Biological Activity of Genus Hypericum Sect. Hypericum Species—H. tetrapterum, H. maculatum subsp. immaculatum, H. triquetrifolium
Abstract
:1. Introduction
2. Results and Discussion
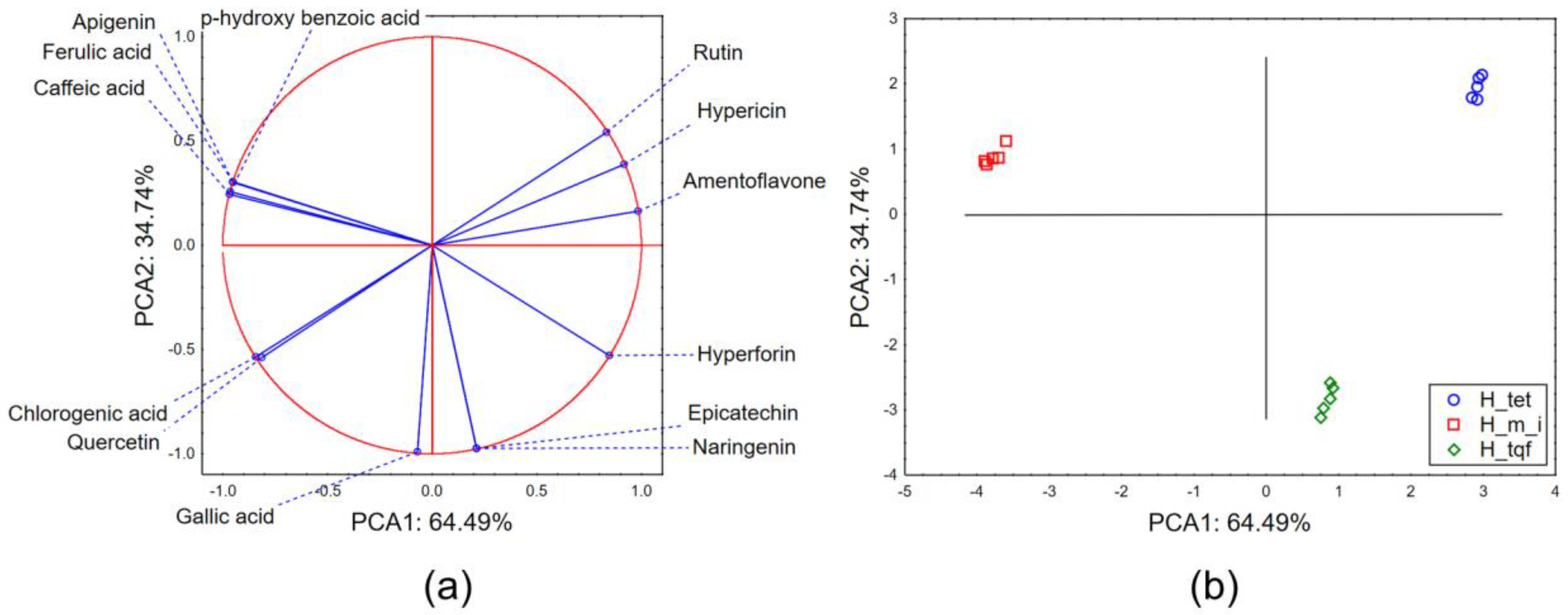
2.1. Chemical Characterization of Hypericum Extracts
2.2. Biological Potential of Evaluated Hypericum Species
2.2.1. Antioxidant Potential
2.2.2. Inhibition of Biologically Important Enzymes
Inhibition of Acetylcholinesterase, Monoamine Oxidases A and B
Antihyperglycemic Potential
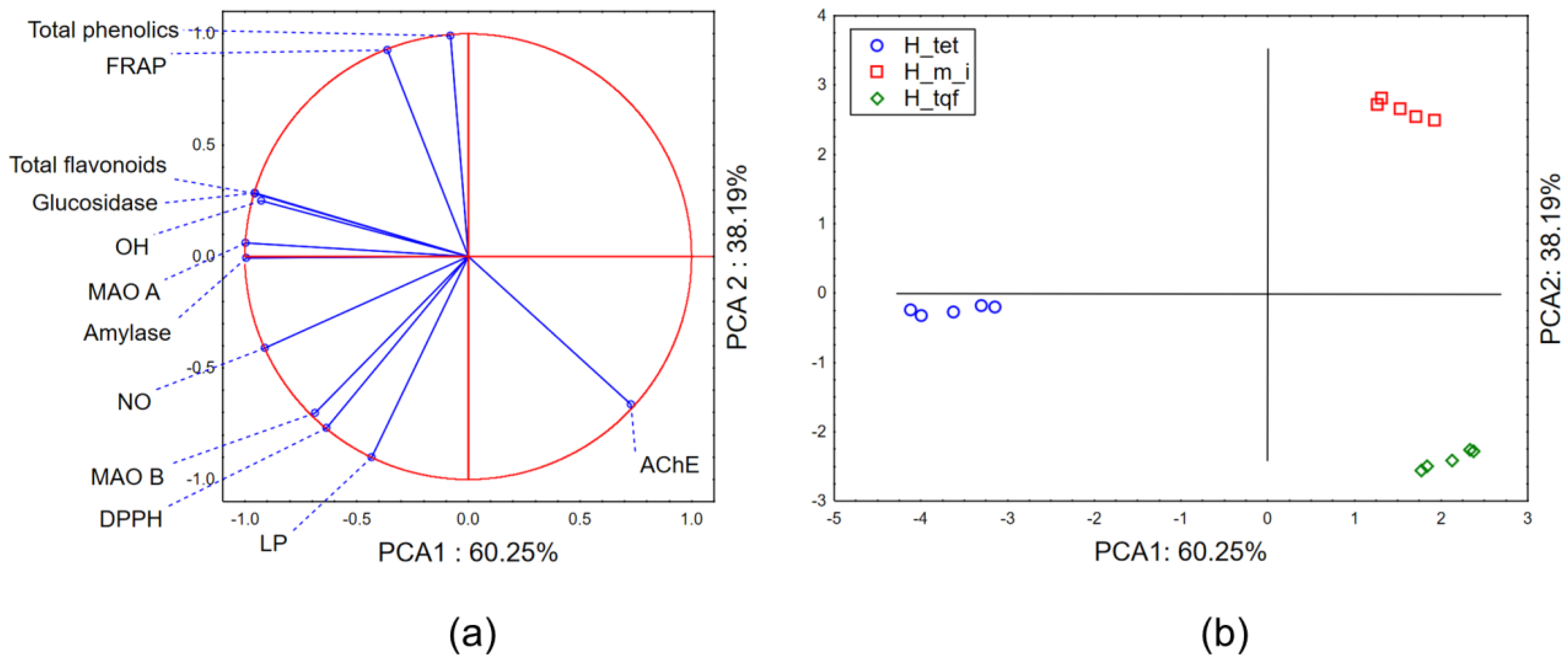
2.2.3. Chemometric Approach—Biological Potential
2.2.4. Antibacterial and Anti-Candida Activity
3. Materials and Methods
3.1. Herbal Material and Extracts Preparation
3.2. Chemical Characterization of Plant Extracts
3.3. Antioxidant Potential
3.3.1. Radical Scavenging Capacity (RSC)
3.3.2. Inhibition of Lipid Peroxidation (LP)
3.3.3. Ferric Reduction Antioxidant Potential
3.4. Inhibition of Biologically Important Enzymes
3.4.1. Inhibition of Acetylcholinesterase
3.4.2. Inhibition of Monoamine Oxidase A (MAO-A) and Monoamine Oxidase B (MAO-B)
3.4.3. Inhibition of α-Amylase
3.4.4. Inhibition of α-Glucosidase
3.4.5. Calculations of Enzymes Inhibitory Activity
3.5. Antimicrobial Activity
3.6. Data Processing
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Robson, N. The genus Hypericum. In Hypericum: The Genus Hypericum; Taylor & Francis: London, UK, 2003; pp. 1–22. [Google Scholar]
- Božin, B.; Kladar, N.; Grujić, N.; Anačkov, G.; Samojlik, I.; Gavarić, N.; Čonić, B.S. Impact of origin and biological source on chemical composition, anticholinesterase and antioxidant properties of some St. John’s wort species (Hypericum spp., Hypericaceae) from the Central Balkans. Molecules 2013, 18, 11733–11750. [Google Scholar] [CrossRef] [PubMed]
- Kladar, N.; Srđenović, B.; Grujić, N.; Rat, M.; Gavarić, N.; Anačkov, G.; St. Božin, B. John’s Wort (Hypericum spp.)–Relation between the Biological Source and Medical Properties. In Hypericum: Botanical Sources, Medical Properties and Health Effects; Nova Science Publishers: New York, NY, USA, 2015; pp. 53–80. [Google Scholar]
- Crockett, S.L.; Robson, N.K. Taxonomy and chemotaxonomy of the genus Hypericum. Med. Aromat. Plant Sci. Biotechnol. 2011, 5, 1–13. [Google Scholar] [PubMed]
- Kladar, N.; Srđenović, B.; Grujić, N.; Bokić, B.; Rat, M.; Anačkov, G.; Božin, B. Ecologically and ontogenetically induced variations in phenolic compounds and biological activities of Hypericum maculatum subsp. maculatum, Hypericaceae. Braz. J. Bot. 2015, 38, 703–715. [Google Scholar] [CrossRef]
- Camas, N.; Radusiene, J.; Ivanauskas, L.; Jakstas, V.; Kayikci, S.; Cirak, C. Chemical composition of Hypericum species from the Taeniocarpium and Drosanthe sections. Plant Syst. Evol. 2013, 300, 953–960. [Google Scholar] [CrossRef]
- Zlatković, B. Hypericaceae Juss. In The Flora of Serbia, 3; Serbian Academy of Sciences and Arts: Belgrade, Serbia, 2022; pp. 287–346. [Google Scholar]
- Nikolić, T. Hypericum L. In Flora Croatica 2; Alfa: Zagreb, Croatia, 2020; pp. 445–450. [Google Scholar]
- Toker, Z. Variation of total hypericin, phenolic and flavonoid compounds in Hypericum triquetrifolium during its phenological cycle. Pharm. Biol. 2009, 47, 285–288. [Google Scholar] [CrossRef]
- Sagratini, G.; Ricciutelli, M.; Vittori, S.; Öztürk, N.; Öztürk, Y.; Maggi, F. Phytochemical and antioxidant analysis of eight Hypericum taxa from Central Italy. Fitoterapia 2008, 79, 210–213. [Google Scholar] [CrossRef] [PubMed]
- Kladar, N.; Anačkov, G.; Srđenović, B.; Gavarić, N.; Hitl, M.; Salaj, N.; Jeremić, K.; Babović, S.; St. Božin, B. John’s Wort Herbal Teas–Biological Potential and Chemometric Approach to Quality Control. Plant Foods Hum. Nutr. 2020, 75, 390–395. [Google Scholar] [CrossRef]
- Kladar, N.; Mrđanović, J.; Anačkov, G.; Šolajić, S.; Gavarić, N.; Srđenović, B.; Božin, B. Hypericum perforatum: Synthesis of active principles during flowering and fruitification—Novel aspects of biological potential. Evid.-Based Complement. Altern. Med. 2017, 2017, 2865610. [Google Scholar] [CrossRef]
- Smelcerovic, A.; Spiteller, M. Phytochemical analysis of nine Hypericum L. species from Serbia and the FYR Macedonia. Die Pharm. Int. J. Pharm. Sci. 2006, 61, 251–252. [Google Scholar]
- Cecchini, C.; Cresci, A.; Coman, M.M.; Ricciutelli, M.; Sagratini, G.; Vittori, S.; Lucarini, D.; Maggi, F. Antimicrobial activity of seven hypericum entities from central Italy. Planta Med. 2007, 73, 564–566. [Google Scholar] [CrossRef]
- Smelcerovic, A.; Zuehlke, S.; Spiteller, M.; Raabe, N.; Özen, T. Phenolic constituents of 17 Hypericum species from Turkey. Biochem. Syst. Ecol. 2008, 36, 316–319. [Google Scholar] [CrossRef]
- Radulović, N.; Stankov-Jovanović, V.; Stojanović, G.; Šmelcerović, A.; Spiteller, M.; Asakawa, Y. Screening of in vitro antimicrobial and antioxidant activity of nine Hypericum species from the Balkans. Food Chem. 2007, 103, 15–21. [Google Scholar] [CrossRef]
- Kızıl, G.; Kızıl, M.; Yavuz, M.; Emen, S.; Hakimoğlu, F. Antioxidant Activities of Ethanol Extracts of Hypericum triquetrifolium and Hypericum scabroides. Pharm. Biol. 2008, 46, 231–242. [Google Scholar] [CrossRef]
- Dall’acqua, S.; Ak, G.; Sinan, K.I.; Elbasan, F.; Ferrarese, I.; Sut, S.; Yıldıztugay, E.; Peron, G.; Schievano, E.; Picot-Allain, M.C.N.; et al. Hypericum triquetrifolium and H. neurocalycinum as Sources of Antioxidants and Multi-Target Bioactive Compounds: A Comprehensive Characterization Combining In Vitro Bioassays and Integrated NMR and LC-MS Characterization by Using a Multivariate Approach. Front. Pharmacol. 2021, 12, 660735. [Google Scholar] [CrossRef]
- Silva, B.A.; Ferreres, F.; Malva, J.O.; Dias, A.C. Phytochemical and antioxidant characterization of Hypericum perforatum alcoholic extracts. Food Chem. 2005, 90, 157–167. [Google Scholar] [CrossRef]
- Hunt, E.J.; Lester, C.E.; Lester, E.A.; Tackett, R.L. Effect of St. John’s wort on free radical production. Life Sci. 2001, 69, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Spiegel, M.; Kapusta, K.; Kołodziejczyk, W.; Saloni, J.; Żbikowska, B.; Hill, G.A.; Sroka, Z. Antioxidant Activity of Selected Phenolic Acids–Ferric Reducing Antioxidant Power Assay and QSAR Analysis of the Structural Features. Molecules 2020, 25, 3088. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Goel, N. Phenolic acids: Natural versatile molecules with promising therapeutic applications. Biotechnol. Rep. 2019, 24, e00370. [Google Scholar] [CrossRef]
- Koroleva, O.; Torkova, A.; Nikolaev, I.; Khrameeva, E.; Fedorova, T.; Tsentalovich, M.; Amarowicz, R. Evaluation of the antiradical properties of phenolic acids. Int. J. Mol. Sci. 2014, 15, 16351–16380. [Google Scholar] [CrossRef]
- Iacopini, P.; Baldi, M.; Storchi, P.; Sebastiani, L. Catechin, epicatechin, quercetin, rutin and resveratrol in red grape: Content, in vitro antioxidant activity and interactions. J. Food Compos. Anal. 2008, 21, 589–598. [Google Scholar] [CrossRef]
- Babotă, M.; Frumuzachi, O.; Mocan, A.; Tămaș, M.; Dias, M.I.; Pinela, J.; Stojković, D.; Soković, M.; Bădărău, A.S.; Crișan, G.; et al. Unravelling phytochemical and bioactive potential of three Hypericum species from Romanian spontaneous flora: H. alpigenum, H. perforatum and H. Rochelii. Plants 2022, 11, 2773. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, K. Minocycline and St. John’s wort as therapeutic drugs for human tauopathy. Biol. Psychiatry 2015, 78, e39. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, M.F.; Falé, P.L.V.; Araújo, M.E.M.; Serralheiro, M.L.M. Acetylcholinesterase inhibition and antioxidant activity of the water extracts of several Hypericum species. Food Chem. 2010, 120, 1076–1082. [Google Scholar] [CrossRef]
- Gnerre, C.; von Poser, G.L.; Ferraz, A.; Viana, A.; Testa, B.; Rates, S.M.K. Monoamine oxidase inhibitory activity of some Hypericum species native to South Brazil. J. Pharm. Pharmacol. 2001, 53, 1273–1279. [Google Scholar] [CrossRef] [PubMed]
- Chhillar, R.; Dhingra, D. Antidepressant-like activity of gallic acid in mice subjected to unpredictable chronic mild stress. Fundam. Clin. Pharmacol. 2013, 27, 409–418. [Google Scholar] [CrossRef]
- Dhiman, P.; Malik, N.; Sobarzo-Sánchez, E.; Uriarte, E.; Khatkar, A. Quercetin and Related Chromenone Derivatives as Monoamine Oxidase Inhibitors: Targeting Neurological and Mental Disorders. Molecules 2019, 24, 418. [Google Scholar] [CrossRef]
- Larit, F.; Elokely, K.M.; Chaurasiya, N.D.; Benyahia, S.; Nael, M.A.; León, F.; Abu-Darwish, M.S.; Efferth, T.; Wang, Y.-H.; Belouahem-Abed, D.; et al. Inhibition of human monoamine oxidase A and B by flavonoids isolated from two Algerian medicinal plants. Phytomedicine 2018, 40, 27–36. [Google Scholar] [CrossRef]
- Nagpal, K.; Singh, S.K.; Mishra, D.N. Nanoparticle mediated brain targeted delivery of gallic acid: In vivo behavioral and biochemical studies for improved antioxidant and antidepressant-like activity. Drug Deliv. 2012, 19, 378–391. [Google Scholar] [CrossRef]
- Herraiz, T.; Guillén, H. Monoamine Oxidase-A Inhibition and Associated Antioxidant Activity in Plant Extracts with Potential Antidepressant Actions. BioMed Res. Int. 2018, 2018, 4810394. [Google Scholar] [CrossRef]
- Wild, S.H.; Roglic, G.; Green, A.; Sicree, R.; King, H. Global prevalence of diabetes: Estimates for the year 2000 and projections for 2030. Diabetes Care 2004, 27, 2569. [Google Scholar] [CrossRef]
- Hamdan, I.I.; Afifi, F.U. Screening of Jordanian Flora for α-Amylase Inhibitory Activity. Pharm. Biol. 2008, 46, 746–750. [Google Scholar] [CrossRef]
- Nowicka, P.; Wojdyło, A.; Laskowski, P. Inhibitory potential against digestive enzymes linked to obesity and type 2 diabetes and content of bioactive compounds in 20 cultivars of the peach fruit grown in Poland. Plant Foods Hum. Nutr. 2018, 73, 314–320. [Google Scholar] [CrossRef] [PubMed]
- Aleixandre, A.; Gil, J.V.; Sineiro, J.; Rosell, C.M. Understanding phenolic acids inhibition of α-amylase and α-glucosidase and influence of reaction conditions. Food Chem. 2022, 372, 131231. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.; Ferruzzi, M.G.; Hamaker, B.R. Structural requirements of flavonoids for the selective inhibition of α-amylase versus α-glucosidase. Food Chem. 2022, 370, 130981. [Google Scholar] [CrossRef] [PubMed]
- Béjaoui, A.; Ben Salem, I.; Rokbeni, N.; M’rabet, Y.; Boussaid, M.; Boulila, A. Bioactive compounds from Hypericum humifusum and Hypericum perfoliatum: Inhibition potential of polyphenols with acetylcholinesterase and key enzymes linked to type-2 diabetes. Pharm. Biol. 2017, 55, 906–911. [Google Scholar] [CrossRef] [PubMed]
- EMA/HMPC. Community Herbal Monograph on Hypericum perforatum L., Herba (Well-Established Medicinal Use). Available online: https://www.ema.europa.eu/en/documents/herbal-monograph/final-community-herbal-monograph-hypericum-perforatum-l-herba-well-established-medicinal-use_en.pdf (accessed on 12 April 2022).
- European Directorate for the Quality of Medicines & Health Care. European Pharmacopoea, 6th ed.; Council of Europe: Strasbourgh, France, 2007. [Google Scholar]
- Bozin, B.; Mimica-Dukic, N.; Samojlik, I.; Goran, A.; Igic, R. Phenolics as antioxidants in garlic (Allium sativum L., Alliaceae). Food Chem. 2008, 111, 925–929. [Google Scholar] [CrossRef]
- Ziaková, A.; Brandšteterová, E. Validation of HPLC determination of phenolic acids present in some Lamiaceae family plants. J. Liq. Chromatogr. Relat. Technol. 2003, 26, 443–453. [Google Scholar] [CrossRef]
- Lesjak, M.M.; Beara, I.N.; Orčić, D.Z.; Anačkov, G.T.; Balog, K.J.; Francišković, M.M.; Mimica-Dukić, N.M. Juniperus sibirica Burgsdorf. as a novel source of antioxidant and anti-inflammatory agents. Food Chem. 2011, 124, 850–856. [Google Scholar] [CrossRef]
- Samoylenko, V.; Rahman, M.M.; Tekwani, B.L.; Tripathi, L.M.; Wang, Y.H.; Khan, S.I.; Khan, I.A.; Miller, L.S.; Joshi, V.C.; Muhammad, I. Banisteriopsis caapi, a unique combination of MAO inhibitory and antioxidative constituents for the activities relevant to neurodegenerative disorders and Parkinson’s disease. J. Ethnopharmacol. 2010, 127, 357–367. [Google Scholar] [CrossRef]
- Mun’im, A.; Andriani, A.; Mahmudah, K.F.; Mashita, M. Screening of α-glucosidase inhibitory activity of some Indonesian medicinal plants. Int. J. Med. Aromat. Plants 2013, 3, 144–150. [Google Scholar]
- M07; Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically: Approved Standard. Clinical & Laboratory Standards Institute: Wayne, PA, USA, 2006.
- M27-A2; Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts, Approved Standard. Clinical & Laboratory Standards Institute: Wayne, PA, USA, 2002.
| Sample | H. tetrapterum | H. maculatum ssp. immaculatum | H. triquetrifolium | |
|---|---|---|---|---|
| Variable | ||||
| Total phenolics (mg GAE)/g d.e. | 137.77 ± 10.1 a | 194.24 ± 14.12 a | 83.52 ± 7.60 a | |
| Total flavonoids (mg QE/g d.e.) | 58.17 ± 4.51 b | 37.01 ± 3.16 b | 24.76 ± 2.36 b | |
| Dry extract yield (%) | 12.77 ± 1.11 c,d | 19.30 ±1.87 c | 19.89 ± 1.98 d | |
| Class of compounds | Compound | µg/g dry herb | ||
| Naphthodianthrones | Hypericin | 450.51 ± 32.11 e | 52.71 ± 4.23 e | 185.16 ± 14.36 e |
| Phloroglucinols | Hyperforin | 1235.02 ± 56.78 f | 278.9 ± 25.64 f | 1563.1 ± 114.65 f |
| Biflavonoids | Amentoflavone | 135.06 ± 11.12 g | n.d. g | 72.26 ± 5.47 g |
| Flavonoids and flavonoid glycosides | Apigenin | n.d. h | 0.82 ± 0.11 h,i | n.d. i |
| Naringenin | n.d. j | n.d. k | 249.83 ± 19.21 j,k | |
| Rutin | 550.93 ± 36.78 l,m | 222.5 ± 23.56 l | 278.07 ± 22.11 m | |
| Quercetin | 150.47 ± 9.45 n | 183.09 ± 14.32 n | 173.88 ± 13.28 | |
| Epicatechin | n.d. o | n.d. p | 390.09 ± 32.06 o,p | |
| Phenolic acids | Ferulic acid | n.d. q | 259.08 ± 23.56 q,r | n.d. r |
| Gallic acid | 62.04 ± 4.15 r | 66.52 ± 6.14 r | 77.07 ± 7.16 | |
| Chlorogenic acid | n.d. s,t | 127.19 ± 16.78 s | 105.35 ± 9.25 t | |
| Caffeic acid | 39.74 ± 3.65 u | 125.12 ± 13.54 u,w | 45.47 ± 4.14 w | |
| p-hydroxybenzoic acid | 46.71 ± 3.78 x | 219.47 ± 22.65 x,y | 56.75 ± 5.14 y | |
| Sample | H. tetrapterum | H. maculatum ssp. immaculatum | H. triquetrifolium | Positive Control |
|---|---|---|---|---|
| Variable | RSC50 (µg/mL) | |||
| DPPH | 3.54 ± 0.33 a | 1.93 ± 0.13 a | 3.14 ± 0.29 a | QDH, RSC50 = 1.01 ± 0.08 PG, RSC50 = 0.65 ± 0.05 |
| NO | 32.17 ± 3.11 b | 12.11 ± 1.98 b | 19.24 ± 1.57 b | PG, RSC50 = 8.87 ± 0.79 |
| OH | 58.74 ± 4.26 c | 55.00 ± 4.87 c | 51.74 ± 4.23 c | BHT, IC50 = 0.03 ± 0.00 AA, IC50 = 2.21 ± 0.17 PG, IC50 = 10.11 ± 0.69 |
| LP | n.d. d | 514.96 ± 36.75 d,e | n.d e | BHT, IC50 = 7.99 ± 0.69 |
| FRAP (mg AAE/g d. e.) | 162.18 ± 12.98 f | 176.75 ± 14.25 f | 113.76 ± 10.58 f | / |
| AChE | 606.03 ± 54.23 g | 774.89 ± 56.92 g | 1304.04 ± 116.88 g | Galantamine IC50 = 9.11 ± 0.64 |
| MAO-A | 11.73± 0.88 h | 5.90± 0.26 h | 4.79 ± 0.32 h | Moclobemide IC50 = 0.71 ± 0.08 |
| MAO-B | 59.25 ± 5.23 i | 47.81 ± 4.23 i | 55.15 ± 4.13 i | Selegiline IC50 = 0.22 ± 0.02 |
| α-amylase | 8440.34 ± 654.28 j | 1270.62 ± 115.32 j | 616.04 ± 53.87 j | Acarbose IC50 = 5.35 ± 0.72 |
| α-glucosidase | 22.43 ± 2.11 k | 14.56 ± 1.12 k | 9.94 ± 0.65 k | Acarbose IC50 = 48.76 ± 3.45 |
| Agent | H. tetrapterum | H. maculatum ssp. immaculatum | H. triquetrifolium | Antibiotics (mm) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Microbe | MIC | MBC | MIC | MBC | MIC | MBC | E (15 µg) | LEV (5 µg) | DA (2 µg) | KF (20 µg) | CIP (5 µg) | CN (10 µg) | OFX (5 µg) | C (30 µg) |
| S. aureus H MRSA | 12.5 | 25 | 12.5 | 12.5 | 12.5 | 12.5 | 22.5 | 27.5 | 25 | 35 | 24.5 | 19 | 26 | 25 |
| E. coli L | 100 | 100 | 25 | 25 | 50 | 100 | / | 35 | / | 16 | 25.5 | 15 | 22.5 | 23.5 |
| P. mirabilis H | 12.5 | 50 | 12.5 | 12.5 | 50 | 50 | / | 20 | / | / | 20 | / | 17 | 10 |
| P. aeruginosa H | 25 | 50 | 25 | 25 | 25 | 25 | / | / | / | / | / | / | / | / |
| Enterococcus sp. L | 100 | 100 | 12.5 | 25 | 12.5 | 25 | 12.5 | 24 | / | 20 | 20.5 | 10.5 | 19.5 | 25.5 |
| P. vulgaris L | ↑100 | ↑100 | 50 | 50 | 12.5 | 12.5 | / | 29.5 | 21 | / | 31 | 16.5 | 22.5 | 20 |
| Candida L | / | / | / | / | / | / | / | / | / | / | / | / | / | / |
| Candida H | / | / | / | / | / | / | / | / | / | / | / | / | / | / |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kladar, N.; Božin, B.; Bijelić, K.; Bogavac, M.; Karaman, M.; Srđenović Čonić, B.; Rat, M.; Anačkov, G. Biological Activity of Genus Hypericum Sect. Hypericum Species—H. tetrapterum, H. maculatum subsp. immaculatum, H. triquetrifolium. Molecules 2023, 28, 6218. https://doi.org/10.3390/molecules28176218
Kladar N, Božin B, Bijelić K, Bogavac M, Karaman M, Srđenović Čonić B, Rat M, Anačkov G. Biological Activity of Genus Hypericum Sect. Hypericum Species—H. tetrapterum, H. maculatum subsp. immaculatum, H. triquetrifolium. Molecules. 2023; 28(17):6218. https://doi.org/10.3390/molecules28176218
Chicago/Turabian StyleKladar, Nebojša, Biljana Božin, Katarina Bijelić, Mirjana Bogavac, Maja Karaman, Branislava Srđenović Čonić, Milica Rat, and Goran Anačkov. 2023. "Biological Activity of Genus Hypericum Sect. Hypericum Species—H. tetrapterum, H. maculatum subsp. immaculatum, H. triquetrifolium" Molecules 28, no. 17: 6218. https://doi.org/10.3390/molecules28176218
APA StyleKladar, N., Božin, B., Bijelić, K., Bogavac, M., Karaman, M., Srđenović Čonić, B., Rat, M., & Anačkov, G. (2023). Biological Activity of Genus Hypericum Sect. Hypericum Species—H. tetrapterum, H. maculatum subsp. immaculatum, H. triquetrifolium. Molecules, 28(17), 6218. https://doi.org/10.3390/molecules28176218








