Antioxidant Effects of Argan Oil and Olive Oil against Iron-Induced Oxidative Stress: In Vivo and In Vitro Approaches
Abstract
1. Introduction
2. Results
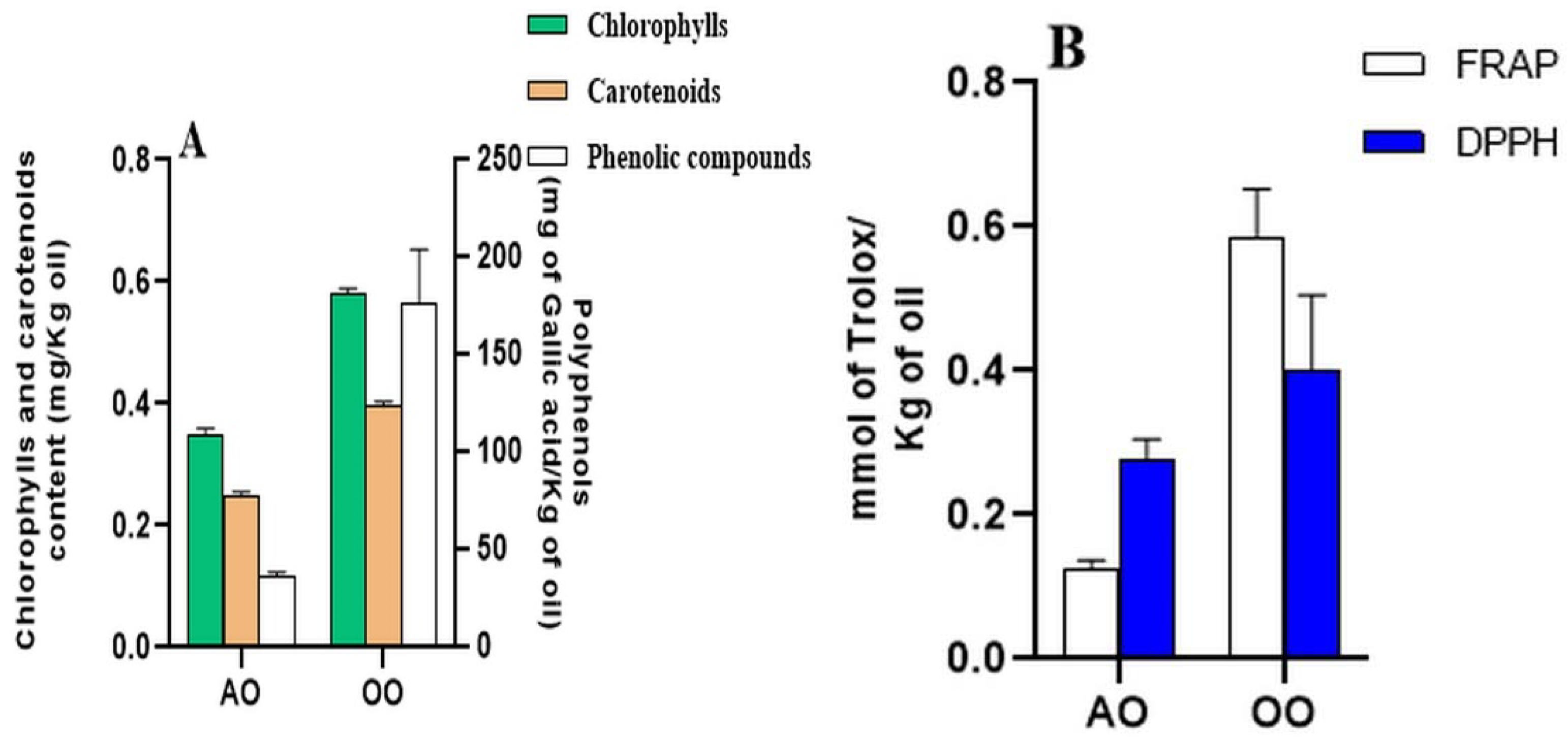
2.1. Total Polyphenol Contents, Pigments Amounts, and Antioxidant Activities
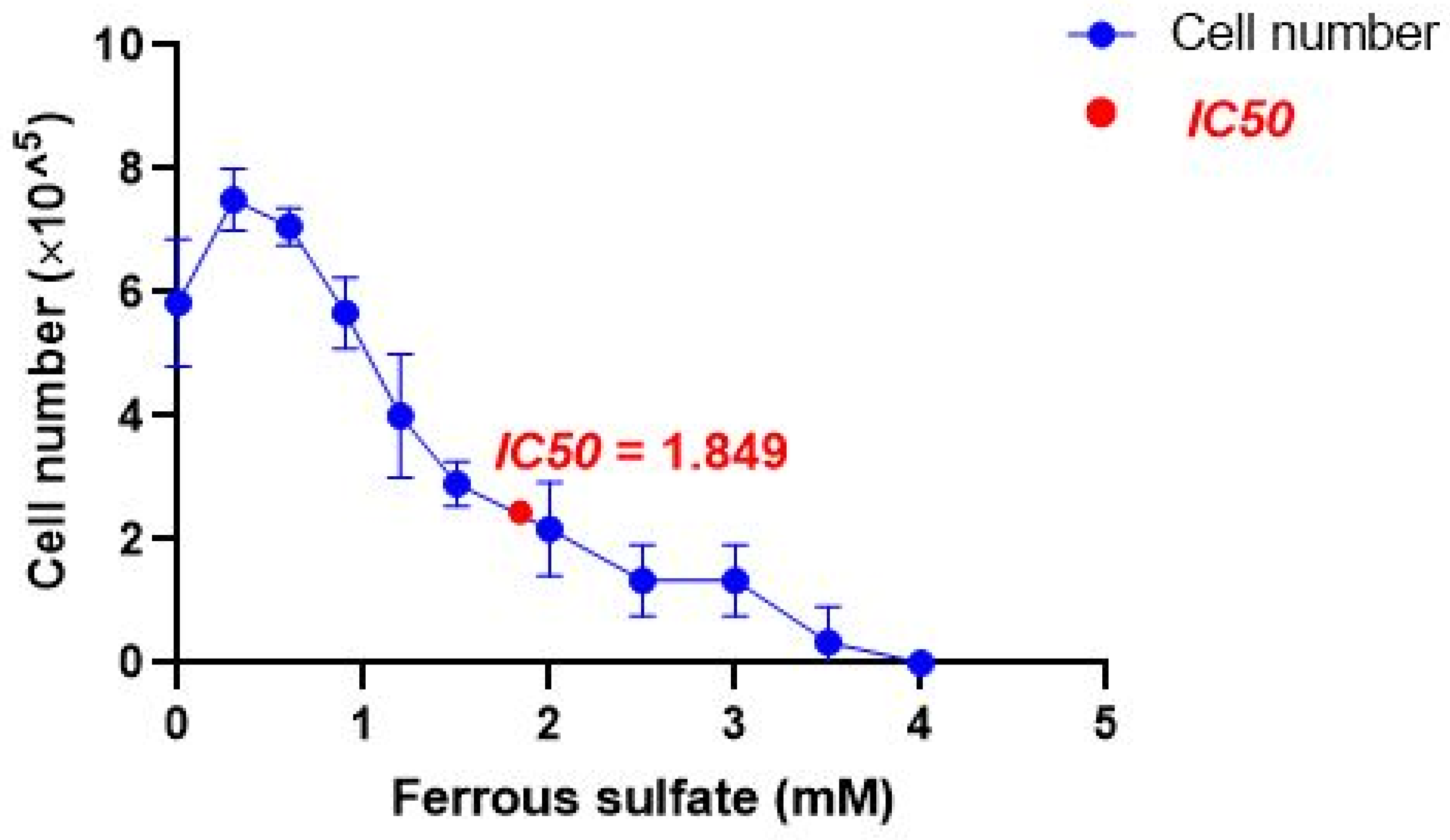
2.2. IC50 of Iron
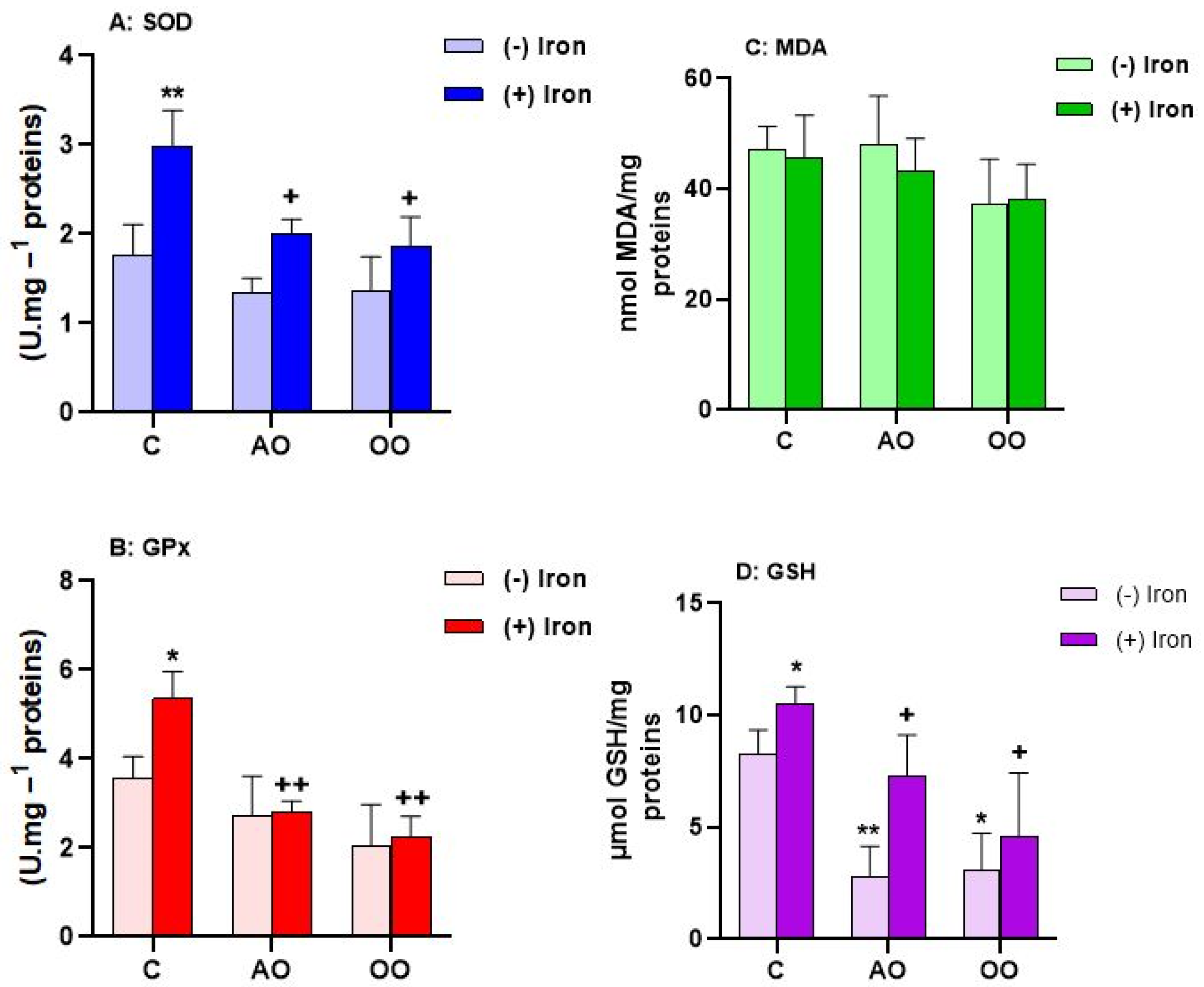
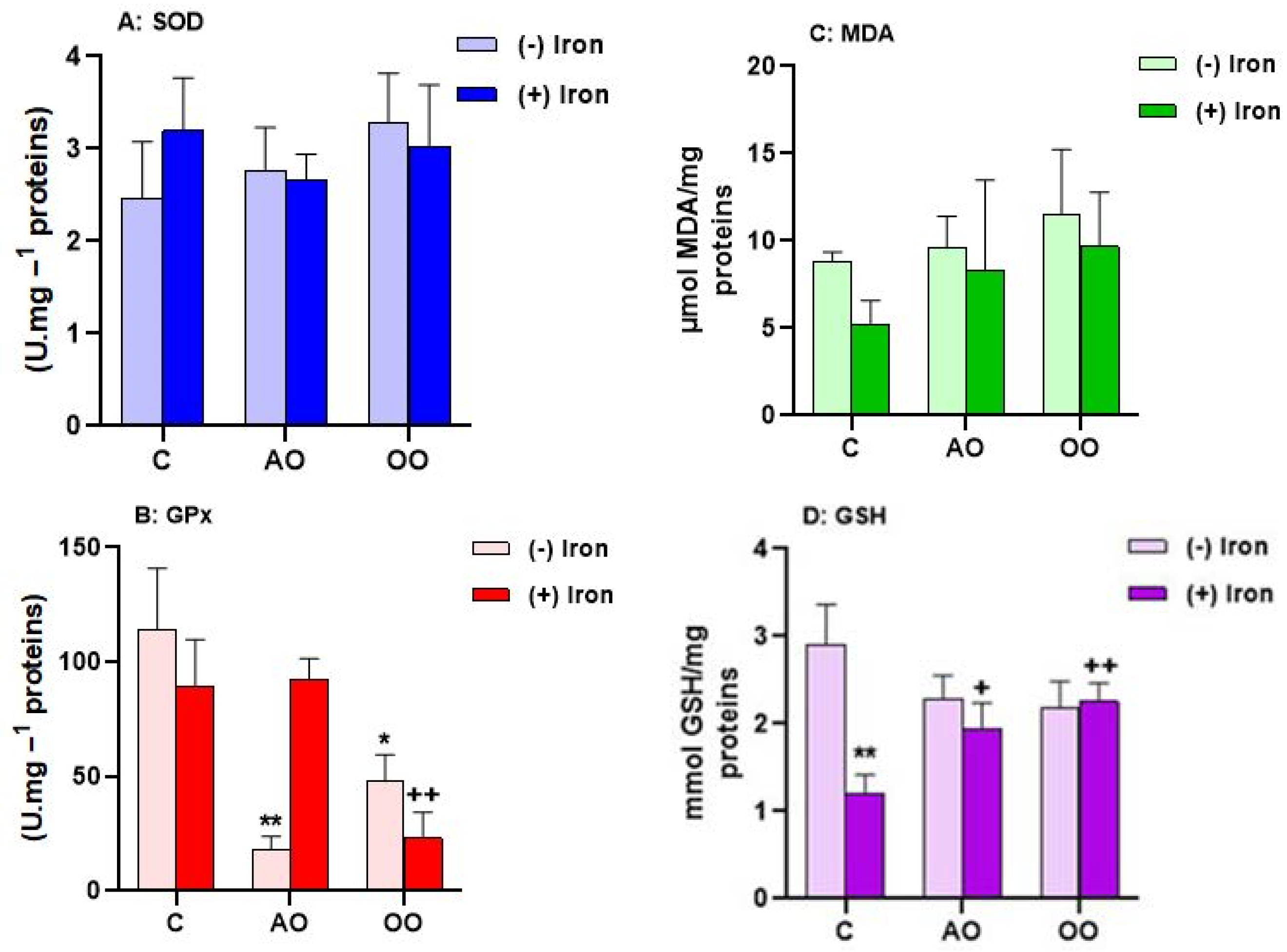
2.3. Argan Oil Protects T. pyriformis from Iron-Disturbed Antioxidant Activities
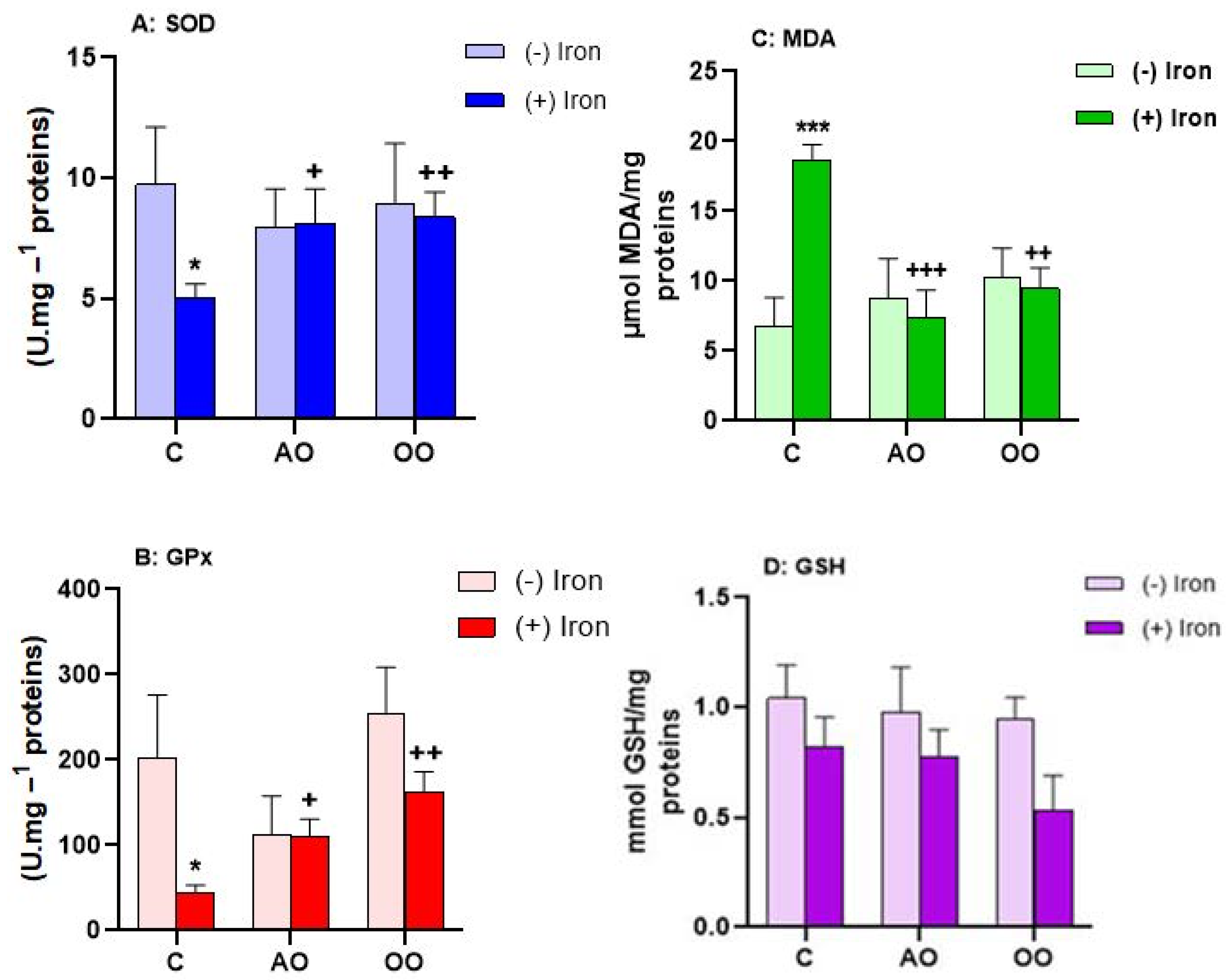
2.4. Argan Oil Protects the Liver from Iron-Disturbed Antioxidant Activities
2.5. Argan Oil Protects the Brain from Iron-Disturbed Antioxidant Activities
2.6. Argan Oil Protects the Kidneys from Iron-Disturbed Antioxidant Activities
| Sample | SOD | GPx | MDA | GSH | |
|---|---|---|---|---|---|
| Tetrahymena pyriformis | C | 1.763 ± 0.346 | 3.572 ± 0.478 | 47.154 ± 4.173 | 8.273 ± 1.068 |
| Iron | 2.992 ± 0.393 ** | 5.358 ± 0.604 * | 45.730 ± 7.603 | 10.521 ± 0.748 * | |
| AO + Iron | 1.994 ± 0.173 + | 2.819 ± 0.239 ++ | 43.243 ± 5.873 | 7.319 ± 1.806 + | |
| OO + Iron | 1.870 ± 0.322 + | 2.263 ± 0.450 ++ | 38.285 ± 6.185 | 4.584 ± 2.845 + | |
| Liver | C | 9.728 ± 2.391 | 202.959 ± 73.156 | 6.737 ± 2.081 | 1.040 ± 0.151 |
| Iron | 5.051 ± 0.550 * | 45.064 ± 8.087 * | 18.638 ± 1.113 *** | 0.817 ± 0.139 | |
| AO + Iron | 8.104 ± 1.448 + | 110.850 ± 19.796 + | 7.442 ± 1.930 +++ | 0.772 ± 0.125 | |
| OO + Iron | 8.400 ± 1.015 ++ | 163.332 ± 22.959 ++ | 9.469 ± 1.487 ++ | 0.536 ± 0.156 | |
| Brain | C | 2.471 ± 0.604 | 114.339 ± 26.456 | 8.801 ± 0.564 | 2.905 ± 0.455 |
| Iron | 3.197 ± 0.570 | 89.673 ± 20.172 | 5.227 ± 1.364 | 1.202 ± 0.212 ** | |
| AO + Iron | 2.657 ± 0.284 | 92.365 ± 9.075 | 8.298 ± 5.173 | 1.938 ± 0.297 + | |
| OO + Iron | 3.027 ± 0.665 | 23.030 ± 11.234 ++ | 9.696 ± 5.173 | 2.255 ± 0.211 ++ | |
| Kidney | C | 2.747 ± 0.634 | 95.221 ± 29.860 | 5.920 ± 1.620 | 2.318 ± 0.268 |
| Iron | 6.333 ± 0.933 ** | 32.101 ± 18.016 * | 5.242 ± 1.693 | 2.190 ± 0.112 | |
| AO + Iron | 2.624 ± 0.401 ++ | 72.961 ± 8.967 + | 6.390 ± 1.010 | 1.134 ± 0.042 | |
| OO + Iron | 3.216 ± 0.615 ++ | 94.682 ± 21.193 ++ | 4.820 ± 2.106 | 2.202 ± 0.185 +++ |
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Origin, Extraction, and Composition of Oils
| Fatty Acids (%) | Argan Oil | Olive Oil |
|---|---|---|
| C14:0 (myristic acid) | 0.1 | 0.0 |
| C15:0 (pentadecanoic acid) | <0.1 | ND a |
| C16:0 (palmitic acid) | 12.6 | 9.7 |
| C17:0 (margaric acid) | 0.1 | 0.0 |
| C17:1 (heptadecenoic acid) | <0.1 | 0.1 |
| C18:0 (stearic acid) | 5.8 | 2.6 |
| C18:1 (oleic acid) | 46.3 | 73.6 |
| C18:2 (linoleic acid) | 34.0 | 11.3 |
| C18:3 (linolenic acid) | 0.1 | 1.0 |
| C20:0 (arachidic acid) | 0.3 | 0.3 |
| C20:1 (gadoleic acid) | 0.3 | 0.4 |
| C22:0 (behenic acid) | 0.1 | 0.1 |
| C24:0 (lignoceric acid) | ND a | 0.2 |
| C18:1t | ND a | 0.02 |
| C18:2t + C18:3t | ND a | 0.04 |
| Argan Oil | Olive Oil | |
|---|---|---|
| Acidity (% as oleic acid) | 0.22 | 0.28 |
| Peroxide value (meq O2/kg oil) | 2.1 | 3.2 |
| K232 | ND a | 1.71 |
| K270 | 0.13 | 0.15 |
| ΔK | 0.003 | 0.00 |
| Moisture and volatile matter (%) | 0.02 | 0.13 |
4.3. Total Polyphenol Contents
4.4. Determination of Chlorophyll and Carotenoid Contents
4.5. Antioxidant Activities
4.5.1. FRAP Assay
4.5.2. DPPH Assay
4.6. Culture and Treatments
4.6.1. Culture Medium
4.6.2. Iron Treatment
4.6.3. Argan Oil and Olive Oil Treatment
4.7. Animals and Experimental Design
4.8. Tetrahymena Pyriformis
4.9. Liver, Brain, and Kidney
4.10. Animals and Experimental Design
4.11. Oxidative Stress Markers and Antioxidants
4.11.1. Lipid Peroxidation
4.11.2. Glutathione
4.12. Enzymatic Activity Measurements
4.12.1. Glutathione Peroxidase
4.12.2. Superoxide Dismutase
4.13. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Weissman, L.; de Souza-Pinto, N.C.; Stevnsner, T.; Bohr, V.A. DNA Repair, Mitochondria, and Neurodegeneration. Neuroscience 2007, 145, 1318–1329. [Google Scholar] [CrossRef] [PubMed]
- Irazabal, M.V.; Torres, V.E. Reactive Oxygen Species and Redox Signaling in Chronic Kidney Disease. Cells 2020, 9, 1342. [Google Scholar] [CrossRef] [PubMed]
- Young, I.S.; Woodside, J.V. Antioxidants in Health and Disease. J. Clin. Pathol. 2001, 54, 176–186. [Google Scholar] [CrossRef] [PubMed]
- Casas, A.I.; Nogales, C.; Mucke, H.A.M.; Petraina, A.; Cuadrado, A.; Rojo, A.I.; Ghezzi, P.; Jaquet, V.; Augsburger, F.; Dufrasne, F.; et al. On the Clinical Pharmacology of Reactive Oxygen Species. Pharmacol. Rev. 2020, 72, 801–828. [Google Scholar] [CrossRef]
- Xue, D.; Zhou, X.; Qiu, J. Emerging Role of NRF2 in ROS-Mediated Tumor Chemoresistance. Biomed. Pharmacother. 2020, 131, 110676. [Google Scholar] [CrossRef]
- Jiménez-Fernández, S.; Gurpegui, M.; Garrote-Rojas, D.; Gutiérrez-Rojas, L.; Carretero, M.D.; Correll, C.U. Oxidative Stress Parameters and Antioxidants in Patients with Bipolar Disorder: Results from a Meta-analysis Comparing Patients, Including Stratification by Polarity and Euthymic Status, with Healthy Controls. Bipolar Disord. 2021, 23, 117–129. [Google Scholar] [CrossRef]
- Khalid, M.; Hassani, S.; Abdollahi, M. Metal-Induced Oxidative Stress: An Evidence-Based Update of Advantages and Disadvantages. Curr. Opin. Toxicol. 2020, 20–21, 55–68. [Google Scholar] [CrossRef]
- Lal, A. Iron in Health and Disease: An Update. Indian J. Pediatr. 2020, 87, 58–65. [Google Scholar] [CrossRef]
- Paunovic, J.; Vucevic, D.; Radosavljevic, T.; Mandić-Rajčević, S.; Pantic, I. Iron-Based Nanoparticles and Their Potential Toxicity: Focus on Oxidative Stress and Apoptosis. Chem.-Biol. Interact. 2020, 316, 108935. [Google Scholar] [CrossRef]
- González-Domínguez, Á.; Visiedo-García, F.M.; Domínguez-Riscart, J.; González-Domínguez, R.; Mateos, R.M.; Lechuga-Sancho, A.M. Iron Metabolism in Obesity and Metabolic Syndrome. Int. J. Mol. Sci. 2020, 21, 5529. [Google Scholar] [CrossRef]
- Juan, C.A.; Pérez de la Lastra, J.M.; Plou, F.J.; Pérez-Lebeña, E. The Chemistry of Reactive Oxygen Species (ROS) Revisited: Outlining Their Role in Biological Macromolecules (DNA, Lipids and Proteins) and Induced Pathologies. Int. J. Mol. Sci. 2021, 22, 4642. [Google Scholar] [CrossRef]
- Saleem, U.; Sabir, S.; Niazi, S.G.; Naeem, M.; Ahmad, B. Role of Oxidative Stress and Antioxidant Defense Biomarkers in Neurodegenerative Diseases. Crit. Rev. Eukaryot. Gene Expr. 2020, 30, 311–322. [Google Scholar] [CrossRef]
- Malik, A.; Firke, S.D.; Patil, R.R.; Shirkhedkar, A.A.; Surana, S.J. Determination of Iron Chelating Agents by Analytical Methods: A Review. Crit. Rev. Anal. Chem. 2020, 50, 254–264. [Google Scholar] [CrossRef]
- Taher, A.T.; Cappellini, M.D.; Aydinok, Y.; Porter, J.B.; Karakas, Z.; Viprakasit, V.; Siritanaratkul, N.; Kattamis, A.; Wang, C.; Zhu, Z.; et al. Optimising Iron Chelation Therapy with Deferasirox for Non-Transfusion-Dependent Thalassaemia Patients: 1-Year Results from the THETIS Study. Blood Cells Mol. Dis. 2016, 57, 23–29. [Google Scholar] [CrossRef]
- El Kebbaj, R.; Andreoletti, P.; El Hajj, H.I.; El Kharrassi, Y.; Vamecq, J.; Mandard, S.; Saih, F.-E.; Latruffe, N.; El Kebbaj, M.S.; Lizard, G.; et al. Argan Oil Prevents Down-Regulation Induced by Endotoxin on Liver Fatty Acid Oxidation and Gluconeogenesis and on Peroxisome Proliferator-Activated Receptor Gamma Coactivator-1α, (PGC-1α), Peroxisome Proliferator-Activated Receptor α (PPARα) and Estrogen Related Receptor α (ERRα). Biochim. Open 2015, 1, 51–59. [Google Scholar] [CrossRef]
- Bouchab, H.; Ishaq, A.; El Kebbaj, R.; Nasser, B.; Saretzki, G. Protective Effect of Argan Oil on DNA Damage in Vivo and in Vitro. Biomarkers 2021, 26, 425–433. [Google Scholar] [CrossRef]
- Badreddine, A.; Zarrouk, A.; Karym, E.M.; Debbabi, M.; Nury, T.; Meddeb, W.; Sghaier, R.; Bezine, M.; Vejux, A.; Martine, L.; et al. Argan Oil-Mediated Attenuation of Organelle Dysfunction, Oxidative Stress and Cell Death Induced by 7-Ketocholesterol in Murine Oligodendrocytes 158N. Int. J. Mol. Sci. 2017, 18, 2220. [Google Scholar] [CrossRef]
- El Kamouni, S.; El Kebbaj, R.; Andreoletti, P.; El Ktaibi, A.; Rharrassi, I.; Essamadi, A.; El Kebbaj, M.S.; Mandard, S.; Latruffe, N.; Vamecq, J.; et al. Protective Effect of Argan and Olive Oils against LPS-Induced Oxidative Stress and Inflammation in Mice Livers. Int. J. Mol. Sci. 2017, 18, 2181. [Google Scholar] [CrossRef]
- Msanda, F.; Mayad, E.H.; Furze, J.N. Floristic Biodiversity, Biogeographical Significance, and Importance of Morocco’s Arganeraie Biosphere Reserve. Environ. Sci. Pollut. Res. 2021, 28, 64156–64165. [Google Scholar] [CrossRef]
- Purba, J.H. The Analysis of European Union’s Vegetable Oil Consumption: “Will The European Parliament Resolution Halt the Consumption of Crude Palm Oil in the European Union in the Future?”. Int. J. Appl. Bus. Econ. Res. 2017, 15, 27–39. [Google Scholar]
- Lucarini, M.; Durazzo, A.; Nicoli, S.F.; Raffo, A.; Santini, A.; Novellino, E.; Souto, E.B.; Romani, A.; Belcaro, M.F.; Vita, C. Cold Pressed Argan (Argania spinose) Oil. In Cold Pressed Oils; Elsevier: Amsterdam, The Netherlands, 2020; pp. 459–465. [Google Scholar]
- Zarrouk, A.; Martine, L.; Grégoire, S.; Nury, T.; Meddeb, W.; Camus, E.; Badreddine, A.; Durand, P.; Namsi, A.; Yammine, A.; et al. Profile of Fatty Acids, Tocopherols, Phytosterols and Polyphenols in Mediterranean Oils (Argan Oils, Olive Oils, Milk Thistle Seed Oils and Nigella Seed Oil) and Evaluation of Their Antioxidant and Cytoprotective Activities. Curr. Pharm. Des. 2019, 25, 1791–1805. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Zhong, Y.; Duan, Y.; Chen, Q.; Li, F. Antioxidant Mechanism of Tea Polyphenols and Its Impact on Health Benefits. Anim. Nutr. 2020, 6, 115–123. [Google Scholar] [CrossRef] [PubMed]
- El Kebbaj, R.; El Kamouni, S.; El Hajj, H.I.; Andreoletti, P.; Gresti, J.; Latruffe, N.; El Kebbaj, M.S.; Vamecq, J.; Lizard, G.; Nasser, B.; et al. Modulation of Peroxisomes Abundance by Argan Oil and Lipopolysaccharides in Acyl-CoA Oxidase 1-Deficient Fibroblasts. Health 2013, 5, 62. [Google Scholar] [CrossRef]
- Badreddine, A.; Zarrouk, A.; Meddeb, W.; Nury, T.; Rezig, L.; Debbabi, M.; Bessam, F.Z.; Brahmi, F.; Vejux, A.; Mejri, M.; et al. Antioxidant and Neuroprotective Properties of Mediterranean Oil. In Oxidative Stress and Dietary Antioxidants in Neurological Diseases; Elsevier: Amsterdam, The Netherlands, 2020; pp. 143–154. ISBN 978-0-12-817780-8. [Google Scholar]
- Maurya, R.; Pandey, A.K. Importance of Protozoa Tetrahymena in Toxicological Studies: A Review. Sci. Total Environ. 2020, 741, 140058. [Google Scholar] [CrossRef] [PubMed]
- Yao, M.-C.; Yao, C.-H.; Halasz, L.M.; Fuller, P.; Rexer, C.H.; Wang, S.H.; Jain, R.; Coyne, R.S.; Chalker, D.L. Identification of Novel Chromatin-Associated Proteins Involved in Programmed Genome Rearrangements in Tetrahymena. J. Cell Sci. 2007, 120, 1978–1989. [Google Scholar] [CrossRef]
- Ruehle, M.D.; Orias, E.; Pearson, C.G. Tetrahymena as a Unicellular Model Eukaryote: Genetic and Genomic Tools. Genetics 2016, 203, 649–665. [Google Scholar] [CrossRef]
- Psomiadou, E.; Tsimidou, M. Stability of Virgin Olive Oil. 1. Autoxidation Studies. J. Agric. Food Chem. 2002, 50, 716–721. [Google Scholar] [CrossRef]
- Abbassi, A.E.; Khalid, N.; Zbakh, H.; Ahmad, A. Physicochemical Characteristics, Nutritional Properties, and Health Benefits of Argan Oil: A Review. Crit. Rev. Food Sci. Nutr. 2014, 54, 1401–1414. [Google Scholar] [CrossRef]
- Wang, T.; Jonsdottir, R.; Ólafsdóttir, G. Total Phenolic Compounds, Radical Scavenging and Metal Chelation of Extracts from Icelandic Seaweeds. Food Chem. 2009, 116, 240–248. [Google Scholar] [CrossRef]
- Dev, S.; Babitt, J.L. Overview of Iron Metabolism in Health and Disease: Iron Metabolism in Health and Disease. Hemodial. Int. 2017, 21, S6–S20. [Google Scholar] [CrossRef]
- Essadek, S.; Bouchab, H.; El Kebbaj, R.; Gondcaille, C.; El Kamouni, S.; Savary, S.; Vamecq, J.; Essamadi, A.; Cherkaoui-Malki, M.; Nasser, B.; et al. Effects of a Short-Term Lipopolysaccharides Challenge on Mouse Brain and Liver Peroxisomal Antioxidant and β-Oxidative Functions: Protective Action of Argan Oil. Pharmaceuticals 2022, 15, 465. [Google Scholar] [CrossRef]
- Essadek, S.; Gondcaille, C.; Savary, S.; Samadi, M.; Vamecq, J.; Lizard, G.; Kebbaj, R.E.; Latruffe, N.; Benani, A.; Nasser, B.; et al. Two Argan Oil Phytosterols, Schottenol and Spinasterol, Attenuate Oxidative Stress and Restore LPS-Dysregulated Peroxisomal Functions in Acox1-/- and Wild-Type BV-2 Microglial Cells. Antioxidants 2023, 12, 168. [Google Scholar] [CrossRef]
- Guillaume, D.; Pioch, D.; Charrouf, Z. Argan [Argania spinosa (L.) Skeels] Oil. In Fruit Oils: Chemistry and Functionality; Ramadan, M.F., Ed.; Springer International Publishing: Cham, Switzerland, 2019; pp. 317–352. ISBN 978-3-030-12472-4. [Google Scholar]
- Ben Mansour, R.; Ben Slema, H.; Falleh, H.; Tounsi, M.; Kechebar, M.S.A.; Ksouri, R.; Megdiche-Ksouri, W. Phytochemical Characteristics, Antioxidant, and Health Properties of Roasted and Unroasted Algerian Argan (Argania spinosa) Oil. J. Food Biochem. 2018, 42, e12562. [Google Scholar] [CrossRef]
- Laincer, F.; Iaccarino, N.; Amato, J.; Pagano, B.; Pagano, A.; Tenore, G.; Tamendjari, A.; Rovellini, P.; Venturini, S.; Bellan, G.; et al. Characterization of Monovarietal Extra Virgin Olive Oils from the Province of Béjaïa (Algeria). Food Res. Int. 2016, 89, 1123–1133. [Google Scholar] [CrossRef]
- Patil, B.S.; Jayaprakasha, G.K.; Chidambara Murthy, K.N.; Vikram, A. Bioactive Compounds: Historical Perspectives, Opportunities, and Challenges. J. Agric. Food Chem. 2009, 57, 8142–8160. [Google Scholar] [CrossRef]
- Siger, A.; Nogala-kalucka, M.; Lampart-Szczapa, E. The Content and Antioxidant Activity of Phenolic Compounds in Cold-Pressed Plant Oils. J. Food Lipids 2008, 15, 137–149. [Google Scholar] [CrossRef]
- Elbir, M.; Es-Safi, N.E.; Amhoud, A.; Mbarki, M. Characterization of Phenolic Compounds in Olive Stones of Three Moroccan Varieties. Maderas. Cienc. Y Tecnol. 2015, 17, 479–492. [Google Scholar] [CrossRef]
- Haouhay, N.E.; Samaniego-Sánchez, C.; Asehraou, A.; Villalón-Mir, M.; Serrana, H.L.-G. de la Microbiological Characterization of Picholine Variety Olives and Analysis of Olive Oil Produced in Traditional Oil Mills in Morocco. CyTA-J. Food 2015, 13, 107–115. [Google Scholar] [CrossRef]
- Miliauskas, G.; Venskutonis, P.R.; Van Beek, T.A. Screening of Radical Scavenging Activity of Some Medicinal and Aromatic Plant Extracts. Food Chem. 2004, 85, 231–237. [Google Scholar] [CrossRef]
- Pérez-Gálvez, A.; Viera, I.; Roca, M. Carotenoids and Chlorophylls as Antioxidants. Antioxidants 2020, 9, 505. [Google Scholar] [CrossRef]
- Garcia, P.M.C. Antioxidantes En La Dietamediterranea. Nutr. Clínica En Me-Dicina 2008, 2, 129–140. [Google Scholar]
- Borges, T.H.; Pereira, J.A.; Cabrera–Vique, C.; Seiquer, I. Study of the Antioxidant Potential of Arbequina Extra Virgin Olive Oils from Brazil and Spain Applying Combined Models of Simulated Digestion and Cell Culture Markers. J. Funct. Foods 2017, 37, 209–218. [Google Scholar] [CrossRef]
- Seiquer, I.; Rueda, A.; Olalla, M.; Cabrera-Vique, C. Assessing the Bioavailability of Polyphenols and Antioxidant Properties of Extra Virgin Argan Oil by Simulated Digestion and Caco-2 Cell Assays. Comparative Study with Extra Virgin Olive Oil. Food Chem. 2015, 188, 496–503. [Google Scholar] [CrossRef] [PubMed]
- Marfil, R.; Giménez, R.; Martínez, O.; Bouzas, P.R.; Rufián-Henares, J.A.; Mesías, M.; Cabrera-Vique, C. Determination of Polyphenols, Tocopherols, and Antioxidant Capacity in Virgin Argan Oil (Argania spinosa, Skeels). Eur. J. Lipid Sci. Technol. 2011, 113, 886–893. [Google Scholar] [CrossRef]
- Samaniego Sánchez, C.; Troncoso González, A.M.; García-Parrilla, M.C.; Quesada Granados, J.J.; López García de la Serrana, H.; López Martínez, M.C. Different Radical Scavenging Tests in Virgin Olive Oil and Their Relation to the Total Phenol Content. Anal. Chim. Acta 2007, 593, 103–107. [Google Scholar] [CrossRef]
- Matera, R.; Gabbanini, S.; Berretti, S.; Amorati, R.; De Nicola, G.R.; Iori, R.; Valgimigli, L. Acylated Anthocyanins from Sprouts of Raphanus Sativus Cv. Sango: Isolation, Structure Elucidation and Antioxidant Activity. Food Chem. 2015, 166, 397–406. [Google Scholar] [CrossRef]
- Xie, D.; Zhou, P.; Liu, L.; Jiang, W.; Xie, H.; Zhang, L.; Xie, D. Protective Effect of Astragaloside IV on Hepatic Injury Induced by Iron Overload. BioMed Res. Int. 2019, 2019, 3103946. [Google Scholar] [CrossRef]
- Rishi, G.; Subramaniam, V.N. The Liver in Regulation of Iron Homeostasis. Am. J. Physiol.-Gastrointest. Liver Physiol. 2017, 313, G157–G165. [Google Scholar] [CrossRef]
- Milic, S.; Mikolasevic, I.; Orlic, L.; Devcic, E.; Starcevic-Cizmarevic, N.; Stimac, D.; Kapovic, M.; Ristic, S. The Role of Iron and Iron Overload in Chronic Liver Disease. Med. Sci. Monit. 2016, 22, 2144. [Google Scholar] [CrossRef]
- Mortimer, M.; Kasemets, K.; Kahru, A. Toxicity of ZnO and CuO Nanoparticles to Ciliated Protozoa Tetrahymena Thermophila. Toxicology 2010, 269, 182–189. [Google Scholar] [CrossRef]
- Jain, S.; Ahmed, R.S.; Arora, V.K.; Banerjee, B.D. Biochemical and Histopathological Studies to Assess Chronic Toxicity of Triazophos in Blood, Liver and Brain Tissue of Rats. Pestic. Biochem. Physiol. 2011, 100, 182–186. [Google Scholar] [CrossRef]
- Allameh, A.; Amini-Harandi, A.; Osati-Ashtiani, F.; O’Brien, P. Iron Overload Induced Apoptotic Cell Death in Isolated Rat Hepatocytes Mediated by Reactive Oxygen Species. Iran. J. Pharm. Res. 2010, 7, 115–121. [Google Scholar]
- Shull, S.; Heintz, N.H.; Periasamy, M.; Manohar, M.; Janssen, Y.M.; Marsh, J.P.; Mossman, B.T. Differential Regulation of Antioxidant Enzymes in Response to Oxidants. J. Biol. Chem. 1991, 266, 24398–24403. [Google Scholar] [CrossRef]
- Ighodaro, O.M.; Akinloye, O.A. First Line Defence Antioxidants-Superoxide Dismutase (SOD), Catalase (CAT) and Glutathione Peroxidase (GPX): Their Fundamental Role in the Entire Antioxidant Defence Grid. Alex. J. Med. 2018, 54, 287–293. [Google Scholar] [CrossRef]
- Tosukhowong, P.; Sangwatanaroj, S.; Jatuporn, S.; Prapunwattana, P.; Saengsiri, A.; Rattanapruks, S.; Srimahachota, S.; Udayachalerm, W.; Tangkijvanich, P. The Correlation between Markers of Oxidative Stress and Risk Factors of Coronary Artery Disease in Thai Patients. Clin. Hemorheol. Microcirc. 2003, 29, 321–329. [Google Scholar]
- Scapagnini, G.; Sonya, V.; Nader, A.G.; Calogero, C.; Zella, D.; Fabio, G. Modulation of Nrf2/ARE Pathway by Food Polyphenols: A Nutritional Neuroprotective Strategy for Cognitive and Neurodegenerative Disorders. Mol. Neurobiol. 2011, 44, 192–201. [Google Scholar] [CrossRef]
- Ashrafizadeh, M.; Ahmadi, Z.; Samarghandian, S.; Mohammadinejad, R.; Yaribeygi, H.; Sathyapalan, T.; Sahebkar, A. MicroRNA-Mediated Regulation of Nrf2 Signaling Pathway: Implications in Disease Therapy and Protection against Oxidative Stress. Life Sci. 2020, 244, 117329. [Google Scholar] [CrossRef]
- Bellezza, I.; Giambanco, I.; Minelli, A.; Donato, R. Nrf2-Keap1 Signaling in Oxidative and Reductive Stress. Biochim. Et Biophys. Acta (BBA)-Mol. Cell Res. 2018, 1865, 721–733. [Google Scholar] [CrossRef]
- Bhakkiyalakshmi, E.; Sireesh, D.; Ramkumar, K.M. Redox Sensitive Transcription via Nrf2-Keap1 in Suppression of Inflammation. In Immunity and Inflammation in Health and Disease; Elsevier: Amsterdam, The Netherlands, 2018; pp. 149–161. ISBN 978-0-12-805417-8. [Google Scholar]
- Lakhan, S.E.; Kirchgessner, A.; Hofer, M. Inflammatory Mechanisms in Ischemic Stroke: Therapeutic Approaches. J. Transl. Med. 2009, 7, 97. [Google Scholar] [CrossRef]
- El Kharrassi, Y.; Samadi, M.; Lopez, T.; Nury, T.; El Kebbaj, R.; Andreoletti, P.; El Hajj, H.I.; Vamecq, J.; Moustaid, K.; Latruffe, N.; et al. Biological Activities of Schottenol and Spinasterol, Two Natural Phytosterols Present in Argan Oil and in Cactus Pear Seed Oil, on Murine Miroglial BV2 Cells. Biochem. Biophys. Res. Commun. 2014, 446, 798–804. [Google Scholar] [CrossRef]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free Radicals, Antioxidants and Functional Foods: Impact on Human Health. Pharmacogn. Rev. 2010, 4, 118. [Google Scholar] [CrossRef] [PubMed]
- Antoine, D. Quand Le Cerveau s’enflamme: Le Rôle Des Enzymes NOX Dans Certaines Maladies Du Cerveau. Available online: https://www.echosciences-grenoble.fr/communautes/atout-cerveau/articles/quand-le-cerveau-s-enflamme-le-role-des-enzymes-nox-dans-certaines-maladies-du-cerveau (accessed on 1 February 2022).
- Vilhardt, F.; Haslund-Vinding, J.; Jaquet, V.; McBean, G. Microglia Antioxidant Systems and Redox Signalling. Br. J. Pharmacol. 2017, 174, 1719–1732. [Google Scholar] [CrossRef] [PubMed]
- Dringen, R. Metabolism and Functions of Glutathione in Brain. Prog. Neurobiol. 2000, 62, 649–671. [Google Scholar] [CrossRef] [PubMed]
- Ndayisaba, A.; Kaindlstorfer, C.; Wenning, G.K. Iron in Neurodegeneration–Cause or Consequence? Front. Neurosci. 2019, 13, 180. [Google Scholar] [CrossRef] [PubMed]
- Rodrigo, R.; Gil, D.; Miranda-Merchak, A.; Kalantzidis, G. Antihypertensive Role of Polyphenols. In Advances in Clinical Chemistry; Elsevier: Amsterdam, The Netherlands, 2012; Volume 58, pp. 225–254. ISBN 978-0-12-394383-5. [Google Scholar]
- Heo, S.; Kim, S.; Kang, D. The Role of Hydrogen Peroxide and Peroxiredoxins throughout the Cell Cycle. Antioxidants 2020, 9, 280. [Google Scholar] [CrossRef]
- El Kharrassi, Y.; Maata, N.; Mazri, M.A.; El Kamouni, S.; Talbi, M.; El Kebbaj, R.; Moustaid, K.; Essamadi, A.K.; Andreoletti, P.; El Mzouri, E.H.; et al. Chemical and Phytochemical Characterizations of Argan Oil (Argania spinosa L. Skeels), Olive Oil (Olea europaea L. cv. Moroccan picholine), Cactus Pear (Opuntia megacantha salm-dyck) Seed Oil and Cactus Cladode Essential Oil. Food Meas. 2018, 12, 747–754. [Google Scholar] [CrossRef]
- Martínez-Huélamo, M.; Rodríguez-Morató, J.; Boronat, A.; de la Torre, R. Modulation of Nrf2 by Olive Oil and Wine Polyphenols and Neuroprotection. Antioxidants 2017, 6, 73. [Google Scholar] [CrossRef]
- Visioli, F.; Galli, C. The Role of Antioxidants in the Mediterranean Diet. Lipids 2001, 36, S49–S52. [Google Scholar] [CrossRef]
- Furumoto, H.; Nanthirudjanar, T.; Kume, T.; Izumi, Y.; Park, S.-B.; Kitamura, N.; Kishino, S.; Ogawa, J.; Hirata, T.; Sugawara, T. 10-Oxo-Trans-11-Octadecenoic Acid Generated from Linoleic Acid by a Gut Lactic Acid Bacterium Lactobacillus Plantarum Is Cytoprotective against Oxidative Stress. Toxicol. Appl. Pharmacol. 2016, 296, 1–9. [Google Scholar] [CrossRef]
- Kinter, M.; Spitz, D.R.; Robert, R.J. Oleic Acid Incorporation Protects Cultured Hamster Fibroblasts from Oxygen-Induced Cytotoxicity. J. Nutr. 1996, 126, 2952–2959. [Google Scholar] [CrossRef][Green Version]
- Sun, T.; Xu, Z.; Wu, C.-T.; Janes, M.; Prinyawiwatkul, W.; No, H.K. Antioxidant Activities of Different Colored Sweet Bell Peppers (Capsicum annuum L.). J. Food Sci. 2007, 72, S98–S102. [Google Scholar] [CrossRef]
- Minguez-Mosquera, M.I.; Gandul-Rojas, B.; Garrido-Fernandez, J.; Gallardo-Guerrero, L. Pigments Present in Virgin Olive Oil. J. Am. Oil Chem. Soc. 1990, 67, 192–196. [Google Scholar] [CrossRef]
- Oyaizu, M. Studies on Products of Browning Reaction Antioxidative Activities of Products of Browning Reaction Prepared from Glucosamine. Jpn. J. Nutr. Diet. 1986, 44, 307–315. [Google Scholar] [CrossRef]
- Koprivnjak, O.; Škevin, D.; Valić, S.; Majetić, V.; Petričević, S.; Ljubenkov, I. The Antioxidant Capacity and Oxidative Stability of Virgin Olive Oil Enriched with Phospholipids. Food Chem. 2008, 111, 121–126. [Google Scholar] [CrossRef]
- Mori, K.; Kashiwagi, A.; Yomo, T. Single-Cell Isolation and Cloning of Tetrahymena Thermophila Cells with a Fluorescence-Activated Cell Sorter. J. Eukaryot. Microbiol. 2011, 58, 37–42. [Google Scholar] [CrossRef]
- Nilsson, J.R. On Cell Organelles in Tetrahymena. With Special Reference to Mitochondria and Peroxisomes. Carlsberg Res. Commun. 1981, 46, 279–304. [Google Scholar] [CrossRef][Green Version]
- World Health Organization. Safety Guidelines for Drinking-Water Quality. Vol. 2, Health Criteria and Other Supporting Information; World Health Organization: Geneva, Switzerland, 1996.
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein Measurement with the Folin Phenol Reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for Lipid Peroxides in Animal Tissues by Thiobarbituric Acid Reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Ellman, G.L. Tissue Sulfhydryl Groups. Arch. Biochem. Biophys. 1959, 82, 70–77. [Google Scholar] [CrossRef]
- Mills, G.C. The Purification and Properties of Glutathione Peroxidase of Erythrocytes. J. Biol. Chem. 1959, 234, 502–506. [Google Scholar] [CrossRef]
- Beyer, W.F.; Fridovich, I. Assaying for Superoxide Dismutase Activity: Some Large Consequences of Minor Changes in Conditions. Anal. Biochem. 1987, 161, 559–566. [Google Scholar] [CrossRef] [PubMed]

| Groups | Treatment |
|---|---|
| Group I | Culture medium with the solvent ethanol |
| Group II | Culture medium (as group I) supplemented with 0.1% of AO solubilized in ethanol |
| Group III | Culture medium (as group I) supplemented with 0.1% of OO solubilized in ethanol |
| Group IV | Culture medium (as group I) supplemented with iron at 1.849 mM dose dissolved in water |
| Group V | Culture medium (as group I) and AO (as group II) |
| Group VI | Culture medium (as group I) and AO (as group III) |
| Groups | Treatment |
|---|---|
| Group I | A standard chow with the solvent acetone |
| Group II | A standard chow (as group I) supplemented with 6% of AO solubilized in acetone |
| Group III | A standard chow (as group I) supplemented with 6% of OO solubilized in acetone |
| Group IV | The reference drug Tardyferon (iron sulphate 3.5 mg2+/liter) was dissolved in drinking water |
| Group V | The animals received iron (as group IV) and AO (as group II) |
| Group VI | The animals received iron (as group IV) and OO (as group III) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bouchab, H.; Essadek, S.; El Kamouni, S.; Moustaid, K.; Essamadi, A.; Andreoletti, P.; Cherkaoui-Malki, M.; El Kebbaj, R.; Nasser, B. Antioxidant Effects of Argan Oil and Olive Oil against Iron-Induced Oxidative Stress: In Vivo and In Vitro Approaches. Molecules 2023, 28, 5924. https://doi.org/10.3390/molecules28155924
Bouchab H, Essadek S, El Kamouni S, Moustaid K, Essamadi A, Andreoletti P, Cherkaoui-Malki M, El Kebbaj R, Nasser B. Antioxidant Effects of Argan Oil and Olive Oil against Iron-Induced Oxidative Stress: In Vivo and In Vitro Approaches. Molecules. 2023; 28(15):5924. https://doi.org/10.3390/molecules28155924
Chicago/Turabian StyleBouchab, Habiba, Soukaina Essadek, Soufiane El Kamouni, Khadija Moustaid, Abdelkhalid Essamadi, Pierre Andreoletti, Mustapha Cherkaoui-Malki, Riad El Kebbaj, and Boubker Nasser. 2023. "Antioxidant Effects of Argan Oil and Olive Oil against Iron-Induced Oxidative Stress: In Vivo and In Vitro Approaches" Molecules 28, no. 15: 5924. https://doi.org/10.3390/molecules28155924
APA StyleBouchab, H., Essadek, S., El Kamouni, S., Moustaid, K., Essamadi, A., Andreoletti, P., Cherkaoui-Malki, M., El Kebbaj, R., & Nasser, B. (2023). Antioxidant Effects of Argan Oil and Olive Oil against Iron-Induced Oxidative Stress: In Vivo and In Vitro Approaches. Molecules, 28(15), 5924. https://doi.org/10.3390/molecules28155924










