Analysis of Lutein Content in Microencapsulated Marigold Flower Extract (Tagetes erecta L.) Using UHPLC-Q-Orbitrap-HRMS and Its Cytotoxicity in ARPE-19 Cells
Abstract
1. Introduction
2. Results and Discussion
2.1. Extraction Yield
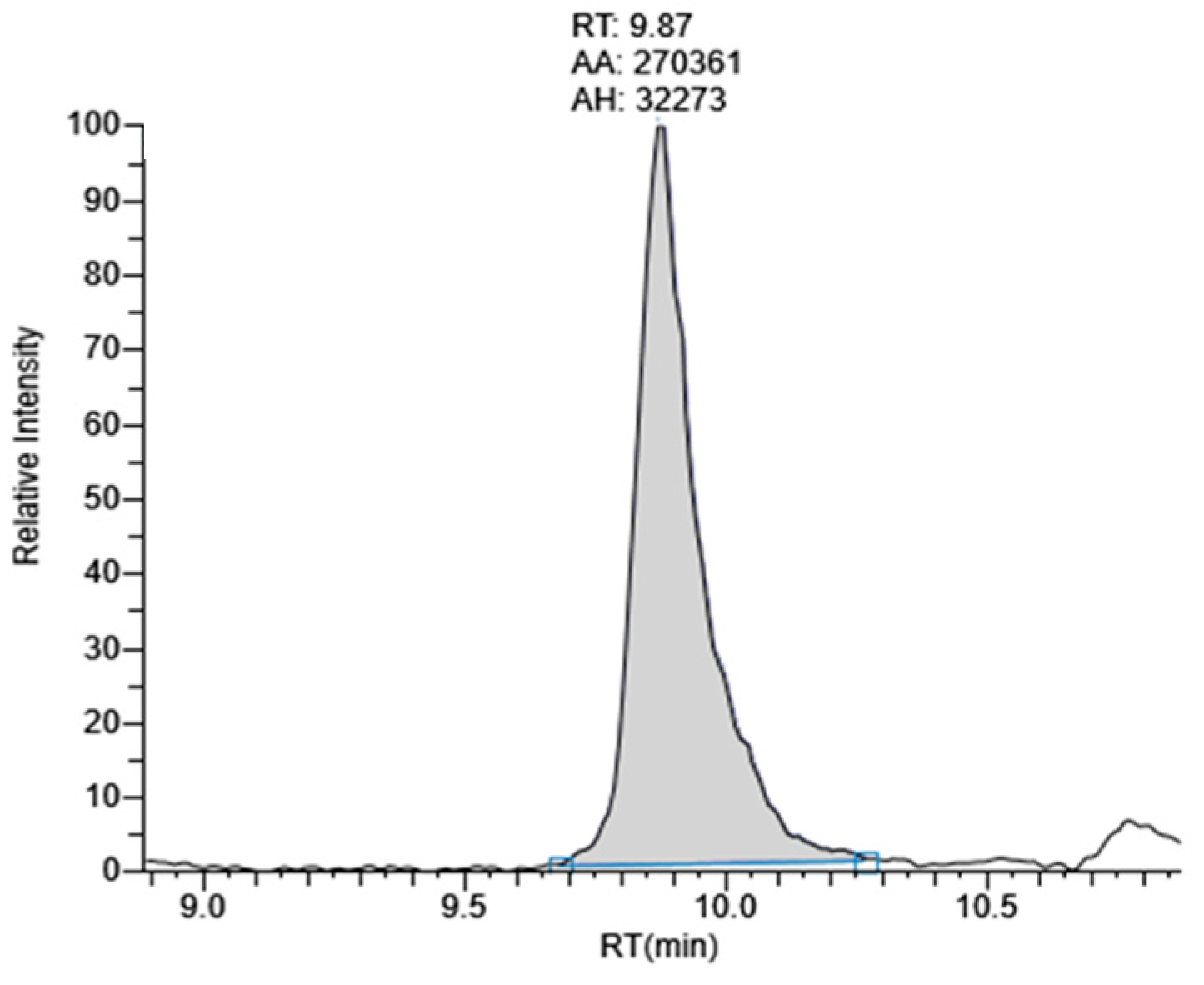
2.2. Lutein Content in Marigold Flower Extract
2.2.1. Method Validation
- Specificity
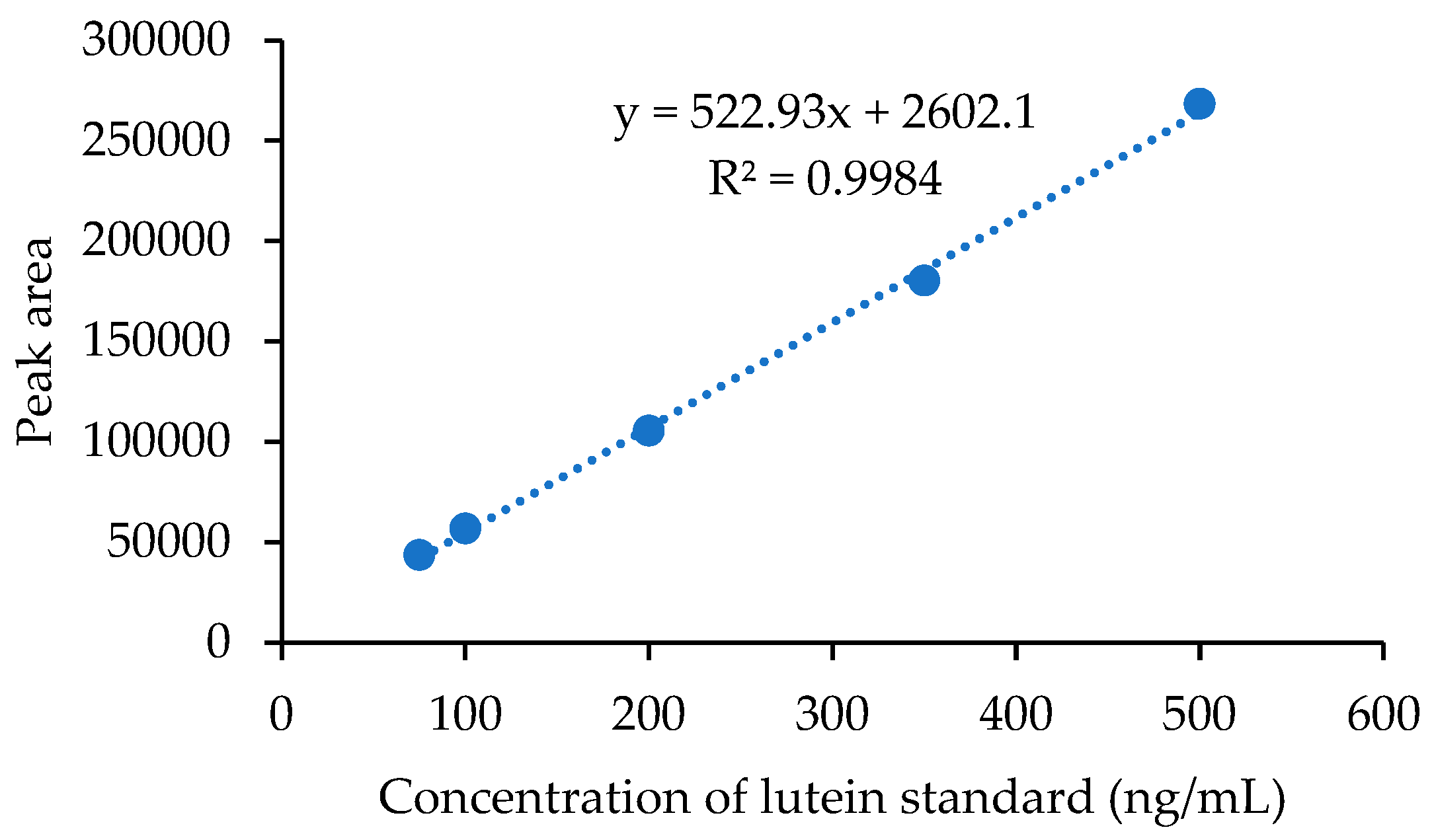
- Linearity
- Sensitivity
- Accuracy
- Precision
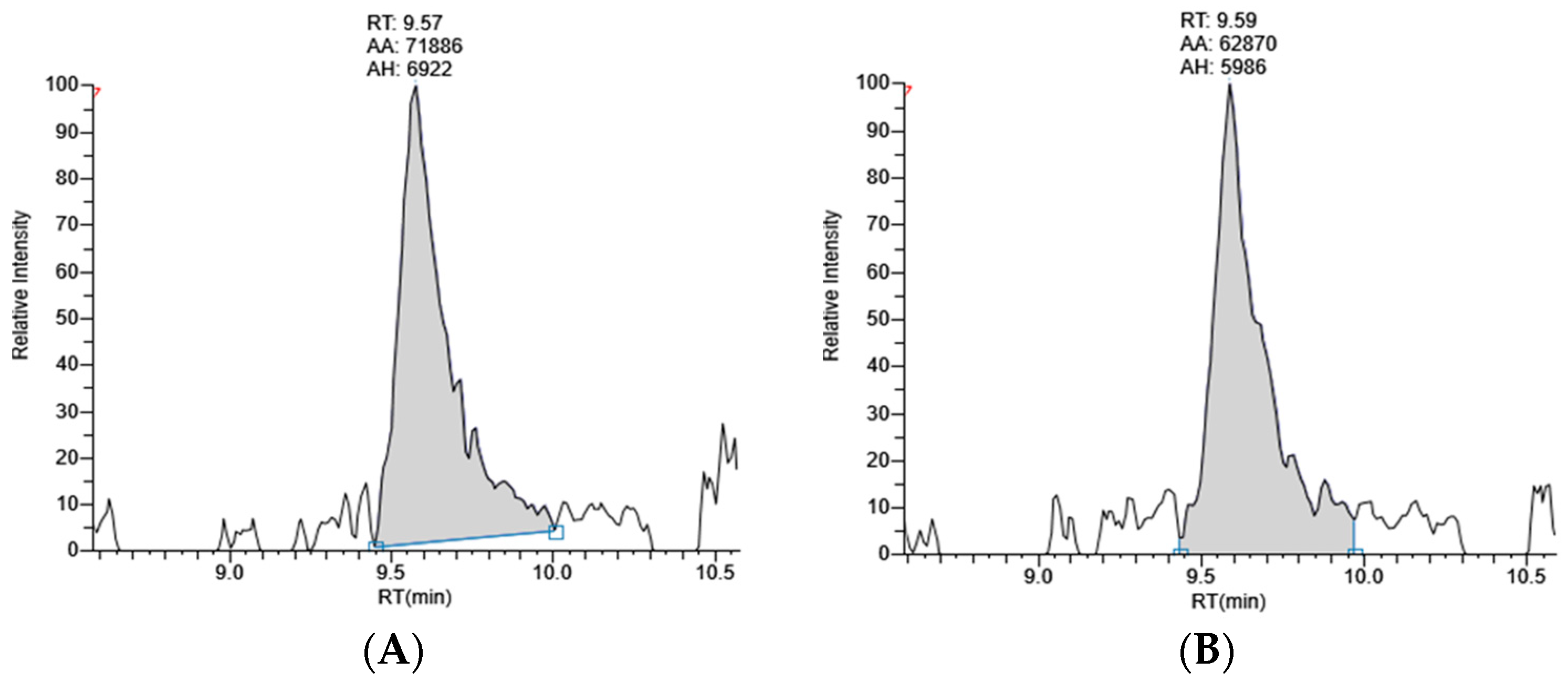
2.2.2. Quantification of Lutein in Marigold Extract
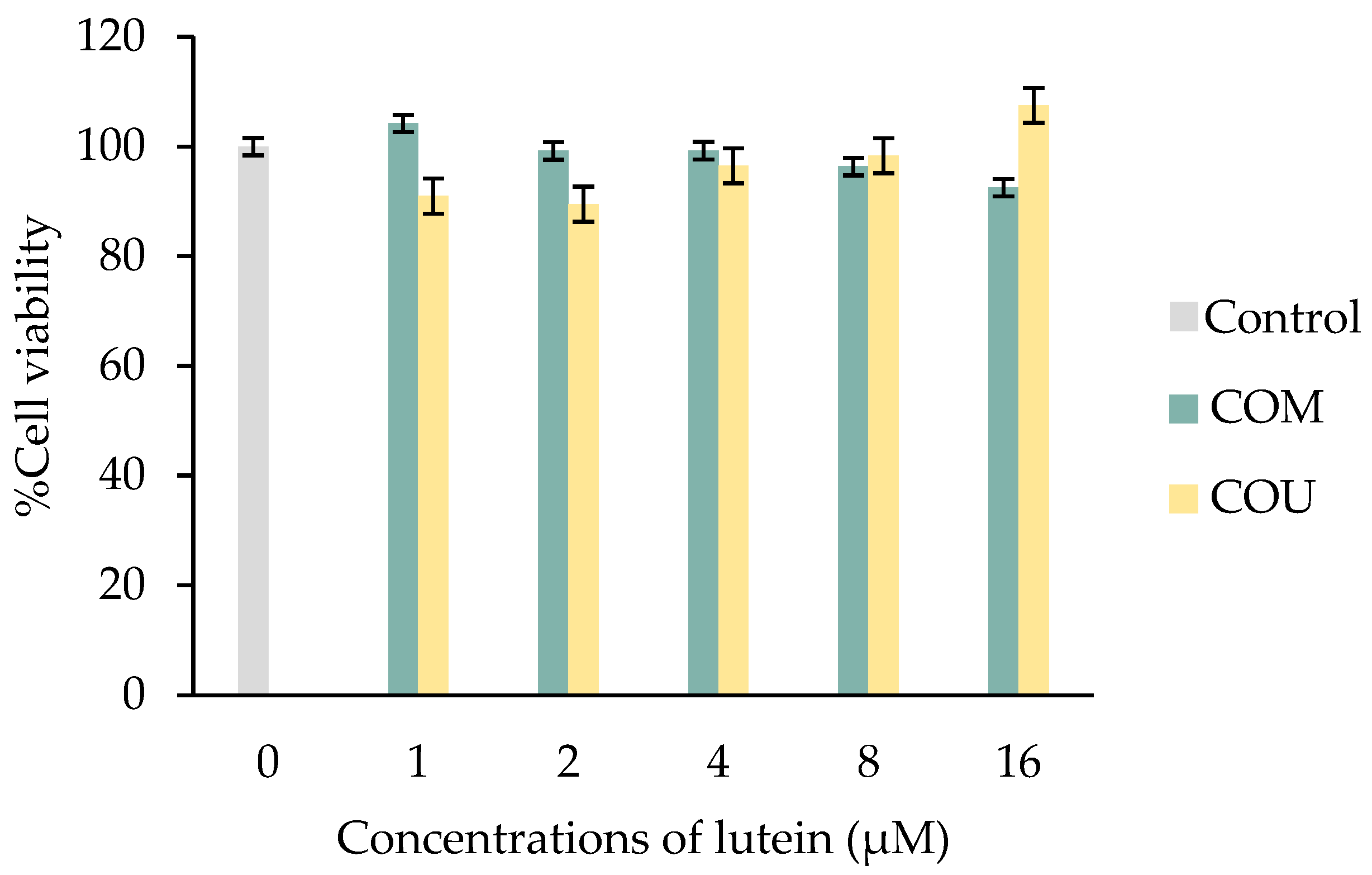
2.3. Cytotoxicity of Lutein-Rich Fraction from Marigold Extracts in ARPE-19 Cell Line
2.4. Microencapsulation of Marigold Extract
3. Materials and Methods
3.1. Plant Materials
3.2. Chemicals and Reagents
3.3. Determination of Suitable Extraction Method
3.3.1. Ultrasonic Extraction
3.3.2. Microwave-Assisted Extraction
3.4. Sample Preparation
3.5. Determination of Lutein Content in Marigold Flower Extract
3.6. Cytotoxicity Study of Marigold Extract in ARPE-19 Cell Line
3.6.1. Cell Culture
3.6.2. Cell Viability Assay
3.6.3. Cytotoxicity Assay
3.7. Development of Microencapsulated Marigold Flower Extract
3.7.1. Emulsion Characterization
3.7.2. Powder Characterization
- Moisture Content
- Microencapsulation Yield
- Microencapsulation Efficiency
- o
- Surface Oil
- o
- Total Oil
- Scanning Electron Microscopy (SEM)
3.7.3. Quantification of Lutein in Microencapsulated Marigold Flower Extract
3.8. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Fu, X.Q.; Ma, N.; Sun, W.P.; Dang, Y.Y. Microwave and enzyme co-assisted aqueous two-phase extraction of polyphenol and lutein from marigold (Tagetes erecta L.) flower. Ind. Crops Prod. 2018, 123, 296–302. [Google Scholar] [CrossRef]
- Indrawati, R.; Kurniawan, J.M.; Wibowo, A.A.; Juliana; Gunawan, I.A.; Heriyanto; Brotosudarmo, T.H. Integrated solvent-free extraction and encapsulation of lutein from marigold petals and its application. CyTA-J. Food 2019, 17, 121–127. [Google Scholar] [CrossRef]
- Hasin, B.M.; Ferdaus, A.J.; Islam, M.A.; Uddin, M.J.; Islam, M.S. Marigold and orange skin as egg yolk color promoting agents. Int. J. Poult. Sci. 2006, 5, 979–987. [Google Scholar] [CrossRef]
- Koushan, K.; Rusovici, R.; Li, W.; Ferguson, L.R.; Chalam, K.V. The role of lutein in eye-related disease. Nutrients 2013, 5, 1823–1839. [Google Scholar] [CrossRef] [PubMed]
- The Age-Related Eye Disease Study 2 (AREDS2) Research Group. Secondary analyses of the effects of lutein/zeaxanthin on age-related macular degeneration progression: AREDS2 Report No. 3. JAMA Ophthalmol. 2014, 132, 142–149. [Google Scholar] [CrossRef]
- Buscemi, S.; Corleo, D.; Di Pace, F.; Petroni, M.L.; Satriano, A.; Marchesini, G. The effect of lutein on eye and extra-eye health. Nutrients 2018, 10, 1321. [Google Scholar] [CrossRef] [PubMed]
- National Statistical Office. Available online: http://www.nso.go.th/sites/2014en/Survey/ICT/Survey%20In%20Household/2022/full_report_q1_2022.pdf (accessed on 8 August 2022).
- Zhao, Z.C.; Zhou, Y.; Tan, G.; Li, J. Research progress about the effect and prevention of blue light on eyes. Int. J. Ophthalmol. 2018, 11, 1999–2003. [Google Scholar] [CrossRef]
- Johnson, E.J. Role of lutein and zeaxanthin in visual and cognitive function throughout the lifespan. Nutr. Rev. 2014, 72, 605–612. [Google Scholar] [CrossRef]
- Kurniawan, R.; Nurkolis, F.; Taslim, N.A.; Subali, D.; Surya, R.; Gunawan, W.B.; Alisaputra, D.; Mayulu, N.; Salindeho, N.; Kim, B. Carotenoids composition of green algae Caulerpa racemosa and their antidiabetic, anti-obesity, antioxidant, and anti-inflammatory properties. Molecules 2023, 28, 3267. [Google Scholar] [CrossRef] [PubMed]
- Kaimainen, M.; Jarvenpaa, E.; Huopalahti, R. Enzyme-assisted oil extraction of lutein from marigold (Tagetes erecta) flowers and stability of lutein during storage. Int. J. Agric. Res. 2015, 4, 11–19. [Google Scholar] [CrossRef]
- Onsaard, E.; Onsaard, W. Microencapsulated vegetable oil powder. In Microencapsulation—Processes, Technologies, and Industrial Applications; Salaün, F., Ed.; IntechOpen: London, UK, 2019; pp. 1–19. [Google Scholar] [CrossRef]
- Bhatnagar, A.S.; Prasanth Kumar, P.K.; Hemavathy, J.; Gopala Krishna, A.G. Fatty acid composition, oxidative stability, and radical scavenging activity of vegetable oil blends with coconut oil. J. Am. Oil Chem. Soc. 2009, 86, 991–999. [Google Scholar] [CrossRef]
- Sivakanthan, S.; Bopitiya, D.; Madhujith, T. A comparative study on stability of different types of coconut (Cocos nucifera) oil against autoxidation and photo-oxidation. Afr. J. Food Sci. 2018, 12, 216–229. [Google Scholar] [CrossRef][Green Version]
- Validation of Analytical Procedures: Text and Methodology Q2(R1); International Conference on Harmonisation: Geneva, Switzerland, 2005.
- Yeong, Y.; Pang, S.F.; Chong, S.Y.; Gimbun, J. Comparison of microwave and ultrasonic assisted extraction of kaempferol from Cassia alata. Int. J. Eng. Technol. 2018, 7, 84–89. [Google Scholar] [CrossRef]
- San, S.M.; Jaturanpinyo, M.; Limwikrant, W. Effects of wall material on medium-chain triglyceride (MCT) oil microcapsules prepared by spray drying. Pharmaceutics 2022, 14, 1281. [Google Scholar] [CrossRef] [PubMed]
- Tonon, R.V.; Grosso, C.R.F.; Hubinger, M.D. Influence of emulsion composition and inlet air temperature on the microencapsulation of flaxseed oil by spray drying. Food Res. Int. 2011, 44, 282–289. [Google Scholar] [CrossRef]
- Goula, A.M.; Adamopoulos, K.G. A method for pomegranate seed application in food industries: Seed oil encapsulation. Food Bioprod. Process. 2012, 90, 639–652. [Google Scholar] [CrossRef]
- Pal, S.; Bhattacharjee, P. Spray dried powder of lutein-rich supercritical carbon dioxide extract of gamma-irradiated marigold flowers: Process optimization, characterization and food application. Powder Technol. 2018, 327, 512–523. [Google Scholar] [CrossRef]
- Kurniawan, J.M.; Yusuf, M.M.; Azmi, S.S.; Salim, K.P.; Utami Prihastyanti, M.N.; Indrawati, R.; Heriyanto; Shioi, Y.; Limantara, L.; Panintingjati Brotosudarmo, T.H. Effect of drying treatments on the contents of lutein and zeaxanthin in orange- and yellow-cultivars of marigold flower and its application for lutein ester encapsulation. IOP Conf. Ser. Mater. Sci. Eng. 2019, 509, 12060. [Google Scholar] [CrossRef]
- Papoutsis, K.; Golding, J.; Vuong, Q.; Pristijono, P.; Stathopoulos, C.; Scarlett, C.; Bowyer, M. Encapsulation of citrus by-product extracts by spray-drying and freeze-drying using combinations of maltodextrin with soybean protein and ι-carrageenan. Foods 2018, 7, 115. [Google Scholar] [CrossRef]
- Bakry, A.M.; Abbas, S.; Ali, B.; Majeed, H.; Abouelwafa, M.Y.; Mousa, A.; Liang, L. Microencapsulation of oils: A comprehensive review of benefits, techniques, and applications. Compr. Rev. Food Sci. Food Saf. 2016, 15, 143–182. [Google Scholar] [CrossRef]
- Tiwary, B.; Kumar, A.; Nanda, A.; Chakraborty, R. A study on optimization of marigold petal yield, pure lutein and formulation of free flowing lutein esters. J. Crop. Sci. Biotechnol. 2014, 17, 175–181. [Google Scholar] [CrossRef]
- Paduano, A.; Caporaso, N.; Santini, A.; Sacchi, R. Microwave and ultrasound-assisted extraction of capsaicinoids from chili peppers (Capsicum annuum L.) in flavored olive oil. J. Food Res. 2014, 3, 51–59. [Google Scholar] [CrossRef]
- Nguyet, N.T.M.; Ha, V.T.T.; Binh, N.T.; Dang, N.M.; Bay, N.T. Quantification of lutein from marigold flower (Tagetes erecta L.) petals by liquid chromatography-tandem mass spectrometry method. Vietnam J. Chem. 2019, 57, 240–244. [Google Scholar] [CrossRef]
- Liu, H.; Liu, W.; Zhou, X.; Long, C.; Kuang, X.; Hu, J.; Tang, Y.; Liu, L.; He, J.; Huang, Z.; et al. Protective effect of lutein on ARPE-19 cells upon H2O2-induced G2/M arrest. Mol. Med. Rep. 2017, 16, 2069–2074. [Google Scholar] [CrossRef]
- Chunhui, Z.H.; Bifei, L.A.; Jiangping, H.O.; Lingyun, C.H. Cytotoxicity of dimethyl sulphoxide on ocular cells in vitro. Chin. J. Exp. Ophthalmol. 2015, 33, 216–220. [Google Scholar] [CrossRef]
- Hee, Y.Y.; Tan, C.P.; Rahman, R.A.; Noranizan, M.; Smith, R.L.; Chong, G.H. Production of virgin coconut oil microcapsules from oil-in-water emulsion with supercritical carbon dioxide spray drying. J. Supercrit. Fluids 2017, 130, 118–124. [Google Scholar] [CrossRef]
- Ogrodowska, D.; Tanska, M.; Brandt, W. The influence of drying process conditions on the physical properties, bioactive compounds and stability of encapsulated pumpkin seed oil. Food Bioproc. Technol. 2017, 10, 1265–1280. [Google Scholar] [CrossRef]
- Kuang, P.; Zhang, H.; Bajaj, P.R.; Yuan, Q.; Tang, J.; Chen, S.; Sablani, S.S. Physicochemical properties and storage stability of lutein microcapsules prepared with maltodextrins and sucrose by spray drying. J. Food Sci. 2015, 80, 359–369. [Google Scholar] [CrossRef] [PubMed]

| Compound | Standard Addition (%) | Recovery Rate (%) |
|---|---|---|
| Lutein | 50 | 90.53 ± 2.00 |
| 100 | 101.96 ± 169 | |
| 150 | 125.20 ± 2.18 |
| Conc. Of Lutein (ng/mL) | RSD (%) | |
|---|---|---|
| Intra-Day | Inter-Day | |
| 75 | 1.78 | 3.15 |
| 200 | 0.52 | 1.64 |
| 500 | 6.46 | 4.62 |
| Sample | Compound | Amount (ng Lutein/µg Marigold Powder) | %RSD | Rt (min) | %RSD |
|---|---|---|---|---|---|
| COM | Lutein | 27.22 ± 1.17 a | 4.31 | 9.58 ± 0.01 | 0.12 |
| COU | Lutein | 23.11 ± 0.49 b | 2.11 | 9.60 ± 0.01 | 0.10 |
| Formula | Oil-to-Wall | Moisture Content (%) | Yield (%) | ME (%) | Lutein Content (µg Lutein/g Powder) |
|---|---|---|---|---|---|
| F1 | 1:2 | 3.83 ± 0.02 b | 62.01 ± 4.35 a | 35.33 ± 3.21 d | 6.11 ± 0.11 a |
| F2 | 1:3 | 4.67 ± 0.10 a | 67.52 ± 5.75 a | 52.16 ± 4.39 c | 5.98 ± 0.11 a |
| F3 | 1:4 | 4.86 ± 0.09 a | 65.71 ± 5.75 a | 72.29 ± 0.96 b | 4.69 ± 0.25 b |
| F4 | 1:5 | 4.88 ± 0.23 a | 70.20 ± 6.22 a | 82.60 ± 1.49 a | 6.18 ± 0.17 a |
| Formula | Oil-to-Wall | Ingredients (%w/w) | |||
|---|---|---|---|---|---|
| Marigold Flower Extract | Maltodextrin | Gum Acacia | Deionized Water | ||
| F1 | 1:2 | 10.00 | 6.00 | 14.00 | 70 |
| F2 | 1:3 | 7.50 | 6.75 | 15.75 | 70 |
| F3 | 1:4 | 6.00 | 7.20 | 16.80 | 70 |
| F4 | 1:5 | 5.00 | 7.50 | 17.50 | 70 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suwanklang, P.; Thilavech, T.; Limwikrant, W.; Kitphati, W.; Supharattanasitthi, W.; Lomarat, P. Analysis of Lutein Content in Microencapsulated Marigold Flower Extract (Tagetes erecta L.) Using UHPLC-Q-Orbitrap-HRMS and Its Cytotoxicity in ARPE-19 Cells. Molecules 2023, 28, 6025. https://doi.org/10.3390/molecules28166025
Suwanklang P, Thilavech T, Limwikrant W, Kitphati W, Supharattanasitthi W, Lomarat P. Analysis of Lutein Content in Microencapsulated Marigold Flower Extract (Tagetes erecta L.) Using UHPLC-Q-Orbitrap-HRMS and Its Cytotoxicity in ARPE-19 Cells. Molecules. 2023; 28(16):6025. https://doi.org/10.3390/molecules28166025
Chicago/Turabian StyleSuwanklang, Pornson, Thavaree Thilavech, Waree Limwikrant, Worawan Kitphati, Wasu Supharattanasitthi, and Pattamapan Lomarat. 2023. "Analysis of Lutein Content in Microencapsulated Marigold Flower Extract (Tagetes erecta L.) Using UHPLC-Q-Orbitrap-HRMS and Its Cytotoxicity in ARPE-19 Cells" Molecules 28, no. 16: 6025. https://doi.org/10.3390/molecules28166025
APA StyleSuwanklang, P., Thilavech, T., Limwikrant, W., Kitphati, W., Supharattanasitthi, W., & Lomarat, P. (2023). Analysis of Lutein Content in Microencapsulated Marigold Flower Extract (Tagetes erecta L.) Using UHPLC-Q-Orbitrap-HRMS and Its Cytotoxicity in ARPE-19 Cells. Molecules, 28(16), 6025. https://doi.org/10.3390/molecules28166025







