Phytochemical Analysis, Antioxidant and Enzyme-Inhibitory Activities, and Multivariate Analysis of Insect Gall Extracts of Picea koraiensis Nakai
Abstract
1. Introduction
2. Results and Discussion
2.1. Qualitative Phytochemical Analysis
2.2. Yields
2.3. Quantitative Phytochemical Analysis
2.3.1. Total Carbohydrate Content
2.3.2. Total Protein Content (TProC)
2.3.3. Total Triterpenoid Content
2.3.4. Total Phenolic Content
2.3.5. Total Flavonoid Content
2.3.6. Total Phenolic Acid Content
2.3.7. Total Tannin Content, Gallotannin Content, and Condensed Tannin Content
2.4. Antioxidant Capacity
2.4.1. DPPH and ABTS
2.4.2. Hydroxyl Radicals, Superoxide Radicals, and Singlet Oxygen
2.4.3. FRAP and CUPRAC
2.4.4. Metal Chelating
2.4.5. H2O2
2.4.6. β-Carotene Bleaching
2.4.7. Hypochlorous Acid
2.4.8. Nitric Oxide
2.5. Enzyme-Inhibitory Activities
2.5.1. α-Amylase and α-Glucosidase
2.5.2. AChE and BChE
2.5.3. Tyrosinase
2.5.4. Xanthine Oxidase
2.5.5. Urease
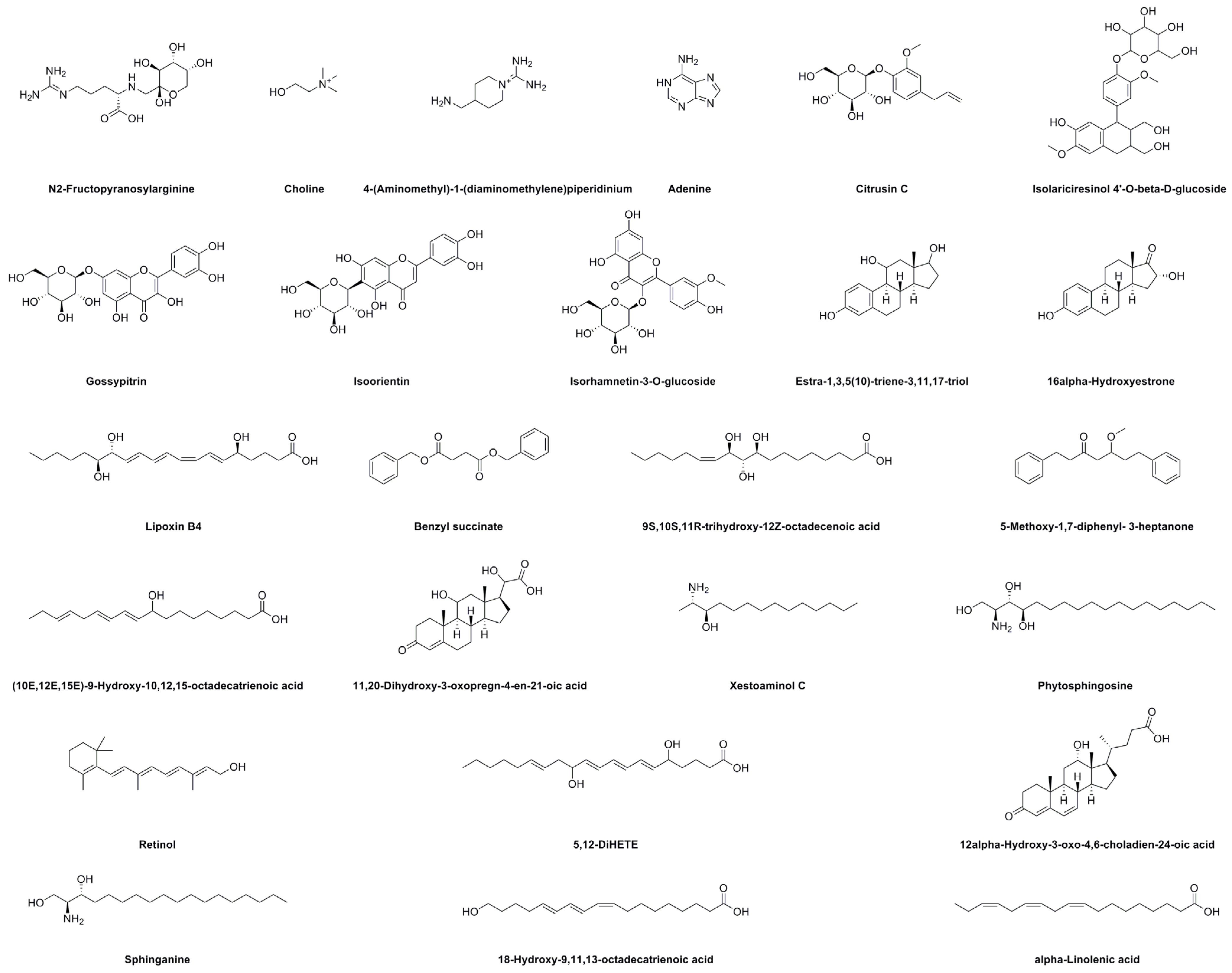
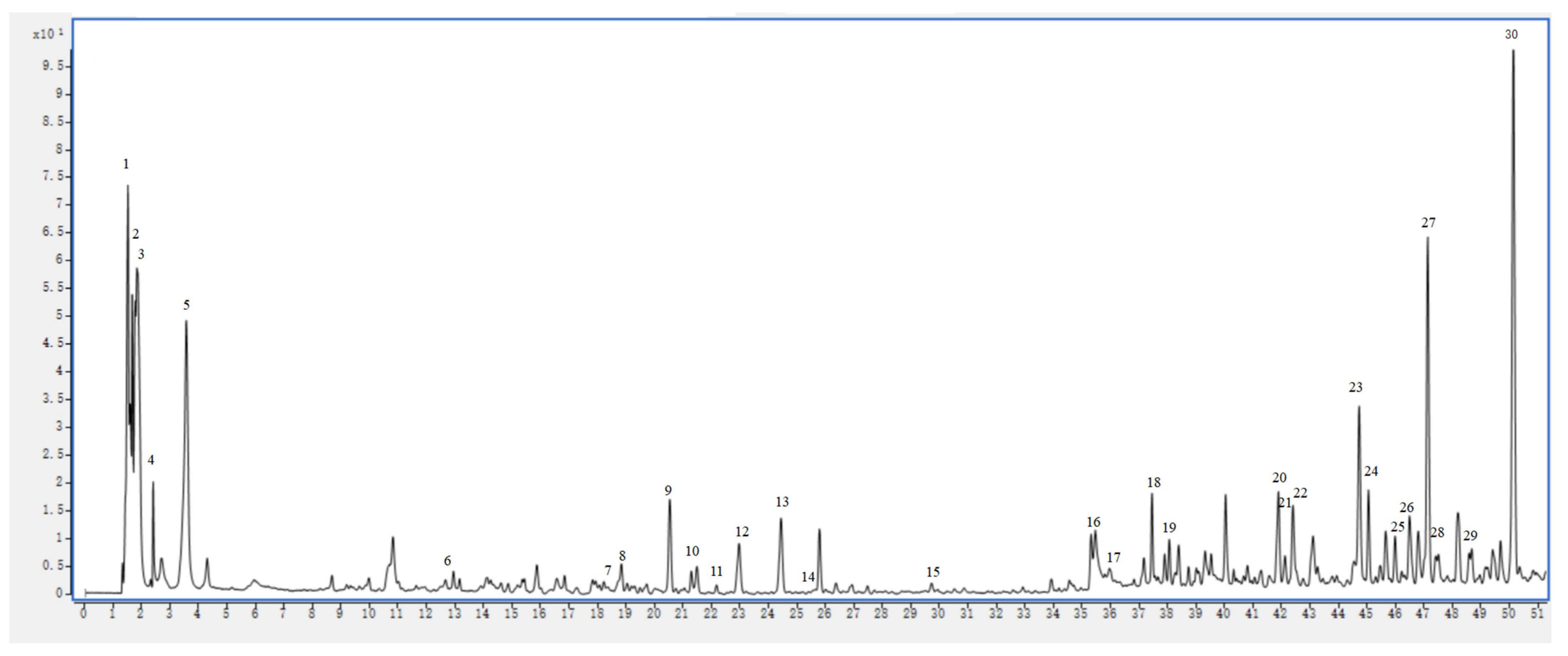
2.6. UHPLC–MS Analysis
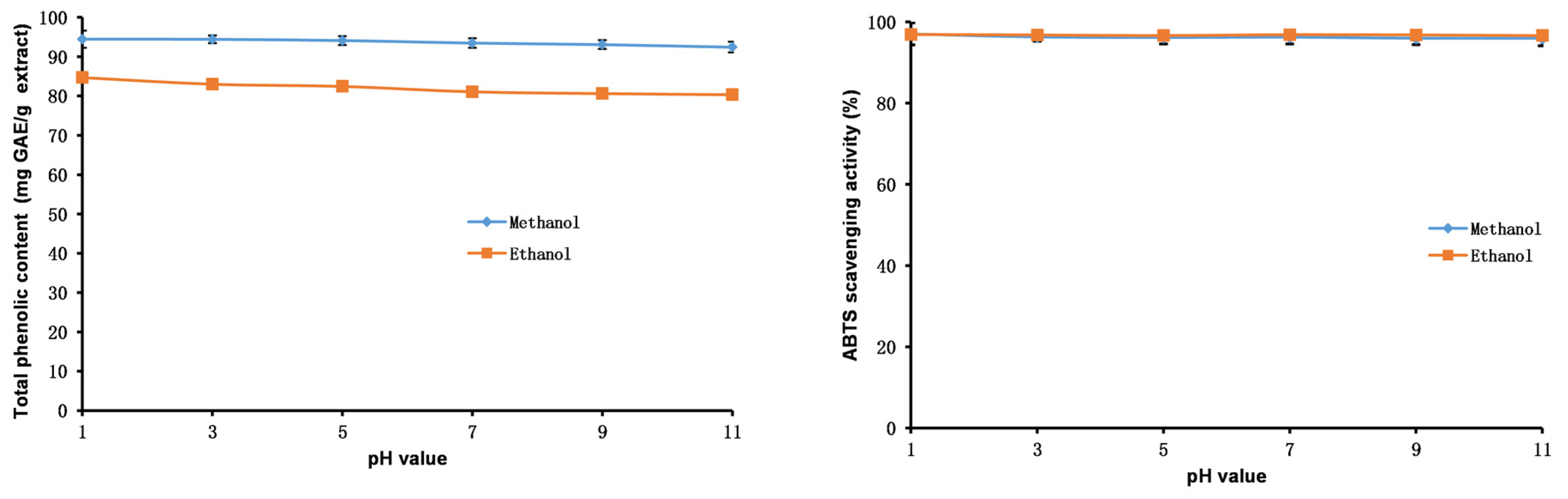
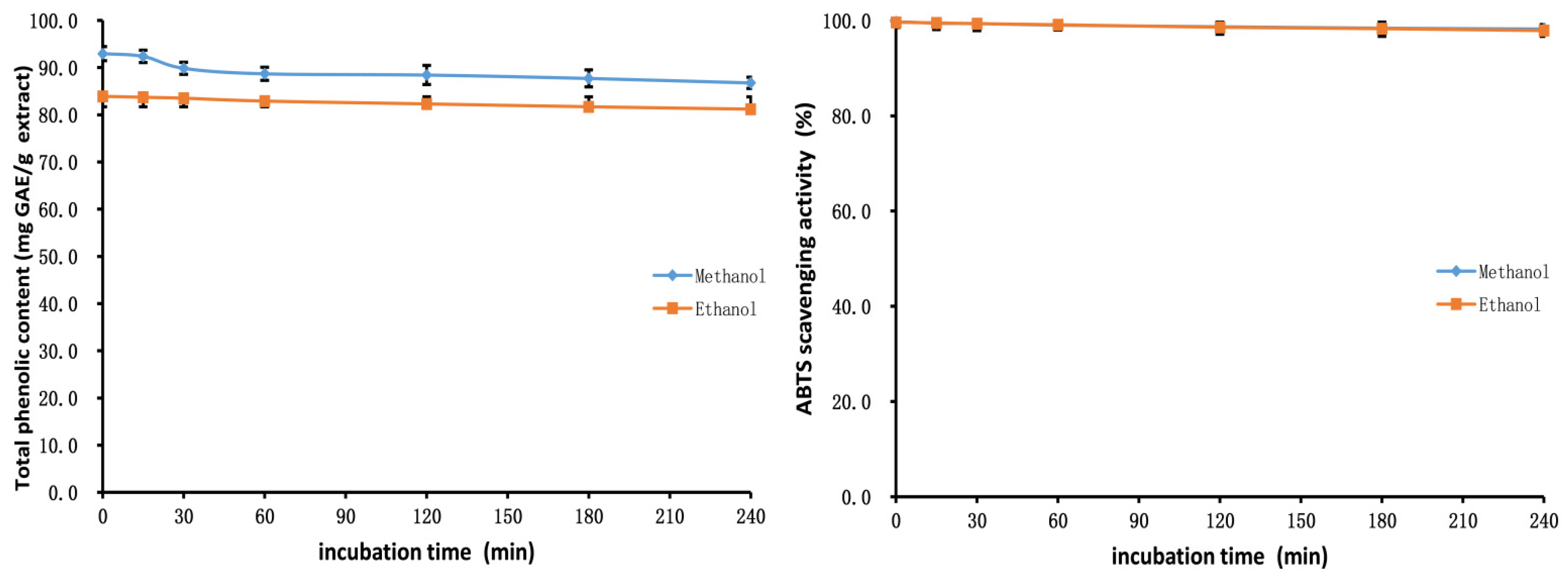
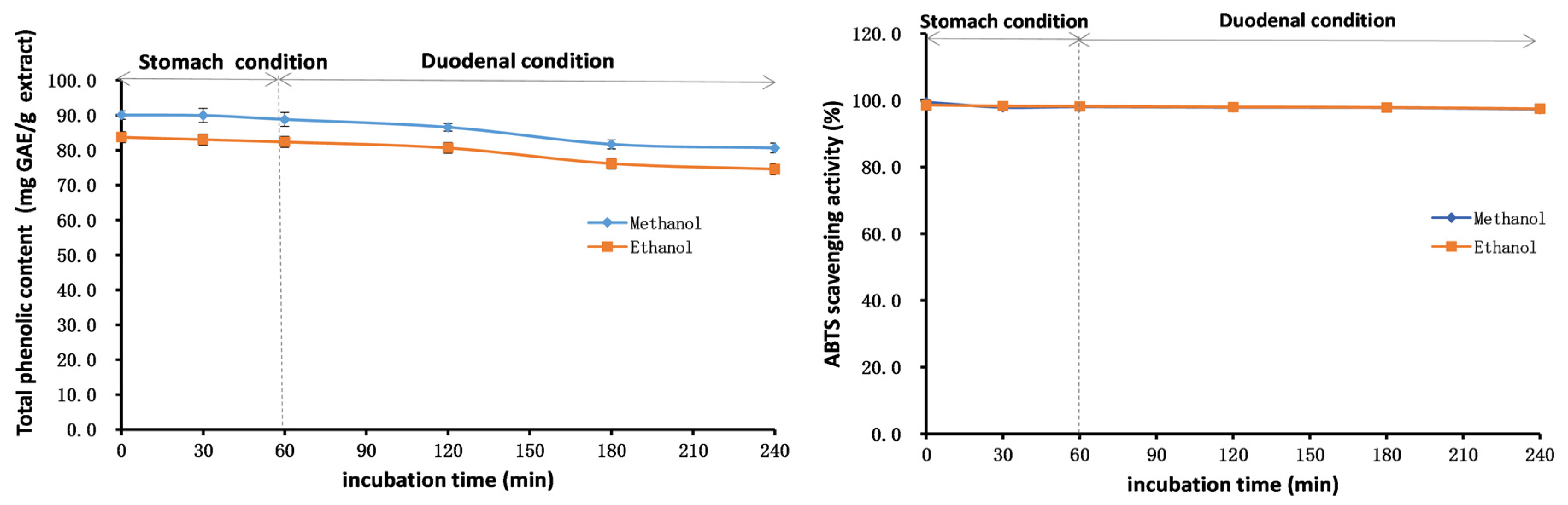
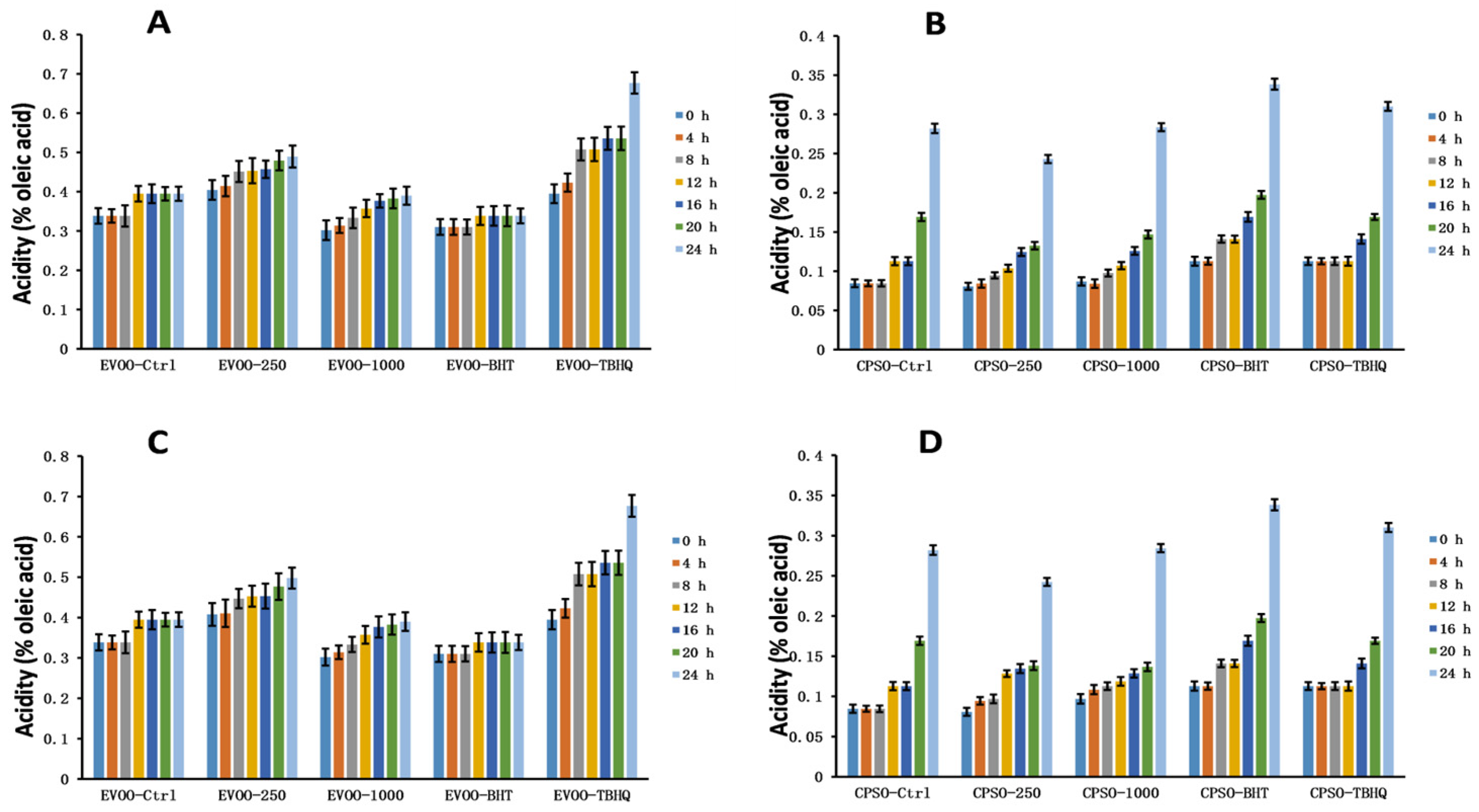
2.7. Stability Studies of Methanol and Ethanol Extracts
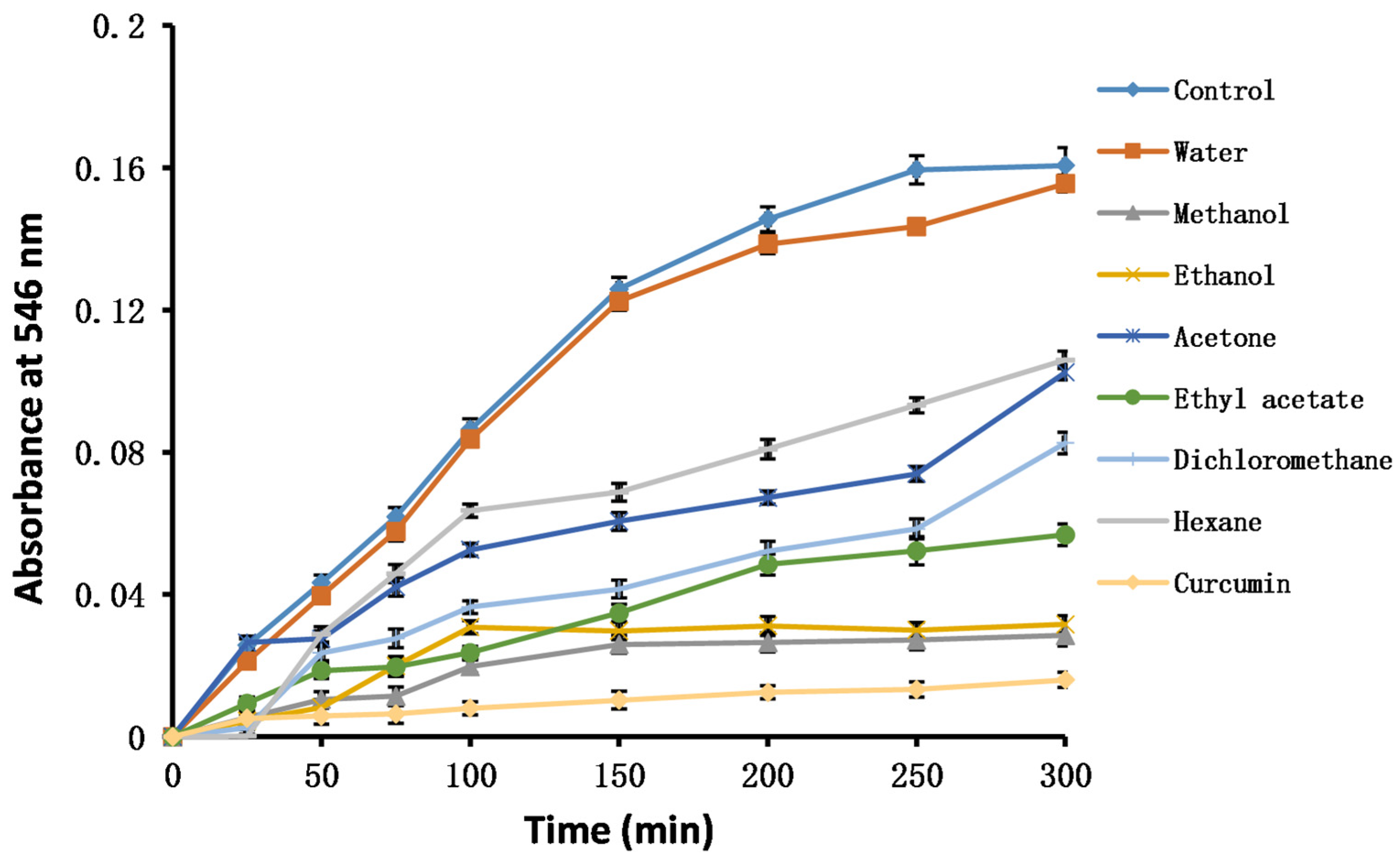
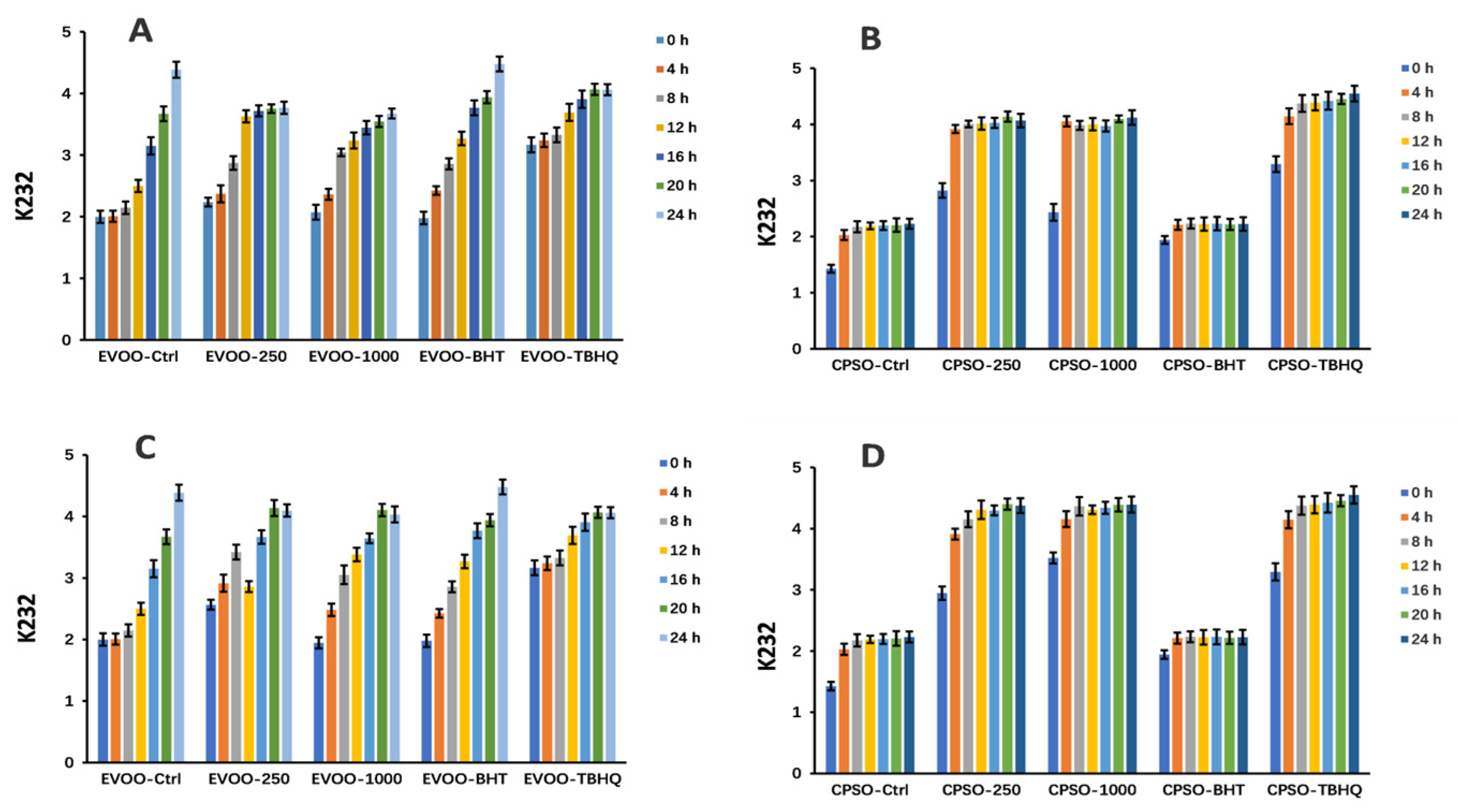
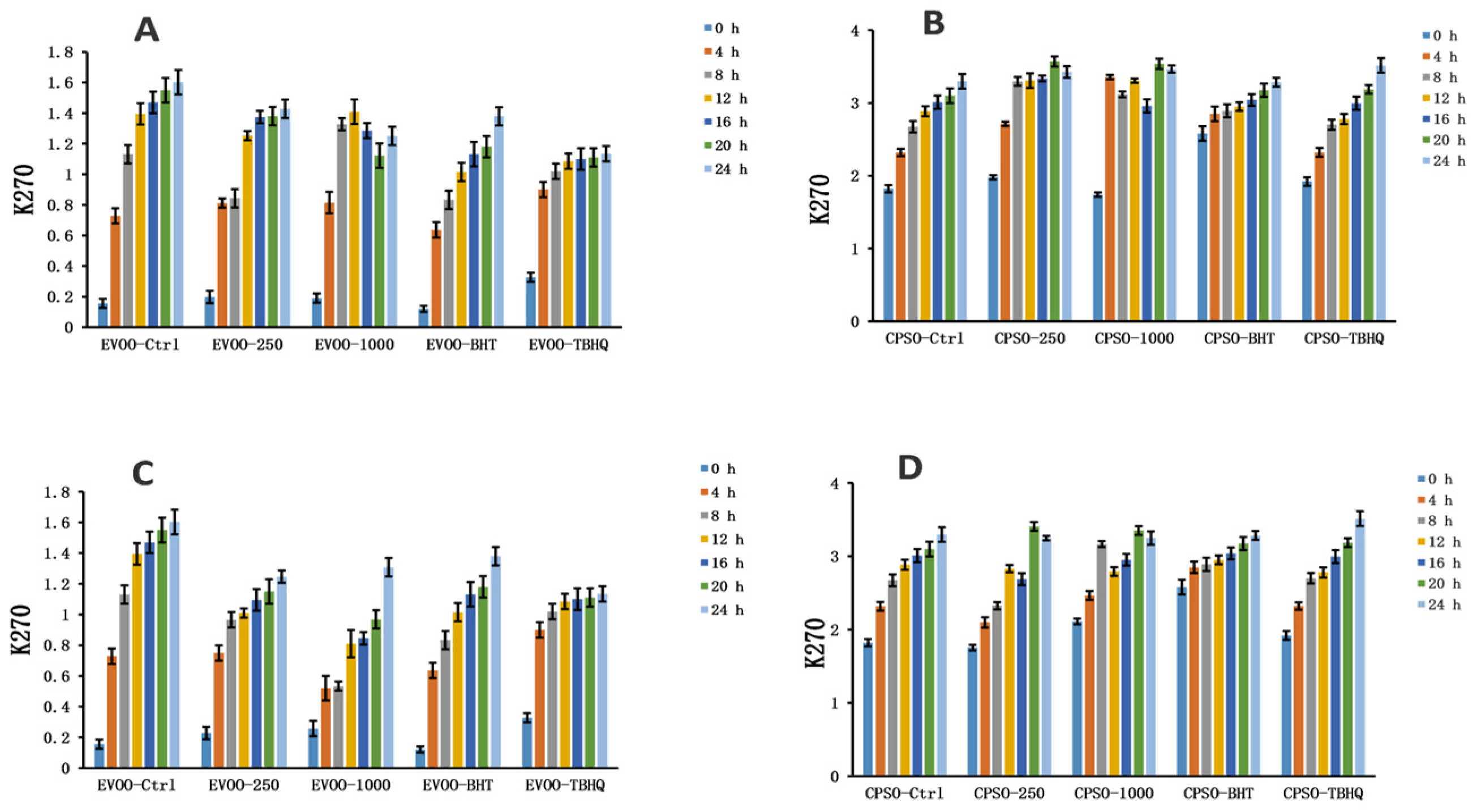
2.8. Oxidative Stability Studies of Oils
2.9. Cell Viability
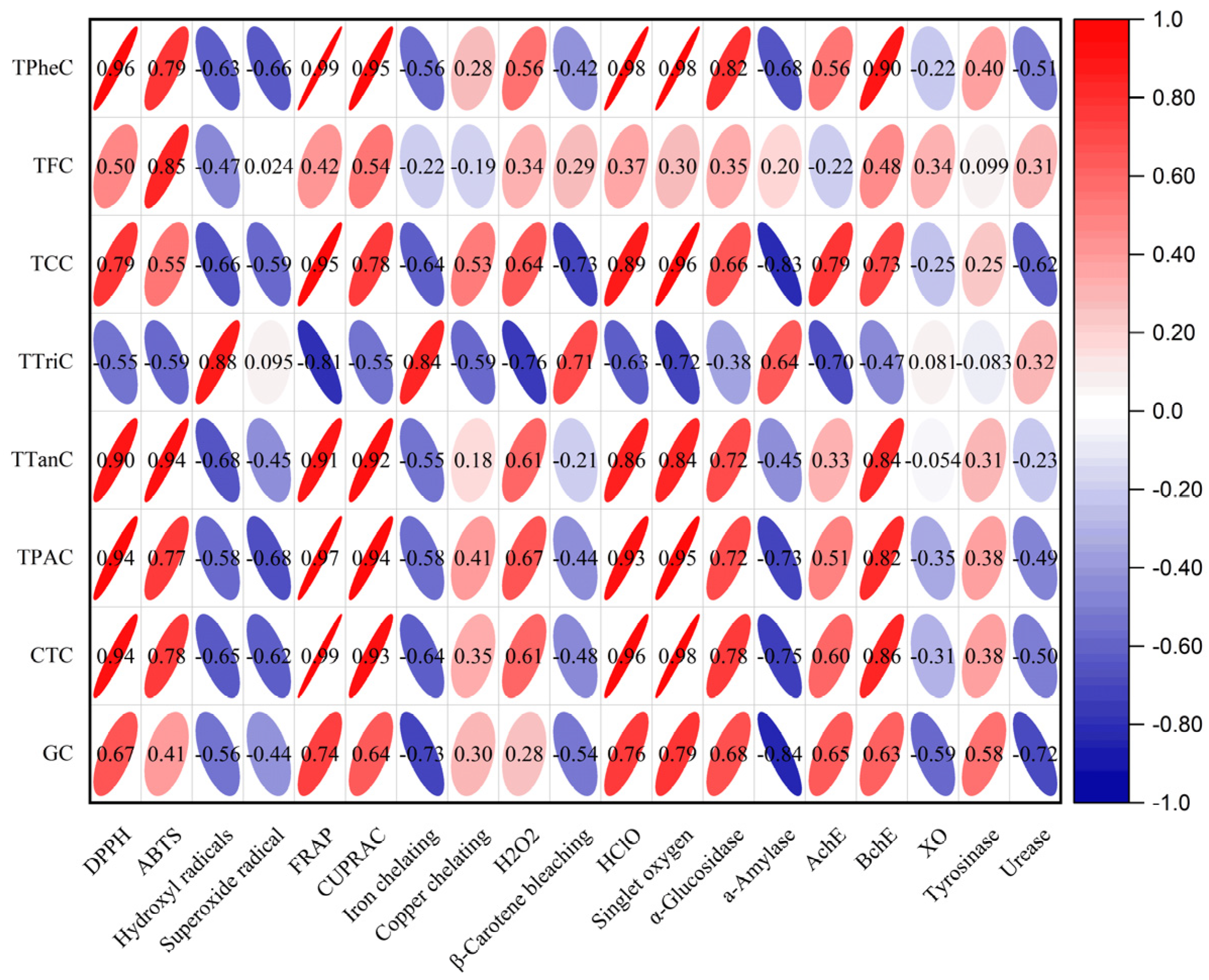
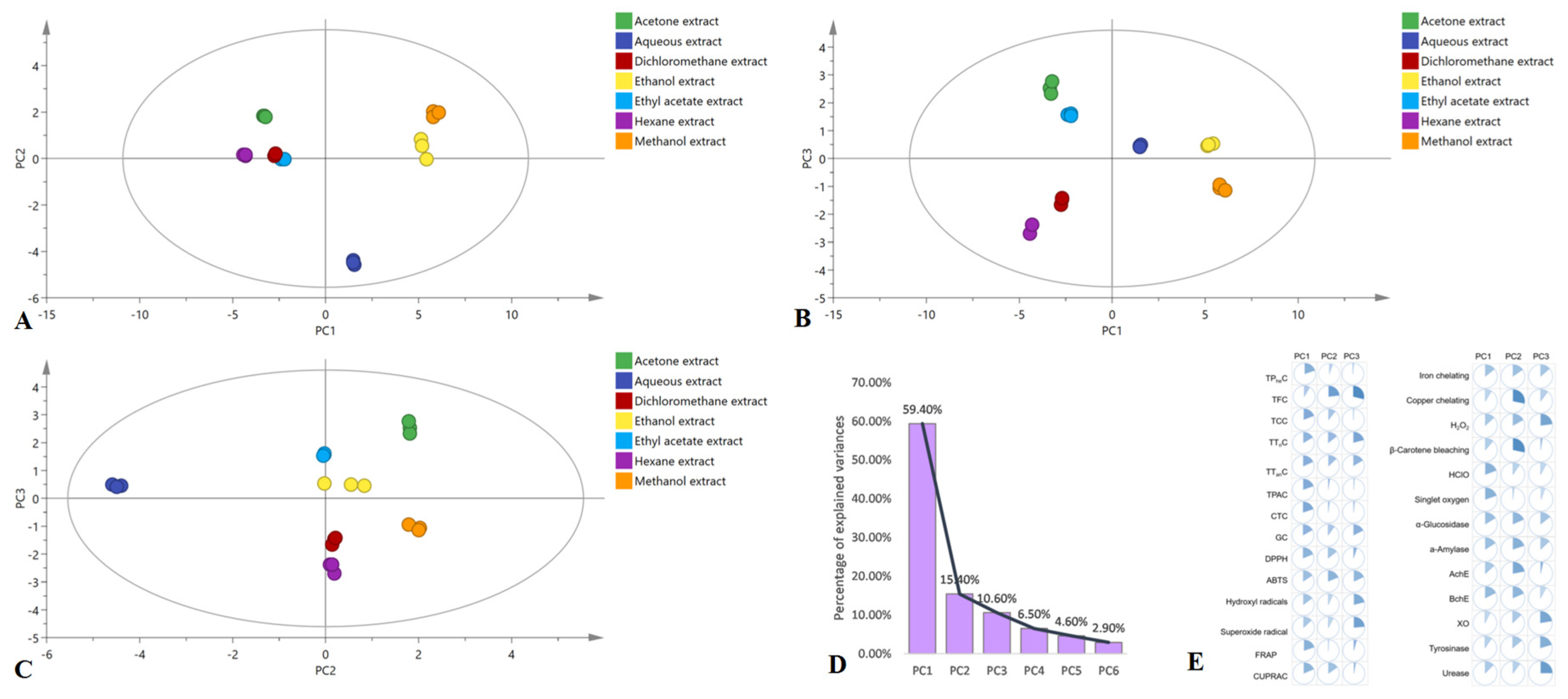
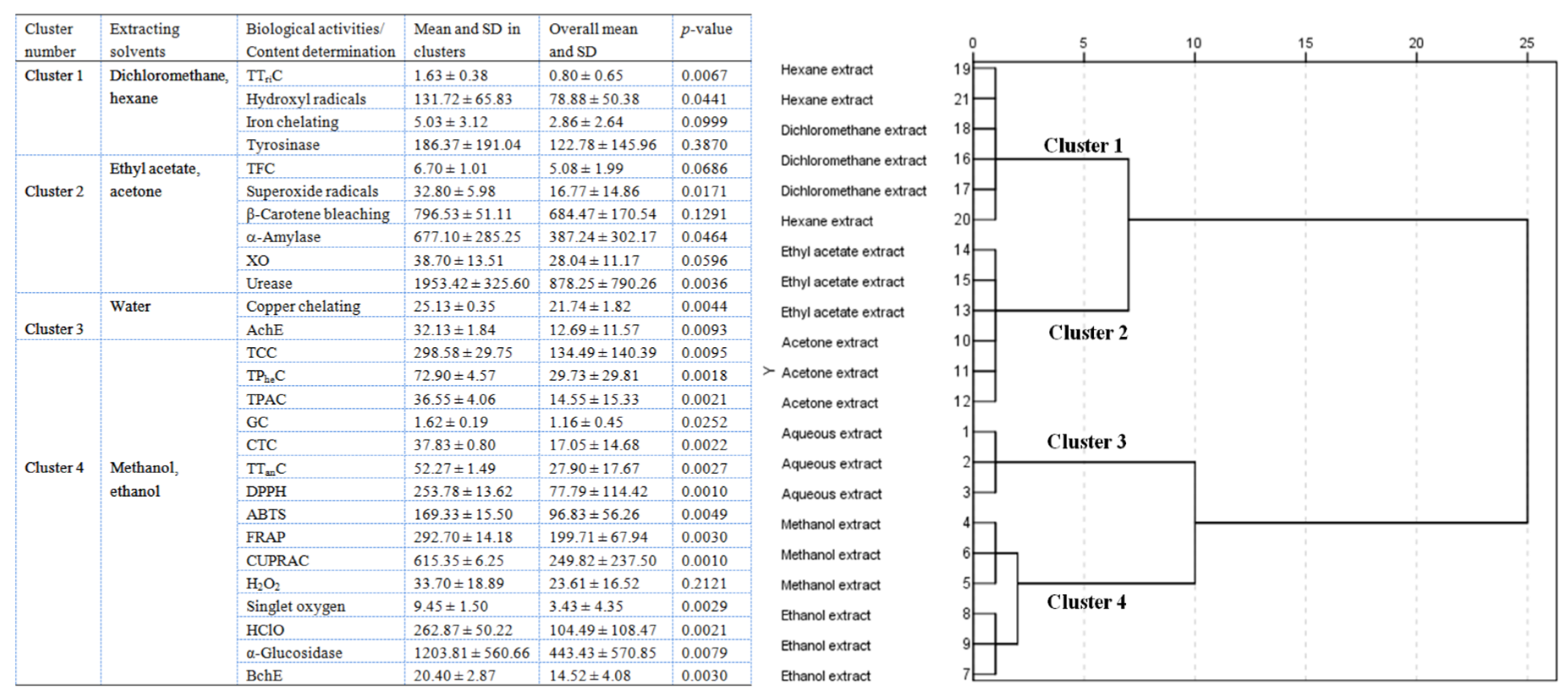
2.10. Multivariate Analysis
3. Material and Methods
3.1. Reagents and Chemicals
3.2. Materials
3.3. Preparation of Different Extracts of PK Insect Gall for Quantitative Phytochemical Analysis and UHPLC–MS Analysis
3.4. Qualitative Phytochemical Analysis
3.5. Quantitative Phytochemical Analysis
3.5.1. Determination of TCC
3.5.2. Determination of TProC
3.5.3. Determination of TTriC
3.5.4. Determination of TPheC
3.5.5. Determination of TFC
3.5.6. Determination of TPAC
3.5.7. Determination of TTanC
3.5.8. Determination of GC
3.5.9. Determination of CTC
3.6. Antioxidant Activity Assays
3.6.1. DPPH Assay
3.6.2. ABTS Assay
3.6.3. Hydroxyl Radical Assay
3.6.4. Superoxide Radical Assay
3.6.5. FRAP Assay
3.6.6. CUPRAC Assay
3.6.7. Iron Chelating Assay
3.6.8. Copper Chelating Assay
3.6.9. H2O2 Assay
3.6.10. Singlet Oxygen Assay
3.6.11. HClO Assay
3.6.12. β-Carotene Bleaching Assay
3.6.13. NO Assay
3.7. Enzyme Inhibition Assays
3.7.1. α-Glucosidase Inhibition Assay
3.7.2. α-Amylase Inhibition Assay
3.7.3. AChE Inhibition Assay
3.7.4. BChE Inhibition Assay
3.7.5. Tyrosinase Inhibition Assay
3.7.6. Urease Inhibition Assay
3.7.7. XO Inhibition Assay
3.8. UHPLC–MS
3.9. Stability of Methanol and Ethanol Extracts
3.9.1. pH Stability
3.9.2. Thermal Stability
3.9.3. Modeling of the Stability in the Gastrointestinal Tract
3.10. Oxidative Stability of Oils
3.11. Cell Viability Assay
3.12. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Hirano, T.; Kimura, S.; Sakamoto, T.; Okamoto, A.; Nakayama, T.; Matsuura, T.; Ikeda, Y.; Takeda, S.; Suzuki, Y.; Ohshima, I.; et al. Reprogramming of the developmental program of Rhus javanica during initial stage of gall induction by Schlechtendalia chinensis. Front. Plant Sci. 2020, 11, 471. [Google Scholar] [CrossRef] [PubMed]
- Fay, P.A.; Hartnett, D.C.; Knapp, A.K. Plant tolerance of gall-insect attack and gall-insect performance. Ecology 1996, 77, 521–534. [Google Scholar] [CrossRef]
- Wang, Z.; Xue, R.; Lv, M.; Qi, Y.; Yu, W.; Xie, Z.; Chen, W.; Wang, X.; Tian, X.; Han, B. Stepwise tracking strategy to screen ingredient from Galla Chinensis based on the “mass spectrometry guided preparative chromatography coupled with systems pharmacology”. J. Ethnopharmacol. 2022, 284, 114533. [Google Scholar] [CrossRef]
- Gao, J.; Yang, X.; Yin, W.; Li, M. Gallnuts: A potential treasure in anticancer drug discovery. Evid. Based Complement. Alternat. Med. 2018, 2018, 4930371. [Google Scholar] [CrossRef]
- Yu, Z.J.; Qi, H.A.; Jiang, Z.L.; Li, G.W. Three-stage and sequential sampling techniques for the emigrant form of Adelges laricis Vall. J. Beijing Forest. Uni. 1998, 20, 47–51. (In Chinese) [Google Scholar]
- Jia, M.; Li, Q.; Hua, J.; Liu, J.; Zhou, W.; Qu, B.; Luo, S. Phytohormones regulate both “fish scale” galls and cones on Picea koraiensis. Front. Plant Sci. 2020, 11, 580155. [Google Scholar] [CrossRef]
- Latos-Brozio, M.; Masek, A.; Chrzescijanska, E.; Podsędek, A.; Kajszczak, D. Characteristics of the polyphenolic profile and antioxidant activity of cone extracts from conifers determined using electrochemical and spectrophotometric methods. Antioxidants 2021, 10, 1723. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Qin, X.; Wang, Q.; Xu, Q.; Wang, J.; Wu, Y.; Chen, W.; Wang, C.; Zhang, T.; Xing, D.; et al. Application of carbohydrates in approved small molecule drugs: A review. Eur. J. Med. Chem. 2021, 223, 113633. [Google Scholar] [CrossRef] [PubMed]
- Bécquer-Viart, M.Á.; Armentero-López, A.; Alvarez-Almiñaque, D.; Fernández-Acosta, R.; Matos-Peralta, Y.; D’Vries, R.F.; Marín-Prida, J.; Pardo-Andreu, G.L. Gossypitrin, A naturally occurring flavonoid, attenuates iron-induced neuronal and mitochondrial damage. Molecules 2021, 26, 3364. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.; Zhang, R.X.; Wei, Y.; Ling, K.; Lu, L.; Wang, J.; Pan, X.C.; Cai, M.Y. Anti-fatigue effects of fermented soybean protein peptides in mice. J. Sci. Food Agric. 2022, 102, 2693–2703. [Google Scholar] [CrossRef] [PubMed]
- Hill, R.A.; Connolly, J.D. Triterpenoids. Nat. Prod. Rep. 2020, 37, 962–998. [Google Scholar] [CrossRef]
- Fraga, C.G.; Croft, K.D.; Kennedy, D.O.; Tomás-Barberán, F.A. The effects of polyphenols and other bioactives on human health. Food Funct. 2019, 10, 514–528. [Google Scholar] [CrossRef] [PubMed]
- de Lima Cherubim, D.J.; Buzanello Martins, C.V.; Oliveira Fariña, L.; da Silva de Lucca, R.A. Polyphenols as natural antioxidants in cosmetics applications. J. Cosmet. Dermatol. 2020, 19, 33–37. [Google Scholar] [CrossRef]
- Wen, K.; Fang, X.; Yang, J.; Yao, Y.; Nandakumar, K.S.; Salem, M.L.; Cheng, K. Recent research on flavonoids and their biomedical applications. Curr. Med. Chem. 2021, 28, 1042–1066. [Google Scholar] [CrossRef]
- Tong, Z.; He, W.; Fan, X.; Guo, A. Biological function of plant tannin and its application in animal health. Front. Vet. Sci. 2022, 8, 803657. [Google Scholar] [CrossRef] [PubMed]
- Gulcin, İ. Antioxidants and antioxidant methods: An updated overview. Arch. Toxicol. 2020, 94, 651–715. [Google Scholar] [CrossRef] [PubMed]
- Jakubczyk, K.; Dec, K.; Kałduńska, J.; Kawczuga, D.; Kochman, J.; Janda, K. Reactive oxygen species—Sources, functions, oxidative damage. Pol. Merkur. Lekarski. 2020, 48, 124–127. [Google Scholar]
- Munteanu, I.G.; Apetrei, C. Analytical methods used in determining antioxidant activity: A review. Int. J. Mol. Sci. 2021, 22, 3380. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.J.; Kim, Y.S.; Kumar, V. Heavy metal toxicity: An update of chelating therapeutic strategies. J. Trace Elem. Med. Biol. 2019, 54, 226–231. [Google Scholar] [CrossRef] [PubMed]
- Murphy, E.C.; Friedman, A.J. Hydrogen peroxide and cutaneous biology: Translational applications, benefits, and risks. J. Am. Acad. Dermatol. 2019, 81, 1379–1386. [Google Scholar] [CrossRef] [PubMed]
- Marcelino, G.; Machate, D.J.; Freitas, K.C.; Hiane, P.A.; Maldonade, I.R.; Pott, A.; Asato, M.A.; Candido, C.J.; Guimarães, R.C.A. β-Carotene: Preventive role for type 2 Diabetes Mellitus and obesity: A review. Molecules 2020, 25, 5803. [Google Scholar] [CrossRef] [PubMed]
- Block, M.S.; Rowan, B.G. Hypochlorous acid: A review. J. Oral Maxillofac. Surg. 2020, 78, 1461–1466. [Google Scholar] [CrossRef]
- Papoutsis, K.; Zhang, J.; Bowyer, M.C.; Brunton, N.; Gibney, E.R.; Lyng, J. Fruit, vegetables, and mushrooms for the preparation of extracts with α-amylase and α-glucosidase inhibition properties: A review. Food Chem. 2021, 338, 128119. [Google Scholar] [CrossRef] [PubMed]
- Pohanka, M. Diagnoses of pathological states based on acetylcholinesterase and butyrylcholinesterase. Curr. Med. Chem. 2020, 27, 2994–3011. [Google Scholar] [CrossRef]
- Zolghadri, S.; Bahrami, A.; Hassan Khan, M.T.; Munoz-Munoz, J.; Garcia-Molina, F.; Garcia-Canovas, F.; Saboury, A.A. A comprehensive review on tyrosinase inhibitors. J. Enzyme Inhib. Med. Chem. 2019, 34, 279–309. [Google Scholar] [CrossRef]
- Mehmood, A.; Ishaq, M.; Zhao, L.; Safdar, B.; Rehman, A.U.; Munir, M.; Raza, A.; Nadeem, M.; Iqbal, W.; Wang, C. Natural compounds with xanthine oxidase inhibitory activity: A review. Chem. Biol. Drug Des. 2019, 93, 387–418. [Google Scholar] [CrossRef]
- Hameed, A.; Al-Rashida, M.; Uroos, M.; Qazi, S.U.; Naz, S.; Ishtiaq, M.; Khan, K.M. A patent update on therapeutic applications of urease inhibitors (2012–2018). Expert Opin. Ther. Pat. 2019, 29, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Lu, Y.M.; Liu, B.Z.; He, H.Y. Electrospray positive ionization tandem mass spectrometry of Amadori compounds. J. Mass Spectrom. 2008, 43, 262–264. [Google Scholar] [CrossRef]
- Tang, H.W.; Wong, M.Y.; Lam, W.; Cheng, Y.C.; Che, C.M.; Ng, K.M. Molecular histology analysis by matrix-assisted laser desorption/ionization imaging mass spectrometry using gold nanoparticles as matrix. Rapid Commun. Mass Spectrom. 2011, 25, 3690–3696. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Miao, X.; Ge, M.; Zhang, M.; Lv, Z.; Wang, W.; Chang, Y.; Ouyang, H.; He, J. Exploration of habitat-related chemical markers for Stephania tetrandra applying multiple chromatographic and chemometric analysis. Molecules 2022, 27, 7224. [Google Scholar] [CrossRef]
- Zahran, E.M.; Abdelmohsen, U.R.; Shalash, M.M.; Salem, M.A.; Khalil, H.E.; Desoukey, S.Y.; Fouad, M.A.; Krischke, M.; Mueller, M.; Kamel, M.S. Local anaesthetic potential, metabolic profiling, molecular docking and in silico ADME studies of Ocimum forskolei, family Lamiaceae. Nat. Prod. Res. 2021, 35, 4757–4763. [Google Scholar] [CrossRef]
- Zhang, Y.; Deng, B.; Cui, Y.; Chen, X.; Bi, J.; Zhang, G. Two new secoiridoid glucosides and a new lignan from the roots of Ilex pubescens. J. Nat. Med. 2018, 72, 946–953. [Google Scholar] [CrossRef] [PubMed]
- Zheleva-Dimitrova, D.; Zengin, G.; Sinan, K.I.; Yıldıztugay, E.; Mahomoodally, M.F.; Ak, G.; Picot-Allain, M.C.N.; Gevrenova, R. Identification of bioactive compounds from Rhaponticoides iconiensis extracts and their bioactivities: An endemic plant to Turkey flora. J. Pharm. Biomed. Anal. 2020, 190, 113537. [Google Scholar] [CrossRef]
- Deseo, M.A.; Elkins, A.; Rochfort, S.; Kitchen, B. Antioxidant activity and polyphenol composition of sugarcane molasses extract. Food Chem. 2020, 314, 126180. [Google Scholar] [CrossRef]
- Guo, Z.; Lai, J.; Wu, Y.; Fang, S.; Liang, X. Investigation on antioxidant activity and different metabolites of mulberry (Morus spp.) leaves depending on the harvest months by UPLC-Q-TOF-MS with multivariate tools. Molecules 2023, 28, 1947. [Google Scholar] [CrossRef]
- Gaikwad, N.W. Ultra performance liquid chromatography-tandem mass spectrometry method for profiling of steroid metabolome in human tissue. Anal. Chem. 2013, 85, 4951–4960. [Google Scholar] [CrossRef] [PubMed]
- Escobar-Wilches, D.C.; Ventura-Bahena, A.; de Lourdes López-González, M.; Torres-Sánchez, L.; Figueroa, M.; Sierra-Santoyo, A. Analysis of testosterone-hydroxylated metabolites in human urine by ultra high performance liquid chromatography-mass spectrometry. Anal. Biochem. 2020, 597, 113670. [Google Scholar] [CrossRef] [PubMed]
- Frank Lee, C.; Brown, C.E.; Nielsen, A.J.; Kim, C.; Livne-Bar, I.; Parsons, P.J.; Boldron, C.; Autelitano, F.; Weaver, D.F.; Sivak, J.M.; et al. A stereocontrolled total synthesis of lipoxin B4 and its biological activity as a pro-resolving lipid mediator of neuroinflammation. Chem.-Eur. J. 2022, 28, e202200360. [Google Scholar] [CrossRef] [PubMed]
- Qiu, W.; Shi, S.; Li, R.; Lin, X.; Rao, L.; Sun, Z. A mild, general, metal-free method for desulfurization of thiols and disulfides induced by visible-light. Chin. J. Chem. 2021, 39, 1255–1258. [Google Scholar] [CrossRef]
- Xu, Z.P.; Algradi, A.M.; Liu, Y.; Wang, S.Y.; Jiang, Y.K.; Guan, W.; Pan, J.; Kuang, H.X.; Yang, B.Y. Bioactive lipids from the fruits of Solanum xanthocarpum and their anti-inflammatory activities. Fitoterapia 2022, 157, 105134. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, X.; Wang, Y.; Chen, F.; Li, Y.; Li, Y.; Tan, Y.; Gong, J.; Zhong, X.; Li, H.; et al. A new diarylheptanoid from Alpinia officinarum promotes the differentiation of 3T3-L1 preadipocytes. Nat. Prod. Res. 2018, 32, 529–535. [Google Scholar] [CrossRef]
- Shah, S.L.; Bashir, K.; Rasheed, H.M.; Rahman, J.U.; Ikram, M.; Shah, A.J.; Majrashi, K.A.; Alnasser, S.M.; Menaa, F.; Khan, T. LC-MS/MS-based metabolomic profiling of constituents from Glochidion velutinum and its activity against cancer cell lines. Molecules 2022, 27, 9012. [Google Scholar] [CrossRef]
- Cho, J.Y.; Matsubara, T.; Kang, D.W.; Ahn, S.H.; Krausz, K.W.; Idle, J.R.; Luecke, H.; Gonzalez, F.J. Urinary metabolomics in fxr-null mice reveals activated adaptive metabolic pathways upon bile acid challenge. J. Lipid Res. 2010, 51, 1063–1074. [Google Scholar] [CrossRef] [PubMed]
- Saleem, H.; Zengin, G.; Khan, K.U.; Ahmad, I.; Waqas, M.; Mahomoodally, F.M.; Rengasamy, K.R.R.; Zainol, N.; Abidin, S.A.Z.; Ahemad, N. New insights into the phytochemical composition, enzyme inhibition and antioxidant properties of desert cotton (Aerva javanica (Bum.f) Shult. -Amaranthaceae). Nat. Prod. Res. 2021, 35, 664–668. [Google Scholar] [CrossRef] [PubMed]
- Bergueiro, J.; Montenegro, J.; Cambeiro, F.; Saá, C.; López, S. Cross-coupling reactions of organosilicon compounds in the stereocontrolled synthesis of retinoids. Chem.-Eur. J. 2012, 18, 4401–4410. [Google Scholar] [CrossRef] [PubMed]
- Horinouchi, M.; Hayashi, T.; Koshino, H.; Malon, M.; Hirota, H.; Kudo, T. Identification of 9α-hydroxy-17-oxo-1,2,3,4,10,19-hexanorandrost-6-en-5-oic acid and β-oxidation products of the C-17 side chain in cholic acid degradation by Comamonas testosteroni TA441. J. Steroid Biochem. Mol. Biol. 2014, 143, 306–322. [Google Scholar] [CrossRef] [PubMed]
- Ji, T.; Wang, J.; Xu, Z.; Cai, H.D.; Su, S.L.; Peng, X.; Ruan, H.S. Combination of mulberry leaf active components possessed synergetic effect on SD rats with diabetic nephropathy by mediating metabolism, Wnt/β-catenin and TGF-β/Smads signaling pathway. J. Ethnopharmacol. 2022, 292, 115026. [Google Scholar] [CrossRef]
- Hopkins, C.Y.; Chisholm, M.J.; Orgodnik, J.A. Identity and configuration of conjugated fatty acids in certain seed oils. Lipids 1969, 4, 89–92. [Google Scholar] [CrossRef]
- Zhang, Z.; Xu, H.; Zhao, H.; Geng, Y.; Ren, Y.; Guo, L.; Shi, J.; Xu, Z. Edgeworthia gardneri (Wall.) Meisn. water extract improves diabetes and modulates gut microbiota. J. Ethnopharmacol. 2019, 239, 111854. [Google Scholar] [CrossRef]
- Chen, M.H.; He, X.; Sun, H.; Sun, Y.; Li, L.; Zhu, J.Y.; Xia, G.Q.; Guo, X.; Zang, H. Phytochemical analysis, UPLC-ESI-Orbitrap-MS analysis, biological activity, and toxicity of extracts from Tripleurospermum limosum (Maxim.) Pobed. Arabian J. Chem. 2022, 15, 103797. [Google Scholar] [CrossRef]
- Zhang, L.Y.; Xu, Q.; Li, L.; Lin, L.; Yu, J.L.; Zhu, J.Y.; Zhang, H.; Xia, G.Q.; Zang, H. Antioxidant and enzyme-inhibitory activity of extracts from Erigeron annuus flower. Ind. Crops Prod. 2020, 148, 112283. [Google Scholar] [CrossRef]
- Guo, X.; Chen, M.H.; He, X.; Zhao, Y.M.; Yu, J.H.; Zhu, J.Y.; Li, L.; Xia, G.Q.; Zang, H. Phytochemical profiling and antioxidant, enzyme-inhibitory, and toxic activities of extracts from Adonis ramosa Franch. Nat. Prod. Res. 2022, 37, 2269–2273. [Google Scholar] [CrossRef] [PubMed]




| Phytochemicals | Type of Tests | Sample Solution | ||
|---|---|---|---|---|
| Water | Methanol | Petroleum Ether | ||
| Proteins/amino acids | 1. Ninhydrin tests | + | ○ | ○ |
| 2. Biuret tests | − | ○ | ○ | |
| Carbohydrates | 1. Fehling’s tests | + | ○ | ○ |
| 2. Benedict’s tests | + | ○ | ○ | |
| 3. Molisch’s tests | + | ○ | ○ | |
| 4. Iodine tests | + | ○ | ○ | |
| Phenolics | 1. FeCl3 tests | + | ○ | ○ |
| 2. FeCl3-K3[Fe(CN)6] tests | + | ○ | ○ | |
| 3. Diazotization tests | + | ○ | ○ | |
| Organic acids | 1. pH tests | + | ○ | ○ |
| 2. Blue litmus paper tests | + | ○ | ○ | |
| 3. Bromocresol green tests | + | ○ | ○ | |
| Tannins | 1. FeCl3 tests | + | ○ | ○ |
| 2. Bromine water tests | + | ○ | ○ | |
| 3. Lead acetate tests | + | ○ | ○ | |
| 4. Lime water tests | + | ○ | ○ | |
| 5. Gelatin tests | + | ○ | ○ | |
| Flavonoids | 1. Shinoda tests | ○ | + | ○ |
| 2. Alkaline reagent tests | ○ | + | ○ | |
| 3. AlCl3 tests | ○ | + | ○ | |
| 4. Lead acetate tests | ○ | + | ○ | |
| Saponins | 1. Foam tests | − | ○ | ○ |
| Steroids and triterpenoids | 1. Liebermann–Burchard tests | ○ | + | ○ |
| 2. Salkowski tests | ○ | + | ○ | |
| Terpenoids | 1. CHCl3-H2SO4 tests | ○ | + | ○ |
| 2. Vanillin-H2SO4 tests | ○ | ○ | + | |
| Alkaloids | 1. Bertrad’s reagent tests | ○ | + | ○ |
| 2. Dragendorff’s reagent tests | ○ | + | ○ | |
| 3. Mayer’s reagent tests | ○ | + | ○ | |
| Anthraquinones | 1. Borntrager’s tests | ○ | − | ○ |
| 2. Magnesium acetate tests | ○ | − | ○ | |
| Coumarins and lactones | 1. Hydroxamic acid iron tests | ○ | + | ○ |
| 2. Diazotization tests | ○ | + | ○ | |
| 3. Fluorescence tests | ○ | + | ○ | |
| Volatile oils and fats | 1. Phosphomolybdic acid tests | ○ | + | ○ |
| 2. Vanillin-H2SO4 tests | ○ | + | ○ | |
| 3. Sudan tests | ○ | + | ○ | |
| Cardiac glycosides | 1. Kedde tests | ○ | − | ○ |
| 2. Raymond tests | ○ | − | ○ | |
| 3. Legal tests | ○ | − | ○ | |
| Cyanogenic glycosides | 1. Prussian blue tests | − | ○ | ○ |
| Extracting Solvents | Yields (%, w/w) |
|---|---|
| Water | 34.32 ± 0.41 a |
| Methanol | 30.45 ± 0.53 b |
| Ethanol | 25.21 ± 1.60 b |
| Acetone | 10.43 ± 1.20 c |
| Ethyl acetate | 8.74 ± 0.79 d |
| Dichloromethane | 7.13 ± 0.64 d |
| Hexane | 5.28 ± 0.21 e |
| Extracting Solvents | TCC (mg GE/g Extract) | TProC (mg BSAE/g Extract) | TTriC (mg GRE/g Extract) | TPheC (mg GAE/g Extract) | TFC (mg QE/g Extract) | TPAC (mg CAE/g Extract) | GC (mg GAE/g Extract) | CTC (mg GAE/g Extract) | TTanC (mg TAE/g Extract) |
|---|---|---|---|---|---|---|---|---|---|
| Water | 277.03 ± 1.61 b | 313.32 ± 11.61 a | NONE | 34.21 ± 0.52 c | 2.92 ± 0.02 e | 17.61 ± 0.83 c | 1.42 ± 0.13 b | 21.21 ± 1.61 c | 23.02 ± 0.21 e |
| Methanol | 325.83 ± 0.47 a | NONE | 0.31 ± 0.01 e | 77.11 ± 0.52 a | 6.22 ± 0.12 b | 33.22 ± 1.43 b | 1.81 ± 0.22 a | 38.54 ± 0.23 a | 50.91 ± 0.52 b |
| Ethanol | 271.52 ± 1.21 c | NONE | 0.29 ± 0.03 e | 68.79 ± 1.19 b | 7.14 ± 0.23 a | 39.92 ± 2.54 a | 1.52 ± 0.11 b | 37.12 ± 0.22 b | 53.64 ± 0.22 a |
| Acetone | 17.02 ± 0.18 e | NONE | 1.02 ± 0.01 c | 12.52 ± 0.02 d | 7.52 ± 0.61 a | 3.61 ± 0.32 d | 0.36 ± 0.01 e | 5.61 ± 0.38 e | 28.74 ± 0.47 c |
| Ethyl acetate | 32.14 ± 1.52 d | NONE | 0.73 ± 0.02 d | 10.31 ± 0.21 e | 5.88 ± 0.42 b | 4.64 ± 0.32 d | 1.02 ± 0.02 d | 10.04 ± 0.81 d | 24.05 ± 0.21 d |
| Dichloromethane | 12.87 ± 0.22 f | NONE | 1.31 ± 0.01 b | 4.54 ± 0.12 f | 3.57 ± 0.23 c | 1.38 ± 0.21 e | 1.34 ± 0.11 c | 4.73 ± 0.44 e | 10.71 ± 0.32 f |
| Hexane | 5.42 ± 0.31 g | NONE | 1.89 ± 0.10 a | 0.92 ± 0.03 g | 2.42 ± 0.22 d | 1.49 ± 0.10 e | 0.84 ± 0.01 f | 2.22 ± 0.23 f | 4.32 ± 0.01 g |
| Extracting Solvents | DPPH (mg TE/g Extract) | ABTS (mg TE/g Extract) | Hydroxyl Radicals (mg TE/g Extract) | Superoxide Radicals (%, 2143 μg/mL) |
|---|---|---|---|---|
| Water | 20.54 ± 0.42 e | 48.53 ± 1.3 f | <39.00 e | 19.81 ± 1.82 d |
| Methanol | 259.43 ± 16.81 b | 156.72 ± 8.4 d | <39.00 e | NONE |
| Ethanol | 248.22 ± 9.32 c | 182.02 ± 7.0 c | 49.72 ± 0.11 e | NONE |
| Acetone | 6.41 ± 0.43 f | 100.81 ± 6.0 e | 77.41 ± 5.42 d | 27.62 ± 2.22 c |
| Ethyl acetate | 7.74 ± 0.42 f | 110.72 ± 2.1 e | 83.62 ± 7.61 d | 38.12 ± 1.61 b |
| Dichloromethane | 2.03 ± 0.22 f | 62.43 ± 2.0 f | 72.61 ± 6.42 d | 28.71 ± 1.52 c |
| Hexane | <0.44 f | 16.71 ± 0.0 g | 190.81 ± 17.63 c | 3.31 ± 0.21 e |
| L-ascorbic acid * | 1111.13 ± 8.01 a | 1118.30 ± 32.89 a | 1119.43 ± 3.91 a | N.T. |
| BHT * | 215.12 ± 2.32 d | 808.51 ± 10.32 b | 468.81 ± 6.31 b | N.T. |
| Curcumin * | N.T. | N.T. | N.T. | 45.62 ± 1.01 a |
| Extracting Solvents | FRAP (mg TE/g Extract) | CUPRAC (mg TE/g Extract) | Iron Chelating (mg EDTAE/g Extract) | Copper Chelating (mg EDTAE/g Extract) |
|---|---|---|---|---|
| Water | 228.02 ± 1.62 c | 118.61 ± 8.31 d | 0.52 ± 0.02 e | 25.12 ± 0.42 a |
| Methanol | 298.31 ± 17.43 b | 615.33 ± 9.41 c | 1.89 ± 0.01 c | 20.31 ± 0.51 c |
| Ethanol | 287.02 ± 10.24 b | 615.27 ± 3.02 c | 0.51 ± 0.02 e | 23.45 ± 1.41 b |
| Acetone | 162.84 ± 1.81 d | 126.89 ± 2.81 d | 5.56 ± 0.13 b | <20.80 c |
| Ethyl acetate | 155.02 ± 1.52 d | 108.32 ±7.67 e | 1.52 ± 0.11 d | <20.80 c |
| Dichloromethane | 142.02 ± 5.14 d | 87.41 ± 4.02 f | 2.17 ± 0.21 c | <20.80 c |
| Hexane | 124.78 ± 2.22 e | 76.78 ± 3.42 f | 7.88 ± 0.37 a | <20.80 c |
| L-ascorbic acid * | 980.03 ± 10.02 a | 1401.56 ± 10.32 b | N.T. | N.T. |
| BHT * | 310.02 ± 0.81 b | 1530.01 ± 11.41 a | N.T. | N.T. |
| Extracting Solvents | H2O2 (mg FAE/g Extract) | β-Carotene Bleaching AAC | Singlet Oxygen (%, 2000 μg/mL) | HClO (mg LAE/g Extract) |
|---|---|---|---|---|
| Water | 42.89 ± 0.11 c | 312.71 ± 2.00 d | 5.12 ± 0.21 d | 97.72 ± 0.13 c |
| Methanol | 16.62 ± 1.42 e | 643.32 ± 10.74 c | 10.61 ± 1.12 b | 307.25 ± 18.22 a |
| Ethanol | 50.81 ± 3.61 b | 659.33 ± 9.03 c | 8.32 ± 0.84 c | 218.52 ± 8.81 b |
| Acetone | 24.42 ± 1.23 d | 823.32 ± 12.61 b | NONE | <27 d |
| Ethyl acetate | 18.45 ± 1.81 e | 769.82 ± 21.62 b | NONE | <27 d |
| Dichloromethane | <6.00 f | 807.24 ± 21.53 b | NONE | <27 d |
| Hexane | <6.00 f | 775.73 ± 11.20 b | NONE | <27 d |
| BHT * | N.T. | 876.94 ± 4.61 a | N.T. | N.T. |
| BHA * | N.T. | 883.03 ± 3.42 a | N.T. | N.T. |
| Ferulic acid * | 1000.03 ± 12.77 a | N.T. | 90.32 ± 1.21 a | N.T. |
| Extracting Solvents | α-Glucosidase (mg AE/g Extract) | α-Amylase (mg AE/g Extract) | AChE (100 μg/mL) | BChE (mg DE/g Extract) | XO (1250 μg/mL) | Tyrosinase (mg ArbE/g Extract) | Urease (mg ThiE/g Extract) |
|---|---|---|---|---|---|---|---|
| Water | <49.02 e | <36.00 g | 32.11 ± 1.81 b | <12.01 e | 27.24 ± 1.52 d | <1.01 c | 256.90 ± 0.03 e |
| Methanol | 1713.62 ± 78.62 a | 173.24 ± 6.02 e | 25.42 ± 2.02 c | 23.02 ± 0.71 b | 32.03 ± 0.54 c | 247.41 ± 9.89 b | 102.04 ± 8.02 e |
| Ethanol | 694.13 ± 11.64 b | 89.78 ± 4.6 f | 10.45 ± 0.89 e | 17.81 ± 0.63 c | 13.41 ± 2.31 f | 236.34 ± 13.92 b | 583.52 ± 12.24 d |
| Acetone | <49.02 e | 940.03 ± 58.89 a | NONE | 12.91 ± 0.22 d | 50.78 ± 3.89 b | <1.01 c | 1672.16 ± 87.53 b |
| Ethyl acetate | 181.82 ± 3.12 d | 416.94 ± 11.56 d | 12.43 ± 0.44 d | <12.01 e | 26.62 ± 1.32 d | <1.01 c | 2234.58 ± 143.24 a |
| Dichloromethane | 240.03 ± 4.82 c | 588.43 ± 11.53 b | NONE | <12.01 e | 20.51 ± 0.89 e | 360.72 ± 5.93 a | 152.78 ± 11.67 e |
| Hexane | 176.61 ± 1.93 d | 469.12 ± 14.72 c | 8.34 ± 0.43 f | <12.01 e | 25.61 ± 1.21 d | 12.03 ± 0.94 c | 1145.81 ± 39.72 c |
| Donepezil * | N.T. | N.T. | 99.81 ± 0.11 a | 1000.02 ± 9.21 a | N.T. | N.T. | N.T. |
| Allopurinol * | N.T. | N.T. | N.T. | N.T. | 95.22 ± 0.11 a | N.T. | N.T. |
| Peak No. | RT (min) | Identification | Molecular Formula | Selective Ion | Full Scan MS (m/z) | Error (ppm) | MS/MS Fragments (m/z) | |
|---|---|---|---|---|---|---|---|---|
| Theory | Measured | |||||||
| 1 | 1.42 | N2-Fructopyranosylarginine | C12H24N4O7 | [M + H]+ | 337.1714 | 337.1717 | −0.9 | 251.0314 |
| 2 | 1.52 | Choline | C5H14NO+ | [M]+ | 104.1070 | 104.1069 | 1.0 | — |
| 3 | 1.85 | 4-(Aminomethyl)-1-(diaminomethylene)piperidinium | C7H17N4+ | [M + H]+ | 158.1526 | 158.1538 | −7.6 | 140.1438 |
| 4 | 2.41 | Adenine | C5H5N5 | [M + H]+ | 136.0623 | 136.0621 | 1.5 | 118.0859 |
| 5 | 3.58 | Unknown | 140.1435 | 98.0964 | ||||
| 6 | 12.66 | Citrusin C | C16H22O7 | [M + Na]+ | 349.1263 | 349.1260 | 0.9 | 344.1706 |
| 7 | 18.85 | Isolariciresinol 4′-O-beta-D-glucoside | C26H34O11 | [M + Na]+ | 545.1999 | 545.1999 | 0 | 540.2458, 285.1132 |
| 8 | 19.05 | Gossypitrin | C21H20O12 | [M + H]+ | 465.1033 | 465.1027 | 1.3 | 303.0507 |
| 9 | 20.55 | Unknown | 369.1537 | 167.1074 | ||||
| 10 | 21.49 | Isoorientin | C21H20O11 | [M + H]+ | 449.1084 | 449.1072 | 2.7 | 287.0551 |
| 11 | 22.17 | Isorhamnetin-3-O-glucoside | C22H22O12 | [M + H]+ | 479.1190 | 479.1180 | 2.1 | 317.0665 |
| 12 | 22.97 | Estra-1,3,5(10)-triene-3,11,17-triol | C18H24O3 | [M + NH4]+ | 306.2069 | 306.2071 | −0.7 | 107.0490 |
| 13 | 24.45 | 16α-Hydroxyestrone | C18H22O3 | [M + NH4]+ | 304.1913 | 304.1915 | −0.7 | 112.1111 |
| 14 | 25.79 | Unknown | 466.2669 | 335.0948 | ||||
| 15 | 29.74 | Lipoxin B4 | C20H32O5 | [M + Na]+ | 375.2147 | 375.2142 | 1.3 | 309.0986 |
| 16 | 35.48 | Benzyl succinate | C18H18O4 | [M + H]+ | 299.1298 | 299.1300 | −0.7 | 91.0419, 77.0386 |
| 17 | 35.96 | 9S,10S,11R-trihydroxy-12Z-octadecenoic acid | C18H34O5 | [M + Na]+ | 353.2304 | 353.2299 | 1.4 | 301.1358 |
| 18 | 37.46 | 5-Methoxy-1,7-diphenyl-3-heptanone | C20H24O2 | [M + H]+ | 297.1855 | 297.1856 | −0.3 | 282.1622 |
| 19 | 38.08 | (10E,12E,15E)-9-Hydroxy-10,12,15-octadecatrienoic acid | C18H30O3 | [M + Na]+ | 317.2093 | 317.2117 | −7.6 | 253.1952 |
| 20 | 41.86 | 11,20-Dihydroxy-3-oxopregn-4-en-21-oic acid | C21H30O5 | [M + H]+ | 363.2171 | 363.2173 | −0.6 | 317.2122 |
| 21 | 42.11 | Xestoaminol C | C14H31NO | [M + H]+ | 230.2484 | 230.2481 | 1.3 | 212.2368, 201.1640 |
| 22 | 42.40 | Phytosphingosine | C18H39NO3 | [M + H]+ | 318.3008 | 318.3006 | 0.6 | 302.2220, 301.2139 |
| 23 | 44.73 | Unknown | 301.2165 | 283.2068, 255.2115 | ||||
| 24 | 45.05 | Unknown | 385.2381 | 128.0620 | ||||
| 25 | 46.29 | Retinol | C20H30O | [M + H]+ | 287.2375 | 287.2371 | 1.4 | 269.2265 |
| 26 | 46.49 | 5,12-DiHETE | C20H32O4 | [M + H]+ | 337.2379 | 337.2362 | 5.0 | 279.2330 |
| 27 | 47.13 | 12α-Hydroxy-3-oxo-4,6-choladien-24-oic acid | C24H34O4 | [M + H]+ | 387.2535 | 387.2533 | 0.5 | 269.2280 |
| 28 | 47.48 | Sphinganine | C18H39NO2 | [M + H]+ | 302.3059 | 302.3048 | 3.6 | 303.3090 |
| 29 | 48.62 | 18-Hydroxy-9,11,13-octadecatrienoic acid | C18H30O3 | [M + Na]+ | 317.2093 | 317.2110 | −5.4 | 318.2156 |
| 30 | 50.14 | α-Linolenic acid | C18H30O2 | [M + Na]+ | 301.2143 | 301.2160 | −5.6 | 183.1169, 169.1012 |
| Methanol Extract (μg/mL) | Cell Survival Rate of TM3 Cells (%) | Ethanol Extract (μg/mL) | Cell Survival Rate of TM3 Cells (%) | ||
|---|---|---|---|---|---|
| 24 h | 48 h | 24 h | 48 h | ||
| 0 | 100.00 ± 0.64 d | 100.03 ± 0.73 a | 0 | 100.00 ± 0.52 b | 100.00 ± 0.63 a |
| 25 | 107.58 ± 2.14 c | 95.12 ± 1.45 b | 25 | 97.31 ± 1.89 c | 96.23 ± 1.56 b |
| 50 | 101.78 ± 1.67 d | 96.78 ± 1.44 b | 50 | 114.49 ± 2.74 a | 111.02 ± 3.21 a |
| 100 | 111.80 ± 2.42 b | 91.64 ± 1.89 c | 100 | 103.52 ± 2.43 b | 111.72 ± 2.56 a |
| 200 | 116.42 ± 2.81 a | 96.71 ± 2.33 b | 200 | 101.53 ± 1.42 b | 108.89 ± 1.63 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Sun, H.; He, X.; Chen, M.; Zang, H.; Liu, X.; Piao, H. Phytochemical Analysis, Antioxidant and Enzyme-Inhibitory Activities, and Multivariate Analysis of Insect Gall Extracts of Picea koraiensis Nakai. Molecules 2023, 28, 6021. https://doi.org/10.3390/molecules28166021
Wang Y, Sun H, He X, Chen M, Zang H, Liu X, Piao H. Phytochemical Analysis, Antioxidant and Enzyme-Inhibitory Activities, and Multivariate Analysis of Insect Gall Extracts of Picea koraiensis Nakai. Molecules. 2023; 28(16):6021. https://doi.org/10.3390/molecules28166021
Chicago/Turabian StyleWang, Yanqiu, Hui Sun, Xu He, Meihua Chen, Hao Zang, Xuekun Liu, and Huri Piao. 2023. "Phytochemical Analysis, Antioxidant and Enzyme-Inhibitory Activities, and Multivariate Analysis of Insect Gall Extracts of Picea koraiensis Nakai" Molecules 28, no. 16: 6021. https://doi.org/10.3390/molecules28166021
APA StyleWang, Y., Sun, H., He, X., Chen, M., Zang, H., Liu, X., & Piao, H. (2023). Phytochemical Analysis, Antioxidant and Enzyme-Inhibitory Activities, and Multivariate Analysis of Insect Gall Extracts of Picea koraiensis Nakai. Molecules, 28(16), 6021. https://doi.org/10.3390/molecules28166021






