Simultaneous Determination of Five Iridoids of Picrorhiza scrophulariiflora in Rat Plasma Using UHPLC-ESI-MS/MS
Abstract
1. Introduction
2. Results and Discussion
2.1. Optimization of Chromatographic and Mass Spectrometric Conditions
2.2. Selection of Internal Standard
2.3. Optimization of Plasma Sample Processing Method
2.4. UHPLC-MS/MS Method Validation
2.4.1. Specificity
2.4.2. Linearity and Lowest Limit of Quantification (LLOQ)
2.4.3. Extraction Recovery and Matrix Effect
2.4.4. Intra-Day and Inter-Day Precision and Accuracy
2.4.5. Stability
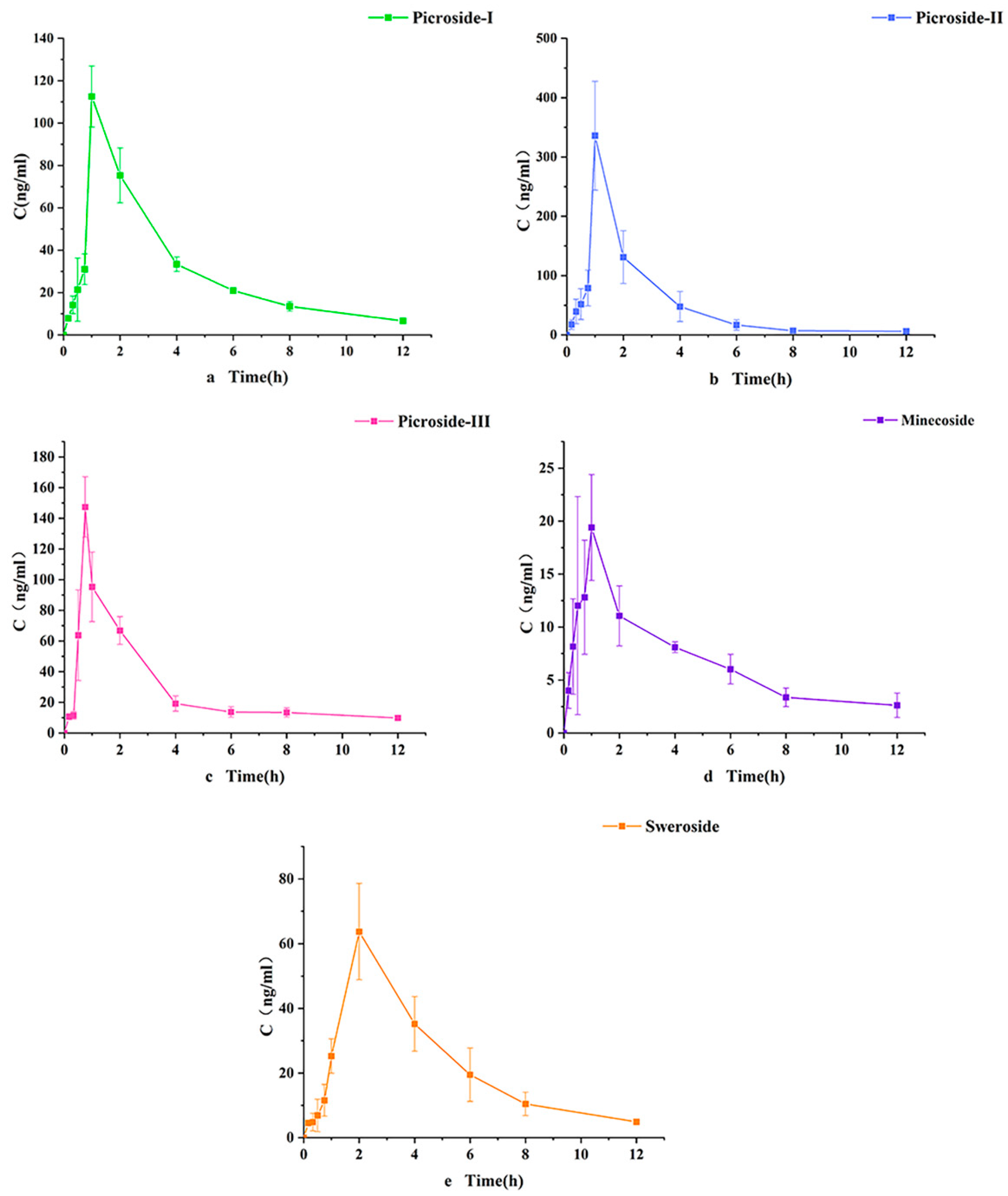
2.5. Pharmacokinetic Characteristics
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Preparation of Standard Solutions
3.3. Processing of Plasma Samples
3.4. Instrumentation and LC–MS/MS Analytical Conditions
3.5. Validation of Analytical Methods for Plasma Samples
3.5.1. Specificity
3.5.2. Linearity and Lowest Limit of Quantification (LLOQ)
3.5.3. Extraction Recovery and Matrix Effects
3.5.4. Intra-Day and Inter-Day Precision and Accuracy
3.5.5. Stability
3.6. Pharmacokinetic and Data Analysis
3.6.1. Animals
3.6.2. Preparation of PS Gavage Solution
3.6.3. Dosing Regimen and Sample Collection and Processing
3.6.4. Plasma Sample Determination
3.6.5. Calculation of Pharmacokinetic Parameters
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Ma, L.-Z.; Kang, L.-P.; Nan, T.-G.; Zhan, Z.-L.; Guo, L.-P. Advances in research on chemical composition of Picrorhiza scrophulariiflora and P. kurroa and their biological activities. China J. Chin. Mater. Med. 2021, 46, 6114–6129. [Google Scholar] [CrossRef]
- Kitagawa, I.; Hino, K.; Nishimura, T.; Mukai, E.; Yosioka, I.; Inouye, H.; Yoshida, T. Picroside I: A bitter principle of picrorhiza kurrooa. Tetrahedron Lett. 1969, 10, 3837–3840. [Google Scholar] [CrossRef]
- Cao, X.; Qiao, J.; Wang, L.; Ye, X.; Zheng, L.; Jiang, N.; Mo, W. Screening of glycoside isomers in P. scrophulariiflora using ionic liquid-based ultrasonic-assisted extraction and ultra-performance liquid chromatography/electrospray ionization quadrupole time-of-flight tandem mass spectrometry. Rapid Commun. Mass. Spectrom. 2012, 26, 740–748. [Google Scholar] [CrossRef]
- Shen, Q.; Dong, W.; Wang, Y.; Gong, L.; Dai, Z.; Cheung, H.-Y. Pipette tip solid-phase extraction and ultra-performance liquid chromatography/mass spectrometry based rapid analysis of picrosides from Picrorhiza scrophulariiflora. J. Pharm. Biomed. Anal. 2013, 80, 136–140. [Google Scholar] [CrossRef]
- Li, T.; Xu, L.; Zheng, R.; Wang, X.; Li, L.; Ji, H.; Hu, Q. Picroside II protects against cholestatic liver injury possibly through activation of farnesoid X receptor. Phytomedicine 2019, 68, 153153. [Google Scholar] [CrossRef] [PubMed]
- Thapa, A.; Kaushik, R.; Arora, S.; Jaglan, S.; Jaswal, V.; Yadav, V.K.; Singh, M.; Bains, A.; Chawla, P.; Khan, A.; et al. Biological Activity of Picrorhiza kurroa: A Source of Potential Antimicrobial Compounds against Yersinia enterocolitica. Int. J. Mol. Sci. 2022, 23, 14090. [Google Scholar] [CrossRef] [PubMed]
- Akkol, E.K.; Tatli, I.I.; Akdemir, Z.S. Antinociceptive and anti-inflammatory effects of saponin and iridoid glycosides from Verbascum pterocalycinum var. mutense Hub.-Mor. Z. Für Naturforschung C 2007, 62, 813–820. [Google Scholar]
- Li, T.; Liu, J.-W.; Zhang, X.-D.; Guo, M.-C.; Ji, G. The Neuroprotective Effect of Picroside II from Hu-Huang-Lian against Oxidative Stress. Am. J. Chin. Med. 2007, 35, 681–691. [Google Scholar] [CrossRef]
- Jia, D.; Wu, N.; Li, W.; Shu, W. Protective effect of picroside II on myocardial ischemia reperfusion injury in rats. Drug Des. Dev. Ther. 2014, 8, 545–554. [Google Scholar] [CrossRef][Green Version]
- Mehrotra, R.; Rawat, S.; Kulshreshtha, D.K.; Patnaik, G.K.; Dhawan, B.N. In vitro studies on the effect of certain natural products against hepatitis B virus. Indian. J. Med. Res. 1990, 92, 133–138. [Google Scholar]
- Najar, I.A.; Sachin, B.S.; Sharma, S.C.; Satti, N.K.; Suri, K.A.; Johri, R.K. Modulation of P-glycoprotein ATPase activity by some phytoconstituents. Phytother. Res. 2009, 24, 454–458. [Google Scholar] [CrossRef]
- Huang, S.-X.; Zhou, Y.; Nie, Q.-J.; Ding, L.-S.; Peng, S.-L. Two new iridoid glucosides from Picrorhiza scrophulariiflora. J. Asian Nat. Prod. Res. 2006, 8, 259–263. [Google Scholar] [CrossRef]
- Zou, L.-C.; Zhu, T.-F.; Xiang, H.; Yu, L.; Yan, Z.-H.; Gan, S.-C.; Wang, D.-C.; Zeng, S.; Deng, X.-M. New secoiridoid Glycosides from the Roots of Picrorhiza Scrophulariiflora. Molecules 2008, 13, 2049–2057. [Google Scholar] [CrossRef]
- Morikawa, T.; Nakanishi, Y.; Inoue, N.; Manse, Y.; Matsuura, H.; Hamasaki, S.; Yoshikawa, M.; Muraoka, O.; Ninomiya, K. Acylated iridoid glycosides with hyaluronidase inhibitory activity from the rhizomes of Picrorhiza kurroa Royle ex Benth. Phytochemistry 2019, 169, 112185. [Google Scholar] [CrossRef]
- Kim, B.; Min, Y.-H.; Park, B. Minecoside Modulates Cell Invasion via Regulation of CXCR4 Expression in Breast and Colon Cancer Cells. Planta Medica 2020, 86, 331–337. [Google Scholar] [CrossRef]
- Sun, H.; Li, L.; Zhang, A.; Zhang, N.; Lv, H.; Sun, W.; Wang, X. Protective effects of sweroside on human MG-63 cells and rat osteoblasts. Fitoterapia 2013, 84, 174–179. [Google Scholar] [CrossRef]
- Choi, L.Y.; Kim, M.H.; Yang, W.M. Promotion of osteogenesis by Sweroside via BMP2-involved signaling in postmenopausal osteoporosis. Phytother. Res. 2021, 35, 7050–7063. [Google Scholar] [CrossRef]
- Wang, R.; Dong, Z.; Lan, X.; Liao, Z.; Chen, M. Sweroside alleviated LPS-induced inflammation via SIRT1 mediating NF-κB and FOXO1 signaling pathways in RAW264. 7 cells. Molecules 2019, 24, 872. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.-Q.; Yu, Y.; Chen, H.; Li, M.; Ihsan, A.; Tong, H.-Y.; Huang, X.-J.; Gao, Y. Sweroside Alleviated Aconitine-Induced Cardiac Toxicity in H9c2 Cardiomyoblast Cell Line. Front. Pharmacol. 2018, 9, 1138. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.L.; Yang, F.; Gong, J.T.; Tang, X.W.; Wang, G.Y.; Wang, Z.T.; Yang, L. Sweroside ameliorates al-pha-naphthylisothiocyanate-induced cholestatic liver injury in mice by regulating bile acids and suppressing pro-inflammatory responses. Acta Pharmacol. Sin. 2016, 37, 1218–1228. [Google Scholar] [CrossRef]
- Zhu, J.; Xue, B.; Ma, B.; Zhang, Q.; Liu, M.; Liu, L.; Yao, D.; Qi, H.; Wang, Y.; Ying, H.; et al. A pre-clinical pharmacokinetic study in rats of three naturally occurring iridoid glycosides, Picroside-I, II and III, using a validated simultaneous HPLC–MS/MS assay. J. Chromatogr. B 2015, 993–994, 47–59. [Google Scholar] [CrossRef]
- Liu, Y.; Chi, S.; Wang, W.; Su, L.; Liu, B. Simultaneous Determination of Seven Components in Rat Plasma by the UPLC-MS/MS Method and Application of Pharmacokinetic Studies to SimiaoYong’an Decoction. Molecules 2017, 22, 1937. [Google Scholar] [CrossRef] [PubMed]
- Sheng, N.; Yuan, L.; Zhi, X.; Cui, C.; Zhang, Z.; Jia, P.; Zhang, X.; Zhang, L.; Wang, X. Application of a liquid chromatog-raphy-tandem mass spectrometry method to the pharmacokinetics, tissue distribution and excretion studies of sweroside in rats. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2014, 969, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Gaikwad, P.S.; Bhope, S.G.; Kuber, V.V.; Patil, M.J. Validated TLC method for Simultaneous Quantitation of Kutkoside and Picroside-I from Kutki Extract. Phytochem. Anal. 2010, 22, 36–41. [Google Scholar] [CrossRef]
- Lv, J.; Pan, L.; Ye, Y.; Zhou, Y. A sensitive and selective RP-HPLC method for simultaneous determination of picroside-I and picroside-II in rat plasma and its application in pharmacokinetics studies. J. Sep. Sci. 2007, 30, 2466–2472. [Google Scholar] [CrossRef]
- Remeur, C.; Borgne, E.L.; Gauthier, L.; Grougnet, R.; Deguin, B.; Poullaina, C.; Litaudon, M. HPLC-ELSD Quantification and Centrifugal Partition Chromatography Isolation of 8-OAcetylharpagide from Oxera coronata (Lamiaceae). Phytochem. Anal. 2016, 28, 242–246. [Google Scholar] [CrossRef]
- Zhao, X.; Wei, J.; Yang, M. Simultaneous Analysis of Iridoid Glycosides and Anthraquinones in Morinda officinalis Using UPLC-QqQ-MS/MS and UPLC-Q/TOF-MSE. Molecules 2018, 23, 1070. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Sun, X.; Wang, J.; Wang, H.; Wang, J. Pharmacokinetics of picroside I and picroside II in rats. J. Shenyang Pharm. Univ. 2013, 30, 724–728. [Google Scholar]



| ID | Regression Equation | Linear Range (ng/mL) | r | LLOQ (ng/mL) |
|---|---|---|---|---|
| Picroside-I | Y = 1.32 × 10−3X + 2.7 × 10−3 | 6.876–764.0 | 0.9973 | 6.876 |
| Picroside-II | Y = 8.51 × 10−3X + 6.2 × 10−2 | 5.193–577.0 | 0.9983 | 5.193 |
| Picroside-III | Y = 0.82 × 10−3X + 0.6 × 10−3 | 5.040–560.0 | 0.9954 | 5.040 |
| Minecoside | Y = 5.32 × 10−3X − 1.3 × 10−1 | 1.260–140.0 | 0.9911 | 1.260 |
| Sweroside | Y = 2.20 × 10−2X + 0.4 × 10−2 | 4.527–503.0 | 0.9989 | 4.527 |
| Compound | QC (ng/mL) | Recovery | Matrix Effect | ||
|---|---|---|---|---|---|
| Mean ± SD (%) | RSD (%) | Mean ± SD (%) | RSD (%) | ||
| Picroside-I | 13.75 | 86.51 ± 4.2 | 4.9 | 96.17 ± 2.6 | 2.7 |
| 382.0 | 85.12 ± 3.6 | 4.2 | 101.9 ± 5.4 | 5.3 | |
| 573.0 | 91.34 ± 2.3 | 2.5 | 98.93 ± 6.4 | 6.5 | |
| Picroside-II | 10.39 | 71.02 ± 8.2 | 11.5 | 101.7 ± 7.6 | 7.5 |
| 288.5 | 67.11 ± 4.0 | 6.0 | 99.71 ± 7.2 | 7.2 | |
| 432.8 | 72.53 ± 4.6 | 6.3 | 97.26 ± 5.1 | 5.2 | |
| Picroside-III | 10.08 | 76.43 ± 6.1 | 8.0 | 95.77 ± 3.7 | 3.9 |
| 280.0 | 80.47 ± 3.3 | 4.1 | 99.32 ± 2.9 | 2.9 | |
| 420.0 | 83.84 ± 2.5 | 3.0 | 101.8 ± 4.8 | 4.7 | |
| Minecoside | 2.520 | 77.62 ± 8.9 | 11.4 | 96.84 ± 5.4 | 5.6 |
| 70.00 | 83.16 ± 6.1 | 7.3 | 97.17 ± 2.6 | 2.7 | |
| 105.00 | 80.45 ± 3.7 | 4.6 | 97.47 ± 5.1 | 5.2 | |
| Sweroside | 9.054 | 82.25 ± 6.8 | 8.3 | 98.61 ± 7.5 | 7.6 |
| 251.5 | 85.36 ± 5.6 | 6.6 | 98.65 ± 6.4 | 6.5 | |
| 377.2 | 79.57 ± 2.0 | 2.5 | 101.5 ± 3.7 | 3.6 | |
| ID | QC (ng/mL) | Inter-Day | Intra-Day | ||
|---|---|---|---|---|---|
| RSD (%) | RE (%) | RSD (%) | RE (%) | ||
| Picroside-I | 6.876 | 5.5 | 1.3 | 7.7 | 6.1 |
| 13.75 | 6.8 | 1.2 | 7.9 | 5.8 | |
| 382.0 | 8.9 | 1.1 | 6.9 | 4.1 | |
| 573.0 | 5.6 | 2.6 | 6.6 | 3.1 | |
| Picroside-II | 5.193 | 5.7 | 12.9 | 9.7 | 6.3 |
| 10.39 | 4.8 | −12.1 | 9.4 | −4.3 | |
| 288.5 | 8.5 | 13.2 | 10.1 | 7.1 | |
| 432.8 | 6.1 | 8.7 | 4.2 | 2.9 | |
| Picroside-III | 5.040 | 6.2 | 11.5 | 8.1 | 6.8 |
| 10.08 | 5.8 | −11.3 | 7.6 | −3.4 | |
| 280.0 | 7.5 | 6.5 | 5.0 | 6.6 | |
| 420.0 | 5.9 | 2.0 | 5.8 | 6.3 | |
| Minecoside | 1.260 | 10.2 | 12.8 | 7.0 | 12.9 |
| 2.520 | 9.7 | −12.3 | 4.1 | 13.2 | |
| 70.00 | 6.5 | 5.6 | 6.5 | 8.7 | |
| 105.00 | 11.1 | 2.2 | 6.7 | −10.3 | |
| Sweroside | 4.527 | 9.1 | 10.8 | 9.4 | 10.7 |
| 9.054 | 7.6 | 11.2 | 7.8 | 6.3 | |
| 251.5 | 9.0 | −4.8 | 9.1 | 3.5 | |
| 377.2 | 6.8 | 8.8 | 8.8 | 11.3 | |
| ID | QC (ng/mL) | Short-Term Stability | Stability after Preparation | Freeze-Thaw | Long-Term Stability | ||||
|---|---|---|---|---|---|---|---|---|---|
| RSD (%) | RE (%) | RSD (%) | RE (%) | RSD (%) | RE (%) | RSD (%) | RE (%) | ||
| Picroside-I | 13.75 | 5.1 | 8.93 | 5.8 | 6.80 | 7.1 | 6.43 | 10.9 | 5.60 |
| 382.0 | 2.9 | 3.83 | 6.8 | 9.73 | 5.0 | −2.74 | 8.1 | −5.69 | |
| 573.0 | 2.9 | 6.58 | 4.7 | 7.02 | 5.3 | −1.94 | 7.7 | 3.59 | |
| Picroside-II | 10.39 | 4.4 | −4.99 | 4.4 | −3.26 | 4.2 | 7.08 | 7.6 | 8.69 |
| 288.5 | 3.6 | 8.53 | 5.0 | 6.66 | 5.9 | −7.21 | 6.6 | 1.37 | |
| 432.8 | 2.7 | 4.47 | 2.8 | −6.58 | 4.3 | −1.47 | 5.0 | −2.19 | |
| Picroside-III | 10.08 | 4.5 | −2.91 | 3.0 | 9.08 | 6.3 | 2.75 | 11.1 | 5.66 |
| 280.0 | 3.2 | 6.82 | 5.5 | 7.95 | 5.1 | −5.33 | 6.3 | −1.92 | |
| 420.0 | 3.1 | 4.51 | 3.4 | −4.87 | 3.5 | −1.64 | 4.2 | −4.99 | |
| Minecoside | 2.520 | 4.5 | 3.33 | 3.3 | 6.38 | 11.1 | −1.90 | 4.5 | 3.31 |
| 70.00 | 3.2 | 5.72 | 5.7 | 5.13 | 6.3 | 7.16 | 2.2 | 5.70 | |
| 105.00 | 3.1 | 3.40 | 3.4 | 3.56 | 4.2 | −6.28 | 2.1 | 3.41 | |
| Sweroside | 9.054 | 1.9 | 3.68 | 4.7 | 9.17 | 5.3 | −1.94 | 7.7 | 3.59 |
| 251.5 | 4.3 | −5.09 | 2.4 | 4.06 | 4.2 | 7.08 | 7.6 | 8.69 | |
| 377.2 | 1.6 | 9.62 | 5.0 | 6.66 | 5.9 | −7.21 | 6.6 | 1.37 | |
| ID | Cmax (ng/mL) | Tmax (h) | t1/2 (h) | AUC0–t (ng h/mL) | AUC0–∞ (ng h/mL) | MRT0–t (h) | Cl (L/h/kg) | Vd (L/kg) |
|---|---|---|---|---|---|---|---|---|
| Picroside-I | 114.2 ± 16.1 | 1.1 ± 0.2 | 2.4 ± 0.3 | 360.9 ± 25.6 | 381.8 ± 29.4 | 3.6 ± 0.2 | 36.43 ± 17.436 | 186.7 ± 17.436 |
| Picroside-II | 335.9 ± 92.3 | 1.1 ± 0.1 | 2.3 ± 1.5 | 603.9 ± 239.4 | 610.8 ± 246.1 | 2.3 ± 0.5 | 172.40 ± 59.96 | 796 ± 503.5 |
| Picroside-III | 148.2 ± 21.6 | 0.8 ± 0.1 | 17.5 ± 3.1 | 339.4 ± 56.1 | 597.6 ± 198.1 | 3.4 ± 0.1 | 90.07 ± 34.33 | 2747 ± 1642 |
| Minecoside | 21.1 ± 7.8 | 1.0 ± 0.2 | 5.0 ± 2.3 | 78.9 ± 10.2 | 93.5 ± 22.6 | 4.1 ± 0.7 | 7.148 ± 2.382 | 19.26 ± 4.77 |
| Sweroside | 63.7 ± 17.6 | 2.1 ± 0.1 | 1.2 ± 0.3 | 261.2 ± 65.3 | 267.9 ± 70.8 | 3.9 ± 0.3 | 120.9 ± 36.95 | 785.1 ± 130.6 |
| ID | Parent Ion (m/z) | Daughter Ion (m/z) | DP (V) | CE (eV) |
|---|---|---|---|---|
| Picroside-I | 491.2 | 147.0 | −75.20 | −12.90 |
| Picroside-II | 511.3 | 234.8 | −96.20 | −33.30 |
| Picroside-III | 537.4 | 261.0 | −101.29 | −30.00 |
| Minecoside | 537.5 | 281.4 | −84.92 | −13.46 |
| Sweroside | 403.2 | 195.0 | −45.86 | −18.75 |
| IS | 525.2 | 449.1 | −59.40 | −20.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Shi, X.; Jiang, S.; Sun, J.; Borjigin, G.; Li, Q.; Mu, Y.; Yang, C.; Wang, Z.; Kuang, H. Simultaneous Determination of Five Iridoids of Picrorhiza scrophulariiflora in Rat Plasma Using UHPLC-ESI-MS/MS. Molecules 2023, 28, 5925. https://doi.org/10.3390/molecules28155925
Wang Z, Shi X, Jiang S, Sun J, Borjigin G, Li Q, Mu Y, Yang C, Wang Z, Kuang H. Simultaneous Determination of Five Iridoids of Picrorhiza scrophulariiflora in Rat Plasma Using UHPLC-ESI-MS/MS. Molecules. 2023; 28(15):5925. https://doi.org/10.3390/molecules28155925
Chicago/Turabian StyleWang, Zhibin, Xuepeng Shi, Shuang Jiang, Jiahui Sun, Gilwa Borjigin, Qi Li, Yuanqiu Mu, Chunjuan Yang, Zhenyue Wang, and Haixue Kuang. 2023. "Simultaneous Determination of Five Iridoids of Picrorhiza scrophulariiflora in Rat Plasma Using UHPLC-ESI-MS/MS" Molecules 28, no. 15: 5925. https://doi.org/10.3390/molecules28155925
APA StyleWang, Z., Shi, X., Jiang, S., Sun, J., Borjigin, G., Li, Q., Mu, Y., Yang, C., Wang, Z., & Kuang, H. (2023). Simultaneous Determination of Five Iridoids of Picrorhiza scrophulariiflora in Rat Plasma Using UHPLC-ESI-MS/MS. Molecules, 28(15), 5925. https://doi.org/10.3390/molecules28155925







