Simultaneous Quantification of Nine Target Compounds in Traditional Korean Medicine, Bopyeo-Tang, Using High-Performance Liquid Chromatography–Photodiode Array Detector and Ultra-Performance Liquid Chromatography–Tandem Mass Spectrometry
Abstract
1. Introduction
2. Results and Discussion
2.1. HPLC–PDA Analysis
2.1.1. Selection of Target Compounds in BPT for Simultaneous Quantification by HPLC–PDA
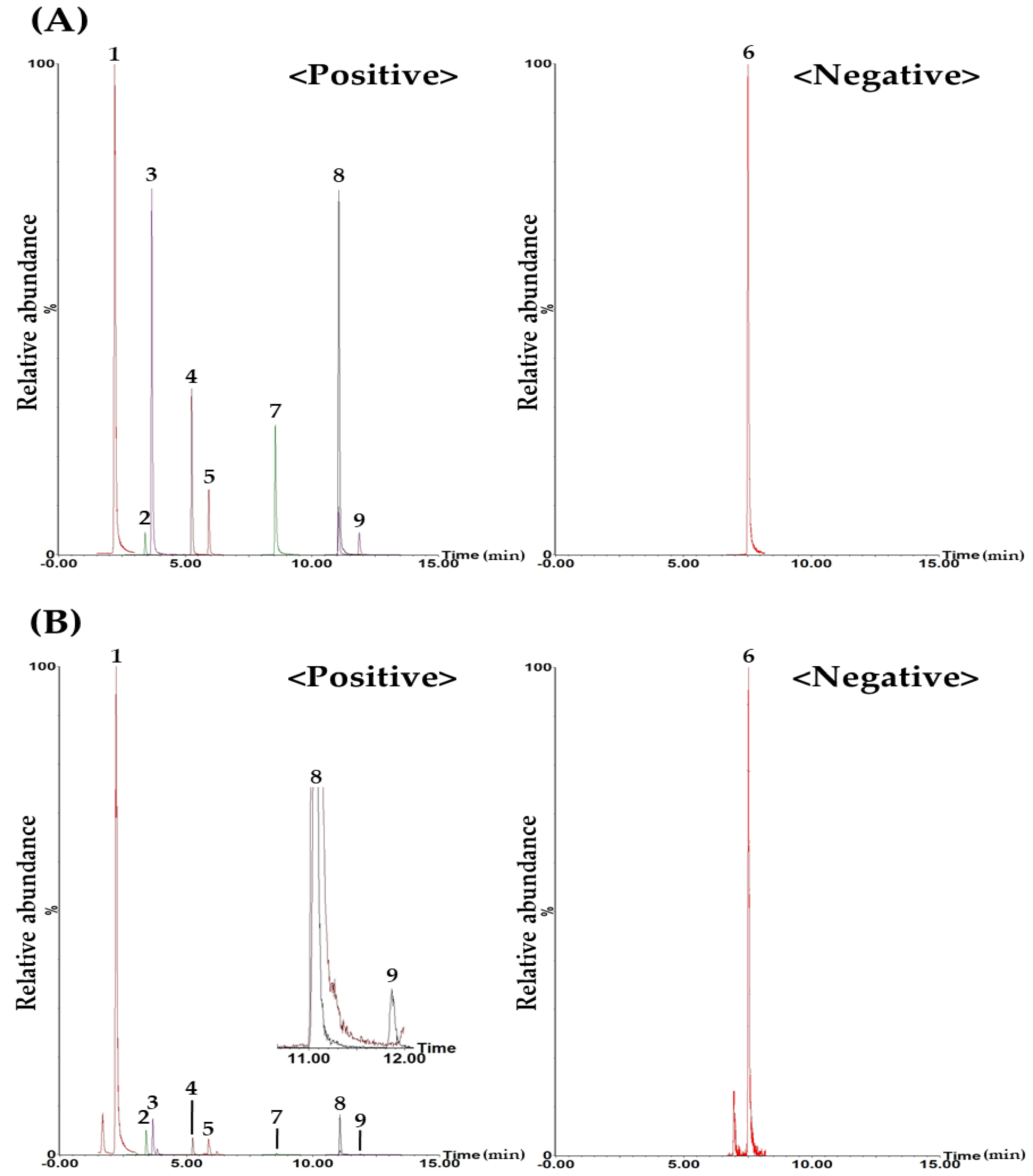
2.1.2. HPLC Operating Conditions for Simultaneous Quantification of BPT
2.1.3. Validation of the Established HPLC–PDA Analytical Method
2.1.4. Simultaneous Quantification of Nine Target Compounds in a BPT Sample by the HPLC–PDA Analytical Method
2.2. UPLC–MS/MS Simultaneous Analysis
2.2.1. UPLC–MS/MS Multiple Reaction Monitoring (MRM) Method for Simultaneous Analysis
2.2.2. Validation of the Developed UPLC–MS/MS Analytical Method
2.2.3. Simultaneous Determination of the Nine Target Components in 70% Ethanol Extract of Freeze-Dried BPT
3. Materials and Methods
3.1. Plant Materials
3.2. Chemicals and Reagents
3.3. Preparation of the BPT Sample
3.4. Equipment and Analytical Conditions for HPLC–PDA Simultaneous Quantification
3.5. Equipment and Analytical Conditions for UPLC–MS/MS Simultaneous Quantification
3.6. Validation of Established Assays in HPLC–PDA and UPLC–MS/MS Systems
4. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, D.; Zhang, Y.; Gao, Y.; Chai, X.; Pi, R.; Chan, G.; Hu, Y. Translating traditional herbal formulas into modern drugs: A network-based analysis of Xiaoyao decoction. Chin. Med. 2020, 15, 25. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Liu, W.; Hu, Z.; Yang, M.; Li, J.; Fan, X.; Pan, H. A systems pharmacology approach for identifying the multiple mechanisms of action of the Wei Pi Xiao decoction for the treatment of gastric precancerous lesions. Evid. Based Complement. Alternat. Med. 2019, 2019, 1562707. [Google Scholar] [CrossRef] [PubMed]
- Xue, Z.; Li, Y.; Zhou, M.; Liu, Z.; Fan, G.; Wang, X.; Zhu, Y.; Yang, J. Traditional herbal medicine discovery for the treatment and prevention of pulmonary arterial hypertension. Front. Pharmacol. 2021, 12, 720873. [Google Scholar] [CrossRef] [PubMed]
- Heo, J. Donguibogan; Namsandang: Seoul, Korea, 2007; p. 470. [Google Scholar]
- Shen, J.; Zhu, X.; Chen, Y.; Li, W.; Liu, H.; Chu, C.; Zhang, Y.; Xu, C.; Tong, P.; Yu, X.; et al. Bufei decoction improves lung-qi deficiency syndrome of chronic obstructive pulmonary disease in rats by regulating the balance of Th17/Treg cells. Evid. Based Complement. Alternat. Med. 2022, 2022, 1459232. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Tian, Y.; Zhao, P.; Feng, S.; Han, X.; Li, J. Network pharmacology analysis uncovers the effect on apoptotic pathway by Bu-Fei formula for COPD treatment. J. Ethnopharmacol. 2022, 289, 115022. [Google Scholar] [CrossRef]
- Timalsina, D.; Pokhrel, K.P.; Bhusal, D. Pharmacologic activities of plant-derived natural products on respiratory diseases and inflammations. BioMed Res. Int. 2021, 2021, 1636816. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wu, H.; Li, Y.; Liu, J.; Jia, Z.; Xu, W.; Xiao, H.; Wang, W. Aster tataricus attenuates asthma efficiently by simultaneously inhibiting tracheal ring contraction and inflammation. Biomed. Pharmacother. 2020, 130, 110616. [Google Scholar] [CrossRef]
- Su, X.D.; Jang, H.J.; Wang, C.Y.; Lee, S.W.; Rho, M.C.; Kim, Y.H.; Yang, S.Y. Anti-inflammatory potential of saponins from Aster tataricus via NF-κB/MAPK activation. J. Nat. Prod. 2019, 82, 1139–1148. [Google Scholar] [CrossRef]
- Yu, J.; Wen, J.; Ying, Y.; Yin, W.; Zhang, S.; Pang, W.; Wang, C.; Bian, Y.; Yuan, J.; Yan, J.; et al. Astragaloside trigger autophage: Implication a potential therapeutic strategy for pulmonary fibrosis. Biomed. Pharmacother. 2022, 154, 113603. [Google Scholar] [CrossRef]
- Jiang, S.T.; Han, S.Y.; Pang, L.N.; Jiao, Y.N.; He, X.R.; Li, P.P. Bu-Fei decoction and modified Bu-Fei decoction inhibit the growth of non-small cell lung cancer, possibly via inhibition of apurinic/apyrimidinic endonuclease 1. Int. J. Mol. Med. 2018, 41, 2128–2138. [Google Scholar] [CrossRef]
- Pang, L.; Han, S.; Jiao, Y.; Jiang, S.; He, X.; Li, P. Bu Fei decoction attenuates the tumor associated macrophage stimulated proliferation, migration, invasion and immunosuppression of non-small cell lung cancer, partially via IL-10 and PD-L1 regulation. Int. J. Oncol. 2017, 51, 25–38. [Google Scholar] [CrossRef] [PubMed]
- He, X.R.; Han, S.Y.; Li, X.H.; Zheng, W.X.; Pang, L.N.; Jiang, S.T.; Li, P.P. Chinese medicine Bu-Fei decoction attenuates epithelial-mesenchymal transition of non-small cell lung cancer via inhibition of transforming growth factor β1 signaling pathway in vitro and in vivo. J. Ethnopharmacol. 2017, 204, 45–57. [Google Scholar] [CrossRef]
- Kim, J.H.; Doh, E.J.; Lee, G. Quantitative comparison of the marker compounds in different medicinal parts of Morus alba L. using high-performance liquid chromatography-diode array detector with chemometric analysis. Molecules 2020, 25, 5592. [Google Scholar] [CrossRef]
- Hwang, S.Y.; Hwang, B.Y.; Choi, W.H.; Jung, H.J.; Huh, J.D.; Lee, K.S.; Ro, J.S. Quantitative determination of 5-hydroxymethyl-2-furaldehyde in the Rehmanniae Radix Preparata samples at various processing stages. Korean J. Pharmacogn. 2001, 32, 116–120. [Google Scholar]
- Wang, H.P.; Zhang, Y.B.; Yang, X.W.; Zhao, D.Q.; Wang, Y.P. Rapid characterization of ginsenosides in the roots and rhizomes of Panax ginseng by UPLC–DAD–QTOF–MS/MS and simultaneous determination of 19 ginsenosides by HPLC–ESI-MS. J. Ginseng Res. 2016, 40, 382–394. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Shi, H.; Zhang, Q.; Liu, Y.; Wan, C.; Zhang, L. Simultaneous determination of five components in Aster tataricus by ultra performance liquid chromatography–tandem mass spectrometry. J. Chromatogr. Sci. 2016, 54, 500–506. [Google Scholar] [CrossRef]
- Song, J.Z.; Yiu, H.H.W.; Qiao, C.F.; Han, Q.B.; Xu, H.X. Chemical comparison and classification of Radix Astragali by determination of isoflanonoids and astragalosides. J. Pharm. Biomed. Anal. 2008, 47, 399–406. [Google Scholar] [CrossRef]
- Chen, S.; Shi, J.; Zou, L.; Liu, X.; Tang, R.; Ma, J.; Wang, C.; Tan, M.; Chen, J. Quality evaluation of wild and cultivated Schisandrae chinensis fructus based on simultaneous determination of multiple bioactive constituents combined with multivariate statistical analysis. Molecules 2019, 24, 1335. [Google Scholar] [CrossRef]
- Ye, J.; Zhang, X.; Dai, W.; Yan, S.; Huang, H.; Liang, X.; Li, Y.; Zhang, W. Chemical fingerprinting of Liuwei Dihuang Pill and simultaneous determination of its major bioactive constituents by HPLC coupled with multiple detections of DAD, ELSD and ESI-MS. J. Pharm. Biomed. Anal. 2009, 49, 638–645. [Google Scholar] [CrossRef]
- Chang, Y.E.; Yao, H.T.; Chien, D.S.; YeH, T.K. High-performance liquid chromatography–electrospray mass spectrometry for the simultaneous determination of multiple active components in Sheng-mai san, a prescription of traditional Chinese medicine. Phytochem. Anal. 2008, 19, 258–265. [Google Scholar] [CrossRef]
- Wang, S.; Liu, X.M.; Zhang, J.; Zhang, Y.Q. An efficient preparation of mulberroside A from the branch bark of mulberry and its effect on the inhibition of tyrosinase activity. PLoS ONE 2014, 9, e109396. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Chem, H.; Su, G.; Song, P.; Jiang, M.; Gong, J. An animal research and a chemical composition analysis of a Chinese prescription for pulmonary fibrosis: Yangfei Huoxue Decoction. J. Ethnopharmacol. 2019, 245, 112126. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Geng, C.A.; Ma, Y.B.; Huang, X.Y.; Chen, H.; Cao, T.W.; He, K.; Wang, H.; Zhang, X.M.; Chen, J.J. UFLC/MS-IT-TOF guided isolation of anti-HBV active chlorogenic acid analogues from Artemisia capillaris as a traditional Chinese herb for the treatment of hepatitis. J. Ethnopharmacol. 2014, 156, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Tsimogiannis, D.; Samiotaki, M.; Panayotou, G.; Oreopoulou, V. Characterization of flavonoid subgroups and hydroxy substitution by HPLC–MS/MS. Molecules 2007, 12, 593–606. [Google Scholar] [CrossRef] [PubMed]
- Szopa, A.; Kokotkiewicz, A.; Marzec-Wroblewska, U.; Bucinski, A.; Luczkiewicz, M.; Ekiert, H. Accumulation of dibenzocyclooctadiene lignans in agar cultures and in stationary and agitated liquid cultures of Schisandra chinensis (Turcz.) Baill. Appl. Microbiol. Biotechnol. 2016, 100, 3965–3977. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Song, F.; Liu, Z.; Liu, S. Studies on lignan constituents from Schisandra chinensis (Turcz.) Baill. fruits using high-performance liquid chromatography/electrospray ionization multiple-stage tandem mass spectrometry. J. Mass Spectrom. 2007, 42, 1148–1161. [Google Scholar] [CrossRef] [PubMed]
- WFO Plant List. Available online: http://www.wfoplantlist.org (accessed on 21 November 2023).
- Seo, C.S.; Lee, M.Y. Simultaneous quantification of eight marker components in traditional herbal formula, Haepyoyijin-tang using HPLC–PDA. Appl. Sci. 2020, 10, 3888. [Google Scholar] [CrossRef]
- Seo, C.S.; Lee, M.Y. Quality assessment of Insamyangpye decoction by liquid chromatography tandem mass spectrometry multiple reaction monitoring. Processes 2021, 9, 831. [Google Scholar] [CrossRef]
- Seo, C.S.; Lee, M.Y. Method development and validation for simultaneous analysis of eleven components for quality control of Geumgwesingihwan using HPLC–DAD and UPLC–MS/MS. Separations 2022, 9, 213. [Google Scholar] [CrossRef]
- International Conference on Harmonisation (ICH). Guidance for Industry, Q2B, Validation of Analytical Procedures: Methodology; Food and Drug Administration: Rockville, MD, USA, 1996. [Google Scholar]

| Analyte 1 | Detected Wavelength (nm) | Linear Range (μg/mL) | Regression Equation 2 | r2 | LOD (μg/mL) | LOQ (μg/mL) |
|---|---|---|---|---|---|---|
| 1 | 280 | 0.47–30.00 | y = 84,054.98x + 12,165.26 | 0.9999 | 0.06 | 0.17 |
| 2 | 325 | 0.78–50.00 | y = 17,148.00x + 2048.13 | 1.0000 | 0.08 | 0.26 |
| 3 | 325 | 0.31–20.00 | y = 37,034.13x + 2053.14 | 1.0000 | 0.02 | 0.05 |
| 4 | 250 | 0.47–30.00 | y = 54,712.87x + 6199.80 | 1.0000 | 0.05 | 0.16 |
| 5 | 325 | 0.31–20.00 | y = 36,855.19x + 938.58 | 1.0000 | 0.03 | 0.10 |
| 6 | 370 | 0.31–20.00 | y = 36,647.35x + 1188.71 | 1.0000 | 0.05 | 0.16 |
| 7 | 365 | 0.31–20.00 | y = 43,096.32x + 2275.32 | 1.0000 | 0.05 | 0.15 |
| 8 | 250 | 0.31–20.00 | y = 20,953.10x + 1163.90 | 1.0000 | 0.01 | 0.04 |
| 9 | 250 | 0.31–20.00 | y = 19,636.01x + 1391.43 | 1.0000 | 0.05 | 0.16 |
| Analyte 1 | Spiked Amount (μg/mL) | Found Amount (μg/mL) | Recovery (%) | SD 2 | RSD 3 (%) |
|---|---|---|---|---|---|
| 1 | 1.00 | 0.98 | 97.62 | 0.51 | 0.53 |
| 2.00 | 2.05 | 102.63 | 0.82 | 0.80 | |
| 4.00 | 4.17 | 104.15 | 1.06 | 1.02 | |
| 2 | 4.00 | 4.09 | 102.34 | 1.98 | 1.93 |
| 10.00 | 10.17 | 101.71 | 1.47 | 1.44 | |
| 20.00 | 21.28 | 106.40 | 0.63 | 0.59 | |
| 3 | 1.00 | 0.99 | 99.00 | 1.77 | 1.78 |
| 2.00 | 2.03 | 101.26 | 1.49 | 1.47 | |
| 4.00 | 4.02 | 100.60 | 1.16 | 1.15 | |
| 4 | 1.00 | 1.02 | 102.13 | 1.28 | 1.25 |
| 2.00 | 2.05 | 102.55 | 0.46 | 0.45 | |
| 4.00 | 4.14 | 103.49 | 0.78 | 0.75 | |
| 5 | 1.00 | 1.00 | 100.45 | 0.89 | 0.89 |
| 2.00 | 2.05 | 102.37 | 1.01 | 0.99 | |
| 4.00 | 4.05 | 101.34 | 0.74 | 0.73 | |
| 6 | 1.00 | 0.99 | 99.32 | 0.77 | 0.77 |
| 2.00 | 1.94 | 96.91 | 0.42 | 0.44 | |
| 4.00 | 3.84 | 95.93 | 0.32 | 0.33 | |
| 7 | 1.00 | 0.99 | 99.07 | 0.65 | 0.66 |
| 2.00 | 2.03 | 101.62 | 0.25 | 0.24 | |
| 4.00 | 4.01 | 100.24 | 0.32 | 0.32 | |
| 8 | 1.00 | 1.01 | 100.51 | 1.49 | 1.48 |
| 2.00 | 2.04 | 102.06 | 0.56 | 0.55 | |
| 4.00 | 4.02 | 100.44 | 0.52 | 0.52 | |
| 9 | 1.00 | 1.01 | 101.20 | 0.70 | 0.69 |
| 2.00 | 2.02 | 101.24 | 1.37 | 1.36 | |
| 4.00 | 4.09 | 102.25 | 0.44 | 0.43 |
| Analyte 1 | Conc. (μg/mL) | Intra-Day (n = 5) | Inter-Day (n = 5) | ||||
|---|---|---|---|---|---|---|---|
| Observed Conc. (μg/mL) | Precision (RSD, %) | Accuracy (%) | Observed Conc. (μg/mL) | Precision (RSD, %) | Accuracy (%) | ||
| 1 | 7.50 | 7.49 | 0.72 | 99.83 | 7.40 | 2.53 | 97.95 |
| 15.00 | 15.12 | 0.76 | 100.83 | 15.17 | 1.36 | 101.14 | |
| 30.00 | 29.51 | 0.16 | 98.36 | 29.67 | 0.77 | 98.89 | |
| 2 | 12.50 | 12.59 | 0.35 | 100.74 | 12.30 | 3.09 | 98.82 |
| 25.00 | 25.19 | 1.55 | 100.78 | 25.68 | 2.38 | 102.70 | |
| 50.00 | 49.64 | 0.34 | 99.27 | 50.27 | 1.61 | 100.54 | |
| 3 | 5.00 | 4.91 | 0.73 | 98.16 | 4.84 | 3.30 | 97.13 |
| 10.00 | 9.96 | 1.06 | 99.63 | 10.09 | 1.93 | 100.87 | |
| 20.00 | 19.65 | 0.22 | 98.23 | 19.89 | 1.34 | 99.47 | |
| 4 | 7.50 | 7.52 | 0.69 | 100.26 | 7.39 | 2.71 | 97.93 |
| 15.00 | 15.16 | 1.34 | 101.09 | 15.44 | 2.33 | 102.94 | |
| 30.00 | 29.90 | 0.41 | 99.67 | 30.24 | 1.61 | 100.79 | |
| 5 | 5.00 | 4.94 | 0.53 | 98.74 | 4.86 | 3.06 | 97.67 |
| 10.00 | 9.98 | 1.33 | 99.77 | 10.18 | 2.42 | 101.83 | |
| 20.00 | 19.86 | 0.36 | 99.29 | 20.07 | 1.48 | 100.35 | |
| 6 | 5.00 | 4.95 | 0.77 | 99.08 | 4.87 | 3.02 | 97.73 |
| 10.00 | 10.02 | 1.16 | 100.23 | 10.17 | 1.97 | 101.66 | |
| 20.00 | 19.88 | 0.32 | 99.42 | 20.09 | 1.36 | 100.44 | |
| 7 | 5.00 | 4.97 | 0.67 | 99.37 | 4.89 | 2.75 | 97.81 |
| 10.00 | 10.08 | 1.05 | 100.76 | 10.21 | 1.95 | 102.11 | |
| 20.00 | 19.94 | 0.51 | 99.69 | 20.15 | 1.51 | 100.76 | |
| 8 | 5.00 | 4.99 | 0.55 | 99.87 | 4.91 | 2.75 | 98.21 |
| 10.00 | 10.08 | 1.18 | 100.82 | 10.20 | 1.92 | 101.98 | |
| 20.00 | 19.96 | 0.43 | 99.81 | 20.17 | 1.45 | 100.85 | |
| 9 | 5.00 | 4.99 | 0.67 | 99.87 | 4.93 | 2.80 | 98.53 |
| 10.00 | 10.09 | 1.09 | 100.86 | 9.58 | 1.90 | 95.78 | |
| 20.00 | 19.95 | 0.42 | 99.77 | 18.89 | 1.40 | 94.46 | |
| Analyte 1 | HPLC−PDA Assay | ||
|---|---|---|---|
| Mean (mg/g) | SD × 10−2 | RSD (%) | |
| 1 | 3.02 | 1.43 | 0.48 |
| 2 | 1.81 | 2.72 | 1.50 |
| 3 | 0.39 | 0.39 | 1.00 |
| 4 | 0.07 | 0.06 | 0.88 |
| 5 | 0.11 | 0.26 | 2.32 |
| 6 | 0.02 | 0.02 | 1.10 |
| 7 | 0.02 | 0.04 | 1.84 |
| 8 | 0.10 | 0.10 | 0.99 |
| 9 | 0.01 | 0.01 | 1.52 |
| Analyte 1 | Ion Mode | Molecular Weight | MRM Transition | Cone Voltage (V) | Collision Energy (eV) | |
|---|---|---|---|---|---|---|
| Precursor Ion | Production Ion | |||||
| 1 | + | 126.0 | 126.9 | 109.0 | 25 | 8 |
| 2 | + | 568.2 | 569.0 | 244.9 | 32 | 16 |
| 3 | + | 354.1 | 355.0 | 162.9 | 36 | 14 |
| 4 | + | 446.1 | 447.0 | 284.9 | 32 | 16 |
| 5 | + | 516.1 | 517.1 | 162.9 | 10 | 22 |
| 6 | − | 302.0 | 300.8 | 150.9 | 52 | 20 |
| 7 | + | 286.1 | 286.9 | 152.9 | 64 | 28 |
| 8 | + | 432.2 | 433.0 | 415.0 | 26 | 8 |
| 9 | + | 416.2 | 417.1 | 341.0 | 44 | 16 |
| Analyte 1 | Retention Time (min) | Linear Range (μg/L) | Regression Equation 2 | r2 | LOD (μg/L) | LOQ (μg/L) |
|---|---|---|---|---|---|---|
| 1 | 2.20 | 78.10−1250.00 | y = 710.12x + 34,295.70 | 0.9983 | 0.45 | 1.36 |
| 2 | 3.41 | 15.60−250.00 | y = 178.53x + 591.23 | 0.9953 | 1.06 | 3.18 |
| 3 | 3.67 | 62.50−1000.00 | y = 335.50x + 2926.29 | 0.9993 | 0.22 | 0.66 |
| 4 | 5.25 | 10.90−175.00 | y = 2309.15x + 1839.68 | 0.9982 | 0.02 | 0.05 |
| 5 | 5.93 | 10.90−175.00 | y = 52.14x + 392.15 | 0.9952 | 0.50 | 1.51 |
| 6 | 7.52 | 46.90−750.00 | y = 9.49x + 429.68 | 0.9989 | 0.92 | 2.77 |
| 7 | 8.56 | 62.50−1000.00 | y = 120.00x − 562.67 | 0.9981 | 0.69 | 2.07 |
| 8 | 11.06 | 23.40−375.00 | y = 1695.32x + 1762.09 | 0.9990 | 0.06 | 0.18 |
| 9 | 11.87 | 23.40−375.00 | y = 179.70x − 860.67 | 0.9994 | 0.26 | 0.78 |
| Analyte 1 | Spiked Amount (μg/L) | Found Amount (μg/L) | Recovery (%) | SD | RSD (%) |
|---|---|---|---|---|---|
| 1 | 90.00 | 79.84 | 88.71 | 9.33 | 2.34 |
| 225.00 | 195.50 | 86.89 | 1.19 | 0.24 | |
| 450.00 | 406.67 | 90.37 | 5.35 | 0.73 | |
| 2 | 15.00 | 14.16 | 94.39 | 2.09 | 1.92 |
| 37.50 | 36.96 | 98.56 | 2.06 | 1.51 | |
| 75.00 | 73.53 | 98.04 | 3.73 | 2.1 | |
| 3 | 70.00 | 70.10 | 100.14 | 15.94 | 4.30 |
| 175.00 | 177.92 | 101.67 | 23.75 | 4.92 | |
| 350.00 | 347.41 | 99.26 | 12.82 | 1.99 | |
| 4 | 9.00 | 7.85 | 87.20 | 0.49 | 1.04 |
| 22.50 | 19.41 | 86.27 | 5.39 | 9.58 | |
| 45.00 | 39.19 | 87.08 | 0.75 | 1.01 | |
| 5 | 11.00 | 10.96 | 99.62 | 2.44 | 3.71 |
| 27.50 | 25.56 | 92.95 | 1.47 | 1.91 | |
| 55.00 | 54.42 | 98.94 | 1.57 | 1.44 | |
| 6 | 80.00 | 68.79 | 85.99 | 14.45 | 4.35 |
| 200.00 | 173.54 | 86.77 | 10.57 | 2.54 | |
| 400.00 | 352.12 | 88.03 | 17.49 | 3.33 | |
| 7 | 100.00 | 87.59 | 87.59 | 17.48 | 4.24 |
| 250.00 | 215.05 | 86.02 | 14.62 | 2.75 | |
| 500.00 | 440.45 | 88.09 | 7.29 | 1.08 | |
| 8 | 30.00 | 26.55 | 88.50 | 0.54 | 0.34 |
| 75.00 | 71.91 | 95.88 | 0.77 | 0.35 | |
| 150.00 | 139.34 | 92.89 | 0.54 | 0.19 | |
| 9 | 34.00 | 29.45 | 86.61 | 0.67 | 0.43 |
| 85.00 | 73.81 | 86.83 | 2.66 | 1.26 | |
| 170.00 | 149.70 | 88.06 | 0.43 | 0.15 |
| Analyte 1 | Conc. (μg/L) | Intra-Day | Inter-Day | ||||
|---|---|---|---|---|---|---|---|
| Observed Conc. (μg/L) | Precision (RSD, %) | Accuracy (%) | Observed Conc. (μg/L) | Precision (RSD, %) | Accuracy (%) | ||
| 1 | 156.25 | 155.12 | 3.79 | 99.28 | 152.89 | 1.27 | 97.85 |
| 312.50 | 338.06 | 9.09 | 108.18 | 338.60 | 1.42 | 108.35 | |
| 1250.00 | 1134.75 | 2.58 | 90.78 | 1194.31 | 4.33 | 95.54 | |
| 2 | 31.25 | 29.09 | 9.14 | 93.08 | 31.81 | 7.60 | 101.78 |
| 62.50 | 66.16 | 3.34 | 105.85 | 65.40 | 2.83 | 104.64 | |
| 250.00 | 237.67 | 3.98 | 95.07 | 241.67 | 1.62 | 96.67 | |
| 3 | 125.00 | 131.20 | 4.02 | 104.96 | 126.59 | 4.77 | 101.27 |
| 250.00 | 266.67 | 4.04 | 106.67 | 258.68 | 3.65 | 103.47 | |
| 1000.00 | 1068.47 | 2.17 | 106.85 | 1020.08 | 4.13 | 102.01 | |
| 4 | 21.88 | 21.53 | 2.55 | 98.43 | 22.16 | 3.10 | 101.29 |
| 43.75 | 42.95 | 2.73 | 98.16 | 45.90 | 5.61 | 104.92 | |
| 175.00 | 188.71 | 1.76 | 107.84 | 174.94 | 6.82 | 99.97 | |
| 5 | 21.88 | 21.73 | 2.99 | 99.33 | 22.72 | 3.77 | 103.85 |
| 43.75 | 44.01 | 5.57 | 100.59 | 44.63 | 5.62 | 102.00 | |
| 175.00 | 177.13 | 4.18 | 101.22 | 172.87 | 3.21 | 98.78 | |
| 6 | 93.75 | 92.98 | 5.89 | 99.17 | 96.59 | 3.67 | 103.03 |
| 187.50 | 172.18 | 7.62 | 91.83 | 187.13 | 6.93 | 99.80 | |
| 750.00 | 712.02 | 4.02 | 94.94 | 730.76 | 2.23 | 97.43 | |
| 7 | 125.00 | 120.87 | 2.29 | 96.70 | 123.54 | 2.01 | 98.83 |
| 250.00 | 239.62 | 3.93 | 95.85 | 252.51 | 4.54 | 101.01 | |
| 1000.00 | 972.64 | 3.05 | 97.26 | 973.85 | 1.05 | 97.38 | |
| 8 | 46.88 | 48.68 | 1.60 | 103.84 | 48.29 | 0.91 | 103.01 |
| 93.75 | 98.97 | 0.81 | 105.57 | 97.99 | 1.16 | 104.52 | |
| 375.00 | 374.83 | 0.57 | 99.95 | 368.78 | 1.43 | 98.34 | |
| 9 | 46.88 | 44.55 | 1.87 | 95.04 | 46.12 | 2.96 | 98.40 |
| 93.75 | 95.89 | 4.07 | 102.28 | 94.64 | 2.51 | 92.53 | |
| 375.00 | 365.02 | 2.68 | 97.34 | 370.17 | 1.45 | 98.71 | |
| Analyte 1 | UPLC−MS/MS MRM Assay | ||
|---|---|---|---|
| Mean (mg/g) | SD × 10−1 | RSD (%) | |
| 1 | 4.70 | 1.88 | 4.01 |
| 2 | 0.74 | 0.61 | 8.26 |
| 3 | 0.30 | 0.01 | 2.57 |
| 4 | 0.05 | 0.01 | 1.85 |
| 5 | 0.05 | 0.01 | 2.00 |
| 6 | 0.04 | 0.02 | 5.40 |
| 7 | 0.05 | 0.04 | 7.09 |
| 8 | 0.15 | 0.02 | 1.05 |
| 9 | 0.17 | 0.01 | 0.50 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seo, C.-S. Simultaneous Quantification of Nine Target Compounds in Traditional Korean Medicine, Bopyeo-Tang, Using High-Performance Liquid Chromatography–Photodiode Array Detector and Ultra-Performance Liquid Chromatography–Tandem Mass Spectrometry. Molecules 2024, 29, 1171. https://doi.org/10.3390/molecules29051171
Seo C-S. Simultaneous Quantification of Nine Target Compounds in Traditional Korean Medicine, Bopyeo-Tang, Using High-Performance Liquid Chromatography–Photodiode Array Detector and Ultra-Performance Liquid Chromatography–Tandem Mass Spectrometry. Molecules. 2024; 29(5):1171. https://doi.org/10.3390/molecules29051171
Chicago/Turabian StyleSeo, Chang-Seob. 2024. "Simultaneous Quantification of Nine Target Compounds in Traditional Korean Medicine, Bopyeo-Tang, Using High-Performance Liquid Chromatography–Photodiode Array Detector and Ultra-Performance Liquid Chromatography–Tandem Mass Spectrometry" Molecules 29, no. 5: 1171. https://doi.org/10.3390/molecules29051171
APA StyleSeo, C.-S. (2024). Simultaneous Quantification of Nine Target Compounds in Traditional Korean Medicine, Bopyeo-Tang, Using High-Performance Liquid Chromatography–Photodiode Array Detector and Ultra-Performance Liquid Chromatography–Tandem Mass Spectrometry. Molecules, 29(5), 1171. https://doi.org/10.3390/molecules29051171





