Decaborane: From Alfred Stock and Rocket Fuel Projects to Nowadays †
Abstract
1. Introduction
2. Synthesis, Structure, and General Properties
3. Halogen Derivatives
4. Derivatives with a B-O Bond
5. Derivatives with a B-S Bond
6. Derivatives with a B-N Bond
7. Derivatives with a B-P Bond
8. Derivatives with a B-As Bond
9. Derivatives with a B-C Bond
10. Derivatives with an exo-Polyhedral B-B Bond
11. Decaborane-Related conjucto-Boranes [B18H22]
12. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Grimes, R.N. Carboranes, 3rd ed.; Academic Press: London, UK, 2016. [Google Scholar] [CrossRef]
- Shmal’ko, A.V.; Sivaev, I.B. Chemistry of carba-closo-decaborate anions [CB9H10]−. Russ. J. Inorg. Chem. 2019, 64, 1726–1749. [Google Scholar] [CrossRef]
- Sivaev, I.B.; Bregadze, V.I.; Sjöberg, S. Chemistry of closo-dodecaborate anion [B12H12]2−: A review. Collect. Czech. Chem. Commun. 2002, 67, 679–727. [Google Scholar] [CrossRef]
- Zhao, X.; Yang, Z.; Chen, H.; Wang, Z.; Zhou, X.; Zhang, H. Progress in three-dimensional aromatic-like closo-dodecaborate. Coord. Chem. Rev. 2021, 444, 214042. [Google Scholar] [CrossRef]
- Körbe, S.; Schreiber, P.J.; Michl, J. Chemistry of the carba-closo-dodecaborate(-) anion, CB11H12−. Chem. Rev. 2006, 106, 5208–5249. [Google Scholar] [CrossRef]
- Douvris, C.; Michl, J. Update 1 of: Chemistry of the carba-closo-dodecaborate(-) anion, CB11H12−. Chem. Rev. 2013, 113, R179–R233. [Google Scholar] [CrossRef]
- Sivaev, I.B.; Prikaznov, A.V.; Naoufal, D. Fifty years of the closo-decaborate anion chemistry. Collect. Czech. Chem. Commun. 2010, 75, 1149–1199. [Google Scholar] [CrossRef]
- Mahfouz, N.; Abi Ghaida, F.; El Hajj, Z.; Diab, M.; Floquet, S.; Mehdi, A.; Naoufal, D. Recent achievements on functionalization within closo-decahydrodecaborate [B10H10]2− clusters. ChemistrySelect 2022, 7, e202200770. [Google Scholar] [CrossRef]
- Nakamura, K. Preparation and properties of amorphous boron films deposited by pyrolysis of decaborane in the molecular flow region. J. Electrochem. Soc. 1984, 131, 2691. [Google Scholar] [CrossRef]
- Kim, Y.G.; Dowben, P.A.; Spencer, J.T.; Ramseyer, G.O. Chemical vapor deposition of boron and boron nitride from decaborane(14). J. Vac. Sci. Technol. A 1989, 7, 2796–2799. [Google Scholar] [CrossRef]
- Perkins, F.K.; Rosenberg, R.A.; Lee, S.; Dowben, P.A. Synchrotron-radiation-induced deposition of boron and boron carbide films from boranes and carboranes: Decaborane. J. Appl. Phys. 1991, 69, 4103–4109. [Google Scholar] [CrossRef]
- Saidoh, M.; Ogiwara, N.; Shimada, M.; Arai, T.; Hiratsuka, H.; Koike, T.; Shimizu, M.; Ninomiya, H.; Nakamura, H.; Jimbou, R. Initial boronization of JT-60U tokamak using decaborane. Jpn. J. Appl. Phys. 1993, 32, 3276–3281. [Google Scholar] [CrossRef]
- Kodama, H.; Oyaidzu, M.; Sasaki, M.; Kimura, H.; Morimoto, Y.; Oya, Y.; Matsuyama, M.; Sagara, A.; Noda, N.; Okuno, K. Studies on structural and chemical characterization for boron coating films deposited by PCVD. J. Nucl. Mater. 2004, 329–333, 889–893. [Google Scholar] [CrossRef]
- Bellott, B.J.; Noh, W.; Nuzzo, R.G.; Girolami, G.S. Nanoenergetic materials: Boron nanoparticles from the pyrolysis of decaborane and their functionalisation. Chem. Commun. 2009, 3214–3215. [Google Scholar] [CrossRef]
- Ekimov, E.A.; Zibrov, I.P.; Zoteev, A.V. Preparation of boron microcrystals via high-pressure, high-temperature pyrolysis of decaborane, B10H14. Inorg. Mater. 2011, 47, 1194–1198. [Google Scholar] [CrossRef]
- Ekimov, E.A.; Lebed, J.B.; Sidorov, V.A.; Lyapin, S.G. High-pressure synthesis of crystalline boron in B-H system. Sci. Technol. Adv. Mater. 2011, 12, 055009. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Luo, Z.; Acerce, M.; Yates, D.M.; Johnson, A.T.C.; Sneddon, L.G. Chemical vapor deposition of boron nitride nanosheets on metallic substrates via decaborane/ammonia reactions. Chem. Mater. 2011, 23, 4414–4416. [Google Scholar] [CrossRef]
- Chatterjee, S.; Kim, M.J.; Zakharov, D.N.; Kim, S.M.; Stach, E.A.; Maruyama, B.; Sneddon, L.G. Syntheses of boron nitride nanotubes from borazine and decaborane molecular precursors by catalytic chemical vapor deposition with a floating nickel catalyst. Chem. Mater. 2012, 24, 2872–2879. [Google Scholar] [CrossRef]
- Kher, S.S.; Spencer, J.T. Chemical vapor deposition precursor chemistry. 3. Formation and characterization of crystalline nickel boride thin films from the cluster-assisted deposition of polyhedral borane compounds. Chem. Mater. 1992, 4, 538–544. [Google Scholar] [CrossRef]
- Kher, S.S.; Tan, Y.; Spencer, J.T. Chemical vapor deposition of metal borides. 4. The application of polyhedral boron clusters to the chemical vapor deposition formation of gadolinium boride thin-film materials. Appl. Organomet. Chem. 1996, 10, 297–304. [Google Scholar] [CrossRef]
- Kher, S.S.; Romero, J.V.; Caruso, J.D.; Spencer, J.T. Chemical vapor deposition of metal borides. 6. The formation of neodymium boride thin film materials from polyhedral boron clusters and metal halides by chemical vapor deposition. Appl. Organomet. Chem. 2008, 22, 300–307. [Google Scholar] [CrossRef]
- Tynell, T.; Aizawa, T.; Ohkubo, I.; Nakamura, K.; Mori, T. Deposition of thermoelectric strontium hexaboride thin films by a low pressure CVD method. J. Cryst. Growth 2016, 449, 10–14. [Google Scholar] [CrossRef]
- Guélou, G.; Martirossyan, M.; Ogata, K.; Ohkubo, I.; Kakefuda, Y.; Kawamoto, N.; Kitagawa, Y.; Ueda, J.; Tanabe, S.; Maeda, K.; et al. Rapid deposition and thermoelectric properties of ytterbium boride thin films using hybrid physical chemical vapor deposition. Materialia 2018, 1, 244–248. [Google Scholar] [CrossRef]
- Ohkubo, I.; Aizawa, T.; Nakamura, K.; Mori, T. Control of competing thermodynamics and kinetics in vapor phase thin-film growth of nitrides and borides. Front. Chem. 2021, 9, 642388. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Peng, Y.; Wan, Q.; Ye, D.; Wang, A.; Zhang, L.; Jiang, W.; Liu, Y.; Li, J.; Zhuang, X.; et al. Fuel cell power source based on decaborane with high energy density and low crossover. Mater. Today Energy 2023, 32, 101244. [Google Scholar] [CrossRef]
- Hawthorne, M.F. Decaborane-14 and its derivatives. In Advances in Inorganic Chemistry and Radiochemistry; Academic Press: New York, NY, USA, 1963; Volume 5, pp. 307–347. [Google Scholar] [CrossRef]
- Stanko, V.I.; Chapovskii, Y.A.; Brattsev, V.A.; Zakharkin, L.I. The chemistry of decaborane and its derivatives. Russ. Chem. Rev. 1965, 34, 424–439. [Google Scholar] [CrossRef]
- Shore, S.G. Nido- and arachno-boron hydrides. In Boron Hydride Chemistry; Muetterties, E.L., Ed.; Academic Press: New York, NY, USA, 1975; pp. 79–174. [Google Scholar] [CrossRef]
- Greenwood, N.N. Boron. In Comprehensive Inorganic Chemistry; Pergamon Press: Oxford, UK, 1973; Volume 1, pp. 818–837. [Google Scholar] [CrossRef]
- Sivaev, I.B. Molecular boron clusters. In Comprehensive Inorganic Chemistry; Elsevier: Amsterdam, The Netherlands, 2023; Volume 1, pp. 740–777. [Google Scholar] [CrossRef]
- Stock, A.; Friederici, K.; Priess, O. Borwasserstoffe. III. Feste Borwasserstoffe: Zur Kenntnis des B2H6. Ber. Dtsch. Chem. Ges. 1913, 46, 3353–3365. [Google Scholar] [CrossRef]
- Stock, A.; Pohland, E. Borwasserstoffe. XII. Zur Kenntnis des B10H14. Ber. Dtsch. Chem. Ges. 1929, 62, 90–99. [Google Scholar] [CrossRef]
- Stock, A. Hydrides of Boron and Silicon; Cornell University Press: Ithaca, NY, USA, 1933. [Google Scholar]
- Stock, A. Borwasserstoffe. XI. Strukturformeln der Borhydride. Ber. Dtsch. Chem. Ges. 1926, 59, 2226–2229. [Google Scholar] [CrossRef]
- Huggins, M.L. Boron hydrides. J. Phys. Chem. 1922, 26, 833–835. [Google Scholar] [CrossRef][Green Version]
- Clark, J.D. Ignition! An Informal History of Liquid Rocket Propellants; Rutgers University Press: New Brunswick, NJ, USA, 1972. [Google Scholar] [CrossRef]
- Boron High Energy Fuels: Hearings Before the Committee on Science and Astronautics U.S. House of Representatives. Eighty-Six Congress, First Session; United States Government Printing Office: Washington, DC, USA, 1959.
- Goodger, E. Unconventional fuels. New Sci. 1957, 27–29. [Google Scholar]
- Siegel, B.; Mack, J.L. The boron hydrides. J. Chem. Ed. 1957, 34, 314–317. [Google Scholar] [CrossRef]
- Martin, D.R. The development of borane fuels. J. Chem. Ed. 1959, 36, 208–214. [Google Scholar] [CrossRef]
- Gold, J.W.; Militscher, C.; Slauson, D.D. Pentaborane Disposal: Taming the Dragon. Available online: https://dokumen.tips/documents/pentaborane-taming-the-dragonpdf.html (accessed on 23 August 2023).
- Kasper, J.S.; Lucht, C.M.; Harker, D. The structure of the decaborane molecule. J. Am. Chem. Soc. 1948, 70, 881. [Google Scholar] [CrossRef]
- Kasper, J.S.; Lucht, C.M.; Harker, M. The crystal structure of decaborane, B10H14. Acta Cryst. 1950, 3, 436–455. [Google Scholar] [CrossRef]
- Moore, E.B.; Dickerson, R.E.; Lipscomb, W.N. Least squares refinements of B10H14, B4H10, and B5H11. J. Chem. Phys. 1957, 27, 209–211. [Google Scholar] [CrossRef]
- Brill, R.; Dietrich, H.; Dierks, H. Distribution of the bonding electrons in decaborane-14. Angew. Chem. Int. Ed. 1970, 9, 524–526. [Google Scholar] [CrossRef]
- Brill, R.; Dietrich, H.; Dierks, H. Die Verteilung der Bindungselektronen im Dekaboran-molekül (B10H14). Acta Cryst. B 1971, 27, 2003–2018. [Google Scholar] [CrossRef]
- Dietrich, H.; Scheringer, C. Refinement of the molecular charge distribution in decaborane(14). Acta Cryst. B 1978, 34, 54–63. [Google Scholar] [CrossRef]
- Tippe, A.; Hamilton, W.C. Neutron diffraction study of decaborane. Inorg. Chem. 1969, 8, 464–470. [Google Scholar] [CrossRef]
- Vilkov, L.V.; Mastryukov, V.S.; Akishin, P.A. An electron-diffraction study of the structure of the decaborane molecule in the vapor state. J. Struct. Chem. 1964, 4, 301–304. [Google Scholar] [CrossRef]
- Mastryukov, V.S.; Dorofeeva, O.V.; Vilkov, L.V. Reexamination of the electron-diffraction data for the decaborane(14) molecule. J. Struct. Chem. 1975, 16, 110–112. [Google Scholar] [CrossRef]
- Eberhardt, W.H.; Crawford, B.; Lipscomb, W.N. The valence structure of the boron hydrides. J. Chem. Phys. 1954, 22, 989–1001. [Google Scholar] [CrossRef]
- Lipscomb, W.N. Valence in the boron hydrides. J. Phys. Chem. 1957, 61, 23–27. [Google Scholar] [CrossRef]
- Lipscomb, W.N. Boron Hydrides; W. A. Benjamin, Inc.: New York, NY, USA, 1963. [Google Scholar]
- Moore, E.B.; Lohr, L.L.; Lipscomb, W.N. Molecular orbitals in some boron compounds. J. Chem. Phys. 1961, 35, 1329–1334. [Google Scholar] [CrossRef]
- Moore, E.B. Molecular orbitals in B10H14. J. Chem. Phys. 1962, 37, 675–677. [Google Scholar] [CrossRef]
- Hoffmann, R.; Lipscomb, W.N. Boron hydrides: LCAO-MO and resonance studies. J. Chem. Phys. 1962, 37, 2872–2883. [Google Scholar] [CrossRef]
- Laws, E.A.; Stevens, R.M.; Lipscomb, W.N. Self-consistent field study of decaborane(14). J. Am. Chem. Soc. 1972, 94, 4467–4474. [Google Scholar] [CrossRef]
- Lipscomb, W.N. Advances in theoretical studies of boron hydrides and carboranes. In Boron Hydride Chemistry; Muetterties, E.L., Ed.; Academic Press: New York, NY, USA, 1975; pp. 39–78. [Google Scholar] [CrossRef]
- Kononova, E.G.; Klemenkova, Z.S. The electronic structure of nido-B10H14 and [6-Ph-nido-6-CB9H11]− in terms of Bader’s theory (AIM). J. Mol. Struct. 2013, 1036, 311–317. [Google Scholar] [CrossRef]
- Shoolery, J.N. The relation of high resolution nuclear magnetic resonance spectra to molecular structures. Discuss. Faraday Soc. 1955, 19, 215–225. [Google Scholar] [CrossRef]
- Williams, R.E.; Shapiro, I. Reinterpretation of nuclear magnetic resonance spectra of decaborane. J. Chem. Phys. 1958, 29, 677–678. [Google Scholar] [CrossRef]
- Phillips, W.D.; Miller, H.C.; Muetterties, E.L. B11 Magnetic resonance study of boron compounds. J. Am. Chem. Soc. 1959, 81, 4496–4500. [Google Scholar] [CrossRef]
- Pilling, R.L.; Tebbe, F.N.; Hawthorne, M.F.; Pier, E.A. Boron-11 nuclear magnetic resonance spectra of two boron hydride derivatives at 60 Mc./sec. Proc. Chem. Soc. 1964, 402–403. [Google Scholar] [CrossRef]
- Keller, P.C.; MacLean, D.; Schaeffer, R. Final assignment of B1 (3) N.M.R. resonance of decaborane. Chem. Commun. 1965, 204. [Google Scholar] [CrossRef]
- Williams, R.L.; Greenwood, N.N.; Morris, J.H. The nuclear magnetic resonance spectrum of decaborane-14. Spectrochim. Acta 1965, 21, 1579–1587. [Google Scholar] [CrossRef]
- Bodner, G.M.; Sneddon, L.G. An assignment of the hydrogen-1 magnetic resonance spectrum of decaborane at 220 MHz. Inorg. Chem. 1970, 9, 1421–1423. [Google Scholar] [CrossRef]
- Onak, T.; Marynick, D. Application of a ring current model to decaborane(14). A correlation of proton and boron-11 nuclear magnetic resonance chemical shifts. Trans. Faraday Soc. 1970, 66, 1843–1847. [Google Scholar] [CrossRef]
- Greenwood, N.N.; Kennedy, J.D. N.M.R. evidence for a transition between isotropic and anisotropic thermal motion of nido-decaborane in an aromatic solvent. J. Chem. Soc. Chem. Commun. 1979, 1099–1101. [Google Scholar] [CrossRef]
- Colquhoun., I.J.; McFarlane, W. Heteronuclear two-dimensional nuclear magnetic resonance: Decaborane. J. Chem. Soc. Dalton Trans. 1981, 2014–2016. [Google Scholar] [CrossRef]
- Venable, T.L.; Hutton, W.C.; Grimes, R.N. Two-dimensional boron-11-boron-11 nuclear magnetic resonance spectroscopy as a probe of polyhedral structure: Application to boron hydrides, carboranes, metallaboranes, and metallacarboranes. J. Am. Chem. Soc. 1984, 106, 29–37. [Google Scholar] [CrossRef]
- Keller, W.E.; Johnston, H.L. A note on the vibrational frequencies and the entropy of decaborane. J. Chem. Phys. 1952, 20, 1749–1751. [Google Scholar] [CrossRef]
- Hanousek, F.; Štíbr, B.; Heřmánek, S.; Plešek, J. Chemistry of boranes. XXXI. Infrared spectra of decaborane and its deuterio derivatives. Collect. Czech. Chem. Commun. 1973, 38, 1312–1320. [Google Scholar] [CrossRef]
- Pimentel, G.C.; Pitzer, K.S. The ultraviolet absorption and luminescence of decaborane. J. Chem. Phys. 1949, 17, 882–884. [Google Scholar] [CrossRef]
- Pondy, P.R.; Beachell, H.C. Near infrared spectra of diborane, pentaborane, and decaborane. J. Chem. Phys. 1956, 25, 238–241. [Google Scholar] [CrossRef]
- Haaland, A.; Eberhardt, W.H. Electronic spectrum of decaborane. J. Chem. Phys. 1962, 36, 2386–2392. [Google Scholar] [CrossRef]
- Lötz, A.; Olliges, J.; Voitländer, J. The nuclear quadrupole double resonance spectrum of decaborane. Chem. Phys. Lett. 1982, 93, 560–563. [Google Scholar] [CrossRef]
- Hiyama, Y.; Butler, L.G.; Brown, T.L. Boron-10 and boron-11 nuclear quadrupole resonance spectrum of decaborane[14]. J. Magn. Res. 1985, 65, 472–480. [Google Scholar] [CrossRef]
- Lloyd, D.R.; Lynaugh, N.; Roberts, P.J.; Guest, M.F. Photoelectron studies of boron compouds. Part 5. Higher boron hydrides B4H10, B5H9 and B10H14. J. Chem. Soc. Faraday Trans. 2 1975, 71, 1382–1394. [Google Scholar] [CrossRef]
- Hitchcock, A.P.; Wen, A.T.; Lee, S.; Glass, J.A.; Spencer, J.T.; Dowben, P.A. Inner-shell excitation of boranes and carboranes. J. Phys. Chem. 1993, 97, 8171–8181. [Google Scholar] [CrossRef]
- Margrave, J.L. Ionization potentials of B5H9, B5H8I, B10H14, and B10H13C2H5 from electron impact studies. J. Chem. Phys. 1960, 32, 1889. [Google Scholar] [CrossRef]
- Kaufman, J.J.; Koski, W.S.; Kuhns, L.J.; Law, R.W. Appearance and ionization potentials of selected fragments from decaborane, B1110H141. J. Am. Chem. Soc. 1962, 84, 4198–4205. [Google Scholar] [CrossRef]
- Bottei, R.S.; Laubengayer, A.W. The dipole moment and magnetic susceptibility of decaborane. J. Phys. Chem. 1962, 66, 1449–1451. [Google Scholar] [CrossRef]
- Johnson, W.H.; Kilday, M.V.; Prosen, E.J. Heat of formation of decaborane. J. Res. Natl. Bur. Stand. A Phys. Chem. 1960, 64, 521–525. [Google Scholar] [CrossRef] [PubMed]
- Kerr, E.C.; Hallett, N.C.; Johnston, H.L. Low temperature heat capacity of inorganic solids. VI. The heat capacity of decaborane, B10H14, from 14 to 305 K. J. Am. Chem. Soc. 1951, 73, 1117–1119. [Google Scholar] [CrossRef]
- Furukawa, G.T.; Park, R.P. Heat capacity, heats of fusion and vaporization, and vapor pressure of decaborane (B10H14). J. Res. Natl. Bur. Stand. 1955, 55, 255–260. [Google Scholar] [CrossRef]
- Miller, G.A. The vapor pressure of solid decaborane. J. Phys. Chem. 1963, 67, 1363–1364. [Google Scholar] [CrossRef]
- Nakano, S.; Hemley, R.J.; Gregoryanz, E.A.; Goncharov, A.F.; Mao, H.-K. Pressure-induced transformations of molecular boron hydride. J. Phys. Condens. Matter 2002, 14, 10453. [Google Scholar] [CrossRef]
- Rozendaal, H.M. Clinical observation on the toxicology of boron hydrides. AMA Arch. Ind. Hyg. Occup. Med. 1951, 4, 257–260. Available online: https://archive.org/details/sim_a-m-a-archives-of-industrial-health_1951-09_4_3/page/256/mode/2up (accessed on 31 July 2023).
- Krackow, E.H. Toxicity and health hazards of boron hydrides. AMA Arch. Ind. Hyg. Occup. Med. 1953, 8, 335–339. Available online: https://archive.org/details/sim_a-m-a-archives-of-industrial-health_1953-10_8_4/page/334/mode/2up (accessed on 31 July 2023).
- Cole, V.V.; Hill, D.L.; Oikemus, A.H. Problems in the study of decaborane and possible therapy of its poisoning. AMA Arch. Ind. Hyg. Occup. Med. 1954, 10, 158–161. Available online: https://archive.org/details/sim_a-m-a-archives-of-industrial-health_1954-08_10_2/page/158/mode/2up (accessed on 31 July 2023).
- Lowe, H.; Freeman, G. Boron hydride (borane) intoxication in man. AMA Arch. Ind. Hyg. Occup. Med. 1957, 12, 523–533. Available online: https://archive.org/details/sim_a-m-a-archives-of-industrial-health_1957-12_16_6/page/522/mode/2up (accessed on 31 July 2023). [CrossRef]
- Cordasco, E.M.; Cooper, R.W.; Murphy, J.V.; Anderson, C. Pulmonary aspects of some toxic experimental space fuels. Dis. Chest 1962, 41, 68–72. [Google Scholar] [CrossRef]
- Merritt, J.H. Pharmacology and toxicology of propellant fuels: Boron hydrides. Aeromed. Rev. 1966, 3, 1–11. [Google Scholar]
- Svirbely, J.L. Acute toxicity studies of decaborane and pentaborane by inhalation. AMA Arch. Ind. Hyg. Occup. Med. 1954, 10, 298–304. Available online: https://archive.org/details/sim_a-m-a-archives-of-industrial-health_1954-10_10_4/page/298/mode/2up (accessed on 31 July 2023).
- Svirbely, J.L. Subacute toxicity of decaborane and pentaborane vapors. AMA Arch. Ind. Hyg. Occup. Med. 1954, 10, 305–311. Available online: https://archive.org/details/sim_a-m-a-archives-of-industrial-health_1954-10_10_4/page/304/mode/2up (accessed on 31 July 2023).
- Svirbely, J.L. Toxicity tests of decaborane for laboratory animals. I. Acute toxicity studies. AMA Arch. Ind. Hyg. Occup. Med. 1955, 11, 132–137. Available online: https://archive.org/details/sim_a-m-a-archives-of-industrial-health_1955-02_11_2/page/132/mode/2up (accessed on 31 July 2023).
- Svirbely, J.L. Toxicity tests of decaborane for laboratory animals. I. Effect of repeated doses. AMA Arch. Ind. Hyg. Occup. Med. 1955, 11, 138–141. Available online: https://archive.org/details/sim_a-m-a-archives-of-industrial-health_1955-02_11_2/page/138/mode/2up (accessed on 31 July 2023).
- Walton, R.P.; Richardson, J.A.; Brodie, O.J. Cardiovascular actions of decaborane. J. Pharmacol. Exp. Ther. 1955, 114, 367–378. [Google Scholar]
- Lamberti, J.M. Review of the toxicological properties of pentaborane, diborane, decaborane, and boric acid. NASA Tech. Rep. 1956, NACA-RM-E56H13a. Available online: https://ntrs.nasa.gov/api/citations/19930090289/downloads/19930090289.pdf (accessed on 31 July 2023).
- Roush, G. The toxicology of the boranes. J. Occup. Med. 1959, 1, 46–52. Available online: https://www.jstor.org/stable/44999044 (accessed on 31 July 2023). [CrossRef]
- Fabre, R.; Chary, R.; Bocquet, P.; Jayot, R. Etat actuel de la toxicologie des hydrides de bore. Arch. Mal. Prof. Med. Trav. Secur. Soc. 1959, 20, 701–712. [Google Scholar]
- Miller, D.F.; Tamas, A.; Robinson, L.; Merriweather, E. Observations on experimental boron hydride exposures. Toxicol. Appl. Pharmacol. 1960, 2, 430–440. [Google Scholar] [CrossRef]
- Miller, D.F.; Tamas, A.A.; Robinson, L.; Merriweather, E. Cumulative effects of borane toxicity as revealed by a clinical test. Tech. Rep. Aerospace Med. Res. Lab. 1960, WADD-60-604. Available online: https://web.archive.org/web/20181030113902/http://www.dtic.mil/dtic/tr/fulltext/u2/247355.pdf (accessed on 31 July 2023).
- Delgado, J.M. Effects of decaborane on brain activity. Tech. Rep. Aerospace Med. Res. Lab. 1963, AMRL-TDR-63-41. Available online: https://web.archive.org/web/20180726163136/http://www.dtic.mil/dtic/tr/fulltext/u2/411769.pdf (accessed on 31 July 2023).
- Delgado, J.M.R.; Back, K.C.; Tamas, A.A. The effect of boranes on the monkey brain. Arch. Int. Pharmacodyn. Thérap. 1963, 141, 262–270. [Google Scholar]
- Lalli, G. Sulla tossicità di alcuni propellenti per missile. Ann. Geophys. 1963, 16, 385–406. [Google Scholar] [CrossRef]
- Merrit, J.A. Methylene blue in the treatment of decaborane toxicity. Arch. Environ. Health 1965, 10, 452–454. [Google Scholar] [CrossRef]
- Reynolds, H.H.; Back, K.C. Effect of decaborane injection on operant behavior of monkeys. Toxicol. Appl. Pharmacol. 1966, 8, 197–209. [Google Scholar] [CrossRef]
- Fairchild, M.D.; Sterman, M.B.; McRae, G.L. The effects of decaborane on cerebral electrical activity and locomotor behavior in the cat. Tech. Rep. Aerosp. Med. Res. Lab. 1972, AMRL-TR-72-80. Available online: https://web.archive.org/web/20180724164936/http://www.dtic.mil/dtic/tr/fulltext/u2/756526.pdf (accessed on 31 July 2023).
- Tadepalli, A.S.; Buckley, J.P. Cardiac and peripheral vascular effects of decaborane. Toxicol. Appl. Pharmacol. 1974, 29, 210–222. [Google Scholar] [CrossRef]
- Dekaboran. The MAK-Collection for Occupational Health and Safety; Greim, G., Ed.; Wiley-VCH Verlag: Berlin, Germany, 2002; Volume 1. [Google Scholar] [CrossRef]
- Merritt, J.H.; Schultz, E.J.; Wykes, A.A. Effect of decaborane on the norepinephrine content of rat brain. Biochem. Pharmacol. 1964, 13, 1364–1366. [Google Scholar] [CrossRef]
- Oliverio, A. Release of cardiac noradrenaline by decaborane in the heart-lung preparation of guinea pig. Biochem. Pharmacol. 1965, 14, 1689–1692. [Google Scholar] [CrossRef]
- von Euler, U.S.; Lishajko, F. Stereospecific catecholamine uptake in rabbit hearts depleted by decaborane. Int. J. Neuropharmacol. 1965, 4, 273–280. [Google Scholar] [CrossRef]
- von Euler, U.S.; Lishajko, F. Catecholamine depletion and uptake in adrenergic nerve vesicles and in rabbit organs after decaborane. Acta Physiol. Scand. 1965, 65, 324–330. [Google Scholar] [CrossRef]
- Byodeman, S.; von Euler, U.S. Neurotransmitter deficiency and reloading in noradrenaline depleted rabbits. Acta Physiol. Scand. 1966, 66, 134–140. [Google Scholar] [CrossRef]
- Merritt, J.H.; Schultz, E.J. The effect of decaborane on the biosynthesis and metabolism of norepinephrine in the rat brain. Life Sci. 1966, 5, 27–32. [Google Scholar] [CrossRef]
- Johnson, D.G. The effect of cold exposure on the catecholamine excretion of rats treated with decaborane. Acta Physiol. Scand. 1966, 68, 129–133. [Google Scholar] [CrossRef]
- Merritt, J.H.; Sulkowski, T.S. Inhibition of aromatic L-amino acid decarboxylation by decaborane. Biochem. Pharmacol. 1967, 16, 369–373. [Google Scholar] [CrossRef]
- Bhattacharya, I.C. Uptake of noradrenaline in the isolated perfused rat heart after depletion with decaborane. Acta Physiol. Scand. 1968, 73, 128–138. [Google Scholar] [CrossRef]
- Medina, M.A.; Landez, J.H.; Foster, L.L. Inhibition of tissue histamine formation by decaborane. J. Pharmacol. Exp. Ther. 1969, 169, 132–137. Available online: https://jpet.aspetjournals.org/content/169/1/132 (accessed on 31 July 2023).
- Scott, W.N.; Landez, J.H.; Cole, H.D. Effects of boranes upon tissues of the rat. I. Aspartate aminotransferase and lactic dehydrogenase. Proc. Soc. Exp. Biol. Med. 1970, 134, 348–352. [Google Scholar] [CrossRef]
- Korty, P.; Scott, W.N. Effects of boranes upon tissues of the rat. II. Tissue amino acid content in rats on a normal diet. Proc. Soc. Exp. Biol. Med. 1970, 135, 629–632. [Google Scholar] [CrossRef]
- Landez, J.H.; Scott, W.N. Effects of boranes upon tissues of the rat. III. Tissue amino acids in rats on a pyridoxine-deficient diet. Proc. Soc. Exp. Biol. Med. 1971, 136, 1389–1393. [Google Scholar] [CrossRef]
- Malmfors, T.; von Euler, U.S. Depletion and repletion of noradrenaline in adrenergic nerves of the rat after decaborane treatment. Experientia 1971, 27, 417–419. [Google Scholar] [CrossRef]
- Shahab, L.; Lishajko, F.; von Euler, U.S. Differentiated storage mechanisms for noradrenaline and dopamine in the rabbit heart. Neuropharmacology 1971, 10, 765–769. [Google Scholar] [CrossRef]
- Menon, M.; Clark, W.G.; Aures, D. Effect of tremorine, oxotremorine and decaborane on brain histamine levels in rats. Pharmacol. Res. Commun. 1971, 3, 345–350. [Google Scholar] [CrossRef]
- Schayer, R.W.; Reilly, M.A. Effect of decaborane on histamine formation in mice. J. Pharmacol. Exp. Ther. 1971, 177, 177–180. Available online: https://jpet.aspetjournals.org/content/177/1/177 (accessed on 31 July 2023).
- Naeger, L.L.; Leibman, K.C. Mechanisms of decaborane toxicity. Toxicol. Appl. Pharmacol. 1972, 22, 517–527. [Google Scholar] [CrossRef]
- Valerino, D.M.; Soliman, M.R.I.; Aurori, K.C.; Tripp, S.L.; Wykes, A.A.; Vesell, E.S. Studies on the interaction of several boron hydrides with liver microsomal enzymes. Toxicol. Appl. Pharmacol. 1974, 29, 358–366. [Google Scholar] [CrossRef]
- Merritt, J.A.; Meyer, H.C.; Greenberg, R.I.; Tanton, G.A. The production of decaborane-14 from diborane by laser induced chemistry. Propellants Explos. Pyrotech. 1979, 4, 78–82. [Google Scholar] [CrossRef]
- Dunks, G.B.; Palmer Ordonez, K. A one-step synthesis of B11H14− ion from NaBH4. Inorg. Chem. 1978, 17, 1514–1516. [Google Scholar] [CrossRef]
- Dunks, G.B.; Palmer Ordonez, K. A simplified preparation of B10H14 from NaBH4. Inorg. Chem. 1978, 17, 2555–2556. [Google Scholar] [CrossRef]
- Dunks, G.B.; Barker, K.; Hedaya, E.; Hefner, C.; Palmer-Ordonez, K.; Remec, P. Simplified synthesis of B10H14 from NaBH4 via B11H14− ion. Inorg. Chem. 1981, 20, 1692–1697. [Google Scholar] [CrossRef]
- Belov, P.P.; Storozhenko, P.A.; Voloshina, N.S.; Kuznetsova, M.G. Synthesis of decaborane by the reaction of sodium undecaborate with mild organic oxidants. Russ. J. Appl. Chem. 2018, 90, 1804–1809. [Google Scholar] [CrossRef]
- Voloshina, N.S.; Belov, P.P.; Storozhenko, P.A.; Shebashova, N.M.; Kozlova, E.E.; Egorova, N.V.; Kuznetsova, M.G.; Gurkova, E.L. Specific features of oxidation of sodium tetradecahydroundecaborate to decaborane with manganese dioxide. Russ. J. Appl. Chem. 2020, 93, 807–812. [Google Scholar] [CrossRef]
- Mongeot, H.; Atchekzai, H.R. Opening of the B10H102− cage to give B10H14. Z. Naturforsch. B 1981, 36, 313–314. [Google Scholar] [CrossRef]
- Guter, G.A.; Schaeffer, G.W. The strong acid behavior of decaborane. J. Am. Chem. Soc. 1956, 78, 3546. [Google Scholar] [CrossRef]
- Norment, H.J. Unit cells and space groups for two etherates of sodium tridecahydrodecaborate(1-). Acta Cryst. 1959, 12, 695. [Google Scholar] [CrossRef]
- Greenwood, N.N.; Sharrocks, D.N. Decaborane anions and the synthesis of polyhedral borane complexes of mercury(II) and cobalt(II). J. Chem. Soc. A 1969, 2334–2338. [Google Scholar] [CrossRef]
- Hawthorne, M.F.; Pitochelli, A.R.; Strahm, R.D.; Miller, J.J. The preparation and characterization of salts which contain the B10H13 anion. J. Am. Chem. Soc. 1960, 82, 1825–1829. [Google Scholar] [CrossRef]
- Hawthorne, M.F. The reaction of phosphine methylenes with boron hydrides. J. Am. Chem. Soc. 1958, 80, 3480–3481. [Google Scholar] [CrossRef]
- Onak, T.; Rosendo, H.; Siwapinyoyos, G.; Kubo, R.; Liauw, L. Reaction of 1,8-bis(dimethylamino)naphthalene, a highly basic and weakly nucleophilic amine, with several polyboranes and with boron trifluoride. Inorg. Chem. 1979, 18, 2943–2945. [Google Scholar] [CrossRef]
- Pérez, S.; Sanz Miguel, P.J.; Macías, R. Decaborane anion tautomerism: Ion pairing and proton transfer control. Dalton Trans. 2018, 47, 5850–5859. [Google Scholar] [CrossRef] [PubMed]
- Heřmánek, S.; Plotová, H.; Plešek, J. On the acidity characteristics of decaborane(14) and its benzyl derivatives in organic solvent-water systems. Collect. Czech. Chem. Commun. 1975, 40, 3593–3601. [Google Scholar] [CrossRef]
- McCrary, P.D.; Barber, P.S.; Kelley, S.P.; Rogers, R.D. Nonaborane and decaborane cluster anions can enhance the ignition delay in hypergolic ionic liquids and induce hypergolicity in molecular solvents. Inorg. Chem. 2014, 53, 4770–4776. [Google Scholar] [CrossRef] [PubMed]
- Sneddon, L.G.; Huffman, J.C.; Schaeffer, R.O.; Streib, W.E. Structure of the B10H13− ion. J. Chem. Soc. Chem. Commun. 1972, 474–475. [Google Scholar] [CrossRef]
- Wynd, A.J.; Welch, A.J. Structure of [PhCH2NMe3]+[B10H13]−. Acta Cryst. C 1989, 45, 615–617. [Google Scholar] [CrossRef]
- Siedle, A.R.; Bodner, G.M.; Todd, L.J. Studies in boron hydrides—V: Assignment of the 11B NMR spectrum of the tridecahydro decaborate(1−) ion. J. Inorg. Nucl. Chem. 1971, 33, 3671–3676. [Google Scholar] [CrossRef]
- Wilks, P.H.; Carter, J.C. Preparation and properties of sodium decaboranate(12,2-). J. Am. Chem. Soc. 1966, 88, 3441. [Google Scholar] [CrossRef]
- Bridges, A.N.; Gaines, D.F. The dianion of nido-decaborane(14), nido-dodecahydrodecaborate(2-), [B10H122−], and its solution behavior. Inorg. Chem. 1995, 34, 4523–4524. [Google Scholar] [CrossRef]
- Hofmann, M.; von Ragué Schleyer, P. Structures of arachno- and hypho-B10 clusters and stability of their possible Lewis base adducts ([B10H12]2−, [B10H12·L]2−, [B10H12·2L]2−, [B10H13]−, [B10H13·L]−, [B10H12·2L]). An ab initio/IGLO/NMR investigation. Inorg. Chem. 1998, 37, 5557–5565. [Google Scholar] [CrossRef]
- Muetterties, E.L. Chemistry of boranes. VI. Preparation and structure of B10H142−. Inorg. Chem. 1963, 2, 647–648. [Google Scholar] [CrossRef]
- Kendall, D.S.; Lipscomb, W.N. Crystal structure of tetramethylammonium tetradecahydrodecaborate. Structure of the Bl0H142− ion. Inorg. Chem. 1973, 12, 546–551. [Google Scholar] [CrossRef]
- Lipscomb, W.N.; Wiersema, R.J.; Hawthorne, M.F. Structural ambiguity of the B10H142− ion. Inorg. Chem. 1972, 11, 651–652. [Google Scholar] [CrossRef]
- Schaeffer, R.; Tebbe, F. Formation of B10H15- as an intermediate in borohydride attack on decaborane-14. Inorg. Chem. 1964, 3, 1638–1640. [Google Scholar] [CrossRef]
- Rietz, R.R.; Siedle, A.R.; Schaeffer, R.O.; Todd, L.J. High-resolution nuclear magnetic resonance study of the pentadecahydro-decaborate(1-) ion. Inorg. Chem. 1973, 12, 2100–2102. [Google Scholar] [CrossRef]
- Zahkarkin, L.I.; Stanko, V.I.; Chapovskii, Y.A. Reactions of acetals and ortho-ethers with decaborane and diacetonitrile decaborane. Bull. Acad. Sci. USSR Div. Chem. Sci. 1962, 11, 1048–1049. [Google Scholar] [CrossRef]
- Lee, S.H.; Park, Y.J.; Yoon, C.M. Reductive etherification of aromatic aldehydes with decaborane. Tetrahedron Lett. 1999, 40, 6049–6050. [Google Scholar] [CrossRef]
- Funke, U.; Jiay, H.; Fischer, S.; Scheunemann, M.; Steinbach, J. One-step reductive etherification of 4-[18F]fluoro-benzaldehyde with decaborane. J. Label Compd. Radiopharm. 2006, 49, 745–755. [Google Scholar] [CrossRef]
- Park, E.S.; Lee, J.H.; Kim, S.J.; Yoon, C.M. One-pot reductive amination of acetals with aromatic amines using decaborane (B10H14) in methanol. Synth. Commun. 2003, 33, 3387–3396. [Google Scholar] [CrossRef]
- Toyosuke, T.; Tsuneo, M.; Katsunori, K.; Yuichi, I. The reaction of decaborane with carbonyl compounds. Bull. Chem. Soc. Jpn. 1978, 51, 1259–1260. [Google Scholar] [CrossRef]
- Bae, J.W.; Lee, S.H.; Jung, Y.J.; Yoon, C.-O.M.; Yoon, C.M. Reduction of ketones to alcohols using a decaborane/pyrrolidine/ cerium(III) chloride system in methanol. Tetrahedron Lett. 2001, 42, 2137–2139. [Google Scholar] [CrossRef]
- Lee, S.H.; Nam, M.H.; Cho, M.Y.; Yoo, B.W.; Rhee, H.J.; Yoon, C.M. Chemoselective reduction of aldehydes using decaborane in aqueous solution. Synth. Commun. 2006, 36, 2469–2474. [Google Scholar] [CrossRef]
- Lee, S.H.; Jung, Y.J.; Cho, Y.J.; Yoon, C.-O.M.; Hwang, H.-J.; Yoon, C.M. Dehalogenation of α-halocarbonyls using decaborane as a transfer hydrogen agent in methanol. Synth. Commun. 2001, 31, 2251–2254. [Google Scholar] [CrossRef]
- Lee, S.H.; Park, J.Y.; Yoon, C.M. Hydrogenation of alkenes or alkynes using decaborane in methanol. Tetrahedron Lett. 2000, 41, 887–889. [Google Scholar] [CrossRef]
- Hawthorne, M.F.; Miller, J.J. Deuterium exchange of decaborane with deuterium oxide and deuterium chloride. J. Am. Chem. Soc. 1958, 80, 754. [Google Scholar] [CrossRef]
- Miller, J.J.; Hawthorne, M.F. The course of base-catalyzed hydrogen exchange in decaborane. J. Am. Chem. Soc. 1959, 81, 4501–4503. [Google Scholar] [CrossRef]
- Dupont, J.A.; Hawthorne, M.F. The nature of the electrophilic deuterium exchange reaction of decaborane with deuterium chloride. J. Am. Chem. Soc. 1962, 84, 1804–1808. [Google Scholar] [CrossRef]
- Dupont, J.A.; Hawthorne, M.F. Deuterium exchange of decaborane with deuterium chloride under electrophilic conditions. J. Am. Chem. Soc. 1959, 81, 4998–4999. [Google Scholar] [CrossRef]
- Dopke, J.A.; Gaines, D.F. Deuteration of decaborane(14) via exchange with deuterated aromatic solvents. Inorg. Chem. 1999, 38, 4896–4897. [Google Scholar] [CrossRef]
- Gaines, D.F.; Beall, H. Hydrogen−deuterium exchange in decaborane(14): Mechanistic studies. Inorg. Chem. 2000, 39, 1812–1813. [Google Scholar] [CrossRef] [PubMed]
- Hillman, M. The chemistry of decaborane. Iodination studies. J. Am. Chem. Soc. 1960, 82, 1096–1099. [Google Scholar] [CrossRef]
- Sprecher, R.F.; Aufderheide, B.E.; Luther, G.W., III; Carter, J.C. Boron-11 nuclear magnetic resonance chemical shift assignments for monohalogenated decaborane(14) isomers. J. Am. Chem. Soc. 1974, 96, 4404–4410. [Google Scholar] [CrossRef]
- Stuchlík, J. Gas chromatographic separation of 1- and 2-halogenodecaboranes. J. Chromatogr. A 1973, 81, 142–143. [Google Scholar] [CrossRef]
- Schaeffer, R.; Shoolery, J.N.; Jones, R. Structures of halogen substituted boranes. J. Am. Chem. Soc. 1958, 80, 2670–2673. [Google Scholar] [CrossRef]
- Williams, R.E.; Onak, T.P. Boron-11 nuclear magnetic resonance spectra (32.1 Mc.) of alkylated derivatives of dicarbahexaborane(8) and 1-iododecaborane(14). J. Am. Chem. Soc. 1964, 86, 3159–3160. [Google Scholar] [CrossRef]
- Hillman, M. The chemistry of decaborane. II. Iodination in solvent. J. Inorg. Nucl. Chem. 1960, 12, 384–385. [Google Scholar] [CrossRef]
- Wallbridge, M.H.G.; Williams, J.; Williams, R.L. Boron hydride derivatives. Part XI. Iodination of decaborane. J. Chem. Soc. A 1967, 132–133. [Google Scholar] [CrossRef]
- Safronov, A.V.; Sevryugina, Y.V.; Jalisatgi, S.S.; Kennedy, R.D.; Barnes, C.L.; Hawthorne, M.F. Unfairly forgotten member of the iodocarborane family: Synthesis and structural characterization of 8-iodo-1,2-dicarba-closo-dodecaborane, its precursors, and derivatives. Inorg. Chem. 2012, 51, 2629–2637. [Google Scholar] [CrossRef] [PubMed]
- Sequeira, A.; Hamilton, W.C. Crystal and molecular structure of monoiododecaborane. Inorg. Chem. 1967, 6, 1281–1286. [Google Scholar] [CrossRef]
- Schaeffer, R. The molecular structure of B10H12I2. J. Am. Chem. Soc. 1957, 79, 2726–2728. [Google Scholar] [CrossRef]
- Plešek, J.; Štíbr, B.; Heřmánek, S. Chemistry of boranes. VI. The reaction of bis-dialkylsulphido-dodecahydrodecaboranes with hydrohalogens. General preparation of 6- (or 5-) halogentridecahydrodecaboranes. Collect. Czech. Chem. Commun. 1966, 31, 4744–4745. [Google Scholar] [CrossRef]
- Sedmera, P.; Hanousek, F.; Samek, Z. Chemistry of boranes. XIII. Determination of structure of some halogenedecaboranes and oxido-bis-tridecahydrodecaborane by means of 11B NMR and IR spectra. Collect. Czech. Chem. Commun. 1968, 33, 2169–2175. [Google Scholar] [CrossRef]
- Štíbr, B.; Plešek, J.; Heřmánek, S. Chemistry of boranes. XV. Synthesis, properties, reactions and mechanism of formation of 5(6)-halogenotridecahydrodecaboranes. Collect. Czech. Chem. Commun. 1969, 34, 194–205. [Google Scholar] [CrossRef]
- Ewing, W.C.; Carroll, P.J.; Sneddon, L.G. Crystallographic characterizations and new high-yield synthetic routes for the complete series of 6-X-B10H13 halodecaboranes (X = F, Cl, Br, I) via superacid-induced cage-opening reactions of closo-B10H102−. Inorg. Chem. 2008, 47, 8580–8582. [Google Scholar] [CrossRef]
- Ewing, W.C.; Carroll, P.J.; Sneddon, L.G. Efficient Syntheses of 5-X-B10H13 halodecaboranes via the photochemical (X = I) and/or base-catalyzed (X = Cl, Br, I) isomerization reactions of 6-X-B10H13. Inorg. Chem. 2010, 49, 1983–1994. [Google Scholar] [CrossRef] [PubMed]
- Zakharkin, L.I.; Kalinin, V.N. Halogenation of decaborane in the presence of aluminum chloride. Zh. Obshch. Khim. 1966, 36, 2160–2162. [Google Scholar]
- Bonnetot, B.; Miele, P.; Naoufal, D.; Mongeot, H. The interaction of the [B10H10]2− cage with Lewis acids and the formation of decaborane derivatives by cage-opening reactions. Collect. Czech. Chem. Commun. 1997, 62, 1273–1278. [Google Scholar] [CrossRef]
- Stuchlík, J.; Heřmánek, S.; Plešek, J.; Štíbr, B. Chemistry of boranes. XVIII. A preparative separation of halogenodecaboranes. The isolation of 1-, 2-, 5-, and 6-bromotridecahydrodecaboranes. Collect. Czech. Chem. Commun. 1970, 35, 339–343. [Google Scholar] [CrossRef]
- Dupont, T.J.; Loffredo, R.E.; Haltiwanger, R.C.; Turner, C.A.; Norman, A.D. Oxidative cleavage of dimethylstanna-undecaborane: Preparation and structural characterization of 5,10-dibromodecaborane(14). Inorg. Chem. 1978, 17, 2062–2067. [Google Scholar] [CrossRef]
- Hillman, M.; Mangold, D.J. Chlorodecaborane. Inorg. Chem. 1965, 4, 1356–1357. [Google Scholar] [CrossRef]
- Williams, R.E.; Pier, E. Chlorodecaboranes identified as 1-ClB10H13 and 2-ClB10H13 by 64.2-Mc. 11B nuclear magnetic resonance spectra. Inorg. Chem. 1965, 4, 1357–1358. [Google Scholar] [CrossRef]
- Mongeot, H.; Atchekzai, J.; Bonnetot, B.; Colombier, M. Preparation du 6-B10H13Cl a partir de melanges AlCl3-(Et4N)2B10H10. Bull. Soc. Chim. Fr. 1987, 75–77. [Google Scholar]
- Bonnetot, B.; Aboukhassib, A.; Mongeot, H. Study of the interaction of AlCl3 with the B10H102− cage in the solid state. Inorg. Chim. Acta 1989, 156, 183–187. [Google Scholar] [CrossRef]
- Hawthorne, M.F.; Miller, J.J. The alkoxylation of decaborane. J. Am. Chem. Soc. 1960, 82, 500. [Google Scholar] [CrossRef]
- Norman, A.D.; Rosell, S.L. Evidence of terminal ethoxy group substitution in ethoxydecaborane(14). Inorg. Chem. 1969, 8, 2818–2820. [Google Scholar] [CrossRef]
- Loffredo, R.E.; Drullinger, L.F.; A. Slater, J.A.; Turner, C.A.; Norman, A.D. Preparation and properties of 6-ethoxy-, 6-phenyl-, and 6-trimethylsiloxydecaborane(14). Inorg. Chem. 1976, 15, 478–480. [Google Scholar] [CrossRef]
- Beachell, H.C.; Schar, W.C. The reaction of decaborane with substituted alcohols. J. Am. Chem. Soc. 1958, 80, 2943–2945. [Google Scholar] [CrossRef]
- Amberger, E.; Leidl, P. Synthese von (CH3)3SiB10H13. J. Organomet. Chem. 1969, 18, 345–347. [Google Scholar] [CrossRef]
- Ewing, W.C.; Carroll, P.J.; Sneddon, L.G. Syntheses and surprising regioselectivity of 5- and 6-substituted decaboranyl ethers via the nucleophilic attack of alcohols on 6- and 5-halodecaboranes. Inorg. Chem. 2011, 50, 4054–4064. [Google Scholar] [CrossRef]
- Hawthorne, M.F.; Mavunkal, I.J.; Knobler, C.B. Electrophilic reactions of protonated closo-Bl0Hl02− with arenes, alkane C-H bonds, and triflate ion forming aryl, alkyl, and triflate nido-6-X-Bl0H13 derivatives. J. Am. Chem. Soc. 1992, 114, 4427–4429. [Google Scholar] [CrossRef]
- Bondarev, O.; Sevryugina, Y.V.; Jalisatgi, S.S.; Hawthorne, M.F. Acid-induced opening of [closo-B10H10]2− as a new route to 6-Substituted nido-B10H13 decaboranes and related carboranes. Inorg. Chem. 2012, 51, 9935–9942. [Google Scholar] [CrossRef]
- Berkeley, E.R.; Ewing, W.C.; Carroll, P.J.; Sneddon, L.G. Synthesis, structural characterization, and reactivity studies of 5-CF3SO3-B10H13. Inorg. Chem. 2014, 53, 5348–5358. [Google Scholar] [CrossRef]
- Wang, Y.; Han, H.; Kang, J.-X.; Peng, J.; Lu, X.; Cao, H.-J.; Liu, Z.; Chen, X. Silylium ion-mediated cage-opening functionalization of closo-B10H102− salts. Chem. Commun. 2022, 58, 11933–11936. [Google Scholar] [CrossRef] [PubMed]
- Naoufal, D.; Kodeih, M.; Cornu, D.; Miele, P. New method of synthesis of 6-hydroxy-nido-decaborane 6-(OH)B10H13 by cage opening of closo-[B10H10]2−. J. Organomet. Chem. 2005, 690, 2787–2789. [Google Scholar] [CrossRef]
- Greenwood, N.N.; Hails, M.J.; Kennedy, J.D.; McDonald, W.S. Reactions of 6,6’-bis(nido-decaboranyl) oxide and 6-hydroxy-nido-decaborane with dihalogenobis(phosphine) complexes of nickel, palladium, and platinum, and some related chemistry; nuclear magnetic resonance investigations and the crystal and molecular structures of bis(dimethylphosphine)-di-µ-(2,3,4-η3-nido-hexaboranyl)-diplatinum(Pt–Pt), [Pt2(µ-η3-B6H9)2(PMe2Ph)2], and of 2,4-dichloro-1,1-bis(dimethylphenylphosphine)-closo-1-nickeladecaborane, [(PhMe2P)2NiB9H7Cl2]. J. Chem. Soc. Dalton Trans. 1985, 953–972. [Google Scholar] [CrossRef]
- Štíbr, B.; Plešek, J.; Hanousek, F.; Heřmánek, S. Chemistry of boranes. XXIII. Reaction of 6,9-bis(dialkylsulfido)-dodecahydrodecaboranes with mercuric salts. Collect. Czech. Chem. Commun. 1971, 36, 1794–1799. [Google Scholar] [CrossRef]
- Kelley, S.P.; Rachiero, G.P.; Titi, H.M.; Rogers, R.D. New reactions for old ions: Cage rearrangements, hydrolysis, and two-electron reduction of nido-decaborane in neat 1-ethyl-3-methylimidazolium acetate. ACS Omega 2018, 3, 8491–8496. [Google Scholar] [CrossRef] [PubMed]
- Plešek, J.; Heřmánek, S.; Štíbr, B. Chemistry of boranes. VIII. Synthesis and reactions of 6,6’-oxido-bis-tridecahydrodecaborane. Collect. Czech. Chem. Commun. 1968, 33, 691–698. [Google Scholar] [CrossRef]
- Kennedy, J.D.; Greenwood, N.N. A proton and boron-l 1 NMR study of icosaborane oxide, B20H16O. Inorg. Chim. Acta 1980, 38, 93–96. [Google Scholar] [CrossRef]
- Greenwood, N.N.; McDonald, W.S.; Spalding, T.R. Crystal and molecular structure of 6,6’-bis(nido-decaboranyl) oxide (B10H13)2O. J. Chem. Soc. Dalton Trans. 1980, 1251–1252. [Google Scholar] [CrossRef]
- Bonnetot, B.; Tangi, A.; Colombier, M.; Mongeot, H. Dehydration of (H3O)2B10H10: An improved preparation of icosaborane oxide, (B10H13)2O. Inorg. Chim. Acta 1985, 105, L15–L16. [Google Scholar] [CrossRef]
- Pace, R.J.; Williams, J.; Williams, R.L. Boron hydride derivatives. Part VII. The characterisation of some decaborane derivatives of the type, B10H12,2M. J. Chem. Soc. 1961, 2196–2204. [Google Scholar] [CrossRef]
- Knoth, W.H.; Muetterties, E.L. Chemistry of boranes. II: Decaborane derivatives based on the B10H12 structural unit. J. Inorg. Nucl. Chem. 1961, 20, 66–72. [Google Scholar] [CrossRef]
- Fein, M.M.; Green, J.; Bobinski, J.; Cohen, M.S. Reaction products from decaborane and amides. Inorg. Chem. 1965, 4, 583–584. [Google Scholar] [CrossRef]
- Cragg, R.H.; Fortuin, M.S.; Greenwood, N.N. Complexes of decaborane. Part I. Ultraviolet spectra of some bis-(ligand) complexes containing phosphorus and sulphur. J. Chem. Soc. A 1970, 1817–1821. [Google Scholar] [CrossRef]
- Janoušek, Z.; Plešek, J.; Plzák, Z. Open cage boranes and heteroborane thiols: Syntheses, structures, and some properties. Collect. Czech. Chem. Commun. 1979, 44, 2904–2907. [Google Scholar]
- Bould, J.; Macháček, J.; Londesborough, M.G.S.; Macías, R.; Kennedy, J.D.; Bastl, Z.; Rupper, P.; Baše, T. Decaborane thiols as building blocks for self-assembled monolayers on metal surfaces. Inorg. Chem. 2012, 51, 1685–1694. [Google Scholar] [CrossRef]
- Zakharkin, L.I.; Stanko, V.I.; Okhlobystin, O.Y. Reaction of decaborane and pentaborane with mercaptans and sulfides. Bull. Acad. Sci. USSR Div. Chem. Sci. 1961, 10, 1942–1943. [Google Scholar] [CrossRef]
- Hawthorne, M.F.; Pilling, R.L.; Grimes, R.N. The mechanism of B10H10-2 formation from B10H12(ligand)2 species. J. Am. Chem. Soc. 1967, 89, 1067–1074. [Google Scholar] [CrossRef]
- Beall, H.; Gaines, D.F. Reactions of 6,9-bis(dimethyl sulfide)-decaborane(14), 6,9-[(CH3)2S]2B10H12: Mechanistic considerations. Inorg. Chem. 1998, 37, 1420–1422. [Google Scholar] [CrossRef]
- Sands, D.E.; Zalkin, A. The crystal structure of B10H12[S(CH3)2]2. Acta Cryst. 1962, 15, 410–417. [Google Scholar] [CrossRef]
- Yumatov, V.D.; Murakhtanov, V.V.; Volkov, V.V.; Il’inchik, E.A.; Volkov, O.V. Comparative study of electronic structure of 6,9-bis(dimethyl sulfide)-arachno-decaborane(12) B10H12[S(CH3)]2 in a series of sulfide derivatives by the X-ray emission method. Russ. J. Inorg. Chem. 1998, 43, 1557–1561. [Google Scholar]
- Yumatov, V.D.; Il’inchik, E.A.; Mazalov, L.N.; Volkov, O.V.; Volkov, V.V. X-Ray and X-ray photoelectron spectroscopy studies of the electronic structure of borane derivatives. J. Struct. Chem. 2001, 42, 281–295. [Google Scholar] [CrossRef]
- Il’inchik, E.A.; Volkov, V.V.; Mazalov, L.N. X-ray photoelectron spectroscopy of boron compounds. J. Struct. Chem. 2005, 46, 523–534. [Google Scholar] [CrossRef]
- Volkov, V.V.; Ikorskii, V.N.; Dunaev, S.T. Diamagnetism of compounds of the B10H12L2 series. Bull. Acad. Sci. USSR Div. Chem. Sci. 1988, 37, 845–848. [Google Scholar] [CrossRef]
- Zakharkin, L.I.; Stanko, V.I.; Klimova, A.I. Exchange reactions of decaborane complexes of the type B10H12[X]2. Bull. Acad. Sci. USSR Div. Chem. Sci. 1964, 13, 857–858. [Google Scholar] [CrossRef]
- Graybill, B.M.; Hawthorne, M.F. The nature of the colored 6,9-bis-pyridine decaborane molecule, B10H12Py2. J. Am. Chem. Soc. 1961, 83, 2673–2676. [Google Scholar] [CrossRef]
- Marshall, M.D.; Hunt, R.M.; Hefferan, G.T.; Adams, R.M.; Makhlouf, J.M. Opening the B10H102-cage to produce B10H12(Et2S)2. J. Am. Chem. Soc. 1967, 89, 3361–3362. [Google Scholar] [CrossRef]
- Guillevic, G.; Dazord, J.; Mongeot, H.; Cueilleron, J. Improved conversion of potassium tetrahydroborate into bis(dialkylsulfide)-decaborane(12), B10H12(R2S)2, via bis(tetraethylammonium) decahydrodecaborate, (Et4N)2B10H10. J. Chem. Res. (S) 1978, 402. [Google Scholar]
- Wang, G.-C.; Lu, Y.-X.; Huang, X.-Y.; Dai, L.-X. A new method for the synthesis of bis(diethylsulfide)decaborane. Acta Chim. Sin. 1981, 39, 251–254. Available online: http://sioc-journal.cn/Jwk_hxxb/EN/Y1981/V39/I3/251 (accessed on 31 July 2023).
- Heying, T.L.; Naar-Colin, C. Some chemistry of substituted decaboranes. Inorg. Chem. 1964, 3, 282–285. [Google Scholar] [CrossRef]
- Ahmad, R.; Crook, J.E.; Greenwood, N.N.; Kennedy, J.D. Synthesis, reactions, and nuclear magnetic resonance studies of some substituted arachno-decaborane and arachno-nonaborane derivatives, and the isolation of novel polyhedral diplatinaboranes. Crystal and molecular structure of [Pt2(PMe2Ph)2(η3-B2H5)(η3-B6H9)]. J. Chem. Soc. Dalton Trans. 1986, 2433–2442. [Google Scholar] [CrossRef]
- V. Petřiček, V.; Cisařova, I.; Subrtova, V. Structure of σ(+)-5-bromo-6,9-bis(dimethylsulphido)-nido-decaborane(12), C4H23B10BrS2, determined with a twinned crystal. Acta Cryst. C 1983, 39, 1070–1072. [Google Scholar] [CrossRef]
- Bould, J.; Dörfler, U.; Thornton-Pett, M.; Kennedy, J.D. A rearrangement of the 10-boron nido/arachno decaboranyl cluster. Inorg. Chem. Commun. 2001, 4, 544–546. [Google Scholar] [CrossRef]
- Bridges, A.N.; Powell, D.R.; Dopke, J.A.; Desper, J.M.; Gaines, D.F. Monoalkyldecaborane(14) syntheses via nucleophilic alkylation and hydroboration. Inorg. Chem. 1998, 37, 503–509. [Google Scholar] [CrossRef]
- Bould, J.; Dörfler, U.; Rath, N.P.; Barton, L.; Kilner, C.A.; Londesborough, M.G.S.; Ormsby, D.L.; Kennedy, J.D. Macropolyhedral boron-containing cluster chemistry. A synthetic approach via the auto-fusion of [6,9-(SMe2)2-arachno-B10H12]. Dalton Trans. 2006, 3752–3765. [Google Scholar] [CrossRef]
- Beachell, H.C.; Hoffman, D.E. The reaction of decaborane with amines and related compounds. J. Am. Chem. Soc. 1962, 84, 180–182. [Google Scholar] [CrossRef]
- Schaeffer, R. A new type of substituted borane. J. Am. Chem. Soc. 1957, 79, 1006–1007. [Google Scholar] [CrossRef]
- van der Maas Reddy, J.; Lipscomb, W.N. Molecular structure of B10H12(CH3CN)2. J. Am. Chem. Soc. 1959, 81, 754. [Google Scholar] [CrossRef]
- van der Maas Reddy, J.; Lipscomb, W.N. Molecular structure of B10H12(CH3CN)2. J. Chem. Phys. 1959, 31, 610–616. [Google Scholar] [CrossRef]
- Mebs, S.; Kalinowski, R.; Grabowsky, S.; Förster, D.; Kickbusch, R.; Justus, E.; Morgenroth, W.; Paulmann, C.; Luger, P.; Gabel, D.; et al. Real-space indicators for chemical bonding. Experimental and theoretical electron density studies of four deltahedral boranes. Inorg. Chem. 2011, 50, 90–103. [Google Scholar] [CrossRef] [PubMed]
- Beall, H. Icosahedral carboranes. XVII. Simplified preparation of o-carborane. Inorg. Chem. 1972, 11, 637–638. [Google Scholar] [CrossRef]
- Hawthorne, M.F.; Pitochelli, A.R. Displacement reactions on the B10H12 unit. J. Am. Chem. Soc. 1958, 80, 6685. [Google Scholar] [CrossRef]
- Hawthorne, M.F.; Pitochelli, A.R. The reactions of bis-acetonitrile decaborane with amines. J. Am. Chem. Soc. 1959, 81, 5519. [Google Scholar] [CrossRef]
- Fein, M.M.; Bobinski, J.; Paustian, J.E.; Grafstein, D.; Cohen, M.S. Reaction of decaborane and its derivatives. II. Addition reactions of 6,9-bis(acetonitrile)decaborane with hydrazine. Inorg. Chem. 1965, 4, 422. [Google Scholar] [CrossRef]
- Froehner, G.; Challis, K.; Gagnon, K.; Getman, T.D.; Luck, R.L. A re-investigation of the reactions of amines and alcohols with 6,9-bis-(acetonitrile)decaborane. Synth. React. Inorg. Metal-Org. Nano-Metal Chem. 2007, 36, 777–785. [Google Scholar] [CrossRef]
- Stogniy, M.Y.; Erokhina, S.A.; Sivaev, I.B.; Bregadze, V.I. Nitrilium derivatives of polyhedral boron compounds (boranes, carboranes, metallacarboranes): Synthesis and reactivity. Phosphorus Sulfur Silicon Relat. Elem. 2019, 194, 983–988. [Google Scholar] [CrossRef]
- Zhdanov, A.P.; Nelyubin, A.V.; Klyukin, I.N.; Selivanov, N.A.; Bortnikov, E.O.; Grigoriev, M.S.; Zhizhin, K.Y.; Kuznetsov, N.T. Nucleophilic addition reaction of secondary amines to acetonitrilium closo-decaborate [2-B10H9NCCH3]−. Russ. J. Inorg. Chem. 2019, 64, 841–846. [Google Scholar] [CrossRef]
- Stogniy, M.Y.; Erokhina, S.A.; Suponitsky, K.Y.; Anisimov, A.A.; Godovikov, I.A.; Sivaev, I.B.; Bregadze, V.I. Synthesis of novel carboranyl amidines. J. Organomet. Chem. 2020, 909, 121111. [Google Scholar] [CrossRef]
- Bogdanova, E.V.; Stogniy, M.Y.; Chekulaeva, L.A.; Anisimov, A.A.; Suponitsky, K.Y.; Sivaev, I.B.; Grin, M.A.; Mironov, A.F.; Bregadze, V.I. Synthesis and reactivity of propionitrilium derivatives of cobalt and iron bis(dicarbollides). New J. Chem. 2020, 44, 15836–15848. [Google Scholar] [CrossRef]
- El Anwar, S.; Růžičková, Z.; Bavol, D.; Fojt, L.; Grüner, B. Tetrazole ring substitution at carbon and boron sites of the cobalt bis(dicarbollide) ion available via dipolar cycloadditions. Inorg. Chem. 2020, 59, 17430–17442. [Google Scholar] [CrossRef] [PubMed]
- Bogdanova, E.V.; Stogniy, M.Y.; Suponitsky, K.Y.; Sivaev, I.B.; Bregadze, V.I. Synthesis of boronated amidines by addition of amines to nitrilium derivative of cobalt bis(dicarbollide). Molecules 2021, 26, 6544. [Google Scholar] [CrossRef] [PubMed]
- Voinova, V.V.; Selivanov, N.A.; Plyushchenko, I.V.; Vokuev, M.F.; Bykov, A.Y.; Klyukin, I.N.; Novikov, A.S.; Zhdanov, A.P.; Grigoriev, M.S.; Rodin, I.A.; et al. Fused 1,2-diboraoxazoles based on closo-decaborate anion—Novel members of diboroheterocycle class. Molecules 2021, 26, 248. [Google Scholar] [CrossRef] [PubMed]
- Stogniy, M.Y.; Erokhina, S.A.; Suponitsky, K.Y.; Markov, V.Y.; Sivaev, I.B. Synthesis and crystal structures of nickel(II) and palladium(II) complexes with o-carboranyl amidine ligands. Dalton Trans. 2021, 50, 4967–4975. [Google Scholar] [CrossRef]
- Nelyubin, A.V.; Selivanov, N.A.; Bykov, A.Y.; Klyukin, I.N.; Novikov, A.S.; Zhdanov, A.P.; Karpechenko, N.Y.; Grigoriev, M.S.; Zhizhin, K.Y.; Kuznetsov, N.T. Primary amine nucleophilic addition to nitrilium closo-dodecaborate [B12H11NCCH3]−: A simple and effective route to the new BNCT drug design. Int. J. Mol. Sci. 2021, 22, 13391. [Google Scholar] [CrossRef]
- Laskova, J.; Ananiev, I.; Kosenko, I.; Serdyukov, A.; Stogniy, M.; Sivaev, I.; Grin, M.; Semioshkin, A.; Bregadze, V.I. Nucleophilic addition reactions to nitrilium derivatives [B12H11NCCH3]− and [B12H11NCCH2CH3]−. Synthesis and structures of closo-dodecaborate-based iminols, amides and amidines. Dalton Trans. 2022, 51, 3051–3059. [Google Scholar] [CrossRef]
- Williams, J.; Williams, R.L.; Wright, J.C. Boron hydride derivatives. Part IX. The reaction of decaborane with ammonia. J. Chem. Soc. 1963, 5816–5824. [Google Scholar] [CrossRef]
- Rozenberg, A.S.; Neehiporenko, G.N.; Alekseev, A.P. Decomposition of nitrogen-hydrogen complexes of decaborane(12). 1. The kinetics of the thermal decomposition of decaborane(12) diammoniate. Bull. Acad. Sci. USSR Div. Chem. Sci. 1978, 27, 284–287. [Google Scholar] [CrossRef]
- Baidina, I.A.; Podberezskaya, N.V.; Alekseev, V.I.; Volkov, V.V.; Borisov, S.V. Crystal structure of 6,9-bis-aminododecahydro-nido-decaborane B10H12(NH3)2. J. Struct. Chem. 1978, 19, 476–479. [Google Scholar] [CrossRef]
- Polyanskaya, T.M.; Volkov, V.V. Crystal and molecular structure of 6,9-bis(trimethylamino)-nido-decaborane(12) B10H12[N(CH3)3]2. J. Struct. Chem. 1989, 30, 629–634. [Google Scholar] [CrossRef]
- Baidina, I.A.; Podberezskaya, N.V.; Volkov, V.V.; Borisov, S.V. Crystal structure of 6,9-bis-(triethylamino)-nido-decaborane B10H12[(C2H5)3N]2. J. Struct. Chem. 1978, 19, 479–482. [Google Scholar] [CrossRef]
- Volkov, V.V.; Il’inchik, E.A.; Khudorozhko, G.F.; Yumatov, V.D.; Mazalov, L.N. Comparative study of B10H12(NH3)2 and B10H12(NEt3)2. Bull. Acad. Sci. USSR Div. Chem. Sci. 1985, 34, 2079–2085. [Google Scholar] [CrossRef]
- Rozenberg, A.S. Decomposition of nitrogen-hydrogen complexes of decaborane(12). 2. An IR study of the mechanism of decomposition of decaborane(12) diammoniate. Bull. Acad. Sci. USSR Div. Chem. Sci. 1978, 27, 287–290. [Google Scholar] [CrossRef]
- Isaenko, L.I.; Myakishev, K.G.; Posnaya, I.S.; Volkov, V.V. About the thermal stability of several derivatives of boron hydrides. Izv. Sib. Otd. Akad. Nauk SSSR Ser. Khim. 1982, 73–78. [Google Scholar]
- Graybill, B.M.; Pitochelli, A.R.; Hawthorne, M.F. The preparation and reactions of B10H13(Ligand) anions. Inorg. Chem. 1962, 1, 622–626. [Google Scholar] [CrossRef]
- Cendrowski-Guillaume, S.M.; O’Loughlin, J.L.; Pelczer, I.; Spencer, J.T. Reactivity of decaborane(14) with pyridine: Synthesis and characterization of the first 6,6-substituted isomer of nido-B10H14, 6,6-(C5H5N)2B10H12, and application of 11B-11B double-quantum NMR spectroscopy. Inorg. Chem. 1995, 34, 3935–3941. [Google Scholar] [CrossRef]
- Bould, J.; Laromaine, A.; Bullen, N.J.; Viñas, C.; Thornton-Pett, M.; Sillanpää, R.; Kivekäs, R.; Kennedy, J.D.; Teixidor, F. Borane reaction chemistry. Alkyne insertion reactions into boron-containing clusters. Products from the thermolysis of [6,9-(2-HC≡C–C5H4N)2-arachno-B10H12]. Dalton Trans. 2008, 1552–1563. [Google Scholar] [CrossRef]
- Volkov, V.V.; Myakishev, K.G.; Potapova, O.G.; Polyanskaya, T.M.; Dunaev, S.T.; Il’inchik, E.A.; Baidina, I.A.; Hudorozhko, G.F. Synthesis and physico-chemical study of 6,9-bis-pyridino-nido-decaborane(14). Izv. Sib. Otd. Akad. Nauk SSSR Ser. Khim. 1988, 3, 20–27. [Google Scholar]
- Il’inchik, E.A.; Volkov, V.V.; Dunaev, S.T. Structural effects in the electron absorption and luminescence spectra of decaborane(14) derivatives B10H12L2. J. Struct. Chem. 1996, 37, 51–57. [Google Scholar] [CrossRef]
- Volkov, V.V.; Il’inchik, E.A.; Volkov, O.V.; Yuryeva, O.P. The luminescence of cluster derivatives of boron hydrides, and some applied aspects. Chem. Sustain. Develop. 2000, 8, 185–191. Available online: https://sibran.ru/upload/iblock/fed/the_luminescence_of_cluster_derivatives_of_boron_hydrides_and_some_applied_aspects.PDF (accessed on 31 July 2023).
- Volkov, V.V.; Il’inchik, E.A.; Yur’eva, O.P.; Volkov, O.V. Luminescence of the derivatives of boranes and adducts of decaborane(14) of the B10H12[Py(X)]2 type. J. Appl. Spectr. 2000, 67, 864–870. [Google Scholar] [CrossRef]
- Volkov, V.V.; Myakishev, K.G.; Dunaev, S.T. Thermal transformations of B10H12(NH3)2, B10H12(C5H5N)2, and B10H12(C9H7N)2. Bull. Acad. Sci. USSR Div. Chem. Sci. 1988, 37, 2234–2236. [Google Scholar] [CrossRef]
- Polyanskaya, T.M.; Volkov, V.V.; Andrianov, V.I.; Il’inchik, E.A. Structures of two modifications of 6,9-bis-pyridine-nido-decaborane(12). Bull. Acad. Sci. USSR Div. Chem. Sci. 1989, 38, 1751–1754. [Google Scholar] [CrossRef]
- Londesborough, M.G.S.; Price, C.; Thornton-Pett, M.; Clegg, W.; Kennedy, J.D. Two potential pyridine-borane oligomer and polymer building blocks. Structural characterisation of [NC5H4·C5H4N·B10H12·NC5H4·C5H4N] and [Me2S·B10H12·NC4H4N·B10H12·SMe2] by conventional and synchrotron X-ray methods. Inorg. Chem. Commun. 1999, 2, 298–300. [Google Scholar] [CrossRef]
- Genady, A.R.; Fayed, T.A.; Gabel, D. Synthesis, characterization, and spectrophotometric studies of novel fluorescent arachno decaborane and nonaborane clusters containing aza-distyrylbenzene derivatives. J. Organomet. Chem. 2008, 693, 1065–1072. [Google Scholar] [CrossRef]
- Hawthorne, M.F.; Pilling, R.L.; Vasavada, R.C. The mechanism of ligand exchange with B10H12(ligand)2 species. J. Am. Chem. Soc. 1967, 89, 1075–1078. [Google Scholar] [CrossRef]
- Rachiero, G.P.; Titi, H.M.; Rogers, R.D. Versatility and remarkable hypergolicity of exo-6, exo-9 imidazole-substituted nido-decaborane. Chem. Commun. 2017, 53, 7736–7739. [Google Scholar] [CrossRef]
- Fetter, N.R. Reaction of decaborane with 2-isopropyl- and 2-methyl-5-(2-chloroethyl) tetrazole. Chem. Ind. 1959, 1548. [Google Scholar]
- Kendall, D.S.; Lipscomb, W.N. Molecular structure and two crystal structures of 6-isothiocyanodecaborane, 6-B10H13NCS. Inorg. Chem. 1973, 12, 2915–2919. [Google Scholar] [CrossRef]
- Müller, J.; Paetzold, P.; Boese, R. The reaction of decaborane with hydrazoic acid: A novel access to azaboranes. Heteroat. Chem. 1990, 1, 461–465. [Google Scholar] [CrossRef]
- Il’inchik, E.A.; Dunaev, S.T.; Myakishev, K.G.; Asanov, I.P. On solvatation and thermal transformations of B10H12(PPh3)2. Zh. Neorg. Khim. 1994, 39, 1071–1074. [Google Scholar]
- Polyanskaya, T.M.; Yumatov, V.D.; Volkov, V.V. Molecular and electronic structure of 6,9-(PPh3)2-arachno-B10H12. Dokl. Chem. 2003, 390, 144–147. [Google Scholar] [CrossRef]
- Fontaine, X.L.R.; Kennedy, J.D. Identification of the endo,exo isomer of 6,9-(PMe2Ph)2-arachno-B10H12 by nuclear magnetic resonance spectroscopy. J. Chem. Soc. Dalton Trans. 1987, 1573–1575. [Google Scholar] [CrossRef]
- Dörfler, U.; McGrath, T.D.; Cooke, P.A.; Kennedy, J.D.; Thornton-Pett, M. exo,endo and exo,exo isomers of 6,9-(PMe2Ph)2-arachno-B10H12 and its halogenated derivatives. Molecular structures of exo,endo- and exo,exo-6,9-(PMe2Ph)2-arachno-B10H12 and exo-6,endo-9-(PMe2Ph)2-2-Br-arachno-B10H11. J. Chem. Soc. Dalton Trans. 1997, 4739–4746. [Google Scholar] [CrossRef]
- Zakharkin, L.I.; Stanko, V.I. Complexes of decaborane with organic phosphorus and arsenic compounds. Bull. Acad. Sci. USSR Div. Chem. Sci. 1961, 10, 1936–1937. [Google Scholar] [CrossRef]
- Polak, R.J.; Heying, T.L. The preparation of phosphite and phosphinite decaboranes. J. Org. Chem. 1962, 27, 1483–1484. [Google Scholar] [CrossRef]
- Stanko, V.I.; Klimova, A.I.; Zakharkin, L.I. Complexes of decaborane with trialkyl-, triaryl-, trialkyltrithiophosphites and trialkyl-, trialkyltrithioarsenites. Bull. Acad. Sci. USSR Div. Chem. Sci. 1962, 11, 856–857. [Google Scholar] [CrossRef]
- Schroeder, H.; Reiner, J.R.; Heying, T.L. Chemistry of decaborane-phosphorus compounds. I. Nucleophilic substitutions of bis-(chlorodiphenylphosphine)-decaborane. Inorg. Chem. 1962, 1, 618–621. [Google Scholar] [CrossRef]
- Schroeder, H.; Reiner, J.R.; Knowles, T.A. Chemistry of decaborane-phosphorus compounds. III. Decaborane-14-phosphine polymers. Inorg. Chem. 1963, 2, 393–396. [Google Scholar] [CrossRef]
- Seyferth, D.; Rees, W.S.; Haggerty, J.S.; Lightfoot, A. Preparation of boron-containing ceramic materials by pyrolysis of the decaborane(14)-derived [B10H12·Ph2POPPh2]x polymer. Chem. Mater. 1989, 1, 45–52. [Google Scholar] [CrossRef]
- Packirisamy, S. Decaborane(14)-based polymers. Progr. Polym. Sci. 1996, 21, 707–773. [Google Scholar] [CrossRef]
- Donaghy, K.J.; Carroll, P.J.; Sneddon, L.G. Reactions of 1,1’-bis(diphenylphosphino)ferrocene with boranes, thiaboranes, and carboranes. Inorg. Chem. 1997, 36, 547–553. [Google Scholar] [CrossRef]
- Schroeder, H. Chemistry of decaborane-phosphorus compounds. II. Synthesis and reactions of diphenylphosphinodecaborane-14. Inorg. Chem. 1963, 2, 390–393. [Google Scholar] [CrossRef]
- Friedman, L.B.; Perry, S.L. Crystal and molecular structure of 5,6-μ-diphenylphosphino-decaborane(14). Inorg. Chem. 1973, 12, 288–293. [Google Scholar] [CrossRef]
- Muetterties, E.L.; Aftandilian, V.D. Chemistry of boranes. IV. Phosphine derivatives of B10H14 and B9H15. Inorg. Chem. 1962, 1, 731–734. [Google Scholar] [CrossRef]
- Thornton-Pett, M.; Beckett, M.A.; Kennedy, J.D. Polyhedral phosphaborane chemistry: Crystal and molecular structure of the diphenylphosphido-bridged arachno-decaboranyl cluster compound [PMePh3][6,9-µ-(PPh2)B10H12]. J. Chem. Soc. Dalton Trans. 1986, 303–308. [Google Scholar] [CrossRef]
- Miller, R.W.; Spencer, J.T. Small heteroborane cluster systems. 7. Reaction of phosphaalkyne t-BuCP with bis-(acetonitrile)decaborane(12). A new synthetic route to a large phosphaborane cluster compound. Polyhedron 1996, 15, 3151–3155. [Google Scholar] [CrossRef]
- Miller, R.W.; Spencer, J.T. Small heteroborane cluster systems. 8. Preparation of phosphaborane clusters from the reaction of polyhedral boranes with low-coordinate phosphorus compounds: Reaction chemistry of phosphaalkynes with decaborane(14). Organometallics 1996, 15, 4293–4300. [Google Scholar] [CrossRef]
- Williams, R.L.; Dunstan, I.; Blay, N.J. Boron hydride derivatives. Part IV. Friedel–Crafts methylation of decaborane. J. Chem. Soc. 1960, 5006. [Google Scholar] [CrossRef]
- Obenland, C.O.; Newberry, J.R.; Schreiner, W.L.; Bartoszek, E.J. Friedel-Crafts methylation of decaborane. Ind. Eng. Chem. Prod. Res. Dev. 1965, 4, 281–283. [Google Scholar] [CrossRef]
- Polak, R.J.; Goodspeed, N.C. Catalyst study in methylation of decaborane. Ind. Eng. Chem. Prod. Res. Dev. 1965, 4, 158–160. [Google Scholar] [CrossRef]
- Holub, J.; Růžička, A.; Růžičková, Z.; Fanfrlík, J.; Hnyk, D.; Štíbr, B. Electrophilic methylation of decaborane(14): Selective synthesis of tetramethylated and heptamethylated decaboranes and their conjugated bases. Inorg. Chem. 2020, 59, 10540–10547. [Google Scholar] [CrossRef] [PubMed]
- Blay, N.J.; Dunstan, I.; Williams, R.L. Boron hydride derivatives. Part III. Electrophilic substitution in pentaborane and decaborane. J. Chem. Soc. 1960, 430–433. [Google Scholar] [CrossRef]
- Cueilleron, J.; Guillot, P. Preparation de quelques composes du decaborane. Bull. Chim. Fr. 1960, 2044–2052. [Google Scholar]
- Perloff, A. The crystal structure of 1-ethyldecaborane. Acta Cryst. 1964, 17, 332–338. [Google Scholar] [CrossRef]
- Dunstan, I.; Williams, R.L.; Blay, N.J. Boron hydride derivatives. Part V. Nucleophilic substitution in decaborane. J. Chem. Soc. 1960, 5012–5015. [Google Scholar] [CrossRef]
- Dunstan, I.; Blay, N.J.; Williams, R.L. Boron hydride derivatives. Part VI. Decaborane Grignard reagent. J. Chem. Soc. 1960, 5016–5019. [Google Scholar] [CrossRef]
- Gallaghan, J.; Siegel, B. Grignard synthesis of alkyl decaboranes. J. Am. Chem. Soc. 1959, 81, 504. [Google Scholar] [CrossRef]
- Siegel, B.; Mack, J.L.; Lowe, J.U.; Gallaghan, J. Decaborane Grignard reagents. J. Am. Chem. Soc. 1958, 80, 4523–4526. [Google Scholar] [CrossRef]
- Palchak, R.J.F.; Norman, J.H.; Williams, R.E. Decaborane, “6-benzyl” B10H13 chemistry. J. Am. Chem. Soc. 1961, 83, 3380–3384. [Google Scholar] [CrossRef]
- Gaines, D.F.; Bridges, A.N. New routes to monoalkyl decaborane(14) derivatives. Organometallics 1993, 12, 2015–2016. [Google Scholar] [CrossRef]
- Tolpin, E.I.; Mizusawa, E.; Becker, D.S.; Venzel, J. Synthesis and chemistry of 9-cyclohexyl-5(7)-(dimethyl sulfide)-nido-decaborane(11), B10H11C6H11S(CH3)2. Inorg. Chem. 1980, 19, 1182–1187. [Google Scholar] [CrossRef]
- Millan, M.D.; Davis, J.H. Hydroboration of (1R)-(+)-α-pinene and (1S)-(−)-β-pinene with B10H12(SMe2)2: A straightforward approach to the preparation of optically active 6-(alkyl)-nido-B10H13 derivatives. Tetrahedron Asymmetry 1998, 9, 709–712. [Google Scholar] [CrossRef]
- Mizusawa, E.; Rudnick, S.E.; Eriks, K. The crystal and molecular structure of 9-cyclohexyl-5(7)-(dimethyl sulfide)-nido-decaborane(11), B10H11C6H11S(CH3)2. Inorg. Chem. 1980, 19, 1188–1191. [Google Scholar] [CrossRef]
- Kusari, U.; Li, Y.; Bradley, M.G.; Sneddon, L.G. Polyborane reactions in ionic liquids: New efficient routes to functionalized decaborane and o-carborane clusters. J. Am. Chem. Soc. 2004, 126, 8662–8663. [Google Scholar] [CrossRef] [PubMed]
- Kusari, U.; Carroll, P.J.; Sneddon, L.G. Ionic-liquid-promoted decaborane olefin-hydroboration: A new efficient route to 6-R-B10H13 derivatives. Inorg. Chem. 2008, 47, 9203–9215. [Google Scholar] [CrossRef]
- Yu, X.-H.; Cao, K.; Huang, Y.; Yang, J.; Li, J.; Chang, G. Platinum catalyzed sequential hydroboration of decaborane: A facile approach to poly(alkenyldecaborane) with decaborane in the mainchain. Chem. Commun. 2014, 50, 4585–4587. [Google Scholar] [CrossRef]
- Boggio, P.; Toppino, A.; Geninatti Crich, S.; Alberti, D.; Marabello, D.; Medana, C.; Prandi, C.; Venturello, P.; Aime, S.; Deagostino, A. The hydroboration reaction as a key for a straightforward synthesis of new MRI-NCT agents. Org. Biomol. Chem. 2015, 13, 3288–3297. [Google Scholar] [CrossRef]
- Naoufal, D.; Laila, Z.; Yazbeck, O.; Hamad, H.; Ibrahim, G.; Aoun, R.; Safa, A.; El Jamal, M. Kanj Synthesis, characterization and mechanism of formation of 6-substituted nido-B10H13 decaboranes by the opening reaction of closo-decahydrodecaborate [B10H10]2- cage. Main Group Chem. 2013, 12, 39–48. [Google Scholar] [CrossRef]
- Pender, M.J.; Wideman, T.; Carroll, P.J.; Sneddon, L.G. Transition metal promoted reactions of boron hydrides. 15. Titanium-catalyzed decaborane−olefin hydroborations: One-step, high-yield syntheses of monoalkyldecaboranes. J. Am. Chem. Soc. 1998, 120, 9108–9109. [Google Scholar] [CrossRef]
- Pender, M.J.; Carroll, P.J.; Sneddon, L.G. Transition-metal-promoted reactions of boron hydrides. 17. Titanium-catalyzed decaborane−olefin hydroborations. J. Am. Chem. Soc. 2001, 123, 12222–12231. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Carroll, P.J.; Sneddon, L.G. New routes to organodecaborane polymers via ruthenium-catalyzed ring-opening metathesis polymerization. Organometallics 2004, 23, 163–165. [Google Scholar] [CrossRef]
- Wei, X.; Carroll, P.J.; Sneddon, L.G. Ruthenium-catalyzed ring-opening polymerization syntheses of poly(organodecaboranes): New single-source boron-carbide precursors. Chem. Mater. 2006, 18, 1113–1123. [Google Scholar] [CrossRef]
- Pender, M.J.; Forsthoefel, K.M.; Sneddon, L.G. Molecular and polymeric precursors to boron carbide nanofibers, nanocylinders, and nanoporous ceramics. Pure Appl. Chem. 2003, 75, 1287–1294. [Google Scholar] [CrossRef]
- Pender, M.J.; Sneddon, L.G. An efficient template synthesis of aligned boron carbide nanofibers using a single-source molecular precursor. Chem. Mater. 2000, 12, 280–283. [Google Scholar] [CrossRef]
- Li, J.; Yang, H.; Liang, Y.; Chen, J.; Xie, M.; Zhou, L.; Xiong, Q.; Li, W. Ruthenium-catalyzed cascade ring-opening polymerization/hydroboration for the synthesis of low cross-linking poly(6-norbornenyldecaborane) and its thermal property. Adv. Mater. Res. 2014, 1035, 288–291. [Google Scholar]
- Zhang, X.; Li, J.; Cao, K.; Yi, Y.; Yang, J.; Li, B. Synthesis and characterization of B-C polymer hollow microspheres from a new organodecaborane preceramic polymer. RSC Adv. 2015, 5, 86214–86218. [Google Scholar] [CrossRef]
- Wang, J.; Gou, Y.; Jian, K.; Huang, J.; Wang, H. Boron carbide ceramic hollow microspheres prepared from poly(6-CH2=CH(CH2)4-B10H13) precursor. Mater. Design 2016, 109, 408–414. [Google Scholar] [CrossRef]
- Wang, J.; Gou, Y.; Zhang, Q.; Jian, K.; Chen, Z.; Wang, H. Linear organodecaborane block copolymer as a single-source precursor for porous boron carbide ceramics. J. Eur. Ceram. Soc. 2017, 37, 1937–1943. [Google Scholar] [CrossRef]
- Li, J.; Cao, K.; Li, J.; Liu, M.; Zhang, S.; Yang, J.; Zhang, Z.; Li, B. Synthesis and ceramic conversion of a new organodecaborane preceramic polymer with high-ceramic-yield. Molecules 2018, 23, 2461. [Google Scholar] [CrossRef]
- Mazighi, K.; Carroll, P.J.; Sneddon, L.G. Transition metal promoted reactions of boron hydrides. 13. Platinum catalyzed synthesis of 6,9-dialkyldecaboranes. Inorg. Chem. 1993, 32, 1963–1969. [Google Scholar] [CrossRef]
- Chatterjee, S.; Carroll, P.J.; Sneddon, L.G. Metal-catalyzed decaborane-alkyne hydroboration reactions: Efficient routes to alkenyldecaboranes. Inorg. Chem. 2010, 49, 3095–3097. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Carroll, P.J.; Sneddon, L.G. Iridium and ruthenium catalyzed syntheses, hydroborations, and metathesis reactions of alkenyl-decaboranes. Inorg. Chem. 2013, 52, 9119–9130. [Google Scholar] [CrossRef] [PubMed]
- Ernest, R.L.; Quintana, W.; Rosen, R.; Carroll, P.J.; Sneddon, L.G. Reactions of decaborane(14) with silylated acetylenes. Synthesis of the new monocarbon carborane 9-Me2S-7-[(Me3Si)2CH]CB10H11. Organometallics 1987, 6, 80–88. [Google Scholar] [CrossRef]
- Burgos-Adorno, G.; Carroll, P.J.; Quintana, W. Synthesis and characterization of a new alkenyldecaborane and alkenyl monocarbon carboranes. Inorg. Chem. 1996, 35, 2568–2575. [Google Scholar] [CrossRef]
- Meyer, F.; Paetzold, P.; Englert, U. Reaktion von Decaboran mit dem Phosphaalkin PCtBu. Chem. Ber. 1994, 127, 93–95. [Google Scholar] [CrossRef]
- Bould, J.; Londesborough, M.G.S.; Ormsby, D.L.; MacBride, J.A.H.; Wade, K.; Kilner, C.A.; Clegg, W.; Teat, S.J.; Thornton-Pett, M.; Greatrex, R.; et al. Macropolyhedral boron-containing cluster chemistry: Models for intermediates en route to globular and discoidal megaloborane assemblies. Structures of [nido-B10H12(nido-B5H8)2] and [(CH2CH2C5H4N)-arachno-B10H10(NC5H4-closo-C2B10H10)] as determined by synchrotron X-ray diffraction analysis. J. Organomet. Chem. 2002, 657, 256–261. [Google Scholar] [CrossRef]
- Kim, S.; Treacy, J.W.; Nelson, Y.A.; Gonzalez, J.A.M.; Gembicky, M.; Houk, K.N.; Spokoyny, A.M. Arene C–H borylation strategy enabled by a non-classical boron cluster-based electrophile. Nat. Commun. 2023, 14, 1671. [Google Scholar] [CrossRef]
- Demel, J.; Kloda, M.; Lang, K.; Škoch, K.; Hynek, J.; Opravil, A.; Novotný, M.; Bould, J.; Ehn, M.; Londesborough, M.G.S. Direct Phenylation of nido-B10H14. J. Org. Chem. 2022, 87, 10034–10043. [Google Scholar] [CrossRef]
- Bůžek, D.; Škoch, K.; Ondrušová, S.; Kloda, M.; Bavol, D.; Mahun, A.; Kobera, L.; Lang, K.; Londesborough, M.G.S.; Demel, J. “Activated Borane”—A porous borane cluster network as an effective adsorbent for removing organic pollutants. Chem. Eur. J. 2022, 28, e202201885. [Google Scholar] [CrossRef]
- Wille, A.E.; Su, K.; Carroll, P.J.; Sneddon, L.G. New synthetic routes to azacarborane clusters: Nitrile insertion reactions of nido-5,6-C2B8H11- and nido-B10H13−. J. Am. Chem. Soc. 1996, 118, 6407–6421. [Google Scholar] [CrossRef]
- Baše, K.; Alcock, N.W.; Howarth, O.W.; Powell, H.R.; Harrison, A.T.; Wallbridge, M.G.H. The structure of the arachno-[B10H13C≡N]2− anion: An example of endo substitution in the decaborane(l4) framework. J. Chem. Soc. Chem. Commun. 1988, 341–342. [Google Scholar] [CrossRef]
- Orlova, A.M.; Sivaev, I.B.; Lagun, V.L.; Katser, S.B.; Solntsev, K.A.; Kuznetsov, N.T. Synthesis and structure of Pb(Bipy)2(6-B10H13CN). Koord. Khim. 1996, 22, 119–124. [Google Scholar]
- Sivaev, I.B. Chemistry of 11-vertex polyhedral boron hydrides (Review). Russ. J. Inorg. Chem. 2019, 64, 955–976. [Google Scholar] [CrossRef]
- Geisberger, G.; Linti, G.; Nöth, H. Beiträge zur Chemie des Bors. 213. Reaktionen eines Amino-imino-borans mit Triboran(7) und Decaboran(14). Chem. Ber. 1992, 125, 2691–2699. [Google Scholar] [CrossRef]
- Bridges, A.N.; Liu, J.; Kultyshev, R.G.; Gaines, D.F.; Shore, S.G. Partial insertion of the 9-BBN unit into the nido-B10 framework: Preparation and structural characterization of (9-BBN)B10H13 and [(9-BBN)B10H12]−. Inorg. Chem. 1998, 37, 3276–3283. [Google Scholar] [CrossRef]
- Greenwood, N.N.; Kennedy, J.D.; Taylorson, D. Mass spectroscopic evidence for icosaborane(26). J. Phys. Chem. 1978, 82, 623–625. [Google Scholar] [CrossRef]
- Greenwood, N.N.; Kennedy, J.D.; Spalding, T.R.; Taylorson, D. Isomers of icosaborane(26): Some synthetic routes and preliminary characterisations in the bis(nido-decaboranyl) system. J. Chem. Soc. Dalton Trans. 1979, 840–846. [Google Scholar] [CrossRef]
- Boocock, S.K.; Cheek, Y.M.; Greenwood, N.N.; Kennedy, J.D. A new route to isomers of icosaborane(26), B20H26. The use of 115.5-MHz 11B and 11B-{1H} nuclear magnetic resonance spectroscopy for the comparison and characterisation of separated isomers and the identification of three further icosaboranes as 1,2’-, 2,5’-, and 5,5’(or 5,7’)-(B10H13)2. J. Chem. Soc. Dalton Trans. 1981, 1430–1437. [Google Scholar] [CrossRef]
- Brown, G.M.; Pinson, J.W.; Ingram, L.L. Crystal and molecular structure of 1,5’-bidecaboran(14)yl: A new borane from γ irradiation of decaborane(14). Inorg. Chem. 1979, 18, 1951–1956. [Google Scholar] [CrossRef]
- Bould, J.; Clegg, W.; Kennedy, J.D.; Teat, S.J. Isomeric icosaboranes B20H26: The synchrotron structure of 1,1’-bis(nido-decaboranyl). Acta Cryst. C 2001, 57, 779–780. [Google Scholar] [CrossRef]
- Barrett, S.A.; Greenwood, N.N.; Kennedy, J.D.; Thornton-Pett, M. The chemistry of isomers of icosaborane(26): Crystal and molecular structure of 1,2’-bi(nido-decaboranyl). Polyhedron 1985, 4, 1981–1984. [Google Scholar] [CrossRef]
- Greenwood, N.N.; Kennedy, J.D.; McDonald, W.S.; Staves, J.; Taylorson, D. Isomers of B20H26: Structural characterisation by X-ray diffraction of 2,2’-bi(nido-decaboranyl). J. Chem. Soc. Chem. Commun. 1979, 17–18. [Google Scholar] [CrossRef]
- Boocock, S.K.; Greenwood, N.N.; Kennedy, J.D.; McDonald, W.S.; Staves, J. The chemistry of isomeric icosaboranes, B20H26. Molecular structures and physical characterization of 2,2’-bi(nido-decaboranyl) and 2,6’-bi(nido-decaboranyl). J. Chem. Soc. Dalton Trans. 1980, 790–796. [Google Scholar] [CrossRef]
- Boocock, S.K.; Greenwood, N.N.; Kennedy, J.D.; Taylorson, D. Isomers of B20H26: Elucidation of the structure of 6,6’-bi(nido-decaboranyl) by 11B-{1H} and 1H-{11B} n.m.r. spectroscopy. J. Chem. Soc. Chem. Commun. 1979, 16–17. [Google Scholar] [CrossRef]
- Bould, J.; Dörfler, U.; Clegg, W.; Teat, S.J.; Thornton-Pett, M.; Kennedy, J.D. Triple linking of the decaboranyl cluster. Structure of [(SMe2)2B10H10(B10H13)2] as determined by synchrotron X-ray diffraction analysis. Chem. Commun. 2001, 1788–1789. [Google Scholar] [CrossRef]
- Olsen, F.P.; Vasavada, R.C.; Hawthorne, M.F. The chemistry of n-B18H22 and i-B18H22. J. Am. Chem. Soc. 1968, 90, 3946–3951. [Google Scholar] [CrossRef]
- Li, Y.; Sneddon, L.G. Improved synthetic route to n-B18H22. Inorg. Chem. 2006, 45, 470–471. [Google Scholar] [CrossRef]
- Londesborough, M.G.S.; Hnyk, D.; Bould, J.; Bould, J.; Serrano-Andres, L.; Sauri, V.; Oliva, J.M.; Kubat, P.; Polivka, T.; Lang, K. Distinct photophysics of the isomers of B18H22 explained. Inorg. Chem. 2012, 51, 1471–1479. [Google Scholar] [CrossRef]
- Londesborough, M.G.S.; Dolanský, J.; Braborec, J.; Cerdán, L. Interaction of anti-B18H22 with light. In Handbook of Boron Science with Applications in Organometallics, Catalysis, Materials and Medicine; Hosmane, N.S., Eagling, R., Eds.; World Scientific Publishing: London, UK, 2019; Volume 3, pp. 115–136. [Google Scholar] [CrossRef]
- Cerdan, L.; Frances-Monerris, A.; Roca-Sanjuan, D.; Bould, J.; Dolandky, J.; Fuciman, M.; Londesborough, M.G.S. Unveiling the role of upper excited electronic states in the photochemistry and laser performance of anti-B18H22. J. Mater. Chem. C 2020, 8, 12806–12818. [Google Scholar] [CrossRef]
- Tan, C.; Zhang, B.; Chen, J.; Zhang, L.; Huang, X.; Meng, H. Study of hydrolysis kinetic of new laser material [anti-B18H22]. Russ. J. Inorg. Chem. 2019, 64, 1359–1364. [Google Scholar] [CrossRef]
- Ševčik, J.; Urbanek, P.; Hanulikova, B.; Čapkova, T.; Urbanek, M.; Antoš, J.; Londesborough, M.G.S.; Bould, J.; Ghasemi, B.; Petřkovsky, L.; et al. The photostability of novel boron hydride blue emitters in solution and polystyrene matrix. Materials 2021, 14, 589. [Google Scholar] [CrossRef] [PubMed]
- Capkova, T.; Hanulikova, B.; Sevcik, J.; Urbanek, P.; Antos, J.; Urbanek, M.; Kuritka, I. Incorporation of the new anti-octadecaborane laser dyes into thin polymer films: A temperature-dependent photoluminescence and infrared spectroscopy study. Int. J. Mol. Sci. 2022, 23, 8832. [Google Scholar] [CrossRef] [PubMed]
- Anderson, K.P.; Rheingold, A.L.; Djurovich, P.I.; Soman, O.; Spokoyny, A.M. Synthesis and luminescence of monohalogenated B18H22 clusters. Polyhedron 2022, 227, 116099. [Google Scholar] [CrossRef]
- Ehn, M.; Bavol, D.; Bould, J.; Strnad, V.; Litecká, M.; Lang, K.; Kirakci, K.; Clegg, W.; Waddell, P.G.; Londesborough, M.G.S. A window into the workings of anti-B18H22 luminescence—Blue-fluorescent isomeric pair 3,3’-Cl2-B18H20 and 3,4’-Cl2-B18H20 (and others). Molecules 2023, 28, 4505. [Google Scholar] [CrossRef]
- Anderson, K.P.; Waddington, M.A.; Balaich, G.J.; Stauber, J.M.; Bernier, N.A.; Caram, J.R.; Djurovich, P.I.; Spokoyny, A.M. A molecular boron cluster-based chromophore with dual emission. Dalton Trans. 2020, 49, 16245–16251. [Google Scholar] [CrossRef] [PubMed]
- Anderson, K.P.; Hua, A.S.; Plumley, J.B.; Ready, A.D.; Rheingold, A.L.; Peng, T.L.; Djurovich, I.; Kerestes, C.; Snyder, N.A.; Andrews, A.; et al. Benchmarking the dynamic luminescence properties and UV stability of B18H22-based materials. Dalton Trans. 2022, 51, 9223–9228. [Google Scholar] [CrossRef]
- Londesborough, M.G.S.; Dolansky, J.; Bould, J.; Braborec, J.; Kirakci, K.; Lang, K.; Cisařova, I.; Kubat, P.; Roca-Sanjuan, D.; Frances-Monerris, A.; et al. Effect of iodination on the photophysics of the laser borane anti-B18H22: Generation of efficient photosensitizers of oxygen. Inorg. Chem. 2019, 58, 10248–10259. [Google Scholar] [CrossRef]
- Anderson, K.P.; Djurovich, P.I.; Rubio, V.P.; Liang, A.; Spokoyny, A.M. Metal-catalyzed and metal-free nucleophilic substitution of 7-I-B18H21. Inorg. Chem. 2022, 61, 15051–15057. [Google Scholar] [CrossRef]
- Bould, J.; Lang, K.; Kirakci, K.; Cerdan, L.; Roca-Sanjuan, D.; Frances-Monerris, A.; Clegg, W.; Waddell, P.G.; Fuciman, M.; Polivka, T.; et al. A series of ultra-efficient blue borane fluorophores. Inorg. Chem. 2020, 59, 17058–17070. [Google Scholar] [CrossRef]
- Londesborough, M.G.S.; Lang, K.; Clegg, W.; Waddell, P.G.; Bould, J. Swollen polyhedral volume of the anti-B18H22 cluster via extensive methylation: Anti-B18H8Cl2Me12. Inorg. Chem. 2020, 59, 2651–2654. [Google Scholar] [CrossRef]
- Sauri, V.; Oliva, J.M.; Hnyk, D.; Bould, J.; Braborec, J.; Merchan, M.; Kubat, P.; Cisařova, I.; Lang, K.; Londesborough, M.G.S. Tuning the Photophysical Properties of anti-B18H22: Efficient intersystem crossing between excited ainglet and triplet states in new 4,4’-(HS)2-anti-B18H20. Inorg. Chem. 2013, 52, 9266–9274. [Google Scholar] [CrossRef]
- Londesborough, M.G.S.; Dolansky, J.; Cerdan, L.; Lang, K.; Jelinek, T.; Oliva, J.M.; Hnyk, D.; Roca-Sanjuan, D.; Frances-Monerris, A.; Martinčik, J.; et al. Thermochromic fluorescence from B18H20(NC5H5)2: An inorganic–organic composite luminescent compound with an unusual molecular geometry. Adv. Opt. Mater. 2017, 5, 1600694. [Google Scholar] [CrossRef]
- Londesborough, M.G.S.; Dolansky, J.; Jelinek, T.; Kennedy, J.D.; Cisařova, I.; Kennedy, R.D.; Roca-Sanjuan, D.; Frances-Monerris, A.; Lang, K.; Clegg, W. Substitution of the laser borane anti-B18H22 with pyridine: A structural and photophysical study of some unusually structured macropolyhedral boron hydrides. Dalton Trans. 2018, 47, 1709–1725. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Xiong, L.; Zhang, L.; Huang, X.; Meng, H.; Tan, C. Synthesis, aggregation-induced emission of a new anti-B18H22-isoquinoline hybrid. Chem. Phys. Lett. 2020, 747, 137328. [Google Scholar] [CrossRef]
- Xiong, L.; Zheng, Y.; Wang, H.; Yan, J.; Huang, X.; Meng, H.; Tan, C. A novel AIEE active anti-B18H22 derivative-based Cu2+ and Fe3+ fluorescence off-on-off sensor. Methods Appl. Fluoresc. 2022, 10, 035004. [Google Scholar] [CrossRef]
- Jelinek, T.; Kennedy, J.D.; Štibr, B.; Thornton-Pett, M. Macropolyhedral boron-containing cluster chemistry. A reductive trimerisation of MeNC to give an imidazole-based carbene stabilized by coordination to boron in an eighteen-vertex cluster compound. J. Chem. Soc. Chem. Commun. 1994, 1999–2000. [Google Scholar] [CrossRef]
- Jelinek, T.; Kilner, C.A.; Štibr, B.; Thornton-Pett, M.; Kennedy, J.D. Macropolyhedral borane reaction chemistry: Reductive oligomerisation of terBuNC by anti-B18H22 to give the boron-coordinated {(terBuNHCH){terBuNHC(CN)}CH2:} carbene residue. Inorg. Chem. Commun. 2005, 8, 491–494. [Google Scholar] [CrossRef]
- Patel, D.K.; Sooraj, B.S.; Kirakci, K.; Macháček, J.; Kučeráková, M.; Bould, J.; Dušek, M.; Frey, M.; Neumann, C.; Ghosh, S.; et al. Macropolyhedral syn-B18H22, the “forgotten” isomer. J. Am. Chem. Soc. 2023, 145, 17975–17986. [Google Scholar] [CrossRef] [PubMed]
- Ehn, M.; Litecká, M.; Londesborough, M.G.S. Unexpected minor products from the thermal auto-fusion of arachno-SB8H12: Luminescent 4-(HS)-syn-B18H21 and 3-(HS)-syn-B18H21. Inorg. Chem. Commun. 2023, 155, 111021. [Google Scholar] [CrossRef]
- Jelínek, T.; Grüner, B.; Císařová, I.; Štíbr, B.; Kennedy, J.D. Macropolyhedral boron-containing cluster chemistry: The reaction of syn-B18H22 with SMe2 and I2 in monoglyme: Structure of [7-(SMe2)-syn-B18H20]. Inorg. Chem. Commun. 2007, 10, 125–128. [Google Scholar] [CrossRef]
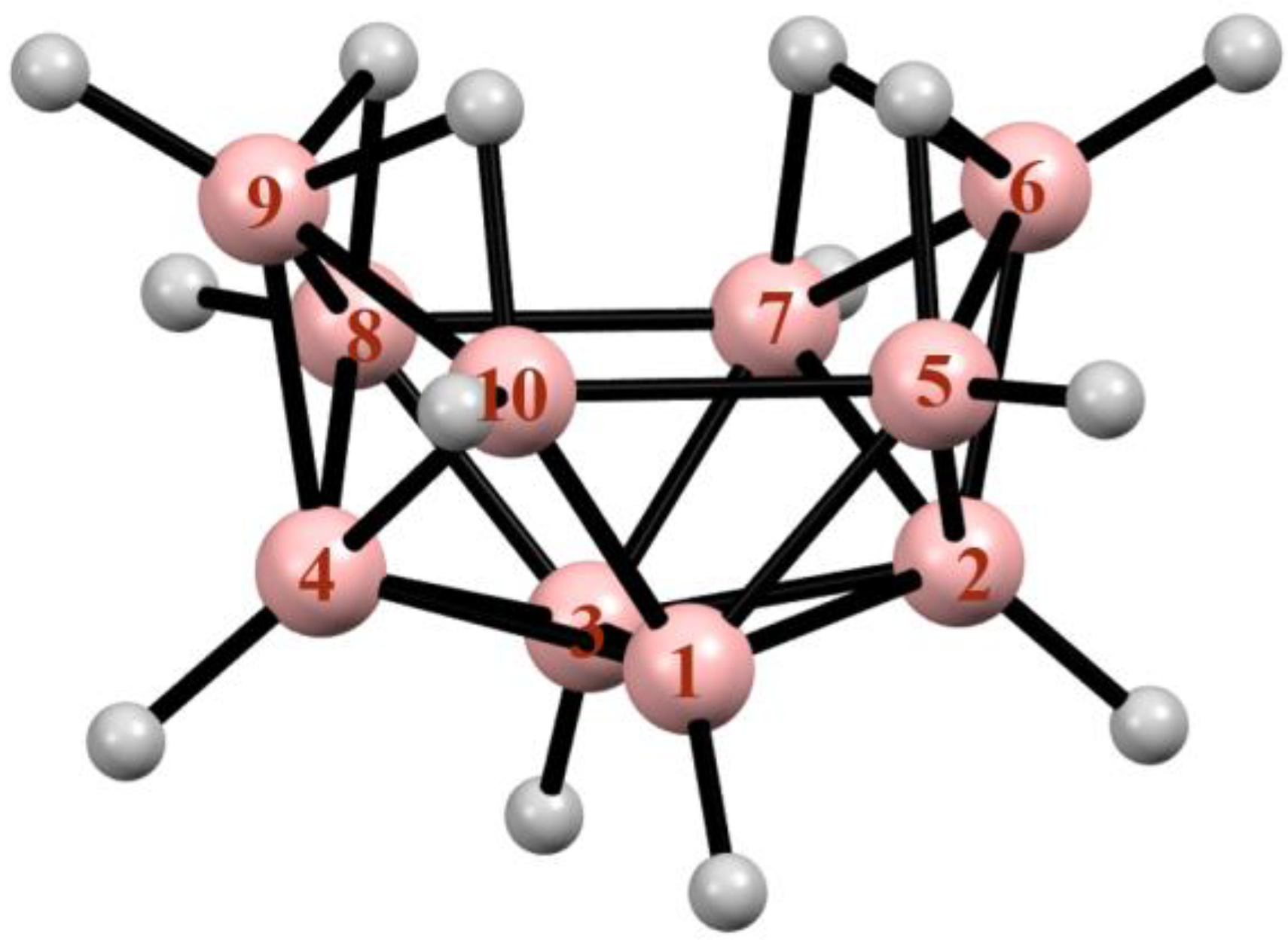

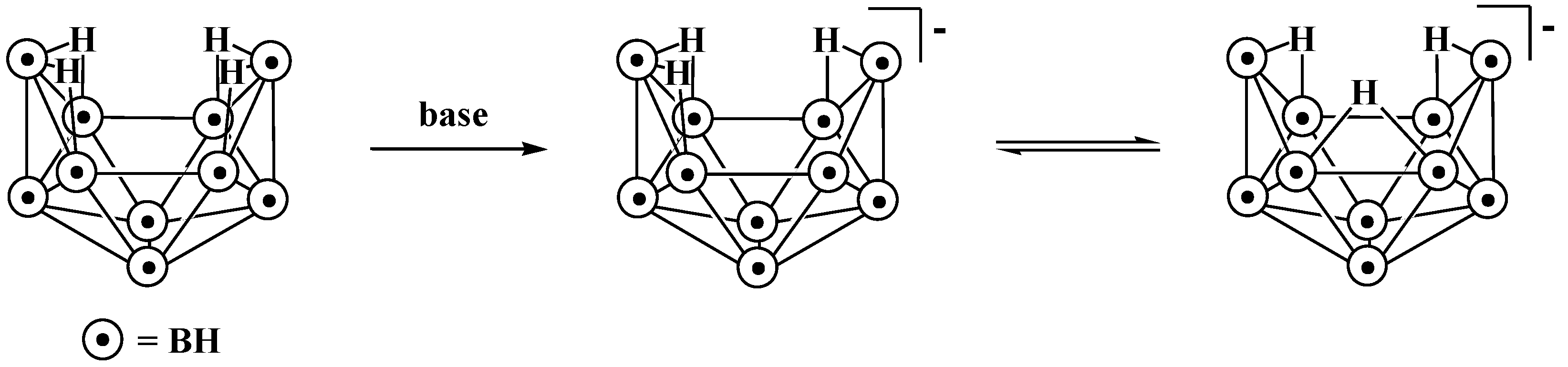
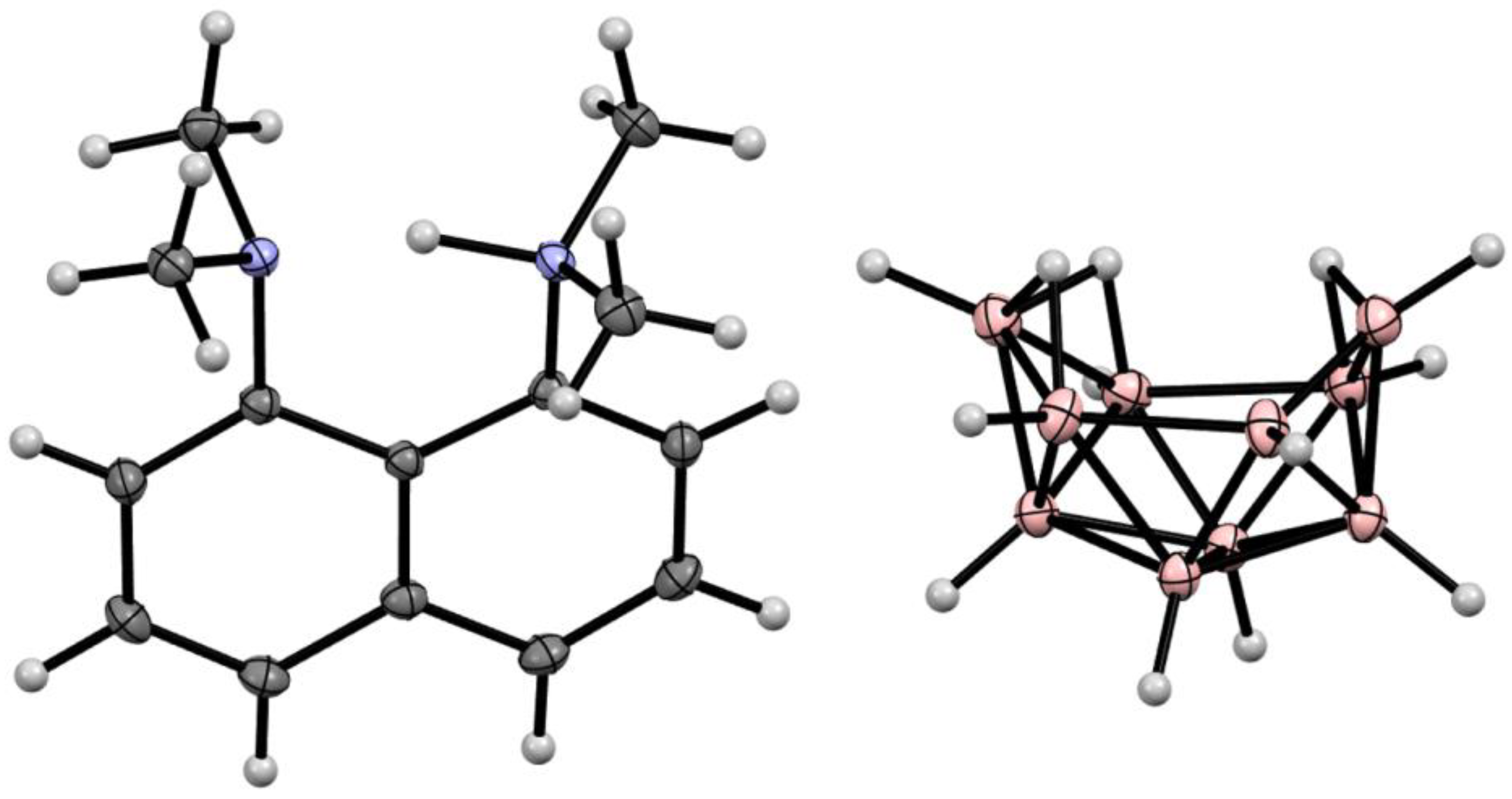
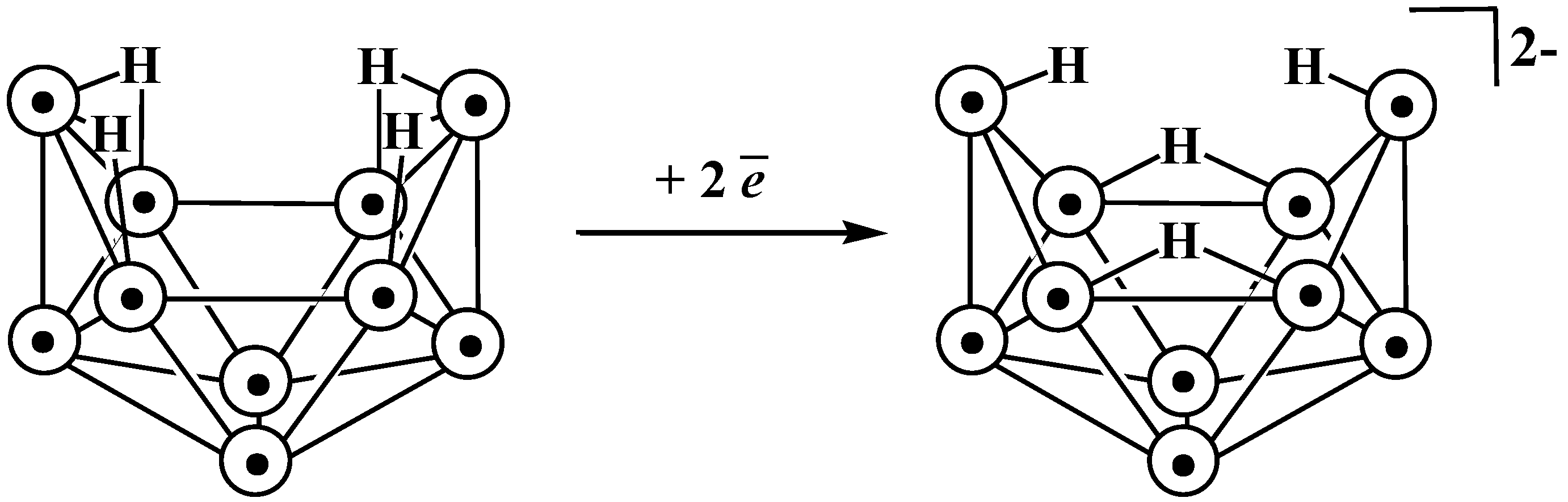
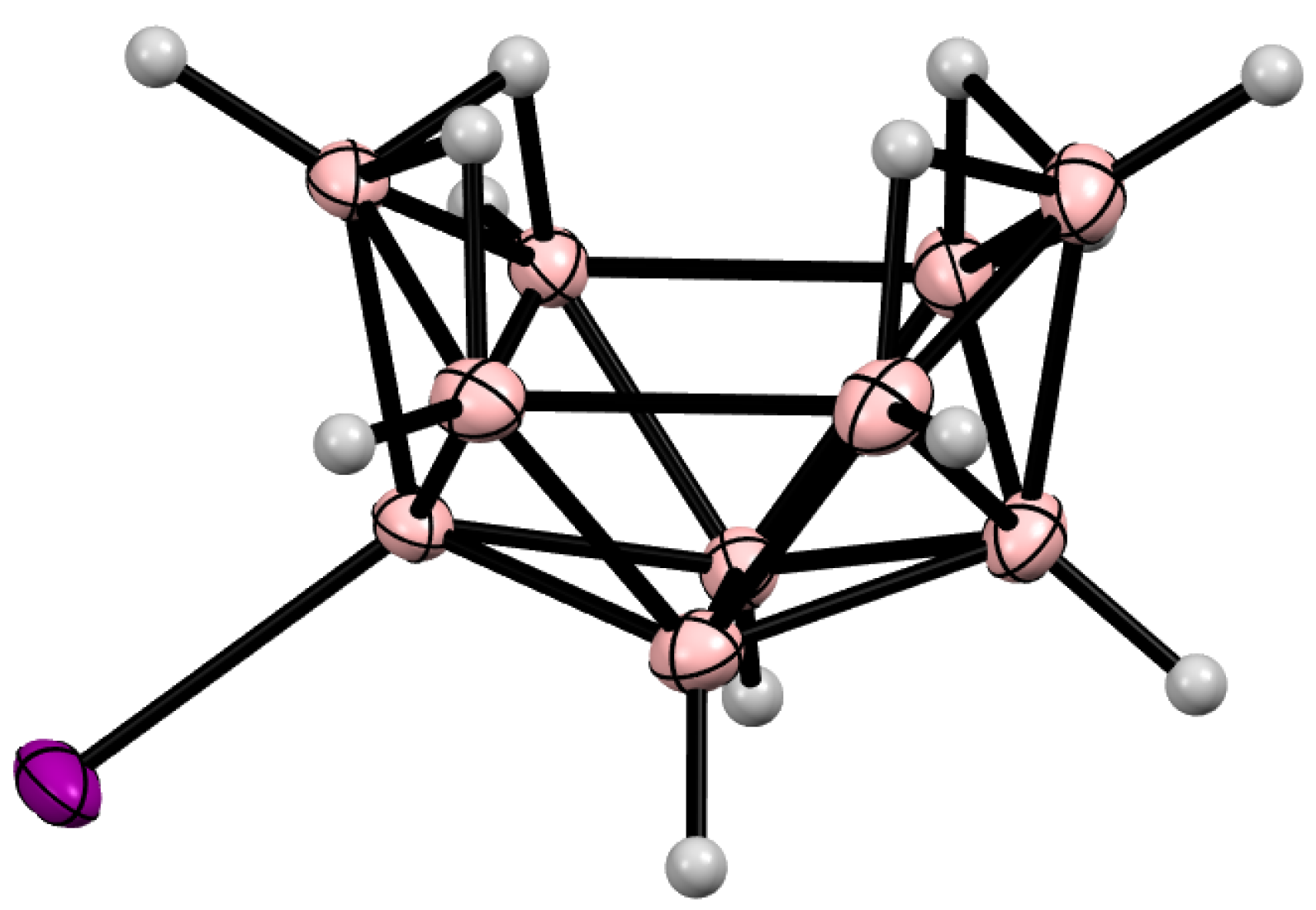
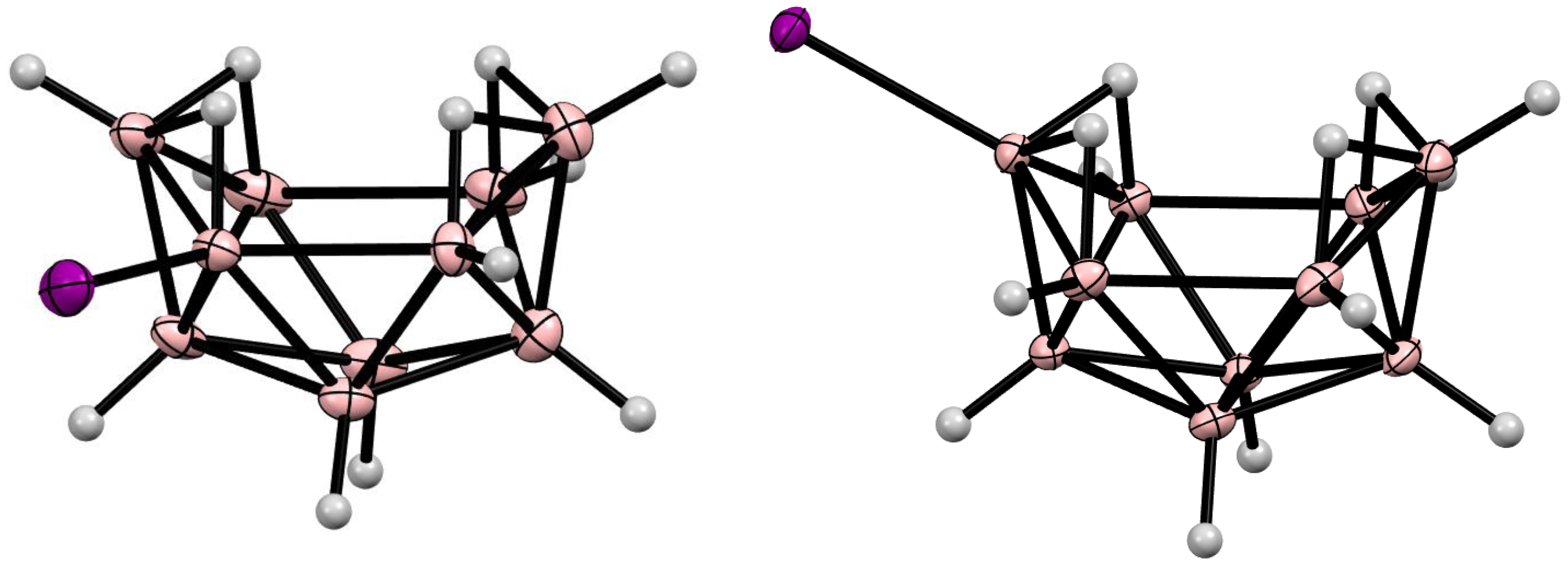
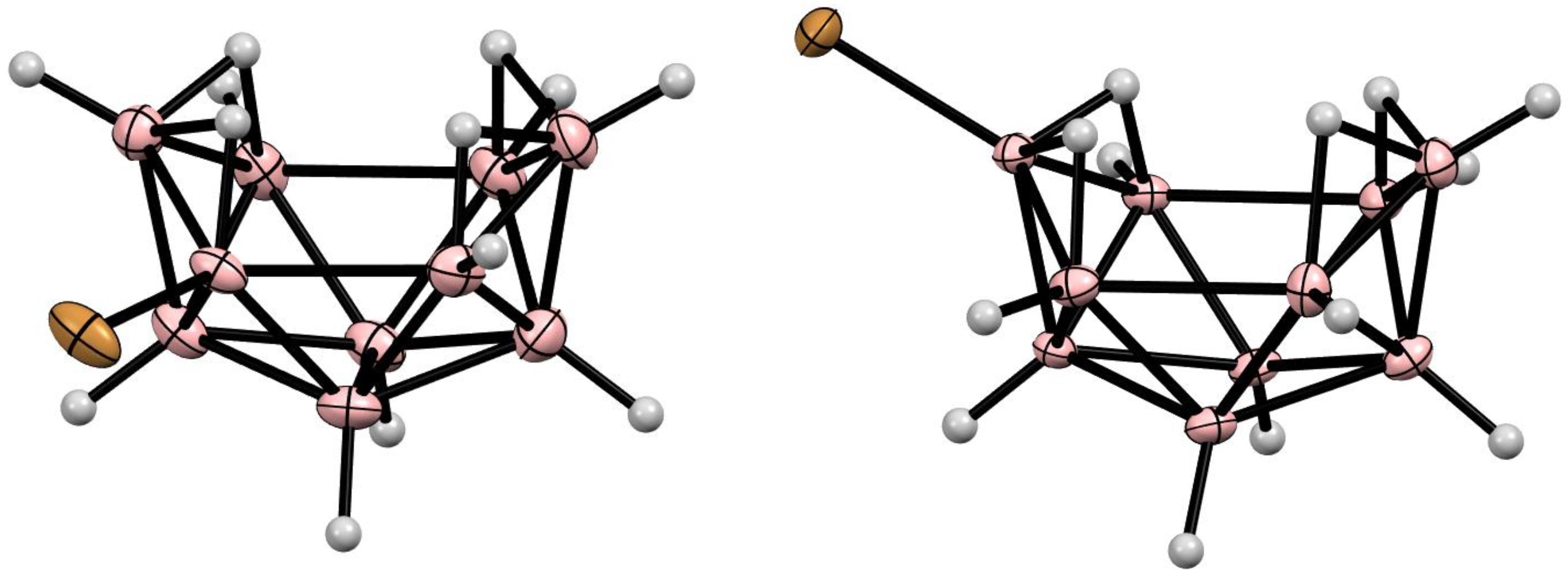
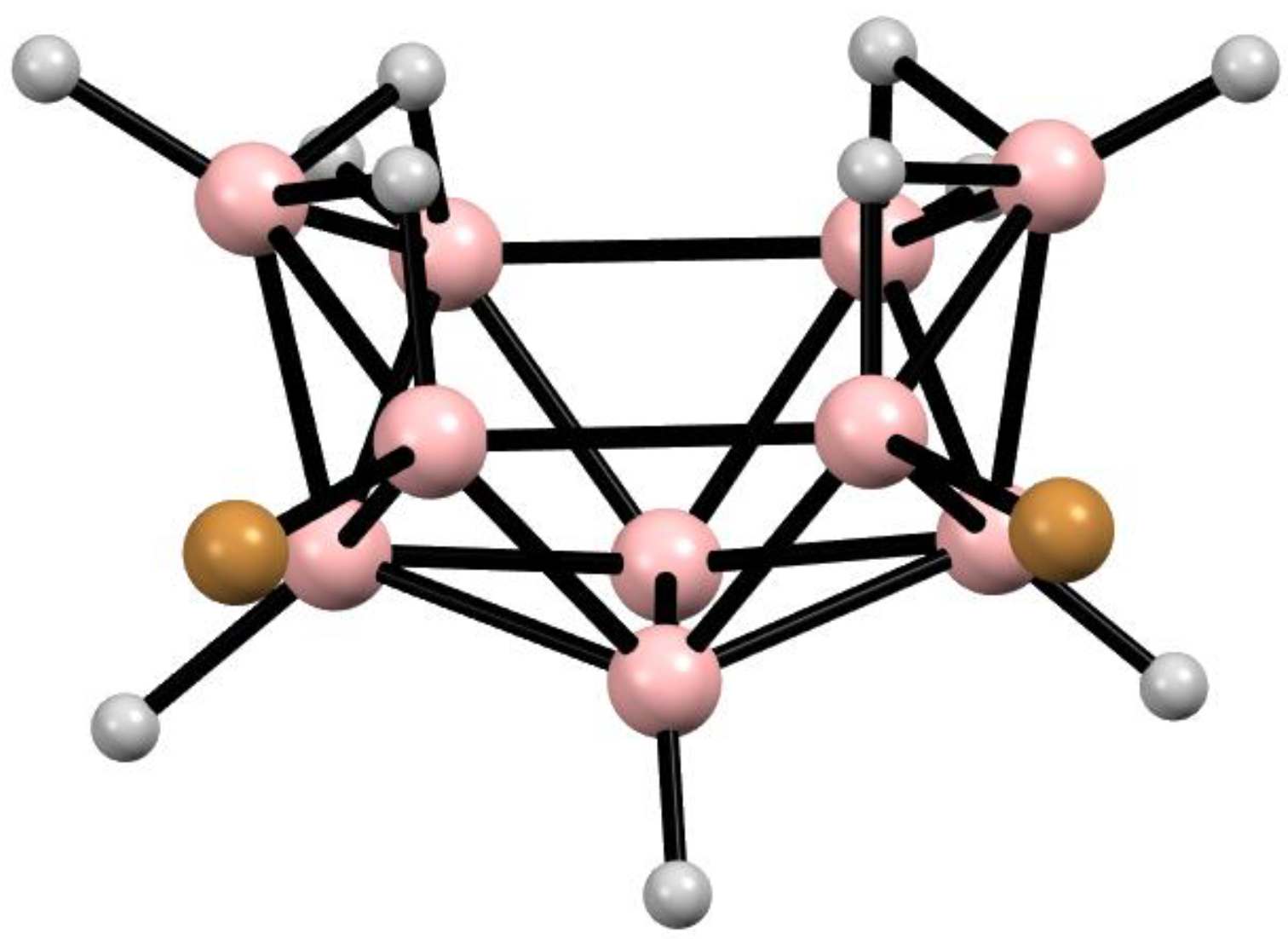

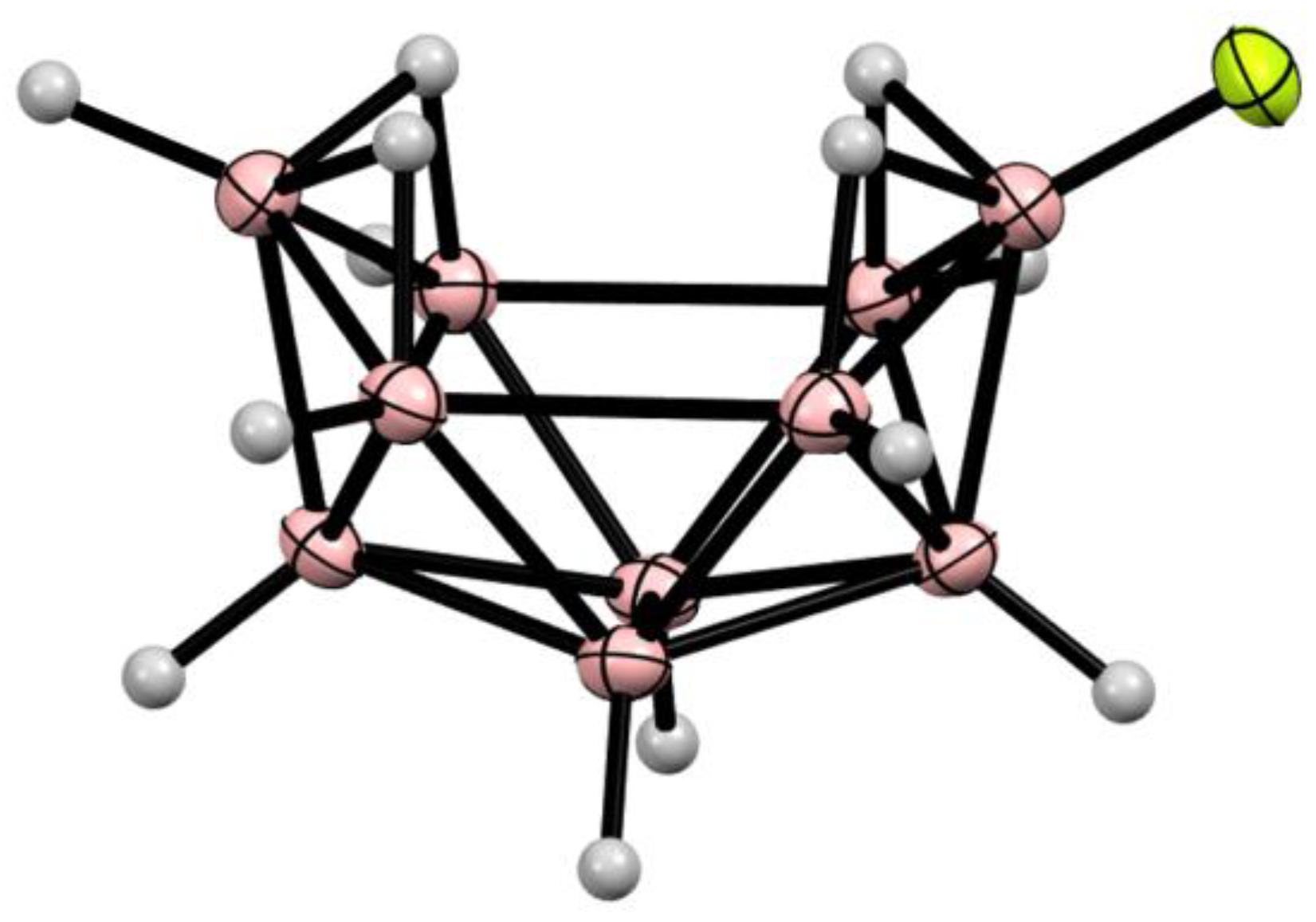

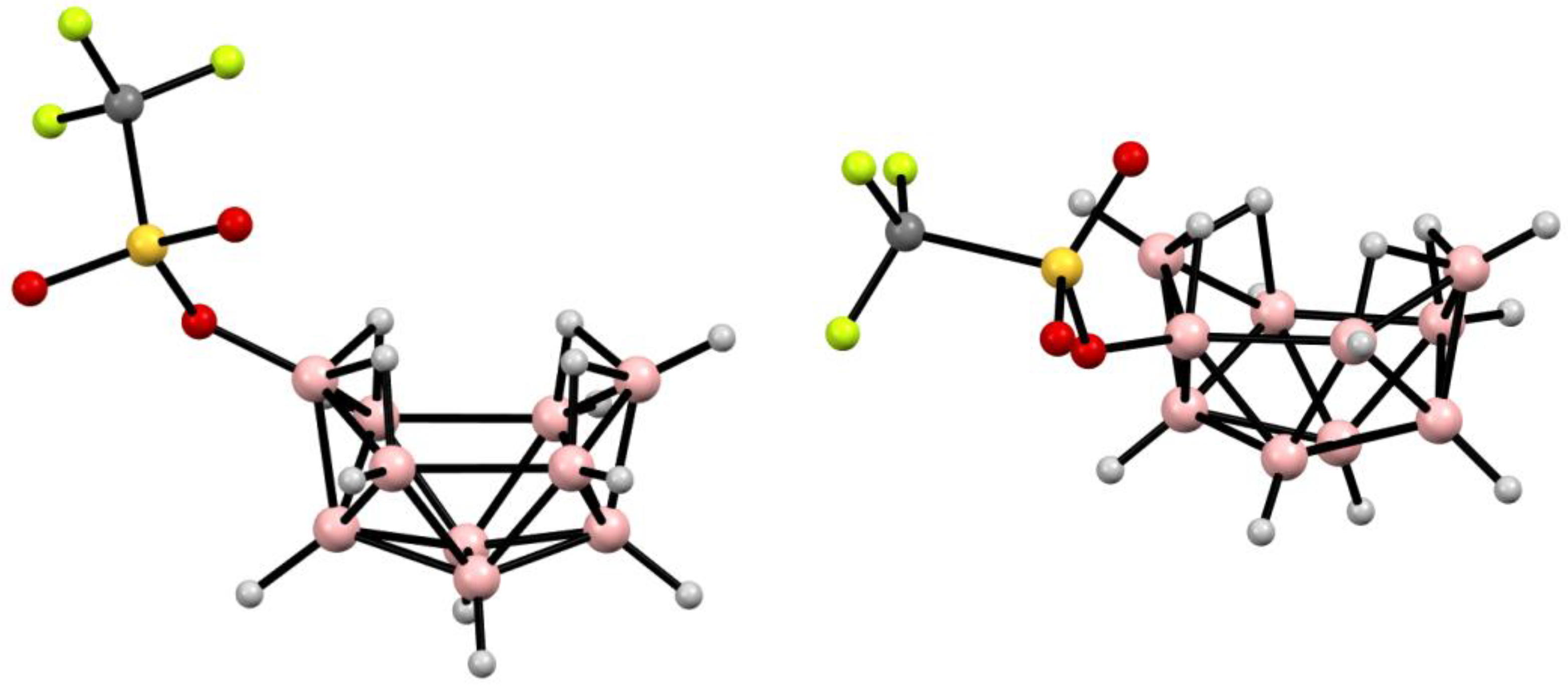
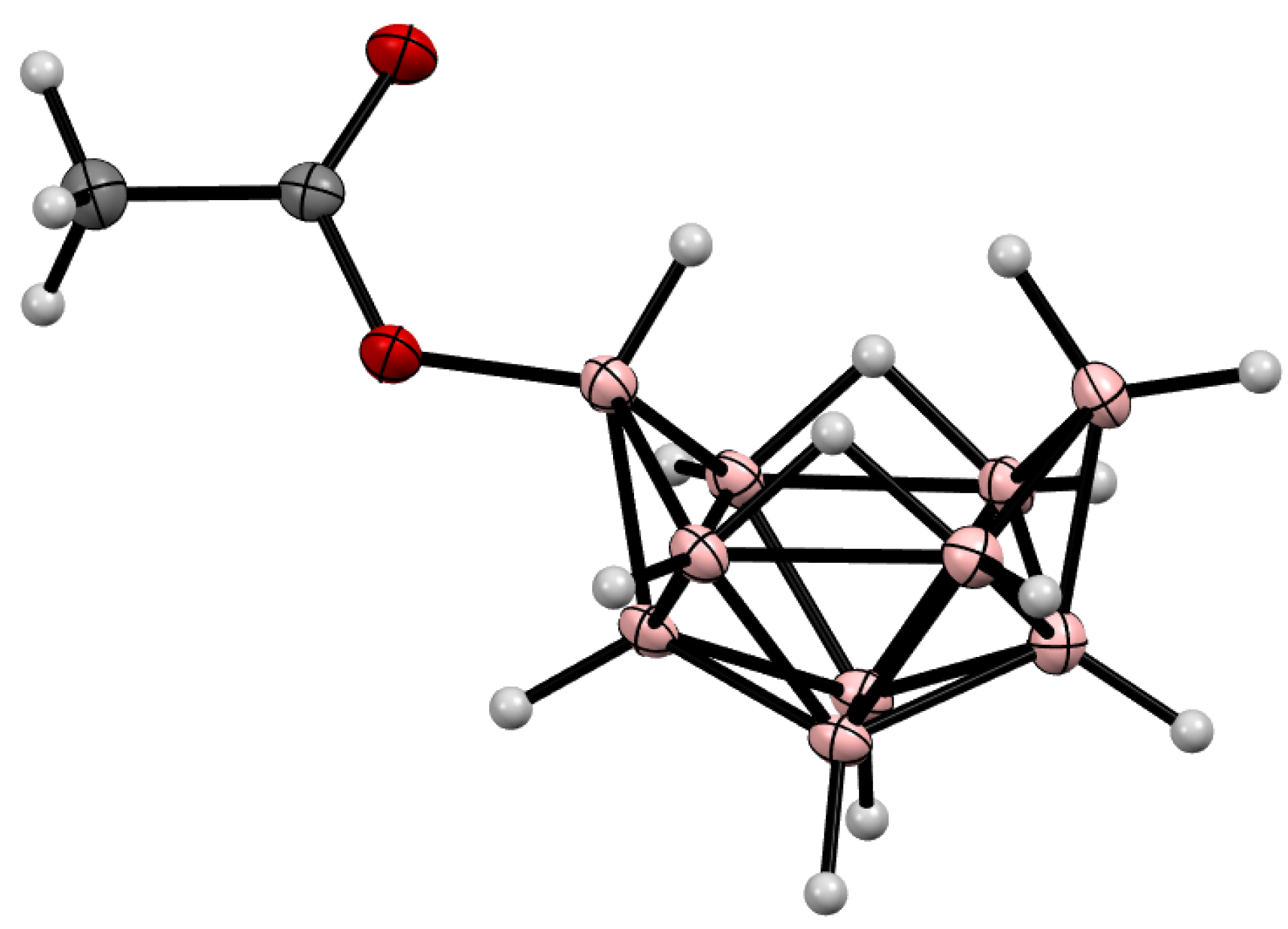
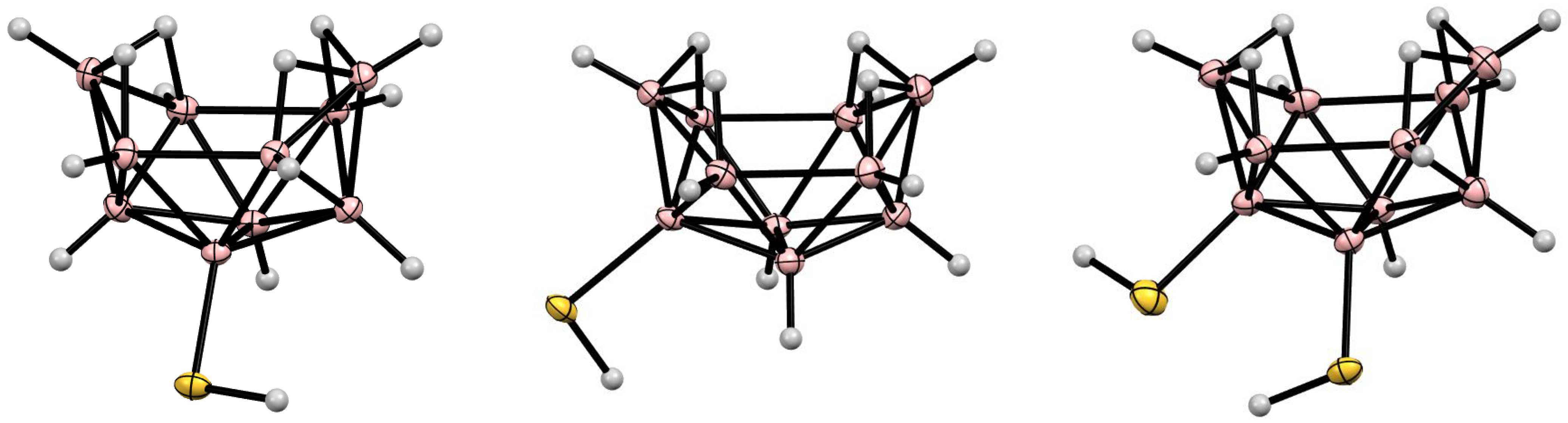

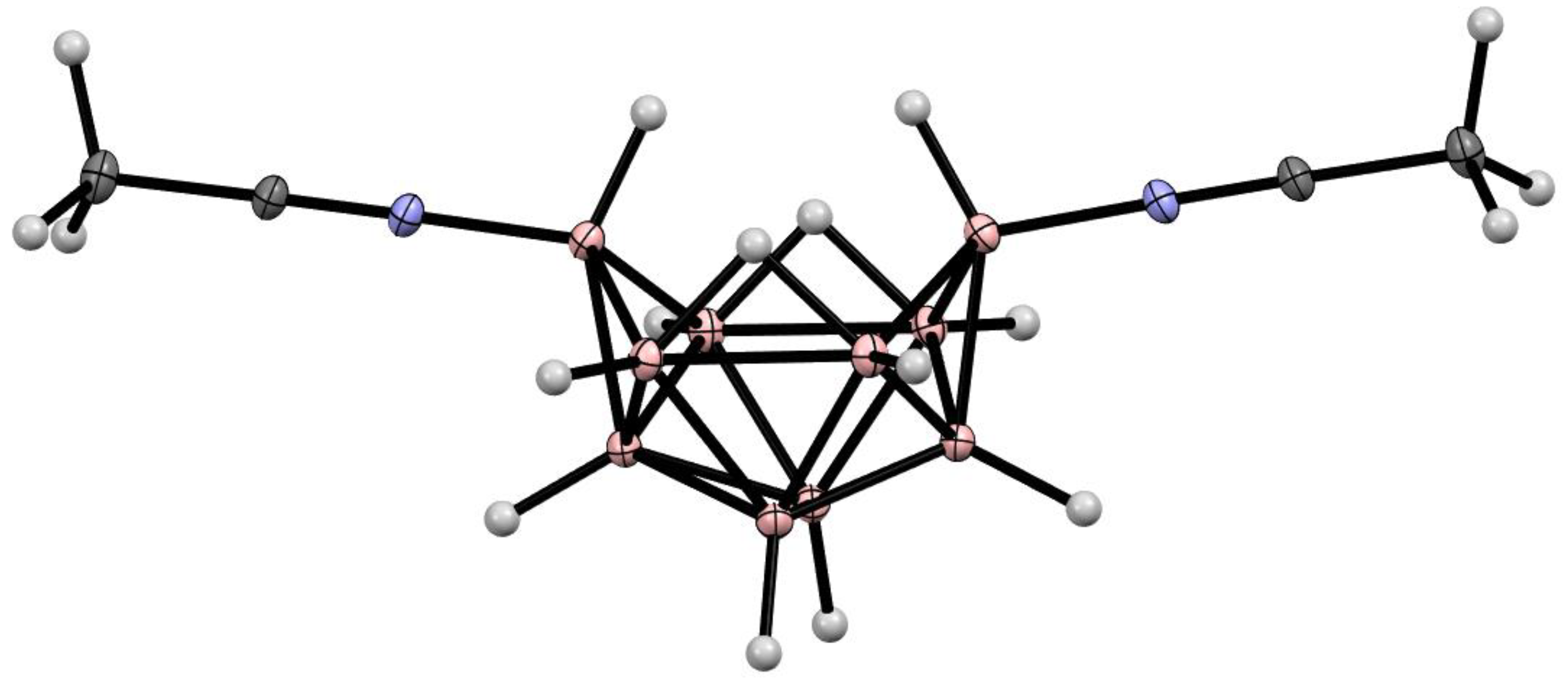


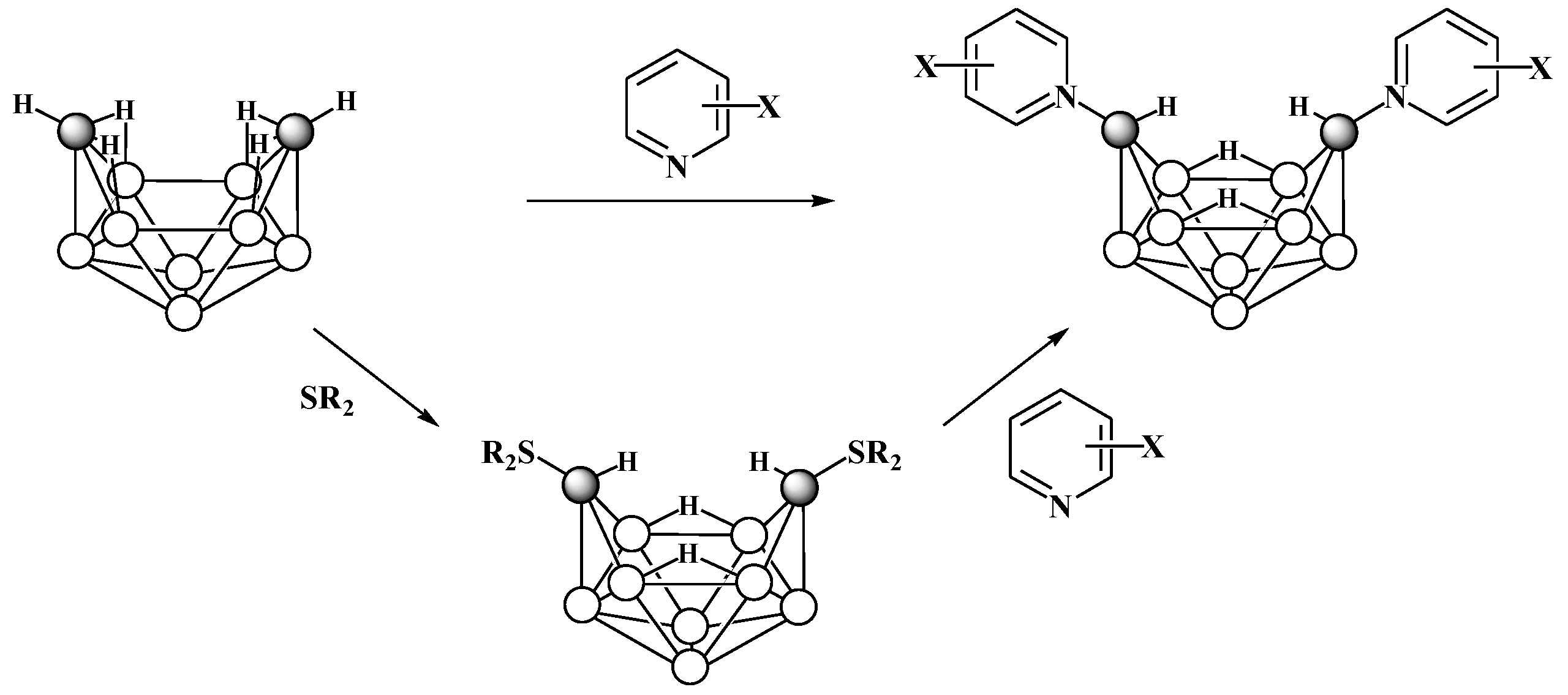
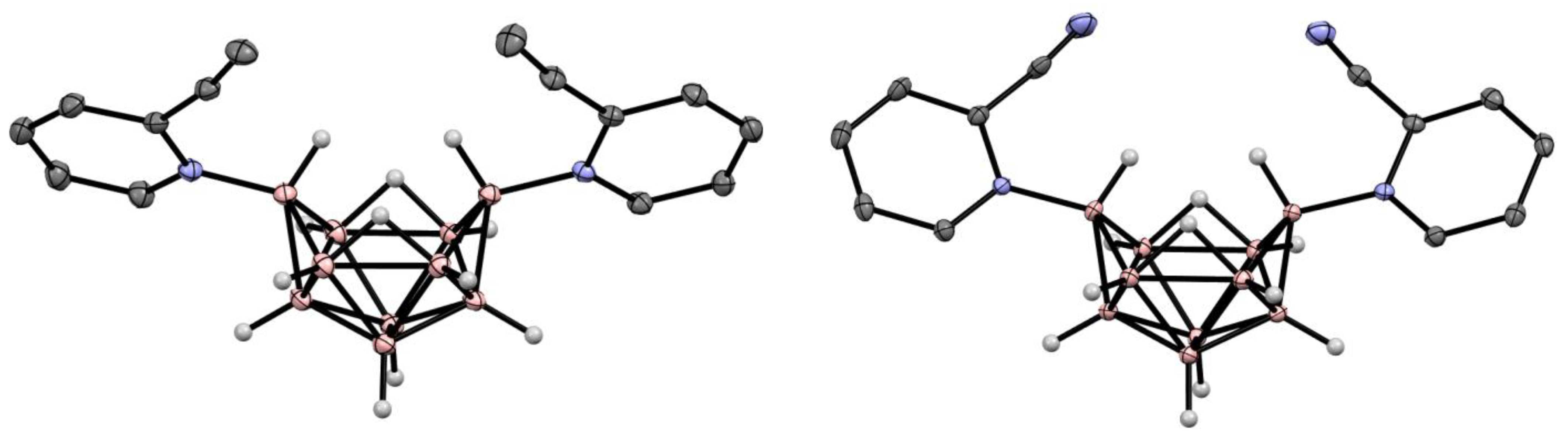
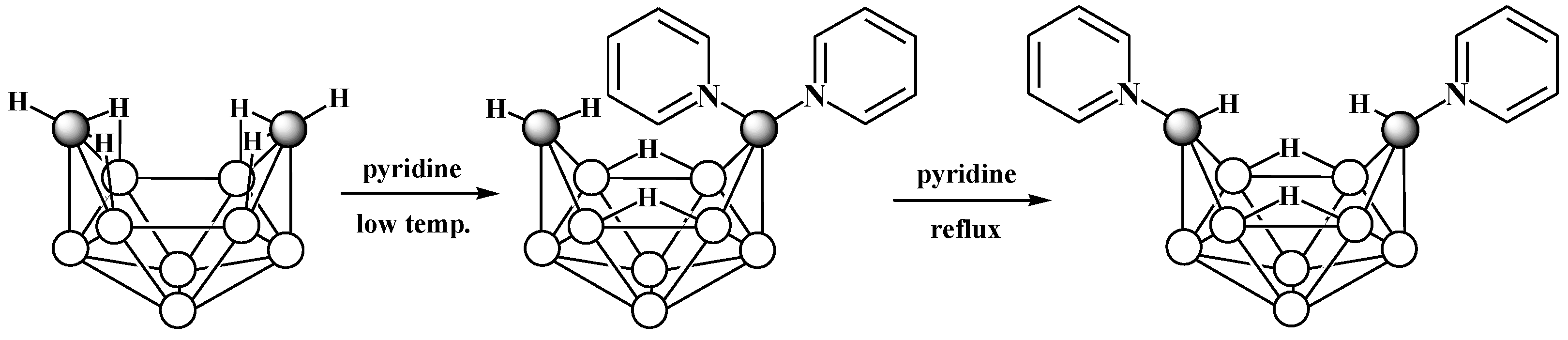

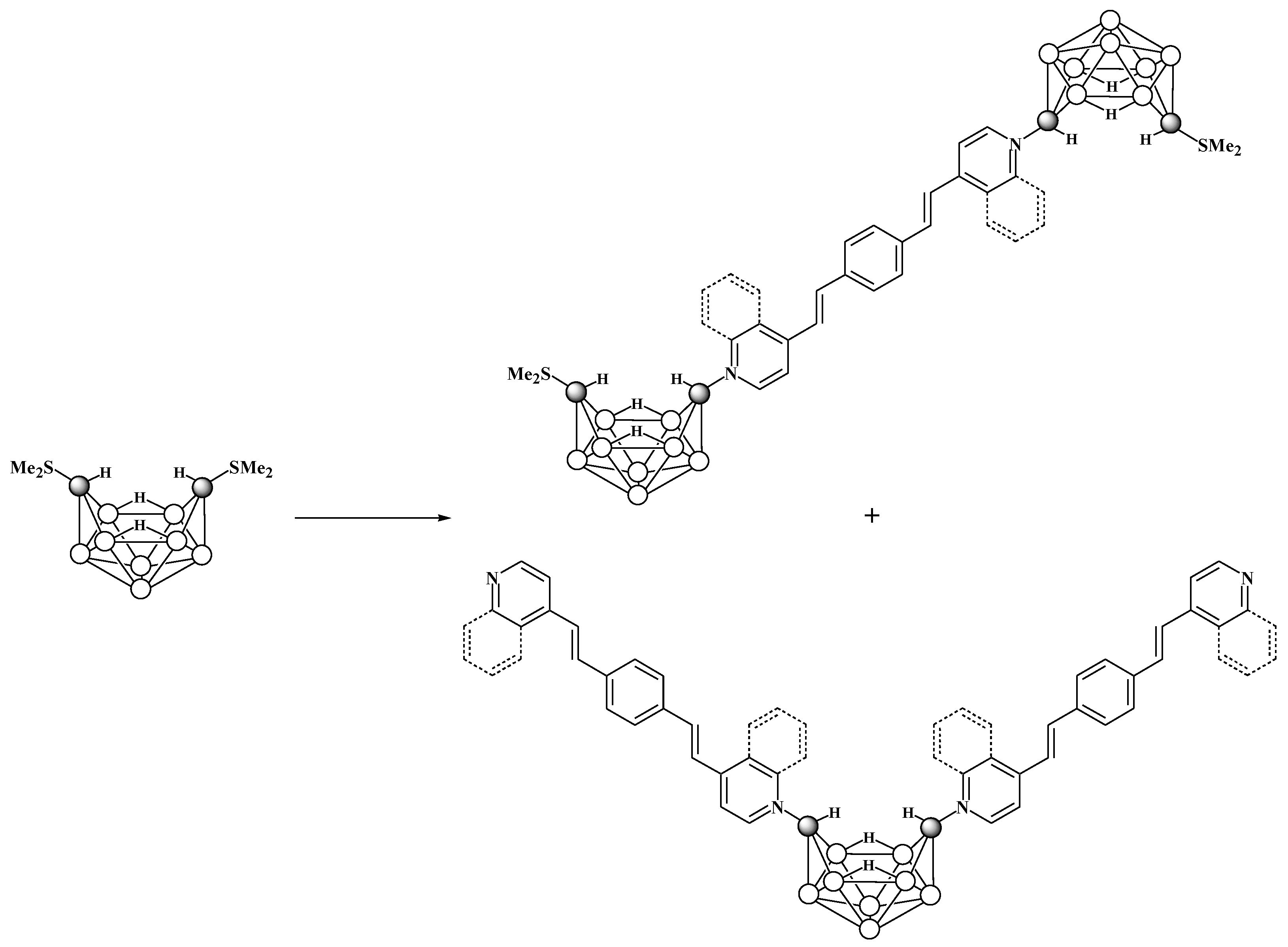
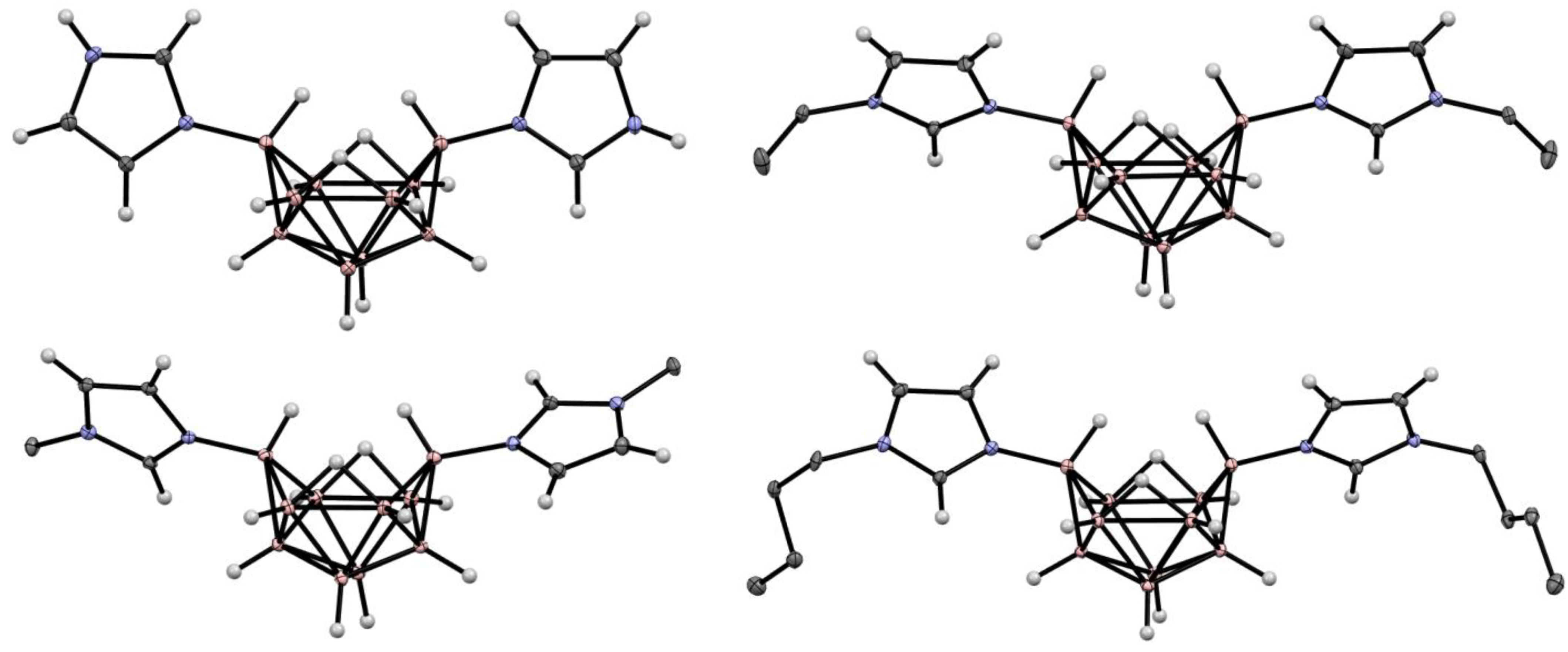
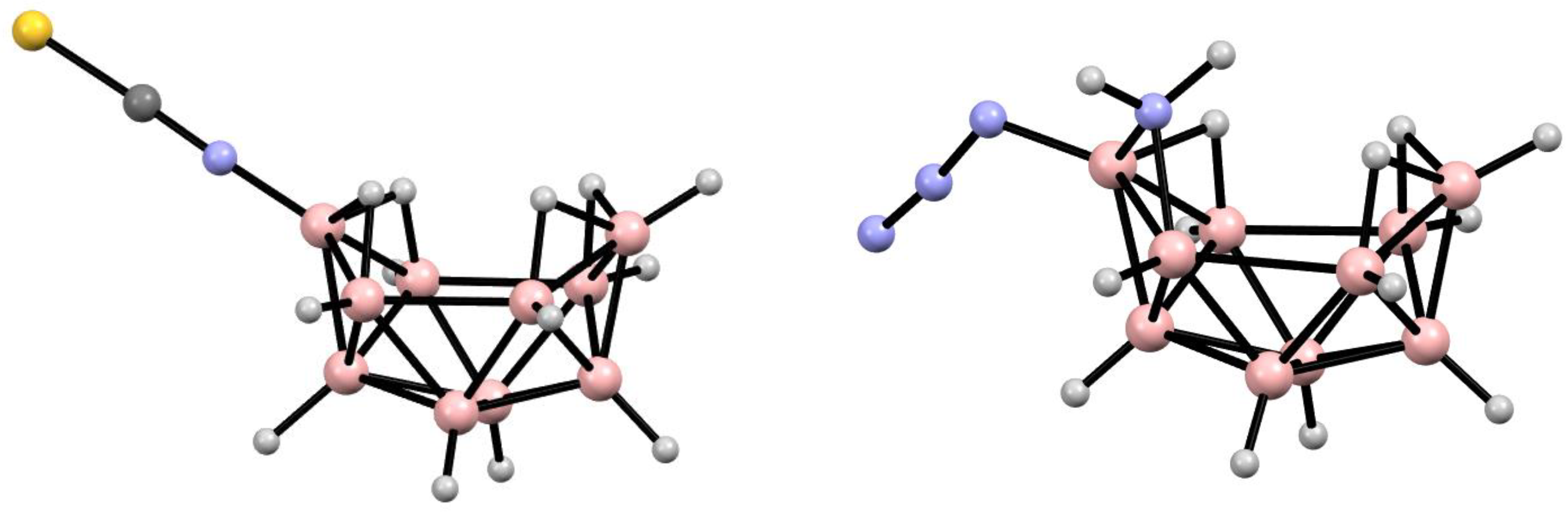




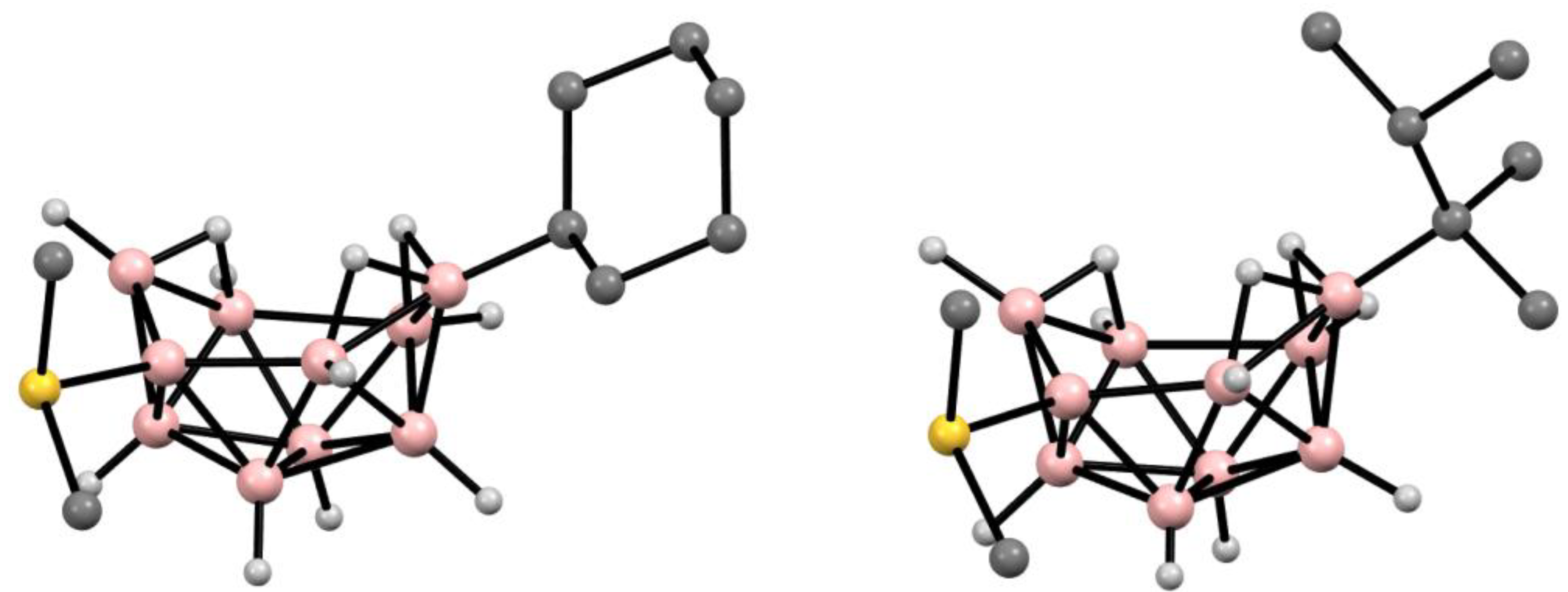
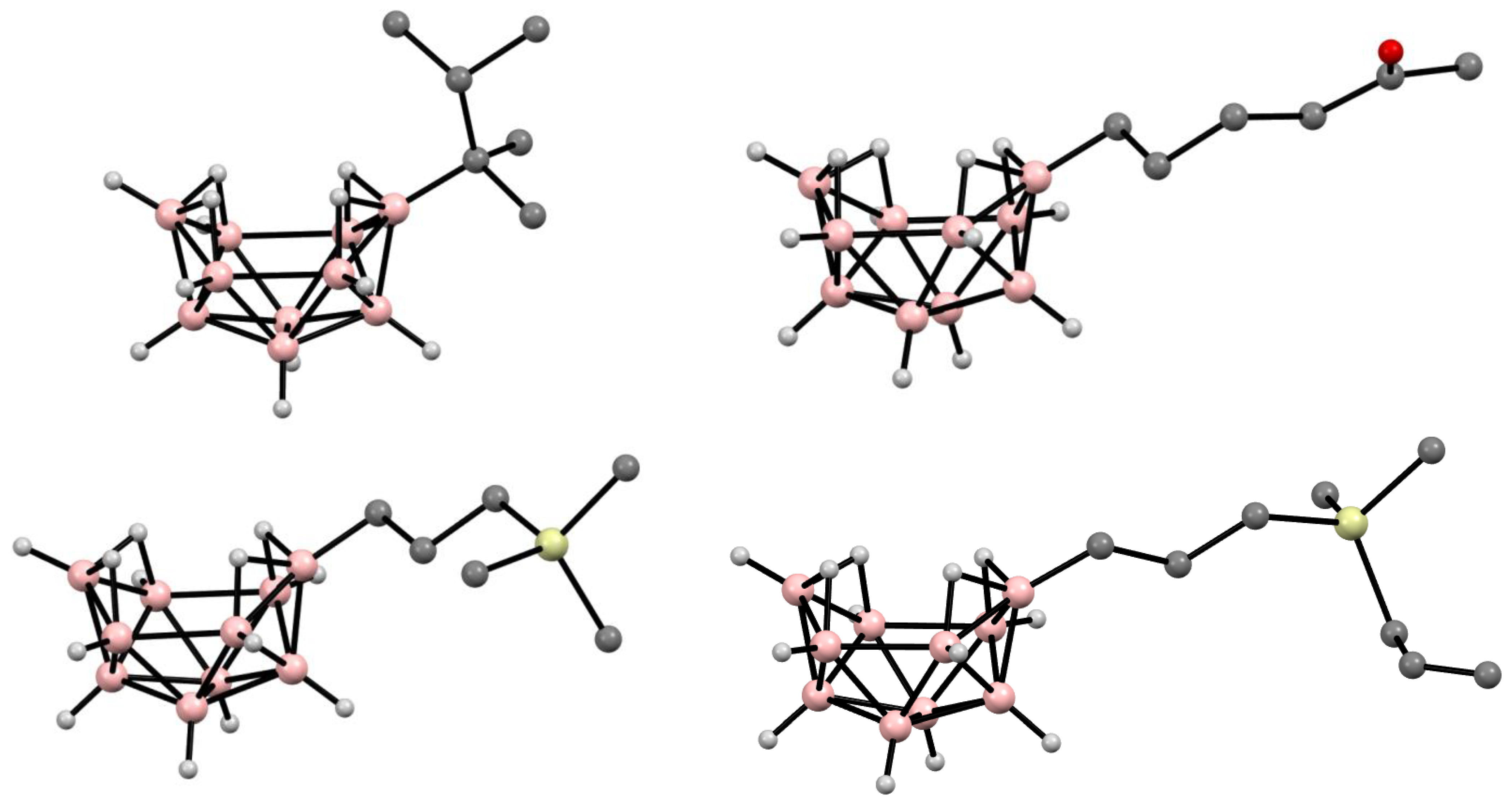
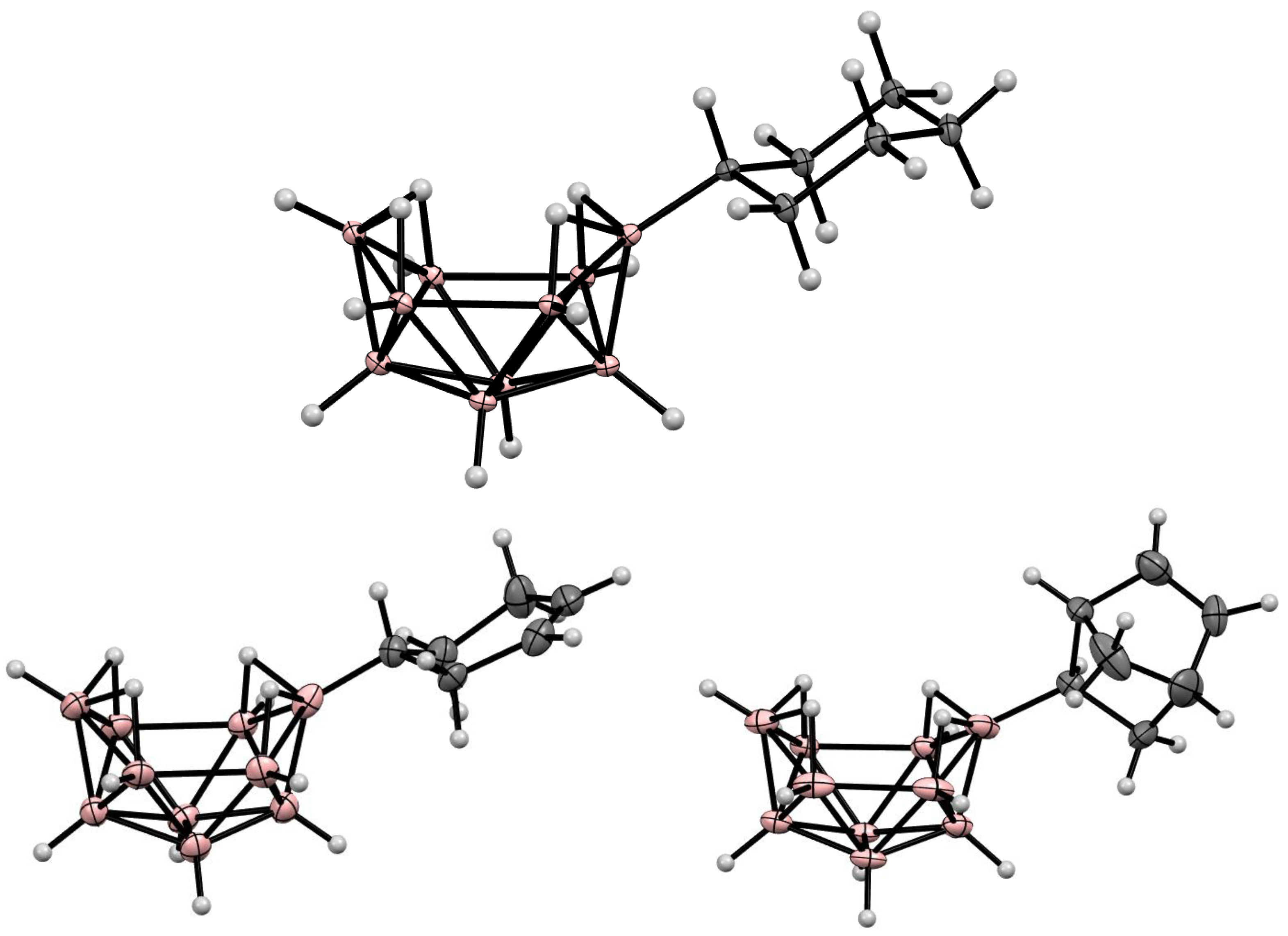

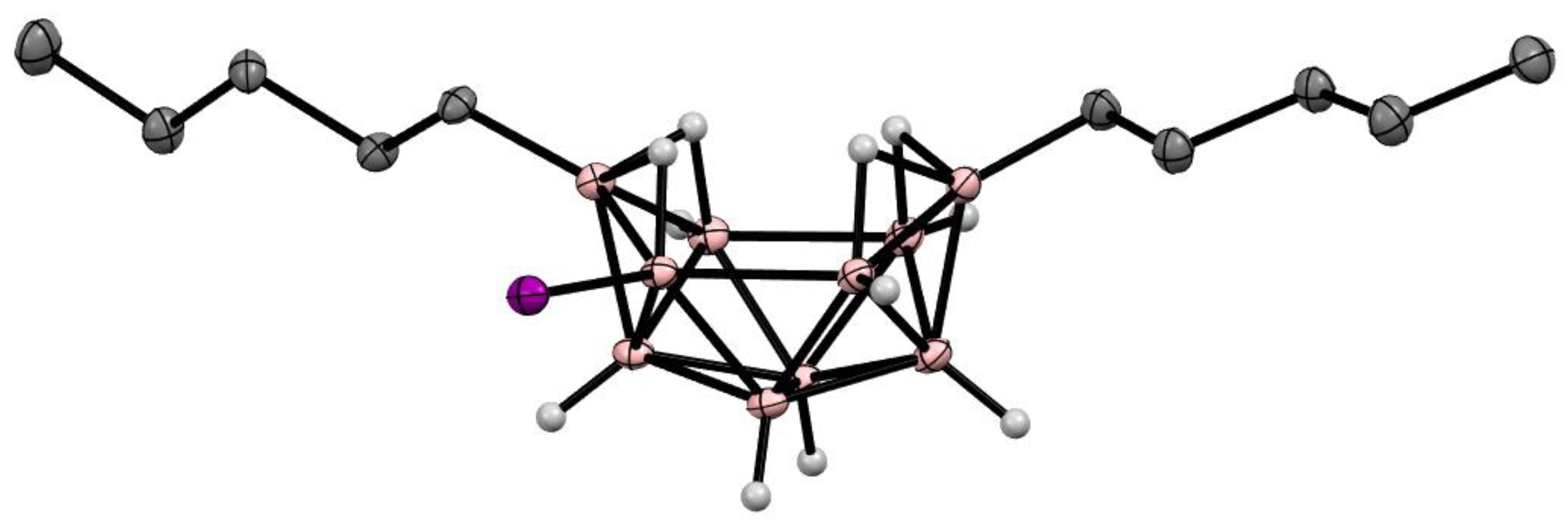

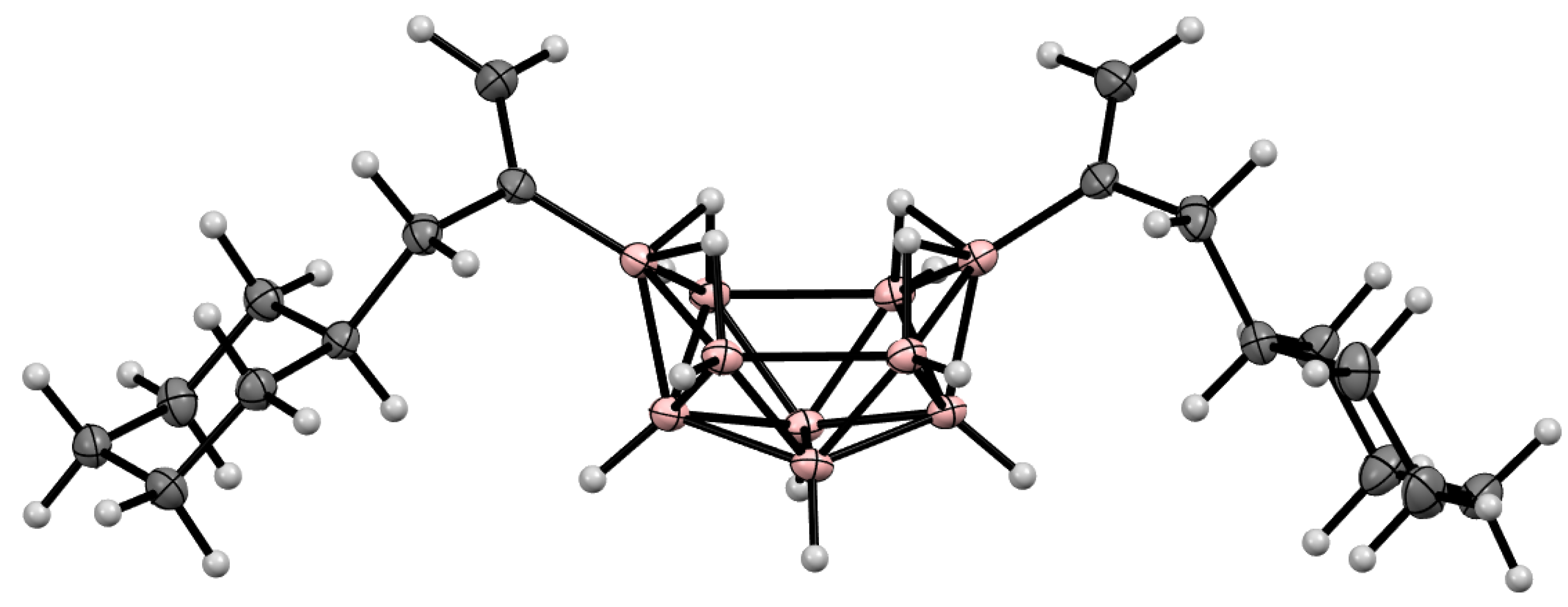
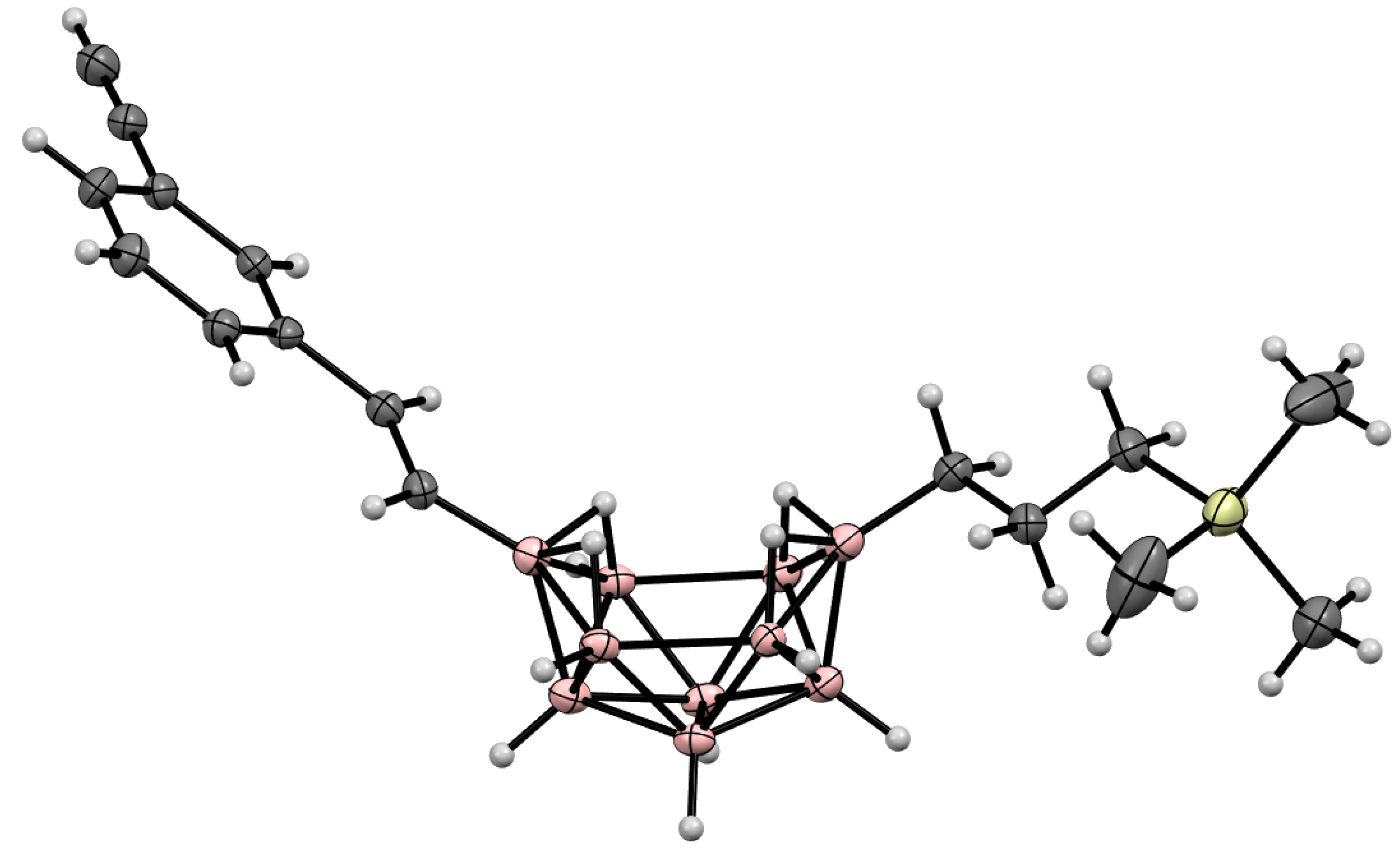
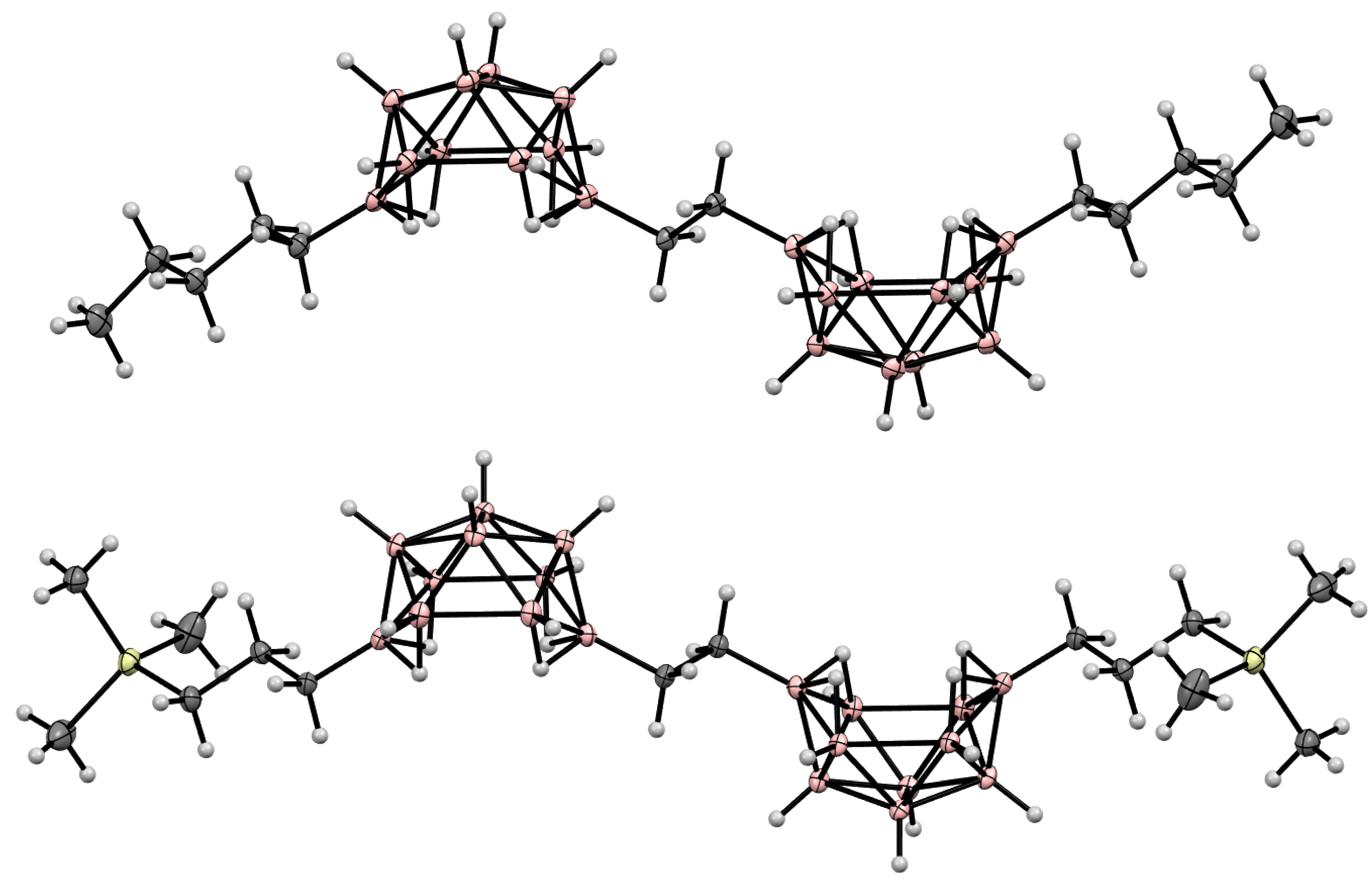
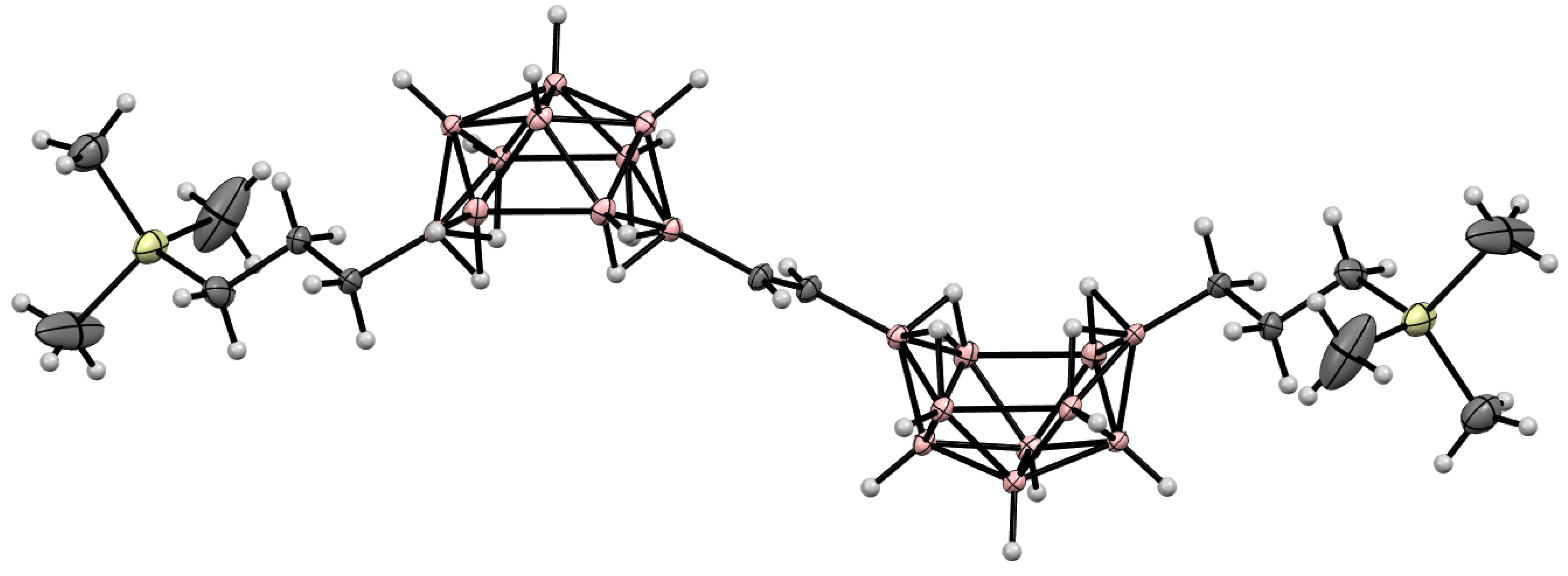
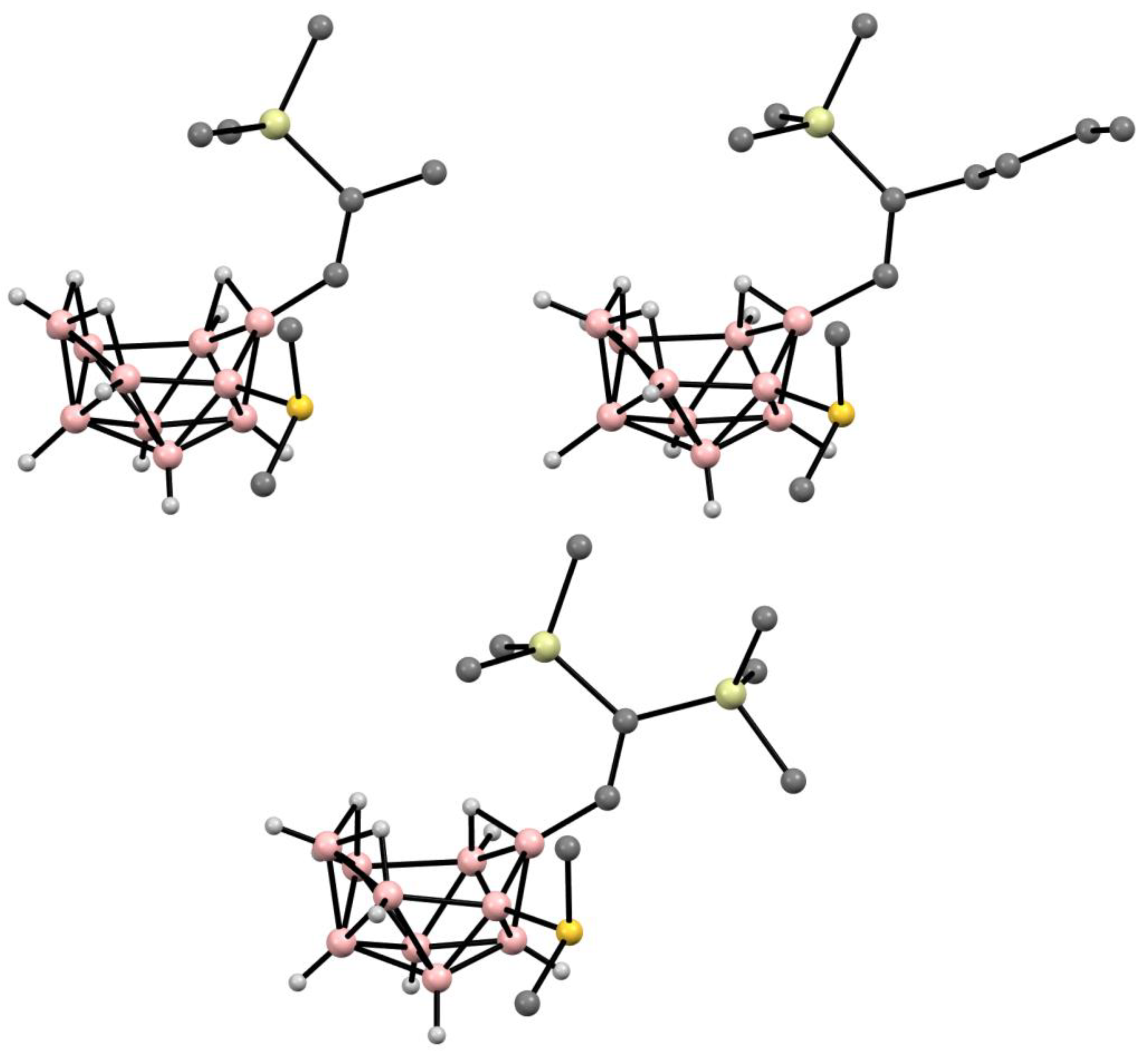
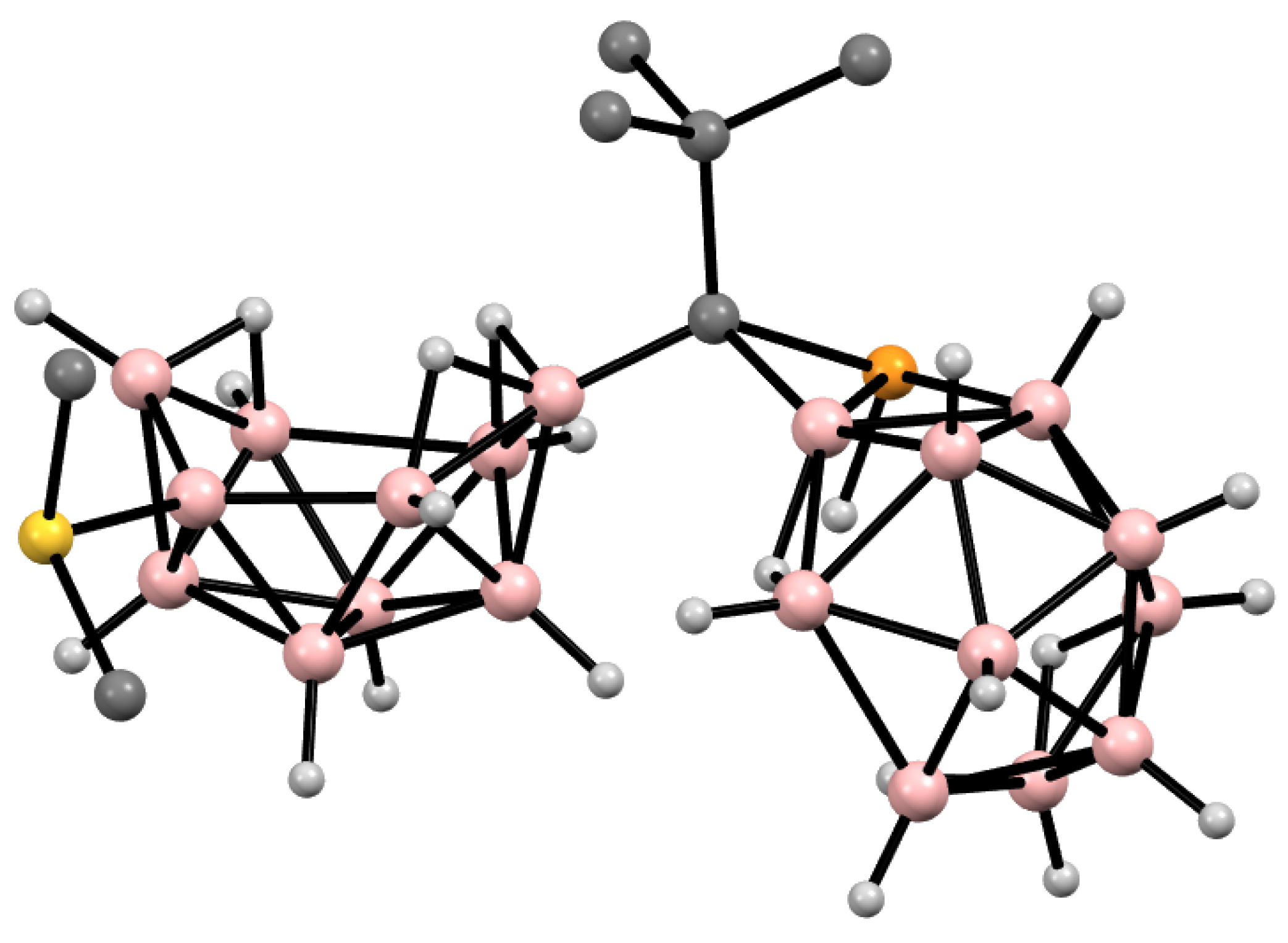
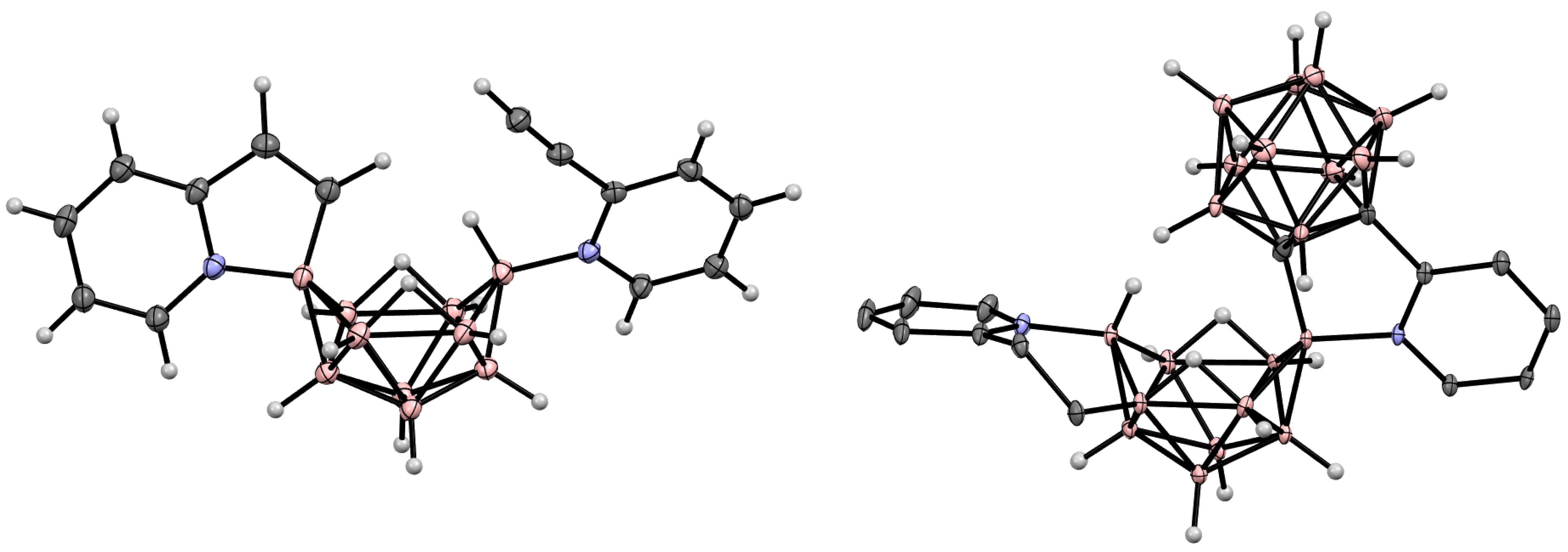
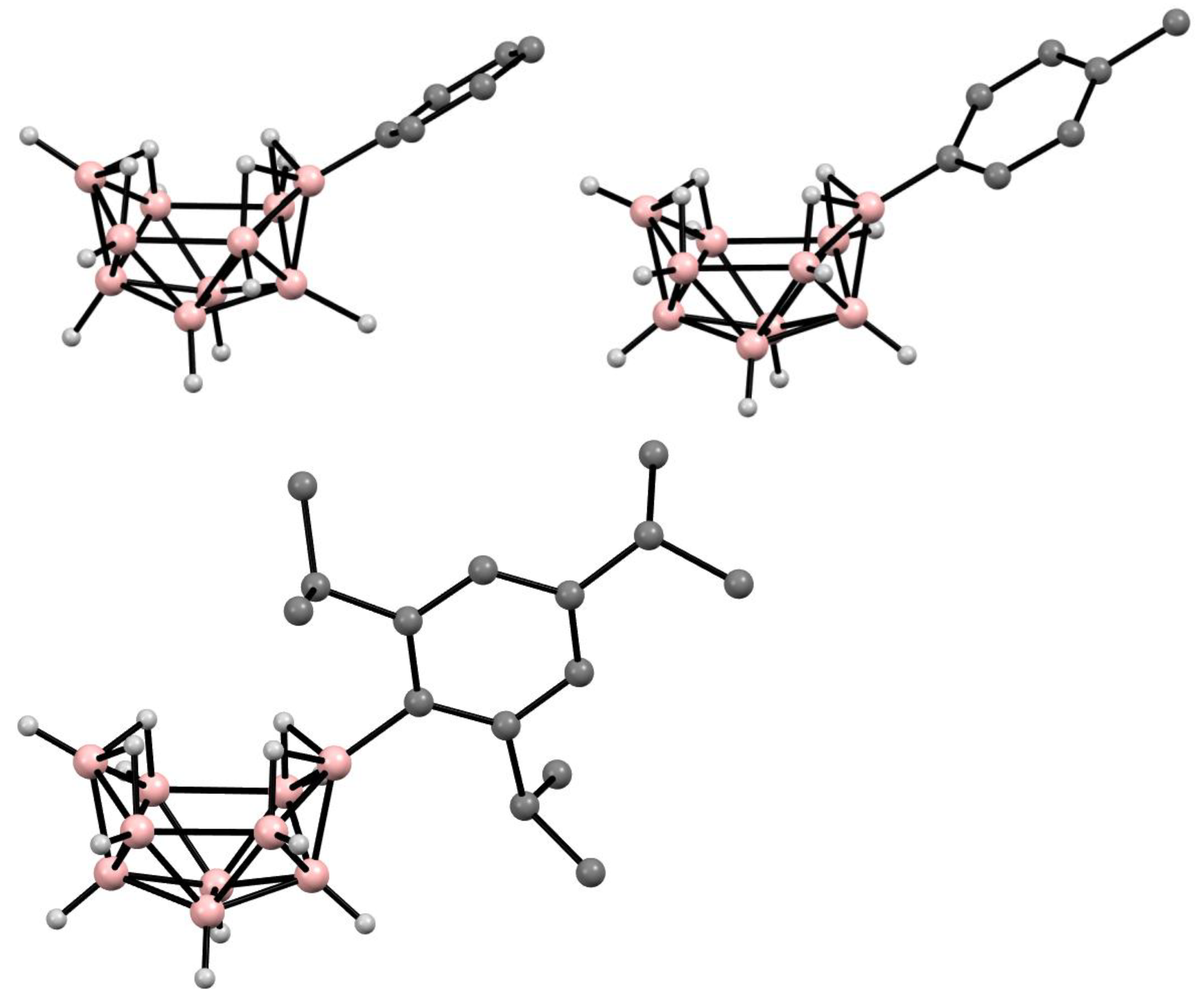
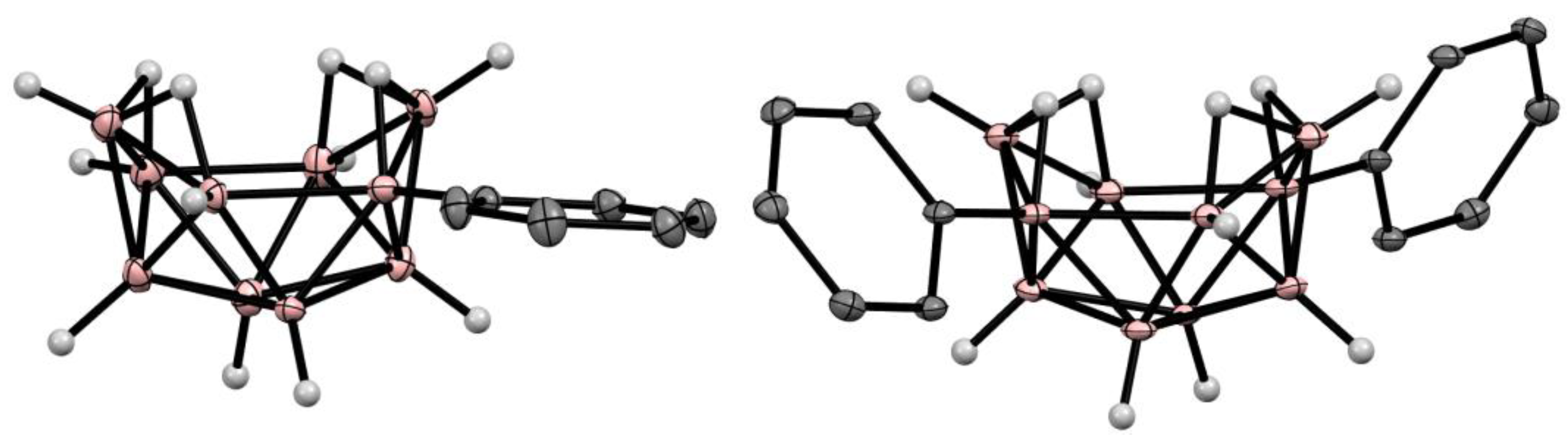
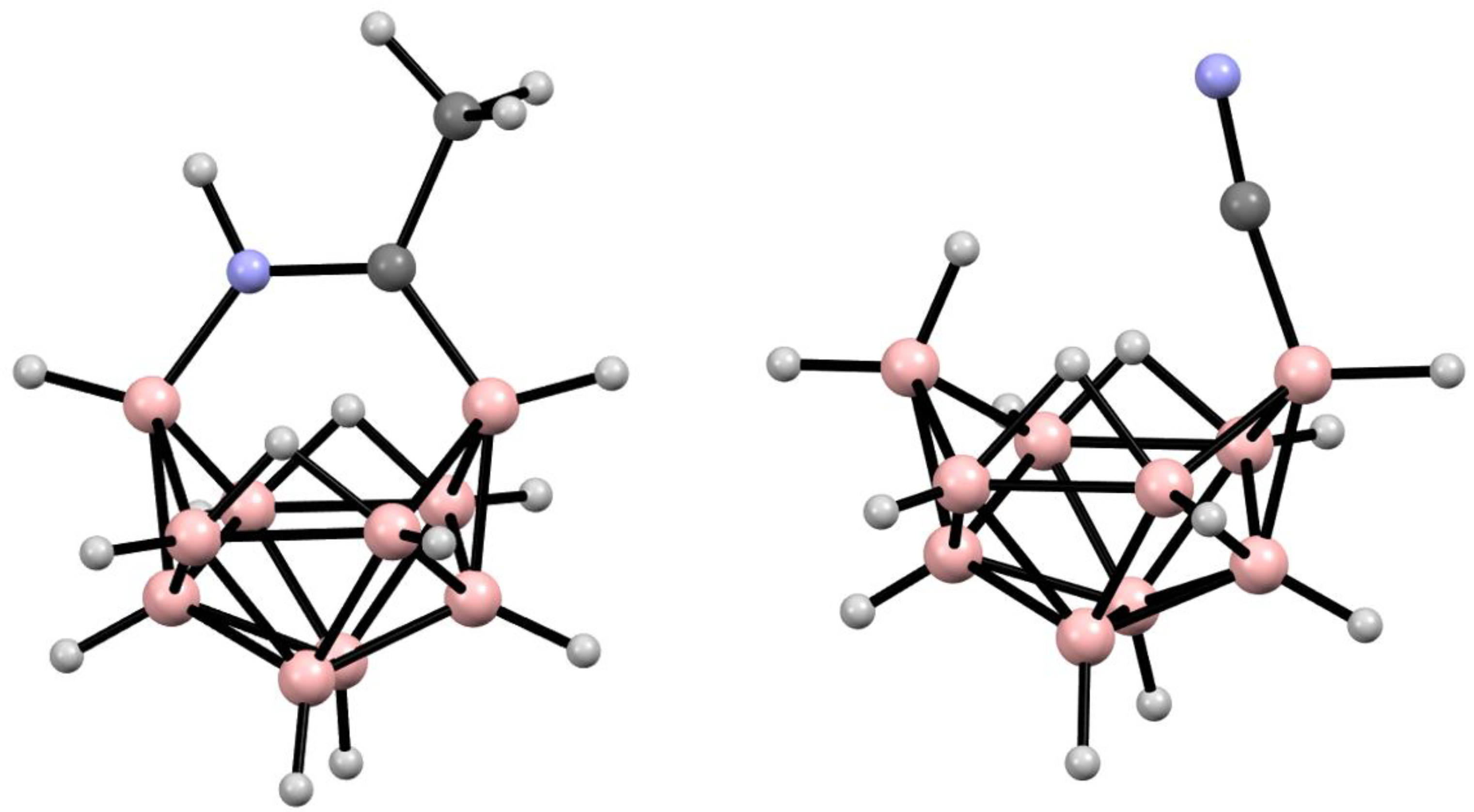
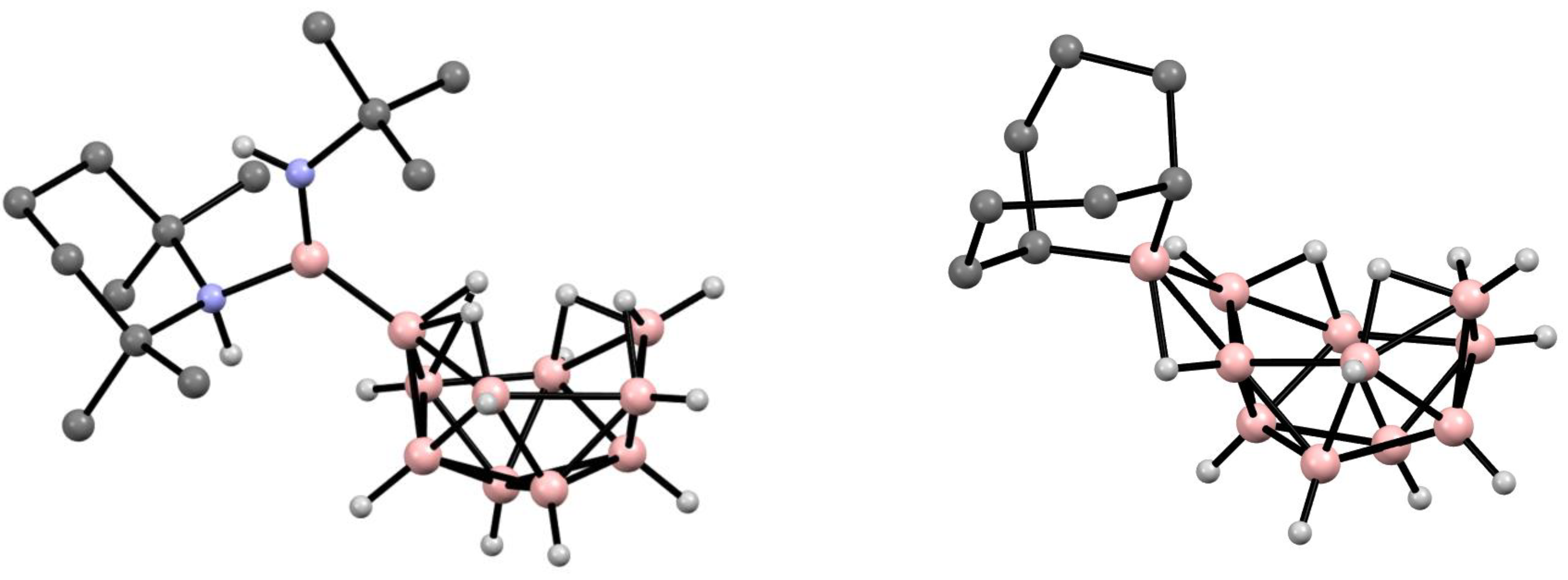
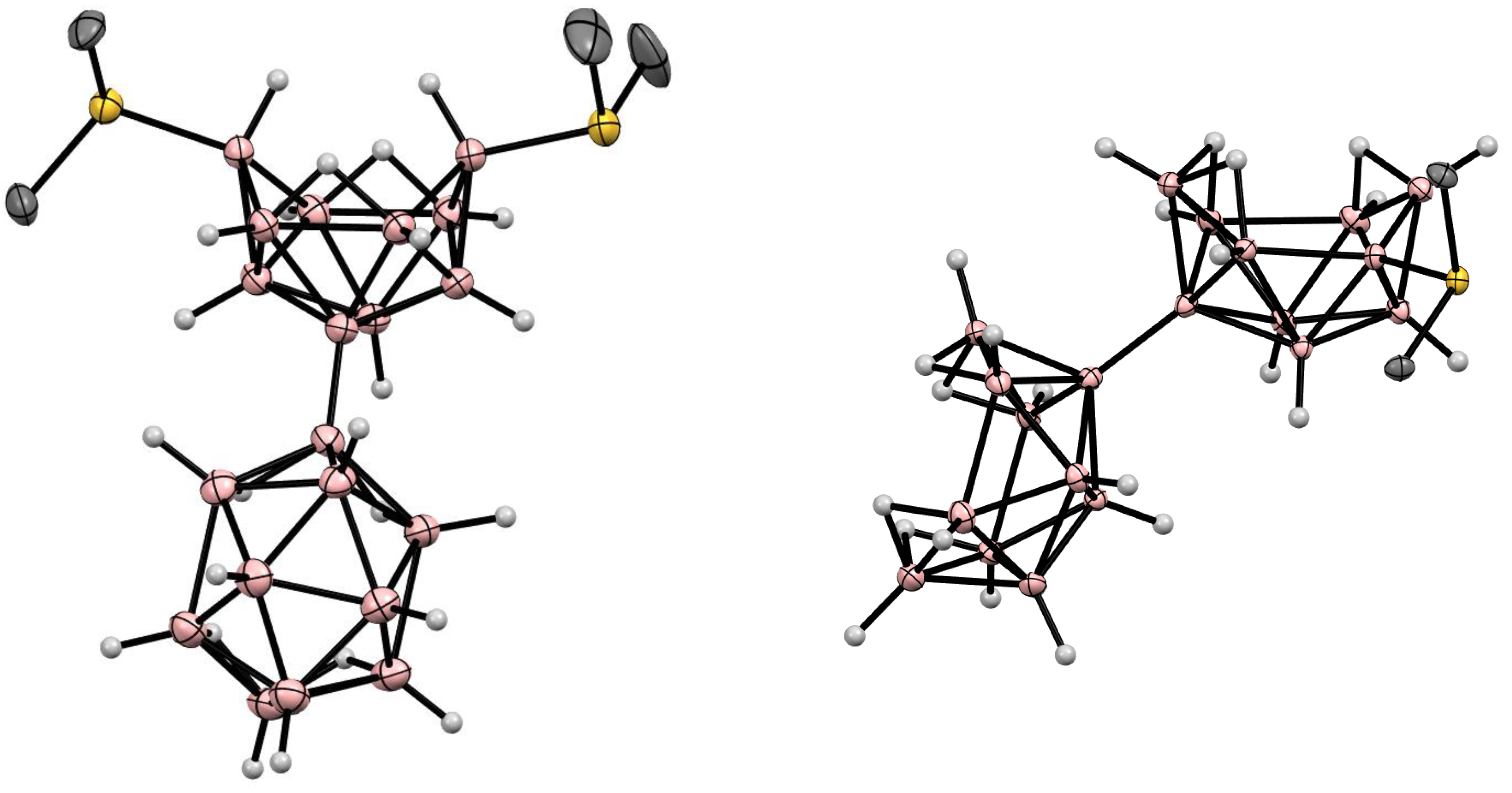
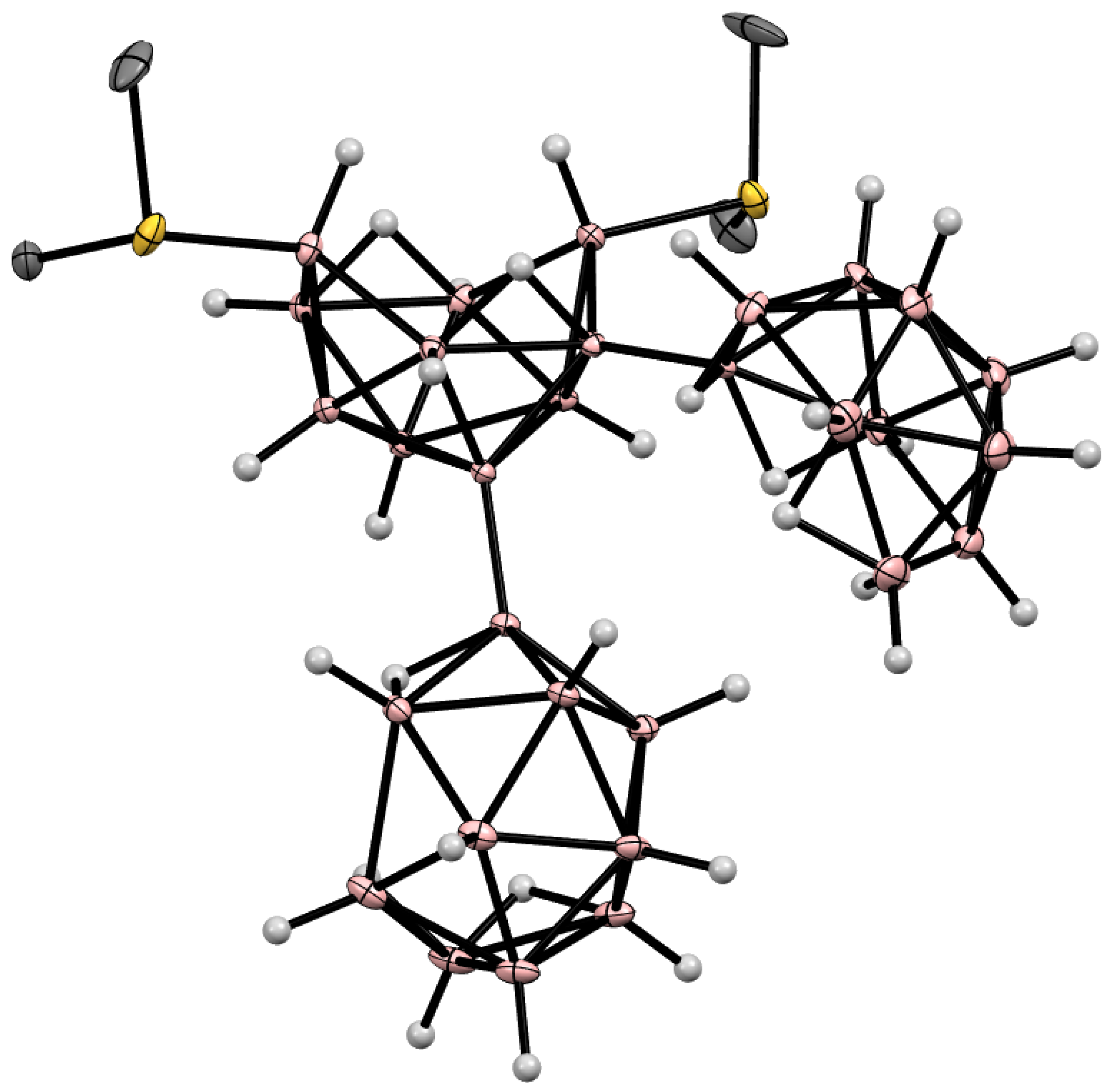

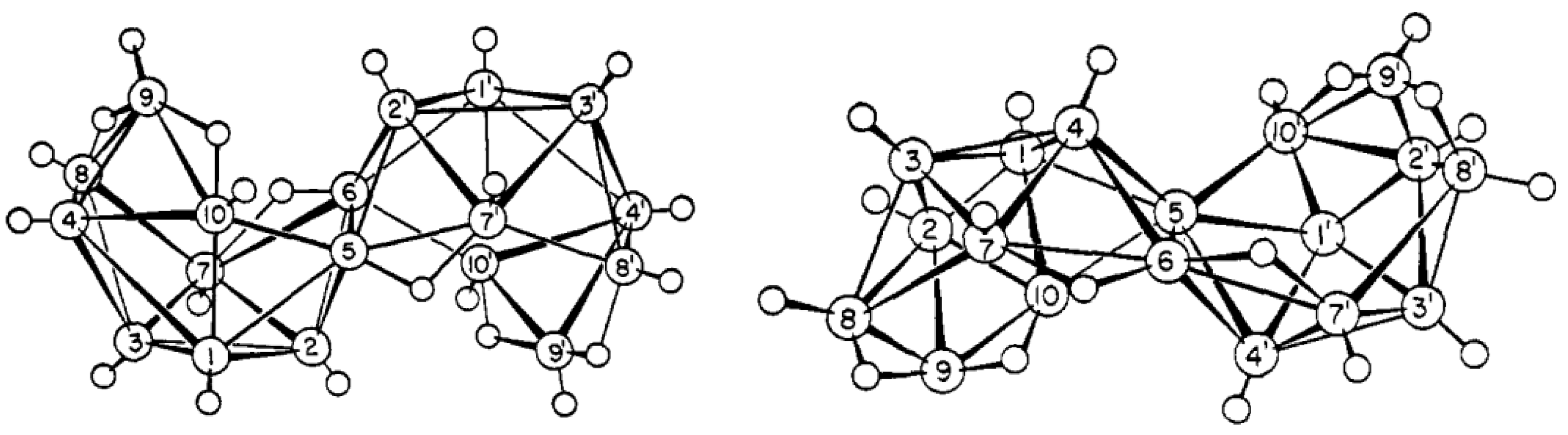
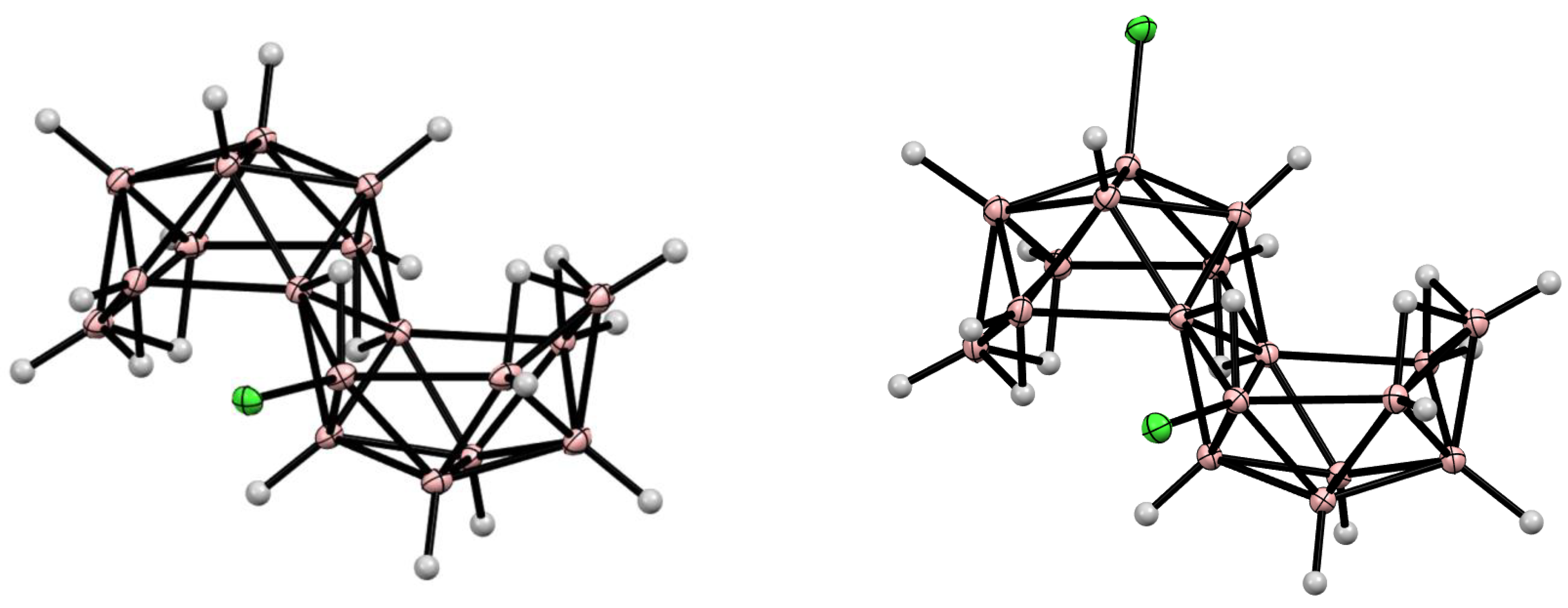
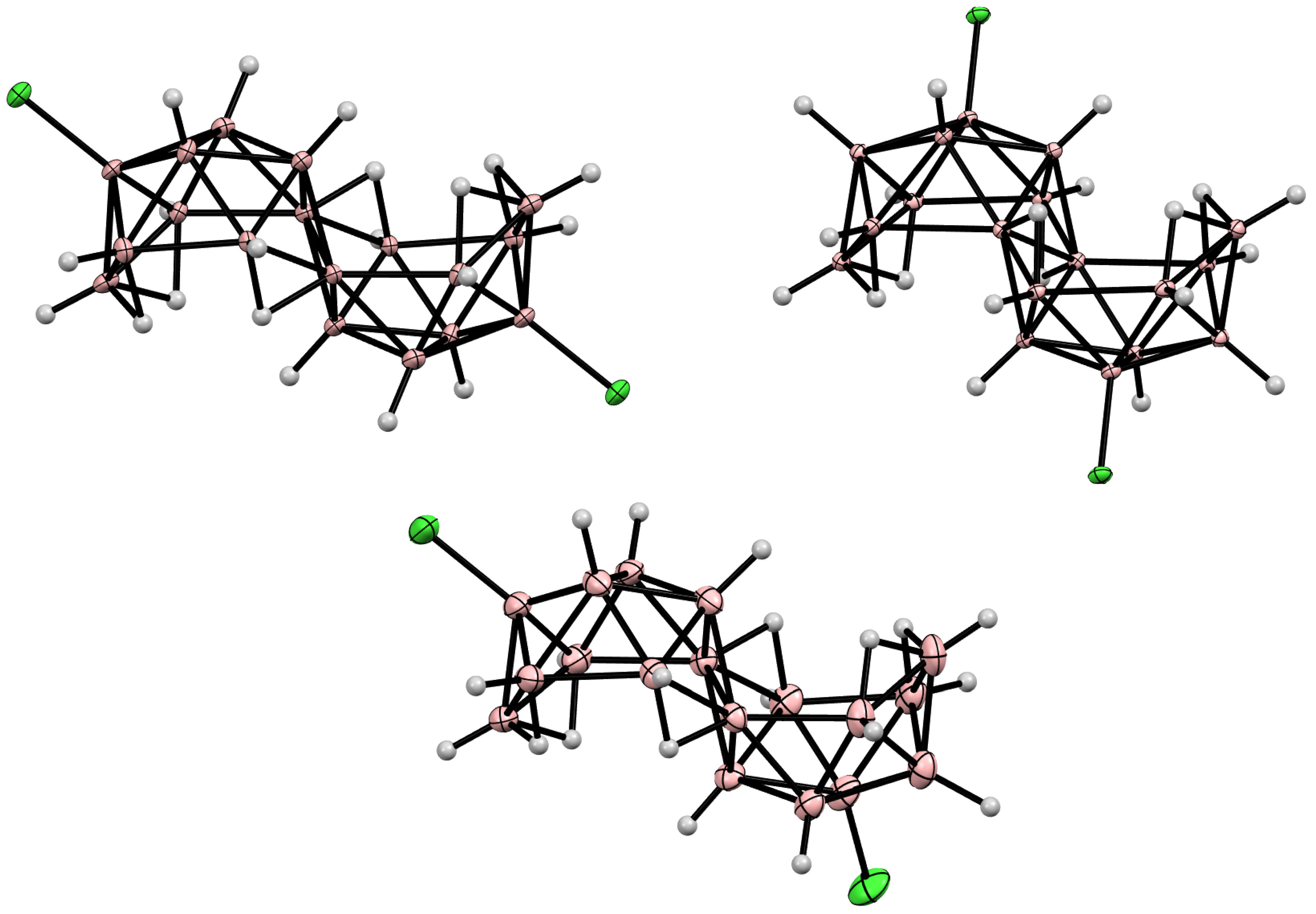

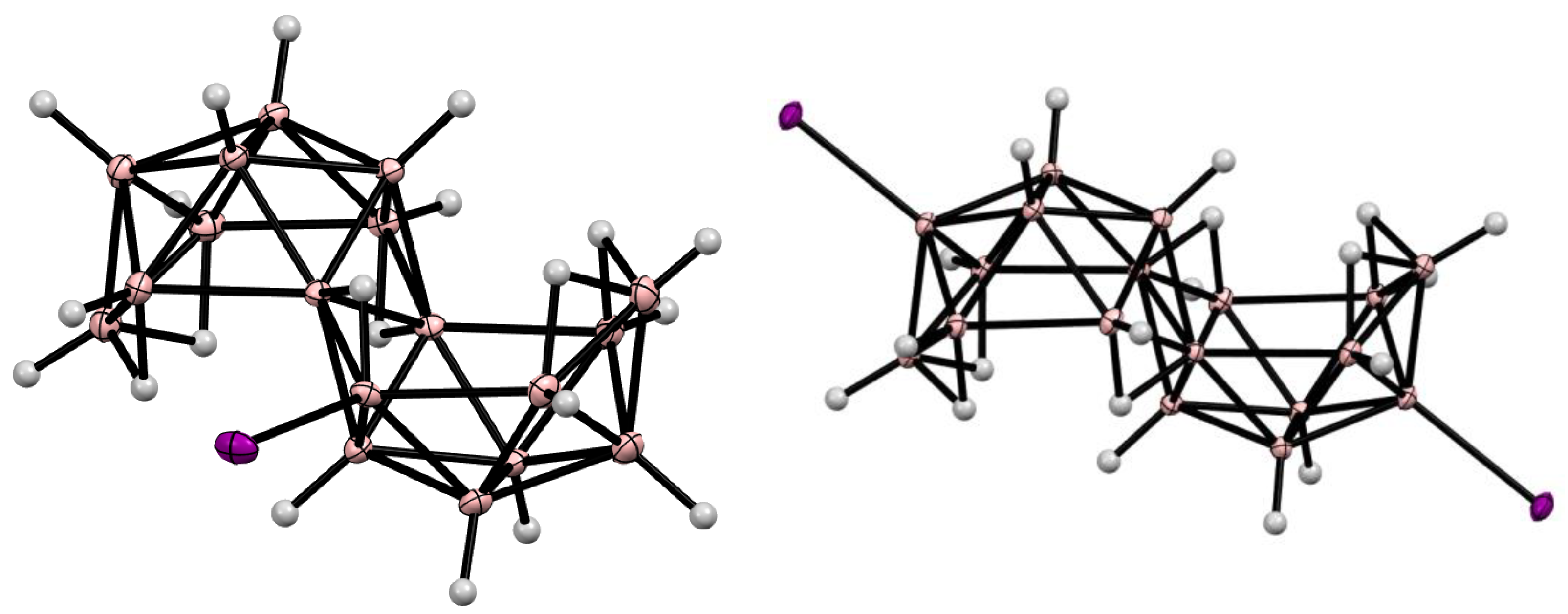
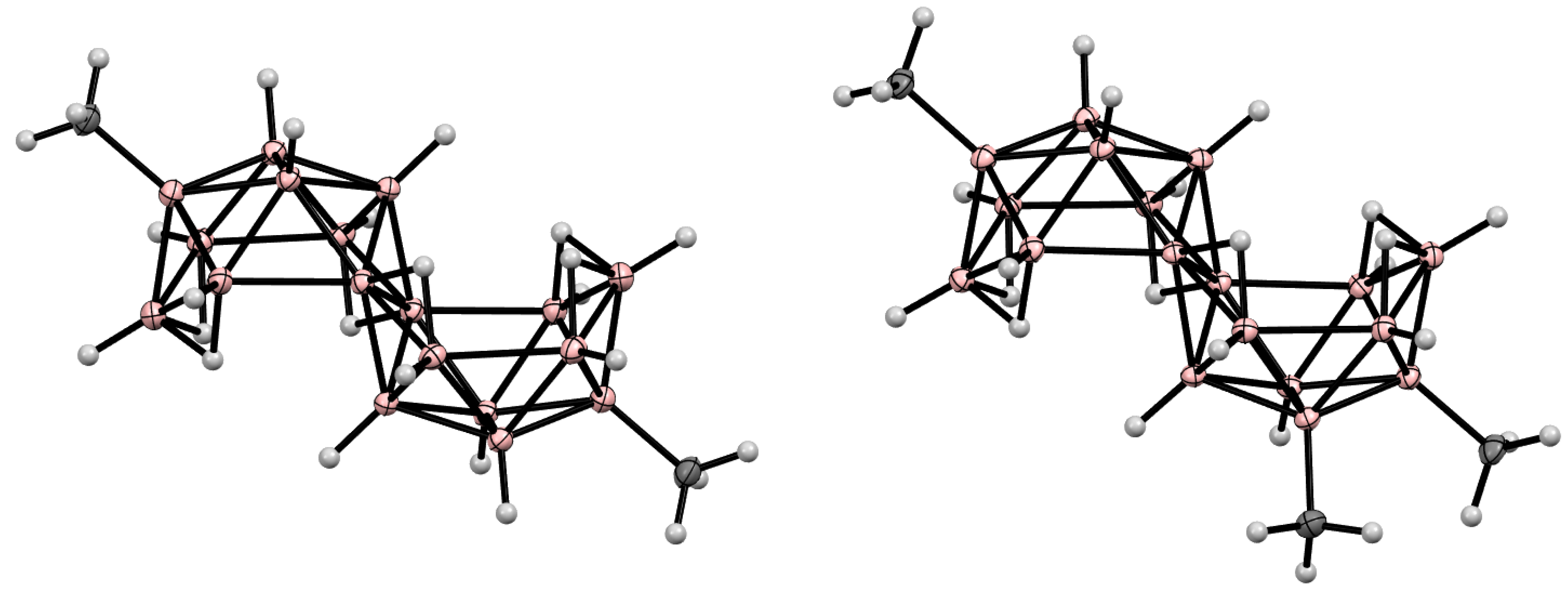
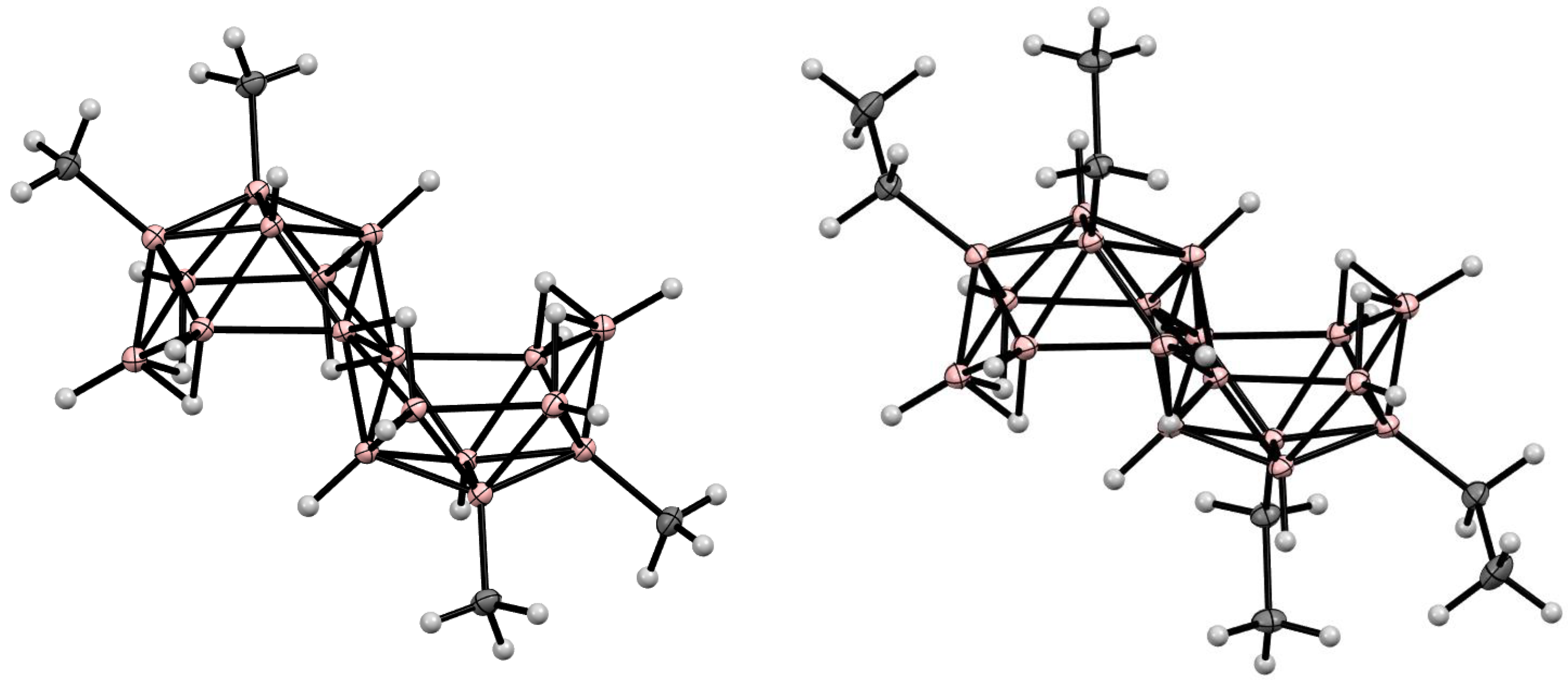
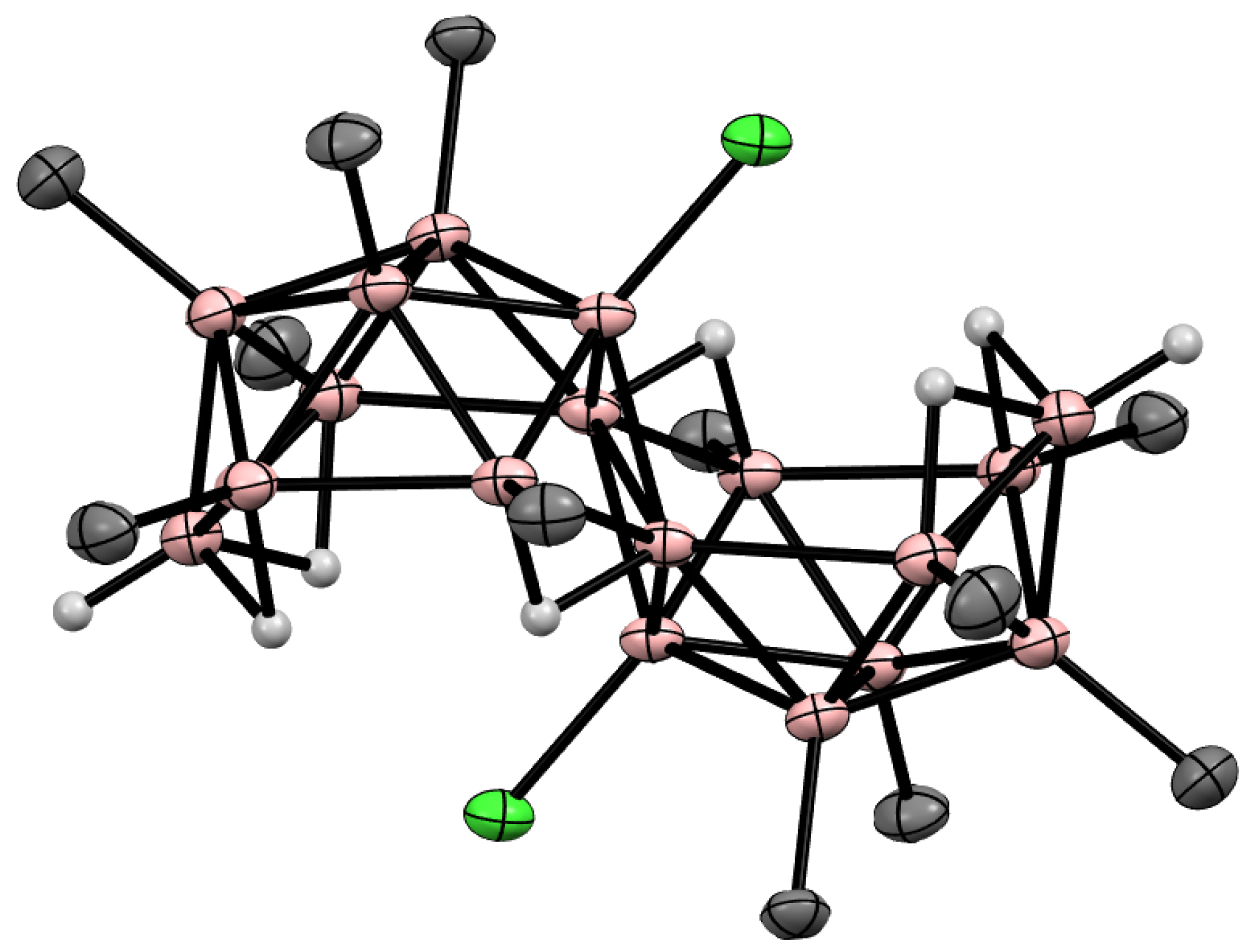
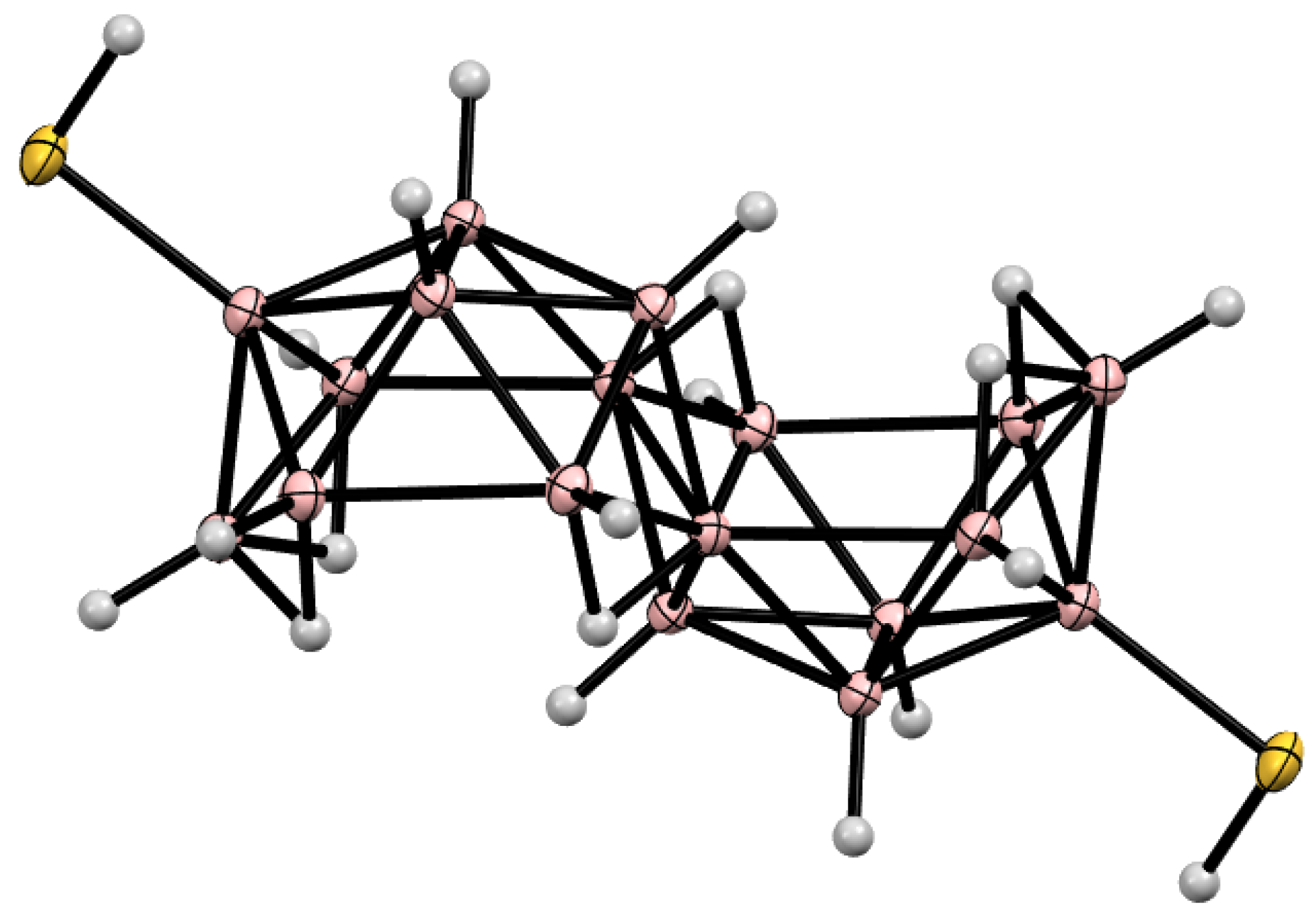

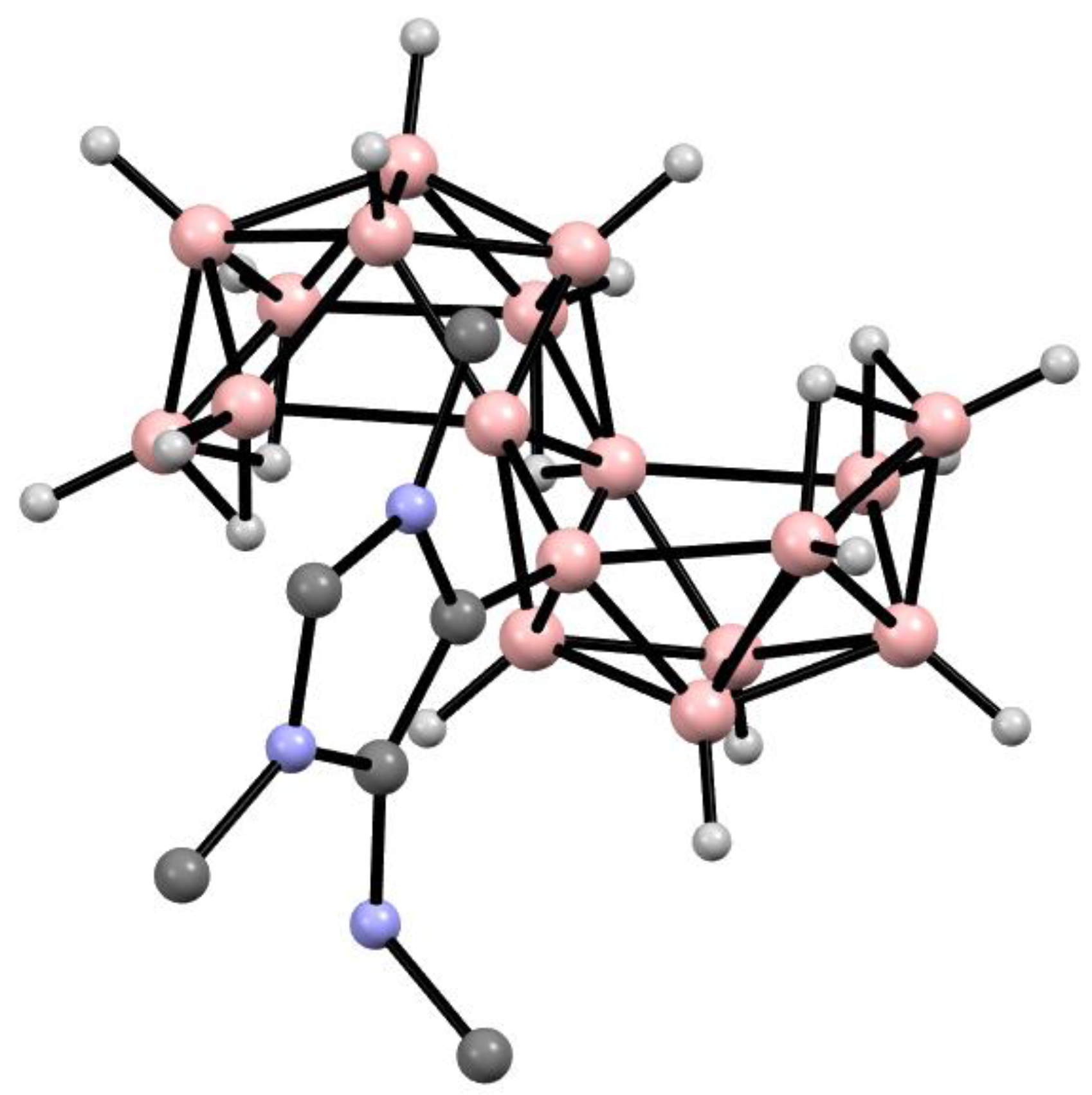



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sivaev, I.B. Decaborane: From Alfred Stock and Rocket Fuel Projects to Nowadays. Molecules 2023, 28, 6287. https://doi.org/10.3390/molecules28176287
Sivaev IB. Decaborane: From Alfred Stock and Rocket Fuel Projects to Nowadays. Molecules. 2023; 28(17):6287. https://doi.org/10.3390/molecules28176287
Chicago/Turabian StyleSivaev, Igor B. 2023. "Decaborane: From Alfred Stock and Rocket Fuel Projects to Nowadays" Molecules 28, no. 17: 6287. https://doi.org/10.3390/molecules28176287
APA StyleSivaev, I. B. (2023). Decaborane: From Alfred Stock and Rocket Fuel Projects to Nowadays. Molecules, 28(17), 6287. https://doi.org/10.3390/molecules28176287






