Understanding the Anti-Obesity Potential of an Avocado Oil-Rich Cheese through an In Vitro Co-Culture Intestine Cell Model
Abstract
1. Introduction
2. Results and Discussion
2.1. Avocado-Rich Cheese Fatty Acid Profile
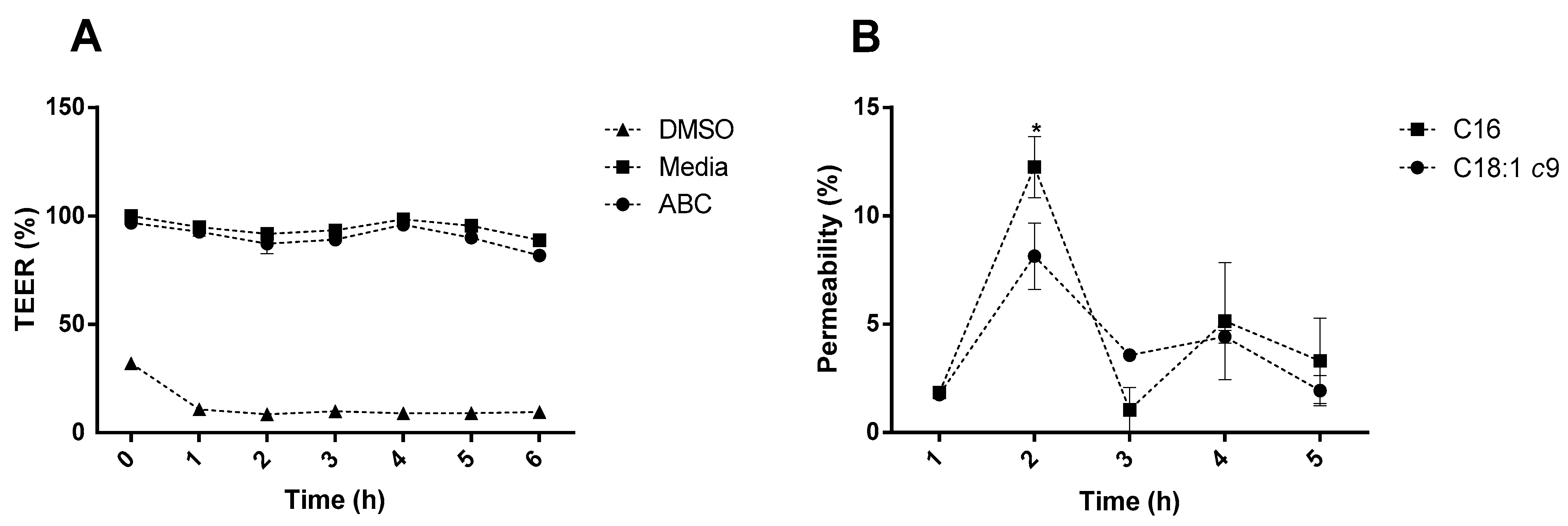
2.2. Fatty Acid Permeability
2.3. Effect on Adipocyte Metabolism
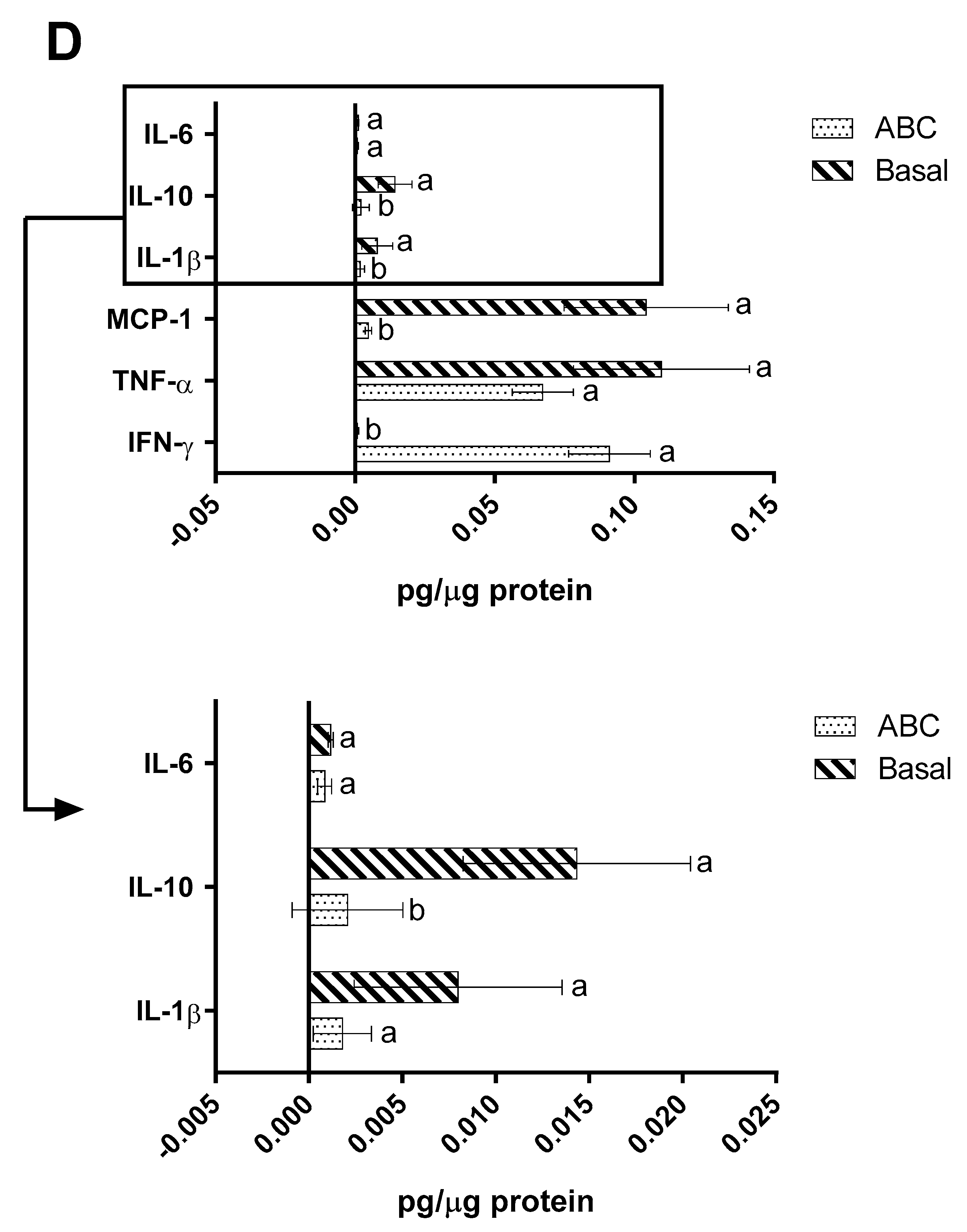
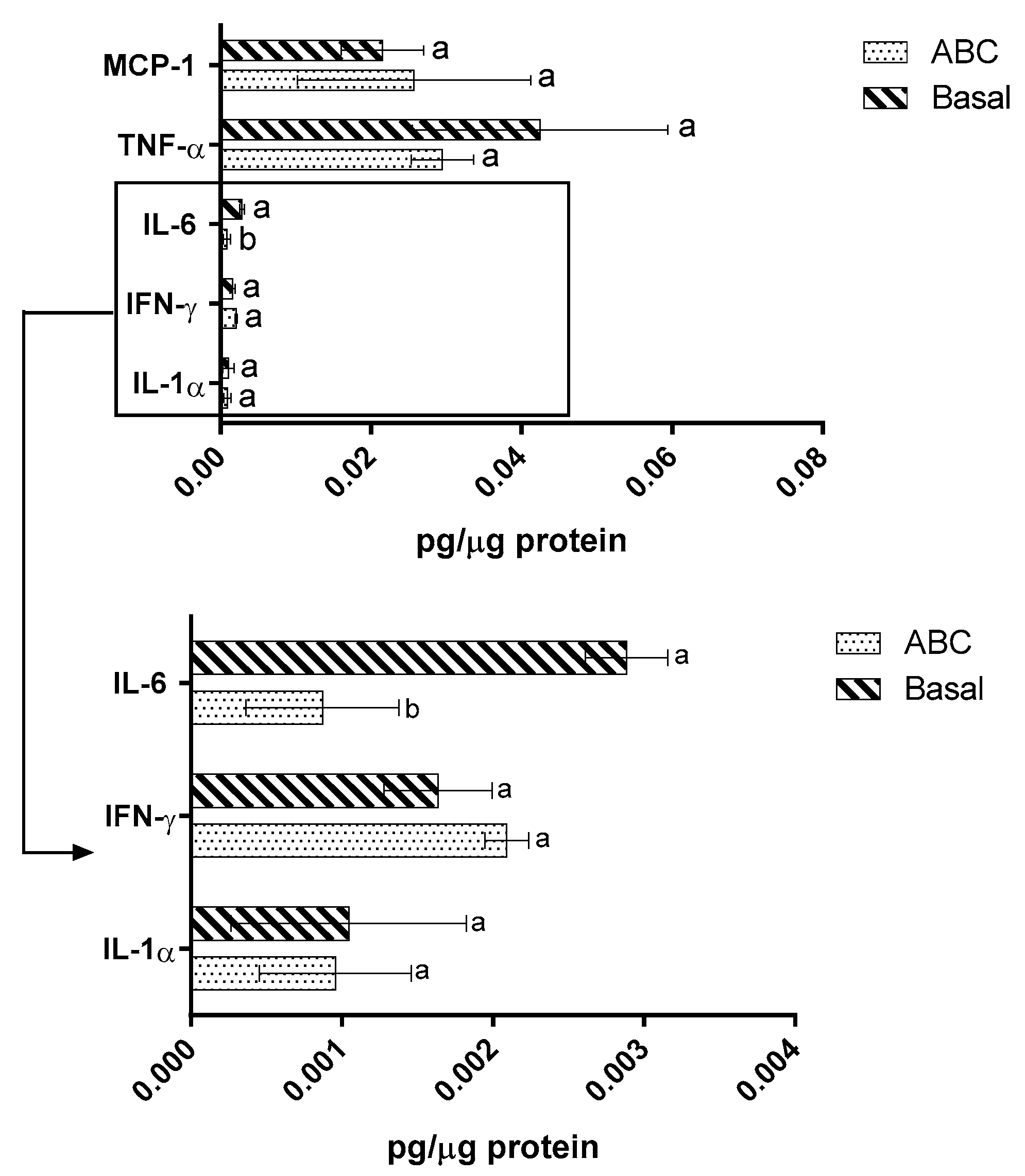
2.4. Immunomodulation Model
3. Materials and Methods
3.1. Avocado Bigel and Cheese Production
3.2. Fatty Acid Profile
3.3. In Vitro Simulation of the GIT
3.4. Cellular Models
3.4.1. Cell Lines
3.4.2. Co-Culture Models
3.4.3. Transepithelial Electrical Resistance (TEER) Measurements
3.4.4. Fatty Acid Permeability Assay
3.4.5. Modulation of Adipocyte Metabolism Using a Co-Culture Model
3.4.6. Raw 264.7 Co-Culture Intestinal Inflammatory Model
3.5. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Finkelstein, E.A.; Khavjou, O.A.; Thompson, H.; Trogdon, J.G.; Pan, L.; Sherry, B.; Dietz, W. Obesity and severe obesity forecasts through 2030. Am. J. Prev. Med. 2012, 42, 563–570. [Google Scholar] [CrossRef]
- Karczewski, J.; Śledzińska, E.; Baturo, A.; Jończyk, I.; Maleszko, A.; Samborski, P.; Begier-Krasińska, B.; Dobrowolska, A. Obesity and inflammation. Eur. Cytokine Netw. 2018, 29, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Jeria, N.; Cornejo, S.; Prado, G.; Bustamante, A.; Garcia-Diaz, D.F.; Jimenez, P.; Valenzuela, R.; Poblete-Aro, C.; Echeverria, F. Beneficial Effects of Bioactive Compounds Obtained from Agro-Industrial By-Products on Obesity and Metabolic Syndrome Components. Food Rev. Int. 2022, 1–30. [Google Scholar] [CrossRef]
- Konstantinidi, M.; Koutelidakis, A.E. Functional Foods and Bioactive Compounds: A Review of Its Possible Role on Weight Management and Obesity’s Metabolic Consequences. Medicines 2019, 6, 94. [Google Scholar] [CrossRef]
- Kamath, R.; Basak, S.; Gokhale, J. Recent trends in the development of healthy and functional cheese analogues—A review. LWT 2022, 155, 112991. [Google Scholar] [CrossRef]
- Chourasia, R.; Abedin, M.M.; Chiring Phukon, L.; Sahoo, D.; Singh, S.P.; Rai, A.K. Biotechnological approaches for the production of designer cheese with improved functionality. Compr. Rev. Food Sci. Food Saf. 2021, 20, 960–979. [Google Scholar] [CrossRef]
- Machado, M.; Rodríguez-Alcalá, L.M.; Maria Gomes, A.; Pintado, M. Does the Nature of Added Bioactive Lipids Affect the Biological Properties of Yogurts?—Case Study Coconut and Avocado Oils. Appl. Sci. 2023, 13, 3101. [Google Scholar] [CrossRef]
- Tan, C.X. Virgin avocado oil: An emerging source of functional fruit oil. J. Funct. Foods 2019, 54, 381–392. [Google Scholar] [CrossRef]
- Konstantinidou, V.; Covas, M.I.; Sola, R.; Fitó, M. Up-to date knowledge on the in vivo transcriptomic effect of the Mediterranean diet in humans. Mol. Nutr. Food Res. 2013, 57, 772–783. [Google Scholar] [CrossRef]
- Konstantinidou, V.; Covas, M.; Muñoz-Aguayo, D.; Khymenets, O.; Torre, R.; Saez, G.; Carmen Tormos, M.; Toledo, E.; Marti, A.; Ruiz-Gutiérrez, V.; et al. In vivo nutrigenomic effects of virgin olive oil polyphenols within the frame of the Mediterranean diet: A randomized controlled trial. FASEB J. 2010, 24, 2546–2557. [Google Scholar] [CrossRef]
- Esposito, K.; Marfella, R.; Ciotola, M.; Di Palo, C.; Giugliano, F.; Giugliano, G.; D’Armiento, M.; D’Andrea, F.; Giugliano, D. Effect of a Mediterranean-style diet on endothelial dysfunction and markers of vascular inflammation in the metabolic syndrome: A randomized trial. J. Am. Med. Assoc. 2004, 292, 1440–1446. [Google Scholar] [CrossRef] [PubMed]
- Ponce de León-Rodríguez, M.d.C.; Guyot, J.-P.; Laurent-Babot, C. Intestinal in vitro cell culture models and their potential to study the effect of food components on intestinal inflammation. Crit. Rev. Food Sci. Nutr. 2019, 59, 3648–3666. [Google Scholar] [CrossRef]
- Chung, H.H.; Mireles, M.; Kwarta, B.J.; Gaborski, T.R. Use of porous membranes in tissue barrier and co-culture models. Lab Chip 2018, 18, 1671–1689. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, Y.-G. 2D- and 3D-Based Intestinal Stem Cell Cultures for Personalized Medicine. Cells 2018, 7, 225. [Google Scholar] [CrossRef] [PubMed]
- Berger, E.; Nassra, M.; Atgié, C.; Plaisancié, P.; Géloën, A. Oleic Acid Uptake Reveals the Rescued Enterocyte Phenotype of Colon Cancer Caco-2 by HT29-MTX Cells in Co-Culture Mode. Int. J. Mol. Sci. 2017, 18, 1573. [Google Scholar] [CrossRef]
- Cullberg, K.B.; Larsen, J.Ø.; Pedersen, S.B.; Richelsen, B. Effects of LPS and dietary free fatty acids on MCP-1 in 3T3-L1 adipocytes and macrophages in vitro. Nutr. Diabetes 2014, 4, e113. [Google Scholar] [CrossRef] [PubMed]
- Ghezzal, S.; Postal, B.G.; Quevrain, E.; Brot, L.; Seksik, P.; Leturque, A.; Thenet, S.; Carrière, V. Palmitic acid damages gut epithelium integrity and initiates inflammatory cytokine production. Biochim. Biophys. Acta-Mol. Cell Biol. Lipids 2020, 1865, 158530. [Google Scholar] [CrossRef]
- Aspenström-Fagerlund, B.; Ring, L.; Aspenström, P.; Tallkvist, J.; Ilbäck, N.G.; Glynn, A.W. Oleic acid and docosahexaenoic acid cause an increase in the paracellular absorption of hydrophilic compounds in an experimental model of human absorptive enterocytes. Toxicology 2007, 237, 12–23. [Google Scholar] [CrossRef]
- Aspenström-Fagerlund, B.; Sundström, B.; Tallkvist, J.; Ilbäck, N.G.; Glynn, A.W. Fatty acids increase paracellular absorption of aluminium across Caco-2 cell monolayers. Chem. Biol. Interact. 2009, 181, 272–278. [Google Scholar] [CrossRef]
- Iqbal, J.; Hussain, M.M. Intestinal lipid absorption. Am. J. Physiol.-Endocrinol. Metab. 2009, 296, E1183–E1194. [Google Scholar] [CrossRef]
- Bermudez, B.; Lopez, S.; Ortega, A.; Varela, L.M.; Pacheco, Y.M.; Abia, R.; Muriana, F.J.G. Oleic Acid in Olive Oil: From a Metabolic Framework Toward a Clinical Perspective. Curr. Pharm. Des. 2011, 17, 831–843. [Google Scholar] [CrossRef] [PubMed]
- Berger, E.; Géloën, A. Adipocytes as lipid sensors of oleic acid transport through a functional Caco-2/HT29-MTX intestinal barrier. Adipocyte 2019, 8, 83–97. [Google Scholar] [CrossRef] [PubMed]
- Malodobra-Mazur, M.; Cierzniak, A.; Dobosz, T. Oleic acid influences the adipogenesis of 3T3-L1 cells via DNA Methylation and may predispose to obesity and obesity-related disorders. Lipids Health Dis. 2019, 18, 230. [Google Scholar] [CrossRef]
- Saraswathi, V.; Kumar, N.; Gopal, T.; Bhatt, S.; Ai, W.; Ma, C.; Talmon, G.A.; Desouza, C. Lauric acid versus palmitic acid: Effects on adipose tissue inflammation, insulin resistance, and non-alcoholic fatty liver disease in obesity. Biology 2020, 9, 346. [Google Scholar] [CrossRef] [PubMed]
- Schaeffler, A.; Gross, P.; Buettner, R.; Bollheimer, C.; Buechler, C.; Neumeier, M.; Kopp, A.; Schoelmerich, J.; Falk, W. Fatty acid-induced induction of Toll-like receptor-4/nuclear factor-κB pathway in adipocytes links nutritional signalling with innate immunity. Immunology 2009, 126, 233–245. [Google Scholar] [CrossRef]
- Granados, N.; Amengual, J.; Ribot, J.; Palou, A.; Luisa Bonet, M. Distinct effects of oleic acid and its trans-isomer elaidic acid on the expression of myokines and adipokines in cell models. Br. J. Nutr. 2011, 105, 1226–1234. [Google Scholar] [CrossRef]
- Ravaut, G.; Légiot, A.; Bergeron, K.-F.; Mounier, C. Monounsaturated Fatty Acids in Obesity-Related Inflammation. Int. J. Mol. Sci. 2020, 22, 330. [Google Scholar] [CrossRef]
- Han, C.Y.; Kargi, A.Y.; Omer, M.; Chan, C.K.; Wabitsch, M.; O’Brien, K.D.; Wight, T.N.; Chait, A. Differential effect of saturated and unsaturated free fatty acids on the generation of monocyte adhesion and chemotactic factors by adipocytes: Dissociation of adipocyte hypertrophy from inflammation. Diabetes 2010, 59, 386–396. [Google Scholar] [CrossRef]
- Bradley, R.L.; Fisher, F.M.; Maratos-Flier, E. Dietary fatty acids differentially regulate production of TNF-α and IL-10 by murine 3T3-L1 adipocytes. Obesity 2008, 16, 938–944. [Google Scholar] [CrossRef]
- Finucane, O.M.; Lyons, C.L.; Murphy, A.M.; Reynolds, C.M.; Klinger, R.; Healy, N.P.; Cooke, A.A.; Coll, R.C.; Mcallan, L.; Nilaweera, K.N.; et al. Monounsaturated fatty acid-enriched high-fat diets impede adipose NLRP3 inflammasome-mediated IL-1β secretion and insulin resistance despite obesity. Diabetes 2015, 64, 2116–2128. [Google Scholar] [CrossRef]
- Montserrat-de la Paz, S.; Naranjo, M.C.; Millan-Linares, M.C.; Lopez, S.; Abia, R.; Biessen, E.A.L.; Muriana, F.J.G.; Bermudez, B. Monounsaturated Fatty Acids in a High-Fat Diet and Niacin Protect from White Fat Dysfunction in the Metabolic Syndrome. Mol. Nutr. Food Res. 2019, 63, 1900425. [Google Scholar] [CrossRef]
- Tamer, F.; Ulug, E.; Akyol, A.; Nergiz-Unal, R. The potential efficacy of dietary fatty acids and fructose induced inflammation and oxidative stress on the insulin signaling and fat accumulation in mice. Food Chem. Toxicol. 2020, 135, 110914. [Google Scholar] [CrossRef] [PubMed]
- Murumalla, R.K.; Gunasekaran, M.K.; Padhan, J.K.; Bencharif, K.; Gence, L.; Festy, F.; Césari, M.; Roche, R.; Hoareau, L. Fatty acids do not pay the toll: Effect of SFA and PUFA on human adipose tissue and mature adipocytes inflammation. Lipids Health Dis. 2012, 11, 175. [Google Scholar] [CrossRef] [PubMed]
- Schwingshackl, L.; Christoph, M.; Hoffmann, G. Effects of olive oil on markers of inflammation and endothelial function—A systematic review and meta-analysis. Nutrients 2015, 7, 7651–7675. [Google Scholar] [CrossRef]
- Malandrino, M.I.; Fucho, R.; Weber, M.; Calderon-Dominguez, M.; Mir, J.F.; Valcarcel, L.; Escoté, X.; Gómez-Serrano, M.; Peral, B.; Salvadó, L.; et al. Enhanced fatty acid oxidation in adipocytes and macrophages reduces lipid-induced triglyceride accumulation and inflammation. Am. J. Physiol.-Endocrinol. Metab. 2015, 308, E756–E769. [Google Scholar] [CrossRef]
- Davanso, M.R.; Crisma, A.R.; Murata, G.; Newsholme, P.; Curi, R. Impact of Dietary Fatty Acids on Macrophage Lipid Metabolism, Signaling and Function. Immunometabolism 2020, 2, e200008. [Google Scholar] [CrossRef]
- Pardo, V.; González-Rodríguez, Á.; Guijas, C.; Balsinde, J.; Valverde, Á.M. Opposite cross-talk by oleate and palmitate on insulin signaling in hepatocytes through macrophage activation. J. Biol. Chem. 2015, 290, 11663–11677. [Google Scholar] [CrossRef]
- Müller, A.K.; Albrecht, F.; Rohrer, C.; Koeberle, A.; Werz, O.; Schlörmann, W.; Glei, M.; Lorkowski, S.; Wallert, M. Olive oil extracts and oleic acid attenuate the lps-induced inflammatory response in murine raw264.7 macrophages but induce the release of prostaglandin e2. Nutrients 2021, 13, 4437. [Google Scholar] [CrossRef]
- Machado, M.; Sousa, S.C.; Rodríguez-Alcalá, L.M.; Pintado, M.; Gomes, A.M. Bigels as Delivery Systems of Bioactive Fatty Acids Present in Functional Edible Oils: Coconut, Avocado, and Pomegranate. Gels 2023, 9, 349. [Google Scholar]
- Machado, M.; Sousa, S.C.; Rodríguez-Alcalá, L.M.; Pintado, M.; Gomes, A.M. Functional lipid enriched probiotic cheese: Gastrointestinal stability and potential health benefits. Int. Dairy J. 2023, 144, 105700. [Google Scholar] [CrossRef]
- Machado, M.; Sousa, S.; Morais, P.; Miranda, A.; Rodriguez-Alcalá, L.M.; Gomes, A.M.; Pintado, M. Novel avocado oil-functionalized yogurt with anti-obesity potential: Technological and nutraceutical perspectives. Food Biosci. 2022, 50, 101983. [Google Scholar] [CrossRef]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assunção, R.; Ballance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carrière, F.; et al. INFOGEST static in vitro simulation of gastrointestinal food digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef]
- Antunes, F.; Andrade, F.; Araújo, F.; Ferreira, D.; Sarmento, B. Establishment of a triple co-culture in vitro cell models to study intestinal absorption of peptide drugs. Eur. J. Pharm. Biopharm. 2013, 83, 427–435. [Google Scholar] [CrossRef] [PubMed]
- Machado, M.; Costa, E.M.; Silva, S.; Rodriguez-Alcalá, L.M.; Gomes, A.M.; Pintado, M. Pomegranate Oil’s Potential as an Anti-Obesity Ingredient. Molecules 2022, 27, 4958. [Google Scholar] [CrossRef] [PubMed]

| Before GIT | After GIT | |
|---|---|---|
| C14 | 0.14 ± 0.001 a | 0.14 ± 0.01 a |
| C16 | 3.63 ± 0.09 a | 3.53 ± 0.42 a |
| C16:1 c9 | 0.77 ± 0.03 a | 0.60 ± 0.09 b |
| C18 | 0.82 ± 0.01 b | 1.22 ± 0.09 a |
| C18:1 c9 | 10.83 ± 0.31 a | 8.17 ± 0.94 b |
| C18:1 c11 | 0.80 ± 0.02 a | 0.61 ± 0.08 b |
| C18:2 c9c12 | 2.26 ± 0.08 a | 2.31 ± 0.34 a |
| C20 | 0.14 ± 0.01 a | 0.12 ± 0.01 a |
| C18:3 c6c9c12 | 0.10 ± 0.01 a | 0.08 ± 0.01 a |
| C20:1 | 0.07 ± 0.01 a | 0.05 ± 0.01 a |
| ∑Fatty acids | 19.57 ± 0.51 a | 16.83 ± 1.98 b |
| Initial (µg/mL) | Apical (µg/mL) | Membrane (µg/mL) | Basolateral (µg/mL) | Papp (cm/s) | |
|---|---|---|---|---|---|
| C16 | 50.5 ± 1.83 a | 1.24 ± 1.18 d | 25.40 ± 1.70 b | 11.89 ± 1.15 c | 0.03 ± 0.01 |
| C18:1 c9 | 116.8 ± 8.33 a | 5.57 ± 0.01 c | 48.04 ± 2.82 b | 23.14 ± 6.84 b | 0.14 ± 0.04 |
| 6 h | 24 h | ||||
|---|---|---|---|---|---|
| Initial | Apical | Membrane | Basolateral | Cells | |
| C16 | 50.5 ± 1.83 b | 6.18 ± 3.71 b | 12.64 ± 1.33 b | 1.12 ± 0.05 b | 26.68 ± 6.88 a |
| C18:1 c9 | 116.8 ± 8.33 a | 19.29 ± 3.71 a | 75.25 ± 24.90 a | 2.45 ± 0.04 a | 19.22 ± 11.30 a |
| 6 h | 24 h | ||||
|---|---|---|---|---|---|
| Initial | Apical | Membrane | Basolateral | Cells | |
| C16 | 50.5 ± 1.83 b | 10.69 ± 5.36 a | 9.43 ± 3.73 b | 3.30 ± 0.15 a | 11.12 ± 1.24 a |
| C18:1 c9 | 116.8 ± 8.33 a | 10.52 ± 4.83 a | 41.94 ± 4.00 a | 2.16 ± 0.21 b | 9.26 ± 0.15 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Machado, M.; Costa, E.M.; Silva, S.; Rodriguez-Alcalá, L.M.; Gomes, A.M.; Pintado, M. Understanding the Anti-Obesity Potential of an Avocado Oil-Rich Cheese through an In Vitro Co-Culture Intestine Cell Model. Molecules 2023, 28, 5923. https://doi.org/10.3390/molecules28155923
Machado M, Costa EM, Silva S, Rodriguez-Alcalá LM, Gomes AM, Pintado M. Understanding the Anti-Obesity Potential of an Avocado Oil-Rich Cheese through an In Vitro Co-Culture Intestine Cell Model. Molecules. 2023; 28(15):5923. https://doi.org/10.3390/molecules28155923
Chicago/Turabian StyleMachado, Manuela, Eduardo M. Costa, Sara Silva, Luís M. Rodriguez-Alcalá, Ana Maria Gomes, and Manuela Pintado. 2023. "Understanding the Anti-Obesity Potential of an Avocado Oil-Rich Cheese through an In Vitro Co-Culture Intestine Cell Model" Molecules 28, no. 15: 5923. https://doi.org/10.3390/molecules28155923
APA StyleMachado, M., Costa, E. M., Silva, S., Rodriguez-Alcalá, L. M., Gomes, A. M., & Pintado, M. (2023). Understanding the Anti-Obesity Potential of an Avocado Oil-Rich Cheese through an In Vitro Co-Culture Intestine Cell Model. Molecules, 28(15), 5923. https://doi.org/10.3390/molecules28155923












