Multiprotein Adsorption from Human Serum at Gold and Oxidized Iron Surfaces Studied by Atomic Force Microscopy and Polarization-Modulation Infrared Reflection Absorption Spectroscopy
Abstract
1. Introduction
2. Results and Discussion
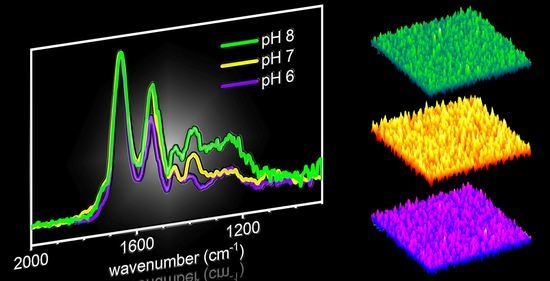
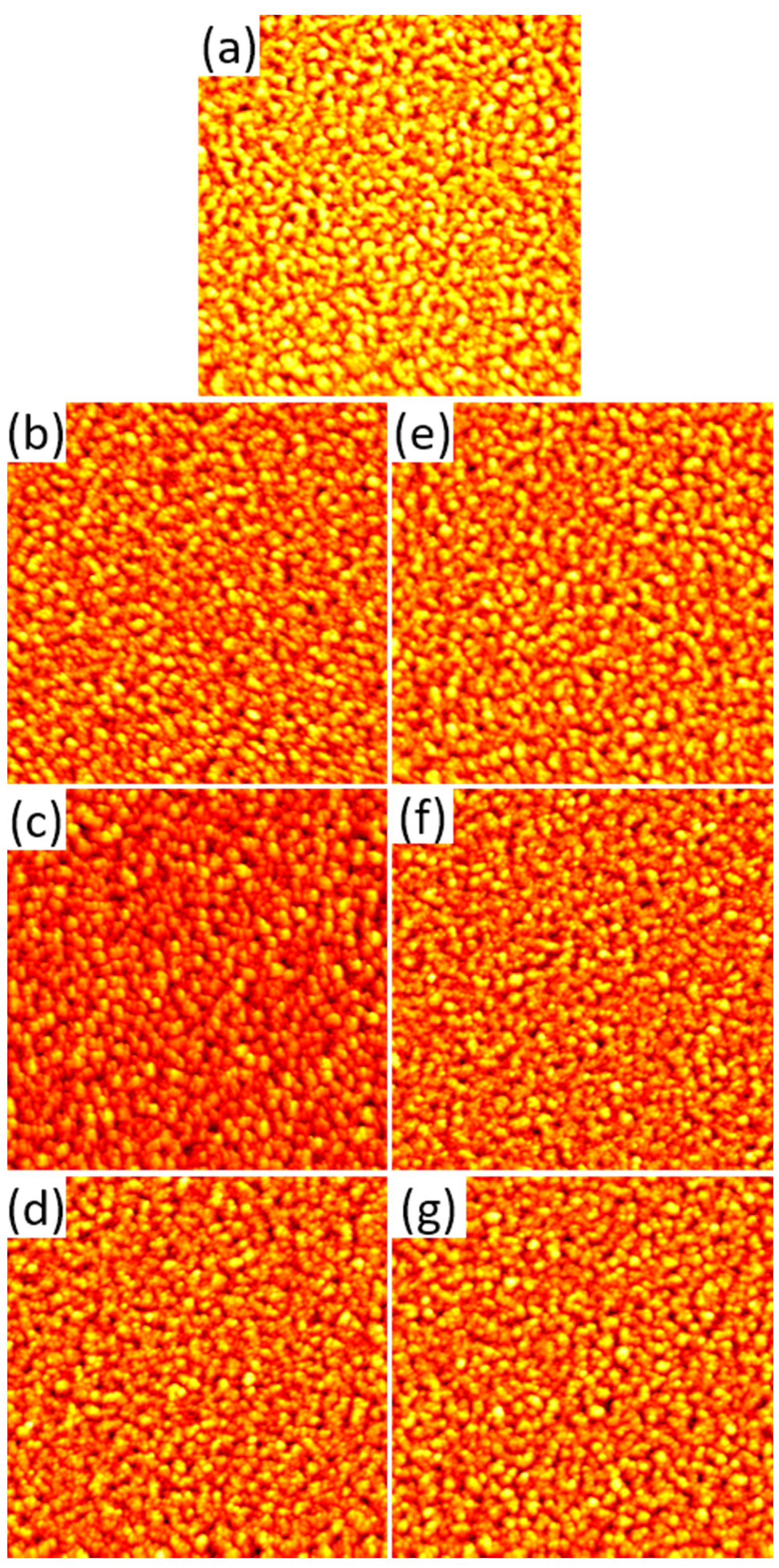
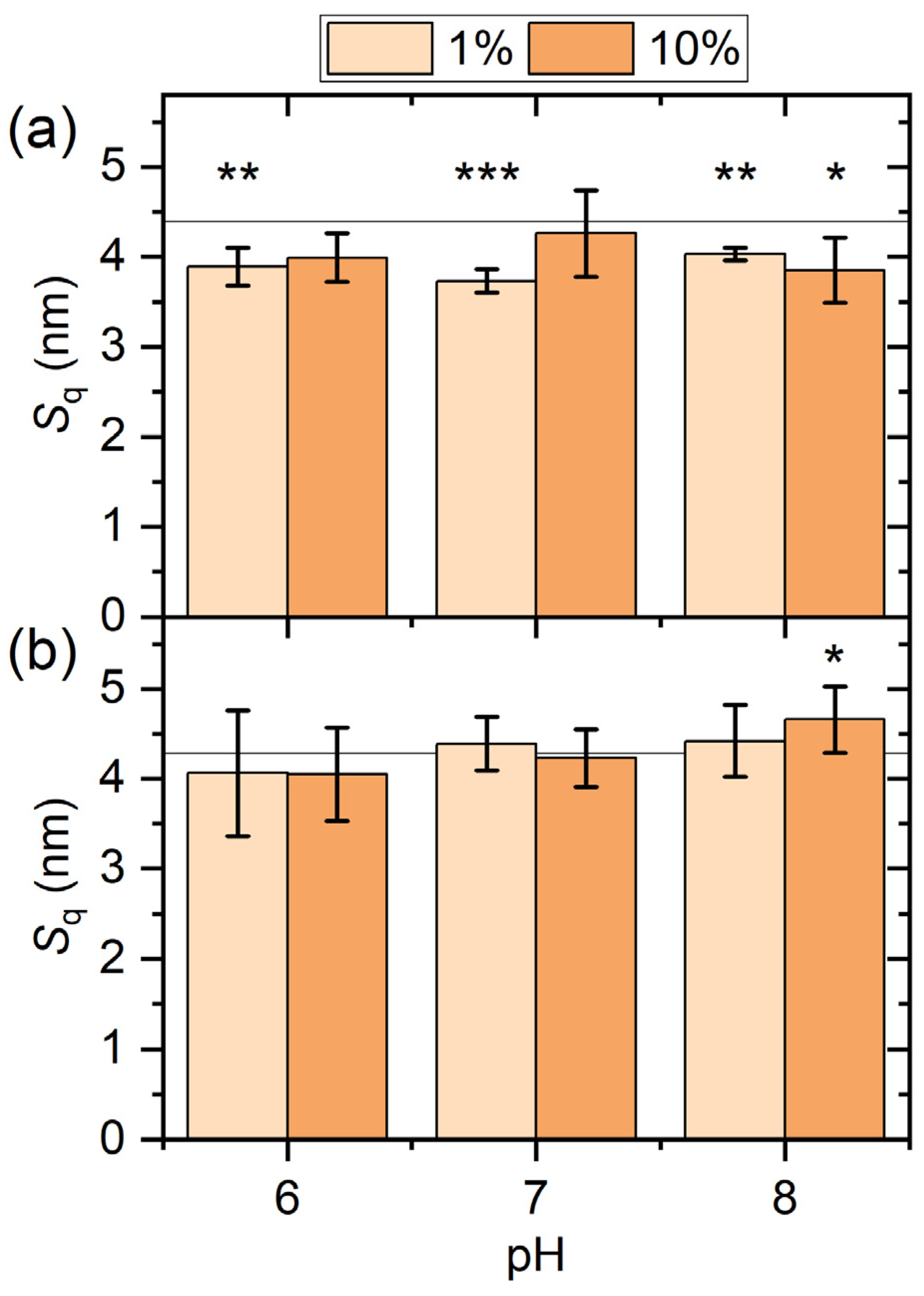
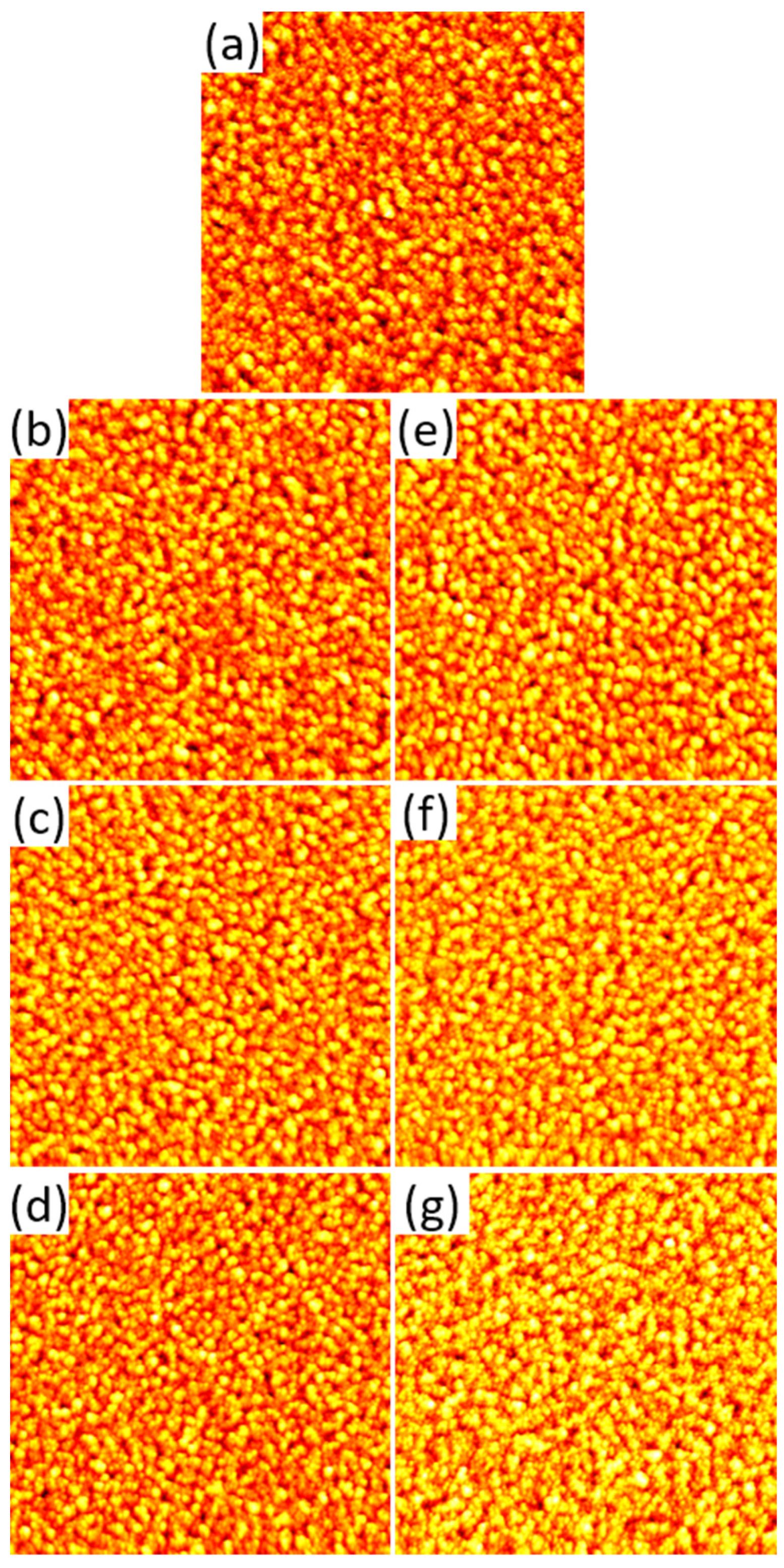
2.1. AFM Results
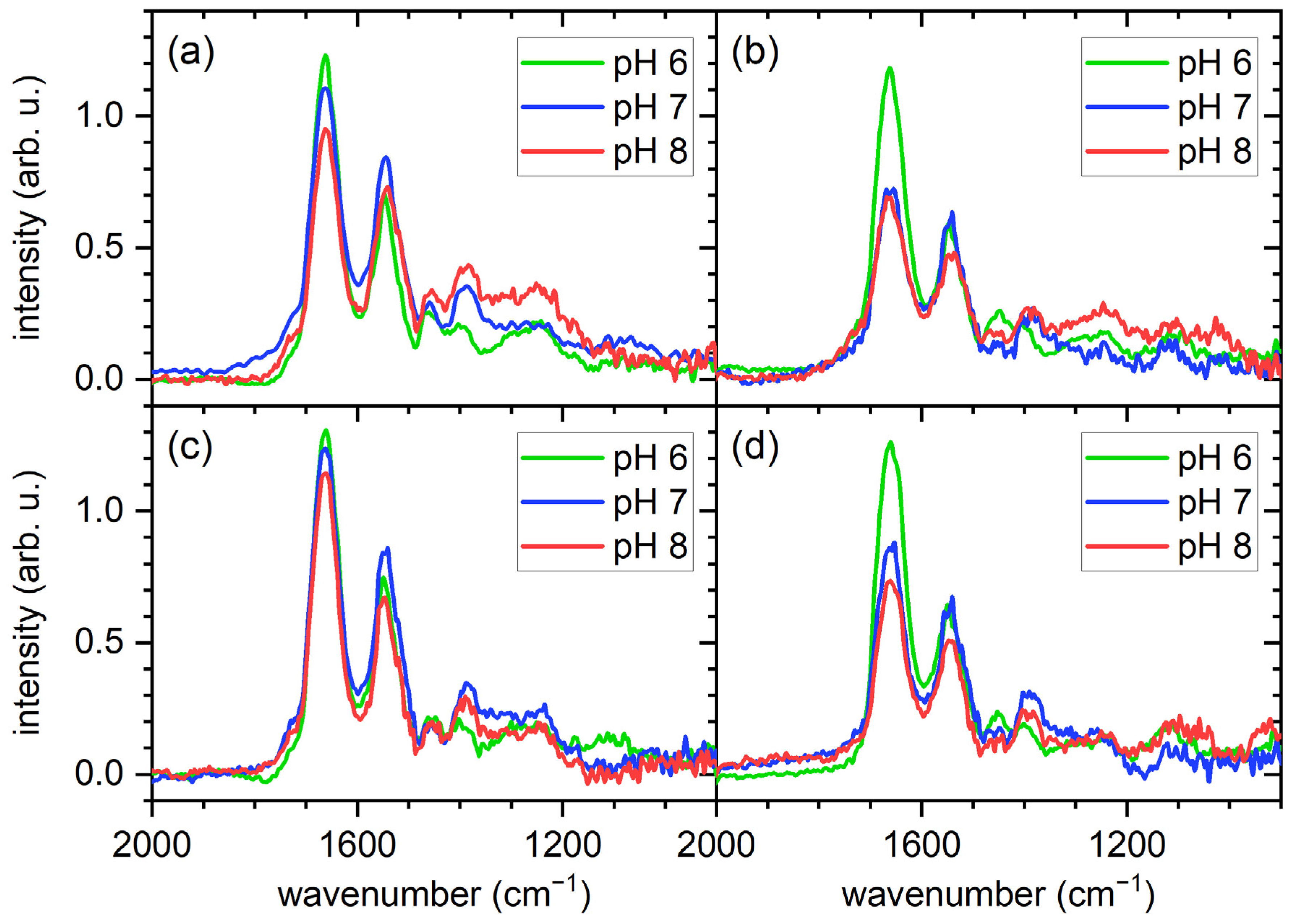
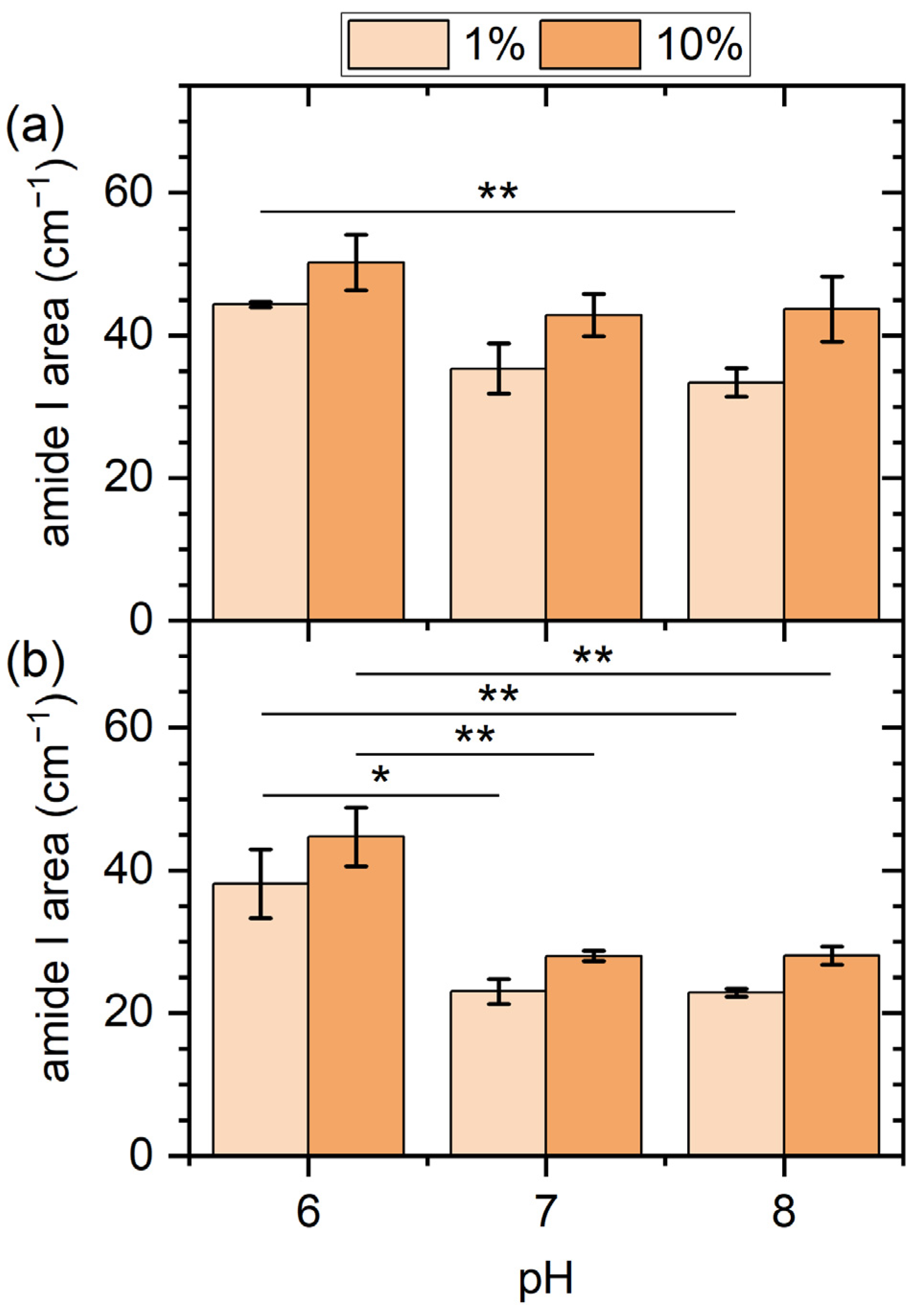
2.2. PM-IRRAS Results
3. Materials and Methods
3.1. Thin Film Deposition
3.2. Serum Exposure
3.3. AFM
3.4. PM-IRRAS
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Jain, A. Body fluid composition. Pediatr. Rev. 2015, 36, 141–152. [Google Scholar] [CrossRef]
- Wilson, C.J.; Clegg, R.E.; Leavesley, D.I.; Pearcy, M.J. Mediation of biomaterial-cell interactions by adsorbed proteins: A review. Tissue Eng. 2005, 11, 1–18. [Google Scholar] [CrossRef]
- Yang, Y.; Yu, M.; Böke, F.; Qin, Q.; Hübner, R.; Knust, S.; Schwiderek, S.; Grundmeier, G.; Fischer, H.; Keller, A. Effect of nanoscale surface topography on the adsorption of globular proteins. Appl. Surf. Sci. 2021, 535, 147671. [Google Scholar] [CrossRef]
- Yang, Y.; Knust, S.; Schwiderek, S.; Qin, Q.; Yun, Q.; Grundmeier, G.; Keller, A. Protein Adsorption at Nanorough Titanium Oxide Surfaces: The Importance of Surface Statistical Parameters beyond Surface Roughness. Nanomaterials 2021, 11, 357. [Google Scholar] [CrossRef]
- Hemmersam, A.G.; Rechendorff, K.; Foss, M.; Sutherland, D.S.; Besenbacher, F. Fibronectin adsorption on gold, Ti-, and Ta-oxide investigated by QCM-D and RSA modelling. J. Colloid Interface Sci. 2008, 320, 110–116. [Google Scholar] [CrossRef]
- Höök, F.; Vörös, J.; Rodahl, M.; Kurrat, R.; Böni, P.; Ramsden, J.; Textor, M.; Spencer, N.; Tengvall, P.; Gold, J.; et al. A comparative study of protein adsorption on titanium oxide surfaces using in situ ellipsometry, optical waveguide lightmode spectroscopy, and quartz crystal microbalance/dissipation. Colloids Surf. B Biointerfaces 2002, 24, 155–170. [Google Scholar] [CrossRef]
- Keller, T.F.; Schönfelder, J.; Reichert, J.; Tuccitto, N.; Licciardello, A.; Messina, G.M.L.; Marletta, G.; Jandt, K.D. How the surface nanostructure of polyethylene affects protein assembly and orientation. ACS Nano 2011, 5, 3120–3131. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.H.K.; Pham, V.T.H.; Al Kobaisi, M.; Bhadra, C.; Orlowska, A.; Ghanaati, S.; Manzi, B.M.; Baulin, V.A.; Joudkazis, S.; Kingshott, P.; et al. Adsorption of Human Plasma Albumin and Fibronectin onto Nanostructured Black Silicon Surfaces. Langmuir 2016, 32, 10744–10751. [Google Scholar] [CrossRef]
- Desroches, M.J.; Chaudhary, N.; Omanovic, S. PM-IRRAS investigation of the interaction of serum albumin and fibrinogen with a biomedical-grade stainless steel 316LVM surface. Biomacromolecules 2007, 8, 2836–2844. [Google Scholar] [CrossRef]
- Bergkvist, M.; Carlsson, J.; Oscarsson, S. Surface-dependent conformations of human plasma fibronectin adsorbed to silica, mica, and hydrophobic surfaces, studied with use of Atomic Force Microscopy. J. Biomed. Mater. Res. A 2003, 64, 349–356. [Google Scholar] [CrossRef]
- Firkowska-Boden, I.; Helbing, C.; Dauben, T.J.; Pieper, M.; Jandt, K.D. How Nanotopography-Induced Conformational Changes of Fibrinogen Affect Platelet Adhesion and Activation. Langmuir 2020, 36, 11573–11580. [Google Scholar] [CrossRef] [PubMed]
- Pasche, S.; Vörös, J.; Griesser, H.J.; Spencer, N.D.; Textor, M. Effects of ionic strength and surface charge on protein adsorption at PEGylated surfaces. J. Phys. Chem. B 2005, 109, 17545–17552. [Google Scholar] [CrossRef]
- Wasilewska, M.; Adamczyk, Z.; Pomorska, A.; Nattich-Rak, M.; Sadowska, M. Human Serum Albumin Adsorption Kinetics on Silica: Influence of Protein Solution Stability. Langmuir 2019, 35, 2639–2648. [Google Scholar] [CrossRef]
- Huang, L.; Shao, D.; Wang, Y.; Cui, X.; Li, Y.; Chen, Q.; Cui, J. Human body-fluid proteome: Quantitative profiling and computational prediction. Brief. Bioinform. 2021, 22, 315–333. [Google Scholar] [CrossRef] [PubMed]
- Dee, K.C.; Puleo, D.A.; Bizios, R. An Introduction to Tissue-Biomaterial Interactions; John Wiley & Sons, Inc.: New York, NY, USA, 2002; ISBN 9780471270591. [Google Scholar]
- Benesch, J.; Svedhem, S.; Svensson, S.C.; Valiokas, R.; Liedberg, B.; Tengvall, P. Protein adsorption to oligo(ethylene glycol) self-assembled monolayers: Experiments with fibrinogen, heparinized plasma, and serum. J. Biomater. Sci. Polym. Ed. 2001, 12, 581–597. [Google Scholar] [CrossRef]
- Ladd, J.; Zhang, Z.; Chen, S.; Hower, J.C.; Jiang, S. Zwitterionic polymers exhibiting high resistance to nonspecific protein adsorption from human serum and plasma. Biomacromolecules 2008, 9, 1357–1361. [Google Scholar] [CrossRef]
- Lestelius, M.; Liedberg, B.; Tengvall, P. In Vitro Plasma Protein Adsorption on ω-Functionalized Alkanethiolate Self-Assembled Monolayers. Langmuir 1997, 13, 5900–5908. [Google Scholar] [CrossRef]
- Yang, W.; Xue, H.; Li, W.; Zhang, J.; Jiang, S. Pursuing “zero” protein adsorption of poly(carboxybetaine) from undiluted blood serum and plasma. Langmuir 2009, 25, 11911–11916. [Google Scholar] [CrossRef]
- Yang, W.; Chen, S.; Cheng, G.; Vaisocherová, H.; Xue, H.; Li, W.; Zhang, J.; Jiang, S. Film thickness dependence of protein adsorption from blood serum and plasma onto poly(sulfobetaine)-grafted surfaces. Langmuir 2008, 24, 9211–9214. [Google Scholar] [CrossRef]
- Tosatti, S.; Paul, S.; Askendal, A.; VandeVondele, S.; Hubbell, J.A.; Tengvall, P.; Textor, M. Peptide functionalized poly(l-lysine)-g-poly(ethylene glycol) on titanium: Resistance to protein adsorption in full heparinized human blood plasma. Biomaterials 2003, 24, 4949–4958. [Google Scholar] [CrossRef]
- Rosengren, Å.; Pavlovic, E.; Oscarsson, S.; Krajewski, A.; Ravaglioli, A.; Piancastelli, A. Plasma protein adsorption pattern on characterized ceramic biomaterials. Biomaterials 2002, 23, 1237–1247. [Google Scholar] [CrossRef]
- Boyd, A.R.; Burke, G.A.; Duffy, H.; Holmberg, M.; O’ Kane, C.; Meenan, B.J.; Kingshott, P. Sputter deposited bioceramic coatings: Surface characterisation and initial protein adsorption studies using surface-MALDI-MS. J. Mater. Sci. Mater. Med. 2011, 22, 71–84. [Google Scholar] [CrossRef]
- Hemmersam, A.G.; Foss, M.; Chevallier, J.; Besenbacher, F. Adsorption of fibrinogen on tantalum oxide, titanium oxide and gold studied by the QCM-D technique. Colloids Surf. B Biointerfaces 2005, 43, 208–215. [Google Scholar] [CrossRef]
- Huang, J.; Gonzalez Orive, A.; Krüger, J.T.; Hoyer, K.-P.; Keller, A.; Grundmeier, G. Influence of proteins on the corrosion of a conventional and selective laser beam melted FeMn alloy in physiological electrolytes. Corros. Sci. 2022, 200, 110186. [Google Scholar] [CrossRef]
- Vlasova, I.M.; Saletsky, A.M. Study of the denaturation of human serum albumin by sodium dodecyl sulfate using the intrinsic fluorescence of albumin. J. Appl. Spectrosc. 2009, 76, 536–541. [Google Scholar] [CrossRef]
- Yang, D.; Kroe-Barrett, R.; Singh, S.; Laue, T. IgG Charge: Practical and Biological Implications. Antibodies 2019, 8, 24. [Google Scholar] [CrossRef]
- Viglio, S.; Iadarola, P.; D’Amato, M.; Stolk, J. Methods of Purification and Application Procedures of Alpha1 Antitrypsin: A Long-Lasting History. Molecules 2020, 25, 4014. [Google Scholar] [CrossRef] [PubMed]
- Satchell, L.; Leake, D.S. Oxidation of low-density lipoprotein by iron at lysosomal pH: Implications for atherosclerosis. Biochemistry 2012, 51, 3767–3775. [Google Scholar] [CrossRef]
- Back, S.A.; Alhadeff, J.A. Differential isoelectric focusing properties of crude and purified human alpha 2-macroglobulin and alpha 2-macroglobulin-proteinase complexes. J. Chromatogr. 1983, 278, 43–51. [Google Scholar] [CrossRef]
- Hovanessian, A.G.; Awdeh, Z.L. Gel isoelectric focusing of human-serum transferrin. Eur. J. Biochem. 1976, 68, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, R.C.; Halbwachs-Mecarelli, L.; Roque-Barreira, M.C.; Noel, L.H.; Berger, J.; Lesavre, P. Charge and size of mesangial IgA in IgA nephropathy. Kidney Int. 1985, 28, 666–671. [Google Scholar] [CrossRef]
- Shibata, K.; Constans, J.; Viau, M.; Matsumoto, H. Polymorphism of the haptoglobin peptides by isoelectric focusing electrophoresis and isoelectric point determinations. Hum. Genet. 1982, 61, 210–214. [Google Scholar] [CrossRef]
- Sundaram, G.S.; Mackenzie, S.L.; Sodhi, H.S. Preparative isoelectric focusing of human serum high-density lipoprotein (HDL3). Biochim. Biophys. Acta 1974, 337, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Nelson, R.A.; Brebner, E. Isoelectric focusing of components of the complement system and certain related proteins in human serum. Immunol. Commun. 1974, 3, 109–132. [Google Scholar] [CrossRef]
- Protein Calculator v3.4. Available online: http://protcalc.sourceforge.net/ (accessed on 8 August 2023).
- Dolatshahi-Pirouz, A.; Kolman, N.; Arpanaei, A.; Jensen, T.; Foss, M.; Chevallier, J.; Kingshott, P.; Baas, J.; Søballe, K.; Besenbacher, F. The adsorption characteristics of osteopontin on hydroxyapatite and gold. Mater. Sci. Eng. C 2011, 31, 514–522. [Google Scholar] [CrossRef]
- Yang, Y.; Schwiderek, S.; Grundmeier, G.; Keller, A. Strain-Dependent Adsorption of Pseudomonas aeruginosa-Derived Adhesin-Like Peptides at Abiotic Surfaces. Micro 2021, 1, 129–139. [Google Scholar] [CrossRef]
- Tidwell, C.D.; Castner, D.G.; Golledge, S.L.; Ratner, B.D.; Meyer, K.; Hagenhoff, B.; Benninghoven, A. Static time-of-flight secondary ion mass spectrometry and x-ray photoelectron spectroscopy characterization of adsorbed albumin and fibronectin films. Surf. Interface Anal. 2001, 31, 724–733. [Google Scholar] [CrossRef]
- Ishida, K.P.; Griffiths, P.R. Comparison of the Amide I/II Intensity Ratio of Solution and Solid-State Proteins Sampled by Transmission, Attenuated Total Reflectance, and Diffuse Reflectance Spectrometry. Appl. Spectrosc. 1993, 47, 584–589. [Google Scholar] [CrossRef]
- Barth, A. The infrared absorption of amino acid side chains. Prog. Biophys. Mol. Biol. 2000, 74, 141–173. [Google Scholar] [CrossRef] [PubMed]
- Belinskaia, D.A.; Voronina, P.A.; Batalova, A.A.; Goncharov, N.V. Serum Albumin. Encyclopedia 2021, 1, 65–75. [Google Scholar] [CrossRef]
- Shaw, A.L.; Mathews, D.W.; Hinkle, J.E.; Petschow, B.W.; Weaver, E.M.; Detzel, C.J.; Klein, G.L.; Bradshaw, T.P. Absorption and safety of serum-derived bovine immunoglobulin/protein isolate in healthy adults. Clin. Exp. Gastroenterol. 2016, 9, 365–375. [Google Scholar] [CrossRef] [PubMed]
- Nečas, D.; Klapetek, P. Gwyddion: An open-source software for SPM data analysis. Open Phys. 2012, 10, 181–188. [Google Scholar] [CrossRef]
- Torun, B.; Giner, I.; Grundmeier, G.; Ozcan, O. In situ PM-IRRAS studies of organothiols and organosilane monolayers-ZnO interfaces at high water activities. Surf. Interface Anal. 2017, 49, 71–74. [Google Scholar] [CrossRef]


| Protein | Concentration (mg/mL) | Relative Portion (%) | Molecular Weight (kDa) | Isoelectric Point |
|---|---|---|---|---|
| Albumin | 40 | 50–67 | 66 | 4.7 [26] |
| IgG | 15 | 19–25 | 150 | 6.2–8.6 [27] |
| α1-Antitrypsin | 3 | 4–5 | 54 | 5.1 [28] |
| Low-density lipoprotein | 3 | 4–5 | 5000 | 5.1–5.8 [29] |
| α2-Macroglobulin | 3 | 4–5 | 725 | 5.3 [30] |
| Transferrin | 2.6 | 3–4 | 77 | 5.2–5.6 [31] |
| IgA | 2.3 | 3–4 | 162 | 4.5–6.8 [32] |
| α2-Haptoglobins | 2 | 3–4 | 100 | 5.55–6.52 [33] |
| High-density lipoprotein | 2 | 3–4 | 195 | 3.8–7.4 [34] |
| Complement 3 | 1.6 | 2–3 | 180 | 5.75 [35] |
| pH | Net Charge |
|---|---|
| 6 | −0.6 |
| 7 | −12.2 |
| 8 | −24.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, J.; Qiu, Y.; Lücke, F.; Su, J.; Grundmeier, G.; Keller, A. Multiprotein Adsorption from Human Serum at Gold and Oxidized Iron Surfaces Studied by Atomic Force Microscopy and Polarization-Modulation Infrared Reflection Absorption Spectroscopy. Molecules 2023, 28, 6060. https://doi.org/10.3390/molecules28166060
Huang J, Qiu Y, Lücke F, Su J, Grundmeier G, Keller A. Multiprotein Adsorption from Human Serum at Gold and Oxidized Iron Surfaces Studied by Atomic Force Microscopy and Polarization-Modulation Infrared Reflection Absorption Spectroscopy. Molecules. 2023; 28(16):6060. https://doi.org/10.3390/molecules28166060
Chicago/Turabian StyleHuang, Jingyuan, Yunshu Qiu, Felix Lücke, Jiangling Su, Guido Grundmeier, and Adrian Keller. 2023. "Multiprotein Adsorption from Human Serum at Gold and Oxidized Iron Surfaces Studied by Atomic Force Microscopy and Polarization-Modulation Infrared Reflection Absorption Spectroscopy" Molecules 28, no. 16: 6060. https://doi.org/10.3390/molecules28166060
APA StyleHuang, J., Qiu, Y., Lücke, F., Su, J., Grundmeier, G., & Keller, A. (2023). Multiprotein Adsorption from Human Serum at Gold and Oxidized Iron Surfaces Studied by Atomic Force Microscopy and Polarization-Modulation Infrared Reflection Absorption Spectroscopy. Molecules, 28(16), 6060. https://doi.org/10.3390/molecules28166060