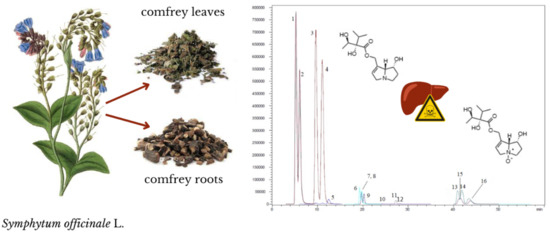
LC-MS/MS Evaluation of Pyrrolizidine Alkaloids Profile in Relation to Safety of Comfrey Roots and Leaves from Polish Sources
Abstract
:1. Introduction
2. Results
2.1. Optimization of Separation Conditions
2.2. Identification of Pyrrolizidine Alkaloids
2.3. Semi-Quantitative Analysis of Pyrrolizidine Alkaloids in Comfrey Root and Leaf
2.4. Quantitative Analysis of Pyrrolizidine Alkaloids in Comfrey Root and Leaf
2.5. Factor Analysis of Pyrrolizidine Alkaloids Distribution in the Roots and Leaves of Comfrey
- the anatomical part of the plant (root–leaf)
- growing conditions (differentiating between garden conditions and those used by producers)
- the geographical origin of the samples (various regions of Poland)
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Plant Material and Extraction
4.3. Separation Conditions
4.4. Qualitative Analysis
4.5. Quantitative and Semi-Quantitative Analysis
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Rode, D. Comfrey toxicity revisited. Trends Pharmacol. Sci. 2002, 23, 497–499. [Google Scholar] [CrossRef] [PubMed]
- Staiger, C. Comfrey root: From tradition to modern clinical trials. Wien. Med. Wochenschr. 2013, 163, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Kimel, K.; Krauze-Baranowska, M. Skuteczność i bezpieczeństwo stosowania korzenia Symphytum officinale L.—Przegląd danych literaturowych [The efficacy and safety of Symphytum officinale L. root—A review of literature data]. Post. Fitoter. 2021, 22, 23–31. [Google Scholar] [CrossRef]
- Salehi, B.; Sharopov, F.; Tumer, T.B.; Ozleyen, A.; Rodriguez-Perez, C.; Ezzat, S.M.; Azzini, E.; Hosseinabadi, T.; Butnariu, M.; Sarac, I.; et al. Symphytum Species: A Comprehensive Review on Chemical Composition, Food Applications and Phytopharmacology. Molecules 2019, 24, 2272. [Google Scholar] [CrossRef] [PubMed]
- EMA. Assessment Report on Symphytum officinale L., Radix; Committee on Herbal Medicine Products (HMPC), EMA: London, UK, 2015. [Google Scholar]
- FDA. FDA Advises Dietary Supplement Manufacturers to Remove Comfrey Products from the Market; Office of Nutritional Products, Labeling, and Dietary Supplements: Washington, DC, USA, 2001. [Google Scholar]
- Frost, R.; O’Meara, S.; MacPherson, H. The external use of comfrey: A practitioner survey. Complement. Ther. Clin. Pract. 2014, 20, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Trifan, A.; Wolfram, E.; Esslinger, N.; Grubelnik, A.; Skalicka-Wozniak, K.; Minceva, M.; Luca, S.V. Globoidnan A, rabdosiin and globoidnan B as new phenolic markers in European-sourced comfrey (Symphytum officinale L.) root samples. Phytochem. Anal. 2021, 32, 482–494. [Google Scholar] [CrossRef] [PubMed]
- Madge, I.; Gehling, M.; Schone, C.; Winterhalter, P.; These, A. Pyrrolizidine alkaloid profiling of four Boraginaceae species from Northern Germany and implications for the analytical scope proposed for monitoring of maximum levels. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess 2020, 37, 1339–1358. [Google Scholar] [CrossRef]
- Liu, F.; Wan, S.Y.; Jiang, Z.J.; Li, S.F.Y.; Ong, E.S.; Osorio, J.C.C. Determination of pyrrolizidine alkaloids in comfrey by liquid chromatography-electrospray ionization mass spectrometry. Talanta 2009, 80, 916–923. [Google Scholar] [CrossRef]
- Altamirano, J.C.; Gratz, S.R.; Wolnik, K.A. Investigation of pyrrolizidine alkaloids and their N-oxides in commercial comfrey-containing products and botanical materials by liquid chromatography electrospray ionization mass spectrometry. J. AOAC Int. 2005, 88, 406–412. [Google Scholar] [CrossRef]
- Wang, Z.Q.; Han, H.L.; Wang, C.; Zheng, Q.Q.; Chen, H.P.; Zhang, X.C.; Hou, R. Hepatotoxicity of Pyrrolizidine Alkaloid Compound Intermedine: Comparison with Other Pyrrolizidine Alkaloids and Its Toxicological Mechanism. Toxins 2021, 13, 849. [Google Scholar] [CrossRef]
- Wang, Z.Q.; Qiao, L.; Zheng, Q.Q.; Han, H.L.; Li, Z.G.; Zhang, X.C.; Chen, H. Combined Hepatotoxicity and Toxicity Mechanism of Intermedine and Lycopsamine. Toxins 2022, 14, 633. [Google Scholar] [CrossRef] [PubMed]
- Jedlinszki, N.; Balazs, B.; Csanyi, E.; Csupor, D. Penetration of lycopsamine from a comfrey ointment through human epidermis. Regul. Toxicol. Pharmacol. 2017, 83, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Kuchta, K.; Schmidt, M. Safety of medicinal comfrey cream preparations (Symphytum officinales L.): The pyrrolizidine alkaloid lycopsamine is poorly absorbed through human skin. Regul. Toxicol. Pharmacol. 2020, 118, 104784. [Google Scholar] [CrossRef] [PubMed]
- Betz, J.M.; Eppley, R.M.; Taylor, W.C.; Andrzejewski, D. Determination of Pyrrolizidine Alkaloids in Commercial Comfrey Products (Symphytum sp.). J. Pharm. Sci. 1994, 83, 649–653. [Google Scholar] [CrossRef]
- Roeder, E. Medicinal Plants in Europe Containing Pyrrolizidine Alkaloids. Pharmazie 1995, 50, 83–98. [Google Scholar]
- Kopp, T.; Abdel-Tawab, M.; Mizaikoff, B. Extracting and Analyzing Pyrrolizidine Alkaloids in Medicinal Plants: A Review. Toxins 2020, 12, 320. [Google Scholar] [CrossRef]
- Casado, N.; Morante-Zarcero, S.; Sierra, I. The concerning food safety issue of pyrrolizidine alkaloids: An overview. Trends Food Sci. Technol. 2022, 120, 123–139. [Google Scholar] [CrossRef]
- Council of Europe. European Pharmacopoeia: Supplement 11.2, 11th ed.; Council of Europe: Strasbourg, France, 2023. [Google Scholar]
- Viteritti, E.; Oliva, E.; Eugelio, F.; Fanti, F.; Palmieri, S.; Bafile, E.; Compagnone, D.; Sergi, M. Analysis of carbazole alkaloids in Murraya koenigii by means of high-performance liquid chromatography coupled to Tandem mass spectrometry with a predictive multi experiment approach. J. Chromatogr. Open. 2022, 2, 100055. [Google Scholar] [CrossRef]
- Oppermann, U.; Moreau, S.; Burkli, A. Determination of Pyrrolizidine Alkaloids in Teas and Herbal Teas by LC-MS/MS. In Proceedings of the Recent Advances in Food Analysis Symposium, Prague, Czech Republic, 5–8 November 2019. [Google Scholar]
- Trifan, A.; Opitz, S.E.; Josuran, R.; Grubelnik, A.; Esslinger, N.; Peter, S.; Bräm, S.; Meier, N.; Wolfram, E. Is comfrey root more than toxic pyrrolizidine alkaloids? Salvianolic acids among antioxidant polyphenols in comfrey (Symphytum officinale L.) roots. Food Chem. Toxicol. 2018, 112, 178–187. [Google Scholar] [CrossRef]
- Mroczek, T.; Ndjoko-Ioset, K.; Glowniak, K.; Miekiewicz-Capala, A.; Hostettmann, K. Investigation of Symphytum cordatum alkaloids by liquid-liquid partitioning, thin-layer chromatography and liquid chromatography-ion-trap mass spectrometry. Anal. Chim. Acta 2006, 566, 157–166. [Google Scholar] [CrossRef]
- Skoneczny, D.; Weston, P.A.; Zhu, X.C.; Gurr, G.M.; Callaway, R.M.; Weston, L.A. Metabolic Profiling of Pyrrolizidine Alkaloids in Foliage of Two Echium spp. Invaders in Australia-A Case of Novel Weapons? Int. J. Mol. Sci. 2015, 16, 26721–26737. [Google Scholar] [CrossRef] [PubMed]
- Wuilloud, J.C.A.; Gratz, S.R.; Gamble, B.M.; Wolnik, K.A. Simultaneous analysis of hepatotoxic pyrrolizidine alkaloids and N-oxides in comfrey root by LC-ion trap mass spectrometry. Analyst 2004, 129, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Sixto, A.; Niell, S.; Heinzen, H. Straightforward Determination of Pyrrolizidine Alkaloids in Honey through Simplified Methanol Extraction (QuPPE) and LC-MS/MS Modes. ACS Omega 2019, 4, 22632–22637. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.W.; Stegelmeier, B.L.; Colegate, S.M.; Gardner, D.R.; Panter, K.E.; Knoppel, E.L.; Hall, J.O. The comparative toxicity of a reduced, crude comfrey (Symphytum officinale) alkaloid extract and the pure, comfrey-derived pyrrolizidine alkaloids, lycopsamine and intermedine in chicks (Gallus gallus domesticus). J. Appl. Toxicol. 2016, 36, 716–725. [Google Scholar] [CrossRef] [PubMed]
- Brauchli, J.; Lüthy, J.; Zweifel, U.; Schlatter, C. Pyrrolizidine alkaloids from Symphytum officinale L. and their percutaneous absorption in rats. Experientia 1982, 38, 1085–1087. [Google Scholar] [CrossRef]
- Avila, C.; Breakspear, I.; Hawrelak, J.; Salmond, S.; Evans, S. A systematic review and quality assessment of case reports of adverse events for borage (Borago officinalis), coltsfoot (Tussilago farfara) and comfrey (Symphytum officinale). Fitoterapia 2020, 142, 104519. [Google Scholar] [CrossRef]
- International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use, ICH Harmonized Tripartite Guideline, Validation of Analytical Procedures: Text and Methodology Q2(R1); ICH Secretariat, Geneva, Switzerland 2005. Available online: https://www.gmp-compliance.org/files/guidemgr/Q2(R1).pdf (accessed on 20 August 2023).
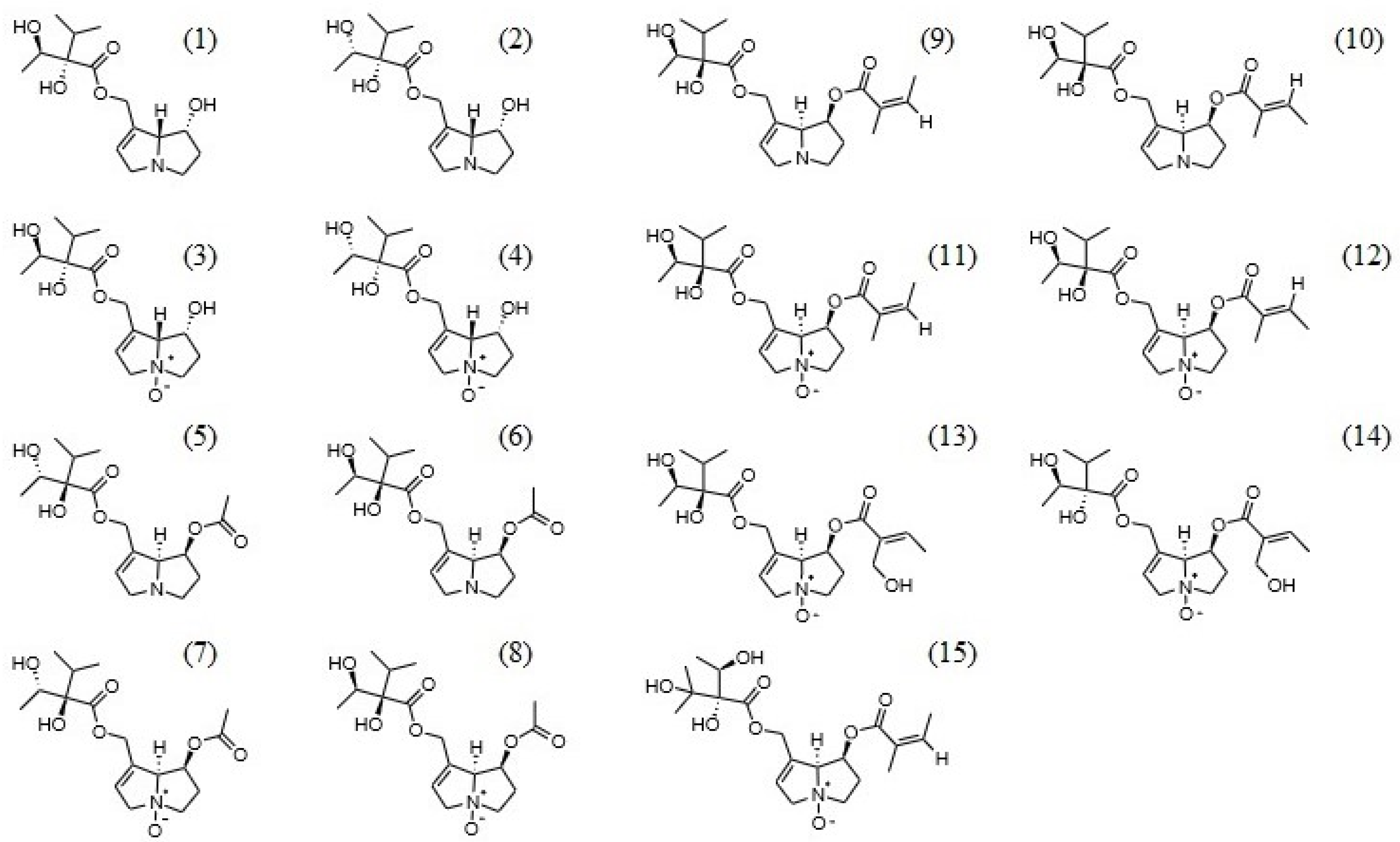


| No. | Compound Name | Retention Time (min) | Precursor Ion [M + H]+ (m/z) | MS2 (m/z) (CE [eV]) |
|---|---|---|---|---|
| 1 | Intermedine * | 5.25 | 300.1 | 94.1 (−28), 138.05 (−19). 156.1 (−30) |
| 2 | Lycopsamine * | 6.07 | 300.1 | 94.1 (−28), 156.05 (−30), 138.1 (−20) |
| 3 | Intermedine N-oxide * | 9.62 | 316.1 | 172.05 (−28), 138.05 (−29), 111 (−42) |
| 4 | Lycopsamine N-oxide | 11.04 | 316.1 | 172.05 (−28), 138.05 (−29), 111 (−42) |
| 5 | Dihydrointermedine N-oxide/ Dihydrolycopsamine N-oxide | 12.48/13.16 | 318.2 | 174.1 (−30), 113.05 (−40), 156.1 (−40) |
| 6 | 7′-acetylintermedine | 19.28 | 342.2 | 120.07 (−25), 180.09 (−15), 198.07 (−30) |
| 7 | 7′-acetyllycopsamine | 19.66 | 342.2 | 120.08 (−25), 180.1 (−15), 198.08 (−30) |
| 8 | 7′-acetylintermedine N-oxide | 19.76 | 358.1 | 214.01 (−30), 180.09 (−30), 137.08 (−30) |
| 9 | 7′-acetyllycopsamine N-oxide | 20.25 | 358.1 | 214.01 (−30), 180.1 (−30), 137.07 (−30) |
| 10 | 7′-sarracinyl-9- trachelanthylretronecine N-oxide | 22.79 | 414.2 | 270.1 (−30), 120.07 (−40), 137.08 (−40) |
| 11 | 7′-sarracinyl-9- viridiflorylretronecine N-oxide | 24.31 | 414.2 | 270.1 (−30), 137.08 (−35), 120.08 (−45) |
| 12 | Echimidine N-oxide | 26.20/27.27/28.14 | 414.2 | 254.1 (−30), 396.07 (−25), 352.1 (−25), 137.08 (−30) |
| 13 | Symphytine | 40.92 | 382.2 | 120.08 (−25), 238.1 (−30), 220.1 (−15), 138.08 (−35) |
| 14 | Symlandine | 41.86 | 382.2 | 120.07 (−25), 220.1 (−15), 238.1 (−30), 138.08 (−35) |
| 15 | Symphytine N-oxide | 41.14 | 398.2 | 254.1 (−30), 137.07 (−35), 220.1 (−15) |
| 16 | Symlandine N-oxide | 43.83 | 398.2 | 254.1 (−30), 137.08 (−35), 220.1 (−15) |
| 17 | 3′-acetylsymphytine N-oxide and its isomers | 51.28/52.79/52.31/52.96 | 440.2 | 254.1 (−30), 220.1 (−30), 137.07 (−40), 120.07 (−35) |
| No. | Compound Name | Roots | Leaves | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| GR1 | GR2 | HR1 | HR2 | HR3 | NR1 | HL2 | HL3 | HL4 | ||
| 1 | Intermedine | + | ++ | +++ | +++ | ++ | ++ | ++++ | +++ | ++ |
| 2 | Lycopsamine | ++ | ++ | +++ | +++ | +++ | ++ | ++++ | ++++ | +++ |
| 3 | Intermedine N-oxide | +++ | ++++ | ++++ | ++++ | ++++ | +++ | ++++ | +++ | +++ |
| 4 | Lycopsamine N-oxide | ++++ | ++++ | ++++ | ++++ | ++++ | ++++ | ++++ | +++ | ++++ |
| 5 | Dihydrointermedine N-oxide/ Dihydrolycopsamine N-oxide | + | + | + | + | + | + | + | + | + |
| 6 | 7′-acetylintermedine | + | + | ++ | ++ | ++ | ++ | + | + | + |
| 7 | 7′-acetyllycopsamine | ++ | + | ++ | ++ | ++ | ++ | + | + | + |
| 8 | 7′-acetylintermedine N-oxide | ++ | +++ | ++ | +++ | +++ | +++ | + | + | + |
| 9 | 7′-acetyllycopsamine N-oxide | +++ | +++ | ++ | +++ | +++ | ++++ | + | + | + |
| 10 | 7′-sarracinyl-9- trachelanthylretronecine N-oxide | + | + | + | + | + | + | + | + | + |
| 11 | 7′-sarracinyl-9- 7′-sarracinyl-9- viridiflorylretronecine N-oxide | + | + | + | + | + | + | + | + | + |
| 12 | Echimidine N-oxide | + | + | + | + | + | + | + | + | ++ |
| 13 | Symphytine | + | + | ++ | + | + | + | + | + | + |
| 14 | Symlandine | + | + | ++ | + | + | + | + | + | + |
| 15 | Symphytine N-oxide | + | + | ++ | + | + | + | + | + | + |
| 16 | Symlandine N-oxide | + | + | ++ | + | + | + | + | + | + |
| 17 | 3′-acetylsymphytine N-oxide and its isomers | – | – | + | + | + | + | – | – | – |
| Plant Material | Intermedine [ng/mL] | Lycopsamine [ng/mL] | Intermedine N-oxide [ng/mL] | Lycopsamine N-oxide [ng/mL] |
|---|---|---|---|---|
| GR1 | 0.307 ± 0.034 | 2.24 ± 0.17 | 5.15 ± 0.30 | 20.42 ± 1.23 |
| GR2 | 0.832 ± 0.085 | 1.54 ± 0.16 | 8.09 ± 0.59 | 13.49 ± 0.4 |
| HR1 | 2.07 ± 0.21 | 2.51 ± 0.12 | 62.30 ± 8.76 | 64.5 ± 2.5 |
| HR2 | 7.83 ± 0.81 | 6.95 ± 0.43 | 8.10 ± 0.81 | 14.65 ± 0.83 |
| HR3 | 5.38 ± 0.52 | 6.31 ± 0.23 | 60.85 ± 2.70 | 58.3 ± 7.9 |
| NR1 | 1.56 ± 0.18 | 2.82 ± 0.28 | 18.80 ± 1.10 | 33.74 ± 1.36 |
| HL2 | 1.49 ± 0.16 | 2.59 ± 0.30 | 2.63 ± 0.15 | 4.500 ± 0.066 |
| HL3 | 3.00 ± 0.10 | 3.15 ± 0.19 | 2.63 ± 0.06 | 2.61 ± 0.11 |
| HL4 | 2.15 ± 0.19 | 4.99 ± 0.44 | 6.22 ± 0.12 | 10.9 ± 0.360 |
| Plant Material Origin | Intermedine [mg/g d.w.] | Lycopsamine [mg/g d.w.] | Intermedine N-oxide [mg/g d.w.] | Lycopsamine N-oxide [mg/g d.w.] | ΣPas * |
|---|---|---|---|---|---|
| GR1 a | 0.0077 ± 0.00090 bcdefghi | 0.0560 ± 0.0042 bdefgh | 0.1287 ± 0.0075 bcdefghi | 0.5105 ± 0.0307 bcdefghi | 0.70 ± 0.043 |
| GR2 b | 0.0208 ± 0.0021 acdefh | 0.0385 ± 0.0040 acdefgi | 0.2022 ± 0.0147 bcefghi | 0.3372 ± 0.0100 acefghi | 0.60 ± 0.31 |
| HR1 c | 0.0517 ± 0.0052 abdefghi | 0.0627 ± 0.0030 bdefghi | 1.5575 ± 0.1150 abdfghi | 1.6125 ± 0.0625 abdefghi | 3.28 ± 0.19 |
| HR2 d | 0.1957 ± 0.0202 abcefghi | 0.1737 ± 0.0107 abcefghi | 0.2057 ± 0.0147 acefghi | 0.3662 ± 0.0207 acefghi | 0.94 ± 0.0665 |
| HR3 e | 0.1345 ± 0.0130 abcdfghi | 0.1577 ± 0.0057 abcdfghi | 1.5212 ± 0.0675 abdfghi | 1.4575 ± 0.1975 abcdfghi | 3.27 ± 0.284 |
| NR1 f | 0.0390 ± 0.0045 abcdeghi | 0.0705 ± 0.0070 abcdeghi | 0.4700 ± 0.0275 abcdeghi | 0.8435 ± 0.0340 abcdeghi | 1.42 ± 0.073 |
| HL2 g | 0.0149 ± 0.0016 acdefh | 0.0259 ± 0.003 abcdefi | 0.0264 ± 0.0017 abcdef | 0.0449 ± 0.0007 abcdef | 0.11 ± 0.0070 |
| HL3 h | 0.0300 ± 0.0010 abcdefgi | 0.0315 ± 0.0019 acdefi | 0.0263 ± 0.0006 abcdef | 0.0261 ± 0.0011 abcdefi | 0.11 ± 0.0046 |
| HL4 i | 0.0215 ± 0.0019 acdefh | 0.0499 ± 0.0044 bcdefgh | 0.0622 ± 0.0012 abcdef | 0.1090 ± 0.0036 abcdefh | 0.24 ± 0.11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kimel, K.; Godlewska, S.; Gleńsk, M.; Gobis, K.; Ośko, J.; Grembecka, M.; Krauze-Baranowska, M. LC-MS/MS Evaluation of Pyrrolizidine Alkaloids Profile in Relation to Safety of Comfrey Roots and Leaves from Polish Sources. Molecules 2023, 28, 6171. https://doi.org/10.3390/molecules28166171
Kimel K, Godlewska S, Gleńsk M, Gobis K, Ośko J, Grembecka M, Krauze-Baranowska M. LC-MS/MS Evaluation of Pyrrolizidine Alkaloids Profile in Relation to Safety of Comfrey Roots and Leaves from Polish Sources. Molecules. 2023; 28(16):6171. https://doi.org/10.3390/molecules28166171
Chicago/Turabian StyleKimel, Katarzyna, Sylwia Godlewska, Michał Gleńsk, Katarzyna Gobis, Justyna Ośko, Małgorzata Grembecka, and Mirosława Krauze-Baranowska. 2023. "LC-MS/MS Evaluation of Pyrrolizidine Alkaloids Profile in Relation to Safety of Comfrey Roots and Leaves from Polish Sources" Molecules 28, no. 16: 6171. https://doi.org/10.3390/molecules28166171
APA StyleKimel, K., Godlewska, S., Gleńsk, M., Gobis, K., Ośko, J., Grembecka, M., & Krauze-Baranowska, M. (2023). LC-MS/MS Evaluation of Pyrrolizidine Alkaloids Profile in Relation to Safety of Comfrey Roots and Leaves from Polish Sources. Molecules, 28(16), 6171. https://doi.org/10.3390/molecules28166171