Abstract
Natural polysaccharides are macromolecular substances with great potential owing to their wide biological activity and low toxicity. However, not all polysaccharides have significant pharmacodynamic activity; hence, appropriate chemical modification methods can be selected according to the unique structural characteristics of polysaccharides to assist in enhancing and promoting the presentation of their biological activities. This review summarizes research progress on modified polysaccharides, including common chemical modification methods, the change in biological activity following modification, and the factors affecting the biological activity of chemically modified polysaccharides. At the same time, the difficulties and challenges associated with the structural modification of natural polysaccharides are also outlined in this review. Thus, research on polysaccharide structure modification is critical for improving the development and utilization of sugar products.
1. Introduction
Polysaccharides are natural macromolecular carbohydrates made up of more than ten monosaccharides joined by different glycosidic bonds. They are one of the essential components of living organisms with an important role in life processes. Polysaccharides are widely found in plants, animals, bacteria and microorganisms [1]. Due to their good antioxidant [2], antitumor [3], immune regulation [4], antiviral [5], anticoagulant [6] and other biological activities, they have been the focus of scholars in the medical field. Since 1943, polysaccharides have been used as medicines to treat diseases [7] and, up until now, have gradually been developed into functional foods with the development of technology, which are well liked by people.
It has been confirmed that the biological activity of polysaccharides is closely related to their monosaccharide composition, category of glycosidic bond, spatial structure, molecular weight and branched chain structure [8]. However, not all natural polysaccharides have good biological activity, and some of them are not biologically active or their biological activity is relatively weak due to their special structure, decreasing their clinical therapeutic potential and efficacy. For example, due to their large molecular weight, some polysaccharides are difficult to be absorbed by the human body through the cell membrane, and thus cannot exert biological activity. It has been reported that appropriate structural modification can enhance the biological activity of polysaccharides for therapeutic purposes [9]. The chemical modification of polysaccharides can change their spatial structure, monosaccharide composition, monosaccharide molar ratio, molecular weight, as well as substituent type, position and number, to achieve the purpose of activity enhancement [10,11,12,13]. Therefore, the study of the chemical modification of polysaccharides has been the focus of polysaccharide analysis in recent years. At present, the commonly chemical modification methods include acetylation [14], sulfation [15], phosphorylation [16], selenization [17], carboxymethylation [18] and other chemical modification methods.
Numerous review articles have reported the methods of chemical modification for polysaccharides and changes in bioactivity [19,20,21,22]. However, there are few reports on the influencing factors of biological activity after chemical modification. The aim of this paper is to show the methods of chemical modification of polysaccharides, changes in bioactivity and factors affecting chemically modified polysaccharides, providing a reference for broadening the application of chemically modified polysaccharides in the field of pharmaceuticals and functional foods.
2. Methods for the Chemical Modification of Polysaccharides
Chemical modification is a method of modifying the structure of polysaccharides by introducing different kinds of reactive groups through chemical reagents to obtain derivatized polysaccharides. The chemical modification will cause the original hydroxyl group of the polysaccharide to be replaced by substituents. For example, the hydroxyl groups are replaced by groups such as acetyl, sulfate, phosphate, selenate and carboxymethyl groups. With the introduction of functional groups, information such as the molar ratio, spatial structure and molecular weight of monosaccharides also undergo corresponding changes in the polysaccharide. This has improved the problem of the low bioactivity of natural polysaccharides due to the shortcomings of physicochemical properties, such as high viscosity, poor water solubility and excessive molecular weight. It is vital for the research of conformational relationships of polysaccharides to select appropriate chemical modification methods, which can enhance or alter the biological activity of polysaccharides.
2.1. Acetylation Modification
Acetylation modification is one of the important branched chain modification methods in polysaccharide chemical modification. Due to the introduction of an acetyl group, the hydroxyl groups have a nucleophilic substitution with acetic acid or acetic anhydride to generate acetate products, which can further change the spatial structure of the polysaccharides. This promotes the full expansion of the branched chains of the polysaccharide and further improves the solubility of the acetylated polysaccharide. This may be one of the reasons why acetylated modified polysaccharides have certain activities enhanced [14,19,23]. The polysaccharide acetylation reaction is shown in Figure 1.
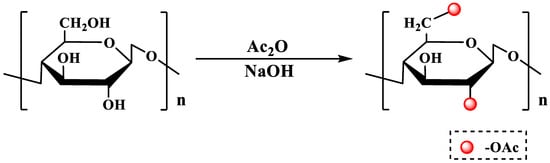
Figure 1.
The acetylation modification of polysaccharides.
Typical acetylation reagents include acetic anhydride or acetic acid. The acetylation reaction is performed by dissolving pre-prepared polysaccharides in organic solvents, such as formamide, dimethylformamide and DMSO, and then adding acetic anhydride or acetic acid reagents. As established, a suitable catalyzator is the key to a successful acetylation reaction. Common traditional acetylation catalysts mainly include pyridine and 4-dimethylaminopyridine (4-DMAP), as well as N-bromosuccinimide (NBS) [24,25]. It is worth noting that pyridine has a strong irritating odor and neurotoxicity, and although 4-DMAP has less toxicity than pyridine, it is more expensive and only suitable for laboratory-level research and development. These issues make them temporarily difficult to apply to large-scale production, resulting in limited application. However, reviewing the updated literature, many polysaccharide acetylation modification experiments do not add catalysts [26,27]. For example, polysaccharides can be directly dissolved in distilled water, and the pH value of the solution is adjusted to 9 with a NaOH reagent. Then, acetic anhydride is added, and NaOH is continued to maintain the pH value at 8–10 for a period of the reaction. Finally, HCl reagent is added to adjust the solution to being neutral, and acetylated polysaccharides are dialyzed to remove reaction by-products, and their concentration increased [28]. There are a lack of corresponding experiments for this phenomenon to clarify the relationship between the addition of catalysts and the degree of acetylation substitution.
2.2. Sulfation Modification
Sulfated polysaccharides refer to polysaccharides that contain sulfate groups on the sugar chain. It has been reported that various marine algal species contain sulfated polysaccharides [6]. In recent years, it has been found that sulfated polysaccharides have higher biological activity in terms of anticoagulation, antitumor and antioxidant activity compared to non-sulfated polysaccharides, which has attracted attention and made it one of the best choices for treating diseases [29,30]. However, in some species of marine polysaccharides, sulfated polysaccharides have better efficacy but lower content, which is difficult for use in large-scale clinical treatments. Therefore, it is urgent to synthesize sulfated polysaccharides using artificial chemical methods. As early as 1988, a Japanese scholar introduced sulfate groups to polysaccharides and found that the antiviral ability was enhanced, which established the basis for the artificial modification of sulfated polysaccharides [31]. At present, researchers’ interest in the synthesis of sulfated polysaccharides is mainly focused on methods such as concentrated sulfuric acid, chlorosulfate–pyridine and sulfur trioxide–pyridine methods [32]. However, after collecting articles from the past decade, it was found that the sulfamic acid method also seems to be helpful for the sulfation modification of polysaccharides [33,34]. In addition, the regional selective sulfation of polysaccharides is a very active research direction, ensuring the controllability and predictability of the introduction of sulfuric acid groups into polysaccharides and clarifying the structure–activity relationship [35].
2.2.1. Sulfur Trioxide–Pyridine Method
The sulfur trioxide–pyridine method is a milder sulfation modification method. First, the polysaccharide is completely dissolved in DMSO by stirring. Then, the esterification reagent prepared by adding a mixture of sulfur trioxide–pyridine dissolved in formamide is added, heated and stirred for a while. After the reaction is completed, the desired product is obtained via dialysis and freeze-drying (Figure 2) [36]. The product obtained using this method has a higher DS and is easy to operate and control. However, sulfur trioxide is relatively expensive and only suitable for small-scale production in the laboratory.
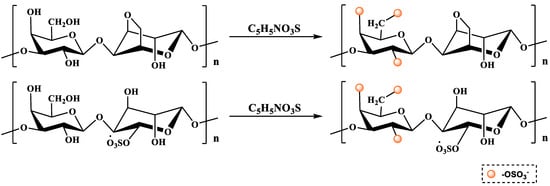
Figure 2.
The sulfation modification of polysaccharide (i.e., the sulfur trioxide–pyridine method).
2.2.2. Concentrated Sulfuric Acid Method
The concentrated sulfuric acid method is one of the classic sulfation methods. First, concentrated sulfuric acid and N-butanol are mixed in proportion, followed by adding ammonium sulfate and stirring in an ice-water bath. Then, the polysaccharide samples are added and stirred at a certain temperature, neutralized, precipitated with alcohol and freeze-dried to obtain the final products [37]. The concentrated sulfuric acid method has the advantages of producing a stable reaction and having low toxicity and low cost. However, it is less used nowadays mainly because concentrated sulfuric acid has strong acidity, which can easily cause polysaccharide carbonization and sugar-chain degradation.
2.2.3. Chlorosulfonic Acid–Pyridine Method
The chlorosulfonic acid–pyridine method (CSA/Pyr) is currently the most widely used sulfation modification method, with the advantages of easy operation, high product yield and high DS. This method involves dissolving polysaccharides in formamide (or DMSO) and reacting with esterification reagents (chlorosulfonic acid–pyridine) under ice-water bath conditions for a period of time to obtain the products [38].
As a strongly oxidizing agent, chlorosulfonic acid is unstable and will react violently when exposed to water. In addition, it is flammable and highly toxic. The pyridine reagent also has a highly irritating odor. Nonetheless, this strategy is the best choice compared to the other two methods. Therefore, it is necessary to develop a less toxic and safer alternative to chlorosulfonic acid–pyridine sulfation.
2.2.4. Sulfamic Acid Method
Compared to the preceding methods of sulfation, sulfamic acid seems to have received less attention. First, the polysaccharide sample is crushed and placed in a beaker, and then sulfamic acid and N, N-dimethylformamide are added. The mixture is then reacted in a water bath at 100 °C for 5 h. After the reaction is completed, the product is placed for cooling, neutralized with NaOH, dialyzed and freeze-dried to obtain the target product [33]. In addition, some scholars have used the amino sulfonic acid method to optimize the Box–Behnken process of guar gum galactomannan. The optimal process occurs upon adding 34 mmol of sulfamic acid to 1 g guar gum galactomannan at 85 °C for 2.6 h [34]. The results indicate that the reaction conditions for preparing the product using the amino sulfonic acid method are mild and that the reagent toxicity is low. However, its drawbacks are still obvious. The disadvantage of the sulfation reaction using the sulfamic acid method is that the reaction activity is low. Therefore, catalysts such as pyridine, urea and acetamide are usually required for catalytic reactions. At the same time, it is easily accompanied by the side reactions of carbamate. In recent years, the development of the amino sulfonic acid method has not been sufficient, so the sample size is smaller compared to the previous three methods.
2.3. Phosphorylation Modification
In nature, phosphorylated polysaccharides are mostly found in animal and plant species, with phosphate esters as the main form. However, because of their low content, limited variety and difficulty in extraction and isolation, they are generally synthesized artificially using chemical modification methods [39]. Under suitable conditions, polysaccharides can react with phosphorylation reagents so that the side chains and phosphorylation groups exist in a covalent manner. In addition, common contemporary phosphorylation modification methods mainly include phosphoric acid and its anhydride method, phosphorus oxychloride method, phosphate salt method and phosphorus pentoxide method.
2.3.1. Acid and Anhydride Methods
The acid and acid anhydride methods were applied earlier in the modification of polysaccharide phosphorylation, whose reaction process is relatively simple. Briefly, polysaccharide powder is dissolved in a mixture of urea and DMSO. Then, phosphoric acid is added, and it is reacted at 100 °C for 6 h. In the end, the product is dialyzed and lyophilized (Figure 3) [40]. This method has the advantages of simple operational steps, universal instrument applicability and low cost. However, due to the intense phosphoric acid reaction process, which generates a large amount of heat, it can lead to the degradation of polysaccharides and lower yield of the target product, so it currently has few applications.
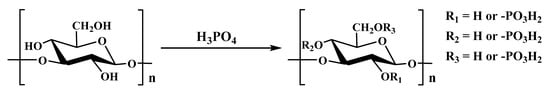
Figure 3.
The phosphorylation modification of polysaccharides (i.e., the acid and anhydride method).
2.3.2. Phosphorus Oxychloride Method
The phosphorus oxychloride method, also known as the phosphoryl chloride method, is a method for synthesizing highly substituted phosphorylated products. Its advantages include a rapid reaction time, simple operation and high DS [41]. First, the polysaccharide powder is dissolved in DMF, and a mixture of phosphorus oxychloride (POCl3) with pyridine is slowly added under the condition of an ice-water bath to react at a specific temperature and time. After that, phosphorylated polysaccharides are obtained through alcohol precipitation, centrifugation and freeze-drying [16]. Although the phosphorus oxychloride method is widely used, it also has certain drawbacks, such as a relatively violent reaction and highly toxic byproducts, and the reaction will also be accompanied by the production of irritating gases.
2.3.3. Phosphate Method
At present, the phosphate method is a commonly used method for phosphorylation modification. Compared with other methods, the phosphate method has the superiority of not easily degrading polysaccharides. However, the disadvantages are also obvious, such as the low reaction activity of polysaccharides when phosphorylated, which results in the DS and yield of products being relatively low. Commonly used phosphates are sodium tripolyphosphate (STPP) and sodium trimetaphosphate (STMP) in phosphorylation modification [42]. Specifically, polysaccharide powder is dissolved in distilled water, and STPP and STMP are added in a 6:1 ratio and according to optimal phosphorylation conditions. The reaction lasts for 6 h at 80 °C, and the target product is obtained via neutralization, dialysis and freeze-drying at the end of the reaction [43].
2.3.4. Phosphorus Pentoxide Method
Chitosan (CSSA) alkylated with stearic acid is mixed with pre-cooled methanesulfonic acid. After process optimization, the best ratio is selected to add to it four times the proportion of phosphorus pentoxide (P2O5) to that of CSSA. Then, the mixture is stirred at 0–5 °C for 1 h. Finally, the target product is obtained via ether precipitation, centrifugation, neutralization or dialysis [44]. However, P2O5 has a strong acidity which can easily cause the degradation of polysaccharides during the reaction process and also lead to a low DS of the products. Therefore, its current application is limited.
2.4. Selenization Modification
Selenided polysaccharides are formed by the combination of polysaccharides and inorganic selenium through covalent bonds. Numerous studies have shown that selenided polysaccharides have stronger biological activity and better absorption properties than original polysaccharides and inorganic selenium [45,46,47]. Selenided polysaccharides exist only in trace amounts in natural microorganisms and plants. However, due to their low content and limited variety, selenium polysaccharides’ development and utilization are limited. Until now, the synthesis of selenium polysaccharides was often carried out through artificial synthesis, with the aim of increasing the bioavailability of selenium polysaccharides and thus expanding their application scope. There are many common types of chemical modification methods for selenium polysaccharides at present, mainly divided into two categories, i.e., the selenate method and the selenium oxychloride method.
2.4.1. Selenate Method
The method of using selenite and its salts as selenide reagents is called the selenate method, and common selenite salts include sodium selenite. Therefore, according to different reaction systems, it can be divided into the nitric acid sodium selenite method (NA-SS) [46], the glacial acetic acid sodium selenite method (GA-SS) [48], the nitric acid selenite method (NA-SA) [49] and the glacial acetic acid selenite method (GA-SA) [48]. BaCl2 is often used as a catalyst in the GA-SA method due to its strong coordination with hydroxyl groups. Among them, the NA-SS method is currently the most widely used selenization method due to its simple operation and high degree of selenization. Briefly, the specific operation process consists of dissolving polysaccharide powder in nitric acid at 25 °C, followed by the addition of Na2SeO3 and maintaining the mixture in an oil bath at 70 °C for 10 h. After the reaction, the selenized polysaccharide is obtained via neutralization, dialysis and freeze-drying [50]. The NA-SS method modifies the polysaccharides as shown in Figure 4.
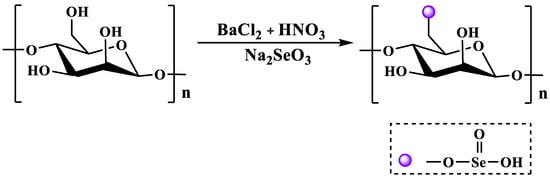
Figure 4.
The selenization modification of polysaccharides (i.e., the NA-SS method).
2.4.2. Other Selenization Methods
In addition to the selenate method, there is also the selenium oxychloride method (SeOCl2) for the chemical modification of polysaccharides [51]. However, the application of this reaction is currently limited because SeOCl2 is unstable and easily decomposes compared with the selenate method and is accompanied by irritating toxic gases. Except for the chemical synthesis of selenated polysaccharides, selenium polysaccharide can also be artificially synthesized via plant [52] and microbial transformations [53].
2.5. Other Methods for the Chemical Modification of Polysaccharides
In addition to the common chemical modification methods mentioned above, other methods for the chemical modification of polysaccharides consist of carboxymethylating [54], benzoylating [55], alkylating [56], hydroxypropylating [57], etc. As is well established, the pharmacological activity of most compounds depends on their structure. Chemical modifications of polysaccharides have become one of the main focuses in the field of polysaccharide research. They are used to modify the internal structure of polysaccharides, thereby improving biological activity and clarifying their conformational relationships. The advantages and disadvantages of chemical modification methods for polysaccharides are presented in Table 1.

Table 1.
The advantages and disadvantages of several common methods for polysaccharide chemical modification.
3. Biological Activities of Chemically Modified Polysaccharides
3.1. Antioxidant Capacity
Free radicals are metabolic products in the body, and active oxygen radicals such as superoxide anion (O2−) and hydroxyl radical (·OH) and –NO groups are produced during normal life activities. An appropriate amount of reactive oxygen radicals will participate during normal life activities, but excessive accumulation of free radicals will lead to the occurrence of lipid peroxidation [58,59,60]. It can lead to a series of diseases such as chronic kidney disease [61], cancer [62] and cardiovascular disease [63]. Therefore, it is of great significance to search for natural antioxidants with low toxicity. In recent years, relevant studies have shown that chemically modified polysaccharides have the advantages of good antioxidant activity, low toxicity and being extensive medicinal resources, which have become a research hotspot in the scientific community.
The DPPH and superoxide anion systems can effectively evaluate the antioxidant activity of substances under in vitro antioxidant conditions. The latest research results show that the ability of polysaccharides (PYPs) from Porphyra yezoensis to scavenge DPPH and hydroxyl radicals increases with the introduction of sulfate groups. The reason may be that with the increase in sulfuric acid groups, the hydrogen atoms on the end-group carbon become active, increasing their own nucleophilic properties and thus enhancing the antioxidant capacity [33]. Due to the introduction of carboxymethyl groups, carboxymethylated polysaccharides (CRNPs) from blackcurrant fruits have stronger anti-lipid peroxidation capacity and free radical-scavenging abilities than original polysaccharides [64]. In addition, chemically modified polysaccharides also have outstanding antioxidant effects in vivo. With the introduction of selenium, selenified Chinese angelica polysaccharides (CAPs) are able to increase superoxide dismutase (SOD) and total antioxidant capacity (T-AOC) activities, significantly reducing the content of malondialdehyde (MDA) and reactive oxygen species (ROS) in liver tissues, and they have a significant antioxidant effect [65]. Sulfated polysaccharides (SMP) from Mesona chinensis Benth have good free radical-scavenging performance, which can reduce the content of MDA and improve the activity of SOD. Therefore, they exhibit excellent results in the ability to protect cells from oxidative stress (Figure 5) [66]. The biological activity and related mechanisms of chemically modified polysaccharides are shown in Table 2.
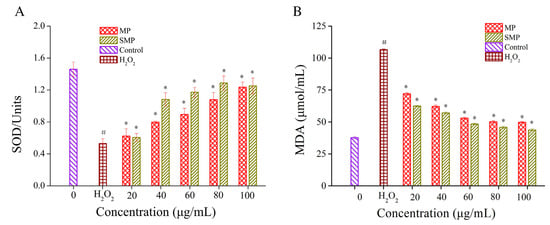
Figure 5.
Effects of polysaccharides (MP) from Mesona chinensis Benth and SMP on the activities of SOD (A) and MDA content (B) in RAW264.7 cells. # p < 0.05 compared with normal group, * p < 0.05 compared with H2O2 group alone. This figure was adapted from Huang et al. [66].
3.2. Antitumor Activity
Tumor disease is one of the most life-threatening and incurable diseases, which has attracted wide attention from researchers [67]. Most existing chemical drugs for cancer treatment also cause very serious damage to normal cells. Natural plant polysaccharides and their derivatives have a greater future prospect due to their good antitumor activity and low toxic side effects [68,69].
In vitro cellular experiments have shown that selenized polysaccharide (EJP90-1-Se) from Eriobotrya japonica with the introduction of the selenium element significantly inhibited cancer cell proliferation by inducing apoptosis. Further verification using a zebrafish model showed that EJP90-1–Se had a stronger inhibition of HepG2 proliferation and angiogenesis than polysaccharide (EJP90-1) from E. japonica [46]. Previous in vitro experiments have shown that sulfated polysaccharides (ASPs) from Artemisia sphaerocephala can significantly inhibit HeLa cells and HepG2. Meanwhile, according to the cell cycle, ASPs can block H22 cells in the S phase, thereby achieving antitumor effects through cell apoptosis. With other antitumor drugs, no direct cytotoxic effect on mouse fibroblast L929 was observed, while ASPs were antitumor [70]. It has been reported that sulfated polysaccharides may also achieve antitumor effects by reducing tumor microvascular density (MVD) and inhibiting the expression of vascular endothelial factor [71]. According to the latest research, phosphorylated polysaccharides are effectively modified to enhance antitumor activity. The mechanism may be to increase the activity of Cyt-c, Caspase-3 and Caspase-9, to induce cell apoptosis and also to arrest the cell cycle in the S phase (Figure 6) [72]. In addition, it has also been shown that sulfated polysaccharides can stimulate the activity of lymphocytes, increase macrophage phagocytosis and improve the production of large amounts of cytokines in macrophages. This can activate the immune response, thus possessing good proliferative activity against HONE1 cells [37]. The reason for enhanced immunity is that sulfuric acid groups increase contact with immune cell receptors by combining oxygen and electrostatic attraction, enhance the immune response and then inhibit the proliferation of tumor cells.
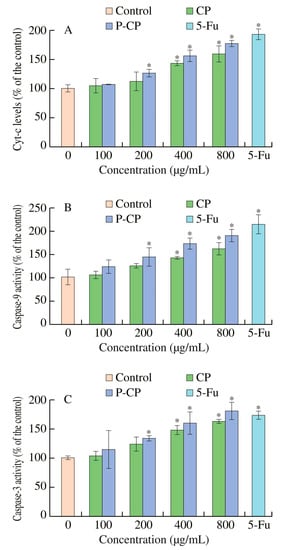
Figure 6.
Polysaccharides (CP) from Cyclocarya paliurus and phosphorylated polysaccharides (P-CP) from Cyclocarya paliurus induced the activation of Cyt-c (A), caspase-9 (B) and caspase-3 (C) in CT-26 cells. * p < 0.05, compared with the untreated group. This figure was adapted from Xie et al. [72].
3.3. Antiviral Activity
The antiviral mechanism of chemically modified polysaccharides mainly operates through the inhibition of virus replication, enhancement of immune function and prevention of virus adsorption and invasion. Sulfated polysaccharide (SP) from Sargassum ilicifolium can prevent the adsorption and entry of viruses, thus achieving an antiviral effect. At the same time, SP is much less toxic compared to other antiviral drugs [73]. Some scholars have successfully synthesized phosphorylated polysaccharides (pCPPSs) from Codonopsis pilosula, and in vitro and in vivo experiments have shown that pCPPS was able to block the formation of autophagosomes, thereby inhibiting the replication of the duck hepatitis A virus (DHAV) genome and achieving antiviral effects [74]. In addition, enhancement of the body’s immune response is also one of the antiviral mechanisms of chemically modified polysaccharides. Sulfated polysaccharides (sCVPSs) from Chuanmingshen violaceum can significantly reduce the virus titer in the thymus, spleen, brain and lungs of diseased chickens. The detection of serum interferon α and γ concentrations allowed for the conclusion that the antiviral effect of sCVPS was due to immune enhancement [75].
3.4. Immunomodulatory Activity
Immune function is a defense system for the body to maintain the relative stability of the internal environment and remove invading body antigens. Plant polysaccharides can perform immunomodulatory functions in the following method: regulating the secretion of cytokines, regulating signaling pathways such as mitogen-activated protein kinase (MAPK) and nuclear factor-κB (NF-κB), regulating intestinal flora and ameliorating organ failure.
In the short term, it has been elucidated by RNA-seq that sulfated polysaccharide (S-CYP) from Chinese yam can achieve immune regulation by regulating the MAPK signaling pathway. At the same time, it can synergistically enhance immune function by increasing the secretion of cytokines [76]. In addition, it has been shown that sulfated polysaccharides also have the ability to stimulate an increase in NF-κBp65 protein and simultaneously block the action of TLR2/4, thereby passing through the NF-κB signaling pathway for immune regulation [77]. The most common ways of immune regulation modalities for chemically modified polysaccharides include the stimulation of macrophage activity and enhancement of cytokine secretion [27,78]. A small amount of research has shown that sulfated polysaccharides can enhance immune function by promoting the repair of intestinal mechanical barriers and regulating intestinal microflora. Compared to unmodified polysaccharides, sulfated modified polysaccharides have significantly increased immune regulatory activity [79]. Similarly, carboxymethylated polysaccharide (CSP) from Schisandra chinensis inhibited the thymic and splenic atrophy induced by dioxins such as 3, 3′, 4, 4′, 5-pentachlorobiphenyl (PCB 126). Moreover, it showed higher immunomodulatory activity compared to unmodified polysaccharides (Figure 7) [80].
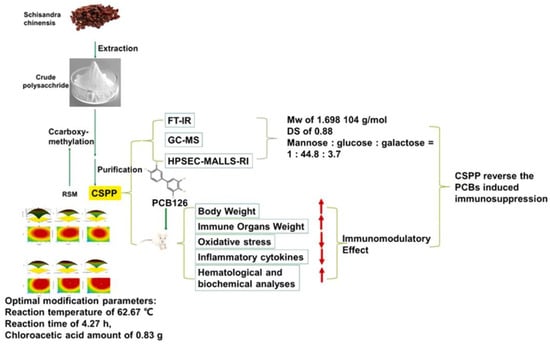
Figure 7.
Preparation and characterization of carboxymethylated polysaccharides and their intervention in the immunotoxicity of polychlorinated biphenyls. This figure was adapted from Zhao et al. [80].
3.5. Anti-Inflammatory Activity
The development of inflammation is an extremely complex process, and inflammation is usually a natural protective response of the body. However, long-term inflammation or inflammation that attacks oneself is harmful and can lead to a series of diseases in the body [81]. Therefore, the development of safe and effective anti-inflammatory drugs currently has a broad clinical application value. Natural macromolecular polysaccharides have received widespread attention from scientists because of their significant anti-inflammatory effects and high safety profile.
It has been reported that phosphorylated polysaccharide (PPN) from Pholiota nameko has anti-inflammatory effects on lipopolysaccharide (LPS)-induced RAW 264.7 cells through inhibition of the PI3K/AKT/mTOR pathway. Furthermore, the anti-inflammatory effect is consistently superior to that of polysaccharides without phosphorylation modification at the same concentration [82]. On the other hand, some studies have shown that acetylated polysaccharides also have good anti-inflammatory activity, for which the mechanism is to enhance anti-inflammatory activity through the NF-κB and p38/MAPK signaling pathways, as well as a strong ability to inhibit nitric oxide (NO) production [26]. Not only do artificially synthesized modified polysaccharides have good anti-inflammatory activity, but naturally occurring sulfated polysaccharides also have the same effect. Sulfated polysaccharide (CFCE-PS) from Codium fragile was able to dose-dependently reduce the levels of inflammatory factors in LPS-induced RAW264.7 cells, including NO, TNF-α, IL-1β, IL-6, etc. [83].

Table 2.
Structure, biological activity and mechanism of chemically modified polysaccharides.
Table 2.
Structure, biological activity and mechanism of chemically modified polysaccharides.
| Bioactivity | Polysaccharide Sources | Monosaccharide Composition | Monosaccharide Composition of Modified Polysaccharide | Structures | Chemical Modification Methods | Mechanism | Ref. |
|---|---|---|---|---|---|---|---|
| Antioxidant | Porphyra haitanensis | N/A | N/A | 3-linked β-D-galactosyl residues alternating with 4-linked 3,6-anhydro-a-L-galactose | Benzoylation | Direct scavenging of free radicals | [25,42,64] |
| Ulva pertusa | Rha:Xyl:Glc:GlcA = 1.00:0.67:0.13:0.15 | Rha:Xyl:Glc:GlcA = 1.00:0.79:0.04:0.19 | β-D-Glcp A-(1→4)-α-L-Rhap3s and α-L-Idup A-(1→4)-α-L-Rhap3s | Phosphorylation | |||
| Blackcurrant fruits | Glc:Rha:Ara:Man:Gal:GalA = 1.00:2.31:13.29:0.95:5.13:1.96 | Glc:Rha:Ara:Man:Gal:GalA = 1.00:4.35:5.65:0.23:6.65:4.35 | There are pyranose rings in polysaccharides | Carboxymethylation | |||
| Ulva pertusa | Rha:Xyl:Glc:GlcA = 1.00:0.67:0.13:0.15 | Rha:Xyl:Glc:GlcA = 1.00:0.79:0.04:0.19 | This structure is the same as that of ref. [42] in the previous table | Phosphorylation | Regulation of antioxidant enzyme activity through the Nrf2/ARE pathway | [42,65] | |
| Chinese angelica | N/A | N/A | [(→4)-a-d-Glcp-(1→4)-a-d-Glcp-(1→6)-a-d-Glcp-(1→4)-a-d-Glcp-(1→4)-a-d-Glcp-(1→)]n | Selenization | |||
| Anti-tumor | E. Japonica | N/A | N/A | →5)-linked-α-L-Araf-(1→, →4)-linked-β-D-Manp-(1→, →2,4)-linked-α-L-Rhap-(1→, →4)-linked-α-D-Xylp-(1→, →4)-linked-β-D-Galp-(1→, →2)-linked-β-D-Galp-(1→, →6)-linked-β-D-Glcp-(1→, α-D-Glcp-(4→, and t-linked-α-L-Araf | Selenization | Blocking tumor angiogenesis | [46] |
| E. Japonica | N/A | N/A | This structure is the same as that of ref. [46] in the previous table | Selenization | Induction of apoptosis in tumor cells | [46] | |
| Alfalfa | Rha:Xyl:Ara:GalA:Man:Glc = 2.13:3.07:2.77:1.00:1.30:1.10 | N/A | 1→2, 1→4, 1→3, and 1→6 or 1→glycosidic bonds | Selenization | Unspecified | [50] | |
| A. sphaerocephala | Ara:Xyl:Man:Glc:Gal = 1.00:4.2:45.9:9.7:11.4 | N/A | N/A | Sulfation | Blocking the tumor cell cycle | [70] | |
| Anti-viral | Sargassum ilicifolium | N/A | N/A | N/A | Sulfation | Resists virus adsorption and invasion | [73] |
| Codonopsis pilosula | N/A | N/A | (1→3)-linked-β-D-galactopyranosyl, (1→2,3)-linked-β-D-galactopyranosyl and (1→3)-linke-α-D-rhamnopyranosyl residues | Phosphorylation | Inhibition of virus replication | [74] | |
| Chuanmingshen violaceum | N/A | N/A | N/A | Sulfation | Activates the immune system and improves resistance to viruses | [75] | |
| Immunomodulation | C. paliurus | Ara:Gal:Glc:Rha:xyl:Man:GalA:GlcA = 1.00:1.59:1.18:0.08:0.35:0.48:0.81:0.31 | Ara:Gal:Glc:Rha:Man:GalA:GlcA = 1.00:1.67:1.07:0.15:0.34:1.58:0.16 | N/A | Acetylation | Effect on cytokines | [27] |
| Cyclocarya paliurus | Rha:Fuc:Ara:Xyl:Man:Glc:Gal = 0.11:0.07:3.11:0.36:0.24:0.275:3.36 | Rha:Fuc:Ara:Xyl:Man:Glc:Gal = 0.27:0.07:3.51:0.25:0.17:2.41:3.32 | N/A | Sulfation | Regulation of signaling pathways such as MAPK and NF-κB | [77] | |
| Cyclocarya paliurus | Rha:Fuc:Ara:Xyl:Man:Glc:Gal = 0.11:0.07:3.11:0.36:0.24:0.275:3.36 | Rha:Fuc:Ara:Xyl:Man:Glc:Gal = 0.27:0.07:3.51:0.25:0.17:2.41:3.32 | N/A | Sulfation | Regulation of intestinal flora | [79] | |
| Schisandra | N/A | Man:Glc:Gal = 1:44.8:3.71 | 1,4-α-D-Glcp and 1,4,6-β-D-Glcp | Carboxymethylation | Improves immune organ failure | [80] | |
| Anti-inflammatory | Morchella angusticeps Peck | Ara:Man:Glc:Gal = 1.00:2.37:4.79:3.09 | N/A | (1→4)-α-D-glucose, (1→6)-α-D-galactose, (1→2)-α-D-mannose, and (1→5)-α-D-arabinose; and the branches were found to be (→2→6)-α-D-mannose, (1→2→6)-α-Dglucose, and (1→2→6)-β-D-galactose | Acetylation | Inhibition of NF-κB and MAPK signaling pathways | [26] |
| Pholiota nameko | Man:Glc:Gal:Ara:Rha = 6.4:38.6:27.1:20.5:7.4 | Man:Glc:Gal:Ara:Rha = 7.3:44.9:23.6:15.7:8.5 | The main chains were1,4-linked Glcp, 1,6-linked Galp, 1,2- linked Rhap, and 1.6-linked Manp with terminals of t-linked Glcp, t-linked Araf The side chains change from 1,4,6-linked Galp, 1,2,5-linked Araf to 1,4,6-linked Galp | Phosphorylation | Inhibition of PI3K/AKT signaling pathway | [82] | |
| Morchella angusticeps Peck | Ara:Man:Glc:Gal = 1.00:2.37:4.79:3.09 | N/A | Its structure is the same as that of ref. [26] in the previous table | Acetylation | Inhibition of NO and PGE2 production | [26] | |
| Codium fragile | N/A | N/A | N/A | Sulfation | Affects cytokine secretion | [83] |
3.6. Other Biological Activities
Many pharmacological experiments have shown that both sulfated polysaccharides naturally present in plants and those obtained via chemical modification have good anticoagulant effects [84,85]. Through testing routine coagulation indicators (APTT, TT, PT), it was found that the products modified by phosphorylation and carboxymethylation have significant anticoagulant activity compared to original polysaccharides [86]. This indicates that in addition to the well-known sulfated polysaccharides, the introduction of other anionic groups into polysaccharides increases their anticoagulant activity, which can greatly enrich the types of anticoagulants and provide a reference in the search for naturally active anticoagulants. In addition to this, chemically modified polysaccharides also have liver-protective effects. Selenizing polysaccharides (sCAPs) from Chinese angelica can significantly reduce the levels of ALP, ALT and AST in the serum of liver-injury mice. Moreover, sCAP significantly alleviates pathological changes in the liver while also inhibiting the expression of p-ERK, indicating that selenization modification can enhance the hepatoprotective effect of sCAP [65]. Sulfated (SLEP) and carboxymethylated (CLEP) extracellular polysaccharides from Lachnum YM240 can both have a hypolipidemic effect, but the hypolipidemic effect of CLEP is more significant [87].
Moreover, the potential of marine polysaccharides should not be overlooked. Marine polysaccharides have attracted much attention because of their abundant sources, special molecular structures and extensive biological activities [88]. The biological activities of marine-derived polysaccharides and their derivatives have proved to be anti-thrombotic, antitumor, antioxidative, immunomodulatory, etc., which have wide application prospects in functional foods, drugs and other fields [6,29,33,89].
4. Factors Affecting the Bioactivity of Chemically Modified Polysaccharides
4.1. Introduction of Different Chemical Modification Groups
As is well known, the structure of substances determines their function, and when the structure of polysaccharides changes, their function tends to change as well. For example, when polysaccharides WPMP-1 and WPMP-2 were extracted from Polygonum multiflorum, both the methylation and NMR results indicated that the main chain structure of WPMP-1 was composed of 1, 4-Glcp. In contrast, the main chain of WPMP-2 is 1, 3, 5-Araf and 1, 2, 4-Rhap. Therefore, the significant differences in structure also lead to functional differences between the two purified polysaccharides, such as WPMP-2, showing stronger immune regulatory activity than WPMP-1 [90].
Recently, a study using 13C NMR technology has characterized the structure of onion polysaccharide. At the same time, phosphate and acetyl groups were introduced in onion polysaccharides, and antioxidant analysis was conducted. The results of in vitro antioxidant activity showed that the antioxidant effect of phosphated onion polysaccharide was the strongest compared to original and acetylated polysaccharides, which approached the activity of vitamin C [91]. In addition, acetylated (AcP) and carboxymethylated polysaccharides (CM-Ps) from bitter gourd were successfully prepared by introducing acetyl and carboxymethyl groups on the main chain of the polysaccharide using bitter gourd polysaccharide (P) as the raw material. Antioxidant tests were conducted on the three polysaccharides, with the results showing that CM-Ps had the strongest antioxidant effect. It was speculated that this may be because, with the introduction of carboxymethyl groups, the negative charge on the surface of polysaccharide increases, resulting in the construction of negatively charged hydrophilic surface structures that increase their water solubility and thus enhance their antioxidant activity [9]. However, the same chemical modifications applied to different polysaccharides often exhibit vastly different activities, which may be closely related to the unique structure of the polysaccharides themselves. Therefore, it is necessary to select appropriate chemical modification methods based on the structure of polysaccharides themselves during chemical modification [92,93].
4.2. DS and Substituent Position
The DS (degree of substitution) is an important indicator for evaluating the success of chemically modified polysaccharides. However, even if polysaccharides with different DS values are prepared using the same chemical modification method, their activity often varies greatly. Generally, it is believed that the biological activity of modified polysaccharides is directly proportional to the size of the DS values, i.e., the larger the DS values are, the greater the activity is. For example, different DS carboxymethylated blackcurrant fruit polysaccharides (CRNPs) were prepared from blackcurrant fruits using the carboxymethylation method. On the other hand, the in vitro antioxidant results indicated that the antioxidant activity of CRNPs is enhanced compared to unmodified polysaccharides. Under the test conditions of the in vitro hemolytic protective effect on red blood cells, the activity of CRNPs also increases with the increase in the DS [64]. In addition, arabinoxylans is extracted from the seed shell of Plantago, and sulfate is added to obtain products with different DS values. After in vitro anti-HSV-1 activity testing, it was found that the polysaccharide with the highest DS had the strongest anti-HSV-1 activity [94]. However, most experiments show that the relationship between DS values and biological activity does not seem to be so simple. Briefly, the fruiting bodies of polysaccharides from Russula virescens (SRVP) with DS values between 0.34 and 0.73 were prepared, and the in vitro activity tests showed that SRVP1-20 with the DS of 0.68 had the strongest antibacterial and antitumor activity [15]. At the same time, other results can prove this viewpoint as well. Researchers have used Sagittaria trifolia as a raw material to prepare three sulfated polysaccharides with different DS values and evaluated their antioxidant activity. The results showed that the antioxidant activity decreased with the increase in the DS value [95].
In addition to the DS value, the biological activity of chemically modified polysaccharides is also closely related to the sites of the substituent introduction. Some scholars modified carrageenans with sulfation via selective sulfation. A total of eleven samples were prepared. Sample 8 has the same DS as sample 11, but sample 8 is replaced by a sulfuric acid group at C4, and sample 11 is replaced by a sulfuric acid group at C2. The results obtained from samples 8 and 11 can indicate that substitution by sulfate groups at C4 appears to have better anticoagulant activity than substitution at C2 [96].
4.3. Monosaccharide Molar Ratio and Glycosidic Bond Link Order
Monosaccharide composition analysis is the basis for studying the structural properties and the structure–activity relationships of polysaccharides. Common analytical methods for determining monosaccharide composition currently include liquid chromatography [10], liquid chromatography–mass spectrometry (Figure 8) [97], gas chromatography [64] and gas chromatography–mass spectrometry [41]. A large number of studies have concluded that the chemical modification of natural polysaccharides generally leads to changes in the molar percentage of monosaccharide composition, but it does not lead to changes in monosaccharide composition [42,64,77,82]. Natural polysaccharide from Undaria pinnatifida (UPPS-B1) and its sulfated derivative (S-UPPS-B1) were used for the determination of monosaccharide composition by GC–MS. The molar ratio of monosaccharides in UPPS-B1 was Glc, Man, Xyl and Gal at 12.0:8.7:7.9:9.8. After sulfation modification, the proportion of Xyl, Glc and Gal were significantly decreased, and the antitumor activity of sulfated polysaccharides was enhanced [98]. However, in rare cases, the chemical modification of polysaccharides can lead to changes in the composition of monosaccharides. For example, monosaccharides in the polysaccharide (CP) from Cyclocarya paliurus were Ara, Gal, Glc, Rha, xyl, Man, GalA and GlcA. Surprisingly, the monosaccharide composition of the acetylated polysaccharide (AC-CP) from Cyclocarya paliurus was Ara, Gal, Glc, Rha, Man, GalA and GlcA, without Xyl composition. At the same time, comparing immunoregulatory activity, AC-CP activity is superior to CP [27].
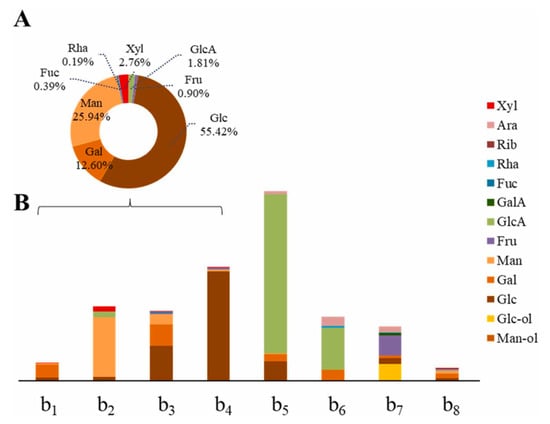
Figure 8.
The polysaccharide components were analyzed using the RPLC–ESI–MRM–MS method. (A) Composition and proportion of monosaccharides in all four fungi polysaccharides. (B) Analysis of monosaccharide composition and content of individual polysaccharides from edible plants and fungi. b1: Ganoderma lucidum polysaccharide; b2: Auricularia auricular-judae polysaccharide; b3: Armillaria mellea polysaccharide; b4: Hericium erinaceus polysaccharide; b5: Panax ginseng polysaccharide; b6: Aralia elata bud polysaccharide; b7: Platycodon grandiflorum polysaccharide; b8: Stigma maydis polysaccharide. This figure was adapted from Gao et al. [97].
Additionally, chemical modifications can also lead to a certain degree of alteration of glycosidic bond linkages. Phosphorylated polysaccharide (PPN) from Pholiota nameko was prepared via chemical modification of polysaccharide (SPN) from Pholiota nameko. The methylation results showed that there was no change in the main chain structure of SPN and PPN, but the side chains of SPN underwent phosphorylation, transitioning from 1, 2, 5-Araf to 1, 2, 4-Glcp linkage. At the same time, the ability of PPN to scavenge free radicals and its anti-inflammatory effect against LPS-induced RAW 264.7 cells are stronger than those of SPN [82]. Therefore, the chemical modification of polysaccharides will lead to changes in monosaccharide composition, molar ratio, glycoside linkage sites and other structures, further leading to changes in biological activity. However, so far, there is no exact evidence to show the relationship between polysaccharide structure and activity, which is still the focus of a future research direction.
4.4. Molecular Weight (MW)
At present, the most common method for the determination of molecular weight in polysaccharides is high-performance gel permeation chromatography (HPGPC), which is widely used in the analysis and preparation of polysaccharides due to the advantages of simple operation and short time [85]. The molecular weights of Enteromorpha prolifera polysaccharide (PEP) and its enzymatic degradation products (LEP) were 147.8 KDa and 44.8 KDa, respectively. By sulfating PEP and LEP, the products were SPEP (176.3 KDa) and SLEP (59.9 KDa), respectively. The molecular weight of polysaccharides increased after sulfated modification. In addition, the antioxidant activities of PEP, LEP, SPEP and SLEP were measured and compared. The results showed that SLEP had the strongest antioxidant activity due to low Mw and high sulfate-group content. Therefore, the molecular weight and sulfate groups have obvious effects on the antioxidant activity of E. prolifera polysaccharide [99]. However, in contrast to the previous example, the molecular weight of polysaccharide (SGP) from Sphallerocarpus gracilis is 743 KDa, and S-SGP is prepared by sulfation using the CSA/Pyr method with SGP as the raw material. The results indicate that the molecular weight of S-SGP is significantly reduced to 212 KDa. In spite of this, the antioxidant activity of the sulfated modification product S-SGP was instead elevated [100]. According to the current evidence, the biological activity of modified polysaccharides is related to molecular weight to some extent, but the chemical modification of polysaccharides often leads to changes in the monosaccharide molar ratio, Mw, degree of substitution, glucoside linkage and other factors. Therefore, there is no direct evidence to show the exact relationship between molecular weight and biological activity, and the structure–activity relationship still needs to be further studied.
5. Conclusions and Prospects
At present, the application of chemically modified polysaccharides has been implemented in the biomedical and food industries, along with the cosmetics and materials industries and antibacterial agent research and development. Therefore, it has high practical and economic value. However, due to the large molecular weight and complex advanced structure of polysaccharides, it is also a great challenge to study the structure–activity relationship of polysaccharides. Changes in the activity of chemically modified polysaccharides are often related to the type, position and quantity of substituent groups (expressed by DS or content), monosaccharide composition and molecular weight, and the sequence of glucoside linkage [93,100,101]. This unpredictability in the structure–activity relationship also leads to the biological activity of some chemical modifications being less than expected and even to the phenomenon of reduced activity [91,102].
Therefore, polysaccharide modification should not overlook decoration through various methods; instead, it should be according to their own unique structure modification. Despite the promising prospects of chemically modified polysaccharides in recent years, the following challenges remain: (1) homogeneous purified polysaccharides are rarely used in polysaccharide modification. A low purity of lead compounds will lead to an unstable DS and substitution sites after chemical modification and poor experimental reproducibility, and it will increase the difficulty of studying the structure–activity relationship. (2) At the same time, the controllability of the reaction is not high, and the DS and molecular weight of different batches of modified products under the same reaction conditions are inconsistent, making products difficult to control. Therefore, increasing the controllability of the DS and molecular weight of the modified products is very important in the study of the structure–activity relationship of modified polysaccharide products. (3) Catalysts used in many chemical modification methods have a strong toxicity and too many by-products that are difficult to separate, resulting in a low yield of target products. (4) At present, the structure–activity relationship of chemically modified polysaccharides is still relatively shallow, and the mechanism research is not clear, so it is necessary to deepen the study of the structure–activity relationship. However, with the continuous development of scientific research equipment and the gradual development of analytical methods, these problems will be solved, and the chemical modification of polysaccharide methods will be gradually improved so that polysaccharides and their modified products will have a wider range of applicability.
Author Contributions
Conceptualization, T.L.; investigation, Q.R., S.W., J.G., C.S. and S.Z.; writing—original draft preparation, T.L.; writing—review and editing, Y.W. and F.G. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (No. 82074025), and the Heilongjiang Touyan Innovation Team Program ([2019] No.5).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Not applicable.
References
- Mu, S.; Yang, W.J.; Huang, G.L. Antioxidant activities and mechanisms of polysaccharides. Chem. Biol. Drug Des. 2021, 97, 628–632. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhao, Y.C.; Jiang, Y.; Chu, W.H. Antioxidant, and Immunomodulatory Properties of Acidic Exopolysaccharide From Marine Rhodotorula RY1801. Front. Nutr. 2021, 8, 710668. [Google Scholar] [CrossRef] [PubMed]
- Kokoulin, M.S.; Kuzmich, A.S.; Romanenko, L.A.; Menchinskaya, E.S.; Mikhailov, V.V.; Chernikov, O.V. Sulfated O-polysaccharide with anticancer activity from the marine bacterium Poseidonocella sedimentorum KMM 9023T. Carbohydr. Polym. 2018, 202, 157–163. [Google Scholar] [CrossRef]
- Kim, Y.S.; Hwang, J.; Lee, S.G.; Jo, H.Y.; Oh, M.J.; Liyanage, N.M.; Je, J.G.; An, H.J.; Jeon, Y.J. Structural characteristics of sulfated polysaccharides from Sargassum horneri and immune-enhancing activity of polysaccharides combined with lactic acid bacteria. Food Funct. 2022, 13, 8214–8227. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Zheng, R.L.; Wu, H.L.; Chen, D.Y.; Su, J.Y.; Xu, T.T.; Wu, H.B.; Xiang, W.Z.; Li, Y.H.; Zhu, B. Inhibition of enterovirus 71 infection by polysaccharides extracted from Picochlorum sp. 122 via the AKT and ATM/ATR signaling pathways. Arch. Virol. 2021, 166, 3269–3274. [Google Scholar] [CrossRef]
- Chagas, F.D.; Lima, G.C.; Santos, V.I.; Costa, L.E.; Sousa, W.M.; Sombra, V.G.; Araújo, D.F.; Barros, F.C.; Soriano, E.M.; Feitosa, J.P.; et al. Sulfated polysaccharide from the red algae Gelidiella acerosa: Anticoagulant, antiplatelet and antithrombotic effects. Int. J. Biol. Macromol. 2020, 159, 415–421. [Google Scholar] [CrossRef]
- Huang, H.L.; Huang, G.L. Extraction, separation, modification, structural characterization, and antioxidant activity of plant polysaccharides. Chem. Biol. Drug Des. 2020, 96, 1209–1222. [Google Scholar] [CrossRef]
- Liu, H.L.; Liu, X.L.; Yue, L.; Jiang, Q.X.; Xia, W.S. Synthesis, characterization and bioactivities of N, O-carbonylated chitosan. Int. J. Biol. Macromol. 2016, 91, 220–226. [Google Scholar] [CrossRef]
- Chen, F.; Huang, G.L. Extraction, derivatization and antioxidant activity of bitter gourd polysaccharide. Int. J. Biol. Macromol. 2019, 141, 14–20. [Google Scholar] [CrossRef]
- Deng, Q.F.; Wang, X.; Chen, H.G.; Zhao, C.; Gong, X.J.; Zhao, X. Structural characterization, modification and hepatoprotective effects of polysaccharide from Mori Fructus. Int. J. Biol. Macromol. 2020, 153, 357–363. [Google Scholar] [CrossRef]
- Li, J.X.; Huang, G.L. Extraction, purification, separation, structure, derivatization and activities of polysaccharide from Chinese date. Process Biochem. 2021, 110, 231–242. [Google Scholar] [CrossRef]
- Zhou, N.; Long, H.R.; Wang, C.H.; Yu, L.; Zhao, M.M.; Liu, X.L. Research progress on the biological activities of selenium polysaccharides. Food Funct. 2020, 11, 4834–4852. [Google Scholar] [CrossRef] [PubMed]
- Chakka, V.P.; Zhou, T. Carboxymethylation of polysaccharides: Synthesis and bioactivities. Int. J. Biol. Macromol. 2020, 165, 2425–2431. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.Y.; Zhang, J.H.; Yang, H.L.; Yang, Z.F.; Xue, H.B.; Wu, F.; Wang, Z.Y.; Xie, L.; Chen, Y.Y. Acetylation modification and antioxidant activity of polysaccharides from Agrocybe cylindracea. J. Food Meas.Charact. 2022, 16, 1911–1919. [Google Scholar] [CrossRef]
- Li, H.; Wang, X.; Xiong, Q.; Yu, Y.; Peng, L.C. Sulfated modification, characterization, and potential bioactivities of polysaccharide from the fruiting bodies of Russula virescens. Int. J. Biol. Macromol. 2020, 154, 1438–1447. [Google Scholar] [CrossRef]
- Xiong, X.; Huang, G.L.; Huang, H.L. The antioxidant activities of phosphorylated polysaccharide from native ginseng. Int. J. Biol. Macromol. 2019, 126, 842–845. [Google Scholar] [CrossRef] [PubMed]
- Fiorito, S.; Epifano, F.; Preziuso, F.; Taddeo, V.A.; Genovese, S. Selenylated plant polysaccharides: A survey of their chemical and pharmacological properties. Phytochemistry 2018, 153, 1–10. [Google Scholar] [CrossRef]
- Duan, Z.H.; Duan, W.W.; Li, F.P.; Li, Y.Q.; Luo, P.; Liu, H.Z. Effect of carboxymethylation on properties of fucoidan from Laminaria japonica: Antioxidant activity and preservative effect on strawberry during cold storage. Postharvest Biol. Technol. 2019, 151, 127–133. [Google Scholar] [CrossRef]
- Xie, L.M.; Shen, M.Y.; Hong, Y.Z.; Ye, H.D.; Huang, L.X.; Xie, J.H. Chemical modifications of polysaccharides and their anti-tumor activities. Carbohydr. Polym. 2020, 229, 115436. [Google Scholar] [CrossRef]
- Xu, Y.; Wu, Y.J.; Sun, P.L.; Zhang, F.M.; Linhardt, R.J.; Zhang, A.Q. Chemically modified polysaccharides: Synthesis, characterization, structure activity relationships of action. Int. J. Biol. Macromol. 2019, 132, 970–977. [Google Scholar] [CrossRef]
- Wang, Z.J.; Xie, J.H.; Shen, M.Y.; Nie, S.P.; Xie, M.Y. Sulfated modification of polysaccharides: Synthesis, characterization and bioactivities. Trends Food Sci. Technol. 2018, 74, 147–157. [Google Scholar] [CrossRef]
- Xue, H.K.; Li, P.C.; Bian, J.Y.; Gao, Y.C.; Song, Y.M.; Tan, J.Q. Extraction, purification, structure, modification, and biological activity of traditional Chinese medicine polysaccharides: A review. Front. Nutr. 2022, 9, 2022–2045. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.H.; Zhang, F.; Wang, Z.J.; Shen, M.Y.; Nie, S.P.; Xie, M.Y. Preparation, characterization and antioxidant activities of acetylated polysaccharides from Cyclocarya paliurus leaves. Carbohydr. Polym. 2015, 133, 596–604. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Yang, Y.; Zhang, Y.Y.; Duan, L.S.; Zhou, C.L.; Ni, Y.Y.; Liao, X.J.; Li, Q.H.; Hu, X.S. Effect of acetylation on antioxidant and cytoprotective activity of polysaccharides isolated from pumpkin (Cucurbita pepo, lady godiva). Carbohydr. Polym. 2013, 98, 686–691. [Google Scholar] [CrossRef]
- Zhang, Z.S.; Zhang, Q.B.; Wang, J.; Shi, X.L.; Song, H.F.; Zhang, J.J. In vitro antioxidant activities of acetylated, phosphorylated and benzoylated derivatives of porphyran extracted from Porphyra haitanensis. Carbohydr. Polym. 2009, 78, 449–453. [Google Scholar] [CrossRef]
- Yang, Y.X.; Chen, J.L.; Lei, L.; Li, F.H.; Tang, Y.; Yuan, Y.; Zhang, Y.Q.; Wu, S.R.; Yin, R.; Ming, J. Acetylation of polysaccharide from Morchella angusticeps peck enhances its immune activation and anti-inflammatory activities in macrophage RAW264.7 cells. Food Chem. Toxicol. 2019, 125, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Xie, J.H.; Jia, S.; Huang, L.X.; Wang, Z.J.; Li, C.; Xie, M.Y. Immunomodulatory effects of an acetylated Cyclocarya paliurus polysaccharide on murine macrophages RAW264.7. Int. J. Biol. Macromol. 2017, 98, 576–581. [Google Scholar] [CrossRef]
- Hu, Z.Y.; Sun, J.F.; Jin, L.; Zong, T.Q.; Duan, Y.Q.; Zhou, H.L.; Zhou, W.; Li, G. Acetylation Modification, Characterization, and Anticomplementary Activity of Polysaccharides from Rhododendron dauricum Leaves. Polymers 2022, 14, 3130. [Google Scholar] [CrossRef]
- Mazepa, E.; Biscaia, S.M.; Bellan, D.L.; Trindade, E.S.; Simas, F.F. Structural characteristics of native and chemically sulfated polysaccharides from seaweed and their antimelanoma effects. Carbohydr. Polym. 2022, 289, 119436. [Google Scholar] [CrossRef]
- Rizkyana, A.D.; Ho, T.C.; Roy, V.C.; Park, J.S.; Kiddane, A.T.; Kim, G.D.; Chun, B.S. Sulfation and characterization of polysaccharides from Oyster mushroom (Pleurotus ostreatus) extracted using subcritical water. J. Supercrit. Fluids 2022, 179, 105412. [Google Scholar] [CrossRef]
- Mizumoto, K.; Sugawara, I.; Ito, W.; Kodama, T.; Hayami, M.; Mori, S. Sulfated homopolysaccharides with immunomodulating activities are more potent anti-HTLV-III agents than sulfated heteropolysaccharides. J. Exp. Med. 1988, 58, 145–151. [Google Scholar]
- Caputo, H.E.; Straub, J.E.; Grinstaff, M.W. Design, synthesis, and biomedical applications of synthetic sulphated polysaccharides. Chem. Soc. Rev. 2019, 48, 2338–2365. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.F.; He, Y.X.; Tang, X.Z.; Li, N.Y. Sulfation, structural analysis, and anticoagulant bioactivity of ginger polysaccharides. J. Food Sci. 2020, 85, 2427–2434. [Google Scholar] [CrossRef] [PubMed]
- Kazachenko, A.S.; Malyar, Y.N.; Vasilyeva, N.Y.; Borovkova, V.S.; Issaoui, N. Optimization of guar gum galactomannan sulfation process with sulfamic acid. Biomass Conv. Bioref. 2021, 13, 10041–10050. [Google Scholar] [CrossRef]
- Bedini, E.; Laezza, A.; Parrilli, M.; Iadonisi, A. A review of chemical methods for the selective sulfation and desulfation of polysaccharides. Carbohydr. Polym. 2017, 174, 1224–1239. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.W.; Huang, W.B.; Sun, X.Y.; Xiong, P.; Ouyang, J.M. Antioxidant activity of sulfated Porphyra yezoensis polysaccharides and their regulating effect on calcium oxalate crystal growth. Mater. Sci. Eng. C 2021, 128, 112338. [Google Scholar] [CrossRef]
- Jiang, J.; Meng, F.Y.; He, Z.; Ning, Y.L.; Li, X.H.; Song, H.; Wang, J.; Rui, J. Sulfated modification of longan polysaccharide and its immunomodulatory and antitumor activity in vitro. Int. J. Biol. Macromol. 2014, 67, 323–329. [Google Scholar] [CrossRef]
- Wang, Z.H.; Cai, T.Q.; He, X.P. Characterization, sulfated modification and bioactivity of a novel polysaccharide from Millettia dielsiana. Int. J. Biol. Macromol. 2018, 117, 108–115. [Google Scholar] [CrossRef]
- Xia, S.L.; Zhai, Y.C.; Wang, X.; Fan, Q.R.; Dong, X.Y.; Chen, M.; Han, T. Phosphorylation of polysaccharides: A review on the synthesis and bioactivities. Int. J. Biol. Macromol. 2021, 184, 946–954. [Google Scholar] [CrossRef]
- Chen, X.Y.; Xu, X.J.; Zhang, L.N.; Zeng, F.B. Chain conformation and anti-tumor activities of phosphorylated (1→3)-β-d-glucan from Poria cocos. Carbohydr. Polym. 2009, 78, 581–587. [Google Scholar] [CrossRef]
- Wang, J.L.; Wang, Y.X.; Xu, L.; Wu, Q.Q.; Wang, Q.; Kong, W.B.; Liang, J.Y.; Yao, J.; Zhang, J. Synthesis and structural features of phosphorylated Artemisia sphaerocephala polysaccharide. Carbohydr. Polym. 2018, 181, 19–26. [Google Scholar] [CrossRef]
- Jiang, N.F.; Li, B.X.; Wang, X.Q.; Xu, X.N.; Liu, X.L.; Li, W.D.; Chang, X.T.; Li, H.; Qi, H.M. The antioxidant and antihyperlipidemic activities of phosphorylated polysaccharide from Ulva pertusa. Int. J. Biol. Macromol. 2020, 145, 1059–1065. [Google Scholar] [CrossRef]
- Li, H.P.; Feng, Y.B.; Sun, W.X.; Kong, Y.; Jia, L. Antioxidation, anti-inflammation and anti-fibrosis effect of phosphorylated polysaccharides from Pleurotus djamor mycelia on adenine-induced chronic renal failure mice. Int. J. Biol. Macromol. 2021, 170, 652–663. [Google Scholar] [CrossRef] [PubMed]
- Le-Vinh, B.; Le, N.M.; Nazir, I.; Matuszczak, B.; Bernkop-Schnürch, A. Chitosan based micelle with zeta potential changing property for effective mucosal drug delivery. Int. J. Biol. Macromol. 2019, 133, 647–655. [Google Scholar] [CrossRef]
- Shao, C.T.; Zhong, J.W.; Liu, J.W.; Yang, Y.Y.; Li, M.L.; Yu, Y.; Xu, Y.Q.; Wang, L.B. Preparation, characterization and bioactivities of selenized polysaccharides from Lonicera caerulea L. fruits. Int. J. Biol. Macromol. 2023, 225, 484–493. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.J.; Zhang, H.; Shi, L.J.; Li, Y.; Tuerhong, M.; Abudukeremu, M.; Cui, J.L.; Li, Y.H.; Jin, D.Q.; Xu, J.; et al. Structure features, selenylation modification, and improved anti-tumor activity of a polysaccharide from Eriobotrya japonica. Carbohydr. Polym. 2021, 273, 118496. [Google Scholar] [CrossRef]
- Zhan, Q.P.; Chen, Y.; Guo, Y.F.; Wang, Q.; Wu, H.; Zhao, L.Y. Effects of selenylation modification on the antioxidative and immunoregulatory activities of polysaccharides from the pulp of Rose laevigata Michx fruit. Int. J. Biol. Macromol. 2022, 206, 242–254. [Google Scholar] [CrossRef]
- Gao, Z.H.; Chen, J.; Qiu, S.L.; Li, Y.Y.; Wang, D.Y.; Liu, C.; Li, X.P.; Hou, R.R.; Yue, C.J.; Liu, J.; et al. Optimization of selenylation modification for garlic polysaccharide based on immune-enhancing activity. Carbohydr. Polym. 2016, 136, 560–569. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.T.; Zhang, J.; Yao, J.; Song, S.; Yin, Z.X.; Gao, Q.Y. Selenylation modification can enhance antioxidant activity of Potentilla anserina L. polysaccharide. Int. J. Biol. Macromol. 2013, 58, 320–328. [Google Scholar] [CrossRef]
- Gao, P.Y.; Bian, J.; Xu, S.S.; Liu, C.F.; Sun, Y.Q.; Zhang, G.L.; Li, D.Q.; Liu, X.G. Structure features, selenylation modification, and improved anti-tumor activity of a polysaccharide from Eriobotrya japonica. Int. J. Biol. Macromol. 2020, 149, 207–214. [Google Scholar] [CrossRef]
- Zhu, S.Y.; Hu, J.H.; Liu, S.; Guo, S.J.; Jia, Y.; Li, M.; Kong, W.B.; Liang, J.Y.; Zhang, J.; Wang, J.L. Synthesis of Se-polysaccharide mediated by selenium oxychloride: Structure features and antiproliferative activity. Carbohydr. Polym. 2020, 246, 116545. [Google Scholar] [CrossRef] [PubMed]
- Miletić, D.; Turło, J.; Podsadni, P.; Sknepnek, A.; Szczepańska, A.; Klimaszewska, M.; Malinowska, E.; Lević, S.; Nedović, V.; Nikšić, M. Production of bioactive selenium enriched crude exopolysaccharides via selenourea and sodium selenite bioconversion using Trametes versicolor. Food Biosci. 2021, 42, 101046. [Google Scholar] [CrossRef]
- Dong, Z.; Dong, G.; Lai, F.; Wu, H.; Zhan, Q.P. Purification and comparative study of bioactivities of a natural selenized polysaccharide from Ganoderma Lucidum mycelia. Int. J. Biol. Macromol. 2021, 190, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liu, J.X.; Wang, X.; Hu, H.W.; Zhang, Y.R.; Liu, T.T.; Zhao, H. Structure characterization and antioxidant activity of carboxymethylated polysaccharide from Pholiota nameko. J. Food Biochem. 2022, 46, e14121. [Google Scholar] [CrossRef]
- Xu, P.; Li, J.L.; Hou, G.H.; Shi, F.; Ye, M. Cardioprotective effect of an exopolysaccharide from Lachnum YM130 and its derivatives on diabetic mice. Process Biochem. 2017, 58, 333–340. [Google Scholar] [CrossRef]
- Akil, Y.; Lorenz, D.; Lehnen, R.; Saake, B. Safe and non-toxic hydroxyalkylation of xylan using propylene carbonate. Eur. Polym. J. 2016, 77, 88–97. [Google Scholar] [CrossRef]
- Sharma, D.; Sharma, P. Taguchi design-based synthesis and structural analysis of Cassia galactomannan hydroxypropyl derivative. Carbohydr. Polym. 2022, 292, 119672. [Google Scholar] [CrossRef]
- DiNardo, A.; Subramanian, J.; Singh, A. Investigation of antioxidant content and capacity in yellow European plums. Int. J. Food Sci. 2018, 18, 99–116. [Google Scholar] [CrossRef]
- Zheng, Y.Z.; Chen, D.F.; Deng, G.; Guo, R.; Fu, Z.M. The antioxidative activity of piceatannol and its different derivatives: Antioxidative mechanism analysis. Phytochemistry 2018, 156, 184–192. [Google Scholar] [CrossRef]
- Neha, K.; Haider, M.R.; Pathak, A.; Yar, M.S. Medicinal prospects of antioxidants: A review. Eur. J. Med. Chem. 2019, 178, 687–704. [Google Scholar] [CrossRef]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative stress, aging, and diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef]
- Farhood, B.; Najafi, M.; Salehi, E.; Goradel, N.H.; Nashtaei, M.S.; Khanlarkhani, N.; Mortezaee, K. Disruption of the redox balance with either oxidative or anti-oxidative overloading as a promising target for cancer therapy. J. Cell. Biochem. 2019, 120, 71–76. [Google Scholar] [CrossRef]
- Chang, C.C.; Chang, Y.C.; Hu, W.L.; Huang, Y.C. Oxidative stress and salvia miltiorrhiza in aging-associated cardiovascular diseases. Oxid. Med. Cell. Longev. 2016, 2016, 4797102. [Google Scholar] [CrossRef]
- Duan, S.Y.; Zhao, M.M.; Wu, B.Y.; Wang, S.J.; Yang, Y.; Xu, Y.Q.; Wang, L.B. Preparation, characteristics, and antioxidant activities of carboxymethylated polysaccharides from blackcurrant fruits. Int. J. Biol. Macromol. 2020, 155, 1114–1122. [Google Scholar] [CrossRef]
- Gao, Z.Z.; Zhang, C.; Tian, W.J.; Liu, K.H.; Hou, R.R.; Yue, C.J.; Wu, Y.; Wang, D.Y.; Liu, J.G.; Hu, Y.L.; et al. The antioxidative and hepatoprotective effects comparison of Chinese angelica polysaccharide (CAP) and selenizing CAP (sCAP) in CCl4 induced hepatic injury mice. Int. J. Biol. Macromol. 2017, 97, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.X.; Huang, M.; Shen, M.Y.; Wen, P.W.; Wu, T.; Hong, Y.Z.; Yu, Q.; Chen, Y.; Xie, J.H. Sulfated modification enhanced the antioxidant activity of Mesona chinensis Benth polysaccharide and its protective effect on cellular oxidative stress. Int. J. Biol. Macromol. 2019, 136, 1000–1006. [Google Scholar] [CrossRef] [PubMed]
- Pandya, U.; Dhuldhaj, U.; Sahay, N.S. Bioactive mushroom polysaccharides as antitumor: An overview. Nat. Prod. Res. 2019, 33, 2668–2680. [Google Scholar] [CrossRef]
- Chen, L.; Huang, G. Antitumor activity of polysaccharides: An overview. Curr. Drug Targets 2018, 19, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.Q.; Li, M.Z.; Yu, M.L.; Shen, M.Y.; Wang, Q.; Yu, Y.; Xie, J.H. Natural polysaccharides exhibit anti-tumor activity by targeting gut microbiota. Int. J. Biol. Macromol. 2019, 121, 743–751. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.L.; Bao, A.J.; Wang, Q.; Guo, H.Y.; Zhang, Y.D.; Liang, J.Y.; Kong, W.B.; Yao, J.; Zhang, J. Sulfation can enhance antitumor activities of Artemisia sphaerocephala polysaccharide in vitro and vivo. Int. J. Biol. Macromol. 2018, 107, 502–511. [Google Scholar] [CrossRef]
- Ding, J.; Jia, W.; Cui, Y.G.; Jin, J.; Zhang, Y.Q.; Xu, L.C.; Liu, Y.H. Anti-angiogenic effect of a chemically sulfated polysaccharide from Phellinus ribis by inhibiting VEGF/VEGFR pathway. Int. J. Biol. Macromol. 2020, 154, 72–81. [Google Scholar] [CrossRef]
- Xie, L.M.; Shen, M.Y.; Huang, R.; Liu, X.; Yu, Y.; Lu, H.Y.; Xie, J.H. Apoptosis of colon cancer CT-26 cells induced polysaccharide from Cyclocarya paliurus and its phosphorylated derivative via intrinsic mitochondrial passway. FSHW 2023, 12, 1545–1556. [Google Scholar] [CrossRef]
- Jyotsna; Vijayakumar, P.; Dhas, T.S.; Mani, R.; Raguraman, V. Antiviral activity of sulfated polysaccharides from Sargassum ilicifolium against fish Betanodavirus infection. Aquac. Int. 2021, 29, 1049–1067. [Google Scholar] [CrossRef]
- Ming, K.; Chen, Y.; Yao, F.K.; Shi, J.T.; Yang, J.J.; Du, H.X.; Wang, X.Y.; Wang, Y.X.; Liu, J.G. Phosphorylated Codonopsis pilosula polysaccharide could inhibit the virulence of duck hepatitis A virus compared with Codonopsis pilosula polysaccharide. Int. J. Biol. Macromol. 2017, 94, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Zhang, Y.T.; Yin, Z.Q.; Zhao, X.H.; Liang, X.X.; He, C.L.; Yin, L.Z.; Lv, C.; Zhao, L.; Ye, G.; et al. Antiviral effect of sulfated Chuanmingshen violaceum polysaccharide in chickens infected with virulent Newcastle disease virus. Virology 2015, 476, 316–322. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.X.; Wu, S.H.; Huang, R.; Liu, X.; Shen, M.Y.; Xie, J.H. Immunomodulatory activity and mechanism of Chinese yam polysaccharide after sulfated modification. Ind. Crops Prod. 2023, 197, 116549. [Google Scholar] [CrossRef]
- Han, Y.; Ouyang, K.H.; Li, J.G.; Liu, X.; An, Q.; Zhao, M.; Chen, S.; Li, X.; Ye, X.M.; Zhao, Z.T.; et al. Sulfated modification, characterization, immunomodulatory activities and mechanism of the polysaccharides from Cyclocarya paliurus on dendritic cells. Int. J. Biol. Macromol. 2020, 159, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.B.; Fan, J.; Lin, L.; Liu, Y.J.; Chai, D.K.; Yang, J. Immunomodulatory effects of phosphorylated radix cyathulae officinalis polysaccharides in immunosuppressed mice. Molecules 2019, 24, 4150. [Google Scholar] [CrossRef]
- Han, Y.; Zhang, Y.; Ouyang, K.H.; Chen, L.L.; Zhao, M.; Wang, W.J. Sulfated Cyclocarya paliurus polysaccharides improve immune function of immunosuppressed mice by modulating intestinal microbiota. Int. J. Biol. Macromol. 2022, 212, 31–42. [Google Scholar] [CrossRef]
- Zhao, T.; Guo, Y.C.; Yan, S.Y.; Li, N.; Ji, H.C.; Hu, Q.H.; Zhang, M.; Li, Q.; Gao, H.; Yang, L.Q.; et al. Preparation, structure characterization of carboxymethylated schisandra polysaccharides and their intervention in immunotoxicity to polychlorinated biphenyls. Process Biochem. 2022, 115, 30–41. [Google Scholar] [CrossRef]
- Hou, C.Y.; Chen, L.L.; Yang, L.Z.; Ji, X.L. An insight into anti-inflammatory effects of natural polysaccharides. Int. J. Biol. Macromol. 2020, 153, 248–255. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, T.T.; Wang, X.; Zhou, L.Y.; Qi, J.; An, S.Y. Structural characterization, antioxidant activity and anti-inflammatory of the phosphorylated polysaccharide from Pholiota nameko. Front. Nutr. 2022, 9, 976552. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Je, J.G.; Huang, C.X.; Oh, J.Y.; Fu, X.T.; Wang, K.Q.; Ahn, G.; Xu, J.C.; Gao, X.; Jeon, Y.J. Anti-inflammatory effect of sulfated polysaccharides isolated from Codium fragile in vitro in RAW 264.7 macrophages and in vivo in zebrafish. Mar. Drugs 2022, 20, 391. [Google Scholar] [CrossRef]
- Li, N.; Liu, X.; He, X.X.; Wang, S.Y.; Cao, S.J.; Xia, Z.; Xian, H.L.; Qin, L.; Mao, W.J. Structure and anticoagulant property of a sulfated polysaccharide isolated from the green seaweed Monostroma angicava. Carbohydr. Polym. 2017, 159, 195–206. [Google Scholar] [CrossRef] [PubMed]
- Gao, N.; Chen, R.; Mou, R.R.; Xiang, J.Y.; Zhou, K.; Li, Z.; Zhao, J.H. Purification, structural characterization and anticoagulant activities of four sulfated polysaccharides from sea cucumber Holothuria fuscopunctata. Int. J. Biol. Macromol. 2020, 164, 3421–3428. [Google Scholar] [CrossRef]
- Liu, H.Z.; Li, F.P.; Luo, P. Effect of carboxymethylation and phosphorylation on the properties of polysaccharides from Sepia esculenta ink: Antioxidation and anticoagulation in vitro. Mar. Drugs 2019, 17, 626. [Google Scholar]
- Wang, Y.F.; Su, N.N.; Hou, G.H.; Li, J.L.; Ye, M. Hypoglycemic and hypolipidemic effects of a polysaccharide from Lachnum YM240 and its derivatives in mice, induced by a high fat diet and low dose STZ. Medchemcomm 2017, 8, 964–974. [Google Scholar] [CrossRef] [PubMed]
- Navvabi, A.; Homaei, A.; Navvabi, N.; Michaud, P. Exopolysaccharides from Marine Microalgae. In Marine Biochemistry, 1st ed.; Kim, S.K., Ed.; CRC Press: Boca Raton, FL, USA, 2022. [Google Scholar]
- Sharifian, S.; Homaei, A. Marine-Derived Polysaccharides: Prospects for Future Pharmaceuticals and Drug Delivery Systems, 1st ed.; Springer: Singapore, 2022; pp. 403–453. [Google Scholar]
- Zhang, Q.; Xu, Y.; Lv, J.J.; Cheng, M.X.; Wu, Y.; Cao, K.; Zhang, X.F.; Mou, X.N.; Fan, Q. Structure characterization of two functional polysaccharides from Polygonum multiflorum and its immunomodulatory. Int. J. Biol. Macromol. 2018, 113, 195–204. [Google Scholar] [CrossRef]
- Zhou, S.Y.; Huang, G.L.; Huang, H.L. Extraction, derivatization and antioxidant activities of onion polysaccharide. Food Chem. 2022, 388, 133000. [Google Scholar] [CrossRef]
- Wen, Y.; Zheng, S.Q.; Su, C.M. A new acidic polysaccharide and its sulfated derivative from cultured Morchella sextelata fruiting bodies and their antioxidant and immunoregulatory activities. Biomass Conv. Bioref. 2022, 1–14. [Google Scholar] [CrossRef]
- Chen, S.H.; Huang, H.L.; Huang, G.L. Extraction, derivatization and antioxidant activity of cucumber polysaccharide. Int. J. Biol. Macromol. 2019, 140, 1047–1053. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Pujol, C.A.; Jana, S.; Damonte, E.B.; Ray, B.; Ray, S. Chemically sulfated arabinoxylans from Plantago ovata seed husk: Synthesis, characterization and antiviral activity. Carbohydr. Polym. 2021, 256, 117555. [Google Scholar] [PubMed]
- Zhang, Y.; Liu, Y.H.; Ni, G.Y.; Xu, J.H.; Tian, Y.P.; Liu, X.Y.; Gao, J.; Gao, Q.; Shen, Y.C.; Yan, Z.W. Sulfated modification, basic characterization, antioxidant and anticoagulant potentials of polysaccharide from Sagittaria trifolia. Arab. J. Chem. 2023, 16, 104812. [Google Scholar] [CrossRef]
- De Araújo, C.A.; Noseda, M.D.; Cipriani, T.R.; Gonçalves, A.G.; Duarte, M.E.R.; Ducatti, D.R.B. Selective sulfation of carrageenans and the influence of sulfate regiochemistry on anticoagulant properties. Carbohydr. Polym. 2013, 91, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.N.; Li, Y.; Liang, J.; Chai, J.H.; Kuang, H.X.; Xia, Y.G. Direct acetylation for full analysis of polysaccharides in edible plants and fungi using reverse phase liquid chromatography-multiple reaction monitoring mass spectrometry. J. Pharm. Biomed. Anal. 2023, 222, 115083. [Google Scholar]
- Wang, F.L.; Ji, Y.B.; Yang, B. Sulfated modification, characterization and monosaccharide composition analysis of Undaria pinnatifida polysaccharides and anti-tumor activity. Exp. Ther. Med. 2020, 20, 630–636. [Google Scholar] [CrossRef] [PubMed]
- Li, J.X.; Chi, Z.; Yu, L.J.; Jiang, F.; Liu, C.G. Sulfated modification, characterization, and antioxidant and moisture absorption/retention activities of a soluble neutral polysaccharide from Enteromorpha prolifera. Int. J. Biol. Macromol. 2017, 105, 1544–1553. [Google Scholar]
- Xu, Y.F.; Song, S.; Wei, Y.X.; Wang, F.X.; Zhao, M.; Guo, J.; Zhang, J. Sulfated modification of the polysaccharide from Sphallerocarpus gracilis and its antioxidant activities. Int. J. Biol. Macromol. 2016, 87, 180–190. [Google Scholar] [CrossRef]
- Xie, J.H.; Wang, Z.J.; Shen, M.Y.; Nie, S.P.; Gong, B.; Li, H.S.; Zhao, Q.; Li, W.J.; Xie, J.H. Sulfated modification, characterization and antioxidant activities of polysaccharide from Cyclocarya paliurus. Food Hydrocoll. 2016, 53, 7–15. [Google Scholar] [CrossRef]
- Qi, K.; Xia, G.D.; Huang, G.L.; Huang, H.L. Extraction, chemical modification, and antioxidant activities of Daucus carota polysaccharide. Chem. Biol. Drug Des. 2021, 98, 1098–1103. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).