Three Iodoargentate-Based Hybrids Decorated by Metal Complexes: Structures, Optical/Photoelectric Properties and Theoretical Studies
Abstract
:1. Introduction
2. Results and Discussion
2.1. Crystal Structures
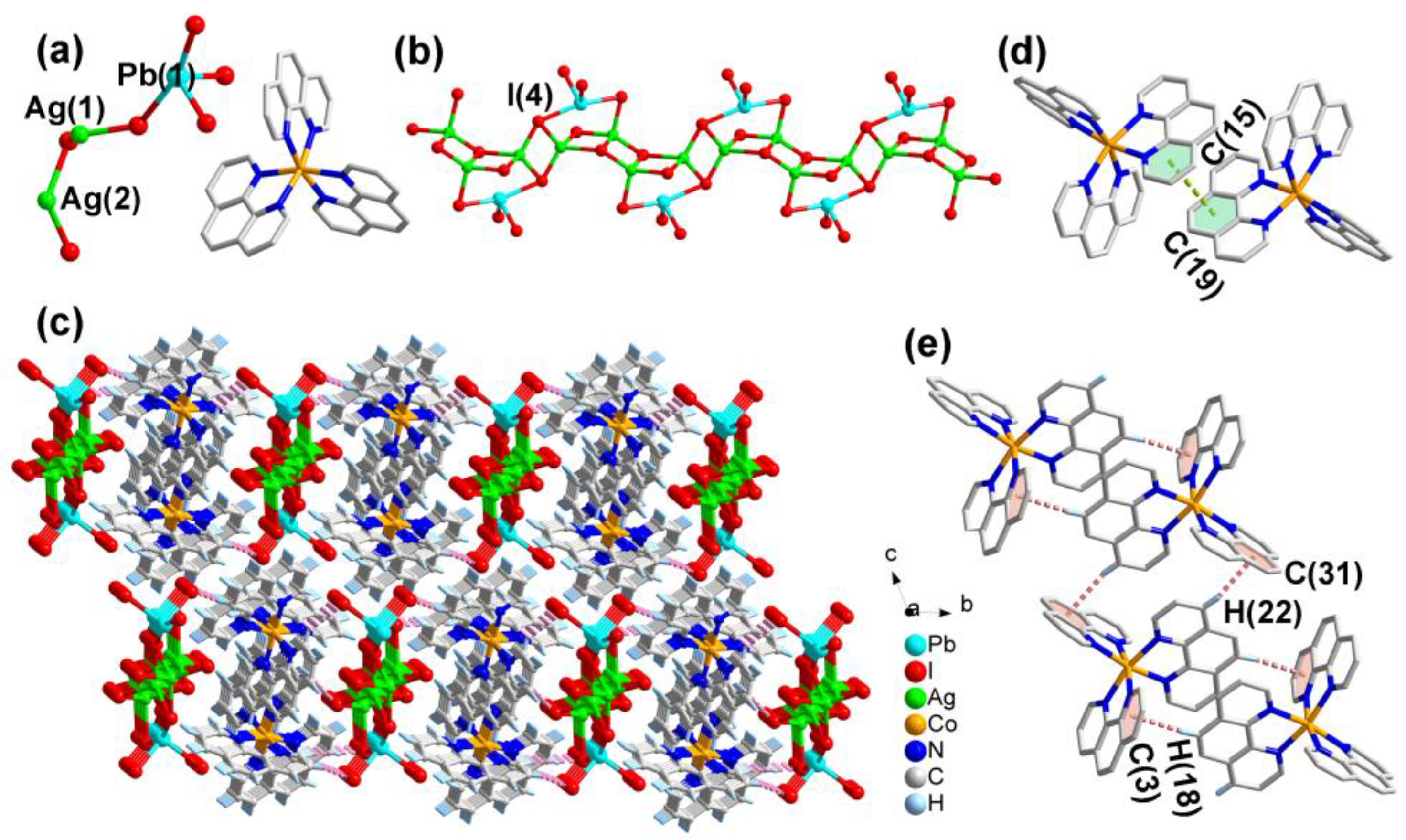
2.1.1. Structural Description of [Co(phen)3]Ag2PbI6 (1)
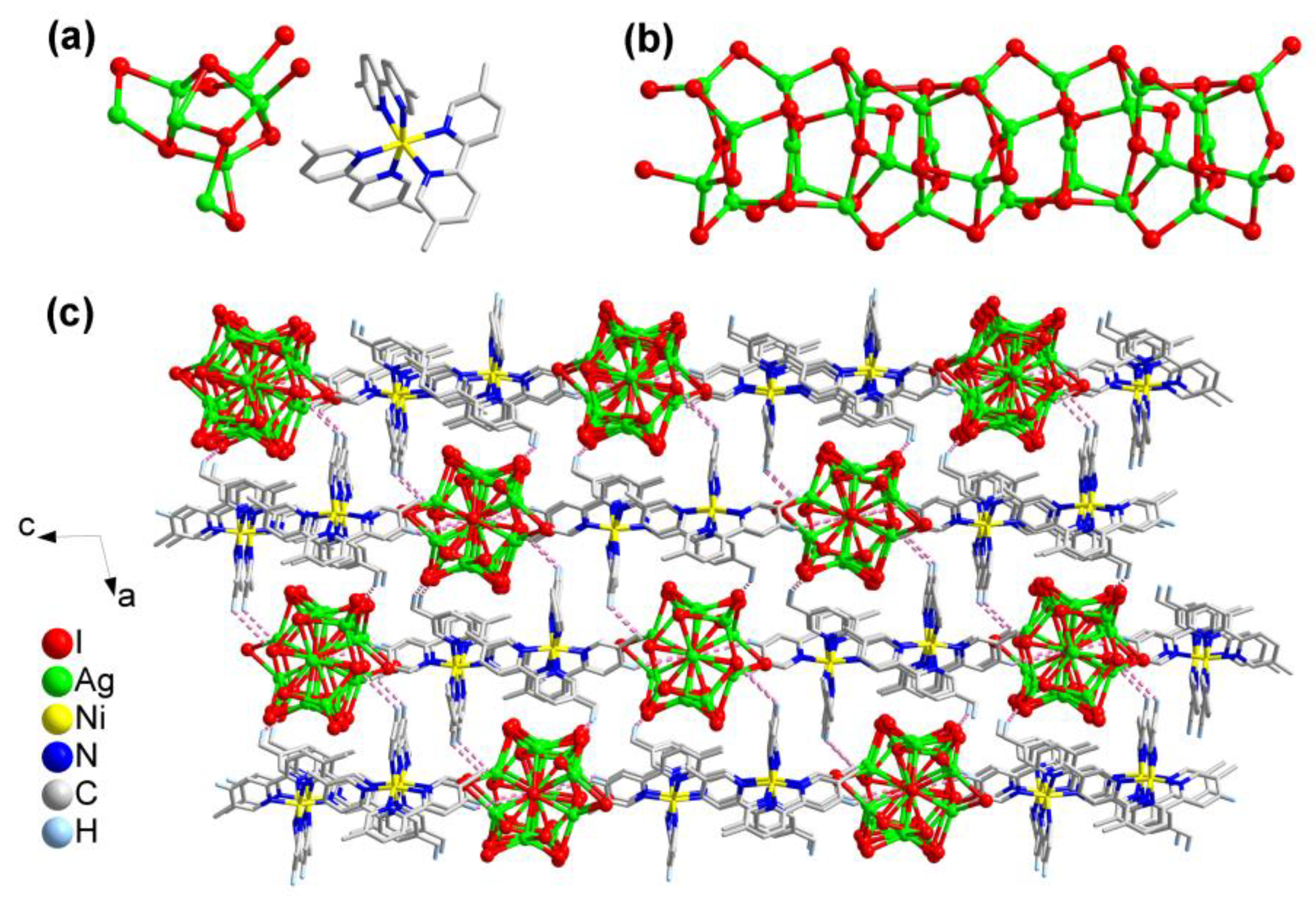
2.1.2. Structural Description of [Ni(5,5-dmpy)3]Ag7I9·CH3CN (2)
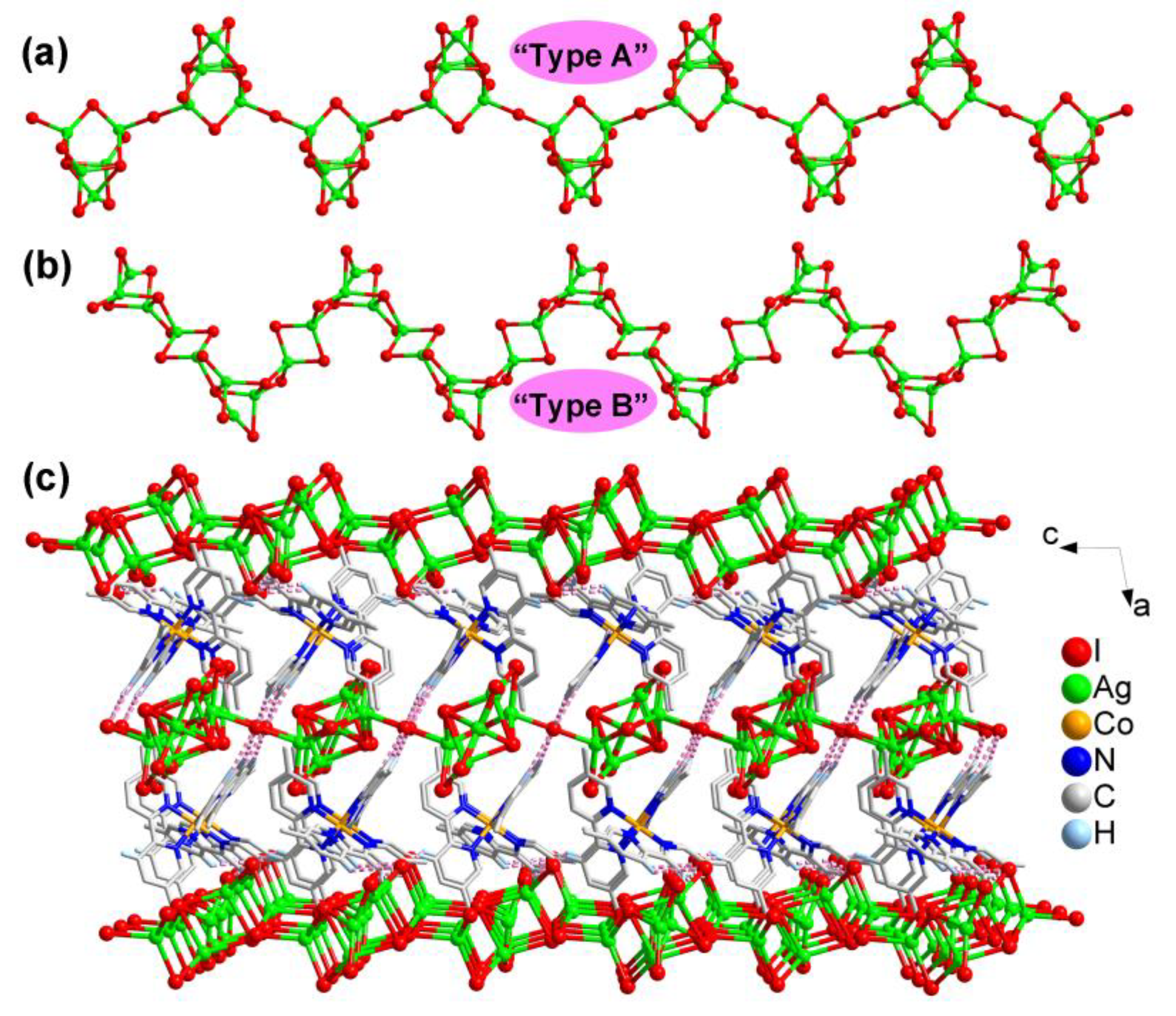
2.1.3. Structural Description of [Co(5,5-dmpy)3]Ag5I8 (3)
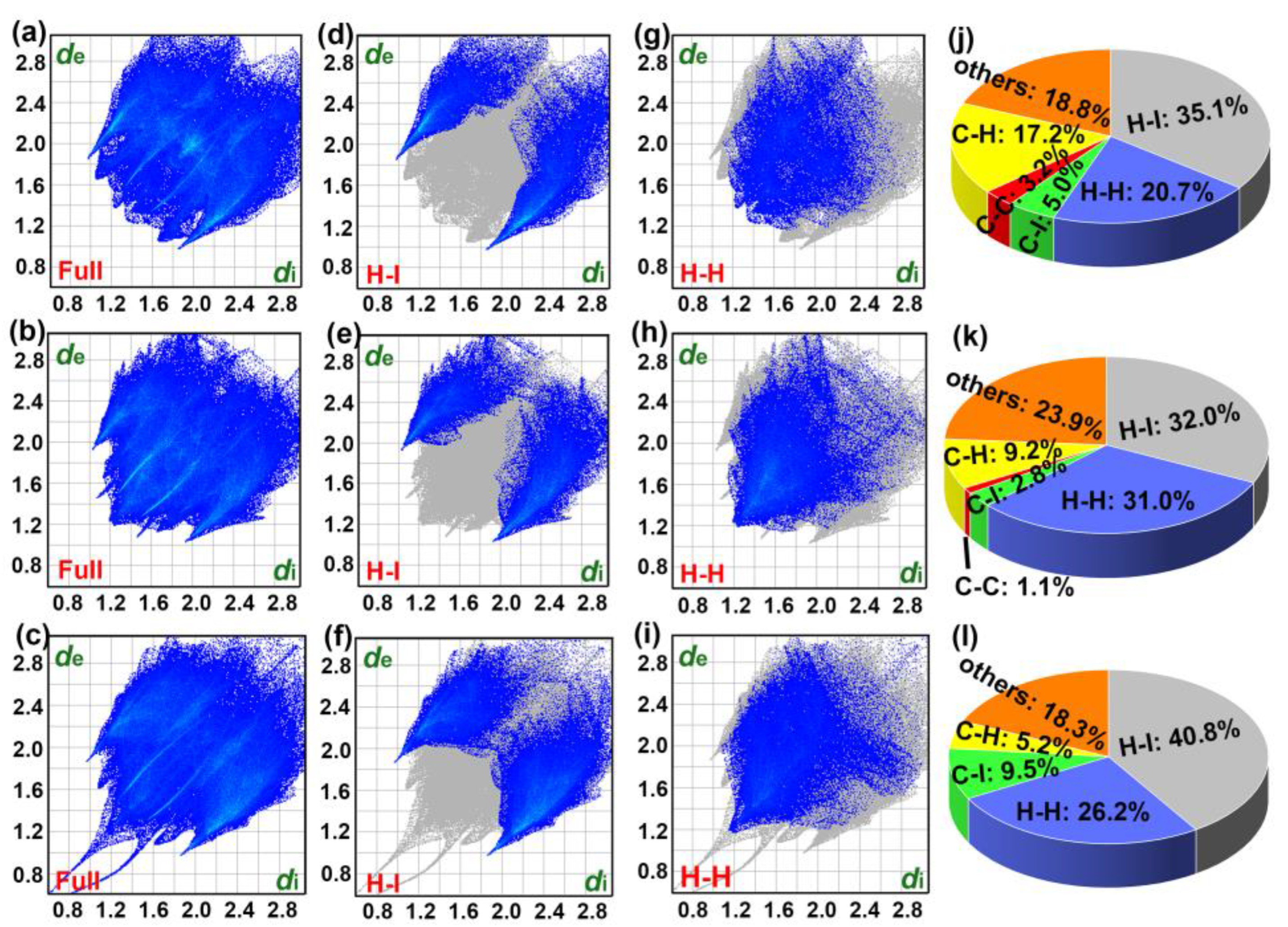
2.2. Hirshfeld Surface Analyses
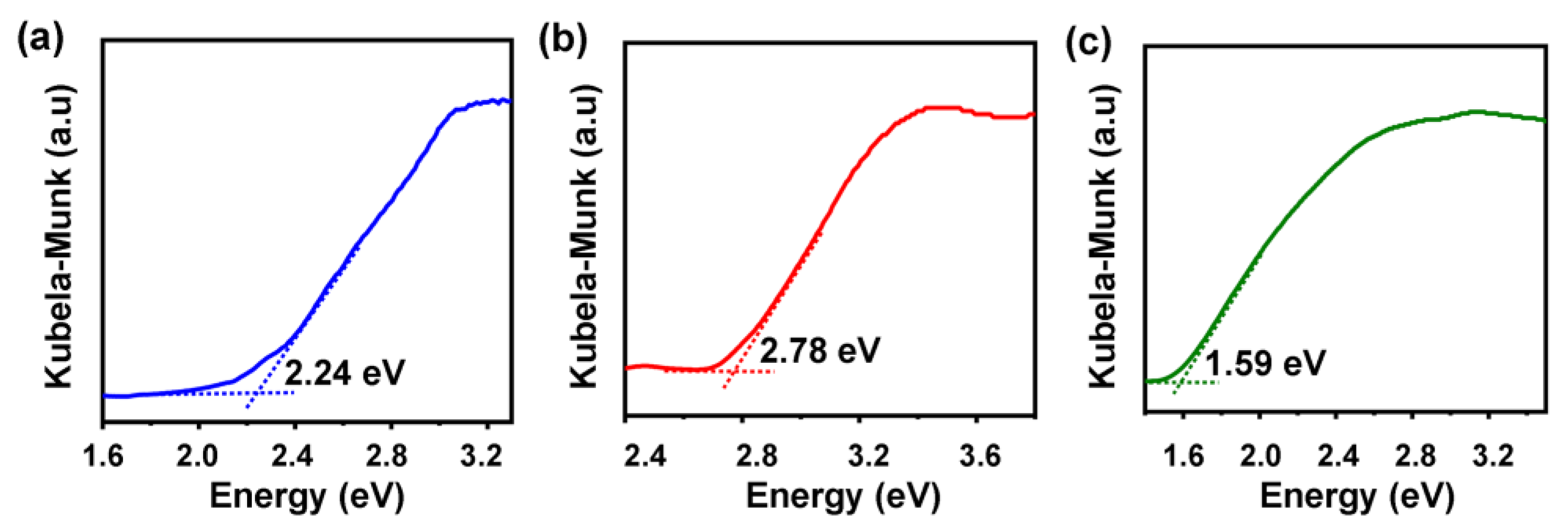
2.3. Optical Properties
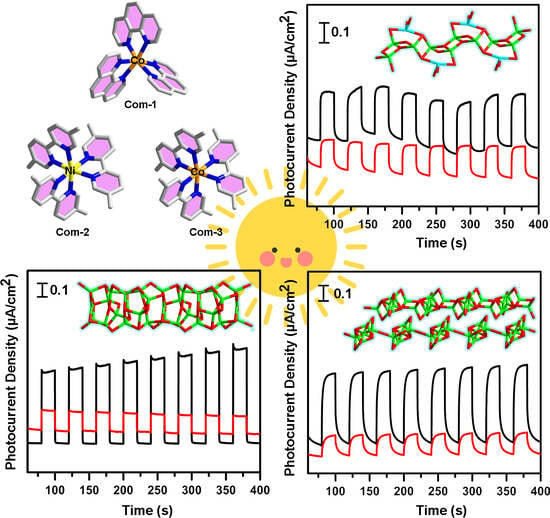
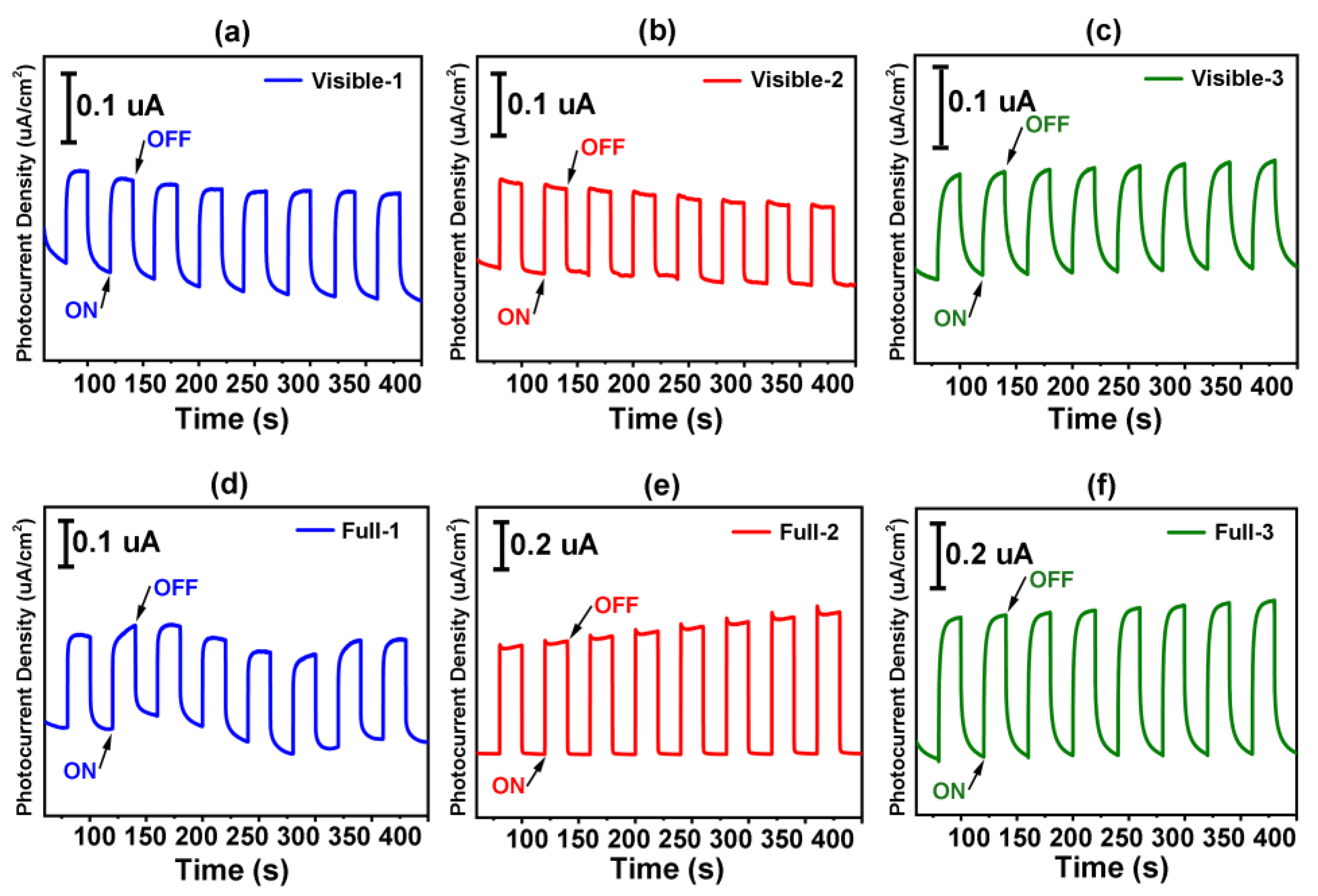
2.4. Photocurrent Responses
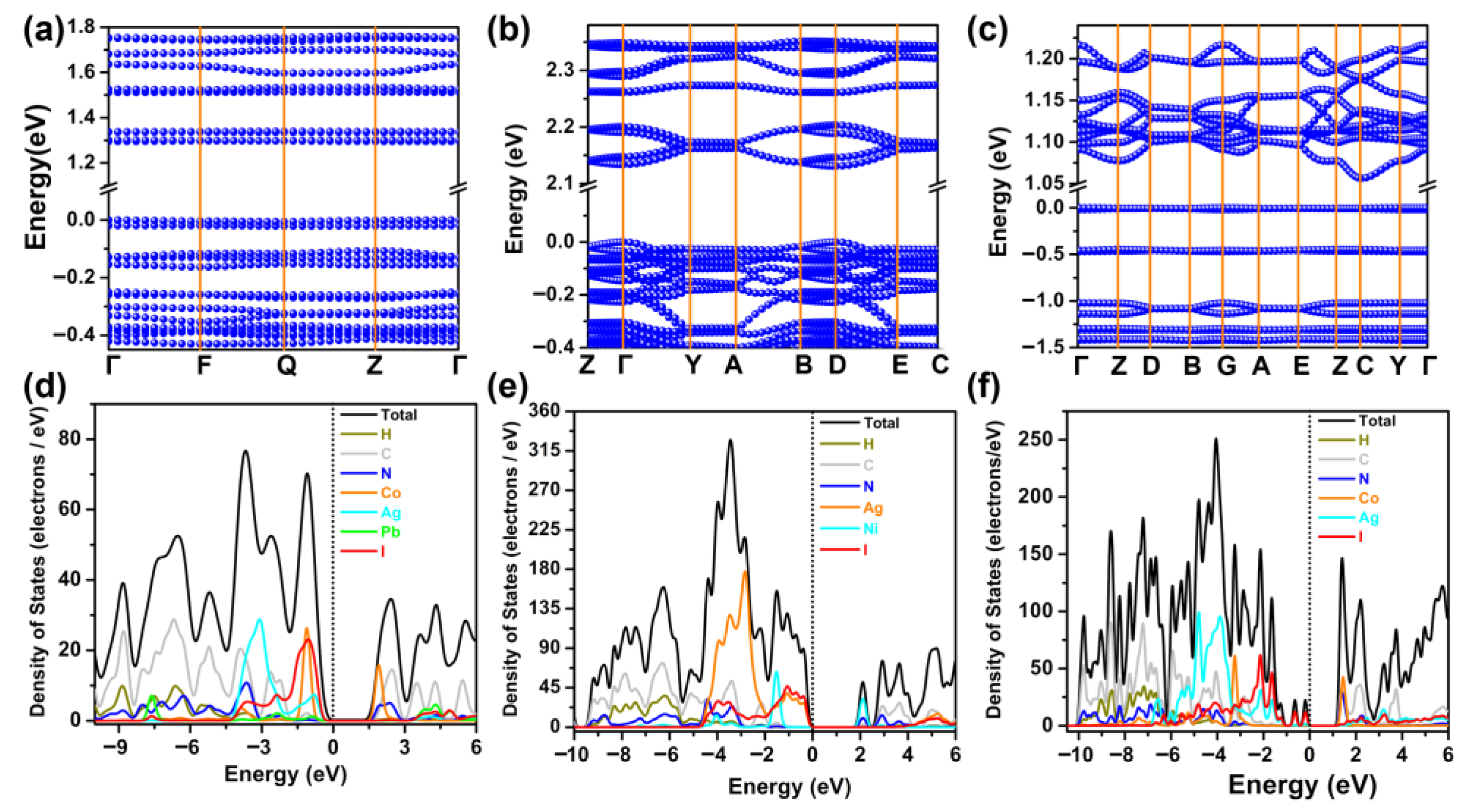
2.5. Theoretical Studies
3. Materials and Methods
3.1. General Information
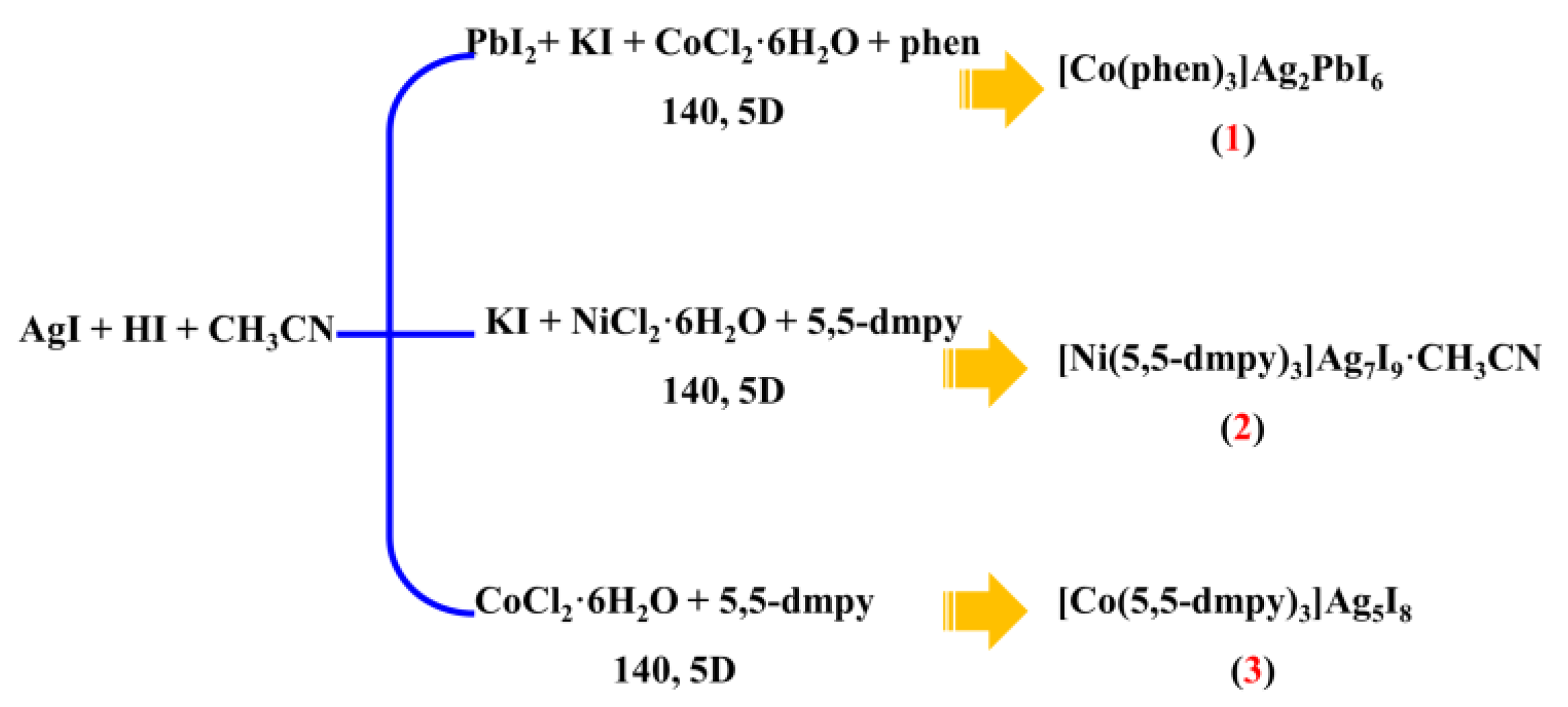
3.2. Syntheses
3.3. X-ray Crystallography
3.4. Photocurrent Measurements
3.5. Calculation Details
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fu, Y.; Zhu, H.; Chen, J.; Hautzinger, M.P.; Zhu, X.Y.; Jin, S. Metal halide perovskite nanostructures for optoelectronic applications and the study of physical properties. Nat. Rev. Mater. 2019, 4, 169–188. [Google Scholar] [CrossRef]
- Han, Y.; Yue, S.; Cui, B.B. Low-dimensional metal halide perovskite crystal materials: Structure strategies and luminescence applications. Adv. Sci. 2021, 8, 2004805. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Lin, H.; He, Q.; Xu, L.; Worku, M.; Chaaban, M.; Lee, S.; Shi, X.; Du, M.H.; Ma, B. Low dimensional metal halide perovskites and hybrids. Mater. Sci. Eng. R Rep. 2019, 137, 38–65. [Google Scholar] [CrossRef]
- Zhang, D.; Xue, Z.Z.; Pan, J.; Shang, M.M.; Mu, Y.; Han, S.D.; Wang, G.M. Solvated lanthanide cationic template strategy for constructing iodoargentates with photoluminescence and white light emission. Cryst. Growth Des. 2018, 18, 7041–7047. [Google Scholar] [CrossRef]
- Yu, T.L.; An, L.; Zhang, L.; Shen, J.J.; Fu, Y.B.; Fu, Y.L. Two thermochromic layered iodoargentate hybrids directed by 4-and 3-cyanopyridinium cations. Cryst. Growth Des. 2014, 14, 3875–3879. [Google Scholar] [CrossRef]
- Zhang, R.C.; Wang, J.J.; Zhang, J.C.; Wang, M.Q.; Sun, M.; Ding, F.; Zhang, D.J.; An, Y.L. Coordination-induced syntheses of two hybrid framework iodides: A thermochromic luminescent thermometer. Inorg. Chem. 2016, 55, 7556–7563. [Google Scholar] [CrossRef]
- Yang, L.L.; Zhou, J.; An, L.T.; Cao, S.M.; Hu, J. A unique formyl iodoargentate exhibiting luminescent and photocurrent response properties. Dalton Trans. 2019, 48, 15762–15766. [Google Scholar] [CrossRef]
- Xue, Z.Z.; Meng, X.D.; Li, X.Y.; Pan, J.; Wang, G.M. Penta-nuclear [Ag5I6] cluster-based photochromic hybrid: Synthesis, structure, dye sorption, and separation. Cryst. Growth Des. 2021, 21, 1055–1061. [Google Scholar] [CrossRef]
- Tang, C.Y.; Yao, J.; Li, Y.Y.; Xia, Z.R.; Liu, J.B.; Zhang, C.Y. Transition-metal-complex-directed synthesis of hybrid iodoargentates with single-crystal to single-crystal structural transformation and photocatalytic properties. Inorg. Chem. 2020, 59, 13962–13971. [Google Scholar] [CrossRef]
- Yu, T.L.; Guo, Y.M.; Wu, G.X.; Yang, X.F.; Xue, M.; Fu, Y.L.; Wang, M.S. Recent progress of d10 iodoargentate(I)/iodocuprate(I) hybrids: Structural diversity, directed synthesis, and photochromic/thermochromic properties. Coord. Chem. Rev. 2019, 397, 91–111. [Google Scholar] [CrossRef]
- Li, X.X.; Zheng, S.T. Three-dimensional metal-halide open frameworks. Coord. Chem. Rev. 2021, 430, 213663. [Google Scholar] [CrossRef]
- Lei, X.W.; Yue, C.Y.; Zhao, J.Q.; Han, Y.F.; Ba, Z.R.; Wang, C.; Liu, X.Y.; Gong, Y.P.; Liu, X.Y. Syntheses, crystal structures, and photocatalytic properties of polymeric iodoargentates [TM(2,2-bipy)3]Ag3I5 (TM = Mn, Fe, Co, Ni, Zn). Eur. J. Inorg. Chem. 2015, 26, 4412–4419. [Google Scholar] [CrossRef]
- Lei, X.W.; Yue, C.Y.; Zhao, J.Q.; Han, Y.F.; Yang, J.T.; Meng, R.R.; Gao, C.S.; Ding, H.; Wang, C.Y.; Chen, W.D.; et al. Two types of 2D layered iodoargentates based on trimeric Ag3I7 secondary building units and hexameric Ag6I12 ternary building units: Syntheses, crystal structures, and efficient visible light responding photocatalytic properties. Inorg. Chem. 2015, 54, 10593–10603. [Google Scholar] [CrossRef] [PubMed]
- Lei, X.W.; Yue, C.Y.; Wu, F.; Jiang, X.Y.; Chen, L.N. Syntheses, crystal structures and photocatalytic properties of transition metal complex directed iodoargentates: [TM(2,2-bipy)3]Ag5I7. Inorg. Chem. Commun. 2017, 77, 64–67. [Google Scholar] [CrossRef]
- Tang, C.Y.; Sun, Y.W.; Liu, J.B.; Xu, Q.F.; Zhang, C.Y. [Co(2,2′-bipy)3]Ag3I6 with a hole structure facilitates dye adsorption and photocatalytic reduction. Dalton Trans. 2022, 51, 16784–16789. [Google Scholar] [CrossRef]
- Li, H.H.; Chen, Z.R.; Sun, L.G.; Lian, Z.X.; Chen, X.B.; Li, J.B.; Li, J.Q. Two iodoargentate hybrid coordination polymers induced by transition-metal complexes: Structures and properties. Cryst. Growth Des. 2010, 10, 1068–1073. [Google Scholar] [CrossRef]
- Yu, T.L.; Fu, Y.B.; Wang, Y.L.; Hao, P.F.; Shen, J.J.; Fu, Y.L. Hierarchical symmetry transfer and flexible charge matching in five [M(phen)3]2+ directed iodoargentates with 1 to 3D frameworks. CrystEngComm 2015, 17, 8752–8761. [Google Scholar] [CrossRef]
- Yue, C.Y.; Lei, X.W.; Lu, X.X.; Li, Y.; Wei, J.C.; Wang, W.; Yin, Y.D.; Wang, N. Comparison studies of hybrid lead halide [MPb2X7]2− (M = Cu, Ag; X = Br, I) chains: Band structures and visible light driven photocatalytic properties. Dalton Trans. 2017, 46, 9235–9244. [Google Scholar] [CrossRef]
- Ren, X.C.; Li, J.; Wang, W.H.; Shao, Y.N.; Zhang, B.; Li, L.Z. Hybrid silver haloplumbates containing metal complexes: Syntheses, structures and photoelectric properties. J. Solid State Chem. 2022, 308, 122912. [Google Scholar] [CrossRef]
- Zhang, B.; Li, J.; Pang, M.; Wang, Y.S.; Liu, M.Z.; Zhao, H.M. Four discrete silver iodobismuthates/bromobismuthates with metal complexes: Syntheses, structures, photocurrent responses, and theoretical studies. Inorg. Chem. 2022, 61, 406–413. [Google Scholar] [CrossRef]
- Zhang, B.; Li, J.; Pang, M.; Chen, X.; Liu, M.Z. Two [Co(bipy)3]3+-templated silver halobismuthate hybrids: Syntheses, structures, photocurrent responses, and theoretical studies. Inorg. Chem. 2022, 61, 9808–9815. [Google Scholar] [CrossRef] [PubMed]
- Yue, C.Y.; Sun, C.; Li, D.Y.; Dong, Y.H.; Wang, C.L.; Zhao, H.F.; Jiang, H.; Jing, Z.H.; Lei, X.W. Organic-inorganic hybrid heterometallic halides with low-dimensional structures and red photoluminescence emissions. Inorg. Chem. 2019, 58, 10304–10312. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.Y.; Wang, F.; Jia, D.X.; Jiang, W.Q.; Zhang, Y. Syntheses and characterizations of polymeric silver iodoplumbates, and iodoplumbates with lanthanide complexes. CrystEngComm 2014, 16, 2016–2024. [Google Scholar] [CrossRef]
- Jansen, M. Homoatomic d10-d10 interactions: Their effects on structure and chemical and physical-properties. Angew. Chem. Int. Ed. Engl. 1987, 26, 1098–1110. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, Y.N.; Li, J.; Ren, X.C.; Yang, Y.; Yang, X.R. Two silver halobismuthate hybrids decorated by photosensitive metal complexes: Syntheses, structures, photoelectric properties, and theoretical studies. Cryst. Growth Des. 2022, 22, 7434–7442. [Google Scholar] [CrossRef]
- Fang, W.; Tang, C.Y.; Chen, R.H.; Jia, D.X.; Jiang, W.Q.; Zhang, Y. Polymeric heterometallic Pb–Ag iodometallates, iodoplumbates and iodoargentates with lanthanide complex cations as templates. Dalton Trans. 2013, 42, 15150–15158. [Google Scholar] [CrossRef]
- Wei, Q.; Wang, D.; Pan, J.; Han, S.D.; Wang, G.M. A series of iodoargentates directed by solvated metal cations featuring uptake and photocatalytic degradation of organic dye pollutants. Chem.-Asian J. 2019, 14, 640–646. [Google Scholar] [CrossRef]
- Zhang, B.; Li, J.; Chen, X.; Yang, M.F.; Shen, H.Y.; Zhu, J.C. [NH4][Fe(bipy)3]2[Ag6Br11]: Synthesis, structure, characterization and photocurrent response. Inorg. Chem. Commun. 2022, 137, 109250. [Google Scholar] [CrossRef]
- Zhang, B.; Li, J.; Yang, Y.; Wang, W.H.; Shen, H.Y.; Shao, Y.N. A new metal complex-templated silver iodobismuthate exhibiting photocurrent response and photocatalytic property. Dalton Trans. 2022, 51, 13361–13367. [Google Scholar] [CrossRef]
- Pang, M.; Ren, X.C.; Chen, X.; Shen, H.Y.; Li, J.; Zhang, B.; Li, L.Z. Two heterometallic silver-iodoplumbates with [Fe(phen)3]2+ complexes: Syntheses, structures, photocurrent responses and theoretical studies. Inorg. Chem. Commun. 2022, 139, 109342. [Google Scholar] [CrossRef]
- Zhang, B.; Li, W.A.; Li, J.; Xu, Y.P.; Xu, Y.R.; Wang, W.H.; Zou, G.D. [Ni(5,5′-dmbpy)3]2Ag4.9I8.9·4H2O: A discrete iodoargentate with transition metal complexes. Inorg. Chem. Commun. 2020, 121, 108219. [Google Scholar] [CrossRef]
- Lei, X.W.; Yue, C.Y.; Feng, L.J.; Han, Y.F.; Meng, R.R.; Yang, J.T.; Ding, H.; Gao, C.S.; Wang, C.Y. Syntheses, crystal structures and photocatalytic properties of four hybrid iodoargentates with zero- and two-dimensional structures. CrystEngComm 2016, 18, 427–436. [Google Scholar] [CrossRef]
- Zheng, W.; Chen, N.N.; Gao, Y.; Wu, B.; Jia, D.X. Heterometallic Pb–Ag iodides from 1-D chains to 2-D layers induced by transition metal complex cations: Syntheses, crystal structures, and photocatalytic properties. Eur. J. Inorg. Chem. 2019, 2019, 4752–4759. [Google Scholar] [CrossRef]
- Mu, Y.; Wang, D.; Meng, X.D.; Pan, J.; Han, S.D.; Xue, Z.Z. Construction of iodoargentates with diverse architectures: Template syntheses, structures, and photocatalytic properties. Cryst. Growth Des. 2020, 20, 1130–1138. [Google Scholar] [CrossRef]
- Huang, L.; Zhou, J. Two hybrid polymeric iodoargentates incorporating aromatic N-heterocycle derivatives as electron acceptors. Inorg. Chem. 2020, 59, 16814–16818. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.H.; Zhao, L.M.; Lin, X.Y.; Wang, Y.K.; Zhang, W.T.; Song, K.Y.; Li, H.H.; Chen, Z.R. Iodoargentate/iodobismuthate-based materials hybridized with lanthanide-containing metalloviologens: Thermochromic behaviors and photocurrent responses. Inorg. Chem. Front. 2018, 5, 1162–1173. [Google Scholar] [CrossRef]
- Zhang, B.; Sun, H.Y.; Li, J.; Xu, Y.R.; Xu, Y.P.; Yang, X.; Zou, G.D. Hybrid iodoplumbates with metal complexes: Syntheses, crystal structures, band gaps and photoelectric properties. Dalton Trans. 2020, 49, 1803–1810. [Google Scholar] [CrossRef]
- Yue, C.Y.; Yue, Y.D.; Sun, H.X.; Li, D.Y.; Lin, N.; Wang, X.M.; Jin, Y.X.; Dong, Y.H.; Jing, Z.H.; Lei, X.W. Transition metal complex dye-sensitized 3D iodoplumbates: Syntheses, structures and photoelectric properties. Chem. Commun. 2019, 55, 6874–6877. [Google Scholar] [CrossRef]
- Zhang, B.; Li, J.; Li, L.Z.; Ren, X.C.; Pang, M.; Shao, Y.N. Two new hybrid iodoplumbates with chain-like cations. Cryst. Growth Des. 2021, 21, 5317–5324. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmuller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Ren, X.C.; Li, J.; Wang, W.H.; Chen, X.; Zhang, B.; Li, L.Z. [NH4][Ni(phen)3]BiI6: Synthesis, structure, photocatalytic property and theoretical study of a discrete iodobismuthate. Inorg. Chem. Commun. 2021, 130, 108714. [Google Scholar] [CrossRef]
- Lei, X.W.; Yue, C.Y.; Wei, J.C.; Li, R.Q.; Li, Y.; Mi, F.Q. Transition metal complex directed lead bromides with tunable structures and visible light driven photocatalytic properties. Dalton Trans. 2016, 45, 19389–19398. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
| 1 | 2 | 3 | |
|---|---|---|---|
| formula | C36H24Ag2CoI6N6Pb | C38H39Ag7I9N7Ni | C36H36Ag5CoI8N6 |
| fw | 1783.87 | 2549.66 | 2166.19 |
| crystal system | triclinic | monoclinic | monoclinic |
| space group | P-1 (No. 2) | P21/n (No. 14) | P2/c (No. 13) |
| a/Ǻ | 12.0376(11) | 17.9597(8) | 22.867(2) |
| b/Ǻ | 13.8472(12) | 13.1590(5) | 13.4056(14) |
| c/Ǻ | 14.8853(13) | 24.1497(11) | 17.2141(18) |
| α/° | 112.785(4) | 90 | 90 |
| β/° | 94.948(2) | 101.825(4) | 100.819(4) |
| γ/° | 101.542(3) | 90 | 90 |
| V/Å3 | 2204.2(3) | 5586.2(4) | 5183.0(9) |
| Z | 2 | 4 | 4 |
| T/K | 298(2) | 293(2) | 293(2) |
| λ/Ǻ | 0.71073 | 0.71073 | 0.71073 |
| ρcalcd/g cm−3 | 2.688 | 3.032 | 2.776 |
| F(000) | 1606 | 4600 | 3920 |
| Rint | 0.0465 | 0.0396 | 0.1065 |
| GOF | 1.091 | 1.032 | 1.029 |
| R1 [I > 2s(I)] | 0.0725 | 0.0315 | 0.0776 |
| wR(F2) [I > 2s(I)] | 0.2053 | 0.0667 | 0.1942 |
| CCDC | 2,282,554 | 2,282,555 | 2,282,556 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, M.; Ren, X.; Wen, W.; Li, B.; Li, J.; Li, J.; Zhang, B. Three Iodoargentate-Based Hybrids Decorated by Metal Complexes: Structures, Optical/Photoelectric Properties and Theoretical Studies. Molecules 2023, 28, 6116. https://doi.org/10.3390/molecules28166116
Liu M, Ren X, Wen W, Li B, Li J, Li J, Zhang B. Three Iodoargentate-Based Hybrids Decorated by Metal Complexes: Structures, Optical/Photoelectric Properties and Theoretical Studies. Molecules. 2023; 28(16):6116. https://doi.org/10.3390/molecules28166116
Chicago/Turabian StyleLiu, Minghui, Xiaochen Ren, Weiyang Wen, Baohan Li, Jiaqi Li, Jun Li, and Bo Zhang. 2023. "Three Iodoargentate-Based Hybrids Decorated by Metal Complexes: Structures, Optical/Photoelectric Properties and Theoretical Studies" Molecules 28, no. 16: 6116. https://doi.org/10.3390/molecules28166116
APA StyleLiu, M., Ren, X., Wen, W., Li, B., Li, J., Li, J., & Zhang, B. (2023). Three Iodoargentate-Based Hybrids Decorated by Metal Complexes: Structures, Optical/Photoelectric Properties and Theoretical Studies. Molecules, 28(16), 6116. https://doi.org/10.3390/molecules28166116