Diffusion-Limited Processes in Hydrogels with Chosen Applications from Drug Delivery to Electronic Components
Abstract
:1. Introduction
2. Diffusion in Hydrogels
3. Permeability of the Hydrogels as a Tool for Understanding Diffusion and Its Practical Application
4. Diffusion-Controlled Drug Delivery by Hydrogel Systems
5. Practical Application of Limited Diffusion Kinetics in Regenerative Medicine
6. Practical Application of Limited Diffusion Kinetics in Agriculture
7. Practical Application of Limited Diffusion Kinetics in Soft Robotics and Microrobotics
8. Future Prospectives
9. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nakayama, K.H.; Shayan, M.; Huang, N.F. Engineering Biomimetic Materials for Skeletal Muscle Repair and Regeneration. Adv. Healthc. Mater. 2019, 8, 1801168. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, L.; Guan, Q.; Guo, Y.; Lou, J.; Lei, D.; Wang, S.; Chen, S.; Sun, L.; Xuan, H.; et al. Biomimetic Materials with Multiple Protective Functionalities. Adv. Funct. Mater. 2019, 29, 1901058. [Google Scholar] [CrossRef]
- Thompson, V.P. The Tooth: An Analogue for Biomimetic Materials Design and Processing. Dent. Mater. 2020, 36, 25–42. [Google Scholar] [CrossRef]
- Cleary, P.W.; Harrison, S.M.; Sinnott, M.D. Flow Processes Occurring within the Body but Still External to the Body’s Epithelial Layer (Gastrointestinal and Respiratory Tracts). In Digital Human Modeling and Medicine; Elsevier: Amsterdam, The Netherlands, 2023; pp. 361–424. [Google Scholar]
- Zarebanadkouki, M.; Fink, T.; Benard, P.; Banfield, C.C. Mucilage Facilitates Nutrient Diffusion in the Drying Rhizosphere. Vadose Zone J. 2019, 18, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Campbell, P.G.C.; Hodson, P.V.; Welbourn, P.M.; Wright, D.A. Contaminant Bioaccumulation: Kinetics and Modelling Uptake Mechanisms. In Ecotoxicology; Cambridge University Press: Cambridge, UK, 2022; ISBN 9781108876605. [Google Scholar]
- Pei, D. How Do Biomolecules Cross the Cell Membrane? Acc. Chem. Res. 2022, 55, 309–318. [Google Scholar] [CrossRef]
- Orrico, F.; Lopez, A.C.; Saliwonczyk, D.; Acosta, C.; Rodriguez-Grecco, I.; Mouro-Chanteloup, I.; Ostuni, M.A.; Denicola, A.; Thomson, L.; Möller, M.N. The Permeability of Human Red Blood Cell Membranes to Hydrogen Peroxide Is Independent of Aquaporins. J. Biol. Chem. 2022, 298, 101503. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.R.; Leadbetter, T.; Camley, B.A. Sensing the Shape of a Cell with Reaction Diffusion and Energy Minimization. Proc. Natl. Acad. Sci. USA 2022, 119, e2121302119. [Google Scholar] [CrossRef] [PubMed]
- Yuan, T.; Gao, L.; Zhan, W.; Dini, D. Effect of Particle Size and Surface Charge on Nanoparticles Diffusion in the Brain White Matter. Pharm. Res. 2022, 39, 767–781. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Q.; Zhu, Z.; Zhao, W.; Yan, C.; Liu, Z.; Liu, M.; Zhao, X.; Tian, H.; Zhu, W.-H. Spatiotemporal Visualization of Cell Membrane with Amphiphilic Aggregation-Induced Emission-Active Sensor. CCS Chem. 2022, 4, 1619–1632. [Google Scholar] [CrossRef]
- Sharsheeva, A.; Iglin, V.A.; Nesterov, P.V.; Kuchur, O.A.; Garifullina, E.; Hey-Hawkins, E.; Ulasevich, S.A.; Skorb, E.V.; Vinogradov, A.V.; Morozov, M.I. Light-Controllable Systems Based on TiO2-ZIF-8 Composites for Targeted Drug Release: Communicating with Tumour Cells. J. Mater. Chem. B 2019, 7, 6810–6821. [Google Scholar] [CrossRef] [PubMed]
- Craciun, A.M.; Mititelu Tartau, L.; Pinteala, M.; Marin, L. Nitrosalicyl-Imine-Chitosan Hydrogels Based Drug Delivery Systems for Long Term Sustained Release in Local Therapy. J. Colloid Interface Sci. 2019, 536, 196–207. [Google Scholar] [CrossRef] [PubMed]
- Ulasevich, S.A.; Melnyk, I.; Andreeva, D.V.; Möhwald, H.; Skorb, E.V. Photomobility and Photohealing of Cellulose-Based Hybrids. EPL Europhys. Lett. 2017, 119, 38003. [Google Scholar] [CrossRef]
- Oh, K.S.; Hwang, C.; Lee, H.-Y.; Song, J.S.; Park, H.-J.; Lee, C.-K.; Song, I.; Lim, T.-H. Preclinical Studies of Ropivacaine Extended-Release from a Temperature Responsive Hydrogel for Prolonged Relief of Pain at the Surgical Wound. Int. J. Pharm. 2019, 558, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Chen, X.; Li, H.; Feng, G.; Nie, Y.; Wei, Y.; Li, N.; Han, Z.; Han, Z.; Kong, D.; et al. A Nitric Oxide-Releasing Hydrogel for Enhancing the Therapeutic Effects of Mesenchymal Stem Cell Therapy for Hindlimb Ischemia. Acta Biomater. 2020, 113, 289–304. [Google Scholar] [CrossRef] [PubMed]
- Ahmadian, E.; Eftekhari, A.; Dizaj, S.M.; Sharifi, S.; Mokhtarpour, M.; Nasibova, A.N.; Khalilov, R.; Samiei, M. The Effect of Hyaluronic Acid Hydrogels on Dental Pulp Stem Cells Behavior. Int. J. Biol. Macromol. 2019, 140, 245–254. [Google Scholar] [CrossRef]
- Zhao, J.; Liu, J.-D.; Shen, H.; Guo, M.; Li, Q.; Wang, C.-F.; Liu, C.; Chen, S. Rapid Preparation of Dual Cross-Linked Mechanical Strengthening Hydrogels via Frontal Polymerization for Use as Shape Deformable Actuators. ACS Appl. Polym. Mater. 2022, 4, 1457–1465. [Google Scholar] [CrossRef]
- Wang, Y.; Nitta, T.; Hiratsuka, Y.; Morishima, K. In Situ Integrated Microrobots Driven by Artificial Muscles Built from Biomolecular Motors. Sci. Robot 2022, 7, eaba8212. [Google Scholar] [CrossRef]
- Takishima, Y.; Yoshida, K.; Khosla, A.; Kawakami, M.; Furukawa, H. Fully 3D-Printed Hydrogel Actuator for Jellyfish Soft Robots. ECS J. Solid State Sci. Technol. 2021, 10, 037002. [Google Scholar] [CrossRef]
- Shiblee, M.N.I.; Ahmed, K.; Kawakami, M.; Furukawa, H. 4D Printing of Shape-Memory Hydrogels for Soft-Robotic Functions. Adv. Mater. Technol. 2019, 4, 1900071. [Google Scholar] [CrossRef]
- Rahaman, S.J.; Samanta, A.; Mir, M.H.; Dutta, B. Metal-Organic Frameworks (MOFs): A Promising Candidate for Stimuli-Responsive Drug Delivery. ES Mater. Manuf. 2022, 19, 792. [Google Scholar] [CrossRef]
- Xu, L.; Chu, Z.; Wang, H.; Cai, L.; Tu, Z.; Liu, H.; Zhu, C.; Shi, H.; Pan, D.; Pan, J.; et al. Electrostatically Assembled Multilayered Films of Biopolymer Enhanced Nanocapsules for On-Demand Drug Release. ACS Appl. Bio Mater. 2019, 2, 3429–3438. [Google Scholar] [CrossRef]
- Imoro, N.; Shilovskikh, V.V.; Nesterov, P.V.; Timralieva, A.A.; Gets, D.; Nebalueva, A.; Lavrentev, F.V.; Novikov, A.S.; Kondratyuk, N.D.; Orekhov, N.D.; et al. Biocompatible PH-Degradable Functional Capsules Based on Melamine Cyanurate Self-Assembly. ACS Omega 2021, 6, 17267–17275. [Google Scholar] [CrossRef] [PubMed]
- Yuan, W.; Wang, H.; Fang, C.; Yang, Y.; Xia, X.; Yang, B.; Lin, Y.; Li, G.; Bian, L. Microscopic Local Stiffening in a Supramolecular Hydrogel Network Expedites Stem Cell Mechanosensing in 3D and Bone Regeneration. Mater. Horiz. 2021, 8, 1722–1734. [Google Scholar] [CrossRef] [PubMed]
- Tang, T.-C.; Tham, E.; Liu, X.; Yehl, K.; Rovner, A.J.; Yuk, H.; de la Fuente-Nunez, C.; Isaacs, F.J.; Zhao, X.; Lu, T.K. Hydrogel-Based Biocontainment of Bacteria for Continuous Sensing and Computation. Nat. Chem. Biol. 2021, 17, 724–731. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Guo, T.; Song, M.; Zhang, B.; Zhang, B. Hemoglobin Recognition by Imprinting in Semi-Interpenetrating Polymer Network Hydrogel Based on Polyacrylamide and Chitosan. Biomacromolecules 2005, 6, 2601–2606. [Google Scholar] [CrossRef]
- Huang, Y.; Morozova, S.M.; Li, T.; Li, S.; Naguib, H.E.; Kumacheva, E. Stimulus-Responsive Transport Properties of Nanocolloidal Hydrogels. Biomacromolecules 2023, 24, 1173–1183. [Google Scholar] [CrossRef]
- Stekolshchikova, A.A.; Radaev, A.V.; Orlova, O.Y.; Nikolaev, K.G.; Skorb, E.V. Thin and Flexible Ion Sensors Based on Polyelectrolyte Multilayers Assembled onto the Carbon Adhesive Tape. ACS Omega 2019, 4, 15421–15427. [Google Scholar] [CrossRef] [Green Version]
- Nikolaev, K.G.; Kalmykov, E.V.; Shavronskaya, D.O.; Nikitina, A.A.; Stekolshchikova, A.A.; Kosareva, E.A.; Zenkin, A.A.; Pantiukhin, I.S.; Orlova, O.Y.; Skalny, A.V.; et al. ElectroSens Platform with a Polyelectrolyte-Based Carbon Fiber Sensor for Point-of-Care Analysis of Zn in Blood and Urine. ACS Omega 2020, 5, 18987–18994. [Google Scholar] [CrossRef]
- Lanchuk, Y.V.; Ulasevich, S.A.; Fedotova, T.A.; Kolpashchikov, D.M.; Skorb, E.V. Towards Sustainable Diagnostics: Replacing Unstable H2O2 by Photoactive TiO2 in Testing Systems for Visible and Tangible Diagnostics for Use by Blind People. RSC Adv. 2018, 8, 37735–37739. [Google Scholar] [CrossRef]
- Nikolaev, K.G.; Ulasevich, S.A.; Luneva, O.; Orlova, O.Y.; Vasileva, D.; Vasilev, S.; Novikov, A.S.; Skorb, E.V. Humidity-Driven Transparent Holographic Free-Standing Polyelectrolyte Films. ACS Appl. Polym. Mater. 2020, 2, 105–112. [Google Scholar] [CrossRef]
- Hussain, S.; Park, S. Sweat-Based Noninvasive Skin-Patchable Urea Biosensors with Photonic Interpenetrating Polymer Network Films Integrated into PDMS Chips. ACS Sens. 2020, 5, 3988–3998. [Google Scholar] [CrossRef]
- Osswald, C.R.; Kang-Mieler, J.J. Controlled and Extended In Vitro Release of Bioactive Anti-Vascular Endothelial Growth Factors from a Microsphere-Hydrogel Drug Delivery System. Curr. Eye Res. 2016, 41, 1216–1222. [Google Scholar] [CrossRef]
- Liu, W.; Lee, B.-S.; Mieler, W.F.; Kang-Mieler, J.J. Biodegradable Microsphere-Hydrogel Ocular Drug Delivery System for Controlled and Extended Release of Bioactive Aflibercept In Vitro. Curr. Eye Res. 2019, 44, 264–274. [Google Scholar] [CrossRef]
- Ulasevich, S.A.; Brezesinski, G.; Möhwald, H.; Fratzl, P.; Schacher, F.H.; Poznyak, S.K.; Andreeva, D.V.; Skorb, E.V. Light-Induced Water Splitting Causes High-Amplitude Oscillation of PH-Sensitive Layer-by-Layer Assemblies on TiO2. Angew. Chem. 2016, 128, 13195–13198. [Google Scholar] [CrossRef]
- Ulasevich, S.A.; Brezhneva, N.; Zhukova, Y.; Möhwald, H.; Fratzl, P.; Schacher, F.H.; Sviridov, D.V.; Andreeva, D.V.; Skorb, E.V. Switching the Stiffness of Polyelectrolyte Assembly by Light to Control Behavior of Supported Cells. Macromol. Biosci. 2016, 16, 1422–1431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Van der Meeren, L.; Verduijn, J.; Parakhonskiy, B.V.; Skirtach, A.G. New All-Nanoparticle Microcapsules for Ultrasound Release and Laser Remote Killing of Cancer Cells. Mater. Today Commun. 2022, 33, 104287. [Google Scholar] [CrossRef]
- Basaran, S.; Dey, S.; Bhusari, S.; Sankaran, S.; Kraus, T. Plasmonic Stimulation of Gold Nanorods for the Photothermal Control of Engineered Living Materials. Biomater. Adv. 2023, 147, 213332. [Google Scholar] [CrossRef]
- Lee, S.; Kim, S.; Kim, D.; You, J.; Kim, J.S.; Kim, H.; Park, J.; Song, J.; Choi, I. Spatiotemporally Controlled Drug Delivery via Photothermally Driven Conformational Change of Self-Integrated Plasmonic Hybrid Nanogels. J. Nanobiotechnol. 2023, 21, 191. [Google Scholar] [CrossRef] [PubMed]
- Amsden, B. Solute Diffusion within Hydrogels. Mechanisms and Models. Macromolecules 1998, 31, 8382–8395. [Google Scholar] [CrossRef]
- Mehrer, H.; Stolwijk, N.A. Heroes and Highlights in the History of Diffusion. Diffus. Fundam. 2009, 11, 1–32. [Google Scholar]
- Bromberg, S.; Dill, K.A. Molecular Driving Forces: Statistical Thermodynamics in Chemistry and Biology; Garland Science: New York, NY, USA, 2003; ISBN 9780815320517. [Google Scholar]
- Sheth, S.; Barnard, E.; Hyatt, B.; Rathinam, M.; Zustiak, S.P. Predicting Drug Release From Degradable Hydrogels Using Fluorescence Correlation Spectroscopy and Mathematical Modeling. Front. Bioeng. Biotechnol. 2019, 7, 410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, H.-Y.; Lin, C.-C. A Diffusion-Reaction Model for Predicting Enzyme-Mediated Dynamic Hydrogel Stiffening. Gels 2019, 5, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, V. Trends and Future Perspectives in Peptide and Protein Drug Delivery; CRC Press: Boca Raton, FL, USA, 1995; Volume 4. [Google Scholar]
- Refojo, M.F. Permeation of Water through Some Hydrogels. J. Appl. Polym. Sci. 1965, 9, 3417–3426. [Google Scholar] [CrossRef]
- Amsden, B. Solute Diffusion in Hydrogels: An Examination of the Retardation Effect. Polym. Gels Netw. 1998, 6, 13–43. [Google Scholar] [CrossRef]
- Caccavo, D.; Cascone, S.; Lamberti, G.; Barba, A.A.; Larsson, A. Swellable Hydrogel-Based Systems for Controlled Drug Delivery. In Smart Drug Delivery System; InTech: London, UK, 2016. [Google Scholar]
- Koetting, M.C.; Peters, J.T.; Steichen, S.D.; Peppas, N.A. Stimulus-Responsive Hydrogels: Theory, Modern Advances, and Applications. Mat. Sci. Eng. R Rep. 2015, 93, 1–49. [Google Scholar] [CrossRef] [Green Version]
- Caccavo, D.; Cascone, S.; Lamberti, G.; Barba, A.A. Hydrogels: Experimental Characterization and Mathematical Modelling of Their Mechanical and Diffusive Behaviour. Chem. Soc. Rev. 2018, 47, 2357–2373. [Google Scholar] [CrossRef]
- Omar, J.; Ponsford, D.; Dreiss, C.A.; Lee, T.; Loh, X.J. Supramolecular Hydrogels: Design Strategies and Contemporary Biomedical Applications. Chem. Asian J. 2022, 17, e202200081. [Google Scholar] [CrossRef]
- Burla, F.; Sentjabrskaja, T.; Pletikapic, G.; van Beugen, J.; Koenderink, G.H. Particle Diffusion in Extracellular Hydrogels. Soft. Matter. 2020, 16, 1366–1376. [Google Scholar] [CrossRef] [Green Version]
- Dai, X.; Zhu, Z.; Li, Y.; Yang, B.; Xu, J.-F.; Dong, Y.; Zhou, X.; Yan, L.-T.; Liu, D. “Shutter” Effects Enhance Protein Diffusion in Dynamic and Rigid Molecular Networks. J. Am. Chem. Soc. 2022, 144, 19017–19025. [Google Scholar] [CrossRef] [PubMed]
- Moncure, P.J.; Simon, Z.C.; Millstone, J.E.; Laaser, J.E. Relationship between Gel Mesh and Particle Size in Determining Nanoparticle Diffusion in Hydrogel Nanocomposites. J. Phys. Chem. B 2022, 126, 4132–4142. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, K.; Liu, T.; Ni, C.; Chen, D.; Guo, J.; Liu, C.; Zhou, J.; Jia, Z.; Zhao, Q.; et al. Differential Diffusion Driven Far-from-Equilibrium Shape-Shifting of Hydrogels. Nat. Commun. 2021, 12, 6155. [Google Scholar] [CrossRef] [PubMed]
- Plou, J.; Molina-Martínez, B.; García-Astrain, C.; Langer, J.; García, I.; Ercilla, A.; Perumal, G.; Carracedo, A.; Liz-Marzán, L.M. Nanocomposite Scaffolds for Monitoring of Drug Diffusion in Three-Dimensional Cell Environments by Surface-Enhanced Raman Spectroscopy. Nano Lett. 2021, 21, 8785–8793. [Google Scholar] [CrossRef] [PubMed]
- Axpe, E.; Chan, D.; Offeddu, G.S.; Chang, Y.; Merida, D.; Hernandez, H.L.; Appel, E.A. A Multiscale Model for Solute Diffusion in Hydrogels. Macromolecules 2019, 52, 6889–6897. [Google Scholar] [CrossRef] [PubMed]
- Babicheva, T.S.; Konduktorova, A.A.; Shmakov, S.L.; Shipovskaya, A.B. Formation of Liesegang Structures under the Conditions of the Spatiotemporal Reaction of Polymer-Analogous Transformation (Salt → Base) of Chitosan. J. Phys. Chem. B 2020, 124, 9255–9266. [Google Scholar] [CrossRef]
- Kukhtenko, E.V.; Lavrentev, F.V.; Shilovskikh, V.V.; Zyrianova, P.I.; Koltsov, S.I.; Ivanov, A.S.; Novikov, A.S.; Muravev, A.A.; Nikolaev, K.G.; Andreeva, D.V.; et al. Periodic Self-Assembly of Poly(Ethyleneimine)–Poly(4-Styrenesulfonate) Complex Coacervate Membranes. Polymers 2022, 15, 45. [Google Scholar] [CrossRef]
- Eltantawy, M.M.; Belokon, M.A.; Belogub, E.V.; Ledovich, O.I.; Skorb, E.V.; Ulasevich, S.A. Self-Assembled Liesegang Rings of Hydroxyapatite for Cell Culturing. Adv. Nanobiomed. Res. 2021, 1, 2000048. [Google Scholar] [CrossRef]
- Farkas, S.; Fonyi, M.S.; Holló, G.; Német, N.; Valletti, N.; Kukovecz, Á.; Schuszter, G.; Rossi, F.; Lagzi, I. Periodic Precipitation of Zeolitic Imidazolate Frameworks in a Gelled Medium. J. Phys. Chem. C 2022, 126, 9580–9586. [Google Scholar] [CrossRef]
- Jo, M.; Oh, Y.; Kim, H.J.; Kim, H.L.; Yang, S.H. Diffusion-Controlled Crystallization of Calcium Carbonate in a Hydrogel. Cryst. Growth Des. 2020, 20, 560–567. [Google Scholar] [CrossRef]
- Shin, Y.S.; Jo, M.; Cho, Y.S.; Yang, S.H. Diffusion-Controlled Crystallization of Calcium Phosphate in a Hydrogel toward a Homogeneous Octacalcium Phosphate/Agarose Composite. ACS Omega 2022, 7, 1173–1185. [Google Scholar] [CrossRef]
- Rizzato, S.; Moret, M.; Merlini, M.; Albinati, A.; Beghi, F. Crystal Growth in Gelled Solution: Applications to Coordination Polymers. CrystEngComm 2016, 18, 2455–2462. [Google Scholar] [CrossRef] [Green Version]
- Saccone, M.A.; Gallivan, R.A.; Narita, K.; Yee, D.W.; Greer, J.R. Additive Manufacturing of Micro-Architected Metals via Hydrogel Infusion. Nature 2022, 612, 685–690. [Google Scholar] [CrossRef]
- Schroeder, T.B.H.; Aizenberg, J. Patterned Crystal Growth and Heat Wave Generation in Hydrogels. Nat. Commun. 2022, 13, 259. [Google Scholar] [CrossRef]
- Dromel, P.C.; Singh, D.; Christoff-Tempesta, T.; Martheswaran, T.; Alexander-Katz, A.; Spector, M.; Young, M. Controlling Growth Factor Diffusion by Modulating Water Content in Injectable Hydrogels. Tissue Eng. Part A 2021, 27, 714–723. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; He, Y.; Liao, S.; Ma, Y.; Tao, X.; Wang, Y. Renatured Hydrogel Painting. Sci. Adv. 2021, 7, eabf9117. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Kang, B.; Kim, K.; Kang, D.; Lee, H.; Ma, S.; Jang, G.; Lee, H.; Moon, J. Hydrogel Protection Strategy to Stabilize Water-Splitting Photoelectrodes. Nat. Energy 2022, 7, 537–547. [Google Scholar] [CrossRef]
- Jia, C.; Zhang, X.; Liang, S.; Fu, Y.; Liu, W.; Chen, J.; Liu, X.; Zhang, L. Environmentally Adaptable Hydrogel Electrolyte with the Triple Interpenetrating Network in the Flexible Zinc-Ion Battery with Ultralong Stability. J. Power Sources 2022, 548, 232072. [Google Scholar] [CrossRef]
- Yuan, C.; Zhong, X.; Tian, P.; Wang, Z.; Gao, G.; Duan, L.; Wang, C.; Shi, F. Antifreezing Zwitterionic-Based Hydrogel Electrolyte for Aqueous Zn Ion Batteries. ACS Appl. Energy Mater. 2022, 5, 7530–7537. [Google Scholar] [CrossRef]
- Slavík, P.; Trowse, B.R.; O’Brien, P.; Smith, D.K. Organogel Delivery Vehicles for the Stabilization of Organolithium Reagents. Nat. Chem. 2023, 15, 319–325. [Google Scholar] [CrossRef]
- Jiang, G.; Zhang, S.; Zhu, Y.; Gao, S.; Jin, H.; Luo, L.; Zhang, F.; Jin, J. Hydrogel-Embedded Tight Ultrafiltration Membrane with Superior Anti-Dye-Fouling Property for Low-Pressure Driven Molecule Separation. J. Mater. Chem. A Mater. 2018, 6, 2927–2934. [Google Scholar] [CrossRef]
- Yang, Z.; Yuan, S.; Shi, Y.; Zhang, Y.; Bian, S.; Liang, S.; Xiao, K.; Yu, W.; Hou, H.; Hu, J.; et al. Nanofibrous Kevlar Hydrogel Ultrafiltration Membrane with High Acid Resistance and Antifouling Properties for Wastewater Treatment. ACS EST Water 2022, 3, 1747–1755. [Google Scholar] [CrossRef]
- Jiang, H.; Liu, S. Construction of Self-Healing Polyethersulfone Ultrafiltration Membrane by Cucurbit[8]Uril Hydrogel via RTIPS Method and Host-Guest Chemistry. Chemosphere 2023, 311, 137079. [Google Scholar] [CrossRef]
- Shao, H.; Qi, Y.; Liang, S.; Qin, S.; Yu, J. Polypropylene Composite Hollow Fiber Ultrafiltration Membranes with an Acrylic Hydrogel Surface by in Situ Ultrasonic Wave-assisted Polymerization for Dye Removal. J. Appl. Polym. Sci. 2019, 136, 47099. [Google Scholar] [CrossRef]
- Levien, M.; Nasri, Z.; Weltmann, K.-D.; Fricke, K. Study on the Interaction of Plasma-Polymerized Hydrogel Coatings with Aqueous Solutions of Different PH. Gels 2023, 9, 237. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Zhan, J.; Chen, L.; Zhao, Y. Preparation of CTS/PAMAM/SA/Ca2+ Hydrogel and Its Adsorption Performance for Heavy Metal Ions. Appl. Surf. Sci. 2023, 607, 155135. [Google Scholar] [CrossRef]
- Sethi, S.; Thakur, S.; Sharma, D.; Singh, G.; Sharma, N.; Kaith, B.S.; Khullar, S. Malic Acid Cross-Linked Chitosan Based Hydrogel for Highly Effective Removal of Chromium (VI) Ions from Aqueous Environment. React. Funct. Polym. 2022, 177, 105318. [Google Scholar] [CrossRef]
- Hamza, M.F.; Hamad, N.A.; Hamad, D.M.; Khalafalla, M.S.; Abdel-Rahman, A.A.-H.; Zeid, I.F.; Wei, Y.; Hessien, M.M.; Fouda, A.; Salem, W.M. Synthesis of Eco-Friendly Biopolymer, Alginate-Chitosan Composite to Adsorb the Heavy Metals, Cd(II) and Pb(II) from Contaminated Effluents. Materials 2021, 14, 2189. [Google Scholar] [CrossRef]
- Wu, C.; Ma, Q.; Ge, W.; Yan, S.; Xia, L.; Song, S. Preparation of Fe-La Montmorillonite Nanosheets Based Composite Hydrogel Beads for As(V) Removal from Water. Chem. Phys. Lett. 2022, 806, 139994. [Google Scholar] [CrossRef]
- Tamaddon, F.; Ahmadi-AhmadAbadi, E.; Khoje-neamah, E. Nano-Carboxymethylcellulose, Polyacrylamide, and γ-Fe2O3-SO3H Cross-Linked to a Hydrophobic Linker: An Organic-Inorganic Hydrogel for Adsorptive Removal of Dyes. J. Mol. Struct. 2022, 1270, 133872. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, R.; Hou, Y.; Qin, Y.; Li, S.; Yang, S.; Gao, Z. DNA Hydrogels Combined with Microfluidic Chips for Melamine Detection. Anal. Chim. Acta 2022, 1228, 340312. [Google Scholar] [CrossRef]
- Zulfiqar, M.; Lee, S.Y.; Mafize, A.A.; Kahar, N.A.M.A.; Johari, K.; Rabat, N.E. Efficient Removal of Pb(II) from Aqueous Solutions by Using Oil Palm Bio-Waste/MWCNTs Reinforced PVA Hydrogel Composites: Kinetic, Isotherm and Thermodynamic Modeling. Polymers 2020, 12, 430. [Google Scholar] [CrossRef] [Green Version]
- Cansu Tarakci, E.; Nihal Gevrek, T. Isocyanate Group Containing Reactive Hydrogels: Facile Synthesis and Efficient Biofunctionalization. Eur. Polym. J. 2022, 175, 111338. [Google Scholar] [CrossRef]
- Hu, Q.; Huang, S.; Wei, T.; Wang, J.; Huo, Z.; Zhu, T.; Wu, C.; Chen, H. Temperature-Regulated Hybrid Protein Hydrogel for Recyclable Extraction of Uranium from Seawater. ACS Appl. Polym. Mater. 2022, 4, 2189–2196. [Google Scholar] [CrossRef]
- Jia, P.; He, X.; Yang, J.; Sun, X.; Bu, T.; Zhuang, Y.; Wang, L. Dual–Emission MOF–Based Ratiometric Platform and Sensory Hydrogel for Visible Detection of Biogenic Amines in Food Spoilage. Sens. Actuators B Chem. 2023, 374, 132803. [Google Scholar] [CrossRef]
- Xie, S.; Li, F.; Liu, F.; Xu, Q.; Zhang, X. Tough Lanthanide Luminescent Hydrogel for Nitroaromatics Detection. J. Rare Earths 2022, in press. [CrossRef]
- Ma, W.; Liu, M.; Xie, S.; Liu, B.; Jiang, L.; Zhang, X.; Yuan, X. CRISPR/Cas12a System Responsive DNA Hydrogel for Label-Free Detection of Non-Glucose Targets with a Portable Personal Glucose Meter. Anal. Chim. Acta 2022, 1231, 340439. [Google Scholar] [CrossRef]
- Geraili, A.; Xing, M.; Mequanint, K. Design and Fabrication of Drug-delivery Systems toward Adjustable Release Profiles for Personalized Treatment. VIEW 2021, 2, 20200126. [Google Scholar] [CrossRef]
- Lee, A.L.Z.; Voo, Z.X.; Chin, W.; Ono, R.J.; Yang, C.; Gao, S.; Hedrick, J.L.; Yang, Y.Y. Injectable Coacervate Hydrogel for Delivery of Anticancer Drug-Loaded Nanoparticles in Vivo. ACS Appl. Mater. Interfaces 2018, 10, 13274–13282. [Google Scholar] [CrossRef]
- Koshani, R.; Tavakolian, M.; van de Ven, T.G.M. Natural Emulgel from Dialdehyde Cellulose for Lipophilic Drug Delivery. ACS Sustain. Chem. Eng. 2021, 9, 4487–4497. [Google Scholar] [CrossRef]
- Lai, W.-F.; Susha, A.S.; Rogach, A.L. Multicompartment Microgel Beads for Co-Delivery of Multiple Drugs at Individual Release Rates. ACS Appl. Mater. Interfaces 2016, 8, 871–880. [Google Scholar] [CrossRef]
- Schmidt, B.V.K.J. Multicompartment Hydrogels. Macromol. Rapid Commun. 2022, 43, 2100895. [Google Scholar] [CrossRef]
- Li, J.; Mooney, D.J. Designing Hydrogels for Controlled Drug Delivery. Nat. Rev. Mater. 2016, 1, 16071. [Google Scholar] [CrossRef]
- Lieleg, O.; Ribbeck, K. Biological Hydrogels as Selective Diffusion Barriers. Trends Cell Biol. 2011, 21, 543–551. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Chen, J.; Zhao, S.; Huang, T.; Ying, H.; Trujillo, C.; Molinaro, G.; Zhou, Z.; Jiang, T.; Liu, W.; et al. High Drug-Loaded Microspheres Enabled by Controlled in-Droplet Precipitation Promote Functional Recovery after Spinal Cord Injury. Nat. Commun. 2022, 13, 1262. [Google Scholar] [CrossRef]
- Li, Z.; Van Zee, N.J.; Bates, F.S.; Lodge, T.P. Polymer Nanogels as Reservoirs To Inhibit Hydrophobic Drug Crystallization. ACS Nano 2019, 13, 1232–1243. [Google Scholar] [CrossRef] [PubMed]
- Torchilin, V. (Ed.) Multifunctional Pharmaceutical Nanocarriers; Springer: New York, NY, USA, 2008; Volume 4, ISBN 978-0-387-76551-8. [Google Scholar]
- Kalyane, D.; Raval, N.; Maheshwari, R.; Tambe, V.; Kalia, K.; Tekade, R.K. Employment of Enhanced Permeability and Retention Effect (EPR): Nanoparticle-Based Precision Tools for Targeting of Therapeutic and Diagnostic Agent in Cancer. Mater. Sci. Eng. C 2019, 98, 1252–1276. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Wu, J.; Sawa, T.; Matsumura, Y.; Hori, K. Tumor Vascular Permeability and the EPR Effect in Macromolecular Therapeutics: A Review. J. Control Release 2000, 65, 271–284. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Mochida, A.; Choyke, P.L.; Kobayashi, H. Nanodrug Delivery: Is the Enhanced Permeability and Retention Effect Sufficient for Curing Cancer? Bioconj. Chem. 2016, 27, 2225–2238. [Google Scholar] [CrossRef] [PubMed]
- Longmire, M.; Choyke, P.L.; Kobayashi, H. Clearance Properties of Nano-Sized Particles and Molecules as Imaging Agents: Considerations and Caveats. Nanomedicine 2008, 3, 703–717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Said, S.S.; Campbell, S.; Hoare, T. Externally Addressable Smart Drug Delivery Vehicles: Current Technologies and Future Directions. Chem. Mater. 2019, 31, 4971–4989. [Google Scholar] [CrossRef]
- Wang, X.; Zheng, Y.; Xue, Y.; Wu, Y.; Liu, Y.; Cheng, X.; Tang, R. PH-Sensitive and Tumor-Targeting Nanogels Based on Ortho Ester-Modified PEG for Improving the in Vivo Anti-Tumor Efficiency of Doxorubicin. Colloids Surf. B Biointerfaces 2021, 207, 112024. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Tian, R.; Chen, L.; Jin, R.; Feng, Y.; Bai, Y.; Chen, X. Redox-Responsive Nanogel with Intracellular Reconstruction and Programmable Drug Release for Targeted Tumor Therapy. Macromol. Rapid. Commun. 2019, 40, 1800824. [Google Scholar] [CrossRef]
- Dong, S.; Jiang, Y.; Qin, G.; Liu, L.; Zhao, H. Methionine-Based PH and Oxidation Dual-Responsive Block Copolymer: Synthesis and Fabrication of Protein Nanogels. Biomacromolecules 2020, 21, 4063–4075. [Google Scholar] [CrossRef] [PubMed]
- Hussain, Z.; Khan, S.; Imran, M.; Sohail, M.; Shah, S.W.A.; de Matas, M. PEGylation: A Promising Strategy to Overcome Challenges to Cancer-Targeted Nanomedicines: A Review of Challenges to Clinical Transition and Promising Resolution. Drug Deliv. Transl. Res. 2019, 9, 721–734. [Google Scholar] [CrossRef]
- Barenholz, Y. (Chezy) Doxil®—The First FDA-Approved Nano-Drug: Lessons Learned. J. Control. Release 2012, 160, 117–134. [Google Scholar] [CrossRef] [PubMed]
- Preman, N.K.; Jain, S.; Johnson, R.P. “Smart” Polymer Nanogels as Pharmaceutical Carriers: A Versatile Platform for Programmed Delivery and Diagnostics. ACS Omega 2021, 6, 5075–5090. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Nakamura, H.; Fang, J. The EPR Effect for Macromolecular Drug Delivery to Solid Tumors: Improvement of Tumor Uptake, Lowering of Systemic Toxicity, and Distinct Tumor Imaging in Vivo. Adv. Drug Deliv. Rev. 2013, 65, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Mura, S.; Nicolas, J.; Couvreur, P. Stimuli-Responsive Nanocarriers for Drug Delivery. Nat. Mater. 2013, 12, 991–1003. [Google Scholar] [CrossRef]
- Zhou, X.; He, X.; Shi, K.; Yuan, L.; Yang, Y.; Liu, Q.; Ming, Y.; Yi, C.; Qian, Z. Injectable Thermosensitive Hydrogel Containing Erlotinib-Loaded Hollow Mesoporous Silica Nanoparticles as a Localized Drug Delivery System for NSCLC Therapy. Adv. Sci. 2020, 7, 2001442. [Google Scholar] [CrossRef]
- Yang, K.; Chen, M.; Wang, Q.; Grebenchuk, S.; Chen, S.; Leng, X.; Novoselov, K.S.; Andreeva, D.V. Electro-Thermo Controlled Water Valve Based on 2D Graphene–Cellulose Hydrogels. Adv. Funct. Mater. 2022, 32, 2201904. [Google Scholar] [CrossRef]
- Qiu, M.; Wang, D.; Liang, W.; Liu, L.; Zhang, Y.; Chen, X.; Sang, D.K.; Xing, C.; Li, Z.; Dong, B.; et al. Novel Concept of the Smart NIR-Light–Controlled Drug Release of Black Phosphorus Nanostructure for Cancer Therapy. Proc. Natl. Acad. Sci. USA 2018, 115, 501–506. [Google Scholar] [CrossRef] [Green Version]
- Lengert, E.; Parakhonskiy, B.; Khalenkow, D.; Zečić, A.; Vangheel, M.; Monje Moreno, J.M.; Braeckman, B.P.; Skirtach, A.G. Laser-Induced Remote Release in Vivo in C. Elegans from Novel Silver Nanoparticles-Alginate Hydrogel Shells. Nanoscale 2018, 10, 17249–17256. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Sun, M.; Bu, Y.; Luo, F.; Lin, C.; Lin, Z.; Weng, Z.; Yang, F.; Wu, D. Microcapsule-Embedded Hydrogel Patches for Ultrasound Responsive and Enhanced Transdermal Delivery of Diclofenac Sodium. J. Mater. Chem. B 2019, 7, 2330–2337. [Google Scholar] [CrossRef]
- Huang, D.; Zhang, X.; Zhao, C.; Fu, X.; Zhang, W.; Kong, W.; Zhang, B.; Zhao, Y. Ultrasound-Responsive Microfluidic Microbubbles for Combination Tumor Treatment. Adv. Ther. 2021, 4, 2100050. [Google Scholar] [CrossRef]
- Manjua, A.C.; Alves, V.D.; Crespo, J.G.; Portugal, C.A.M. Magnetic Responsive PVA Hydrogels for Remote Modulation of Protein Sorption. ACS Appl. Mater. Interfaces 2019, 11, 21239–21249. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, M.; Boudoukhani, M.; Belmonte-Reche, E.; Genicio, N.; Sillankorva, S.; Gallo, J.; Rodríguez-Abreu, C.; Moulai-Mostefa, N.; Bañobre-López, M. Xanthan-Fe3O4 Nanoparticle Composite Hydrogels for Non-Invasive Magnetic Resonance Imaging and Magnetically Assisted Drug Delivery. ACS Appl. Nano Mater. 2021, 4, 7712–7729. [Google Scholar] [CrossRef]
- Park, S.-Y.; Kang, J.-H.; Kim, H.-S.; Hwang, J.-Y.; Shin, U.S. Electrical and Thermal Stimulus-Responsive Nanocarbon-Based 3D Hydrogel Sponge for Switchable Drug Delivery. Nanoscale 2022, 14, 2367–2382. [Google Scholar] [CrossRef]
- Mac Kenna, N.; Calvert, P.; Morrin, A.; Wallace, G.G.; Moulton, S.E. Electro-Stimulated Release from a Reduced Graphene Oxide Composite Hydrogel. J. Mater. Chem. B 2015, 3, 2530–2537. [Google Scholar] [CrossRef]
- Zhang, W.; Jin, X.; Li, H.; Zhang, R.; Wu, C. Injectable and Body Temperature Sensitive Hydrogels Based on Chitosan and Hyaluronic Acid for PH Sensitive Drug Release. Carbohydr. Polym. 2018, 186, 82–90. [Google Scholar] [CrossRef]
- Han, Z.; Wang, P.; Mao, G.; Yin, T.; Zhong, D.; Yiming, B.; Hu, X.; Jia, Z.; Nian, G.; Qu, S.; et al. Dual PH-Responsive Hydrogel Actuator for Lipophilic Drug Delivery. ACS Appl. Mater. Interfaces 2020, 12, 12010–12017. [Google Scholar] [CrossRef] [PubMed]
- Ma, G.; Lin, W.; Yuan, Z.; Wu, J.; Qian, H.; Xu, L.; Chen, S. Development of Ionic Strength/PH/Enzyme Triple-Responsive Zwitterionic Hydrogel of the Mixed l-Glutamic Acid and l-Lysine Polypeptide for Site-Specific Drug Delivery. J. Mater. Chem. B 2017, 5, 935–943. [Google Scholar] [CrossRef]
- Chakroun, R.W.; Sneider, A.; Anderson, C.F.; Wang, F.; Wu, P.; Wirtz, D.; Cui, H. Supramolecular Design of Unsymmetric Reverse Bolaamphiphiles for Cell-Sensitive Hydrogel Degradation and Drug Release. Angew. Chem. 2020, 132, 4464–4472. [Google Scholar] [CrossRef]
- Su, R.S.-C.; Galas, R.J.; Lin, C.; Liu, J.C. Redox-Responsive Resilin-Like Hydrogels for Tissue Engineering and Drug Delivery Applications. Macromol. Biosci. 2019, 19, 1900122. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Wang, L.; Li, Y.; Huang, Z.; Luo, S.; He, Y.; Han, H.; Raza, F.; Wu, J.; Ge, L. Injectable PH and Redox Dual Responsive Hydrogels Based on Self-Assembled Peptides for Anti-Tumor Drug Delivery. Biomater. Sci. 2020, 8, 5415–5426. [Google Scholar] [CrossRef]
- Son, G.-H.; Lee, B.-J.; Cho, C.-W. Mechanisms of Drug Release from Advanced Drug Formulations Such as Polymeric-Based Drug-Delivery Systems and Lipid Nanoparticles. J. Pharm. Investig. 2017, 47, 287–296. [Google Scholar] [CrossRef]
- Liu, Y.; Fan, Q.; Huo, Y.; Liu, C.; Li, B.; Li, Y. Construction of a Mesoporous Polydopamine@GO/Cellulose Nanofibril Composite Hydrogel with an Encapsulation Structure for Controllable Drug Release and Toxicity Shielding. ACS Appl. Mater. Interfaces 2020, 12, 57410–57420. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Liu, H.; Tang, D.; Li, Y.; Li, X.; Xu, F. Bioactuators Based on Stimulus-Responsive Hydrogels and Their Emerging Biomedical Applications. NPG Asia Mater. 2019, 11, 64. [Google Scholar] [CrossRef] [Green Version]
- Cao, M.; Wang, Y.; Hu, X.; Gong, H.; Li, R.; Cox, H.; Zhang, J.; Waigh, T.A.; Xu, H.; Lu, J.R. Reversible Thermoresponsive Peptide–PNIPAM Hydrogels for Controlled Drug Delivery. Biomacromolecules 2019, 20, 3601–3610. [Google Scholar] [CrossRef]
- Choi, Y.H.; Hwang, J.; Han, S.H.; Lee, C.; Jeon, S.; Kim, S. Thermo-Responsive Microcapsules with Tunable Molecular Permeability for Controlled Encapsulation and Release. Adv. Funct. Mater. 2021, 31, 2100782. [Google Scholar] [CrossRef]
- Nita, L.E.; Chiriac, A.P.; Rusu, A.G.; Ghilan, A.; Dumitriu, R.P.; Bercea, M.; Tudorachi, N. Stimuli Responsive Scaffolds Based on Carboxymethyl Starch and Poly(2-Dimethylaminoethyl Methacrylate) for Anti-Inflammatory Drug Delivery. Macromol. Biosci. 2020, 20, 1900412. [Google Scholar] [CrossRef]
- Kim, J.; Francis, D.M.; Sestito, L.F.; Archer, P.A.; Manspeaker, M.P.; O’Melia, M.J.; Thomas, S.N. Thermosensitive Hydrogel Releasing Nitric Oxide Donor and Anti-CTLA-4 Micelles for Anti-Tumor Immunotherapy. Nat. Commun. 2022, 13, 1479. [Google Scholar] [CrossRef]
- Liu, S.; Wang, Y.; Ma, B.; Shao, J.; Liu, H.; Ge, S. Gingipain-Responsive Thermosensitive Hydrogel Loaded with SDF-1 Facilitates In Situ Periodontal Tissue Regeneration. ACS Appl. Mater. Interfaces 2021, 13, 36880–36893. [Google Scholar] [CrossRef] [PubMed]
- Tebcharani, L.; Wanzke, C.; Lutz, T.M.; Rodon-Fores, J.; Lieleg, O.; Boekhoven, J. Emulsions of Hydrolyzable Oils for the Zero-Order Release of Hydrophobic Drugs. J. Control Release 2021, 339, 498–505. [Google Scholar] [CrossRef] [PubMed]
- Nikitina, A.A.; Ulasevich, S.A.; Kassirov, I.S.; Bryushkova, E.A.; Koshel, E.I.; Skorb, E.V. Nanostructured Layer-by-Layer Polyelectrolyte Containers to Switch Biofilm Fluorescence. Bioconj. Chem. 2018, 29, 3793–3799. [Google Scholar] [CrossRef] [PubMed]
- Ulasevich, S.; Ryzhkov, N.V.; Andreeva, D.V.; Özden, D.S.; Piskin, E.; Skorb, E.V. Light-to-Heat Photothermal Dynamic Properties of Polypyrrole-Based Coating for Regenerative Therapy and Lab-on-a-Chip Applications. Adv. Mater. Interfaces 2020, 7, 2000980. [Google Scholar] [CrossRef]
- Tang, S.; Richardson, B.M.; Anseth, K.S. Dynamic Covalent Hydrogels as Biomaterials to Mimic the Viscoelasticity of Soft Tissues. Prog. Mater. Sci. 2021, 120, 100738. [Google Scholar] [CrossRef]
- Nonoyama, T.; Wada, S.; Kiyama, R.; Kitamura, N.; Mredha, M.T.I.; Zhang, X.; Kurokawa, T.; Nakajima, T.; Takagi, Y.; Yasuda, K.; et al. Double-Network Hydrogels Strongly Bondable to Bones by Spontaneous Osteogenesis Penetration. Adv. Mater. 2016, 28, 6740–6745. [Google Scholar] [CrossRef] [Green Version]
- Ma, Z.; Yang, Z.; Gao, Q.; Bao, G.; Valiei, A.; Yang, F.; Huo, R.; Wang, C.; Song, G.; Ma, D.; et al. Bioinspired Tough Gel Sheath for Robust and Versatile Surface Functionalization. Sci. Adv. 2021, 7, eabc3012. [Google Scholar] [CrossRef]
- Xie, H.; Chen, X.; Shen, X.; He, Y.; Chen, W.; Luo, Q.; Ge, W.; Yuan, W.; Tang, X.; Hou, D.; et al. Preparation of Chitosan-Collagen-Alginate Composite Dressing and Its Promoting Effects on Wound Healing. Int. J. Biol. Macromol. 2018, 107, 93–104. [Google Scholar] [CrossRef]
- Jiang, S.; Deng, J.; Jin, Y.; Qian, B.; Lv, W.; Zhou, Q.; Mei, E.; Neisiany, R.E.; Liu, Y.; You, Z.; et al. Breathable, Antifreezing, Mechanically Skin-like Hydrogel Textile Wound Dressings with Dual Antibacterial Mechanisms. Bioact. Mater. 2023, 21, 313–323. [Google Scholar] [CrossRef]
- Fahmy, H.M.; Aly, A.A.; Abou-Okeil, A. A Non-Woven Fabric Wound Dressing Containing Layer–by–Layer Deposited Hyaluronic Acid and Chitosan. Int. J. Biol. Macromol. 2018, 114, 929–934. [Google Scholar] [CrossRef]
- Ahmad, F.; Mushtaq, B.; Butt, F.A.; Zafar, M.S.; Ahmad, S.; Afzal, A.; Nawab, Y.; Rasheed, A.; Ulker, Z. Synthesis and Characterization of Nonwoven Cotton-Reinforced Cellulose Hydrogel for Wound Dressings. Polymers 2021, 13, 4098. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Hu, H. Application of Superabsorbent Spacer Fabrics as Exuding Wound Dressing. Polymers 2018, 10, 210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nawalakhe, R.; Shi, Q.; Vitchuli, N.; Noar, J.; Caldwell, J.M.; Breidt, F.; Bourham, M.A.; Zhang, X.; McCord, M.G. Novel Atmospheric Plasma Enhanced Chitosan Nanofiber/Gauze Composite Wound Dressings. J. Appl. Polym. Sci. 2013, 129, 916–923. [Google Scholar] [CrossRef]
- Huang, W.; Ying, R.; Wang, W.; Guo, Y.; He, Y.; Mo, X.; Xue, C.; Mao, X. A Macroporous Hydrogel Dressing with Enhanced Antibacterial and Anti-Inflammatory Capabilities for Accelerated Wound Healing. Adv. Funct. Mater. 2020, 30, 2000644. [Google Scholar] [CrossRef]
- Mu, Z.; Chen, K.; Yuan, S.; Li, Y.; Huang, Y.; Wang, C.; Zhang, Y.; Liu, W.; Luo, W.; Liang, P.; et al. Gelatin Nanoparticle-Injectable Platelet-Rich Fibrin Double Network Hydrogels with Local Adaptability and Bioactivity for Enhanced Osteogenesis. Adv. Healthc. Mater. 2020, 9, 1901469. [Google Scholar] [CrossRef]
- Taheri, S.; Bao, G.; He, Z.; Mohammadi, S.; Ravanbakhsh, H.; Lessard, L.; Li, J.; Mongeau, L. Injectable, Pore-Forming, Perfusable Double-Network Hydrogels Resilient to Extreme Biomechanical Stimulations. Adv. Sci. 2022, 9, 2102627. [Google Scholar] [CrossRef]
- Khataei, S.H.; Al-Musawi, M.; Asadi, K.; Ramezani, S.; Abbasian, M.; Ghorbani, M. Effect of Molecular Weight and Content of Polyvinylpyrrolidone on Cell Proliferation, Loading Capacity and Properties of Electrospun Green Tea Essential Oil-Incorporated Polyamide-6/Polyvinylpyrrolidone Nanofibers. J. Drug Deliv. Sci. Technol. 2023, 82, 104310. [Google Scholar] [CrossRef]
- Tang, S.; Liu, K.; Chen, J.; Li, Y.; Liu, M.; Lu, L.; Zhou, C.; Luo, B. Dual-Cross-Linked Liquid Crystal Hydrogels with Controllable Viscoelasticity for Regulating Cell Behaviors. ACS Appl. Mater. Interfaces 2022, 14, 21966–21977. [Google Scholar] [CrossRef]
- Zhang, K.; Yuan, W.; Wei, K.; Yang, B.; Chen, X.; Li, Z.; Zhang, Z.; Bian, L. Highly Dynamic Nanocomposite Hydrogels Self-Assembled by Metal Ion-Ligand Coordination. Small 2019, 15, 1900242. [Google Scholar] [CrossRef]
- Galdopórpora, J.M.; Morcillo, M.F.; Ibar, A.; Perez, C.J.; Tuttolomondo, M.V.; Desimone, M.F. Development of Silver Nanoparticles/Gelatin Thermoresponsive Nanocomposites: Characterization and Antimicrobial Activity. Curr. Pharm. Des. 2019, 25, 4121–4129. [Google Scholar] [CrossRef]
- Wang, Y.; Zoneff, E.R.; Thomas, J.W.; Hong, N.; Tan, L.L.; McGillivray, D.J.; Perriman, A.W.; Law, K.C.L.; Thompson, L.H.; Moriarty, N.; et al. Hydrogel Oxygen Reservoirs Increase Functional Integration of Neural Stem Cell Grafts by Meeting Metabolic Demands. Nat. Commun. 2023, 14, 457. [Google Scholar] [CrossRef] [PubMed]
- Priddy, L.B.; Chaudhuri, O.; Stevens, H.Y.; Krishnan, L.; Uhrig, B.A.; Willett, N.J.; Guldberg, R.E. Oxidized Alginate Hydrogels for Bone Morphogenetic Protein-2 Delivery in Long Bone Defects. Acta Biomater. 2014, 10, 4390–4399. [Google Scholar] [CrossRef] [Green Version]
- Zhao, D.; Wang, X.; Cheng, B.; Yin, M.; Hou, Z.; Li, X.; Liu, K.; Tie, C.; Yin, M. Degradation-Kinetics-Controllable and Tissue-Regeneration-Matchable Photocross-Linked Alginate Hydrogels for Bone Repair. ACS Appl. Mater. Interfaces 2022, 14, 21886–21905. [Google Scholar] [CrossRef]
- Harley, B.A.C.; Kim, H.-D.; Zaman, M.H.; Yannas, I.V.; Lauffenburger, D.A.; Gibson, L.J. Microarchitecture of Three-Dimensional Scaffolds Influences Cell Migration Behavior via Junction Interactions. Biophys. J. 2008, 95, 4013–4024. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Feng, Y.; Wang, L.; Liu, D.; Qin, C.; Shi, Y. A Review of Preparation Methods of Porous Skin Tissue Engineering Scaffolds. Mater. Today Commun. 2022, 32, 104109. [Google Scholar] [CrossRef]
- Annabi, N.; Nichol, J.W.; Zhong, X.; Ji, C.; Koshy, S.; Khademhosseini, A.; Dehghani, F. Controlling the Porosity and Microarchitecture of Hydrogels for Tissue Engineering. Tissue Eng. Part B Rev. 2010, 16, 371–383. [Google Scholar] [CrossRef] [Green Version]
- Chiu, Y.-C.; Cheng, M.-H.; Engel, H.; Kao, S.-W.; Larson, J.C.; Gupta, S.; Brey, E.M. The Role of Pore Size on Vascularization and Tissue Remodeling in PEG Hydrogels. Biomaterials 2011, 32, 6045–6051. [Google Scholar] [CrossRef] [PubMed]
- Xue, W.; Lee, D.; Kong, Y.; Kuss, M.; Huang, Y.; Kim, T.; Chung, S.; Dudley, A.T.; Ro, S.; Duan, B. A Facile Strategy for the Fabrication of Cell-Laden Porous Alginate Hydrogels Based on Two-Phase Aqueous Emulsions. Adv. Funct. Mater. 2023, 2214129. [Google Scholar] [CrossRef]
- Luo, Y.; Zhang, T.; Lin, X. 3D Printed Hydrogel Scaffolds with Macro Pores and Interconnected Microchannel Networks for Tissue Engineering Vascularization. Chem. Eng. J. 2022, 430, 132926. [Google Scholar] [CrossRef]
- Prakoso, A.T.; Basri, H.; Adanta, D.; Yani, I.; Ammarullah, M.I.; Akbar, I.; Ghazali, F.A.; Syahrom, A.; Kamarul, T. The Effect of Tortuosity on Permeability of Porous Scaffold. Biomedicines 2023, 11, 427. [Google Scholar] [CrossRef]
- Guerreiro, R.; Pires, T.; Guedes, J.M.; Fernandes, P.R.; Castro, A.P.G. On the Tortuosity of TPMS Scaffolds for Tissue Engineering. Symmetry 2020, 12, 596. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Liu, K.; Chen, J.; Wen, W.; Li, H.; Li, L.; Ding, S.; Liu, M.; Zhou, C.; Luo, B. Bone ECM-Inspired Biomineralization Chitin Whisker Liquid Crystal Hydrogels for Bone Regeneration. Int. J. Biol. Macromol. 2023, 231, 123335. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Liu, Z.; Zhao, Q. Inorganic Ionic Oligomers Induced Organic-Inorganic Synergistic Toughening Enabling Mechanical Robust and Recyclable Nanocomposite Hydrogels. Adv. Funct. Mater. 2023, 33, 2213699. [Google Scholar] [CrossRef]
- Gao, E.; Wang, Y.; Wang, P.; Wang, Q.; Wei, Y.; Song, D.; Xu, H.; Ding, J.; Xu, Y.; Xia, H.; et al. C-Shaped Cartilage Development Using Wharton’s Jelly-Derived Hydrogels to Assemble a Highly Biomimetic Neotrachea for Use in Circumferential Tracheal Reconstruction. Adv. Funct. Mater. 2023, 33, 2212830. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, X.; Yang, M.; Sun, W.; Mao, C. Integration of Genetically Engineered Virus Nanofibers and Fibrin to Form Injectable Fibrous Neuron-Rich Hydrogels and Enable Neural Differentiation. J. Mater. Chem. B 2023, 11, 802–815. [Google Scholar] [CrossRef]
- Wu, K.; Wang, Y.; Yang, H.; Chen, Y.; Lu, K.; Wu, Y.; Liu, C.; Zhang, H.; Meng, H.; Yu, Q.; et al. Injectable Decellularized Extracellular Matrix Hydrogel Containing Stromal Cell-Derived Factor 1 Promotes Transplanted Cardiomyocyte Engraftment and Functional Regeneration after Myocardial Infarction. ACS Appl. Mater. Interfaces 2023, 15, 2578–2589. [Google Scholar] [CrossRef] [PubMed]
- Jíménez-Arias, D.; Morales-Sierra, S.; Silva, P.; Carrêlo, H.; Gonçalves, A.; Ganança, J.F.T.; Nunes, N.; Gouveia, C.S.S.; Alves, S.; Borges, J.P.; et al. Encapsulation with Natural Polymers to Improve the Properties of Biostimulants in Agriculture. Plants 2022, 12, 55. [Google Scholar] [CrossRef]
- Xu, C.; Cao, L.; Bilal, M.; Cao, C.; Zhao, P.; Zhang, H.; Huang, Q. Multifunctional Manganese-Based Carboxymethyl Chitosan Hydrogels for PH-Triggered Pesticide Release and Enhanced Fungicidal Activity. Carbohydr. Polym. 2021, 262, 117933. [Google Scholar] [CrossRef]
- Nörnberg, A.B.; Gehrke, V.R.; Mota, H.P.; Camargo, E.R.; Fajardo, A.R. Alginate-Cellulose Biopolymeric Beads as Efficient Vehicles for Encapsulation and Slow-Release of Herbicide. Colloids Surf. A Physicochem. Eng. Asp. 2019, 583, 123970. [Google Scholar] [CrossRef]
- Xu, C.; Cao, L.; Cao, C.; Chen, H.; Zhang, H.; Li, Y.; Huang, Q. Fungicide Itself as a Trigger to Facilely Construct Hymexazol-Encapsulated Polysaccharide Supramolecular Hydrogels with Controllable Rheological Properties and Reduced Environmental Risks. Chem. Eng. J. 2023, 452, 139195. [Google Scholar] [CrossRef]
- Bajaj, S.R.; Marathe, S.J.; Singhal, R.S. Co-Encapsulation of Vitamins B12 and D3 Using Spray Drying: Wall Material Optimization, Product Characterization, and Release Kinetics. Food Chem. 2021, 335, 127642. [Google Scholar] [CrossRef]
- Sarmah, D.; Karak, N. Biodegradable Superabsorbent Hydrogel for Water Holding in Soil and Controlled-release Fertilizer. J. Appl. Polym. Sci. 2020, 137, 48495. [Google Scholar] [CrossRef]
- Liu, S.; Wu, Q.; Sun, X.; Yue, Y.; Tubana, B.; Yang, R.; Cheng, H.N. Novel Alginate-Cellulose Nanofiber-Poly(Vinyl Alcohol) Hydrogels for Carrying and Delivering Nitrogen, Phosphorus and Potassium Chemicals. Int. J. Biol. Macromol. 2021, 172, 330–340. [Google Scholar] [CrossRef]
- Vinceković, M.; Jalšenjak, N.; Topolovec-Pintarić, S.; Đermić, E.; Bujan, M.; Jurić, S. Encapsulation of Biological and Chemical Agents for Plant Nutrition and Protection: Chitosan/Alginate Microcapsules Loaded with Copper Cations and Trichoderma Viride. J. Agric. Food Chem. 2016, 64, 8073–8083. [Google Scholar] [CrossRef] [PubMed]
- Maluin, F.N.; Hussein, M.Z.; Yusof, N.A.; Fakurazi, S.; Idris, A.S.; Zainol Hilmi, N.H.; Jeffery Daim, L.D. Preparation of Chitosan–Hexaconazole Nanoparticles as Fungicide Nanodelivery System for Combating Ganoderma Disease in Oil Palm. Molecules 2019, 24, 2498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maluin, F.N.; Hussein, M.Z.; Yusof, N.A.; Fakurazi, S.; Idris, A.S.; Hilmi, N.H.Z.; Jeffery Daim, L.D. A Potent Antifungal Agent for Basal Stem Rot Disease Treatment in Oil Palms Based on Chitosan-Dazomet Nanoparticles. Int. J. Mol. Sci. 2019, 20, 2247. [Google Scholar] [CrossRef] [Green Version]
- Maluin, F.N.; Hussein, M.Z.; Yusof, N.A.; Fakurazi, S.; Abu Seman, I.; Zainol Hilmi, N.H.; Jeffery Daim, L.D. Enhanced Fungicidal Efficacy on Ganoderma Boninense by Simultaneous Co-Delivery of Hexaconazole and Dazomet from Their Chitosan Nanoparticles. RSC Adv. 2019, 9, 27083–27095. [Google Scholar] [CrossRef] [PubMed]
- Omer, A.M.; Tamer, T.M.; Hassan, M.E.; Khalifa, R.E.; Abd El-Monaem, E.M.; Eltaweil, A.S.; Mohy Eldin, M.S. Fabrication of Grafted Carboxymethyl Cellulose Superabsorbent Hydrogel for Water Retention and Sustained Release of Ethephon in Sandy Soil. Arab J. Sci. Eng. 2023, 48, 561–572. [Google Scholar] [CrossRef]
- Dispat, N.; Poompradub, S.; Kiatkamjornwong, S. Synthesis of ZnO/SiO2-Modified Starch-Graft-Polyacrylate Superabsorbent Polymer for Agricultural Application. Carbohydr. Polym. 2020, 249, 116862. [Google Scholar] [CrossRef] [PubMed]
- Qiao, D.; Liu, H.; Yu, L.; Bao, X.; Simon, G.P.; Petinakis, E.; Chen, L. Preparation and Characterization of Slow-Release Fertilizer Encapsulated by Starch-Based Superabsorbent Polymer. Carbohydr. Polym. 2016, 147, 146–154. [Google Scholar] [CrossRef]
- Zafar, N.; Niazi, M.B.K.; Sher, F.; Khalid, U.; Jahan, Z.; Shah, G.A.; Zia, M. Starch and Polyvinyl Alcohol Encapsulated Biodegradable Nanocomposites for Environment Friendly Slow Release of Urea Fertilizer. Chem. Eng. J. Adv. 2021, 7, 100123. [Google Scholar] [CrossRef]
- Qi, T.; Lü, S.; Li, T.; Chen, J.; Huang, M.; Ji, Y.; Zhang, S.-F.; Liu, M. A Multielement Compound Fertilizer Used Polydopamine and Sodium Carboxymethyl Starch Matrices as Coatings. Int. J. Biol. Macromol. 2019, 124, 582–590. [Google Scholar] [CrossRef]
- Zheng, L.; Seidi, F.; Wu, W.; Pan, Y.; Xiao, H. Dual-Functional Lignin-Based Hydrogels for Sustained Release of Agrochemicals and Heavy Metal Ion Complexation. Int. J. Biol. Macromol. 2023, 235, 123701. [Google Scholar] [CrossRef]
- Thombare, N.; Mishra, S.; Shinde, R.; Siddiqui, M.Z.; Jha, U. Guar Gum Based Hydrogel as Controlled Micronutrient Delivery System: Mechanism and Kinetics of Boron Release for Agricultural Applications. Biopolymers 2021, 112, e23418. [Google Scholar] [CrossRef] [PubMed]
- Bortoletto-Santos, R.; Cavigelli, M.A.; Montes, S.E.; Schomberg, H.H.; Le, A.; Thompson, A.I.; Kramer, M.; Polito, W.L.; Ribeiro, C. Oil-Based Polyurethane-Coated Urea Reduces Nitrous Oxide Emissions in a Corn Field in a Maryland Loamy Sand Soil. J. Clean. Prod. 2020, 249, 119329. [Google Scholar] [CrossRef]
- Yu, X.; Wang, Z.; Liu, J.; Mei, H.; Yong, D.; Li, J. Preparation, Swelling Behaviors and Fertilizer-Release Properties of Sodium Humate Modified Superabsorbent Resin. Mater. Today Commun. 2019, 19, 124–130. [Google Scholar] [CrossRef]
- Essawy, H.A.; Ghazy, M.B.M.; El-Hai, F.A.; Mohamed, M.F. Superabsorbent Hydrogels via Graft Polymerization of Acrylic Acid from Chitosan-Cellulose Hybrid and Their Potential in Controlled Release of Soil Nutrients. Int. J. Biol. Macromol. 2016, 89, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Arafa, E.G.; Sabaa, M.W.; Mohamed, R.R.; Kamel, E.M.; Elzanaty, A.M.; Mahmoud, A.M.; Abdel-Gawad, O.F. Eco-Friendly and Biodegradable Sodium Alginate/Quaternized Chitosan Hydrogel for Controlled Release of Urea and Its Antimicrobial Activity. Carbohydr. Polym. 2022, 291, 119555. [Google Scholar] [CrossRef]
- Elbarbary, A.M.; Ghobashy, M.M. Controlled Release Fertilizers Using Superabsorbent Hydrogel Prepared by Gamma Radiation. Radiochim. Acta 2017, 105, 865–876. [Google Scholar] [CrossRef]
- Iftime, M.M.; Irimiciuc, S.A.; Agop, M.; Angheloiu, M.; Ochiuz, L.; Vasincu, D. A Theoretical Multifractal Model for Assessing Urea Release from Chitosan Based Formulations. Polymers 2020, 12, 1264. [Google Scholar] [CrossRef]
- Yang, L.-T.; Pan, J.-F.; Hu, N.-J.; Chen, H.-H.; Jiang, H.-X.; Lu, Y.-B.; Chen, L.-S. Citrus Physiological and Molecular Response to Boron Stresses. Plants 2021, 11, 40. [Google Scholar] [CrossRef]
- Thombare, N.; Mishra, S.; Siddiqui, M.Z.; Jha, U.; Singh, D.; Mahajan, G.R. Design and Development of Guar Gum Based Novel, Superabsorbent and Moisture Retaining Hydrogels for Agricultural Applications. Carbohydr. Polym. 2018, 185, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.; Duan, B.; Cai, J.; Zhang, L. Superabsorbent Hydrogels Based on Cellulose for Smart Swelling and Controllable Delivery. Eur. Polym. J. 2010, 46, 92–100. [Google Scholar] [CrossRef]
- Kaczmarek, B.; Nadolna, K.; Owczarek, A. The Physical and Chemical Properties of Hydrogels Based on Natural Polymers. In Hydrogels Based on Natural Polymers; Elsevier: Amsterdam, The Netherlands, 2020; pp. 151–172. [Google Scholar]
- Chen, W.; Xiao, P.; Chen, H.; Zhang, H.; Zhang, Q.; Chen, Y. Polymeric Graphene Bulk Materials with a 3D Cross-Linked Monolithic Graphene Network. Adv. Mater. 2019, 31, 1802403. [Google Scholar] [CrossRef] [PubMed]
- Osi, A.R.; Zhang, H.; Chen, J.; Zhou, Y.; Wang, R.; Fu, J.; Müller-Buschbaum, P.; Zhong, Q. Three-Dimensional-Printable Thermo/Photo-Cross-Linked Methacrylated Chitosan–Gelatin Hydrogel Composites for Tissue Engineering. ACS Appl. Mater. Interfaces 2021, 13, 22902–22913. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Li, Y.; Fang, X.; Zhang, Z.; Li, S.; Sun, J. Healable and Recyclable Polymeric Materials with High Mechanical Robustness. ACS Mater. Lett. 2022, 4, 554–571. [Google Scholar] [CrossRef]
- Supare, K.; Mahanwar, P. Starch-Chitosan Hydrogels for the Controlled-Release of Herbicide in Agricultural Applications: A Study on the Effect of the Concentration of Raw Materials and Crosslinkers. J. Polym. Environ. 2022, 30, 2448–2461. [Google Scholar] [CrossRef]
- Durpekova, S.; Di Martino, A.; Dusankova, M.; Drohsler, P.; Sedlarik, V. Biopolymer Hydrogel Based on Acid Whey and Cellulose Derivatives for Enhancement Water Retention Capacity of Soil and Slow Release of Fertilizers. Polymers 2021, 13, 3274. [Google Scholar] [CrossRef] [PubMed]
- Ai, F.; Yin, X.; Hu, R.; Ma, H.; Liu, W. Research into the Super-Absorbent Polymers on Agricultural Water. Agric. Water Manag. 2021, 245, 106513. [Google Scholar] [CrossRef]
- Feng, G.d.; Ma, Y.; Zhang, M.; Jia, P.y.; Hu, L.h.; Liu, C.g.; Zhou, Y.h. Polyurethane-Coated Urea Using Fully Vegetable Oil-Based Polyols: Design, Nutrient Release and Degradation. Prog. Org. Coat. 2019, 133, 267–275. [Google Scholar] [CrossRef]
- Sohail, M.; Pirzada, T.; Opperman, C.H.; Khan, S.A. Recent Advances in Seed Coating Technologies: Transitioning toward Sustainable Agriculture. Green Chem. 2022, 24, 6052–6085. [Google Scholar] [CrossRef]
- Kaur, P.; Agrawal, R.; Pfeffer, F.M.; Williams, R.; Bohidar, H.B. Hydrogels in Agriculture: Prospects and Challenges. J. Polym. Environ. 2023. [Google Scholar] [CrossRef]
- Pathak, V.; Ambrose, R.P.K. Starch-based Biodegradable Hydrogel as Seed Coating for Corn to Improve Early Growth under Water Shortage. J. Appl. Polym. Sci. 2020, 137, 48523. [Google Scholar] [CrossRef]
- Skrzypczak, D.; Jarzembowski, Ł.; Izydorczyk, G.; Mikula, K.; Hoppe, V.; Mielko, K.A.; Pudełko-Malik, N.; Młynarz, P.; Chojnacka, K.; Witek-Krowiak, A. Hydrogel Alginate Seed Coating as an Innovative Method for Delivering Nutrients at the Early Stages of Plant Growth. Polymers 2021, 13, 4233. [Google Scholar] [CrossRef] [PubMed]
- Skrzypczak, D.; Witek-Krowiak, A.; Dawiec-Liśniewska, A.; Podstawczyk, D.; Mikula, K.; Chojnacka, K. Immobilization of Biosorbent in Hydrogel as a New Environmentally Friendly Fertilizer for Micronutrients Delivery. J. Clean. Prod. 2019, 241, 118387. [Google Scholar] [CrossRef]
- Zhao, X.; Kim, J.; Cezar, C.A.; Huebsch, N.; Lee, K.; Bouhadir, K.; Mooney, D.J. Active Scaffolds for On-Demand Drug and Cell Delivery. Proc. Natl. Acad. Sci. USA 2011, 108, 67–72. [Google Scholar] [CrossRef]
- Huang, H.-W.; Sakar, M.S.; Petruska, A.J.; Pané, S.; Nelson, B.J. Soft Micromachines with Programmable Motility and Morphology. Nat. Commun. 2016, 7, 12263. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.; Song, W.J.; Sun, J.-Y. Hydrogel Soft Robotics. Mater. Today Phys. 2020, 15, 100258. [Google Scholar] [CrossRef]
- Lin, X.; Xu, B.; Zhu, H.; Liu, J.; Solovev, A.; Mei, Y. Requirement and Development of Hydrogel Micromotors towards Biomedical Applications. Research 2020, 2020, 7659749. [Google Scholar] [CrossRef]
- Mou, F.; Chen, C.; Zhong, Q.; Yin, Y.; Ma, H.; Guan, J. Autonomous Motion and Temperature-Controlled Drug Delivery of Mg/Pt-Poly(N-Isopropylacrylamide) Janus Micromotors Driven by Simulated Body Fluid and Blood Plasma. ACS Appl. Mater. Interfaces 2014, 6, 9897–9903. [Google Scholar] [CrossRef]
- Strachota, B.; Strachota, A.; Šlouf, M.; Brus, J.; Cimrová, V. Monolithic Intercalated PNIPAm/Starch Hydrogels with Very Fast and Extensive One-Way Volume and Swelling Responses to Temperature and PH: Prospective Actuators and Drug Release Systems. Soft. Matter. 2019, 15, 752–769. [Google Scholar] [CrossRef] [PubMed]
- Asmolov, E.S.; Nizkaya, T.V.; Vinogradova, O.I. Self-Diffusiophoresis of Janus Particles That Release Ions. Phys. Fluids 2022, 34, 032011. [Google Scholar] [CrossRef]
- Cai, L.; Zhao, C.; Chen, H.; Fan, L.; Zhao, Y.; Qian, X.; Chai, R. Suction-Cup-Inspired Adhesive Micromotors for Drug Delivery. Adv. Sci. 2022, 9, 2103384. [Google Scholar] [CrossRef]
- Wu, J.; Ma, S.; Li, M.; Hu, X.; Jiao, N.; Tung, S.; Liu, L. Enzymatic/Magnetic Hybrid Micromotors for Synergistic Anticancer Therapy. ACS Appl. Mater. Interfaces 2021, 13, 31514–31526. [Google Scholar] [CrossRef]
- Wu, Y.; Wu, Z.; Lin, X.; He, Q.; Li, J. Autonomous Movement of Controllable Assembled Janus Capsule Motors. ACS Nano 2012, 6, 10910–10916. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Jeong, S.H.; Kim, T.Y.; Yi, J.; Hahn, S.K. Bioinspired Urease-Powered Micromotor as an Active Oral Drug Delivery Carrier in Stomach. Bioact. Mater. 2022, 9, 54–62. [Google Scholar] [CrossRef]
- Yuan, K.; Pacheco, M.; Jurado-Sánchez, B.; Escarpa, A. Design and Control of the Micromotor Swarm Toward Smart Applications. Adv. Intell. Syst. 2021, 3, 2100002. [Google Scholar] [CrossRef]
- Szatrowski, T.P.; Nathan, C.F. Production of Large Amounts of Hydrogen Peroxide by Human Tumor Cells. Cancer Res. 1991, 51, 794–798. [Google Scholar]
- Zhao, Y.; Hua, M.; Yan, Y.; Wu, S.; Alsaid, Y.; He, X. Stimuli-Responsive Polymers for Soft Robotics. Annu. Rev. Control Robot Auton. Syst. 2022, 5, 515–545. [Google Scholar] [CrossRef]
- Deegan, R.D. Finessing the Fracture Energy Barrier in Ballistic Seed Dispersal. Proc. Natl. Acad. Sci. USA 2012, 109, 5166–5169. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, M.; Feilich, K.L.; Ellerby, D.J. The Mechanics of Explosive Seed Dispersal in Orange Jewelweed (Impatiens capensis). J. Exp. Bot. 2009, 60, 2045–2053. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mariani, S.; Cecchini, L.; Mondini, A.; Del Dottore, E.; Ronzan, M.; Filippeschi, C.; Pugno, N.M.; Sinibaldi, E.; Mazzolai, B. A Bioinspired Plasmonic Nanocomposite Actuator Sunlight-Driven by a Photothermal-Hygroscopic Effect for Sustainable Soft Robotics. Adv. Mater. Technol. 2023, 8, 2202166. [Google Scholar] [CrossRef]
- Zhang, H.; Guo, X.; Wu, J.; Fang, D.; Zhang, Y. Soft Mechanical Metamaterials with Unusual Swelling Behavior and Tunable Stress-Strain Curves. Sci. Adv. 2018, 4, eaar8535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cai, M.; Nie, S.; Du, Y.; Wang, C.; Song, J. Soft Elastomers with Programmable Stiffness as Strain-Isolating Substrates for Stretchable Electronics. ACS Appl. Mater. Interfaces 2019, 11, 14340–14346. [Google Scholar] [CrossRef]
- Wang, W.; Wang, S.; Rastak, R.; Ochiai, Y.; Niu, S.; Jiang, Y.; Arunachala, P.K.; Zheng, Y.; Xu, J.; Matsuhisa, N.; et al. Strain-Insensitive Intrinsically Stretchable Transistors and Circuits. Nat. Electron. 2021, 4, 143–150. [Google Scholar] [CrossRef]
- Morales, D.; Podolsky, I.; Mailen, R.; Shay, T.; Dickey, M.; Velev, O. Ionoprinted Multi-Responsive Hydrogel Actuators. Micromachines 2016, 7, 98. [Google Scholar] [CrossRef] [Green Version]
- Koo, H.-J.; Velev, O.D. Design and Characterization of Hydrogel-Based Microfluidic Devices with Biomimetic Solute Transport Networks. Biomicrofluidics 2017, 11, 024104. [Google Scholar] [CrossRef] [Green Version]
- Shay, T.; Velev, O.D.; Dickey, M.D. Soft Electrodes Combining Hydrogel and Liquid Metal. Soft. Matter. 2018, 14, 3296–3303. [Google Scholar] [CrossRef]
- Williams, A.H.; Roh, S.; Jacob, A.R.; Stoyanov, S.D.; Hsiao, L.; Velev, O.D. Printable Homocomposite Hydrogels with Synergistically Reinforced Molecular-Colloidal Networks. Nat. Commun. 2021, 12, 2834. [Google Scholar] [CrossRef]
- Morales, D.; Palleau, E.; Dickey, M.D.; Velev, O.D. Electro-Actuated Hydrogel Walkers with Dual Responsive Legs. Soft. Matter. 2014, 10, 1337–1348. [Google Scholar] [CrossRef]
- Cheng, Y.; Chan, K.H.; Wang, X.; Ding, T.; Li, T.; Zhang, C.; Lu, W.; Zhou, Y.; Ho, G.W. A Fast Autonomous Healing Magnetic Elastomer for Instantly Recoverable, Modularly Programmable, and Thermorecyclable Soft Robots. Adv. Funct. Mater. 2021, 31, 2101825. [Google Scholar] [CrossRef]
- Skorb, E.V.; Andreeva, D.V. Layer-by-Layer Approaches for Formation of Smart Self-Healing Materials. Polym. Chem. 2013, 4, 4834. [Google Scholar] [CrossRef]
- Skorb, E.V.; Andreeva, D.V. Surface Nanoarchitecture for Bio-Applications: Self-Regulating Intelligent Interfaces. Adv. Funct. Mater. 2013, 23, 4483–4506. [Google Scholar] [CrossRef]
- Ivanov, A.S.; Pershina, L.V.; Nikolaev, K.G.; Skorb, E.V. Recent Progress of Layer-by-layer Assembly, Free-Standing Film and Hydrogel Based on Polyelectrolytes. Macromol. Biosci. 2021, 21, 2100117. [Google Scholar] [CrossRef]
- Zhang, Y.-F.; Guo, M.-M.; Zhang, Y.; Tang, C.Y.; Jiang, C.; Dong, Y.; Law, W.-C.; Du, F.-P. Flexible, Stretchable and Conductive PVA/PEDOT:PSS Composite Hydrogels Prepared by SIPN Strategy. Polym. Test. 2020, 81, 106213. [Google Scholar] [CrossRef]
- Xu, Z.; Song, J.; Liu, B.; Lv, S.; Gao, F.; Luo, X.; Wang, P. A Conducting Polymer PEDOT:PSS Hydrogel Based Wearable Sensor for Accurate Uric Acid Detection in Human Sweat. Sens. Actuators B Chem. 2021, 348, 130674. [Google Scholar] [CrossRef]
- Niu, F.; Han, X.; Sun, H.; Li, Q.; He, X.; Liu, Z.; Sun, J.; Lei, Z. Connecting PEDOT Nanotube Arrays by Polyaniline Coating toward a Flexible and High-Rate Supercapacitor. ACS Sustain. Chem. Eng. 2021, 9, 4146–4156. [Google Scholar] [CrossRef]
- Du, H.; Zhang, M.; Liu, K.; Parit, M.; Jiang, Z.; Zhang, X.; Li, B.; Si, C. Conductive PEDOT:PSS/Cellulose Nanofibril Paper Electrodes for Flexible Supercapacitors with Superior Areal Capacitance and Cycling Stability. Chem. Eng. J. 2022, 428, 131994. [Google Scholar] [CrossRef]
- Yang, Z.; Ma, J.; Bai, B.; Qiu, A.; Losic, D.; Shi, D.; Chen, M. Free-Standing PEDOT/Polyaniline Conductive Polymer Hydrogel for Flexible Solid-State Supercapacitors. Electrochim. Acta 2019, 322, 134769. [Google Scholar] [CrossRef]
- Wu, Y.; Shen, L.; Zhang, C.; Gao, H.; Chen, J.; Jin, L.; Lin, P.; Zhang, H.; Xia, Y. Polyacid Doping-Enabled Efficient Solar Evaporation of Polypyrrole Hydrogel. Desalination 2021, 505, 114766. [Google Scholar] [CrossRef]
- Bo, J.; Luo, X.; Huang, H.; Li, L.; Lai, W.; Yu, X. Morphology-Controlled Fabrication of Polypyrrole Hydrogel for Solid-State Supercapacitor. J. Power Sources 2018, 407, 105–111. [Google Scholar] [CrossRef]
- Chalmers, E.; Lee, H.; Zhu, C.; Liu, X. Increasing the Conductivity and Adhesion of Polypyrrole Hydrogels with Electropolymerized Polydopamine. Chem. Mater. 2020, 32, 234–244. [Google Scholar] [CrossRef]
- Cai, Y.; Shen, J.; Yang, C.-W.; Wan, Y.; Tang, H.-L.; Aljarb, A.A.; Chen, C.; Fu, J.-H.; Wei, X.; Huang, K.-W.; et al. Mixed-Dimensional MXene-Hydrogel Heterostructures for Electronic Skin Sensors with Ultrabroad Working Range. Sci. Adv. 2020, 6, eabb5367. [Google Scholar] [CrossRef]
- Dai, C.F.; Khoruzhenko, O.; Zhang, C.; Zhu, Q.L.; Jiao, D.; Du, M.; Breu, J.; Zhao, P.; Zheng, Q.; Wu, Z.L. Magneto-Orientation of Magnetic Double Stacks for Patterned Anisotropic Hydrogels with Multiple Responses and Modulable Motions. Angew. Chem. Int. Ed. 2022, 61, e202207272. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Cho, H.; Jeong, S.; Yoon, J.; Jang, D.; Lee, D.K.; Kim, D.; Chung, S.; Hong, Y. Stretchable Hybrid Electronics: Combining Rigid Electronic Devices with Stretchable Interconnects into High-Performance on-Skin Electronics. J. Inf. Disp. 2022, 23, 163–184. [Google Scholar] [CrossRef]
- Amr, A.; Kamel, A.; Almehizia, A.; Sayed, A.; Abd-Rabboh, H. Solid-Contact Potentiometric Sensors Based on Main-Tailored Bio-Mimics for Trace Detection of Harmine Hallucinogen in Urine Specimens. Molecules 2021, 26, 324. [Google Scholar] [CrossRef]
- Peng, X.; Wang, H. Shape Changing Hydrogels and Their Applications as Soft Actuators. J. Polym. Sci. B Polym. Phys. 2018, 56, 1314–1324. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, R.; Wu, Z.L.; Arifuzzaman, M.; Nonoyama, T.; Nakajima, T.; Kurokawa, T.; Gong, J.P. Control Superstructure of Rigid Polyelectrolytes in Oppositely Charged Hydrogels via Programmed Internal Stress. Nat. Commun. 2014, 5, 4490. [Google Scholar] [CrossRef] [Green Version]
- Pan, X.; Wang, Q.; Benetti, D.; Ni, Y.; Rosei, F. Polyelectrolyte Hydrogel: A Versatile Platform for Mechanical-Electric Conversion and Self-Powered Sensing. Nano Energy 2022, 103, 107718. [Google Scholar] [CrossRef]
- Kim, C.-J.; Ercole, F.; Goudeli, E.; Bhangu, S.K.; Chen, J.; Faria, M.; Quinn, J.F.; Caruso, F. Engineering Programmable DNA Particles and Capsules Using Catechol-Functionalized DNA Block Copolymers. Chem. Mater. 2022, 34, 7468–7480. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, R.; Yang, S.; Li, S.; Gao, Z. Design and Application of Stimuli-Responsive DNA Hydrogels: A Review. Mater. Today Bio 2022, 16, 100430. [Google Scholar] [CrossRef] [PubMed]
- Ding, B.; Zeng, P.; Huang, Z.; Dai, L.; Lan, T.; Xu, H.; Pan, Y.; Luo, Y.; Yu, Q.; Cheng, H.-M.; et al. A 2D Material–Based Transparent Hydrogel with Engineerable Interference Colours. Nat. Commun. 2022, 13, 1212. [Google Scholar] [CrossRef]
- Nojoomi, A.; Jeon, J.; Yum, K. 2D Material Programming for 3D Shaping. Nat. Commun. 2021, 12, 603. [Google Scholar] [CrossRef]
- Derakhshi, M.; Daemi, S.; Shahini, P.; Habibzadeh, A.; Mostafavi, E.; Ashkarran, A.A. Two-Dimensional Nanomaterials beyond Graphene for Biomedical Applications. J. Funct. Biomater. 2022, 13, 27. [Google Scholar] [CrossRef]
- Lee, H.-R.; Woo, J.; Han, S.H.; Lim, S.-M.; Lim, S.; Kang, Y.-W.; Song, W.J.; Park, J.-M.; Chung, T.D.; Joo, Y.-C.; et al. A Stretchable Ionic Diode from Copolyelectrolyte Hydrogels with Methacrylated Polysaccharides. Adv. Funct. Mater. 2019, 29, 1806909. [Google Scholar] [CrossRef]
- Yeon, S.Y.; Yun, J.; Yoon, S.; Lee, D.; Jang, W.; Han, S.H.; Kang, C.M.; Chung, T.D. A Miniaturized Solid Salt Reverse Electrodialysis Battery: A Durable and Fully Ionic Power Source. Chem. Sci. 2018, 9, 8071–8076. [Google Scholar] [CrossRef] [Green Version]
- Boydston, A.J.; Cao, B.; Nelson, A.; Ono, R.J.; Saha, A.; Schwartz, J.J.; Thrasher, C.J. Additive Manufacturing with Stimuli-Responsive Materials. J. Mater. Chem. A Mater. 2018, 6, 20621–20645. [Google Scholar] [CrossRef]
- Hao, X.P.; Li, C.Y.; Zhang, C.W.; Du, M.; Ying, Z.; Zheng, Q.; Wu, Z.L. Self-Shaping Soft Electronics Based on Patterned Hydrogel with Stencil-Printed Liquid Metal. Adv. Funct. Mater. 2021, 31, 2105481. [Google Scholar] [CrossRef]
- Park, J.-E.; Kang, H.S.; Baek, J.; Park, T.H.; Oh, S.; Lee, H.; Koo, M.; Park, C. Rewritable, Printable Conducting Liquid Metal Hydrogel. ACS Nano 2019, 13, 9122–9130. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Chen, Z.; Chen, C.; Ur Rehman, H.; Liu, H.; Li, H.; Hedenqvist, M.S. A Gradient-Distributed Liquid-Metal Hydrogel Capable of Tunable Actuation. Chem. Eng. J. 2021, 421, 127762. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, L.; Xie, M.; Zhan, Z.; Yang, D.; Cheng, P.; Duan, H.; Ge, Q.; Wang, Z. Ultra-Fast Programmable Human-Machine Interface Enabled by 3D Printed Degradable Conductive Hydrogel. Mater. Today Phys. 2022, 27, 100794. [Google Scholar] [CrossRef]
- Ahmed, A.; Sharma, S.; Adak, B.; Hossain, M.M.; LaChance, A.M.; Mukhopadhyay, S.; Sun, L. Two-dimensional MXenes: New Frontier of Wearable and Flexible Electronics. InfoMat 2022, 4, e12295. [Google Scholar] [CrossRef]
- Kim, H.; Alshareef, H.N. MXetronics: MXene-Enabled Electronic and Photonic Devices. ACS Mater. Lett. 2020, 2, 55–70. [Google Scholar] [CrossRef] [Green Version]
- Li, K.; Zhao, J.; Zhussupbekova, A.; Shuck, C.E.; Hughes, L.; Dong, Y.; Barwich, S.; Vaesen, S.; Shvets, I.V.; Möbius, M.; et al. 4D Printing of MXene Hydrogels for High-Efficiency Pseudocapacitive Energy Storage. Nat. Commun. 2022, 13, 6884. [Google Scholar] [CrossRef] [PubMed]
- Ge, G.; Zhang, Y.-Z.; Zhang, W.; Yuan, W.; El-Demellawi, J.K.; Zhang, P.; Di Fabrizio, E.; Dong, X.; Alshareef, H.N. Ti 3 C 2 T x MXene-Activated Fast Gelation of Stretchable and Self-Healing Hydrogels: A Molecular Approach. ACS Nano 2021, 15, 2698–2706. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Luo, M.; Zhang, D.; Liu, C.; Li, Z.; Wang, L.; Chen, W.; Zhou, X. Ti3C2T /Carbon Nanotube/Porous Carbon Film for Flexible Supercapacitor. Chem. Eng. J. 2022, 427, 132002. [Google Scholar] [CrossRef]
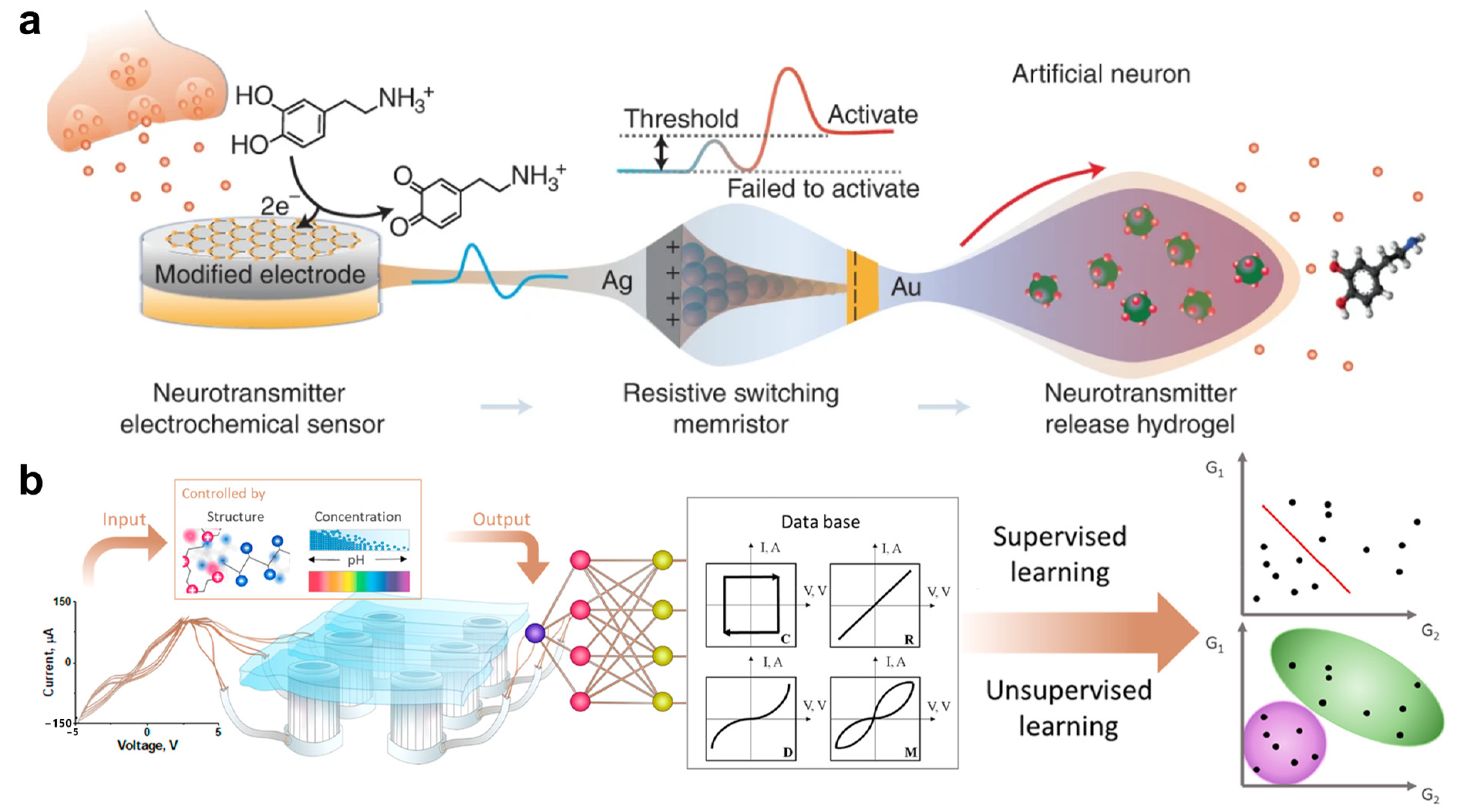
- Wang, T.; Wang, M.; Wang, J.; Yang, L.; Ren, X.; Song, G.; Chen, S.; Yuan, Y.; Liu, R.; Pan, L.; et al. A Chemically Mediated Artificial Neuron. Nat. Electron. 2022, 5, 586–595. [Google Scholar] [CrossRef]
- Lavrentev, F.V.; Rumyantsev, I.S.; Ivanov, A.S.; Shilovskikh, V.V.; Orlova, O.Y.; Nikolaev, K.G.; Andreeva, D.V.; Skorb, E.V. Soft Hydrogel Actuator for Fast Machine-Learning-Assisted Bacteria Detection. ACS Appl. Mater. Interfaces 2022, 14, 7321–7328. [Google Scholar] [CrossRef]
- Ivanov, A.S.; Nikolaev, K.G.; Stekolshchikova, A.A.; Tesfatsion, W.T.; Yurchenko, S.O.; Novoselov, K.S.; Andreeva, D.V.; Rubtsova, M.Y.; Vorovitch, M.F.; Ishmukhametov, A.A.; et al. Tick-Borne Encephalitis Electrochemical Detection by Multilayer Perceptron on Liquid–Metal Interface. ACS Appl. Bio Mater. 2020, 3, 7352–7356. [Google Scholar] [CrossRef]
- Ivanov, A.S.; Nikolaev, K.G.; Novikov, A.S.; Yurchenko, S.O.; Novoselov, K.S.; Andreeva, D.V.; Skorb, E.V. Programmable Soft-Matter Electronics. J. Phys. Chem. Lett. 2021, 12, 2017–2022. [Google Scholar] [CrossRef]
- Zhang, Y.; Jeong, C.K.; Wang, J.; Chen, X.; Choi, K.H.; Chen, L.; Chen, W.; Zhang, Q.M.; Wang, Q. Hydrogel Ionic Diodes toward Harvesting Ultralow-Frequency Mechanical Energy. Adv. Mater. 2021, 33, 2103056. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Lu, M.; Chen, F.; Jia, H.; Zhou, H.; Li, K.; Zeng, X.; Zhao, W.; Lin, P. Multifunctional Hydrogel Hybrid-Gated Organic Photoelectrochemical Transistor for Biosensing. Adv. Funct. Mater. 2022, 32, 2109046. [Google Scholar] [CrossRef]
- Duy, L.T.; Seo, H. Construction of Stretchable Supercapacitors Using Graphene Hybrid Hydrogels and Corrosion-Resistant Silver Nanowire Current Collectors. Appl. Surf. Sci. 2020, 521, 146467. [Google Scholar] [CrossRef]
- Molinnus, D.; Poghossian, A.; Keusgen, M.; Katz, E.; Schöning, M.J. Coupling of Biomolecular Logic Gates with Electronic Transducers: From Single Enzyme Logic Gates to Sense/Act/Treat Chips. Electroanalysis 2017, 29, 1840–1849. [Google Scholar] [CrossRef]
- Ruskowitz, E.R.; Comerford, M.P.; Badeau, B.A.; DeForest, C.A. Logical Stimuli-Triggered Delivery of Small Molecules from Hydrogel Biomaterials. Biomater. Sci. 2019, 7, 542–546. [Google Scholar] [CrossRef]
- Badeau, B.A.; Comerford, M.P.; Arakawa, C.K.; Shadish, J.A.; DeForest, C.A. Engineered Modular Biomaterial Logic Gates for Environmentally Triggered Therapeutic Delivery. Nat. Chem. 2018, 10, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Walther, A. PH Feedback Lifecycles Programmed by Enzymatic Logic Gates Using Common Foods as Fuels. Angew. Chem. Int. Ed. 2021, 60, 11398–11405. [Google Scholar] [CrossRef]
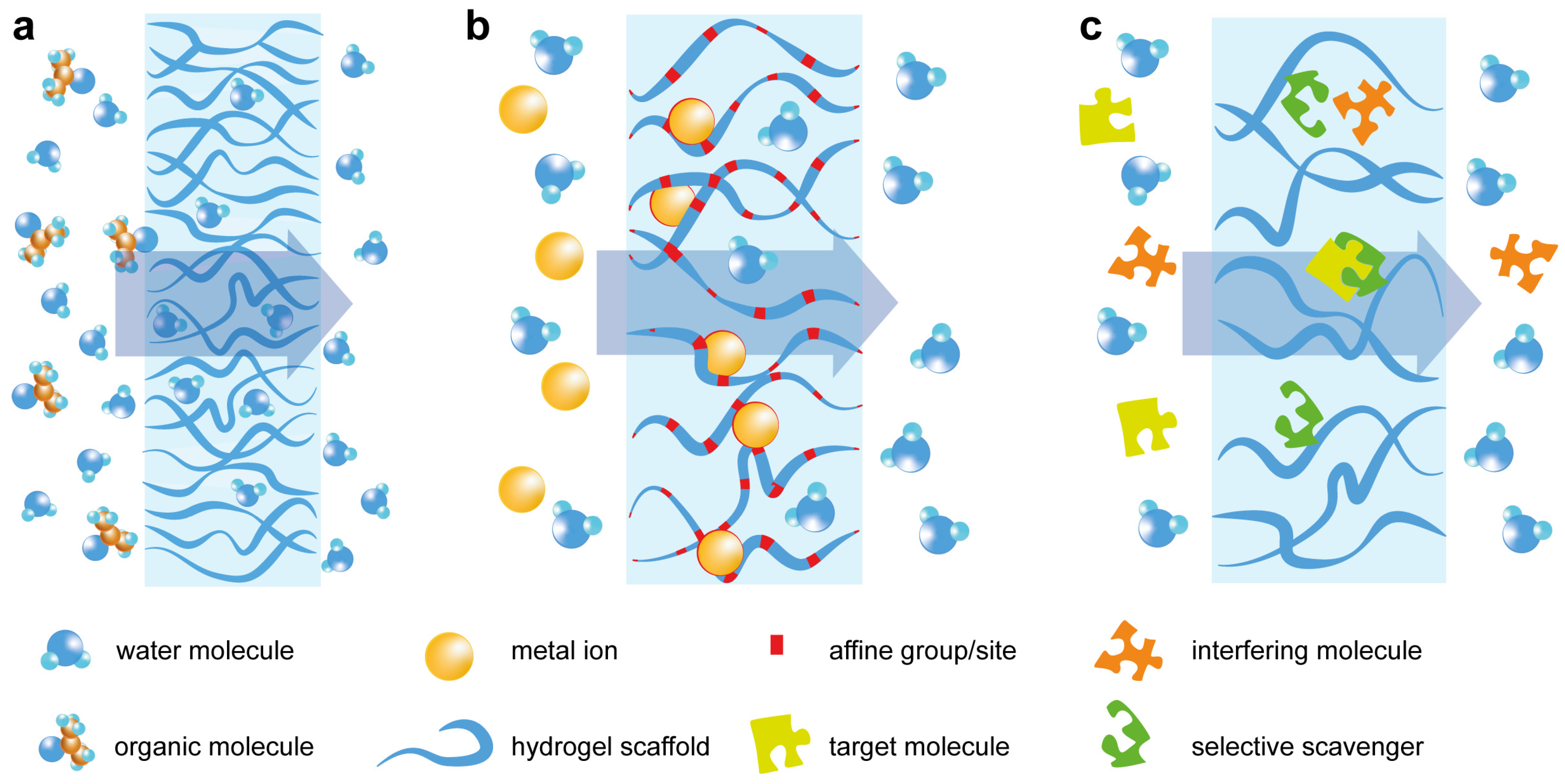

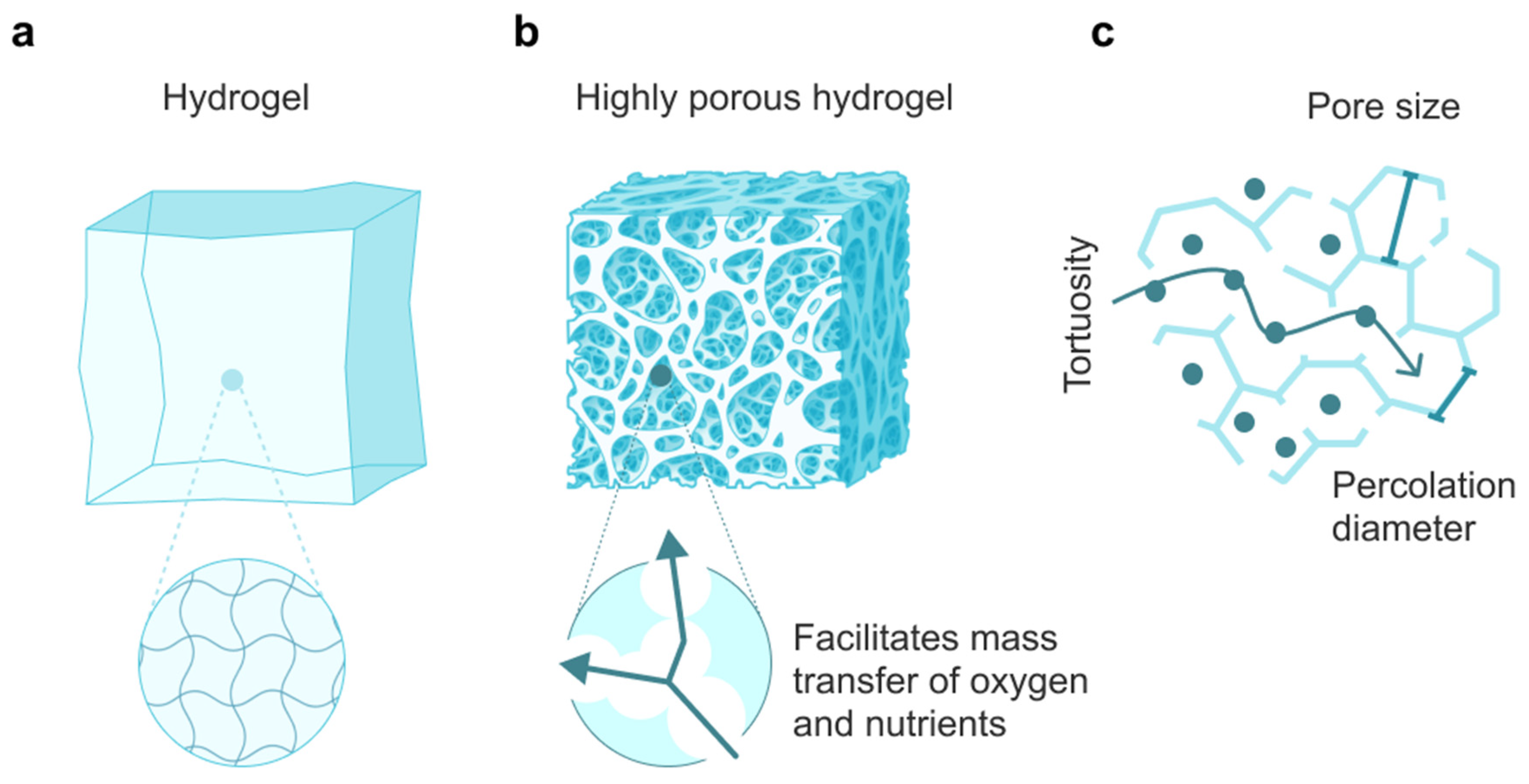

| Type of Hydrogel | Strategies for Changing the Diffusion Rate | Application | References |
|---|---|---|---|
| Composite nanofibrous scaffolds of polyamide-6, polyvinylpyrrolidone, and tea tree oil | Molecular weight of the polymer | Obtaining nanofibers for the manufacture of antimicrobial wound dressings | [153] |
| Chitin whisker/chitosan (CHW/CS) hydrogels | Crosslinking density | Dual-crosslinked liquid crystal hydrogels are good candidates for bone repair applications | [154] |
| Hyaluronic acid nanocomposite hydrogel (HA-BP hydrogel) by coordination bonds with bisphosphonates (BPs) | Doping of metal | Highly dynamic nanocomposite hydrogels that self-assemble by coordinating metal ions and ligands enable new dynamic materials for regenerative medicine | [155] |
| Nanocomposites of silver nanoparticles/gelatin | Thermoresponsive | Silver diffusion-controlled hydrogel is a tool as an effective dosage form for topical wound healing | [156] |
| The functional hydrogel encoding the binding domain of laminin (Fmoc-DDIKVAV) | Addition of myoglobin | The hydrogel can control cell fate in progenitor cell grafts, enabling the successful integration of stem cell grafts to treat nerve damage and illnesses affecting the central and peripheral nervous system | [157] |
| Types of Hydrogel | Crosslinking Agent | Included Fertilizer | References |
|---|---|---|---|
| Alginate–cellulose nanofibers–PVA | Calcium sulfate | Potassium chloride, ammonium dihydrogen phosphate | [179] |
| CS–alginate | Copper sulfate | Trichoderma viride, copper cations | [180] |
| CMC–CS | Manganese sulfate | Prothioconazole | [174] |
| CS | Sodium tripolyphosphate | Hexaconazole, dazomet | [181,182,183] |
| CMC-g-poly(AM-co-AMPS) | N,N’-methylenebisacrylamide | 2-Chloroethylphosphonic acid | [184] |
| MS-g-PA | N,N′-methylenebisacrylamide | ZnO/tetraethyl orthosilicate | [185] |
| Starch–cellulose | N,N′-methylenebisacrylamide | Urea | [186] |
| Starch–PVA | Acrylic acid, citric acid, and maleic acid | Urea | [187] |
| Carboxymethyl starch/polydopamine | Monochloroacetic acid | NH4+, Zn, P, and Fe | [188] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lavrentev, F.V.; Shilovskikh, V.V.; Alabusheva, V.S.; Yurova, V.Y.; Nikitina, A.A.; Ulasevich, S.A.; Skorb, E.V. Diffusion-Limited Processes in Hydrogels with Chosen Applications from Drug Delivery to Electronic Components. Molecules 2023, 28, 5931. https://doi.org/10.3390/molecules28155931
Lavrentev FV, Shilovskikh VV, Alabusheva VS, Yurova VY, Nikitina AA, Ulasevich SA, Skorb EV. Diffusion-Limited Processes in Hydrogels with Chosen Applications from Drug Delivery to Electronic Components. Molecules. 2023; 28(15):5931. https://doi.org/10.3390/molecules28155931
Chicago/Turabian StyleLavrentev, Filipp V., Vladimir V. Shilovskikh, Varvara S. Alabusheva, Veronika Yu. Yurova, Anna A. Nikitina, Sviatlana A. Ulasevich, and Ekaterina V. Skorb. 2023. "Diffusion-Limited Processes in Hydrogels with Chosen Applications from Drug Delivery to Electronic Components" Molecules 28, no. 15: 5931. https://doi.org/10.3390/molecules28155931
APA StyleLavrentev, F. V., Shilovskikh, V. V., Alabusheva, V. S., Yurova, V. Y., Nikitina, A. A., Ulasevich, S. A., & Skorb, E. V. (2023). Diffusion-Limited Processes in Hydrogels with Chosen Applications from Drug Delivery to Electronic Components. Molecules, 28(15), 5931. https://doi.org/10.3390/molecules28155931







