MOF-Based Biosensors for the Detection of Carcinoembryonic Antigen: A Concise Review
Abstract
1. Background
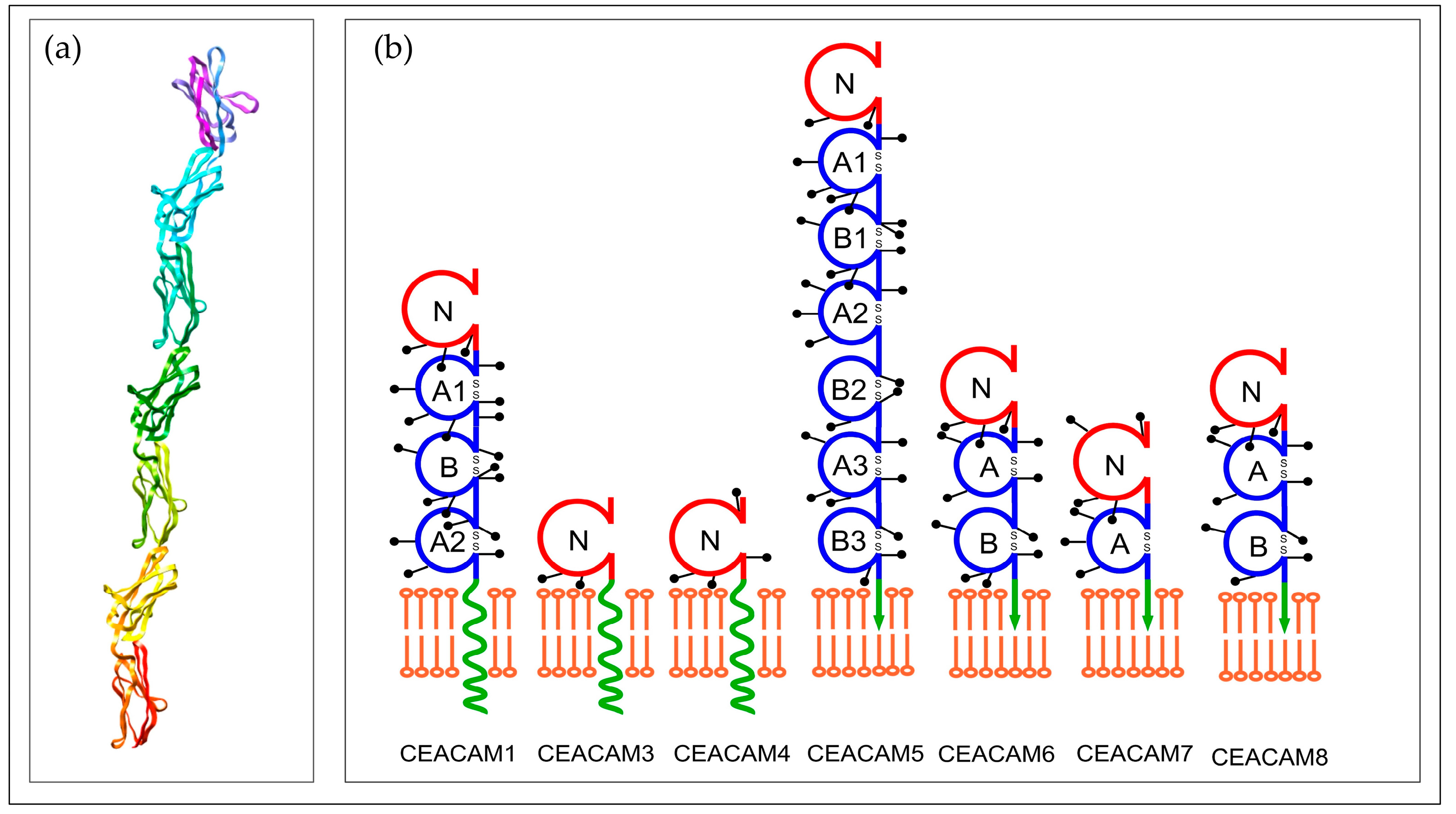
1.1. Structure and Function of CEA
1.2. Detection of CEA Biomarker: Conventional Methods vs. Biosensors
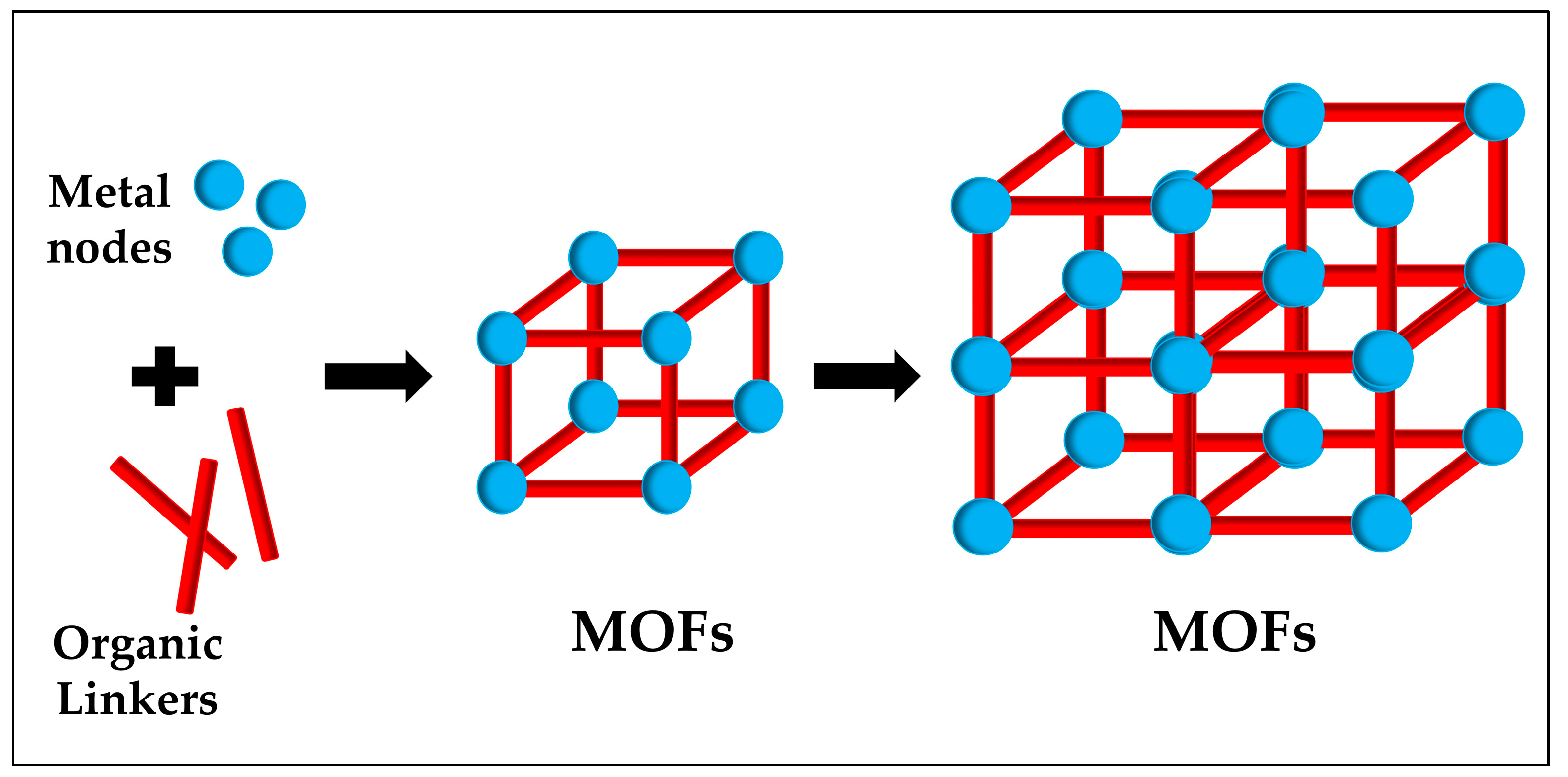
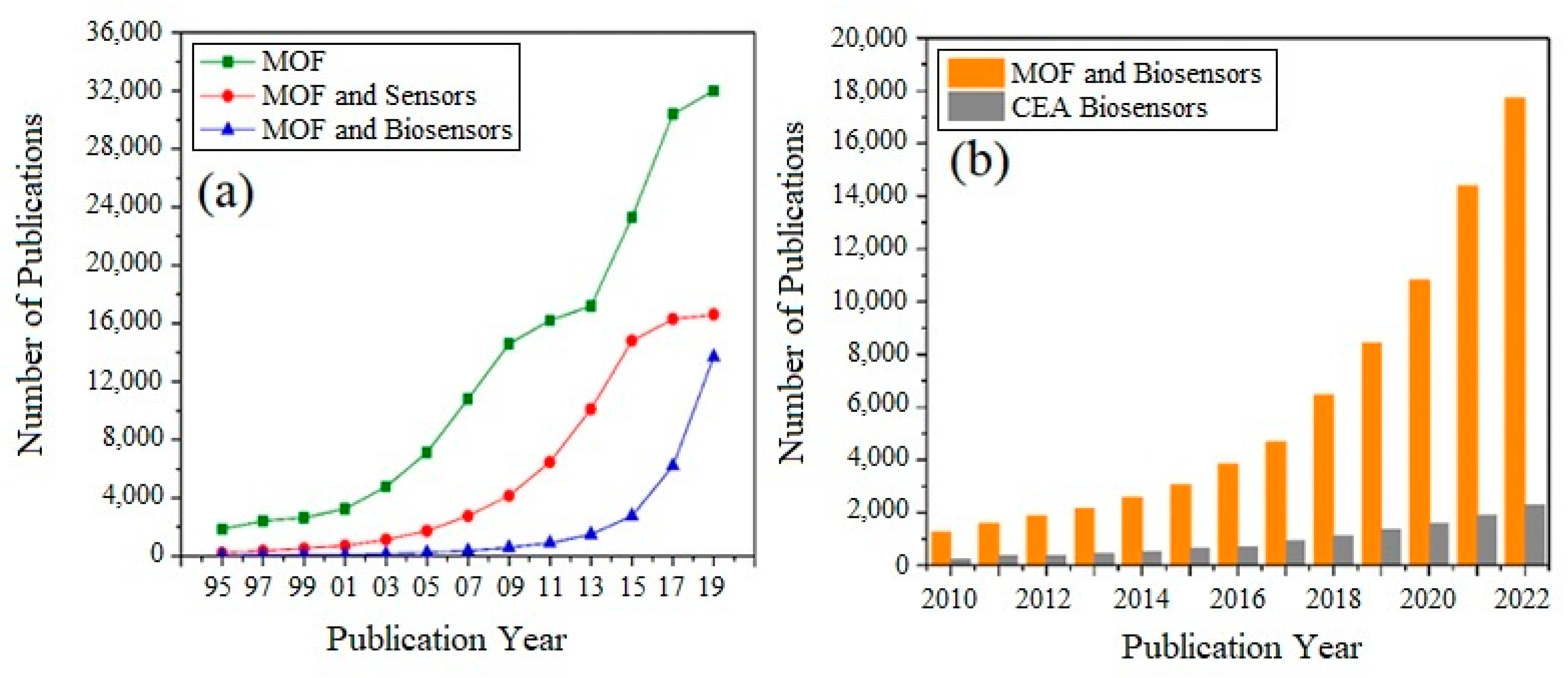
1.3. Why MOFs Are Used for Biosensors
2. Progress in MOF-Based Biosensors for the Detection of CEA
2.1. Electrochemical Approach
2.2. Chemiluminescence Approach
2.3. Electrochemiluminescence Approach
2.4. Fluorescence Approach
2.5. Photoelectrochemical Approach
2.6. Colorimetric Approach
3. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
Abbreviations
| CEA | Carcinoembryonic antigen |
| MOFs | Metal–organic frameworks |
| ELISA | Enzyme-linked immunosorbent assays |
| RIA | Radioimmunoassay |
| IRMA | Immunoradiometric assay |
| RT-PCR | Reverse transcriptase polymerase chain reaction |
| MIPs | Molecular imprinted polymers |
| AuNPs | Gold nanoparticles |
| CNT | Carbon nanotubes |
| CV | Cyclic voltammetry |
| DPV | Differential pulse voltammetry |
| SWV | Square wave voltammetry |
| EIS | Electrochemical impedance spectroscopy |
| GCE | Glass carbon electrode |
| AFP | Alpha fetoprotein |
| AgNPs | Silver nanoparticles |
| LOD | Limit of detection |
| PDA | Polydopamine |
| OMC | Ordered mesoporous carbon |
| MWCNTs | Multiwall carbon nanotubes |
| CL | Chemiluminescence |
| ECL | Electrochemiluminescence |
| GO | Graphene oxide |
| QDs | Quantum dots |
| PAD | Paper-based analytical device |
| PEC | Photoelectrochemical |
| TDN | DNA tetrahedral |
| CDs | Carbon dots |
| SRP | Surface Plasmonic Resonance |
References
- Malhotra, B.D.; Kumar, S.; Pandey, C.M. Nanomaterials based biosensors for cancer biomarker detection. J. Phys. Conf. Ser. 2016, 704, 012011. [Google Scholar] [CrossRef]
- Vinood, P.B.; Victor, P.R. Biomarkers in Disease: Methods, Discoveries and Applications Series. In Textbook of Cancer Epidemiology; Springer: Dordrecht, The Netherlands, 2015. [Google Scholar]
- Henry, N.L.; Hayes, D.F. Cancer biomarkers. Mol. Oncol. 2012, 6, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Gold, P.; Freedman, S.O. Specific carcinoembryonic antigens of the human digestive system. J. Exp. Med. 1965, 122, 467–481. [Google Scholar] [CrossRef] [PubMed]
- Nan, J.; Li, J.; Li, X.; Guo, G.; Wen, X.; Tian, Y. Preoperative serum carcinoembryonic antigen as a marker for predicting the outcome of three cancers. Biomark. Cancer 2017, 9, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Lee, S. The roles of carcinoembryonic antigen in liver metastasis and therapeutic approaches. Gastroenterol. Res. Pract. 2017, 2017, 7521987. [Google Scholar] [CrossRef]
- Öbrink, B. CEA adhesion molecules: Multifunctional proteins with signal-regulatory properties. Curr. Opin. Cell Biol. 1997, 9, 616–626. [Google Scholar] [CrossRef]
- Xiang, W.; Lv, Q.; Shi, H.; Xie, B.; Gao, L. Aptamer-based biosensor for detecting carcinoembryonic antigen. Talanta 2020, 214, 120716. [Google Scholar] [CrossRef]
- Hammarström, S. The carcinoembryonic antigen (CEA) family: Structures, suggested functions and expression in normal and malignant tissues. Semin. Cancer Biol. 1999, 9, 67–81. [Google Scholar] [CrossRef]
- Beauchemin, N.; Arabzadeh, A. Carcinoembryonic antigen-related cell adhesion molecules (CEACAMs) in cancer progression and metastasis. Cancer. Metastasis Rev. 2013, 32, 643–671. [Google Scholar] [CrossRef]
- Hall, C.; Clarke, L.; Pal, A.; Buchwald, P.; Eglinton, T.; Wakeman, C.; Frizelle, F.A. A review of the role of carcinoembryonic antigen in clinical practice. Ann. Coloproctol. 2019, 35, 294–305. [Google Scholar] [CrossRef]
- Saied, G.M.; El-Metenawy, W.H.; Elwan, M.S.; Dessouki, N.R. Urine carcinoembryonic antigen levels are more useful than serum levels for early detection of Bilharzial and non-Bilharzial urinary bladder carcinoma: Observations of 43 Egyptian cases. World. J. Surg. Oncol. 2007, 5, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, S.; Yang, C.F. Electrochemical biosensors for cancer biomarker detection. Electroanalysis 2012, 24, 2213–2229. [Google Scholar] [CrossRef]
- Tang, Z.; Ma, Z. Multiple functional strategies for amplifying sensitivity of amperometric immunoassay for tumor markers: A review. Biosens. Bioelectron. 2017, 98, 100–112. [Google Scholar] [CrossRef] [PubMed]
- Špringer, T.; Homola, J. Biofunctionalized gold nanoparticles for SPR-biosensor-based detection of CEA in blood plasma. Anal. Bioanal. Chem. 2012, 404, 2869–2875. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, Q.; Liu, Y.; Cui, J.; Liu, H.; Wang, P.; Li, Y.; Chen, L.; Zhao, Z.; Dong, Y. A novel label-free electrochemical immunosensor based on functionalized nitrogen-doped graphene quantum dots for carcinoembryonic antigen detection. Biosens. Bioelectron. 2017, 90, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.; Deng, Y.; Yang, G.; Li, S.; Zhang, C.; Liu, X. Molecular Imprinting Polymers Electrochemical Sensor Based on AuNPs/PTh Modified GCE for Highly Sensitive Detection of Carcinomaembryonic Antigen. J. Biomed. Nanotechnol. 2018, 14, 1688–1694. [Google Scholar] [CrossRef] [PubMed]
- Carrasco, S. Metal-Organic Frameworks for the development of biosensors: A current overview. Biosensors 2018, 8, 92. [Google Scholar] [CrossRef] [PubMed]
- Yap, M.H.; Fow, K.L.; Chen, G. Synthesis and applications of MOF-derived porous nanostructures. Green Energy Environ. 2017, 2, 218–245. [Google Scholar] [CrossRef]
- Afreen, S.; He, Z.; Yan, X.; Zhu, J. Nanoscale metal–organic frameworks in detecting cancer biomarkers. J. Mater. Chem. B 2020, 8, 1338–1349. [Google Scholar] [CrossRef]
- Anik, Ü.; Timur, S.; Dursun, Z. Metal organic frameworks in electrochemical and optical sensing platforms: A review. Mikrochim. Acta 2019, 186, 18–24. [Google Scholar] [CrossRef]
- Asefa, T.; Duncan, C.T.; Sharma, K.K. Recent advances in nanostructured chemosensors and biosensors. J. Mater. Chem. 2009, 18, 5604–5614. [Google Scholar] [CrossRef]
- Zhang, Z.; Duan, F.; Tian, J.; He, J.; Yang, L.; Zhao, H.; Zhang, S.; He, L.; Chen, M.; Chen, D.; et al. Aptamer-Embedded Zirconium-Based Metal–Organic Framework Composites Prepared by De Novo Bio-Inspired Approach with Enhanced Biosensing for Detecting Trace Analytes. ACS Sens. 2017, 7, 982–989. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Chen, W.; Zhu, P.; Tian, Y.; Chen, Y.; Wu, C. Applications of functional Metal-Organic frameworks in biosensors. Biotechnol. J. 2020, 16, 1900424. [Google Scholar] [CrossRef]
- Osman, D.; El-Sheikh, S.M.; Sheta, S.M.; Ali, O.; Salem, A.; Shousha, W.G.; El-Khamisy, S.F.; Shawky, S.M. Nucleic acids biosensors based on metal-organic framework (MOF): Paving the way to clinical laboratory diagnosis. Biosens. Bioelectron. 2019, 141, 111451. [Google Scholar] [CrossRef]
- An, H.; Li, M.; Gao, J.; Zhang, Z.; Ma, S.; Chen, Y. Incorporation of biomolecules in Metal-Organic Frameworks for advanced applications. Coord. Chem. Rev. 2019, 384, 90–106. [Google Scholar] [CrossRef]
- Yuan, S.; Wang, K.; Pang, J.; Bosch, M.; Lollar, C.; Sun, Y.; Qin, J.; Yang, X.; Zhang, P.; Wang, Q.; et al. Stable Metal–Organic Frameworks: Design, synthesis, and applications. Adv. Mater. 2018, 30, 1704303. [Google Scholar] [CrossRef]
- Zhang, Y.; Bo, X.; Nsabimana, A.; Han, C.; Li, M.; Guo, L. Electrocatalytically active cobalt-based metal–organic framework with incorporated macroporous carbon composite for electrochemical applications. J. Mater. Chem. 2015, 3, 732–738. [Google Scholar] [CrossRef]
- Zhang, B.; Luo, Y.; Kanyuck, K.; Saenz, N.; Reed, K.; Zavalij, P.Y.; Mowery, J.; Bauchan, G.R. Facile and template-free solvothermal synthesis of mesoporous/macroporous metal–organic framework nanosheets. RSC Adv. 2018, 8, 833059–833064. [Google Scholar] [CrossRef]
- Khan, N.A.; Jhung, S.H. Synthesis of metal-organic frameworks (MOFs) with microwave or ultrasound: Rapid reaction, phase-selectivity, and size reduction. Coord. Chem. Rev. 2015, 285, 11–23. [Google Scholar] [CrossRef]
- Campagnol, N.; Souza, E.R.; De Vos, D.; Binnemans, K.; Fransaer, J. Luminescent terbium-containing metal–organic framework films: New approaches for the electrochemical synthesis and application as detectors for explosives. Chem. Commun. 2014, 50, 12680–12683. [Google Scholar] [CrossRef] [PubMed]
- Masoomi, M.Y.; Morsali, A.; Junk, P.C. Rapid mechanochemical synthesis of two new Cd(ii)-based metal–organic frameworks with high removal efficiency of Congo red. CrystEngComm 2015, 17, 686–692. [Google Scholar] [CrossRef]
- Choi, J.; Son, W.; Kim, J.; Ahn, W. Metal–organic framework MOF-5 prepared by microwave heating: Factors to be considered. Microporous Mesoporous Mater. 2008, 116, 727–731. [Google Scholar] [CrossRef]
- Li, M.; Zhang, G.; Boakye, A.; Chai, H.; Qu, L.; Zhang, X. Recent Advances in Metal-Organic Framework-Based Electrochemical Biosensing Applications. Front. Bioeng. Biotechnol. 2021, 9, 1–8. [Google Scholar] [CrossRef]
- Ma, T.; Li, H.; Ma, J.; Cheng, P. Application of MOF-based materials in electrochemical sensing. Dalton Trans. 2020, 49, 17121–17129. [Google Scholar] [CrossRef] [PubMed]
- Kajal, N.; Singh, V.; Gupta, R.; Gautam, S. Metal organic frameworks for electrochemical sensor applications: A review. Environ. Res. 2022, 204, 112320. [Google Scholar] [CrossRef] [PubMed]
- Ulhakim, M.T.; Rezki, M.; Dewi, K.K.; Abrori, S.A.; Harimurti, S.; Septiani, N.L.W.; Kurnia, K.A.; Setyaningsih, W.; Darmawan, N. RevIew—Recent Trend on Two-Dimensional Metal-Organic Frameworks for Electrochemical Biosensor Application. J. Electrochem. Soc. 2020, 167, 136509. [Google Scholar] [CrossRef]
- Zhang, P.; Huang, H.; Wang, N.; Li, H.; Shen, D.; Ma, H. Duplex voltammetric immunoassay for the cancer biomarkers carcinoembryonic antigen and alpha-fetoprotein by using metal-organic framework probes and a glassy carbon electrode modified with thiolated polyaniline nanofibers. Mikrochim. Acta 2017, 184, 4037–4045. [Google Scholar] [CrossRef]
- Li, W.; Ma, C.; Song, Y.; Qiao, X.; Yin, B. Sensitive detection of carcinoembryonic antigen (CEA) by a sandwich-type electrochemical immunosensor using MOF-Ce@HA/Ag-HRP-Ab2 as a nanoprobe. Nanotechnology 2020, 31, 185605. [Google Scholar] [CrossRef]
- Liu, J.; Shang, Y.; Zhu, Q.; Zhang, X.; Zheng, J. A voltammetric immunoassay for the carcinoembryonic antigen using silver(I)-terephthalate metal-organic frameworks containing gold nanoparticles as a signal probe. Mikrochim. Acta 2019, 186, 509. [Google Scholar] [CrossRef]
- Zhou, X.; Guo, S.; Gao, J.; Zhao, J.; Xue, S.; Xu, W. Glucose oxidase-initiated cascade catalysis for sensitive impedimetric aptasensor based on metal-organic frameworks functionalized with Pt nanoparticles and hemin/G-quadruplex as mimicking peroxidases. Biosens. Bioelectron. 2017, 98, 83–90. [Google Scholar] [CrossRef]
- Guo, C.; Su, F.; Song, Y.; Hu, B.; Wang, M.; He, L.; Peng, D.; Zhang, Z. Aptamer-Templated Silver Nanoclusters Embedded in Zirconium Metal–Organic Framework for Bifunctional Electrochemical and SPR Aptasensors toward Carcinoembryonic Antigen. ACS Appl. Mater. Interfaces 2017, 9, 41188–41199. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, L.; Ai, Y.; Liu, Y.; Sun, H.; Liang, Q. Self-Polymerized Dopamine-Decorated Au NPs and Coordinated with Fe-MOF as a Dual Binding Sites and Dual Signal-Amplifying Electrochemical Aptasensor for the Detection of CEA. ACS Appl. Mater. Interfaces 2020, 12, 5500–5510. [Google Scholar] [CrossRef] [PubMed]
- Bao, T.; Fu, R.; Wen, W.; Zhang, X. Target-Driven Cascade-Amplified Release of Loads from DNA-Gated Metal–Organic Frameworks for Electrochemical Detection of Cancer Biomarker. ACS Appl. Mater. Interfaces 2020, 12, 2087–2094. [Google Scholar] [CrossRef] [PubMed]
- Hong, F.; Wang, Q.; Wang, W.; Chen, X.; Cao, Y.; Dong, Y.; Gan, N.; Wu, D.; Hu, F. Background signal-free and highly sensitive electrochemical aptasensor for rapid detecting tumor markers with Pb-MOF functionalized dendritic DNA probes. J. Electroanal. Chem. 2020, 861, 113956. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Z.; Rong, S.; Yu, H.; Gao, H.; Ding, P.; Dong, C.; Pan, H. Electrochemical immunoassay for the carcinoembryonic antigen based on Au NPs modified zeolitic imidazolate framework and ordered mesoporous carbon. Mikrochim. Acta 2020, 187, 265. [Google Scholar] [CrossRef]
- Li, J.; Shang, Y.; Xu, J.; Chen, Y.; Jia, Y.; Zheng, J. A novel electrochemical immunosensor for carcinoembryonic antigen based on Cu-MOFs-TB/polydopamine nanocarrier. J. Electroanal. Chem. 2020, 877, 114563. [Google Scholar] [CrossRef]
- Biswas, S.; Lan, Q.; Li, C.; Xia, X. Morphologically Flex SM-MOF based electrochemical immunosensor for ultrasensitive detection of a colon cancer biomarker. Anal. Chem. 2022, 94, 3013–3019. [Google Scholar] [CrossRef]
- Liu, M.; Lin, Z.; Lin, J. A review on applications of chemiluminescence detection in food analysis. Anal. Chim. Acta 2010, 670, 1–10. [Google Scholar] [CrossRef]
- Aboul-Enein, H.Y.; Stefan, R.; Van Staden, J.F. Chemiluminescence-Based (Bio)Sensors an Overview. Crit. Rev. Anal. Chem. 1999, 29, 323–331. [Google Scholar] [CrossRef]
- Han, R.; Sun, Y.; Dai, Y.; Gao, D.; Wang, X.; Luo, C. A chemiluminescence aptasensor for sensitive detection of carcinoembryonic antigen based on dual aptamer-conjugates biorecognition. Sens. Actuators B Chem. 2021, 326, 128833. [Google Scholar] [CrossRef]
- Ma, C.; Cao, Y.; Gou, X.; Zhu, J. Recent progress in electrochemiluminescence sensing and imaging. Anal. Chem. 2020, 92, 431–454. [Google Scholar] [CrossRef]
- Zhou, J.; Li, Y.; Wang, W.; Tan, X.; Lu, Z.; Han, H. Metal-organic frameworks-based sensitive electrochemiluminescence biosensing. Biosens. Bioelectron. 2020, 164, 112332. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Hu, G.; Liang, W.; Yao, L.; Huang, W.; Zhang, Y.; Zhang, J.; Wang, J.; Yuan, R.; Xiao, D. An AIEgen-based 2D ultrathin metal–organic layer as an electrochemiluminescence platform for ultrasensitive biosensing of carcinoembryonic antigen. New J. Chem. 2020, 12, 5932–5941. [Google Scholar] [CrossRef]
- Huang, X.; Deng, X.; Qi, W.; Wu, D. A metal–organic framework nanomaterial as an ideal loading platform for ultrasensitive electrochemiluminescence immunoassays. New J. Chem. 2018, 42, 13558–13564. [Google Scholar] [CrossRef]
- Li, Q.; Yang, Y.; Liu, X.; Wei, Y.; Mao, C.; Niu, H.; Song, J.; Zhang, S.; Jin, B.; Jiang, M.D. A facile in situ synthesis of MIL-101-CdSe nanocomposites for ultrasensitive electrochemiluminescence detection of carcinoembryonic antigen. Sens. Actuators B Chem. 2017, 242, 1073–1078. [Google Scholar] [CrossRef]
- Huo, Y.; Li, S.; Gao, Z.; Ning, B.; Wang, Y. State-of-the-art progress of switch fluorescence biosensors based on metal-organic frameworks and nucleic acids. Mikrochim. Acta 2021, 4, 1–29. [Google Scholar] [CrossRef]
- Yang, J.; Ni, W.; Ruan, B.; Tsai, L.; Ma, N.; Shi, D.; Jiang, T.; Tsai, F. Review—Design and Synthesis of Fluorescence Sensing Metal-Organic Frameworks. ECS J. Solid State Sci. Technol. 2021, 10, 056003. [Google Scholar] [CrossRef]
- Zhao, D.; Wu, Z.; Yu, J.; Wang, H.; Li, Y.; Duan, Y. Highly sensitive microfluidic detection of carcinoembryonic antigen via a synergetic fluorescence enhancement strategy based on the micro/nanostructure optimization of ZnO nanorod arrays and in situ ZIF-8 coating. Chem. Eng. J. 2020, 383, 123230. [Google Scholar] [CrossRef]
- Lv, S.; Tang, Y.; Zhang, K.; Tang, D. Wet NH3-Triggered NH2-MIL-125(Ti) Structural Switch for Visible Fluorescence Immunoassay Impregnated on Paper. Anal. Chem. 2018, 90, 14121–14125. [Google Scholar] [CrossRef]
- Devadoss, A.; Sudhagar, P.; Terashima, C.; Nakata, K.; Fujishima, A. Photoelectrochemical biosensors: New insights into promising photoelectrodes and signal amplification strategies. J. Photochem. Photobiol. C Photochem. Rev. 2015, 24, 43–63. [Google Scholar] [CrossRef]
- Liu, X.; Zhao, Y.; Li, F. Nucleic acid-functionalized metal-organic framework for ultrasensitive immobilization-free photoelectrochemical biosensing. Biosens. Bioelectron. 2021, 173, 112832. [Google Scholar] [CrossRef]
- Li, H.; Li, Y.; Zhang, X.; Liu, P.; He, M.; Li, C.; Wang, Y. Near-infrared photoactive Yb-MOF functionalized with a large conjugate ionic liquid: Synthesis and application for photoelectrochemical immunosensing of carcinoma embryonic antigen. Nanoscale 2021, 13, 9757–9765. [Google Scholar] [CrossRef] [PubMed]
- Mauriz, E. Clinical applications of Visual Plasmonic Colorimetric sensing. Sensors 2020, 20, 6214. [Google Scholar] [CrossRef] [PubMed]
- Sha, L.; Zhu, M.; Lin, F.; Yu, X.; Dong, L.; Wu, L.; Rong, D.; Wu, S.; Xu, J. Stable DNA Aptamer–Metal–Organic Framework as Horseradish Peroxidase Mimic for Ultra-Sensitive Detection of Carcinoembryonic Antigen in Serum. Gels 2021, 7, 181. [Google Scholar] [CrossRef]
- Zeng, Y.; Wang, M.; Sun, Z.; Sha, L.; Yang, J.; Li, G. Colorimetric immunosensor constructed using 2D metal–organic framework nanosheets as enzyme mimics for the detection of protein biomarkers. J. Mater. Chem. B 2022, 10, 450–455. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Jiao, L.; Wang, H.; Xu, W.; Wu, Y.; Gu, W.; Du, D.; Lin, Y.; Zhu, C. A “sense-and-treat” ELISA using zeolitic imidazolate framework-8 as carriers for dual-modal detection of carcinoembryonic antigen. Sens. Actuators B Chem. 2019, 297, 126760. [Google Scholar] [CrossRef]






| # | MOFs | Metal Used | Organic Ligand | Surface Modifications and Added Materials | Types of Sensing | LOD/Detection Range | Reference |
|---|---|---|---|---|---|---|---|
| 1 | Pd/Cd MOFs | Pd/Cd | 2-aminoterephthalic acid | Immobilization of labels for secondary anti-CEA and secondary anti-AFP antibody | Electrochemical | 0.03 pg/mL and 0.1 pg/mL | [38] |
| 2 | Ce MOF | Ce | 1,3,5-benzenetricarboxylic acid | Hyaluronic acid was coated on the surface of a Ce MoF that was loaded with silver nanoparticles (AgNPs) and horseradish peroxidase | Electrochemical | 0.2 pg/mL | [39] |
| 3 | Ag MOF | Ag | Terephthalic acid | A Ag MOF was dopped using gold nanoparticles and labelled with anti-CEA | Electrochemical | 8.0 fg/mL | [40] |
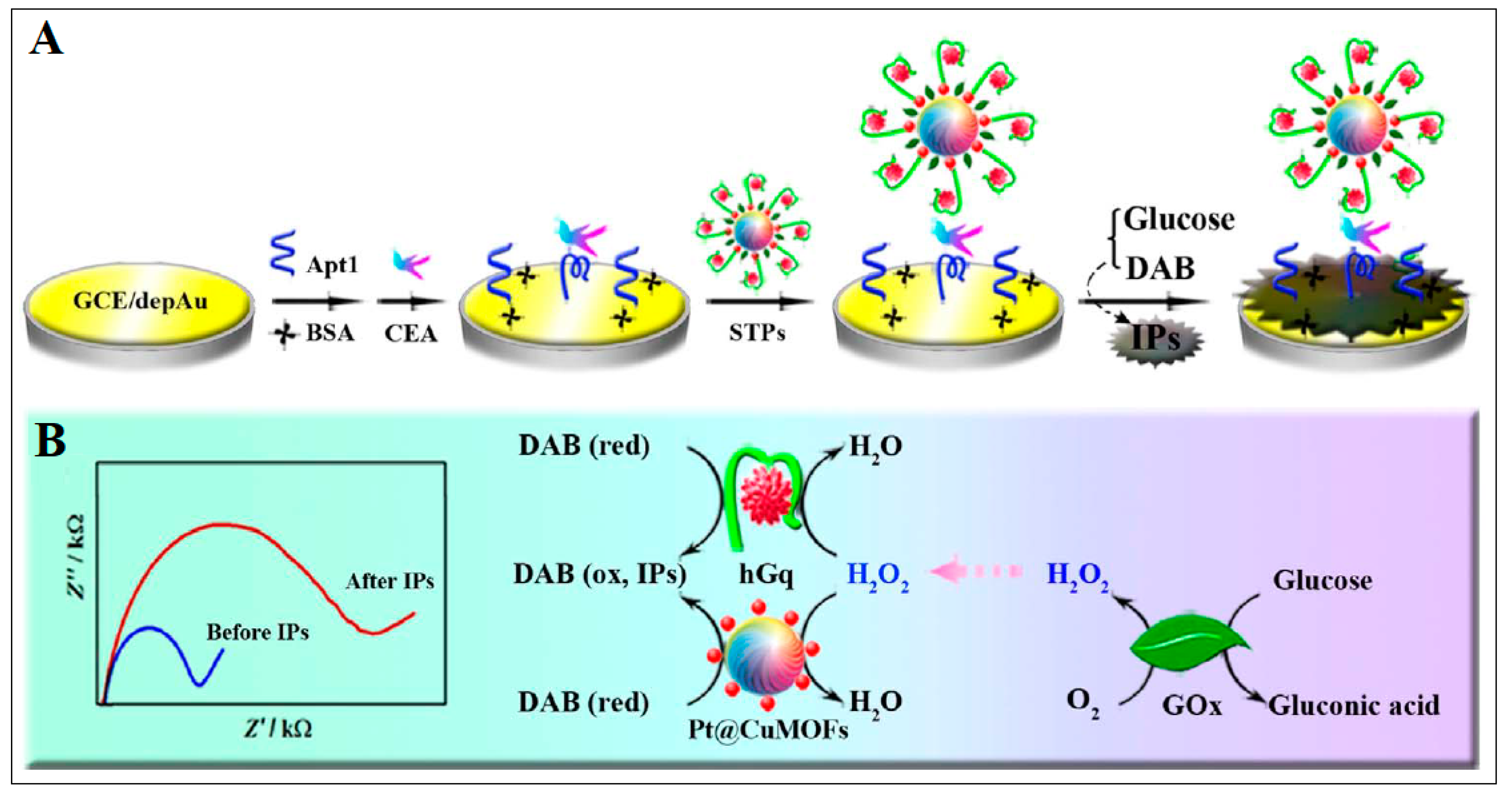
| 4 | Cu MOF | Cu | 2-amino terephthalic acid | Platinum nanoparticles (PtNPs) were linked to a Cu MOF; then, a CEA aptamer was loaded onto Pt@CuMOFs; finally, this was bound with hemin to form hemin@G-quadruplex (hGq) with mimicking peroxidase activity | Electrochemical | 0.023 pg/mL | [41] |
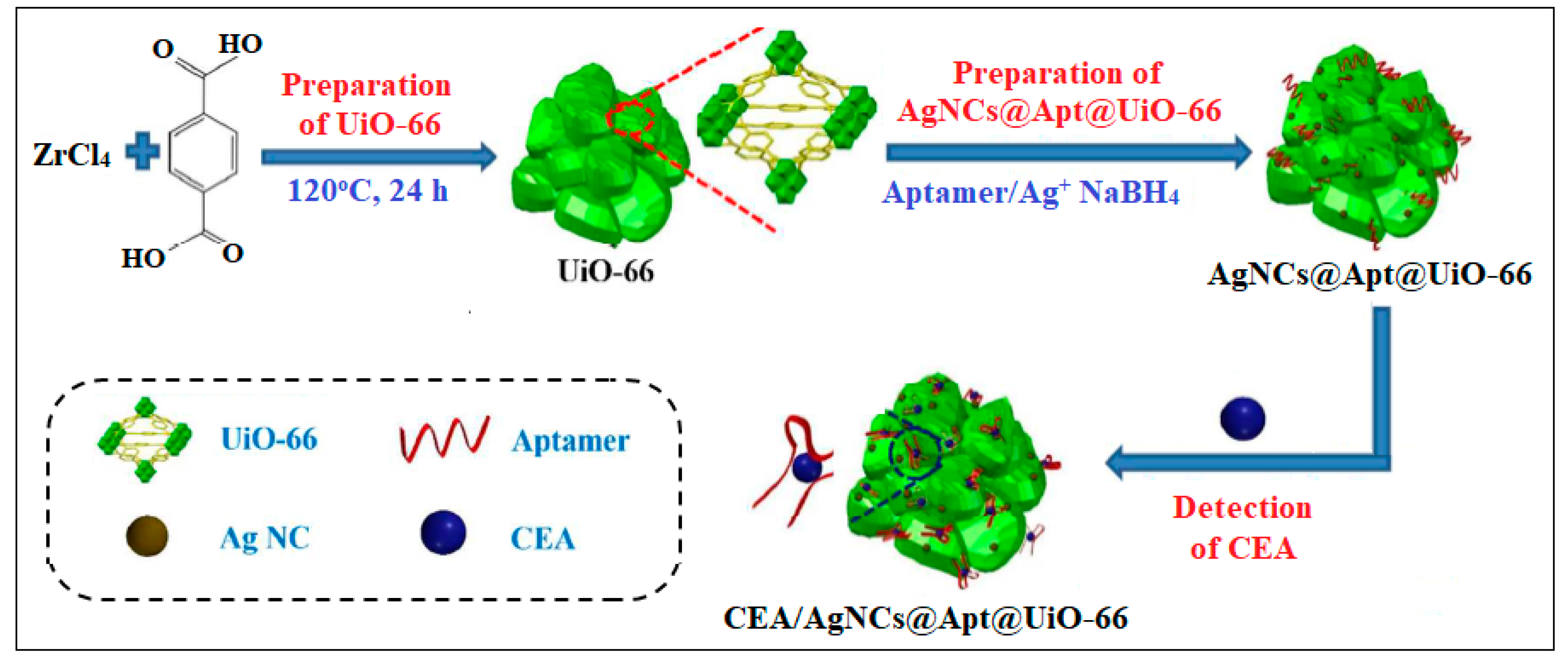
| 5 | UiO-66 | Zr | 2-Aminoterephthalic acid | MOF embedded with silver nanoclusters (AgNCs) using the carcinoembryonic antigen (CEA)-targeted aptamer as template | A. Electrochemical 1—Impedance | 8.88 pg/mL | [42] |
| 2—Differential pulse voltammetry | 4.93 pg/mL | ||||||
| B. SPR | 0.3 ng/mL | ||||||
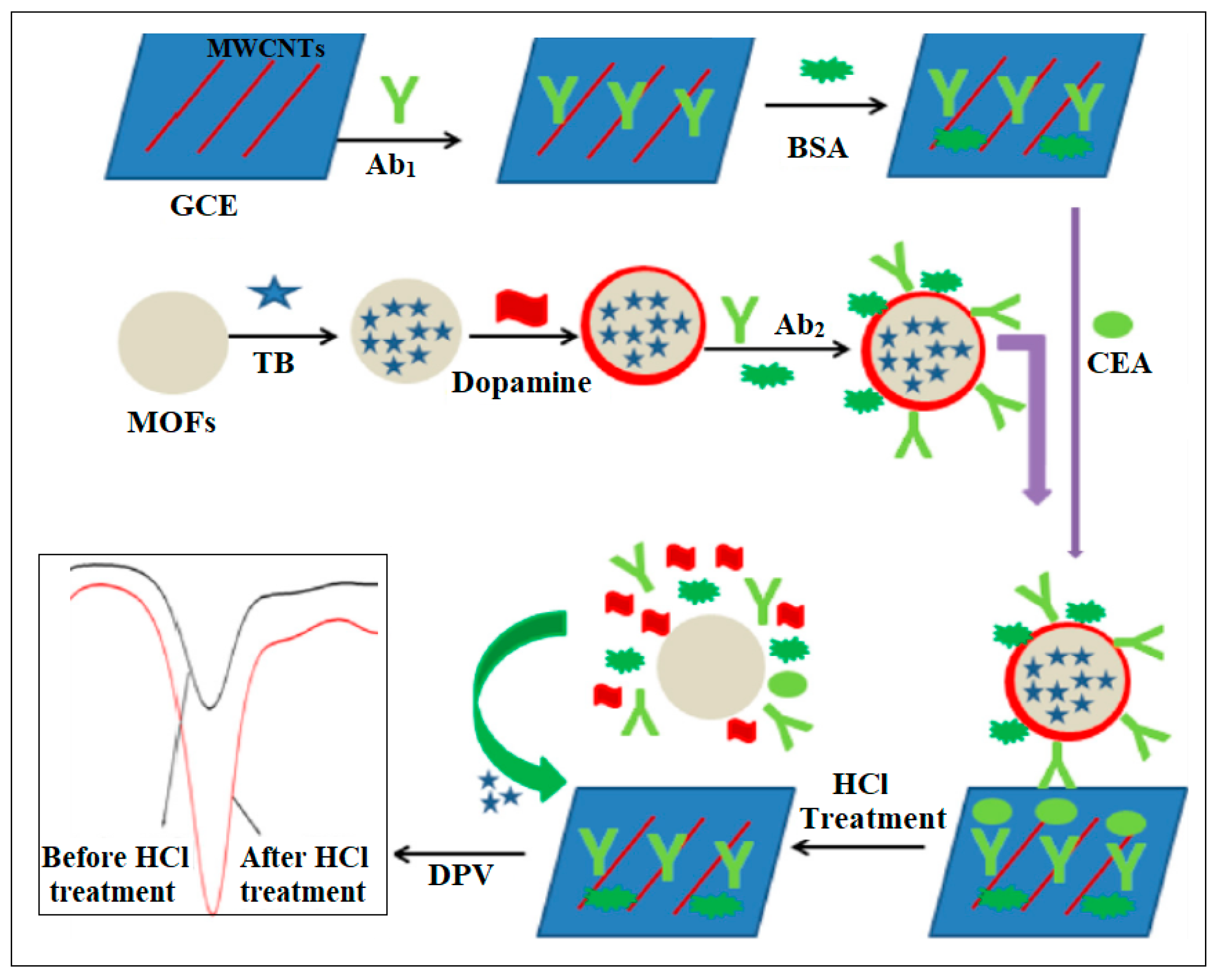
| 6 | Fe MOF | Fe | 1,4-dicarboxybenzene | Self-polymerized dopamine-decorated AuNPs were loaded on an Fe MOF and attached to a CEA aptamer | Electrochemical | 0.33 fg /mL | [43] |
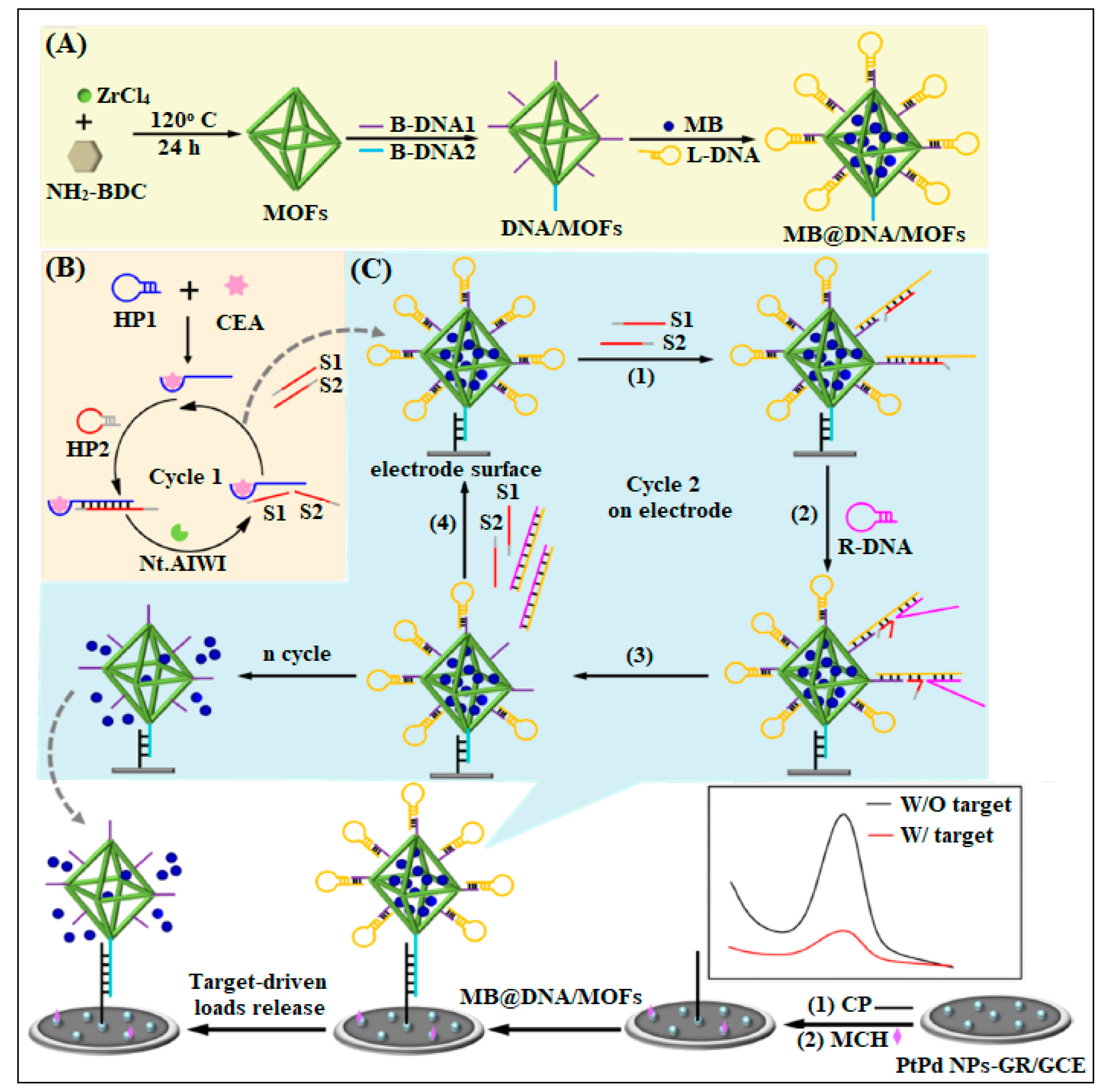
| 7 | UiO-66-NH2 | Zr | 2-Aminoterephthalic acid | By using MOF as nanocarrier of electroactive molecules (methylene blue, MB) and functionalized by the assembled DNA | Electrochemical | 16 fg/mL | [44] |
| 8 | Pd MOF | Pd | 2-amino-1,4-benzenedicarboxylic acid (H2N-BDC) | Dendritic (hybridization chain reaction) HCR-triggered DNA nanostructure was labeled with Pb MOF | Electrochemical | 0.333 pg /mL | [45] |
| 9 | Zif-8 | Zn | Methyl Imidazole | It is based on the use of a Au NP-modified ZIF-8 and ordered mesoporous carbon (OMC) | Electrochemical | 1.3 pg/mL | [46] |
| 10 | Cu MOF | Cu | Terephthalic acid | Toluidine blue (TB) loaded mesoporous Cu MOFs with polydopamine (PDA) coating were employed as a signal probe | Electrochemical | 3.0 fg/mL | [47] |
| 11 | Zr MOF | Zr | 4′,4‴,4′′′′-nitrilotris [1,1′-biphenyl]-4-carboxylic acid (H3NBB) | Aptamers of CEA, thrombin, and kanamycin were separately immobilized on the MOF | Electrochemical | 0.40 pg/mL 0.21 pg/mL 0.37 pg/mL | [23] |
| 12 | Sm MOF | Sm | Trimesic acid (TMA), meso-tetra(4-carboxyphenyl)porphine (TCPP), and 1,3,6,8-tetra(4-carboxylphenyl) pyrene(TBPy) | Anti-CEA immobilization | Electrochemical | SmTMA, SmTBPy, and SmTCPP MOF-based immunosensors are determined to be 0.001, 0.05, and 0.01 U/mL, respectively | [48] |
| 13 | MIL-88B | Fe | 2-aminoterephthalic acid | Hemin-modified Mil-88B Immobilization of CEA aptamer | CL | 1.5 × 10−3 ng/mL | [51] |
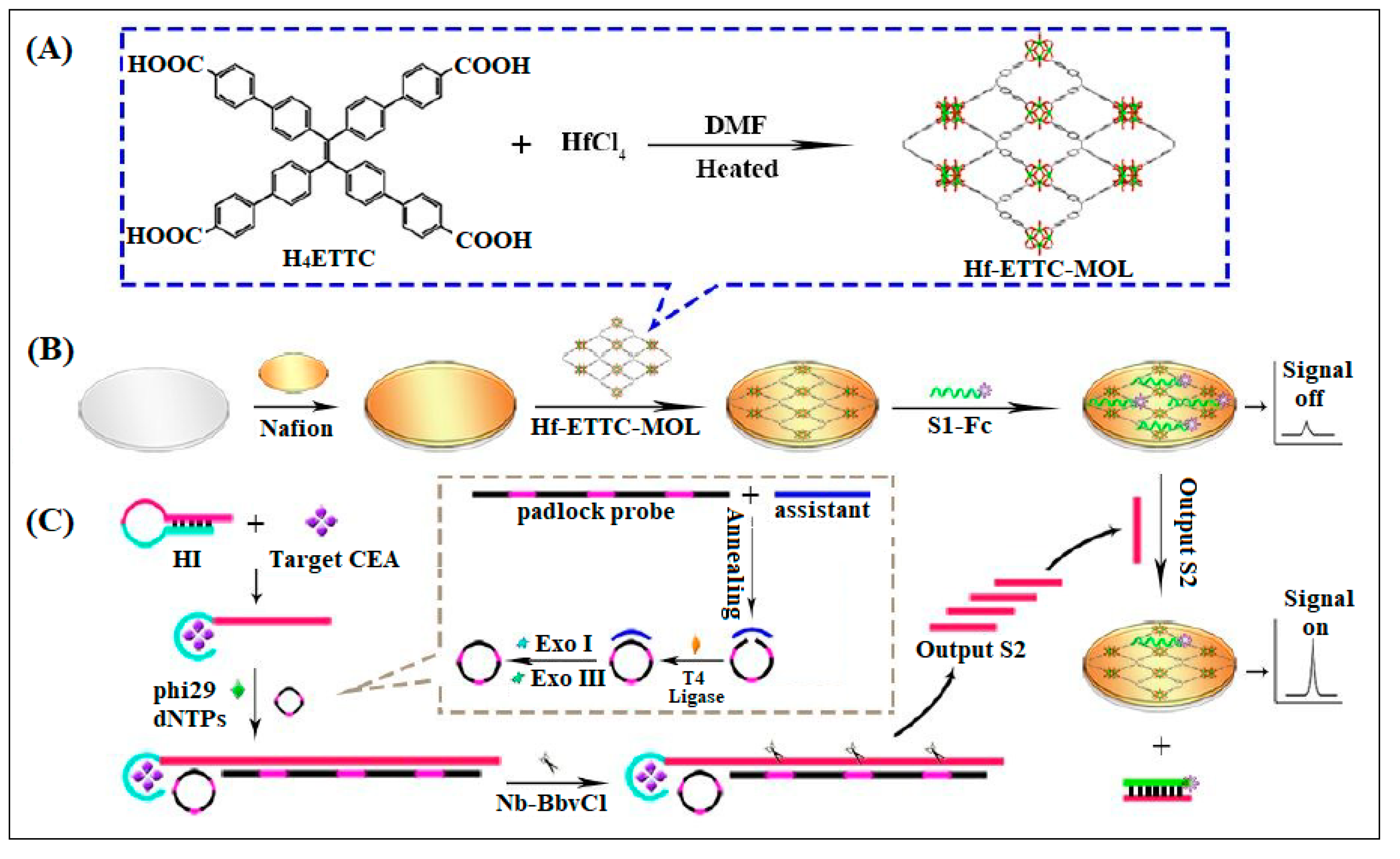
| 14 | Hf-ETTC-MOL | Hf | H4ETTC (H4ETTC = 4′,4′′′,4′′′′′,4′′′′′′′-(ethene-1,1,2,2-tetrayl)tetrakis(([1,1′-biphenyl]-4-carboxylic acid))) | A two-dimensional (2D) ultrathin metal–organic layer (MOL) was used as a platform for CEA detection | ECL | 0.63 fg/mL | [54] |
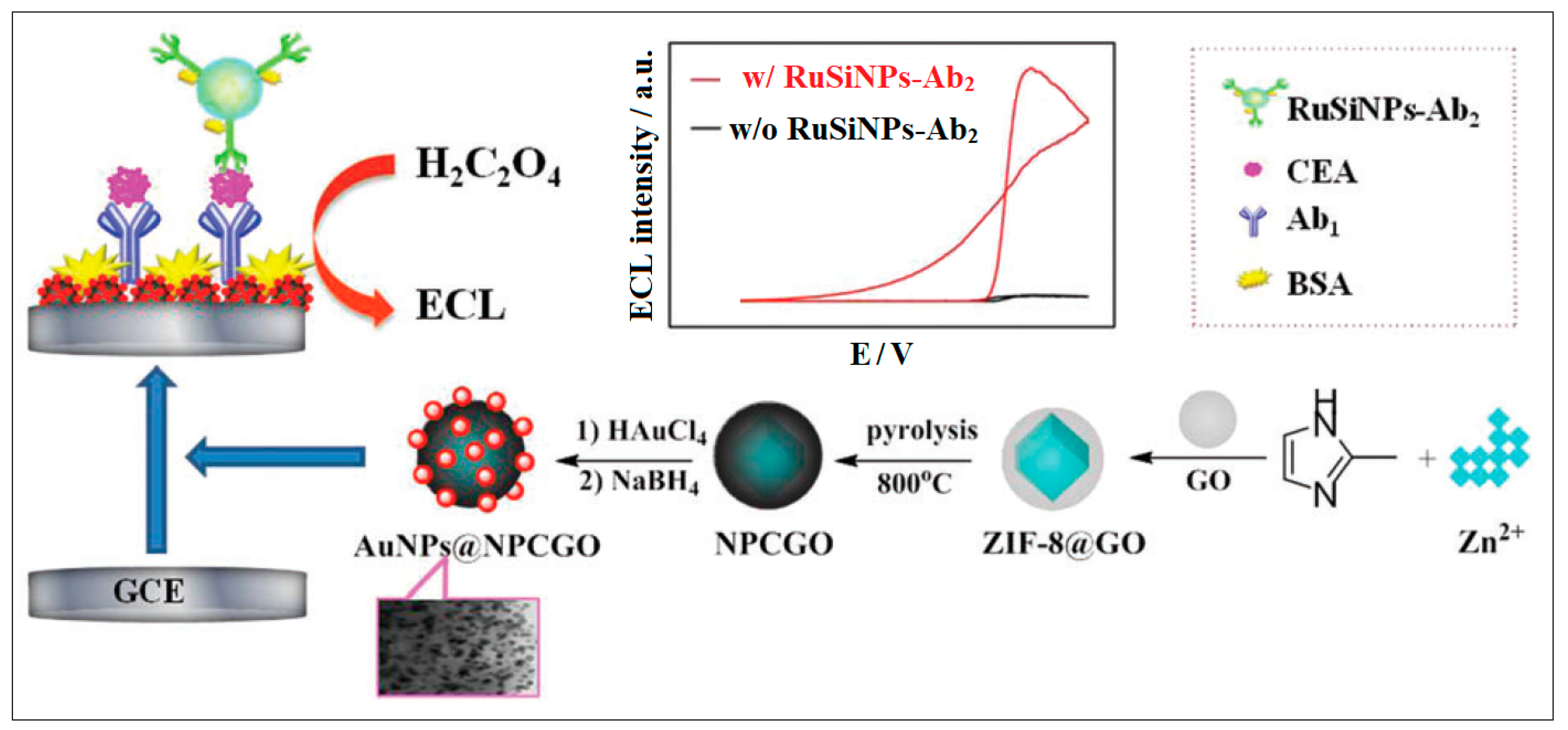
| 15 | Zif-8 | Zn | Methyl imidazole | ZIF-8 and graphene oxide (GO) to form a ZIF-8@GO composite. Then, the in situ growth of AuNPs due to the p–p interaction between AuNPs and Zif-8@GO took place | ECL | 0.003 ng/mL | [55] |
| 16 | MIL-101 | Cr | Terephthalic acid | Prepared MIL-101-CdSe nanocomposites antibodies for CEA was linked | ECL | 0.33 fg/mL | [56] |
| 17 | Zif-8 | Zn | Methyl Imidazole | Formation of sandwich immunoassay by using Zif-8 coated with ZnO/PAA (polyacrylic acid) nanorod arrays | Fluorescence | 0.01 pg/mL | [59] |
| 18 | NH2-MIL-125(Ti) | Ti | 2- amino-1,4-benzenedicarboxylic acid (H2N-BDC) | Gold nanoparticles heavily functionalized with glutamate dehydrogenase (GDH) and secondary antibody were used for generation of wet NH3 | Fluorescence | 0.041 ng/mL | [60] |
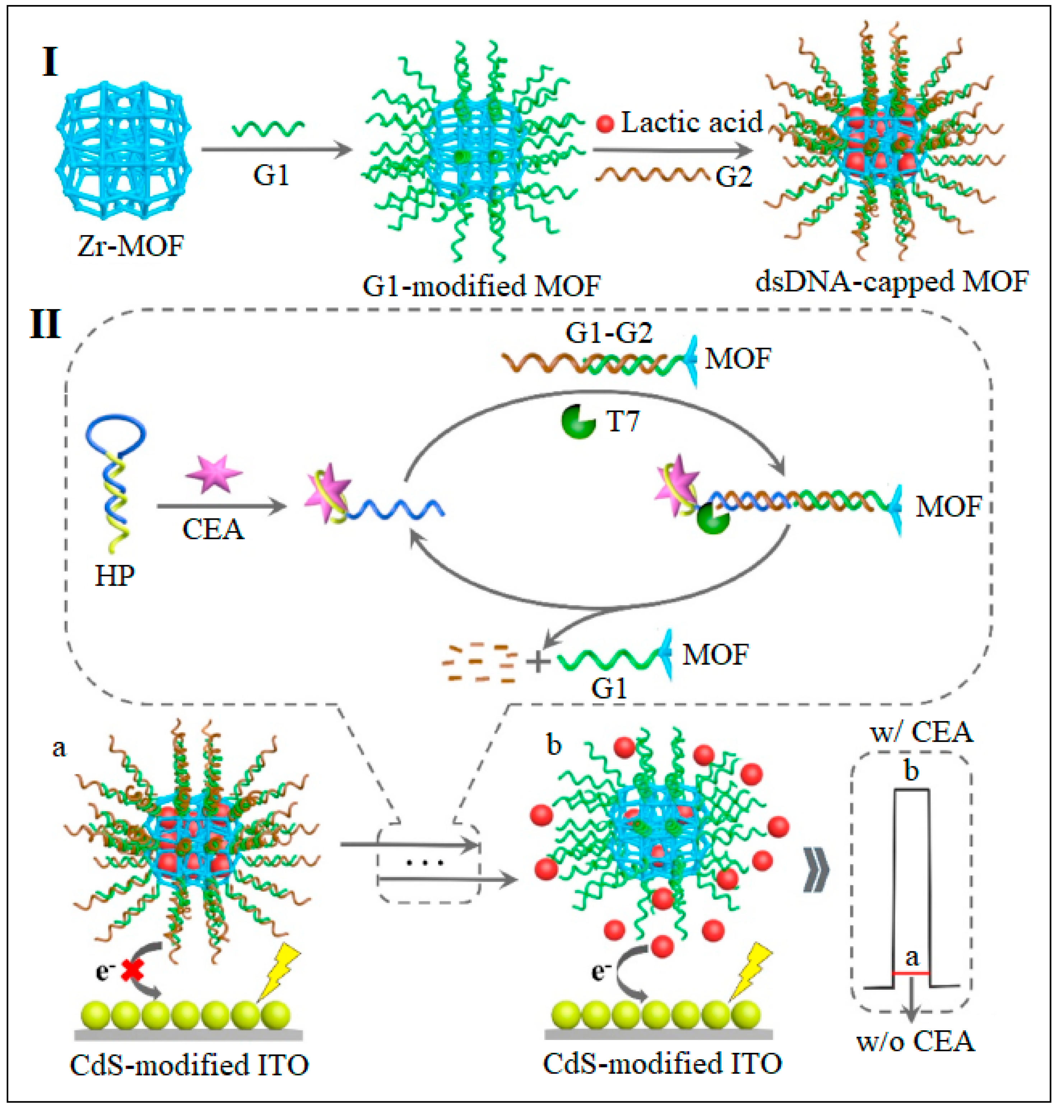
| 19 | UiO-66-NH2 | Zr | 2-Aminoterephthalic acid | MOFs loaded with lactic acid and attached to dsDNA | PEC | 0.36 fg/mL | [62] |
| 20 | Yb MOF | Yb | 1,1′-(1,5-dihydropyrene-2,7-diyl)bis(3-(4-carboxybenzyl)-1H-imidazol-3-ium) bromide [DDPDBCBIm(Br)2] ionic liquid | Combined with gold nanoparticles | PEC | 0.005–15 ng/mL | [63] |
| ch21 | PCN-222 | Zr- and Fe-based MOF | meso-tetra (4-carboxyphenyl) porphine ferric chloride (Fe-TCPP) | A CEA aptamer was immobilized on PCN-222 | Colorimetric | 3.3 pg/mL | [65] |
| 22 | Cu TCPP | Cu-MOF | TCPP | Gold nanoparticle immobilization and aptamer | Colorimetric | 1 pg/mL to 1000 ng/mL | [66] |
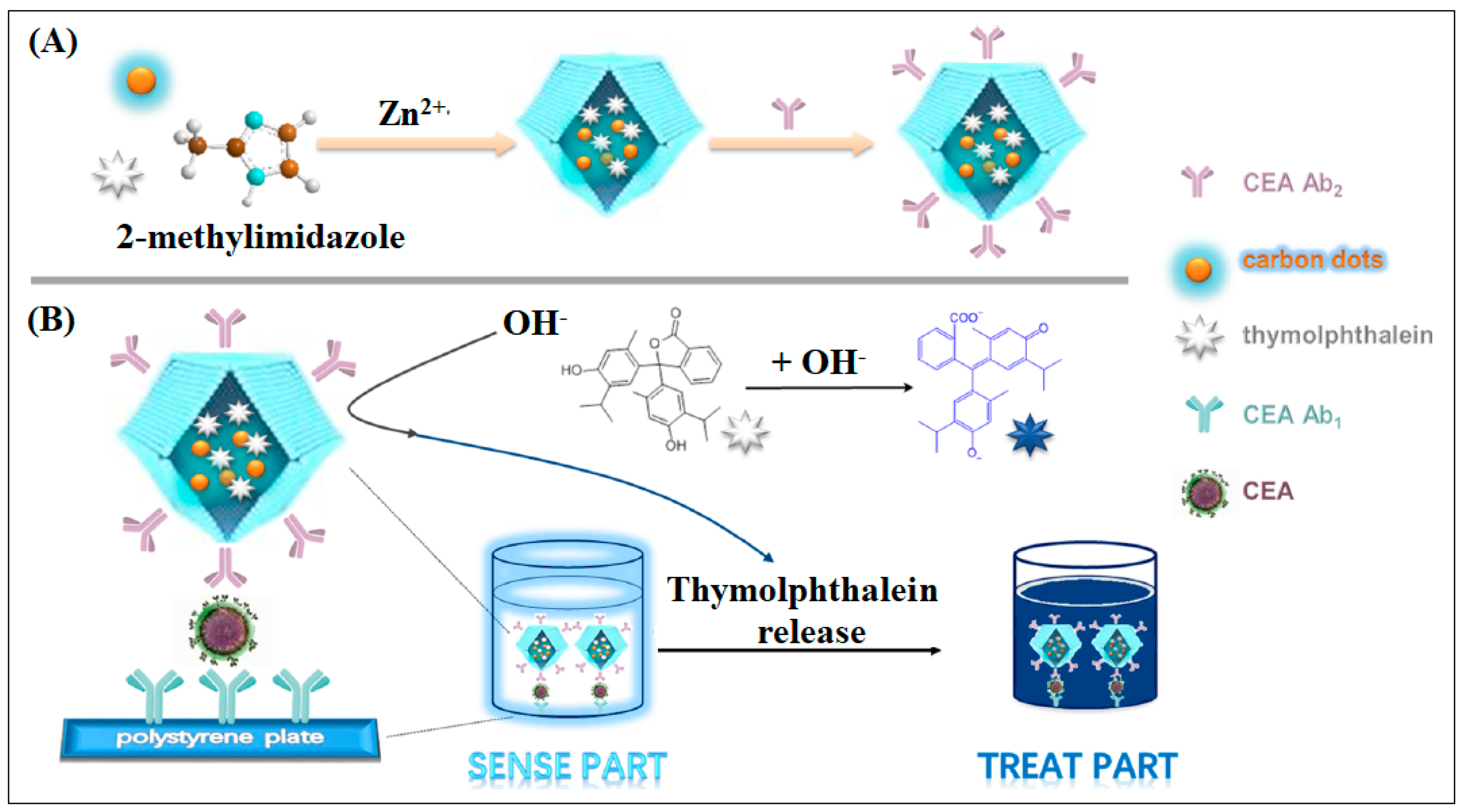
| 23 | Zif-8 | Zn | Methyl Imidazole | ZIF-8 used as the carrier to deliver the tracer agent carbon dots (CDs) and the “drug” thymolphthalein (TP) | ELIZA | 10 pg/mL | [67] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ibrahim, M.R.; Greish, Y.E. MOF-Based Biosensors for the Detection of Carcinoembryonic Antigen: A Concise Review. Molecules 2023, 28, 5970. https://doi.org/10.3390/molecules28165970
Ibrahim MR, Greish YE. MOF-Based Biosensors for the Detection of Carcinoembryonic Antigen: A Concise Review. Molecules. 2023; 28(16):5970. https://doi.org/10.3390/molecules28165970
Chicago/Turabian StyleIbrahim, May R., and Yaser E. Greish. 2023. "MOF-Based Biosensors for the Detection of Carcinoembryonic Antigen: A Concise Review" Molecules 28, no. 16: 5970. https://doi.org/10.3390/molecules28165970
APA StyleIbrahim, M. R., & Greish, Y. E. (2023). MOF-Based Biosensors for the Detection of Carcinoembryonic Antigen: A Concise Review. Molecules, 28(16), 5970. https://doi.org/10.3390/molecules28165970






