Momordica charantia-Derived Extracellular Vesicles Provide Antioxidant Protection in Ulcerative Colitis
Abstract
:1. Introduction
2. Results and Discussion
2.1. MCEV Characterization
2.2. Lipid Composition Analysis of MCEVs
2.3. Protein Domain Analysis of MCEVs
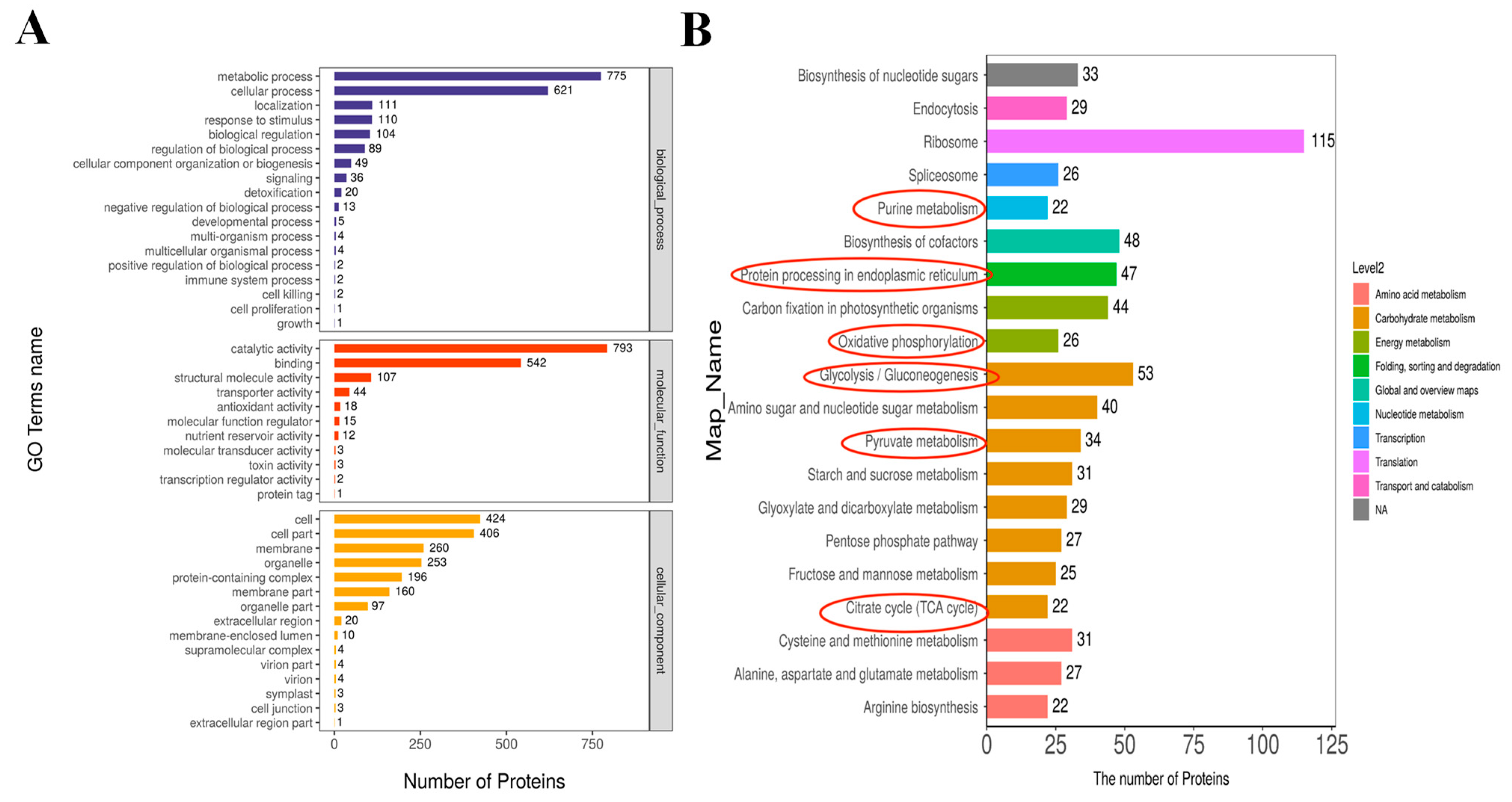
2.4. GO Annotation and KEGG Analysis of MCEVs
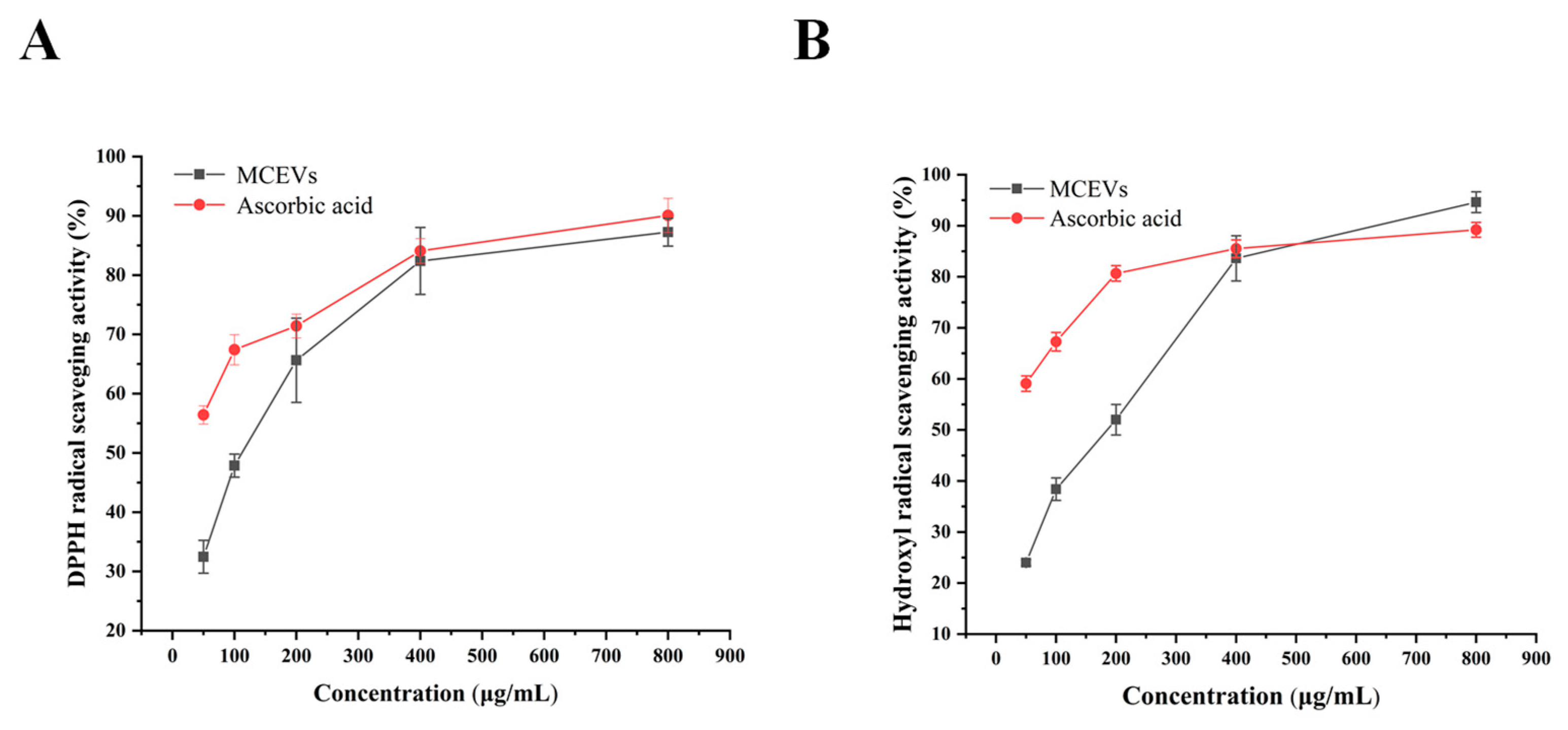
2.5. Antioxidant Activity of MCEVs In Vitro
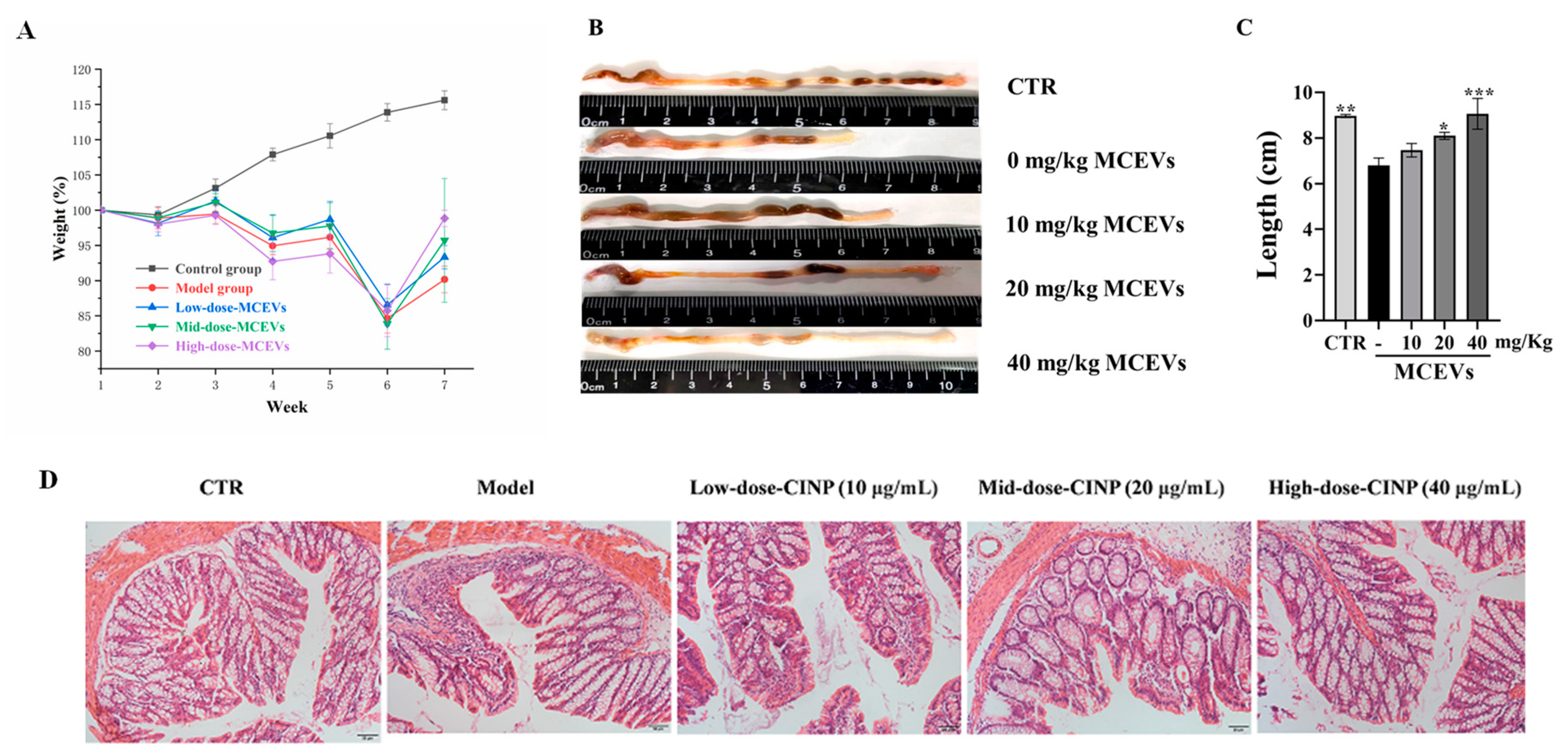
2.6. MCEVs Ease UC in C57BL/6 Mice
2.7. MCEVs Inhibit Oxidative Stress Induced by UC
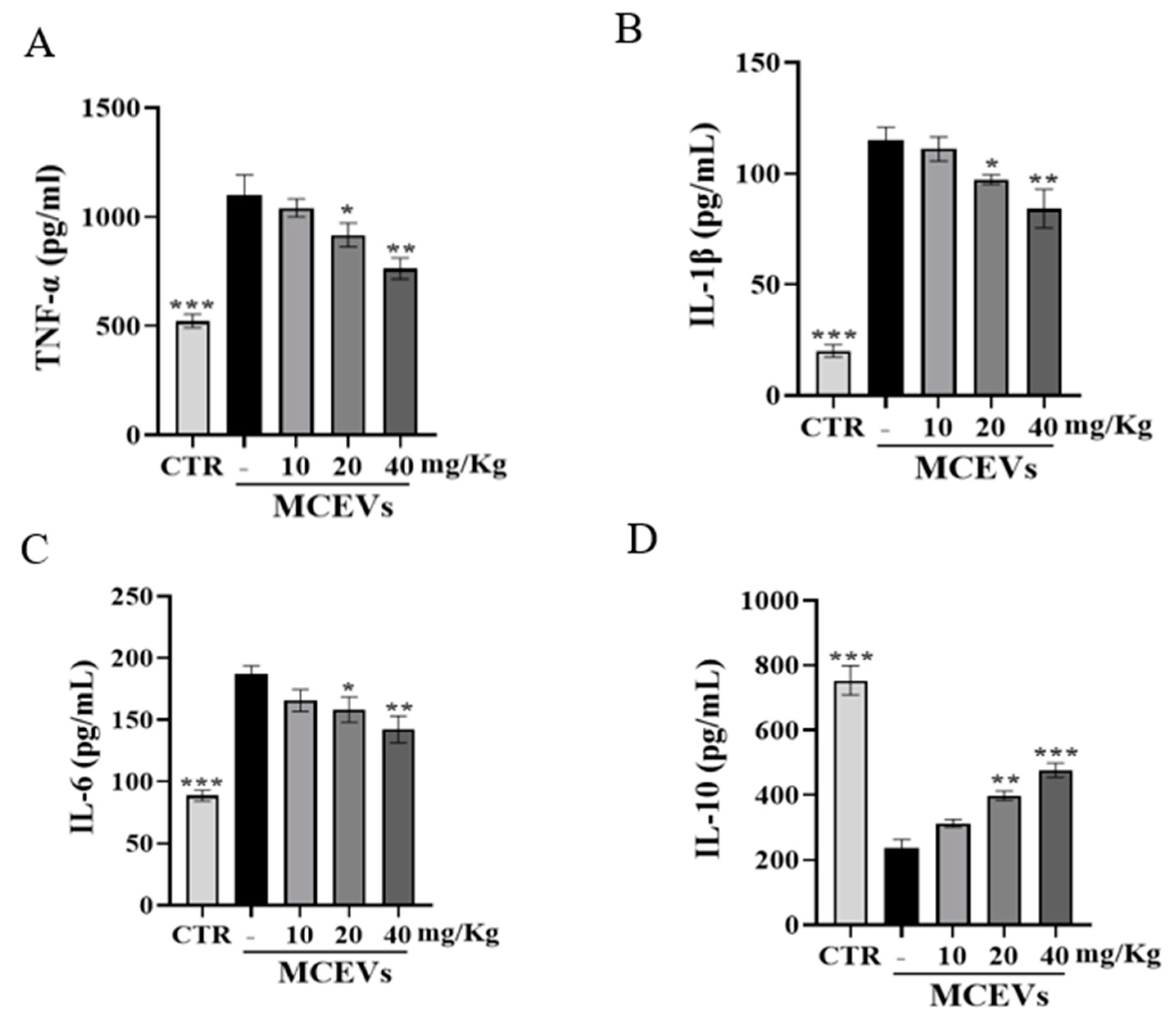
2.8. MCEVs Stimulate Inflammatory Factors in UC
3. Materials and Methods
3.1. Materials
3.2. Preparation of MCEVs
3.3. MCEV Characterization
3.4. Lipid Composition Identification
3.5. Proteomic Identification
3.6. Determination of DPPH Radical Scavenging Activity
3.7. Determination of Hydroxyl Radical Scavenging Activity
3.8. Animals and Treatments
3.9. Histopathologic Analysis of Colon Tissue
3.10. Determination of Antioxidant Indexes and Inflammatory Cytokines in Serum
3.11. Statistics
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Li, M.; Li, M.; Lei, J.; Wu, Y.; Li, Z.; Chen, L.; Zhou, C.; Su, J.; Huang, G.; Huang, X.; et al. Huangqin decoction ameliorates DSS-induced ulcerative colitis: Role of gut microbiota and amino acid metabolism, mTOR pathway and intestinal epithelial barrier. Phytomedicine 2022, 100, 154052. [Google Scholar] [CrossRef]
- Cheng, H.; Liu, J.; Zhang, D.; Wang, J.; Tan, Y.; Feng, W.; Peng, C. Ginsenoside Rg1 alleviates acute ulcerative colitis by modulating gut microbiota and microbial tryptophan metabolism. Front. Immunol. 2022, 13, 817600. [Google Scholar] [CrossRef]
- Ungaro, R.; Mehandru, S.; Allen, P.B.; Peyrin, B.L.; Colombel, J.F. Ulcerative colitis. Lancet 2017, 389, 1756–1770. [Google Scholar] [CrossRef]
- Kaplan, G.G.; Windsor, J.W. The four epidemiological stages in the global evolution ofinflammatory bowel disease. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 56–66. [Google Scholar] [CrossRef]
- Wan, F.; Wang, M.; Zhong, R.; Chen, L.; Han, H.; Liu, L.; Zhao, Y.; Lv, H.; Hou, F.; Yi, B.; et al. Supplementation with chinese medicinal plant extracts from Lonicera hypoglauca and Scutellaria baicalensis mitigates colonic Inflammation by regulating oxidative stress and gut microbiota in a colitis mouse model. Front. Cell. Infect. Microbiol. 2022, 11, 798052. [Google Scholar] [CrossRef]
- Zhao, B.; Xia, B.; Li, X.; Zhang, L.; Liu, X.; Shi, R.; Kou, R.; Liu, Z.; Liu, X. Sesamol supplementation attenuates DSS-induced colitis via mediating gut barrier integrity, inflammatory responses, and reshaping gut microbiome. J. Agric. Food. Chem. 2020, 68, 10697–10708. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Yan, X.; Zhang, Y.; Yang, M.; Ma, Y.; Zhang, Y.; Xu, Q.; Tu, K.; Zhang, M. Oral administration of turmeric-derived exosome-like nanovesicles with anti-inflammatory and pro-resolving bioactions for murine colitis therapy. J. Nanobiotechnol. 2022, 20, 206. [Google Scholar] [CrossRef]
- Zhang, M.; Xu, C.; Liu, D.; Han, M.K.; Wang, L.; Merlin, D. Oral delivery of nanoparticles loaded with ginger active compound, 6-shogaol, attenuates ulcerative colitis and promotes wound healing in a murine model of ulcerative colitis. J. Crohns. Colitis. 2018, 12, 217–229. [Google Scholar] [CrossRef] [PubMed]
- Ordás, I.; Eckmann, L.; Talamini, M.; Baumgart, D.C.; Sandborn, W.J. Ulcerative colitis. Lancet 2012, 380, 1606–1619. [Google Scholar] [CrossRef]
- Han, W.; Xie, B.; Li, Y.; Shi, L.; Wan, J.; Chen, X.; Wang, H. Orally deliverable nanotherapeutics for the synergistic treatment of colitis-associated colorectal cancer. Theranostics 2019, 9, 7458–7473. [Google Scholar] [CrossRef]
- Ginini, L.; Billan, S.; Fridman, E.; Gil, Z. Insight into extracellular vesicle-cell communication: From cell recognition to intracellular fate. Cells 2022, 11, 1375. [Google Scholar] [CrossRef] [PubMed]
- Mu, J.; Zhuang, X.; Wang, Q.; Jiang, H.; Deng, Z.B.; Wang, B.; Zhang, L.; Kakar, S.; Jun, Y.; Miller, D.; et al. Interspecies communication between plant and mousegut host cells through edible plant derived exosome-like nanoparticles. Mol. Nutr. Food. Res. 2014, 58, 1561–1573. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Feng, S.; Wang, X.; Long, K.; Luo, Y.; Wang, Y.; Ma, J.; Tang, Q.; Jin, L.; Li, X.; et al. Identification of exosome-like nanoparticle-derived microRNAs from 11edible fruits and vegetables. PeerJ. 2018, 6, e5186. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Zhang, L.; Zhang, Y.; Lu, R. Plant-derived exosomes as a drug-delivery approach for the treatment of inflammatory bowel disease and colitis-associated cancer. Pharmaceutics 2022, 14, 822. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Xiu, Q.; Huang, Y.; Troyer, Z.; Li, B.; Zheng, L. Plant-derived vesicle-like nanoparticles as promising biotherapeutic tools: Present and future. Adv. Mater. 2023, 35, e2207826. [Google Scholar] [CrossRef]
- Gao, C.; Zhou, Y.; Chen, Z.; Li, H.; Xiao, Y.; Hao, W.; Zhu, Y.; Vong, C.T.; Farag, M.A.; Wang, Y.; et al. Turmeric-derived nanovesicles as novel nanobiologics for targeted therapy of ulcerative colitis. Theranostics 2022, 12, 5596–5614. [Google Scholar] [CrossRef]
- Sriwastva, M.K.; Deng, Z.B.; Wang, B.; Teng, Y.; Kumar, A.; Sundaram, K.; Mu, J.; Lei, C.; Dryden, G.W.; Xu, F.; et al. Exosome-like nanoparticles from Mulberry bark prevent DSS-induced colitis via the AhR/COPS8 pathway. EMBO Rep. 2022, 23, e53365. [Google Scholar] [CrossRef]
- Kilasoniya, A.; Garaeva, L.; Shtam, T.; Spitsyna, A.; Putevich, E.; Moreno-Chamba, B.; Salazar-Bermeo, J.; Komarova, E.; Malek, A.; Valero, M.; et al. Potential of plant exosome vesicles from grapefruit (Citrus × paradisi) and tomato (Solanum lycopersicum) juices as functional ingredients and targeted drug delivery vehicles. Antioxidants 2023, 12, 943. [Google Scholar] [CrossRef]
- Yuwai, K.E.; Rao, K.S.; Kaluwin, C.; Jones, G.P.; Rivett, D.E. Chemical composition of Momordica charantia L. fruits. J. Agric. Food Chem. 1991, 39, 1762–1763. [Google Scholar] [CrossRef]
- Parvathi, S.; Kumar, V.J. Studies on chemical composition and utilization of the wild edible vegetable athalakkai (Momordica tuberosa). Plant Foods Hum. Nutr. 2002, 57, 215–222. [Google Scholar] [CrossRef]
- Sur, S.; Ray, R.B. Bitter melon (Momordica Charantia), a nutraceutical approach for cancer prevention and therapy. Cancers 2020, 12, 2064. [Google Scholar] [CrossRef]
- Dandawate, P.R.; Subramaniam, D.; Padhye, S.B.; Anant, S. Bitter melon: A panacea for inflammation and cancer. Chin. J. Nat. Med. 2016, 14, 81–100. [Google Scholar] [CrossRef]
- Semiz, A.; Ozgun Acar, O.; Cetin, H.; Semiz, G.; Sen, A. Suppression of Inflammatory Cytokines Expression with Bitter Melon (Momordica Charantia) in TNBS-instigated Ulcerative Colitis. J. Transl. Int. Med. 2020, 8, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Ji, S.; Zhang, Q. Momordica charantia polysaccharides alleviate diarrhea-predominant irritable bowel syndrome by regulating intestinal inflammation and barrier via NF-κB pathway. Allergol. Immunopathol. 2022, 50, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Nieto-Veloza, A.; Wang, Z.; Zhong, Q.; Krishnan, H.B.; Dia, V.P. BG-4 from bitter gourd (Momordica charantia) differentially affects inflammation in vitro and in vivo. Antioxidants 2019, 8, 175. [Google Scholar] [CrossRef]
- Li, C.; Song, Q.; Yin, X.; Song, R.; Chen, G. Preparation, characterization, and in vitro anticancer activity evaluation of broccoli-derived extracellular vesicle-coated astaxanthin nanoparticles. Molecules. 2022, 27, 3955. [Google Scholar] [CrossRef]
- Cui, W.W.; Ye, C.; Wang, K.X.; Yang, X.; Zhu, P.Y.; Hu, K.; Lan, T.; Huang, L.Y.; Wang, W.; Gu, B.; et al. Momordica. charantia-derived extracellular vesicles-like nanovesicles protect cardiomyocytes against radiation injury via attenuating DNA damage and mitochondria dysfunction. Front. Cardiovasc. Med. 2022, 9, 864188. [Google Scholar] [CrossRef]
- Feng, T.; Wan, Y.; Dai, B.; Liu, Y. Anticancer activity of bitter melon-derived vesicles extract against breast cancer. Cells 2023, 12, 824. [Google Scholar] [CrossRef]
- Wang, C.; Wang, C.; Liu, F.; Rainosek, S.; Patterson, T.A.; Slikker, W., Jr.; Han, X. Lipidomics reveals changes in metabolism, indicative of anesthetic-induced neurotoxicity in developing brains. Chem. Res. Toxicol. 2018, 31, 825–835. [Google Scholar] [CrossRef]
- Subra, C.; Grand, D.; Laulagnier, K.; Stella, A.; Lambeau, G.; Paillasse, M.; De Medina, P.; Monsarrat, B.; Perret, B.; Silvente-Poirot, S.; et al. Exosomes account for vesicle-mediated transcellular transport of activatable phospholipases and prostaglandins. J. Lipid. Res. 2010, 51, 2105–2120. [Google Scholar] [CrossRef]
- Hadizadeh, N.; Bagheri, D.; Shamsara, M.; Hamblin, M.R.; Farmany, A.; Xu, M.; Liang, Z.; Razi, F.; Hashemi, E. Extracellular vesicles biogenesis, isolation, manipulation and genetic engineering for potential in vitro and in vivo therapeutics: An overview. Front. Bioeng. Biotechnol. 2022, 10, 1019821. [Google Scholar] [CrossRef]
- Nagan, N.; Zoeller, R.A. Plasmalogens: Biosynthesis and functions. Prog. Lipid. Res. 2001, 40, 199–229. [Google Scholar] [CrossRef] [PubMed]
- Manning, G.; Whyte, D.B.; Martinez, R.; Hunter, T.; Sudarsanam, S. The protein kinase complement of the human genome. Science 2002, 298, 1912–1934. [Google Scholar] [CrossRef]
- Roskoski, R.J. The ErbB/HER family of protein-tyrosine kinases and cancer. Pharmacol. Res. 2014, 79, 34–74. [Google Scholar] [CrossRef]
- Zhou, X.; Agazie, Y.M. Molecular mechanism for SHP2 in promoting HER2-induced signaling and transformation. J. Biol. Chem. 2009, 284, 12226–12234. [Google Scholar] [CrossRef]
- Zhang, J.; Li, X.; Zhao, Z.; Cai, W.; Fang, J. Thioredoxin signaling pathways in cancer. Antioxid. Redox. Signal. 2023, 38, 403–424. [Google Scholar] [CrossRef]
- Ndrepepa, G. Myeloperoxidase—A bridge linking inflammation and oxidative stress with cardiovascular disease. Clin. Chim. Acta. 2019, 493, 36–51. [Google Scholar] [CrossRef]
- Wan, W.; Li, H.; Xiang, J.; Yi, F.; Xu, L.; Jiang, B.; Xiao, P. Aqueous extract of black maca prevents metabolism disorder via regulating the glycolysis/gluconeogenesis-TCA cycle and PPARα signaling activation in golden hamsters fed a high-fat, high-fructose diet. Front. Pharmacol. 2018, 9, 333. [Google Scholar] [CrossRef]
- Pathinayake, P.S.; Waters, D.W.; Nichol, K.S.; Brown, A.C.; Reid, A.T.; Hsu, A.C.; Horvat, J.C.; Wood, L.G.; Baines, K.J.; Simpson, J.L.; et al. Endoplasmic reticulum-unfolded protein response signalling is altered in severe eosinophilic and neutrophilic asthma. Thorax 2022, 77, 443–451. [Google Scholar] [CrossRef]
- Hetz, C.; Zhang, K.; Kaufman, R.J. Mechanisms, regulation and functions of the unfolded protein response. Nat. Rev. Mol. Cell. Biol. 2020, 21, 421–438. [Google Scholar] [CrossRef]
- Tian, Y.; Mao, M.; Cao, X.; Zhu, H.; Shen, C. Identification and validation of autophagy-related genes in necrotizing enterocolitis. Front. Pediatr. 2022, 10, 839110. [Google Scholar] [CrossRef] [PubMed]
- Chagnoleau, J.B.; Ferreira, A.M.; Coutinho, J.A.P.; Fernandez, X.; Azoulay, S.; Papaiconomou, N. Sustainable extraction of antioxidants from out-of-caliber kiwifruits. Food Chem. 2023, 401, 133992. [Google Scholar] [CrossRef]
- Ren, B.; Chen, C.; Li, C.; Fu, X.; You, L.; Liu, R.H. Optimization of microwave-assisted extraction of Sargassum thunbergii polysaccharides and its antioxidant and hypoglycemic activities. Carbohydr. Polym. 2017, 173, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Huang, G.; Yang, Z.; Hou, Y. Antioxidant activity of Momordica charantia polysaccharide and its derivatives. Int J Biol. Macromol. 2019, 138, 673–680. [Google Scholar] [CrossRef]
- Aljohi, A.; Sabine, M.N.; Ahmed, N. Antiglycation and Antioxidant Properties of Momordica charantia. PLoS ONE 2016, 11, e0159985. [Google Scholar] [CrossRef] [PubMed]
- Domingueti, C.P.; Dusse, L.M.; Carvalho, M.D.; Sousa, L.P.; Gomes, K.B.; Fernandes, A.P. Diabetes mellitus: The linkage between oxidative stress, inflammation, hypercoagulability and vascular complications. J. Diabetes. Complicat. 2016, 30, 738–745. [Google Scholar] [CrossRef]
- Jiang, N.; Wei, Y.; Cen, Y.; Shan, L.; Zhang, Z.; Yu, P.; Wang, Y.; Xu, L. Andrographolide derivative AL-1 reduces intestinal permeability in dextran sulfate sodium (DSS)-induced mice colitis model. Life Sci. 2020, 241, 117164. [Google Scholar] [CrossRef]
- Guo, H.; Guo, H.; Xie, Y.; Chen, Y.; Lu, C.; Yang, Z.; Zhu, Y.; Ouyang, Y.; Zhang, Y.; Wang, X. Mo3Se4 nanoparticle with ROS scavenging and multi-enzyme activity for the treatment of DSS-induced colitis in mice. Redox Biol. 2022, 56, 102441. [Google Scholar] [CrossRef]
- Chen, S.; Wu, X.; Yu, Z. Juglone suppresses inflammation and oxidative stress in colitis mice. Front. Immunol. 2021, 12, 674341. [Google Scholar] [CrossRef]
- Wang, Z.; Li, S.; Cao, Y.; Tian, X.; Zeng, R.; Liao, D.F.; Cao, D. Oxidative Stress and Carbonyl Lesions in Ulcerative Colitis and Associated Colorectal Cancer. Oxidative Med. Cell. Longev. 2016, 2016, 9875298. [Google Scholar] [CrossRef]
- Zhang, Y.; Tao, M.; Chen, C.; Zhao, X.; Feng, Q.; Chen, G.; Fu, Y. BAFF blockade attenuates DSS-induced chronic colitis via inhibiting NLRP3 inflammasome and NF-κB activation. Front. Immunol. 2022, 13, 783254. [Google Scholar] [CrossRef] [PubMed]
- Barbara, J.A.; Van ostade, X.; Lopez, A. Tumour necrosis factor-alpha (TNF-alpha): The good, the bad and potentially very effective. Immunol. Cell. Biol. 1996, 74, 434–443. [Google Scholar] [CrossRef] [PubMed]
- Ivanova-Todorova, E.; Bochev, I.; Dimitrov, R.; Belemezova, K.; Mourdjeva, M.; Kyurkchiev, S.; Kinov, P.; Altankova, I.; Kyurkchiev, D. Conditioned medium from adiposetissue-derived mesenchymal stem cells induces CD4+FOXP3+ cells and increases IL-10 secretion. J. BioMed Biotechnol. 2012, 2012, 295167. [Google Scholar] [CrossRef] [PubMed]
- Xiang, X.W.; Wang, R.; Yao, L.W.; Zhou, Y.F.; Sun, P.L.; Zheng, B.; Chen, Y.F. Anti-Inflammatory Effects of Mytilus coruscus Polysaccharide on RAW264.7 Cells and DSS-Induced Colitis in Mice. Mar. Drugs 2021, 19, 468. [Google Scholar] [CrossRef]
- Chen, W.; Li, Y.; Yang, S.; Yue, L.; Jiang, Q.; Xia, W. Synthesis and antioxidant properties of chitosan and carboxymethyl chitosan-stabilized selenium nanoparticles. Carbohydr. Polym. 2015, 132, 574–581. [Google Scholar] [CrossRef]
- Chen, X.; He, X.; Sun, J.; Wang, Z. Phytochemical composition, antioxidant activity, α-glucosidase and acetylcholinesterase inhibitory activity of quinoa extract and its fractions. Molecules 2022, 27, 2420. [Google Scholar] [CrossRef]
- Wu, Z.; Huang, S.; Li, T.; Li, N.; Han, D.; Zhang, B.; Xu, Z.Z.; Zhang, S.; Pang, J.; Wang, S.; et al. Gut microbiota from green tea polyphenol-dosed mice improves intestinal epithelial homeostasis and ameliorates experimental colitis. Microbiome 2021, 9, 184. [Google Scholar] [CrossRef]



| Lipid Subclass Names | Lipid Subclass Abbreviations | Content (%) |
|---|---|---|
| Sphingosine | SPH | 55.01 |
| Ceramide | Cer | 11.01 |
| Phosphatidylethanolamines | PE | 8.23 |
| Triglyceride | TG | 7.46 |
| Phosphatidylcholine | PC | 6.36 |
| Diglyceride | DG | 5.21 |
| Phosphatidylglycerols | PG | 1.79 |
| Zymosteryl | ZyE | 1.41 |
| Wax ester | WE | 0.84 |
| Monoglyceride | MG | 0.72 |
| Phosphatidylinositols | PI | 0.6 |
| Phosphatidylserines | PS | 0.42 |
| Sphingomyelin | SM | 0.32 |
| Cardiolipin | CL | 0.29 |
| Phosphatidic acid | PA | 0.071 |
| Lyso-phosphatidylcholine | LPC | 0.07 |
| Lyso-phosphatidylethanolamine | LPE | 0.055 |
| Lyso-phosphatidylinositol | LPI | 0.039 |
| Coenzyme | CO | 0.011 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, F.; Yuan, M.; Shao, C.; Ji, N.; Zhang, H.; Li, C. Momordica charantia-Derived Extracellular Vesicles Provide Antioxidant Protection in Ulcerative Colitis. Molecules 2023, 28, 6182. https://doi.org/10.3390/molecules28176182
Wang F, Yuan M, Shao C, Ji N, Zhang H, Li C. Momordica charantia-Derived Extracellular Vesicles Provide Antioxidant Protection in Ulcerative Colitis. Molecules. 2023; 28(17):6182. https://doi.org/10.3390/molecules28176182
Chicago/Turabian StyleWang, Feng, Meng Yuan, Chenqi Shao, Nan Ji, Haifeng Zhang, and Chunmei Li. 2023. "Momordica charantia-Derived Extracellular Vesicles Provide Antioxidant Protection in Ulcerative Colitis" Molecules 28, no. 17: 6182. https://doi.org/10.3390/molecules28176182
APA StyleWang, F., Yuan, M., Shao, C., Ji, N., Zhang, H., & Li, C. (2023). Momordica charantia-Derived Extracellular Vesicles Provide Antioxidant Protection in Ulcerative Colitis. Molecules, 28(17), 6182. https://doi.org/10.3390/molecules28176182





