Following the Decomposition of Hydrogen Peroxide in On-Site Mixture Explosives: Study of the Effect of the Auxiliary Oxidising Agent and Binder
Abstract
1. Introduction
- Manufacture from non-renewable resources via highly energy-intensive processes;
- Emission of large amounts of toxic and highly corrosive gases (carbon monoxide, nitrogen oxides) upon detonation [12];
- Gradual decomposition, particularly in the case of liquid nitric acid esters, as well as gradual leakage of liquid nitroesters from the explosives, necessitating the use of stabilising and anti-leakage agents;
- Susceptibility to theft and subsequent criminal misuse, due to maintaining the ability to detonate even following improper storage or a misfire during blasting [15];
2. Results and Discussion
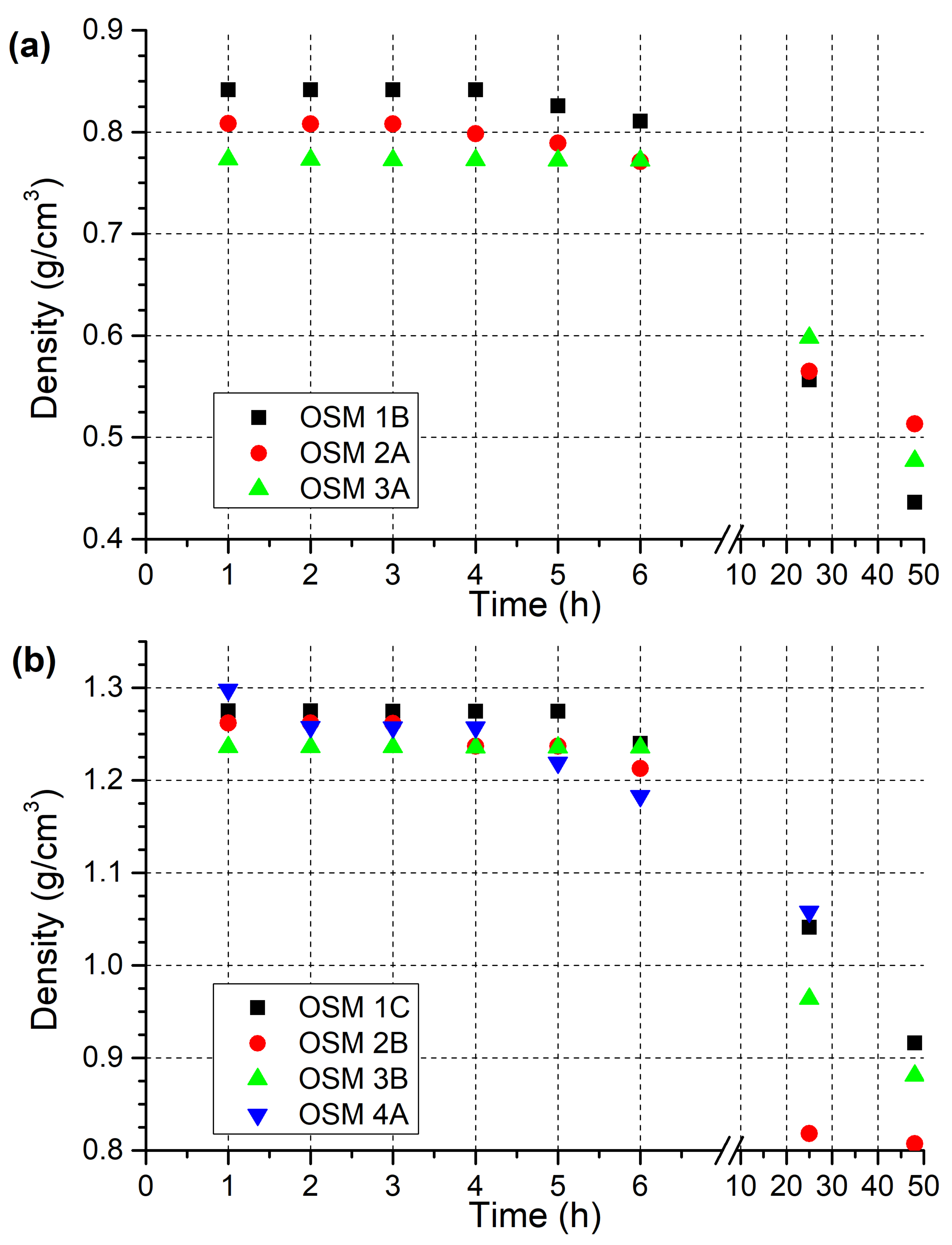
2.1. OSM Density Investigation
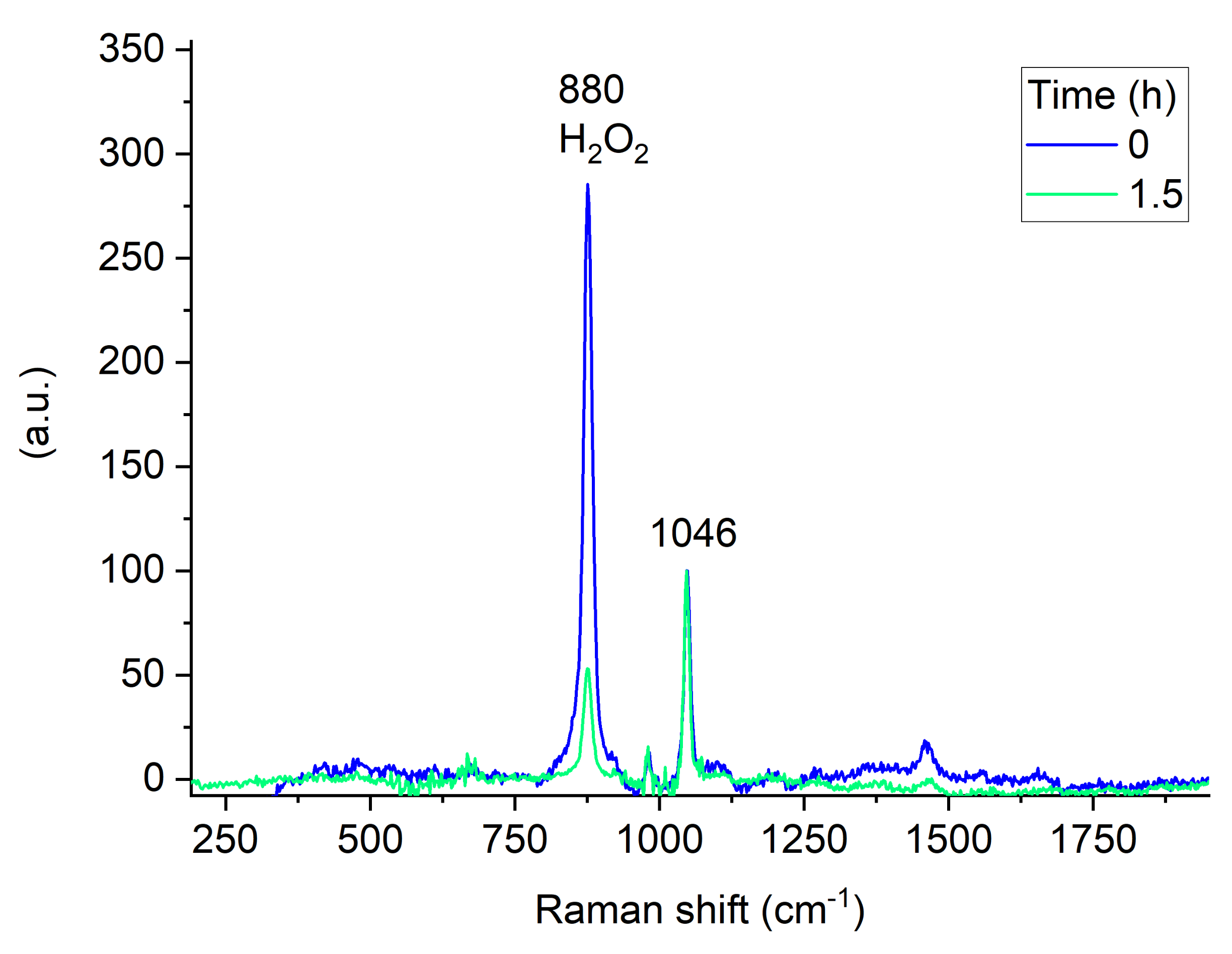
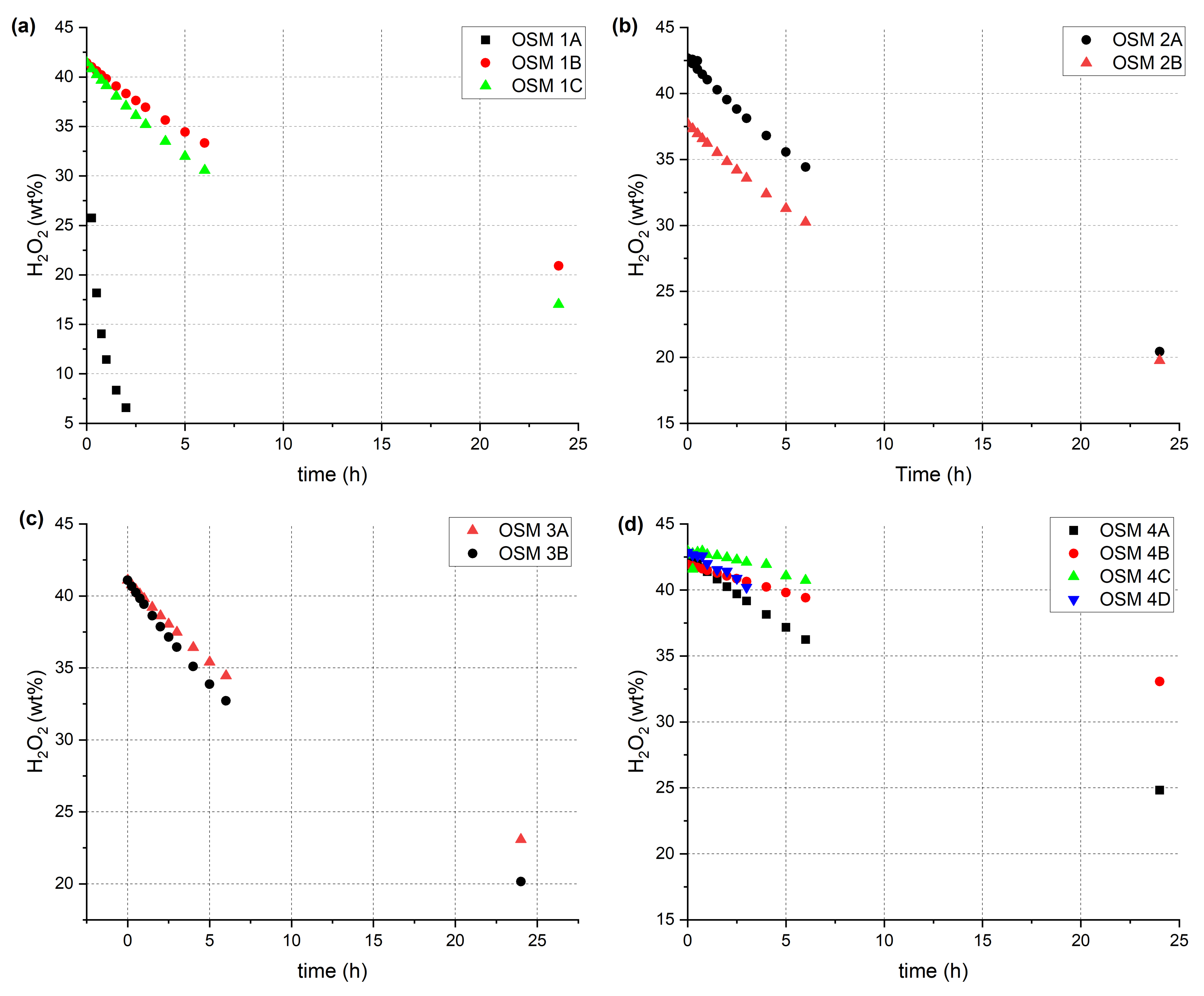
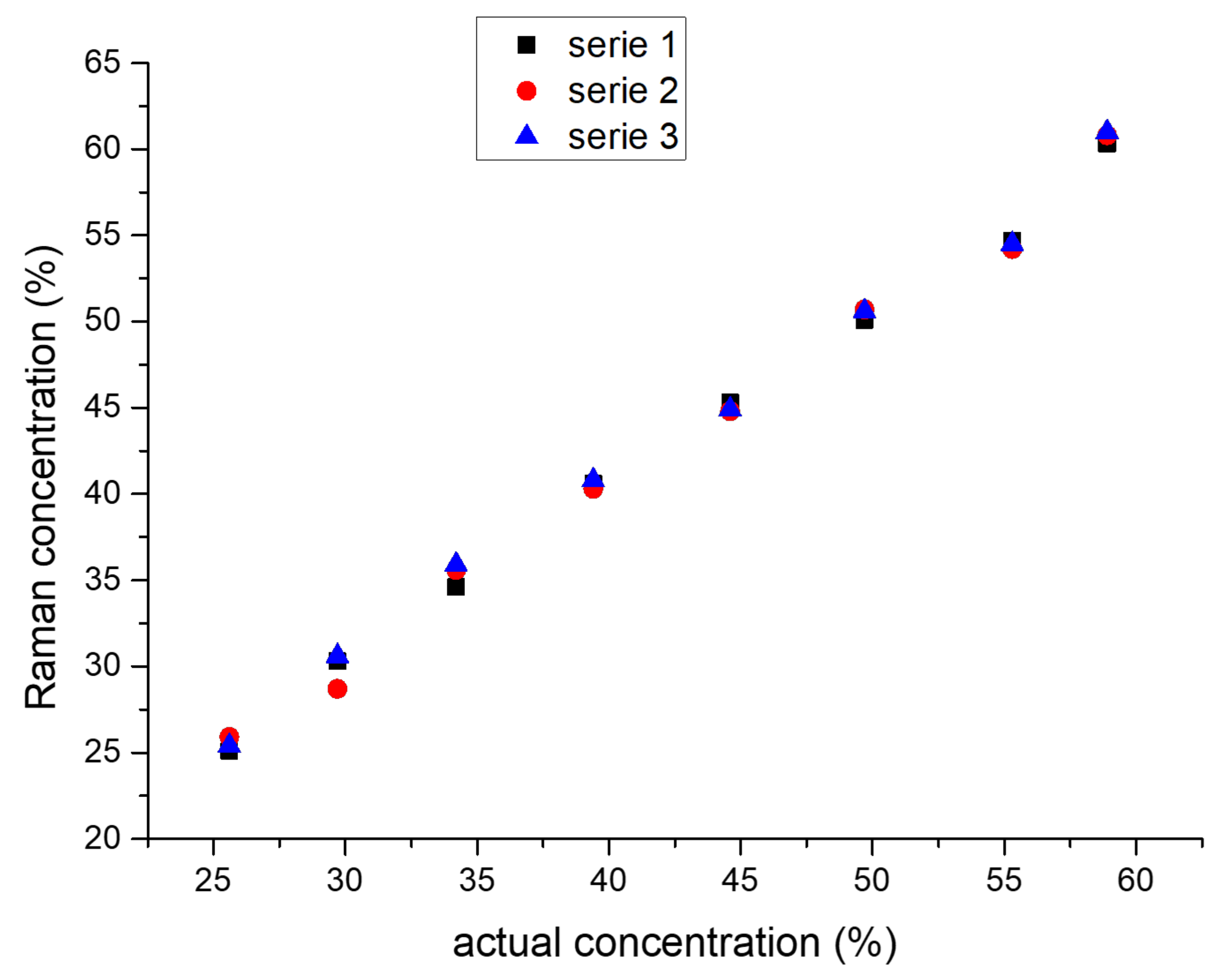
2.2. Time-Resolved Raman Spectroscopy
3. Materials and Methods
3.1. Preparation of On-Site Mixture Samples
3.2. Density Measurement
3.3. Time-Resolved Raman Spectroscopy
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AN | Ammonium nitrate |
| CCD | Charge-coupled device |
| CN | Calcium nitrate |
| EX | Explosives |
| GA | Gum arabic |
| GG | Guar gum |
| HEC | Hydroxyethylcellulose |
| HTP | Concentrated hydrogen peroxide |
| LBG | Locust bean gum |
| MS | Glass microspheres |
| OSM | On-site mixed |
| PM | Polymer microspheres |
| PN | Potassium nitrate |
| RPM | Rotations per minute |
| SN | Sodium nitrate |
| UltrAN | Ammonium nitrate porous prill |
| VoD | Velocity of detonation |
Appendix A

References
- Cardu, M.; Dompieri, M.; Seccatore, J. Complexity analysis of blast-induced vibrations in underground mining: A case study. Int. J. Min. Sci. Technol. 2012, 22, 125–131. [Google Scholar] [CrossRef]
- Leng, Z.; Fan, Y.; Gao, Q.; Hu, Y. Evaluation and optimization of blasting approaches to reducing oversize boulders and toes in open-pit mine. Int. J. Min. Sci. Technol. 2020, 30, 373–380. [Google Scholar] [CrossRef]
- Adushkin, V.; Solov’ev, S.; Spivak, A.; Khazins, V. Open pit mining with blasting: Geoecological aftermath. J. Min. Sci. 2020, 56, 309–321. [Google Scholar] [CrossRef]
- Khomenko, O.; Kononenko, M.; Myronova, I.; Savchenko, M. Application of the emulsion explosives in the tunnels construction. In Proceedings of the E3S Web of Conferences, Berdiansk, Ukraine, 3–7 September 2019; EDP Sciences: Les Ulis, France, 2019; Volume 123, p. 01039. [Google Scholar]
- Singh, R.K.; Sawmliana, C.; Hembram, P. Time-constrained demolition of a concrete cofferdam using controlled blasting. Innov. Infrastruct. Solut. 2021, 6, 20. [Google Scholar] [CrossRef]
- Bataev, I.; Tanaka, S.; Zhou, Q.; Lazurenko, D.; Junior, A.J.; Bataev, A.; Hokamoto, K.; Mori, A.; Chen, P. Towards better understanding of explosive welding by combination of numerical simulation and experimental study. Mater. Des. 2019, 169, 107649. [Google Scholar] [CrossRef]
- Lange, R.L.; Reid, M.S.; Tresch, D.D.; Keelan, M.H.; Bernhard, V.M.; Coolidge, G. Nonatheromatous ischemic heart disease following withdrawal from chronic industrial nitroglycerin exposure. Circulation 1972, 46, 666–678. [Google Scholar] [CrossRef] [PubMed]
- Hogstedt, C.; Davidsson, B. Nitroglycol and nitroglycerine exposure in a dynamite industry 1958–1978. Am. Ind. Hyg. Assoc. J. 1980, 41, 373–375. [Google Scholar] [CrossRef]
- Gori, T.; Parker, J.D. Nitrate-induced toxicity and preconditioning: A rationale for reconsidering the use of these drugs. J. Am. Coll. Cardiol. 2008, 52, 251–254. [Google Scholar] [CrossRef] [PubMed]
- Farahani, M.V.; Soltani, S.; Marashi, S.M. Hyperinsulinemic Euglycemia Therapy For Acute Nitroglycerin Poisoning Poisoning: Case Report. Int. J. Med. Toxicol. Forensic Med. 2022, 12, 37176. [Google Scholar] [CrossRef]
- Tokutake, S.; Minami, M.; Katsumata, M.; Inagaki, H. The effect of organic nitrates on neurogenic release of dopamine: Relevance to toxicity of dynamite ingredients in the central nervous system. Biog. Amines 1993, 10, 19–26. [Google Scholar]
- Zawadzka-Małota, I. Testing of mining explosives with regard to the content of carbon oxides and nitrogen oxides in their detonation products. J. Sustain. Min. 2015, 14, 173–178. [Google Scholar] [CrossRef]
- Liu, L.; Yan, L.; Dong, B.; Liu, W.; Yi, W.; Zhao, K. Detection and Recognition Method of Misfire for Chamber (Deep-Hole) Blasting Based on RFID. IEEE Access 2019, 7, 170144–170156. [Google Scholar] [CrossRef]
- Misfired Explosives Initiated during Excavation. 2020. Available online: https://www.rshq.qld.gov.au/safety-notices/explosives/misfired-explosives-initiated-during-excavation (accessed on 29 June 2023).
- Logrado, L.P.L.; Silva, M.N.; Laboissiere, J.C.A.; Braga, J.W.B. Profile of explosives’s use in ATMs/cash safes robberies in Brazil. J. Forensic Sci. 2022, 67, 1441–1449. [Google Scholar] [CrossRef]
- Guoshun, Z. Causes and lessons of five explosion accidents. J. Loss Prev. Process. Ind. 2000, 13, 439–442. [Google Scholar] [CrossRef]
- Negovanović, M.; Kričak, L.; Milanović, S.; Đokić, N.; Simić, N. Ammonium nitrate explosion hazards. Podzemn. Rad. 2015, 27, 49–63. [Google Scholar] [CrossRef]
- Budakoğlu, E. Seismological investigations of fireworks factory explosions in Hendek-Sakarya (Turkey). J. Seismol. 2022, 26, 283–299. [Google Scholar] [CrossRef]
- Bluhm, H.F. Ammonium Nitrate Emulsion Blasting Agent and Method of Preparing Same. U.S. Patent 3,447,978, 3 August 1969. [Google Scholar]
- Sheffield, S.A.; Dattelbaum, D.M.; Stahl, D.B.; Gibson, L.L.; Bartram, B.D.; Engelke, R. Shock Initiation and Detonation Study on High Concentration H2O2/H2O Solutions Using In-Situ Magnetic Gauges; Technical Report; Los Alamos National Lab. (LANL): Los Alamos, NM, USA, 2010.
- Shanley, E.; Kaufmann, O. Peroxide-Glycerol Explosive. U.S. Patent US2452074A, 26 October 1948. [Google Scholar]
- Jia, X.; Sun, F.; Fei, Y.; Jin, M.; Zhang, F.; Xu, W.; Shi, N.; Lv, Z. Explosion characteristics of mixtures containing hydrogen peroxide and working solution in the anthraquinone route to hydrogen peroxide. Process. Saf. Environ. Prot. 2018, 119, 218–222. [Google Scholar] [CrossRef]
- Rososhek, A.; Efimov, S.; Maler, D.; Virozub, A.; Krasik, Y.E. Shockwave generation by electrical explosion of cylindrical wire arrays in hydrogen peroxide/water solutions. Appl. Phys. Lett. 2020, 116, 243702. [Google Scholar] [CrossRef]
- Halleux, F.; Pons, J.F.; Wilson, I.; Simoens, B.; Van Riet, R.; Lefebvre, M. Detonation performance of urea-hydrogen peroxide (UHP). Propellants Explos. Pyrotech. 2023, 48, e202300011. [Google Scholar] [CrossRef]
- Bouillet, E.; Colery, J.C.; Declerck, C.; Ledoux, P. Process for the Manufacture of Explosive Cartridges, and Explosive Cartridges Obtained Using the Said Process. U.S. Patent 4,942,800, 24 July 1990. [Google Scholar]
- Nikolczuk, K.; Maranda, A.; Mertuszka, P.; Fuławka, K.; Wilk, Z.; Koślik, P. Measurements of the VOD of selected mining explosives and novel “Green Explosives” using the continuous method. Cent. Eur. J. Energetic Mater. 2019, 16, 468–481. [Google Scholar] [CrossRef]
- Araos, M.; Onederra, I. Detonation characteristics of a NOx-free mining explosive based on sensitised mixtures of low concentration hydrogen peroxide and fuel. Cent. Eur. J. Energetic Mater. 2017, 14, 759–774. [Google Scholar] [CrossRef]
- Paszula, J.; Maranda, A.; Nikolczuk, K.; Giercuszkiewicz, A. Modification of the Detonation Parameters of Mining Explosives Containing Hydrogen Peroxide and Aluminium Powder. Cent. Eur. J. Energetic Mater. 2021, 18, 477–491. [Google Scholar] [CrossRef]
- Onederra, I.; Araos, M. Preliminary quantification of the in situ performance of a novel hydrogen peroxide based explosive. Min. Technol. 2017, 126, 113–122. [Google Scholar] [CrossRef]
- Polis, M.; Nikolczuk, K.; Maranda, A.; Stolarczyk, A.; Jarosz, T. Theft-safe explosive mixtures based on hydrogen peroxide: Study of properties and built-in self-deactivation kinetics. Materials 2021, 14, 5818. [Google Scholar] [CrossRef]
- Saxby, J. Minerals in coal. In Organic Matter and Mineralisation: Thermal Alteration, Hydrocarbon Generation and Role in Metallogenesis; Springer: Berlin/Heidelberg, Germany, 2000; pp. 314–328. [Google Scholar]
- Bakalarz, A. Chemical and mineral analysis of flotation tailings from stratiform copper ore from lubin concentrator plant (SW Poland). Miner. Process. Extr. Metall. Rev. 2019, 40, 437–446. [Google Scholar] [CrossRef]
- Shanley, E.S.; Greenspan, F.P. Highly concentrated hydrogen peroxide. Ind. Eng. Chem. 1947, 39, 1536–1543. [Google Scholar] [CrossRef]
- den Otter, H. Derivatives of the oxidation products of glycerol. Recl. Des Trav. Chim. Des Pays Bas 1937, 56, 474–491. [Google Scholar] [CrossRef]
- Faroppa, M.L.; Musci, J.J.; Chiosso, M.E.; Caggiano, C.G.; Bideberripe, H.P.; Fierro, J.L.G.; Siri, G.J.; Casella, M.L. Oxidation of glycerol with H2O2 on Pb-promoted Pd/γ-Al2O3 catalysts. Chin. J. Catal. 2016, 37, 1982–1990. [Google Scholar] [CrossRef]
- Siddiqui, S.; Keswani, M.; Brooks, B.; Fuerst, A.; Raghavan, S. A study of hydrogen peroxide decomposition in ammonia-peroxide mixtures (APM). Microelectron. Eng. 2013, 102, 68–73. [Google Scholar] [CrossRef]
- Ogata, Y.; Tomizawa, K.; Adachi, K. Photo-oxidation of ammonia with aqueous hydrogen peroxide. Mem. Fac. Eng. Nagoya Univ. 1981, 33, 58–65. [Google Scholar]
- Cass, O.W.; Paris, J.P.; Stock, A.M. Research on The Stability of High Strength H2O2; Du Pont de Nemours (ei) and Co.: Wilmington, DE, USA, 1966. [Google Scholar]
- Stewart, S.; Bell, S.; McAuley, D.; Baird, I.; Speers, S.; Kee, G. Determination of hydrogen peroxide concentration using a handheld Raman spectrometer: Detection of an explosives precursor. Forensic Sci. Int. 2012, 216, e5–e8. [Google Scholar] [CrossRef] [PubMed]
- Kramarczyk, B.; Mertuszka, P. Study of the influence of sensitizer content on the density of a bulk emulsion explosive used in underground operations. Cent. Eur. J. Energetic Mater. 2021, 18, 429–447. [Google Scholar]

| Fuels | Auxiliary Substances | VoD [m·s−1] | Ref. |
|---|---|---|---|
| Glycerine | ammonium nitrate, glass microspheres | 5400–5700 | [26] |
| Glycerine | glass microspheres/polymer microspheres/gas bubbles | 3000–5500 | [27] |
| Glycerine, Al | ammonium nitrate, glass microspheres | 3600–5500 | [28] |
| Glycerine | glass microspheres | 2600–5100 | [29] |
| Glycerine, Al | ammonium nitrate, glass microspheres | 4400–5200 | [30] |
| OSM: a | 1A | 1B | 1C | 2A | 2B | 3A | 3B | 4A | 4B | 4C | 4D |
|---|---|---|---|---|---|---|---|---|---|---|---|
| UltrAN | 9.52 | - | - | - | - | - | - | - | - | - | - |
| AN | - | 9.52 | 9.52 | - | - | - | - | - | - | - | - |
| SN | - | - | - | 9.52 | 9.52 | - | - | - | - | - | - |
| PN | - | - | - | - | - | 9.52 | 9.52 | - | - | - | - |
| CN | - | - | - | - | - | - | - | 9.52 | 9.52 | 9.52 | 9.52 |
| GG | 2.91 | 2.91 | 2.91 | 2.91 | 2.91 | 2.91 | 2.91 | 2.91 | - | - | - |
| LBG | - | - | - | - | - | - | - | - | 2.91 | - | - |
| HEC | - | - | - | - | - | - | - | - | - | 2.91 | - |
| GA | - | - | - | - | - | - | - | - | - | - | 2.91 |
| HTP | 68.25 | ||||||||||
| Gl | 14.52 | ||||||||||
| MS | - | - | 0.95 | - | 0.95 | - | 0.95 | 0.95 | 0.95 | 0.95 | 0.95 |
| PM | 0.95 | 0.95 | - | 0.95 | - | 0.95 | - | - | - | - | - |
| MgSO4 | 3.85 | 3.85 | 3.85 | 3.85 | 3.85 | 3.85 | 3.85 | 3.85 | 3.85 | 3.85 | 3.85 |
| Chemical (Code) | Purity Grade | Source |
|---|---|---|
| Ammonium nitrate porous prill (UltrAN) a | >99.4% | Yara (Szczecin, Poland) |
| Ammonium nitrate (AN) | >95% | Nitrogen Plant “Pulawy” (Pulawy, Poland) |
| Sodium nitrate (SN) | >99% | POCH S.A (Gliwice, Poland) |
| Potassium nitrate (PN) | >99% | POCH S.A (Gliwice, Poland) |
| Calcium nitrate tetrahydrate (CN) | >99% | POCH S.A (Gliwice, Poland) |
| Hydrogen peroxide 60 wt.% solution (HTP) | analytical | EnvoLab Chemicals (Dlugomilowice, Poland) |
| Glycerine (Gl) | >99.5% | TechlandLab (Tarnobrzeg. Poland) |
| Guar gum S.C.-406 (GG) | >99% | Meyhall Chemical AG (Kreuzlingen, Germany) |
| Locust bean gum (LBG) | >99% | Sigma-Aldrich (Burlington, MA, USA) |
| Hydroxyethylcellulose (HEC) | >99% | Sigma-Aldrich (Burlington, MA, USA) |
| Gum arabic (GA) | >99% | Sigma-Aldrich (Burlington, MA, USA) |
| Glass microspheres type K-015 (MS) | n/a | 3M (Saint Paul, MN, USA) |
| Polymer microspheres (PM) | n/a | AkzoNobel (Amsterdam, The Netherlands) |
| Magnesium sulfate (MgSO4) | >99% | POCH S.A (Gliwice, Poland) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fabin, M.; Stolarczyk, A.; Zakusylo, R.; Jarosz, T. Following the Decomposition of Hydrogen Peroxide in On-Site Mixture Explosives: Study of the Effect of the Auxiliary Oxidising Agent and Binder. Molecules 2023, 28, 5957. https://doi.org/10.3390/molecules28165957
Fabin M, Stolarczyk A, Zakusylo R, Jarosz T. Following the Decomposition of Hydrogen Peroxide in On-Site Mixture Explosives: Study of the Effect of the Auxiliary Oxidising Agent and Binder. Molecules. 2023; 28(16):5957. https://doi.org/10.3390/molecules28165957
Chicago/Turabian StyleFabin, Magdalena, Agnieszka Stolarczyk, Roman Zakusylo, and Tomasz Jarosz. 2023. "Following the Decomposition of Hydrogen Peroxide in On-Site Mixture Explosives: Study of the Effect of the Auxiliary Oxidising Agent and Binder" Molecules 28, no. 16: 5957. https://doi.org/10.3390/molecules28165957
APA StyleFabin, M., Stolarczyk, A., Zakusylo, R., & Jarosz, T. (2023). Following the Decomposition of Hydrogen Peroxide in On-Site Mixture Explosives: Study of the Effect of the Auxiliary Oxidising Agent and Binder. Molecules, 28(16), 5957. https://doi.org/10.3390/molecules28165957





