Extraction, Purification, and Structural Characterization of Polysaccharides from Sanghuangporus vaninii with Anti-Inflammatory Activity
Abstract
:1. Introduction
2. Results
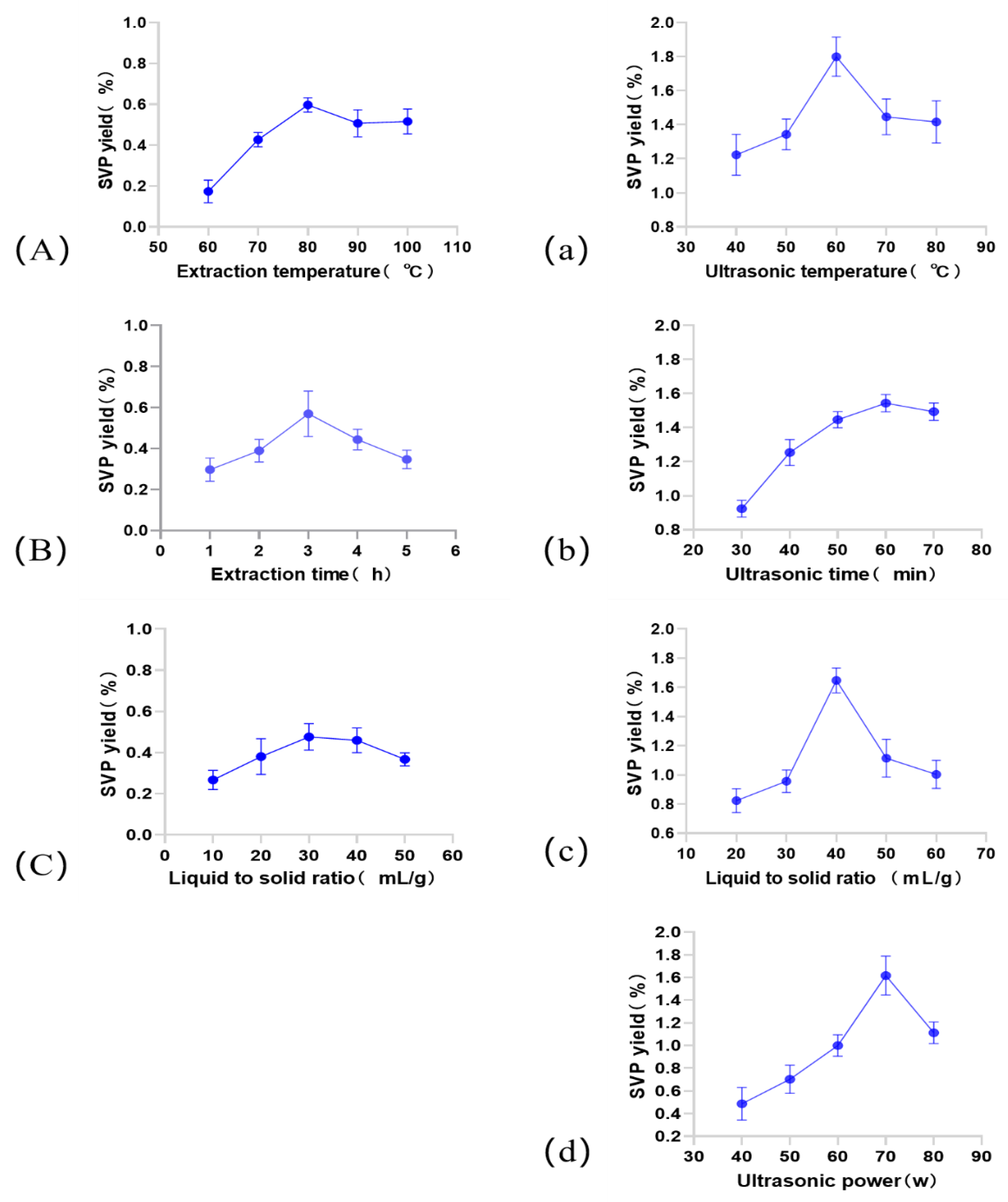
2.1. Single-Factor Experiment Analysis
2.1.1. Influence of the Extraction Temperature on the SVP Extraction Yield
2.1.2. Effect of the Extraction Time on the Extraction Rate of SVP
2.1.3. Effect of the Liquid–Solid Ratio on the Extraction Rate of SVP
2.1.4. Effect of the Ultrasound Power on the SVP Extraction Rate
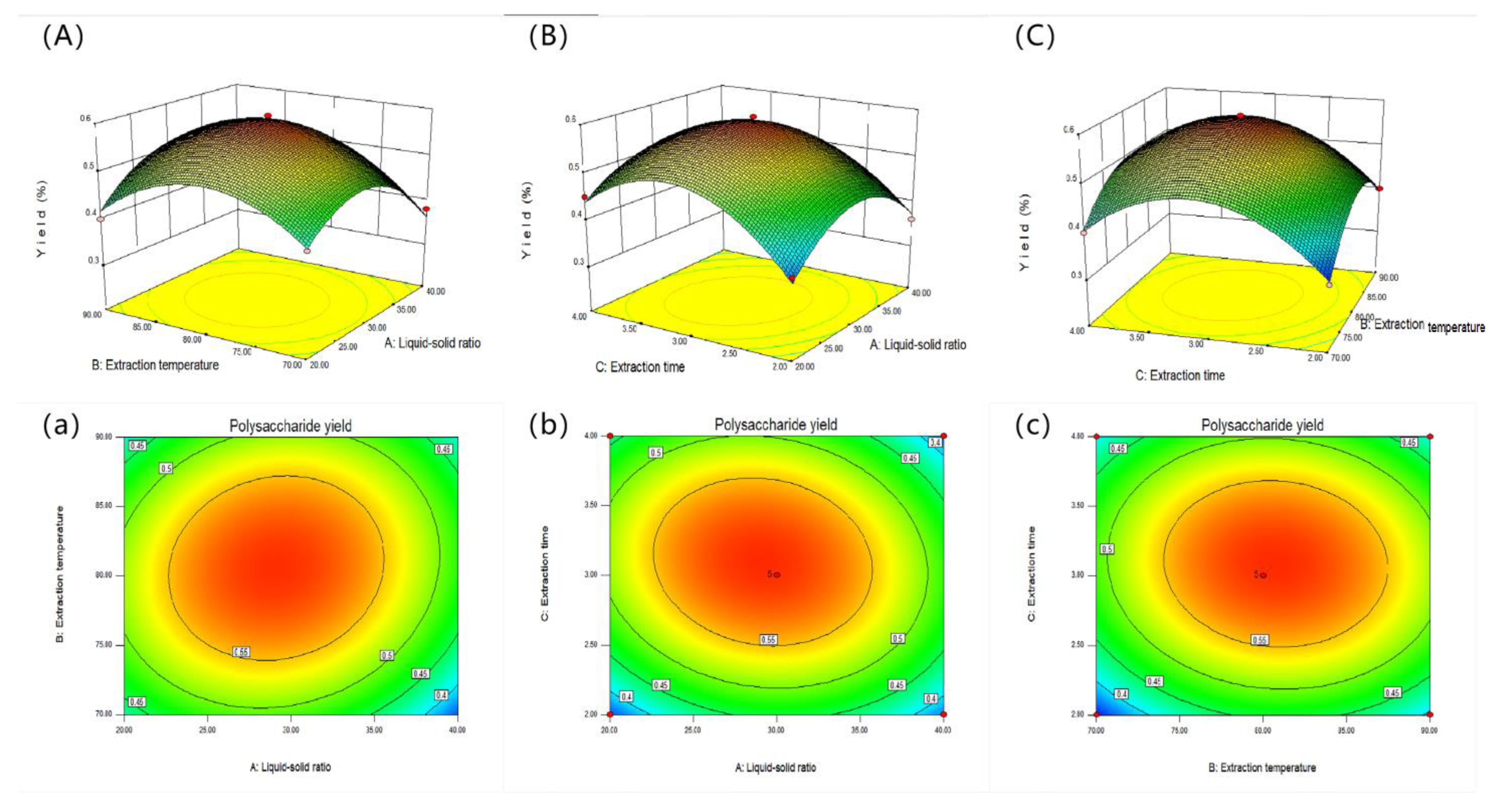
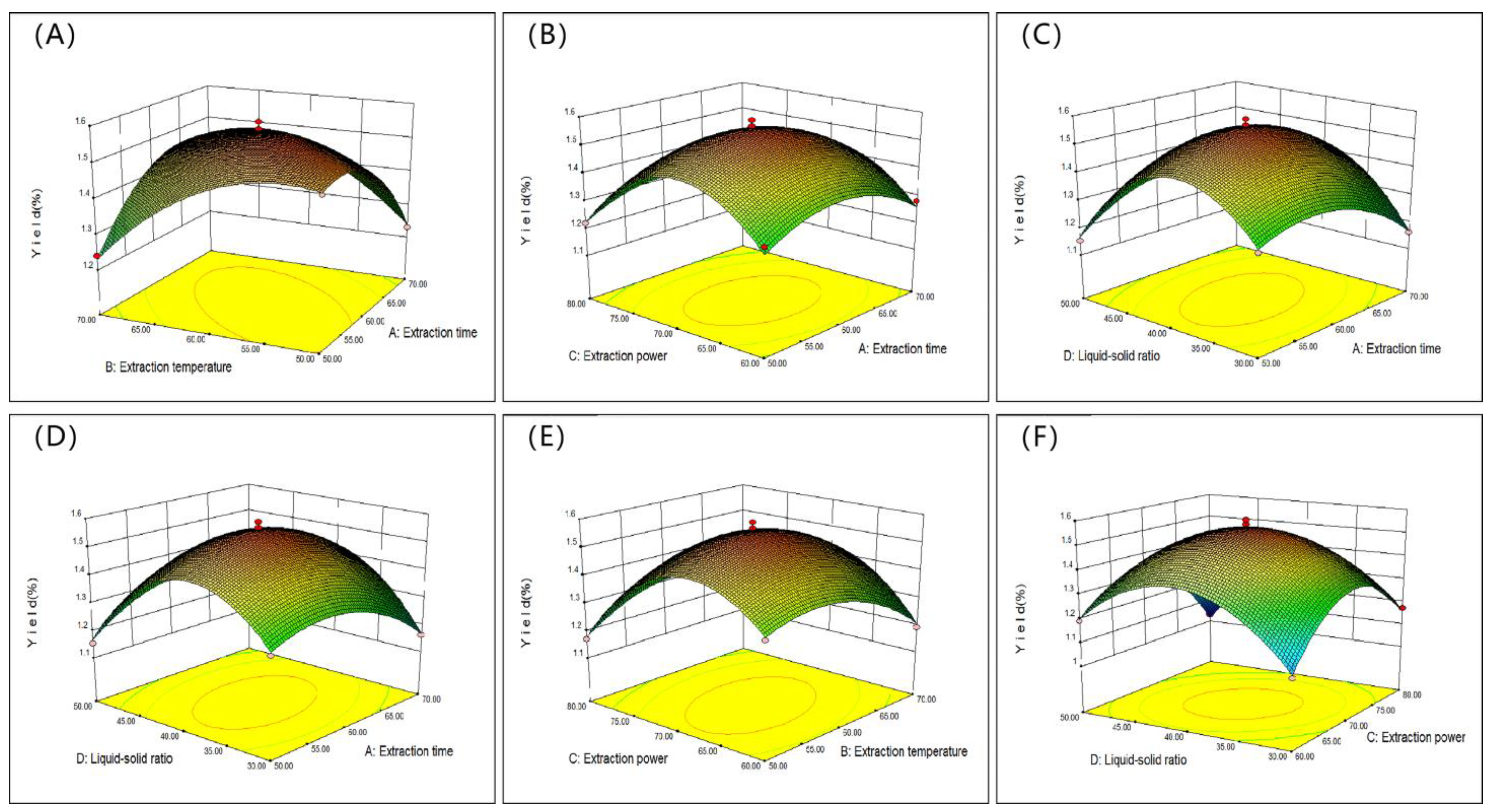
2.2. Optimization of SVP Extraction Parameters
2.2.1. Statistical Analysis and Model Fitting
2.2.2. Response Surface Diagram
2.3. Optimization and Validation
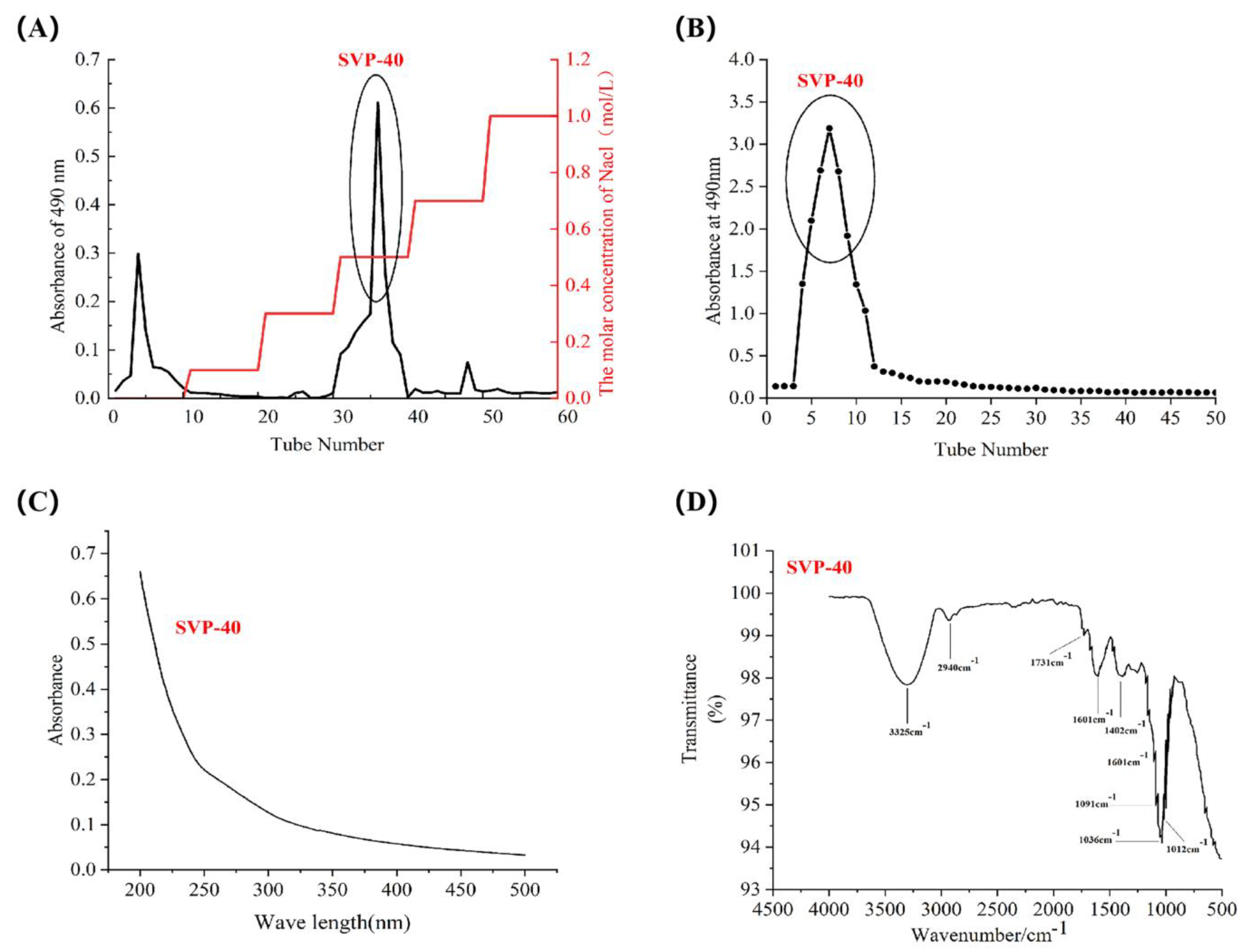
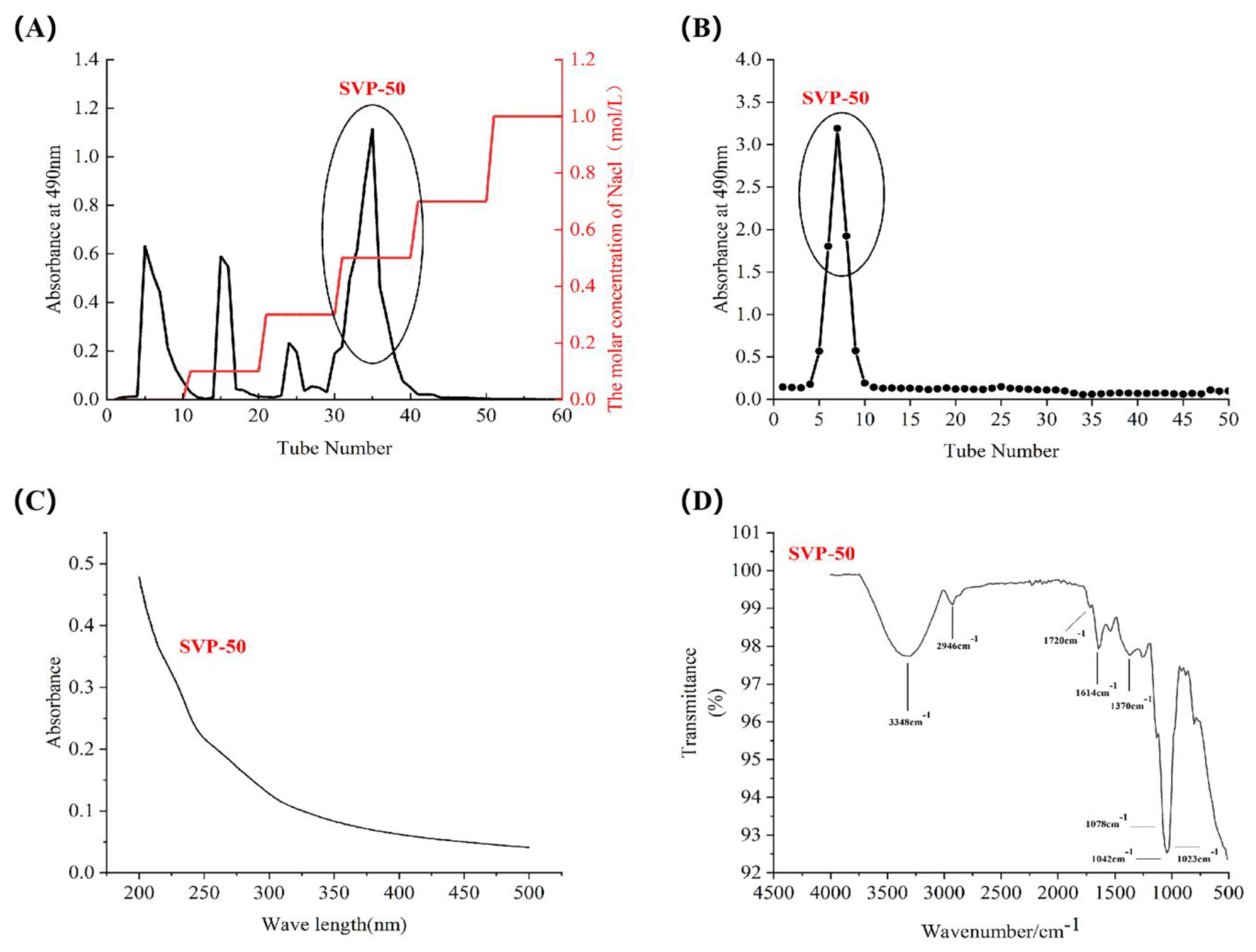
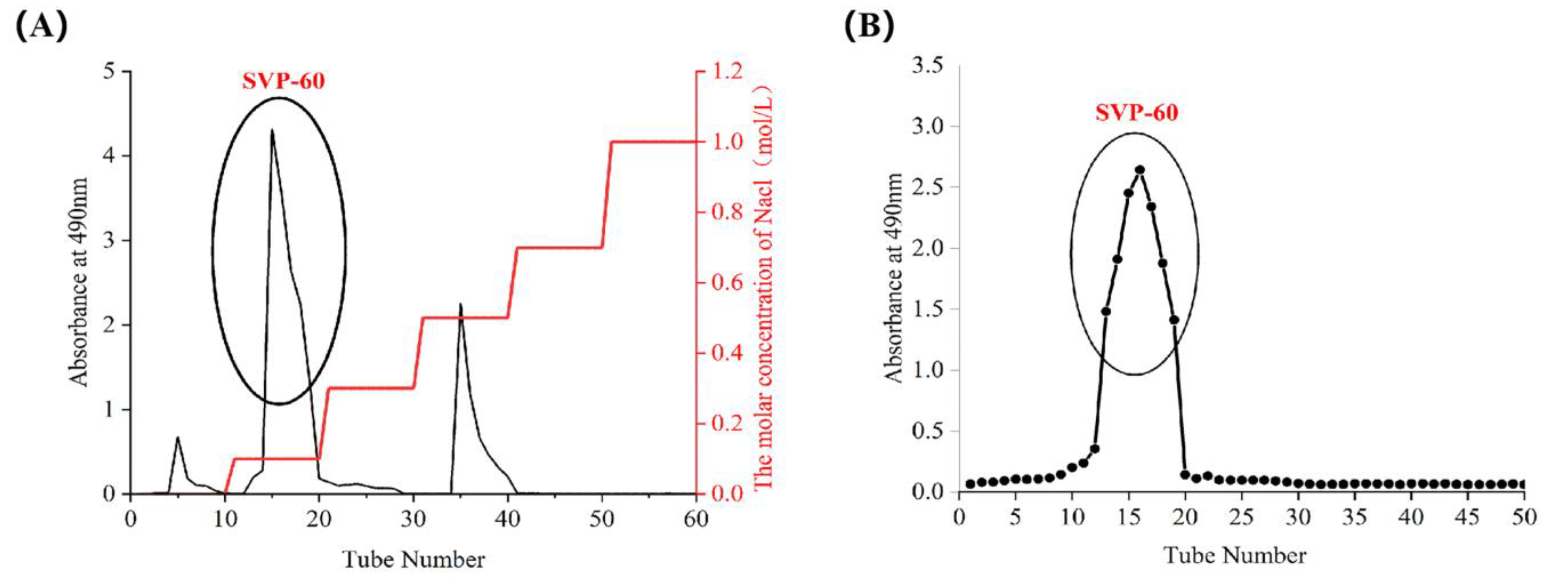
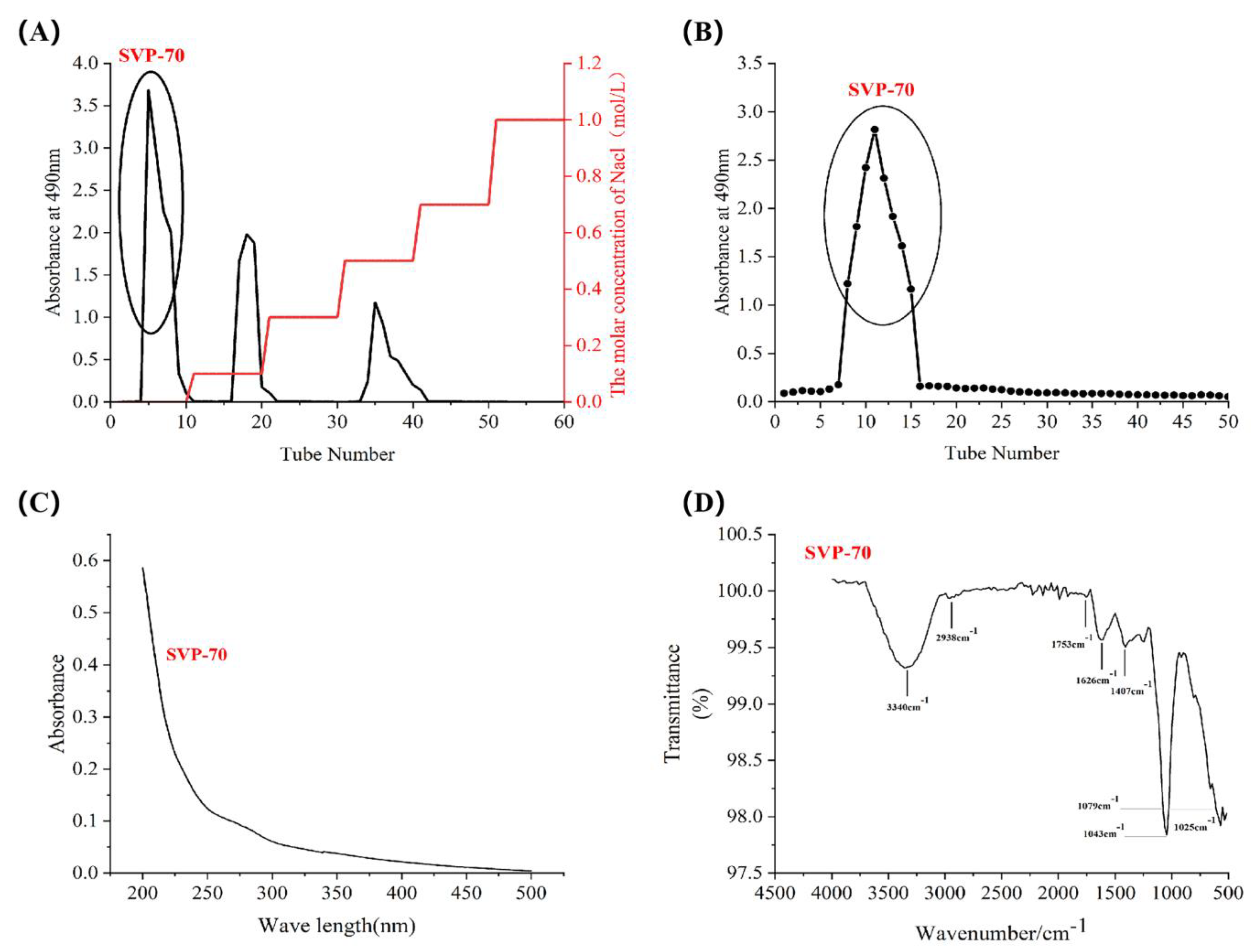
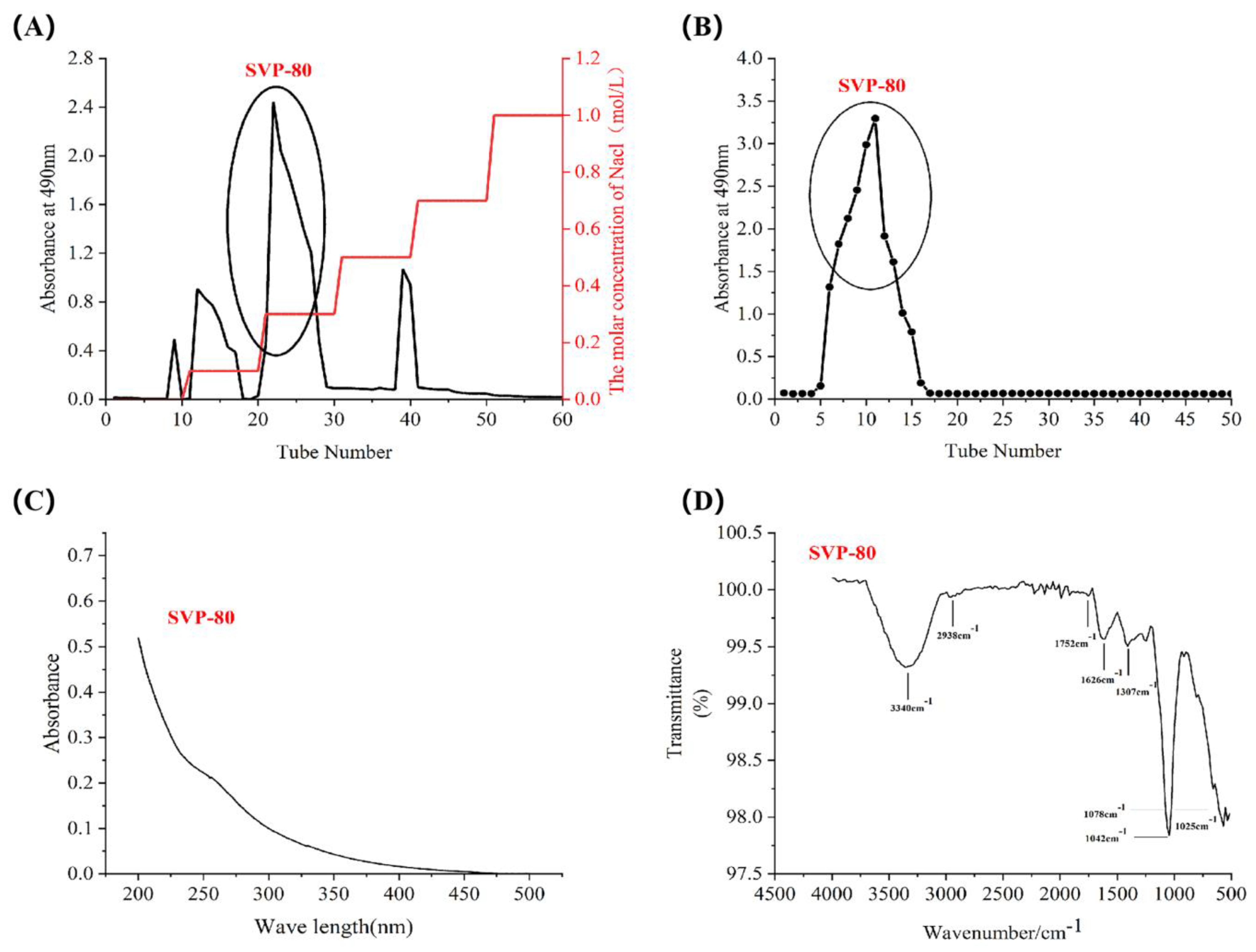
2.4. Isolation and Purification of SVP
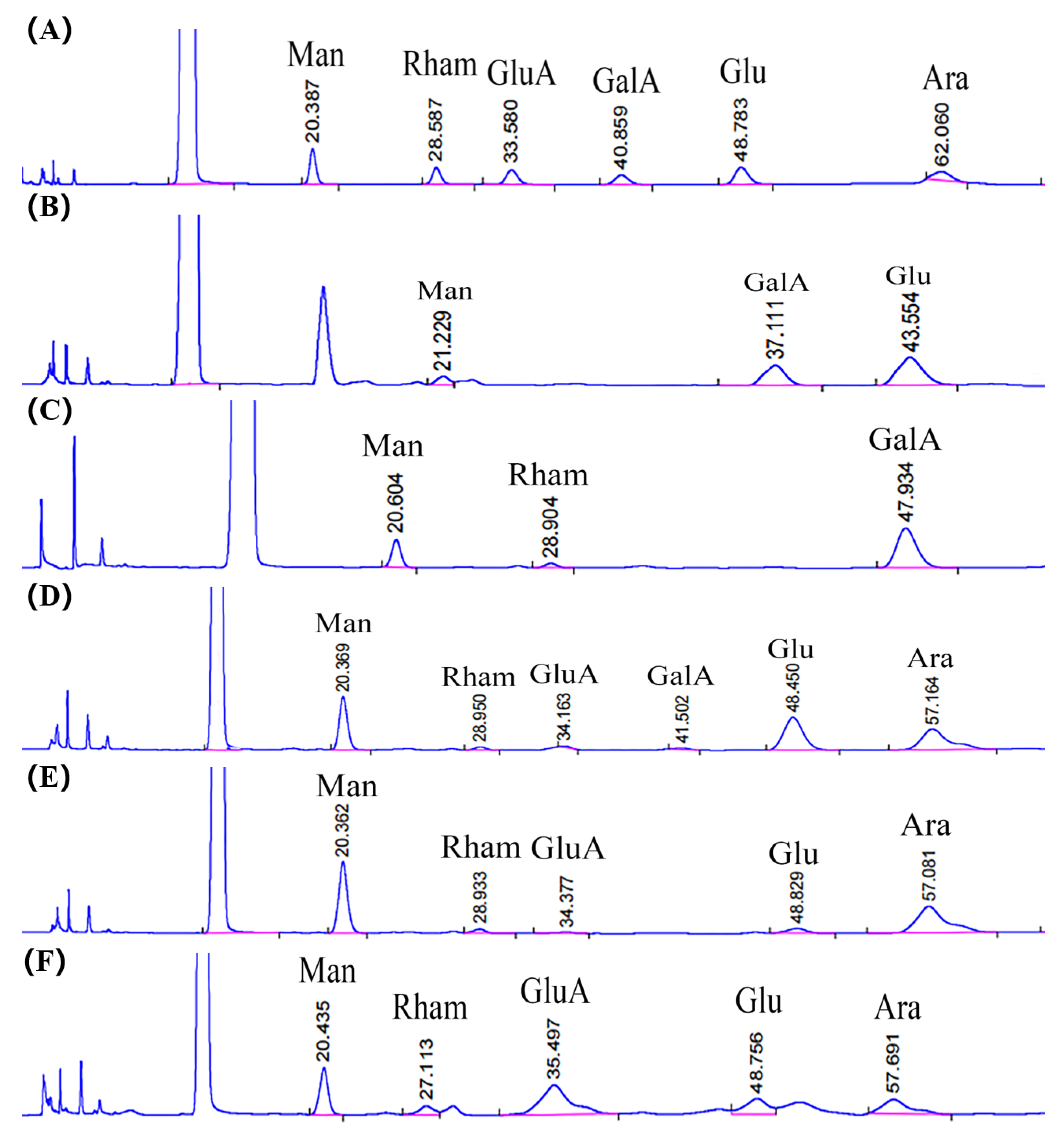
2.5. Monosaccharide Composition, Molar Ratio, and Molecular Weight of SVP
2.6. Uv-vis Spectral Characteristics
2.7. Fourier Transform Infrared Spectral Characteristics
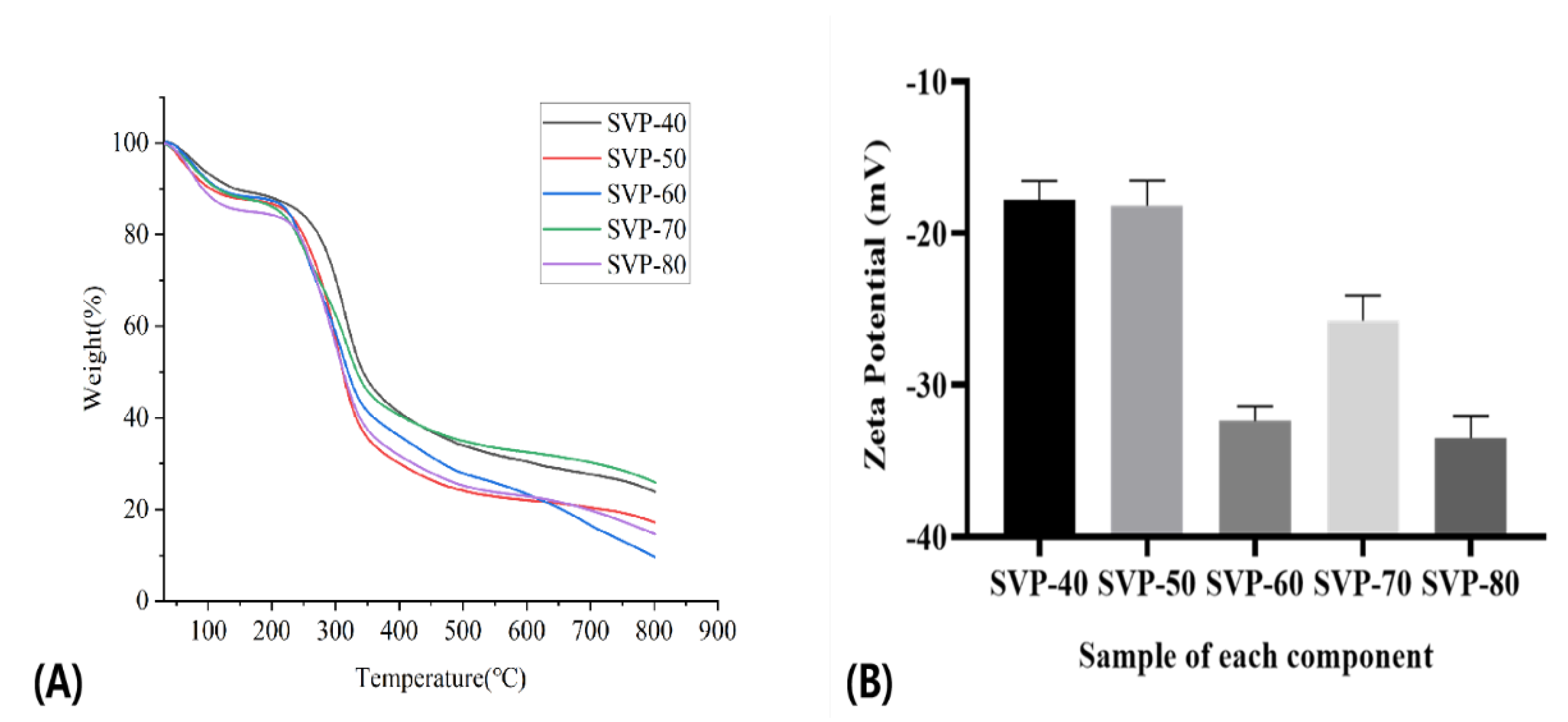
2.8. Results of the TG Analysis
2.9. Zeta Potential
2.10. Antioxidant Activities In Vitro
2.10.1. DPPH Radical Scavenging Capacity
2.10.2. Hydroxyl Radical Scavenging Ability
2.10.3. Superoxide Anion Radical Scavenging Ability
2.10.4. Determination of the Reducing Power
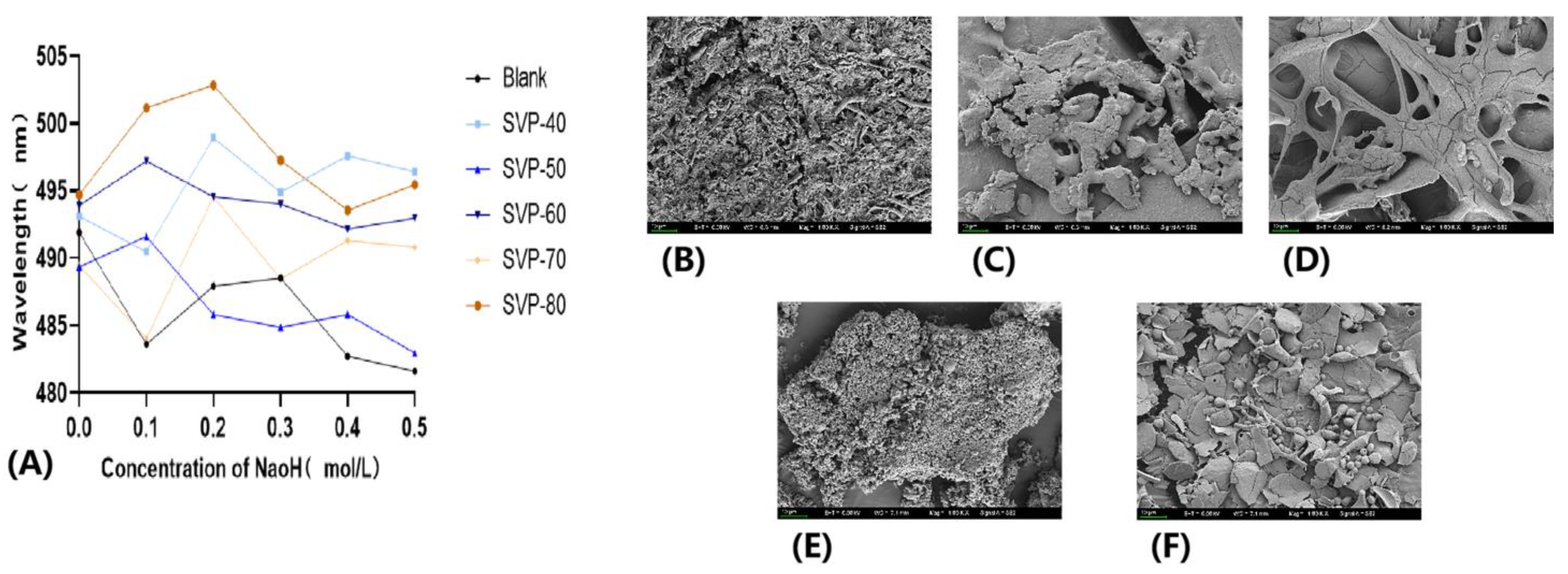
2.11. Congo Red Experimental Analysis
2.12. SEM Electron Microscopy Analysis
2.13. Anti-Inflammatory Activity Analysis
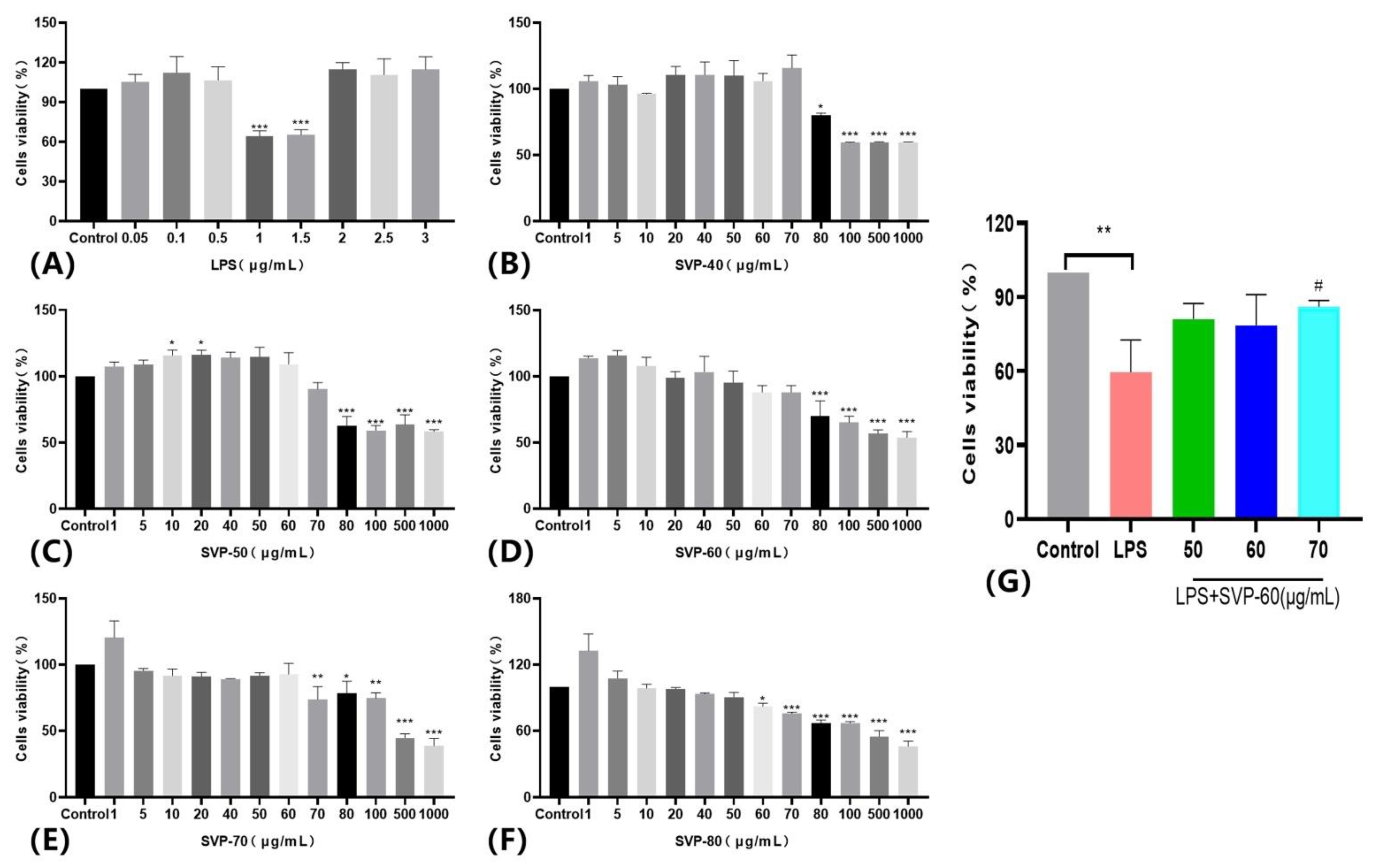
2.13.1. Effects of Five Purified Polysaccharides on the Viability of RAW 264.7 Macrophages and Kupffer Macrophages
2.13.2. Effects of the Purified Polysaccharide SVP-60 on the Viability of LPS-Induced RAW 264.7 Macrophages
2.13.3. Effects of the Purified Polysaccharide SVP-60 on the Viability of LPS-Induced Kupffer Macrophages
2.13.4. Effect of SVP-60 on the Production of LPS-Induced Pro-Inflammatory and Anti-Inflammatory Factor in RAW 264.7 Cells
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Extraction Method
4.2.1. HWE Procedure
4.2.2. UAE Procedure
4.3. Box–Behnken Design (BBD)
4.4. Separation and Purification of S. vaninii Polysaccharide with Different Ethanol Gradients
4.5. Determination of the Chemical Composition and Monosaccharide Compositiont
4.6. Determination of the Molecular Weight
4.7. Uv-vis Spectral Analysis
4.8. FT-IR Spectral Analysis
4.9. TG Analysis
4.10. Zeta Potential
4.11. Determination of the Antioxidant Activity In Vitro
4.12. Congo Red Experiment on Purified Polysaccharides
4.13. Detection of Purified Polysaccharide by Scanning Electron Microscopy (SEM)
4.14. Cell Culture
4.14.1. Cell Viability Test
4.14.2. Anti-Inflammatory Activity of Macrophages
4.14.3. Measurement of IL-6, IL-10, TNF-α, and IL-1β
4.15. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Lu, H.; Shen, M.; Chen, Y.; Yu, Q.; Chen, T.; Xie, J. Alleviative effects of natural plant polysaccharides against DSS-induced ulcerative colitis via inhibiting inflammation and modulating gut microbiota. Food Res. Int. 2023, 167, 112630. [Google Scholar] [CrossRef]
- Meng, X.; Kuang, H.; Wang, Q.; Zhang, H.; Wang, D.; Kang, T. A polysaccharide from Codonopsis pilosula roots attenuates carbon tetrachloride-induced liver fibrosis via modulation of TLR4/NF-kappaB and TGF-beta1/Smad3 signaling pathway. Int. Immunopharmacol. 2023, 119, 110180. [Google Scholar] [CrossRef]
- Wei, X.; Li, N.; Wu, X.; Cao, G.; Qiao, H.; Wang, J.; Hao, R. The preventive effect of Glycyrrhiza polysaccharide on lipopolysaccharide-induced acute colitis in mice by modulating gut microbial communities. Int. J. Biol. Macromol. 2023, 239, 124199. [Google Scholar] [CrossRef]
- Hou, C.Y.; Chen, L.L.; Yang, L.Z.; Ji, X.L. An insight into anti-inflammatory effects of natural polysaccharides. Int. J. Biol. Macromol. 2020, 153, 248–255. [Google Scholar] [CrossRef]
- Alhallaf, W.; Perkins, L.B. The Anti-Inflammatory Properties of Chaga Extracts Obtained by Different Extraction Methods against LPS-Induced RAW 264.7. Molecules 2022, 27, 4207. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Ma, B.; Xue, F.; Xing, Y.; Wu, P.; Li, T.; Shi, F.; Xu, C. Structure Characterization and Anti-Inflammatory Activity of Polysaccharides from Lingzhi or Reishi Medicinal Mushroom Ganoderma lucidum (Agaricomycetes) by Microwave-Assisted Freeze-Thaw Extraction. Int. J. Med. Mushrooms 2022, 24, 49–61. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Wang, J.; Jiang, H.; Wang, G.; Wang, Y. Deep sequencing of the Sanghuangporus vaninii transcriptome reveals dynamic landscapes of candidate genes involved in the biosynthesis of active compounds. Arch. Microbiol. 2021, 203, 2315–2324. [Google Scholar] [CrossRef]
- Guo, Q.; Zhao, L.; Zhu, Y.; Wu, J.; Hao, C.; Song, S.; Shi, W. Optimization of culture medium for Sanghuangporus vaninii and a study on its therapeutic effects on gout. Biomed. Pharmacother. 2021, 135, 111194. [Google Scholar] [CrossRef]
- Wan, X.; Jin, X.; Xie, M.; Liu, J.; Gontcharov, A.A.; Wang, H.; Lv, R.; Liu, D.; Wang, Q.; Li, Y. Characterization of a polysaccharide from Sanghuangporus vaninii and its antitumor regulation via activation of the p53 signaling pathway in breast cancer MCF-7 cells. Int. J. Biol. Macromol. 2020, 163, 865–877. [Google Scholar] [CrossRef] [PubMed]
- Huo, J.; Sun, Y.; Zhong, S.; Li, Y.; Yang, R.; Xia, L.; Wang, J.; Zhang, M.; Zhu, J. Safety evaluation of aqueous extracts of Sanghuangporus vaninii fruiting body in Sprague-Dawley rats. Food Sci. Nutr. 2020, 8, 5107–5113. [Google Scholar] [CrossRef]
- Chen, W.; Tan, H.; Liu, Q.; Zheng, X.; Zhang, H.; Liu, Y.; Xu, L. A Review: The Bioactivities and Pharmacological Applications of Phellinus linteus. Molecules 2019, 24, 1888. [Google Scholar] [CrossRef] [PubMed]
- Yin, C.; Li, Y.; Li, J.; Fan, X.; Yao, F.; Shi, D.; Cheng, Y.; Liu, M.; Lu, Q.; Gao, H. Gastrointestinal digestion, probiotic fermentation behaviors and immunomodulatory effects of polysaccharides from Sanghuangporus vaninii. Int. J. Biol. Macromol. 2022, 223, 606–617. [Google Scholar] [CrossRef]
- Xia, Y.; Yang, C.; Liu, X.; Wang, G.; Xiong, Z.; Song, X.; Yang, Y.; Zhang, H.; Ai, L. Enhancement of triterpene production via in situ extractive fermentation of Sanghuangporus vaninii YC-1. Biotechnol. Appl. Biochem. 2022, 69, 2561–2572. [Google Scholar] [CrossRef]
- Wang, H.; Ma, J.X.; Wu, D.M.; Gao, N.; Si, J.; Cui, B.K. Identifying Bioactive Ingredients and Antioxidant Activities of Wild Sanghuangporus Species of Medicinal Fungi. J. Fungi 2023, 9, 242. [Google Scholar] [CrossRef] [PubMed]
- Cai, C.; Ma, J.; Han, C.; Jin, Y.; Zhao, G.; He, X. Extraction and antioxidant activity of total triterpenoids in the mycelium of a medicinal fungus, Sanghuangporus sanghuang. Sci. Rep. 2019, 9, 7418. [Google Scholar] [CrossRef]
- Lin, W.C.; Deng, J.S.; Huang, S.S.; Wu, S.H.; Chen, C.C.; Lin, W.R.; Lin, H.Y.; Huang, G.J. Anti-Inflammatory Activity of Sanghuangporus sanghuang Mycelium. Int. J. Mol. Sci. 2017, 18, 347. [Google Scholar] [CrossRef]
- Cheng, J.; Wang, Y.; Song, J.; Liu, Y.; Ji, W.; He, L.; Wei, H.; Hu, C.; Jiang, Y.; Xing, Y.; et al. Characterization, immunostimulatory and antitumor activities of a beta-galactoglucofurannan from cultivated Sanghuangporus vaninii under forest. Front. Nutr. 2022, 9, 1058131. [Google Scholar] [CrossRef] [PubMed]
- Badalyan, S.M.; Barkhudaryan, A.; Rapior, S. Medicinal Macrofungi as cosmeceuticals: A Review. Int. J. Med. Mushrooms 2022, 24, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Huang, G. Extraction and antioxidant activities of cushaw polysaccharide. Int. J. Biol. Macromol. 2018, 120, 1646–1649. [Google Scholar] [CrossRef]
- Kumar, C.S.; Sivakumar, M.; Ruckmani, K. Microwave-assisted extraction of polysaccharides from Cyphomandra betacea and its biological activities. Int. J. Biol. Macromol. 2016, 92, 682–693. [Google Scholar] [CrossRef]
- Ma, T.; Sun, X.; Tian, C.; Luo, J.; Zheng, C.; Zhan, J. Polysaccharide extraction from Sphallerocarpus gracilis roots by response surface methodology. Int. J. Biol. Macromol. 2016, 88, 162–170. [Google Scholar] [CrossRef]
- Chen, Z.; Yin, C.; Fan, X.; Ma, K.; Yao, F.; Zhou, R.; Shi, D.; Cheng, W.; Gao, H. Characterization of physicochemical and biological properties of Schizophyllum commune polysaccharide extracted with different methods. Int. J. Biol. Macromol. 2020, 156, 1425–1434. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhang, H.; Du, W.; Qian, L.; Xu, Y.; Huang, Y.; Xiong, Q.; Li, H.; Yuan, J. Comparison of different extraction methods for polysaccharides from Crataegus pinnatifida Bunge. Int. J. Biol. Macromol. 2020, 150, 1011–1019. [Google Scholar] [CrossRef] [PubMed]
- Xue, H.Y.; Li, J.R.; Liu, Y.G.; Gao, Q.; Wang, X.W.; Zhang, J.W.; Tanokura, M.; Xue, Y.L. Optimization of the ultrafiltration-assisted extraction of Chinese yam polysaccharide using response surface methodology and its biological activity. Int. J. Biol. Macromol. 2019, 121, 1186–1193. [Google Scholar] [CrossRef]
- Cai, L.; Chen, B.; Yi, F.; Zou, S. Optimization of extraction of polysaccharide from dandelion root by response surface methodology: Structural characterization and antioxidant activity. Int. J. Biol. Macromol. 2019, 140, 907–919. [Google Scholar] [CrossRef]
- Zhang, W.; Duan, W.; Huang, G.; Huang, H. Ultrasonic-assisted extraction, analysis and properties of mung bean peel polysaccharide. Ultrason. Sonochem. 2023, 98, 106487. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Li, J.; Cao, Y.; Huang, J.; Lin, H.; Zhao, T.; Liu, L.; Shen, P.; Julian McClements, D.; Chen, J.; et al. Extraction and characterization of pectic polysaccharides from Choerospondias axillaris peels: Comparison of hot water and ultrasound-assisted extraction methods. Food Chem. 2023, 401, 134156. [Google Scholar] [CrossRef] [PubMed]
- Samavati, V. Polysaccharide extraction from Abelmoschus esculentus: Optimization by response surface methodology. Carbohydr. Polym. 2013, 95, 588–597. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, Z.; Wang, L.; Walid, E.; Zhang, H. Ultrasonic-assisted extraction of polysaccharides from Hohenbuehelia serotina by response surface methodology. Int. J. Biol. Macromol. 2012, 51, 523–530. [Google Scholar] [CrossRef]
- Wang, H.; Li, Y.; Ren, Z.; Cong, Z.; Chen, M.; Shi, L.; Han, X.; Pei, J. Optimization of the microwave-assisted enzymatic extraction of Rosa roxburghii Tratt. polysaccharides using response surface methodology and its antioxidant and alpha-d-glucosidase inhibitory activity. Int. J. Biol. Macromol. 2018, 112, 473–482. [Google Scholar] [CrossRef]
- Gong, P.; Long, H.; Guo, Y.; Wang, S.; Chen, F.; Chen, X. Isolation, Structural Characterization, and Hypoglycemic Activities In Vitro of Polysaccharides from Pleurotus eryngii. Molecules 2022, 27, 7140. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Cheng, L.; Liu, F.; Li, T.; Yu, Z.; Xu, Y.; Yang, Y. Optimization of Ultrasound-Assisted Extraction and Structural Characterization of the Polysaccharide from Pumpkin (Cucurbita moschata) Seeds. Molecules 2018, 23, 1207. [Google Scholar] [CrossRef]
- Ji, X.; Wang, Z.; Hao, X.; Zhu, Y.; Lin, Y.; Li, G.; Guo, X. Structural characterization of a new high molecular weight polysaccharide from jujube fruit. Front. Nutr. 2022, 9, 1012348. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Zhang, X.; Wang, S.; Guo, Q.; Li, Z.; Liu, H.; Wang, C. Structural characterisation and immunomodulatory activity of polysaccharides from white asparagus skin. Carbohydr. Polym. 2020, 227, 115314. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhou, Y.; Liu, M.; Wang, Q.; Li, Y. Extraction optimization, characterization, antioxidant and immunomodulatory activities of a novel polysaccharide from the wild mushroom Paxillus involutus. Int. J. Biol. Macromol. 2018, 112, 326–332. [Google Scholar] [CrossRef]
- Qu, Y.; Yang, H.; Li, S.; Li, L.; Li, Y.; Wang, D. The involvement of Th1 cell differentiation in the anti-tumor effect of purified polysaccharide from Sanghuangporus vaninii in colorectal cancer via multi-omics analysis. Int. J. Biol. Macromol. 2023, 237, 123927. [Google Scholar] [CrossRef]
- Wei, Q.; Zhang, Y.H. Ultrasound-assisted polysaccharide extraction from Cercis chinensis and properites, antioxidant activity of polysaccharide. Ultrason. Sonochem. 2023, 96, 106422. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, S.C.; Lee, H.Y.; Cho, D.Y.; Jung, J.G.; Kang, D.; Kang, S.S.; Cho, K.M. Changes in nutritional compositions of processed mountain-cultivated ginseng sprouts (Panax ginseng) and screening for their antioxidant and anti-inflammatory properties. J. Funct. Foods 2021, 86, 104668. [Google Scholar] [CrossRef]
- Liao, C.; Wu, L.; Zhong, W.; Zheng, Q.; Tan, W.; Feng, K.; Feng, X.; Meng, F. Cellular Antioxidant Properties of Ischnoderma resinosum Polysaccharide. Molecules 2022, 27, 7717. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chen, Z.; Xiao, Y.; Gao, F.; Zhan, F.; Lu, Z.; Huang, Z.; Wei, X.; Su, F.; Shi, F.; et al. The Keap1-Nrf2 signaling pathway regulates antioxidant defenses of Ctenopharyngodon idella induced by bacterial infection. Fish Shellfish Immunol. 2023, 137, 108686. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Li, Y.; Cao, Q.; Ye, L.; Wang, J.; Guo, M. The Function of Natural Polysaccharides in the Treatment of Ulcerative Colitis. Front. Pharmacol. 2022, 13, 927855. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Lu, X.; Han, Y.; Xing, Y.; Zheng, Y.; Cui, C. Ginseng polysaccharide reduces autoimmune hepatitis inflammatory response by inhibiting PI3K/AKT and TLRs/NF-kappaB signaling pathways. Phytomedicine 2023, 116, 154859. [Google Scholar] [CrossRef] [PubMed]
- Sohrab, S.S.; Raj, R.; Nagar, A.; Hawthorne, S.; Paiva-Santos, A.C.; Kamal, M.A.; El-Daly, M.M.; Azhar, E.I.; Sharma, A. Chronic Inflammation’s Transformation to Cancer: A Nanotherapeutic Paradigm. Molecules 2023, 28, 4413. [Google Scholar] [CrossRef] [PubMed]
- Correia, E.E.M.; Figueirinha, A.; Rodrigues, L.; Pinela, J.; Calhelha, R.C.; Barros, L.; Fernandes, C.; Salgueiro, L.; Goncalves, T. The Chemical Profile, and Antidermatophytic, Anti-Inflammatory, Antioxidant and Antitumor Activities of Withania chevalieri A.E. Gonc. Ethanolic Extract. Plants 2023, 12, 2502. [Google Scholar] [CrossRef]
- Park, J.W.; Kwon, O.K.; Ryu, H.W.; Paik, J.H.; Paryanto, I.; Yuniato, P.; Choi, S.; Oh, S.R.; Ahn, K.S. Anti-inflammatory effects of Passiflora foetida L. in LPS-stimulated RAW 264.7 macrophages. Int. J. Mol. Med. 2018, 41, 3709–3716. [Google Scholar] [CrossRef]
- Han, H.J.; Hyun, C.G. Acenocoumarol Exerts Anti-Inflammatory Activity via the Suppression of NF-kappaB and MAPK Pathways in RAW 264.7 Cells. Molecules 2023, 28, 2075. [Google Scholar] [CrossRef]
- Zhang, Y.; Ma, L.; Peng, M.M.; Wang, L. Effects of the miR-29c/PTEN axis on the PI3 K/Akt/NF-kB pathway in a rat model of severe pneumonia. All Life 2021, 14, 355–364. [Google Scholar] [CrossRef]
- Chen, J.; Tian, S.; Shu, X.; Du, H.; Li, N.; Wang, J. Extraction, Characterization and Immunological Activity of Polysaccharides from Rhizoma gastrodiae. Int. J. Mol. Sci. 2016, 17, 1011. [Google Scholar] [CrossRef]
- Jiang, Y.Y.; Yu, J.; Li, Y.B.; Wang, L.; Hu, L.; Zhang, L.; Zhou, Y.H. Extraction and antioxidant activities of polysaccharides from roots of Arctium lappa L. Int. J. Biol. Macromol. 2019, 123, 531–538. [Google Scholar] [CrossRef]
- Zhang, W.; Huang, J.; Wang, W.; Li, Q.; Chen, Y.; Feng, W.; Zheng, D.; Zhao, T.; Mao, G.; Yang, L.; et al. Extraction, purification, characterization and antioxidant activities of polysaccharides from Cistanche tubulosa. Int. J. Biol. Macromol. 2016, 93, 448–458. [Google Scholar] [CrossRef]
- Wang, K.; Guo, J.; Cheng, J.; Zhao, X.; Ma, B.; Yang, X.; Shao, H. Ultrasound-assisted extraction of polysaccharide from spent Lentinus edodes substrate: Process optimization, precipitation, structural characterization and antioxidant activity. Int. J. Biol. Macromol. 2021, 191, 1038–1045. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Su, F.; Hu, D.; Ruan, C.; Che, P.; Zhang, Y.; Wang, J. Optimization of the extraction process and antioxidant activity of Polysaccharide extracted from Centipeda minima. Chem. Biodivers. 2022, 20, e202200626. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Liu, W.; Zhao, Q.; Li, K.; Zhu, J.; Yao, W.; Gao, X. Combination of polysaccharides from Astragalus membranaceus and Codonopsis pilosula ameliorated mice colitis and underlying mechanisms. J. Ethnopharmacol. 2021, 264, 113280. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Luo, Y.; Sang, Y.; Kan, J. Isolation, purification, structural characterization, and hypoglycemic activity assessment of polysaccharides from Hovenia dulcis (Guai Zao). Int. J. Biol. Macromol. 2022, 208, 1106–1115. [Google Scholar] [CrossRef]
- Tang, Y.; He, X.; Liu, G.; Wei, Z.; Sheng, J.; Sun, J.; Li, C.; Xin, M.; Li, L.; Yi, P. Effects of different extraction methods on the structural, antioxidant and hypoglycemic properties of red pitaya stem polysaccharide. Food Chem. 2023, 405, 134804. [Google Scholar] [CrossRef]
- Liu, Y.J.; Mo, X.L.; Tang, X.Z.; Li, J.H.; Hu, M.B.; Yan, D.; Peng, W.; Wu, C.J. Extraction Optimization, Characterization, and Bioactivities of Polysaccharides from Pinelliae Rhizoma Praeparatum Cum Alumine Employing Ultrasound-Assisted Extraction. Molecules 2017, 22, 965. [Google Scholar] [CrossRef]
- Liu, G.; Kamilijiang, M.; Abuduwaili, A.; Zang, D.; Abudukelimu, N.; Liu, G.; Yili, A.; HA, A.I. Isolation, structure elucidation, and biological activity of polysaccharides from Saussurea involucrata. Int. J. Biol. Macromol. 2022, 222, 154–166. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wu, D.; Li, W.; Chen, W.; Liu, Y.; Zhang, J.; Wan, J.; Yu, H.; Zhou, S.; Yang, Y. Structural elucidation and anti-inflammatory activity of a proteoglycan from spent substrate of Lentinula edodes. Int. J. Biol. Macromol. 2023, 224, 1509–1523. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Abuduaini, G.; Yang, C.; Zhang, S.; Zhang, Y.; Fan, H.; Teng, X.; Bao, C.; Liu, H.; Wang, D.; et al. Isolation, purification, and structural elucidation of Stropharia rugosoannulata polysaccharides with hypolipidemic effect. Front. Nutr. 2022, 9, 1092582. [Google Scholar] [CrossRef]
- Jing, Y.; Zhang, R.; Ma, Y.; Zhang, Y.; Zheng, Y.; Wu, L.; Zhang, D. Structural elucidation, anti-radical and immunomodulatory activities of polysaccharides from the roots of Glehnia littoralis. Nat. Prod. Res. 2022, 36, 4630–4635. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Tian, H.; Xiang, D. Stabilizing the Oil-in-Water Emulsions Using the Mixtures of Dendrobium officinale Polysaccharides and Gum Arabic or Propylene Glycol Alginate. Molecules 2020, 25, 759. [Google Scholar] [CrossRef]
- Khemakhem, I.; Abdelhedi, O.; Trigui, I.; Ayadi, M.A.; Bouaziz, M. Structural, antioxidant and antibacterial activities of polysaccharides extracted from olive leaves. Int. J. Biol. Macromol. 2018, 106, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Pang, H.; Gao, Z.; Zhao, H.; Zhang, J.; Jia, L. Antioxidant and hepatoprotective activities of residue polysaccharides by Pleurotus citrinipileatus. Int. J. Biol. Macromol. 2019, 131, 315–322. [Google Scholar] [CrossRef]
- Tang, Z.; Zhou, C.; Cai, Y.; Tang, Y.; Sun, W.; Yao, H.; Zheng, T.; Chen, H.; Xiao, Y.; Shan, Z.; et al. Purification, characterization and antioxidant activities in vitro of polysaccharides from Amaranthus hybridus L. PeerJ 2020, 8, e9077. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Zou, G.; Li, C. Purification, characterization and in vitro antioxidant activities of polysaccharide from Chaenomeles speciosa. Int. J. Biol. Macromol. 2016, 92, 702–707. [Google Scholar] [CrossRef]
- Jalili Safaryan, M.; Ganjloo, A.; Bimakr, M.; Zarringhalami, S. Optimization of Ultrasound-Assisted Extraction, Preliminary Characterization and In Vitro Antioxidant Activity of Polysaccharides from Green Pea Pods. Foods 2016, 5, 78. [Google Scholar] [CrossRef] [PubMed]
- Mei, X.; Yang, W.; Huang, G.; Huang, H. The antioxidant activities of balsam pear polysaccharide. Int. J. Biol. Macromol. 2020, 142, 232–236. [Google Scholar] [CrossRef]
- Ji, C.; Zhang, Z.; Zhang, B.; Chen, J.; Liu, R.; Song, D.; Li, W.; Lin, N.; Zou, X.; Wang, J.; et al. Purification, characterization, and in vitro antitumor activity of a novel glucan from the purple sweet potato Ipomoea batatas (L.) Lam. Carbohydr. Polym. 2021, 257, 117605. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xu, Y.; Ma, X.; Liu, X.; Yang, M.; Fan, W.; Ren, H.; Efehi, N.; Wang, X.; Zhu, X. Extraction, purification, characterization and antioxidant activities of polysaccharides from Zizyphus jujuba cv. Linzexiaozao. Int. J. Biol. Macromol. 2018, 118, 2138–2148. [Google Scholar] [CrossRef] [PubMed]





| Source | HWE | |||||
|---|---|---|---|---|---|---|
| Sum of Squares | df | Mean Square | F Value | p-Value | Significant * | |
| Model | 0.14 | 9 | 0.016 | 61.69 | <0.0001 | ** |
| A | 0.002 | 1 | 0.002 | 5.87 | 0.0460 | * |
| B | 0.002 | 1 | 0.002 | 5.87 | 0.0460 | * |
| C | 0.003 | 1 | 0.003 | 12.41 | 0.0097 | * |
| AB | 0.001 | 1 | 0.001 | 4.75 | 0.0657 | |
| AC | 0.002 | 1 | 0.002 | 6.20 | 0.0415 | * |
| BC | 0.000 | 1 | 0.000 | 1.55 | 0.2530 | |
| A2 | 0.036 | 1 | 0.036 | 140.47 | <0.0001 | ** |
| B2 | 0.032 | 1 | 0.032 | 125.73 | <0.0001 | ** |
| C2 | 0.051 | 1 | 0.051 | 198.48 | <0.0001 | ** |
| R2 = 0.9875, R2adj = 0.9715, C.V.% = 3.56. | ||||||
| Source | UAE | |||||
|---|---|---|---|---|---|---|
| Sum of Squares | df | Mean Square | F Value | p-Value | Significant * | |
| Model | 0.64 | 14 | 0.046 | 125.97 | <0.0001 | ** |
| A | 0.002 | 1 | 0.002 | 6.13 | 0.0267 | * |
| B | 0.004 | 1 | 0.004 | 10.17 | 0.0066 | ** |
| C | 0.012 | 1 | 0.012 | 33.30 | <0.0001 | ** |
| D | 0.002 | 1 | 0.002 | 4.98 | 0.0425 | * |
| AB | 0.053 | 1 | 0.053 | 146.38 | <0.0001 | ** |
| AC | 0.000 | 1 | 0.000 | 0.000 | 1.0000 | |
| AD | 0.012 | 1 | 0.012 | 32.57 | <0.0001 | ** |
| BC | 0.012 | 1 | 0.012 | 33.48 | <0.0001 | ** |
| BD | 0.007 | 1 | 0.007 | 15.56 | 0.0015 | ** |
| CD | 0.014 | 1 | 0.014 | 39.85 | <0.0001 | ** |
| A2 | 0.069 | 1 | 0.069 | 192.27 | <0.0001 | ** |
| B2 | 0.062 | 1 | 0.062 | 171.06 | <0.0001 | ** |
| C2 | 0.28 | 1 | 0.28 | 764.43 | <0.0001 | ** |
| D2 | 0.33 | 1 | 0.33 | 906.61 | <0.0001 | ** |
| R2 = 0.9921, R2adj = 0.9842, C.V.% = 1.48. | ||||||
| Samples | SVP-40 | SVP-50 | SVP-60 | SVP-70 | SVP-80 |
|---|---|---|---|---|---|
| Yield (%) | 0.35% | 0.39% | 0.77% | 0.26% | 1.08% |
| Total sugar content (%) | 85.53% | 86.90% | 92.40% | 90.12% | 85.65% |
| Protein (%) | 0.29% | 0.19% | 0.91% | 2.43% | 0.32% |
| Uronic acid (%) | 6.03% | 7.87% | 4.39% | 0.95% | 0.47% |
| Monosaccharide composition (mol %) | |||||
| Man (Mannose) | 2.2 | 5.5 | 1.4 | 1.9 | 1.1 |
| Rham (Rhamnose) | _ | 1.2 | 1.1 | 1.5 | 3.5 |
| GluA (Glucuronic acid) | _ | _ | 0.5 | 0.8 | 2.3 |
| GalA (Galactose acid) | 8.5 | 1.8 | 0.6 | _ | _ |
| Glu (Glucose) | 13.7 | _ | 2.0 | 2.7 | 1.0 |
| Ara (Arabinose) | _ | _ | 1.8 | 2.3 | 1.0 |
| Molecular weight (k Da) | 295.06 | 215.36 | 245.09 | 273.75 | 274.77 |
| Test Group | Coded Levels | Response Value | |||
|---|---|---|---|---|---|
| A Liquid-to-Solid Ratio (mL/g) | B Extraction Temperature (℃) | C Extraction Time (h) | Measured Value (%) | Predictive Value (%) | |
| 1 | 40.00 (1) | 70.00 (−1) | 3.00 (0) | 0.38 | 0.36 |
| 2 | 30.00 (0) | 80.00 (0) | 3.00 (0) | 0.58 | 0.59 |
| 3 | 30.00 (0) | 90.00 (1) | 4.00 (1) | 0.42 | 0.41 |
| 4 | 20.00 (−1) | 80.00 (0) | 4.00 (1) | 0.45 | 0.44 |
| 5 | 40.00 (1) | 80.00 (0) | 2.00 (−1) | 0.36 | 0.37 |
| 6 | 20.00 (−1) | 80.00 (0) | 2.00 (−1) | 0.37 | 0.36 |
| 7 | 30.00 (0) | 80.00 (0) | 3.00 (0) | 0.60 | 0.59 |
| 8 | 30.00 (0) | 90.00 (1) | 2.00 (−1) | 0.40 | 0.39 |
| 9 | 30.00 (0) | 70.00 (−1) | 2.00 (−1) | 0.34 | 0.35 |
| 10 | 30.00 (0) | 80.00 (0) | 3.00 (0) | 0.58 | 0.59 |
| 11 | 30.00 (0) | 70.00 (−1) | 4.00 (1) | 0.40 | 0.41 |
| 12 | 40.00 (1) | 80.00 (0) | 4.00 (1) | 0.36 | 0.37 |
| 13 | 20.00 (−1) | 90.00 (1) | 3.00 (0) | 0.40 | 0.42 |
| 14 | 30.00 (0) | 80.00 (0) | 3.00 (0) | 0.58 | 0.59 |
| 15 | 30.00 (0) | 80.00 (0) | 3.00 (0) | 0.60 | 0.59 |
| 16 | 20.00 (−1) | 70.00 (−1) | 3.00 (0) | 0.42 | 0.42 |
| 17 | 40.00 (1) | 90.00 (1) | 3.00 (0) | 0.43 | 0.42 |
| Test Group | Coded Levels | Response Value | ||||
|---|---|---|---|---|---|---|
| A Extraction Time (min) | B Extraction Temperature (℃) | C Liquid-to-Solid Ratio (mL/g) | D Ultrasound Power (W) | Measured Value (%) | Predictive Value (%) | |
| 1 | 70.00 (1) | 60.00 (0) | 70.00 (0) | 50.00 (1) | 1.24 | 1.25 |
| 2 | 60.00 (0) | 60.00 (0) | 70.00 (0) | 40.00 (0) | 1.58 | 1.55 |
| 3 | 60.00 (0) | 60.00 (0) | 70.00 (0) | 40.00 (0) | 1.54 | 1.55 |
| 4 | 60.00 (0) | 70.00 (1) | 80.00 (1) | 40.00 (0) | 1.24 | 1.25 |
| 5 | 60.00 (0) | 70.00 (1) | 70.00 (0) | 50.00 (1) | 1.24 | 1.24 |
| 6 | 60.00 (0) | 60.00 (0) | 70.00 (0) | 40.00 (0) | 1.54 | 1.55 |
| 7 | 70.00 (1) | 60.00 (0) | 80.00 (1) | 40.00 (0) | 1.19 | 1.19 |
| 8 | 50.00 (−1) | 60.00 (0) | 70.00 (0) | 30.00 (−1) | 1.29 | 1.30 |
| 9 | 70.00 (1) | 60.00 (0) | 60.00 (−1) | 40.00 (0) | 1.28 | 1.26 |
| 10 | 60.00 (0) | 60.00 (0) | 80.00 (1) | 50.00 (1) | 1.03 | 1.01 |
| 11 | 60.00 (0) | 50.00 (−1) | 60.00 (−1) | 40.00 (0) | 1.34 | 1.35 |
| 12 | 50.00 (−1) | 60.00 (0) | 80.00 (1) | 40.00 (0) | 1.22 | 1.22 |
| 13 | 70.00 (1) | 70.00 (1) | 70.00 (0) | 40.00 (0) | 1.44 | 1.43 |
| 14 | 70.00 (1) | 50.00 (−1) | 70.00 (0) | 40.00 (0) | 1.23 | 1.24 |
| 15 | 50.00 (−1) | 60.00 (0) | 60.00 (−1) | 40.00 (0) | 1.31 | 1.29 |
| 16 | 60.00 (0) | 50.00 (−1) | 70.00 (0) | 30.00 (−1) | 1.31 | 1.29 |
| 17 | 60.00 (0) | 60.00 (0) | 70.00 (0) | 40.00 (0) | 1.56 | 1.55 |
| 18 | 50.00 (−1) | 50.00 (−1) | 70.00 (0) | 40.00 (0) | 1.49 | 1.49 |
| 19 | 60.00 (0) | 50.00 (−1) | 80.00 (1) | 40.00 (0) | 1.17 | 1.18 |
| 20 | 60.00 (0) | 60.00 (0) | 70.00 (0) | 40.00 (0) | 1.53 | 1.55 |
| 21 | 60.00 (0) | 70.00 (1) | 60.00 (−1) | 40.00 (0) | 1.19 | 1.21 |
| 22 | 70.00 (1) | 60.00 (0) | 70.00 (0) | 30.00 (−1) | 1.16 | 1.17 |
| 23 | 60.00 (0) | 60.00 (0) | 60.00 (−1) | 30.00 (−1) | 1.09 | 1.10 |
| 24 | 50.00 (−1) | 70.00 (1) | 70.00 (0) | 40.00 (0) | 1.24 | 1.23 |
| 25 | 60.00 (0) | 60.00 (0) | 60.00 (−1) | 50.00 (1) | 1.19 | 1.20 |
| 26 | 60.00 (0) | 70.00 (1) | 70.00 (0) | 30.00 (−1) | 1.19 | 1.18 |
| 27 | 50.00 (−1) | 60.00 (0) | 70.00 (0) | 50.00 (1) | 1.15 | 1.17 |
| 28 | 60.00 (0) | 50.00 (−1) | 70.00 (0) | 50.00 (1) | 1.21 | 1.20 |
| 29 | 60.00 (0) | 60.00 (0) | 80.00 (1) | 30.00 (−1) | 1.17 | 1.16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Song, J.; Gao, F.; Chen, W.; Zong, Y.; Li, J.; He, Z.; Du, R. Extraction, Purification, and Structural Characterization of Polysaccharides from Sanghuangporus vaninii with Anti-Inflammatory Activity. Molecules 2023, 28, 6081. https://doi.org/10.3390/molecules28166081
Liu J, Song J, Gao F, Chen W, Zong Y, Li J, He Z, Du R. Extraction, Purification, and Structural Characterization of Polysaccharides from Sanghuangporus vaninii with Anti-Inflammatory Activity. Molecules. 2023; 28(16):6081. https://doi.org/10.3390/molecules28166081
Chicago/Turabian StyleLiu, Jinze, Jinyue Song, Fusheng Gao, Weijia Chen, Ying Zong, Jianming Li, Zhongmei He, and Rui Du. 2023. "Extraction, Purification, and Structural Characterization of Polysaccharides from Sanghuangporus vaninii with Anti-Inflammatory Activity" Molecules 28, no. 16: 6081. https://doi.org/10.3390/molecules28166081
APA StyleLiu, J., Song, J., Gao, F., Chen, W., Zong, Y., Li, J., He, Z., & Du, R. (2023). Extraction, Purification, and Structural Characterization of Polysaccharides from Sanghuangporus vaninii with Anti-Inflammatory Activity. Molecules, 28(16), 6081. https://doi.org/10.3390/molecules28166081







