Characterization and Application of Guar Gum/Polyvinyl Alcohol-Based Food Packaging Films Containing Betacyanins from Pokeweed (Phytolacca acinosa Roxb.) Berries and Silver Nanoparticles
Abstract
:1. Introduction
2. Results and Discussions
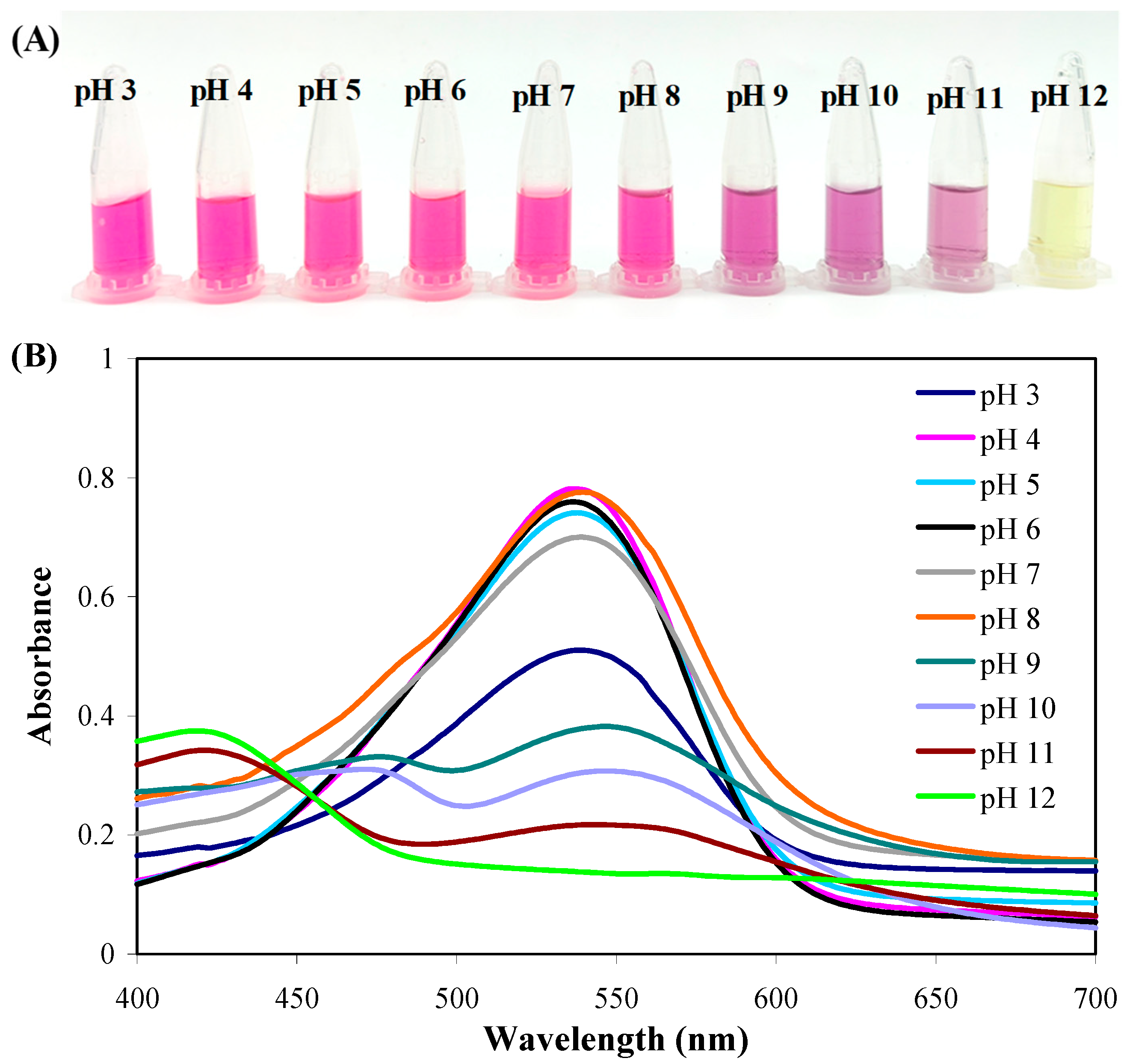
2.1. pH-Sensitive Color-Changing Capacity of PB
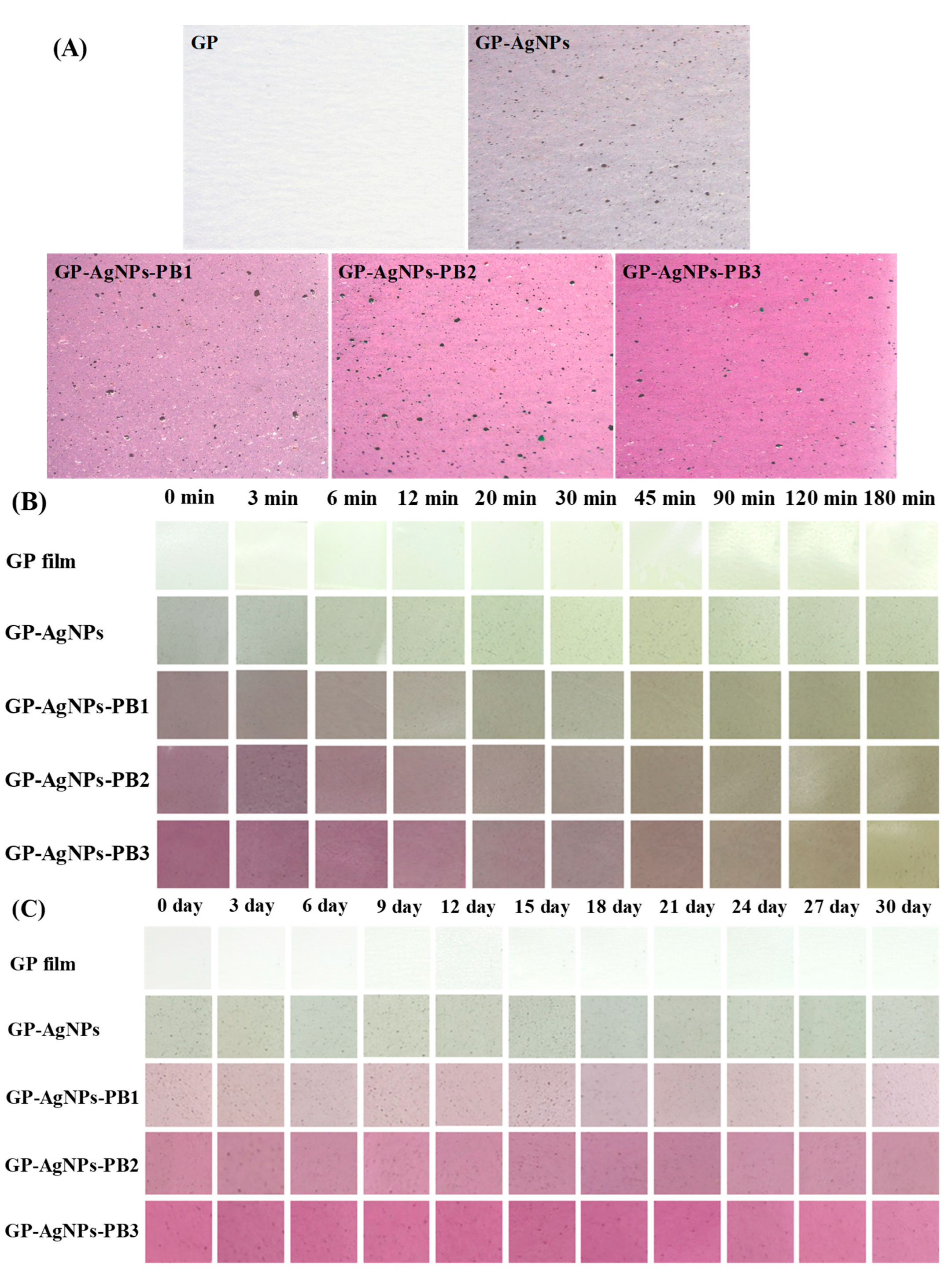
2.2. Structural Characterization of Films
2.3. Color-Related Properties of Films
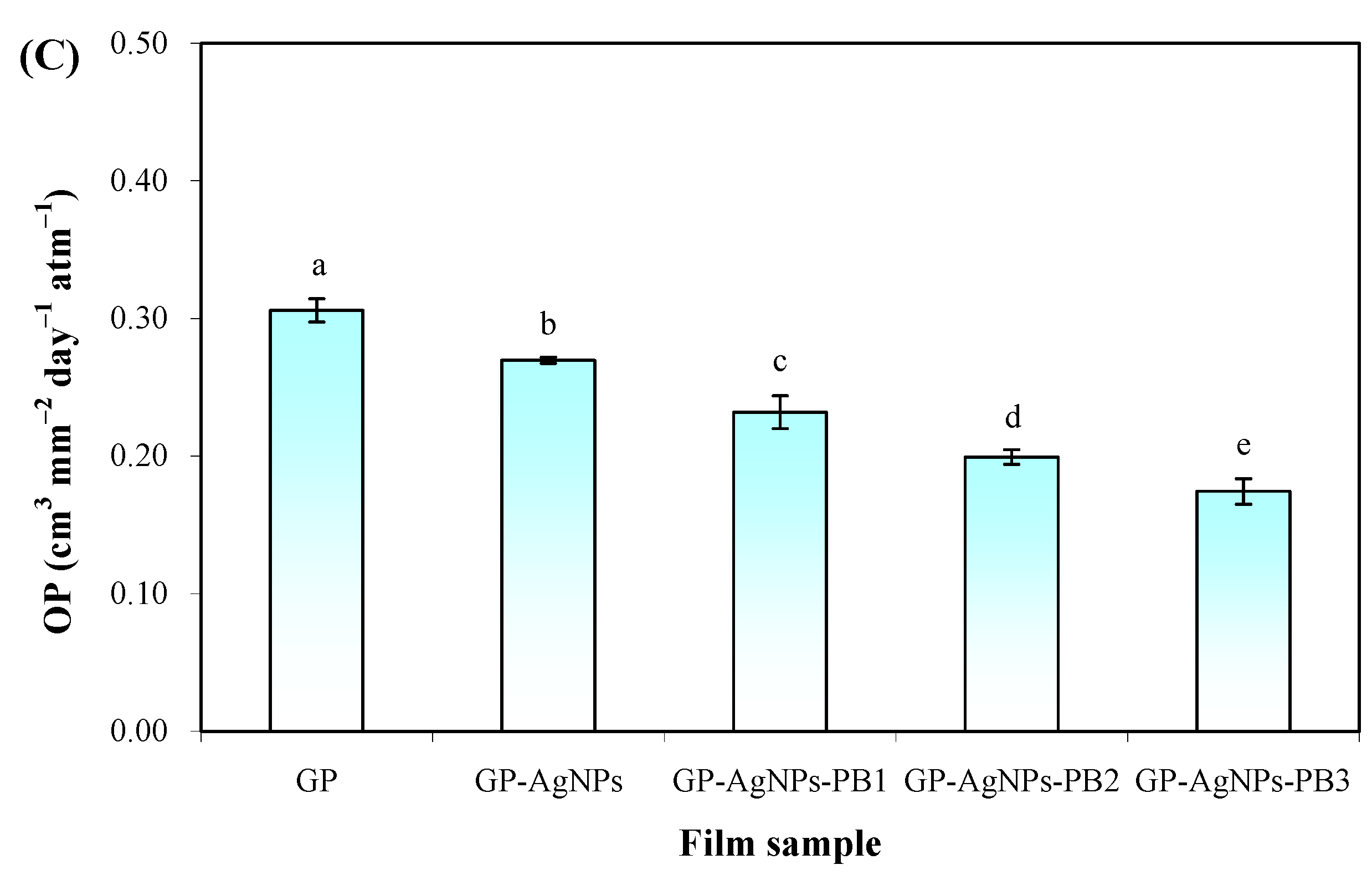
2.4. Barrier Properties of Films
2.5. Mechanical Properties of Films
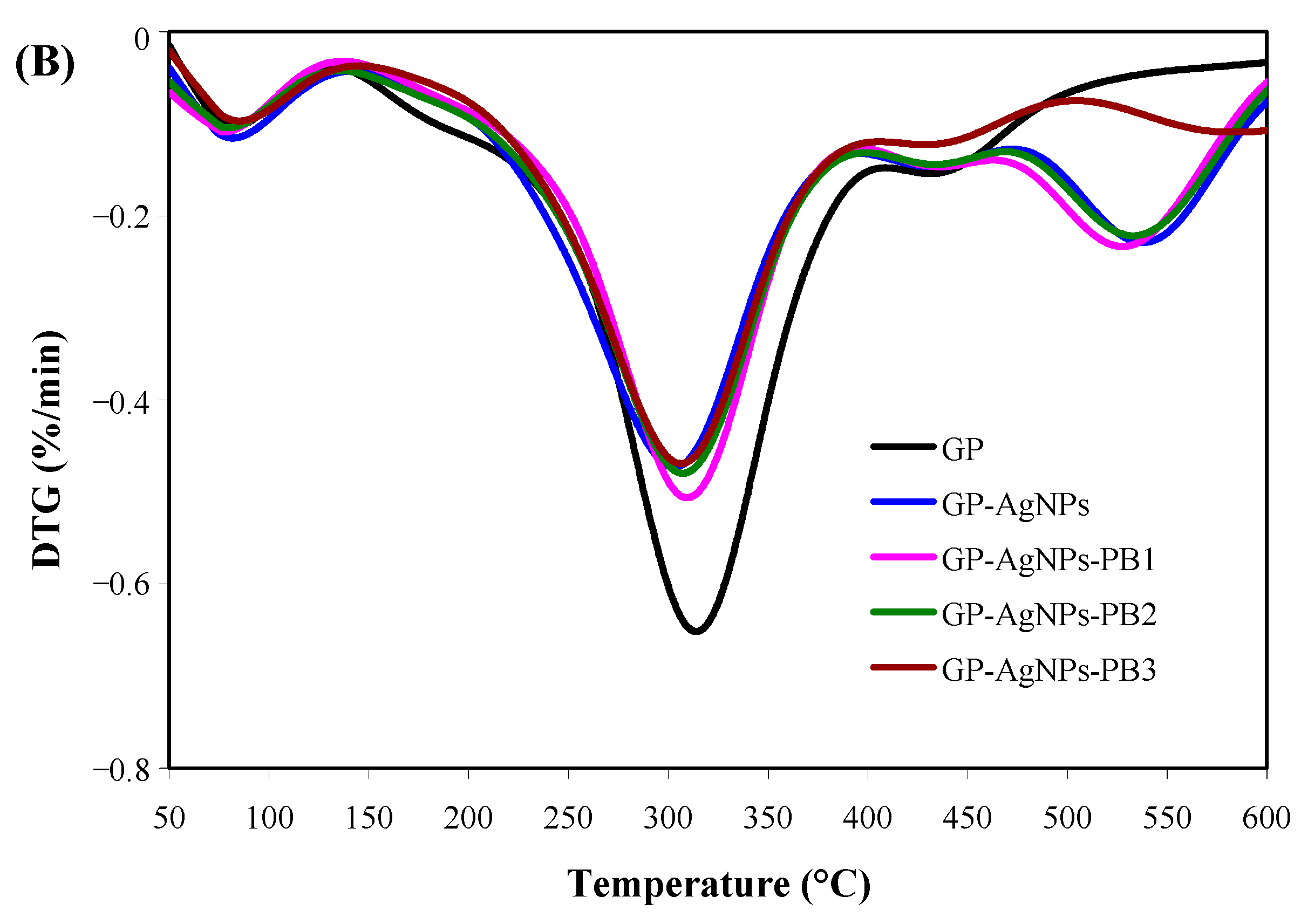
2.6. Thermal Property of Films
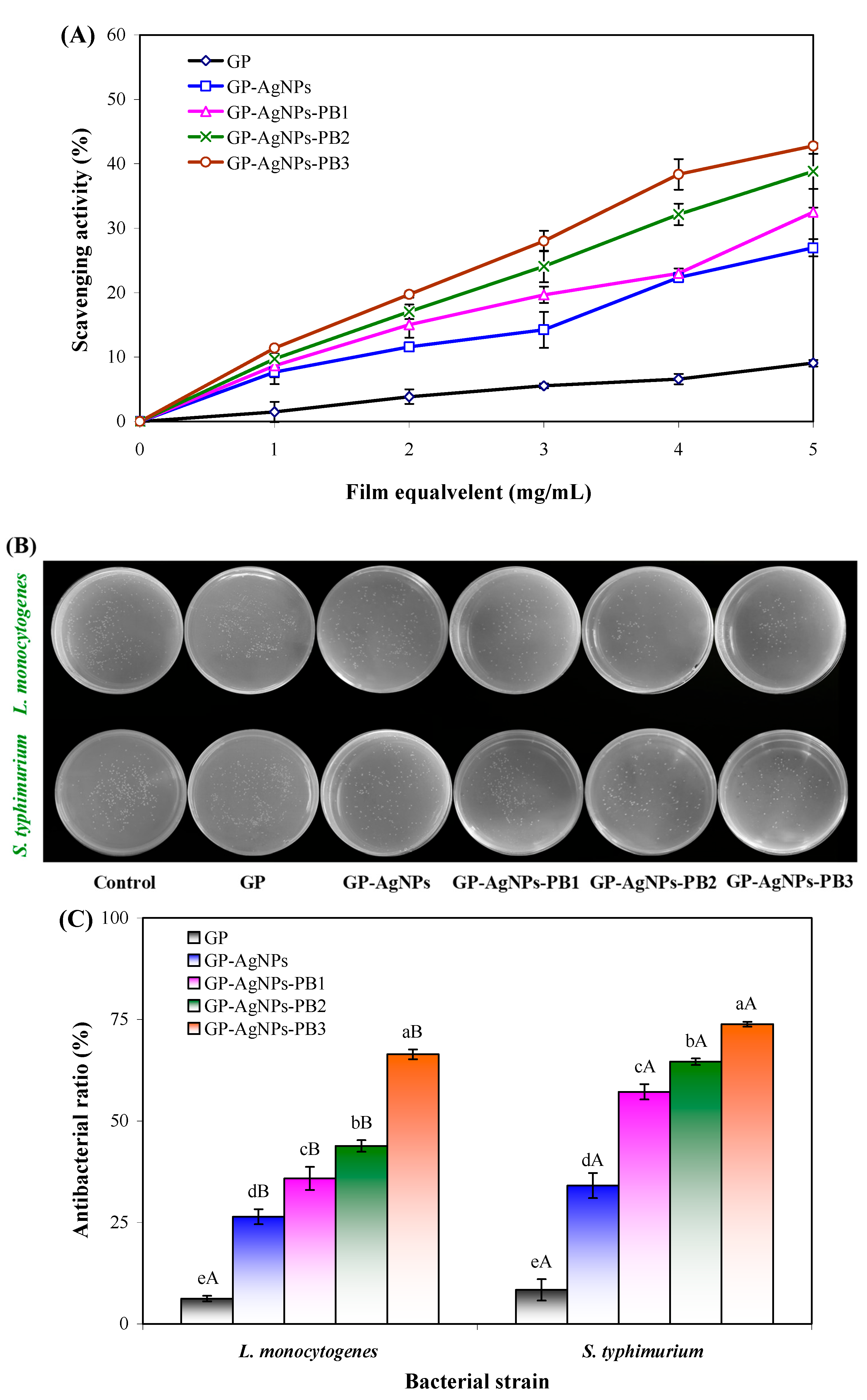
2.7. Antioxidant Activity of Films
2.8. Antimicrobial Activity of Films
2.9. Application of Films in Indicating the Quality of Shrimp
3. Materials and Methods
3.1. Materials, Chemicals and Microbial Strains
3.2. Extraction and Characterization of Pokeweed Betacyanins
3.3. Development of Films
3.4. Characterization of Films
3.4.1. Structural Characterization
3.4.2. Color-Related Properties
3.4.3. Barrier Properties
3.4.4. Mechanical Properties
3.4.5. Thermal Property
3.4.6. Antioxidant Activity
3.4.7. Antibacterial Activity
3.5. Application of Films
3.6. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Asgher, M.; Qamar, S.A.; Bilal, M.; Iqbal, H.M. Bio-based active food packaging materials: Sustainable alternative to conventional petrochemical-based packaging materials. Food Res. Int. 2020, 137, 109625. [Google Scholar] [CrossRef] [PubMed]
- Sani, M.A.; Azizi-Lalabadi, M.; Tavassoli, M.; Mohammadi, K.; McClements, D.J. Recent advances in the development of smart and active biodegradable packaging materials. Nanomaterials 2021, 11, 1331. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Félix, F.; Corte-Tarazón, J.A.; Rochín-Wong, S.; Fernández-Quiroz, J.D.; Garzón-García, A.M.; Santos-Sauceda, I.; Plascencia-Martínez, D.F.; Chan-Chan, L.H.; Vᾴsquez-Lόpez, C.; Barreras-Urbina, C.G.; et al. Physicochemical, structural, mechanical and antioxidant properties of zein films incorporated with no-ultrafiltered and ultrafiltered betalains extract from the beetroot (Beta vulgaris) bagasse with potential application as active food packaging. J. Food Eng. 2022, 334, 111153. [Google Scholar] [CrossRef]
- Mudgil, D.; Barak, S.; Khatkar, B.S. Guar gum: Processing, properties and food applications—A review. J. Food Sci. Tech. 2014, 51, 409–418. [Google Scholar] [CrossRef] [PubMed]
- Thombare, N.; Jha, U.; Mishra, S.; Siddiqui, M.Z. Guar gum as a promising starting material for diverse applications: A review. Int. J. Biol. Macromol. 2016, 88, 361–372. [Google Scholar] [CrossRef]
- Sharma, G.; Sharma, S.; Kumar, A.; Ala’a, H.; Naushad, M.; Ghfar, A.A.; Mola, G.T.; Stadler, F.J. Guar gum and its composites as potential materials for diverse applications: A review. Carbohyd. Polym. 2018, 199, 534–545. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Zhang, W.; Chen, L.; Liu, J.; Cao, J.; Jiang, W. Recent advances in guar gum-based films or coatings: Diverse property enhancement strategies and applications in foods. Food Hydrocolloid. 2023, 136, 108278. [Google Scholar] [CrossRef]
- Constantin, M.; Lupei, M.; Bucatariu, S.M.; Pelin, I.M.; Doroftei, F.; Ichim, D.L.; Daraba, O.M.; Fundueanu, G. PVA/chitosan thin films containing silver nanoparticles and Ibuprofen for the treatment of periodontal disease. Polymers 2023, 15, 4. [Google Scholar] [CrossRef]
- Krzywicka, A.; Megiel, E. Silver-polystyrene (Ag/PS) nanocomposites doped with polyvinyl alcohol (PVA)—Fabrication and bactericidal activity. Nanomaterials 2020, 10, 2245. [Google Scholar] [CrossRef]
- Dorigato, A.; Pegoretti, A. Biodegradable single-polymer composites from polyvinyl alcohol. Colloid Polym. Sci. 2012, 290, 359–370. [Google Scholar] [CrossRef]
- Akhila, K.; Sultana, A.; Ramakanth, D.; Gaikwad, K.K. Monitoring freshness of chicken using intelligent pH indicator packaging film composed of polyvinyl alcohol/guar gum integrated with Ipomoea coccinea extract. Food Biosci. 2023, 52, 102397. [Google Scholar] [CrossRef]
- Gasti, T.; Hiremani, V.D.; Kesti, S.S.; Vanjeri, V.N.; Goudar, N.; Masti, S.P.; Thimmappa, S.C.; Chougale, R.B. Physicochemical and antibacterial evaluation of poly (vinyl alcohol)/guar gum/silver nanocomposite films for food packaging applications. J. Polym. Environ. 2021, 29, 3347–3363. [Google Scholar] [CrossRef]
- Alizadeh-Sani, M.; Mohammadian, E.; McClements, D.J. Eco-friendly active packaging consisting of nanostructured biopolymer matrix reinforced with TiO2 and essential oil: Application for preservation of refrigerated meat. Food Chem. 2020, 322, 126782. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, S.M.; El-Sayed, H.S.; Ibrahim, O.A.; Youssef, A.M. Rational design of chitosan/guar gum/zinc oxide bionanocomposites based on Roselle calyx extract for Ras cheese coating. Carbohyd. Polym. 2020, 239, 116234. [Google Scholar] [CrossRef] [PubMed]
- Kanikireddy, V.; Varaprasad, K.; Rani, M.S.; Venkataswamy, P.; Reddy, B.J.M.; Vithal, M. Biosynthesis of CMC-Guar gum-Ag0 nanocomposites for inactivation of food pathogenic microbes and its effect on the shelf life of strawberries. Carbohyd. Polym. 2020, 236, 116053. [Google Scholar] [CrossRef]
- Liao, C.; Li, Y.; Tjong, S.C. Bactericidal and cytotoxic properties of silver nanoparticles. Int. J. Mol. Sci. 2019, 20, 449. [Google Scholar] [CrossRef]
- Chu, M.; Feng, N.; An, H.; You, G.; Mo, C.; Zhong, H.; Pan, L.; Hu, D. Design and validation of antibacterial and pH response of cationic guar gum film by combining hydroxyethyl cellulose and red cabbage pigment. Int. J. Biol. Macromol. 2020, 162, 1311–1322. [Google Scholar] [CrossRef]
- Emir, A.A.; Yildiz, E.; Aydogdu, Y.; Sumnu, G. Active films based on Faba bean (Vicia faba L.) flour incorporated with Sumac (Rhus coriaria): Assessment of antioxidant and antimicrobial performances of packaging for shelf life of chicken breast. Food Bioprocess Tech. 2023, 16, 327–341. [Google Scholar] [CrossRef]
- Bailly, C. Medicinal properties and anti-inflammatory components of Phytolacca (Shanglu). Digit. Chin. Med. 2021, 4, 159–169. [Google Scholar] [CrossRef]
- Marinas, I.C.; Oprea, E.L.I.Z.A.; Geana, E.I.; Luntraru, C.M.; Gird, C.E.; Chifiriuc, M.C. Chemical composition, antimicrobial and antioxidant activity of Phytolacca americana L. fruits and leaves extracts. Farmacia 2021, 69, 883–889. [Google Scholar] [CrossRef]
- Wu, Y.; Tang, P.; Quan, S.; Zhang, H.; Wang, K.; Liu, J. Preparation, characterization and application of smart packaging films based on locust bean gum/polyvinyl alcohol blend and betacyanins from cockscomb (Celosia cristata L.) flower. Int. J. Biol. Macromol. 2021, 191, 679–688. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Qin, Y.; Zhang, M.; Zhang, J.; Qian, C.; Liu, J. Development of active and smart packaging films based on starch, polyvinyl alcohol and betacyanins from different plant sources. Int. J. Biol. Macromol. 2021, 183, 358–368. [Google Scholar] [CrossRef]
- Qin, Y.; Liu, Y.; Zhang, X.; Liu, J. Development of active and intelligent packaging by incorporating betalains from red pitaya (Hylocereus polyrhizus) peel into starch/polyvinyl alcohol films. Food Hydrocolloid. 2020, 100, 105410. [Google Scholar] [CrossRef]
- Yao, X.; Hu, H.; Qin, Y.; Liu, J. Development of antioxidant, antimicrobial and ammonia-sensitive films based on quaternary ammonium chitosan, polyvinyl alcohol and betalains-rich cactus pears (Opuntia ficus-indica) extract. Food Hydrocolloid. 2020, 106, 105896. [Google Scholar] [CrossRef]
- Hu, H.; Yao, X.; Qin, Y.; Yong, H.; Liu, J. Development of multifunctional food packaging by incorporating betalains from vegetable amaranth (Amaranthus tricolor L.) into quaternary ammonium chitosan/fish gelatin blend films. Int. J. Biol. Macromol. 2020, 159, 675–684. [Google Scholar] [CrossRef] [PubMed]
- Jerz, G.; Skotzki, T.; Fiege, K.; Winterhalter, P.; Wybraniec, S. Separation of betalains from berries of Phytolacca americana by ion-pair high-speed counter-current chromatography. J. Chromatogr. A 2008, 1190, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Kanha, N.; Osiriphun, S.; Rakariyatham, K.; Klangpetch, W.; Laokuldilok, T. On-package indicator films based on natural pigments and polysaccharides for monitoring food quality: A review. J. Sci. Food Agric. 2022, 102, 6804–6823. [Google Scholar] [CrossRef]
- Yao, X.; Liu, J.; Hu, H.; Yun, D.; Liu, J. Development and comparison of different polysaccharide/PVA-based active/intelligent packaging films containing red pitaya betacyanins. Food Hydrocolloid. 2022, 124, 107305. [Google Scholar] [CrossRef]
- Shaikh, H.M.; Anis, A.; Poulose, A.M.; Madhar, N.A.; Al-Zahrani, S.M. Date-palm-derived cellulose nanocrystals as reinforcing agents for poly (vinyl alcohol)/guar-gum-based phase-separated composite films. Nanomaterials 2022, 12, 1104. [Google Scholar] [CrossRef]
- Guo, Q.; Yuan, Y.; He, M.; Zhang, X.; Li, L.; Zhang, Y.; Li, B. Development of a multifunctional food packaging for meat products by incorporating carboxylated cellulose nanocrystal and beetroot extract into sodium alginate films. Food Chem. 2023, 415, 135799. [Google Scholar] [CrossRef]
- Jiang, H.; Zhang, W.; Pu, Y.; Chen, L.; Cao, J.; Jiang, W. Development and characterization of a novel active and intelligent film based on pectin and betacyanins from peel waste of pitaya (Hylocereus undatus). Food Chem. 2023, 404, 134444. [Google Scholar] [CrossRef]
- Jamróz, E.; Kulawik, P.; Guzik, P.; Duda, I. The verification of intelligent properties of furcellaran films with plant extracts on the stored fresh Atlantic mackerel during storage at 2 °C. Food Hydrocolloid. 2019, 97, 105211. [Google Scholar] [CrossRef]
- Arfat, Y.A.; Ejaz, M.; Jacob, H.; Ahmed, J. Deciphering the potential of guar gum/Ag-Cu nanocomposite films as an active food packaging material. Carbohyd. Polym. 2017, 157, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Ge, C.; Popham, J. A review and evaluation of prediction models of gas permeation for a blended flexible packaging film. Packag. Technol. Sci. 2016, 29, 247–262. [Google Scholar] [CrossRef]
- Hajji, S.; Salem, R.B.S.B.; Hamdi, M.; Jellouli, K.; Ayadi, W.; Nasri, M.; Boufi, S. Nanocomposite films based on chitosan-poly (vinyl alcohol) and silver nanoparticles with high antibacterial and antioxidant activities. Process Saf. Environ. 2017, 111, 112–121. [Google Scholar] [CrossRef]
- You, C.; Han, C.; Wang, X.; Zheng, Y.; Li, Q.; Hu, X.; Sun, H. The progress of silver nanoparticles in the antibacterial mechanism, clinical application and cytotoxicity. Mol. Biol. Rep. 2012, 39, 9193–9201. [Google Scholar] [CrossRef]
- Kanatt, S.R. Development of active/intelligent food packaging film containing Amaranthus leaf extract for shelf life extension of chicken/fish during chilled storage. Food Packag. Shelf Life 2020, 24, 100506. [Google Scholar] [CrossRef]
- Takma, D.K.; Korel, F. Active packaging films as a carrier of black cumin essential oil: Development and effect on quality and shelf-life of chicken breast meat. Food Packag. Shelf Life 2019, 19, 210–217. [Google Scholar] [CrossRef]
- Zhang, X.; Lian, H.; Shi, J.; Meng, W.; Peng, Y. Plant extracts such as pine nut shell, peanut shell and jujube leaf improved the antioxidant ability and gas permeability of chitosan films. Int. J. Biol. Macromol. 2020, 148, 1242–1250. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Kim, H.J.; Rhim, J.W. Effect of blended colorants of anthocyanin and shikonin on carboxymethyl cellulose/agar-based smart packaging film. Int. J. Biol. Macromol. 2021, 183, 305–315. [Google Scholar] [CrossRef]




| Film | L* | a* | b* | ΔE |
|---|---|---|---|---|
| GP | 88.86 ± 0.02 a | –0.37 ± 0.11 d | –1.37 ± 0.04 a | 4.98 ± 0.73 e |
| GP-AgNPs | 76.27 ± 0.06 b | –0.02 ± 0.03 d | –1.95 ± 0.08 a | 13.55 ± 0.06 d |
| GP-AgNPs-PB1 | 70.96 ± 0.14 c | 11.18 ± 0.14 c | –5.59 ± 0.34 b | 23.54 ± 0.06 c |
| GP-AgNPs-PB2 | 60.99 ± 0.19 d | 24.78 ± 0.25 b | –11.28 ± 0.16 c | 40.69 ± 0.12 b |
| GP-AgNPs-PB3 | 54.44 ± 0.44 e | 34.53 ± 0.39 a | –14.94 ± 0.28 d | 52.71 ± 0.15 a |
| Time (h) | TVB-N of Shrimp (mg/100 g) | Color of Films | ||||
|---|---|---|---|---|---|---|
| GP | GP-AgNPs | GP-AgNPs-PB1 | GP-AgNPs-PB2 | GP-AgNPs-PB3 | ||
| 0 | 5.08 ± 0.21 g |  |  |  |  |  |
| 8 | 9.10 ± 0.28 f |  |  |  |  |  |
| 16 | 12.69 ± 0.31 e |  |  |  |  |  |
| 24 | 16.94 ± 0.15 d |  |  |  |  |  |
| 32 | 23.22 ± 0.11 c |  |  |  |  |  |
| 40 | 32.01 ± 0.23 b |  |  |  |  |  |
| 48 | 46.02 ± 0.16 a |  |  |  |  |  |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, X.; Song, J.; Xu, F.; Yun, D.; Li, C.; Liu, J. Characterization and Application of Guar Gum/Polyvinyl Alcohol-Based Food Packaging Films Containing Betacyanins from Pokeweed (Phytolacca acinosa Roxb.) Berries and Silver Nanoparticles. Molecules 2023, 28, 6243. https://doi.org/10.3390/molecules28176243
Huang X, Song J, Xu F, Yun D, Li C, Liu J. Characterization and Application of Guar Gum/Polyvinyl Alcohol-Based Food Packaging Films Containing Betacyanins from Pokeweed (Phytolacca acinosa Roxb.) Berries and Silver Nanoparticles. Molecules. 2023; 28(17):6243. https://doi.org/10.3390/molecules28176243
Chicago/Turabian StyleHuang, Xiaoqian, Jiangfeng Song, Fengfeng Xu, Dawei Yun, Chenchen Li, and Jun Liu. 2023. "Characterization and Application of Guar Gum/Polyvinyl Alcohol-Based Food Packaging Films Containing Betacyanins from Pokeweed (Phytolacca acinosa Roxb.) Berries and Silver Nanoparticles" Molecules 28, no. 17: 6243. https://doi.org/10.3390/molecules28176243
APA StyleHuang, X., Song, J., Xu, F., Yun, D., Li, C., & Liu, J. (2023). Characterization and Application of Guar Gum/Polyvinyl Alcohol-Based Food Packaging Films Containing Betacyanins from Pokeweed (Phytolacca acinosa Roxb.) Berries and Silver Nanoparticles. Molecules, 28(17), 6243. https://doi.org/10.3390/molecules28176243







