding structural representatives in the database. It helps match the exact and closely related sequences [50]. Tabs is a different database for companies developing therapeutic antibodies ([51]. The Chinese antibody society, in collaboration with the antibody society, has a tracker (https://chineseantibody.org/covid-19-track/, accessed on 30 June 2023) [52] that records antibody-based therapeutics against COVID-19. This was an effort to support information in fighting the pandemic globally [52]. Information on US-FDA-approved antibodies targeted at different diseases can be found on the US-FDA website [53].
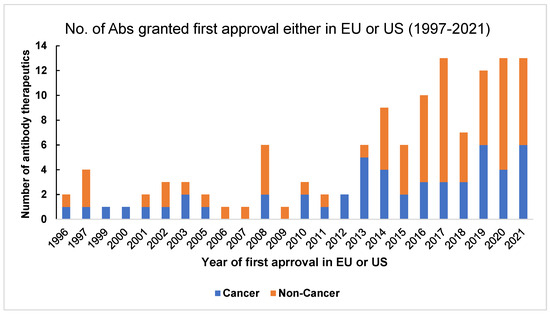
Figure 3.
Number of antibody therapeutics in cancer and non-cancer categories that were granted first approval either in the United States (US) or the European Union (EU) from 1997–2021. This graph is adapted from The Antibody Society. Therapeutic monoclonal antibodies approved or under review in the EU or US. [47] www.antibodysociety.org/resources/approved-antibodies, accessed on 30 June 2023.
2. Antibodies in Different Branches of Medicine
The following sections describe the system-wise utilization or application of antibody therapeutics in treating diseases in different branches of medicine, ranging from hematology, oncology, and autoimmune disease to different organ system-related disorders like vascular, renal, neurological, etc.
2.1. Antibodies in Hematological Diseases
The therapeutic use of monoclonal antibodies is prevalent in hematology conditions, particularly in hematology/oncology. Rituximab represented a breakthrough in cancer treatment as one of the first monoclonal antibodies directed against the CD20 antigen on B lymphocytes [54]. Though first approved for the treatment of relapsed indolent non-Hodgkin’s lymphoma, it has now become a standard component of treatment for follicular lymphoma (FL), diffuse large B-cell lymphoma (DLBCL), and chronic lymphocytic leukemia (CLL) [55]. Since its development, it has spurred the synthesis of additional monoclonal antibody therapies targeting CD20, which now account for over 30% of all current therapeutic monoclonal antibodies for cancer [56]. Each of these anti-CD20 antibodies functions by depleting B cells, albeit through different mechanisms, including direct cell death, antibody-dependent cellular cytotoxicity, phagocytosis, and complement-dependent cytotoxicity [57].
Emicizumab is a bispecific antibody that has emerged as a successful therapy for hemophilia A, and it works by bridging factors IXa and Xa in the coagulation cascade in order to restore the recruitment function of the deficient factor VIII [54,58]. Classic treatment for hemophilia A requires constant prophylactic infusions of factor VIII—at least two infusions a week—contributing to reduced treatment compliance [59]. However, subcutaneous administrations of emicizumab once weekly, or once every 2–4 weeks, have not only shown effectiveness in preventing bleeds but are also well tolerated [60].
Crizanlizumab was approved by the FDA in 2019 for use in preventing vaso-occlusive crises (VOCs) in patients with sickle cell disease [54,61]. Repetitive VOCs in these patients lead to chronic organ damage, making this a major cause of morbidity and mortality [62,63]. The pathophysiology of these crises entails the attachment of sickle cells to the vascular endothelium [61,64], mediated by the adhesion molecule P-selectin, which is also what crizanlizumab inhibits. Although it does not seem to reduce hemolysis, crizanlizumab has a significant impact on the frequency of VOCs with a low rate of adverse effects [64].
Although treatments like rituximab are effective, not all patients are able to take advantage of these treatment options due to excessive costs [65]. Biosimilars are biologic medicines with high similarity to existing biologics that offer a potential solution to this ongoing issue of hematologic monoclonal antibodies [54]. The goal in designing biosimilars is to preserve the safety, efficacy, purity, and mechanism of action of existing biologics [66] while avoiding the complexity of synthesizing large molecules like antibodies. This poses the question of whether new antibodies are true biosimilars [54]. Thus, the current goal of future developments for monoclonal antibody therapies in hematology is to make current therapies more accessible to patients while also improving and developing new treatment options.
2.2. Antibodies for Autoimmune Diseases
Autoimmune diseases are characterized by aberrant immune-cell function, resulting in the destruction of cells and tissues. Though these are often difficult to treat due to a personalized dysfunction of the body’s inherent immune response, a general understanding of immune cellular processes offers insight into the many possible targets for therapy, particularly cytokines and cell signaling receptors [67].
Rheumatoid arthritis (RA) is characterized by chronic inflammation and synovitis, leading to flares of polyarthritis and joint pain. Though the exact mechanism is unknown, pro-inflammatory mediators such as IL-6, IL-1, and TNF-α play a significant role in the synovitis and bone erosion seen in RA. Thus, these cytokines are common targets for treatment with monoclonal antibodies [68]. The first line of treatment for RA is a disease-modifying anti-rheumatic drug (DMARD), such as methotrexate. However, traditional DMARD-resistant RA can be treated using biological DMARDs such as monoclonal antibodies. Combined administration of biologics with traditional DMARDs has shown significant efficacy in randomized control trials [69]. Systemic symptoms of RA can be attributed to IL-6 overactivity, making this cytokine a key target in drug therapy. In RA, aberrant proliferation of macrophage-like synoviocytes (type-A) and fibroblast-like synoviocytes (type-B) leads to uninhibited cytokine release, especially IL-6 and TNF-α [70]. Tocilizumab, a humanized monoclonal antibody (IgG1 κ) against the membrane-bound and soluble IL-6 receptors, is especially useful in minimizing systemic RA symptoms such as fatigue, anemia, and acute phase reactions [71]. Sarilumab is another humanized monoclonal antibody (IgG1) that binds to and inhibits downstream signaling of the IL-6 receptor [72]. Sarilumab has a similar effectiveness and safety profile to tocilizumab when used for the treatment of methotrexate-resistant RA [73]. The TNF-α inhibitors infliximab and adalimumab are also approved for the treatment of RA; however, other biological DMARDs such as tocilizumab seem to be more effective in the treatment of disease symptoms and drug adherence for patients [74,75].
Systemic lupus erythematous (SLE) is an autoimmune disease with widely varying severity and involvement of organ systems. Due to its complex manifestations, treatment of SLE is highly individualized and depends on the specific organ involvement and the magnitude of severity. It is recommended that the majority of SLE patients be treated with hydroxychloroquine, and additional medications like monoclonal antibodies are added to their regimen as needed [76]. Belimumab, a monoclonal antibody (Ig-G1 ɣ) against B lymphocyte stimulator (BLyS), is approved for the treatment of SLE and is effective in cases with renal involvement. Inhibition of BLyS by belimumab leads to autoregulated B-cell apoptosis and a reduction in antibodies to double-stranded forms in serum. Patients treated with belimumab in addition to standard therapy were shown to have a reduction in symptoms, a diminished frequency of disease flares, and fewer adverse events related to SLE [77]. Anifrolumab is a monoclonal antibody (IgG1 κ) targeted against the IFN-1 receptor, approved for the treatment of moderate to severe SLE without lupus nephritis. The activity of type 1 interferons, such as IFN-α, IFN-β, and IFN-κ, is involved in the pathogenesis of SLE. These type 1 interferons are inhibited by anifrolumab [78]. Rituximab has also been successfully used for SLE in patients who fail to respond to other treatments or have severe refractory SLE [79,80]. Other monoclonal antibodies show promise for future use in SLE treatment. Current clinical trials are exploring the use of itolizumab, a monoclonal antibody against CD6, and ustekinumab, a monoclonal antibody against IL-12 and IL-23, for SLE treatment [81,82].
Psoriatic arthritis is a chronic inflammatory arthritis present in patients with psoriasis. It is characterized by peripheral poly- or oligo-arthritis and periarticular disease such as enthesitis, or inflammation of ligament, tendon, and joint capsule attachment sites to the bone. Key cytokines involved in pathogenesis include IL-23, IL-17, and TNF-α. These cytokines ultimately lead to inflammation, bone erosion, and osteoblast production [83]. Treatment of psoriasis is dependent on the presence of skin disease as well as the presence and severity of joint involvement. Methotrexate is often used for patients with psoriasis and psoriatic arthritis, but it does not prevent long-term damage to the joints [84]. The TNF-α inhibitors infliximab and adalimumab are also approved for the treatment of severe psoriatic arthritis and are the preferred treatment over IL-17 and IL-23 inhibitors [83]. IL-17 inhibitors can be used for patients with mild-to-severe psoriatic arthritis. Secukinumab and ixekizumab are both monoclonal antibodies against IL-17 approved for the treatment of psoriatic arthritis. However, patients with inadequate responses to secukinumab have been shown to respond well when switched to ixekizumab, with a reduction in disease symptoms [85,86]. The IL-17 and IL-23 inhibitor, ustekinumab, is also used in the treatment of psoriatic arthritis and has been shown to have a similar safety profile when compared to other monoclonal antibodies, such as secukinumab, with fewer severe adverse events [87].
2.3. Antibodies Specific to Cancer
As the challenge of finding effective small-molecule treatments for many cancers persists, monoclonal antibodies have broken onto the scene to revolutionize cancer treatment [88]. With the discovery of new molecular targets in cancer pathophysiology comes the development of new monoclonal antibody drugs. One crucial aspect of cancer growth is the tumor microenvironment [89], which entails tumor cells as well as immune cells, vasculature, and extracellular matrix. These components offer potential targets for monoclonal antibodies for treatment, especially if the tumor cells themselves are difficult to target in cases like metastasis [90]. However, other than immune checkpoints as targets [91], many clinical trials have failed to show promising efficacy [92].
In some cancers, like metastatic melanoma, aberrant immune cell proliferation and signaling by cancer cells cause immunosuppression, worsening the body’s ability to fight cancer. For example, melanoma cells can express PD-L1, a ligand that neutralizes immune cells by binding to their PD-1 receptor [93]. Nivolumab is a monoclonal antibody that targets PD-1, preventing its binding to the ligand, rendering the cancer’s expression of PD-L1 ineffective, and resulting in a continued immune response to the tumor [90]. Nivolumab showed increased efficacy and positive long-term outcomes when administered with other monoclonal antibodies to treat advanced melanoma, including ipilimumab and relatlimab. Ipilimumab binds the CTLA-4 receptor on the surface of T-cells, preventing immunosuppression by tumor cells and allowing the immune system to appropriately hoist an anti-tumor response [90]. Relatlimab blocks the lymphocyte-activation gene 3 (LAG-3) to also prevent immune suppression and allow for an anti-tumor response [94]. When combined with nivolumab, the length of progression-free survival significantly increased, though there was also an increased rate of treatment-related adverse effects [94].
Using monoclonal antibodies in techniques such as antibody-drug conjugates (ADCs) [95] and bispecific antibodies (BsAbs) [96] has become a promising avenue for cancer therapy. One widely studied ADC treatment for invasive breast cancer is trastuzumab-emtansine, a monoclonal antibody targeting the aberrant HER2 receptor conjugated to a cytotoxin that inhibits microtubules [97]. According to phase III randomized trials, trastuzumab conjugated to deruxtecan, another cytotoxic microtubule-stabilizing drug, has demonstrated a remarkable decrease in disease progression and risk of death when compared to trastuzumab-emtansine [98]. Another ADC treatment called enfortumab vedotin entails a monoclonal antibody that binds to the nectin-4 protein on bladder cancer cells and then releases conjugated monomethylauristatin E (MMAE), a microtubule disrupting agent that kills the cancer cell [99].
Another successful ADC is brentuximab vedotin, a CD30-specific monoclonal antibody [100] used to treat T-cell lymphoma. The addition of brentuximab vedotin to a regimen of doxorubicin, vinblastine, and dacarbazine was successful in increasing progression-free survival in patients with stage III–IV Hodgkin lymphoma in a clinical trial [101], but another clinical trial showed that a monotherapy of the monoclonal antibody pembrolizumab, a PD-1 inhibitor classically used to treat melanoma, was more effective at improving progression-free survival compared to brentuximab vedotin alone [102]. These conflicting studies show that, though there is evidence to support the inclusion of ADCs in cancer treatment, whether they are overall more efficacious than potent monoclonal antibody monotherapy requires further investigation through clinical trials. Further successes in ADCs have been limited [95], but insights show that increased levels of cytotoxic drug conjugation and increased linker stability between drug and antibody may be next for the development of successful ADCs.
There is also research to support ADCs as effective against tumor heterogeneity [103], which has persisted as a common obstacle to cancer treatment. Tumor heterogeneity makes antibody treatment difficult, particularly for ADEPT and ADC, due to the irregular vasculature of the tumors. This feature of solid tumors causes a reduction in the efficacy of antibody therapies [104].
2.4. Antibodies for Pulmonary Diseases
There are more than 120 antibodies targeted at respiratory diseases, mainly asthma, lung cancer, and respiratory infections. While some have shown potential (like immune checkpoint inhibitors and IL-5 pathway inhibitors) and offered hope for treating respiratory diseases, others have failed clinical trials [105]. Moreover, diseases like COPD, asthma, lung cancer, and IPF are complex due to the involvement of different signaling pathways and multiple biomarkers for targeting, with no single particularly dominant cytokine or chemokine target. For this reason, a large proportion of patients with respiratory illnesses are administered one or more antibodies. It is also important to define the optimal antibody combination, route of administration, treatment duration, and biomarkers to improve efficacy in patients [105].
Here are several examples of these antibodies. Omalizumab against the heavy-chain constant (Cε3) domain of IgE was the first biological therapy for asthma [106]. It is also indicated in moderate-to-severe allergic asthma [105]. Ligelizumab, also targeted at the constant domain of IgE, displayed greater efficacy than omalizumab for mild asthma. Quilizumab against the M1 prime segment of IgE reduced acute asthma exacerbations. Mepolizumab and reslizumab against IL-5 were used for eosinophilic asthma and are under phase II evaluation for chronic rhinosinusitis. Its trials are also ongoing for COPD [105]. Benralizumab against IL-5Rα is indicated in severe eosinophilic asthma [107]. It has phase III trials ongoing for COPD and phase II trials ongoing for chronic rhinosinusitis [105,108]. Dupilumab against IL-4Rα is for asthma treatment and should block both IL-3 and IL-4 for therapeutic effect. It is in phase II trials for COPD [107,108]. Tezepelumab, which targetsthymic stromal lymphoproteins, is being tested and is in phase II trials for COPD [108]. Brodalumab, secukinumab, and CJM112, targeted against IL-17, were initially tested for asthma but did not show effective outcomes. They could be used to treat COPD, but this has not yet been tested. Also, no reduction in sputum neutrophil count was observed [105,108,109].
Respiratory syncytial virus (RSV) is another significant pulmonary pathology that can be treated by antibody therapy. RSV’s seropositivity is 100% by the time infants are 2 years old, and half of infants are admitted to having long-term sequalae. Only palivizumab, approved and marketed for RSV, has shown a reduction in hospitalizations of 55%. Apart from palivizumab, which is an antibody with a standard half-life, motavizumab, MPE8, and TLR3D3 are in the preclinical phase. MAbs with extended half-lives such as Motavizumab-YTE, suptavumab, nirsevimab, and MK-1654, are under phase trials. For some of them, the trials have been interrupted [110]. Bevacizumab and ramucirumab against VEGF and VEGF-R2 are used for non-small-cell lung cancer (NSCLC) and show benefits when used in combination with chemotherapy. Necitumumab against EGFR showed benefit as first-line therapy for advanced squamous-cell NSCLC when combined with gemcitabin and cisplatin. However, cetiximab targeted at EFGR showed conflicting results with combined therapy. Tanstmumab (Her2/Eeb2) produced a tumor response in patients with NSCLC. Seribantumab (Her3 signaling) is under testing following a positive phase III trial, but the phase III trial for patritumab (Her3) was terminated due to a lack of efficacy. Nivolumab (PD1), pembrolizumab (PD1), and atezolizumab (PDL-1) have shown survival benefits in NSCLC when combined with first-line chemotherapy. Atezolizumab is also indicated for reducing lung toxicity due to its targeting of only PDL-1. Other antibodies that have shown promise in phase trials for NSCLC include racotumomab, L19-IL-12, MABp1, and sacituzumab [105]. Panobacumab, MEDI3902, and aerucin are antibodies against Psuedomonas aeruginosa and inhibit biofilm formation [105,111]. MEDI3902 received a fast-track designation from the FDA in 2014 [112].
Obiltoxaximab and raxibacumab are antibodies against Bacillus anthracis produced after the US terrorist attacks using anthrax [105]. Lebrikizumab and tralokinumab are against IL-13 and are under phase trials for COPD and asthma exacerbations. The former is also being tested for the treatment of idiopathic pulmonary fibrosis (IPF) [105].
According to the NIH guidelines, antivirals (paxlovid and remdesevir) are the preferred treatment for COVID-19 [113]. Bebtelovimab, against spike protein, was administered to patients aged 17+ with mild to moderate COVID-19 that had the potential to progress. It is now not recommended by the FDA due to omicron resistance [114]. Tixagevimab + cilgavimab (Evusheld) against epitopes of spike protein are recommended as repeat doses every 6 months for the prevention of SARS-CoV-2 infection [115]. Balmlanivimab + etesivimab, casirvimab + imdevimab, and sotrovimab usage have been paused due to omicron variant resistance, according to NIH guidelines. One can receive real-time information about COVID-19 therapeutics by visiting the COVID network data tracker by the CDC and IDSA (https://covid.cdc.gov/covid-data-tracker/#datatracker-home, accessed on 30 June 2023).
2.5. Antibodies for Cardiovascular Diseases
Cardiovascular disease (CVD) is the leading cause of death globally, often accompanied by atherosclerosis. Atherosclerotic plaque erosion/rupture is associated with arterial occlusion (strokes/MI). The risk factors for CVD are high blood pressure, high blood cholesterol (plasma LDL level), inflammation, and diabetes. Statins are the primary treatment for high blood cholesterol but are not effective in statin-resistant patients and have limited efficacy against homozygous familial hypercholesterolemia (HoFH) [116] and statin intolerance, displaying adverse effects like myalgia and rhabdomyolysis (rare but severe) [117]. An alternative is apheresis, but this is difficult and time-consuming for patients.
For primary hyperlipidemia due to heterologous familial hypercholesterolemia (HeFH) and HoFH, statins and PCSK9 inhibitors (that function by increasing the number of LDL receptors) are less effective in treatment due to the absence of LDL receptor expression. Lomitapide and mipomersen work independently of LDL receptors but are limited by adverse effects (such as elevated ALT and hepatic steatosis). Therefore, Ab therapy can be a possible substitute for statins/apheresis [116].
Even if hyperlipidemia is treated, it is important to address vascular inflammation and hypertension to prevent the risk of CV events, especially if inflammation is already present [118]. A potential target for this could be ApoB-100, a protein constituent of low-density lipoprotein (LDL) and a primary driver behind plaque formation. However, no therapeutic antibodies are currently available for this. Alirocumab, an antibody against PCSK9, a serine protease that binds to LDL receptors and promotes their degradation, significantly reduced plasma LDL levels and did not affect very low-density lipoprotein (VLDL) or triglyceride (TG) levels. It is used in the treatment of atherosclerotic cardiovascular disease resistant to diet changes and statin therapy, as well as for the treatment of heterozygous familial hypercholesterolemia [119]. As of April 2021, the FDA has expanded its use to also include the treatment of homozygous familial hypercholesterolemia [120]. It has shown a reduction in CV events [121,122,123] and regression of coronary plaques, particularly when used alongside statins [124]. Evolocumab (also against PCSK9) and alirocumab share a similar safety profile, with no significant difference in efficacy. They have also shown enhanced effects in patients undergoing statin therapy. In phase III trials of bococizumab, also an antibody against PCSK9 and used for the treatment of hyperlipidemia, high levels of anti-bococizumab antibodies developed in patients, reducing overall drug efficacy. Also, an inconsistent reduction in LDL cholesterol was seen, leading to its discontinuation for further development. Evinacumab, an anti-ANGPTL3 (inhibitor of lipoprotein lipase and endothelial lipase) antibody, is involved in the degradation of circulating triglycerides and other lipids. It was developed for the treatment of HoFH. PCSK9 inhibitors and statins were less effective due to the absence of LDL receptors in patients. Evinacumab treated HoFH patients with greater success due to a mechanism of action independent of LDL receptors. Trials demonstrated a significant decrease in LDL cholesterol levels in HoFH patients, but this lacks long-term data. Canakinumab is an antibody that binds to IL-1β, helping reduce inflammation. Models suggest that reducing vascular inflammation (even without addressing cholesterol levels) should decrease the risk of CVD [118,125,126]. Canakinumab is approved for the treatment of several auto-inflammatory diseases: CAPS, TRAPS, HIDS/MKD, FMF, Still’s Disease, and SJIA. The canakinumab anti-inflammatory thrombosis outcome study (CANTOS) trial to determine efficacy against CVD showed that canakinumab was associated with a decrease in CV events but limited by its high cost and risk of fatal sepsis and therefore not approved for CVD treatment. However, it still demonstrated that inflammation—not just hyperlipidemia—was a key component of CVD [125,126]. The CANTOS trial primarily involved patients with elevated inflammatory markers, which could have affected the data. Furthermore, long-term treatment with canakinumab showed a significant reduction in incidental anemia in patients without baseline anemia. Significant increases in mean hemoglobin levels were also seen in patients with anemia [127]. IL-6 induces hepcidin production, thus demonstrating the downstream effect of IL-1 inhibition on anemia [126]. It is associated with an increased risk of infection, mild thrombocytopenia, and mild neutropenia [125,126,127]. Ziltivekimab targets IL-6 and reduces inflammation. Early trials demonstrate a reduction in inflammation without affecting the total/HDL cholesterol ratio (a common adverse effect of IL-6 inhibitors) [126]. Further trials are needed to determine the effects on CV event risk. The roles of IL-6 in angiogenesis entail the induction of VEGF, autoimmunity, and cancer [128]. Also, IL-6 induces hepcidin production, which has a role in iron homeostasis and anemia and is a factor in heart failure. Tocilizumab, an IL-6R-specific antibody that was used as a treatment for rheumatoid arthritis, is not a therapy for CVD. It is contradicted in patients with CVD risk due to a significant increase in lipid levels [129,130]. It shows interaction with CYP450, associated with atorvastatin metabolism. It is associated with pro-atherogenic increases in blood cholesterol and decreased LDL receptor expression. It has a relatively low increase in CV events compared to other bDMARDs, indicating pro-atherogenic effects possibly balanced by anti-inflammatory inhibition of IL-6 [129]. It did not show any significant treatment effect on pulmonary arterial hypertension (PAH), as no changes in vascular resistance or associated inflammatory markers were seen with the treatment response [131].
2.6. Antibodies for Renal Diseases
Rituximab, a chimeric antibody specific for the CD20 antigen on B-cells and initially approved by the FDA for lymphoma, is effective in the treatment of membranous glomerulonephritis, steroid-resistant nephrotic syndromes, and membranoproliferative glomerulonephritis (MPGN) [132]. Clinical studies on rituximab support it as an effective steroid-sparing agent in steroid-dependent idiopathic nephrotic syndrome [133]. In April 2011, it was approved for the treatment of anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitis (including granulomatosis with polyangiitis (Wegener’s granulomatosis) and microscopic polyangiitis) [134]. Additionally, it is used in the treatment of membranous nephropathy and lupus nephritis [134]. Minimal change disease (MCD) or focal segmental glomerulosclerosis (FSGS) are two major causes of nephrotic syndrome and are treated with steroids, antiproteinuric drugs, and immunosuppressants, as suggested by the KDIGO (Kidney Disease: Improving Global Outcomes) guidelines. The monoclonal antibodies shown to have a positive result in reducing proteinuria in small cohorts are rituximab, ofatumumab, adalimumab, fresolimumab, and bleselumab. Out of these, rituximab’s effects were established by larger trials, while other antibodies like ofatumumab, fresolimumab, and adalimubab showed conflicting results in novel therapies for resistant FSGS clinical trials (FONT) and FONT II (phase II) trials. The effectiveness of bleselumab on post-transplant FSGS is currently under investigation [135]. Rituximab is also instrumental in reducing the auto-antibody formation in ANCA-associated vasculitis, lupus nephritis, and mixed cryoglobulinemia. As mentioned earlier, it has been shown to safely decrease proteinuria in patients with nephrotic syndrome secondary to membranous nephropathy, MGS, and FSGS. Eculizumab, an anti-C5 humanized monoclonal antibody, is also documented to be efficient in treating C3 nephropathy, atypical hemolytic uremic syndrome, and membranoproliferative glomerulonephritis [136]. Other antibodies, such as adalimumab, daclizumab, fresolimumab, belimumab, and tocilizumab, some of which are already approved for different medical applications, are under premarketing investigation [132]. Apart from their positive effects, some antibodies also cause renal toxicities. Examples include hypertension and proteinuria caused by bevacizumab, an anti-VEGF antibody, along with other anti-VEGF therapeutics like aflibercept (VEGF trap) and anti-VEGF receptor (VEGFR) tyrosine kinase inhibitors (TKIs) [137]. Cetuximab and panitumumab, monoclonal antibodies against the HER-family of receptors, caused electrolyte imbalances, including hypomagnesemia and hypokalemia, due to the direct nephrotoxic effect of the drug on renal tubules. Cetuximab displayed potential for renal tubular acidosis. It was also mentioned that rituximab caused acute renal failure following the initiation of therapy due to the onset of acute tumor lysis syndrome. Therefore, it is important to discern the adverse effects of the therapeutics to ensure safe treatment strategies, especially in patients with pre-existing renal conditions [137].
2.7. Antibodies for Gastrointestinal Pathologies
While the exact pathogenesis of inflammatory bowel disease (IBD) remains under investigation, aberrant cytokine release is known to play a significant role in the progression of both Crohn’s disease and ulcerative colitis [138,139]. In patients with IBD, elevated levels of TNFα have been associated with inflammatory tissue damage and impaired regulation of intestinal immune cells [138,140,141,142]. Two TNFα inhibitors, infliximab and adalimumab, have been approved by the FDA for the treatment of moderate-to-severe Crohn’s disease and ulcerative colitis [143].
Infliximab, a chimeric mouse/human antibody, binds soluble and transmembrane TNFα, inhibiting its pro-inflammatory activity and inducing lysis of TNFα-producing cells [143,144]. Infliximab has been found to induce mucosal healing and maintain remission of both Crohn’s disease and ulcerative colitis, with a promising safety profile for long-term treatment [145,146,147,148,149]. Adalimumab, a monoclonal human antibody, neutralizes the biological activity of TNFα in a similar manner and has comparable effectiveness in treating IBD [150,151,152,153,154,155,156,157]. Patients treated with infliximab or adalimumab may develop anti-drug antibodies and a resultant loss of response to mAb therapy [158,159]. Switching from one TNFα inhibitor to another was able to restore treatment response in some patients [160,161]. Combination therapy with an immunomodulator (thiopurines or methotrexate) has also been found to improve initial drug efficacy, decrease immunogenicity, and recapture treatment response [162,163,164,165,166]. Immunomodulator use remains controversial, however, due to its association with the development of severe infections and malignancies [148,167,168].
Certolizumab pegol is an anti-TNFα fragment conjugated to polyethylene glycol, humanized to reduce immunogenicity and the development of anti-drug antibodies [169,170,171,172]. This drug was FDA-approved for use in the treatment of Crohn’s disease [173]. PEGylation of certolizumab increases the elimination half-life and possibly further decreases immunogenicity [174,175,176]. Golimumab is another anti-TNFα mAb recently approved for the treatment of ulcerative colitis only [177,178]. Clinical trials and early studies have shown golimumab to be safe and have similar effectiveness to infliximab and adalimumab [179,180]. Both certolizumab pegol and golimumab appear to have lower immunogenicity and decreased anti-drug antibody levels relative to infliximab and adalimumab [181,182].
Beyond TNFα inhibitors, anti-integrin mAb therapy is another promising avenue for IBD treatment. Natalizumab binds α4 integrin, blocking α4β7-integrin/mucosal addressin cell adhesion molecule-1 (MadCAM-1) interactions and α4β1/vascular-cell adhesion molecule-1 (VCAM-1) binding [183,184]. By inhibiting these interactions, natalizumab prevents immune-cell migration to the inflamed intestinal mucosa, preventing further tissue damage. Similarly, vedolizumab limits leukocyte homing to the gut mucosa [185] by directly binding and neutralizing α4β7. Natalizumab is typically reserved as a second-line treatment following failure of TNFα inhibitor treatment due to the risk of progressive multifocal leukoencephalopathy from the reactivated John Cunningham polyoma virus [186]. While both mAbs are approved for the treatment of Crohn’s disease, vedolizumab is additionally approved for ulcerative colitis [187]. In a head-to-head trial involving patients with moderate to severe ulcerative colitis, vedolizumab was found to have higher efficacy than adalimumab [185]. Etrolizumab (anti-β7 subunit of α4β7 and αEβ7 integrins) remains under study for the treatment of IBD. While abandoned as an ulcerative colitis treatment due to mixed phase III trial results, clinical trials involving patients with Crohn’s disease demonstrate high safety and efficacy at remission maintenance [188,189].
With the continued success of mAb IBD treatments, therapeutic antibodies have been additionally explored for other gastrointestinal conditions. Aberrant leukocyte trafficking may be involved in the pathogenesis of IBD-associated primary sclerosing cholangitis (PSC) [190]. Anti-integrin mAbs have been considered for PSC treatment, although vedolizumab treatment failed to provide a clinical benefit to patients [190,191,192,193,194]. Lysyl oxidase like-2 (LOXL2) is known to drive fibrogenesis and is also believed to be involved in the progression of PSC and non-alcoholic steatohepatitis (NASH). Simtuzumab, an antibody directed against LOXL2, was found to be ineffective in alleviating PSC liver damage [195,196]. The monoclonal antibody CM-101 offers a promising therapy for NASH and liver fibrosis. CM-101 selectively targets CCL24, a chemokine involved in mediating inflammation and fibrotic processes, and has demonstrated a significant reduction in liver fibrosis in animal models [197]. Phase II trials are currently ongoing (NCT04595825).
Mepolizumab and reslizumab, anti-IL5 antibodies approved for the treatment of eosinophilic asthma, have been investigated for the treatment of eosinophilic esophagitis. Although IL-5 inhibition suppressed eosinophil accumulation, symptom improvement was inconsistent and not clinically significant [198]. Other pathogenic mechanisms may be involved in the progression of eosinophilic esophagitis beyond IL-5-driven eosinophilia.
2.8. Antibodies for Infectious Diseases
The COVID-19 global pandemic showed the effect of infectious disease on our increasingly connected world, reemphasizing and reprioritizing the focus of infectious diseases for pharmaceutical treatment. COVID-19 caused an estimated 921,000 deaths [199] and cost an estimated USD 163 billion [200] just in the United States. However, globally, the top three infectious diseases (HIV, tuberculosis, and malaria) have contributed to an economic burden of up to USD 7 trillion and over 140 million years of life lost [201]. In response, there has been considerable interest in developing new methods to both prevent the spread of infectious diseases and contain them after infection, especially with the use of monoclonal antibodies.
Derived from humanized IgG1, palivizumab was the first FDA-approved monoclonal antibody treatment for infectious disease—specifically for respiratory syncytial virus (RSV). Palivizumab binds and prevents key conformational changes in the viral F protein, preventing virus attachment and fusion. RSV is a common cause of lower respiratory tract infections, and severe cases require hospitalization, making RSV a significant cause of morbidity for young children [202]. Despite significant efforts [203,204], no vaccines have been made available for RSV, making palivizumab the only prophylaxis for RSV. It has also been effective at reducing hospitalization due to RSV infection [205,206]. Despite its high cost and requirement to be administered every month for five months during RSV season, it has been shown to be cost-effective in patients where treatment is indicated [207].
Another advantage of monoclonal antibody therapy development for infectious diseases is the ability to isolate human immunoglobulins from infected patients to study therapeutic effects or to directly use in treatment. Ansuvimab, an FDA-approved human IgG1 antibody to treat Ebola virus infections, was initially isolated from a survivor of a 1995 Ebola outbreak [208]. The drug binds and inhibits the Ebola virus glycoprotein and the Niemann-Pick C1 (NPC1) receptor, preventing viral membrane fusion and entry. Inmazeb, a cocktail of three fully human monoclonal antibodies obtained from VelocImmune mice, has also been approved to treat Ebola virus infections [209] and has demonstrated protection against escape mutants [210].
Thus far, only seven monoclonal antibodies have been FDA-approved for infectious diseases. In addition to the aforementioned drugs, these include raxibacumab and obiltoxaximab for the treatment of anthrax infection, bezlotoxumab for the prevention of Clostridiodes difficile infection recurrence, and ibalizumab for the treatment of HIV-1 infection [211]. Research into the use of monoclonal antibodies in infectious diseases is ever-growing, and future directions of development are aimed at its integration into vaccine development and the treatment of previously untreatable infections [212].
2.9. Antibodies for Endocrine Disorders
Type 1 diabetes mellitus (T1DM) is an endocrine disease driven by autoimmune destruction of pancreatic β-cells [213,214,215]. The progressive loss of these insulin-producing β-cells results in a declining ability to moderate serum glucose levels. In patients with T1DM, insulin depletion and the resulting chronic elevation of blood glucose are associated with the development of atherosclerosis, neuropathy, and retinopathy [216].
Teplizumab, an anti-CD3 mAb, is currently the only disease-modifying therapy approved for T1DM [217]. By targeting CD3, Teplizumab blocks T-cell receptor interactions and inhibits T-cell activation, an important component of the autoimmune cell-mediated tissue damage in T1DM [218,219]. Anti-CD3 therapy has been associated with partial exhaustion of T-effector cells and an induction of regulatory T-cell activity [218,220,221,222,223]. Treatment has been shown to preserve β-cell function in new-onset T1DM [224,225], significantly slow the long-term progression of overt T1DM [226,227], and delay the onset of T1DM in high-risk individuals [223,228]. Similarly, another anti-CD3 mAb called otelixizumab has been found to protect β-cell function in patients with T1DM and appears safe [229,230,231].
Additional T-cell-targeted therapies include anti-thymocyte globulin (ATG), a polyclonal antibody directed against multiple T-cell antigens. High-dose (6.5 mg/kg) ATG monotherapy was ineffective in preserving β-cell function, although this result was likely due to generalized T-cell depletion without sparing regulatory T-cells [232,233]. Administration of a lower dose of ATG with granulocyte colony stimulating factor (GCSF) appears to deplete T-effector cells without significant reductions in regulatory T-cell levels [234,235]. Low-dose ATG/GCSF combined therapy slowed the decline of C-peptide in individuals with new-onset and established T1DM, indicating preserved β-cell function. However, low-dose ATG monotherapy appears to be more efficacious than low-dose ATG/GCSF, while GCSF alone is ineffective in maintaining β-cell function [236,237,238,239]. Phase II trials are currently ongoing to further evaluate low-dose ATG as a disease-modifying treatment for T1DM (NCT04291703 and NCT04509791).
Rituximab, an anti-CD20 antibody causing B-cell depletion in non-Hodgkin’s lymphoma, chronic lymphocytic leukemia, and rheumatoid arthritis, provides an additional candidate for T1DM treatment. Reflecting the secondary role of autoantibodies in the progression of T1DM, rituximab-mediated B-cell depletion was found to decrease HbA1c, reduce insulin requirements, and slow the decline of C peptide levels in patients with T1DM [240]. Although unable to maintain remission over the long term and overall less effective than teplizumab or low-dose ATG [239,241], rituximab may have greater success when used in a combined therapy. Autologously expanded regulatory T-cells and rituximab were found to have significantly greater efficacy compared to either treatment as a monotherapy.
2.10. Antibodies for Neurological Disorders
Antibody-based therapeutics have proven to be beneficial and are used as a standard in treating a spectrum of neurological and neuroinflammatory diseases. Multiple sclerosis (MS) is a chronic, multifactorial neurological condition that affects the central nervous system via autoimmune-mediated inflammation. Activated T-cells cause demyelination [242] of neurons, leading to clinical symptoms like fatigue, pain, depression, and anxiety. Natalizumab (see Section 2.7, Gastrointestinal) prevents the extravasation of these activated T-cells by binding to integrins, which are leukocyte adhesion molecules [243]. The inhibition of leukocyte extravasation reduces immune-mediated inflammation and slows the progression of MS [244]. This is particularly useful for the long-term management of MS [245]. Though the safety and efficacy of natalizumab have been shown, there has been significant study into one of the main adverse effects of natalizumab treatment—progressive leukoencephalopathy [246]. Current efforts are devoted to identifying, quantifying, and mitigating the risks associated with natalizumab treatment [247].
Other monoclonal antibodies also used to treat MS include alemtuzumab, daclizumab, rituximab, ocrelizumab, and ofatumumab [248]. Alemtuzumab binds the CD52 receptor of lymphocytes, causing lymphocyte lysis and depletion. Daclizumab binds the receptor IL-2R α to prevent IL-2 signaling-mediated inflammation. Rituximab, ocrelizumab, and ofatumumab all bind the CD20 receptor of B cells, leading to cell depletion. All these monoclonal antibodies treat relapsing-remitting multiple sclerosis (RRMS), which is characterized by intervals of symptom remission followed by intervals of severe symptom resurgence.
A major target of monoclonal antibody drug development has been the amyloid-β peptide (Aβ). Alzheimer’s disease is characterized by Aβ aggregates and neurofibrillary tangles in the brain, accompanied by synaptic dysfunction and neurodegeneration. Several antibodies have been developed targeting both the soluble and aggregated forms of the Aβ peptide [249]. Reductions of biomarkers found in Alzheimer’s disease have been shown in clinical trials for monoclonal antibodies but have only minimally improved clinical outcomes in patients. The risk of amyloid-related imaging abnormalities with edema remains a significant risk of these drugs, especially in APOE4 carriers [250]. However, current drugs used for the treatment of Alzheimer’s, such as cholinesterase inhibitors, treat only symptoms and do not show any reduction in the clinical progression of the disease [251]. Due to the pathological complexity of Alzheimer’s disease, further studies are recommended for the use of monoclonal antibodies.
Aducanumab is a human monoclonal antibody (IgG1) that selectively binds to and reduces the soluble and insoluble forms of Aβ found in the brain. Animal studies have shown Aducanumab to be more effective in preventing Aβ aggregation than clearing existing aggregates in the brain, possibly limiting the ability of the drug to improve rather than just slow cognitive impairment [252]. Current phase III clinical trials for Aducanumab have shown a reduction in Aβ peptides in CSF and plasma in Alzheimer’s patients, but further studies are needed to confirm a reduction in clinical decline [253,254]. Lecanemab is a humanized monoclonal antibody (IgG1) that selectively binds to soluble Aβ protofibrils. However, the risk of amyloid-β-related edema limits the strength of the dose used in patients with the ApOE4 allele. In phase II clinical trials, lecanemab was shown to remove Aβ aggregates from the brain, leading to a reduction in cognitive impairment symptoms and diminished Alzheimer’s CSF biomarkers [255]. Bapineuzumab is also a humanized monoclonal antibody (IgG1) against insoluble Aβ that has shown changes in amyloid-related imaging and concentrations of cerebrospinal fluid phospho-tau in carriers of the ApOE4 allele only. However, improvement in clinical outcomes for carriers and non-carriers has not been shown in phase III trials [256,257]. Additional monoclonal antibody candidates for the treatment of Alzheimer’s disease include ponezumab, crenezumab, and gantenerumab [255,258,259].
Parkinson’s disease is a neurodegenerative disorder caused by the aggregation of α-synuclein, which is a neuronal protein integral to presynaptic vesicle trafficking [260]. Prasinezumab is a monoclonal antibody targeted at aggregated α-synuclein. Recent studies have demonstrated no significant therapeutic effect of prasinezumab [261] when compared to placebo groups and, in fact, resulted in infusion site reactions. This has left Parkinson’s disease as a condition that has not yet been successfully treated by monoclonal antibody therapy.
Although often disregarded by many as a true illness [262], chronic migraine is a neurological condition characterized by a severe headache that affects one in nine adults globally [263]. Notably, migraines have arisen as a goal for antibody therapy by targeting calcitonin gene-related peptide (CGRP). Erenumab, fremanezumab, and galcanezumab have all been approved as humanized monoclonal antibody drugs to treat migraines [262]. Though erenumab showed success in significantly reducing the frequency of migraines, its long-term safety has yet to be tested [264]. Similarly, a phase III, randomized clinical trial demonstrated that galcanezumab was significantly more effective at reducing the number of days with migraines per month while also being well tolerated [265]. Fremanezumab also showed decreased headache frequency over a 12-week trial, though patients also presented with injection-site reactions [266]. Nonetheless, fremanezumab was well tolerated and resulted in sustained migraine improvement for up to 12 months in patients, according to a randomized study [267]. An additional meta-analysis of randomized controlled trials also confirmed that fremanezumab was efficacious but was accompanied by only mild adverse effects in some patients [268].
2.11. Antibodies for Ophthalmological Disorders
Ranibizumab is a monoclonal antibody that can treat retinopathy of prematurity (ROP). Ranibizumab targets VEGF-A and is administered via injection to the vitreous humor of the eye. It has shown success at 24 weeks in infants with ROP in a randomized phase III trial. Furthermore, it does not seem to cause systemic suppression of vascular endothelial growth factor, though long-term effects on vision are yet to be studied [269].
Bevacizumab was proposed as another monoclonal antibody that can treat ROP, but a systematic review and meta-analysis of thirteen studies suggested that treatment can cause an increased risk of cognitive impairment and a decreased language score in infants [270]. Naxitamab is an anti-cancer drug used with GM-CSF to treat relapsed or refractory neuroblastoma. Notably, the FDA approved a combined naxitamab-GM-CSF (granulocyte-macrophage colony-stimulating factor) therapy to treat refractory neuroblastoma in pediatric patients about the age of one and in adult patients [271].
2.12. Antibodies for Musculoskeletal Disorders
Musculoskeletal diseases also have great success with monoclonal antibody therapy, largely due to a better understanding of the molecules and pathways involved in musculoskeletal metabolism and regulation. RANK-L is a membrane-bound ligand present on osteoblasts, and it is important for bone turnover. Denosumab is a human IgG2 that binds and inhibits RANK-L, preventing its interaction with the RANK receptor. This inhibition blocks the maturation and function of osteoclasts, resulting in reduced bone resorption [272], making denosumab a suitable treatment for osteoporosis, especially in postmenopausal women at increased risk of bone fractures.
Romosozumab is a humanized IgG2 antibody that binds sclerostin, another key molecule in the bone remodeling pathway expressed by osteocytes. Inhibition of sclerostin by romosozumab inhibits bone resorption and promotes bone formation, allowing romosozumab to also be used as a treatment for osteoporosis in postmenopausal women [273]. According to a systematic review and meta-analysis of randomized controlled trials, romosozumab resulted in a statistically significant reduction in the incidence of vertebral and non-vertebral fractures, as well as a statistically significant increase in bone mineral density of the lumbar spine, hip, and femur [274]. A study comparing denosumab with the newer romosozumab showed that the romosozumab group experienced a significant increase in mean bone mineral density over 12 months when compared with the denosumab group [275]. These differences were present not only in bone mineral density at the lumbar spine but also for the total hip and femoral neck, suggesting that romosozumab may have a higher potential to improve bone mineral density in women with postmenopausal osteoporosis. Burosumab is a human IgG1 that targets fibroblast growth factor 23 to treat X-linked hypophosphatemia in children [276] and adults [277]. A placebo-controlled phase III study demonstrated burosumab’s efficacy in improving stiffness, pain, and fatigue [278].
Though an autoimmune condition [279], myasthenia gravis is a disease presenting with muscle weakness due to aberrant signaling at the neuromuscular junction. In evaluating available treatments, a network meta-analysis showed that eculizumab, a humanized IgG2/4 that binds C5 of the complement cascade, was the most effective and well-tolerated therapy to treat refractory myasthenia gravis when compared to tacrolimus, cyclosporine A, and mycophenolate mofetil.
All the sections listed in Section 2 are summarized in Table 1, which provides an organized overview of antibodies implicated in treating various diseases in different branches of medicine [47].

Table 1.
System-wise list of therapeutic antibodies specific to different targets implicated in different branches of medicine (adapted from the antibody society website) [47]. Asterisk (*) indicates country-specific approval, hash sign (#) indicates that they are either withdrawn or marketing has been discontinued for the first approved indication. NA indicates that they are either not approved or are in review in the EU or not approved/information on review status is not available in the US.
3. Critical Analysis and Adverse Effects
Although mAbs have been implicated in the treatment of various diseases, their use has been associated with immune-mediated adverse reactions, despite continuous efforts to humanize the antibodies. Apart from immune reactions, there have been incidences of an increase in infections and a potential risk for malignancies and secondary autoimmunity. Some of the adverse effects can be predicted by the target specificity and mechanism of action; however, some remain unpredictable (e.g., hepatotoxicity associated with natalizumab) [415]. The immune-related manifestations of mAb treatment include infusion-related reactions (causing a range of symptoms from rash to generalized edema and cardiac arrest), anaphylactic reactions, and complement activation-related pseudo-allergy (CARPA), as was seen in rituximab and infliximab [416,417].
Mabs have also been seen to activate T-cells, causing cytokine release syndrome (CRS). Severe, life-threatening CRS was observed while treating hematological malignancies with rituximab and alemtuzumab [418]. Therefore, prophylactic protocols that include corticosteroids to prevent CRS are used in the cases of rituximab, ocrelizumab, and alemtuzumab. Mabs are occasionally identified as allogenic, resulting in an immunogenic response that tends to neutralize and rapidly eliminate them from the body via the development of anti-drug antibodies (ADA). This leads to allergic reactions, reduced efficacy, and an increased cost of treatment. Chimeric mAbs like infliximab and adalimumab are shown to be more immunogenic, leading to the formation of ADAs, than human mAbs. Examples include natalizumab, an anti-CD49d antibody that resulted in ADAs in 9% of multiple sclerosis (MS) patients, with 6% of patients developing permanent ADAs, as indicated by the high ADA titers [419]. This can be compared to results in the case of alemtuzumab (for B-cell lymphocytic leukemia), with which 29% of patients in CARE-MS I/II showed development of ADAs in serum after one year without loss of efficacy, and erenumab (human anti-CGRP receptor antibody), with which 2–8% of patients developed ADAs without any reduction in efficacy or increased incidence of adverse events [420,421,422].
Mabs that affect immune function by reducing cell populations (e.g., rituximab and ocrelizumab) or blocking cell migration through the endothelium (e.g., natalizumab) have shown associations with opportunistic infections. Treatment with natalizumab was associated with cryptococcal meningitis and activation of latent tuberculosis (TB) [423]. Alemtuzumab has also shown reactivation of latent TB. The development of progressive multifocal leukoencephalopathy (PML) was reported with natalizumab, rituximab, and ocrelizumab. Alemtuzumab and ocrelizumab were also linked to a significant increase in the overall risk of infection [415].
There is also a likelihood of developing malignancy due to the immunosuppressive effects of antibodies. In the phase III trial of progressive MS, 11 cases of malignancy were reported for ocrelizumab, four of which were breast adenocarcinomas. Therefore, women on ocrelizumab are advised to undergo standard breast cancer screenings, according to the European Medicines Agency’s public assessment report on Ocrevus (brand name for ocrelizumab) [424]. However, rituximab, on the other hand, did not show an increase in the incidence of cancer. The potential risk of immunosuppression and immunocompromising effects of mAbs creates a need for further evaluation. Nonetheless, the overall risk-benefit balance of approved mAbs is not significantly impacted.
Mabs specific for immunologic epitopes were seen to be associated with various autoimmune diseases. In 2018, daclizumab was withdrawn due to the development of secondary autoimmune conditions against the central nervous system, liver, and skin [425]. Alemtuzumab was also associated with the development of secondary autoimmunity, with up to 29% of patients developing thyroiditis, followed by idiopathic thrombopenic purpura (ITP) and Goodpasture syndrome. Other autoimmune disorders related to alemtuzumab use are diabetes mellitus type I, autoimmune hemolytic anemia, Still’s disease, myositis, vitiligo, and alopecia areata universalis [415].
The adverse events explain the failure of the treatment of diseases with antibodies. Since adverse reactions are associated with treatment administration and their evolution after treatment discontinuation, safety can only be guaranteed by the development of clinical programs and post-marketing pharmacovigilance monitoring [415].
4. Future Directions of Antibody Therapeutics
Technological advancements in drug development and research opened the doors for numerous strides in antibody design. Several methods to develop antibodies have emerged, including antibody conjugates (e.g., ADC, radioimmunoconjugate, ADEPT, etc.), bispecific or multispecific antibodies, and antibody fragments (scFvs, Fab, VHH, etc.). Antibody engineering strategies can be employed to confer improved affinity for targets and develop multi-targeted antibodies that can simultaneously treat multiple diseases. Even the adjustment of antibody variable domains can be conducted to target molecules characterized by genetic or genomic differences. Bispecific/multispecific antibodies, ADCs, and CAR-T are all emerging as potential antibody therapeutics. Promising approaches include engineering antibodies with one tumor-cell-specific antigen binding domain and one immune-cell-specific antigen binding domain to ideally stimulate the immune response to cancer cells [426].
To make therapeutic antibodies more effective, miniaturization and multi-functionalization are two important antibody development strategies [427]. While the engineered fragmented antibodies offer advantages such as better tissue penetration and enhanced serum clearance that make them suitable candidates for localized targeting and diagnostic applications, multi-functionalization (tagging with a drug/payload) helps with improved biodistribution profiles. Making the antibodies multispecific helps improve the effector function, as it assists with T-cell co-stimulation and recruitment of adaptive and innate immune cells, immune checkpoint blockade, or multiple antigen targeting [427].
Developing personalized antibody-based vaccines can be another avenue to explore in the future. The resources offered by modern advances like deep sequencing, single-cell RNA sequencing, second-generation sequencing, and spatial omics, along with integrated bioinformatics, provide us with the ability to have a comprehensive understanding of diseased cells. This provides an opportunity to venture into and develop novel antibody-based therapeutics for intracellular targeting and precision medicine [427].
The future of antibody therapeutics should also entail the optimization of antibodies to target a wide range of diseases, from cancer to infectious diseases. Though several antibody therapies have been developed for such conditions, an enduring difficulty in immunoglobulin therapy is the delivery of antibodies across biological barriers [428]. Though there is increased literature supporting the development of nanobodies, or smaller antibodies that can better permeate biological barriers, there is still potential for improved delivery of full-size antibodies via bio-conjugation to other molecules or liposomes as a delivery method [429,430,431]. The necessity to administer antibodies intravenously, intramuscularly, and subcutaneously has also evolved, as even intranasal methods are being researched as non-invasive means of antibody drug delivery [432]. Such improvements in delivery can increase the ability of antibody drugs to treat conditions affecting the brain and lungs.
Newer applications of machine learning and artificial intelligence (AI) are improving and increasing the efficiency of antibody therapy discoveries through the identification of common motifs and epitopes. Computational analysis of antibody chemical structures or physical properties can be accomplished in a way that is more efficient than time-intensive methods like X-ray crystallography [433]. Structural prediction of antibody elements like complementarity-determining regions has improved, but de novo design is a persisting challenge [433].
Recent studies have shown that data-trained machine learning tools can be utilized alongside antibody repertoire libraries like the observed antibody space (OAS) to efficiently design new antibodies. Since humanization of murine antibodies remains the predominant method of monoclonal antibody drug synthesis [434], these tools generate and evaluate the humanness of antibodies to reduce adverse immunogenicity reactions while also providing necessary diversity to sequences. Hu-mAb is one such novel computational tool that suggests mutations to reduce immunogenicity, and it has shown that it can produce results similar to those of experimentally deduced mutations yet far more efficiently [435]. AntiBERTa is another such tool that has experimentally outperformed manual and other AI methods of antibody design [436]. More recently, BioPhi is another such computational tool that has novel methods for both humanization (named Sapiens) and evaluation of humanness (named OASis) [434]. Another study used one such design model and further applied an artificial intelligence-based model to generate binders and characterize them by surface plasmon resonance, elucidating three binders with higher binding affinity than the known therapeutic antibody trastuzumab [437]. According to Li et al. (2023) [438], the combination of several different tools like RaptorX, DeepH3, DeepAb, DeepSCAb, ABlooper, Rosetta, and AbAdapt enabled teams to optimize monoclonal antibody design on several levels: prediction of whole antibody variable regions, prediction of antibody rotamer geometry, prediction of rigid docking between antibody and antigen, and even de novo prediction of CDR-H3 loop sequences and structures, which addresses a challenge of AI-powered antibody design mentioned by Kim et al. (2023) [433]. A study published in June 2023 [438] emphasized the combination of tools like Prihoda’s BioPhi with tools like solPredict in order to optimize for antibody tendency to aggregate, solubility, and pharmacokinetics. Such methods are instrumental in the future of high-throughput monoclonal antibody drug production [434,435,437,439]. Furthermore, Hie and coworkers demonstrated the potential of language-model-guided affinity maturation that enhanced the binding affinities of four clinically relevant antibodies and unmatured antibodies by several folds, along with thermostability and viral neutralization activity against Ebola and SARS-CoV-2 pseudo-viruses [440].
A particular focus of AI-supported antibody engineering is the challenge of reducing the immunogenicity of therapeutics, especially in the field of cancer immunotherapy [438]. The AI-powered model used in this study was able to identify and analyze peptide sequences optimized not only for peptide-MHC binding but also for reduced immunogenicity, decreasing the likelihood of ADA formation. Additionally, the study’s analysis of immunotherapeutic responses notably entailed the detection of tumor-specific biomarkers to determine the clinical appropriateness of cancer immunotherapy. This offers a great insight into the potential of AI to transform oncological treatment. Though there is still progress to be made in terms of the volume of shared data that can improve AI and ML models, the integration of AI in a clinical capacity is inevitable for physicians seeking monoclonal antibody therapies for cancer [438].
5. Conclusions
Therapeutic antibodies have emerged as a hot spot in the area of drug development for the treatment of autoimmune diseases, malignant tumors, infectious diseases, and neurological disorders. The rapid expansion of the antibody industry in different therapeutic areas is indicative of its virtually limitless potential. Not only are they beneficial to patients, but they also generate significant revenue for companies invested in antibody therapeutic research and development. However, it is also important to acquire an understanding of their indications, potential side effects, and risk profiles to minimize adverse reactions. Given the myriad of projects completed in the field, future approaches should aim to design novel molecules based on the unmet needs of patients. This can be conducted by assessing clinical needs, investing more in drug discovery and disease mechanism research for continued development, and improving in the field of therapeutic antibody development. With the plethora of resources offered by technological advancements and the application of AI in biomedicine, future antibody therapeutics hold tremendous potential to become a mainstay in the space of personalized medicine.
Author Contributions
P.S. conceived the manuscript. P.S., R.P. and K.X. worked on the initial draft. Data collection and analysis were performed by P.S., R.P., K.X., R.V.J. and M.A.E. P.S., R.P., K.X., R.V.J. and M.A.E. wrote different sections of the manuscript. P.S., R.V.J. and M.A.E. worked on the table. R.V.J. arranged the references. P.S. designed the figures and edited the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors acknowledge the Biomedical Research Club (BMRC), the Medical Research Honors Program, the Summer Research Immersion Program, and the Office of Research and Scholarship at the Geisinger Commonwealth School of Medicine for providing research opportunities for medical students.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Not applicable.
References
- Hudson, P.J.; Souriau, C. Recombinant Antibodies for Cancer Diagnosis and Therapy. Expert Opin. Biol. Ther. 2001, 1, 845–855. [Google Scholar] [CrossRef] [PubMed]
- Köhler, G.; Milstein, C. Continuous Cultures of Fused Cells Secreting Antibody of Predefined Specificity. Nature 1975, 256, 495–497. [Google Scholar] [CrossRef] [PubMed]
- Todd, P.A.; Brogden, R.N. Muromonab CD3. A Review of Its Pharmacology and Therapeutic Potential. Drugs 1989, 37, 871–899. [Google Scholar] [CrossRef] [PubMed]
- Leavy, O. Therapeutic Antibodies: Past, Present and Future. Nat. Rev. Immunol. 2010, 10, 297. [Google Scholar] [CrossRef] [PubMed]
- Stern, M.; Herrmann, R. Overview of Monoclonal Antibodies in Cancer Therapy: Present and Promise. Crit. Rev. Oncol. Hematol. 2005, 54, 11–29. [Google Scholar] [CrossRef] [PubMed]
- Zemlin, M.; Klinger, M.; Link, J.; Zemlin, C.; Bauer, K.; Engler, J.A.; Schroeder, H.W.; Kirkham, P.M. Expressed Murine and Human CDR-H3 Intervals of Equal Length Exhibit Distinct Repertoires that Differ in Their Amino Acid Composition and Predicted Range of Structures. J. Mol. Biol. 2003, 334, 733–749. [Google Scholar] [CrossRef]
- Hudson, P.J.; Souriau, C. Engineered Antibodies. Nat. Med. 2003, 9, 129–134. [Google Scholar] [CrossRef]
- Carter, P.; Presta, L.; Gorman, C.M.; Ridgway, J.B.; Henner, D.; Wong, W.L.; Rowland, A.M.; Kotts, C.; Carver, M.E.; Shepard, H.M. Humanization of an Anti-P185HER2 Antibody for Human Cancer Therapy. Proc. Natl. Acad. Sci. USA 1992, 89, 4285–4289. [Google Scholar] [CrossRef]
- Presta, L.G.; Lahr, S.J.; Shields, R.L.; Porter, J.P.; Gorman, C.M.; Fendly, B.M.; Jardieu, P.M. Humanization of an Antibody Directed against IgE. J. Immunol. 1993, 151, 2623–2632. [Google Scholar] [CrossRef]
- Hamers-Casterman, C.; Atarhouch, T.; Muyldermans, S.; Robinson, G.; Hamers, C.; Songa, E.B.; Bendahman, N.; Hamers, R. Naturally Occurring Antibodies Devoid of Light Chains. Nature 1993, 363, 446–448. [Google Scholar] [CrossRef]
- Asaadi, Y.; Jouneghani, F.F.; Janani, S.; Rahbarizadeh, F. A Comprehensive Comparison between Camelid Nanobodies and Single Chain Variable Fragments. Biomark. Res. 2021, 9, 87. [Google Scholar] [CrossRef] [PubMed]
- Streltsov, V.A.; Carmichael, J.A.; Nuttall, S.D. Structure of a Shark IgNAR Antibody Variable Domain and Modeling of an Early-Developmental Isotype. Protein Sci. Publ. Protein Soc. 2005, 14, 2901–2909. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Sapienza, G.; Rossotti, M.A.; Tabares-da Rosa, S. Single-Domain Antibodies as Versatile Affinity Reagents for Analytical and Diagnostic Applications. Front. Immunol. 2017, 8, 977. [Google Scholar] [CrossRef] [PubMed]
- Vu, K.B.; Ghahroudi, M.A.; Wyns, L.; Muyldermans, S. Comparison of Llama VH Sequences from Conventional and Heavy Chain Antibodies. Mol. Immunol. 1997, 34, 1121–1131. [Google Scholar] [CrossRef] [PubMed]
- Muyldermans, S.; Smider, V.V. Distinct Antibody Species: Structural Differences Creating Therapeutic Opportunities. Curr. Opin. Immunol. 2016, 40, 7–13. [Google Scholar] [CrossRef]
- Mendoza, M.N.; Jian, M.; King, M.T.; Brooks, C.L. Role of a Noncanonical Disulfide Bond in the Stability, Affinity, and Flexibility of a VHH Specific for the Listeria Virulence Factor InlB. Protein Sci. Publ. Protein Soc. 2020, 29, 1004–1017. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, G.; Lu, H.; Li, H.; Tang, M.; Tong, A. Development of Therapeutic Antibodies for the Treatment of Diseases. Mol. Biomed. 2022, 3, 35. [Google Scholar] [CrossRef]
- Carter, P. Improving the Efficacy of Antibody-Based Cancer Therapies. Nat. Rev. Cancer 2001, 1, 118–129. [Google Scholar] [CrossRef]
- Lu, R.-M.; Hwang, Y.-C.; Liu, I.-J.; Lee, C.-C.; Tsai, H.-Z.; Li, H.-J.; Wu, H.-C. Development of Therapeutic Antibodies for the Treatment of Diseases. J. Biomed. Sci. 2020, 27, 1. [Google Scholar] [CrossRef]
- Smith, S.; Sweetenham, J.W. Iodine 131 Tositumomab in the Treatment of Non-Hodgkin’s Lymphoma. Future Oncol. 2007, 3, 255–262. [Google Scholar] [CrossRef]
- Francis, R.J.; Sharma, S.K.; Springer, C.; Green, A.J.; Hope-Stone, L.D.; Sena, L.; Martin, J.; Adamson, K.L.; Robbins, A.; Gumbrell, L.; et al. A Phase I Trial of Antibody Directed Enzyme Prodrug Therapy (ADEPT) in Patients with Advanced Colorectal Carcinoma or Other CEA Producing Tumours. Br. J. Cancer 2002, 87, 600–607. [Google Scholar] [CrossRef] [PubMed]
- Krauss, W.C.; Park, J.W.; Kirpotin, D.B.; Hong, K.; Benz, C.C. Emerging Antibody-Based HER2 (ErbB-2/Neu) Therapeutics. Breast Dis. 2000, 11, 113–124. [Google Scholar] [CrossRef]
- Labrijn, A.F.; Janmaat, M.L.; Reichert, J.M.; Parren, P.W.H.I. Bispecific Antibodies: A Mechanistic Review of the Pipeline. Nat. Rev. Drug Discov. 2019, 18, 585–608. [Google Scholar] [CrossRef] [PubMed]
- June, C.H.; O’Connor, R.S.; Kawalekar, O.U.; Ghassemi, S.; Milone, M.C. CAR T Cell Immunotherapy for Human Cancer. Science 2018, 359, 1361–1365. [Google Scholar] [CrossRef] [PubMed]
- Brudno, J.N.; Kochenderfer, J.N. Chimeric Antigen Receptor T-Cell Therapies for Lymphoma. Nat. Rev. Clin. Oncol. 2018, 15, 31–46. [Google Scholar] [CrossRef]
- Park, J.H.; Rivière, I.; Gonen, M.; Wang, X.; Sénéchal, B.; Curran, K.J.; Sauter, C.; Wang, Y.; Santomasso, B.; Mead, E.; et al. Long-Term Follow-up of CD19 CAR Therapy in Acute Lymphoblastic Leukemia. N. Engl. J. Med. 2018, 378, 449–459. [Google Scholar] [CrossRef] [PubMed]
- Meeker, T.C.; Lowder, J.; Maloney, D.G.; Miller, R.A.; Thielemans, K.; Warnke, R.; Levy, R. A Clinical Trial of Anti-Idiotype Therapy for B Cell Malignancy. Blood 1985, 65, 1349–1363. [Google Scholar] [CrossRef]
- Richards, J.M.; Vogelzang, N.J.; Bluestone, J.A. Neurotoxicity after Treatment with Muromonab-CD3. N. Engl. J. Med. 1990, 323, 487–488. [Google Scholar] [CrossRef]
- Faulds, D.; Sorkin, E.M. Abciximab (C7E3 Fab). A Review of Its Pharmacology and Therapeutic Potential in Ischaemic Heart Disease. Drugs 1994, 48, 583–598. [Google Scholar] [CrossRef]
- Jones, P.T.; Dear, P.H.; Foote, J.; Neuberger, M.S.; Winter, G. Replacing the Complementarity-Determining Regions in a Human Antibody with Those from a Mouse. Nature 1986, 321, 522–525. [Google Scholar] [CrossRef]
- Tsurushita, N.; Hinton, P.R.; Kumar, S. Design of Humanized Antibodies: From Anti-Tac to Zenapax. Methods 2005, 36, 69–83. [Google Scholar] [CrossRef] [PubMed]
- Vincenti, F.; Kirkman, R.; Light, S.; Bumgardner, G.; Pescovitz, M.; Halloran, P.; Neylan, J.; Wilkinson, A.; Ekberg, H.; Gaston, R.; et al. Interleukin-2-Receptor Blockade with Daclizumab to Prevent Acute Rejection in Renal Transplantation. Daclizumab Triple Therapy Study Group. N. Engl. J. Med. 1998, 338, 161–165. [Google Scholar] [CrossRef] [PubMed]
- McCafferty, J.; Griffiths, A.D.; Winter, G.; Chiswell, D.J. Phage Antibodies: Filamentous Phage Displaying Antibody Variable Domains. Nature 1990, 348, 552–554. [Google Scholar] [CrossRef]
- Jaffe, G.J.; Dick, A.D.; Brézin, A.P.; Nguyen, Q.D.; Thorne, J.E.; Kestelyn, P.; Barisani-Asenbauer, T.; Franco, P.; Heiligenhaus, A.; Scales, D.; et al. Adalimumab in Patients with Active Noninfectious Uveitis. N. Engl. J. Med. 2016, 375, 932–943. [Google Scholar] [CrossRef]
- Kimball, A.B.; Okun, M.M.; Williams, D.A.; Gottlieb, A.B.; Papp, K.A.; Zouboulis, C.C.; Armstrong, A.W.; Kerdel, F.; Gold, M.H.; Forman, S.B.; et al. Two Phase 3 Trials of Adalimumab for Hidradenitis Suppurativa. N. Engl. J. Med. 2016, 375, 422–434. [Google Scholar] [CrossRef] [PubMed]
- Keystone, E.C.; Kavanaugh, A.F.; Sharp, J.T.; Tannenbaum, H.; Hua, Y.; Teoh, L.S.; Fischkoff, S.A.; Chartash, E.K. Radiographic, Clinical, and Functional Outcomes of Treatment with Adalimumab (a Human Anti-Tumor Necrosis Factor Monoclonal Antibody) in Patients with Active Rheumatoid Arthritis Receiving Concomitant Methotrexate Therapy: A Randomized, Placebo-Controlled, 52-Week Trial. Arthritis Rheum. 2004, 50, 1400–1411. [Google Scholar] [CrossRef]
- Kempeni, J. Preliminary Results of Early Clinical Trials with the Fully Human Anti-TNFalpha Monoclonal Antibody D2E7. Ann. Rheum. Dis. 1999, 58 (Suppl. S1), I70–I72. [Google Scholar] [CrossRef]
- Menter, A.; Tyring, S.K.; Gordon, K.; Kimball, A.B.; Leonardi, C.L.; Langley, R.G.; Strober, B.E.; Kaul, M.; Gu, Y.; Okun, M.; et al. Adalimumab Therapy for Moderate to Severe Psoriasis: A Randomized, Controlled Phase III Trial. J. Am. Acad. Dermatol. 2008, 58, 106–115. [Google Scholar] [CrossRef]
- Lonberg, N. Human Antibodies from Transgenic Animals. Nat. Biotechnol. 2005, 23, 1117–1125. [Google Scholar] [CrossRef]
- Moroni, M.; Veronese, S.; Benvenuti, S.; Marrapese, G.; Sartore-Bianchi, A.; Di Nicolantonio, F.; Gambacorta, M.; Siena, S.; Bardelli, A. Gene Copy Number for Epidermal Growth Factor Receptor (EGFR) and Clinical Response to AntiEGFR Treatment in Colorectal Cancer: A Cohort Study. Lancet Oncol. 2005, 6, 279–286. [Google Scholar] [CrossRef]
- Ferris, R.L.; Blumenschein, G.; Fayette, J.; Guigay, J.; Colevas, A.D.; Licitra, L.; Harrington, K.; Kasper, S.; Vokes, E.E.; Even, C.; et al. Nivolumab for Recurrent Squamous-Cell Carcinoma of the Head and Neck. N. Engl. J. Med. 2016, 375, 1856–1867. [Google Scholar] [CrossRef]
- Łuksza, M.; Riaz, N.; Makarov, V.; Balachandran, V.P.; Hellmann, M.D.; Solovyov, A.; Rizvi, N.A.; Merghoub, T.; Levine, A.J.; Chan, T.A.; et al. A Neoantigen Fitness Model Predicts Tumour Response to Checkpoint Blockade Immunotherapy. Nature 2017, 551, 517–520. [Google Scholar] [CrossRef] [PubMed]
- INN Bio Review 2019. Available online: https://www.who.int/publications-detail-redirect/who-emp-rht-tsn-2019-1 (accessed on 30 June 2023).
- Revised Monoclonal Antibody (MAb) Nomenclature Scheme. Available online: https://www.who.int/publications/m/item/inn-17-416 (accessed on 30 June 2023).
- New INN Monoclonal Antibody (MAb) Nomenclature Scheme. Available online: https://www.who.int/publications-detail-redirect/inn-21-531 (accessed on 30 June 2023).
- Center for Drug Evaluation and Research. Biosimilar Product Information. FDA. 2023. Available online: https://www.fda.gov/drugs/biosimilars/biosimilar-product-information (accessed on 30 June 2023).
- Antibody Therapeutics Approved or in Regulatory Review in the EU or US. Available online: https://www.antibodysociety.org/resources/approved-antibodies/ (accessed on 30 June 2023).
- Raybould, M. Helpful Resources for People Studying Therapeutic Antibodies; Oxford Protein Informatics Group: Oxford, UK, 2018. [Google Scholar]
- SAbDab: The Structural Antibody Database. Available online: https://opig.stats.ox.ac.uk/webapps/sabdab-sabpred/therasabdab/about/ (accessed on 30 June 2023).
- Raybould, M.I.J.; Marks, C.; Lewis, A.P.; Shi, J.; Bujotzek, A.; Taddese, B.; Deane, C.M. Thera-SAbDab: The Therapeutic Structural Antibody Database. Nucleic Acids Res. 2020, 48, D383–D388. [Google Scholar] [CrossRef] [PubMed]
- TABS Therapeutic Antibody Database. Available online: https://tabs.craic.com/users/sign_in (accessed on 30 June 2023).
- Yang, L.; Liu, W.; Yu, X.; Wu, M.; Reichert, J.M.; Ho, M. COVID-19 Antibody Therapeutics Tracker: A Global Online Database of Antibody Therapeutics for the Prevention and Treatment of COVID-19. Antib. Ther. 2020, 3, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Drugs@FDA: FDA-Approved Drugs. Available online: https://www.accessdata.fda.gov/scripts/cder/daf/ (accessed on 30 June 2023).
- Ghosh, K.; Ghosh, K. Monoclonal Antibodies Used for the Management of Hemataological Disorders. Expert Rev. Hematol. 2022, 15, 443–455. [Google Scholar] [CrossRef]
- Salles, G.; Barrett, M.; Foà, R.; Maurer, J.; O’Brien, S.; Valente, N.; Wenger, M.; Maloney, D.G. Rituximab in B-Cell Hematologic Malignancies: A Review of 20 Years of Clinical Experience. Adv. Ther. 2017, 34, 2232–2273. [Google Scholar] [CrossRef] [PubMed]
- Cuesta-Mateos, C.; Alcaraz-Serna, A.; Somovilla-Crespo, B.; Muñoz-Calleja, C. Monoclonal Antibody Therapies for Hematological Malignancies: Not Just Lineage-Specific Targets. Front. Immunol. 2017, 8, 1936. [Google Scholar] [CrossRef]
- Rougé, L.; Chiang, N.; Steffek, M.; Kugel, C.; Croll, T.I.; Tam, C.; Estevez, A.; Arthur, C.P.; Koth, C.M.; Ciferri, C.; et al. Structure of CD20 in Complex with the Therapeutic Monoclonal Antibody Rituximab. Science 2020, 367, 1224–1230. [Google Scholar] [CrossRef]
- Oldenburg, J.; Mahlangu, J.N.; Kim, B.; Schmitt, C.; Callaghan, M.U.; Young, G.; Santagostino, E.; Kruse-Jarres, R.; Negrier, C.; Kessler, C.; et al. Emicizumab Prophylaxis in Hemophilia A with Inhibitors. N. Engl. J. Med. 2017, 377, 809–818. [Google Scholar] [CrossRef]
- Thornburg, C.D.; Duncan, N.A. Treatment Adherence in Hemophilia. Patient Prefer. Adherence 2017, 11, 1677–1686. [Google Scholar] [CrossRef]
- Callaghan, M.U.; Negrier, C.; Paz-Priel, I.; Chang, T.; Chebon, S.; Lehle, M.; Mahlangu, J.; Young, G.; Kruse-Jarres, R.; Mancuso, M.E.; et al. Long-Term Outcomes with Emicizumab Prophylaxis for Hemophilia A with or without FVIII Inhibitors from the HAVEN 1-4 Studies. Blood 2021, 137, 2231–2242. [Google Scholar] [CrossRef] [PubMed]
- Blair, H.A. Crizanlizumab: First Approval. Drugs 2020, 80, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Gardner, R.V. Sickle Cell Disease: Advances in Treatment. Ochsner J. 2018, 18, 377–389. [Google Scholar] [CrossRef]
- Gutsaeva, D.R.; Parkerson, J.B.; Yerigenahally, S.D.; Kurz, J.C.; Schaub, R.G.; Ikuta, T.; Head, C.A. Inhibition of Cell Adhesion by Anti–P-Selectin Aptamer: A New Potential Therapeutic Agent for Sickle Cell Disease. Blood 2011, 117, 727–735. [Google Scholar] [CrossRef]
- Ataga, K.I.; Kutlar, A.; Kanter, J.; Liles, D.; Cancado, R.; Friedrisch, J.; Guthrie, T.H.; Knight-Madden, J.; Alvarez, O.A.; Gordeuk, V.R.; et al. Crizanlizumab for the Prevention of Pain Crises in Sickle Cell Disease. N. Engl. J. Med. 2017, 376, 429–439. [Google Scholar] [CrossRef] [PubMed]
- Jurczak, W.; Długosz Danecka, M.; Buske, C. Rituximab Biosimilars for Lymphoma in Europe. Expert Opin. Biol. Ther. 2019, 19, 1045–1056. [Google Scholar] [CrossRef]
- Nava-Parada, P.; Shelbaya, A.; Nabhan, C. Rituximab Biosimilars in Hematologic Malignancies: The Need for a Real-World Approach. Future Oncol. 2020, 16, 2017–2027. [Google Scholar] [CrossRef]
- Jung, S.M.; Kim, W.-U. Targeted Immunotherapy for Autoimmune Disease. Immune Netw. 2022, 22, e9. [Google Scholar] [CrossRef]
- McInnes, I.B.; Schett, G. The Pathogenesis of Rheumatoid Arthritis. N. Engl. J. Med. 2011, 365, 2205–2219. [Google Scholar] [CrossRef]
- Benjamin, O.; Goyal, A.; Lappin, S.L. Disease-Modifying Antirheumatic Drugs (DMARD). In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Mousavi, M.J.; Karami, J.; Aslani, S.; Tahmasebi, M.N.; Vaziri, A.S.; Jamshidi, A.; Farhadi, E.; Mahmoudi, M. Transformation of Fibroblast-like Synoviocytes in Rheumatoid Arthritis; from a Friend to Foe. Autoimmun. Highlights 2021, 12, 3. [Google Scholar] [CrossRef]
- Ogata, A.; Kato, Y.; Higa, S.; Yoshizaki, K. IL-6 Inhibitor for the Treatment of Rheumatoid Arthritis: A Comprehensive Review. Mod. Rheumatol. 2019, 29, 258–267. [Google Scholar] [CrossRef]
- Fleischmann, R.; van Adelsberg, J.; Lin, Y.; da Rocha Castelar-Pinheiro, G.; Brzezicki, J.; Hrycaj, P.; Graham, N.M.H.; van Hoogstraten, H.; Bauer, D.; Burmester, G.R. Sarilumab and Nonbiologic Disease-Modifying Antirheumatic Drugs in Patients With Active Rheumatoid Arthritis and Inadequate Response or Intolerance to Tumor Necrosis Factor Inhibitors. Arthritis Rheumatol. 2017, 69, 277–290. [Google Scholar] [CrossRef]
- Paccaly, A.J.; Kovalenko, P.; Parrino, J.; Boyapati, A.; Xu, C.; van Hoogstraten, H.; Ishii, T.; Davis, J.D.; DiCioccio, A.T. Pharmacokinetics and Pharmacodynamics of Subcutaneous Sarilumab and Intravenous Tocilizumab Following Single-Dose Administration in Patients with Active Rheumatoid Arthritis on Stable Methotrexate. J. Clin. Pharmacol. 2021, 61, 90–104. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, S.; Bujkiewicz, S.; Wailoo, A.J.; Sutton, A.J.; Scott, D. The Effectiveness of Anti-TNF-Alpha Therapies When Used Sequentially in Rheumatoid Arthritis Patients: A Systematic Review and Meta-Analysis. Rheumatology 2010, 49, 2313–2321. [Google Scholar] [CrossRef][Green Version]
- Kaufmann, J.; Feist, E.; Roske, A.-E.; Schmidt, W.A. Monotherapy with Tocilizumab or TNF-Alpha Inhibitors in Patients with Rheumatoid Arthritis: Efficacy, Treatment Satisfaction, and Persistence in Routine Clinical Practice. Clin. Rheumatol. 2013, 32, 1347–1355. [Google Scholar] [CrossRef] [PubMed]
- Costedoat-Chalumeau, N.; Dunogué, B.; Morel, N.; Le Guern, V.; Guettrot-Imbert, G. Hydroxychloroquine: A Multifaceted Treatment in Lupus. Presse Med. 2014, 43, e167–e180. [Google Scholar] [CrossRef] [PubMed]
- Furie, R.; Rovin, B.H.; Houssiau, F.; Malvar, A.; Teng, Y.K.O.; Contreras, G.; Amoura, Z.; Yu, X.; Mok, C.-C.; Santiago, M.B.; et al. Two-Year, Randomized, Controlled Trial of Belimumab in Lupus Nephritis. N. Engl. J. Med. 2020, 383, 1117–1128. [Google Scholar] [CrossRef] [PubMed]
- Kalunian, K.C.; Furie, R.; Morand, E.F.; Bruce, I.N.; Manzi, S.; Tanaka, Y.; Winthrop, K.; Hupka, I.; Zhang, L.; Werther, S.; et al. A Randomized, Placebo-Controlled Phase III Extension Trial of the Long-Term Safety and Tolerability of Anifrolumab in Active Systemic Lupus Erythematosus. Arthritis Rheumatol. 2023, 75, 253–265. [Google Scholar] [CrossRef] [PubMed]
- Beckwith, H.; Lightstone, L. Rituximab in Systemic Lupus Erythematosus and Lupus Nephritis. Nephron Clin. Pract. 2014, 128, 250–254. [Google Scholar] [CrossRef]
- Wu, S.; Wang, Y.; Zhang, J.; Han, B.; Wang, B.; Gao, W.; Zhang, N.; Zhang, C.; Yan, F.; Li, Z. Efficacy and Safety of Rituximab for Systemic Lupus Erythematosus Treatment: A Meta-Analysis. Afr. Health Sci. 2020, 20, 871–884. [Google Scholar] [CrossRef]
- van Vollenhoven, R.F.; Hahn, B.H.; Tsokos, G.C.; Wagner, C.L.; Lipsky, P.; Touma, Z.; Werth, V.P.; Gordon, R.M.; Zhou, B.; Hsu, B.; et al. Efficacy and Safety of Ustekinumab, an IL-12 and IL-23 Inhibitor, in Patients with Active Systemic Lupus Erythematosus: Results of a Multicentre, Double-Blind, Phase 2, Randomised, Controlled Study. Lancet 2018, 392, 1330–1339. [Google Scholar] [CrossRef] [PubMed]
- Kalunian, K.; Furie, R.; Radhakrishnan, J.; Mathur, V.; Polu, K.; Connelly, S.; Rothman, J.; Ng, C.; Chinn, L.; Fung, M.; et al. Itolizumab, a Novel Anti-CD6 Therapy, in Systemic Lupus Erythematosus Patients: Interim Safety Results from the Phase 1b EQUALISE Dose-Escalation Study. Arthritis Rheumatol. 2021, 73. [Google Scholar]
- Tiwari, V.; Brent, L.H. Psoriatic Arthritis. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Ogdie, A.; Coates, L.C.; Gladman, D.D. Treatment Guidelines in Psoriatic Arthritis. Rheumatology 2020, 59, i37–i46. [Google Scholar] [CrossRef]
- Kolbinger, F.; Di Padova, F.; Deodhar, A.; Hawkes, J.E.; Huppertz, C.; Kuiper, T.; McInnes, I.B.; Ritchlin, C.T.; Rosmarin, D.; Schett, G.; et al. Secukinumab for the Treatment of Psoriasis, Psoriatic Arthritis, and Axial Spondyloarthritis: Physical and Pharmacological Properties Underlie the Observed Clinical Efficacy and Safety. Pharmacol. Ther. 2022, 229, 107925. [Google Scholar] [CrossRef] [PubMed]
- Berman, J.; Furer, V.; Berman, M.; Isakov, O.; Zisman, D.; Haddad, A.; Elkayam, O. Treatment with Ixekizumab Following Secukinumab Failure in Patients with Psoriatic Arthritis: Real-Life Experience from a Resistant Population. Biol. Targets Ther. 2021, 15, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Kawalec, P.; Holko, P.; Moćko, P.; Pilc, A. Comparative Effectiveness of Abatacept, Apremilast, Secukinumab and Ustekinumab Treatment of Psoriatic Arthritis: A Systematic Review and Network Meta-Analysis. Rheumatol. Int. 2018, 38, 189–201. [Google Scholar] [CrossRef]
- Bayer, V. An Overview of Monoclonal Antibodies. Semin. Oncol. Nurs. 2019, 35, 150927. [Google Scholar] [CrossRef]
- Arneth, B. Tumor Microenvironment. Medicina 2019, 56, 15. [Google Scholar] [CrossRef]
- Koppolu, V.; Rekha Vasigala, V.K. Checkpoint Immunotherapy by Nivolumab for Treatment of Metastatic Melanoma. J. Cancer Res. Ther. 2018, 14, 1167–1175. [Google Scholar] [CrossRef]
- Xiao, Y.; Yu, D. Tumor Microenvironment as a Therapeutic Target in Cancer. Pharmacol. Ther. 2021, 221, 107753. [Google Scholar] [CrossRef]
- Jin, M.-Z.; Jin, W.-L. The Updated Landscape of Tumor Microenvironment and Drug Repurposing. Signal Transduct. Target. Ther. 2020, 5, 166. [Google Scholar] [CrossRef]
- Tang, F.; Zheng, P. Tumor Cells versus Host Immune Cells: Whose PD-L1 Contributes to PD-1/PD-L1 Blockade Mediated Cancer Immunotherapy? Cell Biosci. 2018, 8, 34. [Google Scholar] [CrossRef]
- Tawbi, H.A.; Schadendorf, D.; Lipson, E.J.; Ascierto, P.A.; Matamala, L.; Castillo Gutiérrez, E.; Rutkowski, P.; Gogas, H.J.; Lao, C.D.; De Menezes, J.J.; et al. Relatlimab and Nivolumab versus Nivolumab in Untreated Advanced Melanoma. N. Engl. J. Med. 2022, 386, 24–34. [Google Scholar] [CrossRef]
- Beck, A.; Goetsch, L.; Dumontet, C.; Corvaïa, N. Strategies and Challenges for the next Generation of Antibody—Drug Conjugates. Nat. Rev. Drug Discov. 2017, 16, 315–337. [Google Scholar] [CrossRef]
- Wei, J.; Yang, Y.; Wang, G.; Liu, M. Current Landscape and Future Directions of Bispecific Antibodies in Cancer Immunotherapy. Front. Immunol. 2022, 13, 1035276. [Google Scholar] [CrossRef]
- von Minckwitz, G.; Huang, C.-S.; Mano, M.S.; Loibl, S.; Mamounas, E.P.; Untch, M.; Wolmark, N.; Rastogi, P.; Schneeweiss, A.; Redondo, A.; et al. Trastuzumab Emtansine for Residual Invasive HER2-Positive Breast Cancer. N. Engl. J. Med. 2019, 380, 617–628. [Google Scholar] [CrossRef]
- Cortés, J.; Kim, S.-B.; Chung, W.-P.; Im, S.-A.; Park, Y.H.; Hegg, R.; Kim, M.H.; Tseng, L.-M.; Petry, V.; Chung, C.-F.; et al. Trastuzumab Deruxtecan versus Trastuzumab Emtansine for Breast Cancer. N. Engl. J. Med. 2022, 386, 1143–1154. [Google Scholar] [CrossRef]
- Enfortumab Vedotin Approved for Recurrent Bladder Cancer—NCI. Available online: https://www.cancer.gov/news-events/cancer-currents-blog/2020/enfortumab-vedotin-bladder-cancer-fda-approval (accessed on 29 June 2023).
- Van Der Weyden, C.; Dickinson, M.; Whisstock, J.; Prince, H.M. Brentuximab Vedotin in T-Cell Lymphoma. Expert Rev. Hematol. 2019, 12, 5–19. [Google Scholar] [CrossRef]
- Ansell, S.M.; Radford, J.; Connors, J.M.; Długosz-Danecka, M.; Kim, W.-S.; Gallamini, A.; Ramchandren, R.; Friedberg, J.W.; Advani, R.; Hutchings, M.; et al. Overall Survival with Brentuximab Vedotin in Stage III or IV Hodgkin’s Lymphoma. N. Engl. J. Med. 2022, 387, 310–320. [Google Scholar] [CrossRef]
- Kuruvilla, J.; Ramchandren, R.; Santoro, A.; Paszkiewicz-Kozik, E.; Gasiorowski, R.; Johnson, N.A.; Fogliatto, L.M.; Goncalves, I.; de Oliveira, J.S.R.; Buccheri, V.; et al. Pembrolizumab versus Brentuximab Vedotin in Relapsed or Refractory Classical Hodgkin Lymphoma (KEYNOTE-204): An Interim Analysis of a Multicentre, Randomised, Open-Label, Phase 3 Study. Lancet Oncol. 2021, 22, 512–524. [Google Scholar] [CrossRef]
- Yamazaki, C.M.; Yamaguchi, A.; Anami, Y.; Xiong, W.; Otani, Y.; Lee, J.; Ueno, N.T.; Zhang, N.; An, Z.; Tsuchikama, K. Antibody-Drug Conjugates with Dual Payloads for Combating Breast Tumor Heterogeneity and Drug Resistance. Nat. Commun. 2021, 12, 3528. [Google Scholar] [CrossRef]
- Pedley, R.B.; Sharma, S.K.; Boxer, G.M.; Boden, R.; Stribbling, S.M.; Davies, L.; Springer, C.J.; Begent, R.H. Enhancement of Antibody-Directed Enzyme Prodrug Therapy in Colorectal Xenografts by an Antivascular Agent. Cancer Res. 1999, 59, 3998–4003. [Google Scholar] [PubMed]
- Sécher, T.; Guilleminault, L.; Reckamp, K.; Amanam, I.; Plantier, L.; Heuzé-Vourc’h, N. Therapeutic Antibodies: A New Era in the Treatment of Respiratory Diseases? Pharmacol. Ther. 2018, 189, 149–172. [Google Scholar] [CrossRef]
- Pelaia, C.; Calabrese, C.; Terracciano, R.; de Blasio, F.; Vatrella, A.; Pelaia, G. Omalizumab, the First Available Antibody for Biological Treatment of Severe Asthma: More than a Decade of Real-Life Effectiveness. Ther. Adv. Respir. Dis. 2018, 12, 1753466618810192. [Google Scholar] [CrossRef]
- Agache, I.; Rocha, C.; Beltran, J.; Song, Y.; Posso, M.; Solà, I.; Alonso-Coello, P.; Akdis, C.; Akdis, M.; Canonica, G.W.; et al. Efficacy and Safety of Treatment with Biologicals (Benralizumab, Dupilumab and Omalizumab) for Severe Allergic Asthma: A Systematic Review for the EAACI Guidelines—Recommendations on the Use of Biologicals in Severe Asthma. Allergy 2020, 75, 1043–1057. [Google Scholar] [CrossRef] [PubMed]
- Cazzola, M.; Ora, J.; Cavalli, F.; Rogliani, P.; Matera, M.G. An Overview of the Safety and Efficacy of Monoclonal Antibodies for the Chronic Obstructive Pulmonary Disease. Biol. Targets Ther. 2021, 15, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Lyly, A.; Laulajainen-Hongisto, A.; Gevaert, P.; Kauppi, P.; Toppila-Salmi, S. Monoclonal Antibodies and Airway Diseases. Int. J. Mol. Sci. 2020, 21, 9477. [Google Scholar] [CrossRef]
- Rodriguez-Fernandez, R.; Mejias, A.; Ramilo, O. Monoclonal Antibodies for Prevention of Respiratory Syncytial Virus Infection. Pediatr. Infect. Dis. J. 2021, 40, S35–S39. [Google Scholar] [CrossRef]
- Que, Y.-A.; Lazar, H.; Wolff, M.; François, B.; Laterre, P.-F.; Mercier, E.; Garbino, J.; Pagani, J.-L.; Revelly, J.-P.; Mus, E.; et al. Assessment of Panobacumab as Adjunctive Immunotherapy for the Treatment of Nosocomial Pseudomonas Aeruginosa Pneumonia. Eur. J. Clin. Microbiol. Infect. Dis. Off. Publ. Eur. Soc. Clin. Microbiol. 2014, 33, 1861–1867. [Google Scholar] [CrossRef]
- Chastre, J.; François, B.; Bourgeois, M.; Komnos, A.; Ferrer, R.; Rahav, G.; De Schryver, N.; Lepape, A.; Koksal, I.; Luyt, C.-E.; et al. Safety, Efficacy, and Pharmacokinetics of Gremubamab (MEDI3902), an Anti-Pseudomonas Aeruginosa Bispecific Human Monoclonal Antibody, in P. Aeruginosa-Colonised, Mechanically Ventilated Intensive Care Unit Patients: A Randomised Controlled Trial. Crit. Care 2022, 26, 355. [Google Scholar] [CrossRef]
- Anti-SARS-CoV-2 Monoclonal Antibodies. Available online: https://www.covid19treatmentguidelines.nih.gov/therapies/antivirals-including-antibody-products/anti-sars-cov-2-monoclonal-antibodies/ (accessed on 30 June 2023).
- What’s New. Available online: https://www.covid19treatmentguidelines.nih.gov/about-the-guidelines/whats-new/ (accessed on 30 June 2023).
- Evusheld. HHS/ASPR. Available online: https://aspr.hhs.gov:443/COVID-19/Therapeutics/Products/Evusheld/Pages/default.aspx (accessed on 30 June 2023).
- Sahebkar, A.; Watts, G.F. New Therapies Targeting ApoB Metabolism for High-Risk Patients with Inherited Dyslipidaemias: What Can the Clinician Expect? Cardiovasc. Drugs Ther. 2013, 27, 559–567. [Google Scholar] [CrossRef] [PubMed]
- Fitchett, D.H.; Hegele, R.A.; Verma, S. Statin Intolerance. Circulation 2015, 131, e389–e391. [Google Scholar] [CrossRef] [PubMed]
- Bohula, E.A.; Giugliano, R.P.; Leiter, L.A.; Verma, S.; Park, J.-G.; Sever, P.S.; Lira Pineda, A.; Honarpour, N.; Wang, H.; Murphy, S.A.; et al. Inflammatory and Cholesterol Risk in the FOURIER Trial. Circulation 2018, 138, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Manniello, M.; Pisano, M. Alirocumab (Praluent): First in the New Class of PCSK9 Inhibitors. Pharm. Ther. 2016, 41, 28–53. [Google Scholar]
- Center for Drug Evaluation and Research. FDA Approves Add-on Therapy for Patients with Genetic Form of Severely High Cholesterol. FDA. 2021. Available online: https://www.fda.gov/drugs/news-events-human-drugs/fda-approves-add-therapy-patients-genetic-form-severely-high-cholesterol (accessed on 30 June 2023).
- Schwartz, G.G.; Steg, P.G.; Szarek, M.; Bhatt, D.L.; Bittner, V.A.; Diaz, R.; Edelberg, J.M.; Goodman, S.G.; Hanotin, C.; Harrington, R.A.; et al. Alirocumab and Cardiovascular Outcomes after Acute Coronary Syndrome. N. Engl. J. Med. 2018, 379, 2097–2107. [Google Scholar] [CrossRef]
- Guedeney, P.; Giustino, G.; Sorrentino, S.; Claessen, B.E.; Camaj, A.; Kalkman, D.N.; Vogel, B.; Sartori, S.; De Rosa, S.; Baber, U.; et al. Efficacy and Safety of Alirocumab and Evolocumab: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Eur. Heart J. 2022, 43, e17–e25. [Google Scholar] [CrossRef]
- Guedeney, P.; Sorrentino, S.; Giustino, G.; Chapelle, C.; Laporte, S.; Claessen, B.E.; Ollier, E.; Camaj, A.; Kalkman, D.N.; Vogel, B.; et al. Indirect Comparison of the Efficacy and Safety of Alirocumab and Evolocumab: A Systematic Review and Network Meta-Analysis. Eur. Heart J. Cardiovasc. Pharmacother. 2021, 7, 225–235. [Google Scholar] [CrossRef]
- Räber, L.; Ueki, Y.; Otsuka, T.; Losdat, S.; Häner, J.D.; Lonborg, J.; Fahrni, G.; Iglesias, J.F.; van Geuns, R.-J.; Ondracek, A.S.; et al. Effect of Alirocumab Added to High-Intensity Statin Therapy on Coronary Atherosclerosis in Patients with Acute Myocardial Infarction: The PACMAN-AMI Randomized Clinical Trial. JAMA 2022, 327, 1771–1781. [Google Scholar] [CrossRef]
- Ridker, P.M.; Everett, B.M.; Thuren, T.; MacFadyen, J.G.; Chang, W.H.; Ballantyne, C.; Fonseca, F.; Nicolau, J.; Koenig, W.; Anker, S.D.; et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N. Engl. J. Med. 2017, 377, 1119–1131. [Google Scholar] [CrossRef]
- Ridker, P.M.; Rane, M. Interleukin-6 Signaling and Anti-Interleukin-6 Therapeutics in Cardiovascular Disease. Circ. Res. 2021, 128, 1728–1746. [Google Scholar] [CrossRef]
- Vallurupalli, M.; MacFadyen, J.G.; Glynn, R.J.; Thuren, T.; Libby, P.; Berliner, N.; Ridker, P.M. Effects of Interleukin-1β Inhibition on Incident Anemia. Ann. Intern. Med. 2020, 172, 523–532. [Google Scholar] [CrossRef]
- Sheppard, M.; Laskou, F.; Stapleton, P.P.; Hadavi, S.; Dasgupta, B. Tocilizumab (Actemra). Hum. Vaccines Immunother. 2017, 13, 1972–1988. [Google Scholar] [CrossRef]
- Castagné, B.; Viprey, M.; Martin, J.; Schott, A.-M.; Cucherat, M.; Soubrier, M. Cardiovascular Safety of Tocilizumab: A Systematic Review and Network Meta-Analysis. PLoS ONE 2019, 14, e0220178. [Google Scholar] [CrossRef]
- Effects of Tofacitinib and Other DMARDs on Lipid Profiles in Rheumatoid Arthritis: Implications for the Rheumatologist—ClinicalKey. Available online: https://www-clinicalkey-com.gcsom.idm.oclc.org/#!/content/playContent/1-s2.0-S0049017216000949?returnurl=https:%2F%2Flinkinghub.elsevier.com%2Fretrieve%2Fpii%2FS0049017216000949%3Fshowall%3Dtrue&referrer= (accessed on 30 June 2023).
- Toshner, M.; Church, C.; Harbaum, L.; Rhodes, C.; Moreschi, S.S.V.; Liley, J.; Jones, R.; Arora, A.; Batai, K.; Desai, A.A.; et al. Mendelian Randomisation and Experimental Medicine Approaches to Interleukin-6 as a Drug Target in Pulmonary Arterial Hypertension. Eur. Respir. J. 2022, 59, 2002463. [Google Scholar] [CrossRef]
- Santoro, D.; Pellicanò, V.; Visconti, L.; Trifirò, G.; Cernaro, V.; Buemi, M. Monoclonal Antibodies for Renal Diseases: Current Concepts and Ongoing Treatments. Expert Opin. Biol. Ther. 2015, 15, 1119–1143. [Google Scholar] [CrossRef] [PubMed]
- Ravani, P.; Bonanni, A.; Rossi, R.; Caridi, G.; Ghiggeri, G.M. Anti-CD20 Antibodies for Idiopathic Nephrotic Syndrome in Children. Clin. J. Am. Soc. Nephrol. 2016, 11, 710–720. [Google Scholar] [CrossRef] [PubMed]
- Kattah, A.G.; Fervenza, F.C. Rituximab: Emerging Treatment Strategies of Immune-Mediated Glomerular Disease. Expert Rev. Clin. Immunol. 2012, 8, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Siligato, R.; Cernaro, V.; Nardi, C.; De Gregorio, F.; Gembillo, G.; Costantino, G.; Conti, G.; Buemi, M.; Santoro, D. Emerging Therapeutic Strategies for Minimal Change Disease and Focal and Segmental Glomerulosclerosis. Expert Opin. Investig. Drugs 2018, 27, 839–879. [Google Scholar] [CrossRef] [PubMed]
- Manrique, J.; Cravedi, P. Role of Monoclonal Antibodies in the Treatment of Immune-Mediated Glomerular Diseases. Nefrol. Publ. Soc. Esp. Nefrol. 2014, 34, 388–397. [Google Scholar] [CrossRef]
- Abbas, A.; Mirza, M.M.; Ganti, A.K.; Tendulkar, K. Renal Toxicities of Targeted Therapies. Target. Oncol. 2015, 10, 487–499. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, M.; Pohin, M.; Powrie, F. Cytokine Networks in the Pathophysiology of Inflammatory Bowel Disease. Immunity 2019, 50, 992–1006. [Google Scholar] [CrossRef]
- de Souza, H.S.P.; Fiocchi, C. Immunopathogenesis of IBD: Current State of the Art. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 13–27. [Google Scholar] [CrossRef] [PubMed]
- Geremia, A.; Biancheri, P.; Allan, P.; Corazza, G.R.; Di Sabatino, A. Innate and Adaptive Immunity in Inflammatory Bowel Disease. Autoimmun. Rev. 2014, 13, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Van Deventer, S.J. Tumour Necrosis Factor and Crohn’s Disease. Gut 1997, 40, 443–448. [Google Scholar] [CrossRef] [PubMed]
- Brynskov, J.; Nielsen, O.H.; Ahnfelt-Rønne, I.; Bendtzen, K. Cytokines (Immunoinflammatory Hormones) and Their Natural Regulation in Inflammatory Bowel Disease (Crohn’s Disease and Ulcerative Colitis): A Review. Dig. Dis. 2008, 12, 290–304. [Google Scholar] [CrossRef]
- Liang, S.; Dai, J.; Hou, S.; Su, L.; Zhang, D.; Guo, H.; Hu, S.; Wang, H.; Rao, Z.; Guo, Y.; et al. Structural Basis for Treating Tumor Necrosis Factor α (TNFα)-Associated Diseases with the Therapeutic Antibody Infliximab. J. Biol. Chem. 2013, 288, 13799–13807. [Google Scholar] [CrossRef]
- Knight, D.M.; Trinh, H.; Le, J.; Siegel, S.; Shealy, D.; McDonough, M.; Scallon, B.; Moore, M.A.; Vilcek, J.; Daddona, P. Construction and Initial Characterization of a Mouse-Human Chimeric Anti-TNF Antibody. Mol. Immunol. 1993, 30, 1443–1453. [Google Scholar] [CrossRef]
- Hanauer, S.B.; Feagan, B.G.; Lichtenstein, G.R.; Mayer, L.F.; Schreiber, S.; Colombel, J.F.; Rachmilewitz, D.; Wolf, D.C.; Olson, A.; Bao, W.; et al. Maintenance Infliximab for Crohn’s Disease: The ACCENT I Randomised Trial. Lancet 2002, 359, 1541–1549. [Google Scholar] [CrossRef]
- Rutgeerts, P.; Sandborn, W.J.; Feagan, B.G.; Reinisch, W.; Olson, A.; Johanns, J.; Travers, S.; Rachmilewitz, D.; Hanauer, S.B.; Lichtenstein, G.R.; et al. Infliximab for Induction and Maintenance Therapy for Ulcerative Colitis. N. Engl. J. Med. 2005, 353, 2462–2476. [Google Scholar] [CrossRef]
- Rutgeerts, P.; D’Haens, G.; Targan, S.; Vasiliauskas, E.; Hanauer, S.B.; Present, D.H.; Mayer, L.; Van Hogezand, R.A.; Braakman, T.; DeWoody, K.L.; et al. Efficacy and Safety of Retreatment with Anti-Tumor Necrosis Factor Antibody (Infliximab) to Maintain Remission in Crohn’s Disease. Gastroenterology 1999, 117, 761–769. [Google Scholar] [CrossRef]
- Lichtenstein, G.R.; Rutgeerts, P.; Sandborn, W.J.; Sands, B.E.; Diamond, R.H.; Blank, M.; Montello, J.; Tang, L.; Cornillie, F.; Colombel, J.-F. A Pooled Analysis of Infections, Malignancy, and Mortality in Infliximab- and Immunomodulator-Treated Adult Patients with Inflammatory Bowel Disease. Am. J. Gastroenterol. 2012, 107, 1051–1063. [Google Scholar] [CrossRef]
- Fidder, H.; Schnitzler, F.; Ferrante, M.; Noman, M.; Katsanos, K.; Segaert, S.; Henckaerts, L.; Van Assche, G.; Vermeire, S.; Rutgeerts, P. Long-Term Safety of Infliximab for the Treatment of Inflammatory Bowel Disease: A Single-Centre Cohort Study. Gut 2009, 58, 501–508. [Google Scholar] [CrossRef] [PubMed]
- Hanauer, S.B.; Sandborn, W.J.; Rutgeerts, P.; Fedorak, R.N.; Lukas, M.; MacIntosh, D.; Panaccione, R.; Wolf, D.; Pollack, P. Human Anti-Tumor Necrosis Factor Monoclonal Antibody (Adalimumab) in Crohn’s Disease: The CLASSIC-I Trial. Gastroenterology 2006, 130, 323–333, quiz 591. [Google Scholar] [CrossRef] [PubMed]
- Colombel, J.-F.; Sandborn, W.J.; Rutgeerts, P.; Enns, R.; Hanauer, S.B.; Panaccione, R.; Schreiber, S.; Byczkowski, D.; Li, J.; Kent, J.D.; et al. Adalimumab for Maintenance of Clinical Response and Remission in Patients with Crohn’s Disease: The CHARM Trial. Gastroenterology 2007, 132, 52–65. [Google Scholar] [CrossRef] [PubMed]
- Sandborn, W.J.; Hanauer, S.B.; Rutgeerts, P.; Fedorak, R.N.; Lukas, M.; MacIntosh, D.G.; Panaccione, R.; Wolf, D.; Kent, J.D.; Bittle, B.; et al. Adalimumab for Maintenance Treatment of Crohn’s Disease: Results of the CLASSIC II Trial. Gut 2007, 56, 1232–1239. [Google Scholar] [CrossRef] [PubMed]
- Sandborn, W.J.; van Assche, G.; Reinisch, W.; Colombel, J.; D’Haens, G.; Wolf, D.C.; Kron, M.; Tighe, M.B.; Lazar, A.; Thakkar, R.B. Adalimumab Induces and Maintains Clinical Remission in Patients with Moderate-to-Severe Ulcerative Colitis. Gastroenterology 2012, 142, 257–265.e3. [Google Scholar] [CrossRef]
- Reinisch, W.; Sandborn, W.J.; Hommes, D.W.; D’Haens, G.; Hanauer, S.; Schreiber, S.; Panaccione, R.; Fedorak, R.N.; Tighe, M.B.; Huang, B.; et al. Adalimumab for Induction of Clinical Remission in Moderately to Severely Active Ulcerative Colitis: Results of a Randomised Controlled Trial. Gut 2011, 60, 780–787. [Google Scholar] [CrossRef]
- Afif, W.; Leighton, J.A.; Hanauer, S.B.; Loftus, E.V., Jr.; Faubion, W.A.; Pardi, D.S.; Tremaine, W.J.; Kane, S.V.; Bruining, D.H.; Cohen, R.D.; et al. Open-Label Study of Adalimumab in Patients with Ulcerative Colitis Including Those with Prior Loss of Response or Intolerance to Infliximab. Inflamm. Bowel Dis. 2009, 15, 1302–1307. [Google Scholar] [CrossRef]
- Osterman, M.T.; Haynes, K.; Delzell, E.; Zhang, J.; Bewtra, M.; Brensinger, C.; Chen, L.; Xie, F.; Curtis, J.R.; Lewis, J.D. Comparative Effectiveness of Infliximab and Adalimumab for Crohn’s Disease. Clin. Gastroenterol. Hepatol. 2014, 12, 811–817.e3. [Google Scholar] [CrossRef]
- Lee, Y.I.; Park, Y.; Park, S.J.; Kim, T.I.; Kim, W.H.; Cheon, J.H. Comparison of Long-Term Outcomes of Infliximab versus Adalimumab Treatment in Biologic-Naïve Patients with Ulcerative Colitis. Gut Liver 2021, 15, 232–242. [Google Scholar] [CrossRef]
- Baert, F.; Kondragunta, V.; Lockton, S.; Casteele, N.V.; Hauenstein, S.; Singh, S.; Karmiris, K.; Ferrante, M.; Gils, A.; Vermeire, S. Antibodies to Adalimumab Are Associated with Future Inflammation in Crohn’s Patients Receiving Maintenance Adalimumab Therapy: A Post Hoc Analysis of the Karmiris Trial. Gut 2016, 65, 1126–1131. [Google Scholar] [CrossRef] [PubMed]
- Baert, F.; Noman, M.; Vermeire, S.; Van Assche, G.; D’ Haens, G.; Carbonez, A.; Rutgeerts, P. Influence of Immunogenicity on the Long-Term Efficacy of Infliximab in Crohn’s Disease. N. Engl. J. Med. 2003, 348, 601–608. [Google Scholar] [CrossRef]
- Gisbert, J.P.; Marín, A.C.; McNicholl, A.G.; Chaparro, M. Systematic Review with Meta-Analysis: The Efficacy of a Second Anti-TNF in Patients with Inflammatory Bowel Disease Whose Previous Anti-TNF Treatment Has Failed. Aliment. Pharmacol. Ther. 2015, 41, 613–623. [Google Scholar] [CrossRef] [PubMed]
- Roblin, X.; Vérot, C.; Paul, S.; Duru, G.; Williet, N.; Boschetti, G.; Del Tedesco, E.; Peyrin-Biroulet, L.; Phelip, J.M.; Nancey, S.; et al. Is the Pharmacokinetic Profile of a First Anti-TNF Predictive of the Clinical Outcome and Pharmacokinetics of a Second Anti-TNF? Inflamm. Bowel Dis. 2018, 24, 2078–2085. [Google Scholar] [CrossRef]
- Ong, D.E.H.; Kamm, M.A.; Hartono, J.L.; Lust, M. Addition of Thiopurines Can Recapture Response in Patients with Crohn’s Disease Who Have Lost Response to Anti-Tumor Necrosis Factor Monotherapy. J. Gastroenterol. Hepatol. 2013, 28, 1595–1599. [Google Scholar] [CrossRef]
- Ben-Horin, S.; Waterman, M.; Kopylov, U.; Yavzori, M.; Picard, O.; Fudim, E.; Awadie, H.; Weiss, B.; Chowers, Y. Addition of an Immunomodulator to Infliximab Therapy Eliminates Antidrug Antibodies in Serum and Restores Clinical Response of Patients with Inflammatory Bowel Disease. Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc. 2013, 11, 444–447. [Google Scholar] [CrossRef] [PubMed]
- Colombel, J.F.; Sandborn, W.J.; Reinisch, W.; Mantzaris, G.J.; Kornbluth, A.; Rachmilewitz, D.; Lichtiger, S.; D’Haens, G.; Diamond, R.H.; Broussard, D.L.; et al. Infliximab, Azathioprine, or Combination Therapy for Crohn’s Disease. N. Engl. J. Med. 2010, 362, 1383–1395. [Google Scholar] [CrossRef]
- Panaccione, R.; Ghosh, S.; Middleton, S.; Márquez, J.R.; Scott, B.B.; Flint, L.; van Hoogstraten, H.J.F.; Chen, A.C.; Zheng, H.; Danese, S.; et al. Combination Therapy with Infliximab and Azathioprine Is Superior to Monotherapy with Either Agent in Ulcerative Colitis. Gastroenterology 2014, 146, 392–400.e3. [Google Scholar] [CrossRef]
- Feuerstein, J.D.; Isaacs, K.L.; Schneider, Y.; Siddique, S.M.; Falck-Ytter, Y.; Singh, S.; Chachu, K.; Day, L.; Lebwohl, B.; Muniraj, T.; et al. AGA Clinical Practice Guidelines on the Management of Moderate to Severe Ulcerative Colitis. Gastroenterology 2020, 158, 1450–1461. [Google Scholar] [CrossRef]
- Kirchgesner, J.; Lemaitre, M.; Carrat, F.; Zureik, M.; Carbonnel, F.; Dray-Spira, R. Risk of Serious and Opportunistic Infections Associated with Treatment of Inflammatory Bowel Diseases. Gastroenterology 2018, 155, 337–346.e10. [Google Scholar] [CrossRef]
- Lemaitre, M.; Kirchgesner, J.; Rudnichi, A.; Carrat, F.; Zureik, M.; Carbonnel, F.; Dray-Spira, R. Association between Use of Thiopurines or Tumor Necrosis Factor Antagonists Alone or in Combination and Risk of Lymphoma in Patients with Inflammatory Bowel Disease. JAMA 2017, 318, 1679–1686. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, H.; So, R.; Matsuoka, K.; Kobayashi, T.; Shinzaki, S.; Matsuura, M.; Okabayashi, S.; Kataoka, Y.; Tsujimoto, Y.; Furukawa, T.A.; et al. Certolizumab Pegol for Induction of Remission in Crohn’s Disease. Cochrane Database Syst. Rev. 2019, 8, CD012893. [Google Scholar] [CrossRef]
- Goel, N.; Stephens, S. Certolizumab Pegol. mAbs 2010, 2, 137–147. [Google Scholar] [CrossRef]
- Sandborn, W.J.; Feagan, B.G.; Stoinov, S.; Honiball, P.J.; Rutgeerts, P.; Mason, D.; Bloomfield, R.; Schreiber, S. Certolizumab Pegol for the Treatment of Crohn’s Disease. N. Engl. J. Med. 2007, 357, 228–238. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, S.; Khaliq-Kareemi, M.; Lawrance, I.C.; Thomsen, O.Ø.; Hanauer, S.B.; McColm, J.; Bloomfield, R.; Sandborn, W.J. PRECISE 2 Study Investigators Maintenance Therapy with Certolizumab Pegol for Crohn’s Disease. N. Engl. J. Med. 2007, 357, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Lang, L. FDA Approves Cimzia to Treat Crohn’s Disease. Gastroenterology 2008, 134, 1819. [Google Scholar] [CrossRef]
- Chapman, A.P.; Antoniw, P.; Spitali, M.; West, S.; Stephens, S.; King, D.J. Therapeutic Antibody Fragments with Prolonged in Vivo Half-Lives. Nat. Biotechnol. 1999, 17, 780–783. [Google Scholar] [CrossRef]
- Blick, S.K.A.; Curran, M.P. Certolizumab Pegol. BioDrugs 2007, 21, 195–201. [Google Scholar] [CrossRef]
- de Bourayne, M.; Meunier, S.; Bitoun, S.; Correia, E.; Mariette, X.; Nozach, H.; Maillère, B. Pegylation Reduces the Uptake of Certolizumab Pegol by Dendritic Cells and Epitope Presentation to T-Cells. Front. Immunol. 2022, 13, 808606. [Google Scholar] [CrossRef]
- Sandborn, W.J.; Feagan, B.G.; Marano, C.; Zhang, H.; Strauss, R.; Johanns, J.; Adedokun, O.J.; Guzzo, C.; Colombel, J.-F.; Reinisch, W.; et al. Subcutaneous Golimumab Induces Clinical Response and Remission in Patients with Moderate-to-Severe Ulcerative Colitis. Gastroenterology 2014, 146, 85–95, quiz e14–15. [Google Scholar] [CrossRef]
- Flamant, M.; Paul, S.; Roblin, X. Golimumab for the Treatment of Ulcerative Colitis. Expert Opin. Biol. Ther. 2017, 17, 879–886. [Google Scholar] [CrossRef]
- Danese, S.; Fiorino, G.; Peyrin-Biroulet, L.; Lucenteforte, E.; Virgili, G.; Moja, L.; Bonovas, S. Biological Agents for Moderately to Severely Active Ulcerative Colitis: A Systematic Review and Network Meta-Analysis. Ann. Intern. Med. 2014, 160, 704–711. [Google Scholar] [CrossRef]
- Stidham, R.W.; Lee, T.C.H.; Higgins, P.D.R.; Deshpande, A.R.; Sussman, D.A.; Singal, A.G.; Elmunzer, B.J.; Saini, S.D.; Vijan, S.; Waljee, A.K. Systematic Review with Network Meta-Analysis: The Efficacy of Anti-TNF Agents for the Treatment of Crohn’s Disease. Aliment. Pharmacol. Ther. 2014, 39, 1349–1362. [Google Scholar] [CrossRef]
- Thomas, S.S.; Borazan, N.; Barroso, N.; Duan, L.; Taroumian, S.; Kretzmann, B.; Bardales, R.; Elashoff, D.; Vangala, S.; Furst, D.E. Comparative Immunogenicity of TNF Inhibitors: Impact on Clinical Efficacy and Tolerability in the Management of Autoimmune Diseases. A Systematic Review and Meta-Analysis. BioDrugs 2015, 29, 241–258. [Google Scholar] [CrossRef]
- Vermeire, S.; Gils, A.; Accossato, P.; Lula, S.; Marren, A. Immunogenicity of Biologics in Inflammatory Bowel Disease. Ther. Adv. Gastroenterol. 2018, 11, 1756283X17750355. [Google Scholar] [CrossRef]
- Nakamura, K.; Honda, K.; Mizutani, T.; Akiho, H.; Harada, N. Novel Strategies for the Treatment of Inflammatory Bowel Disease: Selective Inhibition of Cytokines and Adhesion Molecules. World J. Gastroenterol. 2006, 12, 4628–4635. [Google Scholar] [CrossRef]
- Guagnozzi, D.; Caprilli, R. Natalizumab in the Treatment of Crohn’s Disease. Biol. Targets Ther. 2008, 2, 275–284. [Google Scholar]
- Sandborn, W.J.; Feagan, B.G.; Rutgeerts, P.; Hanauer, S.; Colombel, J.-F.; Sands, B.E.; Lukas, M.; Fedorak, R.N.; Lee, S.; Bressler, B.; et al. Vedolizumab as Induction and Maintenance Therapy for Crohn’s Disease. N. Engl. J. Med. 2013, 369, 711–721. [Google Scholar] [CrossRef]
- Nelson, S.M.; Nguyen, T.M.; McDonald, J.W.; MacDonald, J.K. Natalizumab for Induction of Remission in Crohn’s Disease. Cochrane Database Syst. Rev. 2018, 2018, CD006097. [Google Scholar] [CrossRef]
- Rubin, D.T.; Ananthakrishnan, A.N.; Siegel, C.A.; Sauer, B.G.; Long, M.D. ACG Clinical Guideline: Ulcerative Colitis in Adults. Off. J. Am. Coll. Gastroenterol. ACG 2019, 114, 384. [Google Scholar] [CrossRef]
- Sandborn, W.J.; Panés, J.; Danese, S.; Sharafali, Z.; Hassanali, A.; Jacob-Moffatt, R.; Eden, C.; Daperno, M.; Valentine, J.F.; Laharie, D.; et al. Etrolizumab as Induction and Maintenance Therapy in Patients with Moderately to Severely Active Crohn’s Disease (BERGAMOT): A Randomised, Placebo-Controlled, Double-Blind, Phase 3 Trial. Lancet Gastroenterol. Hepatol. 2023, 8, 43–55. [Google Scholar] [CrossRef]
- Laurel, P.I.; Gardenia, P.I.; Hickory, P.I.; Bergamot, P.I. Genentech Provides Update on Phase III Studies of Etrolizumab in People with Moderately to Severely Active Ulcerative Colitis; Bloomberg.com: New York, NY, USA, 2020; Available online: https://www.businesswire.com/news/home/20200809005022/en/Genentech-Provides-Update-on-Phase-III-Studies-of-Etrolizumab-in-People-With-Moderately-to-Severely-Active-Ulcerative-Colitis (accessed on 30 June 2023).
- Caron, B.; Peyrin-Biroulet, L.; Pariente, B.; Bouhnik, Y.; Seksik, P.; Bouguen, G.; Caillo, L.; Laharie, D.; Carbonnel, F.; Altwegg, R.; et al. Vedolizumab Therapy Is Ineffective for Primary Sclerosing Cholangitis in Patients with Inflammatory Bowel Disease: A GETAID Multicentre Cohort Study. J. Crohns Colitis 2019, 13, 1239–1247. [Google Scholar] [CrossRef]
- Lynch, K.D.; Chapman, R.W.; Keshav, S.; Montano-Loza, A.J.; Mason, A.L.; Kremer, A.E.; Vetter, M.; de Krijger, M.; Ponsioen, C.Y.; Trivedi, P.; et al. Effects of Vedolizumab in Patients with Primary Sclerosing Cholangitis and Inflammatory Bowel Diseases. Clin. Gastroenterol. Hepatol. 2020, 18, 179–187.e6. [Google Scholar] [CrossRef]
- Laborda, T.J.; Ricciuto, A.; Aumar, M.; Carman, N.; DiGuglielmo, M.; Draijer, L.G.; Furuya, K.N.; Gupta, N.; Koot, B.G.P.; Loomes, K.M.; et al. Vedolizumab Therapy in Children With Primary Sclerosing Cholangitis: Data from the Pediatric Primary Sclerosing Cholangitis Consortium. J. Pediatr. Gastroenterol. Nutr. 2020, 71, 459. [Google Scholar] [CrossRef]
- Eksteen, B.; Grant, A.J.; Miles, A.; Curbishley, S.M.; Lalor, P.F.; Hübscher, S.G.; Briskin, M.; Salmon, M.; Adams, D.H. Hepatic Endothelial CCL25 Mediates the Recruitment of CCR9+ Gut-Homing Lymphocytes to the Liver in Primary Sclerosing Cholangitis. J. Exp. Med. 2004, 200, 1511–1517. [Google Scholar] [CrossRef]
- Grant, A.J.; Lalor, P.F.; Hübscher, S.G.; Briskin, M.; Adams, D.H. MAdCAM-1 Expressed in Chronic Inflammatory Liver Disease Supports Mucosal Lymphocyte Adhesion to Hepatic Endothelium (MAdCAM-1 in Chronic Inflammatory Liver Disease). Hepatology 2001, 33, 1065–1072. [Google Scholar] [CrossRef]
- Muir, A.J.; Levy, C.; Janssen, H.L.A.; Montano-Loza, A.J.; Shiffman, M.L.; Caldwell, S.; Luketic, V.; Ding, D.; Jia, C.; McColgan, B.J.; et al. Simtuzumab for Primary Sclerosing Cholangitis: Phase 2 Study Results with Insights on the Natural History of the Disease. Hepatology 2019, 69, 684–698. [Google Scholar] [CrossRef]
- Harrison, S.A.; Abdelmalek, M.F.; Caldwell, S.; Shiffman, M.L.; Diehl, A.M.; Ghalib, R.; Lawitz, E.J.; Rockey, D.C.; Schall, R.A.; Jia, C.; et al. Simtuzumab Is Ineffective for Patients With Bridging Fibrosis or Compensated Cirrhosis Caused by Nonalcoholic Steatohepatitis. Gastroenterology 2018, 155, 1140–1153. [Google Scholar] [CrossRef]
- Segal-Salto, M.; Barashi, N.; Katav, A.; Edelshtein, V.; Aharon, A.; Hashmueli, S.; George, J.; Maor, Y.; Pinzani, M.; Haberman, D.; et al. A Blocking Monoclonal Antibody to CCL24 Alleviates Liver Fibrosis and Inflammation in Experimental Models of Liver Damage. JHEP Rep. 2020, 2, 100064. [Google Scholar] [CrossRef]
- Roufosse, F. Targeting the Interleukin-5 Pathway for Treatment of Eosinophilic Conditions Other than Asthma. Front. Med. 2018, 5, 49. [Google Scholar] [CrossRef]
- Cases, Data, and Surveillance. Available online: https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/burden.html (accessed on 30 June 2023).
- Richards, F.; Kodjamanova, P.; Chen, X.; Li, N.; Atanasov, P.; Bennetts, L.; Patterson, B.J.; Yektashenas, B.; Mesa-Frias, M.; Tronczynski, K.; et al. Economic Burden of COVID-19: A Systematic Review. Clin. Outcomes Res. 2022, 14, 293–307. [Google Scholar] [CrossRef]
- Bloom, D.E.; Kuhn, M.; Prettner, K. Modern Infectious Diseases: Macroeconomic Impacts and Policy Responses. J. Econ. Lit. 2022, 60, 85–131. [Google Scholar] [CrossRef]
- Young, M.; Smitherman, L. Socioeconomic Impact of RSV Hospitalization. Infect. Dis. Ther. 2021, 10, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Crank, M.C.; Ruckwardt, T.J.; Chen, M.; Morabito, K.M.; Phung, E.; Costner, P.J.; Holman, L.A.; Hickman, S.P.; Berkowitz, N.M.; Gordon, I.J.; et al. A Proof of Concept for Structure-Based Vaccine Design Targeting RSV in Humans. Science 2019, 365, 505–509. [Google Scholar] [CrossRef] [PubMed]
- Boyoglu-Barnum, S.; Chirkova, T.; Anderson, L.J. Biology of Infection and Disease Pathogenesis to Guide RSV Vaccine Development. Front. Immunol. 2019, 10, 1675. [Google Scholar] [CrossRef]
- Garegnani, L.; Styrmisdóttir, L.; Rodriguez, P.R.; Liquitay, C.M.E.; Esteban, I.; Franco, J.V. Palivizumab for Preventing Severe Respiratory Syncytial Virus (RSV) Infection in Children. Cochrane Database Syst. Rev. 2021, 11, CD013757. [Google Scholar] [CrossRef]
- Scott, L.J.; Lamb, H.M. Palivizumab. Drugs 1999, 58, 305–311. [Google Scholar] [CrossRef]
- Mac, S.; Sumner, A.; Duchesne-Belanger, S.; Stirling, R.; Tunis, M.; Sander, B. Cost-Effectiveness of Palivizumab for Respiratory Syncytial Virus: A Systematic Review. Pediatrics 2019, 143, e20184064. [Google Scholar] [CrossRef]
- Lee, A. Ansuvimab: First Approval. Drugs 2021, 81, 595–598. [Google Scholar] [CrossRef]
- Markham, A. REGN-EB3: First Approval. Drugs 2021, 81, 175–178. [Google Scholar] [CrossRef]
- Rayaprolu, V.; Fulton, B.; Rafique, A.; Arturo, E.; Williams, D.; Hariharan, C.; Callaway, H.; Parvate, A.; Schendel, S.L.; Parekh, D.; et al. Structure of the Inmazeb Cocktail and Resistance to Escape against Ebola Virus. bioRxiv 2022. [Google Scholar] [CrossRef]
- Pantaleo, G.; Correia, B.; Fenwick, C.; Joo, V.S.; Perez, L. Antibodies to Combat Viral Infections: Development Strategies and Progress. Nat. Rev. Drug Discov. 2022, 21, 676–696. [Google Scholar] [CrossRef] [PubMed]
- Andreano, E.; Seubert, A.; Rappuoli, R. Human Monoclonal Antibodies for Discovery, Therapy, and Vaccine Acceleration. Curr. Opin. Immunol. 2019, 59, 130–134. [Google Scholar] [CrossRef] [PubMed]
- Tisch, R.; McDevitt, H. Insulin-Dependent Diabetes Mellitus. Cell 1996, 85, 291–297. [Google Scholar] [CrossRef]
- Van Belle, T.L.; Coppieters, K.T.; Von Herrath, M.G. Type 1 Diabetes: Etiology, Immunology, and Therapeutic Strategies. Physiol. Rev. 2011, 91, 79–118. [Google Scholar] [CrossRef]
- Eisenbarth, G.S. Type 1 Diabetes: Molecular, Cellular and Clinical Immunology. Adv. Exp. Med. Biol. 2004, 552, 306–310. [Google Scholar]
- Deshpande, A.D.; Harris-Hayes, M.; Schootman, M. Epidemiology of Diabetes and Diabetes-Related Complications. Phys. Ther. 2008, 88, 1254–1264. [Google Scholar] [CrossRef]
- Evans-Molina, C.; Oram, R.A. Teplizumab Approval for Type 1 Diabetes in the USA. Lancet Diabetes Endocrinol. 2023, 11, 76–77. [Google Scholar] [CrossRef]
- Long, S.A.; Thorpe, J.; DeBerg, H.A.; Gersuk, V.; Eddy, J.A.; Harris, K.M.; Ehlers, M.; Herold, K.C.; Nepom, G.T.; Linsley, P.S. Partial Exhaustion of CD8 T Cells and Clinical Response to Teplizumab in New-Onset Type 1 Diabetes. Sci. Immunol. 2016, 1, eaai7793. [Google Scholar] [CrossRef]
- Tooley, J.E.; Vudattu, N.; Choi, J.; Cotsapas, C.; Devine, L.; Raddassi, K.; Ehlers, M.R.; McNamara, J.G.; Harris, K.M.; Kanaparthi, S.; et al. Changes in T-Cell Subsets Identify Responders to FcR-Nonbinding Anti-CD3 MAb (Teplizumab) in Patients with Type 1 Diabetes. Eur. J. Immunol. 2016, 46, 230–241. [Google Scholar] [CrossRef]
- Kuhn, C.; You, S.; Valette, F.; Hale, G.; van Endert, P.; Bach, J.-F.; Waldmann, H.; Chatenoud, L. Human CD3 Transgenic Mice: Preclinical Testing of Antibodies Promoting Immune Tolerance. Sci. Transl. Med. 2011, 3, 68ra10. [Google Scholar] [CrossRef] [PubMed]
- You, S.; Leforban, B.; Garcia, C.; Bach, J.-F.; Bluestone, J.A.; Chatenoud, L. Adaptive TGF-β-Dependent Regulatory T Cells Control Autoimmune Diabetes and Are a Privileged Target of Anti-CD3 Antibody Treatment. Proc. Natl. Acad. Sci. USA 2007, 104, 6335–6340. [Google Scholar] [CrossRef]
- Waldron-Lynch, F.; Henegariu, O.; Deng, S.; Preston-Hurlburt, P.; Tooley, J.; Flavell, R.; Herold, K.C. Teplizumab Induces Human Gut-Tropic Regulatory Cells in Humanized Mice and Patients. Sci. Transl. Med. 2012, 4, 118ra12. [Google Scholar] [CrossRef] [PubMed]
- Sims, E.K.; Bundy, B.N.; Stier, K.; Serti, E.; Lim, N.; Long, S.A.; Geyer, S.M.; Moran, A.; Greenbaum, C.J.; Evans-Molina, C.; et al. Teplizumab Improves and Stabilizes Beta Cell Function in Antibody-Positive High-Risk Individuals. Sci. Transl. Med. 2021, 13, eabc8980. [Google Scholar] [CrossRef]
- Perdigoto, A.L.; Preston-Hurlburt, P.; Clark, P.; Long, S.A.; Linsley, P.S.; Harris, K.M.; Gitelman, S.E.; Greenbaum, C.J.; Gottlieb, P.A.; Hagopian, W.; et al. Treatment of Type 1 Diabetes with Teplizumab: Clinical and Immunological Follow-up after 7 Years from Diagnosis. Diabetologia 2019, 62, 655–664. [Google Scholar] [CrossRef]
- Nourelden, A.Z.; Elshanbary, A.A.; El-Sherif, L.; Benmelouka, A.Y.; Rohim, H.I.; Helmy, S.K.; Sayed, M.K.; Ismail, A.; Ali, A.S.; Ragab, K.M.; et al. Safety and Efficacy of Teplizumab for Treatment of Type One Diabetes Mellitus: A Systematic Review and Meta-Analysis. Endocr. Metab. Immune Disord.-Drug Targets (Former. Curr. Drug Targets Immune Endocr. Metab. Disord.) 2021, 21, 1895–1904. [Google Scholar] [CrossRef]
- Herold, K.C.; Gitelman, S.E.; Ehlers, M.R.; Gottlieb, P.A.; Greenbaum, C.J.; Hagopian, W.; Boyle, K.D.; Keyes-Elstein, L.; Aggarwal, S.; Phippard, D.; et al. Teplizumab (Anti-CD3 MAb) Treatment Preserves C-Peptide Responses in Patients with New-Onset Type 1 Diabetes in a Randomized Controlled Trial: Metabolic and Immunologic Features at Baseline Identify a Subgroup of Responders. Diabetes 2013, 62, 3766–3774. [Google Scholar] [CrossRef]
- Sherry, N.; Hagopian, W.; Ludvigsson, J.; Jain, S.M.; Wahlen, J.; Ferry, R.J.; Bode, B.; Aronoff, S.; Holland, C.; Carlin, D.; et al. Teplizumab for Treatment of Type 1 Diabetes (Protégé Study): 1-Year Results from a Randomised, Placebo-Controlled Trial. Lancet 2011, 378, 487–497. [Google Scholar] [CrossRef] [PubMed]
- Herold, K.C.; Bundy, B.N.; Long, S.A.; Bluestone, J.A.; DiMeglio, L.A.; Dufort, M.J.; Gitelman, S.E.; Gottlieb, P.A.; Krischer, J.P.; Linsley, P.S.; et al. An Anti-CD3 Antibody, Teplizumab, in Relatives at Risk for Type 1 Diabetes. N. Engl. J. Med. 2019, 381, 603–613. [Google Scholar] [CrossRef]
- Vlasakakis, G.; Napolitano, A.; Barnard, R.; Brown, K.; Bullman, J.; Inman, D.; Keymeulen, B.; Lanham, D.; Leirens, Q.; MacDonald, A.; et al. Target Engagement and Cellular Fate of Otelixizumab: A Repeat Dose Escalation Study of an Anti-CD3ε MAb in New-Onset Type 1 Diabetes Mellitus Patients. Br. J. Clin. Pharmacol. 2019, 85, 704–714. [Google Scholar] [CrossRef]
- Keymeulen, B.; van Maurik, A.; Inman, D.; Oliveira, J.; McLaughlin, R.; Gittelman, R.M.; Roep, B.O.; Gillard, P.; Hilbrands, R.; Gorus, F.; et al. A Randomised, Single-Blind, Placebo-Controlled, Dose-Finding Safety and Tolerability Study of the Anti-CD3 Monoclonal Antibody Otelixizumab in New-Onset Type 1 Diabetes. Diabetologia 2021, 64, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Keymeulen, B.; Vandemeulebroucke, E.; Ziegler, A.G.; Mathieu, C.; Kaufman, L.; Hale, G.; Gorus, F.; Goldman, M.; Walter, M.; Candon, S.; et al. Insulin Needs after CD3-Antibody Therapy in New-Onset Type 1 Diabetes. N. Engl. J. Med. 2005, 352, 2598–2608. [Google Scholar] [CrossRef] [PubMed]
- Gitelman, S.E.; Gottlieb, P.A.; Rigby, M.R.; Felner, E.I.; Willi, S.M.; Fisher, L.K.; Moran, A.; Gottschalk, M.; Moore, W.V.; Pinckney, A.; et al. Antithymocyte Globulin Treatment for Patients with Recent-Onset Type 1 Diabetes: 12-Month Results of a Randomised, Placebo-Controlled, Phase 2 Trial. Lancet Diabetes Endocrinol. 2013, 1, 306–316. [Google Scholar] [CrossRef] [PubMed]
- Gitelman, S.E.; Gottlieb, P.A.; Felner, E.I.; Willi, S.M.; Fisher, L.K.; Moran, A.; Gottschalk, M.; Moore, W.V.; Pinckney, A.; Keyes-Elstein, L.; et al. Antithymocyte Globulin Therapy for Patients with Recent-Onset Type 1 Diabetes: 2 Year Results of a Randomised Trial. Diabetologia 2016, 59, 1153–1161. [Google Scholar] [CrossRef]
- Haller, M.J.; Gitelman, S.E.; Gottlieb, P.A.; Michels, A.W.; Rosenthal, S.M.; Shuster, J.J.; Zou, B.; Brusko, T.M.; Hulme, M.A.; Wasserfall, C.H.; et al. Anti-Thymocyte Globulin/G-CSF Treatment Preserves β Cell Function in Patients with Established Type 1 Diabetes. J. Clin. Investig. 2015, 125, 448–455. [Google Scholar] [CrossRef]
- Haller, M.J.; Gitelman, S.E.; Gottlieb, P.A.; Michels, A.W.; Perry, D.J.; Schultz, A.R.; Hulme, M.A.; Shuster, J.J.; Zou, B.; Wasserfall, C.H.; et al. Antithymocyte Globulin Plus G-CSF Combination Therapy Leads to Sustained Immunomodulatory and Metabolic Effects in a Subset of Responders With Established Type 1 Diabetes. Diabetes 2016, 65, 3765–3775. [Google Scholar] [CrossRef]
- Haller, M.J.; Atkinson, M.A.; Wasserfall, C.H.; Brusko, T.M.; Mathews, C.E.; Hulme, M.; Cintron, M.; Shuster, J.; McGrail, K.; Posgai, A.; et al. Mobilization without Immune Depletion Fails to Restore Immunological Tolerance or Preserve Beta Cell Function in Recent Onset Type 1 Diabetes. Clin. Exp. Immunol. 2016, 183, 350–357. [Google Scholar] [CrossRef]
- Haller, M.J.; Schatz, D.A.; Skyler, J.S.; Krischer, J.P.; Bundy, B.N.; Miller, J.L.; Atkinson, M.A.; Becker, D.J.; Baidal, D.; DiMeglio, L.A.; et al. Low-Dose Anti-Thymocyte Globulin (ATG) Preserves β-Cell Function and Improves HbA1c in New-Onset Type 1 Diabetes. Diabetes Care 2018, 41, 1917–1925. [Google Scholar] [CrossRef]
- Haller, M.J.; Long, S.A.; Blanchfield, J.L.; Schatz, D.A.; Skyler, J.S.; Krischer, J.P.; Bundy, B.N.; Geyer, S.M.; Warnock, M.V.; Miller, J.L.; et al. Low-Dose Anti-Thymocyte Globulin Preserves C-Peptide, Reduces HbA1c, and Increases Regulatory to Conventional T-Cell Ratios in New-Onset Type 1 Diabetes: Two-Year Clinical Trial Data. Diabetes 2019, 68, 1267–1276. [Google Scholar] [CrossRef]
- Jacobsen, L.M.; Bundy, B.N.; Greco, M.N.; Schatz, D.A.; Atkinson, M.A.; Brusko, T.M.; Mathews, C.E.; Herold, K.C.; Gitelman, S.E.; Krischer, J.P.; et al. Comparing Beta Cell Preservation across Clinical Trials in Recent-Onset Type 1 Diabetes. Diabetes Technol. Ther. 2020, 22, 948–953. [Google Scholar] [CrossRef]
- Pescovitz, M.D.; Greenbaum, C.J.; Krause-Steinrauf, H.; Becker, D.J.; Gitelman, S.E.; Goland, R.; Gottlieb, P.A.; Marks, J.B.; McGee, P.F.; Moran, A.M.; et al. Rituximab, B-Lymphocyte Depletion, and Preservation of Beta-Cell Function. N. Engl. J. Med. 2009, 361, 2143–2152. [Google Scholar] [CrossRef] [PubMed]
- Pescovitz, M.D.; Greenbaum, C.J.; Bundy, B.; Becker, D.J.; Gitelman, S.E.; Goland, R.; Gottlieb, P.A.; Marks, J.B.; Moran, A.; Raskin, P.; et al. B-Lymphocyte Depletion With Rituximab and β-Cell Function: Two-Year Results. Diabetes Care 2014, 37, 453–459. [Google Scholar] [CrossRef]
- Garg, N.; Smith, T.W. An Update on Immunopathogenesis, Diagnosis, and Treatment of Multiple Sclerosis. Brain Behav. 2015, 5, e00362. [Google Scholar] [CrossRef] [PubMed]
- Selewski, D.T.; Shah, G.V.; Segal, B.M.; Rajdev, P.A.; Mukherji, S.K. Natalizumab (Tysabri). AJNR Am. J. Neuroradiol. 2010, 31, 1588–1590. [Google Scholar] [CrossRef] [PubMed]
- Bates, D.; Bartholomé, E. Treatment Effect of Natalizumab on Relapse Outcomes in Multiple Sclerosis Patients despite Ongoing MRI Activity. J. Neurol. Neurosurg. Psychiatry 2012, 83, 55–60. [Google Scholar] [CrossRef]
- Clerico, M.; Artusi, C.A.; Liberto, A.D.; Rolla, S.; Bardina, V.; Barbero, P.; Mercanti, S.F.D.; Durelli, L. Natalizumab in Multiple Sclerosis: Long-Term Management. Int. J. Mol. Sci. 2017, 18, 940. [Google Scholar] [CrossRef]
- Ho, P.-R.; Koendgen, H.; Campbell, N.; Haddock, B.; Richman, S.; Chang, I. Risk of Natalizumab-Associated Progressive Multifocal Leukoencephalopathy in Patients with Multiple Sclerosis: A Retrospective Analysis of Data from Four Clinical Studies. Lancet Neurol. 2017, 16, 925–933. [Google Scholar] [CrossRef]
- Morrow, S.A.; Clift, F.; Devonshire, V.; Lapointe, E.; Schneider, R.; Stefanelli, M.; Vosoughi, R. Use of Natalizumab in Persons with Multiple Sclerosis: 2022 Update. Mult. Scler. Relat. Disord. 2022, 65, 103995. [Google Scholar] [CrossRef]
- Rommer, P.S.; Dudesek, A.; Stüve, O.; Zettl, U. Monoclonal Antibodies in Treatment of Multiple Sclerosis. Clin. Exp. Immunol. 2014, 175, 373–384. [Google Scholar] [CrossRef]
- Rostagno, A.A. Pathogenesis of Alzheimer’s Disease. Int. J. Mol. Sci. 2023, 24, 107. [Google Scholar] [CrossRef]
- Lacorte, E.; Ancidoni, A.; Zaccaria, V.; Remoli, G.; Tariciotti, L.; Bellomo, G.; Sciancalepore, F.; Corbo, M.; Lombardo, F.L.; Bacigalupo, I.; et al. Safety and Efficacy of Monoclonal Antibodies for Alzheimer’s Disease: A Systematic Review and Meta-Analysis of Published and Unpublished Clinical Trials. J. Alzheimers Dis. 2022, 87, 101–129. [Google Scholar] [CrossRef] [PubMed]
- Vaz, M.; Silvestre, S. Alzheimer’s Disease: Recent Treatment Strategies. Eur. J. Pharmacol. 2020, 887, 173554. [Google Scholar] [CrossRef] [PubMed]
- Kastanenka, K.V.; Bussiere, T.; Shakerdge, N.; Qian, F.; Weinreb, P.H.; Rhodes, K.; Bacskai, B.J. Immunotherapy with Aducanumab Restores Calcium Homeostasis in Tg2576 Mice. J. Neurosci. Off. J. Soc. Neurosci. 2016, 36, 12549–12558. [Google Scholar] [CrossRef] [PubMed]
- Budd Haeberlein, S.; Aisen, P.S.; Barkhof, F.; Chalkias, S.; Chen, T.; Cohen, S.; Dent, G.; Hansson, O.; Harrison, K.; von Hehn, C.; et al. Two Randomized Phase 3 Studies of Aducanumab in Early Alzheimer’s Disease. J. Prev. Alzheimers Dis. 2022, 9, 197–210. [Google Scholar] [CrossRef]
- Sevigny, J.; Chiao, P.; Bussière, T.; Weinreb, P.H.; Williams, L.; Maier, M.; Dunstan, R.; Salloway, S.; Chen, T.; Ling, Y.; et al. The Antibody Aducanumab Reduces Aβ Plaques in Alzheimer’s Disease. Nature 2016, 537, 50–56. [Google Scholar] [CrossRef]
- Shi, M.; Chu, F.; Zhu, F.; Zhu, J. Impact of Anti-Amyloid-β Monoclonal Antibodies on the Pathology and Clinical Profile of Alzheimer’s Disease: A Focus on Aducanumab and Lecanemab. Front. Aging Neurosci. 2022, 14, 870517. [Google Scholar] [CrossRef]
- Panza, F.; Frisardi, V.; Imbimbo, B.P.; Seripa, D.; Paris, F.; Santamato, A.; D’Onofrio, G.; Logroscino, G.; Pilotto, A.; Solfrizzi, V. Anti-β-Amyloid Immunotherapy for Alzheimer’s Disease: Focus on Bapineuzumab. Curr. Alzheimer Res. 2011, 8, 808–817. [Google Scholar] [CrossRef]
- Salloway, S.; Sperling, R.; Fox, N.C.; Blennow, K.; Klunk, W.; Raskind, M.; Sabbagh, M.; Honig, L.S.; Porsteinsson, A.P.; Ferris, S.; et al. Two Phase 3 Trials of Bapineuzumab in Mild-to-Moderate Alzheimer’s Disease. N. Engl. J. Med. 2014, 370, 322–333. [Google Scholar] [CrossRef]
- La Porte, S.L.; Bollini, S.S.; Lanz, T.A.; Abdiche, Y.N.; Rusnak, A.S.; Ho, W.-H.; Kobayashi, D.; Harrabi, O.; Pappas, D.; Mina, E.W.; et al. Structural Basis of C-Terminal β-Amyloid Peptide Binding by the Antibody Ponezumab for the Treatment of Alzheimer’s Disease. J. Mol. Biol. 2012, 421, 525–536. [Google Scholar] [CrossRef]
- Landen, J.W.; Andreasen, N.; Cronenberger, C.L.; Schwartz, P.F.; Börjesson-Hanson, A.; Östlund, H.; Sattler, C.A.; Binneman, B.; Bednar, M.M. Ponezumab in Mild-to-Moderate Alzheimer’s Disease: Randomized Phase II PET-PIB Study. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2017, 3, 393–401. [Google Scholar] [CrossRef]
- Burré, J.; Sharma, M.; Südhof, T.C. Cell Biology and Pathophysiology of α-Synuclein. Cold Spring Harb. Perspect. Med. 2018, 8, a024091. [Google Scholar] [CrossRef] [PubMed]
- Pagano, G.; Taylor, K.I.; Anzures-Cabrera, J.; Marchesi, M.; Simuni, T.; Marek, K.; Postuma, R.B.; Pavese, N.; Stocchi, F.; Azulay, J.-P.; et al. Trial of Prasinezumab in Early-Stage Parkinson’s Disease. N. Engl. J. Med. 2022, 387, 421–432. [Google Scholar] [CrossRef] [PubMed]
- Peters, G.L. Migraine Overview and Summary of Current and Emerging Treatment Options. Am. J. Manag. Care 2019, 25, S23–S34. [Google Scholar] [PubMed]
- Nappi, R.E.; Tiranini, L.; Sacco, S.; De Matteis, E.; De Icco, R.; Tassorelli, C. Role of Estrogens in Menstrual Migraine. Cells 2022, 11, 1355. [Google Scholar] [CrossRef] [PubMed]
- Goadsby, P.J.; Reuter, U.; Hallström, Y.; Broessner, G.; Bonner, J.H.; Zhang, F.; Sapra, S.; Picard, H.; Mikol, D.D.; Lenz, R.A. A Controlled Trial of Erenumab for Episodic Migraine. N. Engl. J. Med. 2017, 377, 2123–2132. [Google Scholar] [CrossRef]
- Detke, H.C.; Goadsby, P.J.; Wang, S.; Friedman, D.I.; Selzler, K.J.; Aurora, S.K. Galcanezumab in Chronic Migraine: The Randomized, Double-Blind, Placebo-Controlled REGAIN Study. Neurology 2018, 91, e2211. [Google Scholar] [CrossRef]
- Silberstein, S.D.; Dodick, D.W.; Bigal, M.E.; Yeung, P.P.; Goadsby, P.J.; Blankenbiller, T.; Grozinski-Wolff, M.; Yang, R.; Ma, Y.; Aycardi, E. Fremanezumab for the Preventive Treatment of Chronic Migraine. N. Engl. J. Med. 2017, 377, 2113–2122. [Google Scholar] [CrossRef]
- Goadsby, P.J.; Silberstein, S.D.; Yeung, P.P.; Cohen, J.M.; Ning, X.; Yang, R.; Dodick, D.W. Long-Term Safety, Tolerability, and Efficacy of Fremanezumab in Migraine: A Randomized Study. Neurology 2020, 95, e2487–e2499. [Google Scholar] [CrossRef]
- Gao, B.; Sun, N.; Yang, Y.; Sun, Y.; Chen, M.; Chen, Z.; Wang, Z. Safety and Efficacy of Fremanezumab for the Prevention of Migraine: A Meta-Analysis from Randomized Controlled Trials. Front. Neurol. 2020, 11, 435. [Google Scholar] [CrossRef]
- Lee, A.; Shirley, M. Ranibizumab: A Review in Retinopathy of Prematurity. Paediatr. Drugs 2021, 23, 111–117. [Google Scholar] [CrossRef]
- Kaushal, M.; Razak, A.; Patel, W.; Pullattayil, A.K.; Kaushal, A. Neurodevelopmental Outcomes Following Bevacizumab Treatment for Retinopathy of Prematurity: A Systematic Review and Meta-Analysis. J. Perinatol. Off. J. Calif. Perinat. Assoc. 2021, 41, 1225–1235. [Google Scholar] [CrossRef] [PubMed]
- Markham, A. Naxitamab: First Approval. Drugs 2021, 81, 291–296. [Google Scholar] [CrossRef]
- Hanley, D.A.; Adachi, J.D.; Bell, A.; Brown, V. Denosumab: Mechanism of Action and Clinical Outcomes. Int. J. Clin. Pract. 2012, 66, 1139–1146. [Google Scholar] [CrossRef]
- Cosman, F.; Crittenden, D.B.; Adachi, J.D.; Binkley, N.; Czerwinski, E.; Ferrari, S.; Hofbauer, L.C.; Lau, E.; Lewiecki, E.M.; Miyauchi, A.; et al. Romosozumab Treatment in Postmenopausal Women with Osteoporosis. N. Engl. J. Med. 2016, 375, 1532–1543. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Dutta, S.; Khasbage, S.; Kumar, T.; Sachin, J.; Sharma, J.; Varthya, S.B. A Systematic Review and Meta-Analysis of Efficacy and Safety of Romosozumab in Postmenopausal Osteoporosis. Osteoporos. Int. J. Establ. Result Coop. Eur. Found. Osteoporos. Natl. Osteoporos. Found. USA 2022, 33, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Kobayakawa, T.; Miyazaki, A.; Saito, M.; Suzuki, T.; Takahashi, J.; Nakamura, Y. Denosumab versus Romosozumab for Postmenopausal Osteoporosis Treatment. Sci. Rep. 2021, 11, 11801. [Google Scholar] [CrossRef]
- Imel, E.A. Burosumab for Pediatric X-Linked Hypophosphatemia. Curr. Osteoporos. Rep. 2021, 19, 271–277. [Google Scholar] [CrossRef]
- Schindeler, A.; Biggin, A.; Munns, C.F. Clinical Evidence for the Benefits of Burosumab Therapy for X-Linked Hypophosphatemia (XLH) and Other Conditions in Adults and Children. Front. Endocrinol. 2020, 11, 338. [Google Scholar] [CrossRef] [PubMed]
- Brandi, M.L.; Jan de Beur, S.; Briot, K.; Carpenter, T.; Cheong, H.I.; Cohen-Solal, M.; Crowley, R.K.; Eastell, R.; Imanishi, Y.; Imel, E.A.; et al. Efficacy of Burosumab in Adults with X-Linked Hypophosphatemia (XLH): A Post Hoc Subgroup Analysis of a Randomized Double-Blind Placebo-Controlled Phase 3 Study. Calcif. Tissue Int. 2022, 111, 409–418. [Google Scholar] [CrossRef]
- Tannemaat, M.R.; Verschuuren, J.J.G.M. Emerging Therapies for Autoimmune Myasthenia Gravis: Towards Treatment without Corticosteroids. Neuromuscul. Disord. 2020, 30, 111–119. [Google Scholar] [CrossRef]
- Shapiro, A.D. Concizumab: A Novel Anti-TFPI Therapeutic for Hemophilia. Blood Adv. 2021, 5, 279. [Google Scholar] [CrossRef] [PubMed]
- Röth, A.; Barcellini, W.; D’Sa, S.; Miyakawa, Y.; Broome, C.M.; Michel, M.; Kuter, D.J.; Jilma, B.; Tvedt, T.H.A.; Fruebis, J.; et al. Sutimlimab in Cold Agglutinin Disease. N. Engl. J. Med. 2021, 384, 1323–1334. [Google Scholar] [CrossRef] [PubMed]
- Scully, M.; Cataland, S.R.; Peyvandi, F.; Coppo, P.; Knöbl, P.; Kremer Hovinga, J.A.; Metjian, A.; de la Rubia, J.; Pavenski, K.; Callewaert, F.; et al. Caplacizumab Treatment for Acquired Thrombotic Thrombocytopenic Purpura. N. Engl. J. Med. 2019, 380, 335–346. [Google Scholar] [CrossRef]
- Stern, R.M.; Connell, N.T. Ravulizumab: A Novel C5 Inhibitor for the Treatment of Paroxysmal Nocturnal Hemoglobinuria. Ther. Adv. Hematol. 2019, 10, 2040620719874728. [Google Scholar] [CrossRef] [PubMed]
- Nooka, A.K.; Kaufman, J.L.; Hofmeister, C.C.; Joseph, N.S.; Heffner, T.L.; Gupta, V.A.; Sullivan, H.C.; Neish, A.S.; Dhodapkar, M.V.; Lonial, S. Daratumumab in Multiple Myeloma. Cancer 2019, 125, 2364–2382. [Google Scholar] [CrossRef]
- Dimopoulos, M.A.; Richardson, P.G.; Bahlis, N.J.; Grosicki, S.; Cavo, M.; Beksaç, M.; Legieć, W.; Liberati, A.M.; Goldschmidt, H.; Belch, A.; et al. Addition of Elotuzumab to Lenalidomide and Dexamethasone for Patients with Newly Diagnosed, Transplantation Ineligible Multiple Myeloma (ELOQUENT-1): An Open-Label, Multicentre, Randomised, Phase 3 Trial. Lancet Haematol. 2022, 9, e403–e414. [Google Scholar] [CrossRef] [PubMed]
- Pollack, C.V.; Reilly, P.A.; van Ryn, J.; Eikelboom, J.W.; Glund, S.; Bernstein, R.A.; Dubiel, R.; Huisman, M.V.; Hylek, E.M.; Kam, C.-W.; et al. Idarucizumab for Dabigatran Reversal—Full Cohort Analysis. N. Engl. J. Med. 2017, 377, 431–441. [Google Scholar] [CrossRef]
- Brodsky, R.A.; Young, N.S.; Antonioli, E.; Risitano, A.M.; Schrezenmeier, H.; Schubert, J.; Gaya, A.; Coyle, L.; de Castro, C.; Fu, C.-L.; et al. Multicenter Phase 3 Study of the Complement Inhibitor Eculizumab for the Treatment of Patients with Paroxysmal Nocturnal Hemoglobinuria. Blood 2008, 111, 1840–1847. [Google Scholar] [CrossRef]
- Griffin, M.M.; Morley, N. Rituximab in the Treatment of Non-Hodgkin’s Lymphoma—A Critical Evaluation of Randomized Controlled Trials. Expert Opin. Biol. Ther. 2013, 13, 803–811. [Google Scholar] [CrossRef]
- Grosicki, S.; Bednarczyk, M.; Kociszewska, K. Elranatamab: A New Promising BispAb in Multiple Myeloma Treatment. Expert Rev. Anticancer Ther. 2023, 23, 775–782. [Google Scholar] [CrossRef]
- Chari, A.; Minnema, M.C.; Berdeja, J.G.; Oriol, A.; van de Donk, N.W.C.J.; Rodríguez-Otero, P.; Askari, E.; Mateos, M.-V.; Costa, L.J.; Caers, J.; et al. Talquetamab, a T-Cell-Redirecting GPRC5D Bispecific Antibody for Multiple Myeloma. N. Engl. J. Med. 2022, 387, 2232–2244. [Google Scholar] [CrossRef] [PubMed]
- Thieblemont, C.; Phillips, T.; Ghesquieres, H.; Cheah, C.Y.; Clausen, M.R.; Cunningham, D.; Do, Y.R.; Feldman, T.; Gasiorowski, R.; Jurczak, W.; et al. Epcoritamab, a Novel, Subcutaneous CD3xCD20 Bispecific T-Cell-Engaging Antibody, in Relapsed or Refractory Large B-Cell Lymphoma: Dose Expansion in a Phase I/II Trial. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2023, 41, 2238–2247. [Google Scholar] [CrossRef]
- Dickinson, M.J.; Carlo-Stella, C.; Morschhauser, F.; Bachy, E.; Corradini, P.; Iacoboni, G.; Khan, C.; Wróbel, T.; Offner, F.; Trněný, M.; et al. Glofitamab for Relapsed or Refractory Diffuse Large B-Cell Lymphoma. N. Engl. J. Med. 2022, 387, 2220–2231. [Google Scholar] [CrossRef] [PubMed]
- Coles, A.J.; Twyman, C.L.; Arnold, D.L.; Cohen, J.A.; Confavreux, C.; Fox, E.J.; Hartung, H.-P.; Havrdova, E.; Selmaj, K.W.; Weiner, H.L.; et al. Alemtuzumab for Patients with Relapsing Multiple Sclerosis after Disease-Modifying Therapy: A Randomised Controlled Phase 3 Trial. Lancet 2012, 380, 1829–1839. [Google Scholar] [CrossRef]
- Fox, E.; Lovett-Racke, A.E.; Gormley, M.; Liu, Y.; Petracca, M.; Cocozza, S.; Shubin, R.; Wray, S.; Weiss, M.S.; Bosco, J.A.; et al. A Phase 2 Multicenter Study of Ublituximab, a Novel Glycoengineered Anti-CD20 Monoclonal Antibody, in Patients with Relapsing Forms of Multiple Sclerosis. Mult. Scler. Houndmills Basingstoke Engl. 2021, 27, 420–429. [Google Scholar] [CrossRef]
- Moreau, P.; Garfall, A.L.; van de Donk, N.W.C.J.; Nahi, H.; San-Miguel, J.F.; Oriol, A.; Nooka, A.K.; Martin, T.; Rosinol, L.; Chari, A.; et al. Teclistamab in Relapsed or Refractory Multiple Myeloma. N. Engl. J. Med. 2022, 387, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Budde, L.E.; Sehn, L.H.; Matasar, M.; Schuster, S.J.; Assouline, S.; Giri, P.; Kuruvilla, J.; Canales, M.; Dietrich, S.; Fay, K.; et al. Safety and Efficacy of Mosunetuzumab, a Bispecific Antibody, in Patients with Relapsed or Refractory Follicular Lymphoma: A Single-Arm, Multicentre, Phase 2 Study. Lancet Oncol. 2022, 23, 1055–1065. [Google Scholar] [CrossRef]
- Kim, Y.H.; Bagot, M.; Pinter-Brown, L.; Rook, A.H.; Porcu, P.; Horwitz, S.M.; Whittaker, S.; Tokura, Y.; Vermeer, M.; Zinzani, P.L.; et al. Mogamulizumab versus Vorinostat in Previously Treated Cutaneous T-Cell Lymphoma (MAVORIC): An International, Open-Label, Randomised, Controlled Phase 3 Trial. Lancet Oncol. 2018, 19, 1192–1204. [Google Scholar] [CrossRef]
- Lin, M.; Zhang, J.; Zhang, Y.; Luo, J.; Shi, S. Ocrelizumab for Multiple Sclerosis. Cochrane Database Syst. Rev. 2022, 5, CD013247. [Google Scholar] [CrossRef]
- Reagan, J.L.; Castillo, J.J. Ofatumumab as Front-Line Therapy in Untreated Chronic Lymphocytic Leukemia. Future Oncol. 2014, 10, 1147–1155. [Google Scholar] [CrossRef]
- Caimi, P.F.; Ai, W.; Alderuccio, J.P.; Ardeshna, K.M.; Hamadani, M.; Hess, B.; Kahl, B.S.; Radford, J.; Solh, M.; Stathis, A.; et al. Loncastuximab Tesirine in Relapsed or Refractory Diffuse Large B-Cell Lymphoma (LOTIS-2): A Multicentre, Open-Label, Single-Arm, Phase 2 Trial. Lancet Oncol. 2021, 22, 790–800. [Google Scholar] [CrossRef]
- Offidani, M.; Corvatta, L.; Morè, S.; Olivieri, A. Belantamab Mafodotin for the Treatment of Multiple Myeloma: An Overview of the Clinical Efficacy and Safety. Drug Des. Devel. Ther. 2021, 15, 2401–2415. [Google Scholar] [CrossRef] [PubMed]
- Salles, G.; Duell, J.; González Barca, E.; Tournilhac, O.; Jurczak, W.; Liberati, A.M.; Nagy, Z.; Obr, A.; Gaidano, G.; André, M.; et al. Tafasitamab plus Lenalidomide in Relapsed or Refractory Diffuse Large B-Cell Lymphoma (L-MIND): A Multicentre, Prospective, Single-Arm, Phase 2 Study. Lancet Oncol. 2020, 21, 978–988. [Google Scholar] [CrossRef]
- Moreau, P.; Dimopoulos, M.-A.; Mikhael, J.; Yong, K.; Capra, M.; Facon, T.; Hajek, R.; Špička, I.; Baker, R.; Kim, K.; et al. Isatuximab, Carfilzomib, and Dexamethasone in Relapsed Multiple Myeloma (IKEMA): A Multicentre, Open-Label, Randomised Phase 3 Trial. Lancet 2021, 397, 2361–2371. [Google Scholar] [CrossRef] [PubMed]
- Sehn, L.H.; Herrera, A.F.; Flowers, C.R.; Kamdar, M.K.; McMillan, A.; Hertzberg, M.; Assouline, S.; Kim, T.M.; Kim, W.S.; Ozcan, M.; et al. Polatuzumab Vedotin in Relapsed or Refractory Diffuse Large B-Cell Lymphoma. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2020, 38, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Lin, A.Y.; Dinner, S.N. Moxetumomab Pasudotox for Hairy Cell Leukemia: Preclinical Development to FDA Approval. Blood Adv. 2019, 3, 2905–2910. [Google Scholar] [CrossRef]
- Al-Salama, Z.T. Inotuzumab Ozogamicin: A Review in Relapsed/Refractory B-Cell Acute Lymphoblastic Leukaemia. Target. Oncol. 2018, 13, 525–532. [Google Scholar] [CrossRef]
- Kantarjian, H.; Stein, A.; Gökbuget, N.; Fielding, A.K.; Schuh, A.C.; Ribera, J.-M.; Wei, A.; Dombret, H.; Foà, R.; Bassan, R.; et al. Blinatumomab versus Chemotherapy for Advanced Acute Lymphoblastic Leukemia. N. Engl. J. Med. 2017, 376, 836–847. [Google Scholar] [CrossRef]
- Reda, G.; Orofino, N.; Cassin, R.; Sciumè, M.; Fattizzo, B.; Cortelezzi, A. Treating Chronic Lymphocytic Leukemia with Obinutuzumab: Safety and Efficacy Considerations. Expert Opin. Drug Saf. 2016, 15, 865–873. [Google Scholar] [CrossRef]
- Straus, D.J.; Długosz-Danecka, M.; Connors, J.M.; Alekseev, S.; Illés, Á.; Picardi, M.; Lech-Maranda, E.; Feldman, T.; Smolewski, P.; Savage, K.J.; et al. Brentuximab Vedotin with Chemotherapy for Stage III or IV Classical Hodgkin Lymphoma (ECHELON-1): 5-Year Update of an International, Open-Label, Randomised, Phase 3 Trial. Lancet Haematol. 2021, 8, e410–e421. [Google Scholar] [CrossRef]
- Cheung, M.C.; MacEachern, J.A.; Haynes, A.E.; Meyer, R.M.; Imrie, K. 131I–Tositumomab in Lymphoma. Curr. Oncol. 2009, 16, 32–47. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mondello, P.; Cuzzocrea, S.; Navarra, M.; Mian, M. 90 Y-Ibritumomab Tiuxetan: A Nearly Forgotten Opportunity. Oncotarget 2015, 7, 7597–7609. [Google Scholar] [CrossRef] [PubMed]
- Baron, J.; Wang, E.S. Gemtuzumab Ozogamicin for the Treatment of Acute Myeloid Leukemia. Expert Rev. Clin. Pharmacol. 2018, 11, 549–559. [Google Scholar] [CrossRef]
- Baum, P.; Visvanathan, S.; Garcet, S.; Roy, J.; Schmid, R.; Bossert, S.; Lang, B.; Bachelez, H.; Bissonnette, R.; Thoma, C.; et al. Pustular Psoriasis: Molecular Pathways and Effects of Spesolimab in Generalized Pustular Psoriasis. J. Allergy Clin. Immunol. 2022, 149, 1402–1412. [Google Scholar] [CrossRef]
- Morand, E.F.; Furie, R.; Tanaka, Y.; Bruce, I.N.; Askanase, A.D.; Richez, C.; Bae, S.-C.; Brohawn, P.Z.; Pineda, L.; Berglind, A.; et al. Trial of Anifrolumab in Active Systemic Lupus Erythematosus. N. Engl. J. Med. 2020, 382, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Reich, K.; Warren, R.B.; Lebwohl, M.; Gooderham, M.; Strober, B.; Langley, R.G.; Paul, C.; De Cuyper, D.; Vanvoorden, V.; Madden, C.; et al. Bimekizumab versus Secukinumab in Plaque Psoriasis. N. Engl. J. Med. 2021, 385, 142–152. [Google Scholar] [CrossRef]
- Wollenberg, A.; Blauvelt, A.; Guttman-Yassky, E.; Worm, M.; Lynde, C.; Lacour, J.-P.; Spelman, L.; Katoh, N.; Saeki, H.; Poulin, Y.; et al. Tralokinumab for Moderate-to-Severe Atopic Dermatitis: Results from Two 52-Week, Randomized, Double-Blind, Multicentre, Placebo-Controlled Phase III Trials (ECZTRA 1 and ECZTRA 2). Br. J. Dermatol. 2021, 184, 437–449. [Google Scholar] [CrossRef]
- Papp, K.A.; Blauvelt, A.; Bukhalo, M.; Gooderham, M.; Krueger, J.G.; Lacour, J.-P.; Menter, A.; Philipp, S.; Sofen, H.; Tyring, S.; et al. Risankizumab versus Ustekinumab for Moderate-to-Severe Plaque Psoriasis. N. Engl. J. Med. 2017, 376, 1551–1560. [Google Scholar] [CrossRef]
- Locatelli, F.; Jordan, M.B.; Allen, C.; Cesaro, S.; Rizzari, C.; Rao, A.; Degar, B.; Garrington, T.P.; Sevilla, J.; Putti, M.-C.; et al. Emapalumab in Children with Primary Hemophagocytic Lymphohistiocytosis. N. Engl. J. Med. 2020, 382, 1811–1822. [Google Scholar] [CrossRef]
- Banerji, A.; Riedl, M.A.; Bernstein, J.A.; Cicardi, M.; Longhurst, H.J.; Zuraw, B.L.; Busse, P.J.; Anderson, J.; Magerl, M.; Martinez-Saguer, I.; et al. Effect of Lanadelumab Compared With Placebo on Prevention of Hereditary Angioedema Attacks: A Randomized Clinical Trial. JAMA 2018, 320, 2108–2121. [Google Scholar] [CrossRef]
- Reich, K.; Papp, K.A.; Blauvelt, A.; Tyring, S.K.; Sinclair, R.; Thaçi, D.; Nograles, K.; Mehta, A.; Cichanowitz, N.; Li, Q.; et al. Tildrakizumab versus Placebo or Etanercept for Chronic Plaque Psoriasis (ReSURFACE 1 and ReSURFACE 2): Results from Two Randomised Controlled, Phase 3 Trials. Lancet 2017, 390, 276–288. [Google Scholar] [CrossRef]
- Beccari, M.V.; Mogle, B.T.; Sidman, E.F.; Mastro, K.A.; Asiago-Reddy, E.; Kufel, W.D. Ibalizumab, a Novel Monoclonal Antibody for the Management of Multidrug-Resistant HIV-1 Infection. Antimicrob. Agents Chemother. 2019, 63, e00110-19. [Google Scholar] [CrossRef]
- Chiricozzi, A.; Costanzo, A.; Fargnoli, M.C.; Malagoli, P.; Piaserico, S.; Amerio, P.; Argenziano, G.; Balato, N.; Bardazzi, F.; Bianchi, L.; et al. Guselkumab: An Anti-IL-23 Antibody for the Treatment of Moderate-to-Severe Plaque Psoriasis. Eur. J. Dermatol. 2021, 31, 3–16. [Google Scholar] [CrossRef] [PubMed]
- Lamb, Y.N.; Deeks, E.D. Sarilumab: A Review in Moderate to Severe Rheumatoid Arthritis. Drugs 2018, 78, 929–940. [Google Scholar] [CrossRef] [PubMed]
- Beck, L.A.; Thaçi, D.; Hamilton, J.D.; Graham, N.M.; Bieber, T.; Rocklin, R.; Ming, J.E.; Ren, H.; Kao, R.; Simpson, E.; et al. Dupilumab Treatment in Adults with Moderate-to-Severe Atopic Dermatitis. N. Engl. J. Med. 2014, 371, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Gisondi, P.; Girolomoni, G. Brodalumab in the Treatment of Chronic Plaque Psoriasis. Expert Opin. Biol. Ther. 2020, 20, 1175–1186. [Google Scholar] [CrossRef]
- Gordon, K.B.; Blauvelt, A.; Papp, K.A.; Langley, R.G.; Luger, T.; Ohtsuki, M.; Reich, K.; Amato, D.; Ball, S.G.; Braun, D.K.; et al. Phase 3 Trials of Ixekizumab in Moderate-to-Severe Plaque Psoriasis. N. Engl. J. Med. 2016, 375, 345–356. [Google Scholar] [CrossRef]
- Blair, H.A. Secukinumab: A Review in Moderate to Severe Pediatric Plaque Psoriasis. Paediatr. Drugs 2021, 23, 601–608. [Google Scholar] [CrossRef]
- Barquero, N. Siltuximab: A New Option for the Management of Castleman’s Disease. Drugs Today Barc. Spain 1998 2015, 51, 21–28. [Google Scholar] [CrossRef]
- Singh, J.A.; Shah, N.P.; Mudano, A.S. Belimumab for Systemic Lupus Erythematosus. Cochrane Database Syst. Rev. 2021, 2, CD010668. [Google Scholar] [CrossRef]
- Gabay, C.; Emery, P.; van Vollenhoven, R.; Dikranian, A.; Alten, R.; Pavelka, K.; Klearman, M.; Musselman, D.; Agarwal, S.; Green, J.; et al. Tocilizumab Monotherapy versus Adalimumab Monotherapy for Treatment of Rheumatoid Arthritis (ADACTA): A Randomised, Double-Blind, Controlled Phase 4 Trial. Lancet 2013, 381, 1541–1550. [Google Scholar] [CrossRef] [PubMed]
- Dhimolea, E. Canakinumab. mAbs 2010, 2, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Epstein, D.; Bojke, L.; Craig, D.; Light, K.; Bruce, I.; Sculpher, M.; Woolacott, N. Golimumab for the Treatment of Psoriatic Arthritis. Health Technol. Assess. Winch. Engl. 2011, 15 (Suppl. 1), 87–95. [Google Scholar] [CrossRef]
- Pelechas, E.; Voulgari, P.V.; Drosos, A.A. Golimumab for Rheumatoid Arthritis. J. Clin. Med. 2019, 8, 387. [Google Scholar] [CrossRef]
- Yiu, Z.Z.; Warren, R.B. Ustekinumab for the Treatment of Psoriasis: An Evidence Update. Semin. Cutan. Med. Surg. 2018, 37, 143–147. [Google Scholar] [CrossRef]
- Gupta, A.K.; Cherman, A.M. Efalizumab in the Treatment of Psoriasis. J. Cutan. Med. Surg. 2006, 10, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Voulgari, P.V.; Drosos, A.A. Adalimumab for Rheumatoid Arthritis. Expert Opin. Biol. Ther. 2006, 6, 1349–1360. [Google Scholar] [CrossRef]
- Onrust, S.V.; Wiseman, L.R. Basiliximab. Drugs 1999, 57, 207–213; discussion 214. [Google Scholar] [CrossRef]
- Wiseman, L.R.; Faulds, D. Daclizumab: A Review of Its Use in the Prevention of Acute Rejection in Renal Transplant Recipients. Drugs 1999, 58, 1029–1042. [Google Scholar] [CrossRef]
- Silverberg, J.I.; Guttman-Yassky, E.; Thaçi, D.; Irvine, A.D.; Stein Gold, L.; Blauvelt, A.; Simpson, E.L.; Chu, C.-Y.; Liu, Z.; Gontijo Lima, R.; et al. Two Phase 3 Trials of Lebrikizumab for Moderate-to-Severe Atopic Dermatitis. N. Engl. J. Med. 2023, 388, 1080–1091. [Google Scholar] [CrossRef]
- Hanna, K.S. Enfortumab Vedotin to Treat Urothelial Carcinoma. Drugs Today Barc. Spain 1998 2020, 56, 329–335. [Google Scholar] [CrossRef]
- Frampton, J.E. Catumaxomab: In Malignant Ascites. Drugs 2012, 72, 1399–1410. [Google Scholar] [CrossRef] [PubMed]
- Culver, M.E.; Gatesman, M.L.; Mancl, E.E.; Lowe, D.K. Ipilimumab: A Novel Treatment for Metastatic Melanoma. Ann. Pharmacother. 2011, 45, 510–519. [Google Scholar] [CrossRef] [PubMed]
- Damato, B.E.; Dukes, J.; Goodall, H.; Carvajal, R.D. Tebentafusp: T Cell Redirection for the Treatment of Metastatic Uveal Melanoma. Cancers 2019, 11, 971. [Google Scholar] [CrossRef]
- Paik, J. Nivolumab Plus Relatlimab: First Approval. Drugs 2022, 82, 925–931. [Google Scholar] [CrossRef]
- Keam, S.J. Tremelimumab: First Approval. Drugs 2023, 83, 93–102. [Google Scholar] [CrossRef]
- Kang, C. Retifanlimab: First Approval. Drugs 2023, 83, 731–737. [Google Scholar] [CrossRef]
- Zheng, Y.; Mislang, A.R.A.; Coward, J.; Cosman, R.; Cooper, A.; Underhill, C.; Zhu, J.; Xiong, J.; Jiang, O.; Wang, H.; et al. Penpulimab, an Anti-PD1 IgG1 Antibody in the Treatment of Advanced or Metastatic Upper Gastrointestinal Cancers. Cancer Immunol. Immunother. 2022, 71, 2371–2379. [Google Scholar] [CrossRef]
- Mai, H.-Q.; Chen, Q.-Y.; Chen, D.; Hu, C.; Yang, K.; Wen, J.; Li, J.; Shi, Y.-R.; Jin, F.; Xu, R.; et al. Toripalimab or Placebo plus Chemotherapy as First-Line Treatment in Advanced Nasopharyngeal Carcinoma: A Multicenter Randomized Phase 3 Trial. Nat. Med. 2021, 27, 1536–1543. [Google Scholar] [CrossRef]
- Wang, Z.-X.; Cui, C.; Yao, J.; Zhang, Y.; Li, M.; Feng, J.; Yang, S.; Fan, Y.; Shi, J.; Zhang, X.; et al. Toripalimab plus Chemotherapy in Treatment-Naïve, Advanced Esophageal Squamous Cell Carcinoma (JUPITER-06): A Multi-Center Phase 3 Trial. Cancer Cell 2022, 40, 277–288.e3. [Google Scholar] [CrossRef]
- Shen, L.; Kato, K.; Kim, S.-B.; Ajani, J.A.; Zhao, K.; He, Z.; Yu, X.; Shu, Y.; Luo, Q.; Wang, J.; et al. Tislelizumab Versus Chemotherapy as Second-Line Treatment for Advanced or Metastatic Esophageal Squamous Cell Carcinoma (RATIONALE-302): A Randomized Phase III Study. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2022, 40, 3065–3076. [Google Scholar] [CrossRef] [PubMed]
- Clingan, P.R.; Brungs, D.; Arnold, S.; Coward, J.; Fourie, S.J.; Harris, D.L.; Kurochkin, A.; Ladwa, R.; Malan, N.; Mant, A.M.; et al. Efficacy and Safety of Cosibelimab, an Anti–PD-L1 Antibody, in Patients with Metastatic Cutaneous Squamous Cell Carcinoma. J. Clin. Oncol. 2022, 40, 9537. [Google Scholar] [CrossRef]
- Banerji, U.; van Herpen, C.M.L.; Saura, C.; Thistlethwaite, F.; Lord, S.; Moreno, V.; Macpherson, I.R.; Boni, V.; Rolfo, C.; de Vries, E.G.E.; et al. Trastuzumab Duocarmazine in Locally Advanced and Metastatic Solid Tumours and HER2-Expressing Breast Cancer: A Phase 1 Dose-Escalation and Dose-Expansion Study. Lancet Oncol. 2019, 20, 1124–1135. [Google Scholar] [CrossRef]
- Heo, Y.-A. Mirvetuximab Soravtansine: First Approval. Drugs 2023, 83, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Coleman, R.L.; Lorusso, D.; Gennigens, C.; González-Martín, A.; Randall, L.; Cibula, D.; Lund, B.; Woelber, L.; Pignata, S.; Forget, F.; et al. Efficacy and Safety of Tisotumab Vedotin in Previously Treated Recurrent or Metastatic Cervical Cancer (InnovaTV 204/GOG-3023/ENGOT-Cx6): A Multicentre, Open-Label, Single-Arm, Phase 2 Study. Lancet Oncol. 2021, 22, 609–619. [Google Scholar] [CrossRef] [PubMed]
- Park, K.; Haura, E.B.; Leighl, N.B.; Mitchell, P.; Shu, C.A.; Girard, N.; Viteri, S.; Han, J.-Y.; Kim, S.-W.; Lee, C.K.; et al. Amivantamab in EGFR Exon 20 Insertion-Mutated Non-Small-Cell Lung Cancer Progressing on Platinum Chemotherapy: Initial Results from the CHRYSALIS Phase I Study. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2021, 39, 3391–3402. [Google Scholar] [CrossRef]
- Mirza, M.R.; Chase, D.M.; Slomovitz, B.M.; dePont Christensen, R.; Novák, Z.; Black, D.; Gilbert, L.; Sharma, S.; Valabrega, G.; Landrum, L.M.; et al. Dostarlimab for Primary Advanced or Recurrent Endometrial Cancer. N. Engl. J. Med. 2023, 388, 2145–2158. [Google Scholar] [CrossRef]
- Markham, A. Margetuximab: First Approval. Drugs 2021, 81, 599–604. [Google Scholar] [CrossRef]
- Bardia, A.; Hurvitz, S.A.; Tolaney, S.M.; Loirat, D.; Punie, K.; Oliveira, M.; Brufsky, A.; Sardesai, S.D.; Kalinsky, K.; Zelnak, A.B.; et al. Sacituzumab Govitecan in Metastatic Triple-Negative Breast Cancer. N. Engl. J. Med. 2021, 384, 1529–1541. [Google Scholar] [CrossRef]
- Nakada, T.; Sugihara, K.; Jikoh, T.; Abe, Y.; Agatsuma, T. The Latest Research and Development into the Antibody-Drug Conjugate, [Fam-] Trastuzumab Deruxtecan (DS-8201a), for HER2 Cancer Therapy. Chem. Pharm. Bull. 2019, 67, 173–185. [Google Scholar] [CrossRef]
- Migden, M.R.; Khushalani, N.I.; Chang, A.L.S.; Lewis, K.D.; Schmults, C.D.; Hernandez-Aya, L.; Meier, F.; Schadendorf, D.; Guminski, A.; Hauschild, A.; et al. Cemiplimab in Locally Advanced Cutaneous Squamous Cell Carcinoma: Results from an Open-Label, Phase 2, Single-Arm Trial. Lancet Oncol. 2020, 21, 294–305. [Google Scholar] [CrossRef] [PubMed]
- Lavaud, P.; Hamilou, Z.; Loriot, Y.; Massard, C. Durvalumab in Urothelial Cancers. Expert Rev. Anticancer Ther. 2018, 18, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, H.L.; Russell, J.; Hamid, O.; Bhatia, S.; Terheyden, P.; D’Angelo, S.P.; Shih, K.C.; Lebbé, C.; Linette, G.P.; Milella, M.; et al. Avelumab in Patients with Chemotherapy-Refractory Metastatic Merkel Cell Carcinoma: A Multicentre, Single-Group, Open-Label, Phase 2 Trial. Lancet Oncol. 2016, 17, 1374–1385. [Google Scholar] [CrossRef]
- Hamilou, Z.; Lavaud, P.; Loriot, Y. Atezolizumab in Urothelial Bladder Carcinoma. Future Oncol. 2018, 14, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Pender, A.; Jones, R.L. Olaratumab for the Treatment of Soft-Tissue Sarcoma. Future Oncol. 2017, 13, 2151–2157. [Google Scholar] [CrossRef]
- Hoy, S.M. Dinutuximab: A Review in High-Risk Neuroblastoma. Target. Oncol. 2016, 11, 247–253. [Google Scholar] [CrossRef]
- Luke, J.J.; Rutkowski, P.; Queirolo, P.; Del Vecchio, M.; Mackiewicz, J.; Chiarion-Sileni, V.; de la Cruz Merino, L.; Khattak, M.A.; Schadendorf, D.; Long, G.V.; et al. Pembrolizumab versus Placebo as Adjuvant Therapy in Completely Resected Stage IIB or IIC Melanoma (KEYNOTE-716): A Randomised, Double-Blind, Phase 3 Trial. Lancet 2022, 399, 1718–1729. [Google Scholar] [CrossRef]
- Shitara, K.; Ohtsu, A. Ramucirumab for Gastric Cancer. Expert Rev. Gastroenterol. Hepatol. 2015, 9, 133–139. [Google Scholar] [CrossRef]
- Verma, S.; Miles, D.; Gianni, L.; Krop, I.E.; Welslau, M.; Baselga, J.; Pegram, M.; Oh, D.-Y.; Diéras, V.; Guardino, E.; et al. Trastuzumab Emtansine for HER2-Positive Advanced Breast Cancer. N. Engl. J. Med. 2012, 367, 1783–1791. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Iwata, H.; Taira, N.; Masuda, N.; Takahashi, M.; Yoshinami, T.; Ueno, T.; Toyama, T.; Yamanaka, T.; Takano, T.; et al. Pertuzumab Retreatment for HER2-Positive Advanced Breast Cancer: A Randomized, Open-Label Phase III Study (PRECIOUS). Cancer Sci. 2022, 113, 3169–3179. [Google Scholar] [CrossRef]
- Sartore-Bianchi, A.; Pietrantonio, F.; Lonardi, S.; Mussolin, B.; Rua, F.; Crisafulli, G.; Bartolini, A.; Fenocchio, E.; Amatu, A.; Manca, P.; et al. Circulating Tumor DNA to Guide Rechallenge with Panitumumab in Metastatic Colorectal Cancer: The Phase 2 CHRONOS Trial. Nat. Med. 2022, 28, 1612–1618. [Google Scholar] [CrossRef] [PubMed]
- Welch, S.; Spithoff, K.; Rumble, R.B.; Maroun, J. Gastrointestinal Cancer Disease Site Group Bevacizumab Combined with Chemotherapy for Patients with Advanced Colorectal Cancer: A Systematic Review. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2010, 21, 1152–1162. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Mo, J.; Dai, J.; Ye, C.; Cen, W.; Zheng, X.; Jiang, L.; Ye, L. Cetuximab Promotes RSL3-Induced Ferroptosis by Suppressing the Nrf2/HO-1 Signalling Pathway in KRAS Mutant Colorectal Cancer. Cell Death Dis. 2021, 12, 1079. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Zhang, B.; Yang, J. Evinacumab for the Treatment of Homozygous Familial Hypercholesterolemia. Expert Rev. Clin. Pharmacol. 2022, 15, 139–145. [Google Scholar] [CrossRef]
- Sabatine, M.S.; Giugliano, R.P.; Keech, A.C.; Honarpour, N.; Wiviott, S.D.; Murphy, S.A.; Kuder, J.F.; Wang, H.; Liu, T.; Wasserman, S.M.; et al. Evolocumab and Clinical Outcomes in Patients with Cardiovascular Disease. N. Engl. J. Med. 2017, 376, 1713–1722. [Google Scholar] [CrossRef]
- De Luca, G.; Suryapranata, H.; Grimaldi, R.; Chiariello, M. Coronary Stenting and Abciximab in Primary Angioplasty for ST-Segment-Elevation Myocardial Infarction. QJM Mon. J. Assoc. Physicians 2005, 98, 633–641. [Google Scholar] [CrossRef][Green Version]
- Douglas, R.S.; Kahaly, G.J.; Patel, A.; Sile, S.; Thompson, E.H.Z.; Perdok, R.; Fleming, J.C.; Fowler, B.T.; Marcocci, C.; Marinò, M.; et al. Teprotumumab for the Treatment of Active Thyroid Eye Disease. N. Engl. J. Med. 2020, 382, 341–352. [Google Scholar] [CrossRef]
- Ozen, A.; Chongsrisawat, V.; Sefer, A.P.; Kolukisa, B.; Jalbert, J.J.; Miller, J.L.; Meagher, K.A.; Brackin, T.; Feldman, H.B.; Adiv, O.E.; et al. A Phase 2/3 Study Evaluating the Efficacy and Safety of Pozelimab in Patients with CD55 Deficiency with Hyperactivation of Complement, Angiopathic Thrombosis, and Protein-Losing Enteropathy (CHAPLE Disease). Lancet 2023. [Google Scholar] [CrossRef]
- D’Haens, G.; Dubinsky, M.; Kobayashi, T.; Irving, P.M.; Howaldt, S.; Pokrotnieks, J.; Krueger, K.; Laskowski, J.; Li, X.; Lissoos, T.; et al. Mirikizumab as Induction and Maintenance Therapy for Ulcerative Colitis. N. Engl. J. Med. 2023, 388, 2444–2455. [Google Scholar] [CrossRef]
- Ha, C.; Kornbluth, A. Vedolizumab as a Treatment for Crohn’s Disease and Ulcerative Colitis. Gastroenterol. Hepatol. 2014, 10, 793–800. [Google Scholar]
- Hemperly, A.; Vande Casteele, N. Clinical Pharmacokinetics and Pharmacodynamics of Infliximab in the Treatment of Inflammatory Bowel Disease. Clin. Pharmacokinet. 2018, 57, 929–942. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Ren, Y.; Chen, L.; Sun, T.; Zhang, W.; Sun, B.; Zhu, L.; Xiong, F.; Zheng, C. Transarterial Chemoembolization Combined with Camrelizumab for Recurrent Hepatocellular Carcinoma. BMC Cancer 2022, 22, 270. [Google Scholar] [CrossRef] [PubMed]
- Haller, D.G. Update of Clinical Trials with Edrecolomab: A Monoclonal Antibody Therapy for Colorectal Cancer. Semin. Oncol. 2001, 28, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Wilcox, M.H.; Gerding, D.N.; Poxton, I.R.; Kelly, C.; Nathan, R.; Birch, T.; Cornely, O.A.; Rahav, G.; Bouza, E.; Lee, C.; et al. Bezlotoxumab for Prevention of Recurrent Clostridium Difficile Infection. N. Engl. J. Med. 2017, 376, 305–317. [Google Scholar] [CrossRef]
- Kummerfeldt, C.E. Raxibacumab: Potential Role in the Treatment of Inhalational Anthrax. Infect. Drug Resist. 2014, 7, 101–109. [Google Scholar] [CrossRef]
- Romano, M.J.; Kearns, G.L.; Kaplan, S.L.; Jacobs, R.F.; Killian, A.; Bradley, J.S.; Moss, M.M.; Van Dyke, R.; Rodriguez, W.; Straube, R.C. Single-Dose Pharmacokinetics and Safety of HA-1A, a Human IgM Anti-Lipid-A Monoclonal Antibody, in Pediatric Patients with Sepsis Syndrome. J. Pediatr. 1993, 122, 974–981. [Google Scholar] [CrossRef]
- Mintun, M.A.; Lo, A.C.; Duggan Evans, C.; Wessels, A.M.; Ardayfio, P.A.; Andersen, S.W.; Shcherbinin, S.; Sparks, J.; Sims, J.R.; Brys, M.; et al. Donanemab in Early Alzheimer’s Disease. N. Engl. J. Med. 2021, 384, 1691–1704. [Google Scholar] [CrossRef]
- van Dyck, C.H.; Swanson, C.J.; Aisen, P.; Bateman, R.J.; Chen, C.; Gee, M.; Kanekiyo, M.; Li, D.; Reyderman, L.; Cohen, S.; et al. Lecanemab in Early Alzheimer’s Disease. N. Engl. J. Med. 2023, 388, 9–21. [Google Scholar] [CrossRef]
- Kramer, K.; Pandit-Taskar, N.; Kushner, B.H.; Zanzonico, P.; Humm, J.L.; Tomlinson, U.; Donzelli, M.; Wolden, S.L.; Haque, S.; Dunkel, I.; et al. Phase 1 Study of Intraventricular 131I-Omburtamab Targeting B7H3 (CD276)-Expressing CNS Malignancies. J. Hematol. Oncol. 2022, 15, 165. [Google Scholar] [CrossRef]
- Nicolò, M.; Ferro Desideri, L.; Vagge, A.; Traverso, C.E. Faricimab: An Investigational Agent Targeting the Tie-2/Angiopoietin Pathway and VEGF-A for the Treatment of Retinal Diseases. Expert Opin. Investig. Drugs 2021, 30, 193–200. [Google Scholar] [CrossRef]
- Yamamura, T.; Kleiter, I.; Fujihara, K.; Palace, J.; Greenberg, B.; Zakrzewska-Pniewska, B.; Patti, F.; Tsai, C.-P.; Saiz, A.; Yamazaki, H.; et al. Trial of Satralizumab in Neuromyelitis Optica Spectrum Disorder. N. Engl. J. Med. 2019, 381, 2114–2124. [Google Scholar] [CrossRef] [PubMed]
- Cree, B.A.C.; Bennett, J.L.; Kim, H.J.; Weinshenker, B.G.; Pittock, S.J.; Wingerchuk, D.M.; Fujihara, K.; Paul, F.; Cutter, G.R.; Marignier, R.; et al. Inebilizumab for the Treatment of Neuromyelitis Optica Spectrum Disorder (N-MOmentum): A Double-Blind, Randomised Placebo-Controlled Phase 2/3 Trial. Lancet 2019, 394, 1352–1363. [Google Scholar] [CrossRef] [PubMed]
- Ashina, M.; Saper, J.; Cady, R.; Schaeffler, B.A.; Biondi, D.M.; Hirman, J.; Pederson, S.; Allan, B.; Smith, J. Eptinezumab in Episodic Migraine: A Randomized, Double-Blind, Placebo-Controlled Study (PROMISE-1). Cephalalgia Int. J. Headache 2020, 40, 241–254. [Google Scholar] [CrossRef] [PubMed]
- Dugel, P.U.; Koh, A.; Ogura, Y.; Jaffe, G.J.; Schmidt-Erfurth, U.; Brown, D.M.; Gomes, A.V.; Warburton, J.; Weichselberger, A.; Holz, F.G.; et al. HAWK and HARRIER: Phase 3, Multicenter, Randomized, Double-Masked Trials of Brolucizumab for Neovascular Age-Related Macular Degeneration. Ophthalmology 2020, 127, 72–84. [Google Scholar] [CrossRef] [PubMed]
- Frerichs, L.M.; Friedman, D.I. Galcanezumab for the Prevention of Migraine. Pain Manag. 2021, 11, 101–112. [Google Scholar] [CrossRef]
- Reuter, U.; Ehrlich, M.; Gendolla, A.; Heinze, A.; Klatt, J.; Wen, S.; Hours-Zesiger, P.; Nickisch, J.; Sieder, C.; Hentschke, C.; et al. Erenumab versus Topiramate for the Prevention of Migraine—A Randomised, Double-Blind, Active-Controlled Phase 4 Trial. Cephalalgia Int. J. Headache 2022, 42, 108–118. [Google Scholar] [CrossRef] [PubMed]
- Dhoot, D.S.; Kaiser, P.K. Ranibizumab for Age-Related Macular Degeneration. Expert Opin. Biol. Ther. 2012, 12, 371–381. [Google Scholar] [CrossRef]
- Khoy, K.; Mariotte, D.; Defer, G.; Petit, G.; Toutirais, O.; Le Mauff, B. Natalizumab in Multiple Sclerosis Treatment: From Biological Effects to Immune Monitoring. Front. Immunol. 2020, 11, 549842. [Google Scholar] [CrossRef]
- Cheng, Y.; Han, L.; Wu, L.; Chen, J.; Sun, H.; Wen, G.; Ji, Y.; Dvorkin, M.; Shi, J.; Pan, Z.; et al. Effect of First-Line Serplulimab vs Placebo Added to Chemotherapy on Survival in Patients With Extensive-Stage Small Cell Lung Cancer: The ASTRUM-005 Randomized Clinical Trial. JAMA 2022, 328, 1223–1232. [Google Scholar] [CrossRef]
- Zhou, C.; Wang, Z.; Sun, Y.; Cao, L.; Ma, Z.; Wu, R.; Yu, Y.; Yao, W.; Chang, J.; Chen, J.; et al. Sugemalimab versus Placebo, in Combination with Platinum-Based Chemotherapy, as First-Line Treatment of Metastatic Non-Small-Cell Lung Cancer (GEMSTONE-302): Interim and Final Analyses of a Double-Blind, Randomised, Phase 3 Clinical Trial. Lancet Oncol. 2022, 23, 220–233. [Google Scholar] [CrossRef]
- Zhang, L.; Lin, W.; Tan, F.; Li, N.; Xue, Q.; Gao, S.; Gao, Y.; He, J. Sintilimab for the Treatment of Non-Small Cell Lung Cancer. Biomark. Res. 2022, 10, 23. [Google Scholar] [CrossRef] [PubMed]
- Hammitt, L.L.; Dagan, R.; Yuan, Y.; Baca Cots, M.; Bosheva, M.; Madhi, S.A.; Muller, W.J.; Zar, H.J.; Brooks, D.; Grenham, A.; et al. Nirsevimab for Prevention of RSV in Healthy Late-Preterm and Term Infants. N. Engl. J. Med. 2022, 386, 837–846. [Google Scholar] [CrossRef] [PubMed]
- ACTIV-3–Therapeutics for Inpatients with COVID-19 (TICO) Study Group. Tixagevimab-Cilgavimab for Treatment of Patients Hospitalised with COVID-19: A Randomised, Double-Blind, Phase 3 Trial. Lancet Respir. Med. 2022, 10, 972–984. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Gonzalez-Rojas, Y.; Juarez, E.; Crespo Casal, M.; Moya, J.; Falci, D.R.; Sarkis, E.; Solis, J.; Zheng, H.; Scott, N.; et al. Early Treatment for Covid-19 with SARS-CoV-2 Neutralizing Antibody Sotrovimab. N. Engl. J. Med. 2021, 385, 1941–1950. [Google Scholar] [CrossRef]
- Syed, Y.Y. Regdanvimab: First Approval. Drugs 2021, 81, 2133–2137. [Google Scholar] [CrossRef]
- Deeks, E.D. Casirivimab/Imdevimab: First Approval. Drugs 2021, 81, 2047–2055. [Google Scholar] [CrossRef]
- Menzies-Gow, A.; Corren, J.; Bourdin, A.; Chupp, G.; Israel, E.; Wechsler, M.E.; Brightling, C.E.; Griffiths, J.M.; Hellqvist, Å.; Bowen, K.; et al. Tezepelumab in Adults and Adolescents with Severe, Uncontrolled Asthma. N. Engl. J. Med. 2021, 384, 1800–1809. [Google Scholar] [CrossRef]
- Kavanagh, J.E.; Hearn, A.P.; Dhariwal, J.; d’Ancona, G.; Douiri, A.; Roxas, C.; Fernandes, M.; Green, L.; Thomson, L.; Nanzer, A.M.; et al. Real-World Effectiveness of Benralizumab in Severe Eosinophilic Asthma. Chest 2021, 159, 496–506. [Google Scholar] [CrossRef]
- Deeks, E.D.; Brusselle, G. Reslizumab in Eosinophilic Asthma: A Review. Drugs 2017, 77, 777–784. [Google Scholar] [CrossRef]
- Greig, S.L. Obiltoxaximab: First Global Approval. Drugs 2016, 76, 823–830. [Google Scholar] [CrossRef]
- Díaz-Serrano, A.; Sánchez-Torre, A.; Paz-Ares, L. Necitumumab for the Treatment of Advanced Non-Small-Cell Lung Cancer. Future Oncol. 2019, 15, 705–716. [Google Scholar] [CrossRef] [PubMed]
- Ortega, H.G.; Liu, M.C.; Pavord, I.D.; Brusselle, G.G.; FitzGerald, J.M.; Chetta, A.; Humbert, M.; Katz, L.E.; Keene, O.N.; Yancey, S.W.; et al. Mepolizumab Treatment in Patients with Severe Eosinophilic Asthma. N. Engl. J. Med. 2014, 371, 1198–1207. [Google Scholar] [CrossRef] [PubMed]
- Provencio, M.; Serna-Blasco, R.; Nadal, E.; Insa, A.; García-Campelo, M.R.; Casal Rubio, J.; Dómine, M.; Majem, M.; Rodríguez-Abreu, D.; Martínez-Martí, A.; et al. Overall Survival and Biomarker Analysis of Neoadjuvant Nivolumab Plus Chemotherapy in Operable Stage IIIA Non-Small-Cell Lung Cancer (NADIM Phase II Trial). J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2022, 40, 2924–2933. [Google Scholar] [CrossRef]
- Dell’Aquila, E.; Armento, G.; Iuliani, M.; Simonetti, S.; D’Onofrio, L.; Zeppola, T.; Madaudo, C.; Russano, M.; Citarella, F.; Ribelli, G.; et al. Denosumab for Cancer-Related Bone Loss. Expert Opin. Biol. Ther. 2020, 20, 1261–1274. [Google Scholar] [CrossRef]
- Bril, V.; Drużdż, A.; Grosskreutz, J.; Habib, A.A.; Mantegazza, R.; Sacconi, S.; Utsugisawa, K.; Vissing, J.; Vu, T.; Boehnlein, M.; et al. Safety and Efficacy of Rozanolixizumab in Patients with Generalised Myasthenia Gravis (MycarinG): A Randomised, Double-Blind, Placebo-Controlled, Adaptive Phase 3 Study. Lancet Neurol. 2023, 22, 383–394. [Google Scholar] [CrossRef]
- Gklinos, P.; Papadopoulou, M.; Stanulovic, V.; Mitsikostas, D.D.; Papadopoulos, D. Monoclonal Antibodies as Neurological Therapeutics. Pharmaceuticals 2021, 14, 92. [Google Scholar] [CrossRef] [PubMed]
- Szebeni, J. Complement Activation-Related Pseudoallergy: A New Class of Drug-Induced Acute Immune Toxicity. Toxicology 2005, 216, 106–121. [Google Scholar] [CrossRef]
- Szebeni, J. Complement Activation-Related Pseudoallergy: A Stress Reaction in Blood Triggered by Nanomedicines and Biologicals. Mol. Immunol. 2014, 61, 163–173. [Google Scholar] [CrossRef]
- Bugelski, P.J.; Achuthanandam, R.; Capocasale, R.J.; Treacy, G.; Bouman-Thio, E. Monoclonal Antibody-Induced Cytokine-Release Syndrome. Expert Rev. Clin. Immunol. 2009, 5, 499–521. [Google Scholar] [CrossRef]
- Butzkueven, H.; Kappos, L.; Wiendl, H.; Trojano, M.; Spelman, T.; Chang, I.; Kasliwal, R.; Jaitly, S.; Campbell, N.; Ho, P.-R.; et al. Long-Term Safety and Effectiveness of Natalizumab Treatment in Clinical Practice: 10 Years of Real-World Data from the Tysabri Observational Program (TOP). J. Neurol. Neurosurg. Psychiatry 2020, 91, 660–668. [Google Scholar] [CrossRef]
- Dodick, D.W.; Ashina, M.; Brandes, J.L.; Kudrow, D.; Lanteri-Minet, M.; Osipova, V.; Palmer, K.; Picard, H.; Mikol, D.D.; Lenz, R.A. ARISE: A Phase 3 Randomized Trial of Erenumab for Episodic Migraine. Cephalalgia Int. J. Headache 2018, 38, 1026–1037. [Google Scholar] [CrossRef] [PubMed]
- Tepper, S.; Ashina, M.; Reuter, U.; Brandes, J.L.; Doležil, D.; Silberstein, S.; Winner, P.; Leonardi, D.; Mikol, D.; Lenz, R. Safety and Efficacy of Erenumab for Preventive Treatment of Chronic Migraine: A Randomised, Double-Blind, Placebo-Controlled Phase 2 Trial. Lancet Neurol. 2017, 16, 425–434. [Google Scholar] [CrossRef] [PubMed]
- Ashina, M.; Dodick, D.; Goadsby, P.J.; Reuter, U.; Silberstein, S.; Zhang, F.; Gage, J.R.; Cheng, S.; Mikol, D.D.; Lenz, R.A. Erenumab (AMG 334) in Episodic Migraine: Interim Analysis of an Ongoing Open-Label Study. Neurology 2017, 89, 1237–1243. [Google Scholar] [CrossRef]
- Valenzuela, R.M.; Pula, J.H.; Garwacki, D.; Cotter, J.; Kattah, J.C. Cryptococcal Meningitis in a Multiple Sclerosis Patient Taking Natalizumab. J. Neurol. Sci. 2014, 340, 109–111. [Google Scholar] [CrossRef]
- EMA Ocrevus. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/ocrevus (accessed on 4 August 2023).
- Lancet, T. End of the Road for Daclizumab in Multiple Sclerosis. Lancet 2018, 391, 1000. [Google Scholar] [CrossRef]
- Hosseini, S.S.; Khalili, S.; Baradaran, B.; Bidar, N.; Shahbazi, M.-A.; Mosafer, J.; Hashemzaei, M.; Mokhtarzadeh, A.; Hamblin, M.R. Bispecific Monoclonal Antibodies for Targeted Immunotherapy of Solid Tumors: Recent Advances and Clinical Trials. Int. J. Biol. Macromol. 2021, 167, 1030–1047. [Google Scholar] [CrossRef]
- Jin, S.; Sun, Y.; Liang, X.; Gu, X.; Ning, J.; Xu, Y.; Chen, S.; Pan, L. Emerging New Therapeutic Antibody Derivatives for Cancer Treatment. Signal Transduct. Target. Ther. 2022, 7, 39. [Google Scholar] [CrossRef]
- Pandit, R.; Chen, L.; Götz, J. The Blood-Brain Barrier: Physiology and Strategies for Drug Delivery. Adv. Drug Deliv. Rev. 2020, 165–166, 1–14. [Google Scholar] [CrossRef]
- Jovčevska, I.; Muyldermans, S. The Therapeutic Potential of Nanobodies. BioDrugs Clin. Immunother. Biopharm. Gene Ther. 2020, 34, 11–26. [Google Scholar] [CrossRef]
- Cavaco, M.; Frutos, S.; Oliete, P.; Valle, J.; Andreu, D.; Castanho, M.A.R.B.; Vila-Perelló, M.; Neves, V. Conjugation of a Blood Brain Barrier Peptide Shuttle to an Fc Domain for Brain Delivery of Therapeutic Biomolecules. ACS Med. Chem. Lett. 2021, 12, 1663–1668. [Google Scholar] [CrossRef]
- Di, J.; Xie, F.; Xu, Y. When Liposomes Met Antibodies: Drug Delivery and Beyond. Adv. Drug Deliv. Rev. 2020, 154–155, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Parray, H.A.; Shukla, S.; Perween, R.; Khatri, R.; Shrivastava, T.; Singh, V.; Murugavelu, P.; Ahmed, S.; Samal, S.; Sharma, C.; et al. Inhalation Monoclonal Antibody Therapy: A New Way to Treat and Manage Respiratory Infections. Appl. Microbiol. Biotechnol. 2021, 105, 6315–6332. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; McFee, M.; Fang, Q.; Abdin, O.; Kim, P.M. Computational and Artificial Intelligence-Based Methods for Antibody Development. Trends Pharmacol. Sci. 2023, 44, 175–189. [Google Scholar] [CrossRef] [PubMed]
- Prihoda, D.; Maamary, J.; Waight, A.; Juan, V.; Fayadat-Dilman, L.; Svozil, D.; Bitton, D.A. BioPhi: A Platform for Antibody Design, Humanization, and Humanness Evaluation Based on Natural Antibody Repertoires and Deep Learning. mAbs 2022, 14, 2020203. [Google Scholar] [CrossRef]
- Marks, C.; Hummer, A.M.; Chin, M.; Deane, C.M. Humanization of Antibodies Using a Machine Learning Approach on Large-Scale Repertoire Data. Bioinformatics 2021, 37, 4041–4047. [Google Scholar] [CrossRef]
- Choi, Y. Artificial Intelligence for Antibody Reading Comprehension: AntiBERTa. Patterns 2022, 3, 100535. [Google Scholar] [CrossRef]
- Shanehsazzadeh, A.; Bachas, S.; McPartlon, M.; Kasun, G.; Sutton, J.M.; Steiger, A.K.; Shuai, R.; Kohnert, C.; Rakocevic, G.; Gutierrez, J.M.; et al. Unlocking de Novo Antibody Design with Generative Artificial Intelligence. bioRxiv 2023. [Google Scholar] [CrossRef]
- Li, T.; Li, Y.; Zhu, X.; He, Y.; Wu, Y.; Ying, T.; Xie, Z. Artificial Intelligence in Cancer Immunotherapy: Applications in Neoantigen Recognition, Antibody Design and Immunotherapy Response Prediction. Semin. Cancer Biol. 2023, 91, 50–69. [Google Scholar] [CrossRef]
- Bachas, S.; Rakocevic, G.; Spencer, D.; Sastry, A.V.; Haile, R.; Sutton, J.M.; Kasun, G.; Stachyra, A.; Gutierrez, J.M.; Yassine, E.; et al. Antibody Optimization Enabled by Artificial Intelligence Predictions of Binding Affinity and Naturalness. bioRxiv 2022. [Google Scholar] [CrossRef]
- Hie, B.L.; Shanker, V.R.; Xu, D.; Bruun, T.U.J.; Weidenbacher, P.A.; Tang, S.; Wu, W.; Pak, J.E.; Kim, P.S. Efficient Evolution of Human Antibodies from General Protein Language Models. Nat. Biotechnol. 2023, 1–9. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).