Experimental Characterization and Evaluation of Crude Spiking Influence on Oil/Water Dispersed Flow in Pipe
Abstract
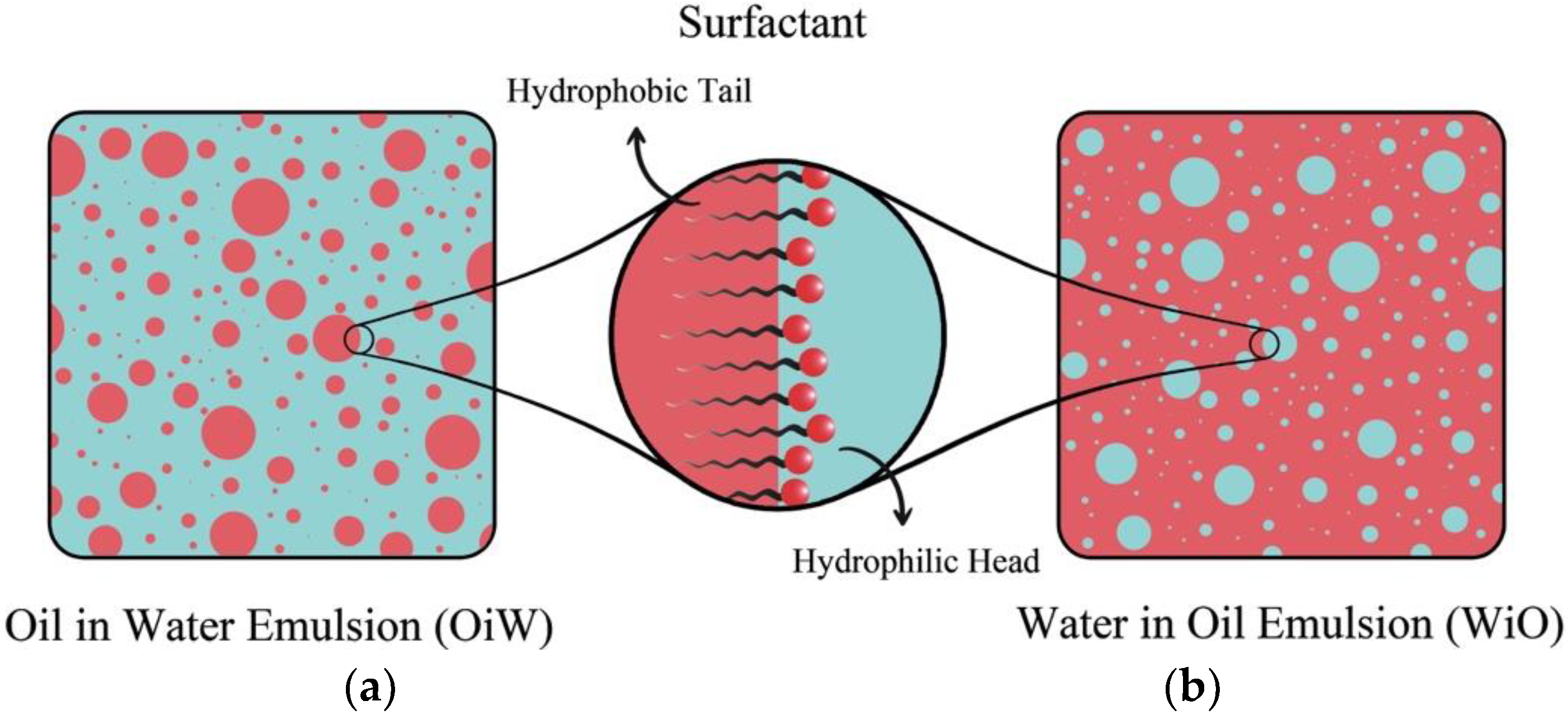
1. Introduction
2. Materials and Method
2.1. Emulsion Preparation
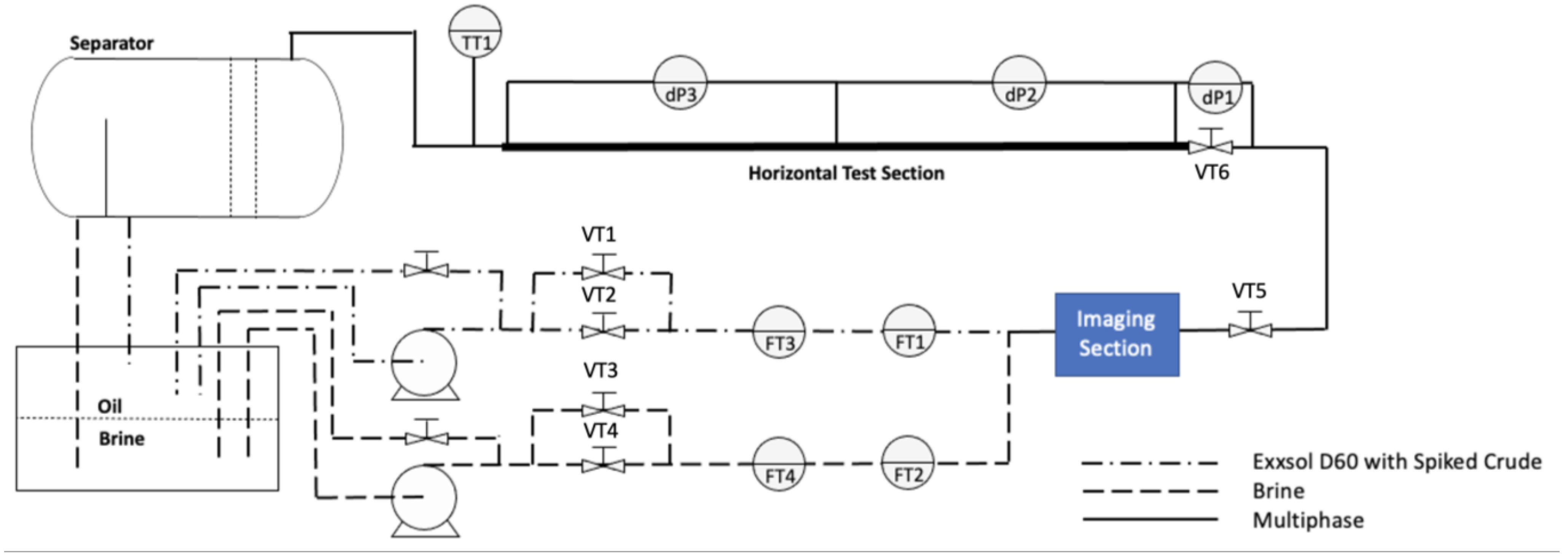
2.2. Test Mini-Flow Loop
2.3. Imaging Section (CANTY Particle Sizing Analyzer)
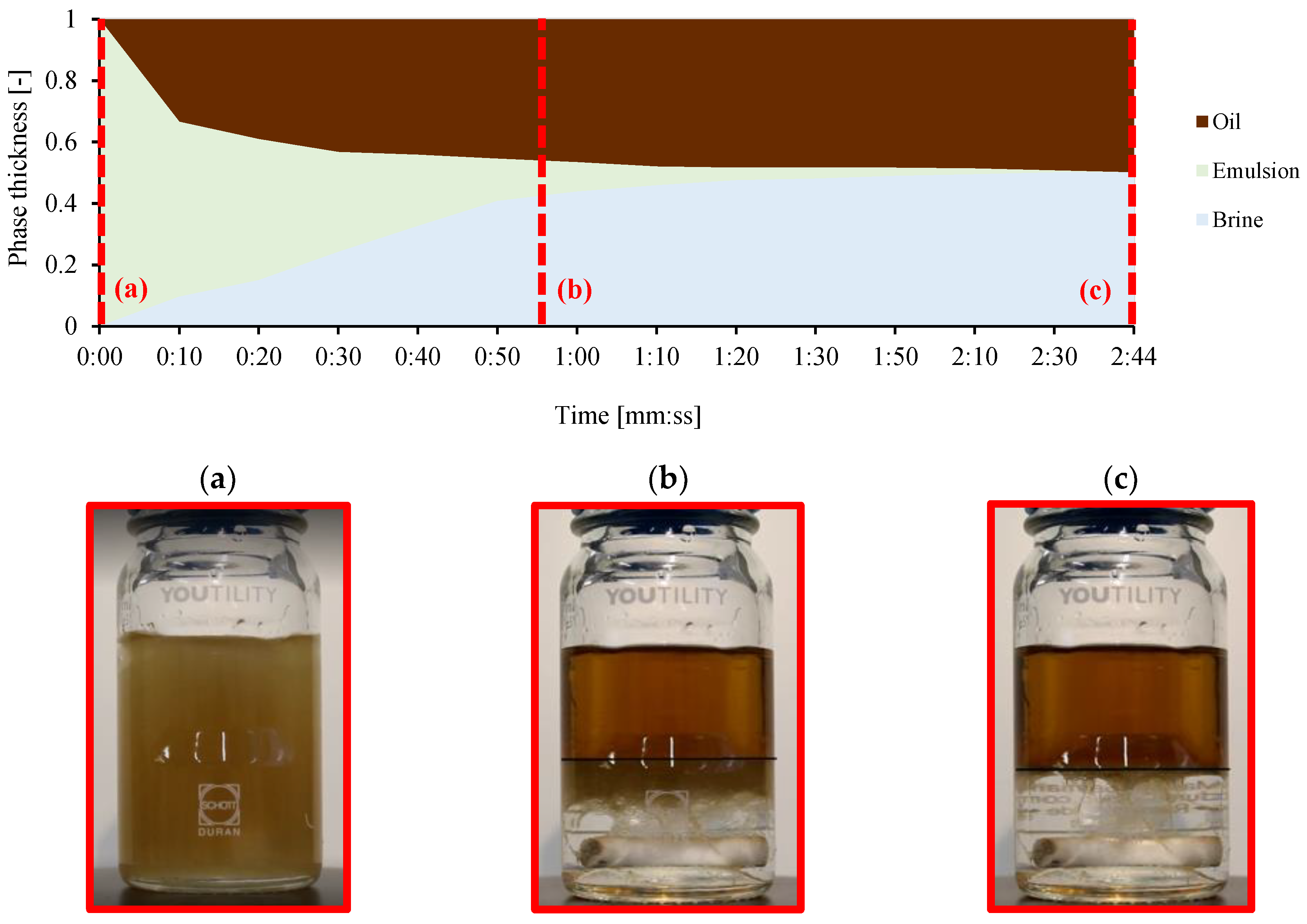
2.4. Separation Time Using Bottle Tests with Spiked Exxsol D60 and Water
3. Results and Discussion
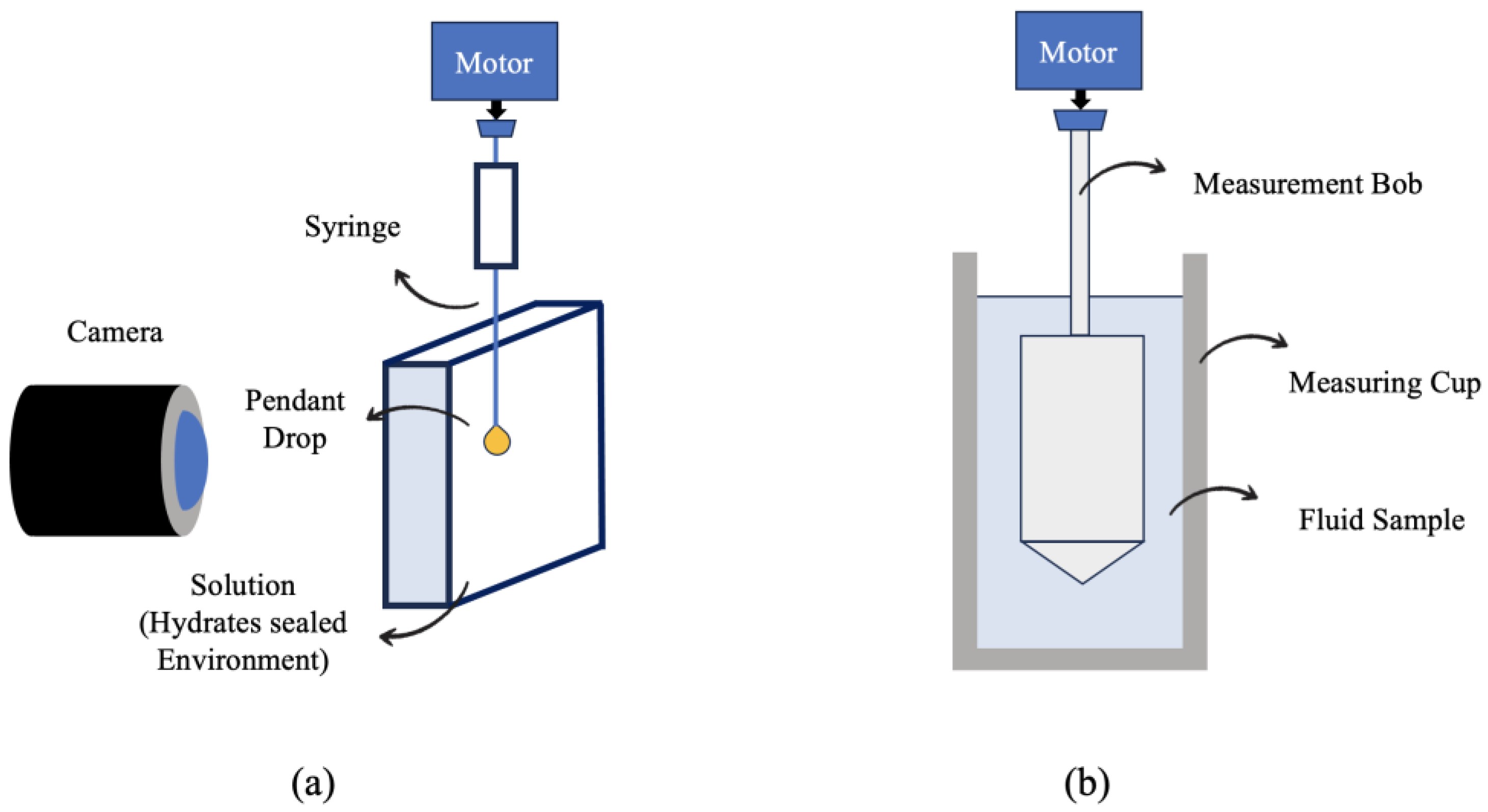
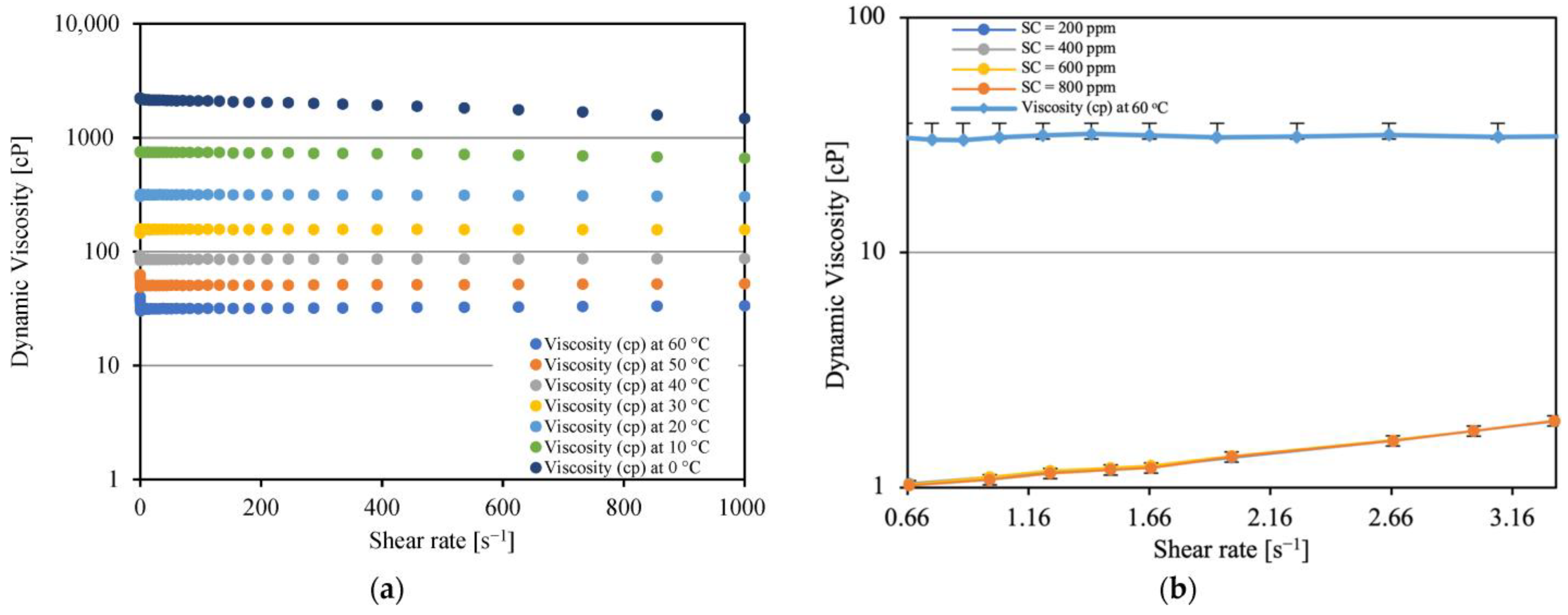
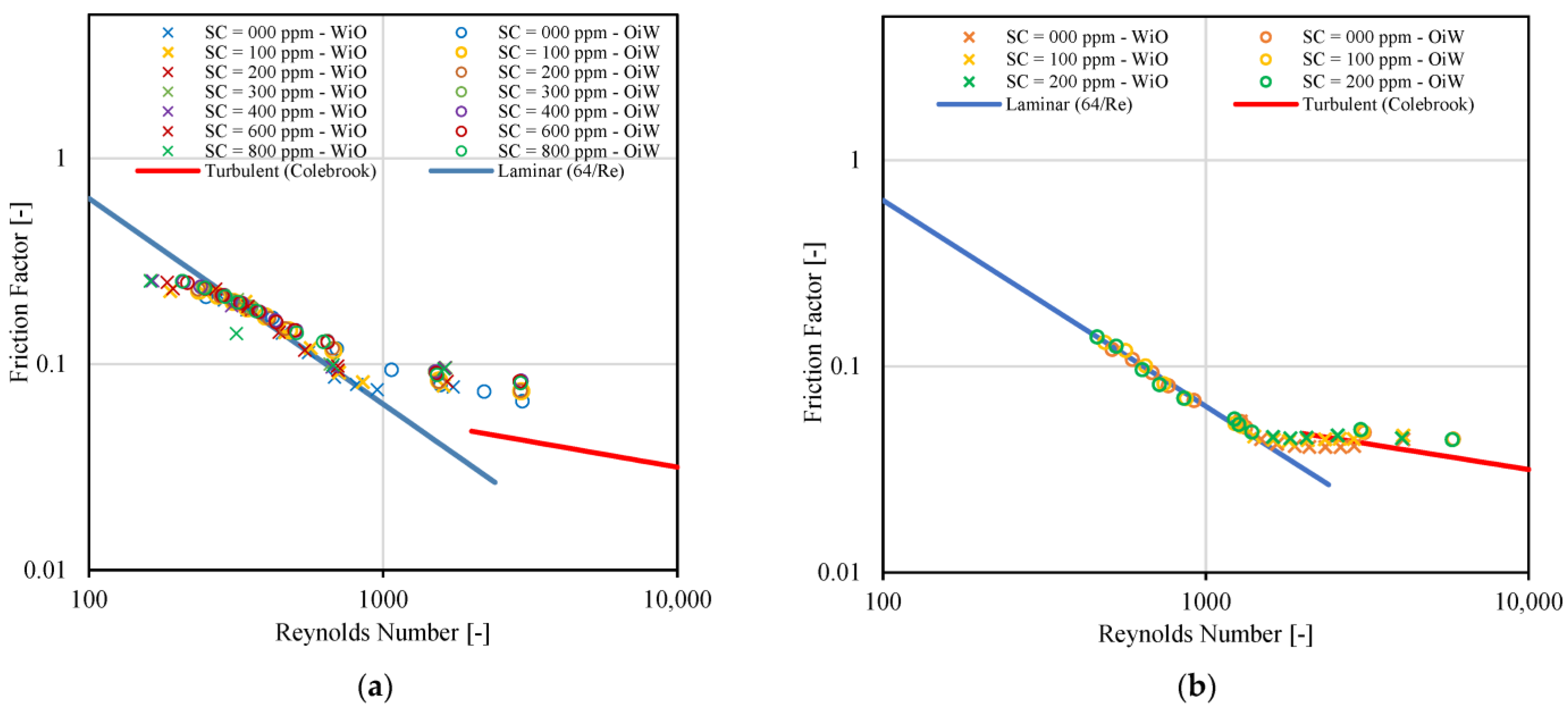
3.1. Density, Viscosity, and Interfacial Tension
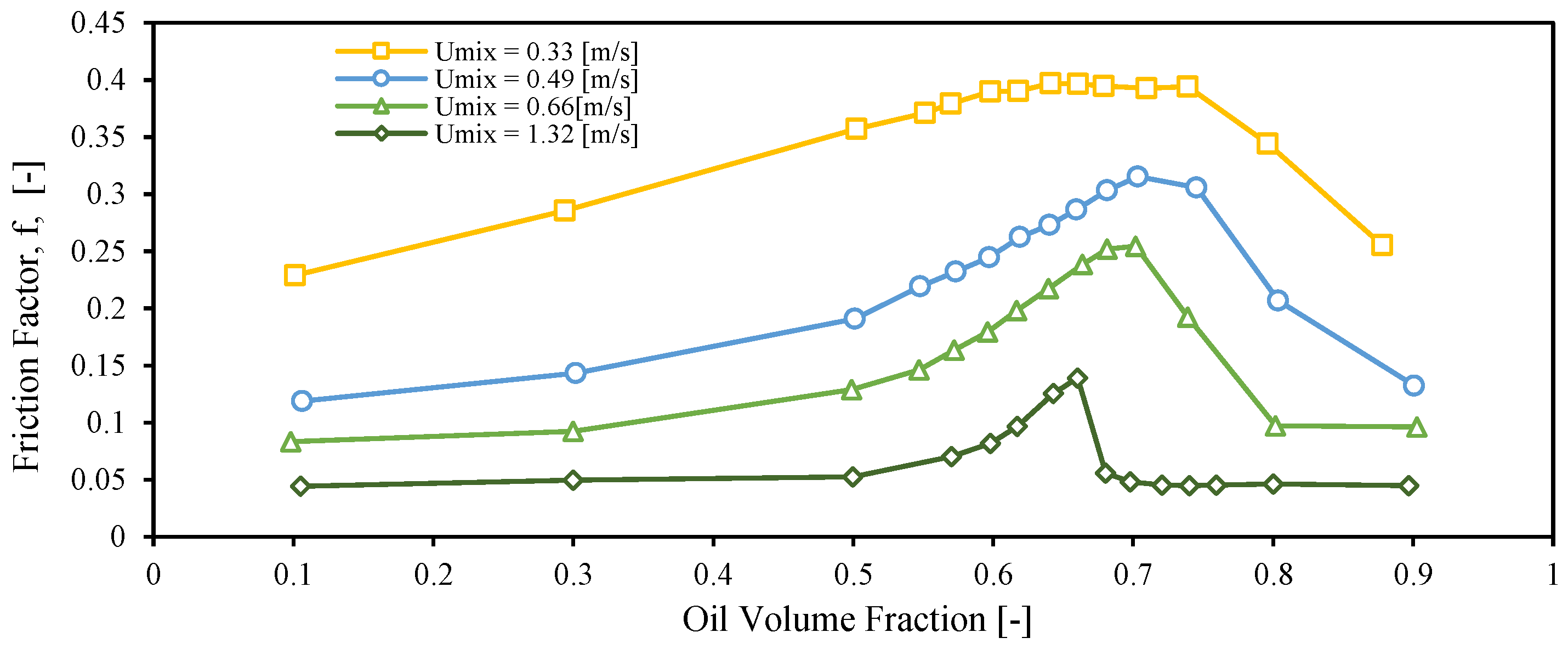
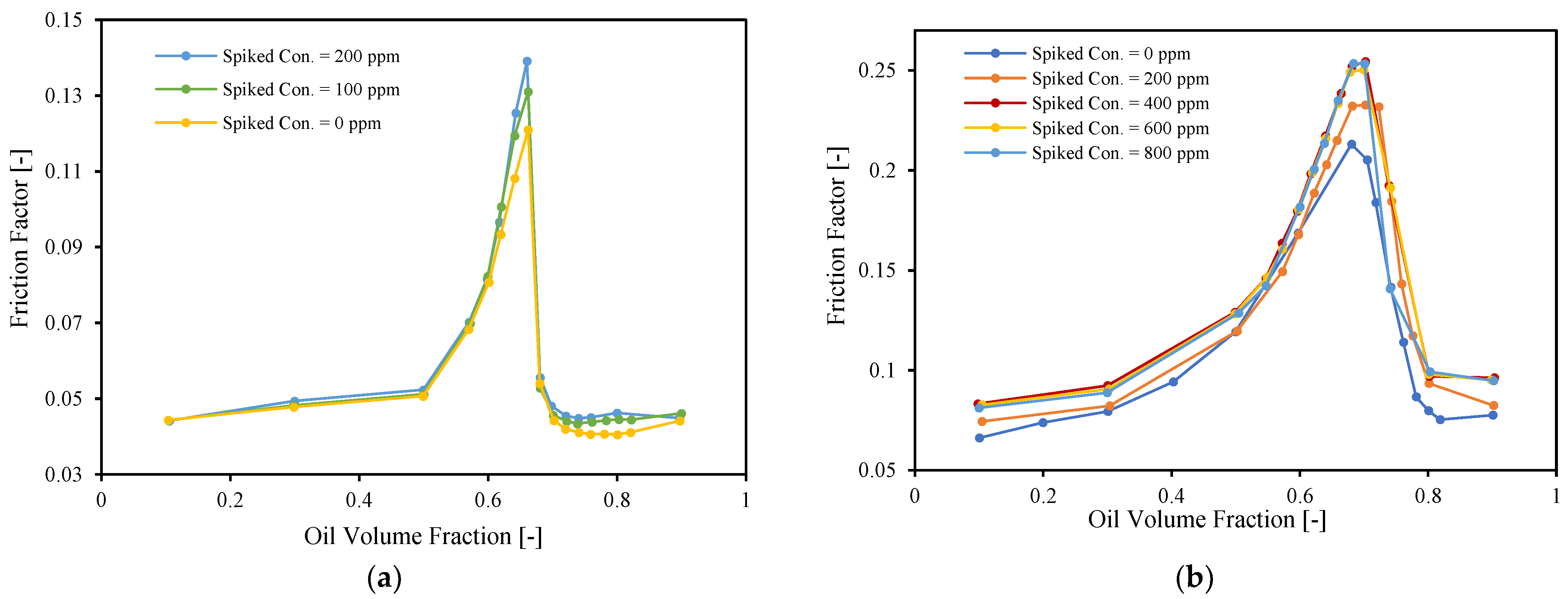
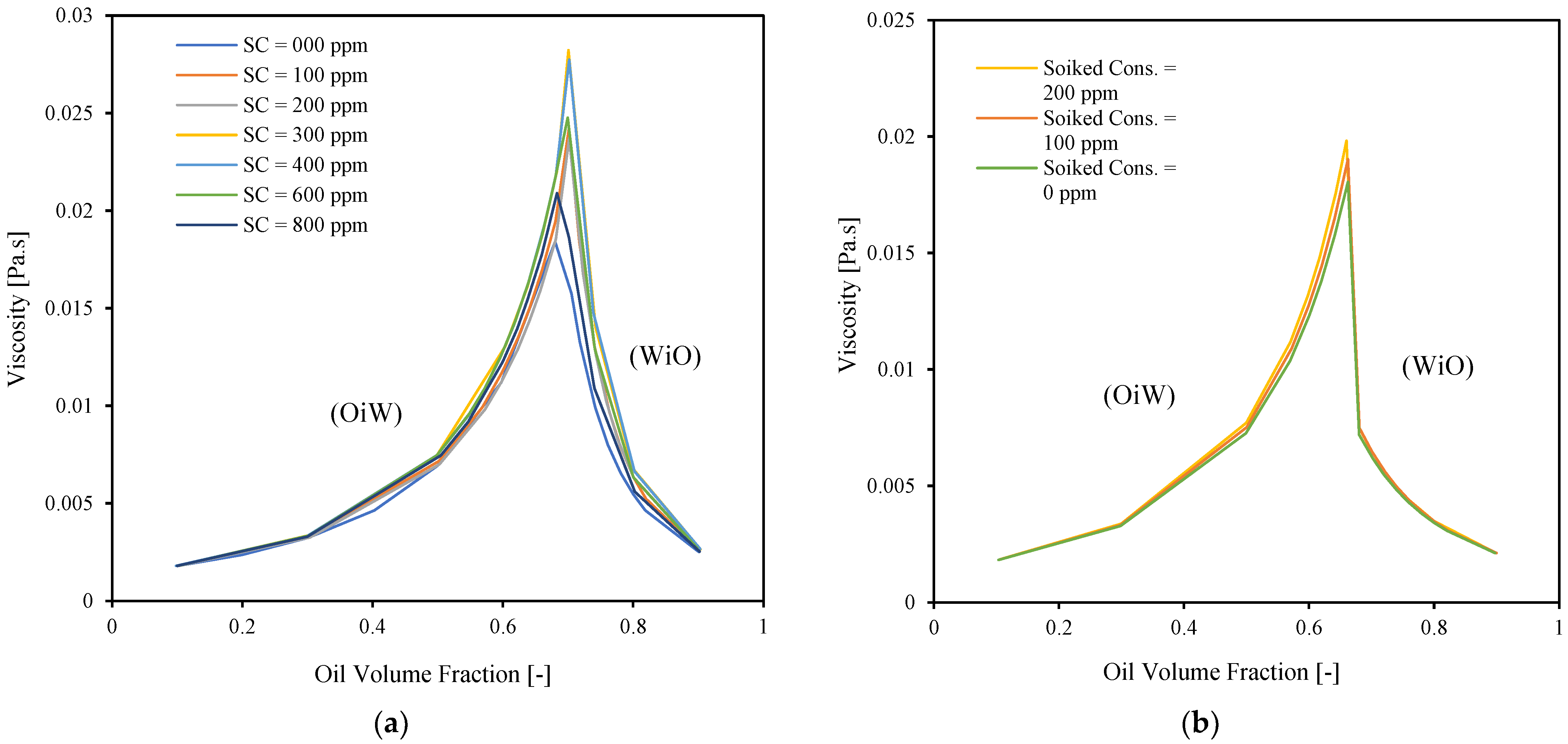
3.2. Inversion Point
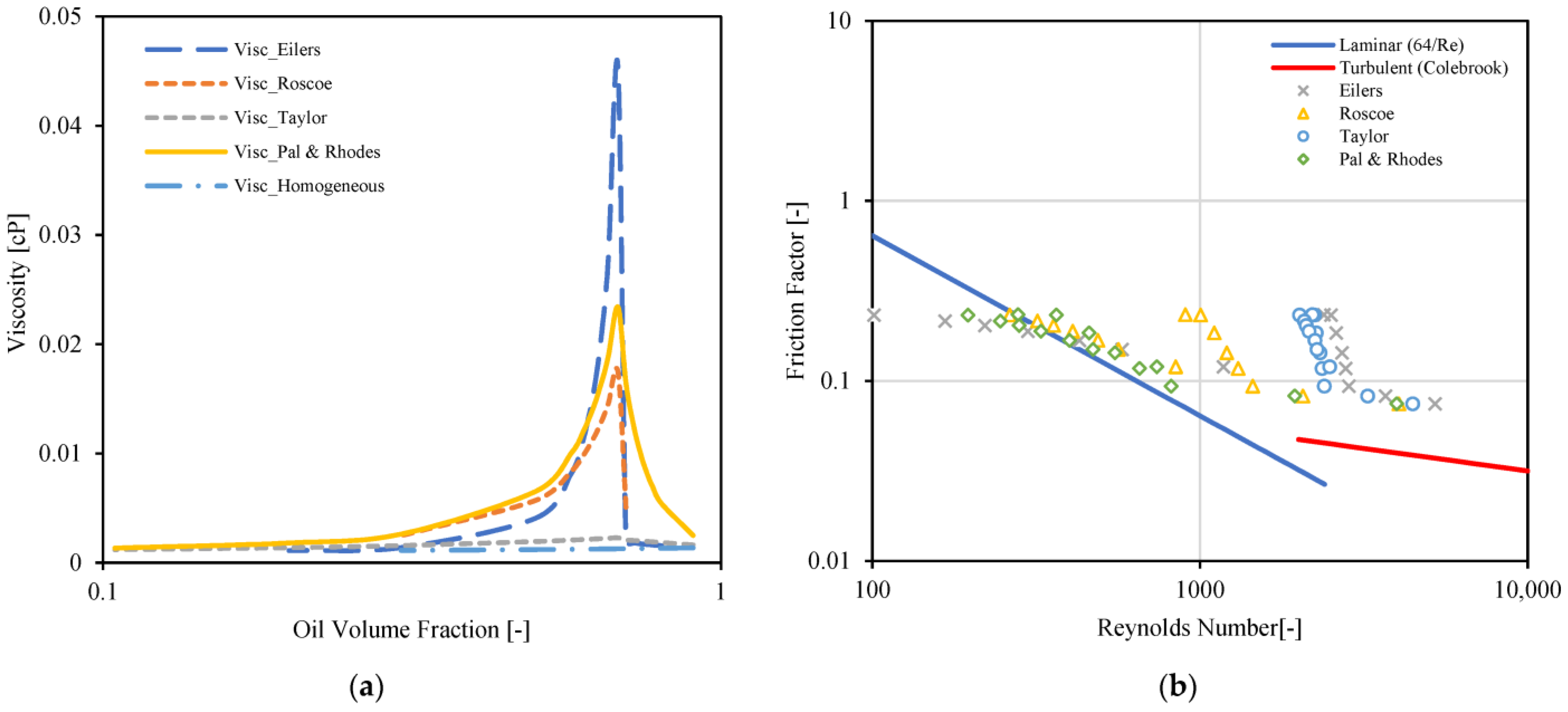
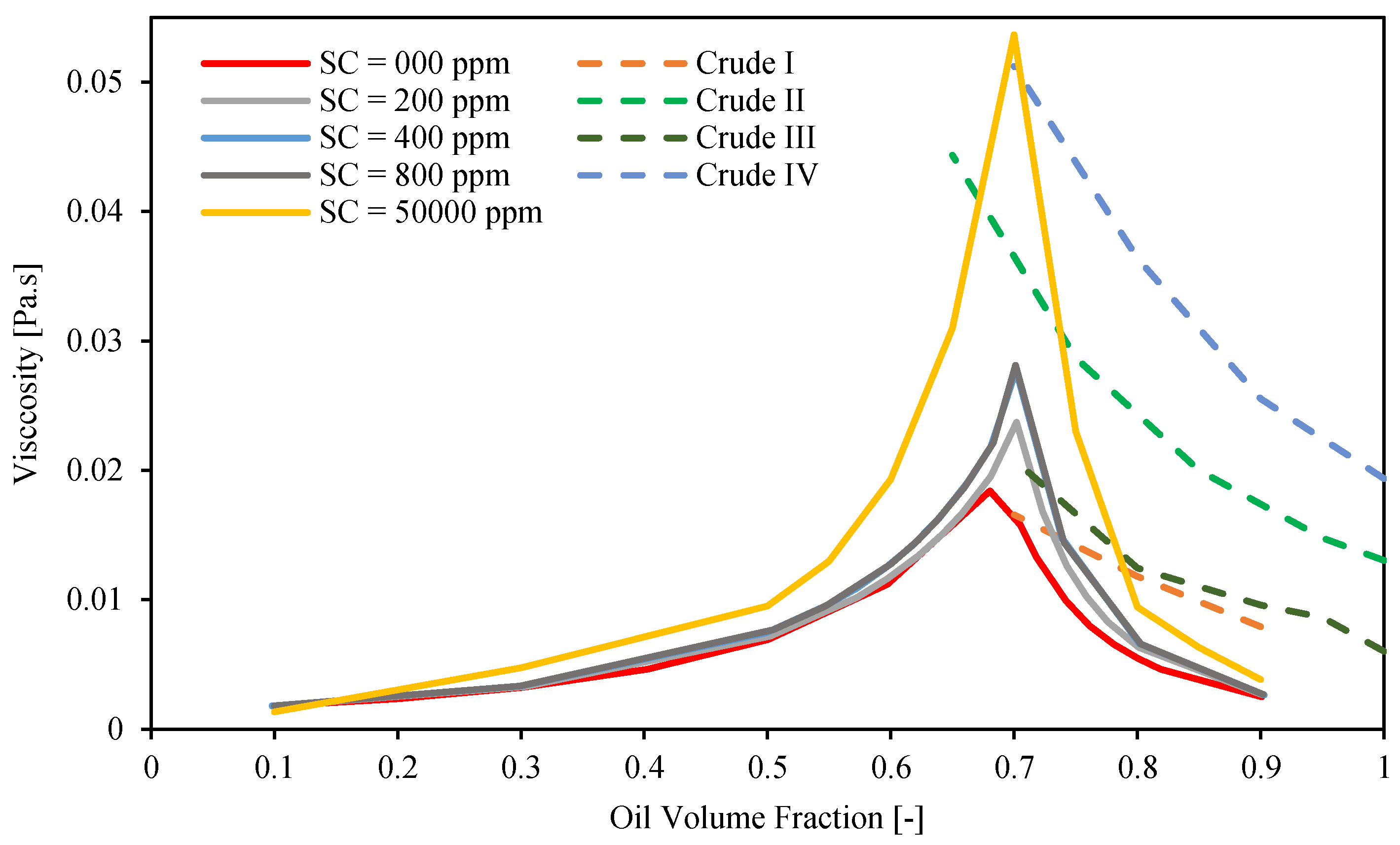
3.3. Emulsion Viscosity
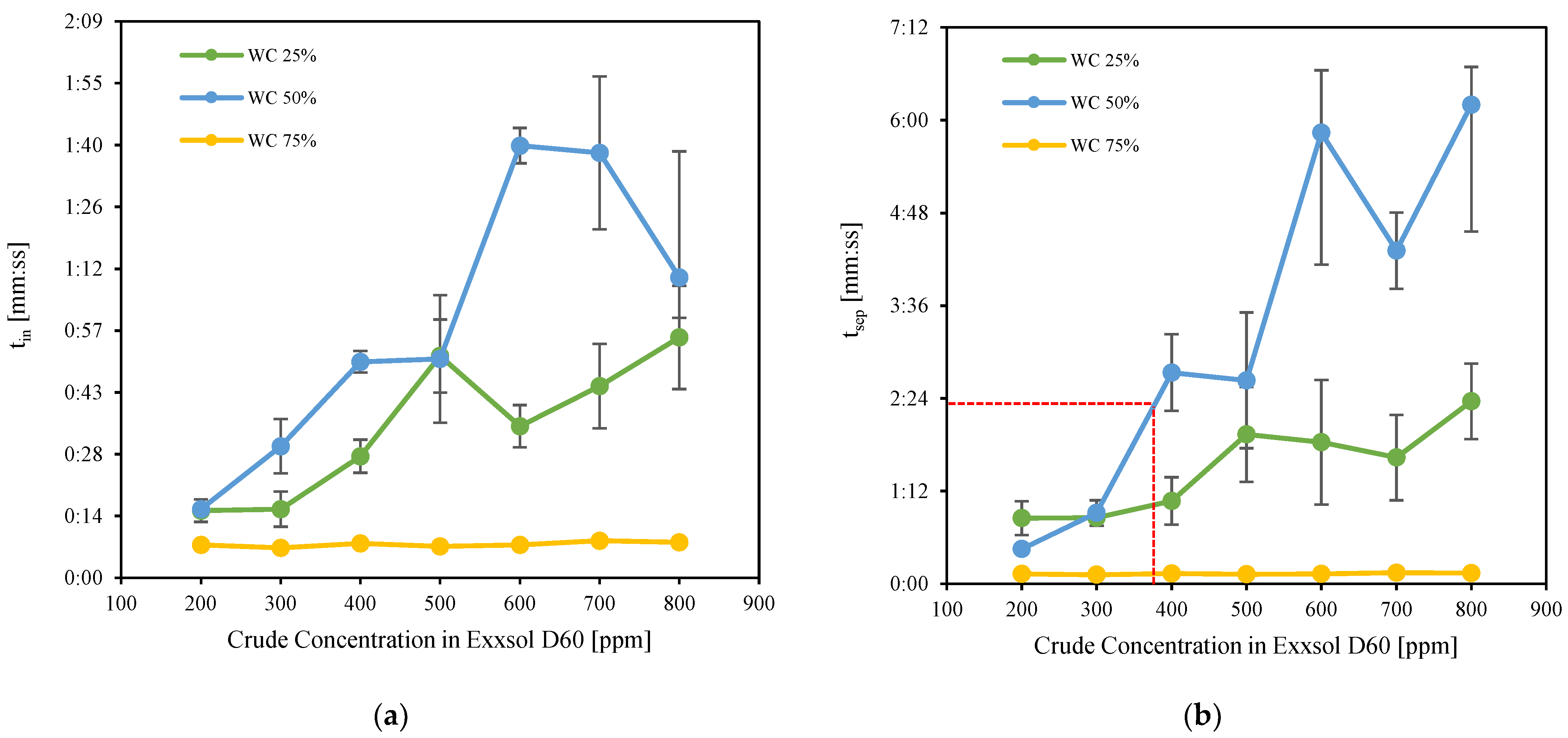
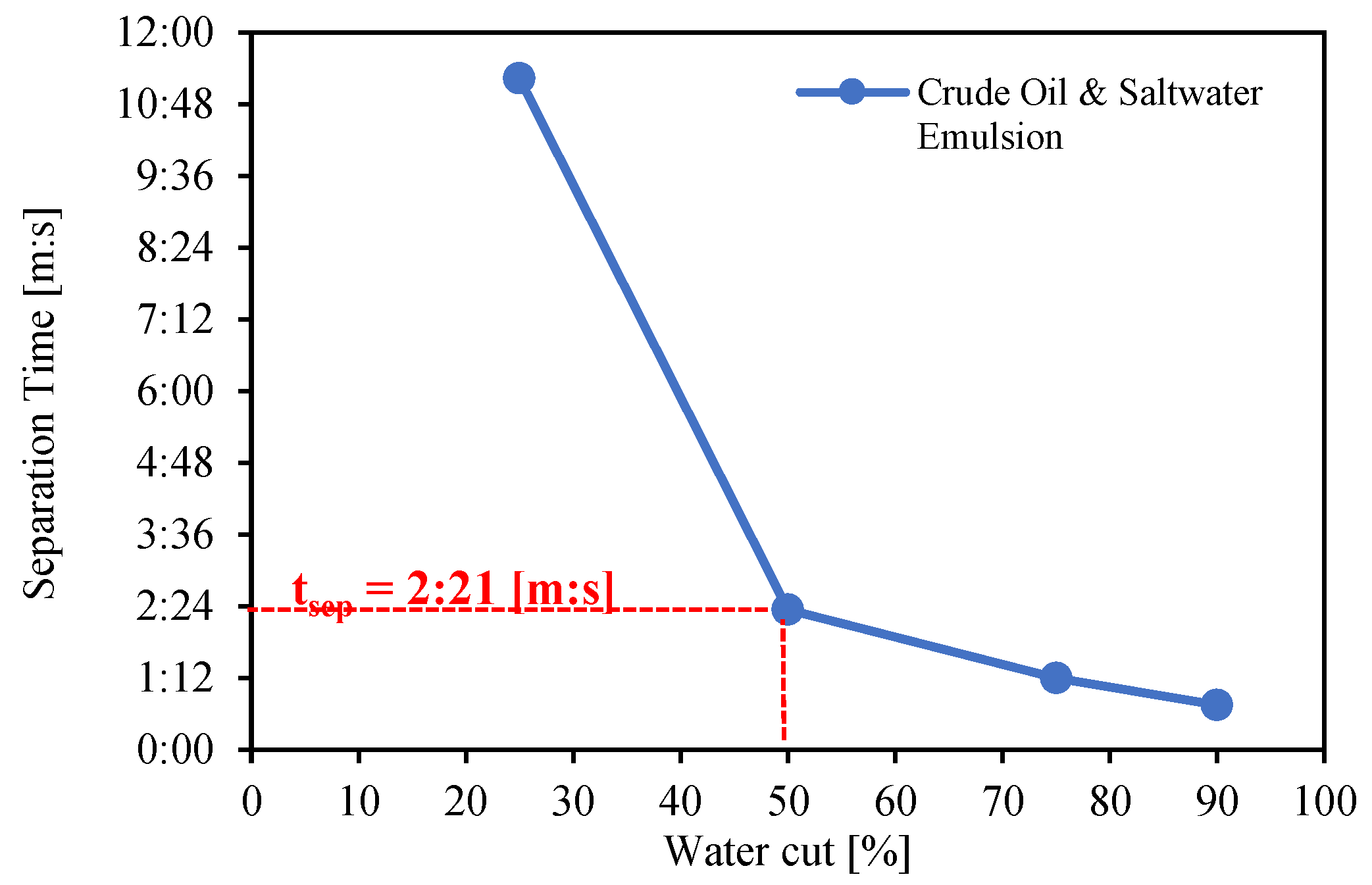
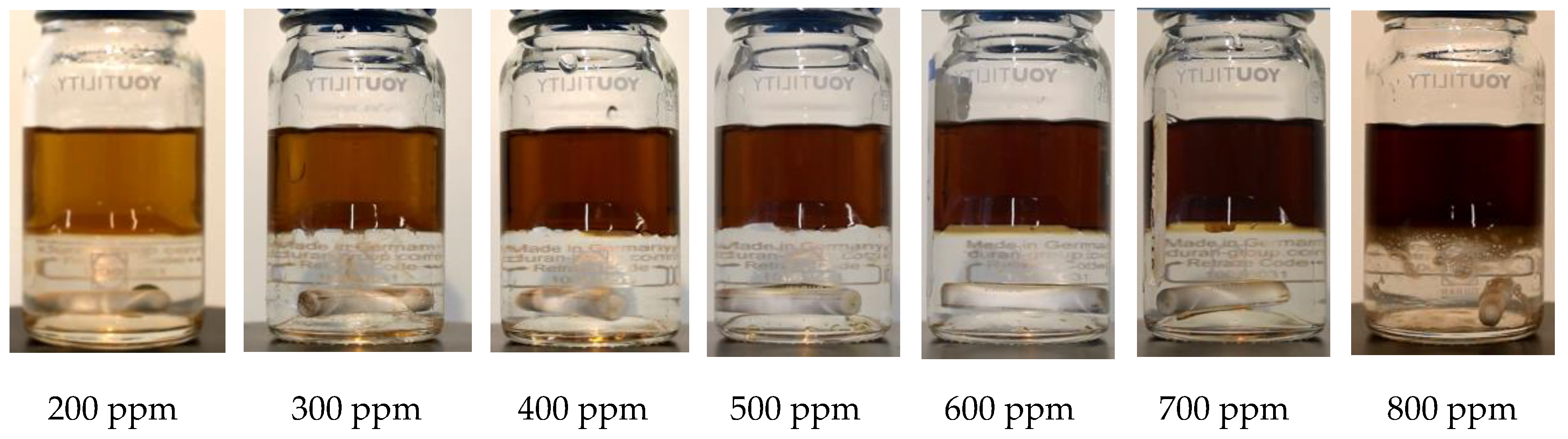
3.4. Bottle Separation Tests
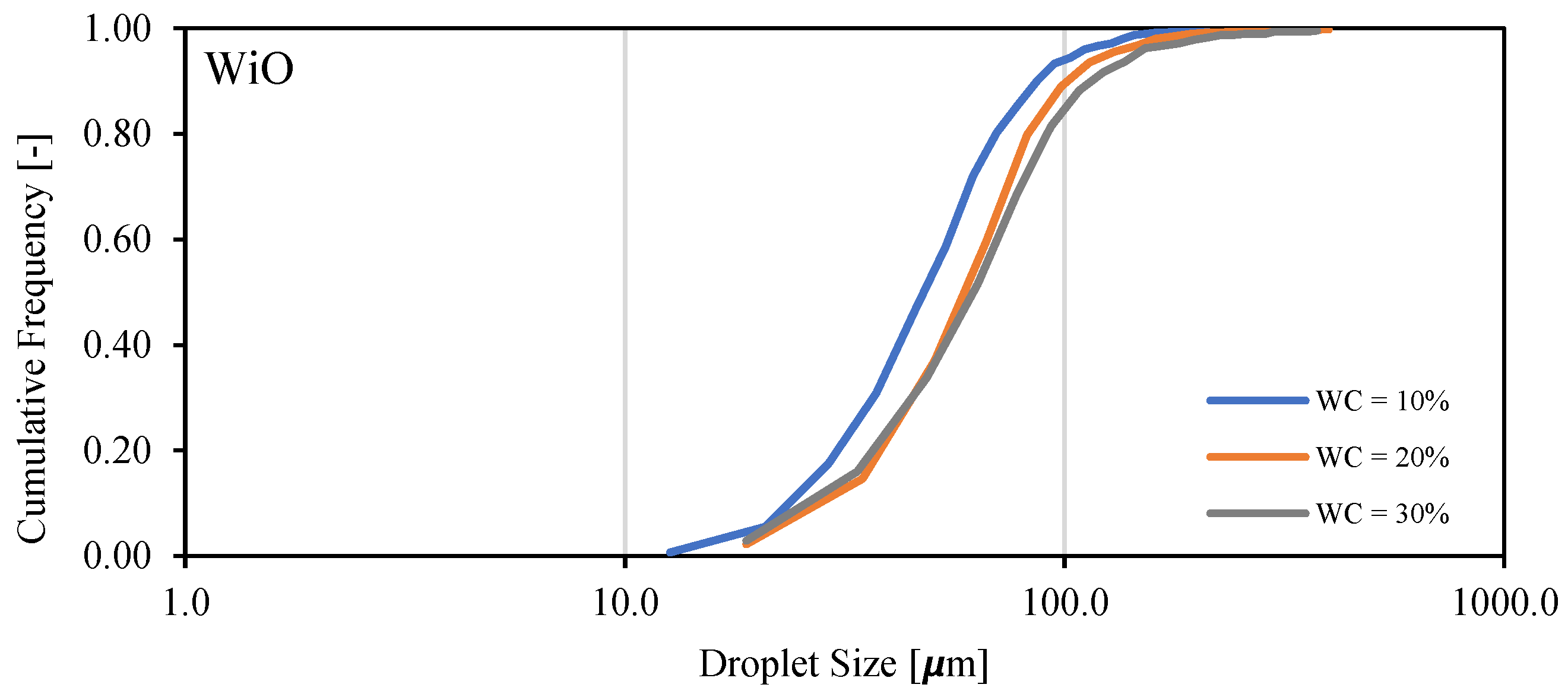
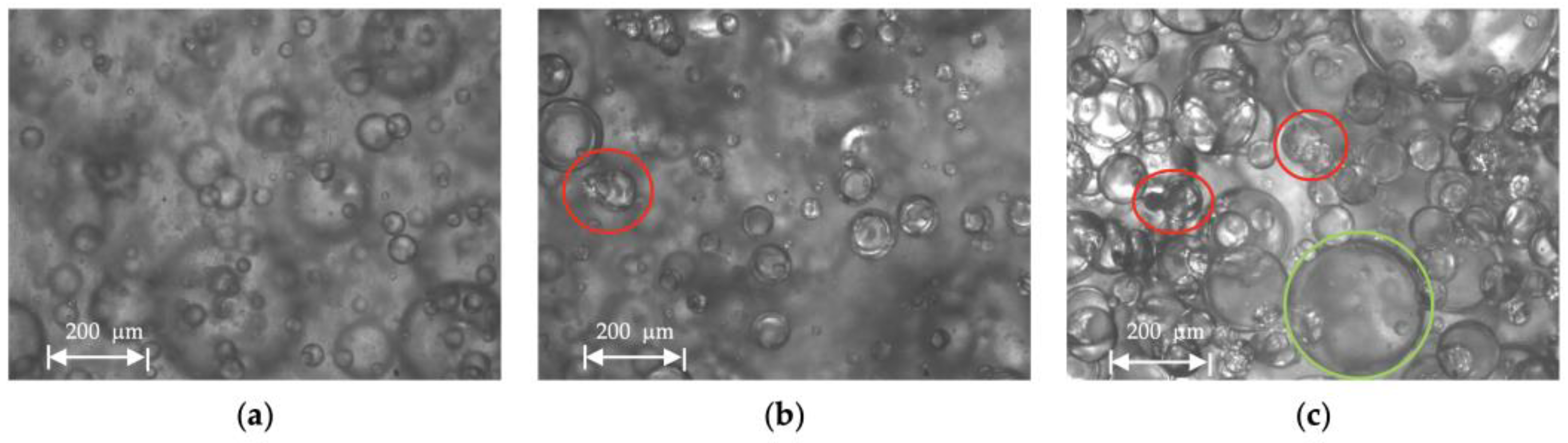
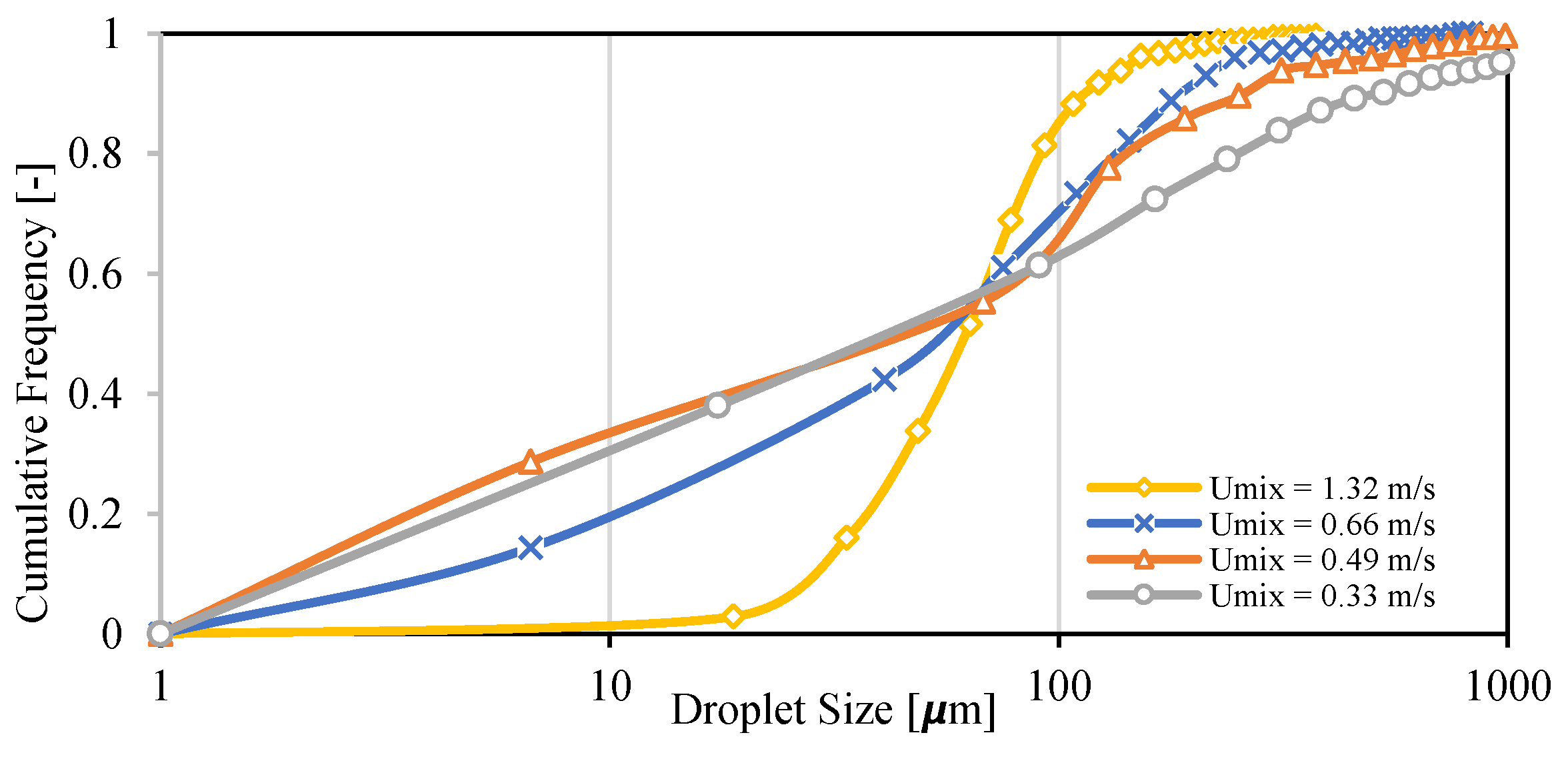
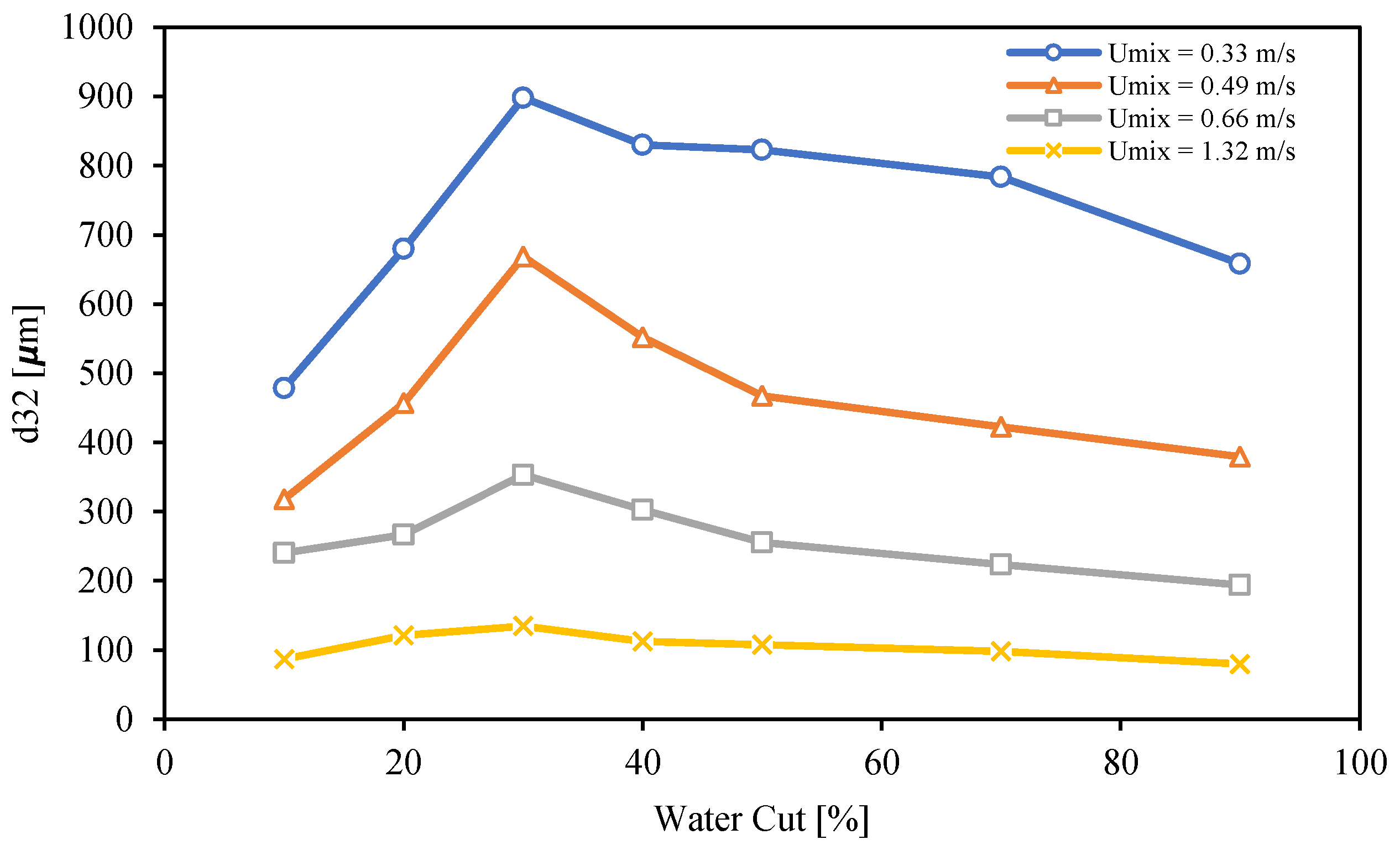
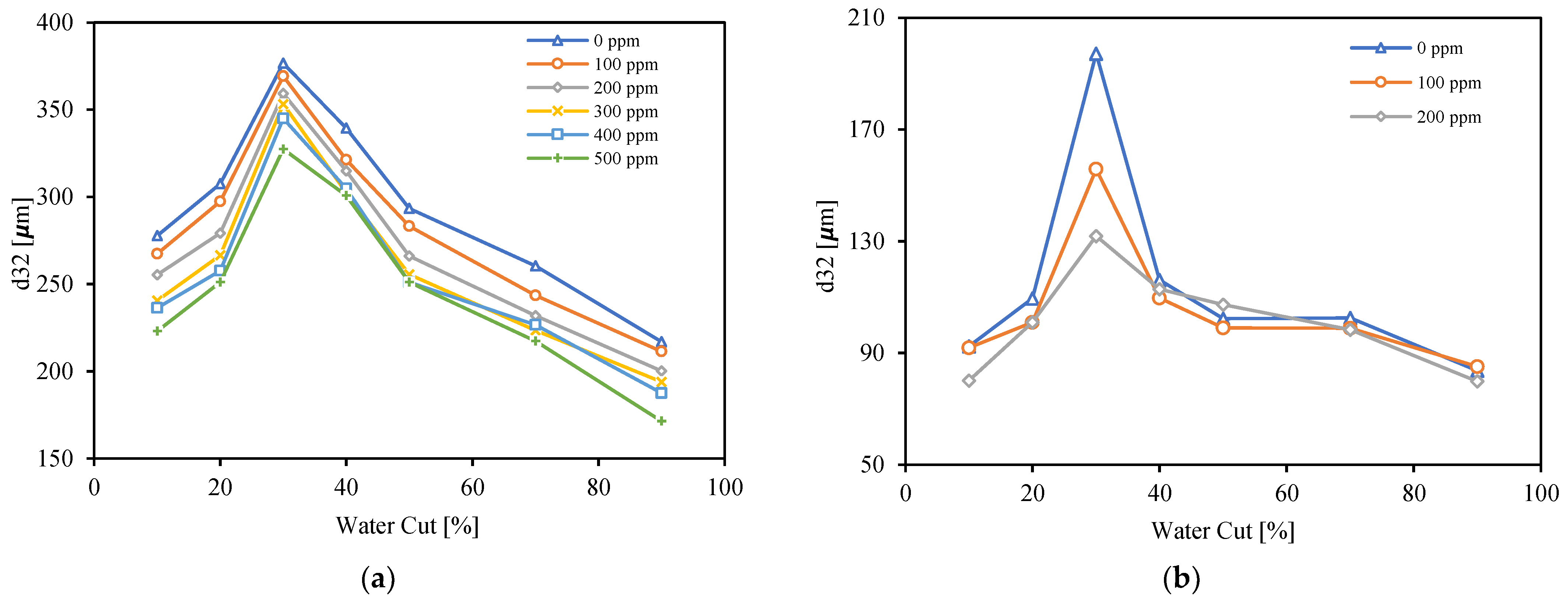
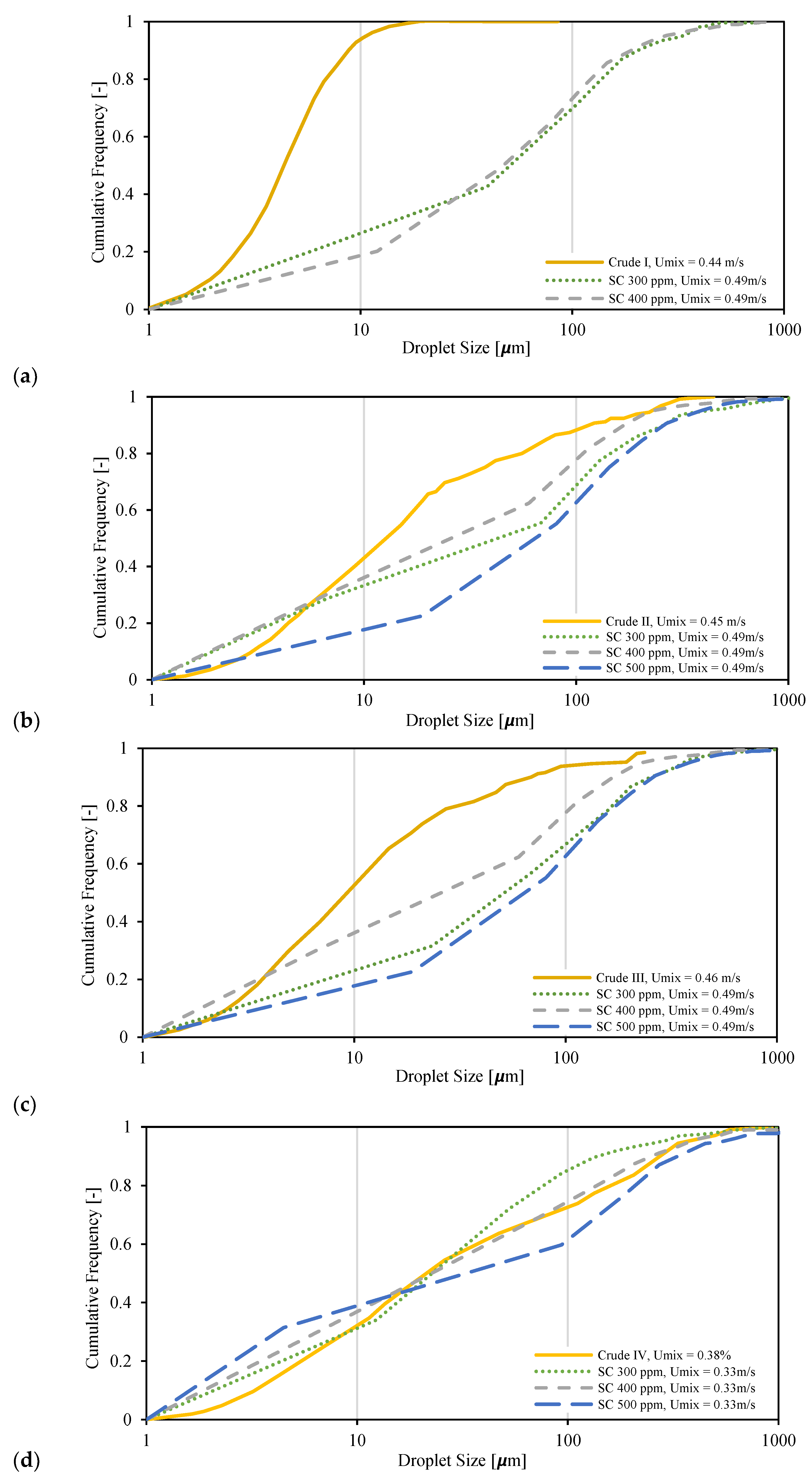
3.5. Droplet Size Distribution
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
Abbreviations
| D | Pipe inner diameter [m] |
| K | Viscosity model constant [-] |
| L | Pipe length [m] |
| Re | Reynolds Number [-] |
| T | Temperature [°C] |
| U | Mixture velocity [m/s] |
| e | Pipe roughness [mm] |
| f | Friction factor [-] |
| Density [kg/m3] | |
| t | Time [s] |
| 𝜇 | Viscosity [cP] |
| 𝛾 | Shear rate [s−1] |
| d | Droplet size distribution [μm] |
| 𝜂 | Viscosity [cP] |
| Shear stress [Pa] | |
| Ω | Volume fraction [-] |
| Pressure drops [Pa] | |
| Subscript | |
| PR | Pal and Rhodes model |
| c | continuous phase |
| d | dispersed phase |
| e | emulsion |
| eff | effective |
| in | interface stabilization |
| m | mixture |
| r | relative |
| sep | separation |
References
- Schümann, H.; Tutkun, M.; Nydal, O.J. Experimental study of dispersed oil-water flow in a horizontal pipe with enhanced inlet mixing, Part 2: In-Situ droplet measurements. J. Pet. Sci. Eng. 2016, 145, 753–762. [Google Scholar] [CrossRef][Green Version]
- Lim, J.; Wong, S.; Law, M.; Samyudia, Y.; Dol, S. A review on the effects of emulsions on flow behaviours and common factors affecting the stability of emulsions. J. Appl. Sci. 2015, 15, 167–172. [Google Scholar] [CrossRef]
- Chen, G.; Tao, D. An experimental study of stability of oil–water emulsion. Fuel Process. Technol. 2005, 86, 499–508. [Google Scholar] [CrossRef]
- Yarranton, H.W.; Sztukowski, D.M.; Urrutia, P. Effect of interfacial rheology on model emulsion coalescence: I. Interfacial rheology. J. Colloid Interface Sci. 2007, 310, 246–252. [Google Scholar] [CrossRef]
- Sjoblom, J. Encyclopedic Handbook of Emulsion Technology; CRC Press: Boca Raton, FL, USA, 2001. [Google Scholar]
- Bibette, J.; Leal-Calderon, F.; Schmitt, V.; Poulin, P. Emulsion Science: Basic Principles. An Overview; Springer: Berlin/Heidelberg, Germany, 2003. [Google Scholar]
- Speight, J.G. The Chemistry and Technology of Petroleum, 4th ed.; CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar]
- Ancheyta, J.; Speight, J.G. Heavy Oils and Residua. Hydroprocessing of Heavy Oils and Residua; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Asaadian, H.; Soulgani, B.S.; Karimi, A. An experimental study on electrical effect on asphaltene deposition. Pet. Sci. Technol. 2017, 35, 2255–2261. [Google Scholar] [CrossRef]
- Ahmadi, P.; Asaadian, H.; Kord, S.; Khadivi, A. Investigation of the simultaneous chemicals influences to promote oil-in-water emulsions stability during enhanced oil recovery applications. J. Mol. Liq. 2019, 275, 57–70. [Google Scholar] [CrossRef]
- Sjöblom, J.; Aske, N.; Auflem, I.H.; Brandal, Ø.; Havre, T.E.; Sæther, Ø.; Westvik, A.; Johnsen, E.E.; Kallevik, H. Our current understanding of water-in-crude oil emulsions: Recent characterization techniques and high pressure performance. Adv. Colloid Interface Sci. 2003, 100, 399–473. [Google Scholar] [CrossRef]
- Aske, N.; Kallevik, H.; Sjöblom, J. Determination of saturate, aromatic, resin, and asphaltenic (SARA) components in crude oils by means of infrared and near-infrared spectroscopy. Energy Fuels 2001, 15, 1304–1312. [Google Scholar] [CrossRef]
- Belt, R.; Duret, E.; Larrey, D.; Djoric, B.; Kalali, S. Comparison of commercial multiphase flow simulators with experimental and field databases. In Proceedings of the 15th International Conference on Multiphase Production Technology, Cannes, France, 15–17 June 2011; OnePetro: Richardson, TX, USA, 2011. [Google Scholar]
- Lakehal, D. Advanced simulation of transient multiphase flow & flow assurance in the oil & gas industry. Can. J. Chem. Eng. 2013, 91, 1201–1214. [Google Scholar]
- Lee, R.F. Agents which promote and stabilize water-in-oil emulsions. Spill Sci. Technol. Bull. 1999, 5, 117–126. [Google Scholar] [CrossRef]
- Langevin, D.; Poteau, S.; Hénaut, I.; Argillier, J.F. Crude oil emulsion properties and their application to heavy oil transportation. Oil Gas Sci. Technol. 2004, 59, 511–521. [Google Scholar] [CrossRef]
- Ahmadi, P.; Asaadian, H.; Khadivi, A.; Kord, S. A new approach for determination of carbonate rock electrostatic double layer variation towards wettability alteration. J. Mol. Liq. 2019, 275, 682–698. [Google Scholar] [CrossRef]
- Moghadasi, R.; Kord, S.; Moghadasi, J.; Asaadian, H. On the Effects of Salinity, Asphaltenes and Resins on Interfacial Tension: Applications to Low Salinity Water Injection. In Proceedings of the 80th EAGE Conference and Exhibition 2018, Copenhagen, Denmark, 11–14 June 2018; European Association of Geoscientists & Engineers: Utrecht, The Netherlands, 2018. [Google Scholar]
- Mansouri, M.; Ahmadi, Y. Applications of zeolite-zirconia-copper nanocomposites as a new asphaltene inhibitor for improving permeability reduction during CO2 flooding. Sci. Rep. 2022, 12, 6209. [Google Scholar] [CrossRef]
- Ahmadi, Y.; Aminshahidy, B. Inhibition of asphaltene precipitation by hydrophobic CaO and SiO2 nanoparticles during natural depletion and CO2 tests. Int. J. Oil Gas Coal Technol. 2020, 24, 394–414. [Google Scholar]
- Schramm, L.L. Fundamentals and applications in the petroleum Industry. Adv. Chem. 1992, 231, 3–24. [Google Scholar]
- Pal, R. Rheology of Particulate Dispersions and Composites; CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar]
- Schuster, D. Encyclopedia of Emulsion Technology; CRC Press: Boca Raton, FL, USA, 1996; Volume 4. [Google Scholar]
- Gafonova, O.V.; Yarranton, H.W. The stabilization of water-in-hydrocarbon emulsions by asphaltenes and resins. J. Colloid Interface Sci. 2001, 241, 469–478. [Google Scholar] [CrossRef]
- Kilpatrick, P.K. Water-in-crude oil emulsion stabilization: Review and unanswered questions. Energy Fuels 2012, 26, 4017–4026. [Google Scholar] [CrossRef]
- Keleşoğlu, S.; Rodionova, G.; Pettersen, B.H.; Foss, M.; Sjöblom, J. Preparation and characterization of reference fluids to mimic flow properties of crude oil emulsions (w/o). J. Dispers. Sci. Technol. 2015, 36, 1458–1464. [Google Scholar] [CrossRef]
- Rodionova, G.; Pettersen, B.; Kelesoğlu, S.; Sjöblom, J. Preparation and characterization of reference fluid mimicking behavior of North Sea heavy crude oil. Fuel 2014, 135, 308–314. [Google Scholar] [CrossRef]
- Brown, D.E.; Pitt, K. Drop size distribution of stirred non-coalescing liquid—Liquid system. Chem. Eng. Sci. 1972, 27, 577–583. [Google Scholar]
- Calabrese, R.V.; Chang, T.P.K.; Dang, P.T. Drop breakup in turbulent stirred-tank contactors. Part I: Effect of dispersed-phase viscosity. AIChE J. 1986, 32, 657–666. [Google Scholar] [CrossRef]
- Boxall, J.A.; Koh, C.A.; Sloan, E.D.; Sum, A.K.; Wu, D.T. Measurement and calibration of droplet size distributions in water-in-oil emulsions by particle video microscope and a focused beam reflectance method. Ind. Eng. Chem. Res. 2010, 49, 1412–1418. [Google Scholar] [CrossRef]
- Fossen, M.; Schümann, H. Experimental study of the relative effect of pressure drop and flow rate on the droplet size downstream of a pipe restriction. J. Dispers. Sci. Technol. 2017, 38, 826–831. [Google Scholar] [CrossRef]
- Ahmadi, Y. Improving fluid flow through low permeability reservoir in the presence of nanoparticles: An experimental core flooding WAG tests. Iran. J. Oil Gas Sci. Technol. 2021, 12, 41. [Google Scholar]
- Mansouri, M.; Ahmadi, Y.; Jafarbeigi, E. Introducing a new method of using nanocomposites for preventing asphaltene aggregation during real static and dynamic natural depletion tests. Energy Sources Part A Recovery Util. Environ. Eff. 2022, 44, 7499–7513. [Google Scholar] [CrossRef]
- Plasencia, J.; Pettersen, B.; Nydal, O.J. Pipe flow of water-in-crude oil emulsions: Effective viscosity, inversion point and droplet size distribution. J. Pet. Sci. Eng. 2013, 101, 35–43. [Google Scholar] [CrossRef]
- Ronningsen, H.P. Correlations for predicting viscosity of W/O-emulsions based on North Sea crude oils. In Proceedings of the SPE International Symposium on Oilfield Chemistry, San Antonio, TX, USA, 14–17 February 1995; OnePetro: Richardson, TX, USA, 1995. [Google Scholar]
- Eilers, V.H. Die viskosität von emulsionen hochviskoser stoffe als funktion der konzentration. Kolloid Z. 1941, 97, 313–321. [Google Scholar] [CrossRef]
- Roscoe, R. The end correction for rotation viscometers. Br. J. Appl. Phys. 1962, 13, 362. [Google Scholar] [CrossRef]
- Taylor, G.I. The viscosity of a fluid containing small drops of another fluid. In Proceedings of the Royal Society of London. Ser. A Contain. Pap. A Math. Phys. Character 1932, 138, 41–48. [Google Scholar]
- Pal, R.; Rhodes, E. Viscosity/concentration relationships for emulsions. J. Rheol. 1989, 33, 1021–1045. [Google Scholar] [CrossRef]







| Reference with Year | Examples of Stabilizer |
|---|---|
| Brown et al. (1972) [28] | Kerosene and salty water |
| Calabrese et al. (1986) [29] | Silicon oil and water system |
| Boxall et al. (2010) [30] | Crude and mineral oil mixes to change the viscosity w/o altering the interfacial tension |
| Rodionova et al. (2014) [27] | Primol 352 and Exxsol D60 mixtures with or without SPAN® 83 |
| Keleşoğlu et al. (2015) [26] | Aerosil® R104 and myristic acids |
| Fossen et al. (2016) [31] | Primol 352 and polyisobutylene mixture, Primol 352 and polyisobutylene mixture |
| Ahmadi (2021) [32] | Crude oil and Calcium Oxide Nanoparticles |
| Mansouri et al. (2022) [33] | Crude oil and zirconia- zinc-copper nanocomposite |
| Fluid | Density [kg/m3] | Viscosity [cP] |
|---|---|---|
| Distilled Water w/wt% 3.4 NaCl | 1020.7 | 0.99 |
| Exxsol D60 w/0.015 g/L Oil Red O | 787.45 | 1.31 |
| Viscosity [cP] | Density [kg/m3] | TAN [mg KOH/g] | TBN [mg KOH/g] | Asphaltenes Content |
|---|---|---|---|---|
| 24.01 | 939.3 | 2.15 | 2.81 | 2.5 wt% hexane insoluble |
| Crude | Viscosity [cP] | Density [kg/m3] | TAN [mg KOH/g] | Asphaltene Content [wt%] | Interfacial Tension [mN/m] | Temperature [°C] |
|---|---|---|---|---|---|---|
| I | 7.5 | 865 | 1.7 | 0.6 | 4.5 | 60 |
| II | 15.9 | 885 | 1.4 | 7.3 | 12.5 | 70 |
| III | 7.1 | 834 | 0.5 | 6.9 | 12.3 | 70 |
| IV | 23.5 | 901 | 4.3 | 2.8 | 11.1 | 70 |
| Mixture Velocity [m/s] | Oil Volume Fractions [-] | Spiking Concentration [ppm] |
|---|---|---|
| 0.33 | 10/20/30/40/50/55/57/60/62/64/ 68/70/72/74/78/80/90 | 300/400/500/600/700/800/900 |
| 0.49 | 10/20/30/40/50/55/57/60/62/64/ 68/70/72/74/78/80/90 | 300/400/500/600/700/800/900 |
| 0.66 | 10/20/30/40/50/55/57/60/62/64/ 68/70/72/74/78/80/90 | 0/100/200/300/400/500/600/700/800/900/50,000 |
| 1.32 | 10/20/30/40/50/55/57/60/62/64/ 68/70/72/74/78/80/90 | 0/100/200 |
| Crude Concentration [ppm] | WC [%] |
|---|---|
| 200/300/400/500/600/700/800 | 25 |
| 200/300/400/500/600/700/800 | 50 |
| 200/300/400/500/600/700/800 | 75 |
| Mixture Velocity [m/s] | Oil Volume Fraction at Inversion Point [-] |
|---|---|
| 0.33 | 71 |
| 0.49 | 70 |
| 0.66 | 69 |
| 1.32 | 67 |
| Reference with Year | Model Equation | Liquids | Equation Considerations |
|---|---|---|---|
| Eilers [36] (1941) | Bayol-35 with 0.5% (v/v) Triton x-100 | 0.19 < < 0.68 | |
| Roscoe [37] (1952) | Shell vitrea-220 with 0.5% (v/v) Triton x-100 | 0.3 < < 0.69 | |
| Taylor [38] (1932) | CCl4/Bayol mix. (26.55% (v/v) CCl4) with 0.5% (v/v) Triton x-100 | and are the dispersed-phase and continuous-phase viscosities, respectively. | |
| Pal and Rhodes [39] (1989) | Diesel with 2% (v/v) Span 85 | < 0.74 | |
| Homogenous model | --- | --- |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asaadian, H.; Stanko, M. Experimental Characterization and Evaluation of Crude Spiking Influence on Oil/Water Dispersed Flow in Pipe. Molecules 2023, 28, 6363. https://doi.org/10.3390/molecules28176363
Asaadian H, Stanko M. Experimental Characterization and Evaluation of Crude Spiking Influence on Oil/Water Dispersed Flow in Pipe. Molecules. 2023; 28(17):6363. https://doi.org/10.3390/molecules28176363
Chicago/Turabian StyleAsaadian, Hamidreza, and Milan Stanko. 2023. "Experimental Characterization and Evaluation of Crude Spiking Influence on Oil/Water Dispersed Flow in Pipe" Molecules 28, no. 17: 6363. https://doi.org/10.3390/molecules28176363
APA StyleAsaadian, H., & Stanko, M. (2023). Experimental Characterization and Evaluation of Crude Spiking Influence on Oil/Water Dispersed Flow in Pipe. Molecules, 28(17), 6363. https://doi.org/10.3390/molecules28176363





