Accelerator-Based Production of Scandium Radioisotopes for Applications in Prostate Cancer: Toward Building a Pipeline for Rapid Development of Novel Theranostics
Abstract
1. Introduction
2. Results and Discussion
2.1. Cyclotron-Based Production of 43Sc
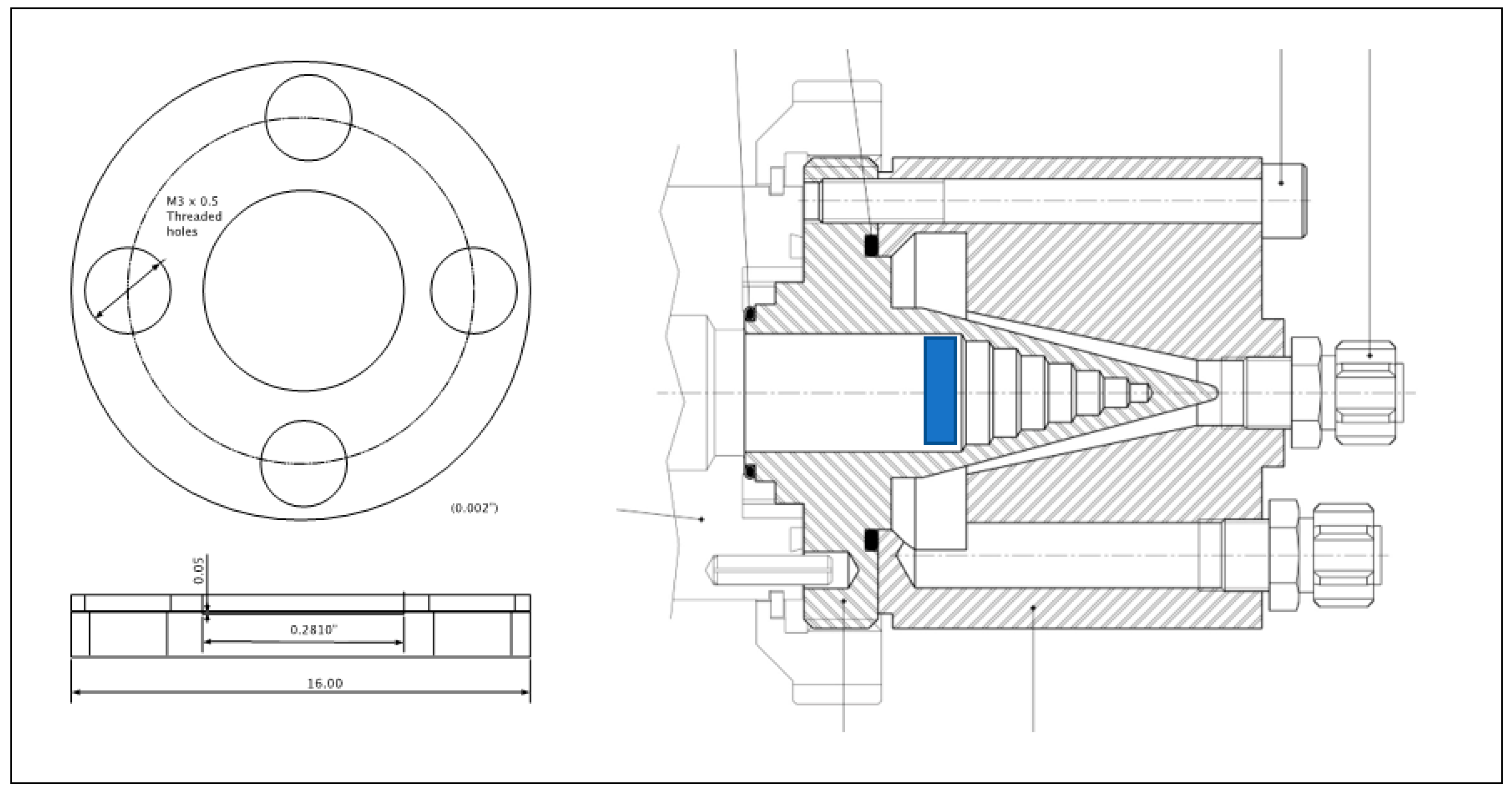
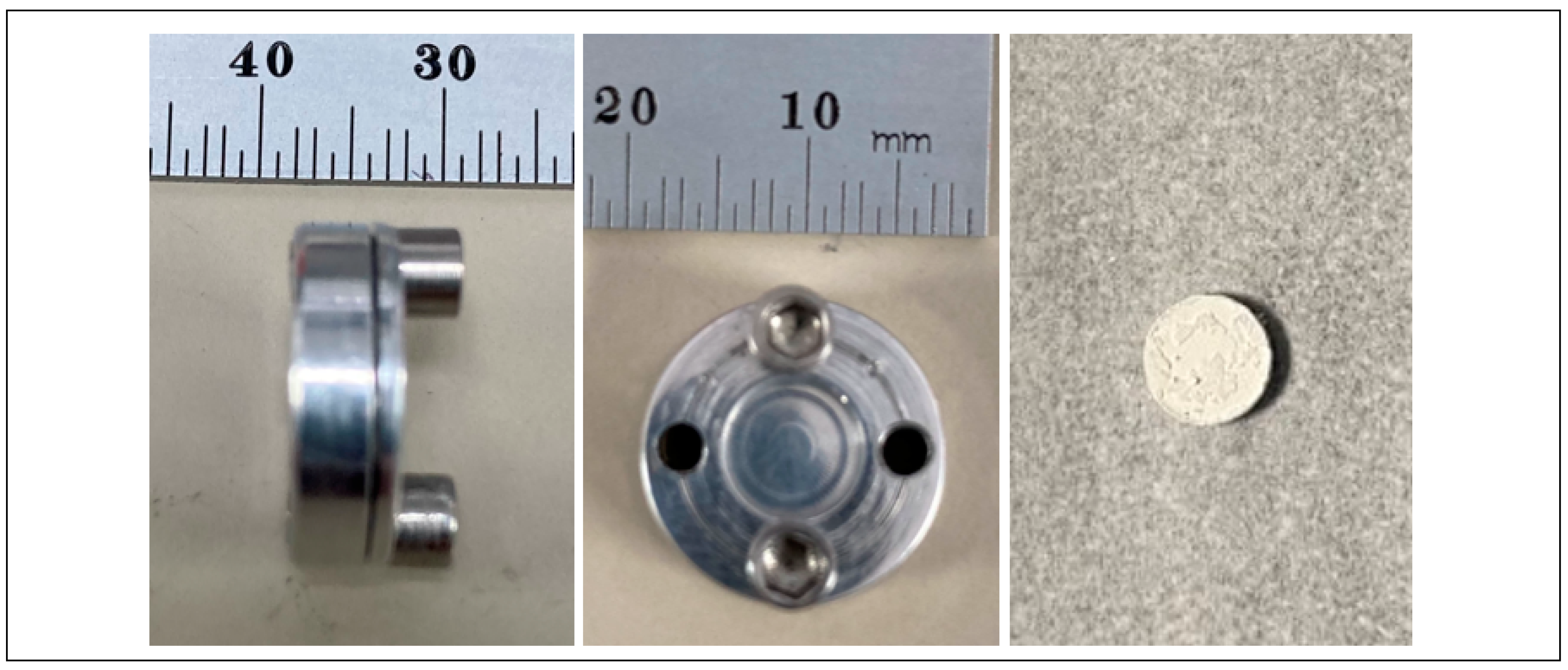
2.1.1. Beam-Stop as a Target Holder
2.1.2. Target Materials and Preparation
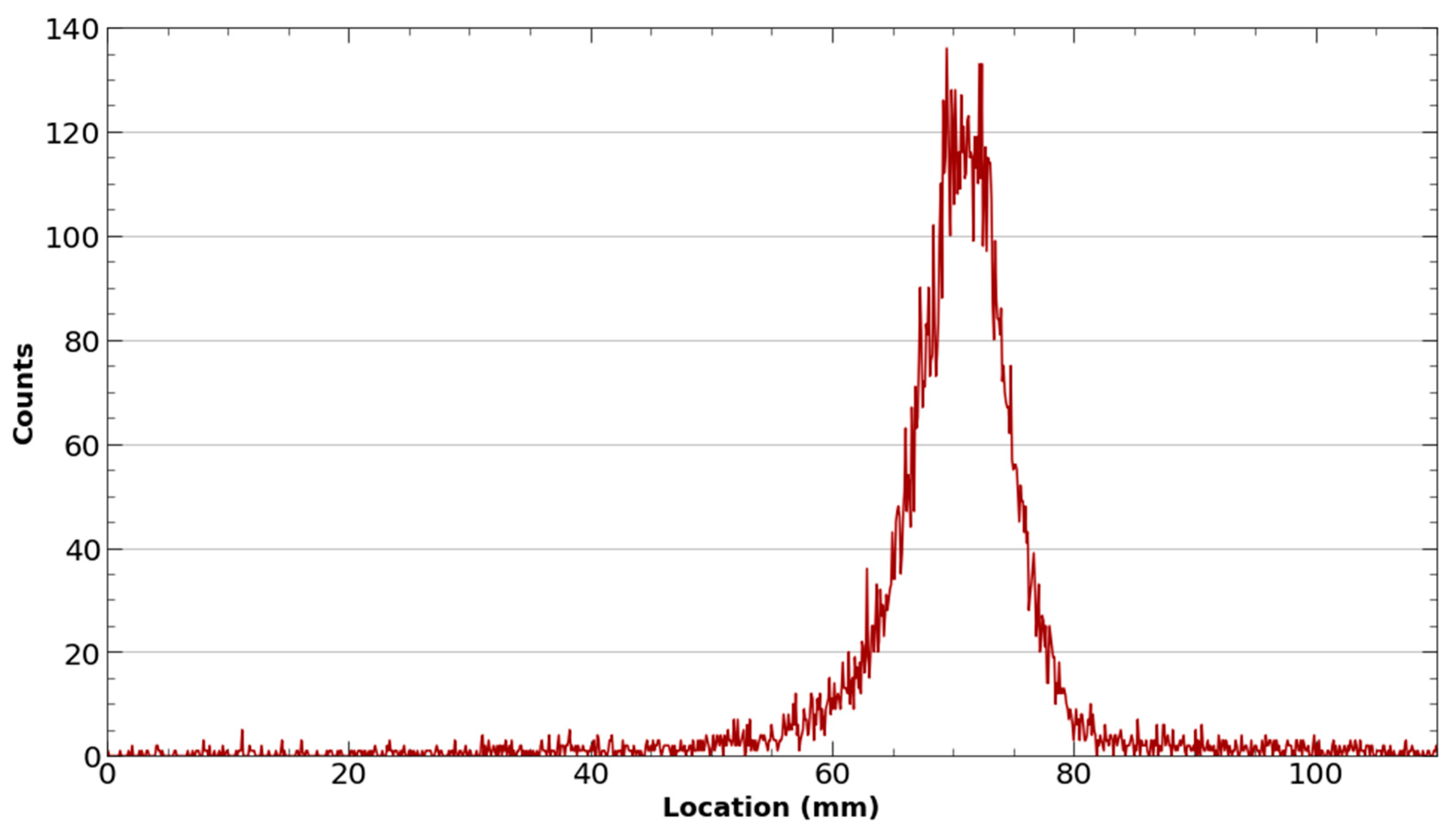
2.1.3. Target Irradiation
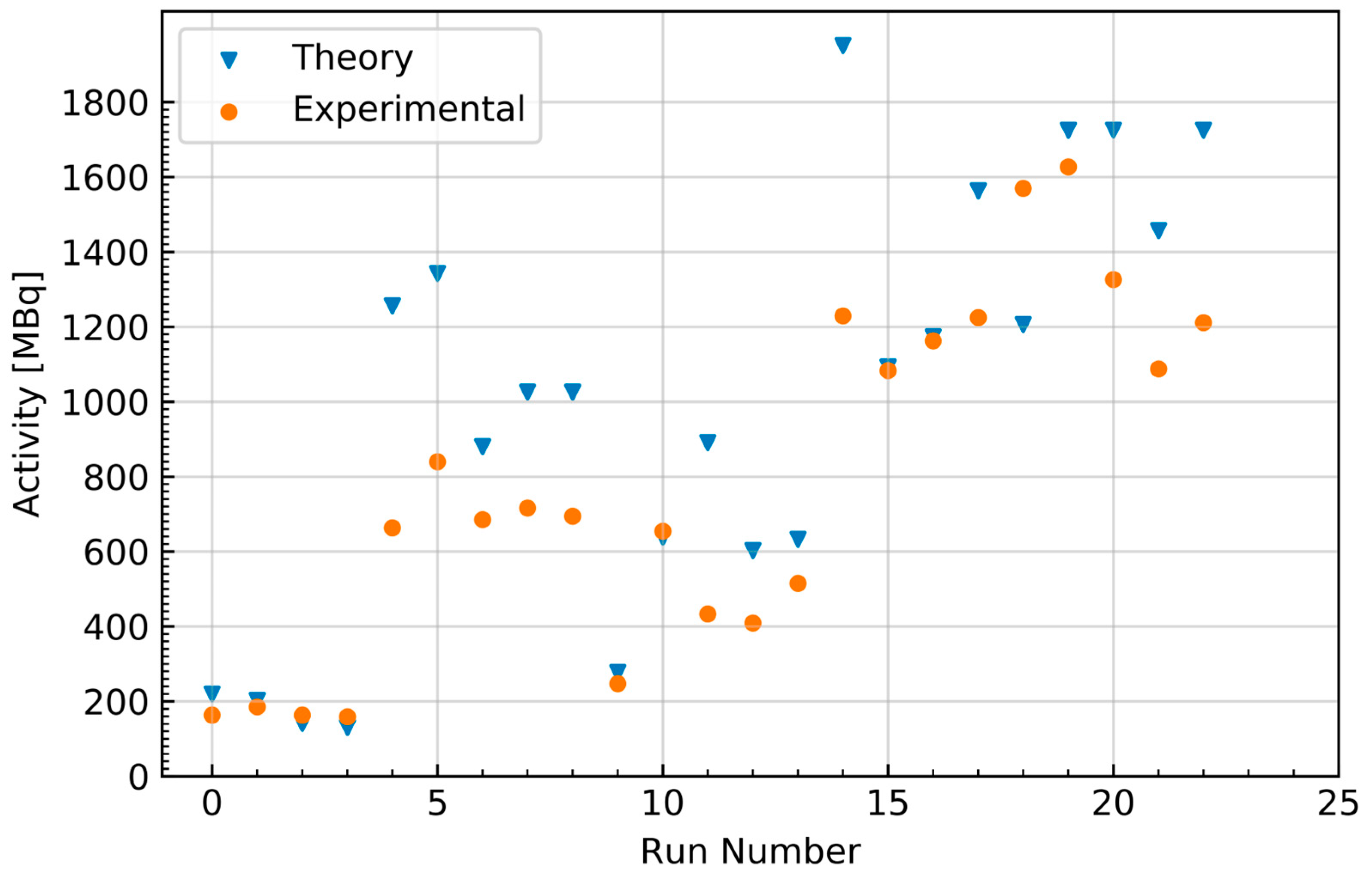
2.1.4. Comparison to Theoretical Yield Calculations
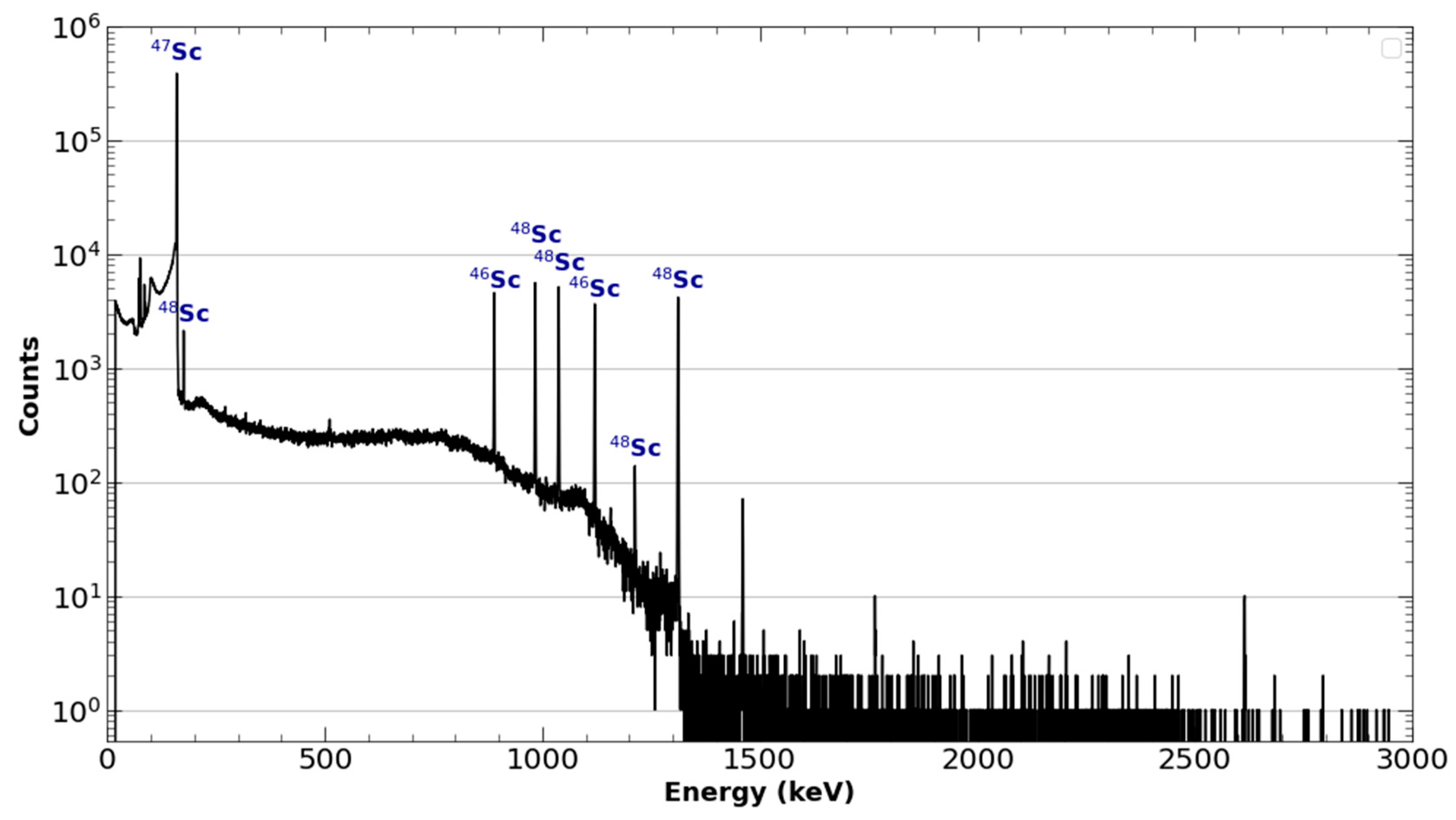
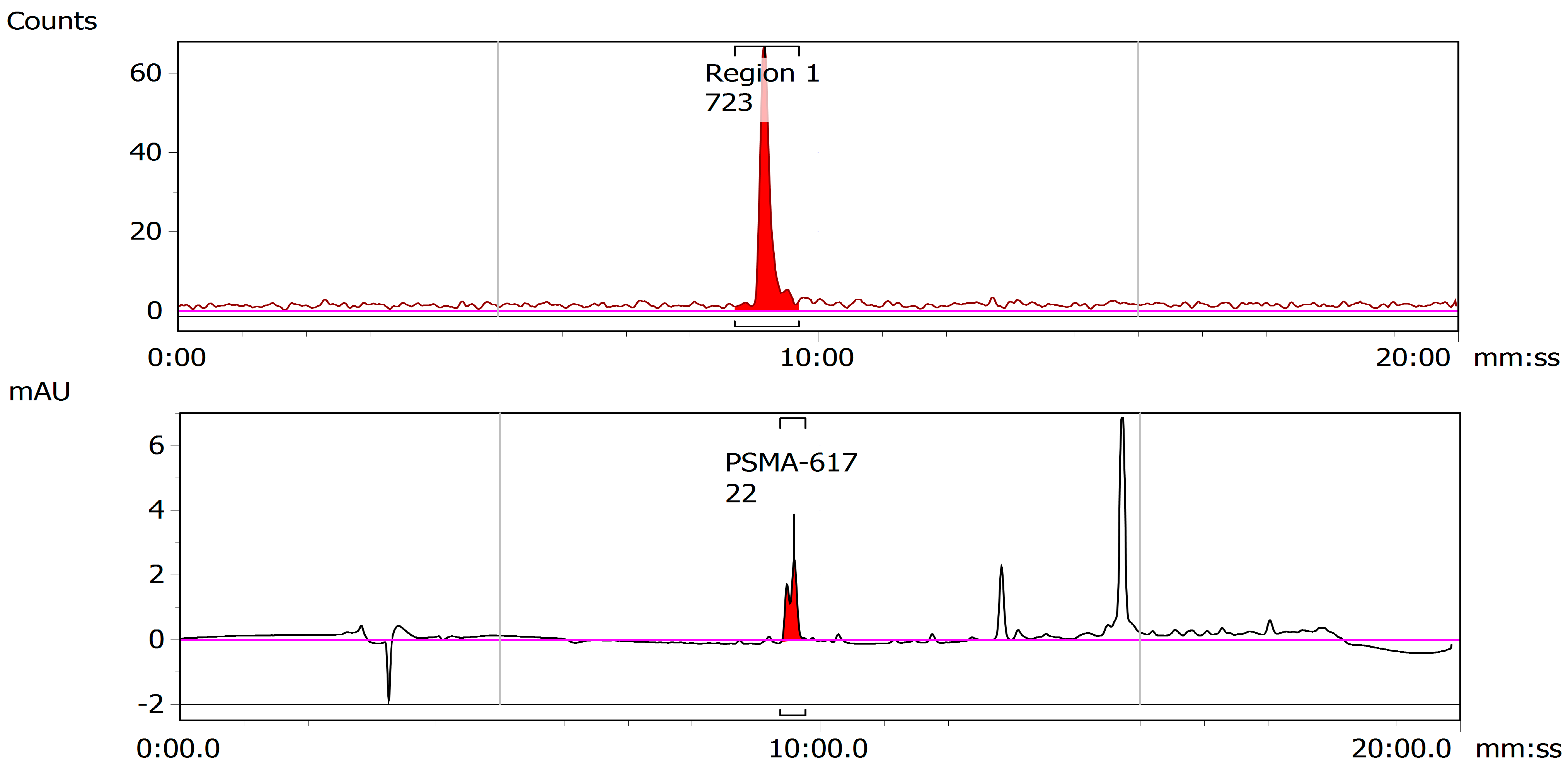
2.1.5. Separation and Purification
2.2. Linear Accelerator-Based Production of 47Sc
2.2.1. Target Preparation
2.2.2. Irradiation
2.2.3. Chemical Separation
2.3. Radiosynthesis
2.3.1. 43Sc-PSMA-617
2.3.2. 47Sc-PSMA-617
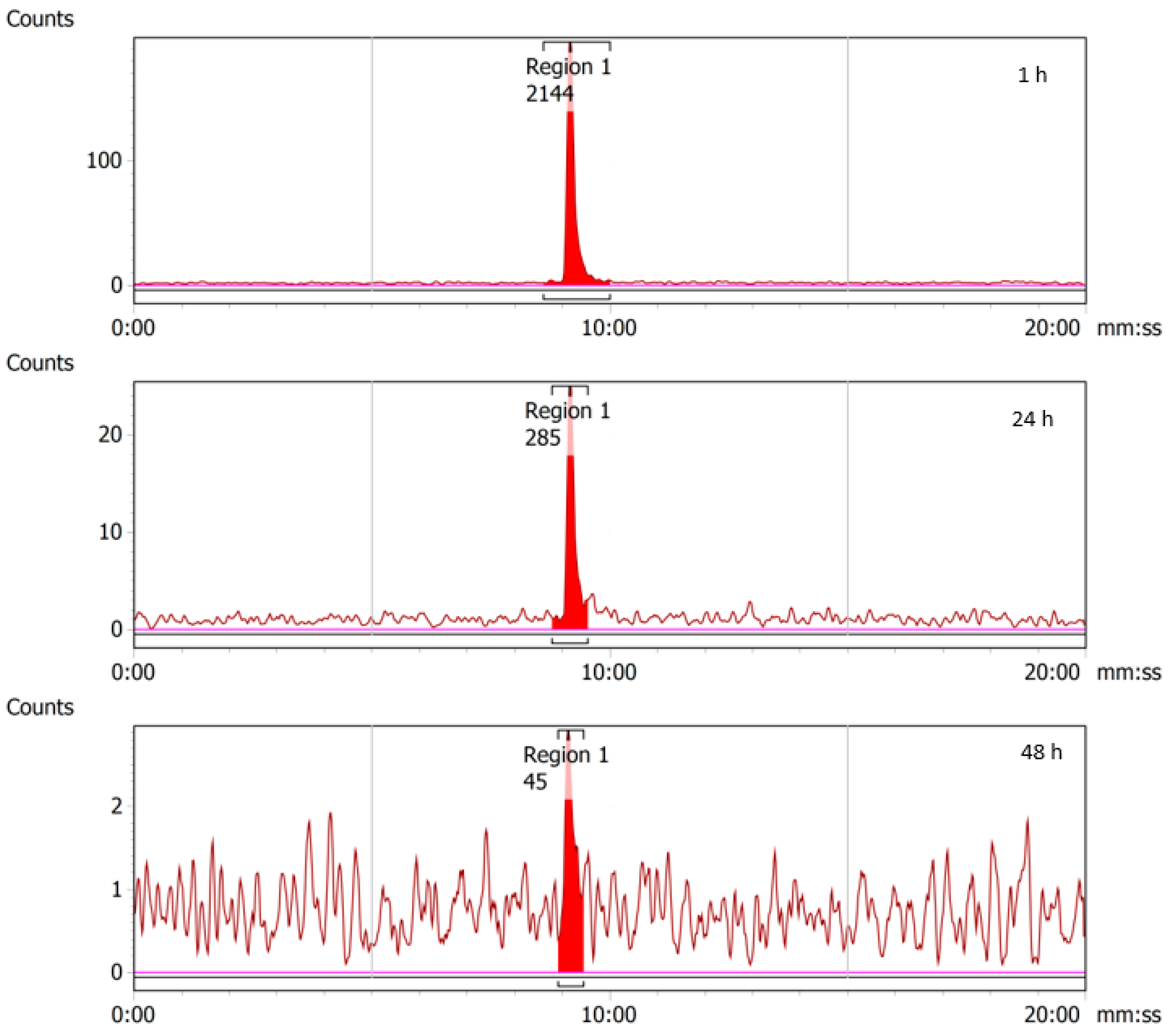
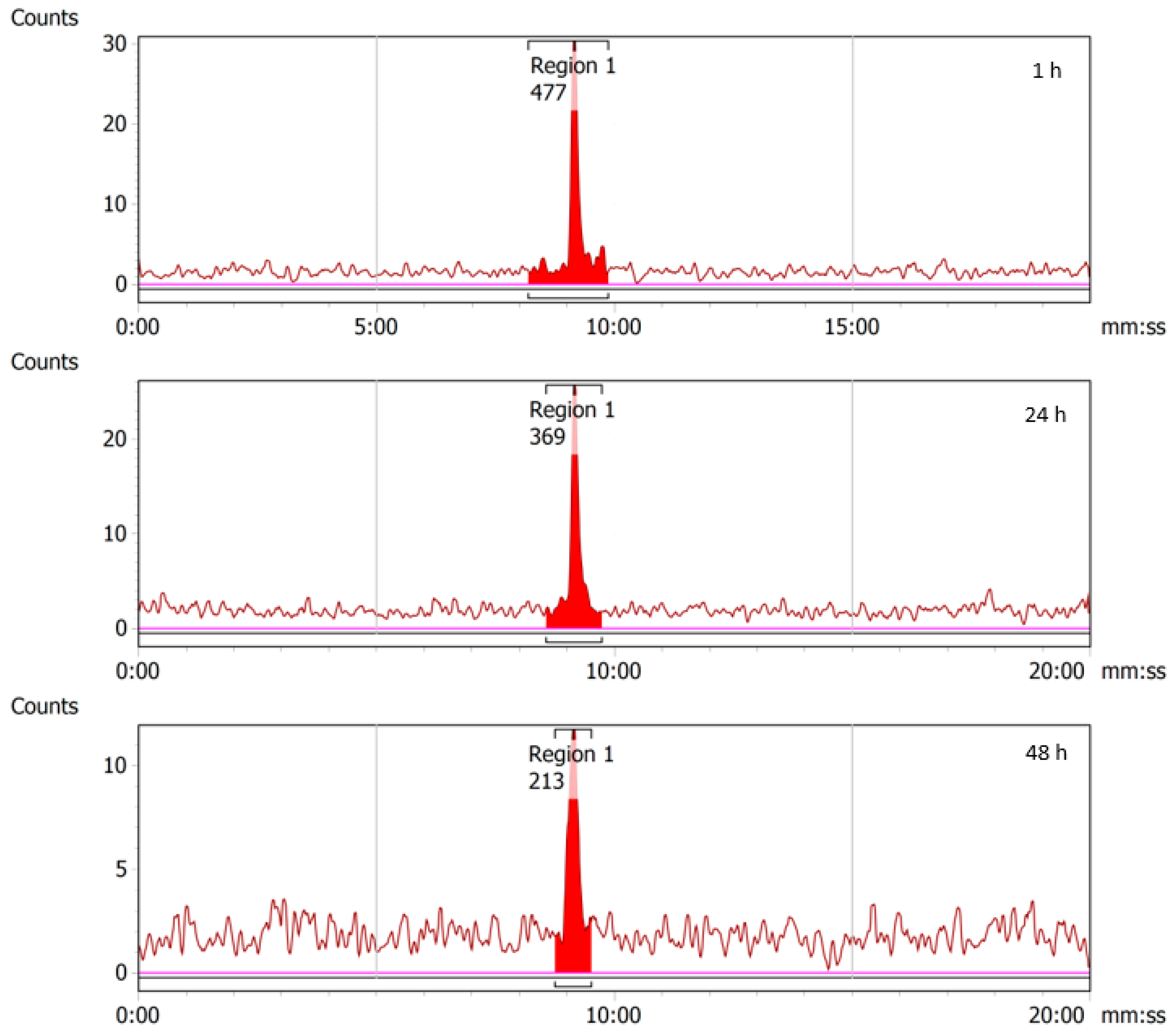
2.4. Stability of 43/47Sc-PSMA-617
2.5. In Vivo Animal Studies
2.5.1. 43Sc-PSMA-617
2.5.2. 47Sc-PSMA-617
3. Materials and Methods
3.1. Cyclotron-Based Production and Purification of 43Sc
3.1.1. Beam-Stop as A Target Holder
3.1.2. Target Materials and Preparation
3.1.3. Target Irradiation
3.2. Accelerator Production and Purification of 47Sc
3.2.1. Argonne National Laboratory LEAF
3.2.2. Irradiation
3.2.3. Ti Metal Target Processing
3.2.4. DOTA Titrations
3.3. Radiolabeling of PSMA-617 with 43Sc and 47Sc
3.4. Quality Control of 43Sc and 47Sc Labeled Radioligands
3.5. Cell Culture for Xenograft
3.6. Animal Preparation
3.7. In Vivo Imaging of Tumor-Bearing Mouse Models with 43Sc/47Sc-PSMA-617
3.7.1. Static microPET/CT Imaging with 43Sc-PSMA-617
3.7.2. Static microSPECT/CT Imaging with 47Sc-PSMA-617
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Gomes Marin, J.F.; Nunes, R.F.; Coutinho, A.M.; Zaniboni, E.C.; Costa, L.B.; Barbosa, F.G.; Queiroz, M.A.; Cerri, G.G.; Buchpiguel, C.A. Theranostics in Nuclear Medicine: Emerging and Re-Emerging Integrated Imaging and Therapies in the Era of Precision Oncology. Radiographics 2020, 40, 1715–1740. [Google Scholar] [CrossRef] [PubMed]
- Langbein, T.; Weber, W.A.; Eiber, M. Future of Theranostics: An Outlook on Precision Oncology in Nuclear Medicine. J. Nucl. Med. 2019, 60, 13S–19S. [Google Scholar] [CrossRef]
- O’Dwyer, E.; Bodei, L.; Morris, M.J. The Role of Theranostics in Prostate Cancer. Semin. Radiat. Oncol. 2021, 31, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Yordanova, A.; Eppard, E.; Kürpig, S.; Bundschuh, R.; Schönberger, S.; Gonzalez-Carmona, M.; Feldmann, G.; Ahmadzadehfar, H.; Essler, M. Theranostics in Nuclear Medicine Practice. OncoTargets Ther. 2017, 10, 4821–4828. [Google Scholar] [CrossRef]
- Mandiwana, V.; Kalombo, L.; Hayeshi, R.; Zeevaart, J.R.; Ebenhan, T. Preclinical Assessment Addressing Intravenous Administration of a [(68)Ga]Ga-PSMA-617 Microemulsion: Acute In Vivo Toxicity, Tolerability, PET Imaging, and Biodistribution. Molecules 2021, 26, 2650. [Google Scholar] [CrossRef]
- Wester, H.J.; Schottelius, M. PSMA-Targeted Radiopharmaceuticals for Imaging and Therapy. Semin. Nucl. Med. 2019, 49, 302–312. [Google Scholar] [CrossRef] [PubMed]
- Zha, Z.; Choi, S.R.; Li, L.; Zhao, R.; Ploessl, K.; Yao, X.; Alexoff, D.; Zhu, L.; Kung, H.F. New PSMA-Targeting Ligands: Transformation from Diagnosis (Ga-68) to Radionuclide Therapy (Lu-177). J. Med. Chem. 2022, 65, 13001–13012. [Google Scholar] [CrossRef]
- Wang, J.; Zang, J.; Wang, H.; Liu, Q.; Li, F.; Lin, Y.; Huo, L.; Jacobson, O.; Niu, G.; Fan, X.; et al. Pretherapeutic 68Ga-PSMA-617 PET May Indicate the Dosimetry of 177Lu-PSMA-617 and 177Lu-EB-PSMA-617 in Main Organs and Tumor Lesions. Clin. Nucl. Med. 2019, 44, 431–438. [Google Scholar] [CrossRef]
- Frost, S.H.; Frayo, S.L.; Miller, B.W.; Orozco, J.J.; Booth, G.C.; Hylarides, M.D.; Lin, Y.; Green, D.J.; Gopal, A.K.; Pagel, J.M.; et al. Comparative Efficacy of 177Lu and 90Y for Anti-CD20 Pretargeted Radioimmunotherapy in Murine Lymphoma Xenograft Models. PLoS ONE 2015, 10, e0120561. [Google Scholar] [CrossRef]
- Milot, M.C.; Benesty, O.B.; Dumulon-Perreault, V.; Ait-Mohand, S.; Richard, P.O.; Rousseau, E.; Guerin, B. (64)Cu-DOTHA(2)-PSMA, a Novel PSMA PET Radiotracer for Prostate Cancer with a Long Imaging Time Window. Pharmaceuticals 2022, 15, 996. [Google Scholar] [CrossRef]
- Muller, C.; Bunka, M.; Reber, J.; Fischer, C.; Zhernosekov, K.; Turler, A.; Schibli, R. Promises of Cyclotron-Produced 44Sc as a Diagnostic Match for Trivalent Beta--Emitters: In Vitro and in Vivo Study of a 44Sc-DOTA-Folate Conjugate. J. Nucl. Med. 2013, 54, 2168–2174. [Google Scholar] [CrossRef] [PubMed]
- Van der Meulen, N.P.; Hasler, R.; Talip, Z.; Grundler, P.V.; Favaretto, C.; Umbricht, C.A.; Muller, C.; Dellepiane, G.; Carzaniga, T.S.; Braccini, S. Developments toward the Implementation of (44)Sc Production at a Medical Cyclotron. Molecules 2020, 25, 4706. [Google Scholar] [CrossRef] [PubMed]
- Loveless, C.S.; Blanco, J.R.; Diehl, G.L.; Elbahrawi, R.T.; Carzaniga, T.S.; Braccini, S.; Lapi, S.E. Cyclotron Production and Separation of Scandium Radionuclides from Natural Titanium Metal and Titanium Dioxide Targets. J. Nucl. Med. 2021, 62, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Chernysheva, M.; Loveless, S.C.; Brossard, T.; Becker, K.; Cingoranelli, S.; Aluicio-Sarduy, E.; Song, J.; Ellison, P.; Nolen, J.; Rotsch, D.A.; et al. Accelerator Production of Scandium Radioisotopes: Sc-43, Sc-44, and Sc-47. Curr. Radiopharm. 2021, 14, 359–373. [Google Scholar] [CrossRef]
- Muller, C.; Bunka, M.; Haller, S.; Koster, U.; Groehn, V.; Bernhardt, P.; van der Meulen, N.; Turler, A.; Schibli, R. Promising Prospects for 44Sc-/47Sc-Based Theragnostics: Application of 47Sc for Radionuclide Tumor Therapy in Mice. J. Nucl. Med. 2014, 55, 1658–1664. [Google Scholar] [CrossRef]
- Eppard, E.; de la Fuente, A.; Benesova, M.; Khawar, A.; Bundschuh, R.A.; Gartner, F.C.; Kreppel, B.; Kopka, K.; Essler, M.; Rosch, F. Clinical Translation and First In-Human Use of [(44)Sc]Sc-PSMA-617 for PET Imaging of Metastasized Castrate-Resistant Prostate Cancer. Theranostics 2017, 7, 4359–4369. [Google Scholar] [CrossRef]
- Umbricht, C.A.; Benesova, M.; Schmid, R.M.; Turler, A.; Schibli, R.; van der Meulen, N.P.; Muller, C. (44)Sc-PSMA-617 for Radiotheragnostics in Tandem with (177)Lu-PSMA-617-Preclinical Investigations in Comparison with (68)Ga-PSMA-11 and (68)Ga-PSMA-617. EJNMMI Res. 2017, 7, 9. [Google Scholar] [CrossRef]
- Lima, T.V.M.; Gnesin, S.; Strobel, K.; Pérez, M.D.S.; Roos, J.E.; Müller, C.; Van Der Meulen, N.P. Fifty Shades of Scandium: Comparative Study of PET Capabilities Using Sc-43 and Sc-44 with Respect to Conventional Clinical Radionuclides. Diagnostics 2021, 11, 1826. [Google Scholar] [CrossRef]
- Mikolajczak, R.; Huclier-Markai, S.; Alliot, C.; Haddad, F.; Szikra, D.; Forgacs, V.; Garnuszek, P. Production of Scandium Radionuclides for Theranostic Applications: Towards Standardization of Quality Requirements. EJNMMI Radiopharm. Chem. 2021, 6, 19. [Google Scholar] [CrossRef]
- Huclier-Markai, S.; Alliot, C.; Kerdjoudj, R.; Mougin-Degraef, M.; Chouin, N.; Haddad, F. Promising Scandium Radionuclides for Nuclear Medicine: A Review on the Production and Chemistry up to In Vivo Proofs of Concept. Cancer Biother. Radiopharm. 2018, 33, 316–329. [Google Scholar] [CrossRef]
- Rotsch, D.A.; Brown, M.A.; Nolen, J.A.; Brossard, T.; Henning, W.F.; Chemerisov, S.D.; Gromov, R.G.; Greene, J. Electron Linear Accelerator Production and Purification of Scandium-47 from Titanium Dioxide Targets. Appl. Radiat. Isot. 2018, 131, 77–82. [Google Scholar] [CrossRef]
- Loveless, C.S.; Radford, L.L.; Ferran, S.J.; Queern, S.L.; Shepherd, M.R.; Lapi, S.E. Photonuclear Production, Chemistry, and in Vitro Evaluation of the Theranostic Radionuclide (47)Sc. EJNMMI Res. 2019, 9, 42. [Google Scholar] [CrossRef] [PubMed]
- Carzaniga, T.S.; van der Meulen, N.P.; Hasler, R.; Kottler, C.; Peier, P.; Turler, A.; Vermeulen, E.; Vockenhuber, C.; Braccini, S. Measurement of the (43)Sc Production Cross-Section with a Deuteron Beam. Appl. Radiat. Isot. 2019, 145, 205–208. [Google Scholar] [CrossRef]
- Domnanich, K.A.; Eichler, R.; Muller, C.; Jordi, S.; Yakusheva, V.; Braccini, S.; Behe, M.; Schibli, R.; Turler, A.; van der Meulen, N.P. Production and Separation of (43)Sc for Radiopharmaceutical Purposes. EJNMMI Radiopharm. Chem. 2017, 2, 14. [Google Scholar] [CrossRef] [PubMed]
- Otuka, N.; Takács, S. Definitions of Radioisotope Thick Target Yields. Radiochim. Acta 2015, 103, 1–6. [Google Scholar] [CrossRef]
- Koning, A.J.; Rochman, D.; Sublet, J.C.; Dzysiuk, N.; Fleming, M.; van der Marck, S. TENDL: Complete Nuclear Data Library for Innovative Nuclear Science and Technology. Nucl. Data Sheets 2019, 155, 1–55. [Google Scholar] [CrossRef]
- Valdovinos, H.F.; Hernandez, R.; Barnhart, T.E.; Graves, S.; Cai, W.; Nickles, R.J. Separation of Cyclotron-Produced (44)Sc from a Natural Calcium Target Using a Dipentyl Pentylphosphonate Functionalized Extraction Resin. Appl. Radiat. Isot. 2015, 95, 23–29. [Google Scholar] [CrossRef]
- Meyer, C.; Prasad, V.; Stuparu, A.; Kletting, P.; Glatting, G.; Miksch, J.; Solbach, C.; Lueckerath, K.; Nyiranshuti, L.; Zhu, S.; et al. Comparison of PSMA-TO-1 and PSMA-617 Labeled with Gallium-68, Lutetium-177 and Actinium-225. EJNMMI Res. 2022, 12, 65. [Google Scholar] [CrossRef]
- Benesova, M.; Schafer, M.; Bauder-Wust, U.; Afshar-Oromieh, A.; Kratochwil, C.; Mier, W.; Haberkorn, U.; Kopka, K.; Eder, M. Preclinical Evaluation of a Tailor-Made DOTA-Conjugated PSMA Inhibitor with Optimized Linker Moiety for Imaging and Endoradiotherapy of Prostate Cancer. J. Nucl. Med. 2015, 56, 914–920. [Google Scholar] [CrossRef]
- Kratochwil, C.; Giesel, F.L.; Leotta, K.; Eder, M.; Hoppe-Tich, T.; Youssoufian, H.; Kopka, K.; Babich, J.W.; Haberkorn, U. PMPA for Nephroprotection in PSMA-Targeted Radionuclide Therapy of Prostate Cancer. J. Nucl. Med. 2015, 56, 293–298. [Google Scholar] [CrossRef]
- Kratochwil, C.; Schmidt, K.; Afshar-Oromieh, A.; Bruchertseifer, F.; Rathke, H.; Morgenstern, A.; Haberkorn, U.; Giesel, F.L. Targeted Alpha Therapy of MCRPC: Dosimetry Estimate of (213)Bismuth-PSMA-617. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Fendler, W.P.; Stuparu, A.D.; Evans-Axelsson, S.; Luckerath, K.; Wei, L.; Kim, W.; Poddar, S.; Said, J.; Radu, C.G.; Eiber, M.; et al. Establishing (177)Lu-PSMA-617 Radioligand Therapy in a Syngeneic Model of Murine Prostate Cancer. J. Nucl. Med. 2017, 58, 1786–1792. [Google Scholar] [CrossRef] [PubMed]
- Kregel, S.; Chen, J.L.; Tom, W.; Krishnan, V.; Kach, J.; Brechka, H.; Fessenden, T.B.; Isikbay, M.; Paner, G.P.; Szmulewitz, R.Z.; et al. Acquired Resistance to the Second-Generation Androgen Receptor Antagonist Enzalutamide in Castration-Resistant Prostate Cancer. Oncotarget 2016, 7, 26259–26274. [Google Scholar] [CrossRef] [PubMed]






| Calcium Isotope | Abundance (ORNL) | Abundance (Isoflex USA) | Reaction | Product Half-Life | IAEA ENDF Cross-Section at 9 MeV (×10−27 cm−1) |
|---|---|---|---|---|---|
| 40Ca | 5.01% | 3.03% | 40Ca(d,p)41Ca 40Ca(d,n)41Sc 40Ca(d,α)38K | 9.94 × 104 y 596.3 ms 924.4 ms | 369.00 41.68 30.70 |
| 42Ca | 94.37% | 96.30(±0.30)% | 42Ca(d,p)43Ca 42Ca(d,n)43Sc 42Ca(d,α)40K | Stable 3.89 h 1.25 × 109 y | 266.74 104.73 60.08 |
| 43Ca | 0.06% | 0.10% | 43Ca(d,p)44Ca 43Ca(d,n)44Sc 43Ca(d,2n)43Sc 43Ca(d,α)41K | Stable 3.97 h 3.89 h Stable | 105.31 128.42 228.04 65.85 |
| 44Ca | 0.55% | 0.55% | 44Ca(d,p)45Ca 44Ca(d,n)45Sc 44Ca(d,2n)44Sc 44Ca(d,α)42K | 162.61 d Stable 3.97 h 12.36 h | 84.75 262.34 264.24 24.45 |
| 46Ca | 0.001% | <0.01% | 46Ca(d,p)47Ca 46Ca(d,n)47Sc 46Ca(d,2n)46Sc 46Ca(d,α)44K | 4.54 d 3.35 d 83.79 d 22.13 m | 215.50 155.62 620.39 7.11 |
| 48Ca | 0.01% | 0.02% | 48Ca(d,p)49Ca 48Ca(d,n)49Sc 48Ca(d,2n)48Sc 48Ca(d,α)46K | 8.72 m 57.18 m 43.67 h 105 s | 40.09 93.64 1116.59 2.12 |
| Target Mass (mg) | Beam Current (μA) | Irradiation Time (h) | EOB Yield (MBq) |
|---|---|---|---|
| 16.4 | 1 | 8 | 163.54 |
| 9.3 | 2 | 10 | 185.37 |
| 5.8 | 3 | 10 | 163.54 |
| 4.6 | 3 | 7.5 | 159.10 |
| 18.2 | 5 | 7.5 | 663.04 |
| 21.9 | 4 | 8.0 | 839.90 |
| 16.4 | 4 | 8.0 | 685.24 |
| 19.3 | 4 | 8.0 | 715.95 |
| 19.0 | 4 | 8.0 | 694.12 |
| 7.0 | 4 | 8.0 | 247.53 |
| 13.3 | 4 | 10.0 | 654.53 |
| 16.5 | 4 | 9.0 | 433.27 |
| 13.4 | 4 | 8.0 | 408.85 |
| 12.3 | 4.2 | 8.0 | 515.04 |
| 22.4 | 6 | 7.72 | 1229.14 |
| 14.5 | 6 | 8 | 1083.36 |
| 13.4 | 7.75 | 8.12 | 1162.91 |
| 17.0 | 7.25 | 8 | 1224.70 |
| 13.4 | 8 | 8 | 1569.54 |
| 17.0 | 8 | 8 | 1627.26 |
| 17.2 | 8 | 8 | 1326.45 |
| 14.5 | 8 | 8 | 1087.80 |
| 17.6 | 8 | 8 | 1211.88 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meier, J.P.; Zhang, H.J.; Freifelder, R.; Bhuiyan, M.; Selman, P.; Mendez, M.; Kankanamalage, P.H.A.; Brossard, T.; Pusateri, A.; Tsai, H.-M.; et al. Accelerator-Based Production of Scandium Radioisotopes for Applications in Prostate Cancer: Toward Building a Pipeline for Rapid Development of Novel Theranostics. Molecules 2023, 28, 6041. https://doi.org/10.3390/molecules28166041
Meier JP, Zhang HJ, Freifelder R, Bhuiyan M, Selman P, Mendez M, Kankanamalage PHA, Brossard T, Pusateri A, Tsai H-M, et al. Accelerator-Based Production of Scandium Radioisotopes for Applications in Prostate Cancer: Toward Building a Pipeline for Rapid Development of Novel Theranostics. Molecules. 2023; 28(16):6041. https://doi.org/10.3390/molecules28166041
Chicago/Turabian StyleMeier, Jason P., Hannah J. Zhang, Richard Freifelder, Mohammed Bhuiyan, Phillip Selman, Megan Mendez, Pavithra H. A. Kankanamalage, Thomas Brossard, Antonino Pusateri, Hsiu-Ming Tsai, and et al. 2023. "Accelerator-Based Production of Scandium Radioisotopes for Applications in Prostate Cancer: Toward Building a Pipeline for Rapid Development of Novel Theranostics" Molecules 28, no. 16: 6041. https://doi.org/10.3390/molecules28166041
APA StyleMeier, J. P., Zhang, H. J., Freifelder, R., Bhuiyan, M., Selman, P., Mendez, M., Kankanamalage, P. H. A., Brossard, T., Pusateri, A., Tsai, H.-M., Leoni, L., Penano, S., Ghosh, K., Broder, B. A., Markiewicz, E., Renne, A., Stadler, W., Weichselbaum, R., Nolen, J., ... Chen, C.-T. (2023). Accelerator-Based Production of Scandium Radioisotopes for Applications in Prostate Cancer: Toward Building a Pipeline for Rapid Development of Novel Theranostics. Molecules, 28(16), 6041. https://doi.org/10.3390/molecules28166041






